Chattanooga Fluidotherapy Series, Fluidotherapy FLU110D, Fluidotherapy FLU115D, Fluidotherapy FLU110DE, Fluidotherapy FLU115DE User Manual
Page 1

ISO 13485 Certified
Dry Heat Therapy Units
User Manual
Models:
FLU110D
FLU110DE
FLU115D
FLU115DE
Page 2

Page 3

Page 4

TABLE OF CONTENTS
Fluidotherapy® - Dry Heat Therapy
Foreword .......................................................................................1
About Dry Heat Therapy .........................................2-5
Precautionary Instructions .................................2-4
Description of Device Markings ........................... 2
Indications & Contraindications ...........................5
Nomenclature ......................................................................... 6-8
FLU110D & FLU110DE Unit
Familiarization .............................................................6
FLU115D & FLU115DE Unit
Familiarization .............................................................7
Operating Controls ....................................................8
Specifications .........................................................9-14
FLU110D & FLU110DE .............................................. 9
FLU115D & FLU115DE ............................................10
Electromagnetic Compatibility Tables ........ 11-14
Setup .....................................................................................15-23
Treatment Mode Parameters........................ 15-17
Time Controlled Parameters ................................18
Preference Mode Default Parameters ..........18-23
©2009 DJO, LLC Vista, California, USA. Any use of editorial, pictorial, or layout composition of this publication without express written consent from DJO, LLC is strictly prohibited. This publication was
written, illustrated, and prepared for distribution by DJO, LLC.
Operation ....................................................................................24
Patient Preparation ..................................................24
Starting Treatment ...................................................24
Stopping Treatment .................................................24
Preventive Maintenance ................................................25-32
Daily Maintenance ....................................................25
Weekly Maintenance ........................................26-29
ADDITIONAL FLUIDOTHERAPY PREVENTIVE
MAINTENANCE REQUIREMENTS
Quarterly ...................................................................... 30
Bi-Annual ..................................................................... 30
Annual ...........................................................................30
As Needed ................................................................... 30
Fluidotherapy Maintenance Record ..................31
Cleaning .......................................................................32
Service ..........................................................................32
Accessories ..................................................................................33
Replacement Accessories ......................................33
Warranty ......................................................................................34
i
Page 5

FOREWORD
Thank you for purchasing a Fluidotherapy Dry Heat Therapy Unit.
This manual contains general safety, operating, maintenance, and care instructions for the owners and operators of the
Fluidotherapy Dry Heat Therapy Units.
The specifications put forth in this manual were in effect at the time of the publication. However, owing to DJO, LLC's
policy of continuous improvement, changes to these specifications may be made at any time without obligation on the
part of DJO, LLC.
Read, understand, and follow the information contained in this manual.
Stay current with the latest clinical developments in the field of Dry Heat Therapy and observe all applicable
precautionary measures for treatment.
Keep informed on appropriate indications and contraindications for the use of Dry Heat Therapy.
Fluidotherapy® - Dry Heat Therapy
This equipment is to be used only under the prescription and supervision of a licensed practitioner.
1
1
Page 6

ABOUT DRY HEAT THERAPY
Fluidotherapy® - Dry Heat Therapy
PRECAUTIONARY INSTRUCTIONS
The precautionary instructions found in this section and throughout
this manual are indicated by specific symbols. Understand these
symbols and their definitions before operating this equipment. The
definitions of these symbols are as follows:
= CAUTION - Text with a “CAUTION” indicator will explain
possible safety infractions that could have the potential to
cause minor to moderate injury or damage to equipment.
= WARNING - Text with a “WARNING” indicator will explain
possible safety infractions that will potentially cause serious
injury and equipment damage.
= DANGER - Text with a “DANGER” indicator will explain
possible safety infractions that are imminently hazardous
situations that would result in death or serious injury.
= EXPLOSION HAZARD - Text with an “Explosion Hazard”
indicator will explain possible safety infractions if this
equipment is used in the presence of flammable anesthetics.
= NON-IONIZING ELECTROMAGNETIC RADIATION - Text with a
Non-Ionizing Electromagnetic Radiation" indicator informs
the user of possible hazards resulting from elevated,
potentially dangerous, levels of non-ionizing radiation.
= Protective Earth (ground)
NOTE: Helpful information marked as "NOTE" may be found
throughout this manual. Each "NOTE" is beneficial in the aid of
a particular area or function being described for this product.
DESCRIPTION OF DEVICE MARKINGS
Degree of Protection Against Electrical Shock ...........................
Complies with UL 2601-1 CSA C22.2 No. 601.1
Medical Electrical Equipment ............ ..............................................
Refer to Instruction Manual/Booklet ......... ....................................
2
Page 7
ABOUT DRY HEAT THERAPY
Fluidotherapy® - Dry Heat Therapy
Read, understand, and practice the precautionary and operating •
instructions found in this manual. Know the limitations and hazards
associated with using any electrical device. Observe the precautionary
and operational decals placed on the unit.
•
DO NOT operate this unit in an environment where other devices
are being used that intentionally radiate electromagnetic energy in
an unshielded manner. Medical Electrical Equipment needs special
precautions regarding EMC and needs to be installed and put into
service according to the EMC information provided in this manual.
•
Portable and Mobile RF Communications equipment can affect Medical
Electrical Equipment.
•
This unit generates, uses, and can radiate radio frequency energy and, if
not installed and used in accordance with the instructions, may cause
harmful interference to other devices in the vicinity. However, there is
no guarantee that interference will not occur in a particular installation.
Harmful interference to other devices can be determined by turning
this unit on and off. Try to correct the interference using one or more
of the following: reorient or relocate the receiving device, increase the
separation between the equipment, connect the equipment to an outlet
on a different circuit from that which the other device(s) are connected
and consult the DJO, LLC Service Department for help.
•
Clean Inlet Filter(s) daily before unit startup.
Turn unit OFF before positioning a patient or removing a patient from •
the unit.
•
Place the patient in a comfortable position allowing for correct
placement of the limb being treated.
•
Secure all entry ports before turning the unit ON.
Use only Cellex® Dry Heat Medium in the Fluidotherapy units.•
Refill unit daily to proper fill level with Chattanooga Cellex Dry Heat •
Medium.
Use only fingers to operate button controls on the control panel(s). Use of sharp •
objects such as pencils or pens will result in damage to the unit.
Check unit temperature before treating patient to ensure correct temperature.•
This unit should be transported and stored in temperatures between -40 °F and •
158 °F (-40 °C and -70 °C) with Relative Humidity between 10% - 100% to prevent
damage to the unit or its components.
This unit should be operated in temperatures between 110 °F and 125 °F (43 °C and •
52 °C) to prevent damage to the unit or its components.
DO NOT operate the unit when connected to any unit other than Chattanooga •
devices.
Change Cellex Dry Heat Medium every six (6) months.•
Do not use accessories other than those supplied with the unit, or recommended •
by DJO, LLC. The safety of other products has not been established, and their use
could result in injury to the patient.
3
Page 8
ABOUT DRY HEAT THERAPY
Fluidotherapy® - Dry Heat Therapy
Federal law restricts this device to sale by, or on the order of, a physician or licensed •
practitioner. This device should be used only under the continued supervision of a
physician or licensed practitioner.
Make certain the unit is electrically grounded by connecting only to a grounded •
electrical service receptacle conforming to the applicable national and local
electrical codes.
Before administering any treatment to a patient, you should become acquainted •
with the operating procedures for each mode of treatment available, as well as the
indications, contraindications, warnings, and precautions. Consult other resources
for additional information regarding the application of Dry Heat Therapy.
The Fluidotherapy Dry Heat Therapy Unit should not be used adjacent to or stacked •
with other equipment. If adjacent or stacked use is necessary, the Fluidotherapy Dry
Heat Therapy Unit should be observed to verify normal operation in the configuration
in which it will be used.
For continued protection against fire hazard, replace fuses only with ones of the •
same type and rating.
This device should be kept away from children.•
To prevent electrical shock, disconnect the unit from the power source before •
attempting any maintenance procedures.
Use only processed dry heat medium in the unit such as Cellex to prevent excessive •
dusting.
Use only accessories that are specially designed for this • unit. Do not use accessories
manufactured by other companies on this unit. DJO, LLC is not responsible for any
consequence resulting from using products manufactured by other companies. The use
of other accessories or cables may result in increased emissions or decreased immunity
of this unit.
Dispose of all products in accordance with local and national regulations and codes.•
Fluidotherapy equipment not in use should be protected against unqualified use.•
The Fluidotherapy Dry Heat Therapy Unit is not suitable in the •
presence of a flammable anesthetic mixture with air, oxygen, or nitrous
oxide.
Perform all Preventive Maintenance as described in this manual. Failure to perform •
the Preventive Maintenance could result in the Cellex medium entering the heat
chamber of the unit and cause severe injury to patients as well as smoke damage
to the facility and the Fluidotherapy unit.
The solvents of adhesives and flammable solutions used for cleaning and •
disinfecting the unit should be allowed to evaporate before the unit is used.
4
Page 9

ABOUT DRY HEAT THERAPY
Indications & Contraindications
Fluidotherapy® - Dry Heat Therapy
Indications
• Relief of local pain
• Treatment of local blood flow insufficiency
• Treatment in range of motion when combined with exercise
• Treatment for symptoms of non-rheumatoid arthritis
Contraindications
• This device should not be used for symptomatic pain relief unless
etiology is established or unless a pain syndrome has been
diagnosed.
• This device should not be used when cancerous lesions or open
wounds are present in the treatment area.
• Other counterindications are patients suspected of carrying
serious infectious disease or disease where it is advisable, for
general medical purposes, to suppress heat or fevers.
Adequate precautions should be taken when treating individuals •
with suspected or diagnosed medical conditions or diseases such as
heart problems, epilepsy, diabetes, etc.
Prior to treatment, consult a medical professional familiar with the •
precautionary measures to be taken for patients that may experience
allergic reactions to dust and pollen.
55
Page 10

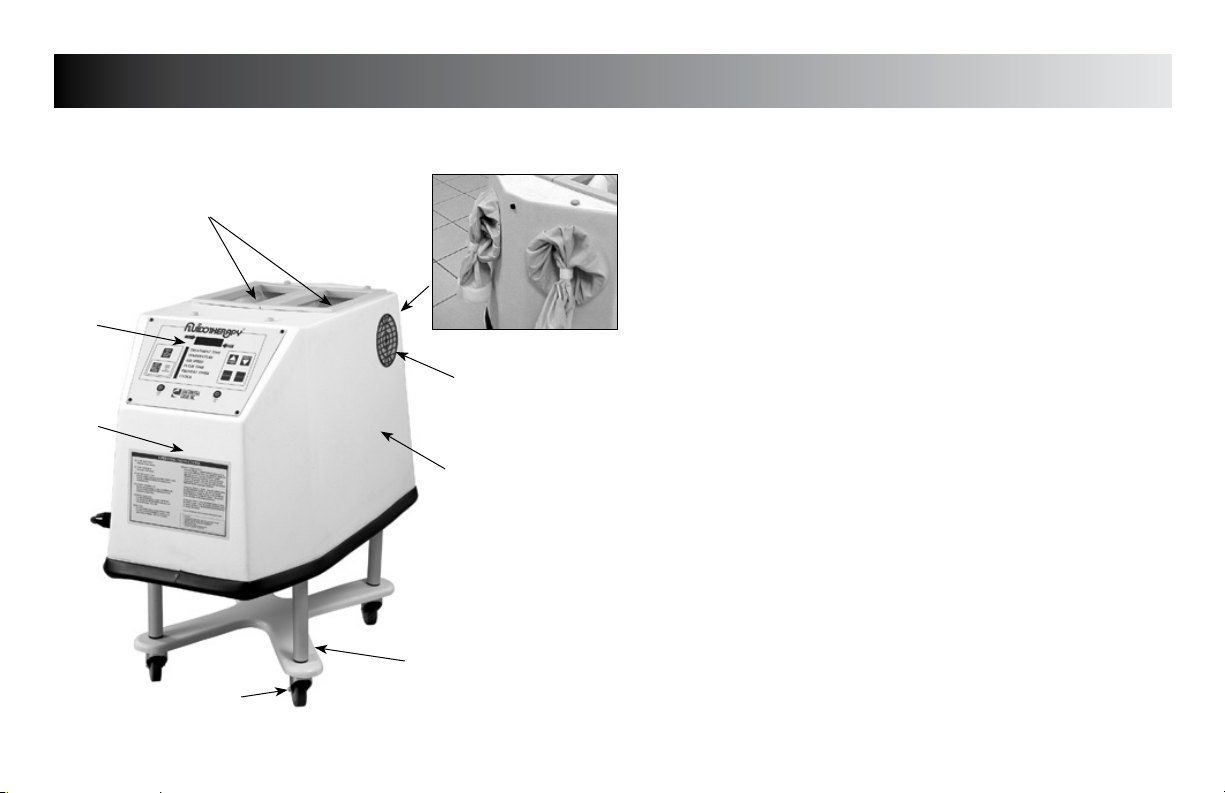
NOMENCLATURE
FLU110D & 110DE Unit Familiarization
1
8
2
Fluidotherapy® - Dry Heat Therapy
1. RESERVOIR LID & TREATMENT VIEWING WINDOW
Access to add/change medium, view patient treatment, and
clinician access to patient treatment area
2. MEDIUM/TREATMENT RESERVOIR
Treatment access and medium reservoir
3
3. HAND/ARM ACCESS PORT
Treatment port for Hand/Arm Treatment (end of unit)
7
4. REPLACEABLE INLET FILTERS
Filters room air entering the Fluidotherapy unit (1 each side)
5. FOUR POINT BASE
Casters included to convert to a Mobile Base
6. BLOWER HOUSING
6
4
Houses Blower & Heater
7. CONTROL PANEL
See page 8 for detailed description of controls
8. ELBOW/FOOT TREATMENT ACCESS PORT
Treatment port for Elbow and Foot
5
6
Page 11
NOMENCLATURE
FLU115D & FLU115DE Unit Familiarization
Fluidotherapy® - Dry Heat Therapy
1
2
8
3
1. ELBOW/FOOT ACCESS PORT
Treatment port for Elbow and Foot Treatment, access to add/
change medium, clinician access to patient treatment area
2. HAND/ARM ACCESS PORTS
Treatment ports for Hand/Arm Treatment (end of unit)
3. INLET FILTERS
Filters room air entering the Fluidotherapy unit (1 each side)
4. MEDIUM/TREATMENT TUB
Treatment access and medium reservoir
7
5. MOBILE BASE
4
6. LOCKING CASTERS
7. BLOWER HOUSING
Houses Blower & Heater
8. CONTROL PANEL
See page 8 for detailed description of controls
5
6
77
Page 12
NOMENCLATURE
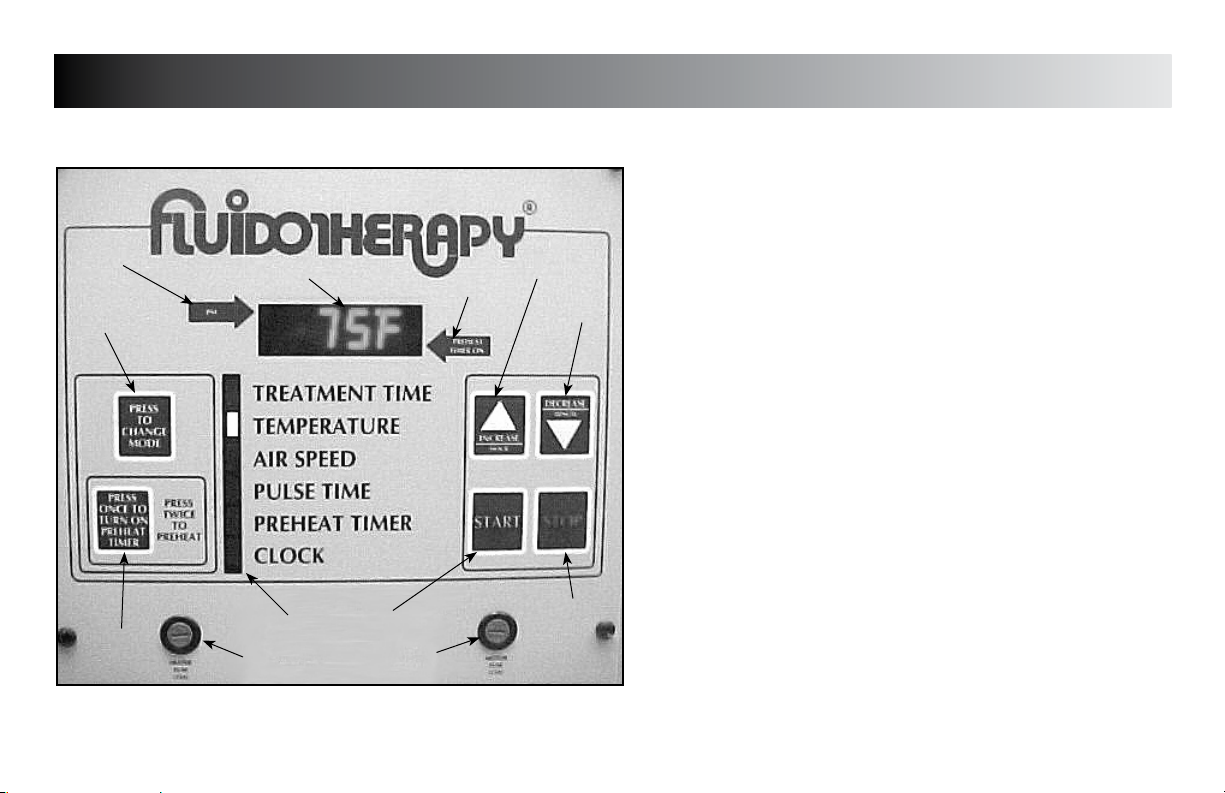
Operating Controls (All Units)
12
1
11
78
10
9
Fluidotherapy® - Dry Heat Therapy
1. DISPLAY
Displays Treatment Time, Temperature, Airspeed, Pulse Time,
Preheat Timer, and Clock when the respective Mode is indicated
2. PREHEAT TIMER ON
2
3
4
5
6
Indicator light for Preheat Timer
3. INCREASE/HOUR
Use to increase Mode parameters and use to set the Hour when
setting Clock
4. DECREASE/MINUTE
Use to decrease Mode parameters and use to set the Minutes
when setting Clock
5. STOP
Use to stop treatment and Preheat Timer
6. MOTOR FUSE
7. START
Use to start treatment
8. INDICATOR BAR
Indicates Mode as they are chosen
9. HEATER FUSE
10. PREHEAT TIMER BUTTON
Press once to turn Preheat Timer On. Press twice to start
Preheat function.
11. MODE SELECT
Use to select desired Mode
12. PM
PM indicator for Clock
8
Page 13

SPECIFICATIONS
FLU110D & FLU110DE
Length
Height
Fluidotherapy® - Dry Heat Therapy
MODES OF OPERATION
Continuous (Default)
Variable Adjustments for Time, Temp, and Air Speed
Pulse Mode.....................................................................OFF to 6 Sec ON/OFF
TREATMENT TIME........................................................................1 to 99 minutes
OPERATING TEMPERATURE...............................110 °F (43.3 °C) to 125 °F (51.6 °C)
AIR SPEED.............................................................0% to 100% (5% increments)
PREHEAT TIMER........................................115 °F (46.1 °C) with 50% Air Flow
MEDIUM CAPACITY.........................................Approximately 30 lb (13.6 kg)
INPUT POWER (FLU110D)..............................................120V, 50/60 Hz, 12A
INPUT POWER (FLU110DE).....................................230-240V, 50/60 Hz, 8A
PHYSICAL DIMENSIONS
Cabinet Length..........................................................................34.0” (86.4 cm)
Cabinet Width............................................................................11.5” (29.2 cm)
Height............................................................................................33.0” (83.8 cm)
Weight...............................................................70 lbs (31.7 kg) Less Medium
Shipping Weight....................................................................100 lbs (45.4 kg)
DEGREE OF PROTECTION AGAINST INGRESS OF WATER...............IPX0
ELECTRICAL CLASS.....................................................................................CLASS I
DEGREE OF PROTECTION AGAINST ELECTRICAL SHOCK......TYPE B
POWER CORD......................................2 meter cable length, 14 AWG, shielded
Width
Make certain the unit is electrically grounded by connecting only to a
grounded electrical service receptacle conforming to the applicable
national and local electrical codes.
99
Page 14

SPECIFICATIONS
FLU115D & FLU115DE
Width
Height
Length
Fluidotherapy® - Dry Heat Therapy
MODES OF OPERATION
Continuous (Default)
Variable Adjustments for Time, Temp, and Air Speed
Pulse Mode.....................................................................OFF to 6 Sec ON/OFF
TREATMENT TIME........................................................................1 to 99 minutes
OPERATING TEMPERATURE..............................110 °F (43.3 °C) to 125 °F (51.6 °C)
AIR SPEED.............................................................0% to 100% (5% increments)
PREHEAT TIMER........................................115 °F (46.1 °C) with 50% Air Flow
MEDIUM CAPACITY.......................................Approximately 40 lbs (18.1 kg)
INPUT POWER (FLU115D)...............................................120V, 50/60 Hz, 12A
INPUT POWER (FLU115DE)...................................230-240V, 50/60 Hz, 10A
PHYSICAL DIMENSIONS
Cabinet Length..........................................................................34.0” (86.4 cm)
Cabinet Width.............................................................................18.5” (47.0 cm)
Height.............................................................................................33.0” (83.8 cm)
Weight................................................................60 lbs (27.2kg) Less Medium
Shipping Weight....................................................................100 lbs (45.4 kg)
DEGREE OF PROTECTION AGAINST INGRESS OF WATER. ..............IPX0
ELECTRICAL CLASS.....................................................................................CLASS I
DEGREE OF PROCTECTION AGAINST ELECTRICAL SHOCK....TYPE B
POWER CORD......................................2 meter cable length, 14 AWG, shielded
Make certain the unit is electrically grounded by connecting only to a
grounded electrical service receptacle conforming to the applicable
national and local electrical codes.
10
Page 15
SPECIFICATIONS
Fluidotherapy® - Dry Heat Therapy
ELECTROMAGNETIC COMPATIBILITY (EMC) TABLES
TABLE 1: GUIDANCE AND MANUFACTURER'S DECLARATION - ELECTROMAGNETIC EMISSIONS
The Fluidotherapy - Dry Heat Therapy Unit is intended for use in the electromagnetic environment specified in the table below. The user
of the Fluidotherapy - Dry Heat Therapy Unit should assure that it is used in such an environment.
Emission Tests Compliance Electromagnetic Environment - Guidance
RF Emissions
CISPR 11
RF Emissions
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations
IEC 61000-3-3
Group 1
Class A
Class A
Complies
The Fluidotherapy - Dry Heat Therapy Unit
cause any interference in nearby electronic equipment.
The Fluidotherapy - Dry Heat Therapy Unit
to the public low-voltage power supply network that supplies buildings used for domestic purposes.
uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to
is suitable for use in all establishments, including domestic establishments and those directly connected
11
Page 16
SPECIFICATIONS
Fluidotherapy® - Dry Heat Therapy
ELECTROMAGNETIC COMPATIBILITY (EMC) TABLES (CONTINUED)
TABLE 2 & 3: GUIDANCE AND MANUFACTURER'S DECLARATION - ELECTROMAGNETIC IMMUNITY
The Fluidotherapy - Dry Heat Therapy Unit is intended for use in the electromagnetic environment specified in the table below. The user of the
Fluidotherapy - Dry Heat Therapy Unit should assure that it is used in such an environment.
Immunity
Test
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
NOTE 1: At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.
a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be
predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the
location in which the Fluidotherapy - Dry Heat Therapy Unit is used exceeds the applicable RF compliance level above, the Fluidotherapy - Dry Heat Therapy Unit should be observed to verify normal
operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the Fluidotherapy - Dry Heat Therapy Unit.
b
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
IEC 60601
Test Level
3 Vrms
150 kHz to 80 MHz
3 V/m
80 MHz to 2.5 GHz
Compliance Level Electromagnetic Environment - Guidance
Portable and mobile RF communications equipment should be used no closer to any part of the Fluidotherapy - Dry Heat Therapy Unit,
including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter.
Recommended separation distance:
3 V
d = [3.5]√P
V
1
d = [3.5]√P 80 MHz to 800 MHz
E
3 V/m
1
d = [7]√P 800 MHz to 2.5 GHz
E
1
where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the
recommended separation distance in meters (m).
Field strengths from fixed RF transmitters, as determined by an electromagnetic site surveya, should be less than the compliance level in each
frequency rangeb.
Interference may occur in the vicinity of equipment marked with the following symbol:
12
Page 17
SPECIFICATIONS
Fluidotherapy® - Dry Heat Therapy
ELECTROMAGNETIC COMPATIBILITY (EMC) TABLES (CONTINUED)
TABLE 2 & 3: GUIDANCE AND MANUFACTURER'S DECLARATION - ELECTROMAGNETIC IMMUNITY
The Fluidotherapy - Dry Heat Therapy Unit is intended for use in the electromagnetic environment specified in the table below. The user of the
Fluidotherapy - Dry Heat Therapy Unit should assure that it is used in such an environment.
Immunity Test
Electrostatic discharge (ESD)
IEC 61000-4-2
Electrical fast transient/burst
IEC 61000-4-4
(1)
Surge
IEC 61000-4-5
Voltage dips, short
interruptions and voltage
variations on power supply
input lines
IEC 61000-4-11
Power frequency (50/60Hz)
IEC 60601
Test Level
±6 kV contact
±8 kV air
±2 kV for power supply lines
±1 kV for input/output lines
±1 kV differential mode
±2 kV common mode
(>95% dip in UT) for 0.5 cycle
<5% U
T
40% U
(60% dip in UT) for 5 cycles
T
70% U
(30% dip in UT) for 25 cycles
T
<5% U
(>95% dip in UT) for 5 sec
T
3 A/m 3 A/m Power frequency magnetic fields should be at levels characteristic of a typical
Compliance Level Electromagnetic Environment - Guidance
±2 kV for power supply lines
±1 kV for input/output lines
±1 kV differential mode
±2 kV common mode
(>95% dip in UT) for 0.5 cycle
<5% U
T
40% U
T
70% U
(30% dip in UT) for 25 cycles
T
<5% U
magnetic field
IEC 61000-4-8
NOTE: UT is the a.c. mains voltage prior to application of the test level.
±6 kV contact
±8 kV air
(60% dip in UT) for 5 cycles
(>95% dip in UT) for 5 sec
T
13
Floors should be wood, concrete, or ceramic tile. If floors are covered with
synthetic material, the relative humidity should be at least 30%.
Mains power quality should be that of a typical commercial or hospital
environment.
Mains power quality should be that of a typical commercial or hospital
environment.
Mains power quality should be that of a typical commercial or hospital
environment. If the user of the Fluidotherapy - Dry Heat Therapy Unit requires
continued operation during power mains interruptions, it is recommended that
the Fluidotherapy - Dry Heat Therapy Unit be powered from an uninterruptible
power supply or a battery.
location in a typical commercial or hospital environment.
Page 18

SPECIFICATIONS
Fluidotherapy® - Dry Heat Therapy
ELECTROMAGNETIC COMPATIBILITY (EMC) TABLES (CONTINUED)
TABLE 4: RECOMMENDED SEPARATION DISTANCES BETWEEN PORTABLE AND MOBILE RF COMMUNICATIONS EQUIPMENT
AND THE FLUIDOTHERAPY - DRY HEAT THERAPY UNIT (CONTINUED)
The Fluidotherapy - Dry Heat Therapy Unit is intended for use in an electromagnetic environment in which radiated RF disturbances
are controlled. The customer or the user of the Fluidotherapy - Dry Heat Therapy Unit can help prevent electromagnetic interference by
maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the Fluidotherapy - Dry
Heat Therapy Unit as recommended below.
Separation Distance According to Frequency of Transmitter
Rated Maximum Output Power of
Transmitter
150 kHz to 80 MHz
W
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be estimated using the equation applicable
to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects, and people.
d = [3.5]√P
V
1
14
m
80 MHz to 800 MHz
d = [3.5]√P
E
1
800 MHz to 2.5 GHz
d = [7]√P
E
1
Page 19
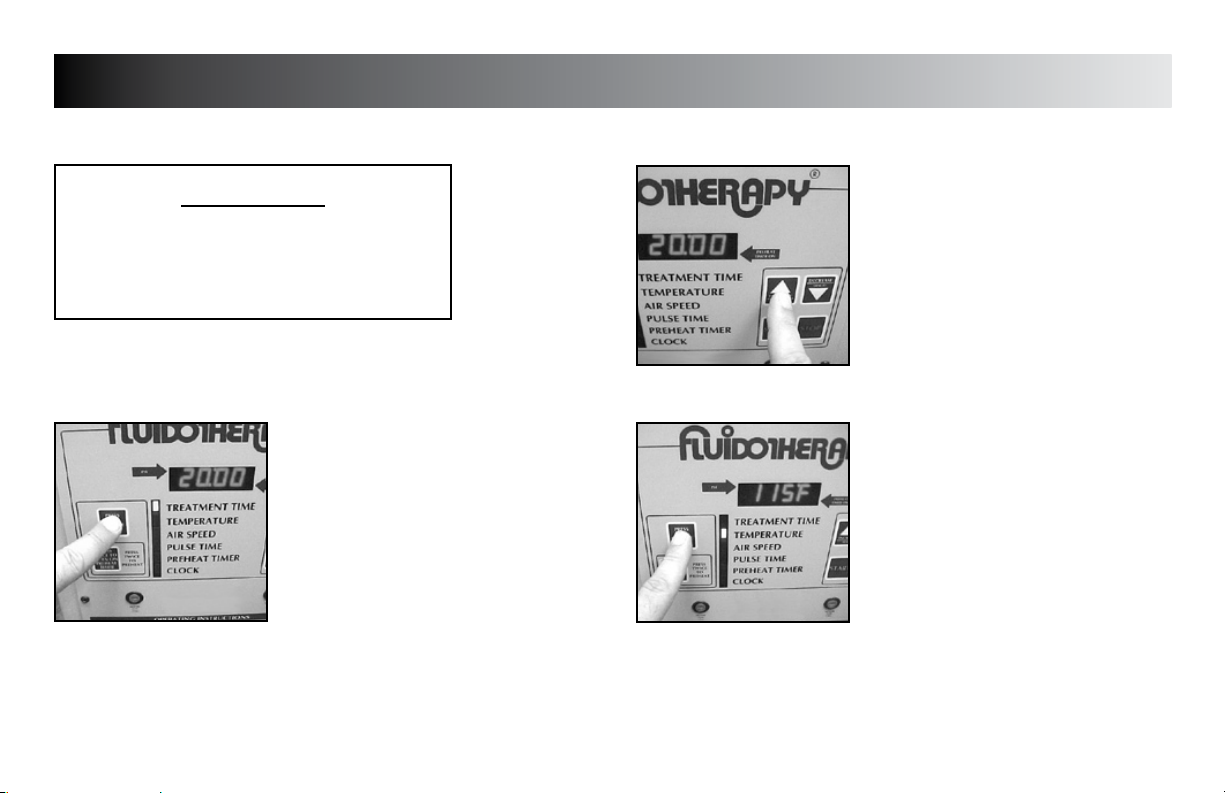
SETUP
TREATMENT MODE PARAMETERS
ATTENTION!!!
ATTRITION OF PARTICLES DURING SHIPMENT
MAY RESULT IN INITIAL DUSTING AND SMALL
PARTICLE RELEASE. DUST AND ODOR WILL
DISSIPATE WITH USE.
Use the following instructions to set the various Mode Parameters to
the desired settings.
TREATMENT TIME
Press the “PRESS TO CHANGE MODE”
button until the indicator light is beside
“TREATMENT TIME”.
NOTE:
The default time of “20:00” will be
displayed.
Fluidotherapy® - Dry Heat Therapy
Press the “INCREASE” or “DECREASE”
buttons until desired Treatment Time is
displayed.
NOTE:
The “TREATMENT TIME” can be adjusted in
one minute increments from 1 to 99.
TREATMENT TEMPERATURE
Press the “PRESS TO CHANGE MODE”
button until the indicator light is beside
“TEMPERATURE”.
NOTE:
The default temperature of “115 °F (46.1 °C)
will be displayed when unit is first plugged
into wall outlet.
The “F” or “C” and “TEMPERATURE” indicator
will flash while the programmed Treatment
Temperature is being displayed.
When the Bed Temperature is displayed, the
“F” or “C” and the “TEMPERATURE” indicator
will illuminate steadily.
15
15
Page 20

SETUP
TREATMENT MODE PARAMETERS (CONTINUED)
Fluidotherapy® - Dry Heat Therapy
TREATMENT TEMPERATURE (CONTINUED)
Press the “INCREASE” or “DECREASE”
buttons until desired Treatment Temperature
is displayed.
NOTE:
°F can be changed to °C and vice versa as
desired. See page 20 for instructions.
Treatment Temperature can be adjusted in 1°
increments from 88 °F to 130 °F
°C).
AIR SPEED
Press the “PRESS TO CHANGE MODE”
button until the indicator light is beside
“AIR SPEED”.
NOTE:
The default speed of “50” will be displayed.
(31.1 °C to 54
Press the “INCREASE” or “DECREASE”
buttons until desired Air Speed is
displayed.
NOTE:
The “AIR SPEED” is adjusted in increments
of 5 from 0 to 100.
PULSE TIME
Press the “PRESS TO CHANGE MODE”
button until the indicator light is beside
“PULSE TIME”.
NOTE:
The “PULSE TIME” allows the unit to
operate by pulsing the medium during
treatment. If selected and set, the medium
will pulse on and off in equal increments.
The Factory Default is “OFF”.
EXAMPLE: “PULSE TIME” is set at “5”.
The unit will pulse the medium during
treatment, five seconds on and five
seconds off.
16
Page 21
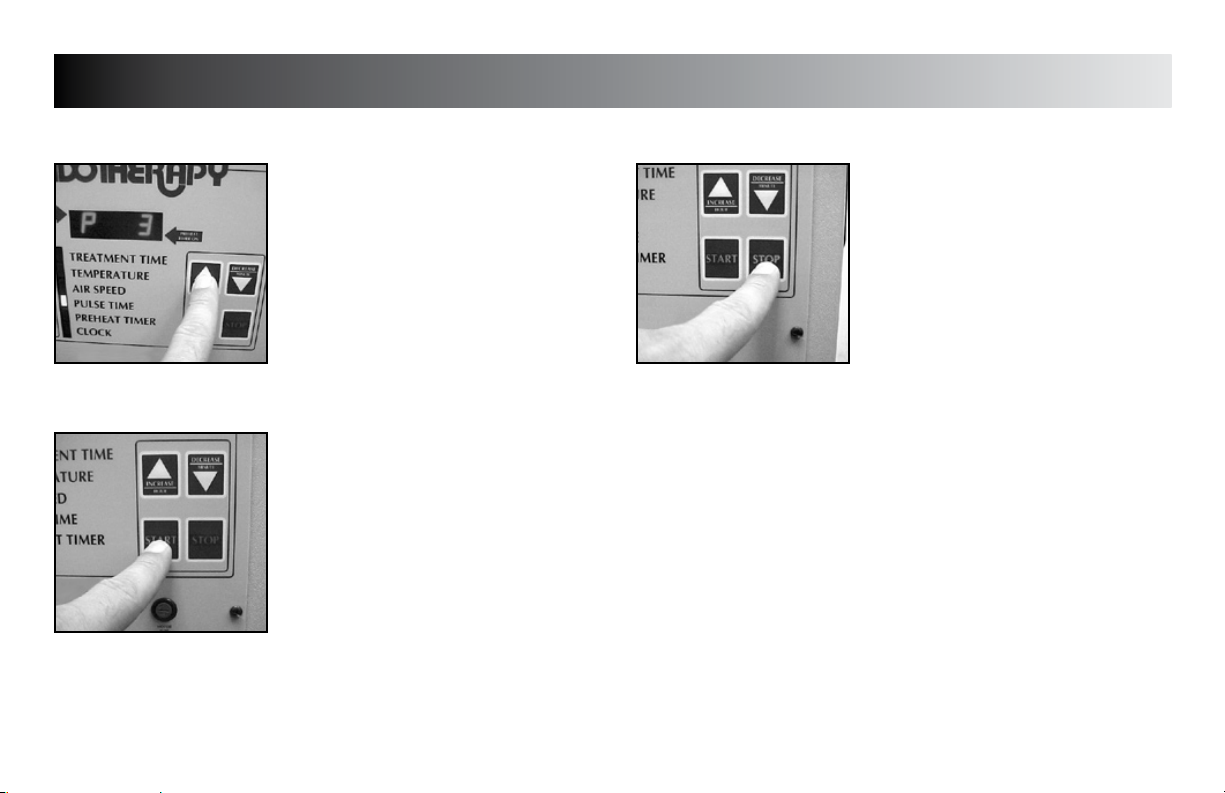
SETUP
TREATMENT MODE PARAMETERS (CONTINUED)
Fluidotherapy® - Dry Heat Therapy
PULSE TIME (CONTINUED)
Press the “INCREASE” or “DECREASE”
buttons until desired Pulse Time is
displayed.
NOTE:
“PULSE TIME” is adjusted in one second
increments from “OFF” to 6 seconds.
START TREATMENT
Press the “START” button.
17
17
STOP TREATMENT
Press the “STOP” button.
Page 22
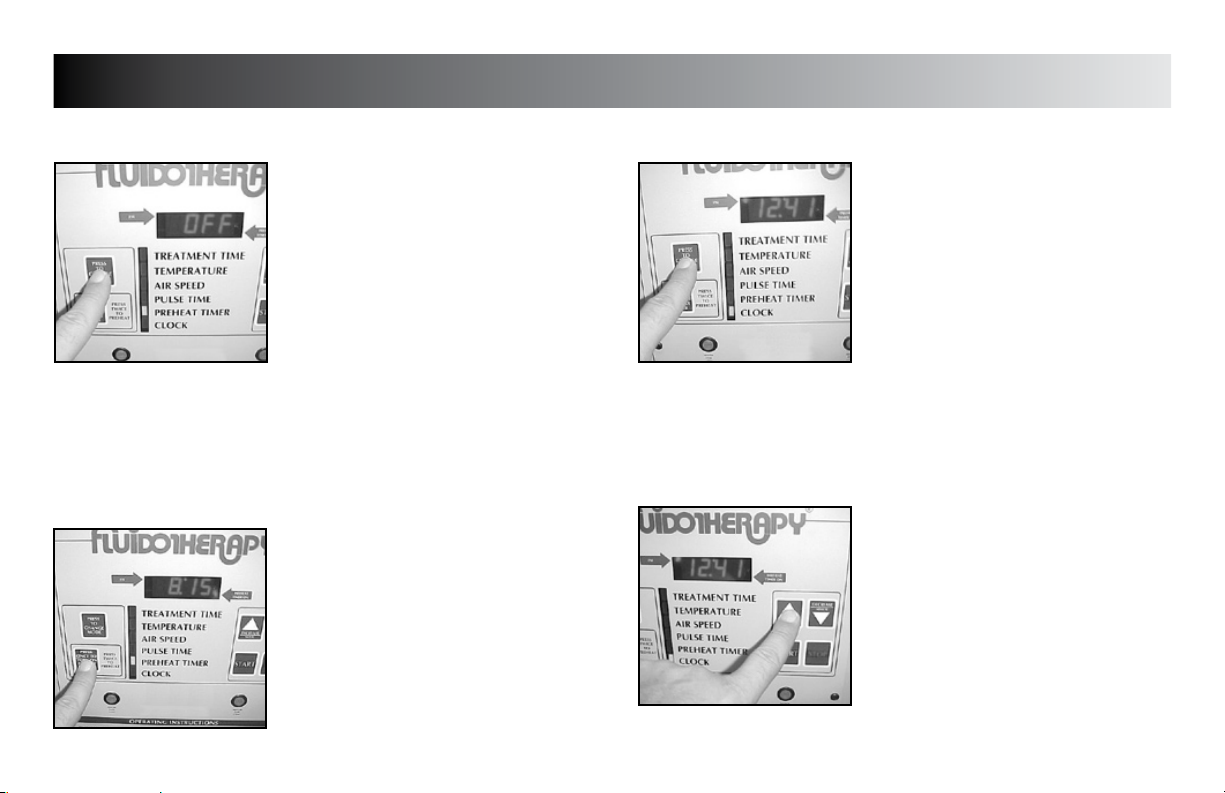
SETUP
TIME CONTROLLED PARAMETERS
Fluidotherapy® - Dry Heat Therapy
PREHEAT TIMER
Press the “PRESS TO CHANGE MODE”
button until the indicator light is beside
“PREHEAT TIMER”.
NOTE:
The “PREHEAT TIMER” allows the unit to
preheat the medium. The unit will run at 50%
Air Speed until the unit reaches the default
preheat temperature or 90 minutes, whichever
comes first. The “PREHEAT TIMER” will operate
Monday through Friday only and can be set
to automatically start at a predetermined time
and heat the medium to a predetermined
temperature. See page 21 for setting the
default parameters of the “PREHEAT TIMER.
Press the “PRESS ONCE TO TURN ON PREHEAT
TIMER” button to illuminate the “PREHEAT
TIMER ON” indicator light. Press again to start
the “PREHEAT TIMER”.
NOTE:
The “PREHEAT TIMER” indicator light must be
set at the end of each day in order for it to
automatically come on the next day.
The “STOP” button is used to turn off the
Preheat if desired.
CLOCK
Press the “PRESS TO CHANGE MODE” button
until the indicator light is beside “CLOCK”.
NOTE:
The “PREHEAT TIMER” will not function until
the Clock is set.
Press the “INCREASE” button to set hours.
Press the “DECREASE” button to set minutes.
NOTE:
The “PM” indicator light will illuminate when
PM hours are reached.
18
Page 23

SETUP
PREFERENCE MODE DEFAULT PARAMETERS
Fluidotherapy® - Dry Heat Therapy
ENTERING “PrEF” MODE (PREFERENCE MODE)
Simultaneously press the “PRESS TO CHANGE MODE”, “INCREASE”, and
“DECREASE” buttons. “PrEF” will be displayed.
NOTE:
The “PrEF” mode allows the user to change the default settings.
TREATMENT TIME DEFAULT
Press the “PRESS TO CHANGE MODE” button
until the indicator light is flashing beside
“TREATMENT TIME”.
Press the “INCREASE” and “DECREASE”
buttons to set unit to the desired default
treatment time.
Press the “PRESS TO CHANGE MODE” button
to save the new setting.
NOTE:
The default setting is 20:00 minutes.
1919
Page 24
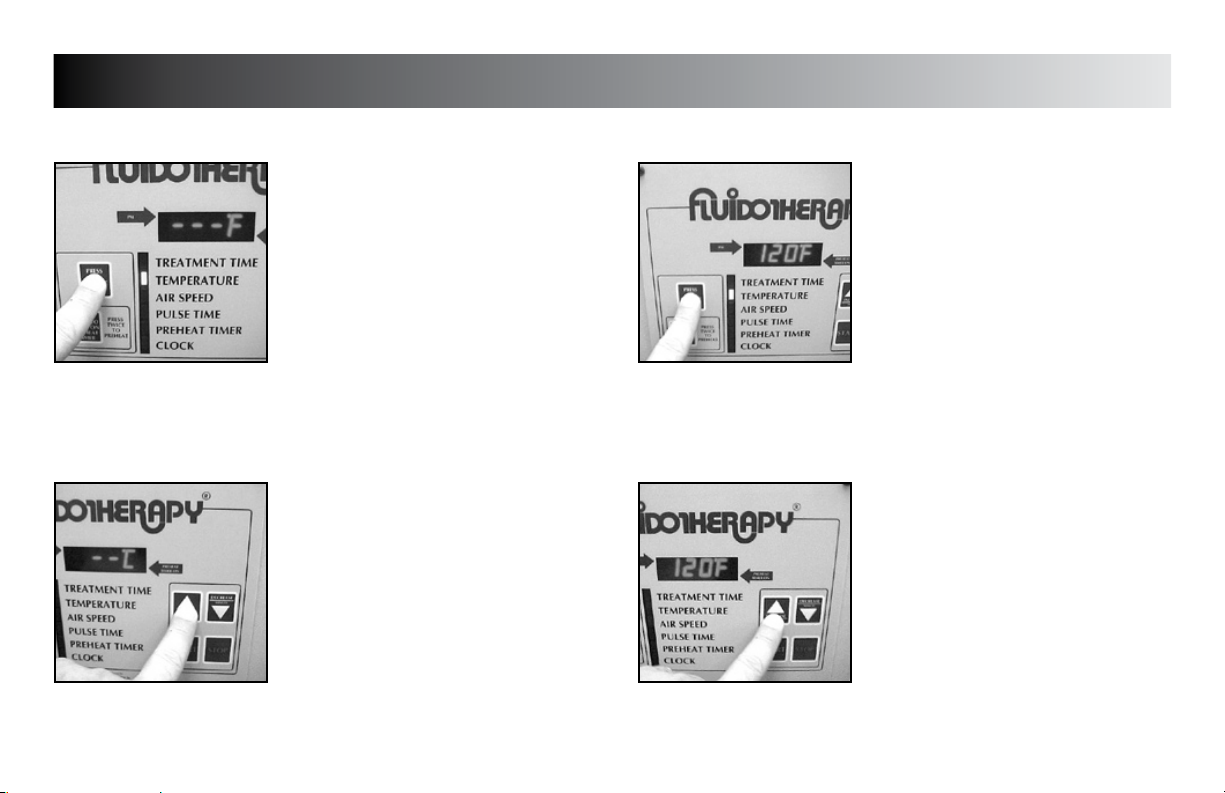
SETUP
PREFERENCE MODE DEFAULT PARAMETERS (CONTINUED)
TEMPERATURE READING DEFAULT
Fluidotherapy® - Dry Heat Therapy
TREATMENT TEMPERATURE DEFAULT
Press the “PRESS TO CHANGE MODE”
button until the indicator light is flashing
beside “TEMPERATURE”.
Press the “INCREASE” button to set unit
to display °F or °C as desired for the
default setting.
Press the “PRESS TO CHANGE MODE”
button to save the new setting.
NOTE:
The default setting is °F.
Press the “PRESS TO CHANGE MODE”
button until the indicator light is flashing
beside “TEMPERATURE” and the existing
default temperature is displayed.
Press the “INCREASE” and “DECREASE”
buttons to set unit to the desired default
treatment temperature.
Operating Temperature Range is:
110 °F - 125 °F (43.3 °C - 51.6 °C)
Press the “PRESS TO CHANGE MODE”
button to save the new setting.
20
Page 25
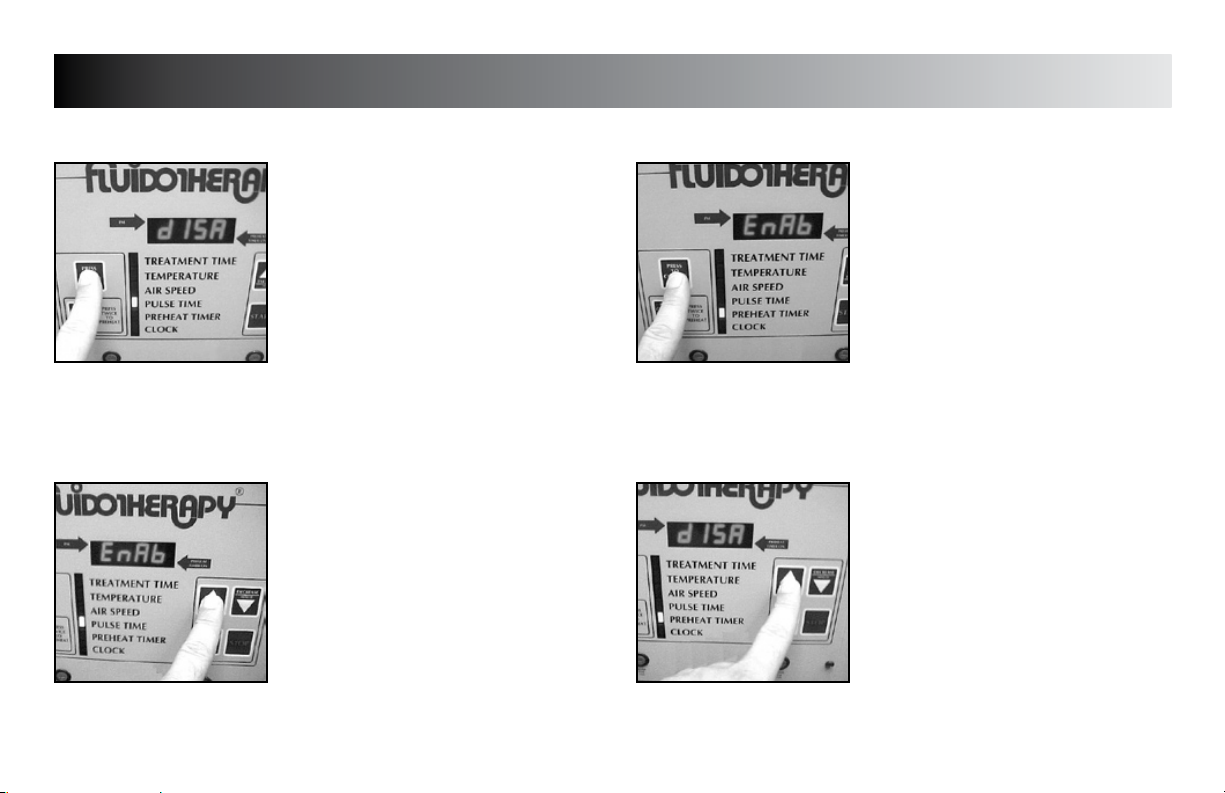
SETUP
PREFERENCE MODE DEFAULT PARAMETERS (CONTINUED)
PULSE MODE (Enable/Disable)
Fluidotherapy® - Dry Heat Therapy
PREHEAT TIMER MODE (Enable/Disable)
Press the “PRESS TO CHANGE MODE”
button until the indicator light is flashing
beside “PULSE TIME”.
“EnAb” (enable) or “dISA” (disable)
will display.
Press the “INCREASE” button to set unit to
display “EnAb” or “dISA” as desired.
Press the “PRESS TO CHANGE MODE”
button to save the new setting.
Press the “PRESS TO CHANGE MODE”
button until the indicator light is flashing
beside “PREHEAT TIMER”.
“EnAb” (enable) or “dISA” (disable)
will display.
Press the “INCREASE” button to set unit to
display “EnAb” or “dISA” as desired.
Press the “PRESS TO CHANGE MODE”
button to save the new setting.
NOTE:
If Clock is disabled, the “PREHEAT TIMER”
will automatically be disabled.
2121
Page 26
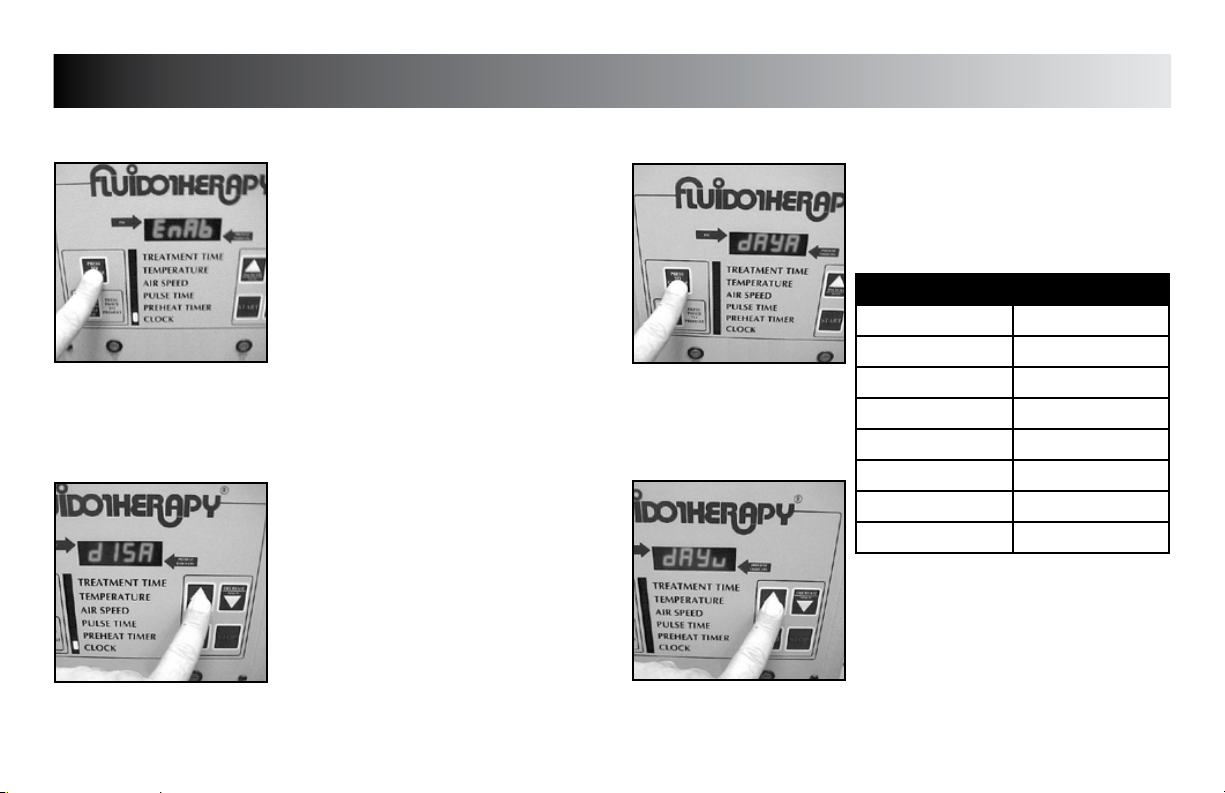
SETUP
PREFERENCE MODE DEFAULT PARAMETERS (CONTINUED)
CLOCK (Enable/Disable)
Fluidotherapy® - Dry Heat Therapy
SETTING DAY OF WEEK
Press the “PRESS TO CHANGE MODE”
button until the indicator light is flashing
beside “CLOCK”.
“EnAb” (enable) or “dISA” (disable)
will display.
Press the “INCREASE” button to set unit to
display “EnAb” or “dISA” as desired.
Press the “PRESS TO CHANGE MODE”
button to save the new setting.
NOTE:
If Clock is disabled, the “PREHEAT TIMER”
will automatically be disabled.
Press the “PRESS TO CHANGE MODE”
button until “dAy__” (__= day code) is
displayed.
DAY CODE CHART
WEEKDAY DAY CODE
Sunday dAyu
Monday dAyn
Tuesday dAyE
Wednesday dAyd
Thursday dAyr
Friday dAyF
Saturday dAyA
Press the “INCREASE” or “DECREASE” button
to set unit to display the desired day code.
Press the “PRESS TO CHANGE MODE” button
to save the new setting.
22
Page 27

SETUP
PREFERENCE MODE DEFAULT PARAMETERS (CONTINUED)
EXITING “PrEF” MODE
Press the “PRESS TO CHANGE MODE” buttons until “PrEF” is displayed.
Simultaneously press the “INCREASE”, “DECREASE”, and “STOP” buttons. The
default treatment time will display and the indicator will be beside “TREATMENT
TIME”.
Fluidotherapy® - Dry Heat Therapy
2323
Page 28
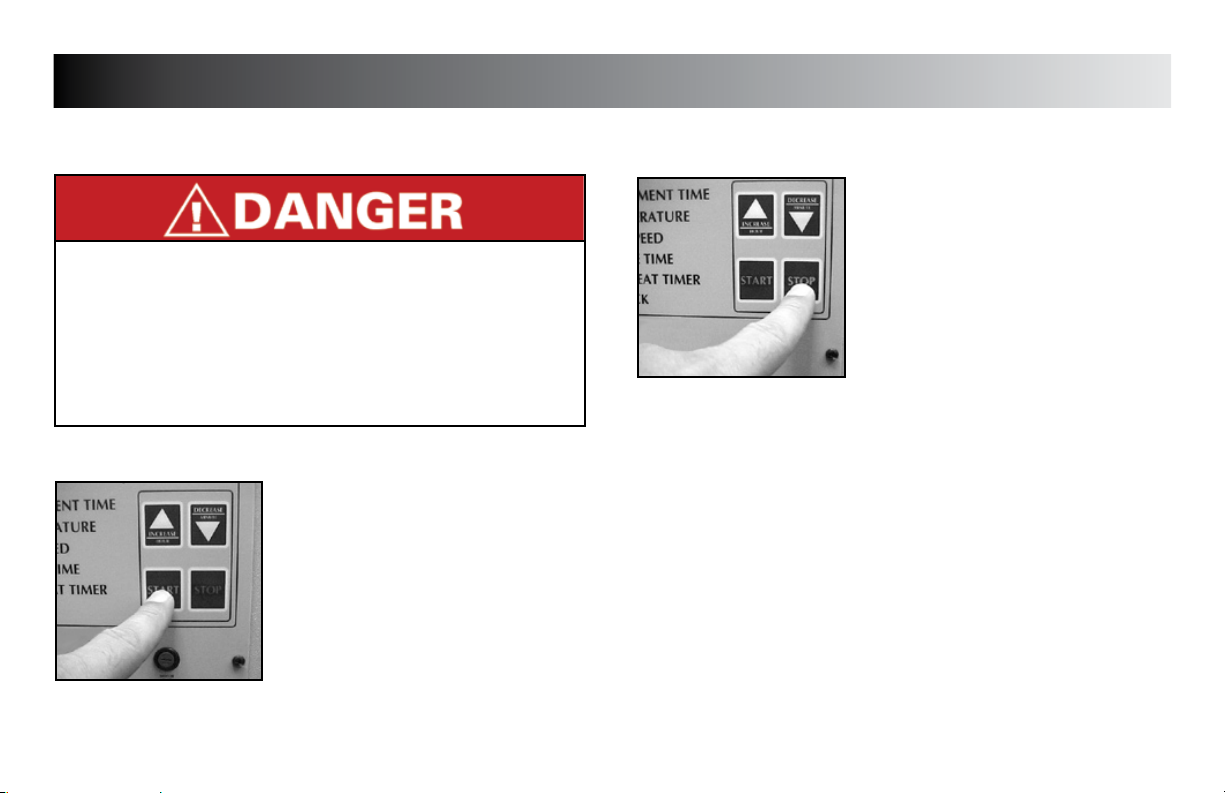
OPERATION
Fluidotherapy® - Dry Heat Therapy
PATIENT PREPARATION
Clean the patient treatment area thoroughly with an antimicrobial •
soap and clean water as per industry, facility, regulatory standards,
and universal skin washing procedures.
Following the skin washing procedure, apply a hospital grade •
antiseptic skin cleanser, according to the cleanser manufacturer's
recommended instructions for use.
Reference the maintenance schedule and make certain the •
preventive maintenance has been performed prior to starting the
unit.
STARTING TREATMENT
STARTING
Make certain all control panel settings have
been made, unit is preheated, and patient
is in proper position with sleeve(s) secure
prior to starting the treatment. Refer to
pages 15 - 17 for setup of the unit.
Press “START” button to begin treatment.
STOPPING TREATMENT
STOPPING
Treatment will automatically stop when the
treatment time reaches zero.
Should it be desired to stop treatment
before the timer reaches zero, push the
“STOP” button.
NOTE:
Should the treatment be stopped before
the timer reaches zero, it will be necessary
to re-set the “TREATMENT TIME” in order to
complete the prescribed treatment.
24
Page 29

PREVENTIVE MAINTENANCE
Fluidotherapy® - Dry Heat Therapy
DAILY MAINTENANCE
Before any maintenance is performed or attempted, unplug the unit
from the power source to prevent the possibility of electrical shock.
CLEAN INLET FILTERS
At the end of each work day, unplug the
unit and clean the Inlet Filters on the unit.
Carefully remove the filter retainer and
wash the filter and screen with a mild
antibacterial soap and water. Thoroughly
dry the filter and screen before placing
back on the unit.
NOTE:
Should your unit have the earlier style inlet
filters, gently clean the filter using a soft
bristle brush. Be careful not to puncture or
damage the filter.
Should the filter become damaged, torn, or
punctured, call your dealer for replacement
of the filter before resuming operation.
REFILL WITH CELLEX MEDIUM
Refill the unit with Cellex Dry Heat Medium to
approximately one (1) inch above the bottom of
the arm treatment ports of the unit.
NOTE:
Use of other than Cellex Medium may cause
premature failure of the Fluidotherapy unit(s).
INSPECT SLEEVE CONDITION
Inspect the port sleeves for tears, rips, and weak seams. Replace all
sleeves that show signs of tears, rips, weak, or loose seams, or excessive
wear. Keeping the sleeves in excellent condition prevents excessive
spillage of the Cellex medium and prevents the medium from entering
the heat chamber of the unit.
ARM PREHEAT TIMER
Plug the unit back into an approved power
source. If the “Preheat Timer” is used, press
the “PREHEAT TIMER” button once and the
“PREHEAT TIMER ON” indicator will illuminate.
NOTE:
The "PREHEAT TIMER ON" indicator must be
illuminated in order for the Preheat Timer to
automatically come on at the default time the
next morning.
The Fluidotherapy Preheat Timer does not
operate on Saturday or Sunday. However,
for Monday morning preheat, set the unit as
25
25
described above on Friday evening.
Page 30
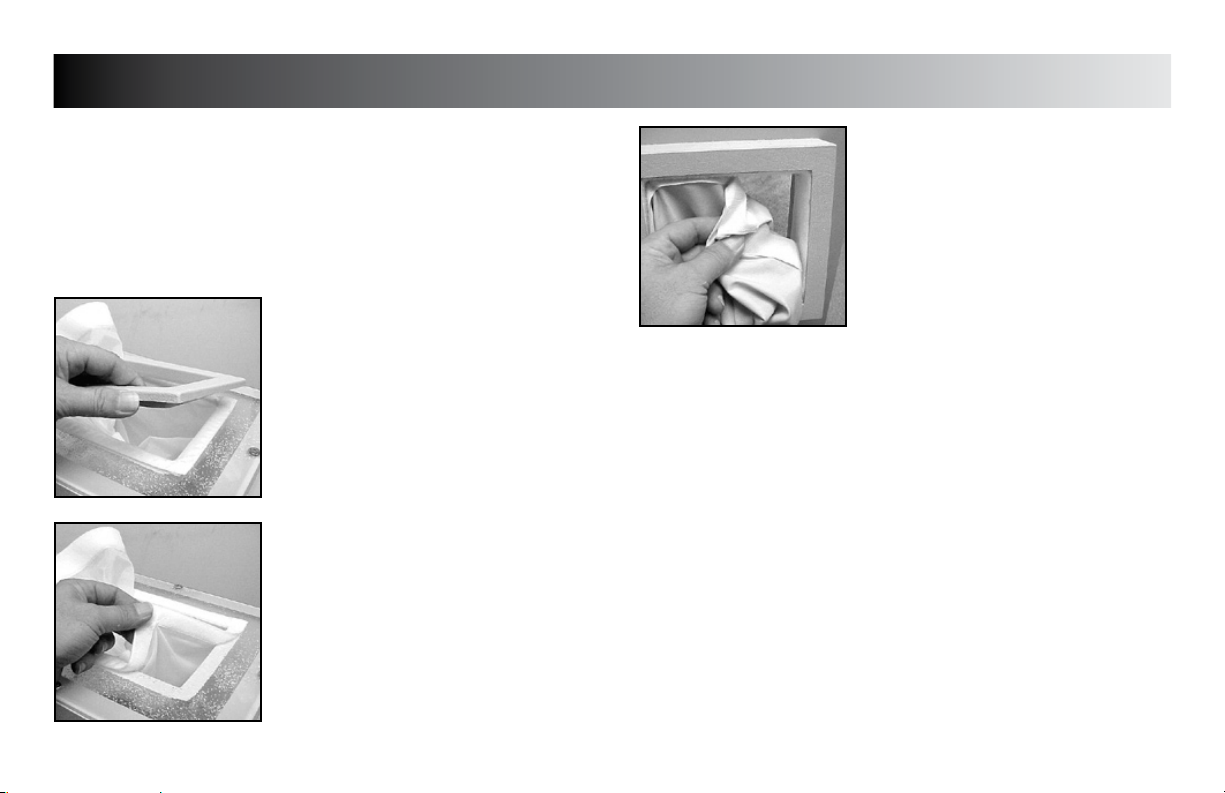
PREVENTIVE MAINTENANCE
Fluidotherapy® - Dry Heat Therapy
WEEKLY MAINTENANCE
Each week all sleeves of the Fluidotherapy unit should be laundered in a
mild antibacterial detergent. Allow the sleeves to air dry or dry on a low
temperature setting. Drying the sleeves in high temperatures could cause
the sleeves to shrink or become distorted, resulting in the sleeve(s) not
properly fitting when placing them back onto the unit.
REMOVING TOP SLEEVES (All Models)
Unplug the unit from the power source.
Carefully remove the rectangular bezel(s)
from the top of the unit.
Carefully pull the hook and loop fasteners
apart to remove the sleeve from the unit.
Replace in reverse order making certain
the corners are well seated to form the seal
required to prevent excessive escape of
medium.
REMOVING END SLEEVE
(Model FLU110D & FLU110DE)
Carefully pull the hook and loop fastener on
the sleeve to remove sleeve from unit.
Replace in reverse order making certain
the corners are well seated to form the seal
required to prevent excessive escape of
medium.
26
Page 31

PREVENTIVE MAINTENANCE
Fluidotherapy® - Dry Heat Therapy
WEEKLY MAINTENANCE (CONTINUED)
REMOVING END SLEEVES
(Model FLU115D & FLU115DE)
NOTE:
The sleeve in these photos has been
modified (cut away) for clarity.
Remove the top sleeves to gain access to
the inside of the unit. Refer to page 26.
Pull the medium back and away from the
arm ports.
NOTE:
It is extremely important to move the medium away from the ports to
prevent the medium entering the space between the inner and outer
walls of the unit when the sleeves are removed and replaced.
Dust away all Cellex medium from inside
the sleeve.
Pull the retainer ring from the sleeve and
lay aside for re-installation.
2727
Pull the sleeve away from the sleeve
extrusion in the inner housing of the unit.
NOTE:
There is a ring folded into the sleeve that
fits over the sleeve extrusion on the inside
of the unit.
From the outside of the unit, reach into the
sleeve and remove the other sleeve retainer
ring.
Cellex medium entering the heat
chamber of the unit can cause
severe injury to patients as well as
smoke damage to the facility and
the Fluidotherapy unit.
Carefully pull the sleeve and ring out of the
unit, avoiding Cellex medium from entering
the cavity between the two housings of the
unit.
Page 32

PREVENTIVE MAINTENANCE
Fluidotherapy® - Dry Heat Therapy
WEEKLY MAINTENANCE (CONTINUED)
Remove the ring from the folded end of the
sleeve.
Launder sleeves as described on page 26.
Vacuum out any of the Cellex medium that
may have entered the cavity between the
inner and outer walls of the unit.
Removal of all Cellex medium that has entered the cavity is essential to
maintain proper and safe operation of the unit. If the medium reaches
the heating chamber of the unit, immediately call a certified technician
for removal of any suspected medium in the heat chamber. Do not place
the unit into service if medium is suspected to be in the heat chamber.
INSTALLING 115 Model SLEEVE
Fold the elastic end of the sleeve over
the ring.
Place sleeve through port hole.
Place the ring/sleeve assembly over the
sleeve extrusion on the inside of the unit.
NOTE:
Position the sleeve seam toward the top of
the unit and push the ring/sleeve assembly
completely onto the sleeve extrusion.
Install the inner sleeve retainer ring making
certain the ring split will be toward the top of
the unit.
Push the retainer ring over the ring/sleeve
assembly until it is completely seated.
NOTE:
The retainer ring should fit tightly over the
sleeve. If necessary, use a pair of pliers to
tighten the ring in the area where it slides over
the sleeve.
28
Page 33

PREVENTIVE MAINTENANCE
WEEKLY MAINTENANCE (CONTINUED)
From the outside of the unit, reach through
the sleeve and install the outer sleeve
retaining ring.
NOTE:
Start installation of the outer ring at the top
of the unit. As the ring is being seated, pull
the area of the sleeve tight. This will aid in the
prevention of excessive medium spillage and
patient comfort.
Fluidotherapy® - Dry Heat Therapy
2929
Page 34

PREVENTIVE MAINTENANCE
Fluidotherapy® - Dry Heat Therapy
ADDITIONAL FLUIDOTHERAPY PREVENTIVE MAINTENANCE REQUIREMENTS
The following additional preventive maintenance requirements must be scheduled and performed as described to ensure that the unit is operating
efficiently, safely, and functioning at optimum level. A blank Maintenance Record is provided on page 31 to aid in the scheduling and record keeping of
this prescribed preventive maintenance program. The following preventive maintenance procedures must be performed by a DJO, LLC qualified service
technician trained in the maintenance requirements of the Chattanooga Fluidotherapy units.
QUARTERLY (Every 3 Months)
The following Preventive Maintenance must be performed on all
Fluidotherapy units quarterly by a certified service technician.
• INTERNAL CAVITY INSPECTION AND CLEANING
• FULL FUNCTIONAL AND PERFORMANCE TESTS
BI-ANNUAL (Every 6 Months)
The following Preventive Maintenance must be performed on all
Fluidotherapy units every six months in addition to the Quarterly
Maintenance requirements by a certified service technician.
• CHANGE CELLEX MEDIUM
ANNUAL (Once per Year)
The following Preventive Maintenance must be performed on all
Fluidotherapy units annually in addition to the Quarterly and Bi-Annual
Maintenance requirements by a certified service technician.
• CALIBRATION
AS NEEDED
The following Preventive Maintenance must be performed only if
performance test results indicate replacement is necessary.
• INTAKE FILTER REPLACEMENT
• DISTRIBUTOR REPLACEMENT
• BLOWER MOTOR(S) REPLACEMENT
If the level of Cellex medium suddenly drops one inch or more •
below the operating level of the unit, immediately pull the unit out
of service and contact a certified service technician.
A sudden level drop in the Cellex medium may indicate that •
medium is leaking into the heating chamber of the unit and must
be repaired before the unit is placed back into service.
30
Page 35

PREVENTIVE MAINTENANCE
Fluidotherapy® - Dry Heat Therapy
FLUIDOTHERAPY MAINTENANCE RECORD
UNIT SERIAL NUMBER UNIT MODEL NUMBER DATE PLACED IN SERVICE
DEALER PHONE CONTACT
DATE MAINTENANCE PERFORMED TECH INITIALS DATE MAINTENANCE PERFORMED TECH INITIALS
Completing this form:
“DATE”-Date Service is performed “MAINTENANCE PERFORMED”- Quarterly, Bi-Annual or Annual “TECH INITIALS”- Certified Tech’s Initials
3131
Page 36

PREVENTIVE MAINTENANCE
Fluidotherapy® - Dry Heat Therapy
CLEANING THE FLUIDOTHERAPY UNIT
After each use, clean the unit and its accessories using a soft, clean cloth dampened with water and a mild antibacterial detergent.
Avoid the use of abrasive materials and cleaning solvents.
SERVICE
When the Fluidotherapy Dry Heat Therapy unit requires service or
preventive maintenance, contact the selling dealer or DJO, LLC Service
Department.
All units returned to the factory for service must include the following:
WARRANTY REPAIR/OUT OF WARRANTY REPAIR
1. Written statement containing the following information:
·
2. Copy of original invoice issued at purchase of the unit.
3. Ship unit to Factory in the original container with all
accessories and information as required in item 1 above to:
DJO, LLC
Chattanooga Repair Center
47492 SD Hwy 22
PO Box 709
Clear Lake, SD 57226 USA
chattgroup.com
Service to these units should be performed only by service technicians
certified by DJO, LLC.
RA Number- Obtain from Factory
·
Unit Model Number
·
Unit Serial Number
·
Contact person with Phone and Fax Numbers
·
Billing Address (for Out of Warranty Repair)
·
Shipping Address (Where to Ship Unit after Repair)
·
Detailed Description of Problem or Symptoms
Council Directive 2002/96/EC concerning Waste Electrical
and Electronic Equipment (WEEE). Indicates a requirement
not to dispose of WEEE as municipal waste. Contact your local
distributor for information regarding disposal of the unit
and accessories.
32
Page 37

ACCESSORIES
REPLACEMENT ACCESSORIES
When ordering additional accessories for Chattanooga Fluidotherapy units,
use the following part numbers and descriptions.
HENLEY No. CGI No.
MODEL FLU110D & FLU110DE
TOP SLEEVE SLE0003 31775
TOP SLEEVE BEZEL FRA0003 31456
ARM SLEEVE SLE0002 31774
MODEL FLU115D & FLU115DE
TOP SLEEVE SLE0003 31775
TOP SLEEVE BEZEL FRA0003 31456
INNER SLEEVE RETAINER TRI0034 31883
SLEEVE RING PPL0013 31679
OUTER SLEEVE RETAINER TRI0035 31834
ARM SLEEVE SLE0001 31773
CELLEX Dry Heat Medium 10 lbs (4.5 kg) MED0001
Fluidotherapy® - Dry Heat Therapy
MEDIUM
Use only Chattanooga’s Cellex Dry Heat Medium in the Fluidotherapy
unit. The Cellex medium is designed specifically for use in the
Chattanooga Fluidotherapy units to ensure optimal and efficient
operation of all the Fluidotherapy products.
3333
Page 38

WARRANTY
DJO ("Company") warrants that the Fluidotherapy Dry Heat Therapy units ("Product") are free of defects in material and workmanship. This warranty shall remain in effect for one
year (12 months) from the date of original consumer purchase. If this Product fails to function during the one year warranty period due to a defect in material or workmanship, at
the Company's option, the Company or the selling dealer will repair or replace this Product without charge within a period of thirty (30) days from the date on which the Product
is returned to the Company or the dealer.
All repairs to the Product must be performed by a service center certified by the Company. Any modifications or repairs performed by unauthorized centers or groups will void
this warranty.
The warranty period for accessories is 90 days. Accessories consist of sleeves and use replaceable intake filter(s).
The warranty period for the motor(s) and distributor is 180 days.
To participate in warranty coverage, this Product's warranty registration card (included with Product) must be filled out and returned to the Company by the original owner
within ten (10) business days of purchase.
This Warranty Does Not Cover:
Replacement parts or labor furnished by anyone other than the Company, the selling dealer, or a certified Company service technician.
Defects or damage caused by labor furnished by someone other than Company, the selling dealer, or a certified Company service technician.
Any malfunction or failure in the Product caused by product misuse, including, but not limited to, the failure to provide reasonable and preventive maintenance or any use that
is inconsistent with the Product User's Manual.
Some states do not allow the exclusion or limitation of incidental or consequential damages, so the above limitation or exclusion may not apply to you.
To Obtain Service From Company or the selling dealer under this warranty:
1. A written claim must be made within the warranty period to the Company or the selling dealer. Written claims made to the Company should be sent to:
DJO, LLC
1430 Decision St.
Vista, CA 92081 USA
Phone: 1-800-592-7329 USA
Phone: 1-423-870-2281 or 1-317-406-2250
Fax: 1-317-406-2014
and
2. The Product must be returned to the Company or the selling dealer by the owner.
This warranty gives you specific legal rights and you may also have other rights which vary from state to state.
The Company does not authorize any person or representative to create for it any other obligation or liability in connection with the sale of the Product.
Any representation or agreement not contained in the warranty shall be void and of no effect.
COMPANY SHALL NOT BE LIABLE IN ANY EVENT FOR INCIDENTAL OR CONSEQUENTIAL DAMAGES.
THE FOREGOING WARRANTY IS IN LIEU OF ALL OTHER WARRANTIES, EXPRESSED OR IMPLIED,
INCLUDING ANY WARRANTY OR MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE.
Fluidotherapy® - Dry Heat Therapy
34
Page 39

Page 40

INDICE DE CONTENIDOS
Fluidotherapy® - Terapia de calor seco
Introducción ........................................................................................1
Acerca de la terapia de calor seco ........................................2-5
Instrucciones de Precaución ......................................2-5
Descripción de las marcas del dispositivo ................2
Indicaciones & Contraindicaciones .............................5
Nomenclatura .................................................................................6-8
Descripción de las unidades
FLU110D y FLU110DE .......................................................6
Descripción de las unidades
FLU115D y FLU115DE .......................................................7
Descripción de las unidades
Controles de Funcionamiento .....................................8
Especificaciones ......................................................................... 9-14
FLU110D & FLU110DE ......................................................9
FLU115D & FLU115DE ................................................... 10
Tablas de compatibilidad electromagnética ..... 11-14
Configuración ............................................................15-23
Parámetros del Modo de Tratamiento ..............15-17
Parámetros Controladores del Tiempo ................... 18
Funcionamiento ...............................................................24
Preparación del Paciente ..............................................24
Inicio del Tratamiento .................................................... 24
Parar el Tratamiento ........................................................ 24
Mantenimiento preventivo ..................................................25-32
Mantenimiento Diario ..............................................25-26
Mantenimiento Semanal.........................................26-29
R E Q U I S I T O S A D I C I O N A L E S D E M A N T E N I M I E N T O
PREVENTIVO DE FLUIDOTERAPIA
Trimestral ............................................................................ 30
Semestral ............................................................................30
Anual .................................................................................... 30
Según sea necesario .......................................................30
Registro del Mantenimiento de Fluidoterapia ............31
Limpieza ................................................................................32
Servicio ................................................................................ 32
Accesorios ........................................................................33
Recambios de accesorios .............................................. 33
Garantía ............................................................................34
Parámetros por Defecto de Modo Preferido ............. 19-23
©2009 DJO, LLC, Vista, CA, EE. UU. Está estrictamente prohibido cualquier uso de la composición editorial, ilustraciones o esquemas de esta publicación sin el consentimiento expreso por escrito de DJO, LLC Esta
publicación la escribió, ilustró y preparó para imprenta DJO, LLC.
i
Page 41

INTRODUCCIÓN
Gracias por adquirir la Unidad de Terapia de Calor Seco del Chattanooga.
Este manual contiene las instrucciones generales de seguridad, funcionamiento, mantenimiento y cuidado para los
propietarios y usuarios de los Aparatos de Terapia de Calor Seco Fluidoterapia.
Las especificaciones recogidas en este manual estaban en vigor en el momento de su publicación. No obstante, la
política de mejora continua de DJO, LLC permite introducir cambios en estas especificaciones en cualquier momento,
sin que esto suponga obligación alguna por parte de DJO, LLC. Lea, entienda y siga la información contenida en este
manual.
Permanezca al corriente de los últimos desarrollos en el campo de la Terapia de Calor Seco y respete todas las medidas
de precaución para el tratamiento.
Mantengasé informado de las indicaciones y contraindicaciones apropiadas para el uso de Terapia de Calor Seco.
Este equipo es para utilizarse solo bajo la prescripción y supervisión de un profesional autorizado.
Fluidotherapy® - Terapia de calor seco
1
1
Page 42

ACERCA DE LA TERAPIA DE CALOR SECO
Fluidotherapy® - Terapia de calor seco
Instrucciones de Precaución
Las instrucciones de precaución que encontrará en esta sección y a lo
largo de este manual se indican con símbolos específicos. Debe entender
estos símbolos y sus definiciones antes de manejar este equipo. Las
definiciones de estos símbolos son las siguientes: :
=CUIDADO - El texto con el indicador "CUIDADO" explicará
posibles infracciones de Seguridad que podrían
causar un daño potencial menor o moderado o
dañar el equipo.
=ATENCION- El texto con un indicador "ATENCION" explicará
posibles infracciones de Seguridad que causarán
potencialmente daños serios y averías al equipo.
=PELIGRO- El texto con el indicador "PELIGRO" explicará
las posibles infracciones de Seguridad que son
situaciones inminentemente peligrosas que darían
como resultado la muerte o daños serios.
=PELIGRO DE EXPLOSION - El texto con un indicador "Peligro de
Explosión" explicará posibles infracciones de Seguridad si
se utiliza este equipo en presencia de anestésicos
inflamables.
=RADIACIÓN ELECTROMAGNÉTICA NO IONIZANTE: El texto
marcado como "Radiación electromagnética no
ionizante" informa al usuario de los posibles
peligros como resultado de la exposición a niveles
de radiación no ionizante elevados y
potencialmente peligrosos.
= Protective Earth (ground)
NOTA: A lo largo de este manual se encontrará "NOTA". Estas Notas son
información útil para ayudar en un área particular o para la
descripción de una función.
DESCRIPCIÓN DE LAS MARCAS DEL DISPOSITIVO
Grado de protección contra descargas eléctricas ...........
Cumple con las normas sobre equipo eléctrico
médico UL 2601-1 CSA C22.2 No. 601.1 ........... ................
Consulte el folleto/manual de instrucciones ...................
2
2
Page 43

ACERCA DE LA TERAPIA DE CALOR SECO
Fluidotherapy® - Terapia de calor seco
Lea, entienda, y ponga en práctica las instrucciones de precaución y funcionamiento que •
aparecen en este manual. Conozca las limitaciones y los peligros relacionados con el uso
de cualquier dispositivo eléctrico. Observe los rótulos de precaución y de funcionamiento
colocados en el aparato.
NO utilice esta unidad en un entorno en que se estén utilizando otros aparatos que radien •
a propósito energía electromagnética de una forma no protegida. El equipo eléctrico
médico precisa unas precauciones especiales en lo relativo a EMC y debe ser instalado y
puesto en servicio según la información sobre EMC incluida en este manual.
Los equipos de comunicación por RF portátiles y móviles pueden afectar el funcionamiento •
del equipo eléctrico médico.
Esta unidad genera, utiliza y puede radiar energía de radiofrecuencia y, si no se instala •
y se utiliza de acuerdo con las instrucciones pertinentes, puede causar interferencias
perjudiciales en otros aparatos próximos. No obstante, no se garantiza que no se
produzcan interferencias en una instalación concreta. La existencia de interferencias
perjudiciales para otros aparatos se puede determinar encendiendo y apagando esta
unidad. Intente corregir la interferencia aplicando una o más de las medidas siguientes:
cambie la orientación o la posición del aparato receptor, aumente la separación entre
los equipos, conecte el equipo a una toma de corriente eléctrica distinta a la que está
conectado el otro aparato o aparatos, y consulte al Departamento de servicio técnico de
DJO, LLC para que le ayude.
NO haga funcionar el aparato conectado a otros dispositivos que no sean de •
Chattanooga
Rellene el aparato a diario para tener un nivel apropiado de llenado con Chattanooga •
Cellex Medio de Calor Seco.
Cambie el Medio de Calor Seco Cellex cada seis (6) meses.•
Use solo el Medio de Calor Seco Cellex en los aparatos de Fluidoterapia.•
Limpie el Filtro(s) Intel diariamente antes de poner en marcha el aparato.•
Use solo los dedos para funcionar los botones de control del panel(es) de control. Utilizar •
objetos puntiagudos como bolígrafos o lapiceros dañaría al aparato.
APAGUE el aparato antes de colocar a un paciente o quitar a un paciente de la unidad.•
Fije todos los puertos de entrada antes de encender el aparato.•
Compruebe la temperatura del aparato antes de tratar al paciente para asegurarse de que •
está a la temperatura correcta.
Coloque al paciente en una posición cómoda teniendo en cuenta una colocación correcta •
del miembro tratado.
Esta unidad sólo debe transportarse y almacenarse a temperaturas de entre -40 °C y -70 °C •
(-40 °F y 158 °F) con una humedad relativa de entre el 10% y el 100% para evitar daños en la
unidad o sus componentes.
Esta unidad debe funcionar a temperaturas de entre 43 °C y 52 °C (110 °F y 125 °F) para evitar •
daños en la unidad o sus componentes.
No use accesorios que no se hayan suministrado con la unidad, o que no estén recomendados •
por DJO, LLC. No se han realizado pruebas que determinen la seguridad de esos otros
productos y su uso podría provocar lesiones al paciente.
3
Page 44

ACERCA DE LA TERAPIA DE CALOR SECO
Instrucciones de Precaución (CONTINUACION)
Fluidotherapy® - Terapia de calor seco
La ley federal restringe la venta de este dispositivo a, o bajo la orden de, un medico o •
profesional autorizado. Este dispositivo se debe usar solo bajo la supervisión continuada
de, un médico o un profesional autorizado.
Para una protección continua contra el riesgo de fuego, cambiar los fusibles solo con •
otros del mismo tipo y potencia.
Asegúrese de que el aparato está conectado a tierra enchufándolo solamente a un •
enchufe de servicio eléctrico de tierra conforme con los códigos eléctricos nacionales y
locales aplicables.
La unidad Fluidotherapy para terapia de calor seco no debe colocarse ni encima ni junto •
a ningún otro equipo. Si es necesario usarla apilada o junto a otro equipo, habrá que
vigilar la unidad Fluidotherapy para terapia de calor seco para verificar que funciona con
normalidad en la configuración establecida.
Este dispositivo se debe mantener lejos de los niños.•
Elimine todos los productos de acuerdo con las normativas y códigos locales y •
nacionales.
Use solamente medio de calor seco tratado en la unidad como Cellex para prevenir el •
polvo excesivo.
Antes de administrar cualquier tratamiento a un paciente debe estar familiarizado con los •
procedimientos de funcionamiento de cada modo de tratamiento disponible, así como
de las indicaciones, contraindicaciones, avisos, y precauciones. Consulte otros recursos
para información adicional con respecto a la aplicación de Terapia de Calor Seco.
Para prevenir una descarga eléctrica, desconectar el aparato de fuente de energía antes •
de intentar cualquier procedimiento de mantenimiento.
Use exclusivamente los accesorios que han sido especialmente diseñados para ser •
utilizados con esta unidad. No use con esta unidad accesorios fabricados por otras
empresas. DJO, LLC no se responsabiliza de ninguna consecuencia derivada del uso
de productos fabricados por otras empresas. El uso de otros accesorios o cables podría
provocar un aumento de las emisiones o una reducción de la inmunidad de esta
unidad.
Elimine todos los productos de acuerdo con las normativas y códigos locales y •
nacionales.
Cuando no se esté utilizando, el equipo de fluidoterapia debe estar protegido para que •
no lo use nadie sin la cualificación correspondiente.
La unidad Fluidotherapy para terapia de calor seco no está diseñada •
para ser utilizada en entornos donde exista una mezcla de anestésicos
inflamables y aire, oxígeno u óxido nitroso.
Realice todas las tareas de mantenimiento preventivo tal como se explica en este •
manual. Si no realiza el mantenimiento preventivo oportuno, podría suceder que
el medio Cellex entre en la cámara de calor de la unidad y provoque lesiones
graves a los pacientes, además de provocar daños por humo en las instalaciones
y la unidad de fluidoterapia.
Hay que dar tiempo a que los disolventes de adhesivos y soluciones inflamables •
utilizados para limpiar y desinfectar la unidad se evaporen antes de poner esta
última en funcionamiento.
44
Page 45

ACERCA DE LA TERAPIA DE CALOR SECO
Indicaciones & Contraindicaciones
Indicaciones
• Alivio del dolor local.
• Tratamiento de la insuficiencia del flujo de sangre local.
• Tratamiento del campo de movimiento cuando se combina con
el ejercicio.
• Tratamiento de los síntomas de artritis no reumática.
Contraindicaciones
Este dispositivo no se debería usar para aliviar un dolor sintomático •
a menos que la etiología esté establecida o a menos que se haya
diagnosticado el síndrome del dolor.
Este dispositivo no se debería usar cuando se presenten lesiones •
cancerosas o heridas abiertas en la zona de tratamiento.
Otras contraindicaciones a considerar es cuando se sospecha que el •
paciente tiene una enfermedad infecciosa grave o enfermedad para
la que se aconseja, con fines médicos generales, suprimir el calor o
fiebre.
• Se deben tomar precauciones adecuadas cuando se trate
a las personas con condiciones o enfermedades medicas
sospechadas o diagnosticadas como problemas cardiacos,
epilepsia, diabetes, etc.
• Antes del tratamiento, consultar a un profesional médico
familiarizado con las medidas de precaución que se deben
tomar para pacientes que pueden tener reacciones alérgicas al
polvo y al polen.
Fluidotherapy® - Terapia de calor seco
55
Page 46

NOMENCLATURA
Descripción de las unidades FLU110D y FLU110DE
1. TAPA DEL DEPOSITO & VENTANA DE VISUALIZACION DEL
1
8
TRATAMIENTO
Acceso a añadir/cambiar medio, ver tratamiento del paciente, y
acceso clínico a a zona de tratamiento del paciente
Fluidotherapy® - Terapia de calor seco
2
3
7
6
4
5
2. DEPOSITO DEL MEDIO/TRATAMIENTO
Acceso al tratamiento y deposito del medio
3. PUERTO DE ACCESO A LA MANO/BRAZO
Puerto de tratamiento para el Tratamiento de Mano/Brazo (extremo
del aparato)
4. FILTROS DE ENTRADA REEMPLAZABLES
Filtros de la entrada de aire de la habitación en el aparato de
Fluidoterapia (1 a cada lado)
5. BASE DE CUATRO PUNTOS
Ruedecillas incluidas para convertirlo en una Base Móvil
6. ALOJAMIENTO DEL VENTILADOR
Alojamiento del Ventilador & Calentador
7. PANEL DE CONTROL
Ver página 8 para la descripción de los detalles de los controles
8. PUERTO DE ACCESO AL TRATAMIENTO DE CODO/PIE
Puerto del tratamiento para Codo y Pie
66
Page 47

NOMENCLATURA
Descripción de las unidades FLU115D y FLU115DE
1
2
8
3
7
4
5
1. PUERTO DE ACCESO A CODO/PIE
Puerto de tratamiento para el Tratamiento de Codo y Pie. Acceso a
añadir/cambiar medio. Acceso clínico a la zona de tratamiento de
pacientes.
2. PUERTOS DE ACCESO A MANO/BRAZO
Puerto de tratamiento para el Tratamiento de Mano/Brazo (extremo
del aparato).
3. FILTROS DE ENTRADA
Filtros de entrada del aire de la habitación al aparato de
Fluidoterapia (1 a cada lado).
4. MEDIO/CUBA DE TRATAMIENTO
Acceso al tratamiento y deposito del medio
5. BASE MOVIL
6. BLOQUEADOR DE LAS RUEDECILLAS
7. ALOJAMIENTO DEL VENTILADOR
Alojamiento del Ventilador & Calentador
8. PANEL DE CONTROL
Ver página 8 para la descripción de los detalles de los controles
Fluidotherapy® - Terapia de calor seco
6
77
Page 48

NOMENCLATURA
Controles de Funcionamiento (Todos los Aparatos)
1. VISUALIZACION
Muestra el Tiempo, Temperatura, Velocidad de Aire, Tiempo del
12
11
10
1
2
3
4
78
9
6
5
2. ENCENDIDO DEL CRONOMETRO DE PRECALENTAMIENTO
Indicador de luz para el Cronómetro de Precalentamiento
3. AUMENTO/HORA
Se utiliza para aumentar los parámetros Modo y para fijar la Hora
4. DISMINUCION/MINUTO
Se usa para disminuir los parámetros Modo y para fijar los
5. PARAR
Se usa para parar el tratamiento y el Cronómetro de Precalentamiento
6. FUSIBLE DEL MOTOR
7. INICIO
Se usa para empezar el tratamiento
8. BARRA INDICADORA
Indica los Modos según se eligen
9. FUSIBLE DEL CALENTADOR
10. BOTON DEL CRONOMETRO DE PRECALENTAMIENTO
Presionar una vez para Encender el Cronómetro de
11. ELEGIR MODO
Se utiliza para elegir el Modo deseado
12. PM
Indicador PM para el Reloj
Fluidotherapy® - Terapia de calor seco
Pulso, Cronómetro del Precalentador, y Reloj del Tratamiento
cuando se indica el Modo respectivo
cuando se pone el Reloj
Minutos cuando se pone el Reloj
Precalentamiento. Presionar dos veces para iniciar la función de
Precalentamiento
88
Page 49

ESPECIFICACIONES
FLU110D & FLU110DE
Longitud
de la Caja
Altura
Fluidotherapy® - Terapia de calor seco
MODOS DE FUNCIONAMIENTO
Continuo (Por Defecto)
Ajuste de Variables para Tiempo, Temperatura, y Velocidad del Aire
Modo Pulsación.............................................................OFF para 6 seg. ON/OFF
TIEMPO DE TRATAMIENTO..............................................................1 a 99 minutos
TEMPERATURA DE FUNCIONAMIENTO............................110 °F (43.3 °C) 125 °F(51.6 °C)
VELOCIDAD DEL AIRE............................................0% a 100% (incrementos 5%)
CRONOMETRO DE PRECALENTAMIENTO.................................115 °F (46.1 °C)
con 50% Corriente de Aire
CAPACIDAD DEL MEDIO..............................Aproximadamente 30 lb (13.6 kg)
ENERGÍA ENTRANTE (FLU110D).........................................120V, 50/60 Hz, 12A
ENERGÍA ENTRANTE (FLU110DE)................................230-240V, 50/60 Hz, 8A
DIMENSIONES FÍSICAS
Longitud de la Caja.........................................................................34.0” (86.4 cm)
Anchura de la caja...........................................................................11.5” (29.2 cm)
Altura.....................................................................................................33.0” (83.8 cm)
Peso..........................................................................70 lbs (31.7 kg) Menos Medio
Peso de Transporte .....................................................................100 lbs (45.4 kg)
GRADO DE PROTECCIÓN CONTRA LA ENTRADA DE AGUA..................IPX0
CLASE ELÉCTRICA...............................................................................................CLASE I
GRADO DE PROTECCIÓN CONTRA DESCARGAS ELÉCTRICAS......TIPO B
CABLE DE ALIMENTACIÓN............2 metros de longitud, 14 AWG, blindado
Anchura de la caja
Asegúrese de que el aparato está conectado a tierra
enchufándolo solamente a un enchufe de servicio eléctrico de
tierra conforme con los códigos eléctricos nacionales y locales
aplicables.
99
Page 50

ESPECIFICACIONES
FLU115D & FLU115DE
Anchura de la caja
Altura
Longitud de
la Caja
Fluidotherapy® - Terapia de calor seco
MODOS DE FUNCIONAMIENTO
Continuo (Por Defecto)
Ajuste de Variables para Tiempo, Temperatura, y Velocidad del Aire
Modo Pulsación..............................................................OFF para 6 seg ON/OFF
TIEMPO DE TRATAMIENTO...............................................................1 a 99 minutos
TEMPERATURA DE FUNCIONAMIENTO.....................110 °F (43.3 °C) to 125 °F (51.6 °C)
VELOCIDAD DEL AIRE..........................................0% to 100% (incrementos 5%)
CRONOMETRO DE PRECALENTAMIENTO..............................115 °F (46.1 °C)
con 50% Corriente de Aire
CAPACIDAD DEL MEDIO..................................Aproximadamente 40 lbs (18.1 kg)
ENERGÍA ENTRANTE (FLU115D).........................................120V, 50/60 Hz, 12A
ENERGÍA ENTRANTE (FLU115DE)..............................230-240V, 50/60 Hz, 10A
DIMENSIONES FÍSICAS
Longitud de la caja........................................................................ 34.0” (86.4 cm)
Anchura de la caja...........................................................................18.5” (47.0 cm)
Altura.....................................................................................................33.0” (83.8 cm)
Peso............................................................................60 lbs (27.2kg) Menos Medio
Peso de Transporte .....................................................................100 lbs (45.4 kg)
GRADO DE PROTECCIÓN CONTRA LA ENTRADA DE AGUA. ................IPX0
CLASE ELÉCTRICA...............................................................................................CLASE I
GRADO DE PROTECCIÓN CONTRA DESCARGAS ELÉCTRICAS.....TIPO B
CABLE DE ALIMENTACIÓN............2 metros de longitud, 14 AWG, blindado
1010
Asegúrese de que el aparato está conectado a tierra
enchufándolo solamente a un enchufe de servicio eléctrico
de tierra conforme con los códigos eléctricos nacionales y
locales aplicables.
Page 51

ESPECIFICACIONES
Fluidotherapy® - Terapia de calor seco
TABLAS DE COMPATIBILIDAD ELECTROMAGNÉTICA (EMC)
TABLA 1: DIRECTRICES Y DECLARACIÓN DEL FABRICANTE, EMISIONES ELECTROMAGNÉTICAS
El Fluidotherapy - Terapia de calor seco destinadas a su uso en entornos electromagnéticos, como se especica a continuación. El cliente o
el usuario de las Fluidotherapy - Terapia de calor seco debe asegurarse de que se utilice en dichos entornos.
Pruebas de emisiones Cumplimiento Entornos electromagnéticos: indicaciones
Emisiones de RF
CISPR 11
Emisiones de RF
CISPR 11
Emisiones armónicas
IEC 61000-3-2
Fluctuaciones de tensión
IEC 61000-3-3
Grupo 1
Clase A
Clase A
Cumplimiento
La unidad Fluidotherapy para terapia de calor seco utiliza la energía de RF únicamente para su función interna. Por lo tanto, sus emisiones de RF son muy bajas
y no es probable que causen ninguna interferencia en los equipos electrónicos cercanos.
El Fluidotherapy - Terapia de calor seco
directamente conectados a la red de alimentación de baja tensión pública que ofrece suministro a edificios con fines domésticos.
son aptas para su uso en cualquier tipo de establecimientos, incluidos establecimientos domésticos y aquéllos
11
Page 52

ESPECIFICACIONES
Fluidotherapy® - Terapia de calor seco
TABLAS DE COMPATIBILIDAD ELECTROMAGNÉTICA (EMC) (CONTINUACIÓN)
TABLAS 2 Y 3: DIRECTRICES Y DECLARACIÓN DEL FABRICANTE, INMUNIDAD ELECTROMAGNÉTICA
El Fluidotherapy - Terapia de calor seco están destinadas a su uso en entornos electromagnéticos, como se especica a continuación. El
cliente o el usuario de Fluidotherapy - Terapia de calor seco debe asegurarse de que se utilice en dichos entornos.
Prueba de
inmunidad
Conducida por RF
IEC 61000-4-6
RF radiada
IEC 61000-4-3
NO TA : A 80 MHz y 800 MHz, se aplica el mayor rango de frecuencia.
NO TA : Estas directrices no se aplican en todas las situaciones. La propagación electromagnética está afectada por la absorción y reflexión de las estructuras los objetos y
la gente.
a
Las fuerzas de campo de los transmisores fijos, como estaciones base de radioteléfonos (móviles/inalámbricos) y radios móviles terrestres, equipos de radioaficionado,
emisiones de radio AM y FM y emisiones de TV no se pueden predecir con precisión en la teoría. Para evaluar el entorno electromagnético causado por transmisores
de RF fijos, se debe realizar una inspección de lugares electromagnéticos. Si la fuerza de campo medida en la ubicación en que se utilizan las plataformas de terapia
física Fluidotherapy thermothérapie sèche supera el nivel de cumplimiento RF aplicable anterior, debe vigilarse que el funcionamiento de las plataformas sea normal.
Si se detecta un rendimiento anormal, puede resultar necesario tomar medidas adicionales, como la reorientación o reubicación de las plataformas de terapia física
Fluidotherapy thermothérapie sèche.
b
Por encima del rango de frecuencias de 150 kHz a 80 MHz, las fuerzas de campo deben ser inferiores a 3 V/m.
IEC 60601
Nivel de prueba
3 Vrms
De 150 kHz a 80 MHz
3 V/m
De 80 MHz a 2.5 GHz
Nivel de
cumplimiento
3
3 V/m d = [3.5]√P 80 MHz a 800 MHz E
Entornos electromagnéticos: indicaciones
Los equipos de comunicaciones mediante RF portátiles no deben utilizarse a una distancia menor de cualquier pieza de las plataformas de terapia física FluidotherapyTerapia de calor seco (cables incluidos) que la distancia de separación recomendada calculada a partir de la ecuación aplicable a la frecuencia del transmisor.
Distancia de separación recomendada:
d = [3.5]√P V
d = [7]√P 800 MHz a 2.5 GHz E
1
1
donde P es la potencia de salida máxima del transmisor en vatios (W) según el fabricante del transmisor y d es la distancia de separación
recomendada en metros (m).
Las fuerzas de campo de los trasmisores de RF fijos, determinadas en una inspección de los lugares electromagnéticos
al nivel de cumplimiento de los rangos de frecuencias
Es posible que se produzcan interferencias en las cercanías de los equipos marcados con el siguiente símbolo:
12
1
a
b
.
deben ser inferiores
Page 53

ESPECIFICACIONES
Fluidotherapy® - Terapia de calor seco
TABLAS DE COMPATIBILIDAD ELECTROMAGNÉTICA (EMC) (CONTINUACIÓN)
TABLAS 2 Y 3: DIRECTRICES Y DECLARACIÓN DEL FABRICANTE, INMUNIDAD ELECTROMAGNÉTICA CONTINUACIÓN
Prueba de inmunidad
Descarga electrostática (ESD)
IEC 61000-4-2
Transitorios rápidos en
ráfagas
IEC 61000-4-4
Sobretensión
IEC 61000-4-5
Huecos de tensión, interrup-
ciones breves y variaciones
de tensión en líneas de
entrada de la fuente de
alimentación
IEC 61000-4-11
Campo magnético de
frecuencia de alimentación
(50/60 Hz)
IEC 61000-4-8
NO TA: U
es el voltaje de corriente c.a. anterior a la aplicación del nivel de prueba.
T
IEC 60601
Nivel de prueba
Contacto de ±6 kV
Aire de ±8 kV
±2 kV para líneas de fuentes de
alimentación
±1 kV para líneas de entrada/salida
±1 kV en modo diferencial
±2 kV en modo común
<5% U
(hueco de >95% en UT) para
T
0,5 ciclos
<40% U
(hueco de >60% en UT)
T
para 5 ciclos
<70% U
(hueco de >30% en UT) para
T
25 ciclos
<5% U
(hueco de >95% en UT) para
T
5 seg.
Nivel de cumplimiento Entornos electromagnéticos: indicaciones
Contacto de ±6 kV
Aire de ±8 kV
±2 kV para líneas de fuentes de
Los suelos deben ser de madera, hormigón o de baldosas de cerámica. Si el suelo no está
cubierto con un material sintético, la humedad relativa debe ser de al menos un 30%.
La calidad de la alimentación debe ser la de un entorno comercial u hospitalario normal.
alimentación
±1 kV para líneas de entrada/salida
±1 kV en modo diferencial
La calidad de la alimentación debe ser la de un entorno comercial u hospitalario normal.
±2 kV en modo común
<5% U
(hueco de >95% en UT) para
T
0,5 ciclos
<40% U
(hueco de >60% en UT) para
T
5 ciclos
<70% U
(hueco de >30% en UT) para
T
25 ciclos
<5% U
(hueco de >95% en UT) para
T
5 seg.
La calidad de la corriente eléctrica debe ser la típica de un entorno comercial u
hospitalario. Si el usuario de Fluidotherapy - Terapia de calor seco necesita que el
funcionamiento no se detenga durante las interrupciones de la red de alimentación,
se recomienda utilizar una fuente de alimentación ininterrumpida o una batería para
suministrar energía a Fluidotherapy - Terapia de calor seco.
3 A/m 3 A/m Los campos magnéticos de frecuencia de alimentación deben tener los niveles típicos de
un entorno comercial u hospitalarios normal.
13
Page 54

ESPECIFICACIONES
Fluidotherapy® - Terapia de calor seco
TABLAS DE COMPATIBILIDAD ELECTROMAGNÉTICA (EMC) (CONTINUACIÓN)
Tabla 4: DisTancias De separación recomenDaDas enTre equipos De comunicaciones rF porTáTiles y
móviles y las plaTaFormas De Terapia Física FluiDoTherapy - Terapia De calor seco
Las Fluidotherapy - Terapia de calor seco están destinada a un uso en un entorno electromagnético en el que las perturbaciones de RF radiada estén
controladas. El cliente o el usuario de Fluidotherapy - Terapia de calor seco puede evitar las interferencias electromagnéticas si mantiene una distancia
mínima entre los equipos de comunicaciones RF portátiles y móviles (transmisores) y Fluidotherapy - Terapia de calor seco, como se recomienda a
continuación.
Potencia de salida máxima nominal
del transmisor
W
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
Para transmisores con una potencia de salida máxima no incluida en la lista anterior, la distancia de separación recomendada d en metros (m) se puede estimar usando la
ecuación aplicable a la frecuencia del transmisor, en la que P es la es la potencia de salida máxima del transmisor en vatios (W) según el fabricante del transmisor.
NO TA : A 80 MHz y 800 MHz, se aplica la distancia de separación del mayor rango de frecuencia.
NO TA : Estas directrices no se aplican en todas las situaciones. La propagación electromagnética está afectada por la absorción y reflexión de las estructuras, los objetos y
la gente.
Distancia de separación según la frecuencia del transmisor
m
De 150 kHz a 80 MHz
d = [3,5]√P V
14
1
80 MHz a 800 MHz
d = [3,5]√P E
De 800 MHz a 2,5 GHz
1
d = [7]√P E
1
Page 55

CONFIGURACIÓN
PARÁMETROS DEL MODO DE TRATAMIENTO
ATENCIÓN
EL DESGASTE DE LAS PARTÍCULAS DURANTE EL
TRANSPORTE PUEDE DERIVAR EN LA LIBERACIÓN
INICIAL DE POLVO Y DE PARTÍCULAS PEQUEÑAS. EL
POLVO Y EL OLOR SE DISIPARÁN CON EL USO.
Utilizar las siguientes Instrucciones para fijar los distintos Parámetros
de Modo en la posición deseada.
Fluidotherapy® - Terapia de calor seco
Presionar los botones de "AUMENTO" o
"DISMINUCION" hasta que se visualice el
Tiempo de Tratamiento deseado.
NOTA:
El "TIEMPO DE TRATAMIENTO" se puede
ajustar en incrementos de un minuto
desde 1 a 99.
TIEMPO DE TRATAMIENTO
Presionar el botón "PRESIONAR PARA
CAMBIAR EL MODO" hasta que el
indicador luminoso esté al lado de
"TIEMPO DE TRATAMIENTO".
NOTA:
Se visualizará el tiempo por defecto de
"20:00".
TEMPERATURA DE TRATAMIENTO
Presionar el botón "PRESIONAR PARA
CAMBIAR MODO" hasta que el indicador
luminoso esté al lado de "TEMPERATURA".
NOTA:
Cuando la unidad se enchufa por primera
vez a la toma de corriente de la pared
mostrará una temperatura predeterminada
de 46,1 °C (115 °F).
Los indicadores “F” o “C”, y “TEMPERATURA”
parpadearán mientras la temperatura
de tratamiento programada esté siendo
visualizada.
Cuando se indique la temperatura de base,
los indicadores “F” o “C”, y “TEMPERATURA”
permanecerán iluminados.
1515
Page 56

CONFIGURACIÓN
PARÁMETROS DEL MODO DE TRATAMIENTO (CONTINUACIÓN)
TEMPERATURA DE TRATAMIENTO
(CONTINUACIÓN)
Presionar los botones de "AUMENTAR"
o "DISMINUIR" hasta que se visualice la
Temperatura de Tratamiento deseada.
NOTA:
Se puede cambiar °F por °C y vice
versa según se desee. Ver la página 20
para las instrucciones. La Temperatura
de Tratamiento se puede ajustar en
incrementos de 1º desde 31,1 °C a 54 °C (88
°F a 130 °F).
VELOCIDAD DEL AIRE
Presionar el botón "PRESIONAR PARA
CAMBIAR MODO" hasta que el indicador
luminoso esté al lado de "VELOCIDAD DEL
AIRE".
NOTA:
Se visualizará la velocidad por defecto de
"50".
1616
Fluidotherapy® - Terapia de calor seco
Presionar los botones de "AUMENTAR"
o "DISMINUIR" hasta que se visualice la
Velocidad del Aire deseada.
NOTA:
La "VELOCIDAD DEL AIRE" se ajusta en
incrementos de 5 desde 0 a 100.
TIEMPO DE PULSO
Presionar el botón de "PRESIONAR PARA
CAMBIAR MODO" hasta que el indicador
luminoso esté al lado de "TIEMPO DE
PULSO".
NOTA:
El "TIEMPO DE PULSO" permite al aparato
funcionar pulsando el medio durante
el tratamiento. Si se elige y fija, el
medio pulsará encendido y apagado en
incrementos iguales.
La elección de fábrica por defecto es
"APAGADO".
EJEMPLO: "TIEMPO DE PULSO" se fija en
"5". La unidad pulsará el medio durante el
tratamiento, cinco segundos encendido y
cinco segundos apagado.
Page 57

CONFIGURACIÓN
PARÁMETROS DEL MODO DE TRATAMIENTO (CONTINUACIÓN)
TIEMPO DE PULSO
(CONTINUACIÓN)
Fluidotherapy® - Terapia de calor seco
PARAR EL TRATAMIENTO
Presionar los botones de "AUMENTAR"
O "DISMINUIR" hasta que se visualice el
Tiempo de Pulso deseado.
NOTA:
El "TIEMPO DE PULSO" se ajusta en
incrementos de un segundo desde
"APAGADO" hasta 6 segundo.
INICIAR EL TRATAMIENTO
Presionar el botón "INICIAR".
Presionar el botón "PARAR".
1717
Page 58

CONFIGURACIÓN
PARÁMETROS CONTROLADORES DEL TIEMPO
CRONOMETRO DE PRECALENTAMIENTO
Presionar el botón de "PRESIONAR PARA CAMBIAR
MODO" hasta que el indicador luminoso esté al lado
de "CRONOMETRO DE PRECALENTAMIENTO".
NOTA:
El "CRONOMETRO DE PRECALENTAMIENTO"
permite que el aparato precaliente el medio. El
aparato funcionará a una Velocidad del Aire del
50% hasta que la unidad alcance la temperatura de
precalentamiento por defecto o 90 minutos, lo que
alcance antes.
El "CRONOMETRO DE PRECALENTAMIENTO"
funcionará solo de lunes a viernes y se puede
poner para que se inicie automáticamente a una
hora predeterminada y caliente el medio a una
temperatura predeterminada. Ver página 21 para
fijar los parámetros por defecto del "CRONOMETRO
DE PRECALENTAMIENTO".
Presionar el botón "PRESIONAR UNA VEZ
PARA ENCENDER EL CRONOMETRO DE
PRECALENTAMIENTO" para iluminar el
indicador luminoso de "CRONOMETRO DE
PRECALENTAMIENTO ENCENDIDO". Presionar
otra vez para empezar el "CRONOMETRO DE
PRECALENTAMIENTO".
NOTA:
El indicador luminoso de "CRONOMETRO DE
PRECALENTAMIENTO" se debe poner al final de cada
día para que se encienda automáticamente al día
siguiente. El botón "PARAR" se usa para apagar el
Precalentamiento si se desea.
Fluidotherapy® - Terapia de calor seco
RELOJ
Presionar el botón "PRESIONAR PARA
CAMBIAR MODO" hasta que el indicador
luminoso esté al lado de "RELOJ".
NOTA:
El "CRONOMETRO DE PRECALENTAMIENTO"
no funcionará hasta que se ponga el reloj.
Presionar el botón "AUMENTAR" para poner
la hora.
Presionar el botón "DISMINUIR" para poner
los minutos.
NOTA:
El indicador luminoso "PM" se iluminará
cuando se alcance las horas PM.
1818
Page 59

CONFIGURACIÓN
PARÁMETROS POR DEFECTO DE MODO PREFERIDO
INTRODUCIR MODO "PrEF" (MODO PREFERENCIA)
Presionar simultáneamente los botones "PRESIONAR PARA CAMBIAR
MODO", "AUMENTAR" y "DISMINUIR". Se visualizará "PrEF".
NOTA:
El modo de preferencia “PrEF” permite al usuario cambiar los ajustes por
defecto.
Fluidotherapy® - Terapia de calor seco
TIEMPO DE TRATAMIENTO POR DEFECTO
Presionar el botón "PRESIONAR PARA
CAMBIAR MODO" hasta que el indicador
luminoso esté intermitente al lado de
"TIEMPO DE TRATAMIENTO".
Presionar los botones de "AUMENTAR"
y "DISMINUIR" para poner en el aparato
el tiempo de tratamiento por defecto
deseado.
Presionar el botón "PRESIONAR PARA
CAMBIAR MODO" para guardar la nueva
posición.
NOTA:
El ajuste por defecto es de 20:00 minutos.
1919
Page 60

CONFIGURACIÓN
PARÁMETROS POR DEFECTO DE MODO PREFERIDO (CONTINUACIÓN)
INDICACION DE LA TEMPERATURA POR
DEFECTO
Fluidotherapy® - Terapia de calor seco
TEMPERATURA DE TRATAMIENTO POR
DEFECTO
Presionar el botón "PRESIONAR PARA
CAMBIAR MODO" hasta que el indicador
luminoso esté intermitente al lado de
"TEMPERATURA".
Presionar el botón "AUMENTAR" para
establecer que se visualice en el aparato °F
o °C según se desee para la posición por
defecto.
Presionar el botón "PRESIONAR PARA
CAMBIAR MODO" para guardar la posición
nueva.
NOTA:
Al posición de fábrica por defecto es ºF.
20
20
Presionar el botón "PRESIONAR PARA
CAMBIAR MODO" hasta que el indicador
luminoso esté intermitente al lado
de "TEMPERATURA" y se visualice la
temperatura por defecto existente.
Presionar los botones de "AUMENTAR"
y "DISMINUIR" para fijar en el aparato la
temperatura de tratamiento por defecto
deseada.
El Rango de Temperaturas de
Funcionamiento es: 43,3 °C - 51,6 °C (110
°F - 125 °F).
Presionar el botón "PRESIONAR PARA
CAMBIAR MODO" para guardar la posición
nueva.
Page 61

CONFIGURACIÓN
PARÁMETROS POR DEFECTO DE MODO PREFERIDO (CONTINUACIÓN)
MODO PULSO (Activado/Desactivado)
Presionar el botón "PRESIONAR PARA
CAMBIAR MODO" hasta que el indicador
luminoso esté intermitente al lado de
"TIEMPO DE PULSO".
Se visualizará "EnAb" (activado) o "dISA"
(desactivado).
Fluidotherapy® - Terapia de calor seco
MODO CRONOMETRO DE
PRECALENTAMIENTO (Activado/
Desactivado)
Presionar el botón "PRESIONAR PARA
CAMBIAR MODO" hasta que el indicador
luminoso esté intermitente al lado de
"CRONOMETRO DE PRECALENTAMIENTO".
Se visualizará "EnAb" (activado) o "dISA"
(desactivado).
Presionar el botón "AUMENTAR" para fijar
en el aparato la visualización de "EnAb" o
"dISA" deseada.
Presionar el botón "PRESIONAR PARA
CAMBIAR MODO" para guardar la posición
nueva.
Presionar el botón "AUMENTAR" para fijar
en el aparato la visualización de "EnAb" o
"dISA" deseada.
Presionar el botón "PRESIONAR PARA
CAMBIAR MODO" para guardar la posición
nueva.
NOTA:
Si el Reloj está desactivado, el
"CRONOMETRO DE PRECALENTAMIENTO"
se desactivará automáticamente.
2121
Page 62
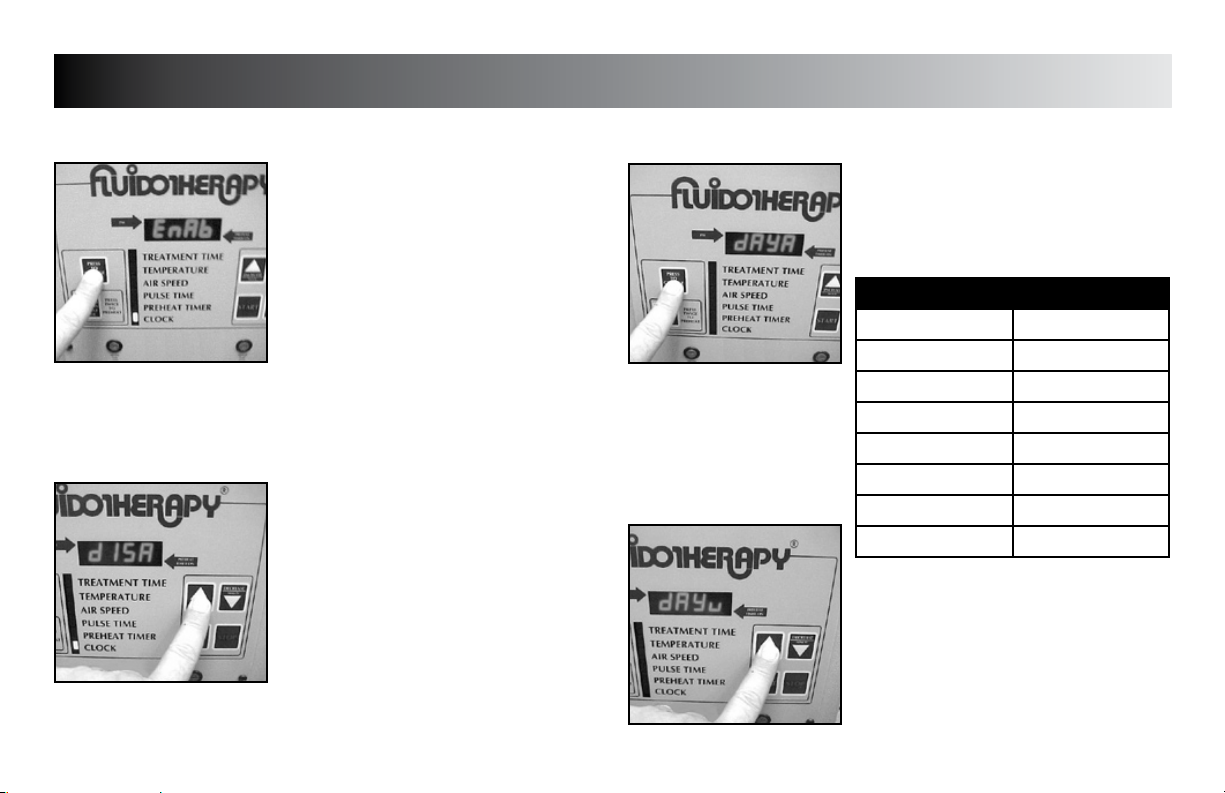
CONFIGURACIÓN
PARÁMETROS POR DEFECTO DE MODO PREFERIDO (CONTINUACIÓN)
RELOJ (Activado/Desactivado)
Fluidotherapy®- Terapia de calor seco
PONER EL DIA DE LA SEMANA
Presionar el botón "PRESIONAR PARA
CAMBIAR MODO" hasta que el indicador
luminoso esté intermitente al lado de
"RELOJ".
Se visualizará "EnAb" (activado) o "dISA"
(desactivado).
Presionar el botón "AUMENTAR" para fijar
en el aparato la visualización de "EnAb" o
"dISA" deseada.
Presionar el botón "PRESIONAR PARA
CAMBIAR MODO" para guardar la posición
nueva.
NOTA:
Si el Reloj está desactivado, el
"CRONOMETRO DE PRECALENTAMIENTO"
se desactivará automáticamente.
Presionar el botón "PRESIONAR PARA
CAMBIAR MODO" hasta que se visualice
"dAy__" (__= código del día).
DAY CODE CHART
WEEKDAY DAY CODE
Sunday dAyu
Monday dAyn
Tuesday dAyE
Wednesday dAyd
Thursday dAyr
Friday dAyF
Saturday dAyA
Presionar el botón de "AUMENTAR" o
"DISMINUIR" para fijar en el aparato la
visualización del código del día deseado.
Presionar el botón "PRESIONAR PARA
CAMBIAR MODO" para guardar la posición
nueva.
2222
Page 63

CONFIGURACIÓN
PARÁMETROS POR DEFECTO DE MODO PREFERIDO (CONTINUACIÓN)
SALIR DE MODO "PrEF"
Presionar el botón "PRESIONAR PARA CAMBIAR MODO" hasta que se visualice "PrEF".
Presionar simultáneamente los botones "AUMENTAR", "DISMINUIR", y "PARAR". Se visualizará el tiempo
de tratamiento por defecto y el indicador estará al lado de "TIEMPO DE TRATAMIENTO".
Fluidotherapy® - Terapia de calor seco
2323
Page 64

FUNCIONAMIENTO
Fluidotherapy® - Terapia de calor seco
PREPARACION DEL PACIENTE
LIMPIAR A FONDO LA ZONA DE TRATAMIENTO DEL PACIENTE CON UN JABON •
ANTIMICROBIANO Y AGUA LIMPIA SEGÚN LAS NORMAS DE INDUSTRIA,
FABRICA, REGULADORAS, Y LOS PROCEDIMIENTOS DE LAVADO DE PIEL
GENERALES.
SEGUIR EL PROCEDIMIENTO DE LAVADO DE PIEL, APLICAR UN LIMPIADOR •
DE PIEL ANTISEPTICO DE TIPO HOSPITALARIO, SEGÚN LAS INSTRUCCIONES
DE USO DE LIMPIEZA RECOMENDADAS POR EL FABRICANTE.
TOME COMO REFERENCIA EL PROGRAMA DE MANTENIMIENTO Y ASEGÚRESE •
DE REALIZAR EL MANTENIMIENTO PREVENTIVO ANTES DE PONER EN
FUNCIONAMIENTO LA UNIDAD.
INICIO DEL TRATAMIENTO
INICIAR
Aseguresé de que se han fijado todos
los paneles de control, el aparato está
precalentado y el paciente está en la
posición correcta con la(s) manga(s)
aseguradas antes de empezar el
tratamiento. Consultar las páginas 15 - 17
para configurar el aparato.
Presionar el botón de "INICIAR" para
empezar el tratamiento
PARAR EL TRATAMIENTO
PARAR
El tratamiento se parará automáticamente
cuando el tiempo de tratamiento alcance
el cero. Si se desea parar el tratamiento
antes de que el cronómetro alcance el cero,
pulsar el botón de "PARAR".
NOTA:
Si el tratamiento se parara antes de que el
cronómetro alcance el cero, será necesario
reiniciar el "TIEMPO DE TRATAMIENTO" para
completar el tratamiento prescrito.
2424
Page 65

MANTENIMIENTO PREVENTIVO
Fluidotherapy® - Terapia de calor seco
MANTENIMIENTO DIARIO
Antes de realizar o intentar cualquier Mantenimiento, desenchufar el aparato
de la fuente de energía para evitar la posibilidad de una descarga eléctrica.
LIMPIEZA DE LOS FILTROS DE ENTRADA
Al final de cada día de trabajo, desenchufar
el aparato y limpiar los Filtros de Entrada
del aparato.
Quitar con cuidado el soporte del filtro
y lavar el filtro y la rejilla con un jabón
antibacteriano suave y agua. Secar
completamente el filtro y la rejilla antes de
volverlo a colocar en el aparato.
NOTA:
Si su aparato tiene el modelo antiguo de
filtros de entrada, limpiar suavemente el
filtro usando un cepillo de cerda blanda.
Cuidado con no perforar o dañar el filtro.
Si se llega a dañar, rasgar o perforar el filtro,
llame a su distribuidor para que sustituya el
filtro antes de reanudar el funcionamiento.
RELLENAR CON MEDIO CELLEX
Rellenar el aparato con Medio Cellex Caliente
Seco hasta aproximadamente una (1) pulgada
por encima del fondo de los puertos de
tratamiento del brazo del aparato.
NO TA:
La utilización de otro que no sea Medio
Cellex puede causar un fallo prematuro en el
aparato(s) de fluidoterapia.
REVISION DEL ESTADO DE LA MANGA
Revisar los desgastes, rasgaduras y las junturas débiles de las mangas
del puerto. Cambiar todas las mangas que muestren signos de desgaste,
rasgaduras o junturas débiles o flojas o uso excesivo. Mantener las
mangas en estado excelente previene el derramamiento excesivo del
medio Cellex y previene el medio de la entrada en la cámara caliente del
aparato.
25
25
Page 66

MANTENIMIENTO PREVENTIVO
Fluidotherapy® - Terapia de calor seco
MANTENIMIENTO DIARIO (CONTINUACIÓN)
TEMPORIZADOR DE PRECALENTAMIENTO
DEL BRAZO
Vuelva a enchufar la unidad en una fuente
de alimentación aprobada. Si se usa el
“Temporizador de precalentamiento”,
pulse el botón “TEMPORIZADOR DE
PRECALENTAMIENTO” una vez y el indicador
“TEMPORIZADOR DE PRECALENTAMIENTO
ENCENDIDO” se iluminará.
NOTA:
El indicador "TEMPORIZADOR DE PRECALENTAMIENTO
ENCENDIDO" debe estar iluminado para que el temporizador
de precalentamiento se encienda automáticamente a la hora
predeterminada a la mañana siguiente.
El temporizador de precalentamiento de fluidoterapia no funciona los
sábados ni los domingos. No obstante, para que el precalentamiento sí
se produzca la mañana del lunes, hay que ajustar la unidad como se ha
explicado anteriormente para la noche del viernes
.
MANTENIMIENTO SEMANAL
Cada semana todas las mangas del aparato de Fluidoterapia se lavarán
con un detergente antibacteriano suave. Dejar que las mangas se
sequen al aire o en un sitio a baja temperatura. Secar las mangas a alta
temperatura podría hacer que las mangas encogieran o que llegaran a
deformarse haciendo que la(s) manga(s) no encajaran cuando se colocan
otra vez en el aparato.
CAMBIO DE LA TAPA DE LAS MANGAS
(Todos los Modelos)
Desenchufar el aparato de la fuente de
energía.
Quitar con cuidado el anillo(s) rectangular
de la tapa del aparato.
Separar con cuidado de los cierres de
gancho y trabilla para quitar la manga del
aparato.
Reponer en orden inverso asegurando que
las esquinas están bien colocadas para
formar la junta necesaria para evitar el
escape excesivo de medio.
2626
Page 67

MANTENIMIENTO PREVENTIVO
MANTENIMIENTO SEMANAL (CONTINUACIÓN)
QUITAR EL EXTREMO DE LA MANGA
(Modelos FLU110D & FLU110DE)
Tirar con cuidado de los cierres de gancho
y trabilla en la manga para quitar la manga
del aparato.
Reponer en orden inverso asegurando que
las esquinas están bien colocadas para
formar la junta necesaria para evitar el
escape excesivo de medio.
QUITAR EL EXTREMO DE LA MANGA
(Modelos FLU115D & FLU115DE)
NOTA:
La manga de estas fotos se ha modificado
(quitado) para mayor claridad.
Quitar la tapa de las mangas para mejorar
el acceso al interior del aparato. Consultar la
página 26.
Retroceder y sacar el medio de los puertos
del brazo.
Fluidotherapy® - Terapia de calor seco
NOTA:
Es extremadamente importante quitar el medio de los puertos para
evitar que entre el medio en el espacio entre las paredes interna y
externa del aparato cuando se quitan o sustituyen las mangas.
Desempolvar todos los medios Cellex del
interior de la manga.
Sacar el anillo de retención de la manga y
dejar a un lado para reinstalarlo.
2727
Page 68

MANTENIMIENTO PREVENTIVO
MANTENIMIENTO SEMANAL (CONTINUACIÓN)
Sacar la manga de la extrusión de manga
en el alojamiento interno del aparato.
NOTA:
Hay un anillo doblado dentro de la manga
que encaja sobre la extrusión de la manga
en el interior del aparato.
Fluidotherapy® - Terapia de calor seco
Quitar el anillo del extremo doblado de la
manga.
Lavar las mangas según se describe en la
página 26.
Desde el exterior del aparato, llegar al
interior de la manga y quitar el otro anillo
de retención de la manga.
La entrada del medio Cellex en
la cámara caliente del aparato(s)
puede causar daños graves a
los pacientes además del daño
del humo para la instalación y el
aparato(s) de Fluidoterapia.
Sacar con cuidado la manga y el anillo del
aparato, evitando que el medio Cellex entre
en la cavidad entre los dos alojamientos del
aparato.
Limpiar con aspiradora el medio Cellex que
pueda haber entrado en la cavidad entre las
paredes interna y externa del aparato.
Retirar todo el medio Cellex que ha entrado en la cavidad es esencial para
mantener un funcionamiento correcto y seguro del aparato. Si el medio
alcanza la cámara de calentamiento del aparato llamar inmediatamente a un
técnico certificado para que quite cualquier medio que se sospeche que está
en la cámara caliente.
2828
Page 69

MANTENIMIENTO PREVENTIVO
MANTENIMIENTO SEMANAL (CONTINUACIÓN)
INSTALACIÓN DE LA MANGA DEL Modelo 115
Doblar el extremo elástico de la manga sobre
el anillo.
Colocar la manga a través de agujero del
puerto.
Colocar la junta anillo/manga sobre la extrusión
de la manga en el interior del aparato.
NOTA:
Situar la costura de la manga hacia la parte de
arriba del aparato y empujar la junta anillo/
manga completamente sobre la extrusión de
la manga.
Instalar la manga interna del anillo de retención
asegurándose de que la raja del anillo está hacia la
parte de arriba del aparato.
Empujar el anillo de retención sobre la junta anillo/
manga hasta que asiente completamente.
NOTA:
El anillo de retención deberá encajar firmemente
en la manga. Si es necesario, usar un par de alicates
para encajar el anillo en la zona sobre la que se
desliza la manga.
Fluidotherapy® - Terapia de calor seco
Desde el exterior del aparato, entrar a
través de la manga e instalar el anillo de
retención de la manga externa.
NOTA:
Comenzar la instalación del anillo
externo en la parte superior del aparato.
Mientras se está asentando el anillo sacar
la superficie de la manga apretada. Esto
ayudará a prevenir el derramamiento del
medio y a la comodidad del paciente.
2929
Page 70

MANTENIMIENTO PREVENTIVO
Fluidotherapy® - Terapia de calor seco
REQUISITOS ADICIONALES DE MANTENIMIENTO PREVENTIVO DE FLUIDOTERAPIA
Los siguientes requisitos de mantenimiento preventivo adicionales se deben programar y realizar según las indicaciones para garantizar que la unidad funciona de forma eficaz,
segura y a su nivel óptimo. En la página 31 encontrará un registro de mantenimiento en blanco para facilitar la planificación y el seguimiento del programa de mantenimiento
preventivo recomendado. Los siguientes procedimientos de mantenimiento preventivo deben ser realizados por un técnico de servicio cualificado de DJO, LLC con la formación
pertinente en los requisitos de mantenimiento de las unidades Fluidotherapy de Chattanooga.
TRIMESTRAL (Cada 3 Meses)
El siguiente Mantenimiento Preventivo se debe realizar en todos los aparatos de
Fluidoterapia trimestralmente por un servicio técnico certificado.
• INSPECCION Y LIMPIEZA DE LA CAVIDAD INTERNA
• PRUEBAS DE PLENO FUNCIONAMIENTO Y RENDIMIENTO
SEMESTRAL (Cada 6 Meses)
El siguiente Mantenimiento Preventivo lo debe realizar un servicio técnico
certificado en todos los aparatos de Fluidoterapia cada seis meses además de los
requisitos de Mantenimiento Trimestral.
• CAMBIO DEL MEDIO CELLEX
ANUAL (Una vez al Año)
El siguiente Mantenimiento Preventivo lo debe realizar un servicio técnico
certificado en todos los aparatos de Fluidoterapia una vez al año además de los
requisitos de Mantenimiento Trimestral y Semestral.
• CALIBRACION
SEGÚN SEA NECESARIO
El siguiente mantenimiento preventivo debe realizarse únicamente si los
resultados de la prueba de rendimiento indican que la sustitución es necesaria.
• SUSTITUCIÓN DEL FILTRO DE ADMISIÓN
• SUSTITUCIÓN DEL DISTRIBUIDOR
• SUSTITUCIÓN DEL MOTOR (O MOTORES) DEL VENTILADOR
Si el nivel de medio Cellex cae de repente una pulgada o más por debajo •
del nivel de funcionamiento del aparato, inmediatamente retirar el
aparato de servicio y contactar con un servicio técnico certificado.
Una repentina caída de nivel en el medio Cellex indica que el medio está •
en la cámara caliente del aparato y se debe reparar antes de volver a
poner el aparato en servicio.
3030
Page 71

MANTENIMIENTO PREVENTIVO
Fluidotherapy® - Terapia de calor seco
REGISTRO DE MANTENIMIENTO DE FLUIDOTERAPIA
NUMERO DE SERIE DEL APARATO NUMERO DE MODELO DEL APARATO FECHA DE PUESTA EN SERVICIO
DISTRIBUIDOR TELEFONO CONTACTO
FECHA MANTENIMIENTO REALIZADO INICIALES TECNICAS FECHA MANTENIMIENTO REALIZADO INICIALES TECNICAS
Rellenar este impreso:
“FECHA”-Fecha en que se realiza el servicio “MANTENIMIENTO REALIZADO”- Trimestral, Semestral o Anual “INICIALES TECNICAS”- Iniciales del Certificado Técnico
3131
Page 72

MANTENIMIENTO PREVENTIVO
Fluidotherapy® - Terapia de calor seco
LIMPIEZA DE LA UNIDAD DE FLUIDOTERAPIA
Después de cada uso, limpie la unidad y sus accesorios con un paño suave y limpio, humedecido con agua y un detergente antibacteriano suave.
Evite el uso de productos abrasivos y disolventes de limpieza.
SERVICIO
Cuando el aparato de Terapia de Calor Seco Fluidoterapia necesite reparación o
mantenimiento preventivo, contactar con el distribuidor de ventas o con el Departamento
de Reparaciones de DJO, LLC.
Todos los aparatos devueltos a la fábrica para su reparación deben incluir lo siguiente:
GARANTIA EN LA REPARACION/FUERA DE LA GARANTIA DE REPARACION
1. Informe por escrito que contenga la información siguiente:
Número RA- Obtenido de la Fábrica
Número de Modelo de la Unidad
Número de Serie de la Unidad
Persona de contacto con Números de Teléfono y Fax
Dirección de Facturación (si está Fuera de la Garantía de Reparación)
Dirección de Envío (Donde Enviar la Unidad después de Repararla)
Descripción Detallada del Problema o Síntomas
2. Copia de la factura original emitida al adquirir la unidad.
3. Enviar la unidad a Fábrica en la caja original con todos los accesorios e
información según se requiere en el punto uno 1 anterior a:
DJO, LLC
Chattanooga Repair Center
47492 SD Hwy 22
PO Box 709
Clear Lake, SD 57226 USA
chattgroup.com
La reparación de estas unidades solo se realizarán en los
Servicios Técnicos Certificados por DJO, LLC.
Directiva 2002/96/CE del Consejo Europeo
sobre Residuos de aparatos eléctricos y
electrónicos (RAEE). Indica la obligación de no
eliminar los RAEE como residuos municipales.
Póngase en contacto con su distribuidor local
para obtener más información sobre cómo
desechar la unidad y sus accesorios.
32
32
Page 73

ACCESORIOS
RECAMBIO DE ACCESORIOS
Cuando se soliciten accesorios adicionales para las unidades Fluidotherapy de
Chattanooga, use los siguientes números de pieza y descripciones.
HENLEY No. CGI No.
MODELO FLU110D & FLU110DE
SUPERFICIE DE LA MANGA BEZEL FRA0003 31456
MANGA DE BRAZO SLE0002 31774
MODELO FLU115D & FLU115DE
SUPERFICIE DE LA MANGA BEZEL FRA0003 31456
RETENCION DE MANGA INTERNA TRI0034 31883
ANILLO DE MANGA PPL0013 31679
RETENCION DE MANGA EXTERNA TRI0035 31834
MANGA DE BRAZO SLE0001 31773
CELLEX Medio de Calor Seco 10 lbs (4.5 kg) MED0001
SUPERFICIE DE LA MANGA SLE0003 31775
SUPERFICIE DE LA MANGA SLE0003 31775
Fluidotherapy® - Terapia de calor seco
MEDIO
Usar solo el Medio Cellex de Calor Seco del Grupo Chattanooga en el
aparato de Fluidoterapia. El medio Cellex está diseñado específicamente
para los aparatos de Fluidoterapia del Grupo Chattanooga para asegurar un
funcionamiento óptimo y eficiente de todos los productos de Fluidoterapia.
3333
Page 74

GARANTIA
DJO, LLC ("Compañía") garantiza que las aparatos de Terapia de Calor Seco Fluidoterapia ("Producto") no tiene defectos materiales ni de ejecución. Esta garantía tendrá efecto
durante un año (12 meses) desde la fecha de compra del cliente original. Si el Producto deja de funcionar durante el periodo de garantía de un año debido a un defecto material
o de ejecución, en la opción de la compañía, la Compañía o el distribuidor de venta reparará o reemplazará este Producto dentro de un periodo de treinta (30) días desde la fecha
en que se devuelva el Producto a la Compañía o al distribuidor.
Todas las reparaciones del Producto se deben realizar por un centro de reparación certificado por la Compañía. Cualquier modificación o reparación realizada por centros o
grupos no autorizados invalidará la garantía.
El periodo de garantía de los accesorios es de 90 días. Los accesorios lo componen las mangas y el filtro(s) que se utiliza de entrada reemplazable.
El periodo de garantía del motor(es) y distribuidor es de 180 días.
Para participar de la cobertura de la garantía, el propietario original debe rellenar y devolver a la Compañía la tarjeta de registro de la garantía del Producto (incluida con el
Producto) en los diez (10) días laborables siguiente a la fecha de la adquisición.
Esta Garantía No Cubre:
La sustitución de partes o mano de obra por cualquier persona ajena a la Compañía, el distribuidor de ventas, o un servicio técnico certificado por la Compañía.
Defectos o daños causados por la mano de obra por cualquier persona ajena a la Compañía, el distribuidor de ventas, o un servicio técnico certificado por la Compañía.
Cualquier funcionamiento incorrecto o fallo en el Producto causado por el mal uso del producto, incluidos, pero sin limitación, la no aplicación de un mantenimiento razonable y
preventivo, o cualquier uso que no esté de acuerdo con el Manual del usuario del Producto.
LA COMPAÑÍA NO SE RESPONSABILIZARA EN TODO CASO POR ACCIDENTE O EL DAÑO CONSIGUIENTE
Algunos estados no permiten la exclusión o limitación de daños por accidente o daños consiguientes, por lo tanto la limitación o exclusión anterior no será de aplicación.
Para Obtener Reparación De la Compañía o del distribuidor de ventas bajo esta garantía:
1. Se debe hacer una reclamación por escrito dentro del periodo de garantía a la Compañía o al distribuidor de ventas. Las reclamaciones escritas hechas a la
Compañía se deben enviar a:
DJO, LLC
1430 Decision St.
Vista, CA 92081 USA
Phone: 1-800-592-7329 USA
Phone: 1-423-870-2281 or 1-317-406-2250
Fax: 1-317-406-2014
y
2. El propietario debe devolver el Producto a la Compañía o al distribuidor de ventas.
Esta garantía le da los derechos legales específicos y puede tener otros derechos que varían de unos estados a otros.
La Empresa no autoriza a ninguna persona o representante a crear, en su nombre, ninguna otra obligación o responsabilidad relacionada con la venta del Producto.
Cualquier representación o acuerdo no contenido en la garantía se considerará nulo y no será aplicable.
LA GARANTÍA ANTERIOR SUSTITUYE A TODAS LAS GARANTÍAS, EXPRESAS O IMPLÍCITAS, INCLUIDA
CUALQUIER GARANTÍA DE COMERCIABILIDAD O IDONEIDAD PARA UN FIN DETERMINADO.
Fluidotherapy® - Terapia de calor seco
3434
Page 75

Page 76

TABLE DES MATIÈRES
Fluidotherapy® - thermothérapie sèche
Avant-propos .......................................................................................1
À propos de la thermothérapie sèche .................................2-5
Instructions de précaution ........................................2-4
Description Des Marquages De L'apparei .................2
Indications et contre indications ..................................5
Nomenclature ..................................................................................6-8
Se familiariser avec les appareils
FLU110D et FLU110DE ....................................................6
Se familiariser avec les appareils
FLU115D et FLU115DE ....................................................7
Se familiariser avec les appareils Commandes ...........8
Spécifications ............................................................................... 9-14
FLU110D et FLU110DE ....................................................9
FLU115D et FLU115DE ................................................. 10
Tableaux de compatibilité
électromagnétique (CEM) ......................................11-14
Configuration .............................................................15-23
Paramètres du mode Traitement .........................15-17
Paramètres régis par le temps .................................... 18
Fonctionnement ..............................................................24
Préparation du patient .................................................24
Commencer le traitement ...........................................24
Arrêter le traitement ......................................................24
Maintenance préventive ...................................................... 25-32
Maintenance quotidienne ............................................25
Maintenance hebdomadaire ................................ 26-29
CONDITIONS SUPPLÉMENTAIRES DE MAINTENANCE
PRÉVENTIVE POUR LA FLUIDOTHÉRAPIE
Trimestrielle .......................................................................30
Semestrielle ......................................................................30
Annuelle .............................................................................30
Selon les besoins .............................................................30
Dossier de maintenance de la fluidothérapie ............ 31
Nettoyage ...........................................................................32
Entretien .............................................................................32
Accessoires ........................................................................................33
Accessoires de remplacement ...................................33
Garantie .......................................................................... 34
Paramètres par défaut du
mode de préférence ..................................................19-23
©2009 DJO, LLC, Vista, CA, USA. Toute utilisation de textes, d’illustrations ou de mise en page de la présente publication sans l’autorisation écrite expresse de DJO, LLC. est strictement interdite. La présente publication a
été rédigée, illustrée et préparée pour l’impression DJO, LLC.
i
Page 77

AVANT-PROPOS
Nous vous remercions d'avoir choisi un appareil de fluidothérapie - thermothérapie sèche.
Ce guide contient des instructions générales concernant la sécurité, le fonctionnement, la maintenance et l'entretien à
l'intention des propriétaires et utilisateurs d'appareils de fluidothérapie - thermothérapie sèche.
Les caractéristiques indiquées dans ce guide étaient en vigueur au moment de la publication. Néanmoins, compte
tenu de la politique d'amélioration continue de DJO, LLC, ces caractéristiques sont à tout moment susceptibles de
modifications, sans obligation de la part de DJO, LLC.
Lisez, assimilez et suivez les renseignements contenus dans le présent guide.
Tenez-vous au courant des derniers développements cliniques dans le domaine de la thérapie par chaleur sèche et
observez toutes les mesures de précaution applicables lors du traitement.
Tenez-vous informé des indications et contre-indications appropriées concernant l'utilisation de la thérapie par chaleur
sèche.
Fluidotherapy® - thermothérapie sèche
Le présent équipement ne doit être utilisé que sur prescription et sous la supervision d’un praticien
licencié.
1
Page 78

À PROPOS DE LA THERMOTHÉRAPIE SÈCHE
Fluidotherapy® - thermothérapie sèche
INSTRUCTIONS DE PRÉCAUTION
Les précautions d'utilisation figurant dans ce chapitre et dans
l'ensemble du guide sont indiquées par des symboles spécifiques.
Veillez à comprendre ces symboles et leur définition avant d'utiliser
cet équipement. Les définitions de ces symboles sont les suivantes :
= MISE EN GARDE - Un texte affecté de l'indicateur « MISE
EN GARDE » explique les infractions potentielles à la sécurité
qui risquent de causer des blessures mineures à modérées ou
d’endommager l’équipement.
=AVERTISSEMENT- Le texte d'un symbole « AVERTISSEMENT »
explique les infractions à la sécurité éventuelles susceptibles de
causer des blessures sérieuses et d'endommager l'équipement.
= DANGER - Un texte affecté de l'indicateur « DANGER » explique
les infractions potentielles à la sécurité qui sont des situations de
danger imminent qui pourraient avoir comme conséquence la mort
ou des blessures graves.
= RISQUE D'EXPLOSION- Le texte portant la mention « Risque
d'explosion » explique les infractions à la sécurité éventuelles si cet
équipement est utilisé en présence d'anesthésiques inflammables.
= RADIATION ÉLECTROMAGNÉTIQUE NON IONISANTE - Le texte
accompagné d’un indicateur « Radiations électromagnétiques non
ionisantes » informe l'utilisateur des risques éventuels liés à des
niveaux élevés et potentiellement dangereux de radiations non
ionisantes.
= Protective Earth (ground)
REMARQUE : Le présent guide contient des renseignements utiles
identifiés par l'indicateur « REMARQUE ». Chaque « REMARQUE » aide
à la compréhension de la description d'un domaine ou d'une fonction
spécifique du produit.
DESCRIPTION DES MARQUAGES DE L'APPAREIL
Degré de protection contre les chocs électriques ........
Conforme aux normes UL 2601-1
CSA C22.2 No. 601.1
Matériel électromédical ..........................................................
Consultez le mode d'emploi/la brochure .........................
2
Page 79

À PROPOS DE LA THERMOTHÉRAPIE SÈCHE
Fluidotherapy® - thermothérapie sèche
Lisez, assimilez et pratiquez les consignes d'utilisation et de précautions •
figurant dans ce guide. Il est important de connaître les limites et les
risques associés à l'utilisation de tout appareil électrique. Vous devez
suivre les décalcomanies de précaution et d'utilisation apposées sur
l'appareil.
•
NE PAS utiliser l’appareil dans un environnement où d’autres dispositifs
sont utilisés et qui dégagent intentionnellement de l’énergie
électromagnétique de manière non protégée. Le matériel médical
électrique requiert une manipulation particulièrement prudente et doit
être installé et mis en service conformément aux informations de la CEM
fournies dans le présent guide.
•
L'équipement de communication portable et mobile à radiofréquences
peut perturber l'équipement médical électrique.
•
Cet appareil génère, utilise et peut rayonner de l’énergie radio-électrique.
S’il n’est pas installé ou utilisé conformément aux instructions précisées,
il peut être à l’origine d’interférences nuisibles avec d'autres dispositifs
voisins. Toutefois, rien ne garantit que des interférences ne surviendront
pas dans le cadre d'une installation particulière. Les interférences
préjudiciables à d’autres dispositifs peuvent être mises en évidence en
mettant cet appareil sous tension et hors tension. Essayez de corriger ces
interférences comme suit : réorientez ou déplacez l’appareil récepteur,
augmentez la séparation entre les équipements, branchez l’équipement
sur une prise d'un circuit différent de celui auquel les autres appareils
sont connectés et consultez le service d'assistance de DJO, LLC pour
obtenir de l'aide.
•
Nettoyez le(s) filtre(s) d'aspiration avant la mise en marche de l'appareil.
Mettez l'appareil à l'ARRÊT avant de positionner ou de retirer un patient •
de l'appareil.
•
Placez le patient dans une position confortable permettant le placement
correct du membre traité.
Sécurisez tous les orifices d'entrée avant de mettre l'appareil en MARCHE.•
Utilisez exclusivement un support à chaleur sèche Cellex® dans les appareils de •
fluidothérapie.
Rechargez quotidiennement l'appareil avec le support à chaleur sèche Cellex de •
Chattanooga.
Les boutons de commande situés sur le(s) panneau(x) de commande doivent •
uniquement être actionnés avec les doigts. N'utilisez pas d'objets pointus comme
des stylos ou des crayons au risque d'endommager l'appareil.
Avant de commencer à traiter vos patients, vérifiez la température de l'appareil •
pour s'assurer qu'elle est correcte.
Cet appareil doit être transporté et stocké à des températures situées entre -40 °C •
et 70 °C (-40 °F et 158 °F) avec une humidité relative comprise entre 10 % et
100 % pour éviter d'endommager l'appareil ou ses composants.
Cet appareil doit fonctionner à des températures situées entre 43 °C et 52 °C •
(110 °F et 125 °F) pour éviter d'endommager l'appareil ou ses composants.
NE PAS utiliser l'appareil lorsqu'il est connecté à un appareil autre que les •
dispositifs de Chattanooga.
Changez le support à chaleur sèche Cellex tous les six (6) mois.•
N'utilisez pas d'accessoires autres que ceux fournis avec l'appareil ou •
recommandés par DJO, LLC. La sécurité des autres produits n'a pas été établie et
leur utilisation pourrait blesser le patient.
3
Page 80

À PROPOS DE LA THERMOTHÉRAPIE SÈCHE
Fluidotherapy® - thermothérapie sèche
Selon la loi fédérale américaine, cet appareil ne peut être vendu que par ou sur •
ordre d'un médecin ou d'un praticien licencié. Le présent équipement ne doit être
utilisé que sous la supervision permanente d’un médecin ou d’un praticien agréé.
Assurez-vous que l'appareil est relié à la terre en le branchant uniquement sur une •
prise électrique reliée à la terre conformément à la législation nationale et locale en
vigueur en matière de codes d'électricité.
Avant d’administrer un traitement à un patient, vous devez vous familiariser •
avec les procédures de fonctionnement pour chaque mode de traitement
disponible ; vous devez également prendre connaissance des indications, des
contre-indications, des avertissements et des précautions d’usage. Consultez
également d'autres sources pour obtenir des renseignements complémentaires sur
l'application de la thérapie par chaleur sèche.
L'appareil de fluidothérapie - thermothérapie sèche ne doit pas être utilisé ni •
stocké à proximité d’un autre équipement. Lorsque l'utilisation à proximité ou
sur un autre équipement est nécessaire, vérifier que l'appareil de fluidothérapie thermothérapie sèche fonctionne normalement dans la configuration dans laquelle
il sera utilisé.
Pour une protection continue contre les risques d’incendie, ne remplacez les •
fusibles que par des fusibles du même type et de la même puissance.
Cet appareil doit être tenu hors de portée des enfants.•
Pour prévenir les chocs électriques, déconnectez l’appareil de la source •
d’alimentation avant d’entamer les procédures de maintenance.
Utilisez exclusivement un support à chaleur sèche traité de type Cellex dans •
l'appareil pour éviter la formation excessive de poussières.
Utilisez exclusivement les accessoires spécifiquement conçus pour cet appareil. •
N'utilisez pas d'accessoires fabriqués par d'autres entreprises sur cet appareil. DJO,
LLC n'est pas responsable des éventuelles conséquences résultant de l'utilisation de
produits fabriqués par d'autres sociétés. L'utilisation d'autres accessoires ou câbles
pourrait provoquer une augmentation des émissions ou une baisse de l'immunité
de cet appareil.
Évacuez tous les produits conformément aux réglementations et codes locaux et •
nationaux.
Lorsqu’il n’est pas utilisé, l’équipement de fluidothérapie doit être protégé contre •
toute utilisation non prévue.
L'appareil de fluidothérapie - thermothérapie sèche n'est pas approprié •
en présence d'un mélange entre un anesthésique inflammable et l'air,
de l'oxygène ou de l'oxyde de diazote.
Effectuez toutes les mesures de maintenance préventive décrites dans le présent •
guide. Le non-respect des mesures de maintenance préventive peut entraîner la
pénétration du support Cellex dans la chambre de chauffe de l'appareil et blesser
gravement les patients, et la fumée émise pourrait endommager les locaux ainsi
que l'appareil.
Avant d'utiliser l'appareil, laissez les solvants des adhésifs et des solutions •
inflammables utilisées pour le nettoyage et la désinfection de l'appareil s'évaporer.
44
Page 81

À PROPOS DE LA THERMOTHÉRAPIE SÈCHE
Indications et contre-indications
Fluidotherapy® - thermothérapie sèche
Indications
Soulagement des douleurs localisées
• Traitement de l'insuffisance circulatoire localisée
• Traitement de l'amplitude articulaire en association avec de l'exercie
• Traitement des symptômes de l'arthrite non rhumatoïde
Contre-indications
• Cet appareil ne doit pas être utilisé pour le soulagement de la
douleur symptomatique sauf si l'étiologie est établie ou si un
syndrome de douleur a été diagnostiqué.
• Cet appareil ne doit pas être utilisé lorsque des lésions cancéreuses
ou des plaies ouvertes sont présentes dans la zone de traitement.
• Les autres contre-indications sont les suivantes : les patients
suspectés d’être porteurs d’une maladie infectieuse grave ou d’une
maladie où il est recommandé, à des fins médicales générales, de
supprimer la chaleur ou la fièvre.
Des précautions adaptées doivent être prises lors du traitement •
de patients qui présentent des affections médicales suspectées ou
diagnostiquées telles que des problèmes cardiaques, l'épilepsie, le
diabète, etc.
Avant le traitement, consultez un médecin familiarisé avec les •
mesures de précaution à prendre chez les patients susceptibles de
présenter des réactions allergiques à la poussière et au pollen.
55
Page 82

NOMENCLATURE
Se familiariser avec les appareils FLU110D et 110DE
1. COUVERCLE DU RÉSERVOIR ET FENÊTRE D'OBSERVATION DU
1
8
2
3
7
TRAITEMENT
Accès pour ajouter/changer le support, observer le traitement du
patient et permettre au clinicien d'accéder à la zone de traitement
du patient
2. RÉSERVOIR DU SUPPORT/TRAITEMENT
Accès au traitement et réservoir du support
3. ORIFICE D'ACCÈS À LA MAIN/AU BRAS
Orifice de traitement pour le traitement de la main/du bras
(extrémité de l'appareil)
4. FILTRES D'ASPIRATION REMPLAÇABLES
Filtre l'air ambiant aspiré dans l'appareil de fluidothérapie (1 de
chaque côté)
Fluidotherapy® - thermothérapie sèche
6
5. BASE À QUATRE POINTS
Des roulettes sont incluses pour la conversion en base mobile
4
6. LOGEMENT DU VENTILATEUR
Contient le ventilateur et le chauffage
7. PANNEAU DE COMMANDE
Voir la description détaillée des commandes en page 8.
5
8. ORIFICE D'ACCÈS ET DE TRAITEMENT DU COUDE/DU PIED
Orifice de traitement pour le coude et le pied
66
Page 83

NOMENCLATURE
Se familiariser avec les appareils FLU115D et FLU115DE
Fluidotherapy® - thermothérapie sèche
1
2
8
3
1. ORIFICE D'ACCÈS DU COUDE/DU PIED
Orifice de traitement pour le traitement du coude et du pied, accès
pour ajouter/changer le support, permet au clinicien d'accéder à la
zone de traitement du patient
2. ORIFICES D'ACCÈS À LA MAIN/AU BRAS
Orifices de traitement pour le traitement de la main/du bras
(extrémité de l'appareil)
3. FILTRES D'ASPIRATION
Filtre l'air ambiant aspiré dans l'appareil de fluidothérapie (1 de
chaque côté)
7
4. CONTENEUR DU SUPPORT/TRAITEMENT
4
5
Accès au traitement et réservoir du support
5. BASE MOBILE
6. ROULETTES DE VERROUILLAGE
7. LOGEMENT DU VENTILATEUR
Contient le ventilateur et le chauffage
8. PANNEAU DE COMMANDE
Voir la description détaillée des commandes en page 8.
6
77
Page 84

NOMENCLATURE
Commandes (tous les appareils)
Fluidotherapy® - thermothérapie sèche
1. AFFICHAGE
Affiche la durée du traitement, la température, la vitesse de l'air,
la durée d'impulsion, la minuterie du préchauffage et l'horloge
lorsque le mode respectif est indiqué.
2. MINUTERIE DU PRÉCHAUFFAGE ACTIVÉE
Indicateur lumineux de la minuterie du préchauffage
3. AUGMENTER/HEURE
Sert à augmenter les paramèters d'un mode et à régler les heures
de l'horloge
4. DIMINUER/MINUTE
Sert à diminuer les paramèters d'un mode et à régler les minutes
de l'horloge
5. STOP
Sert à arrêter le traitement et la minuterie du préchauffage
6. FUSIBLE DU MOTEUR
7. DÉMARRER
Sert à démarrer le traitement
8. BARRE D'INDICATION
Indique les modes choisis
9. FUSIBLE DU CHAUFFAGE
10. BOUTON DE LA MINUTERIE DU PRÉCHAUFFAGE
Appuyez une fois pour activer la minuterie du préchauffage.
Appuyez deux fois pour démarrer
Fonction de préchauffage.
11. SÉLECTION DES MODES
Sert à sélectionner le mode souhaité
12. PM
Indicateur PM (après-midi) pour l'horloge
8
Page 85

SPÉCIFICATIONS
FLU110D et FLU110DE
Longueur
Largeur
Hauteur
Fluidotherapy® - thermothérapie sèche
MODES DE FONCTIONNEMENT
Continu (par défaut)
Ajustements variables pour le temps, la température et la vitesse de l'air
Mode d'impulsion............................................ARRÊT à 6 s MARCHE/ARRÊT
DURÉE DU TRAITEMENT.........................................................de 1 à 99 minutes
TEMPÉRATURE DE SERVICE...............de 43,3 °C (110 °F) à 51,6 °C (125 °F)
VITESSE DE L'AIR............................de 0 % à 100 % (par incréments de 5 %)
MINUTERIE DU PRÉCHAUFFAGE
CAPACITÉ DU SUPPORT.........................................environ 13,6 kg (30 livres)
Puissance d'entrée (FLU110D)......................................120 V, 50/60 Hz, 12 A
Puissance d'entrée (FLU110DE).............................230-240 V, 50/60 Hz, 8 A
DIMENSIONS PHYSIQUES
Longueur de la cabine........................................................86,4 cm (34,0 po.)
Largeur de la cabine.............................................................29,2 cm (11,5 po.)
Hauteur.......................................................................................83,8 cm (33,0 po.)
Poids..........................................................31,7 kg (70 livres) moins le support
Poids d'expédition..............................................................45,4 kg (100 livres)
DEGRÉ DE PROTECTION CONTRE LA PÉNÉTRATION D'EAU.............IPX0
CLASSE ÉLECTRIQUE...................................................................................CLASSE I
DEGRÉ DE PROTECTION CONTRE LES CHOCS ÉLECTRIQUES..TYPE B
CORDON D'ALIMENTATION..câble blindé de 2 mètres de long, 14 AWG
Assurez-vous que l'appareil est relié à la terre en le branchant
uniquement sur une prise électrique reliée à la terre conformément
à la législation nationale et locale en vigueur en matière de codes
d'électricité.
9
.......
46,1 °C (115 °F) avec débit d'air de 50 %
Page 86

SPÉCIFICATIONS
FLU115D et FLU115DE
Largeur
Hauteur
Longueur
Fluidotherapy® - thermothérapie sèche
MODES DE FONCTIONNEMENT
Continu (par défaut)
Ajustements variables pour le temps, la température et la vitesse de l'air
Mode d'impulsion.............................................ARRÊT à 6 s MARCHE/ARRÊT
DURÉE DU TRAITEMENT..........................................................de 1 à 99 minutes
TEMPÉRATURE DE SERVICE................de 43,3 °C (110 °F) à 51,6 °C (125 °F)
VITESSE DE L'AIR.............................de 0 % à 100 % (par incréments de 5 %)
MINUTERIE DU PRÉCHAUFFAGE
CAPACITÉ DU SUPPORT...........................................environ 18,1 kg (40 livres)
Puissance d'entrée (FLU115D)......................................120 V, 50/60 Hz, 12 A
Puissance d'entrée (FLU115DE)...........................230-240 V, 50/60 Hz, 10 A
DIMENSIONS PHYSIQUES
Longueur de la cabine.........................................................86,4 cm (34,0 po.)
Largeur de la cabine.............................................................47,0 cm (18,5 po.)
Hauteur.......................................................................................83,8 cm (33,0 po.)
Poids..........................................................27,2 kg (60 livres) moins le support
Poids d'expédition...............................................................45,4 kg (100 livres)
DEGRÉ DE PROTECTION CONTRE LA PÉNÉTRATION D'EAU.............IPX0
CLASSE ÉLECTRIQUE...................................................................................CLASSE I
DEGRÉ DE PROTECTION CONTRE LES CHOCS ÉLECTRIQUES..TYPE B
CORDON D'ALIMENTATION.......
Assurez-vous que l'appareil est relié à la terre en le branchant
uniquement sur une prise électrique reliée à la terre conformément
à la législation nationale et locale en vigueur en matière de codes
d'électricité.
10
.........
46,1 °C (115 °F) avec débit d'air de 50 %
câble blindé de 2 mètres de long, 14 AWG
Page 87

SPÉCIFICATIONS
Fluidotherapy® - thermothérapie sèche
TABLEAUX DE COMPATIBILITÉ ÉLECTROMAGNÉTIQUE (CEM)
TABLEAU 1 : CONSEILS ET DÉCLARATION DU FABRICANT – ÉMISSIONS ÉLECTROMAGNÉTIQUES
Les Fluidotherapy - thermothérapie sèche est conçues pour être utilisées dans l'environnement électromagnétique spécié ci-dessous.
Le client ou l'utilisateur des Fluidotherapy - thermothérapie sèche doivent s'assurer qu'elles sont utilisées dans un tel environnement.
Tests d’émission Conformité Environnement électromagnétique - conseils
RF Émissions
CISPR 11
RF Émissions
CISPR 11
Émissions harmoniques
IEC 61000-3-2
Fluctuations de tension
IEC 61000-3-3
Groupe 1
Classe A
Classe A
Conforme
L'énergie des utilisations rf d'Fluidotherapy - thermothérapie sèche seulement pour son fonction interne. Par conséquent, ses émissions de rf sont très basses
et ne sont pas susceptibles de causer n'importe quelle interférence dans l'équipement électronique voisin.
Les Fluidotherapy - thermothérapie sèche
directement raccordés au réseau d'alimentation électrique public basse tension qui alimente les immeubles d'habitation.
est adaptées à une utilisation dans tous les établissements, notamment les domiciles et les établissements
11
Page 88

SPÉCIFICATIONS
Fluidotherapy® - thermothérapie sèche
TABLEAUX DE COMPATIBILITÉ ÉLECTROMAGNÉTIQUE (CEM) (SUITE)
Tableau 2 & 3 : conseils eT DÉclaraTion Du FabricanT – immuniTÉ ÉlecTromaGnÉTique
Les Fluidotherapy- thermothérapie sèche est conçues pour être utilisées dans l'environnement électromagnétique spécié ci-dessous. Le client ou
l'utilisateur des Fluidotherapy- thermothérapie sèche doivent s'assurer qu'elles sont utilisées dans un tel environnement.
Test
d’immunité
RF conduite
IEC 61000-4-6
RF rayonnée
IEC 61000-4-3
REMARQUE : A 80 MHz et 800 MHz la plage de fréquence la plus haute s’applique.
REMARQUE : Ces directives peuvent ne pas s’appliquer à toutes les situations. La propagation électromagnétique est affectée par l’absorption et la réflexion contre les
a
Il n’est pas possible de prédire avec exactitude l’intensité des champs de transmetteurs fixes, tels que les postes de base de téléphones radio (cellulaires/sans fil) et les
radios terrestres mobiles, les radios de radio-amateur, les ondes radios AM et FM et les émissions de télévision. Pour évaluer l’environnement électromagnétique dû aux
émetteurs RF fixes, une enquête sur le site électromagnétique devrait être envisagée. Si l’intensité de champ mesurée là où les Fluidotherapy- thermothérapie sèche est
utilisées dépasse le niveau de conformité RF applicable indiqué ci-dessus, il convient de vérifier que les Fluidotherapy- thermothérapie sèche fonctionnent
normalement. Si l’on observe des performances anormales, des mesures supplémentaires peuvent s’avérer nécessaires, notamment la modification de l’orientation ou
du positionnement des Fluidotherapy - thermothérapie sèche.
b
Dans la plage de fréquences 150 kHz à 80 MHz, la puissance des champs doit être inférieure à 3 V/m.
IEC 60601
Niveau du
test
3 Vrms
150 kHz à 80 MHz
3 V/m
80 MHz à 2.5 GHz
structures, objets et personnes.
Niveau de
conformité
Environnement électromagnétique - conseils
L’équipement de communications RF portable et mobile ne doit pas être utilisé plus près de toute partie des Fluidotherapy- thermothérapie sèche, y
compris les câbles, que la distance de séparation recommandée, calculée à partir de l’équation applicable à la fréquence de l’émetteur.
Distance recommandée de séparation :
3
d = [3.5]√P V
1
3 V/m d = [3.5]√P 80 MHz à 800 MHz E
d = [7]√P 800 MHz à 2.5 GHz E
1
où P correspond au taux d’alimentation de sortie maximum de l’émetteur en watts (W) en fonction du fabricant de l’émetteur et d correspond à la séparation en mètres (m).
L’intensité de champ des émetteurs de radiofréquences fixes, telle que déterminée par une étude de site électromagnétique
inférieure au niveau de conformité de chaque gamme de fréquence
Des interférences peuvent survenir à proximité d’un équipement marqué du symbole suivant :
12
1
a
b
.
, doit être
Page 89

SPÉCIFICATIONS
Fluidotherapy® - thermothérapie sèche
TABLEAUX DE COMPATIBILITÉ ÉLECTROMAGNÉTIQUE (CEM) (SUITE)
Tableau 2 & 3 : conseils eT DÉclaraTion Du FabricanT – immuniTÉ ÉlecTromaGnÉTique (suiTe)
Test d’immunité
Décharge électrostatique
(ESD)
IEC 61000-4-2
Transitions électriques
rapides/rafales
IEC 61000-4-4
Surtension
IEC 61000-4-5
Baisses de tension, brèves
interruptions et variations
de tension sur les lignes
d’entrée d’alimentation
IEC 61000-4-11
Champ magnétique à la
fréquence d’alimentation
(50/60 Hz)
IEC 61000-4-8
REMARQUE : U
IEC 60601
Niveau du test
Contact ±6 kV
Air ±8 kV
±2 kV pour les lignes d’alimentation
électrique
±1 kV pour les lignes d’entrée/sortie
±1 kV mode différentiel
±2 kV mode courant
< 5% U
(> 95% de baisse de UT)
T
pendant 0.5 cycle
< 40% U
(> 60% de baisse de UT)
T
pendant 5 cycles
< 70% U
(> 30% de baisse de UT)
T
pendant 25 cycles
< 5% U
(> 95% de baisse de UT)
T
pendant 5 sec
Niveau de conformité Environnement électromagnétique - conseils
Contact ±6 kV
Air ±8 kV
±2 kV pour les lignes d’alimentation
électrique
±1 kV pour les lignes d’entrée/sortie
±1 kV mode différentiel
±2 kV mode courant
< 5% U
(> 95% de baisse de UT)
T
pendant 0,5 cycle
< 40% U
(> 60% de baisse de UT)
T
pendant 5 cycles
< 70% U
(> 30% de baisse de UT)
T
pendant 25 cycles
< 5% U
(> 95% de baisse de UT)
T
pendant 5 sec
3 A/m 3 A/m Les champs magnétiques de la fréquence d’alimentation doivent être à des niveaux
correspond à la tension CA principale avant l’application du niveau de test.
T
13
Le sol doit être en bois, en béton ou en carrelage de céramique. Si le sol est couvert d’un
revêtement synthétique, l’humidité relative doit être d’au moins 30 %.
La qualité de l’alimentation électrique principale doit être celle d’un environnement
commercial ou hospitalier typique.
La qualité de l’alimentation électrique principale doit être celle d’un environnement
commercial ou hospitalier typique.
La qualité de l’alimentation électrique principale doit être celle d’un environnement
commercial ou hospitalier typique. Si l'utilisateur des Fluidotherapy - thermothérapie
sèche requiert un fonctionnement sans interruption lors des coupures de courant, il est
recommandé d'alimenter les Fluidotherapy - thermothérapie sèche au moyen d’un
module d'alimentation ininterrompue ou d’une batterie.
caractéristiques d'un emplacement type dans un cabinet privé ou un environnement
hospitalier.
Page 90

SPÉCIFICATIONS
Fluidotherapy® - thermothérapie sèche
TABLEAUX DE COMPATIBILITÉ ÉLECTROMAGNÉTIQUE (CEM) (SUITE)
Tableau 4 : DisTances recommanDÉes De sÉparaTion enTre les ÉquipemenTs De communicaTion rF
porTable eT mobiles eT les FluiDoTherapy - ThermoThÉrapie sèche
Les Fluidotherapy- thermothérapie sèche est conçues pour être utilisées dans un environnement électromagnétique où les perturbations
RF rayonnées sont contrôlées. Le client ou l’utilisateur des Fluidotherapy- thermothérapie sèche peut contribuer à prévenir les
interférences électromagnétiques en maintenant une distance minimale entre les équipements de communication RF portables et
mobiles (émetteurs) et les Fluidotherapy- thermothérapie sèche conformément aux recommandations ci-dessous.
Puissance maximale de sortie nominale
Distance de séparation selon la fréquence de l’émetteur
del’émetteur
150 kHz à 80 MHz
De 80 MHz à 800 MHz
M
800 MHz à 2,5 GHz
W
d = [3.5]√P V
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
Pour les émetteurs évalués à une puissance de sortie maximale non répertoriée ci-dessus, la distance de séparation recommandée d en mètres (m) peut être évaluée à
l'aide de l'équation applicable à la fréquence de l'émetteur, où P correspond à la puissance maximale de sortie de l’émetteur en watts (W) selon le fabricant de
l’émetteur.
REMARQUE : A 80 MHz et 800 MHz, la distance de séparation pour les plages de fréquences les plus hautes s’appliquent.
REMARQUE : Ces directives peuvent ne pas s’appliquer à toutes les situations. La propagation électromagnétique est affectée par l’absorption et la réflexion
contre les structures, objets et personnes.
1
1414
d = [3.5]√P E
1
d = [7]√P E
1
Page 91

CONFIGURATION
PARAMÈTRES DU MODE TRAITEMENT
ATTENTION !!!
L'ATTRITION DE PARTICULES PENDANT LE
TRANSPORT PEUT ENTRAÎNER LA PRÉSENCE
INITIALE DE POUSSIÈRES ET LA LIBÉRATION
DE PETITES PARTICULES. LA POUSSIÈRE ET LES
ODEURS SE DISSIPERONT AVEC L'UTILISATION.
Fluidotherapy® - thermothérapie sèche
Appuyez sur les boutons « INCREASE »
(AUGMENTER) ou « DECREASE »
(DIMINUER) jusqu'à ce que la durée du
traitement souhaité soit affiché.
REMARQUE :
Le paramètre « TREATMENT TIME » (DURÉE
DU TRAITEMENT) s'ajuste par incréments
d'une minute de 1 à 99.
Utilisez les instructions suivantes pour régler les différents paramètres
du mode selon les valeurs souhaitées.
DURÉE DU TRAITEMENT
Appuyez sur le bouton « PRESS TO
CHANGE MODE » (APPUYER POUR
CHANGER DE MODE) jusqu'à ce
que l'indicateur s'allume à côté de
« TREATMENT TIME » (DURÉE DU
TRAITEMENT).
REMARQUE :
La durée par défaut « 20:00 » s'affiche alors.
1515
TEMPÉRATURE DE TRAITEMENT
Appuyez sur le bouton « PRESS TO
CHANGE MODE » (APPUYER POUR
CHANGER DE MODE)
jusqu'à ce que l'indicateur s'allume à côté
de « TEMPERATURE » (TEMPÉRATURE).
REMARQUE :
La température par défaut « 46,1 °C » (115 °F)
s'affiche lorsque l'appareil est branché pour
la première fois sur une prise électrique
murale.
Les indicateurs « F » ou « C » et
« TEMPERATURE » (TEMPÉRATURE) clignotent
lorsque la températue de traitement
programmée est affichée.
Lorsque la température du lit est affichée, les
indicateurs « F » ou « C » et « TEMPERATURE »
(TEMPÉRATURE) s'allument en continu.
Page 92

CONFIGURATION
Fluidotherapy® - thermothérapie sèche
PARAMÈTRES DU MODE TRAITEMENT (SUITE)
TEMPÉRATURE DE TRAITEMENT (SUITE)
Appuyez sur les boutons « INCREASE »
(AUGMENTER) ou « DECREASE »
(DIMINUER) jusqu'à ce que la température
de traitement souhaitée soit affichée.
REMARQUE :
Les °F peuvent être changés en °C et
inversement si nécessaire. Plus des
instructions à ce sujet, voir la page 20.
La température de traitement peut être
ajustée par incréments de 1° de 31,1 °C à
54 °C (88 °F à 130 °F).
VITESSE DE L'AIR
Appuyez sur le bouton « PRESS TO
CHANGE MODE » (APPUYER POUR
CHANGER DE MODE) jusqu'à ce que
l'indicateur s'allume à côté de « AIR
SPEED » (VITESSE DE L'AIR).
REMARQUE :
La vitesse par défaut « 50 » s'affiche alors.
Appuyez sur les boutons « INCREASE »
(AUGMENTER) ou « DECREASE » (DIMINUER)
jusqu'à ce que la vitesse de l'air souhaitée
soit affichée.
REMARQUE :
Le paramètre « AIR SPEED » (VITESSE DE
L'AIR) s'ajuste par incréments de 5 de 0 à
100.
DURÉE D'IMPULSION
Appuyez sur le bouton « PRESS TO CHANGE
MODE » (APPUYER POUR CHANGER
DE MODE) jusqu'à ce que l'indicateur
s'allume à côté de « PULSE TIME » (DURÉE
D'IMPULSION).
REMARQUE :
Le paramètre « PULSE TIME » (DURÉE
D'IMPULSION) permet le fonctionnement
de l'appareil par l'impulsion du support pendant le traitement. Si
ce paramètre est sélectionné et réglé, le support sera impulsé par
intermittence selon des incréments d'intervalle égal.
La valeur d'usine par défaut est « OFF » (DESACTIVÉ).
EXEMPLE : Le paramètre « PULSE TIME » (DURÉE D'IMPULSION) est réglée
sur « 5 ». L'appareil impulse le support pendant le traitement, selon un
rythme de cinq secondes d'impulsion et cinq secondes d'arrêt.
1616
Page 93

CONFIGURATION
PARAMÈTRES DU MODE TRAITEMENT (SUITE)
DURÉE D'IMPULSION (SUITE)
Appuyez sur les boutons « INCREASE »
(AUGMENTER) ou « DECREASE »
(DIMINUER) jusq'à ce que la durée
d'impulsion souhaitée s'affiche.
REMARQUE :
Le paramètre « PULSE TIME » s'ajuste par
incréments d'une seconde entre « OFF »
(DÉSACTIVÉ) et 6 secondes.
DÉMARRER LE TRAITEMENT
Appuyez sur le bouton « START »
(DÉMARRAGE).
Fluidotherapy® - thermothérapie sèche
ARRÊTER LE TRAITEMENT
Appuyez sur le bouton « STOP ».
1717
Page 94

CONFIGURATION
PARAMÈTRES RÉGIS PAR LE TEMPS
MINUTERIE DU PRÉCHAUFFAGE
Appuyez sur le bouton « PRESS TO
CHANGE MODE » (APPUYER POUR
CHANGER DE MODE)
jusqu'à ce que l'indicateur s'allume à côté
de « PREHEAT TIMER » (MINUTERIE DU
PRÉCHAUFFAGE).
REMARQUE :
La fonction « PREHEAT TIMER » (MINUTERIE
DU PRÉCHAUFFAGE) permet à l'appareil
de préchauffer le support. L'appareil
jusqu'à ce qu'il atteigne la température de préchauffage par défaut ou au
bout de 90 minutes, selon ce qui se produit en premier. La fonction « PREHEAT
TIMER » (MINUTERIE DU PRÉCHAUFFAGE) fonctionne uniquement du lundi
au vendredi, il peut être configuré pour démarrer automatiquement à une
heure prédéfinie et pour chauffer le support à une température prédéfinie.
Pour obtenir des informations sur le réglage des paramètres par défaut de la
MINUTERIE DU PRÉCHAUFFAGE,
fonctionnera avec une vitesse d'air de 50 %
consulter la page 21.
Appuyez sur le bouton « PRESS ONCE TO
TURN ON PREHEAT TIMER » (APPUYER UNE
FOIS POUR ACTIVER LA MINUTERIE DU
PRÉCHAUFFAGE) pour allumer l'indicateur
d'activation « PREHEAT TIMER » (MINUTERIE
DU PRÉCHAUFFAGE). Appuyez une
nouvelle fois pour démarrer la MINUTERIE
DU PRÉCHAUFFAGE.
REMARQUE :
L'indicateur « PREHEAT TIMER » (MINUTERIE
DU PRÉCHAUFFAGE) doit être configuré à
la fin de chaque journée pour qu'il s'active
automatiquement le lendemain..
Le bouton « STOP » sert à éteindre le
préchauffage, si nécessaire.
Fluidotherapy® - thermothérapie sèche
HORLOGE
Appuyez sur le bouton « PRESS TO
CHANGE MODE » (APPUYER POUR
CHANGER DE MODE) jusqu'à ce que
l'indicateur s'allume à côté de « CLOCK »
(HORLOGE).
REMARQUE :
La fonction « PREHEAT TIMER » (MINUTERIE
DU PRÉCHAUFFAGE) ne fonctionne pas
tant que l'horloge n'est pas réglée.
Appuyez sur le bouton « INCREASE »
(AUGMENTER) pour régler les heures.
Appuyez sur le bouton « DECREASE »
(DIMINUER) pour régler les minutes.
REMARQUE :
L'indicateur « PM » (APRÈS-MIDI) s'allume
lorsque les heures de l'après-midi
s'affichent.
1818
Page 95

CONFIGURATION
PARAMÈTRES PAR DÉFAUT DU MODE DE PRÉFÉRENCE
PASSER AU MODE « PrEF » (MODE DE PRÉFÉRENCE)
Fluidotherapy® - thermothérapie sèche
DURÉE DU TRAITEMENT PAR DÉFAUT
Appuyez simultanément sur les boutons « PRESS TO CHANGE MODE »
(APPUYER POUR CHANGER DE MODE), « INCREASE » (AUGMENTER) et
« DECREASE » (DIMINUER). Le message « PrEF » s'affiche.
REMARQUE :
Le mode « PrEF » permet à l'utilisateur de changer les réglages par défaut.
Appuyez sur le bouton « PRESS TO CHANGE
MODE » (APPUYER POUR CHANGER DE
MODE) jusqu'à ce que l'indicateur clignote
à côté de « TREATMENT TIME » (DURÉE DU
TRAITEMENT).
Appuyez sur les boutons « INCREASE »
(AUGMENTER) et « DECREASE » (DIMINUER)
pour régler la durée du traitement par
défaut sur l'appareil.
Appuyez sur le bouton « PRESS TO CHANGE
MODE » (APPUYER POUR CHANGER DE
MODE) pour enregistrer le nouveau
réglage.
REMARQUE :
Le réglage par défaut est de 20:00 minutes.
1919
Page 96

CONFIGURATION
PARAMÈTRES PAR DÉFAUT DU MODE DE PRÉFÉRENCE (SUITE)
AFFICHAGE PAR DÉFAUT DE LA
TEMPÉRATURE
Fluidotherapy® - thermothérapie sèche
TEMPÉRATURE DE TRAITEMENT PAR
DÉFAUT
Appuyez sur le bouton « PRESS TO
CHANGE MODE » (APPUYER POUR
CHANGER DE MODE) jusqu'à ce
que l'indicateur clignote à côté de
« TEMPERATURE » (TEMPÉRATURE).
Appuyez sur le bouton « INCREASE »
(AUGMENTER) pour régler l'appareil
pour qu'il affiche °F ou °C comme
réglage par défaut.
Appuyez sur le bouton « PRESS TO
CHANGE MODE » (APPUYER POUR
CHANGER DE MODE) pour enregistrer le
nouveau réglage.
REMARQUE :
Le paramètre par défaut est °F.
Appuyez sur le bouton « PRESS TO
CHANGE MODE » (APPUYER POUR
CHANGER DE MODE) jusqu'à ce
que l'indicateur clignote à côté de
« TEMPERATURE » (TEMPÉRATURE). et
que la température par défaut actuelle
s'affiche.
Appuyez sur les boutons « INCREASE »
(AUGMENTER) et « DECREASE »
(DIMINUER) pour régler la température de
traitement par défaut de l'appareil.
Les températures de service sont
comprises entre :
43,3 °C et 51,6 °C (110 °F et 125 °F)
Appuyez sur le bouton « PRESS TO
CHANGE MODE » (APPUYER POUR
CHANGER DE MODE) pour enregistrer le
nouveau réglage.
2020
Page 97

CONFIGURATION
PARAMÈTRES PAR DÉFAUT DU MODE DE PRÉFÉRENCE (SUITE)
MODE PAR IMPULSIONS (Activer/
Désactiver)
Fluidotherapy® - thermothérapie sèche
MODE DE MINUTERIE DU
PRÉCHAUFFAGE (Activer/Désactiver)
Appuyez sur le bouton « PRESS TO
CHANGE MODE » (APPUYER POUR
CHANGER DE MODE) jusqu'à ce que
l'indicateur clignote à côté de « PULSE
TIME » (DURÉE D'IMPULSION).
Le message « EnAb » (activer) ou « dISA »
(désactiver)
s'affiche alors.
Appuyez sur le bouton « INCREASE » pour
régler l'appareil afin qu'il affiche « EnAb »
ou « dISA , au choix.
Appuyez sur le bouton « PRESS TO
CHANGE MODE » (APPUYER POUR
CHANGER DE MODE) pour enregistrer le
nouveau réglage.
2121
Appuyez sur le bouton « PRESS TO
CHANGE MODE » (APPUYER POUR
CHANGER DE MODE) jusqu'à ce que
l'indicateur clignote à côté de « PREHEAT
TIMER » (MINUTERIE DU PRÉCHAUFFAGE).
Le message « EnAb » (activer) ou « dISA »
(désactiver)
s'affiche alors.
Appuyez sur le bouton « INCREASE » pour
régler l'appareil afin qu'il affiche « EnAb »
ou « dISA , au choix.
Appuyez sur le bouton « PRESS TO
CHANGE MODE » (APPUYER POUR
CHANGER DE MODE) pour enregistrer le
nouveau réglage.
REMARQUE :
Lorsque l'horloge est désactivée, la
fonction « PREHEAT TIMER » (MINUTERIE
DU PRÉCHAUFFAGE) est automatiquement
désactivée.
Page 98

CONFIGURATION
PARAMÈTRES PAR DÉFAUT DU MODE DE PRÉFÉRENCE (SUITE)
HORLOGE (Activer/Désactiver)
Appuyez sur le bouton « PRESS TO
CHANGE MODE » (APPUYER POUR
CHANGER DE MODE) jusqu'à ce que
l'indicateur clignote à côté de « CLOCK »
(HORLOGE).
Le message « EnAb » (activer) ou « dISA »
(désactiver)
s'affiche alors.
Appuyez sur le bouton « INCREASE » pour
régler l'appareil afin qu'il affiche « EnAb »
ou « dISA , au choix.
Appuyez sur le bouton « PRESS TO
CHANGE MODE » (APPUYER POUR
CHANGER DE MODE) pour enregistrer le
nouveau réglage.
REMARQUE :
Lorsque l'horloge est désactivée, la
fonction « PREHEAT TIMER » (MINUTERIE
DU PRÉCHAUFFAGE) est automatiquement
désactivée.
2222
Fluidotherapy® - thermothérapie sèche
RÉGLAGE DU JOUR DE LA SEMAINE
Appuyez sur le bouton « PRESS TO
CHANGE MODE » (APPUYER POUR
CHANGER DE MODE) jusqu'à ce que le
message « dAy__ » (__= code du jour)
s'affiche.
TABLEAU DES CODES DE JOUR
JOUR DE LA
CODE DU JOUR
SEMAINE
Dimanche dAyu
Lundi dAyn
Mardi dAyE
Mercredi dAyd
Jeudi dAyr
Vendredi dAyF
Samedi dAyA
Appuyez sur les boutons « INCREASE »
(AUGMENTER) ou « DECREASE » (DIMINUER)
pour afficher le code du jour souhaité sur
l'appareil.
Appuyez sur le bouton « PRESS TO CHANGE
MODE » (APPUYER POUR CHANGER DE
MODE) pour enregistrer le nouveau réglage.
Page 99

CONFIGURATION
PARAMÈTRES PAR DÉFAUT DU MODE DE PRÉFÉRENCE (SUITE)
SORTIE DU MODE « PrEF »
Appuyez sur le bouton « PRESS TO CHANGE MODE » (APPUYER POUR
CHANGER DE MODE) jusqu'à ce que le message « PrEF » s'affiche.
Appuyez simultanément sur les boutons « INCREASE » (AUGMENTER),
« DECREASE » (DIMINUER) et « STOP ». La durée de traitement par défaut
s'affiche alors et l'indicateur se trouvera à côté de « TREATMENT TIME »
(DURÉE DU TRAITEMENT).
Fluidotherapy® - thermothérapie sèche
2323
Page 100

FONCTIONNEMENT
Fluidotherapy® - thermothérapie sèche
PRÉPARATION DU PATIENT
Nettoyez soigneusement la zone à traiter sur le patient avec •
un savon antimicrobien et de l'eau propre, conformément aux
normes industrielles, institutionnelles et réglementaires ainsi qu'aux
procédures universelles de nettoyage de la peau.
Après le nettoyage de la peau, appliquez un nettoyant antiseptique •
hospitalier pour la peau, en suivant les recommandations
d'utilisation du fabricant.
Consultez le programme de maintenance et assurez-vous que
•
la maintenance préventive a bien été effectuée avant de mettre
l'appareil en marche.
COMMENCER LE TRAITEMENT
DÉBUT
Assurez-vous que tous les réglages du
panneau de commande ont été définis, que
l'appareil a été préchauffé et que le patient
se trouve dans une position correcte,
manchon(s) sécurisé(s), avant de débuter le
traitement. Pour obtenir des informations
sur la configuration de l'appareil, consultez
les pages 15 à 17.
Appuyez sur le bouton « START »
(DÉMARRAGE) pour commencer le
traitement.
ARRÊTER LE TRAITEMENT
ARRÊT
Le traitement s'arrête automatiquement
lorsque la durée du traitement atteint la
valeur zéro.
Si vous souhaitez arrêter le traitement
avant que la minuterie n'atteigne la valeur
zéro, appuyez sur le bouton « STOP ».
REMARQUE :
Si vous arrêtez le traitement avant
que la minuterie n'atteigne la valeur
zéro, il sera nécessaire de redéfinir le
paramètre « TREATMENT TIME » (DURÉE
DU TRAITEMENT) afin de poursuivre le
traitement prescrit.
2424
 Loading...
Loading...