Cisa 200 User and service manual

CISA S.p.A. – User manual - Version 2.0/08
CISA Autoclaves
Page 1
SSTTEEAAM
M SSTTEERRIILLIIZZIINNGG AAUUTTOOCCLLAAVVEESS
MMAANNUUAALL OOFF UUSSEE AANNDD MMAAIINNTTEENNAANNCCE
E
SSEERRIIEESS 220000
CCIISSAA SS..pp..AA..
VViiaa PPoonnttiinnaa KKmm..2288
0000004400 PPOOMMEEZZIIAA ((RRMM)) -- IITTAALLIIAA --
TTeell.
.
++3399 ((0066)) 9911 1144 3377..11
FFaax
x
++3399 ((0066)) 9911 0077 556655
OOffffiiccee AAssssiissttaanncce
e
.. ++3399 ((0066)) 9911 2222 228888

CISA S.p.A. – User manual - Version 2.0/08
CISA Autoclaves
Page 2

CISA S.p.A. – User manual - Version 2.0/08
CISA Autoclaves
Page 3
CONTENTS
S
S
AAFFEETTYY IINNSSTTRRUUCCTTIIOONNS
S
S
S
EECCTTIIOONN
GGEENNEERRAALL IINNFFOORRMMAATTIIOON
N
S
S
EECCTTIIOONN
A
A
UUTTOOCCLLAAVVEE MMAANNAAGGEEMMEENNT
T
3
1.1 GUARANTEE 9
1.2 OTHER CONDITIONS 9
1.3 TYPES OF CYCLES 10
1.4 WASHING AND PACKING OF MATERIAL BEFORE STERILIZATION 10
1.5 STERILIZATION CYCLES 15
1.6 SAFETY DEVICES 16
1.7 SET-POINT OF SAFETY DEVICES AND PRACTICAL ASPECTS 17
1.8 ALARMS 18
1.0
9
2.1 PRELIMINARY OPERATIONS 20
2.2 STARTING OF AUTOCLAVE 21
2.3 CONTROL PANEL 22
2.4 MAIN MENU 23
2.5 CYCLE STARTING PROCEDURE 23
2.6 CHECKING OF CONDITIONS FOR CYCLE STRATING 25
2.6 CURRENT PHASE 26
2.7 LEVEL CODE 27
2.8 VALUES PAGE 28
2.9 END OF CYCLE 28
2.10 CANCELLATION OF THE CYCLE 29
2.11 TECHNICAL MENU 29
2.12 CYCLE MODIFICATION 30
2.13 CALIBRATION 32
2.14 CHANGING OF DATE AND TIME 35
2.15 CHANGING OF LEVEL CODE 35
2.0
20
CISA S.p.A. – User manual - Version 2.0/08
CISA Autoclaves
Page 4
S
S
EECCTTIIOONN
3
3
M
M
AAIINNTTEENNAANNCCEE
I
I
MMMMAAGGIINNI
I
Pay attention to the components, units and points
where the “GENERAL DANGER” sign is placed.
Pay attention when opening electric board and
connector blocks, where the “DANGER: ELECTRIC
CURRENT” sign is placed.
WARNING! The calibration procedure must be carried out by
professionals. Incorrect use of this function can damage the
sterilization process or workers health.
3.1 GENERAL INFORMATION 36
3.1 MAINTENANCE ADVICES 36
.0
36
PIC.1 – PHOTO AUTOCLAVE SERIES 200 9
PIC.2 – AUTOCLAVE SERIES 200 21
PIC.3 – CONTROL PANEL 22
PIC.4 – OPEN DOOR VIEW 26
PIC.5 – INTERNAL VIEW 27
PIC.6 – PHOTO OF OPEN DOOR VIEW 36
SAFETY WARNINGS
This instructions aim to reduce the risks for employees and to avoid making the
device unsafe because of bad maintenance. All workers and service engineer
have to follow the rules posted in this manual for use and maintenance of this
sterilizer.
CISA S.p.A. – User manual - Version 2.0/08
CISA Autoclaves
Page 5
The operators using sterilizer must be qualified for this job.
Sterilizer maintenance and repair must be carried out by
technical experts.
Sterilizer loading area has be kept clean to avoid dangerous
situations due to slippery floor.
Racks, containers, trays, other packaging, internal trolleys
must be used only with special gloves to avoid burns at the
end of the sterilization cycle.
Wear protective gloves every time when you come into contact with hot
sterilization chamber.
Pay attention to all internal unprotected parts of sterilizer, they can
cause burns during maintenance or repair procedure of the hot
sterilizer.
Wear protective gloves when checking the safety valve.
Turn the power supply off before to start the sterilizer maintenance or
repair.
Do not change or tamper with any safety devices of the sterilizer.
Keep clean the sterilizer front panels using a smooth cloth and non
aggressive cleaning solution.
Keep clean the chamber using a smooth cloth and non aggressive
cleaning solution for stainless steel.
Do not use sharp objects to put in or remove the chamber seals from its
place.
CISA S.p.A. - User Manual - Version 2.0/08
CISA Autoclaves
Page 7
NOTES ABOUT THIS MANUAL
This manual was planned to help all users of CISA sterilization systems.
INTENDED USE
CISA steam autoclaves are used to sterilize all temperature resistant
materials like textiles, steal instruments, rubber materials, fittings and
others.
This equipment is a Medical Device according to Directive 93/42 EEC
concerning Medical Devices, adopted in Italy by DLgs. 46/97 and furter
updates.
CISA steam autoclaves and the full set of attachments are produced also in
according with directive 97/23 CEE (PED).

CISA S.p.A. - User Manual - Version 2.0/08
CISA Autoclaves
Page 8
CCIISSAA AAUUTTOOCCLLAAVVEESS
TTEECCHHNNIICCAALL DDEESSCCRRIIPPTTIIOONN

CISA S.p.A. - User manual Version 2.0/08
CISA Autoclaves
Pag.9
1.0 GENERAL INFORMATION
1.1 - Guarantee
Guarantee is valid for 12 months from the testing date but not over 15 months
from the delivery date. Guarantee includes only possible faults or defects of the
manufacturing or of the raw materials; all the parts, that could be damaged due
to carelessness or incorrect use, are excluded.
1.2 – Other conditions
Other conditions have to be agreed upon with CISA S.p.A.
Pic.1 – Photo Autoclave Series 200
CISA S.p.A. - User manual Version 2.0/08
CISA Autoclaves
Pag.10
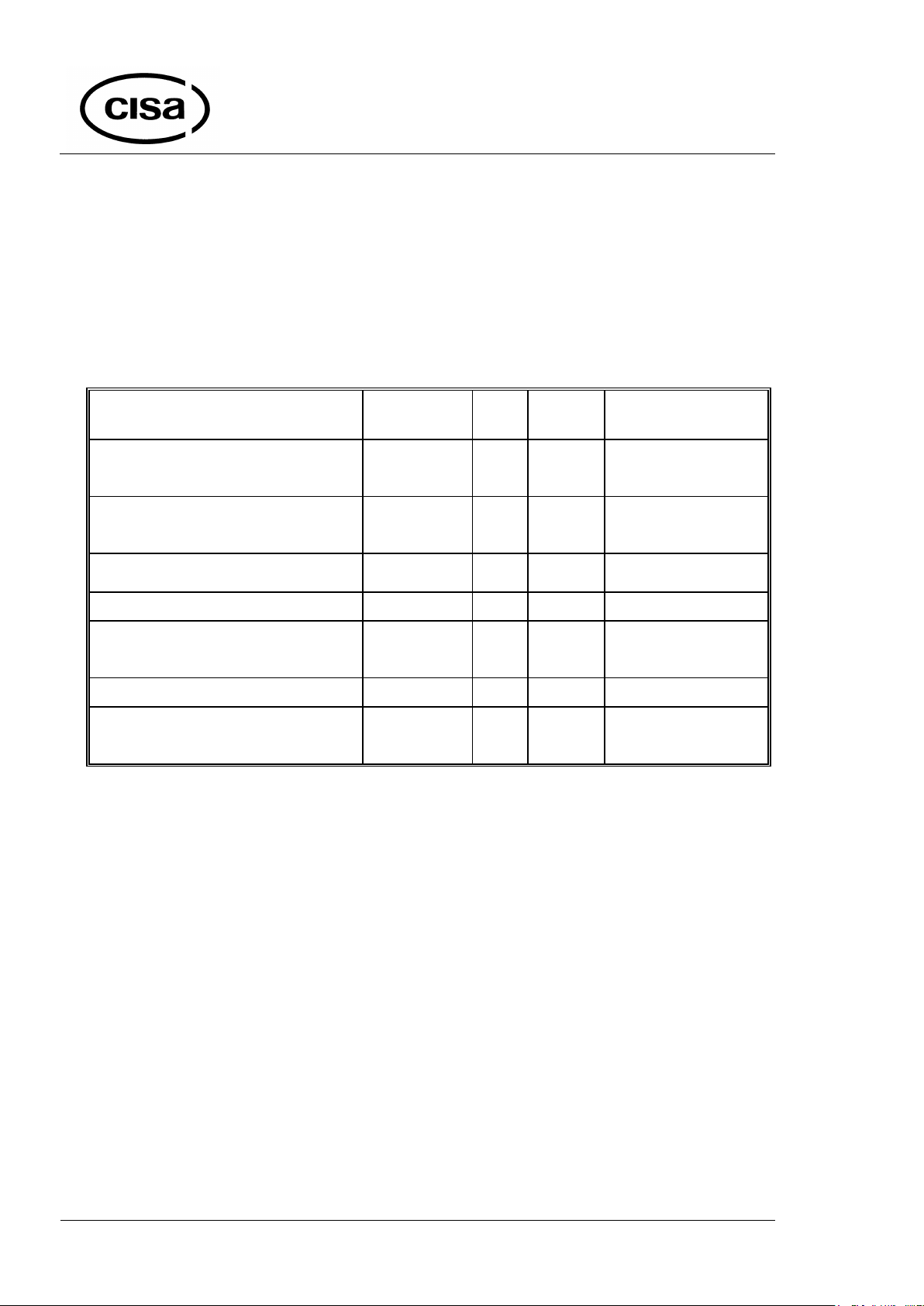
Material
Cycle
°C
min.
Packaging type
Gloves, catheters and other rubber
materials
GLOVES
121°C
15
Containers, envelopes,
baskets, paper
Surgical instruments, empty glassware or
others
INSTRUMENTS
134°C
5
Containers, envelopes,
baskets, paper
Textiles, porous materials, empty
glassware or others
TEXTILES
134°C
5
Containers, envelopes,
baskets, paper
Liquids open
LIQUIDS
121°C
25
Open containers
Surgical instruments, empty glassware or
others
FLASH
134°C 5 Baskets
Bowie & Dick Test
B&D
134°C
210 sec
Special packaging
Vacuum leak Test
VACUUM TEST
10
1.3 –Cycle types
The autoclave provides several tested and programmed cycles, suitable for various
materials (see the table below). Moreover in order to improve the sterilization quality the
operator can choose a packaging type to be put into the autoclave. In such a way the cycle
will be adjusted automatically to characteristics of materials to clean, so that will be
avoided such inconveniences like residual condensate inside of containers, wet or
damaged medical paper, exploded envelopes or tubulars.
The sterilizer loading has to be carried out in the way to allow the steam to circulate
freely. The autoclave load has to be distributed evenly and shouldn’t touch the inner side
of the autoclave. All the objects to sterilize should be put in the way to expose all the
surfaces to the sterilizing agent during the programmed time with the programmed
temperature level.
Put the load in suitable racks.
You can start the sterilization by choosing the sterilization cycle from the display, when
the autoclave is loaded up and the temperature is right. You can control the parameters
and the phases during the sterilization cycle.
At the cycle end, if it has been carried out regularly, you can open the autoclave to
unload the objects.

CISA S.p.A. - User manual Version 2.0/08
CISA Autoclaves
Pag.11
1.4 – Washing and packing of the material before sterilisation
The material, before undergoing a sterilisation process, must be correctly washed in all its parts,
rinsed possibly with demineralized water and dried so as to reduce the microbiological level present in
the material, thereby eliminating the presence of dirt, oily substances and organic material, because
they can interfere with the sterilisation process.
The factors which determine a correct cleaning process are: chemical (detergent) thermal (the
temperature of the water), time (immersion and soaking time) and mechanical (use of machines or
brushes).
The objects to be sterilised must be cleaned using a detergent with a neutral pH. For operating
theatre instruments it is necessary to use a deproteinizing detergents in conformance with the
instructions of the manufacturers. The detergents must be used in accordance with the instructions for
use, in particular in relation to dilution. A product which needs to be diluted before use must not be
used pure, it is necessary to prepare it in an appropriate container in accordance with the defined
instructions.
Cleaning operations must be carried out separately from packaging operations so as not to cause
adulteration of the packaging materials, in practical terms the material requiring sterilisation must be
packaged in a separate area from the cleaning area.
The personnel involved in this operation must be protected during all cleaning operations to avoid
possible wounds, contact with organic material or detergent products, and they must also be
particularly careful when dealing with pointed or sharp instruments.
The precautions they need to take are: remove the obvious dirt from the object: immerse the
instrument (open or dismantled) into a container in which the proteolytic or detergent has already
been poured for the amount of time indicated by the manufacturers; scrub the object with appropriate
sponges, brushes or cleaning rods internally and externally. Do not use metal brushes or abrasive
products; rinse with running water, preferably demineralized water; dry; check the instrument to
ensure cleaning effectiveness and finally lubricate it with appropriate products with a neutral pH; after
lubrication the surgical instrument must be sprayed with a compressed air pistol to remove excess
lubricant.
When cleaning has to be done by a machine it is necessary to ensure that the instruments do not
move from the instruments holder, thereby eliminating the possibility of them knocking into one
another or being damaged. To avoid coagulation of albuminous substances in the cleaning stage, the
water temperature must only be superior to 45°C if a sufficiently high volume of cleansing product is
used. Disinfection can be of a chemical-thermal or thermal type. In using the cleaning products, or in
the combined use of cleaning and disinfection products, the instructions of the manufacturers must be
followed very precisely (time required to achieve the desired cleansing, concentration, temperature).
Only the exact dosage guarantees a perfect cleaning and disinfection result, as well as treating the
materials in the best possible way.
An insufficient dosage of an alkaline product (which is a false cost saving) brings the risk of corrosion
and must be avoided for pH values superior to 10,5. When using acid based cleaning products
corrosion can be caused by the presence of chlorine. This can be avoided only by using
demineralised water. Instruments made from coloured anodized aluminium lose their colour when the
usual machine cleaning methods are used, and they therefore lose their functional value. In the case
of extremely dirty surgical instruments, which are encrusted with material (coagulated blood,
secretions or other materials), it can be necessary to carry out further manual or ultrasound cleaning.
In this case the instructions for delicate manual handling of microsurgical instruments must be
scrupulously followed.
When the cleaning is done by machine, the residues of the cleaning phase must be eliminated during
the rising phase, otherwise stains or colouration can be caused to the instruments. The use of
neutralising product can help this process and improve the results of rinsing.
In practice, the best rinsing temperature for the treatment of surgical instruments by cleaning and
disinfection machines, has turned out to be between 70 and 75°C.

CISA S.p.A. - User manual Version 2.0/08
CISA Autoclaves
Pag.12
If any corrosion takes place the rinsing temperature must be limited to between 70 and 75°C. By
using demineralized water for rinsing it is possible to avoid stains and corrosion without limiting the
temperature.
Surgical instruments must be taken out of the machine immediately after the cleaning programme
has finished. If instruments are immersed in a cleaning and disinfecting solution before being
cleaned in a machine, they must be rinsed to prevent foam being formed in the machine or a
product must be used to avoid the formation of foam. Instruments with long or tight cavities, as for
example metal catheters, metallic vacuum cleaners, special cannula tubes etc, must be rinsed
internally as well. Machine cleaning of micro-surgical and similarly delicate dental instruments must
be carried out in automated machines only if a secure fastening of the instruments is possible during
the cleaning process. Knobs and complicated parts can be machine cleaned if that is one of the
methods considered by the manufacturer and a secure fastening is assured.
As soon as the machine programme has finished it is necessary to carry out the necessary
treatment of the instrument using appropriate sprays to eliminate any humidity that has penetrated
to the inside. Rotating tools (drills, cutters and abrasive tools) are only suitable for machine cleaning
to a limited extent. Treatment with ultrasound is preferable. The same goes for roots instruments for
roots.
Mirrors covered in rhodium vapours can be machine cleaned. Rigid endoscopes need to be
dismantled in accordance with the manufacturers instructions to allow machine cleaning. All seals
must be removed. Machine cleaning must only be carried out for the endoscope parts expressly
indicated by manufacturers (excluding, for example, the optical part). In any case for machine
cleaning the endoscope parts need to be appropriately supported whilst in the machine.
It is necessary to ensure that even instruments with internal cavities are properly cleaned internally.
Machine cleaning of flexible endoscopes necessitates cleaning and disinfection to be carried out
whilst the machine is closed. The usual apparatus for disinfection and cleaning must not be used.
For chemical-thermal processes the temperature must not exceed 60°C. For endoscopes which
require preparatory cleaning and disinfection before being put into a machine, it is necessary to only
use disinfectants, detergents and detergent additives which produce very little foam. Before
proceeding with other treatment the capacity tests indicated by the manufacturer must be carried
out. In this way it is possible to see perforations and cracks, caused by liquids which have
penetrated to the inside of an instrument, on a timely basis, thereby permitting capacity testing
before or during the cleaning and disinfection programme.
A damaged flexible endoscope must be immediately sent to the manufacturer attaching a
description of the cause of the defect. For machine cleaning, only detergents and disinfectants
suitable for the specific treatment required must be used.
A flexible endoscope must be placed inside the machine in a safe manner. Specific equipment must
be used to ensure the complete rinsing of all canals and external surfaces. If the cleaning is by
machine the final rinsing should be done using sterile demineralized water. Also, it is appropriate to
be equipped with a drying instrument. For cleaning in an ultrasound vessel, surgical instruments
must be placed in an open position on the specific sterilisation baskets. Care must be taken to
ensure that neither the sterilisation baskets nor instruments with large surface areas (e.g. other
types of basket) create shadow zones for the ultrasound. Hot water without any additives does not
give satisfactory cleaning results. It is therefore necessary to add an appropriate cleaning product.
The instructions of the manufacturer regarding concentration and temperature must be followed.
The temperature of cleaning solutions in an ultrasound vessel should be at least 40°C, otherwise the
cleaning effect is not assured. A higher temperature assists the de-gasification of the cleaning
solution ad the effect of the ultrasound treatment. The appropriate use of a suitable product does not
result in the coagulation of albuminous substances even at higher temperatures. At temperatures
between 20 and 25°C only cleaning and disinfection products suitable for use at these temperatures
must be used. An excessive concentration of dirt in the ultrasound vessel harms cleaning
effectiveness. Therefore, the solution must be renewed according to the time intervals indicated by
the manufacturer. In practice an ultrasound treatment duration of 3 to 5 minutes with frequencies of
at least 35 Khz has proved sufficient (in accordance with those prescribed by the manufacturer).
 Loading...
Loading...