Chison ECO 6 Operation Manual
Digital Color Doppler Ultrasound System
Model
ECO 6
V1.2
Sep 12, 2017
OPERATION MANUAL
Direction: 57-00594-00
CHISON Medical Technologies Co., Ltd.
We reserve the rights to make changes to this manual without prior notice.
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL

WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL

ECO 6 Digital Color Doppler Ultrasound System
Regulatory Requirement
This product conforms to the essential requirements of the
Medical Device Directive 93/42/EEC. Accessories without the CE mark are not
guaranteed to meet the Essential Requirements of the Medical Device Directive.
This manual is a reference for the ECO 6. Please verify that you are using the latest
revision of this document. If you need the latest revision, contact your distributor.
NOTE:
Important
1. No part of this manual may be reduced, modified, copied or reprinted, in whole or in part,
without written permission from CHISON.
2. The contents of this manual are subject to change without prior notice and without our legal
obligation.
3. Before operating the system, please read and understand this manual. After reading, keep this
manual in an easily accessible place. If you have any question or doubt, please contact CHISON's
authorized service engineer.
4. CHISON’s Warranty only cover material and parts costs for repairing, but do not cover any
labor cost or onsite service cost at end user's side.
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL

ECO 6 Digital Color Doppler Ultrasound System
NOTE:
Important information
1. It is the customer’s responsibility to maintain and manage the system after
delivery.
2. The warranty does not cover the following items, even during the warranty period:
a) Damage or loss due to misuse or abuse with system and probes, for example,
drop the probe, the liquid or the metal part fall into the system.
b) Damage or loss caused by Acts of God such as fires, earthquakes, floods,
lightning, etc.
c) Damage or loss caused by failure to meet the specified conditions for this
system, such as inadequate power supply, improper installation or environmental
conditions.
d) Damage or loss caused by non-approved transportation by CHISON.
e) Damage or loss due to use the system outside the region where the system was
originally sold.
f) Damage or loss involving the system purchased from a source other than
CHISON or its authorized agents.
3. Do not make changes or modifications to the software or hardware of this system
and probes.
4. During operate the system, if user has any doubt, difficulty or any unclear, please
contact CHISON's authorized service engineer immediately. Please describe the
situation clearly to solve the question in time. Before solve the question, please don’t
operate the system.
5. This system shall not be used by persons other than fully qualified and certified
medical personnel.
6. It is prohibited to use the device for fetal sex examination, except for necessary
medical needs. The device can only be sold to qualified medical institutions or doctors.
The users shall fully understand and master the devices before operating. The users
shall have got the qualification, and shall comply with the local laws and regulations,
the local religion and customs, etc.
7. The System modified or repaired by people other than CHISON’s qualified service
engineers, CHISON shall not be liable for the system.
8. The purpose of this system is to provide physicians with data for clinical diagnosis.
It is the physician’s responsibility for diagnostic procedures. CHISON shall not be
liable for the results of diagnostic procedures
9. This manual contains warnings regarding foreseeable potential dangers, but user
shall always be alert to dangers other than those indicated as well. CHISON shall not
be liable for damage or loss that results from negligence or from ignoring the
precautions and operating instructions described in this operation manual.
10. Due to negligence not following operation manual, CHISON shall not be liable
for the results.
11. Each time before and after ultrasound examination, please check the probe
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL
ECO 6 Digital Color Doppler Ultrasound System
Caution: It is prohibited to use the device for fetal sex examination, except for necessary
medical needs. The device can only be sold to qualified medical institutions or doctors. The users
shall fully understand and master the devices before operating. The users shall have got the
qualification, and shall comply with the local laws and regulations, the local religion and customs,
etc.
Caution: The users should read the operation manual carefully before operating the devices.
Turning on the device means the users have read the operation manual and accept the listed
cautions, warnings, and notes in the manuals. If the users disagree and cannot accept the cautions,
the users can ask for returning the device.
surface, probe cable and sheath whether they are abnormal, such as cracking, peeling
and deformation. Also check whether the lens is strongly fixed. Abnormal probes may
cause electric shock and injure the patient. Once any abnormal, user must stop using
and contact CHISON's authorized service engineer.
12. If the probe is dropped or scratched by hard part, please stop using the probe
immediately. And contact CHISON's authorized service engineer to make sure the
safety and effectiveness is in good condition before use.
13. If there is any liquid or metal to enter to the system, please power off the system
and stop using it immediately. Please first contact CHISON’s authorized service
engineer to make sure it’s safe before restart using it.
14. Please don't use solvents (such as paint thinner, benzine, or alcohol) or abrasive
cleansers for cleaning the system (including monitor and probes, etc). It may corrode
the system and probes.
15. While the system or probe is over life time, please refer to operation manual
section 9.4
16. Important data must be backed up on external memory media. CHISON shall not
be liable for loss of data stored in the memory of this system caused by operator error
or accidents.
17. Please put this operation manual with the system to ensure operator and manager
can reach it at any time.
18. LCD display screen may have some dark or light dots, it is normal for the LCD. It
does not mean that LCD screen is defective.
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL

ECO 6 Digital Color Doppler Ultrasound System
Content
Chapter 1 Introduction .............................................................................................................................. 1
1.1 System Overview ...................................................................................................................... 1
1.2 Contact Information ................................................................................................................ 1
Chapter 2 System Safety........................................................................................................................... 2
2.1 Safety Overview ........................................................................................................................ 2
2.2 Electrical Safety ........................................................................................................................ 3
2.3 Label.. ................................ ................................................................ ................................ ........ 5
2.3.1 Warning Symbols ............................................................................................................ 5
2.4 Patient Environmental Devices ............................................................................................... 6
2.5 Biological Safety ....................................................................................................................... 8
2.6 Scanning Patients and Education ........................................................................................... 9
2.6.1 Safe Scanning Guidelines ............................................................................................... 9
2.6.2 Understanding the MI/TI Display ................................................................................. 11
2.7 Battery Handling Instructions .............................................................................................. 14
Chapter 3 System Introduction ............................................................................................................... 16
3.1 Consol Overview ..................................................................................................................... 16
3.2 Physical Specification ............................................................................................................. 16
3.3 System View in Different Views ............................................................................................ 17
3.4 Function Introduction ............................................................................................................ 18
3.4.1 Image Modes ................................................................................................................. 18
3.4.2 Accessories.................................................................................................................... 19
3.5 Installation Procedures .......................................................................................................... 20
3.5.1 Environment Condition ................................................................................................. 20
3.5.2 Powering the System..................................................................................................... 23
3.5.3 Probe Installation .......................................................................................................... 23
3.5.4 Accessories Installation ................................................................................................. 24
Chapter 4 Control Panel ......................................................................................................................... 25
4.1 Keyboard Appearance ........................................................................................................... 25
1
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL

ECO 6 Digital Color Doppler Ultrasound System
4.2 Alphanumeric Keyboard ....................................................................................................... 25
4.3 Function Keys/Knob .............................................................................................................. 26
4.4 Parameter Control key .......................................................................................................... 28
4.5 STC ................................................................................................................................ .......... 28
4.6 Central Control ...................................................................................................................... 28
4.7 Information Area Indicating Machine Status ...................................................................... 29
4.7.1 Indicator Light .............................................................................................................. 29
Chapter 5 Operation and Exam Mode .................................................................................................... 31
5.1 Preparing the System for Use ................................................................................................ 31
5.1.1 The Device Inspection .................................................................................................. 31
5.1.2 Power On ...................................................................................................................... 31
5.2 Choose Exam Mode ................................................................................................................ 31
5.2.1 The Probe Identification................................................................................................ 31
5.2.2 Mode Selection ............................................................................................................. 31
5.3 Patient Data Entry ................................................................................................................. 32
5.4 Image Interface Display ......................................................................................................... 33
5.5 Display Mode .......................................................................................................................... 33
5.5.1 B Mode ......................................................................................................................... 33
5.5.2 B/B Mode ...................................................................................................................... 33
5.5.3 4B Mode ....................................................................................................................... 33
5.5.4 B/M Mode ..................................................................................................................... 34
5.5.5 M Mode ........................................................................................................................ 34
5.5.6 CFM Mode .................................................................................................................... 34
5.5.7 B/BC Mode ................................................................................................................... 35
5.5.8 CPA (PD) Mode ............................................................................................................ 35
5.5.9 DPD Mode .................................................................................................................... 35
5.5.10 CW Mode .................................................................................................................... 35
5.5.11 TDI Mode .................................................................................................................... 35
5.5.12 B steer ......................................................................................................................... 35
5.5.13 Trapezoidal Mode ....................................................................................................... 35
2
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL

ECO 6 Digital Color Doppler Ultrasound System
5.5.14 Biospy and Super Needle ............................................................................................ 36
5.5.15 PW Mode .................................................................................................................... 36
5.6 B Image Adjustment .............................................................................................................. 37
5.6.1 Parameters Adiustment ................................................................................................. 37
5.6.2 B Menu Adjustment ...................................................................................................... 38
5.63 Accessories..................................................................................................................... 40
5.7 CFM Image Adjustment ........................................................................................................ 40
5.8 PW / CW Image Adjustment ................................................................................................. 41
5.8.1 Parameters in PW Mode ............................................................................................... 41
5.8.2 Parameters in CW Mode ............................................................................................... 42
5.9 M Image Menu & Parameters Adjustment ......................................................................... 43
5.10 CPA/DPD/TDI Image Adjustment ..................................................................................... 43
5.10.1 CPA & DPD Parameter and Menu Adjustment ........................................................... 43
5.10.2 TDI Image Adjustment................................................................................................ 44
5.11 Full Screen Show .................................................................................................................. 45
5.12 Edit Comment ....................................................................................................................... 45
5.12.1 Overview ..................................................................................................................... 45
5.12.2 Input Characters .......................................................................................................... 45
5.12.3 Input Comment Library Characters ............................................................................ 46
5.12.4 Edit Quick Comments ................................................................................................. 46
5.12.5 Input Quick Comments ............................................................................................... 46
5.12.6 Move Comments ......................................................................................................... 46
5.12.7 Edit Comments ........................................................................................................... 46
5.12.8 Delete Comments ........................................................................................................ 46
5.12.9 Set the Position of Default Comment.......................................................................... 47
5.13 Set Body Mark ................................ ................................ ...................................................... 47
5.13.1 General Description .................................................................................................... 47
5.13.2 Body Mark Operation ................................................................................................. 51
5.14 Image and Cine Disposition ................................................................................................. 51
5.14.1 The Principle of Cine Storage ..................................................................................... 51
3
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL

ECO 6 Digital Color Doppler Ultrasound System
5.14.2 Manual Loop ................................................................ ............................................... 52
5.14.3 Automatic Loop .......................................................................................................... 52
5.14.4 Save and Recall Image ................................................................................................ 52
5.14.5 Save and Recall Cine .................................................................................................. 52
5.14.6 Delete images .............................................................................................................. 52
5.14.7 Send images ................................................................................................................ 52
5.15 Image Browse ....................................................................................................................... 53
5.16 Archive Management ........................................................................................................... 53
5.17 Report .................................................................................................................................... 54
5.18 DICOM ................................................................................................................................. 56
5.18.1 DICOM Worklist ......................................................................................................... 56
5.18.2 DICOM Storage .......................................................................................................... 56
5.18.3 DICOM Print .............................................................................................................. 57
5.18.4 DICOM SR ................................................................................................................. 57
Chapter 6 Measurement and Calculation ................................................................................................ 58
6.1 Keyboard for Measurement .................................................................................................. 58
6.1.1 Trackball ....................................................................................................................... 58
6.1.2 [ENTER] ................................ ................................ ....................................................... 58
6.1.3 [UPDATE] .................................................................................................................... 58
6.1.4 [DEL] ............................................................................................................................ 59
6.1.5 [Change] ....................................................................................................................... 59
6.1.6 [Exit] ............................................................................................................................. 59
6.1.7 Parameters control button ............................................................................................. 59
6.2 B Mode general Measurement methods ............................................................................... 59
6.2.1 Meas. Distance .............................................................................................................. 59
6.2.2 Ellipse ........................................................................................................................... 59
6.2.3 Trace ............................................................................................................................. 60
6.2.4 Histogram...................................................................................................................... 60
6.2.5 Profile ........................................................................................................................... 61
6.3 B Fast Measurement .............................................................................................................. 61
4
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL

ECO 6 Digital Color Doppler Ultrasound System
6.4 B General Measurement ........................................................................................................ 62
6.5 ABD Measurement ................................................................................................................. 63
6.6 OB Measurement ................................................................................................................... 65
6.6.1 Twins Measurement ...................................................................................................... 67
6.6.2 EDD (estimated date of delivery) Estimation ............................................................... 67
6.6.3 Growth curves ................................................................ ............................................... 67
6.7 Pediatric Measurement .......................................................................................................... 68
6.7.1 HIP Angle...................................................................................................................... 68
6.8 GYN Measurement ................................................................................................................ 69
6.9 Small Parts Measurement ..................................................................................................... 69
6.10 B Mode Vessel Measurement .............................................................................................. 70
6.11 Urology Measurement .......................................................................................................... 70
6.12 Cardiac Measurement .......................................................................................................... 71
6.13 Normal Measurement in M, B/M mode ............................................................................. 74
6.13.1 Distance ...................................................................................................................... 74
6.13.2 Time ............................................................................................................................ 74
6.13.3 Heart rate..................................................................................................................... 75
6.13.4 Velocity ....................................................................................................................... 75
6.14 General Measurement in M mode ...................................................................................... 75
6.15 M Abdomen Measurement .................................................................................................. 75
6.16 M OB Measurement ............................................................................................................. 76
6.17 M GYN Measurement .......................................................................................................... 76
6.18 M Mode Cardiac Measurement .......................................................................................... 76
6.19 M Urology Measurement ..................................................................................................... 78
6.20 M Small Parts Measurement ............................................................................................... 78
6.21 M Pediatric Measurement ................................................................................................... 78
6.22 PW mode measurement methods ........................................................................................ 78
6.22.1 Velocity ....................................................................................................................... 79
6.22.2 Time ............................................................................................................................ 79
6.22.3 HR ............................................................................................................................... 79
5
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL

ECO 6 Digital Color Doppler Ultrasound System
6.22.4 Auto Trace ................................................................................................................... 79
6.22.5 Manual Trace .............................................................................................................. 79
6.23 PW Fast Measurement ......................................................................................................... 79
6.24 PW Genearl Measurement .................................................................................................. 81
6.25 PW Abdomen Measurement ............................................................................................... 83
6.26 PW OB Measurement .......................................................................................................... 84
6.27 PW GYN Measurement ....................................................................................................... 84
6.28 PW Cardiology Measurement ............................................................................................. 85
6.29 PW Vascular Measurement ................................................................................................. 90
6.30 PW Urology Measurement .................................................................................................. 91
6.31 PW Small parts Measurement ............................................................................................ 91
6.32 PW Pediatric Measurement ................................................................................................ 91
Chapter 7 Preset ...................................................................................................................................... 92
7.1 General setting ........................................................................................................................ 92
7.2 Measurement .......................................................................................................................... 93
7.2.1 General measurement setting ........................................................................................ 93
7.2.2 Measurement formula setting........................................................................................ 95
7.3 Comment ............................................................................................................................... 102
7.3.1 Comment Library ........................................................................................................ 102
7.3.2 Edit Comment ............................................................................................................. 103
7.3.3 Comment and Arrow Option ....................................................................................... 103
7.4 Body marks ........................................................................................................................... 104
7.4.1 Body Marks Library .................................................................................................... 104
7.4.2 Bodymark edition ....................................................................................................... 104
7.4.3 BodyMark Option ....................................................................................................... 105
7.5 Exam Mode ........................................................................................................................... 105
7.5.1 Exam Mode Edit ......................................................................................................... 105
7.5.2 Exam Mode Selection ................................................................................................. 105
7.5.3 ExamMode config ....................................................................................................... 106
7.5.4 Image Freeze Config ................................................................................................... 107
6
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL

ECO 6 Digital Color Doppler Ultrasound System
7.6 Keyboard ............................................................................................................................... 108
7.7 DICOM ................................................................................................................................. 109
7.7.1 Add/Edit DICOM Function ......................................................................................... 110
7.8 NET Work ............................................................................................................................ 110
7.9 System ................................................................................................................................... 111
7.9.1 System information ..................................................................................................... 111
7.9.2 Update ......................................................................................................................... 111
7.9.3 Function Setting .......................................................................................................... 111
7.9.4 Installment setting ....................................................................................................... 111
7.9.5 Video VGA ................................................................................................................. 112
7.9.6 Hardware function ...................................................................................................... 112
7.9.7 System Maintenance ................................................................................................... 112
7.9.8 USB Video Printer Option .......................................................................................... 112
Chapter 8 System Maintenance ............................................................................................................ 113
8.1 Machine Cleaning ................................................................................................................. 113
8.2 Probe Maintenance .............................................................................................................. 113
8.3 Safety Check ......................................................................................................................... 114
8.4 Malfunction Check ............................................................................................................... 115
Chapter 9 Probes................................................................................................................................... 116
9.1 General Description ............................................................................................................. 116
9.2 Care and Maintenance ......................................................................................................... 116
9.2.1 Inspecting Probes ........................................................................................................ 116
9.2.2 Cleaning and Disinfecting ........................................................................................... 117
9.3 Probe Operation Instructions.............................................................................................. 125
9.3.1 Scanning the Patient .................................................................................................... 125
9.3.2 Operating Transvaginal probe ..................................................................................... 125
9.3.3 Cleaning and Disinfecting TV and TR Probes ............................................................ 126
9.4 Service Responsibility .......................................................................................................... 127
Appendix A: The Information of EC Representative ........................................................................... 129
Appendix B: Acoustic Output Report Table ......................................................................................... 130
7
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL

ECO 6 Digital Color Doppler Ultrasound System
Appendix C: Guidance and Manufacturer’s Declaration ...................................................................... 179
Appendix D: Measurement Results Summary...................................................................................... 183
Appendix E: Display Accuracy and Acoustic Measurement Uncertainties .......................................... 184
Appendix F: Transducer Maximum Surface Temperature .................................................................... 185
Appendix G: Procedures of set network sharing in ECO series ........................................................... 186
8
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL

ECO 6 Digital Color Doppler Ultrasound System
CHISON website
www.chison.com
Service Support
CHISON Medical Technologies Co., Ltd.
Tel:0086-0510-85311707
Fax: 0086-0510-85310726
E-mail: service@chison.com.cn
Placing an Order
CHISON Medical Technologies Co., Ltd.
Tel: 0086-0510-8531-0593/0937
Fax: 0086-0510-85310726
Email: export@chison.com.cn
Manufacturer
CHISON Medical Technologies Co., Ltd.
No.228, Changjiang East Road, Block 51 and 53, Phase 5, Shuofang Industrial
Park, Xinwu District, Wuxi, Jiangsu, China, 214142
No.9, Xinhuihuan Road, Xinwu District, Wuxi, Jiangsu, China 214028
Chapter 1 Introduction
This manual contains necessary information for safe system operation.
Read and understand all instructions in this manual before operating the system. Always keeping this manual
with the equipment, and periodically review the procedures for operation and safety precautions.
1.1 System Overview
Indications for Use
The device is a general-purpose ultrasonic imaging instrument intended for use by a qualified physician for
evaluation of Fetal/OB; Abdominal (GYN & Urology); Pediatric; Small Organ(breast, testicle, thyroid);
Cardiac (adult & pediatric); Peripheral Vascular; Musculo-skeletal Conventional & Superficial; Transrectal and
Transvaginal.
Contraindication
The system is NOT intended for ophthalmic use or any use that causes the acoustic beam to pass through the
eye.
1.2 Contact Information
For additional information or assistance, please contact your local distributor or the appropriate support
resource shown below:
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL
1

ECO 6 Digital Color Doppler Ultrasound System
Chapter 2 System Safety
2.1 Safety Overview
This section discusses measures to ensure the safety of both the operator and patient. To ensure the safety of
both operator and patient, please read the relevant details in this chapter carefully before operating this system.
Disregarding the warnings or violation of relevant rules may result in personal injury or even loss of life
for operator or patient.
Users should observe the following precautions:
This system complies with Type BF general equipment, and the IEC standard.
Do not modify this system in any way. Necessary modifications must be made only by the manufacturer or
its designated agents.
This system has been fully adjusted at the factory. Do not adjust any fixed adjustable parts.
In the event of a malfunction, turn off the system immediately and inform the manufacturer or its
designated agents.
The power cable of the system should only be connected to a grounded power socket. Do not remove the
ground cable for any reason.
Only connect this system, either electronically or mechanically, with devices that comply with the
EN60601-1 standard. Recheck the leakage current and other safety performance indices of the entire system to
avoid potential system damage caused by leakage from a current superposition.
The system does not incorporate any specialized protective measures in the event it is configured with
high-frequency operation devices. The operator should use caution in these types of applications.
The system should be installed only by personnel authorized by the manufacturer. Do not attempt to install
the system by yourself.
Only an authorized service engineer may perform maintenance.
Only a qualified operator, or someone under qualified supervision, should use the system.
Do not use this system in the presence of flammable substances, otherwise an explosion may occur.
Do not continuously scan the same part of a patient or expose the patient to prolonged scanning; otherwise
it may harm the patient.
When using the system for ultrasound testing, use only qualified ultrasound gel that complies with system
standards.
Do not unplug probe when the system is in active operation. Always go to EXAM screen when need to
remove the probe.
To prevent from arm or neck injury, the operator should not stay at the same position for too long during
patient scanning without taking break.
Do not put liquid on top of the main unit.
NOTE
*The system has built-in screen saver to avoid the tic mark on the display. It is not recommended to constantly
turn on and off the unit.
*To dispose of this product properly, please call your local service department.
2
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL
ECO 6 Digital Color Doppler Ultrasound System
2.2 Electrical Safety
Type of protection against electric shock
Class I Equipment
CLASS I EQUIPMENT in which protection against electric shock does not rely on BASIC INSULATION
only, but includes a protective earth ground. This additional safety precaution prevents exposed metal parts
from becoming LIVE in the event of an insulation failure.
NOTE: The mains supply shall be cut off after disconnecting the power line and the net power.
Degree of protection against electric shock
Type BF Applied part (for Probes marked with BF symbol)
TYPE BF APPLIED PART providing a specified degree of protection against electric shock, with particular
regard to allowable LEAKAGE CURRENT
Level of protection against harmful ingress of water
Parts of probe likely to come into contact with operator or patient meet the requirements of drip-proof
equipment (IPX1)
Parts of probe intended to be immersed in normal use meet the requirements of watertight equipment (IPX7)
The IP Classification of System is Ordinary Equipment (IPX0)
Safety level when used in the presence of FLAMMABLE ANAESTHETIC
MIXED WITH AIR (or WITH OXYGEN or WITH NITROUS OXIDE):
The Equipment is not suitable for use in the environment with FLAMMABLE ANAESTHETIC MIXED
WITH AIR (or WITH OXYGEN or WITH NITROUS OXIDE)
Mode of operation
Continuous Operation
For maximum safety, always follow these guidelines:
Proper grounding of the system is critical to avoid electrical shock. For protection, ground the chassis with
a three-wire cable and plug, and plug the system into a hospital-grade, three-hole outlet.
Do not remove or circumvent the grounding wire.
Do not remove the protective covers on the system. These covers protect users from hazardous voltages.
Cabinet panels must remain in place while the system is in use. A qualified electronic technician must make all
internal replacements.
Do not operate this system in the presence of flammable gases or anesthetics.
All peripheral devices (unless certified as medical grade) that are connected to the system must be powered
through the electrical outlet through an optional isolation transformer.
3
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL

ECO 6 Digital Color Doppler Ultrasound System
Notice upon Installation of Product
Separation distance and effect from fixed radio communications equipment: field strengths from fixed
transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur
radio, AM and FM radio broadcast, and TV broadcast transmitter cannot be predicted theoretically with
accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site
survey should be considered. If the measured field strength in the location in which the ultrasound system is
used exceeds the applicable RF compliance level as stated in the immunity declaration, the ultrasound system
should be observed to verify normal operation. If abnormal operation is observed, additional measures may be
necessary, such as re-orienting or relocating the ultrasound system or using an RF shielded examination room
may be necessary.
Use either power supply cords provided or designated by CHISON. Products equipped with a power source
plug should be plugged into the fixed power socket which has the protective grounding conductor. Never use
any adaptor or converter to connect with a power source plug (e.g. three-prong-to-two-prong converter).
Locate the equipment as far away as possible from other electronic equipment.
Be sure to use only the cables provided by or designated by CHISON. Connect these cables following the
installation procedures (e.g. wire power cables separately from signal cables).
Lay out the main equipment and other peripherals following the installation procedures described in this
manual.
Notice against User Modification
The user should never modify this product.
User modifications may cause degradation in Electrical Safety. Modification of the product includes changes
in:
Cables (length, material, wiring, etc.)
System configuration/components
User modifications may cause degradation in EMC performance. Modification of the product includes changes
in:
Cables (length, material, wiring, etc.)
System installation/layout
System configuration/components
Securing system parts (cover open/close, cover screwing)
4
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL
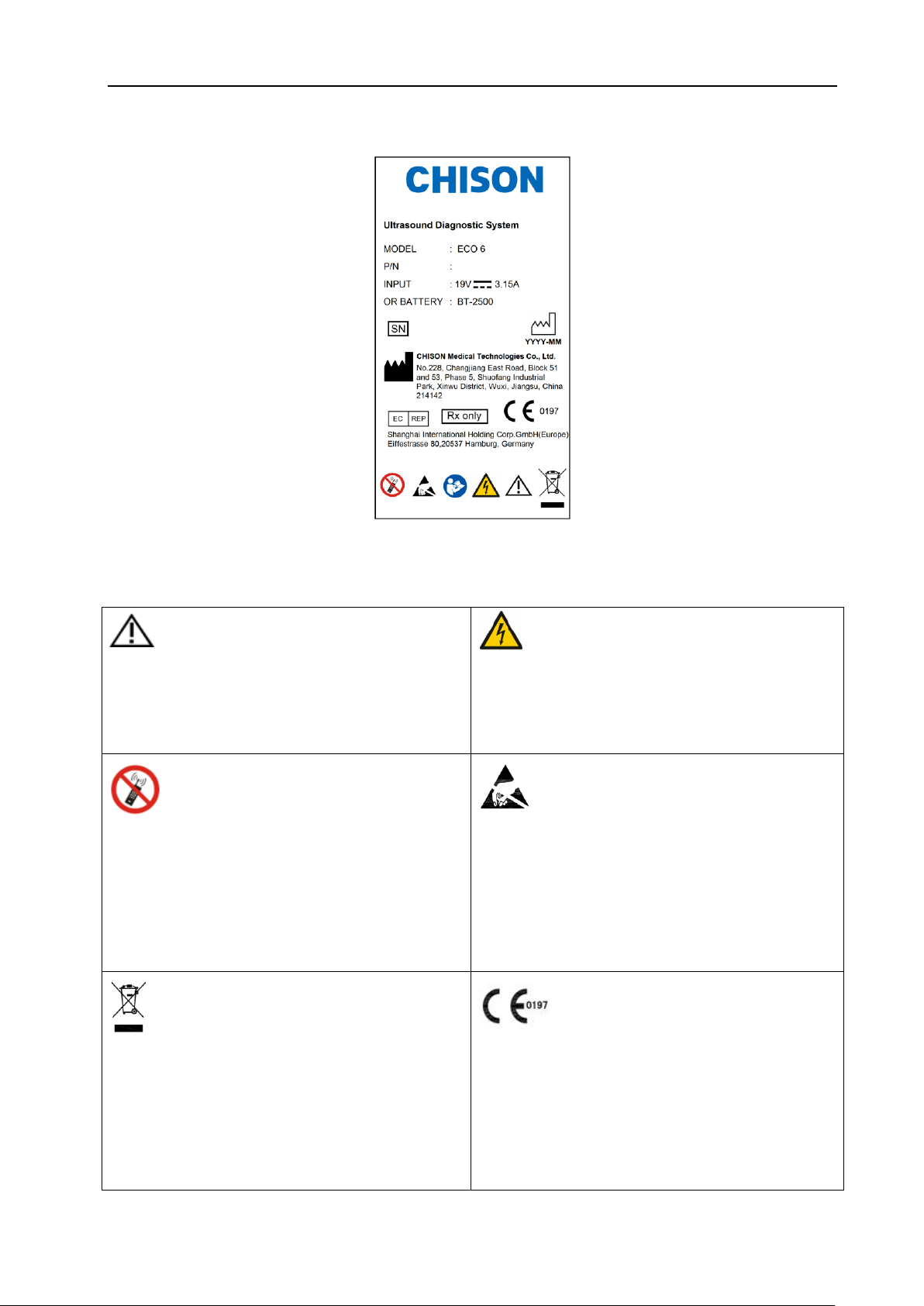
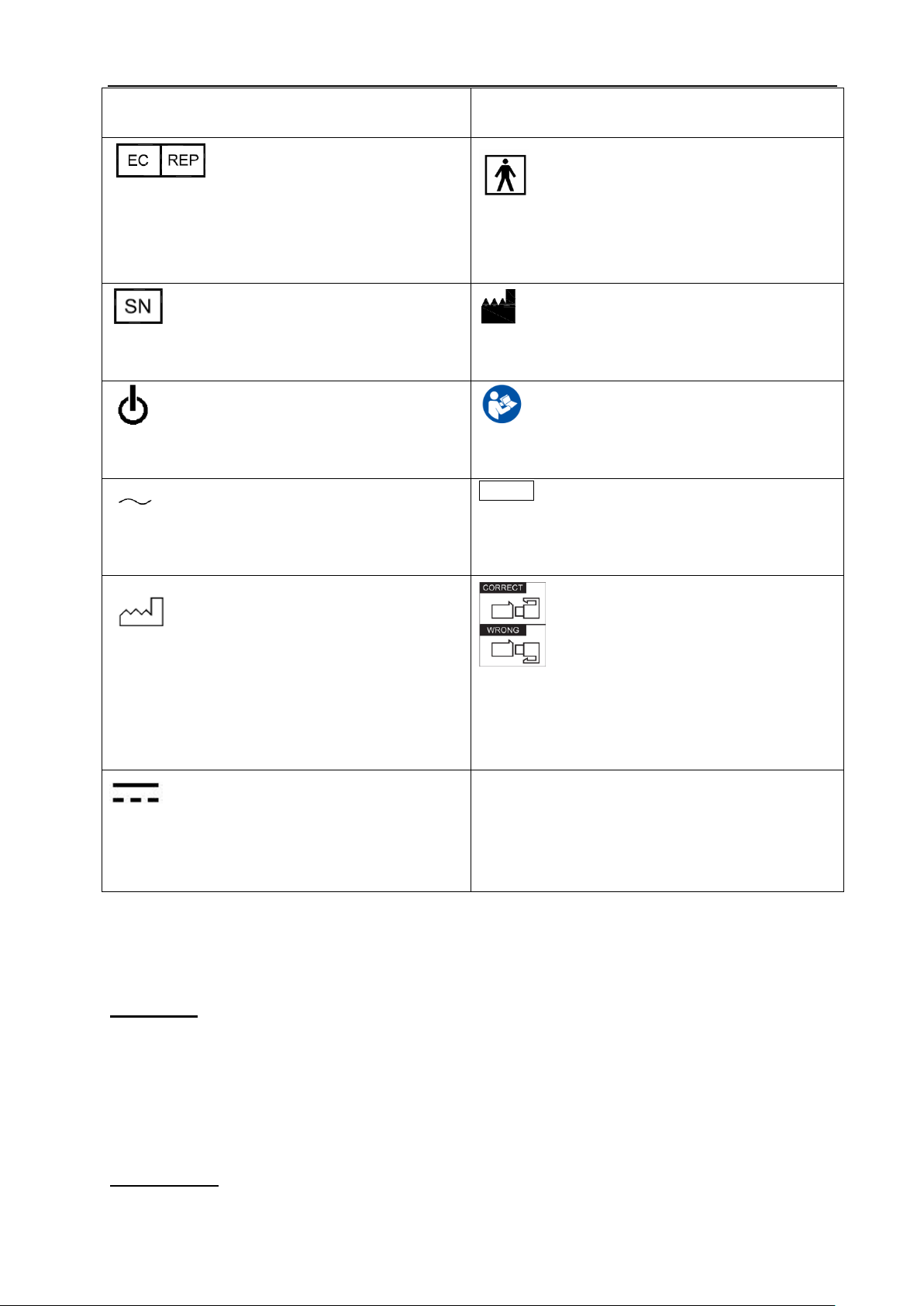
2.3 Label
Caution, consult accompanying documents.
This symbol advises the reader to consult the
accompanying documents for important safety related
information such as warnings and pre-cautions that
cannot be presented on the device itself.
Dangerous electric voltage. Unplug the main
plug before opening the system!
Do not use the following devices near this
equipment: cellular phone, radio receiver, and mobile
radio transmitter, radio controlled toy, etc. Use of
these devices near this equipment could cause this
equipment to perform outside the published
specifications. Keep power to these devices turned off
when near this equipment.
Be careful of static.
WASTE OF ELECTRICAL AND
ELECTRONIC EQUIPMENT (WEEE): This symbol
is used for Environment Protection, it indicates that
the waste of electrical and electronic equipment must
not be disposed as unsorted waste and must be
collected separately. Please contact your local
Authority or distributor of the manufacturer for
The CE mark of Conformity indicates this
equipment conforms with the Council Directive
93/42/EEC
ECO 6 Digital Color Doppler Ultrasound System
Fig. 1 Real panel label
2.3.1 Warning Symbols
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL
5
ECO 6 Digital Color Doppler Ultrasound System
information concerning the decommissioning of your
equipment.
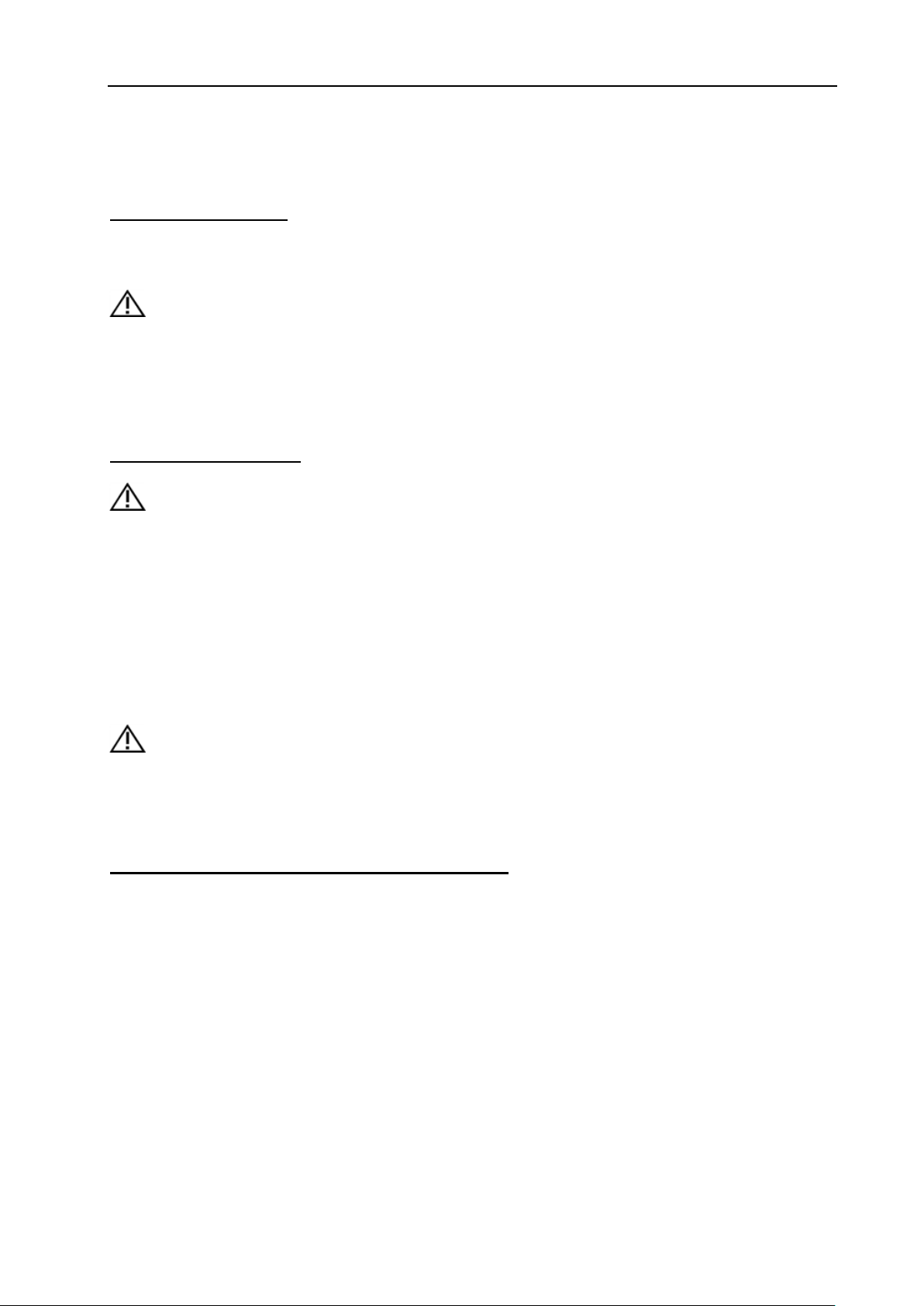
AUTHORIZED REPRESENTATIVE
IN THE EUROPEAN COMMUNITY: This symbol is
accompanied by the name and the address of the
authorized representative in the European
Community.
Type-BF applied part
This symbol is followed by the serial
number of the device.
MANUFACTURER: This symbol is
accompanied by the name and the address of the
manufacturer.
Power On/off.
CAUTION: This Power Switch cannot isolate Mains
Supply completely.
Refer to instruction manual/booklet.
The “Alternating current” symbol indicates
that the equipment is suitable for alternating current
only.
Rx only
This symbol indicates that in the united states of
America, Federal law restricts the device to sale by or
on the order of a licensed practitioner or therapist.
This symbol is followed by the manufacturing date of
the device in the form YYYY-MM.
CORRECT: The correct connection of the battery
connector
WRONG: The wrong connection of the battery
connector
Direct current
To indicate on the rating plate that the equipment is
suitable for direct current only; to identify relevant
terminals.
2.4 Patient Environmental Devices
Left side:
1 LAN port
1 VGA port: External monitor
2 USB ports
1 Footswitch port
1 Power in port
Rear panel:
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL
6
ECO 6 Digital Color Doppler Ultrasound System
2 Probe ports
1 USB port
1 Video out port
1 Remote port
Acceptable Devices
The Patient Environmental devices shown above are specified to be suitable for use within the PATIENT
ENVIRONMENT.
CAUTION:
DO NOT connect any probes or accessories without approval by CHISON within the PATIENT
ENVIRONMENT.
DO NOT touch patient and devices without IEC/EN 60601-1 approval to avoid the leakage current risk
within the PATIENT ENVIRONMENT.
Unapproved Devices
CAUTION:
DO NOT use unapproved devices.
If devices are connected without the approval of CHISON, the warranty will be INVALID.
The system can’t be used with HF surgical equipment; otherwise the burns to patient may occur.
Any device connected to this system must conform to one or more of the requirements listed below:
IEC standard or equivalent standards appropriate to devices.
The devices shall be connected to PROTECTIVE EARTH (GROUND).
CAUTION:
Unsafe operation or malfunction may result. Use only the accessories, options and supplies approved or
recommended in these instructions for use.
Peripheral used in the patient environment
The system has been verified for overall safety, compatibility and compliance with the following on-board
image recording devices:
B/W video printer: SONY UP-X898MD
The system may also be used safely while connected to devices other than those recommended above if the
devices and their specifications, installation, and interconnection with the system conform to the requirements
of IEC/EN 60601-1.
Adapter is considered as a part of ME equipment
The connection of equipment or transmission networks other than as specified in the user instructions can
result in an electric shock hazard or equipment malfunction. Substitute or alternate equipment and connections
require verification of compatibility and conformity to IEC/EN 60601-1 by the installer. Equipment
modifications and possible resulting malfunctions and electromagnetic interference are the responsibility of the
owner.
7
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL

ECO 6 Digital Color Doppler Ultrasound System
General precautions for installing an alternate off-board, remote device or a network would include:
The added device(s) must have appropriate safety standard conformance and CE Marking.
There must be adequate mechanical mounting of the device and stability of the combination.
Risk and leakage current of the combination must comply with IEC/EN 60601-1.
Electromagnetic emissions and immunity of the combination must conform to IEC/EN 60601-1-2.
Peripheral used in the non-patient environment
The system has been verified for compatibility, and compliance for connection to a local area network (LAN)
via a wire LAN, provided the LAN components are IEC/EN 60601-1 compliant.
General precautions for installing an alternate off-board, remote device or a network would include:
The added device(s) must have appropriate safety standard conformance and CE Marking.
The added device(s) must be used for their intended purpose having a compatible interface.
CAUTION: Make sure using ONLY the dedicated USB disk or removable media to save or back up data.
Before connecting to the ultrasound system, make sure using the latest antivirus software on the USB disk or
removable media to clean any virus. It is user’s responsibility to ensure the USB disk or removable media is
virus-free. Improper use of USB disk or removable media may cause the virus infections of system and
eventually malfunction may occur. Such malfunction may impact the stability, effectiveness and safety of the
system and probes, and users should immediately stop using the system and probes until CHISON authorized
engineer has checked the system and confirm the effectiveness and safety of the system and probes.
CAUTION: Use only secure Local Area Network connection. Don’t connect the ultrasound system to
Internet. Make sure your hospital’s firewall software is configured correctly, thus blocking incoming
connection requests from Internet. Improper use of network connection may cause the virus infections of
system and eventually malfunction may occur.
2.5 Biological Safety
This product, as with all diagnostic ultrasound equipment, should be used only for valid reasons and should be
used both for the shortest period of time and at the lowest power settings necessary (ALARA - As Low As
Reasonably Achievable) to produce diagnostically acceptable images. The AIUM offers the following
guidelines:
Clinical Safety Quoted from AIUM
Approved March 26, 1997
Diagnostic ultrasound has been in use since the late 1950s. Given its known benefits and recognized efficacy
for medical diagnosis, including use during human pregnancy, the American Institute of Ultrasound in
Medicine herein addresses the clinical safety of such use:
There are no confirmed biological effects on patients or instrument operators caused by exposures from
present diagnostic ultrasound instruments. Although the possibility exists that such biological effects may be
identified in the future, current data indicate that the benefits to patients of the prudent use of diagnostic
ultrasound outweigh the risks, if any that may be present.
8
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL

ECO 6 Digital Color Doppler Ultrasound System
Heating: Elevating tissue temperature during obstetrical examinations creates medical concerns. At the embryo
development stage, the rise in temperature and the length of time exposed to heat combine to determine
potential detrimental effects. Exercise caution particularly during Doppler/Color exams. The Thermal Index
(TI) provides a statistical estimate of the potential temperature elevation (in centigrade) of tissue temperature.
Three forms of TI are available: Soft Tissue Thermal Index (TIS), Bone Thermal Index (TIB) and Cranial
Bone Thermal Index (TIC).
Soft Tissue Thermal Index (TIS). Used when imaging soft tissue only, it provides an estimate of potential
temperature increase in soft tissue.
Bone Thermal Index (TIB). Used when bone is near the focus of the image as in the third trimester OB
examination, it provides an estimate of potential temperature increase in the bone or adjacent soft tissue.
Cranial Bone Thermal Index (TIC). Used when bone is near the skin surface as in transcranial examination, it
provides an estimate of potential temperature increase in the bone or adjacent soft tissue.
Cavitations: Cavitations may occur when sound passes through an area that contains a cavity, such as a gas
bubble or air pocket (in the lung or intestine, for example). During the process of cavitations, the sound wave
may cause the bubble to contract or resonate. This oscillation may cause the bubbles to explode and damage
the tissue. The Mechanical Index (MI) has been created to help users accurately evaluate the likelihood of
cavitations and the related adverse effects.
MI recognizes the importance of non-thermal processes, cavitations in particular, and the Index is an attempt to
indicate the probability that they might occur within the tissue.
2.6 Scanning Patients and Education
The Track-3 or IEC60601-2-37 output display standard allows users to share the responsibility for the safe use
of this ultrasound system. Follow these usage guidelines for safe operation:
In order to maintain proper cleanliness of the probes, always clean them between patients.
Always use a disinfected sheath on all EV/ER probes during every exam.
Continuously move the probe, rather than staying in a single spot, to avoid elevated temperatures in one
part of the patient’s body.
Move probe away from the patient when not actively scanning.
Understand the meaning of the TI, TIS, TIB, TIC and MI output display, as well as the relationship between
these parameters and the thermal/cavitation bioeffect to the tissue.
Expose the patient to only the very lowest practical transmit power levels for the shortest possible time to
achieve a satisfactory diagnosis (ALARA - As Low As Reasonably Achievable).
2.6.1 Safe Scanning Guidelines
Ultrasound should only be used for medical diagnosis and only by trained medical personnel.
Diagnostic ultrasound procedures should be done only by personnel fully trained in the use of the
equipment, in the interpretation of the results and images, and in the safe use of ultrasound (including
education as to potential hazards).
Operators should understand the likely influence of the machine controls, the operating mode (e.g. B mode)
and probe frequency on thermal and cavitation hazards.
9
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL
ECO 6 Digital Color Doppler Ultrasound System
TI
Maximum exposure time
(minutes)
0.7
60
1.0
30
1.5
15
2.0
4
2.5
1
Select a low setting for each new patient. Output should only be increased during the examination if
penetration is still required to achieve a satisfactory result, and after the Gain control has been moved to its
maximum value.
Maintain the shortest examination time necessary to produce a useful diagnostic result.
Do not hold the probe in a fixed position for any longer than is necessary. The frozen frame and Cine loop
capabilities allow images to be reviewed and discussed without exposing the patient to continuous scanning.
Do not use endo-cavitary probes if there is noticeable self heating of the probe when operating in the air.
Although applicable to any probe, take particular care during trans- vaginal exams during the first eight weeks
of gestation.
Take particular care to reduce output and minimize exposure time of an embryo or fetus when the
temperature of the mother is already elevated.
Take particular care to reduce the risk of thermal hazard during diagnostic ultrasound when exposing: an
embryo less than eight weeks after gestation; or the head, brain or spine of any fetus or neonate.
Operators should continually monitor the on-screen thermal index (TI) and mechanical index (MI) values
and use control settings that keep these settings as low as possible while still achieving diagnostically useful
results. In obstetric examinations, TIS (soft tissue thermal index) should be monitored during scans carried out
in the first eight weeks after gestation, and TIB (bone thermal index) thereafter. In applications where the
probe is very close to bone (e.g. trans-cranial applications), TIC (cranial bone thermal index) should be
monitored.
MI> 0.3 there is a possibility of minor damage to neonatal lung or intestine. If such exposure is necessary,
reduce the exposure time as much as possible.
MI> 0.7 there is a risk of cavitations if an ultrasound contrast agent containing gas micro-spheres is being
used. There is a theoretical risk of cavitations without the presence of ultrasound contrast agents. The risk
increases with MI values above this threshold.
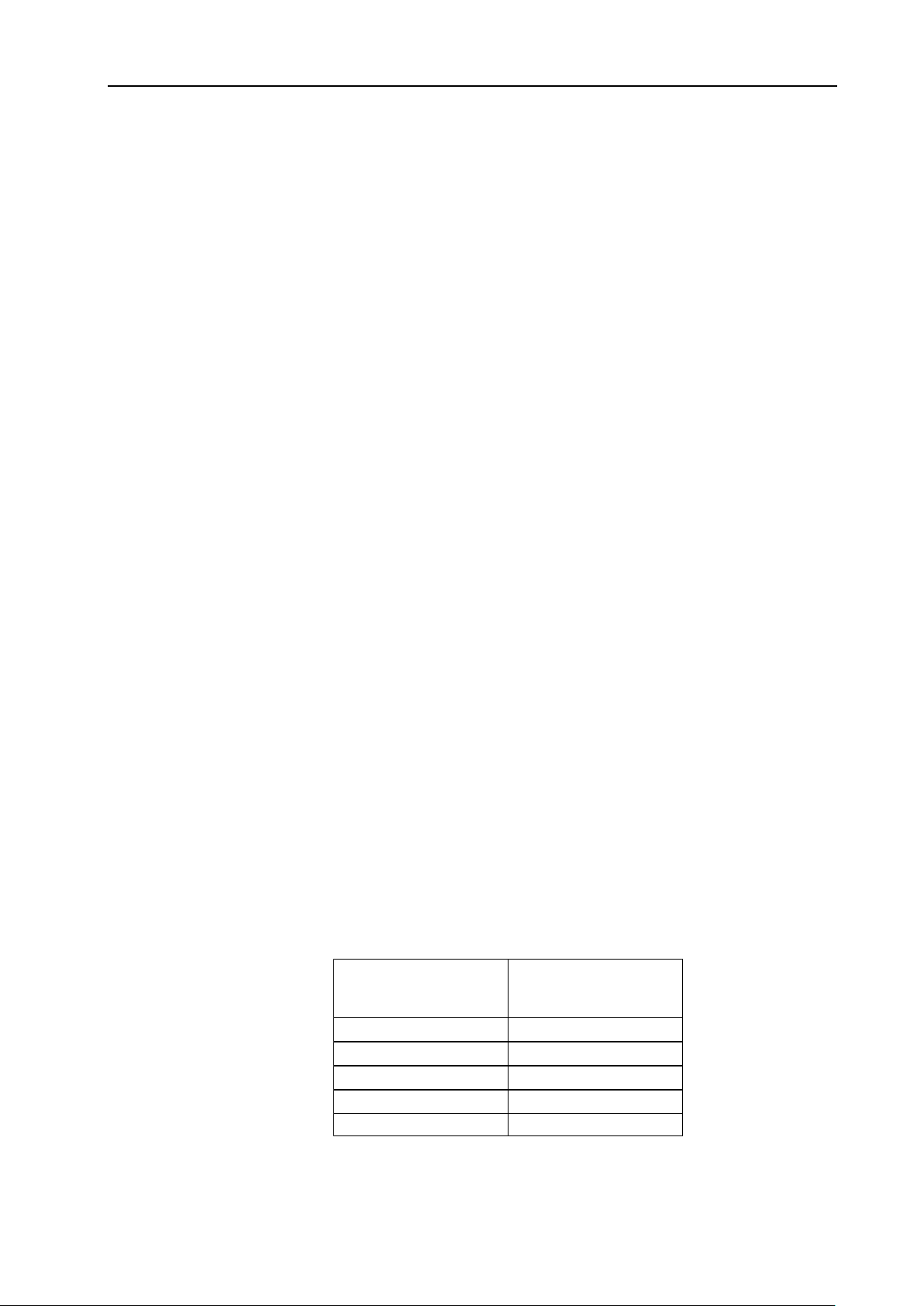
TI> 0.7 the overall exposure time of an embryo or fetus should be restricted in accordance with Table 2-2
below as a reference:
Maximum recommended exposure times for an embryo or fetus
10
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL

ECO 6 Digital Color Doppler Ultrasound System
Non-diagnostic use of ultrasound equipment is not generally recommended. Examples of non-diagnostic
uses of ultrasound equipment include repeated scans for operator training, equipment demonstration using
normal subjects, and the production of souvenir pictures or videos of a fetus. For equipment of which the
safety indices are displayed over their full range of values, the TI should always be less than 0.5 and the MI
should always be less than 0.3. Avoid frequent repeated exposure of any subject. Scans in the first trimester of
pregnancy should not be carried out for the sole purpose of producing souvenir videos or photographs, nor
should their production involve increasing the exposure levels or extending the scan times beyond those
needed for clinical purposes.
Diagnostic ultrasound has the potential for both false positive and false negative results. Misdiagnosis is far
more dangerous than any effect that might result from the ultrasound exposure. Therefore, diagnostic
ultrasound system should be performed only by those with sufficient training and education.
2.6.2 Understanding the MI/TI Display
Track-3 follows the Output Display Standard for systems that include fetal Doppler applications. The acoustic
output will not be evaluated on an application-specific basis, but the global maximum de-rated Ispta must be ≤
720 mW/ cm2 and either the global maximum MI must be ≤ 1.9 or the global maximum de-rated Isppa must be
≤ 190 W/cm
1.0; Ispta.3 ≤50mW/cm
power for a specific exam, and still limit output acoustic power within the global maximum de-rated Ispta ≤
720 mW/cm2 under an Output Display Standard.
For any diagnostic ultrasonic systems, Track-3 provides an Output Indices Display Standard. The diagnostic
ultrasound systems and its operation manual contain the information regarding an ALARA (As Low As
Reasonably Achievable) education program for the clinical end-user and the acoustic output indices, MI and TI.
The MI describes the likelihood of cavitations, and the TI offers the predicted maximum temperature rise in
tissue as a result of the diagnostic examination. In general, a temperature increase of 2.5°C must be present
consistently at one spot for 2 hours to cause fetal abnormalities. Avoiding a local temperature rise above 1°C
should ensure that no thermally induced biologic effect occurs. When referring to the TI for potential thermal
effect, a TI equal to 1 does not mean the temperature will rise 1 degree C. It only means an increased potential
for thermal effects can be expected as the TI increases. A high index does not mean that bioeffects are
occurring, but only that the potential exists and there is no consideration in the TI for the scan duration, so
minimizing the overall scan time will reduce the potential for effects. These operator control and display
features shift the safety responsibility from the manufacturer to the user. So it is very important to have the
Ultrasound systems display the acoustic output indices correctly and the education of the user to interpret the
value appropriately.
RF: (De-rating factor)
In Situ intensity and pressure cannot currently be measured. Therefore, the acoustic power measurement is
normally done in the water tank, and when soft tissue replaces water along the ultrasound path, a decrease in
intensity is expected. The fractional reduction in intensity caused by attenuation is denoted by the de-rating
factor (RF),
RF = 10 (-0.1 a f z)
2
. An exception is for ophthalmic use, in which case the TI = max (TIS_as, TIC) is not to exceed
2
, and MI ≤ 0.23. Track-3 gives the user the freedom to increase the output acoustic
11
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL

ECO 6 Digital Color Doppler Ultrasound System
Distance
Frequency (MHz)
(cm) 1 3 5 7.5
1
0.9332
0.8128
0.7080
0.5957
2
0.8710
0.6607
0.5012
0.3548
3
0.8128
0.5370
0.3548
0.2113
4
0.7586
0.4365
0.2512
0.1259
5
0.7080
0.3548
0.1778
0.0750
6
0.6607
0.2884
0.1259
0.0447
7
0.6166
0.2344
0.0891
0.0266
8
0.5754
0.1903
0.0631
0.0158
Thermal Models
Composition
Mode
Specification
Application
1
TIS
Soft tissue
Unscanned
Large aperture
(>1cm2)
Liver PW
2
TIS
Soft tissue
Unscanned
Small aperture
(<1cm2)
Pencil Probe
3
TIS
Soft tissue
Scanned
Evaluated at
surface
Breast color
4
TIB
Soft tissue and bone
Scanned
Soft tissue at
surface
Muscle color
5
TIB
Soft tissue and bone
Unscanned
Bone at focus
Fetus head PW
6
TIC
Soft tissue and bone
Unscanned/scanned
Bone at surface
Transcranial
Where a is the attenuation coefficient in dB cm-1 MHz-1, f is the transducer center frequency, and z is the
distance along the beam axis between the source and the point of interest.
De-rating factor RF for the various distances and frequencies with attenuation coefficient 0.3dB cm-1 MHz-1
in homogeneous soft tissue is listed in the following table. An example is if the user uses 7.5MHz frequency,
the power will be attenuated by .0750 at 5cm, or 0.3x7.5x5=-11.25dB. The De- rated Intensity is also referred
to as ‘.3’ at the end (e.g. Ispta.3).
I’=I*RF Where I’ is the intensity in soft tissue, I is the time-averaged intensity measured in water.
Tissue Model:
Tissue temperature elevation depends on power, tissue type, beam width, and scanning mode. Six models are
developed to mimic possible clinical situations.
Soft tissue:
Describes low fat content tissue that does not contain calcifications or large gas-filled spaces.
Scanned: (auto-scan)
Refers to the steering of successive burst through the field of view, e.g. B and color mode.
Unscanned:
Emission of ultrasonic pulses occurs along a single line of sight and is unchanged until the transducer is moved
to a new position. For instance, the PW and M mode.
12
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL

ECO 6 Digital Color Doppler Ultrasound System
TI:
TI is defined as the ratio of the In Situ acoustic power (W.3) to the acoustic power required to raise tissue
temperature by 1°C (Wdeg), TI = W.3/Wdeg.
Three TIs corresponding to soft tissue (TIS) for abdominal; bone (TIB) for fetal and neonatal cephalic; and
cranial bone (TIC) for pediatric and adult cephalic, have been developed for applications in different exams.
An estimate of the acoustic power in milli-watts necessary to produce a 1°C temperature elevation in soft
tissue is:
Wdeg = 210/fc,for model 1 to 4, where fc is the center frequency in MHz.
Wdeg = 40 K Dfor model 5 and 6, where K (beam shape factor) is 1.0, D is the aperture diameter in cm at
the depth of interest.
MI:
Cavitation is more likely to occur at high pressures and low frequencies in pulse ultrasound wave in the tissue,
which contains the bubble or air pocket (for instance, the lung, intestine, or scan with gas contrast agents). The
threshold under optimum conditions of pulsed ultrasound is predicted by the ration of the peak pressure to the
square root of the frequency.
MI = Pr’ / sqrt(fc)
Pr’ is the de-rated (0.3) peak rare-fractional pressure in Mpa at the point where PII is the maximum, and fc is
the center frequency in MHz. PII is the Pulse Intensity Integral that the total energy per unit area carried by the
wave during the time duration of the pulse. The peak rare- fractional pressure is measured in hydrophone
maximum negative voltage normalized by the hydrophone calibration parameter.
Display Guideline:
For different operation modes, different indices must be displayed. However, only one index needs to be
shown at a time. Display is not required if maximum MI is less than 1.0 for any setting of the operating mode,
or if maximum TI is less than 1.0 for any setting of the operating mode. For TI, if the TIS and TIB are both
greater than 1.0, the scanners need not be capable of displaying both indices simultaneously. If the index falls
below 0.4, no display is needed. The display increments are no greater than 0.2 for index value less than one
and no greater than 1.0 for index values greater than one (e.g. 0.4, 0.6, 0.8, 1, 2, and 3).
Display and Report
Located on the upper middle section of the system display monitor, the acoustic output display provides the
operator with real-time indication of acoustic levels being generated by the system.
For Scan
Only display and report MI, and start from 0.4 if maximum MI > 1.0, display in increments of 0.2.
Below is a simple guideline for the user when TI exceeds one limit exposure time to 4(6-TI) minutes based on
the ‘National Council on Radiation Protection. Exposure Criteria for Medical Diagnostic Ultrasound: I.
Criteria Based on Thermal Mechanisms. Report No.113 1992’.
13
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL
ECO 6 Digital Color Doppler Ultrasound System
Operator Control Features:
The user should be aware that certain operator controls may affect the acoustic output. It is recommended to
use the default (or lowest) output power setting and compensate using Gain control to acquire an image. Other
than the output power setting in the soft-menu, which has the most direct impact on the power; the PRF, image
sector size, frame rate, depth, and focal position also slightly affect the output power. The default setting is
normally around 70% of the allowable power depending on the exam application mode.
Controls Affecting Acoustic Output
The potential for producing mechanical bioeffects (MI) or thermal bioeffects (TI) can be influenced by certain
controls.
Direct: The Acoustic Output control has the most significant effect on Acoustic Output.
Indirect: Indirect effects may occur when adjusting controls. Controls that can influence MI and TI are detailed
under the bioeffect portion of each control in the Optimizing the Image chapter.
Always observe the Acoustic Output display for possible effects.
Best practices while scanning
HINTS: Raise the Acoustic Output only after attempting image optimization with controls that have no effect
on Acoustic Output, such as Gain and STC.
WARNING: Be sure to have read and understood control explanations for each mode used before
attempting to adjust the Acoustic Output control or any control that can affect Acoustic Output.
Use the minimum necessary acoustic output to get the best diagnostic image or measurement during an
examination. Begin the exam with the probe that provides an optimum focal depth and penetration.
Acoustic Output Default Levels
In order to assure that an exam does not start at a high output level, the system initiates scanning at a reduced
default output level. This reduced level is preset programmable and depends upon the exam icon and probe
selected. It takes effect when the system is powered on or New Patient is selected. To modify acoustic output,
adjust the Power Output level on the Soft Menu.
2.7 Battery Handling Instructions
Caution: Read and observe the following warnings and precautions to ensure correct and safe use of
Li-ion batteries.
Do not immerse the battery in water or allow it to get wet.
Do not use or store the battery near sources of heat such as a fire or heater.
Do not use any chargers other than those recommended.
Do not reverse the positive (+) and negative (-) terminals.
Do not connect the battery directly to wall outlets or car cigarette-lighter sockets.
Do not put the battery into a fire or apply direct heat to it.
Do not short-circuit the battery by connecting wires or other metal objects to the positive (+) and negative
(-) terminals.
14
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL

ECO 6 Digital Color Doppler Ultrasound System
Do not pierce the battery casing with a nail or other sharp object, break it open with a hammer, or step on it.
Do not strike, throw or subject the battery to sever physical shock.
Do not directly solder the battery terminals.
Do not attempt to disassemble or modify the battery in any way.
Do not place the battery in a microwave oven or pressurized container.
Do not use the battery in combination with primary batteries (such as dry-cell batteries) or batteries of
different capacity, type or brand.
Do not use the battery if it gives off an odor, generates heat, becomes discolored or deformed, or appears
abnormal in any way. If the battery is in use or being recharged, remove it from the device or charger
immediately and discontinue use.
Do not use or store the battery where is exposed to extremely hot, such as under window of a car in direct
sunlight in a hot day. Otherwise, the battery may be overheated. This can also reduce battery performance
and/or shorten service life.
If the battery leaks and electrolyte gets in your eyes, do not rub them. Instead, rinse them with clean
running water and immediately seek medical attention. If left as is, electrolyte can cause eye injury.
15
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL

Chapter 3 System Introduction
3.1 Console Overview
ECO 6 Digital Color Doppler Ultrasound System
Fig. 2 Console Overview
3.2 Physical Specification
Dimensions of main unit (approx.): 335mm(Length)×155mm(Width)×350mm(Height)
Net weight of main unit (approx.): 6.5kg
16
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL
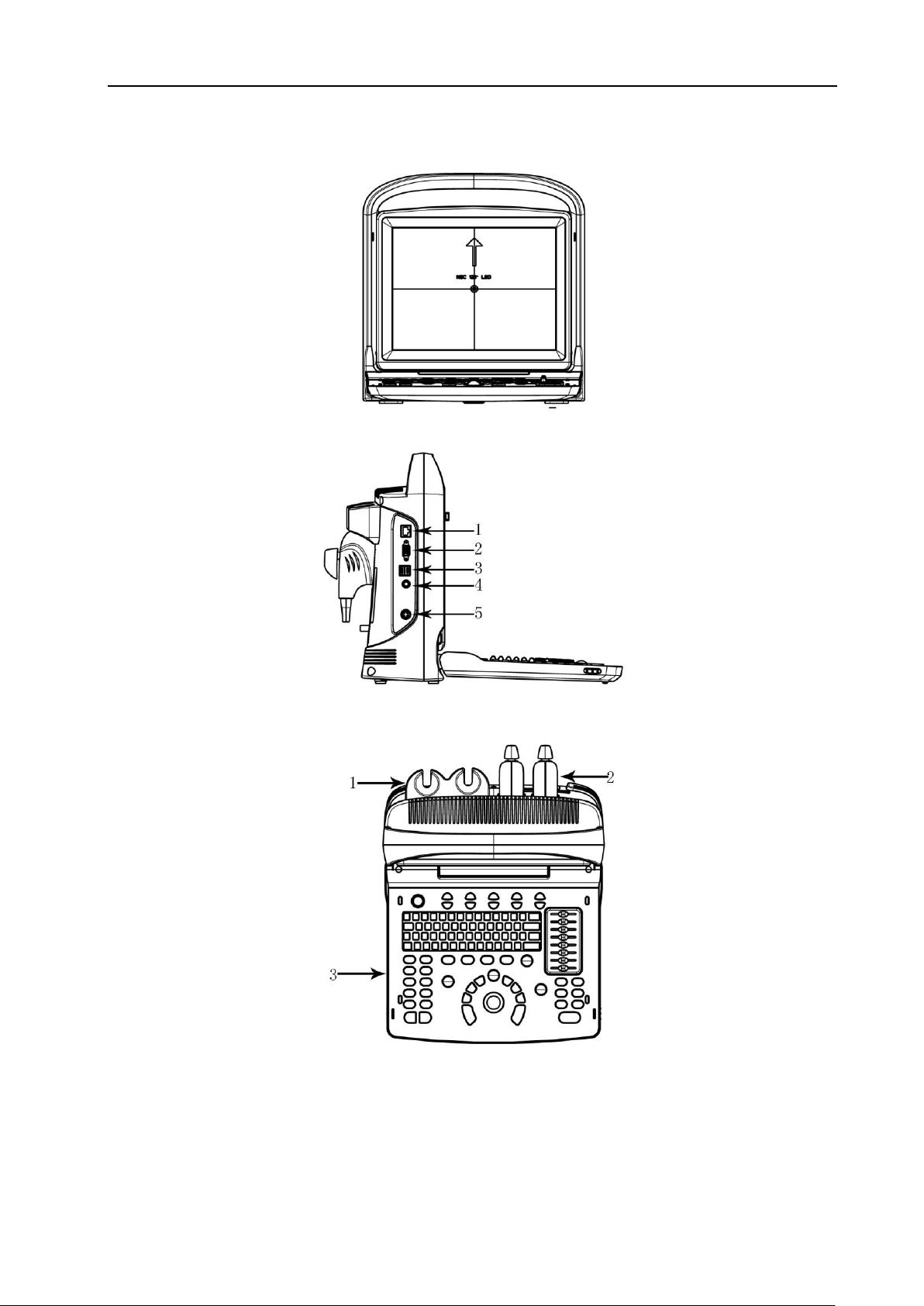
3.3 System View in Different Views
Fig. 3 System Front View
ECO 6 Digital Color Doppler Ultrasound System
Fig. 4 System Side View
1. Ethernet 2. VGA 3.USB 4.FOOT SWITCH 5.DC IN
Fig. 5 Console Overview
1. Probe Holder 2.Probe 3.Keyboard
17
WWW.CFS.IT - CFS PRODOTTI MEDICALI SRL
 Loading...
Loading...