Page 1

NOTE: Federal (USA) law restricts this device to sale by, or on the order
of, a physician or other appropriately licensed medical professional.
USER’S MANUAL
COGENTIX MEDICAL
Flexible Video Esophagoscope
TNE-5000
and Slide-On® EndoSheath® Technology
www.cogentixmedical.com
Page 2

©2008, 2009, 2011, 2012, 2013, 2015 Cogentix Medical, Inc. All rights
reserved. Printed in the United States of America.
The information contained herein is the exclusive and confidential property of
Cogentix Medical, Inc. No part of this manual may be disclosed or reproduced
in whole or in part without permission from Cogentix Medical, Inc.
Page 3

How to Use This Manual
This User’s Manual contains the recommended procedures for preparing and using
the Cogentix Medical TNE-5000 Flexible Video Esophagoscope and the Slide-On®
EndoSheath® Technology. It is intended for physicians and other medical
personnel who will come in contact with the equipment before, during, and after
any patient procedures performed with it. The manual also contains pertinent
information on the proper care and handling of the endoscope. Please read and
become familiar with this entire manual before using the equipment.
This manual contains the following information:
The endoscope’s intended use
Description of the endoscope and Slide-On
Components and features of the endoscope and peripheral equipment used
in conjunction with the endoscope
Complete instructions on endoscope preparation, inspection, operation,
reprocessing, and storage
Warning and Caution statements that must be observed by endoscope
users to ensure patient and user safety
If you are a first time endoscope user, Cogentix Medical strongly recommends
that you read this manual from beginning to end and become intimately familiar
with the endoscope and its use.
If you are an experienced endoscope user, select specific chapters and/or
sections that pertain to features and procedures that you are using.
®
EndoSheath® Technology
Organization of this Manual
Following is a list of the chapters included in this User’s Manual. Each chapter’s title
is listed at the top of all pages after the title page, so that you can quickly access
the information you need.
Chapter 1, Symbols and Terms
endoscope and peripheral equipment. There is also a brief list of the terms that are
commonly used in the manual.
Chapter 2, Important Information
summary of critical Warning and Caution statements in the manual. This
information is essential to the safe operation and reprocessing of the endoscope.
Cogentix Medical strongly recommends that this chapter be read thoroughly and
completely understood by all users before working with the endoscope.
Chapter 3, Endoscope and Accessories
Video Esophagoscope and compatible peripheral equipment. This chapter includes
instrument diagrams, identifies components, and defines their functions.
Chapter 4, Installing and Removing Slide-On® EndoSheath® Technology
Slide-On® EndoSheath® Technology is a sterile, single-use barrier placed over
– This chapter defines the symbols on the
– The information in this chapter is a
– Introduces the TNE-5000 Flexible
–
TNE-5000 Flexible Video Esophagoscope User’s Manual i
Page 4

How to Use This Manual
the endoscope’s Insertion Tube before the procedure, and removed and discarded
after the procedure is completed. This chapter includes the procedures for installing
the sheath prior to the procedure and removing it when the procedure is over.
Chapter 5, Preparation, Inspection and Operation
how to prepare the endoscope and peripheral equipment for use, and how to
assemble the equipment into a system. The chapter also leads you through a
detailed inspection procedure to confirm that the equipment is undamaged and
working properly before it is used in a procedure.
Chapter 6, Reprocessing
proper cleaning, disinfection, and sterilization of the endoscope before its first use
and after each subsequent use. Strict adherence to the instructions in this chapter
will render the endoscope “patient-ready” for each procedure.
Chapter 7, Care and Storage
prolonged period, refer to this chapter for instructions on safe, secure storage.
Chapter 8, Troubleshooting
encountered with the endoscopic system, and suggests corrective actions to take
towards resolving minor problems.
Chapter 9, Warranty and Service
Cogentix Medical warranty on the endoscope, any restrictions that apply and user
actions that may void the warranty if taken. This chapter also includes shipping
instructions in case the endoscope must be returned to Cogentix Medical for repair.
The
Appendix
Video Esophagoscope and infection control information.
contains the technical specifications for the TNE-5000 Flexible
– This chapter contains important instructions on the
– If the equipment will not be used for a
– Describes possible problems that may be
– This chapter contains the terms of the
– This chapter describes
Additional Information
The information in this User’s Manual is subject to change without notice. If you
have any questions regarding any of the material contained in this manual, or wish
to confirm that this is the most-comprehensive information available for these
products, please contact your local distributor or Cogentix Medical Customer
Service Department at 866 258-2182 (toll-free in U.S.) or (+1) 952-426-6189
(international).
ii TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 5

Table of Contents
How to Use This Manual ....................................................................... i
Organization of this Manual ..................................................................... i
Additional Information ............................................................................ ii
1 Symbols and Terms ........................................................................ 1
Symbols ................................................................................................1
Terms ...................................................................................................2
2 Important Information ................................................................... 3
Intended Use ........................................................................................3
User Qualifications .................................................................................3
Reprocessing .........................................................................................3
Maintenance and Repair .........................................................................4
Signal Words .........................................................................................4
Important Safety Precautions ..................................................................4
Preparation, Inspection, and Assembly ............................................... 4
During Use ....................................................................................... 6
Reprocessing ................................................................................... 7
3 Endoscope and Accessories ............................................................ 9
Inspect the Standard Set ...................................................................... 10
Equipment Diagrams ............................................................................ 11
TNE-5000 Flexible Video Esophagoscope .......................................... 11
TV-2.1 Slide-On® EndoSheath® Technology ...................................... 14
TV-1.5 Slide-On® EndoSheath® Technology ...................................... 16
DPU-5000/7000 Series Digital Video Processor .................................. 18
Installation Stand ........................................................................... 19
Accessories ......................................................................................... 20
Video Processor .............................................................................. 20
Light Sources ................................................................................. 20
Leak Testing .................................................................................. 20
Reprocessing ................................................................................. 20
Therapeutic Accessories .................................................................. 20
4 Installing and Removing the Slide-On® EndoSheath®
Technology ................................................................................... 21
Install the Slide-On® EndoSheath® Technology ...................................... 21
Prepare the Endoscope and Sheath .................................................. 22
TNE-5000 Flexible Video Esophagoscope User’s Manual iii
Page 6

Table of Contents
Insert the Endoscope Into the Sheath .............................................. 22
Connect Tubing / Complete Sheath Attachment ................................ 24
Complete System Assembly ............................................................. 24
Observe the Endoscopic Image ........................................................ 24
Remove the Slide-On® EndoSheath® Technology .................................... 25
5 Preparation, Inspection and Operation ........................................ 27
Preparation and Inspection ................................................................... 27
Select an Installation Site ................................................................ 27
Endoscope Operation ........................................................................... 29
Insufflation .......................................................................................... 30
Suction ............................................................................................... 30
Inserting Accessories ............................................................................ 30
Electrosurgical Devices/Accessories ....................................................... 31
Laser Devices/Accessories .................................................................... 32
6 Reprocessing ................................................................................ 33
Reprocessing Steps .............................................................................. 34
Leak Testing ........................................................................................ 34
Attach the Leak Tester to the Endoscope .......................................... 34
Pressurize the Endoscope ................................................................ 35
Cleaning, Disinfection, and Sterilization .................................................. 37
Use of the Vent Cap ........................................................................ 37
Cleaning After Slide-On® EndoSheath® Technology Usage ................. 37
High-Level Disinfection and Sterilization ................................................. 38
Recommended Disinfection and Sterilization Procedures .................... 38
Acceptable Reprocessing Materials ........................................................ 39
Incompatible Methods .......................................................................... 39
High-Level Disinfection Protocol ............................................................ 40
Pre-Cleaning .................................................................................. 40
Disinfection .................................................................................... 40
Rinsing .......................................................................................... 40
Ethylene Oxide (EtO) Gas Sterilization ................................................... 41
EtO Gas Sterilization Parameters ...................................................... 41
After EtO Gas Sterilization ............................................................... 41
STERRAD® and STERIS® Sterilization ..................................................... 42
7 Care and Storage .......................................................................... 43
Storage ............................................................................................... 43
Disposal .............................................................................................. 44
iv TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 7
Table of Contents
8 Troubleshooting ........................................................................... 45
9 Warranty and Service ................................................................... 53
Warranty Information ........................................................................... 53
Cogentix Medical Service Information .................................................... 53
Shipping to Cogentix Medical ................................................................ 54
Appendix............................................................................................ 55
Specifications TNE-5000 ...................................................................... 55
Infection Control Information ................................................................ 56
Electromagnetic Compatibility Declarations ............................................ 57
Index of Figures
Figure 2-1: Incorrect and Correct Sheath Alignment ...................................... 5
Figure 3-1: TNE-5000 Flexible Video Esophagoscope ................................... 11
Figure 3-2: TNE-5000 Flexible Video Esophagoscope – Control Body ............ 12
Figure 3-3: TV-2.1 Slide-On® EndoSheath® Technology ............................... 14
Figure 3-4: TV-1.5 Slide-On® EndoSheath® Technology ............................... 16
Figure 3-5: DPU-5000/7000 Series Digital Video Processor ........................... 18
Figure 3-6: Installation Stand .................................................................... 19
Figure 4-1: Incorrect and Correct Sheath Alignment .................................... 23
Figure 6-1: Leak Tester Connection ........................................................... 35
Figure 6-2: Opening the Vent Valve ........................................................... 37
Index of Tables
Table 3-1: TNE-5000 Flexible Video Esophagoscope – Components .............. 10
Table 6-1: STERRAD® and STERIS® Validated Systems/Cycles ..................... 42
Table 8-1: Troubleshooting ....................................................................... 46
Table A-1: Specifications ........................................................................... 55
Table A-2: Electromagnetic Emissions Declaration ....................................... 57
Table A-3: Electromagnetic Immunity Declaration ....................................... 58
Table A-4: Electromagnetic Immunity Declaration ....................................... 59
Table A-5: Recommended Separation Distances.......................................... 60
TNE-5000 Flexible Video Esophagoscope User’s Manual v
Page 8

Page 9
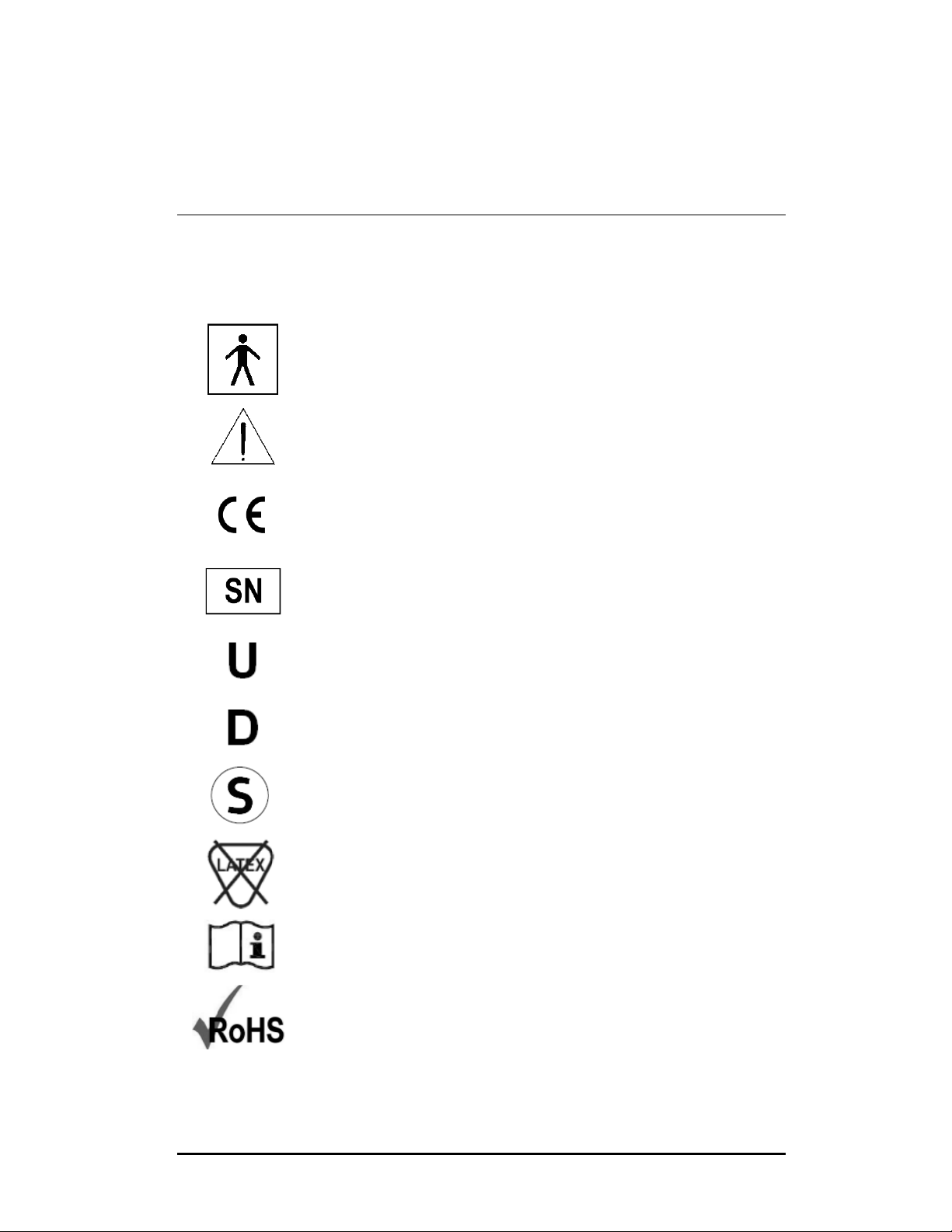
Type BF applied part (Safety degree specified by IEC 60601-1)
Alerts the user to the presence of important operating,
maintenance, and/or service instructions. Refer to the user’s
manuals for warnings and safety precautions associated with
equipment used in the procedure.
The equipment has been designed, tested, and certified as
essentially compliant with all applicable European Union (EU)
regulations and recommendations.
Serial number of the endoscope
Up position for the Angulation Lever
Down position for the Angulation Lever
STERIS® and STERRAD® Reprocessing Compatibility
Refer to Chapter 6, Reprocessing (Endoscope must feature the
○
S symbol for STERIS
®
/ STERRAD® compatibility)
Products do not contain natural rubber latex
Consult Instructions for Use
The presence of this symbol on the product or packaging
indicates that the device is RoHS compliant.
1 Symbols and Terms
Symbols
The symbols listed below can be found on the TNE-5000 Flexible Video
Esophagoscope and on other components of the endoscopic system.
TNE-5000 Flexible Video Esophagoscope User’s Manual 1
Page 10

Symbols and Terms
Terms
The following terms are used throughout this User’s Manual:
“Esophagoscope”, “Endoscope”, or “Videoscope” - refers to the Cogentix
Medical TNE-5000 Flexible Video Esophagoscope.
“Slide-On® EndoSheath® Technology” or “Sheath” refers to the disposable
TV-2.1 or TV-1.5 Slide-On® EndoSheath® Technology for the TNE-5000
Flexible Video Esophagoscope.
“Processor” refers to the DPU-5000/7000 Series Video Processors.
2 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 11

2 Important Information
The information in this chapter is essential for the correct and safe operation of the
TNE-5000 Flexible Video Esophagoscope. Please read and understand this
information before preparing or using the endoscope or any peripheral equipment with
which it will be used.
Intended Use
The Cogentix Medical TNE-5000 Flexible Video Esophagoscope with Slide-On®
EndoSheath® Technology is intended to be used for endoscopic access and
examination of the larynx, esophagus, and gastro-esophageal junction. The equipment
may also be used to assist in intubation.
Do not use the equipment for any purpose other than these intended uses.
User Qualifications
This equipment should only be used in a medical facility by or under the supervision of
a physician trained in endoscopy. Use of the system does not require any deviation
from standard esophagoscopy technique. However, the operator should have
complete familiarity with the operation of the entire system prior to clinical use.
Only use the endoscope and peripheral equipment according to the instructions and
operating conditions described in this manual. Failure to do so could result in
compromised safety, equipment malfunction and/or instrument damage.
For preparation of the device before use, and disassembly and proper cleaning after
use, users should be adequately trained in the proper procedures. Failure to
thoroughly understand these details, such as – but not limited to – EndoSheath®
Technology installation and authorized disinfection protocols, may pose an infection
control risk and/or cause equipment damage.
If training assistance is desired from either the manufacturer or a local distributor,
please contact Cogentix Medical Customer Service at 866 258-2182 (toll-free in U.S.)
or (+1) 952-426-6189 (international).
Reprocessing
The endoscope must be thoroughly cleaned, disinfected, and/or sterilized before its
first use and after each subsequent use. This is the only way to ensure that a “patient-
ready” endoscope is used in every procedure. See Chapter 6, Reprocessing, for
information on all reprocessing equipment and procedures.
TNE-5000 Flexible Video Esophagoscope User’s Manual 3
Page 12

Important Information
Alerts the user to situations which, if not avoided, could result
in death or serious injury.
Alerts the user to situations which, if not avoided, could result in
moderate or minor injury to the user or patient. It is also used to
alert the user to conditions and actions that could cause
equipment damage.
The TNE-5000 and Slide-On® EndoSheath® Technology are
designed to operate as an integrated system. Neither component
can be used independently of the other.
The Slide-On® EndoSheath® Technology is designed for a single
patient use only. Do not reuse or attempt to re-sterilize the
Sheath. For installing the Sheath in a sterile environment, users
should wear two (2) pairs of sterile gloves. Refer to Chapter 4,
Installing and Removing the Slide-On® EndoSheath®
Technology, for complete details.
NOTE: Indicates additional helpful information.
Maintenance and Repair
The endoscope contains no user-serviceable parts; never attempt to modify or repair
it. Doing so may cause further equipment damage and/or compromise patient safety if
the endoscope is subsequently used in a procedure. The endoscope may only be
serviced / repaired at an authorized Cogentix Medical facility.
The endoscope should be thoroughly inspected before each procedure; it should also
be periodically inspected to determine if there is damage or wear that requires
attention.
Signal Words
Information included in this manual to warn users of the possibility of patient injury
and/or equipment damage is signified by the Warning and Caution symbols below.
Warnings, Cautions and Notes will appear throughout this manual; carefully read and
follow all statements.
Important Safety Precautions
The following precautions should always be exercised when using the endoscope and
all medical equipment to ensure safety to all involved parties – user(s), patient(s), etc.
They are summarized here in the order of the stages of the endoscope’s use.
Preparation, Inspection, and Assembly
4 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 13

Important Information
Never drop the equipment or subject it to severe impact, as it could
compromise the functionality and/or safety of the equipment or
system. Should the equipment be mishandled or dropped, do not
use it. Immediately return it to an authorized Cogentix Medical
service facility for inspection and repair.
While the Sheath is being installed, the endoscope should move
freely without the application of force. Should ANY resistance be
encountered, verify that the Sheath’s channel is properly aligned.
Carefully inspect all equipment before using it in a procedure, and
do not use any equipment that is damaged or excessively worn.
Doing so could lead to patient injury and/or further damage to the
equipment.
If inspection reveals difficulty in articulation of the endoscope’s
Distal Bending Section, the endoscope may be damaged. Do not use
a damaged endoscope; doing so could cause patient injury, and
may result in further damage to the endoscope.
If the channel of the sheath is misaligned, straighten out the
channel before continuing scope insertion, as shown in Figure 2-1
below. Also refer to Chapter 8, Troubleshooting, for further
information. If the suggestions given in that chapter do not solve
the alignment or insertion problems, contact your local distributor or
Cogentix Medical Customer Service Department.
NOTE: The Cogentix Medical TNE-5000 Flexible Video Esophagoscope and
Slide-On® EndoSheath® Technology are not made with natural rubber
latex.
MISALIGNED
ALIGNED PROPERLY
TNE-5000 Flexible Video Esophagoscope User’s Manual 5
Figure 2-1: Incorrect and Correct Sheath Alignment
Page 14
Important Information
All devices that are connected to the TNE-5000 Flexible Video
Esophagoscope and DPU-5000/7000 Series Video Processor must be
Classified Medical Equipment. Before using any additional equipment,
confirm that it complies with the appropriate end-product safety standard
(such as IEC 60950-1) and the Standards for Medical Electrical
Equipment, UL 60601-1 or IEC 60601-1, and Safety Requirements for
Medical Electrical Equipment, IEC 60601-1-1.
DO NOT expose the Sheath to alcohol or other cleaning agents prior to
use.
The Working Channel of the TV-2.1 Slide-On® EndoSheath®
Technology accommodates instrumentation indicated by the accessory
manufacturer to be compatible with a 2.1 mm or smaller working
channel. All instrumentation must be tested for compatibility with the
channel prior to clinical use. If assistance is needed to determine
compatibility, contact your local distributor or Cogentix Medical Customer
Service.
DO NOT allow liquids to get inside the Sheath prior to use. Doing so
could damage the Sheath and/or the endoscope.
Do not use this equipment in the presence of a flammable anesthetic
mixture containing air, oxygen or nitrous oxide. There is a possibility of
fire or explosion.
If any of the components of the endoscopic system malfunction during
the procedure, or if the endoscopic image is lost or compromised,
immediately move the endoscope’s Distal Bending Section to the neutral
position and slowly withdraw the endoscope from the patient. Using an
endoscope that is not functioning properly could cause patient injury
and/or further damage to the equipment.
Always wear appropriate personal protective equipment when using the
endoscope and/or sheath, such as a gown, gloves, and face and eye
shields.
Avoid excessive bending or twisting of the endoscope’s Insertion Tube
and Videoscope Cable. Although they are designed to bend, excessive
bending can damage the fiber bundles and internal components. Should
the endoscope develop a severe kink or bend, do not attempt to
straighten the Insertion Tube. Contact Cogentix Medical Customer
Service for assistance.
Do not apply excessive pressure to the endoscope’s Angulation Lever, as
it could damage the endoscope and lead to patient injury.
Do not look directly at the intense light emitted from the endoscope tip
to avoid the possibility of eye injury.
During Use
6 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 15
The endoscope must be properly reprocessed, by cleaning,
disinfecting and/or sterilizing, before its first use and after each
subsequent use. Using an endoscope in a procedure that has not
been properly reprocessed presents an acute infection-control risk to
both the patient and medical personnel performing or assisting in the
procedure.
Always wear appropriate personal protective equipment when
reprocessing the endoscope, such as a gown, gloves, and face and
eye shields.
Use extreme care when reprocessing the endoscope. Do not
forcefully pull, push, or drag wipes, towels, or cloths along the
Insertion Tube. The use of excessive force could damage the
endoscope.
DO NOT immerse the endoscope in disinfectant solution for long
periods of time (>1 hour). Prolonged immersions may damage the
outer coverings of the endoscope and allow fluid infiltration.
DO NOT place the endoscope in or near contaminated areas after
it has been reprocessed. Doing so can recontaminate the
endoscope and require reprocessing to be repeated.
DO NOT place the endoscope in awkward or confining areas
between procedures as this could result in equipment damage.
Reprocessing
Important Information
If resistance is encountered when inserting an instrument into the
Sheath, do not force it, as it could damage the instrument, the
endoscope and/or the Slide-On® EndoSheath® Technology.
A thorough understanding of the principles and techniques involved
in laser, electrosurgical and ultrasonic procedures is essential to
avoid shock and burn hazards to both patient and medical personnel
and damage to the device and other medical instruments. Ensure
that insulation or grounding is not compromised.
TNE-5000 Flexible Video Esophagoscope User’s Manual 7
Page 16

Page 17

3 Endoscope and Accessories
The Cogentix Medical TNE-5000 Flexible Video Esophagoscope and Slide-On®
EndoSheath® Technology are designed to perform safe, sterile, and efficient
esophagoscopy procedures.
The two major components of the endoscopic system are:
The TNE-5000 Flexible Video Esophagoscope, which is shown in Figures 3-1
and 3-2 on pages 11 and 12. The endoscope’s Insertion Tube has no working
channel; it contains the video camera module and illumination bundles.
The disposable Slide-On
disposable sheaths are installed over the endoscope’s Insertion Tube, acting
as a protective barrier to protect patients and users from the spread of
potentially pathogenic materials. The sheaths also contain working channels
through which insufflation, suction, and/or accessory equipment can be used.
There are two types of sheaths available for use with the TNE-5000:
The TV-2.1 Slide-On
14). This Sheath contains a 2.1mm (6.3Fr) channel through which air
insufflation and suction can be performed, or accessory instruments inserted.
The TV-1.5 Slide-On
page 16) has a 1.5mm (4.5Fr) channel that supports insufflation and
suction, but cannot accommodate the insertion of accessory devices.
®
EndoSheath® Technology. The sterile,
®
EndoSheath® Technology (see Figure 3-3 on page
®
EndoSheath® Technology (see Figure 3-4 on
TNE-5000 Flexible Video Esophagoscope User’s Manual 9
Page 18
Endoscope and Accessories
TNE-5000 ESOPHAGOSCOPE STANDARD SET
COGENTIX MEDICAL
CATALOG NO.
DESCRIPTION
03-5201
OR
TNE-5000 Flexible Video Esophagoscope, NTSC
03-5202
TNE-5000 Flexible Video Esophagoscope, PAL
07-6182
Cogentix Medical TNE-5000 Carrying Case
07-6015
Vent Cap
TNE-5000 User’s Manual (this document)
VIDEO PROCESSORS (NOT SHIPPED WITH ENDOSCOPE)
07-5050
DPU-5050 Video Processor with LCD Display*
07-5051
DPU-5050A Video Processor with Air Pump and LCD
Display*
07-7001
DPU-7000A Video Processor with LCD Display
OPTIONAL ITEMS AND ACCESSORIES (NOT SHIPPED WITH ENDOSCOPE)
07-6160
Installation Stand, Floor Model
07-6161
Installation Stand, Clamp Model
07-6162
Installation Stand, Wall Mount
07-6010
Videoscope Leak Tester
ENDOSHEATH
®
TECHNOLOGY
03-5101
TV-2.1 Slide-On® EndoSheath® Technology with
2.1mm (6.3Fr) Working Channel for the TNE-5000
Flexible Video Esophagoscope
03-5102
TV-1.5 Slide-On® EndoSheath® Technology with
1.5mm (4.5Fr) Air/Suction Channel for the TNE-5000
Flexible Video Esophagoscope
Do not use any equipment that is observed to be damaged or
excessively worn. Doing so could lead to patient injury and/or further
damage to the equipment.
Inspect the Standard Set
When the endoscope is received from Cogentix Medical, immediately confirm that all
of the ordered items listed in Table 3-1 have been shipped, and inspect them for
damage. If any item is missing or damaged, do not use the endoscope. Contact
Cogentix Medical to obtain replacement parts.
Table 3-1: TNE-5000 Flexible Video Esophagoscope – Components
*Available in select markets only
10 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 19
Equipment Diagrams
TNE-5000 Flexible Video Esophagoscope
Endoscope and Accessories
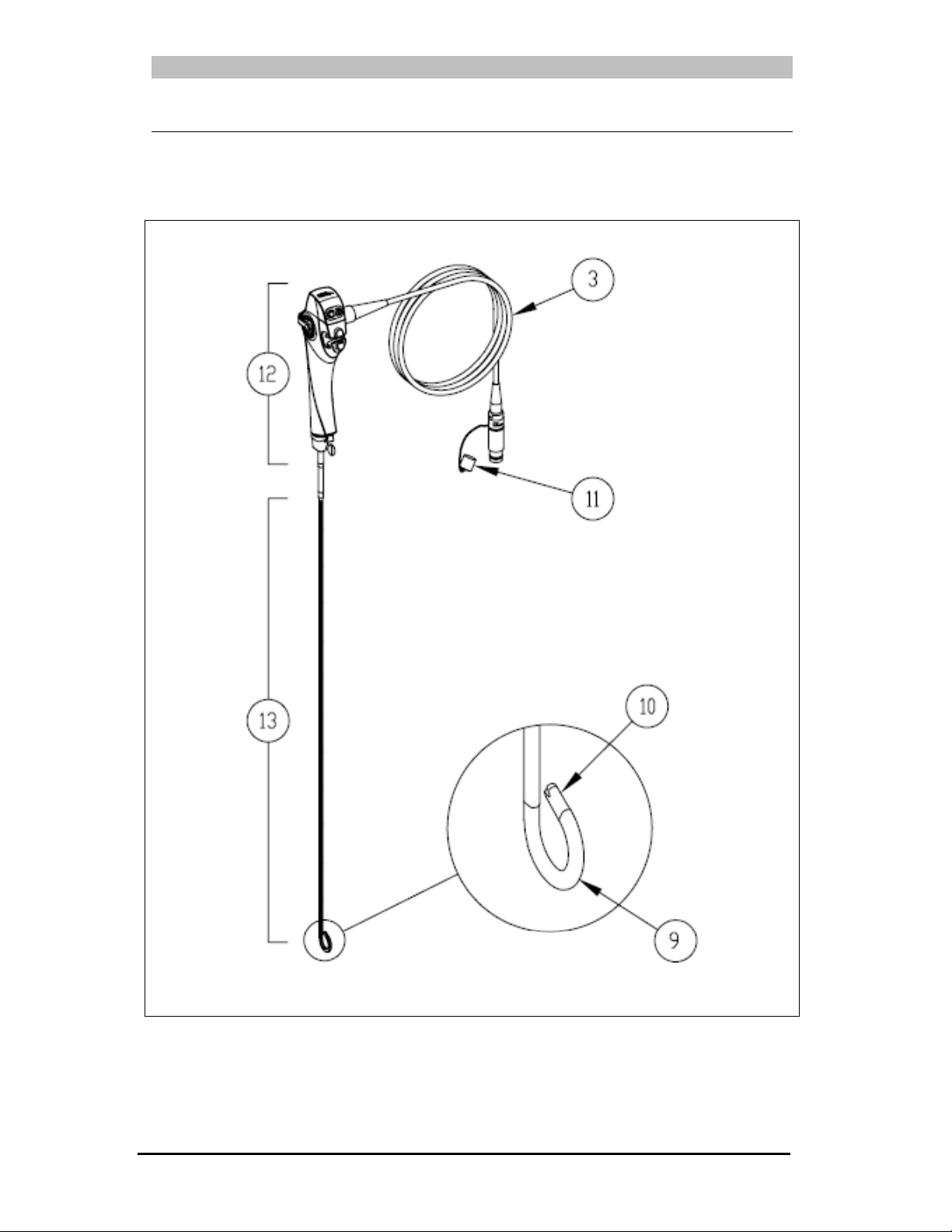
Figure 3-1: TNE-5000 Flexible Video Esophagoscope
TNE-5000 Flexible Video Esophagoscope User’s Manual 11
Page 20
Endoscope and Accessories
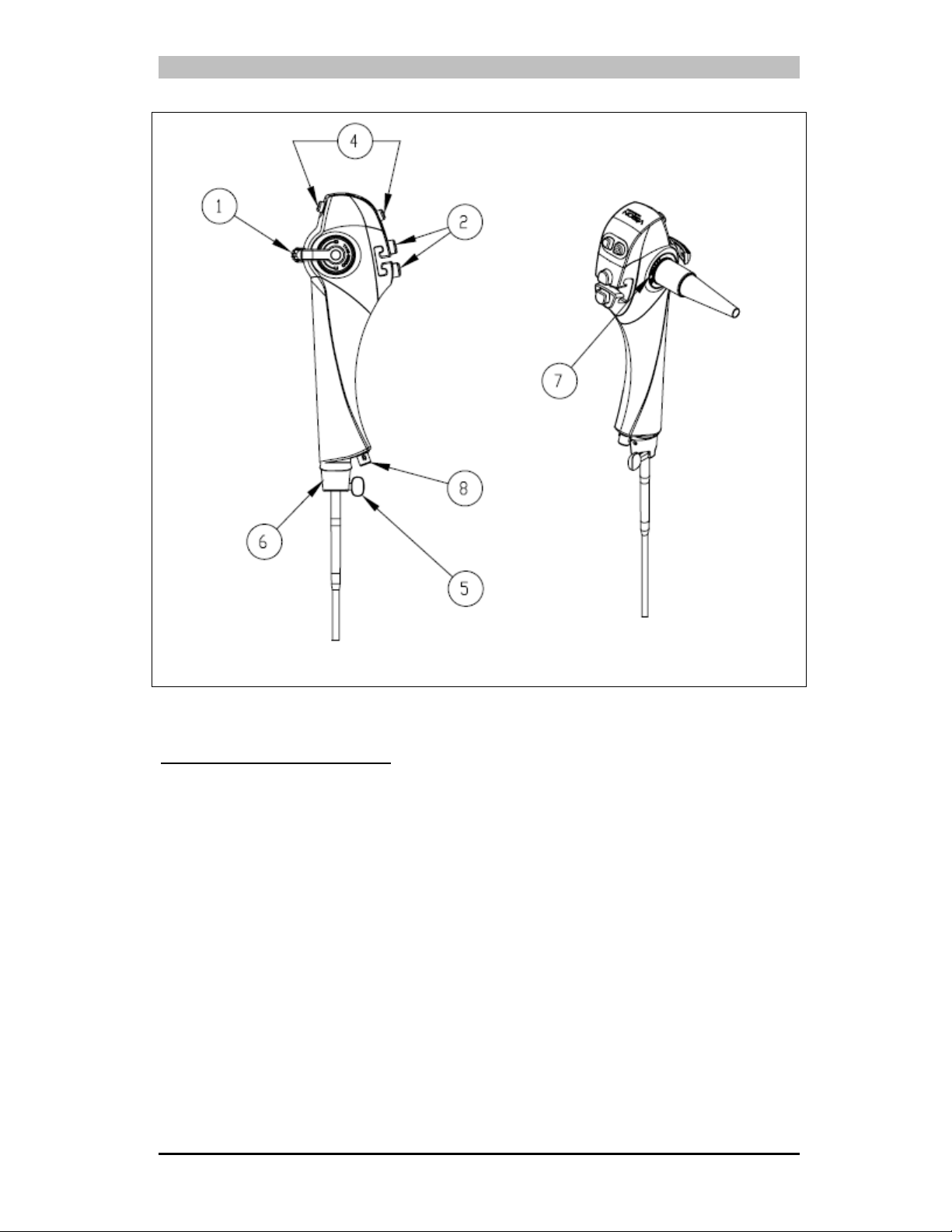
Figure 3-2: TNE-5000 Flexible Video Esophagoscope – Control Body
Instrument Components
1. Angulation Lever: Controls deflection of the endoscope’s Distal Bending Section.
2. Dual Pinch Valves: Attach the Insufflation/Suction Tubing to the valves to
regulate insufflation and suction during use.
3. Videoscope Cable: The connector (plug) at the end of the cable connects to the
DPU-5000/7000 Series Digital Video Processor.
4. Control Buttons: Four programmable function buttons which allow the user to
activate different functions of the video system. Consult the DPU-5000/7000
Series Video Processor User’s Manual for instructions regarding the control
functions and how to program the buttons.
5. Locking Knob: Mates with the EndoSheath
On® EndoSheath® Technology to the endoscope.
®
Rigid Connector to secure the Slide-
12 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 21

Endoscope and Accessories
6. EndoSheath Interface: Secures the disposable EndoSheath
®
cover to the
endoscope body.
7. Identification Ring: Includes the Serial Number, which is a unique number
identifying the endoscope; and the ○S symbol, which indicates the endoscope can
be sterilized using a validated STERIS® or STERRAD® system. The endoscope must
feature this symbol on the Identification Ring in order for STERIS® / STERRAD®
compatibility to apply.
8. Vent Valve: When the Vent Cap is connected, this valve allows access to the
interior of the endoscope for EtO and STERRAD® gas sterilization, and should be
connected during transport. The Vent Cap must be attached to the valve prior to
EtO and STERRAD® gas sterilization and prior to shipping. The valve is also used as
a Leak Tester Connector for Leak Testing.
9. Distal Bending Section: Deflects up and down when the Angulation Lever is
actuated.
10. Distal Tip: The terminating point of the video camera module and the light guide
fiber bundles [Light Guides].
11. Sealing Cap: Seals the plug prior to soaking for leak testing or disinfection.
12. Control Body: This segment allows physician control over endoscopic functions.
13. Insertion Tube: This component, along with the Distal Tip, is the part of the
endoscope that is inserted into the patient.
TNE-5000 Flexible Video Esophagoscope User’s Manual 13
Page 22

Endoscope and Accessories
TV-2.1 Slide-On® EndoSheath® Technology
The TV-2.1 Slide-On® EndoSheath® Technology shown in Figure 3-3 is a sterile,
disposable barrier for the Cogentix Medical TNE-5000 Flexible Video Esophagoscope.
The Sheath isolates the endoscope from contact with patient fluid and material during
the procedure.
Figure 3-3: TV-2.1 Slide-On® EndoSheath® Technology
1. Control Body Cover: Covers and protects the endoscope’s Control Body from
contaminants during the procedure.
2. Slot for Locking Knob: Slides past the Locking Knob to secure the Sheath to
the endoscope.
3. Accessory Port with Removable Seal: Allows rapid fluid injection and
passage of accessories during procedures. A removable Accessory Port Valve
Seal allows for secure Luer-Lock syringe attachment for fluid withdrawal.
4. EndoSheath
5. Working Channel: A solid, impermeable tubing that runs the length of the
Sheath, allowing for maximum air insufflation and suction while also allowing the
passage of accessory devices.
6. Insertion Tube Barrier: A flexible elastomeric sleeve that creates a barrier
covering for the endoscope’s Insertion Tube.
®
Connector: Secures the Sheath to the endoscope.
14 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 23

Endoscope and Accessories
7. Sheath Window: A patented, optically clear window that covers the Distal Tip
of the endoscope.
8. Protector Tube: Protects the Sheath from external damage during shipping,
storage, and Sheath loading.
9. Control Body Cover Clips (2): Secure the Drape Bag on the Installation
Stand, and secure the Control Body Cover to the Videoscope Cable and
Suction/Insufflation Tubes.
10. Air Insufflation Tube: Provides air to the endoscope’s Distal Tip for insufflation
during the procedure.
11. Luer Lock/Air Connector: A standard Luer-Lock Fitting Cap to couple with the
Air Pump Unit’s tubing.
12. Suction Tube: Passes aspirated fluids out to a vacuum source.
13. Suction Tube Connector: Connect suction tubing from a vacuum source here.
14. Drape Bag: Covers the Installation Stand to prevent contamination. The Drape
Bag can also be used to conveniently dispose of the Sheath.
TNE-5000 Flexible Video Esophagoscope User’s Manual 15
Page 24

Endoscope and Accessories
TV-1.5 Slide-On® EndoSheath® Technology
The TV-1.5 Slide-On® EndoSheath® Technology shown in Figure 3-4 is a sterile,
disposable barrier for the Cogentix Medical TNE-5000 Flexible Video Esophagoscope.
The Sheath isolates the endoscope from contact with patient fluid and material during
the procedure, and supports air insufflation and suction.
Figure 3-4: TV-1.5 Slide-On® EndoSheath® Technology
1. Slot for Locking Knob: Slides past the Locking Knob to secure the Sheath to
the endoscope.
2. Sealed Accessory Port: On the TV-1.5 Slide-On
®
EndoSheath®
Technology, the Accessory Port is sealed because standard accessory tools
cannot be passed through the channel.
3. EndoSheath
®
Connector: Secures the Sheath to the endoscope.
4. Insufflation Channel: A 1.5mm channel that supports air insufflation and fluid
aspiration. This channel does not support the passage of accessory tools.
5. Insertion Tube Barrier: A flexible, elastomeric sleeve that creates a barrier
covering for the endoscope’s Insertion Tube.
6. Sheath Window: A patented, optically clear window that covers the Distal Tip
of the endoscope.
16 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 25

Endoscope and Accessories
7. Protector Tube: Protects the Sheath from external damage during shipping,
storage, and Sheath loading.
8. Air Insufflation Tube: Provides air to the endoscope’s Distal Tip for insufflation
during the procedure.
9. Luer Lock/Air Connector: A standard Luer-Lock Fitting Cap to couple with the
Air Pump Unit’s tubing.
10. Suction Tube: Passes aspirated fluids out to a vacuum source.
11. Suction Tube Connector: Connect suction tubing from a vacuum source here.
12. Twist-Lock Clips (2): Secures the Suction/Insufflation Tubing to the
endoscope’s Videoscope Cable.
13. Drape Bag: Covers the Installation Stand to prevent contamination. The Drape
Bag can also be used to conveniently dispose of the Sheath.
TNE-5000 Flexible Video Esophagoscope User’s Manual 17
Page 26

Endoscope and Accessories
The TNE-5000 Flexible Video Esophagoscope is not compatible with
any other manufacturers’ video processors. Attempting to connect the
endoscope to or use it in conjunction with another manufacturer’s
video processor could cause damage to the endoscope and/or the
video processor.
DPU-5000/7000 Series Digital Video Processor
The TNE-5000 Flexible Video Esophagoscope must be used in conjunction with a
DPU-5000/7000 Series Video Processor (shown in Figure 3-5). Refer to the DPU5000/7000 Series User’s Manual for complete instructions on the operation of the
unit.
Figure 3-5: DPU-5000/7000 Series Digital Video Processor
18 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 27

Endoscope and Accessories
Installation and removal of the Slide-On® EndoSheath®
Technology onto and from the Esophagoscope should always be
performed using the Installation Stand. Failure to do so may cause
difficulty in installation and/or equipment damage.
Installation Stand
Refer to Figure 3-6 below and to the Installation Stand’s Instructions-for-Use for
information on the correct preparation and use of the stand.
Figure 3-6: Installation Stand
1. EndoSheath
Connector, which is designed to fit with its Accessory Port facing out.
2. Endoscope Storage Slots: Post-procedure slots for hanging endoscopes (not
intended for long-term storage unless the Installation Stand is wall-mounted).
3. Cable Storage Slots: For securing the Videoscope Cable when the endoscope
is being stored.
4. Wall-Mounting Bracket/Holes: For mounting the Installation Stand on a wall
or inside a cabinet for storage purposes.
®
Slot/Holder: The slot that securely holds the EndoSheath®
TNE-5000 Flexible Video Esophagoscope User’s Manual 19
Page 28

Endoscope and Accessories
Do not use any accessories that are not in compliance with the
equivalent safety requirements of this equipment. Doing so may
reduce the operational safety of the system and could cause
patient and/or user injury. For all accessories, confirm that safety
certifications have been performed in accordance with the
appropriate standard (IEC 60601-1 and/or IEC 60601-1-1).
The use of accessories not specified in this manual or sold by
Cogentix Medical may result in increased electromagnetic
emissions or decreased immunity of the equipment or system.
Accessories
Video Processor
The TNE-5000 Flexible Video Esophagoscope is designed to work with the
Cogentix Medical DPU-5000/7000 Series Video Processors. The endoscope is not
compatible with any other manufacturers’ video processors.
Light Sources
The TNE-5000 Flexible Video Esophagoscope has an integrated, solid-state light
source which is controlled by the DPU-5000/7000 Series Video Processors. No
external light source is required for the TNE-5000 Flexible Video Esophagoscope.
Leak Testing
The TNE-5000 Flexible Video Esophagoscope may only be leak tested with a
Cogentix Medical V1 Endoscope Leak Tester.
Reprocessing
The TNE-5000 Flexible Video Esophagoscope may be reprocessed by a variety of
methods. Refer to Chapter 6, Reprocessing, for the accessories that will be used
when reprocessing the endoscope. Contact Cogentix Medical Customer Service for
advice on compatibility issues.
Therapeutic Accessories
The TV-2.1 Slide-On® EndoSheath® Technology features a 2.1mm working
channel for the passage of therapeutic tools. Laryngoscopy tools and accessories that
are compatible with a 2.1mm channel should be compatible with this system.
The TV-1.5 Slide-On® EndoSheath® Technology has a 1.5mm Insufflation/ Suction
Channel which supports aspiration and air insufflation, but does not support the
passage of accessory tools.
For further information regarding Tools and Accessories, please contact your local
distributor or Cogentix Medical Customer Service Department.
20 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 29

Installation and removal of the Slide-On® EndoSheath®
Technology onto and from the Esophagoscope should always be
performed with the Installation Stand. Failure to utilize the
Installation Stand for setup and preparation may lead to
difficulty in installation and ultimately equipment damage.
Ensure that the endoscope is clean and dry prior to installing the
Slide-On® EndoSheath® Technology. Any moisture on the
endoscope prior to installation may result in difficulty removing the
endoscope from the Slide-On® EndoSheath® Technology after
the procedure.
Wear appropriate protective gear when using the Slide-On®
EndoSheath® Technology and Esophagoscope, including gown,
gloves, and face and eye shields. To maintain a sterile field during
installation, it is recommended that users wear two (2) pairs of
sterile gloves.
Before installing the Sheath, carefully inspect the endoscope’s
Insertion Tube for any damage or defects. If any irregularities are
found, do not use the endoscope. Using a damaged or defective
endoscope could damage the Sheath, cause further damage to
the endoscope itself, and/or cause patient or user injury.
The Slide-On® EndoSheath® Technology is supplied sterile,
and is intended for a single use only. Do not reuse or attempt to
re-sterilize the Sheath, as it could become damaged, which could
in turn cause damage to the endoscope and/or present an
infection-control risk to the patient and/or user.
Do not expose the interior or exterior of the Sheath to alcohol or
other cleaning agents prior to use.
NOTE: Refer to Instructions for Use for TV-2.1 and TV-1.5 Sheaths.
4 Installing and Removing the
Slide-On® EndoSheath®
Technology
The endoscope and Sheath do not have any user-serviceable parts.
Do not attempt any repairs. If malfunction occurs, refer to the
Troubleshooting section of this manual or call Cogentix Medical for
assistance.
Install the Slide-On® EndoSheath® Technology
TNE-5000 Flexible Video Esophagoscope User’s Manual 21
Page 30

Installing and Removing the Slide-On® EndoSheath® Technology
Do not use this equipment in the presence of a flammable
anesthetic mixture containing air, oxygen or nitrous oxide.
There is a possibility of fire or explosion.
Prepare the Endoscope and Sheath
1. Before installing the Sheath, clean the endoscope’s lens on the Distal Tip with an
alcohol prep pad. Do not use abrasive materials to clean any part of the endoscope,
particularly the lens at the Distal Tip of the scope. Doing so could damage the lens
and impair the endoscope’s imaging capability.
2. Connect the TNE-5000 video cable to the DPU-5000/7000 Series video
processor, and turn the processor on.
3. Check the EndoSheath
4. Put on two pairs of sterile gloves.
5. Open the Sheath’s package carefully, remove the plastic Drape Bag, unfold the
Drape Bag, remove adhesive strip backing, and secure the Drape Bag to the
Installation Stand with a plastic clip if necessary.
6. Remove the Sheath from the package. The Sheath should remain in the Protector
Tube during installation.
7. Remove the two Control Body Cover Clips from the package and place them on a
clean surface.
8. Place the Sheath into the Installation Stand with the Accessory Port facing outward
(towards you). Note: the sheath fits into the stand in only one direction.
9. Fold back the Control Body Cover to expose the top opening of the EndoSheath
Connector.
®
disposable packaging for defects or damage.
®
Insert the Endoscope Into the Sheath
10. Take the endoscope in hand; make sure that the Distal Bending section of the
endoscope is straight.
11. Hold the endoscope vertically above the sheath. Align the endoscope’s D-shaped
Insertion Tube with the D-shaped opening of the EndoSheath® Connector – the
flat portion of the Insertion Tube should face toward the Sheath’s
Accessory Port (toward you).
12. Gently slide the endoscope’s Insertion Tube into the Sheath. Keep the Insertion
Tube as straight as possible, aligned with the shape of the insertion hole. DO NOT
TWIST the insertion tube during insertion, doing so will cause resistance.
IMPORTANT NOTE: If there is resistance in loading the endoscope into the
sheath, remove the protective tube to verify that the sheath channel is properly
aligned. If the channel is misaligned, straighten channel and continue scope
insertion.
22 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 31

Installing and Removing the Slide-On® EndoSheath® Technology
When inserting the endoscope into the Sheath, avoid excessive
bending of the Insertion Tube, as it could damage the Sheath and/or
the endoscope.
Do not apply excessive force to install the Sheath onto the
endoscope if insertion is found to be difficult. If you experience
difficulty installing the Sheath, refer to Chapter 8, Trouble-
shooting. If the recommended actions given there do not ease
endoscope insertion, call your local distributor or Cogentix Medical
Customer Service at 866 258-2182 (toll-free in U.S.) or
(+1) 952-426-6189 (international) for further instruction.
NOTE: It is extremely important to maintain the alignment
between the flat portion of the Insertion Tube and the Biopsy Port
on the EndoSheath® Connector during installation and removal. If
there is resistance in loading the endoscope into the Sheath,
remove the protective tube to verify proper alignment of the
channel. If the channel is misaligned, straighten the channel and
continue endoscope insertion.
MISALIGNED
ALIGNED PROPERLY
Figure 4-1: Incorrect and Correct Sheath Alignment
13. Make sure the endoscope’s Locking Knob is in a vertical position.
14. Align the Locking Knob on the endoscope with the vertical slot on the EndoSheath
Connector, and continue to slide the endoscope into the Sheath until the Locking
Knob is fully seated at the base of the slot.
15. Rotate the Locking Knob on the endoscope to a horizontal position. This will ensure
a secure fit between the EndoSheath® Connector and the endoscope.
TNE-5000 Flexible Video Esophagoscope User’s Manual 23
®
Page 32

Installing and Removing the Slide-On® EndoSheath® Technology
Connect Tubing / Complete Sheath Attachment
16. Connect the Air Insufflation Tubing to the front of the Pinch Valve Mechanism by
pressing the front button and stretching the tubing against the tubing slot. When
the tubing is seated in the valve, release the button. This will squeeze the Air
Insufflation Channel closed.
17. Connect the Air Insufflation Tubing to the Cogentix Medical Air Source.
18. Connect the Suction Tubing to the scope’s Pinch Valve Mechanism by pressing the
button and stretching the tubing against the tubing slot. When the tubing is seated
in the valve, release the button. This will squeeze the Suction Channel closed.
19. Connect the Suction Tubing to the external suction source.
20. Remove outer pair of gloves.
Complete System Assembly
21. Pull the Control Body Cover up over the endoscope’s Control Body.
22. Secure the Control Body Cover, Air Insufflation Tubing and Suction Tubing to the
videoscope cable using the Cover Clips.
23. Press the Pinch Valve to turn on and off; confirm that it operates properly.
24. Carefully remove the Sheath from the protective tube and discard the tube. DO
25. Visually inspect the window of the Sheath to confirm that the endoscope’s Distal Tip
26. Turn on the external Air Source and Suction Source. Verify systems are functioning
27. The system is ready for use.
NOT reinstall the protective tube onto the Sheath, or damage to the Sheath may
result.
is flush with the Sheath’s window; if a gap is observed between the Distal Tip and
the Sheath’s window, move the angulation lever to articulate the Distal Bending
Section. This process should properly seat the Sheath. In some cases, it may be
necessary to hold the edge of the optical window (while wearing gloves) and move
it gently to fully seat the Sheath. If the Sheath window is not fully seated, the
image may be impaired.
CAUTION: DO NOT articulate the Bending Section while the protective tube is on.
properly.
Observe the Endoscopic Image
The Sheath’s window should now be in direct contact with the Insertion Tube’s Distal
Tip. To confirm this, view an endoscopic image with illumination on; there should be no
glare.
If a gap is observed between the Distal Tip and the Sheath’s window, or if glare is
observed in the endoscopic image, move the Angulation Lever up and down several
times to articulate the Distal Bending Section. This process should properly seat the
Sheath. In some cases, it may be necessary to hold the edge of the optical window
(while wearing gloves) and move it gently to fully seat the Sheath.
24 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 33

Installing and Removing the Slide-On® EndoSheath® Technology
Wear appropriate personal protective equipment when removing the
Sheath from the endoscope, to prevent the possibility of infection
from contact with patient material. Cogentix Medical strongly
recommends that a double set of gloves be worn for this procedure.
Always use the Installation Stand to remove the endoscope from the
Sheath. Attempting to remove the endoscope without using the
Installation Stand could cause damage to the endoscope.
Remove the Slide-On® EndoSheath® Technology
When the endoscopic procedure is complete, the Slide-On® EndoSheath®
Technology must be removed and disposed of properly to eliminate the possibility of
infection-control risks.
1. Put on two (2) pairs of sterile gloves, and assure the Drape Bag is completely
covering the Installation Stand.
2. Slide the sheathed endoscope into the draped Installation Stand (port facing
you).
3. Disconnect the Air Insufflation Luer from the Air Source Tubing before
disconnecting the Suction Tubing.
4. Disconnect the Suction Connector from the Suction Source.
5. Remove the Control Body Cover Clips and discard them. Keeping your hands on
the outside of the contaminated Control Body Cover, pull the Cover forward and
down off of the endoscope.
6. Remove the outer set of gloves. Do not handle the endoscope’s Control Body
with contaminated gloves.
7. Remove the Air Tube from the Pinch Valve Mechanism by pressing the front
button and pulling the tubing free.
8. Remove the Suction Tubing from the Pinch Valve Mechanism by pressing the
back button and pulling the tubing free.
9. Rotate the Locking Knob into the vertical position so that it is aligned with the
slot on the EndoSheath® Connector.
10. Using the angulation lever, articulate the Distal Bending Section of the
endoscope into the straight/neutral position.
11. Ensure the working channel is not wrapped around the insertion tube by
straightening the channel with gloved fingers.
12. Hold the endoscope’s Control Body in one hand. Slowly and gently withdraw the
scope from the Sheath. If resistance is encountered, use the hanging outside
portion of the Drape Bag as a barrier between your fingers and the Sheath, and
gently grasp the Sheath’s window. Then slowly and gently continue to withdraw
the endoscope from the Sheath.
TNE-5000 Flexible Video Esophagoscope User’s Manual 25
Page 34

Installing and Removing the Slide-On® EndoSheath® Technology
Do not rotate the endoscope when removing it from the Sheath.
Doing so can damage the Insertion Tube.
If you experience difficulty removing the endoscope from the
Sheath, do not use excessive force in trying to remove it. Refer to
Chapter 8, Troubleshooting, for further instructions.
13. Place the endoscope in a non-contaminated area. Power off the Video Processor,
Air Source and Suction Pump.
14. Inspect the Insertion Tube and Distal Tip, and confirm that these areas are dry.
If moisture is observed, there may have been a leak into the Sheath during the
procedure, providing that the endoscope was dry when the Sheath was attached.
In this case, the endoscope must be high-level disinfected and/or sterilized
following the instructions given in Chapter 6, Reprocessing.
15. Collect the contaminated Sheath in the Drape Bag and remove it from the
Installation Stand. Carefully discard the Drape Bag and Sheath per
hospital/facility policy.
16. Proceed to the Recommended Cleaning Procedure detailed in Chapter 6,
Reprocessing, to prepare the endoscope for the next procedure.
26 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 35

If an abnormality is detected during endoscope preparation, do not
use the equipment; refer to the tables in Chapter 8,
Troubleshooting. If the problem cannot be solved using the
information in that chapter, contact your Regional Distributor,
Territory Manager, or Cogentix Medical Customer Service.
When using the Slide-On® EndoSheath® Technology with the endoscope, refer
to the instructions-for-use that are shipped with the system. These instructions will
provide complete details on preparing, installing and removing the disposable
Sheath.
During the procedure, the temperature at the distal end of endoscope may exceed
41oC (106
o
F) due to the intense endoscopic illumination. Surface temperatures
over 41oC (106
o
F) may cause mucosal burns. Always use the minimum level of
illumination necessary for adequate viewing. Whenever possible, avoid close
stationary viewing and do not leave the distal end of the endoscope in close
proximity to mucous membranes for a long time.
The Slide-On® EndoSheath® Technology is shipped sterile and intended for a
single use only; do not reuse it. When the procedure is complete, remove the
Sheath from the endoscope and dispose of it. Reusing the Sheath can damage it,
and in turn cause endoscope damage. In addition, a reused Sheath presents an
acute infection-control risk to the next patient.
A complete review and understanding of the DPU-5000/7000 Series User’s
Manual is recommended before using the TNE-5000 Flexible Video
Esophagoscope.
5 Preparation, Inspection and
Operation
Preparation and Inspection
Follow the inspection steps listed below before connecting any equipment or using the
system. Do not use the equipment if abnormalities are detected:
Select an Installation Site
It is important to select an appropriate location in which to install the Video Processor.
Place the Video Processor on a stable rigid surface such as a cart, counter-top,
or solid stand.
The location must not contain explosive or flammable gases.
TNE-5000 Flexible Video Esophagoscope User’s Manual 27
Page 36

Preparation, Inspection and Operation
Do not apply excessive pressure to the endoscope’s Insertion Tube.
Doing so can damage the internal components of the Insertion
Tube.
Avoid applying excessive pressure when using the Angulation Lever.
Doing so could damage the angulation mechanism.
Do not use abrasive materials to clean the Lens. Doing so could
damage the Lens and impair the endoscope’s imaging capability.
Turn off the Video Processor’s power switch before connecting or
disconnecting the Videoscope Cable. Connecting or disconnecting
the cable with the power on could damage both the endoscope and
the Video Processor.
Place the TNE-5000 Videoscope and Video Processor away from radios,
televisions, cell phones, or any other devices that emit electromagnetic
energy. These can interfere with proper operation. Avoid stacking the
videoscope or the Video Processor on other equipment to avoid possible
electromagnetic interference.
Place the Video Processor in a dry place, and avoid contact with liquids.
Do not allow the Video Processor’s vents to be obstructed; full ventilation is
necessary for proper operation. Vents are located on the bottom and back of
the unit.
1. Check the Insertion Tube for holes, superficial cuts, or abrasions.
2. Lightly run your fingertips over the entire length of the Insertion Tube to confirm
that it is smooth and does not exhibit looseness or bagging.
3. Check for full Distal Tip deflection by actuating the Angulation Lever up and down.
4. Clean the Lens on the endoscope’s Distal Tip with an alcohol prep pad.
5. The DPU-5000/7000 Series Video Processor should be powered on and ready for
the procedure.
6. If not already connected, connect the Air Pump Unit’s tubing to the Sheath’s Air
Tube. Turn on the Air Pump, press the Air Flow Button on the endoscope’s Control
Body, and confirm that air flows from the nozzle at the Distal Tip.
7. If not already connected, connect the Suction Tubing to the Suction Pump. Turn on
the Suction Pump, press the Suction Button, and confirm that there is suction at the
Distal Tip. If the tubing is not seated properly in the valve, suction will not be
available.
28 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 37

Preparation, Inspection and Operation
Avoid excessive bending or twisting of the endoscope’s insertion
tube, particularly at the distal end. While the tube is designed to
bend, excessive pressure can damage the fiber bundles and internal
components.
Excessive angulation or excessive pressure placed on the Angulation
Lever may cause equipment damage. Do not exert force to move
the lever beyond its natural limits.
NOTE: The four (4) buttons on the endoscope’s Control Body may be
programmed on the Video Processor to perform designated image-control
functions. These functions include:
Image Freeze
Image Capture
Image Enhancement
Remote Activation (Copy/Print)
Gain
Refer to the DPU-5000/7000 Series Video Processor User’s Manual for
further information on available control functions and instructions on
programming the Control Buttons.
Endoscope Operation
1. Hold the endoscope so that the Control Body fits comfortably in your hand, allowing
easy manipulation of the Angulation Lever. The other hand is free to manipulate the
Insertion Tube and pass Accessory Devices.
2. The DPU-5000/7000 Series Digital Video Processor should be on; adjust the
settings to the desired level using the Processor’s controls.
3. Perform the White Balance procedure.
4. Prepare the patient using normally acceptable clinical practice prior to endoscope
insertion.
5. If the endoscope will be inserted into the patient’s mouth, first place a bite block
into the patient’s mouth to prevent bite damage.
6. Lubricate the outside of the Sheath before inserting the endoscope into the patient.
Cogentix Medical recommends that the Sheath be lubricated with water or a waterbased lubricant just prior to insertion.
7. Introduce the sheathed endoscope into the patient using normally acceptable clinical
practice. Operate the Angulation Lever as necessary for advancement and
observation.
8. When using the system through an endotrachial tube (for intubation assistance), it
is extremely important to maintain proper alignment between the tube and the
endoscope’s Distal Bending Section.
TNE-5000 Flexible Video Esophagoscope User’s Manual 29
Page 38

Preparation, Inspection and Operation
This section only applies to the TV-2.1 Slide-On® EndoSheath®
Technology. The TV-1.5 Slide-On® EndoSheath® Technology
does not support tool passage.
Do not continue advancing an accessory if excessive resistance is
encountered during insertion. Excessive force may result in damage to
the Sheath, endoscope, and/or accessory.
Confirm that the accessory is compatible with the Sheath’s 2.1 mm
Working Channel prior to insertion. If the accessory is too large, it
could damage the Sheath and may compromise the integrity of the
barrier.
NOTE: Recommended Suction Pressure Settings: 160 – 180 mmHg (6.3–7.1 inHg
or 3.1–3.5 psi) for optimal levels, increasing in slight increments if stronger
suction power is needed.
Depending on the gauge design, the suction pressure level may be displayed as
either a negative (true) or positive number. Due to differences in suction
equipment and accessories, suction settings may need to be adjusted to meet
specific procedural needs. However, excessive suction pressure settings may
collapse tubing, which will impair suction performance. The Slide-On®
EndoSheath® Technology is validated to an upper limit of 580 mmHg (22.8
inHg or 11.2 psi).
9. When the procedure is completed, withdraw the endoscope under direct
visualization without holding the Angulation Lever. This will allow the Distal
Bending Section to move freely during withdrawal.
Insufflation
Press the Air Button on the endoscope’s Control Body to activate insufflation. Release the
Air Button to stop insufflation.
Suction
Press the Suction Button on the endoscope’s Control Body to activate suction. Release the
Suction Button to stop suction.
Inserting Accessories
1. Before inserting an accessory, straighten the endoscope’s Distal Bending Section.
2. Insert the tip of the accessory through the Sheath’s Accessory Port. Using straight,
steady strokes, pass the accessory through the Working Channel until the tip of the
30 TNE-5000 Flexible Video Esophagoscope User’s Manual
accessory is visible on the video monitor.
3. If resistance is encountered while inserting the accessory, withdraw the accessory,
straighten the endoscope’s Distal Bending Section, and attempt to insert the
accessory again. If resistance is still felt, confirm again that the accessory’s
diameter is compatible with the size of the Working Channel. Refer to Chapter 8,
Troubleshooting for additional suggestions.
Page 39

Preparation, Inspection and Operation
Before using any electrosurgical devices for high-frequency
cauterization, users should be thoroughly familiar with all
guidelines, safety precautions, and proper use of the equipment.
Follow all manufacturer instructions on proper equipment
preparation and use. Accessories should be inspected for damage
before and after each procedure.
Before electrosurgery, inspect the endoscope for any physical damage to
surfaces and components. If damage is discovered, discontinue use and
contact Cogentix Medical for repair. Continued use of damaged equipment
during electrosurgery may lead to equipment damage and/or patient injury.
A thorough understanding of the principles and techniques involved in
electrosurgical procedures is essential to avoid shock and burn hazards to both
patient and medical personnel and prevent damage to the device and other
medical instruments. Ensure that insulation or grounding is not compromised.
Do not immerse electrosurgical instruments in liquids, unless the instruments
are specifically designed and labeled to function in liquids.
Always confirm that the electrode section of the electrosurgical accessory is an
appropriate distance from the Distal Tip of the endoscope and that the
electrode is clearly in view. If the electrode is in close proximity to the Distal
Tip or still within the Slide-On® EndoSheath® Technology during use, the
endoscope and/or Slide-On® EndoSheath® Technology may be damaged
and patient injury may occur.
Set the high-frequency (peak) voltage level of the electrosurgical unit no
higher than the voltages given below for the respective operating modes:
CUT: 560 V
p
COAG: 775 V
p
SPRAY: 1,700 V
p
Always utilize the lowest output setting necessary on the Electrosurgical Unit.
This reduces the potential for patient injury or equipment damage.
When the recommended voltages shown in the instructions for electrosurgical
accessories differ from the limits given above, always use the lowest
recommended voltage.
Do not supply oxygen or use in the presence of combustible gases during
electrosurgery. There is the potential for combustion during cauterization.
Electrosurgical Devices/Accessories
The TNE-5000 Flexible Video Esophagoscope and Slide-On® EndoSheath®
Technology may be used with high-frequency (HF) electrosurgical devices. Operators
utilizing HF devices and accessories should follow all manufacturer and facility guidelines
for proper and safe use. Refer to the user manuals of all HF devices being used in the
procedure, and closely follow all indications, instructions, and safety precautions.
TNE-5000 Flexible Video Esophagoscope User’s Manual 31
Page 40

Preparation, Inspection and Operation
Cogentix Medical recommends the use of isolated electrosurgical accessories.
Use of non-isolated accessories may result in operator injury.
To best determine the necessary minimum output, operators should conduct
basic tests before electrosurgery according to the User’s Manual of the
Electrosurgical Unit.
Before inserting or removing the laser fiber accessory, ensure that the
endoscope’s Distal Bending Section is in the neutral position and
straight. If the Distal Bending Section is articulated, there is a risk of
damaging the Instrument Channel of the Slide-On® EndoSheath®
Technology.
Before using any laser devices, users should be thoroughly familiar
with all guidelines, safety precautions, and proper use of the
equipment. This includes, but is not limited to, proper eye and skin
safety guidelines.
Always confirm that the Distal Tip section of the laser fiber accessory
is an appropriate distance from the Distal Tip of the endoscope and
that the laser fiber tip is clearly in view. If the Distal Tip of the fiber
is in close proximity to the endoscope’s Distal Tip, or not advanced
far enough beyond the Sheath, or still within the Channel during use,
the endoscope and Slide-On® EndoSheath® Technology may be
damaged and patient injury may occur.
Do not use a damaged laser fiber accessory. Using a probe with a
damaged cover or distal end may result in patient injury and/or
equipment damage.
Follow all manufacturer instructions on proper equipment
preparation and use. Accessories should be inspected for damage
before and after each procedure.
Do not supply oxygen or use in the presence of combustible gases
during laser surgery. There is the potential for combustion during
cauterization.
Laser Devices/Accessories
32 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 41

The endoscope must be properly reprocessed by cleaning,
disinfection and/or sterilization, before its first use and after
each subsequent use, according to the protocols in this section.
Using an endoscope that has not been properly reprocessed
presents an acute infection-control risk to both the patient and
medical personnel performing or assisting in the procedure.
Some methods of disinfection and sterilization may be harmful
to the endoscope and exposure to them could result in
extensive equipment damage. Please contact Cogentix Medical
Customer Service Dept. to verify the compatibility of a cleaning
method not listed in this manual and/or a complete list of
functionally compatible agents.
The endoscope must be cleaned immediately after use in a
procedure. Failure to do so may allow patient debris to harden
on the endoscope’s external surfaces, which can become
difficult to remove and could inhibit subsequent
disinfection/sterilization processes.
Do not use an endoscope that has been determined to
have a leak, and do not immerse such an endoscope in
fluids. Fluid entry into the endoscope can cause
equipment damage and render the endoscope unfit for
patient use.
Always wear appropriate personal protective equipment when
reprocessing the endoscope or any of its components.
Appropriate protective equipment includes items such as a
gown, gloves, and face and eye shields.
6 Reprocessing
The TNE-5000 endoscopic system works in tandem with the Slide-On®
EndoSheath® Technology. The Slide-On® EndoSheath® Technology is a sterile,
disposable, protective covering which limits the need for elaborate chemical
disinfection or sterilization procedures after every endoscopy procedure. The complete
system enables the user to implement a fast and effective method of reprocessing an
endoscope and ensures an Insertion Tube is covered with a sterile Sheath for every
procedure, thus providing optimal benefit for medical personnel and patients. See
Cleaning After EndoSheath® Technology Usage on page 37 for cleaning/
disinfection procedures when using the Slide-On® EndoSheath® Technology.
Complete and thorough reprocessing of the endoscope is the
only way to ensure that a “patient-ready” endoscope is used in all
patient procedures. Closely adhere to the reprocessing
instructions given in this chapter.
TNE-5000 Flexible Video Esophagoscope User’s Manual 33
Page 42

Reprocessing
Reprocessing Steps
The endoscope reprocessing procedure is made up of a series of discrete steps, each
of which is essential to successful reprocessing. The steps are listed below in their
proper order, and the complete instructions for each step are given in greater detail in
this chapter.
Leak Testing
surface to and immersing the endoscope itself in fluids. If there is a leak in any
part of the endoscope, the internal components of the endoscope are vulnerable
and will likely be damaged by fluid infiltration. Before cleaning, disinfecting and/or
sterilizing the endoscope, it is essential to perform a leak test to ensure the interior
of the endoscope is resistant to fluid invasion.
Cleaning
procedure, which uses water and an instrument-grade detergent. When the SlideOn® EndoSheath® Technology is used and inspection after the procedure
confirms that the sheath was not compromised, surface cleaning and intermediate
level disinfection of the endoscope should be sufficient to prepare it for the next
procedure.
Intermediate-Level Disinfection
Technology and proper cleaning, the endoscope should undergo intermediate-
level disinfection. For the complete routine, see Cleaning After Slide-On®
EndoSheath® Technology Use on page 37.
High-Level Disinfection
contaminated, it will be necessary to immerse the endoscope in a high-level
disinfectant.
Sterilization
sterilized using ethylene oxide (EtO) gas. It must then be thoroughly aerated to
ensure that all residues have been removed. The endoscope may also be
sterilized using a validated STERRAD® or STERIS® system. Refer to the
STERRAD®/STERIS® section in this chapter.
– The reprocessing procedure requires exposing the endoscope’s
– Visible debris is removed from the surface of the endoscope in this
– After use with an EndoSheath®
– If the user suspects the endoscope has become
– In addition to high-level disinfection, the endoscope may be
Leak Testing
The TNE-5000 Flexible Video Esophagoscope must be evaluated for possible leaks in
the Control Body and/or the Insertion Tube before immersion in any fluid. The Leak
Tester accessory should be used for this test (Cogentix Medical Leak Tester is
required). Follow the steps given below.
Attach the Leak Tester to the Endoscope
Connect the Leak Tester to the endoscope’s Vent Valve (see Figure 6-1). Align the slot
on the Leak Tester’s connector with the pin on the Vent Valve, then push down and
rotate the Leak Tester’s connector clockwise until it locks.
34 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 43

Reprocessing
It is essential that gloves be worn when performing the leak test
procedure, in case the endoscope’s Insertion Tube has been
contaminated and requires further disinfection or sterilization.
An endoscope in this condition can present an infection-control
risk to the person(s) reprocessing the endoscope.
The Videoscope Cable’s Sealing Cap must be attached to the plug
end of the Videoscope Cable Connector prior to leak testing or
immersion of the endoscope into water or disinfecting solution.
Figure 6-1: Leak Tester Connection
Leak Tester Connection Components
1. Connector
2. Vent Valve
3. Alignment Pin (align with slot in Connector)
Pressurize the Endoscope
1. Make sure that the Leak Tester’s Pressure-Relief Valve is closed by moving the
TNE-5000 Flexible Video Esophagoscope User’s Manual 35
button to the “out” position.
2. Pump the hand bulb of the Leak Tester until the pressure gauge’s needle
reaches the green zone. Due to the size of the internal space of the
endoscope, 2-3 pumps of the hand bulb may be required to pressurize the
entire chamber. After the first pump, the needle may drop out of the green
zone and reach a stable position in the white zone. Continue with additional
pumps until the needle no longer falls back into the white zone.
Page 44

Reprocessing
Do not over-pressurize the endoscope (do not allow the needle to
go above the green area on the gauge). Over-pressurizing the
interior of the endoscope can damage the light-transmission
and/or optical system components.
Do not continue to use an endoscope if leaks are detected.
Contact your local distributor or the Cogentix Medical Customer
Service Center to arrange for evaluation and/or repair. When
returning the endoscope to Cogentix Medical, follow the
instructions given in Chapter 9, Warranty and Service.
NOTE: Do not mistake the release of trapped air from the crevices
on the endoscope's outer surface for a leak. Trapped air can be
released by tapping the endoscope gently after immersing it in
water.
Failure to discharge/depressurize the endoscope after leak testing
may place stress on the soft covering of the Insertion Tube,
potentially producing a “rolling over” of the covering.
3. Maintain the pressure for ten (10) seconds, observing the position of the needle
on the pressure gauge. If the pressure decreases, the Sealing Cap of the
Videoscope Cable may not be secured onto the plug; or the Leak Tester to
endoscope connection may be loose. Check the Pressure-Relief Valve on the
Leak Tester, it may still be open, and should be closed. Make sure the Sealing
Cap is securely placed on the plug; remove and reattach the Leak Tester to the
endoscope and repeat Steps 1 through 3.
If the pressure decreases after the connections are restored, the endoscope has
a damaged seal. Do not continue to use the endoscope or immerse it in
fluids in this condition. Contact your regional distributor or the Cogentix
Medical Customer Service Center to arrange for evaluation and/or repair. When
returning the endoscope to Cogentix Medical, follow the instructions given in
Chapter 9, Warranty and Service.
4. If the needle’s position remains steady on the Leak Tester, immerse the entire
endoscope in water, and observe it for thirty (30) seconds. Angulate the Distal
Bending Section up and down while it is immersed, as holes in the soft covering
of the Distal Bending Section may not be evident while it is in a relaxed position.
5. A steady stream of air bubbles at a given location indicates a small leak in the
endoscope that was not detected by the pressure gauge. If a leak is detected,
the air pressure in the endoscope will prevent water from entering through the
leak. However, immediately remove the endoscope from the water and do not
immerse it in any more fluids.
6. The absence of air bubbles confirms that the endoscope is watertight. Remove
36 TNE-5000 Flexible Video Esophagoscope User’s Manual
it from the water and open the Leak Tester’s Valve.
7. Make sure that the needle on the pressure gauge falls to zero (0), and
disconnect the Leak Tester from the endoscope. The endoscope can now be
safely immersed in cleaning solutions.
Page 45

Reprocessing
Failure to follow the instructions given in this section regarding the
use of the Vent Cap may result in damage to the endoscope. Any such
damage will void the product warranty.
The Slide-On® EndoSheath® Technology is intended for a
single use only; do not reuse it. When the procedure is complete,
remove the Sheath from the endoscope and dispose of it as
described in Chapter 4, Installing and Removing the Slide-
On® EndoSheath® Technology. Reusing the Sheath can present
an acute infection-control risk to the user and the next patient on
whom the endoscope is used.
Cleaning, Disinfection, and Sterilization
Use of the Vent Cap
The Vent Cap is to be attached to the endoscope prior to all of the following
procedures in order to prevent damage to the endoscope caused by changes in
pressure and temperature:
Gas Sterilization
Aeration
The Vent Cap is to be removed from the endoscope prior to:
Shipping
Patient Procedures
Immersion in Fluids
Figure 6-2: Opening the Vent Valve
Cleaning After Slide-On® EndoSheath® Technology Usage
TNE-5000 Flexible Video Esophagoscope User’s Manual 37
Page 46

Reprocessing
NOTE: Because the possibility exists that a Sheath could be torn, or
that the endoscope or Sheath could come into contact with
contaminated surfaces, the user should develop and follow a
prophylactic routine which includes exercising care when handling
a sheathed or unsheathed endoscope.
Because of the possibility that a Sheath could be torn, or that the
endoscope or Sheath could come in contact with contaminated
surfaces, the user should exercise care when handling the
endoscope, whether sheathed or unsheathed. It could present an
infection-control risk if not handled properly.
After a procedure in which the Slide-On® EndoSheath® Technology was attached
over the endoscope’s Insertion Tube, Cogentix Medical recommends performing the
following prophylactic and cleaning routine between endoscopic procedures:
1. After removing the Sheath, inspect the Insertion Tube and Distal Bending
Section, and confirm that these areas are dry. If moisture is observed, there
may have been a leak into the Sheath during the procedure, providing the
endoscope was dry when the Sheath was attached. In this case, the endoscope
must be high-level disinfected or sterilized following the instructions given in
High-Level Disinfection and Sterilization
In the event that the endoscope is contaminated, it should be high-level disinfected or
sterilized after cleaning. Use caution when cleaning and then high-level disinfecting or
sterilizing the endoscope.
Recommended Disinfection and Sterilization Procedures
The following procedures have been determined by Cogentix Medical to be compatible
with the TNE-5000 Flexible Video Esophagoscope.
38 TNE-5000 Flexible Video Esophagoscope User’s Manual
this chapter.
2. For Cleaning: Gently wash all external surfaces of the endoscope with an
appropriate instrument-grade detergent. An ample size basin must be used for
cleaning the endoscope. If the basin is too small, the endoscope may
inadvertently be kinked or damaged during cleaning.
3. After washing, thoroughly rinse the outside of the endoscope with clean,
lukewarm water and place it on a clean, dry surface.
4. For Disinfection: Wipe down the entire endoscope with a soft, lint-free cloth
or gauze soaked in 70% ethyl or isopropyl alcohol.
5. Ensure that all external surfaces of the endoscope are thoroughly dried prior to
attaching another Sheath or storing the endoscope.
High-Level Disinfection in Glutaraldehyde: All Cogentix Medical
Endoscopes are validated for high-level disinfection in 2.4% glutaraldehyde
solutions. Perform the High-Level Disinfection Protocol described in this
chapter.
Sterilization by Ethylene Oxide (EtO) Gas: The endoscope may be
sterilized using a validated EtO protocol. The acceptable processing parameters
and procedure are given in the Ethylene Oxide (EtO) Gas Sterilization
section on page 41.
Page 47

Reprocessing
Disinfection and sterilization methods not listed here may be harmful
to the endoscope and could cause extensive equipment damage.
Please contact Cogentix Medical Customer Service to determine the
compatibility of a disinfection or sterilization method not listed in this
manual and/or a complete list of functionally compatible agents.
Cleaning
Soft Material Lint-Free Gauze (4x4)
Enzymatic Cleaner
Instrument Grade Detergent
Intermediate Level
Disinfection
70% Isopropyl Alcohol or
70% Ethyl Alcohol
High Level
Disinfection
2.4% Glutaraldehyde-based solution
Sterilization
EtO Gas Sterilization
STERRAD
®
100S, NX, 100NX*
STERIS
®
System 1E*
Incompatible High Level Disinfection and Sterilization Methods/Chemicals
High Level Disinfection
Chemicals
DO
NOT
USE
Chlorines
Formaldehyde
Iodophors
Sterilization Methods
DO
NOT
USE
Autoclave
Ultrasonic
Sterilization by STERRAD
validated STERRAD® protocol. Refer to Table 6-1 on page 42 for the
approved systems / cycles suitable for use with this endoscope.
Sterilization by STERIS
validated STERIS® protocol. Refer to Table 6-1 on page 42 for the
approved system suitable for use with this endoscope.
®:
The endoscope may be sterilized using a
®
: The endoscope may be sterilized using a
Acceptable Reprocessing Materials
* Endoscope must feature the
S symbol for STERIS
○
®
/ STERRAD® compatibility
Incompatible Methods
The high-level disinfection and sterilization chemicals and methods shown below are
not compatible with the TNE-5000 Flexible Video Esophagoscope; DO NOT USE
THEM, as they could cause extensive damage to the endoscope. If you have any
questions regarding the compatibility of a given disinfection or sterilization method,
please contact your local distributor or the Cogentix Medical Customer Service Center.
TNE-5000 Flexible Video Esophagoscope User’s Manual 39
Page 48

Reprocessing
It is imperative that the endoscope be leak tested and cleaned prior to
immersion in high-level disinfectant. Failure to do so may not detect
leaks that could allow fluid ingress and damage the endoscope. Failure
to clean the endoscope may allow gross debris to remain on external
surfaces, which could interfere with proper disinfection. In the event
that the endoscope fails the leak test, do not immerse the
endoscope in liquids and do not use it in a procedure. Return
the endoscope to the manufacturer for repair.
Make sure that the sealing cap is securely attached to the Videoscope
Cable prior to immersion. Failure to do so will lead to fluid
invasion and severe equipment damage.
High-Level Disinfection Protocol
If the endoscope was determined to be free of leaks, it may be immersed in a
glutaraldehyde solution for the amount of time recommended by the disinfectant
manufacturer to achieve high-level disinfection.
Pre-Cleaning
1. Gently pat down the Insertion Tube and wipe the Distal Bending Section with a
soft, lint-free cloth or gauze (4x4) to remove gross debris.
2. Perform the Leak Test procedure.
3. Gently wash down all external surfaces with an enzymatic cleaning solution
and soak the endoscope in the enzymatic cleaning solution for the time
recommended by the enzymatic solution’s manufacturer.
4. Remove the endoscope from the cleaning solution and rinse it thoroughly with
clean, lukewarm water.
5. Dry all external surfaces of the endoscope.
Disinfection
1. Immerse the endoscope in the disinfectant solution at the temperature
recommended by the disinfectant manufacturer.
2. Allow the endoscope to remain immersed in the disinfectant solution for the
period of time recommended by the disinfectant manufacturer.
3. Following disinfection, remove the endoscope from the solution.
Rinsing
1. Immerse the endoscope in a container of clean, lukewarm water.
2. Thoroughly rinse the outside of the endoscope with clean, lukewarm water and
place it on a clean, dry surface.
3. Wipe all external surfaces of the endoscope with a soft, lint-free cloth or gauze
(4x4) until it is completely dry.
40 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 49

Reprocessing
If the Vent Valve is not opened during gas sterilization, the increased
heat and pressure from the sterilization process will cause pressure to
build up inside the endoscope and could rupture the watertight seals
and/or softer materials of the endoscope.
The Videoscope Cable’s Sealing Cap must be removed from the
Videoscope Cable Connector prior to EtO gas sterilization.
NOTE: EtO gas sterilization at the above parameters has been
validated by Cogentix Medical, and will sterilize the device to a
sterility assurance level (SAL) of 10-6.
Failure to properly pre-clean the endoscope may inhibit the EtO gas
sterilization process.
4. Confirm that the Lens at the endoscope’s Distal Tip is free of disinfectant
residue.
Ethylene Oxide (EtO) Gas Sterilization
The TNE-5000 Flexible Video Esophagoscope may be sterilized using a validated
ethylene oxide (EtO) gas sterilization protocol, following the processing parameters
given below.
EtO Gas Sterilization Parameters
Temperature: 125o 5oF (52oC 3oC)
Relative Humidity: 50% 10%
EtO Concentration: 600 mg/liter
Exposure Time: 3 hours + 1/-0 hour
Post-Sterilization Aeration: 12 hours at 130oF (55oC) or 72 hours
at 75oF (24oC)
Prior to EtO Gas Sterilization, the endoscope must be leak tested, precleaned and dried as described for the High-Level Disinfection Protocol.
Prior to gas sterilization, the Vent Valve must be opened as shown in Fig 6-2 (page 37)
to accommodate the heat and pressure changes of the gas sterilization process. To
open the Valve, press the red Vent Cap onto the Vent Valve, and rotate it clockwise
until it is seated and locked.
After EtO Gas Sterilization
Effective aeration must be completed after EtO gas sterilization. Cogentix Medical
recommends following the instructions-for-use supplied by the manufacturer of the gas
sterilizer, and that a biological indicator is used to confirm sterilization efficacy.
TNE-5000 Flexible Video Esophagoscope User’s Manual 41
Page 50

Reprocessing
Short and Long
Cycles
Short and Long
Cycles
Flex Cycle
Only
Failure to properly clean the videoscope may inhibit the
STERRAD® or STERIS® sterilization process.
The Vent Cap must be attached for the STERRAD® sterilization
process. The Vent Cap must be removed for the STERIS®
sterilization process.
Compatibility applies only to endoscopes which feature the ○S
symbol located on the Identification Ring on the Control Body.
STERRAD® and STERIS® Sterilization
Prior to STERRAD® and STERIS® Sterilization, the endoscope must
be leak tested, cleaned and dried as described in this chapter.
The TNE-5000 Flexible Video Esophagoscope has been validated for material and
functional compatibility with the following sterilization systems/cycles:
STERRAD® STERRAD
100S NX 100NX System 1E
®
STERRAD
®
STERIS
®
Table 6-1: STERRAD
®
and STERIS® Validated Systems/Cycles
Refer to the STERRAD® or STERIS® Sterilization User’s Manual for complete
details on instructions for use.
42 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 51

The endoscope should NEVER be stored in areas where it could be
exposed to liquids or environmental conditions such as high
temperature, humidity, direct sunlight, dust, salt, etc., which could
adversely affect its operation.
The endoscope should NEVER be stored in the presence of
flammable or explosive gases or chemicals.
7 Care and Storage
Follow the instructions in this chapter if you anticipate that the endoscope will not
be used for a prolonged period of time. Do not leave the endoscope exposed to the
elements in such circumstances.
Storage
Follow the instructions below when storing the TNE-5000 Flexible Video
Esophagoscope:
DO NOT store the endoscope with the Slide-On
Technology installed on the Insertion Tube. Over time, the Sheath
material may adhere to the Insertion Tube and become difficult to remove.
When storing the endoscope, be sure to keep the Insertion Tube as straight
as possible. The Videoscope Cable may be stored either straight or neatly
coiled to prevent kinking or bending.
The endoscope should be completely clean and dry before storing.
This equipment should be maintained in a clean condition during storage so
that it is ready for subsequent use.
The endoscope should be stored in a dry, well ventilated environment -
avoid high humidity, direct sunlight, and temperatures below -10oC or
above 60oC.
Do not store the endoscope in its carrying case. This case is only intended
for endoscope transport; it is not properly ventilated for storage.
Avoid storing the endoscope in heavily trafficked areas where there is a
chance that it may sustain physical damage.
®
EndoSheath®
TNE-5000 Flexible Video Esophagoscope User’s Manual 43
Page 52

Care and Storage
Disposal
The equipment should be returned to Cogentix Medical for disposal. Contact your
local Cogentix Medical representative or service facility for further information.
44 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 53

If the problem persists even after the corrective action has
been taken, or a problem occurs that is not covered in the
tables, do not use the endoscope. Contact Cogentix Medical
for repairs using the information given in Chapter 9,
Warranty and Service.
8 Troubleshooting
The information in this chapter is intended to help users diagnose problems
that may occur during operation of the endoscope. The tables include some of
the problems that could arise during operation, possible causes for those
problems, and suggested corrective action.
The TNE-5000 Flexible Video Esophagoscope requires a video processor to
process and display images. In order to identify issues related to image
problems, you may also have to refer to the Troubleshooting chapter in the
DPU-5000/7000 Series Video Processors User’s Manual.
TNE-5000 Flexible Video Esophagoscope User’s Manual 45
Page 54

Troubleshooting
PROBLEM
PROBABLE CAUSE
SUGGESTED ACTION
Angulation feels
stiff
Damaged Distal Bending
Section causing impaired
angulation.
Return the endoscope to
Cogentix Medical for
repair. Refer to Chapter 9,
Warranty and Service.
Angulation
alignment is no
longer up/down
Insertion Tube has become
twisted.
Return the endoscope to
Cogentix Medical for
repair. Refer to Chapter 9,
Warranty and Service.
Loss of angulation
Angulation wires have been
stretched or broken during
use.
Return the endoscope to
Cogentix Medical for
repair. Refer to Chapter 9,
Warranty and Service.
Cloudy or foggy
images when the
endoscope is
unsheathed
Patient debris or other
material on the Objective
Lens.
Fluid incursion into the
videoscope’s optical system.
The lens at the Distal Tip has
become damaged.
Clean the Objective Lens
with an alcohol prep pad to
remove material or stain.
Excess staining may not be
correctable and the lens
may require replacement.
Return the endoscope to
Cogentix Medical for
repair. Refer to Chapter 9,
Warranty and Service.
Return the endoscope to
Cogentix Medical for
repair. Refer to Chapter 9,
Warranty and Service.
Table 8-1: Troubleshooting
46 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 55

Troubleshooting
PROBLEM
PROBABLE CAUSE
ACTION
No image
Video processor is not
powered on.
Connection between the
endoscope and the Video
Processor is lost.
No video output signal to a
monitor.
Check the power cord
connection and fuses, or
connect the Video Processor
to a different mains outlet.
Check the cable connection
between the endoscope and
the Video Processor.
Check the video output cable
connections when using an
external monitor. Replace the
cable if necessary.
If the problem cannot be
corrected, send the
endoscope and Video
Processor to Cogentix
Medical for repair.
Poor quality image
from an
unsheathed
endoscope
Patient debris or other
material on the Objective
Lens.
Improper settings on the
Video Processor or display.
Damaged optics, sensors or
electronics in the endoscope.
Clean the Distal Tip with an
alcohol prep pad to remove
material or stain. Excess
staining may not be
correctable and the lens may
require replacement.
Adjust the settings on the
Video Processor or display.
Perform a White Balance
procedure on the Video
Processor.
Return the endoscope to
Cogentix Medical for repair.
Refer to Chapter 9, Warranty
and Service.
Table 8-1: Troubleshooting (cont’d)
TNE-5000 Flexible Video Esophagoscope User’s Manual 47
Page 56

Troubleshooting
Loss of illumination
Patient material or other
substance on the Light
Guides.
Light Intensity is set too low.
Damaged light guide fiber
bundles.
Internal light source is
deteriorating.
Clean the Distal Tip with an
alcohol prep pad to remove
material or stains. Excess
staining may not be
correctable and the lens may
require replacement.
Adjust the Light Intensity
setting.
Return the endoscope to
Cogentix Medical for repair.
Refer to Chapter 9, Warranty
and Service.
Return the endoscope to
Cogentix Medical for repair.
Refer to Chapter 9, Warranty
and Service.
Wrinkles and/or
folds in the
Insertion Tube
These may be a result of
excessive force applied to the
Insertion Tube during
cleaning or Sheath removal,
or the long-term effects of
repeated immersion in
chemical disinfecting
solutions, which could stretch
and weaken the outer
coverings.
Return the endoscope to
Cogentix Medical for repair.
Refer to Chapter 9, Warranty
and Service.
Insertion Tube is
dented
Dents can be caused by
physical trauma to the
endoscope [e.g., closing the
case on the Insertion Tube].
Return the endoscope to
Cogentix Medical for repair.
Refer to Chapter 9, Warranty
and Service.
Loss of pressure
during the leakage
test
The Leak Tester is not
connected properly to the
Vent Valve.
The Leak Tester’s Pressure-
Relief Valve is open.
A hole or crack has broken
the endoscope’s watertight
seal.
Re-connect the Leak Tester
and perform the test again.
Close the Pressure-Relief
Valve.
Return the endoscope to
Cogentix Medical for repair.
Refer to Chapter 9, Warranty
and Service.
Table 8-1: Troubleshooting (cont’d)
48 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 57

Troubleshooting
Cannot insert
endoscope into the
Sheath
The Sheath’s channel is
misaligned.
The endoscope’s Locking
Knob is not aligned with the
slot.
The endoscope’s Insertion
Tube has been damaged and
its diameter has increased.
The Angulation Lever has
been actuated.
The Sheath’s Window is not
aligned with the Insertion
Tube.
The flat portion of the D-
shaped Insertion Tube is not
aligned with the Biopsy Port
during insertion.
The Sheath is torn or
punctured.
Straighten the channel before
continuing scope insertion.
Make sure that the Locking
Knob is positioned vertically.
Align the Locking Knob with the
slot and fully seat the
endoscope into the Sheath.
Rotate the Locking Knob
clockwise until it is horizontal.
Return the endoscope to
Cogentix Medical for repair.
Refer to Chapter 9, Warranty
and Service.
Place the Angulation Lever in
the neutral position so the
Distal Bending Section of the
endoscope is straight.
Articulate the Insertion Tube
back and forth several times to
fully seat the Sheath.
Gently manipulate the edges of
the Sheath’s Window to fully
seat it.
Align the flat side of the
Insertion Tube with the
Sheath’s Accessory Port.
Replace the Sheath.
Cannot remove the
endoscope from the
Sheath
The Locking Knob not rotated.
Rotate the Locking Knob to the
vertical position.
The endoscope has become
twisted during removal.
Sheath is torn or punctured.
The Sheath’s channel is wrapped
around the Insertion Tube.
Align the flat side of the
endoscope’s Insertion Tube
with the Sheath’s Accessory
Port during removal.
Contact your regional
distributor or Cogentix Medical
Customer Service Department
for removal instructions.
Straighten the channel before
gently removing the Sheath.
Table 8-1: Troubleshooting (cont’d)
TNE-5000 Flexible Video Esophagoscope User’s Manual 49
Page 58

Troubleshooting
Cannot remove the
endoscope from the
Sheath (cont’d)
The Sheath has been attached
to the endoscope for an
extended period of time,
causing it to adhere to the
Insertion Tube.
The Distal head is lodged in the
Sheath.
Use a syringe without a needle
to introduce small amounts of
70% alcohol into the opening at
the proximal end of the
EndoSheath® Connector. When
the liquid reaches the Distal Tip
of the endoscope/Sheath,
carefully attempt to remove the
endoscope from the Sheath. If
necessary, contact your
Regional Distributor for removal
instructions. Disinfect / sterilize
the endoscope.
Gently grasp the Sheath’s
Window using the hanging
Drape Bag as a barrier between
your fingers and the Sheath.
Then slowly and gently
withdraw the endoscope from
the Sheath.
Glare in the
endoscopic image
The sheath’s Window is not in
contact with the Distal Tip of the
endoscope due to:
- Incomplete insertion into the
Sheath.
OR
- The endoscope’s tip is damaged
and/or enlarged.
Move the Distal Bending Section
back and forth several times to
fully seat the sheath.
Gently manipulate the edges of
the Sheath’s Window to
properly seat the endoscope.
Remove the endoscope from
the Sheath. Return the endoscope to Cogentix Medical for
repair. Refer to Chapter 9,
Warranty and Service.
Suction or
insufflation is
observed without
the Pinch Valve
Button pressed
The Control Body Cover is
draped too tightly around the
Suction Valve.
Suction/Insufflation Tubing is
not properly positioned in the
Pinch Valves.
The Sheath’s Window is
damaged.
Relieve tension on the Suction
Valve by adjusting the Control
Body Cover.
Properly position the
Suction/Insufflation Tubing in
the Pinch Valve.
Remove the Sheath and install
a new one.
Table 8-1: Troubleshooting (cont’d)
50 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 59

Troubleshooting
Insufficient or no
suction during
procedure
Suction level is set too low or
the unit is malfunctioning.
Suction/Insufflation Tubing is
too tight in the Pinch Valve.
Suction tubing is kinked or
crushed.
Suction tubing is open to the
atmosphere.
The wall of the Working
Channel is occluded with foreign
matter.
Confirm that the suction
pump is set properly.
Disconnect the suction line
from the Sheath and test the
suction level through the
tubing. Reconnect the
suction line.
Relieve tension on the
tubing by repositioning it
to create more slack.
Straighten out the suction
tubing. Make sure that the
plastic clips are holding the
tubes to the umbilical. Take
care not to apply tension to
the suction tubes.
The suction tubing may have
become disconnected. Reattach the tubing to the
suction pump.
Stop applying suction.
Attempt to clear the channel
by passing air through it. Retry suction. OR:
Remove the endoscope from
patient and inject 10cc of
saline through the channel.
If, after repeated attempts,
the channel remains blocked,
remove the endoscope from
the Sheath and install a new
Sheath.
Table 8-1: Troubleshooting (cont’d)
TNE-5000 Flexible Video Esophagoscope User’s Manual 51
Page 60

Troubleshooting
Insufficient or no
air during the
procedure
Pressure level of air source
set too low or the unit is
malfunctioning.
Tubing is not seated properly
in the Pinch valve.
The Sheath’s Air Channel is
occluded.
Confirm the settings of your
air pump. Disconnect the air
line from the Sheath and test
the pressure level through the
tubing.
Seat the tubing properly.
Actuate the air valve to clear
the nozzle.
Check air tubing for leaks or
blockages.
If, after repeated attempts,
the channel remains blocked,
remove the Sheath from
endoscope and install a new
Sheath.
Accessory will not
pass through the
Sheath’s Suction/
Working Channel
Accessory is too large.
The channel is occluded by patient
debris.
The endoscope’s Distal
Bending Section is angulated.
Check the diameter of
accessory. Make sure that
the accessory is compatible
with a 2.1 mm Working
Channel.
Remove the endoscope from
the patient and inject 10cc of
saline through the channel.
If, after repeated attempts,
the channel remains blocked,
remove the Sheath from the
endoscope and install a new
one. Do not force the
accessory through the
working channel as this may
damage the channel and
compromise the integrity of
the barrier.
Maneuver the endoscope’s
Distal Tip to an area of the
anatomy in which it can
safely be straightened out.
Insert the accessory until its
tip can be seen on the
endoscopic image. Now
angulate the endoscope’s
Distal Bending Section and
proceed to the desired area.
52 TNE-5000 Flexible Video Esophagoscope User’s Manual
Table 8-1: Troubleshooting (cont’d)
Page 61

NOTE: Alterations or repairs done by persons not authorized by
Cogentix Medical will void this warranty.
Cogentix Medical is not liable for any damages to the TNE-5000
Flexible Video Esophagoscope resulting from misuse, negligence, or
improper cleaning or storage. The warranty defined herein shall apply
only to the original buyer. In no event shall Cogentix Medical be liable
for anticipated profits, consequential damages or loss of time incurred
by the buyer with the purchase or use of this equipment.
NOTE: Cogentix Medical sells many of its products through regional
distributors. Before sending equipment to Cogentix Medical, contact
your regional distributor for repair/return procedures.
9 Warranty and Service
Warranty Information
Cogentix Medical warrants that the TNE-5000 Flexible Video Esophagoscope and
its accessories will be free from defects in materials and workmanship for a period
of one year from the date of the invoice. Replacement parts are warranted for
a period of ninety (90) days from the date of the invoice.
All non-warranty repairs will be warranted to be free from defects in materials and
workmanship for a period of ninety (90) days from the date of the invoice.
Upon receipt of the TNE-5000 Flexible Video Esophagoscope for repair, Cogentix
Medical will evaluate the instrument and make the final decision as to the warranty
status.
The above warranties are in lieu of all other warranties, either expressed
or implied, including warranties of fitness or merchantability.
Cogentix Medical Service Information
TNE-5000 Flexible Video Esophagoscopes are serviced at authorized Cogentix
Medical repair facilities only. Use the following procedure to expedite returned
goods for repair or replacement:
1. Telephone your Regional Distributor, Territory Manager, or Cogentix Medical
Customer Service Monday through Friday from 8:00 AM to 7:00 PM EST.
USA customers call 866-258-2182 (toll free in U.S.)
TNE-5000 Flexible Video Esophagoscope User’s Manual 53
International customers call (+1) 952-426-6189 for Cogentix Medical
Customer Service or call your regional distributor.
Fax 866 255-4522 (toll free in U.S.)
Fax (+1) 952 426-6199 (international)
Email: customercare@cogentixmedical.com
Page 62

Warranty and Service
If the TNE-5000 Flexible Video Esophagoscope has been used in
a clinical setting, disinfect all system components before shipping
as described in Chapter 6, Reprocessing. Shipping contaminated
equipment could present an acute infection-control risk for
those handling the endoscope, both during shipping and at
Cogentix Medical or authorized facility.
If the TNE-5000 Flexible Video Esophagoscope has been used in
a clinical setting but cannot be disinfected before shipping, place a
red biohazard label on the shipping container to indicate that
the contents are contaminated, in accordance with OSHA
standards 29 CFR 1910.1030.
NOTE: The customer will be contacted and advised of the estimated
repair costs. Repairs will not begin on any equipment until authorization
or a purchase order has been issued indicating approval of the charges.
2. Provide a detailed description of the problem.
3. If troubleshooting cannot solve the problem, a Returned Goods
Authorization (RGA) number will be issued.
4. Complete an Incident Report Form and send it to Cogentix Medical along
with the returned goods. Returned merchandise will only be accepted with
an RGA number.
Shipping to Cogentix Medical or Distributor
Observe the following precautions before shipping the endoscope:
1. Attach the Vent Cap to the endoscope’s Vent Valve in preparation for
Regardless of warranty status, all shipping charges to and from an authorized
Cogentix Medical facility are the responsibility of the customer.
shipping.
2. If the endoscope has a leak or tear or fails the leak test, or for some other
reason cannot be disinfected properly, as described in Chapter 6,
Reprocessing. Wipe down the endoscope with 70% alcohol to remove
debris. Indicate on the outer package that the contents are contaminated.
3. Ship the endoscope in its carrying case. Place the carrying case inside a
corrugated box containing protective shipping material to prevent damage
during shipment.
54 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 63

Insertion Tube Diameter
(Nominal)
TV-2.1 Sheath
(Cat. No. 03-5101)
5.6 mm (with sheath)
TV-1.5 Sheath
(Cat. No. 03-5102)
5.2 mm (with sheath)
Insertion Tube Working Length
650 mm
Field of View
110°
Direction of View
Forward
Depth of Field
3 - 50 mm
Environmental Effects on Optical Performance
None
Angulation
215° down/140° up
Working Channel Diameter
TV-2.1 Sheath
(Cat. No. 03-5101)
2.1 mm (6.3 Fr.)
TV-1.5 Sheath
(Cat. No. 03-5102)
1.5 mm (4.5 Fr.)
Operating Environment
Temperature
Relative Humidity
Air Pressure
50° to 104° F (10° to 40° C)
30 to 85%
700 to 1060 hPa
Storage Environment
Temperature
Relative Humidity
Air Pressure
14° to 140° F (-10° to +60° C)
0 to 95%
700 to 1060 hPa
Mode of Operation
Continuous
Electrical Safety
IEC 60601-1 & IEC 60601-2-18
Thermal Safety
IEC 60601-1 & IEC 60601-2-18
Electromagnetic Compatibility
IEC 60601-1-2
Degree of Protection Against Electrical Shock
Type BF
Degree of Protection Against Ingress of Liquids
Fully Immersible (as per
Reprocessing Instructions)
Appendix
Specifications TNE-5000
Table A-1: Specifications
TNE-5000 Flexible Video Esophagoscope User’s Manual 55
Page 64

Appendix
1
2
Infection Control Information
The Slide-On® EndoSheath® Technology for the TNE-5000 Flexible Video
Esophagoscope is designed to offer practitioners the ability to perform safe,
efficient endoscopy. The Slide-On® EndoSheath® Technology has been
proven to be an effective barrier to microorganisms as small as 27
nanometers1. Efficacy testing for barrier qualities has been performed by an
independent laboratory per FDA required guidelines2. All Slide-On®
EndoSheath® Barriers undergo a rigorous Quality Assurance process to
ensure the utmost in product quality and efficacy.
Please contact Cogentix Medical Customer Service for a detailed information
packet regarding Infection Control and the Slide-On® EndoSheath®
Technology.
Viral challenge testing performed with bacteriophage 27 nanometers in size, per FDA guidance.
Per FDA Clearance requirements, barriers must be tested by the guidelines set within the “FDA Guidance for
Manufacturers Seeking Marketing Clearance of … Endoscope Sheaths Used as Protective Barriers”
56 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 65

Use of accessories not specified in this manual or sold by
Cogentix Medical may result in increased electromagnetic
emissions or decreased immunity of the equipment or system.
Guidance and manufacturer’s declaration – electromagnetic emissions
The TNE-5000 Flexible Video Esophagoscope with the DPU-5000/7000 Series Video
Processor [the “System”] is intended for use in the electromagnetic environments specified
below. The customer or the user of the System should ensure that it is always used in such
environments.
Emissions Test
Compliance
Electromagnetic environment – guidance
RF emissions
CISPR 11
Group 1
The System uses RF energy only for its internal
function. Therefore, its RF emissions are very
low and are not likely to cause any interference
in nearby electronic equipment.
RF emissions
CISPR 11
Class A
The System is suitable for use in all nondomestic establishments, excepting those
directly connected to a public low-voltage power
supply network that supplies buildings used for
domestic purposes.
Harmonic emissions
IEC 61000-3-2
Class A
Voltage
fluctuations/flicker
emissions
IEC 61000-3-3
Complies
Electromagnetic Compatibility Declarations
Appendix
Table A-2: Electromagnetic Emissions Declaration
TNE-5000 Flexible Video Esophagoscope User’s Manual 57
Page 66

Appendix
Guidance and manufacturer’s declaration – electromagnetic immunity
The TNE-5000 Flexible Video Esophagoscope with the DPU-5000/7000 Series Video Processor
[the “System”] is intended for use in the electromagnetic environments specified below. The
customer or the user of the System should ensure that it is always used in such environments.
Immunity Test
IEC 60601 test
level
Compliance
level
Electromagnetic environment –
guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
±6kV contact
±8kV air
±6kV contact
±8kV air
Floors should be wood, concrete or
ceramic tile. If floors are covered with
synthetic material, the relative
humidity should be at least 30%.
Electrical fast
transient/burst
IEC 61000-4-4
±2kV for power
supply lines
±1 kV for
input/output
lines
±2kV for
power supply
lines
±1 kV for
input/output
lines
Mains power quality should be the
equivalent of that in a typical
commercial or hospital environment.
Surge
IEC 61000-4-5
±1 kV
differential mode
±2 kV
common mode
±1 kV
differential
mode
±2 kV
common mode
Mains power quality should be the
equivalent of that in a typical
commercial or hospital environment.
Voltage dips, short
interruptions and
voltage variations
on power supply
input lines
IEC 61000-4-11
>95% dip
U
T
for 0.5 cycle
60% dip
U
T
for 5
cycles
30% dip
U
T
for
25 cycles
95% dip
U
T
for 5
sec
Compliant with
all levels of
voltage dips
for
U
T
= 100 VAC
and
U
T
= 240 VAC
Mains power quality should be the
equivalent of that in a typical
commercial or hospital environment. If
the user of the System requires
continued operation during power
mains interrupts, it is recommended
that the System be powered from an
uninterruptible power supply or a
battery.
Power frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
3 A/m
3 A/m
Power frequency magnetic fields
should be at levels characteristic of a
typical location in a standard
commercial or hospital environment.
Table A-3: Electromagnetic Immunity Declaration
58 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 67

Appendix
Guidance and manufacturer’s declaration – electromagnetic immunity
The TNE-5000 Flexible Video Esophagoscope connected to the DPU-5000/7000 Series Video
Processor [the “System”] is intended for use in the electromagnetic environment specified below. The
customer or the user of the System should assure that it is always used in such environments.
Immunity Test
IEC 60601 test
level
Compliance
level
Electromagnetic environment –
guidance
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 Vrms
150 kHz to 80
MHz
3 V/m
80 MHz to 2.5
GHz
3 V
3 V/m
Portable and mobile RF communications
equipment should be used no closer to any
part of the System, including cables, than
the recommend separation distance
calculated from the equation applicable to
the frequency of the transmitter.
Recommend separation distance
d = 1.17√P
d = 1.17√P 80MHz to 800MHz
d = 2.33√P 800MHz to 2.5GHz
where P is the maximum output power
rating of the transmitter in watts (W)
according to the transmitter manufacturer
and d is the recommended separation
distance in meters (m).
Field strengths from fixed RF transmitters,
as determined by an electromagnetic site
survey, a should be less than the
compliance level in each frequency range. b
Interference may occur in the vicinity of
equipment marked with the following
symbol:
NOTE 1: At 80 MHz and 800 MHz the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected
by absorption and reflection from structures, objects and people.
a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless)
telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast
cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to
fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field
strength in the location in which the System is used exceeds the applicable RF compliance level
above, the System should be observed to verify normal operation. If abnormal performance is
observed, additional measures may be necessary such as re-orienting or relocating the System.
b
Over the frequency range 150 KHz to 80 MHz, field strengths should be less than 3 v/m.
Table A-4: Electromagnetic Immunity Declaration
TNE-5000 Flexible Video Esophagoscope User’s Manual 59
Page 68

Appendix
Recommended separation distances betweenportable and mobile RF
communications equipment and the System
The TNE-5000 Flexible Video Esophagoscope connected to the DPU-5000/7000 Series Video
Processor [the “System”] is intended for use in the electromagnetic environment in which radiated
RF disturbances are controlled. The customer or the user of the System can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF
communications equipment (transmitters) and the System as recommended below according to
the maximum output power of the communications equipment.
Radiated maximum
output power of
transmitter
W
Separation distance according to frequency of transmitter
m
150 KHz to 80 MHz
D = [3.5/
V
1
]√
P
80 MHz to 800 MHz
D = [3.5/
E
1
]√P
800 MHz to 2.5 GHz
D = [7/
E
1
]√P
0.01
D = 0.12
D = 0.12
D = 0.23
0.1
D = 0.37
D = 0.37
D = 0.74
1
D = 1.17
D = 1.17
D = 2.33
10
D = 3.69
D = 3.69
D = 7.38
100
D = 11.67
D = 11.67
D = 23.33
For transmitters rated at a maximum output power not listed above, the recommended separation
distance, d, in meters (m) can be estimated using the equation applicable to the frequency of the
transmitter, where P is the maximum output power rating of the transmitter in watts (W) according
to the transmitter manufacturer.
NOTE 1: At 80 MHz and 800 MHz the separation distance for the higher frequency range
applies.
NOTE 2: These guidances may not apply in all situations. Electromagnetic propagation is
affected by absorption and reflection from structures, objects and people.
Table A-5: Recommended Separation Distances
60 TNE-5000 Flexible Video Esophagoscope User’s Manual
Page 69

Page 70

Page 71

Page 72

Cogentix Medical, Inc.
40 Ramland Road South
Orangeburg, NY 10962 USA
Tel.: 866 258-2182 (toll free in U.S.)
Tel.: (+1) 952 426-6189 (international)
Fax: 866 255-4522 (toll free in U.S.)
Fax: (+1) 952 426-6199 (international)
email: customercare@cogentixmedical.com
www.cogentixmedical.com
MedPass International Ltd.
Windsor House, Bretforton
Evesham, Worcestershire
WR11 7JJ, United Kingdom
0843
For additional product information or questions pertaining to
sales and service, please contact the local distributor or the
manufacturer.
© 2008, 2009, 2011, 2012, 2013, 2015 Cogentix Medical, Inc. Slide-On and EndoSheath are
registered trademarks of Cogentix Medical, Inc.
EndoSheath® products in this manual are covered by one or more of the following U.S. Patents:
6,350,231; 6,530,881; 6,733,440. Other U.S. and international patents pending.
(10/15) 09646-EN Rev. E (12315)
 Loading...
Loading...