Page 1

™
Forte CPS Series
USER MANUAL
200 STIM
200 COMBO
400 STIM
400 COMBO
IISSOO 1133448855 CCEERRTTIIFFIIEEDD
Page 2

Page 3

Table of Contents
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
Features of the Forte
Foreword . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
Forte CPS Series Product Matrix . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2
Liability Disclaimer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2
Precautionary Instructions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Principles of Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
Initial Setup Instructions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
System Components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4
Patient Control Center (PCC) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4
Optional Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4
System Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5
Operator Interface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5
Control Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6
Indications/Contraindications/Adverse Effects for Electrical Stimulation . . . . . . . . . . . . . . . . . . . . . . . .12
Interferential Current . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Premodulated Current . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13
VMS, Russian, HIVOLT . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .14
Microamperage Pulsed Current (Microcurrent) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15
Microcurrent Probes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .16
HIVOLT Probe . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .16
IFC – Interferential Current (Sine Wave) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .17
Quick Start . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .17
Detailed Treatment Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .18
Modifying Treatment Parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .20
Interferential Parameter Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Technical Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .21
™
CPS Electotherapy System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
PREMOD – Premodulated (Sine Wave) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .22
Quick Start . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .22
Detailed Treatment Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .23
Modifying Treatment Parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .24
Premod Parameter Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .25
Technical Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .26
MICRO – Microcurrent (Microamperage Pulsed Current) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .26
Quick Start . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .27
Detailed Treatment Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .28
Modifying Treatment Parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .29
Microcurrent Parameter Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .29
Microcurrent Application with Hand Held Probe (Optional) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .30
Technical Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .31
Page 4

RUSSIAN (Interrupted Sine Wave) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .32
Quick Start . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .33
Detailed Treatment Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .34
Modifying Treatment Parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .35
Russian Parameter Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .36
Technical Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .36
HIVOLT – High Volt (Monophasic Twin-Peak Pulsed Current) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .37
Quick Start . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .37
Detailed Treatment Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .38
Modifying Treatment Parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .40
HIVOLT Parameter Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .40
HIVOLT Application with Hand Held Probes (Optional) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .41
Technical Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .42
VMS – Symmetrical Biphasic Square Wave . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .42
Quick Start . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .43
Detailed Treatment Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .44
Modifying Treatment Parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .45
VMS Parameter Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .46
Technical Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .46
US – Ultrasound . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .47
Quick Start . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .48
Indications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .48
Contraindications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .49
Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .49
Detailed Treatment Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .50
Modifying Treatment Parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .51
US Parameter Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .51
Technical Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .52
COMBO – Combination Therapy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .53
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .53
Quick Start . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .54
Detailed Treatment Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .55
Setting Up a Second Treatment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .57
Quick Start . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .57
Clinical Protocols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .59
Appendix . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .59
Forte CPS Two Year Limited Warranty . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .60
System Utilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .61
Patient Control Center . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .61
Maintenance Instructions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .62
Screen Prompts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .62
Glossary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .64
Page 5

Introduction
• Welcome to the Forte™CPS
• Features of the Forte CPS
• Foreword
• Forte CPS Series Product Matrix
• Liability Disclaimer
• Precautionary Instructions
Welcome to the Forte CPS
The Forte CPS designed and manufactured by Chattanooga Group offers a new dimension in
electrotherapy, combination and ultrasound treatments. This technology is made possible by advanced
software design and digital signal processing. The result is a series of products with extraordinary
versatility based on simplicity of operation. A clinical electrotherapy system should not be self-limiting
in any price range; as your needs as a clinician change, so can your Forte CPS.
Features of the Forte CPS Electrotherapy System
Clinical Protocol System – An efficient approach for setting up a treatment using preset parameters. The
Clinical Protocol library includes over 100 presets for pain management, muscle contraction or
Ultrasound.
Upgradeable – The world’s only system that can be upgraded from two to four Channels or to which
ultrasound can be added after purchase.
™
Electronic Signature
head.
Combo Systems only – Automatically calibrates the system to any size Forte sound
Patient Control Center – A unique method of Interferential stimulation control allowing the patient to
pinpoint their stimulation needs throughout the treatment session.
Easy as One-Two-Go – In two steps you are ready to start therapy: Select “Waveform” – Set “Intensity”
– Select “Start.”
Programmable Start-Up Presets – All power-up presets can be individually customized to meet the needs
of the clinician.
Foreword
This manual has been written for the owners and operators of the Forte CPS. It contains general
instructions for operation, precautionary instructions and maintenance recommendations. In order to
obtain maximum life and efficiency from your Forte CPS and to assist in the proper operation of the
unit, read and understand this manual thoroughly and become familiar with the controls on the panel
as well as the various accessories that come with the unit before operating it. This manual includes
operator information and instructions for the Forte CPS Stim and/or Forte CPS Combo systems. The
sections that discuss ultrasound and combination treatments apply only to the Forte CPS Combo unit.
All other sections in this manual apply to both the Stim and Combo devices.
To inquire about upgrading options of the Forte CPS product line, consult your Chattanooga Group
representative or call 1-800-592-7329.
The specifications put forth in this manual were in effect at the time of publication. However, owing
to Chattanooga Group’s policy of continuous improvement, changes to these specifications may be
made at any time without obligation on the part of Chattanooga Group.
1
Page 6
Forte™CPS Series Product Matrix
PRODUCT 200 STIM 200 COMBO 400 STIM 400 COMBO
Channels 2 2 & Ultrasound 4 4 & Ultrasound
Interferential Channels 1 & 2 Channels 1 & 2 Channels 1 & 2 or 3 & 4 Channels 1 & 2 or 3 & 4
Premod Channels 1 & 2 Channels 1 & 2 Channels 1, 2, 3, 4 Channels 1, 2, 3, 4
Russian Channels 1 & 2 Channels 1 & 2 Channels 1, 2, 3, 4 Channels 1, 2, 3, 4
Microcurrent Channels 1 & 2 Channels 1 & 2 Channels 1, 2, 3, 4 Channels 1, 2, 3, 4
VMS Channels 1 & 2 Channels 1 & 2 Channels 1, 2, 3, 4 Channels 1, 2, 3, 4
Hivolt Channels 1 & 2 Channels 1 & 2 Channels 1, 2, 3, 4 Channels 1, 2, 3, 4
Ultrasound Not Available 1 & 3.3 MHz Not Available 1 & 3.3 MHz
Combination Not Available Available Not Available Available
NOTE: The above features are not available on all units. Please note the type of Forte CPS system you
are operating and reference those sections of this user manual that are applicable.
Liability Disclaimer
Before administering any treatment to a patient you should become acquainted with the operating
procedures for each mode of treatment available, as well as the indications, contraindications,
warnings and precautions. Consult other resources for additional information regarding the application
of electrotherapy.
Precautionary Instructions
1. CAUTION: Read, understand and practice the precautionary and operating instructions. Know the
limitations and hazards associated with using any electrical stimulation or ultrasound
device. Observe the precautionary and operational decals placed on the unit.
2. CAUTION: DO NOT operate the Forte CPS when connected to any unit other than Chattanooga
Group devices. Do not operate the unit in an environment of short-wave diathermy
use.
3. WARNING: Federal law restricts this device to sale by, or on the order of, a physician or licensed
practitioner. This device should be used only under the continued supervision of a
physician or licensed practitioner.
WARNING: Keep electrodes separated during treatment. Electrodes in contact with each other
could result in improper stimulation or skin burns.
4. CAUTION: This unit is not designed to prevent the ingress of water or liquids. Ingress of water
or liquids could cause malfunction of internal components of the system and
therefore, create a risk of injury to the patient.
CAUTION: The Ultrasound generator should be routinely checked before each use to determine
that all controls function normally; especially that the intensity control does properly
adjust the intensity of the ultrasonic power output in a stable manner. Also,
determine that the treatment time control does actually terminate ultrasonic power
output when the timer reaches zero.
2
Page 7
5. CAUTION: Use of controls or adjustments or performance of procedures other than those
!
specified herein may result in hazardous exposure to ultrasonic energy.
6. CAUTION: This unit generates, uses and can radiate radio frequency energy, and if not installed
and used in accordance with the instructions, may cause harmful interference to
other devices in the vicinity. However, there is no guarantee that interference will not
occur in a particular installation. Harmful interference to other devices can be
determined by turning this unit on and off or trying to correct the interference using
one or more of the following: Reorient or relocate the receiving device, increase the
separation between the equipment, connect the unit to an outlet on a different circuit
from that to which the other device(s) are connected and/or consult the factory field
service technician for help.
CAUTION: DO NOT use sharp objects such as a pencil point or ballpoint pen to operate the
buttons on the control panel as damage may result.
7. WARNING: Explosion hazard if used in the presence of flammable anesthetics. The warning
symbol for this hazard is prominently displayed on the cabinet.
8. WARNING: For continued protection against fire hazard, replace fuses only with ones of the
same type and rating.
9. WARNING: Make certain that the unit is electrically grounded by connecting only to a grounded
electrical service receptacle conforming to the applicable national and local electrical
codes.
10. WARNING: This device should be kept out of the reach of children.
11. WARNING: Dispose of all products in accordance with local and national regulations and codes.
DANGER: Patients with an implanted neurostimulation device must not be treated with or be in
close proximity to any shortwave diathermy, microwave diathermy, therapeutic
ultrasound diathermy or laser diathermy anywhere on their body. Energy from
diathermy (shortwave, microwave, ultrasound and laser) can be transferred through
the implanted neurostimulation system, can cause tissue damage and can result in
severe injury or death. Injury, damage or death can occur during diathermy therapy
even if the implanted neurostimulation system is turned "off."
Principles of Operation
• Initial Setup Instructions
• System Components
- Patient Control Center
• Warranty Registration
• Optional Accessories
• System Operation
- Operating Controls
- Quick Start
- General Setup Steps
- Screen Layout
3
Page 8

Initial Setup Instructions
Remove the Forte™CPS unit and any additional items ordered from the carton and inspect for damage
that may have occurred during shipment. Check the voltage rating on the serial decal located on the
bottom of the unit. Plug the system power supply in to a 100 Volt to 220/240 Volt AC outlet, as
required. DO NOT attempt to use Direct Current (DC). DO NOT attempt to use the unit if it is not
properly grounded. DO NOT place unit in a location where the power cord could be tripped over or
pulled out during treatment. Follow the procedures listed in the precautionary instructions located
later in this section.
System Components
The following accessories are included (standard) with your Forte CPS.
Item Part# Description
1 78047 Applicator, Ultrasound 5 cm2(Combo Only)
2 4264 Ultrasound Gel (Combo Only)
3 12213 Lead, 120”, Red/Black 1 & 2
4 12214 Lead, 120”, Red/Black 3 & 4
5 78022 Patient Control Center
6 78082 Forte CPS Power Supply
7 78121 Forte CPS Power Cord
8 78081 Operator’s Manual
Patient Control Center (PCC)
This accessory may be used for the following:
• To alter the patient’s perception of interferential stimulation. The PCC can only be utilized with
Interferential Stimulation and by one patient at a time.
• The Patient Control Center can also be used as a patient interrupt switch.
Warranty Registration
Complete the warranty registration card and return it to Chattanooga Group within 10 days of
purchase. The warranty registration card should be filled out complete with the system serial number
and serial number of the included ultrasound applicator if applicable. Warranty registration will ensure
that you will not be billed for services that are covered by the warranty policy.
4
Page 9

Optional Accessories
The following is a list of optional accessories available for the Forte™CPS Series.
Item Part# Description
1 78046 Applicator, Ultrasound 10 cm
2 78048 Applicator, Ultrasound 2 cm
3 79976 Microcurrent Probe Kit
4 79977 HIVOLT Probe Kit
5 10832 Strap, Nylatex, Long 21/2”x 48”
6 10648 Strap, Nylatex, Medium, 21/2”x 24”
7 10828 Strap, Nylatex, Short, 21/2”x 18”
8 4264 Ultrasound Gel
9 76910 Service Manual
2
2
System Operation
Prior to operating the Forte CPS become familiar with the following:
• Control Panel
- LCD and LED displays
- Modes of operation
- Modes of Treatment Available
• Indications / Contraindications for treatment.
- Specific instructions for performing each treatment.
- Clinical Protocols
• System Components
- Patient Control Center (PCC)
- Probes
- Ultrasound Applicator
• Accessories
Operator Interface
The operator interface consists of an illustrated control panel with light emitting diodes (LED) and a
liquid crystal display (LCD). The operator is able to view parameter options on the LED and LCD
readouts and make selections by touching the designated area of the control panel. The displays will
provide continual information during the treatments concerning amplitude and elapsed time.
Ultrasound and stimulation intensities are adjusted with control panel buttons adjacent to the
corresponding LED display. The stimulation / ultrasound output can be stopped by pressing the
“PAUSE” or “STOP” buttons located at the bottom of the control panel or using the Patient Control
Center (PCC).
5
Page 10
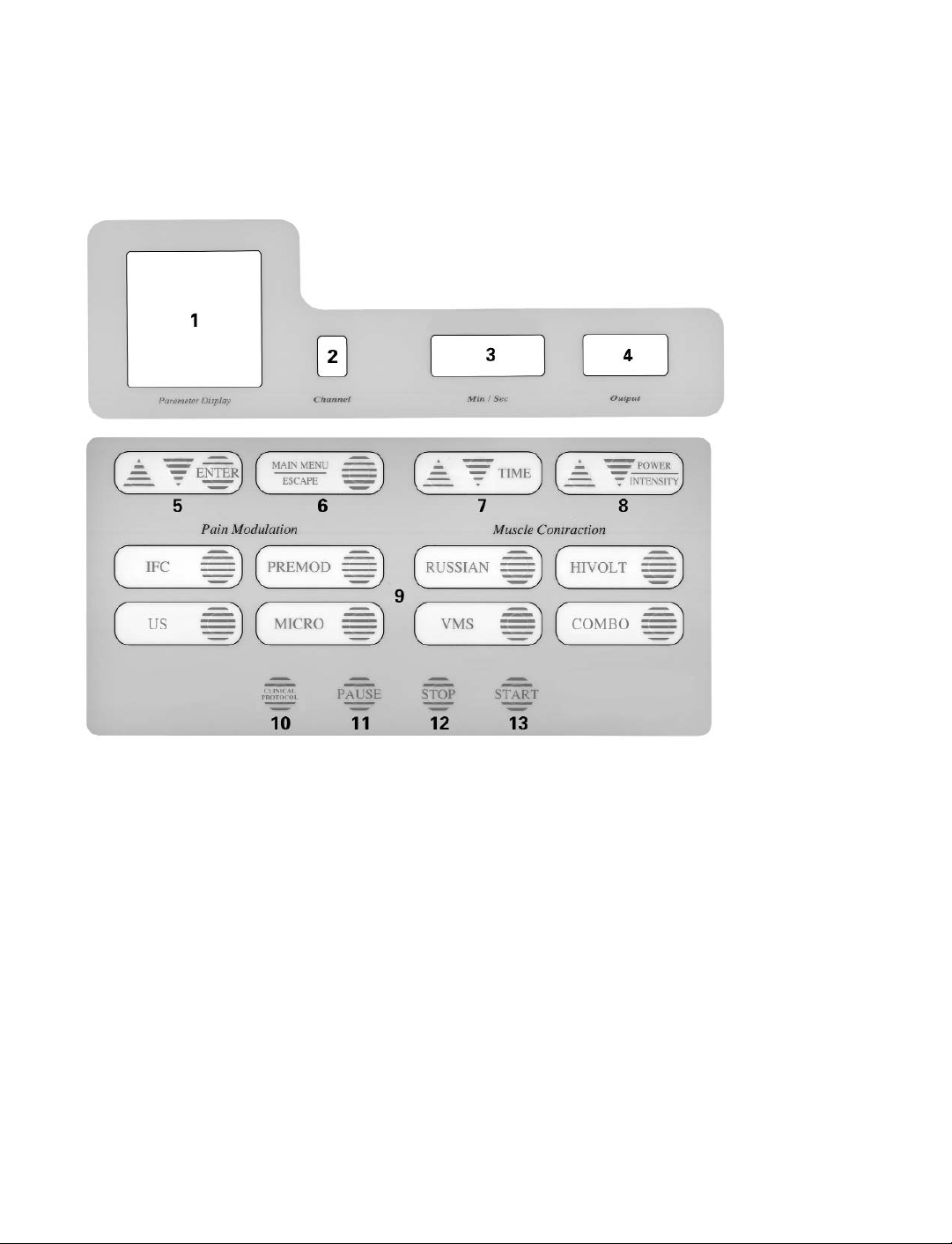
Control Panel
The Forte 200 Stim and 400 Stim do not include Ultrasound or Combo. To upgrade to Ultrasound and
Combo please see your Chattanooga Group representative or call:
1-800-592-7329 or 423-870-2281.
Operating Controls
1. LCD Screen Display
• Provides visual display of parameters selected during all phases of treatment set up.
• Main Menu provides a constant update of available Channels as well as treatments and time
remaining for Channels in use.
2. LED Channel Indicator (displayed continuously)
• Displays the current Channel selected.
• Information presented on the screen display, as well as treatment time and power/ intensity
as related to the specific Channel selected.
3. LED Treatment Time Remaining (displayed continuously)
• Displays the amount of time remaining for the Channel selected.
4. Amplitude/Output Level (displayed continuously)
• Displays output intensity of the selected Channel.
6
Page 11

5. ENTER – Parameter Selection Control
• Select this prompt to change a treatment parameter to a value other than the default setting.
Preset treatment parameters will be displayed on the screen; use the up or down arrow to
highlight a parameter and press ENTER. Use the up and down arrows to navigate through the
list of options and press ENTER to accept.
• When using Clinical Protocols, use the selection control to navigate through the options.
6. Main Menu/ Escape
• Select this prompt to return to the Main Menu or escape from a pop-up menu.
7. Treatment Time Controls
• Select this prompt to set or modify treatment time in minutes.
8. Power/Intensity Control
• Select this prompt to set or modify output intensity or amplitude.
9. Waveform Selection Buttons
• IFC - Interferential Stimulation: This mode of therapy, delivered with two Channels and four
electrodes, includes the Chattanooga Group unique (Quad Balance) Patient Control Center, for
easy location of the treatment area. Choose the high frequency range, the low frequency
range or both, along with Scan to deliver current to a more general area.
• PREMOD - Premodulated Stimulation: Excellent for treating small areas or where the
placement of four electrodes is not practical. This mode is quite versatile as it offers a
premodulated interferential current using one Channel and two electrodes. You may choose
to set up two separate premodulated treatments at the same time using two Channels.
• RUSSIAN - Russian Stimulation: Through this mode, you can deliver muscle stimulation
treatments choosing from a list of options including Single, Reciprocal and Co-Contraction.
Choose from various cycle times and ramp times or customize the burst frequency.
• HIVOLT - High Volt Stimulation: The Forte Twin-Peak HIVOLT is designed to deliver very short
duration pulses, which are very low in charge or power output. Treatment can be
administered using either pads or probes. Intensity displayed in Volts or Peak Current, to aid
in documentation.
• MICRO - Microcurrent Stimulation: Deliver Microcurrent using hand-held probes for manual
attended treatment or two electrodes. You may choose to modify the frequency, polarity and
intensity.
• VMS - Symmetrical Biphasic Square waveform: Symmetrical biphasic rectangular pulse with
interphase interval between the positive and negative phase. This waveform can be
administered in Single, Reciprocal or Co-Contraction cycle times. Convenient presets can
easily be altered for each patient need.
• US - Ultrasound Therapy: (Combo units only) The Forte CPS gives you the choice of 1 or 3.3
2
MHz frequency output. Ultrasound applicators are available in 2 cm
, 5 cm2and 10 cm2and
include the Electronic Signature feature. Duty cycle may be set at 10%, 20%, 50% or
Continuous.
• COMBO - Combination Therapy: (Combo units only) This option allows you to combine
ultrasound therapy with Premodulated, Interferential, VMS or High Volt stimulation. The
ultrasound head becomes one half of the electrical circuit. You can also select from the
available options for both modalities used during a given treatment.
7
Page 12

10. Constant Current (CC) or Constant Voltage (CV) - Output is available on Premod, VMS™, VMS™
Burst (CPS equilivants) and Russian current only. These waveforms are commonly used to elicit
a muscle contraction and clinically one may wish for current output to be in either format. High
Volt and IFC are set on CV and Microcurrent on CC without the ability to change this setting.
Constant Current (CC) - The output waveform maintains its set current amplitude level as
prescribed by the clinician. If resistance increases during a treatment, the unit will
automatically increase the voltage to maintain the current amplitude. The CC setting is
commonly used when a goal of the selected waveform is to elicit a muscle contraction. When
CC is selected, the clinician needs to monitor the skin and be sure good electrode contact is
made throughout the course of therapy as any change in electrode contact or the conducting
medium will cause the voltage and current density to increase. Self-adhesive electrodes are
recommended when CC is selected.
Constant Voltage (CV) - If the output waveform encounters resistance, the set voltage remains
the same, causing the current amplitude to decrease. The CV setting is commonly used when
a goal of therapy is traditional pain management or sensory level stimulation. With Constant
Voltage, as changes in electrode contact or levels of impedance increase during the course of
therapy, the output level can decrease, potentially limiting the desired physiologic effect.
11. Clinical Protocol System
• Select Clinical Protocol button to access the internal library of over 100 preset parameters.
• Presets are grouped by pain management, muscle contraction or ultrasound.
• Use the up or down arrow keys to navigate through the preset library.
12. Output Pause Control
• Select this prompt to pause a treatment session.
13. Output Stop Control
• Select this prompt to stop a treatment session.
14. Output Start Control
• Select this prompt to begin a treatment session.
General Setup Steps
The unique design of the front panel allows you to setup a treatment faster than ever. Careful
grouping of treatment options allows you to easily identify and select from the appropriate option.
Two simple steps are all that is needed prior to pressing START and initiating treatment.
8
Page 13

Quick Start
The following is a general quick step by step procedure for using the Forte™CPS. Before proceeding
refer to the cautions related to the mode of use selected.
Procedure Comments
Turn power on The unit will go through self diagnostics, followed by the main menu.
Prepare treatment site Follow steps in preparation for treatment.
Position electrodes or apply Ultrasound gel Secure electrodes in the area to be treated
Select Mode of use Select Waveform, Ultrasound or Combo.
Set “Intensity” Set intensity of the Channel(s) selected.
Press “Start” To begin treatment.
End treatment Turn power off and remove electrodes and inspect treatment area or remove
residual gel.
Screen Layout
There are three types of screen displays: Main Menu, Active Parameter Display and Parameter
Change screens. Each of these screens is formatted for easy navigation.
1. Main Menu
The Main Menu serves as the opening screen when the unit is first powered-up. It will display
available Channels as well as basic text help at the bottom of the screen to prompt you to action.
The Main Menu will reappear after you have initiated a treatment providing a status of the Channels
in use and available, waveforms selected and elapsed treatment time.
2. Active Parameter Display Screen
Active Parameter Display Screen: When a waveform is selected for use, that waveforms preset
parameters will be displayed on the LCD. Basic text help is displayed at the bottom to prompt the
user to action.
3. Parameter Change Screen
Parameter Change Screen: In this example, moving the highlight bar to Beat Frequency and
pressing ENTER will display the available Beat Frequency parameter options. Moving the highlight
bar to Fixed Frequency and pressing ENTER will display the Fixed Frequency change box.
9
Page 14
Parameter Change Box and Display of Inactive Parameters.
The once Inactive Beat Fixed option is active highlighted with a change box surrounding its preset
value of 100 Hz. At this point the user can modify this value using the up or down arrow keys.
After making a modification you must press ENTER to validate the change.
NOTE: This same sequence of active and inactive parameter options and change sequences is
repeated for all waveforms.
Parameter Adjustment
Each mode of treatment offers preset treatment parameters for quick set up. Refer to individual
sections for descriptions of preset treatment parameters. Change the presets to match your own
most common treatment protocols or utilize the internal library of Clinical Protocols. Refer to Clinical
Protocol section for more information concerning Clinical Protocols.
Parameter adjustment ranges for pain modulation modes of stimulation.
Interferential Premodulated Microcurrent
Function Electrodes Electrodes Electrodes, Probes
Carrier Frequency 5000 Hz 5000 Hz N/A
Beat Frequency 0-200 Hz 0-200 Hz 0.1-1000 Hz
Scan Mode On/Off N/A N/A
Scan Time 15 sec N/A N/A
Sweep Time 15 sec 15 sec N/A
Quadrant Balance Patient Control Center N/A N/A
Duty Cycle N/A Continuous, 5/5 N/A
Ramp Up/Down N/A N/A 1 sec. (+/- only)
Cycle Time 15 sec Continuous, 5/5 N/A
Alternating Time in
Polarity N/A N/A 2.5 sec
Polarity N/A N/A +,-, +/-
Intensity CC N/A 0-50 mA Peak 0-995 uA
Intensity CV 0-50 Volts Peak 0-50 Volts Peak N/A
Treatment Time 1 to 60 min 1 to 60 min 1 to 60 min
N/A = Not Applicable
10
Page 15

Parameter adjustment ranges for muscle contraction modes of stimulation
Russian HIVOLT VMS
Function Electrodes Electrodes, Probes Electrodes
Mode Single, Reciprocal Single, Reciprocal Single, Reciprocal
Co-Contraction Co-Contraction Co-Contraction
Carrier Frequency 2500 Hz N/A N/A
Pulse Frequency N/A 0-120 pps 5-200 pps
Burst Frequency 20-100 Hz N/A N/A
Phase Duration N/A N/A 20-300 microseconds
Interphase Interval N/A N/A 100 microseconds
Duty Cycle 10 - 50% N/A N/A
Ramp Up/Down .5, 1, 2, 5 sec. N/A .5, 1, 2, 5 sec.
Cycle Time 5/5, 10/10, 10/20, 4/12, 5/5, 10/10, 10/20, 4/12, 5/5, 10/10, 10/20, 4/12,
10/30, 10/50 10/30, 10/50 10/30, 10/50
Continuous Continuous Continuous
Polarity N/A Pos.(+), Neg.(-) N/A
Intensity CC 0-100 mA Peak N/A 0-200 mA Peak
Intensity CV 0-100 Volts Peak 0-500 Volts Peak 0-200 Volts Peak
Treatment Time 0-60 min 0-99 min 0-60 min
N/A = Not Applicable
Parameter adjustment ranges for ultrasound mode.
Function Ultrasound
Frequency 1 MHz & 3.3 MHz
Duty Cycle 10%, 20%, 50% & Continuous
Applicator Sizes 2 cm2, 5 cm2& 10 cm
Ultrasonic Power Variable from 1-20 watts
Treatment Timer 0-15 min
Class I BF
2
11
Page 16

Indications/Contraindications/Adverse Effects for Electrical
Stimulation
• Interferential / Premodulated
- Indications / Contraindications
- Warnings / Precautions / Adverse Effects
• VMS, Russian, High Volt
- Indications / Contraindications
- Warnings / Precautions / Adverse Effects
• Microcurrent
- Indications / Contraindications
- Warnings / Precautions / Adverse Effects
• Ultrasound
- Indications / Contraindications
- Warnings / Precautions / Adverse Effects
Interferential Current
Indications
• Symptomatic relief of chronic, intractable pain.
• Management of pain associated with post-traumatic or postoperative conditions.
Contraindications
• This device should not be used for symptomatic pain relief unless etiology is established or
unless a pain syndrome has been diagnosed.
• This device should not be used on patients with demand type cardiac pacemakers.
• This device should not be used over cancerous lesions.
• Electrode placements must be avoided that apply current to the carotid sinus region (anterior
neck) or transcereberally (through the head).
Warnings
• The long term effects of chronic electrical stimulation are unknown.
• Safety has not been established for the use of therapeutic electrical stimulation during
pregnancy.
• Adequate precautions should be taken when treating individuals with suspected or diagnosed
heart problems or epilepsy.
• Benefits of Interferential stimulation have not been established for pain of central origin.
• This device is to be used as a symptomatic treatment for pain and has no curative value.
Patients should be cautioned and their activities regulated if pain is suppressed that would
otherwise serve as a protective mechanism.
• Electronic monitoring equipment (such as ECG monitors and ECG alarms) may not operate
properly when electrical stimulation is being utilized.
Precautions
• Isolated cases of skin rash may occur at the site of electrode placement following long term
applications. The irritation may be reduced by use of an alternate conductive medium or an
alternative electrode placement.
• Effectiveness of this treatment is dependent upon patient selection.
12
Page 17

Adverse Effects
• Skin irritation and burns beneath the electrodes have been reported with the use of therapeutic
electrical stimulation.
Premodulated Current
Indications
• Symptomatic relief of chronic, intractable pain.
• Management of pain associated with post-traumatic or post-operative conditions.
Contraindications
• This device should not be used for symptomatic pain relief unless etiology is established or
unless a pain syndrome has been diagnosed.
• This device should not be used on patients with demand type cardiac pacemakers.
• This device should not be used over cancerous lesions.
• Electrode placements must be avoided that apply current to the carotid sinus region (anterior
neck) or transcereberally (through the head).
Warnings
• Long term effects of chronic electrical stimulation are unknown.
• Safety has not been established for the use of therapeutic electrical stimulation during
pregnancy.
• Adequate precautions should be taken when treating individuals with suspected or diagnosed
heart problems or epilepsy.
• Benefits of Premodulated stimulation have not been established for pain of central origin.
• This device is to be used as a symptomatic treatment for pain and has no curative value.
Patients should be cautioned and their activities regulated if pain is suppressed that would
otherwise serve as a protective mechanism.
• Electronic monitoring equipment (such as ECG monitors and ECG alarms) may not operate
properly when electrical stimulation is being utilized.
Precautions
• Isolated cases of skin rash may occur at the site of electrode placement following long term
applications. The irritation can usually be reduced by use of an alternate conductive medium or
an alternative electrode placement.
• Effectiveness of this treatment is dependent upon patient selection.
Adverse Effects
• Skin irritation and burns beneath the electrodes have been reported with the use of therapeutic
electrical stimulation.
13
Page 18

VMS, Russian, HIVOLT
Indications
• Relaxation of muscle spasms
• Prevention or retardation of disuse atrophy
• Increasing local blood circulation
• Muscle re-education
• Maintaining or increasing range of motion
• Immediate postsurgical stimulation of calf muscles to prevent venous thrombosis
Contraindications
• This device should not be used on patients with demand type cardiac pacemakers.
• This device should not be used on cancer patients.
Warnings
• The long term effects of chronic electrical stimulation are unknown.
• Safety has not been established for the use of therapeutic electrical stimulation during
pregnancy.
• Adequate precautions should be taken when treating individuals with suspected or diagnosed
heart problems.
• Adequate precautions should be taken in the cases of persons with suspected or diagnosed
epilepsy.
• DO NOT stimulate over the carotid sinus nerve, especially in persons with a known sensitivity to
the carotid sinus reflex.
• Severe spasm of the laryngeal and pharyngeal muscles may occur if the electrodes are placed
over the neck or mouth. The contractions may be strong enough to cause breathing difficulty or
even close the airway.
• DO NOT perform therapeutic electrical stimulation transcerebrally (through the head).
• Therapeutic electrical stimulation should not be applied over swollen, infected or inflamed areas
of skin eruptions (e.g., phlebitis, thrombophlebitis and varicose veins).
• Use extreme caution in transthoracic application of therapeutic electrical stimulation, introduction
of electrical current into the heart may cause arrhythmia.
• This device should only be used under medical supervision for adjunctive therapy for the
treatment of medical diseases and conditions.
• This device should be kept out of the reach of children.
Precautions should be observed in the presence of the following:
• Following recent surgical procedures especially when muscle contractions could disrupt the
healing process.
• Where sensory nerve damage is present by a loss of normal skin sensation.
• When there is a tendency to hemorrhage following acute trauma or fracture.
• Over the menstruating uterus.
• Some patients may experience skin irritation or hypersensitivity due to the electrical stimulation
or the electrical conductive medium. The irritations can usually be reduced by the use of an
alternate conductive medium or alternative electrode placement.
Adverse Effects
• Skin irritation and burns beneath the electrodes have been reported with the use of therapeutic
electrical stimulation.
14
Page 19

Microamperage Pulsed Current (Microcurrent)
Indications
• Symptomatic relief of chronic, intractable pain.
• Management of pain associated with post-traumatic or postoperative conditions.
Contraindications
• This device should not be used for symptomatic pain relief unless etiology is established or
unless a pain syndrome has been diagnosed.
• This device should not be used on patients with demand type cardiac pacemakers.
• This device should not be used over cancerous lesions.
• Electrode placements must be avoided that apply current to the carotid sinus region (anterior
neck) or transcereberally (through the head).
Warnings
• Long-term effects of chronic electrical stimulation are unknown.
• Safety has not been established for the use of transcutaneous nerve stimulation during
pregnancy.
• Adequate precautions should be taken when treating individuals with suspected or diagnosed
heart problems or epilepsy.
• Benefits of microcurrent have not been established for pain of central origin.
• This device is to be used as a symptomatic treatment for pain and has no curative value.
Patients should be cautioned and their activities regulated if pain is suppressed that would
otherwise serve as a protective mechanism.
• Electronic monitoring equipment (such as ECG monitors and ECG alarms) may not operate
properly when electrical stimulation is being utilized.
Precautions
• Isolated cases of skin rash may occur at the site of electrode placement, following long term
applications. The irritation can usually be reduced by use of an alternate conductive medium or
an alternative electrode placement.
• Effectiveness of this treatment is dependent upon patient selection.
Adverse Effects
• Skin irritation and burns beneath the electrodes has been reported with the use of
transcutaneous nerve stimulation. The microamperage current levels of this device may
minimize this possibility.
15
Page 20

Hand Held Probes (Optional)
Microcurrent Probes
Active Probe (with switch)
• Plug the Microcurrent probe (with switch) into the port marked Microcurrent Probe.
• To deliver Microcurrent to the patient, hold the active probe as you would hold a pencil, press
the button near the tip of the probe to start delivery of current.
• You must also use a reference probe or a reference electrode (the Channel One Black lead wire,
as described below).
• The active probe should touch the patient’s skin at the treatment site and the reference probe
should touch the patient’s skin elsewhere to complete the circuit.
• Once you press and release the button, current will be delivered for the treatment time, which
has been set in the Microcurrent parameter change screen. When the time has elapsed, a tone
will sound and the device will return to the GSR mode (default).
• The active/treating Microcurrent probe senses skin impedance levels when not delivering
stimulation. This method of skin impedance sensing is referred to as the GSR mode. When low
levels of impedance are found, the number of audible tones per second will increase.
Reference Probe
• The purpose of the reference probe is to complete the circuit allowing flow of current through
patient tissue. The reference probe should touch the patient’s skin away from the location of the
active probe.
• Attach the
a reference.
BBllaacckk wwiirree
CChhaannnneell OOnnee
from
to the reference probe or you may use an electrode as
NOTE: Place the reference device as close to the treatment site as possible where it will not
interfere with placement of the active/treating electrode; for example, do not place the reference
electrode on the leg if you are treating the arm.
• Use the end of a (moistened) cotton swab inserted into the ends of both the active/treating
probe and the reference probe. The cotton must touch the probe’s metal ring; use water to wet
the cotton swab before starting the treatment.
HIGH VOLT Probe
The High Volt probe is used to deliver stimulation manually. Select the High Volt Waveform then
simply plug the Red lead wire into the connector of the Probe. The Black lead wire from the same
Channel should be attached to an electrode and placed neat the treatment site.
• Select the parameters you wish to change then press the start button to begin treatment.
NOTE: Place the reference electrode as close to the treatment site as possible where it will not
interfere with placement of the active electrode; for example, do not place the reference
electrode on the leg if you are treating the arm.
16
Page 21

Treatment Timer
Once you press the start button, the timer will count from the preset time parameter to zero. When
the current is stopped, the timer will reset to the previous time setting. Press the start button again to
begin a new treatment; continue in this way until the session is completed.
IFC - Interferential Current (Sine Wave)
• Introduction
• Quick Start
• Setup Procedure
- Modifying Power-up Presets Parameters
• Preparation for Treatment
• Detailed Treatment Procedures
- Treatment Cautions
• Modifying Treatment Parameters
• Technical Specifications
Introduction to Interferential
Interferential current consists of two Channels with two sinusoidal waveforms: one of fixed frequency
and one of variable frequency. When the four electrodes are positioned so the two Channels cross
each other, the waveforms mix within the tissues to produce a train of pulses whose frequency and
amplitude are dependent on the sweep mode, beat frequency and amplitude settings, respectively.
This mode of therapy also includes Chattanooga Group’s unique Patient Control Center for easy
location of the treatment site and delivery of the full interferential beat where it is needed. You can
choose the high frequency range, the low frequency range or both, along with Scan when you want
to deliver the therapeutic current over a more general area.
Quick Start
The following is a quick step by step procedure for using interferential stimulation. Before proceeding
refer to treatment cautions.
Procedure Comments
Turn power on The unit will go through self diagnosis, followed by the main menu.
Prepare treatment site Follow steps in preparation for treatment.
Position electrodes Secure electrodes around area to be treated.
Press “IFC” To select interferential stimulation.
Set “Intensity” Set intensity level for both Channels.
Press “Start” To begin treatment.
End Treatment Remove electrodes and inspect treatment area.
17
Page 22

Setup Procedure: INTERFERENTIAL (IFC)
The following sequence of events are followed to begin an Interferential treatment:
Step 1. Press IFC and set intensity of both Channels.
Ch. Select
Beat Freq.
Step 2. Press START to begin treatment
Modifying Power-up Preset Parameters
With the exception of output intensity all power-up preset parameters can be modified using the
following sequence of steps:
1. Select and change the desired parameter setting, then press ENTER.
2. Next press and hold the PAUSE button, then press the ENTER button.
Preparation for Treatment
Refer to section three to become familiar with the following:
Indications, Contraindications, Warnings, Precautions and Adverse Effects for Interferential current.
Detailed Treatment Procedures
Remember that most patients are unfamiliar with electrical stimulation and may be anxious or
apprehensive during the initial sensation of current. If the patient is extremely apprehensive, either
discontinue the treatment or set the “Output Intensity” at a level where the patient just feels the
current. Allow the individual to become accustomed to the current before increasing the intensity.
Treatment Cautions
• Never turn the power on or off while the unit is connected to the individual.
• Stop the treatment before removing or attaching electrodes or leads.
• Never use leads or electrodes that are damaged or worn, this may result in injury to the patient.
• Consult published sources for electrode placements, settings and treatment duration.
• Make sure all electrodes make full contact with the patient’s skin.
18
Page 23

1. Place the power switch in the “ON” position.
The display screen will indicate the machine is going through its power-up procedures and
system checks. When completed, the main menu will appear on the screen.
2. Connect electrodes to lead wires and plug into jacks for Channels selected.
3. Adhere electrodes to the location to be treated.
Secure electrodes in the area to be treated. Refer to guidelines under preparation for treatment
earlier in this section.
4. Select “IFC” for interferential current.
5. View and verify the preset parameters for interferential current.
Preset parameters for interferential current; Beat Frequency: 80-150 Hz, Amplitude Modulation:
40% Scan, Treatment Time: 15 minutes.
6. To modify treatment parameters prior to starting treatment:
• Use the up and down arrows located next to ENTER button to navigate through the
parameters available for interferential current. Time and output intensity is modified with the
keys below their LED display.
• Once you have highlighted a desired parameter press ENTER to display a pop-up menu of
options available for this mode of treatment.
• Use the up and down arrows to highlight the desired option, then press ENTER.
7. Set “Power/Intensity” using the controls located on the control panel.
Set the output intensity for both Channels in use using the arrow keys located next to the
POWER/INTENSITY button. The amplitude is displayed digitally on the LED adjacent the
intensity control button.
8. Press “Start” to initiate the treatment.
The stimulation will ramp up to the set values and the Main Menu will reappear, displaying the
Channels and waveforms in use as well as the treatment time remaining for each Channel.
9. To modify settings during treatment: It is recommended that you Pause the treatment session prior to
implementing a parameter change.
• Select the Channel to modify from the Main Menu.
• Press ENTER to display parameters of that Channel on both the LCD and LED displays.
• You may want to choose “Pause” to interrupt treatment or lower the intensity prior to making
changes in treatment parameters to prevent the possibility of undesirable sensation that could
be perceived with a change in parameters.
• Once you have highlighted a desired parameter, press ENTER to display a list of options
available for this mode of treatment.
• Use the arrows once again to highlight a desired option and then press ENTER to accept.
• Use the Treatment Time Control to alter treatment time from the arrows on the control panel.
• Press START to resume treatment.
10. Terminating Treatment
When treatment ends, a tone will sound and the intensity will ramp to 0. Remove electrodes
from patient and inspect area of skin treated.
19
Page 24

Modifying Treatment Parameters
Treatment parameters can be modified prior to or during a treatment session. To modify interferential
or any session parameters, the session parameters must be displayed on the LCD and LED
respectively.
Modify LED functions: Treatment Time and Output Intensity
Select the Channel you wish to modify, Treatment Time and Output will be displayed.
Modify LCD functions: INTERFERENTIAL
Prior to or while a session is in progress, you can modify any of the LCD waveform parameters as
desired. The following describes the general progression for changing parameters:
The left side of the screen lists the parameters that can be modified.
Move the highlight box to the desired parameter and press ENTER, a list of options* is displayed on
the right side of the screen.
Move the highlight box to the desired option and press ENTER.
*If no list of options is displayed, this indicates that there are less than four choices and you may
select them by continuing to press ENTER.
Interferential Parameter Options
Ch. Select: Both Channels, Channel 1 or Channel 2
Amp. Mod: Quad Balance*, 40% Scan, 100% Scan or Static
Beat Freq: 1-10 Hz, 80-150 Hz, 1-150 Hz, Variable or Fixed
Ch. Select
Variable Beat Frequency options: Beat Low 1-150 Hz
Beat High 1-150 Hz
Fixed Beat Frequency options: 0-200 Hz
20
Page 25

Suggestion – Some patients may not tolerate parameter changes during a session unless the PAUSE
key is used to ramp stimulation down prior to making a change.
Technical Tip – Quad. Bal. (Quadrant Balance) - A unique and proprietary balance control system. This
allows you to define the patient’s area of stimulation in continuous fashion prior to and during a
treatment session. Note: The option of Quad Balance will only appear in the parameter change box if
it is properly mounted on the back of the unit.
Setting Channel Output
The power-up preset delivers stimulation from both Channels simultaneously. This can be modified so
stimulation can be set one Channel at a time if so desired. To make this selection, follow these
commands: With the highlight bar positioned over Ch. Select parameter, press Enter. This will change
output intensity settings to Channel 1. At this point raise the intensity of Channel 1 using the
Power/Intensity keys. When at desired levels, press Enter to set Channel 2. The intensity level of
Channel 1 will remain on as Channel 2 intensity level is being set.
Technical Specifications for Interferential Current
Output Channels: 1- 4
Intensity CV: 0-50 Volts Peak
Carrier Frequency: 5000 Hz
Channels: 1 & 2 for CPS 200 Series, 1, 2, 3 and 4 for CPS 400 Series.
Beat Frequencies: 0-200 Hz
Maximum RMS Current Density: 3” x 5” Electrode: 5.2 mA/mm
2” Diameter Electrode: 24.7 mA/mm
Maximum Power Density: 3” x 5” Electrode: .365 mw/mm
2” Diameter Electrode: 1.74 mw/mm
2
2
2
2
Area of Conductive Surface: 3” x 5” Electrode: 15 in2 (9,677 mm2)
1 5/8” x 2” Electrode: 3.25 in2 (2,096 mm2)
2” Diameter Electrode: 3.14 in2 (2,026 mm2)
21
Page 26

PREMOD - Premodulated (Sine Wave)
• Introduction
• Quick Start
• Setup Procedure
- Modifying Power-up Presets Parameters
• Preparation for Treatment
• Detailed Treatment Procedures
- Treatment Cautions
• Modifying Treatment Parameters
• Technical Specifications
Introduction to PREMOD
Premodulated is a single sine wave that has modulated amplitude. This waveform is similar to the
beat frequency, the pattern created by the interferential current, but can be delivered through one
Channel. In some cases, premodulated therapy provides a good alternative for interferential treatment
especially when treating small areas of the body where four electrodes could not be utilized.
Quick Start
The following is a quick step by step procedure for using Premodulated stimulation. Before
proceeding refer to treatment cautions.
Procedure Comments
Turn power on The unit will go through self diagnostics, followed by the main menu.
Prepare treatment site Follow steps in preparation for treatment.
Position electrodes Secure electrodes around area to be treated.
Press “PREMOD” To select Premodulated stimulation.
Set “Intensity” Adjusting intensity of the displayed Channel.
Press “Start” To begin treatment.
End treatment Remove electrodes and inspect treatment area.
22
Page 27

Setup procedure: PREMOD
The following sequence of events is followed to begin a Premod treatment.
Step 1. Press PREMOD and set intensity of displayed Channel.
Cycle Time
CC/CV
Step 2. Press Start to begin treatment.
Channel 1
CV
Modifying Power-up Preset Parameters
With the exception of output intensity all power-up preset parameters can be modified using the
following sequence of steps:
1. Select and change the desired parameter setting, then press ENTER.
2. Next press and hold the PAUSE button, then press the ENTER button.
Preparation for Treatment
Refer to section three to become familiar with the following:
Indications, Contraindications, Warnings, Precautions and Adverse Effects for Premodulated current.
Detailed Treatment Procedures
Remember that most patients are unfamiliar with electrical stimulation and may be anxious or
apprehensive during the initial sensation of current. If the patient is extremely apprehensive, either
discontinue the treatment or set the “Output Intensity” at a level where the patient just feels the
current. Allow the individual to become accustomed to the current before increasing the intensity.
Treatment Cautions:
• Never turn the power on or off while the unit is connected to the individual.
• Stop the treatment before removing or attaching electrodes or leads. Leads and electrodes
should be applied to the patient before a treatment is initiated.
• Never use leads or electrodes that are damaged or worn, this may result in injury to the patient.
• Consult published sources for electrode placements, settings and treatment duration.
• Make sure all electrodes make full contact with the patient’s skin.
1. Place the power switch in the “On” position.
The display screen will indicate the machine is going through its power-up procedures and
system checks. When completed, the main menu will appear on the screen.
23
Page 28

2. Connect electrodes to lead wires and plug into jacks for Channels selected.
3. Adhere electrodes to the location to be treated
Refer to guidelines under preparation for treatment earlier in this section.
4. Press “PREMOD” for premodulated current.
Refer to the information on indications and contraindications, in section three.
5. View and verify the preset parameters for Premodulated current.
Preset Parameters for Premod: Beat Frequency: 80 - 150 Hz, Treatment Time: 15 minutes.
6. To modify treatment parameters prior to starting treatment:
• Use the up and down arrows located next to the ENTER button to navigate through the
parameters available for Premodulated current.
• Once you have highlighted a desired parameter, press ENTER to display a list of options
available for this mode of treatment.
• Use the arrows once again to highlight a desired option and then press ENTER.
7. Set “Power / Intensity” using the controls located on the control panel.
The amplitude is displayed digitally on the LED adjacent the intensity control button.
8. Press “Start” to initiate the treatment.
The stimulation will ramp up to the set values and the Main Menu will reappear, displaying the
Channels and waveforms in use as well as the treatment time remaining for each Channel.
9. To modify settings during treatment: It is recommended that you Pause the treatment session prior to
implementing a parameter change.
• Select the Channel to modify from the Main Menu.
• Press ENTER to display parameters of that Channel on both the LCD and LED displays.
• You may want to choose PAUSE to interrupt treatment or lower the intensity prior to making
changes in treatment parameters to prevent the possibility of undesirable sensation that could
be perceived with a change in parameters.
• Once you have highlighted a desired parameter, press ENTER to display a list of options
available for this mode of treatment.
• Use the arrows once again to highlight a desired option and then press ENTER to accept.
• Press START to resume treatment.
10. Terminating Treatment
When treatment ends, a tone will sound and the intensity will ramp to 0. Remove electrodes
from patient and inspect area of skin treated.
Modifying Treatment Parameters
Treatment parameters can be modified prior to or during a treatment session. To modify Premod or
any session parameters, the session parameters must be displayed on the LCD and LED respectively.
24
Page 29

Modify LED functions: Treatment Time and Output Intensity
Select the Channel you wish to modify and Treatment Time and Output will be displayed.
Suggestion – Some patients may not tolerate parameter changes during a session unless the PAUSE
key is used to ramp stimulation down prior to making a change.
Modify LCD functions: PREMOD
Prior to or while a session is in progress, you can modify any of the LCD waveform parameters as
desired. The following describes the general progression for changing parameters:
The left side of the screed lists the parameters that can be modified.
Move the highlight box to the desired parameter and press ENTER, a list of options* is displayed on
the right side of the screen.
Move the highlight box to the desired option and press ENTER.
*If no list of options is displayed, this indicates that there are less than four choices and you may
select them by continuing to press ENTER.
Premod Parameter Options
Cycle time Continuous or 5/5
Beat Freq. 1-10 Hz, 80-150 Hz, 1-150 Hz, Variable or Fixed
Current: CC or CV (Default)
Cycle Time
CC/CV
Channel 1
CV
Variable Beat Frequency options: Beat Low 1-150 Hz
Beat High 1-150 Hz
Fixed Beat Frequency options: 0-200 Hz
Suggestion – Some patients may not tolerate parameter changes during a session unless the PAUSE
key is used to ramp stimulation down prior to making a change.
25
Page 30

Technical Specifications for Premodulated Current
Channels: 1 - 4
Intensity CC: 0-50 mA Peak
Intensity CV: 0-50 Volts Peak
Carrier Frequency: 5000 Hz
Beat Frequency: 0-200 Hz
Maximum RMS Current Density: 3” x 5” Electrode: 5.2 mA/mm
2” Diameter Electrode: 24.7 mA/mm
Maximum Power Density: 3” x 5” Electrode: .365 mw/mm
2” Diameter Electrode: 1.74 mw/mm
2
2
2
2
Area of Conductive Surface: 3” x 5” Electrode: 15 in2 (9,677 mm2)
1 5/8” x 2” Electrode: 3.25 in2 (2,096 mm
2” Diameter Electrode: 3.14 in2 (2,026 mm2)
2
)
MICRO - Microcurrent (Microamperage Pulsed Current)
• Introduction
• Quick Start
• Setup Procedure
- Modifying Power-up Presets Parameters
• Preparation for Treatment
• Detailed Treatment Procedures
- Treatment Cautions
• Modifying Treatment Parameters
• Technical Specifications
Introduction to MICROCURRENT
Monophasic rectangular wave with selectable or alternating polarity. 50% duty cycle is fixed so pulse
duration is inversely affected by changes in frequency. Some clinicians prefer microcurrent because of
the low current utilized. With such low current, the individual feels no discomfort and quite often feels
no sensation at all during the treatment. You can provide attended or unattended microcurrent
therapy. Use electrodes for longer unattended therapy sessions, pads are usually placed on opposite
sides of the affected area so treatment is “through” the affected area. If attended therapy is
preferred, you can use the microcurrent probes for hands-on treatment.
Probes are used to treat very small areas or treat a number of different areas in succession. This
therapy utilizes an active/treating probe and a reference probe. If desired, a ground electrode may be
substituted for the reference probe.
26
Page 31

Quick Start
The following is a quick step procedure for using Microcurrent stimulation. Before proceeding refer to
treatment cautions.
Procedure Comment
Turn power on The unit will go through self diagnostics, followed by the main menu.
Prepare treatment site Follow steps in preparation for treatment.
Position electrodes Secure electrodes in the around area to be treated.
Press “MICRO” To select Microcurrent stimulation.
Set “Intensity” Set intensity of selected Channel.
Press “Start” To begin treatment.
End treatment Remove electrodes and inspect treatment area.
Setup procedure: MICROCURRENT
The following sequence of events are followed to begin Microcurrent treatment using electrodes:
Step 1. Press MICRO and set intensity of displayed Channel.
Positive
Step 2. Press Start to begin treatment.
Modifying Power-up Preset Parameters
With the exception of output intensity all power-up preset parameters can be modified using the
following sequence of steps:
1. Select and change the desired parameter setting, then press ENTER.
2. Next press and hold the PAUSE button, then press the ENTER button.
Preparation for Treatment
Refer to section three to become familiar with the following:
Indications, Contraindications, Warnings, Precautions and Adverse Effects for Microcurrent current.
27
Page 32

Detailed Treatment Procedures
Remember that most patients are unfamiliar with electrical stimulation and may be anxious or
apprehensive during the initial sensation of current. If the patient is extremely fearful, either
discontinue the treatment or set the “Amplitude” at a level where the patient just feels the current.
Allow the individual to become accustomed to the current before increasing the intensity.
Treatment Cautions:
• Never turn the power on or off while the unit is connected to the individual.
• Stop the treatment before removing or attaching electrodes or leads. Leads and electrodes
should be applied to the patient before a treatment is initiated.
• Never use leads or electrodes that are damaged or worn; this may result in injury to the patient.
• Consult published sources for electrode placements, settings and treatment duration.
• Make sure all electrodes make full contact with the patient’s skin.
Pads/Electrode Application of Microcurrent
1. Place the power switch in the “On” position.
The display screen will indicate the machine is going through its power-up procedures and
system checks. When completed, the main menu will appear on the screen.
2. Connect electrodes to lead wires and plug into jacks for Channels selected.
All Channels are available for Microcurrent treatment.
3. Adhere electrodes to the location to be treated.
Refer to guidelines under preparation for treatment earlier in this section.
4. Press “MICRO” for Microcurrent current.
Refer to the information on indications and contraindications in section three.
5. View and verify the preset parameters for Microcurrent (electrode) therapy.
Preset parameters for Microcurrent Therapy; Method: Pads, Waveform: Bipolar, Frequency
Steps: Fine, Frequency: 0.1 Hz.
6. To modify treatment parameters prior to starting treatment:
• Use the up and down arrows located next to the ENTER button to navigate through the
parameters available for Microcurrent.
• Once you have highlighted a desired parameter, press ENTER to display a list of options
available for this mode of treatment.
• Use the arrows once again to highlight a desired option and then press ENTER.
7. Set “Power / Intensity” using the controls located on the control panel.
8. Press “Start” to initiate the treatment.
The stimulation will ramp up to the set values and the Main Menu will reappear, displaying the
Channels and waveforms in use as well as the treatment time remaining for each Channel.
9. To modify settings during treatment: It is recommended that you Pause the treatment session prior to
implementing a parameter change.
• Select the Channel to modify from the Main Menu.
• Press ENTER to display parameters of that Channel on both the LCD and LED displays.
28
Page 33

• You may want to choose PAUSE to interrupt treatment or lower the intensity prior to making
changes in treatment parameters to prevent the possibility of undesirable sensation that could
be perceived with a change in parameters.
• Use the up and down arrows located next to the ENTER button to navigate through the
parameters available for Microcurrent.
• Once you have highlighted a desired parameter, press ENTER to display a list of options
available for this mode of treatment.
• Use the arrows once again to highlight a desired option and then press ENTER to accept.
• Use the Treatment Time Control to alter treatment time.
• Press START to resume treatment.
10. Terminating Treatment
When treatment ends, a tone will sound and the intensity will ramp to 0. Remove electrodes
from patient and inspect area of skin treated.
Modifying Treatment Parameters
Treatment parameters can be modified prior to or during a treatment session. To modify Microcurrent
or any session parameters the session parameters must be displayed on the LCD and LED
respectively.
Modify LED functions: Treatment Time and Output Intensity
Select the Channel you wish to modify and Treatment Time and Output will be displayed.
Modify LCD functions: MICRO
Prior to or while a session is in progress, you can modify any of the LCD waveform parameters as
desired. The following describes the general progression for changing parameters:
The left side of the screed lists the parameters that can be modified.
Move the highlight box to the desired parameter and press ENTER, a list of options* is displayed on
the right side of the screen.
Move the highlight box to the desired option and press ENTER.
*If no list of options is displayed, this indicates that there are less than four choices and you may
select them by continuing to press ENTER.
Microcurrent Parameter Options
Method Pads or Probes
Waveform Negative, Positive or Bipolar
Freq. Step Fine or Coarse
Frequency .1-1000 Hz
29
Page 34

Positive
Technical Tip – Waveform options: The current flow may be directed in either a positive, negative or
alternating (Bipolar) direction.
Bipolar: Alternating through a Positive and Negative direction.
Technical Tip – Frequency Step: Options are Fine and Coarse. This is a parameter convenience that
allows you to scroll through your frequency options with fine sensitivity or course sensitivity. Use Fine
sensitivity when frequencies between 0.01 and 1.0 Hz are desired. Use the Coarse sensitivity setting
to make increases in frequency of greater magnitude.
Hand Held Probes (Optional) application of Microcurrent
1. Place the power switch in the “ON” position.
The display screen will indicate the machine is going through its power-up procedures and
system check. When completed, the main menu will appear on the screen.
2. Plug in probes
Plug the active probe (with the start switch) into the multiple-pin jack labeled “Probe.” The
reference probe (without start switch) should be plugged into Channel 1 black lead wire. You
may also use a reference electrode adhered to the patient’s body instead of the probe. This
electrode is connected to the black lead wire pin plugged into Channel 1.
3. Press “MICRO” for Microcurrent current.
Refer to the information on indications and contraindications in section three.
4. View and verify the preset parameters for microcurrent current probe therapy.
Preset parameters for Microcurrent Probe Therapy: Method: Pads, Waveform: Bipolar,
Frequency Steps: Fine, Frequency: 0.1 Hz.
5. Specific parameters for Microcurrent Waveform include:
• Frequency: The number of cycles delivered per second. The waveform is pulsed at a 50%
duty cycle so the lower frequency, the longer the pulse duration.
• Polarity: The current flow may be directed in either a positive, negative or alternating (Bipolar)
direction. The “Ramp” is an amplitude increase from 0 to the preset-level in one second
when alternating polarity is selected. The lead wire marked with a dot near the pin is the
polarity selected.
• Treatment Time: The time in minutes (seconds when using the probe) that the stimulation will
be delivered. Treatment will automatically cease and a tone will sound when this time expires.
• Amplitude: The intensity of the output is measured in microamps. In general, higher
amplitudes are used with larger electrodes. The amplitude is adjusted to be subsensory for
this application.
30
Page 35

6. To modify treatment parameters prior to starting treatment:
• Use the up and down arrows located next to the ENTER button to navigate through the
parameters available for Microcurrent current.
• Once you have highlighted a desired parameter press ENTER to display a list of options
available for this mode of treatment.
• Use the arrows once again to highlight a desired option and then press ENTER.
7. Set “Power / Intensity” using the controls located on the control panel.
The amplitude is displayed digitally on the LED adjacent to the intensity control button.
8. Press “Start” to initiate the treatment.
Main Menu will reappear updated with waveforms and Channels in use as well as elapsed time.
9. To modify settings during treatment:
• Parameters for the current Channel will be displayed on the LCD and LED read outs.
• To modify waveforms, frequency or amplitude:
• You may want to choose “Pause” to interrupt treatment or lower the intensity prior to making
changes in treatment parameters to prevent an undesirable sensation of current by you
patient.
• From the Main menu select the Channel to be altered.
• Use the up and down arrows located next to the ENTER button to navigate through the
parameters available for Microcurrent.
• Once you have highlighted a desired parameter, press ENTER to display a list of options
available for this mode of treatment.
• Use the arrows once again to highlight a desired option and then press ENTER to accept.
• Use the Treatment Time Control to alter treatment time.
• Press START to resume treatment.
10. Terminating Treatment:
• When treatment ends, a tone will sound and the intensity will ramp to 0.
• Remove electrodes from patient and inspect area of skin treated.
Technical Specifications for Microcurrent
Channel: 1 & 2 only in the Forte CPS 200 Stim and Combo, Channels 1, 2, 3 & 4 in the
Forte CPS 400 Stim and Combo (Probe Channel 1 Only)
Current: 0-995 microamperes CC
Compliance Voltage: 60 Volts
Frequency: 0.1-1000 Hz
Polarity: Either Positive, Negative or Alternating, selectable as follows:
Negative (-) rectangular wave output or
Positive (+) rectangular wave output
Alternating: 2.5 Seconds in each polarity
31
Page 36

Frequency: 2 Hz
Alternate: 2.5 sec.
Ramp: 0 sec.
Amplitude: 600 µA
Load: 500 ohm
Frequency: 2 Hz
Alternate: 2.5 sec.
Ramp: 1 sec.
Amplitude: 600 µA
Load: 500 ohm
RUSSIAN (Interrupted Sine Wave)
• Introduction
• Quick Start
• Setup Procedure
- Modifying Power-up Presets Parameters
• Preparation for Treatment
• Detailed Treatment Procedures
- Treatment Cautions
• Modifying Treatment Parameters
• Technical Specifications
Introduction to RUSSIAN
The Russian current is a 2,500 Hz sinusoidal carrier wave, interrupted to create pulse trains or
“bursts.” The number of bursts per second is set by the burst frequency and the length of the burst
is determined by the duty cycle.
Russian stimulation currents produce strong muscle contractions. Three modes of treatment are
available including single Channel, reciprocal and co-contraction. You have the option to choose from a
wide variety of parameters to selectively generate the level of contraction(s) specific for your patient’s
needs. Adjustable parameters include: Mode (single Channel, reciprocal or co-contract), Cycle Time,
Frequency, Ramp and Duty Cycle.
32
Page 37

Quick Start
The following is a quick step by step procedure for using Russian stimulation. Before proceeding refer
to treatment cautions.
PPrroocceedduurree CCoommmmeenntt
Turn power on The unit will go through self diagnostics, followed by the main menu.
Prepare treatment site Follow steps in preparation for treatment.
Position electrodes Secure electrodes around area to be treated.
Press “Russian” To select Russian stimulation.
Set “Intensity” Set the output intensity for the selected Channel.
Press “Start” To begin treatment.
End Treatment Remove electrodes and inspect treatment area.
Setup procedure: RUSSIAN
Follow this sequence of events to begin single Channel Russian stimulation:
Step 1. Press RUSSIAN and set intensity of displayed Channel.
CC/CV
Step 2. Press START to begin treatment.
CC
Modifying Power-up Preset Parameters
With the exception of output intensity all power-up preset parameters can be modified using the
following sequence of steps:
1. Select and change the desired parameter setting, then press ENTER.
2. Next press and hold the PAUSE button, then press the ENTER button.
Preparation for Treatment
Refer to section three to become familiar with the following:
Indications, Contraindications, Warnings, Precautions and Adverse Effects for Russian current.
33
Page 38

Detailed Treatment Procedures
Remember that most patients are unfamiliar with electrical stimulation and may be anxious or
apprehensive during the initial sensation of current. If the patient is extremely fearful, either
discontinue the treatment or set the “Amplitude” at a level where the patient just feels the current.
Allow the individual to become accustomed to the current before increasing the intensity.
Treatment cautions:
• Never turn the power on or off while the unit is connected to the individual.
• Stop the treatment before removing or attaching electrodes or leads.
• Leads and electrodes should be applied to the patient before a treatment is initiated.
• Never use leads or electrodes that are damaged or worn; this may result in injury to the patient.
• Consult published sources for electrode placements, settings and treatment duration.
• Make sure all electrodes make full contact with the patient’s skin.
1. Place the power switch in the “ON” position.
The display screen will indicate the machine is going through its power-up procedures and
system checks. When completed, the main menu will appear on the screen.
2. Connect electrodes to lead wires and plug into jacks for Channels selected.
Certain Channels are designated for specific modes of treatment.
3. Adhere electrodes to the location to be treated.
Refer to guidelines under preparation for treatment earlier in this section.
4. Press “Russian” for Russian current.
Refer to the information on indications and contraindications in section three.
5. View and verify the preset parameters for Russian current.
Preset parameters for Russian current; Mode: Single Channel, Cycle Time: 10/50, Frequency:
50bps, Ramp: 2 sec., Duty Cycle: 50%
6. To modify treatment parameters prior to starting treatment:
• Use the up and down arrows located next to the ENTER button to navigate through the
parameters available for Russian current.
• Once you have highlighted a desired parameter, press ENTER to display a list of options
available for this mode of treatment.
• Use the arrows once again to highlight a desired option and then press ENTER.
7. Set “Power / Intensity” using the controls located on the control panel.
The amplitude is displayed digitally on the LED adjacent the intensity control button. Set the
output intensity for the displayed Channel using the arrow keys located next to the
Power/Intensity button.
8. Press “Start” to initiate the treatment.
The stimulation will ramp up to the set values and the Main Menu will reappear, displaying the
Channels and waveforms in use as well as the treatment remaining for each Channel.
34
Page 39

9. To modify settings during treatment: It is recommended that you Pause the treatment session prior to
implementing a parameter change.
• Select the Channel to modify from the Main Menu.
• Press ENTER to display parameters of that Channel on both the LCD and LED displays.
• You may want to choose PAUSE to interrupt treatment or lower the intensity prior to making
changes in treatment parameters to prevent the possibility of undesirable sensation that could
be perceived with a change in parameters.
• Once you have highlighted a desired parameter, press ENTER to display a list of options
available for this mode of treatment.
• Use the arrows once again to highlight a desired option and then press ENTER to accept.
• Use the Treatment Time Control to alter treatment time with the arrows on the control panel.
• Press START to resume treatment.
10. Terminating Treatment
When treatment ends, a tone will sound and the intensity will ramp to 0. Remove electrodes
from patient and inspect area of skin treated.
Modifying Treatment Parameters
Treatment parameters can be modified prior to or during a treatment session. To modify Russian or
any session parameters the session parameters must be displayed on the LCD and LED respectively.
Modify LED functions: Treatment Time and Output Intensity
Select the Channel you wish to modify and Treatment Time and Output will be displayed.
Suggestion:
Some patients may not tolerate parameter changes during a session unless the PAUSE key is used to
ramp stimulation down prior to making a change.
Modify LCD functions: RUSSIAN
Prior to or while a session is in progress, you can modify any of the LCD waveform parameters as
desired.
The following describes the general progression for changing parameters:
The left side of the screed lists the parameters that can be modified.
Move the highlight box to the desired parameter and press ENTER, a list of options* is displayed on
the right side of the screen.
Move the highlight box to the desired option and press ENTER.
*If no list of options is displayed, this indicates that there are less than four choices and you may
select them by continuing to press ENTER.
35
Page 40

Russian Parameter Options
Mode Single, Reciprocal* or CoContract*
Cycle Time 5/5, 10/10, 10/20, 4/12, 10/30, 10/50 or Continuous
Frequency 20-100 bps
Ramp 0.5, 1, 2 or 5 sec.
Duty Cycle 10%, 20%, 30%, 40% or 50%
CC/CV
*Note-Ch. Select is available only when Reciprocal or CoContract is selected. This allows amplitude
adjustment of each Channel independently if desired. To make this selection follow these commands.
With the highlight bar positioned over Ch. Select, press Enter. This will change output intensity of
Channel 1. At this point raise the intensity of Channel 1 using the Power / Intensity keys. When at
desired levels, press Enter to set Channel 2. The intensity level of Channel 1 will remain on as
Channel 2 intensity level is being set.
Technical Tip – Single Channel: One Channel (two electrodes) positioned to stimulate a muscle or
muscle group. Reciprocal: Two Channels (four electrodes) positioned to stimulate the same or
opposing muscle groups. CoContract: Two Channels (four electrodes) positioned to stimulate the
opposing muscle groups simultaneously.
Suggestion– Some patients may not tolerate parameter changes during a session unless the PAUSE
key is used to ramp stimulation down prior to making a change.
Technical Tip – Cycle Time: The contraction / rest cycle time. Options are displayed with the
contraction ON time first followed by the rest time. A 10 / 50 cycle time means a 10 second
contraction time followed by a 50 second rest time. A continuous cycle time may be used when using
this waveform for relaxation of muscle spasms.
CC
Technical Specifications for Russian Current
Channels: 1 - 4
Intensity CC: 100 mA Peak
Intensity CV: 100 Volts Peak
Carrier Frequency: 2500 Hz
Burst Frequency: 20-100 Hz
Duty Cycle: 10% to 50%
Russian Waveform: Sine Wave Bursts
36
Page 41

High Voltage Pulsed Current (HVPC) - Monophasic
Twin-Peak
• Introduction
• Quick Start
• Setup Procedure
- Modifying Power-up Presets Parameters
• Preparation for Treatment
• Detailed Treatment Procedures
- Treatment Cautions
• Modifying Treatment Parameters
• Technical Specifications
1
Introduction to HVPC
HVPC stimulators have output ranges between 300-500 volts. Knowledge of the voltage alone does
not inform the user about the physiological responses attainable. The ability to assess peak
current can help determine tissue response, as too much average current can cause negative effects.
The Forte CPS HVPC is designed to deliver very short duration pulses which are very low in charge or
power output. Treatment can be administered using either pads or probes and
intensity displays are available in Volts or Peak Current.
Twin-Peak High Volt current is available from Channel 1 & 2 from Forte 200 series products and all
Channels with the 400 series of products.
1
Quick Start
The following is a quick step by step procedure for using HVPC stimulation. Before proceeding refer
to treatment cautions.
Procedure Comment
Turn power on The unit will go through self diagnostics, followed by the main menu.
Prepare treatment site Follow steps in preparation for treatment.
Position electrodes Secure electrodes around area to be treated.
Press “HIVOLT” To select High Volt stimulation.
Set “Intensity” Adjusting intensity will affect amplitude for both Channels.
Press “Start” To begin treatment.
End Treatment Remove electrodes and inspect treatment area.
37
Page 42

Setup procedure: High Voltage Pulsed Current (HVPC)
Follow this sequence of events to begin single Channel HVPC stimulation:
Step 1. Press “HIVOLT” and set intensity of displayed Channel
100 pps
Step 2. Press “START” to begin treatment.
Modifying Power-up Preset Parameters
With the exception of output intensity all power-up preset parameters can be modified using the
following sequence of steps:
1. Select and change desired parameter setting, next press “ENTER”.
2. Next press and hold the “PAUSE” button, then press the “ENTER” button.
Preparation for Treatment
Refer to section three to become familiar with the following:
Indications, Contraindications, Warnings, Precautions and Adverse Effects for HVPC current.
Detailed Treatment Procedures
Remember that most patients are unfamiliar with electrical stimulation and may be anxious or
apprehensive during the initial sensation of current. If the patient is extremely fearful, either
discontinue the treatment or set the “Amplitude” at a level where the patient just feels the current.
Allow the individual to become accustomed to the current before increasing the intensity.
Treatment Cautions:
• Never turn the power on or off while the unit is connected to the individual.
• Stop the treatment before removing or attaching electrodes or leads.
• Leads and electrodes should be applied to the patient before a treatment is initiated.
• Never use leads or electrodes that are damaged or worn; this may result in injury to the patient.
• Consult published sources for electrode placements, settings and treatment duration.
• Make sure all electrodes make full contact with the patient’s skin.
38
Page 43

Application of High Voltage Pulsed Current (HVPC) Using Electrodes
1. Place the power switch in the “On” position.
The display screen will indicate the machine is going through its power-up procedures and
system checks. When completed, the main menu will appear on the screen.
2. Adhere electrodes to the location to be treated.
Refer to guidelines under preparation for treatment earlier in this section.
The Red lead wire is the Treating/Active electrode. Example: If the polarity is set on Positive, the
Red lead wire is Positive and if the polarity is set on Negative, then the Red lead wire now has a
Negative polarity.
3. Press “HIVOLT” for HVPC.
Refer to the information on indications and contraindications in section three.
4. View and verify the preset parameters for HVPC.
Preset parameters for single channel HVPC; Method: Pads, Polarity: Positive, Cycle Time:
Continuous, Sweep: Continuous, Frequency: 50 pps, Ramp: 2 sec., Display: Voltage.
5. To modify treatment parameters prior to starting treatment:
• Use the up and down arrows located next to the “ENTER” button to navigate through the
parameters available for HVPC.
• Once you have highlighted a desired parameter, press “ENTER” to display a list of options
available for this mode of treatment.
• Use the arrows once again to highlight a desired option and then press “ENTER”.
6. Set “POWER/INTENSITY” using the controls located on the control panel.
The amplitude is displayed digitally on the LED adjacent the intensity control button.
7. Press “START” to initiate the treatment.
LED treatment timer will begin to display elapsed time and Main Menu will reappear updated
with waveform, Channels in use and available as well as elapsed time.
8. To modify settings during treatment: It is recommended that you Pause the treatment session prior to
implementing a parameter change.
• Select the Channel to modify from the Main Menu.
• Press “ENTER” to display parameters of that Channel on both the LCD and LED displays.
• You may want to choose “PAUSE” to interrupt treatment or lower the intensity prior to
making changes in treatment parameters to prevent the possibility of undesirable sensation
that could be perceived with a change in parameters.
• Once you have highlighted a desired parameter, press “ENTER” to display a list of options
available for this mode of treatment.
• Use the arrows once again to highlight a desired option and then press “ENTER” to accept.
• Use the Treatment Time Control to alter treatment time from the arrows on the control panel.
• Press “START” to resume treatment.
9. Terminating Treatment
When treatment ends, a tone will sound and the intensity will ramp to 0. Remove electrodes
from patient and inspect area of skin treated.
39
Page 44

Modifying Treatment Parameters
Treatment parameters can be modified prior to or during a treatment session. To modify High Voltage
Pulsed Current (HVPC) or any session parameters, the session parameters must be displayed on the
LCD and LED respectively.
Modify LED functions: Treatment Time and Output Intensity
Select the channel you wish to modify and Treatment Time and Output will be displayed digitally on
the display LEDs.
1
1
Suggestion:
Some patients may not tolerate parameter changes during a session unless the “PAUSE” key is used
to ramp stimulation down prior to making a change.
Modify LCD functions:HVPC
Prior to or while a session is in progress, you can modify any of the LCD waveform parameters as
desired.
The following describes the general progression for changing parameters:
The left side of the screed lists the parameters that can be modified.
Move the highlight box to the desired parameter and press “ENTER”, a list of options* is displayed
on the right side of the screen.
Move the highlight box to the desired option and press “ENTER”.
*If no list of options is displayed, this indicates that there are less than four choices and you may
select them by continuing to press “ENTER”.
HVPC Parameter Options
Method Pads, Probes
Polarity Positive or Negative
Cycle Time 5/5, 10/10, 10/20, 4/12, 10/30, 10/50 or Continuous
Sweep Continuous,1-10 Hz, 80-150 Hz or 1-150 Hz
Frequency 0-120 pps
Ramp 0.5, 1, 2 or 5 sec.
Display (Voltage or Peak Current)
40
Page 45

100 pps
Technical Tip – Polarity: You can set the polarity of the red active lead wire in relation to the black
reference lead wire. The red active lead wire delivers the treatment current while the black
reference lead wire completes the patient circuit.
Suggestions – Some patients may not tolerate parameter changes during a session unless the PAUSE
key is used to ramp stimulation down prior to making a change.
Technical Tip – Cycle Time: The contraction / rest cycle time. Options are displayed with the
contraction ON time first followed by the rest cycle. The continuous cycle time option gives
continuous stimulation output. A 10 / 50 cycle time means a 10 second contraction time followed by
a 50 second rest time.
Sweep: The system automatically changes the frequency with in a selected range.
Display: A unique feature that will display output as Voltage or Peak Current.
High Voltage Pulsed Current (HVPC) Application with Hand Held Probes
(Optional)
1. Place the power switch in the “On” position.
The display screen will indicate the machine is going through its power-up procedures and
system checks. When completed, the main menu will appear on the screen.
2. Press “HIVOLT” as the desired treatment from the control panel.
3. Attach the probe to the red lead wire from active channel.
4. Apply an electrode to the black lead wire and place on the patient in an area near the treatment site.
5. Change method from Pads (preset) to probes:
Use the up and down arrows located next to the “ENTER” button to navigate through the
parameters available for HVPC.
Once you have highlighted a desired parameter, press “ENTER” to display a list of options
available for this mode of treatment.
Use the arrows once again to highlight a desired option and then press “ENTER”.
6. Set “POWER/INTENCITY” using the controls located on the control panel.
The amplitude is displayed digitally on the LED adjacent to the intensity control button.
7. Press “START” to initiate the treatment.
LED treatment timer will begin to display elapsed time and Main Menu will reappear updated
with waveform, Channels in use and available as well as elapsed time.
41
Page 46

8. To modify settings during treatment:
• Refer to “modify settings during treatment” described earlier in this section.
9. Terminating Treatment:
• When treatment ends, a tone will sound and the intensity will ramp to 0.
• Remove electrodes from patient and inspect area of skin treated.
Technical Specifications for High Voltage Pulsed Current (HVPC)
Description: The output is a fast rising twin-peak pulse with approximately 75 microseconds spacing
between peaks.
Pulse duration: The first peak is 5 microseconds at one-half pulse height and second peak is 8
microseconds at one-half pulse height. These measurements are with load impedance of 1,000 ohms.
Pulse duration varies according to body impedance (load resistance).
Channel: 1 & 2 (Forte 200 Stim and Combo) and 1, 2, 3 & 4 (Forte 400 Stim and Combo)
Voltage Current: 0-500 Volts CV
Frequency: 1-120 pulses per second
Maximum Average Current Density at Electrodes: 23 microamps/mm2(3” electrode)
Maximum Power Density at Electrode: 69 microwatts/mm2(3” electrode)
High Voltage Pused Waveform:
VMS™ - Symmetrical Biphasic Square Wave
• Introduction
• Quick Start
• Setup Procedure
- Modifying Power-up Presets Parameters
• Preparation for Treatment
• Detailed Treatment Procedures
- Treatment Cautions
• Modifying Treatment Parameters
• Technical Specifications
42
Page 47

Introduction to VMS™
A symmetrical biphasic square wave that treats the tissue under each electrode equally. Its single
pulses have the same physiologic function as the beat frequency of the interferential current. Low
total current makes this waveform good for comfortable submaximal muscle contractions.
Quick Start
The following is a quick step by step procedure for using VMS™ stimulation. Before proceeding refer
to treatment cautions.
Procedure Comments
Turn power on The unit will go through self diagnostics, followed by the main menu.
Prepare treatment site Follow steps in preparation for treatment.
Position electrodes Secure electrodes in a criss-cross fashion around area to be treated.
Press “VMS” To select VMS™ stimulation.
Set “Intensity” Set the output intensity for the selected channel.
Press “Start” To begin treatment.
End Treatment Remove electrodes and inspect treatment area.
Setup procedure: VMS™
Follow this sequence of events to begin single channel VMS™ stimulation:
Step 1. Press “VMS” and set intensity of displayed Channel
CC/CV
Step 2. Press “START” to begin treatment.
CC
Modifying Power-up Preset Parameters
With the exception of output intensity all power-up preset parameters can be modified using the
following sequence of steps:
1. Select and change desired parameter setting, next press “ENTER”.
2. Next press and hold the “PAUSE” button, then press the “ENTER” button.
43
Page 48

Preparation for Treatment
Refer to section three to become familiar with the following:
Indications, Contraindications, Warnings, Precautions and Adverse Effects for High Voltage Pulsed
Current (HVPC).
Detailed Treatment Procedures
Remember that most patients are unfamiliar with electrical stimulation and may be anxious or
apprehensive during the initial sensation of current. If the patient is extremely fearful, either
discontinue the treatment or set the “Amplitude” at a level where the patient just feels the current.
Allow the individual to become accustomed to the current before increasing the intensity.
Treatment Cautions:
• Never turn the power on or off while the unit is connected to the individual.
• Stop the treatment before removing or attaching electrodes or leads.
• Leads and electrodes should be applied to the patient before a treatment is initiated.
• Never use leads or electrodes that are damaged or worn; this may result in injury to the patient.
• Consult published sources for electrode placements, settings and treatment duration.
• Make sure all electrodes make full contact with the patient’s skin.
1. Place the power switch in the “On” position.
The display screen will indicate the machine is going through its power-up procedures and
system checks. When completed, the main menu will appear on the screen.
2. Connect electrodes to lead wires and plug into jacks for Channels selected.
Certain Channels are designated for specific modes of treatment.
3. Adhere electrodes to the location to be treated.
Refer to guidelines under preparation for treatment earlier in this section.
4. Select “VMS” for a symmetrical biphasic square wave current.
Refer to the information on indications and contraindications in section three.
5. View and verify the preset parameters for VMS™ current.
Preset parameters for VMS™ stimulation; Mode: Single Channel, Cycle Time: 10/30, Frequency:
50 pps, Ramp: 2 sec, Phase Duration: 200 mSec.
6. To modify treatment parameters prior to starting treatment:
• Use the up and down arrows located next to the “ENTER” button to navigate through the
parameters available for VMS™ current.
• Once you have highlighted a desired parameter, press “ENTER” to display a list of options
available for this mode of treatment.
• Use the arrows once again to highlight a desired option and then press “ENTER”.
7. Set “POWER/INTENCITY” using the controls located on the control panel.
The amplitude is displayed digitally on the LED adjacent the intensity control button.
8. Press “START” to initiate the treatment.
VMS™ stimulation will ramp up to set output values. The Main Menu will reappear, displaying
Channel(s), waveform(s) in use with elapsed time.
44
Page 49

9. To modify settings during treatment: It is recommended that you Pause the treatment session prior to
implementing a parameter change.
• Select the Channel to modify from the Main Menu.
• Press “ENTER” to display parameters of that Channel on both the LCD and LED displays.
• You may want to choose “PAUSE” to interrupt treatment or lower the intensity prior to
making changes in treatment parameters to prevent the possibility of undesirable sensation
that could be perceived with a change in parameters.
• Once you have highlighted a desired parameter, press “ENTER” to display a list of options
available for this mode of treatment.
• Use the arrows once again to highlight a desired option and then press “ENTER” to accept.
• Use the Treatment Time Control to alter treatment time with the arrows on the control panel.
• Press “START” to resume treatment.
10. Terminating Treatment
When treatment ends, a tone will sound and the intensity will ramp to 0. Remove electrodes
from patient and inspect area of skin treated.
Modifying Treatment Parameters
Treatment parameters can be modified prior to or during a treatment session. To modify VMS™ or any
session parameters, the session parameters must be displayed on the LCD and LED respectively.
Modify LED functions: Treatment Time and Output Intensity
Select the Channel you wish to modify and Treatment Time and Output will be displayed digitally on
the display LEDs.
Suggestion
Some patients may not tolerate parameter changes during a session unless the “PAUSE” key is used
to ramp stimulation down prior to making a change.
Modify LCD functions: VMS™
Prior to or while a session is in progress, you can modify any of the LCD waveform parameters as
desired.
The following describes the general progression for changing parameters:
The left side of the screed lists the parameters that can be modified.
Move the highlight box to the desired parameter and press “ENTER”, a list of options* is displayed
on the right side of the screen.
*If no list of options is displayed, this indicates that there are less than four choices and you may
select them by continuing to press “ENTER”.
45
Page 50

VMS™ Parameter Options
Mode Single, Reciprocal* or CoContract*
Cycle Time 5/5, 10/10, 10/20, 4/12, 10/30, 10/50 or Continuous
Frequency 5-200 pps
Ramp 0.5, 1, 2 or 5 sec.
Phase Dur. 20-300 msec.
CC/CV
Technical Tip – Single Channel: One Channel (two electrodes) positioned to stimulate a muscle or
muscle group. Reciprocal: Two Channels (four electrodes) positioned to stimulate the same or opposing
muscle groups. CoContract: Two Channels (four electrodes) positioned to stimulate the opposing
muscle groups simultaneously.
Suggestion – Some patients may not tolerate parameter changes during a session unless the “PAUSE”
key is used to ramp stimulation down prior to making a change.
Technical Tip – Cycle Time: The contraction/rest cycle time. Options are displayed with the contraction
ON time first followed by the rest cycle. A 10/30 cycle time means a 10 second contraction time
followed by a 30 second rest time. A continuous cycle time may be used when using this waveform for
relaxation of muscle spasms.
CC
Technical Specifications for VMS™
Symmetrical Biphasic Description: Symmetrical biphasic rectangular pulse with interphase interval
between the positive and negative phases
Channels: 0-200mA Peak
Intensity CC: 0-200 mA Peak
Intensity CV: 0-200 Volts Peak
Pulse Frequency: 5-200 Biphasic Pairs / Second
Phase Duration: 20-300 microseconds
Interphase Interval: 100 microseconds
VMS Wave Forms: Symmetrical Biphasic Rectangular Waveform:
46
Page 51

VVMMSS™™ WWaavvee FFoorrmmss:: SSyymmmmeettrriiccaall BBiipphhaassiicc RReeccttaanngguullaarr WWaavveeffoorrmm
PPhhaassee DDuurraattiioonn
PPuullssee RRaattee
US - Ultrasound
• Introduction
• Quick Start
• Setup Procedure
- Modifying Power-up Preset Parameters
• Indication
• Contraindications
• Precautions
- Ultrasound Applicators
- Electronic Signature
- Head Warming
• Detailed Treatment Procedures
• Modifying Treatment Parameters
• Technical Specifications
™
IInntteerrpphhaassee IInntteerrvvaall
PPuullssee TTrraaiinn
Introduction to Ultrasound Therapy
Utilizing ultrasound waves through muscle, nerve and connective tissue has been well documented as
effective in reducing pain, muscle spasms and joint contractures.
There are 3 ultrasound applicators available with the Forte CPS Combo: 2 cm2, 5 cm2and 10 cm2.
Frequency of 1 MHz or 3.3 MHz may be selected both before and during treatment.
47
Page 52

Quick Start
The following is a quick step by step procedure for using ultrasound (US). Before proceeding refer to
treatment cautions.
Procedure Comments
Turn power on The unit will go through self diagnostics, followed by the main menu.
Apply ultrasound gel Follow steps in preparation for treatment.
Press “US” To select Ultrasound.
Set “Intensity” Set desired intensity for your treatment.
Press “Start” To begin treatment.
End Treatment Clean skin area of residual gel.
Setup procedure: ULTRASOUND
Follow this sequence of events to begin a 1 MHz Ultrasound application:
Step 1. Press “US” for ultrasound. Set ultrasound output intensity
Step 2. Press “START” to begin treatment.
Modifying Power-up Preset Parameters
With the exception of output intensity all power-up preset parameters can be modified using the
following sequence of steps:
1. Select and change the desired parameter setting, then press “ENTER”.
2. Next press and hold the “PAUSE” button, then press the “ENTER” button.
Indications
Ultrasound for use in applying deep heat can be used for treatment of selected medical conditions
such as the relief of pain, muscle spasms and joint contractures. These conditions may be associated
with adhesive capsulitis, bursitis with slight calcification, myositis and soft tissue injuries. The Forte
CPS Combo can provide therapeutic deep heating between 40°C and 45°C in all of its operating
modes, while using any of the applicators available for this device.
48
Page 53

Contraindications
••
This device should not be used for symptomatic local pain relief unless etiology is established or
unless a pain syndrome has been diagnosed.
••
This device should not be used when cancerous lesions are present in the treatment area.
••
This device should not be used when open wounds are present in the treatment area.
••
Other contraindications are patients suspected of carrying serious infectious disease and or disease
where it is advisable, for general medical purposes, to suppress heat or fevers.
••
This device should not be used over or near bone growth centers until bone growth is complete.
••
This device should not be used over the thoracic area if the patient is using a cardiac pacemaker.
••
This device should not be used over a healing fracture.
••
This device should not be used over or applied to the eye.
••
This device should not be used over a pregnant uterus.
••
This device should not be used on ischemic tissues in individuals with vascular disease where the
blood supply would be unable to follow the increase in metabolic demand and tissue necrosis
might result.
••
Patients with an implanted neurostimulation device must not be treated with or be in close
proximity to any shortwave diathermy, microwave diathermy, therapeutic ultrasound diathermy or
laser diathermy anywhere on their body. Energy from diathermy (shortwave, microwave, ultrasound
and laser) can be transferred through the implanted neurostimulation system, can cause tissue
damage and can result in severe injury or death. Injury, damage or death can occur during
diathermy therapy even if the implanted neurostimulation system is turned "off."
Precautions
Precautions should be taken when used:
• For acute conditions of bursitis and tendonitis that can be exacerbated by the use of ultrasound.
• Over an area of the spinal cord following a laminectomy (i.e., when major covering tissues have
been removed).
• On patient’s with a tendency toward hemorrhaging.
Handle the ultrasound applicator(s) with care:
• Do not drop the sound head on hard surfaces.
• Do not allow the sound head to reach maximum temperatures repeatedly.
• Do not cool an overheated sound head with ice water or ice packs.
• All of these conditions are likely to damage the sound head crystal.
• Damage resulting from these conditions is not covered under the warranty.
Electronic Signature
Chattanooga Group’s electronic signature allows you to interchange sound heads without the need for
a lengthy calibration procedure.
™
Head Warming
Head Warm is a unique feature standard in both the Forte 200 Combo and 400 Combo. When
activated, the applicator head will warm to a comfortable temperature setting slightly above normal
skin temperature and maintain that range for as long as it is left on or the unit powered down. Head
warm is fully activated after it is turned on from the ultrasound parameter screen and the user returns
to the Main Menu. At the Main Menu you will see WARM adjacent to the Ultrasound Channel.
Additionally, you will notice the LED output displaying 2 watts of output to rapidly warm the applicator
to slightly above skin temp. Output will drop to a minimal maintenance level to keep applicator head
warm.
49
Page 54

Detailed Treatment Procedures
1. Connect sound head to ultrasound by pressing cable plug into the receptacle marked US.
2. Place the power switch in the “ON” position.
The display screen will indicate the machine is going through its power-up procedures and
system checks. When completed, the main menu will appear on the screen.
3. Select “Ultrasound” from the control panel.
4. View and verify the preset parameters for Ultrasound.
Preset parameters for ultrasound; Frequency: 1 MHz, Duty Cycle: Continuous, Display: w/cm
Head Warm: Off
5. To modify parameter settings prior to starting treatment:
• Use the up and down arrows located next to the “ENTER” button to navigate through the
parameters available for Ultrasound.
• Once you have highlighted a desired parameter, press “ENTER” to display a list of options
available for this mode of treatment.
• Use the arrows once again to highlight a desired option and then press “ENTER”.
6. Prepare the area to be treated by applying an ultrasound coupling agent to the patient’s skin.
7. Place the sound head in contact with the patient’s body with a firm, uniform pressure.
You must keep the sound head moving during the treatment. Failure to keep the sound head
moving may result in hazardous exposure to the ultrasound energy.
2
,
8. Set “POWER/INTENCITY” using the controls located on the control panel.
Use the intensity control buttons located on the control panel to increase the ultrasound
intensity to the desired level.
9. Press “START” to initiate the treatment.
If desired, changes may be made to treatment parameters by moving the highlight to the
Channel marked U. The operator then can proceed with changes as desired.
10. To modify settings during treatment:
• Select the channel to modify from the Main Menu.
• Press “ENTER” to display parameters of that channel on both the LCD and LED displays.
• Choose “PAUSE” to interrupt treatment or lower intensity prior to making changes in the
treatment parameters to prevent an undesirable sensation by your patient.
• Once you have highlighted a desired parameter press “ENTER” to display a list of options
available for this mode of treatment. When the highlight is placed around Frequency or Display
pressing “ENTER” will toggle to the next selection.
• Use the arrows once again to highlight a desired option and then press “ENTER” to accept.
• Use the Treatment Time Control to alter treatment time.
• Press “START” to resume treatment.
11. When treatment time has expired a tone will sound.
• Wipe excess coupling media from patient’s skin and ultrasound head.
• Press the “STOP” button to end treatment early.
50
Page 55

Modifying Treatment Parameters
Treatment parameters can be modified prior to or during a treatment session.
Modify LED functions: Treatment Time and Output Intensity
Select the Channel you wish to modify and Treatment Time and Output will be displayed.
Modify LCD functions: ULTRASOUND
Prior to or while a session is in progress, you can modify any of the LCD waveform parameters as
desired.
The following describes the general progression for changing parameters:
The left side of the screed lists the parameters that can be modified.
Move the highlight box to the desired parameter and press “ENTER”, a list of options* is displayed
on the right side of the screen.
*If no list of options is displayed, this indicates that there are less than four choices and you may
select them by continuing to press “ENTER”.
Ultrasound Parameter Options
Frequency 1 MHz or 3.3 MHz
Duty Cycle 10%, 20%, 50% or Continuous
2
Display W/cm
or Watts
Head Warm Off or On
Technical Tip – Frequency: The ultrasound frequency is measured in cycles per second and is
determined by the size and shape of the crystal. All the available Forte CPS sound heads (2 cm
2
5 cm2and 10 cm2are capable of providing 1 or 3.3 MHz frequencies.
Duty Cycle: Continuous or pulsed settings are available.
,
51
Page 56

Technical Tip – Display: Ultrasound output can be displayed in Watts or Watts/cm2(W/cm2). You will
see a value change in the Output LED read out when you toggle the display from the preset
watts/cm
displayed as 6.0 Watts if display is changed.
Technical Note – Head Warm: From the parameter screen head warm is turned on and off. Head warm
is activated when you return to the main menu, where the display will say WARM. You will notice the
LED output displaying 2 watts of output to rapidly warm the applicator to slightly above skin temp.
Output will drop to a 2-watt maintenance level to keep applicator head warm.
2
to Watts. For example, when using a 5 cm2head, an output value of 1.5 W/cm2will be
Technical Specifications for Ultrasound
Channel: U (Ultrasound)
Ultrasound Frequency: 1.0 MHz ± 5%
3.3 MHz ± 5%
Duty Cycle: 100% (Continuous Mode)
50% (Pulsed Mode)
20% (Pulsed Mode)
10% (Pulsed Mode)
Pulse Duration: 5 msec ± 20% (50% duty cycle, pulsed mode)
2 msec ± 20% (20% duty cycle, pulsed mode)
Ultrasonic Power: Variable from 1 watt to 20 watts, 10 cm2crystal
Variable from 0.4 watt to 10 watts, 5 cm2crystal
Variable from 0.2 watt to 4 watts, 2 cm2crystal
Output Meter Accuracy: ±20% for any output above 10% of maximum
Temporal Peak/Average Intensity Ratio: 2:1 ± 20% for 50% Duty Cycle
5:1 ± 20% for 20% Duty Cycle
9:1 ± 20% for 10% Duty Cycle
Output: Continuous: 1 MHz or 3.3 MHz nominal signal that is
activated as long as the timer is operating.
Pulsed: 1 MHz or 3 MHz signal, modulated 100% by
the 100 Hz rectangular wave with the selected
Duty Cycle.
Timer Accuracy: ±0.2 minute
Sound Head: Effective Radiating Area:
8.5 cm2± 1.5 cm2for the 10 cm2crystal
4.0 cm2± 1.0 cm2for the 5 cm2crystal
1.8 cm2-0.4/+0.2 cm2for the 2 cm2crystal
Maximum beam non-uniformity ratio: < 5.0:1
Beam type: Collimating
52
Page 57

Description of Ultrasonic Field
The spatial distribution of the radiated field is essentially a collimated beam of the ultrasonic energy
having a cross sectional area of 8.5 cm
the transducer face.
The energy distribution within the radiated field is 2.4 W/cm
shape having decreasing intensity at progressively increasing distance from the face of the transducer.
This field distribution applies for the radiation emitted into the equivalent of an infinite medium of
distilled, degassed water at 30° C and with the line voltage variations in the range of 10% of the rated
line voltage.
2
for 10 cm2sound head when measured at a point 5 mm from
2
maximum and it takes a generally conic
Abbreviations
The following abbreviations are used on the sound head of the Forte CPS ultrasound:
Area = Effective Radiating Area
Coll. = Collimating
BNR = Beam Non-Uniformity Ratio
Freq. = Frequency
COMBO - Combination Therapy
• Introduction
• Quick Start
• Indications/ Contraindications
• Warnings/ Precautions
• Preparation for Treatment
• Detailed Treatment Procedures
• Modify Treatment Parameters
• Setting Up a Second Treatment
- Quick Start
• Simultaneous Treatments
Introduction to COMBO
The “Combo” mode of treatment includes an ultrasound treatment combined with Premodulated
(PREMOD), Interferential (IFC), High Voltage Pulsed Current (HIVOLT) or VMS. In this treatment the
aluminum face of the ultrasound head becomes one half of the electrical circuit. The circuit is completed by an electrode attached to the
rreedd lleeaadd wwiirree
plugged into
53
““CChhaannnneell 22..””
Page 58

Quick Start
The following is a quick step by step procedure for using Combo with PREMOD stimulation. Before
proceeding refer to treatment cautions.
Procedure Comments
Turn power on The unit will go through self diagnostics, followed by the main menu.
Prepare treatment site Follow steps in preparation for treatment.
Position electrodes Secure electrodes around area to be treated. (Channel 2 Red lead wire)
Press “Combo” To select Combination mode.
Set “Waveform” Preset to Premod on Channel 2.
Coupling Agent Apply Ultrasound gel to the area to be treated.
Set “Intensity” Set ultrasound output intensity, followed by premod intensity.
Press “Start” To begin treatment.
End Treatment Remove electrodes, clean and inspect treatment area.
Combination Therapy Parameter Options
The following sequence of events are followed to begin Combination treatment with PREMOD:
Step 1. Press “COMBO” set Ultrasound Output intensity on Ch. U and press “ENTER”.
Step 2. Set Premod output intensity displayed on Channel 2 and press “START”.
CC/CV
Step 3. Press “START” to begin treatment.
CV
54
Page 59

Modifying Power-up Preset Parameters
With the exception of output intensity all power-up preset parameters can be modified using the
following sequence of steps:
1. Select and change the desired parameter setting, then press “ENTER”.
2. Next press and hold the “PAUSE” button, then press the “ENTER” button.
Indications/Contraindications
The operator should be familiar with the operating procedures for ultrasound and therapeutic electrical
stimulation as well as indications, contraindications, precautions and warnings prior to using the
combination set up.
Warnings/Precautions
• Do not use combination therapy for underwater treatments. Placing active electrodes
underwater poses a serious hazard to the patient.
• Just as with therapeutic electrical stimulation treatments, current travels between the two
electrodes. In the case of combination stim / sound the ultrasound head becomes one of the
active electrodes. As a result the ground or dispersive electrode should not be placed in a
location that will cause current to travel through a contraindicated area.
• Make sure the sound head is making contact with the patient’s skin before pressing “START”.
Otherwise, the stim current may cause the patient some discomfort upon initial contact.
Maintain contact of the sound head with the skin surface during the entire combination
treatment; this will prevent an increase in current density which may be uncomfortable to the
individual.
• Be on the alert for signs of periosteal (bone) pain or any other unusual reactions to this method
of treatment.
Preparation for Treatment
Refer to section three to become familiar with the following:
• Indications and contraindications for interferential, premodulated, VMS™ and High Voltage
Pulsed Current.
• Preventing adverse effects of therapeutic electrical stimulation.
Detailed Treatment Procedures
1. Place the power switch in the “ON” position.
The display screen will indicate the machine is going through its power-up procedures and
system checks. When completed, the main menu will appear on the screen.
2. Select “COMBO” from the control panel.
3. Select one of the combo preset waveforms; “PREMOD” (Preset), “IFC”, “HIVOLT” or “VMS”.
• After selecting a waveform, view the electrical stim summary screen to confirm your selection.
Channel 2 will automatically be selected for “COMBO”, but you will also use Channel 1 if you
choose Interferential.
• Attach the electrode Red #2 lead wire to the patient. It may be placed in the treatment area if it
is similar in size to the sound head surface area (bipolar technique) or in a non-treatment area if
it is larger than the sound head and acts to disperse the current (monopolar technique).
55
Page 60

4. View and verify the preset parameters for Combination treatments.
•
Ultrasound
Cycle: Continuous, Display: W/cm
•
Premodulated
– Ultrasound preset parameters used in Combo mode; Frequency: 1 MHz, Duty
2
, Head Warm: Off, Treatment Time: 5 minutes.
– Premodulated preset parameters used in Combo mode; Channel used: Ch. 2,
Beat Frequency: 80-150 Hz, Treatment time: 5 minutes.
•
Interferential
– Interferential preset parameters used in Combo mode; Amplitude modulation:
40% Scan, Beat
Frequency: 80-150 Hz, Treatment Time: 5 minutes
•
HIVOLT
– Preset parameters for single Channel High Volt current; Method: Pads, Polarity:
Positive, Cycle Time: Continuous, Sweep: Continuous, Frequency: 50 pps, Ramp: 2 sec.,
Display: Voltage Time: 5 minutes.
•
VMS
– Preset parameters for VMS stimulation; Mode: Single Channel, Cycle Time: 10/30,
Frequency: 50 pps, Ramp: 2 sec, Phase Duration: 200 msec., Treatment Time: 5 minutes.
5. To modify parameter settings prior to starting treatment:
• Use the up and down arrows located next to the “ENTER” button to navigate through the
parameters available for the selected current.
• Once you have highlighted a desired parameter, press “ENTER” to display a list of options
available for this mode of treatment.
• Use the arrows once again to highlight a desired option, then press “ENTER”.
• Highlight Ch. Select and press “ENTER”. Ultrasound parameters should be displayed.
6. Apply an ultrasound coupling agent to the patient’s skin.
Place the sound head in contact with the patient’s body with a firm, uniform pressure. You must
keep the sound head moving during the treatment. Failure to keep the sound head moving may
result in hazardous exposure to the ultrasound energy.
7. Set “INTENCITY” in the following order:
Use the “POWER/INTENCITY controls located on the control panel to set the ultrasound level.
Keep the sound head moving as you increase the power setting. Next press “ENTER”.
Set output intensity of the selected waveform and press “START”.
8. Press “START” to initiate the treatment timer.
9. To modify settings during treatment: It is recommended that you Pause the treatment session prior to
implementing a parameter change.
• Press the channel to modify from the Main Menu.
• Press “ENTER” to display parameters of that channel on both the LCD and LED displays.
• You may want to choose “PAUSE” to interrupt treatment or lower the intensity prior to
making changes in treatment parameters to prevent the possibility of undesirable sensation
that could be perceived with a change in parameters.
• Once you have highlighted a desired parameter, press “ENTER” to display a list of options
available for this mode of treatment.
• Use the arrows once again to highlight a desired option and then press “ENTER” to accept.
• Use the Treatment Time Control to alter treatment time.
• Press “START” to resume treatment.
10. When treatment time has expired, a tone will sound.
The amplitude will ramp to zero, remove electrodes from patient and wipe excess coupling
agent from skin and sound head.
56
Page 61

Modifying Treatment Parameters
Please refer to Ultrasound Modify Treatment Parameters section and Premod Modify
Treatment Parameters section for modification parameters.
Setting Up a Second Treatment
After setting up the first treatment, you may wish to set up a second (or third) treatment. Select the
waveform you wish to use by pressing the appropriate button on the control panel. You may choose
the same waveform s selected for the first treatment (for example, PREMOD and PREMOD) or a
different waveform. When you select a mode of treatment, the Main Menu will be displayed on the
LCD screen to let you know if this mode is available and which Channel(s) to use.
Once you have chosen a waveform and have plugged the lead wires into the appropriate Channels,
follow the quick start instructions (below); or you may want to refer to the designated section in this
manual (for the current selected) for more details concerning setting up your patient.
Quick Start
The following is a quick step by step procedure for setting up a second treatment. Before proceeding,
refer to the treatment cautions of the selected treatment.
Procedure Comments
Prepare treatment site Follow steps in preparation for treatment.
Position electrodes Secure electrodes around area to be treated.
Set “Intensity” Adjust amplitude for the Channel selected.
Press “Start” To begin treatment.
End Treatment Remove electrodes, clean and inspect treatment area.
To modify treatment parameters prior to starting treatment:
• Use the up and down arrows located next to the “ENTER” button to navigate through the
parameters available for the selected current.
• Once you have highlighted a desired parameter, press “ENTER” to display a list of options
available for this mode of treatment.
• Use the arrows once again to highlight a desired option, then press “ENTER”.
Stop One Treatment
• If you press the “STOP” button on the control panel, the treatment highlighted on the Main
Menu will be stopped immediately. If you want to stop a specific treatment, allowing the other
treatments to continue, then perform the following:
- Select the specific treatment from the Main Menu.
- Press “STOP” to discontinue the selected treatment.
57
Page 62

Simultaneous Treatments
The Forte™ CPS Stim and Combo allows you to perform a variety of simultaneous treatments.
Simultaneous treatments should not be mistaken for combination treatments. Combination
treatments blend ultrasound with Premodulated, Interferential, VMS™ or High Voltage Pulesed
Current (HVPC) into a single treatment. Simultaneous treatments are separate and independent which
may be delivered to one or more patients at the same time.
As many as 5 different treatments may be performed at the same time when using the 400 Combo
and 3 when using the 200 combo. Simultaneous treatments available through the Forte CPS are listed
below:
Note: Any reference to Channel 3 or 4 is associated with the Forte CPS (Stim or Combo) 400,
reference to Ultrasound is associated with the Forte CPS Combo 200 or 400 unit.
IFC - Interferential
• Channels 1 & 2
• Channels 3 & 4
• Ultrasound (sound output)
PREMOD - Premodulated
• Channel 1, 2, 3 or 4
• Ultrasound (sound output)
MICRO - Microcurrent
• Channel 1, 2, 3 or 4 (pads) (Probes available on Channel 1 only)
• Ultrasound (sound output)
RUSSIAN
• Channel 1, 2, 3 or 4 (single)
• Channels 1 & 2 (reciprocal or co-contraction)
• Channels 3 & 4 (reciprocal or co-contraction)
• Ultrasound (sound output)
High Voltage Pulsed Current (HVPC)
• Channel 1, 2, 3 or 4 (probes or pads)
• Ultrasound (sound output)
VMS™
• Channel 1, 2, 3 or 4 (single)
• Channels 1 & 2 (reciprocal or co-contraction)
• Channels 3 & 4 (reciprocal or co-contraction)
• Ultrasound (sound output)
US - Ultrasound
• In combination with interferential, Premodulated, VMS™ or HVPC
• As a stand alone treatment
58
Page 63

Clinical Protocols™
• Introduction
• Setup procedure
Introduction
Clinical Protocols provides the clinician with a library of general application presets. To access the
Clinical Protocols, press the Clinical Protocol button. Select the desired preset from the provided list
and press ENTER. The user is given the opportunity to alter the preset parameters to the patient’s
needs to prior to initiating treatment.
The protocols contained in this section are to be used only as guidelines. Each patient should be
individually assessed to determine the appropriateness of the parameter settings prior to use.
Setup Procedure
Navigation example
1. Press “Clinical Protocol” button to access the library.
2. Choose Anatomical Location, press “ENTER”.
3. Choose Indication or Modallity, press “ENTER”.
4. Make a selection from on-screen choices, press “ENTER”.
5. Set Intensity, press “START” button.
All parameters can still be changed before or during a treatment.
Appendix
• Warranty
• System Utilities
• System Trouble Shooting
• Maintenance
• Screen Prompts
59
Page 64

Forte CPS Two Year Limited Warranty
The Chattanooga Group (“Company) warrants that the Forte™ CPS Model 200 or 400 series
(“Product”) excluding accessories is free of defects in material and workmanship. This warranty shall
remain in effect for two(2) years from the date of the original consumer purchase of this and extends
to any owner of the product during the warranty period. Accessories that are included as standard
with the product (as listed in the users manual) are warranted for 90 days. Ultrasound applicators (2
2
, 5 cm2or 10 cm2) as included with the Combo (Combination) units are warranted for one(1) year.
cm
If this product fails to function during the two year warranty period because of a defect in material or
workmanship, the company or the selling dealer will replace or repair this product without charge
within a period of 30 days from the date on which the defective product is returned to the company
or the dealer. The company or the dealer will ship the replacement or the repaired product to the
owner.
All repairs must be performed by a service center authorized by the Chattanooga Group. Any
modifications or repairs performed by unauthorized centers or groups will void this warranty. To
participate in warranty coverage, the products warranty registration card (included with the product)
must be filled out and returned to the Chattanooga Group by the original owner within 10
business days of purchase.
This warranty does not cover:
Replacement parts or labor furnished by anyone other than the Company, the dealer or an approved
Company service agent.
Defects or damage caused by labor furnished by someone other than Company, the dealer or an
approved Company service agent.
Any malfunction or failure in the Product while it is in the possession of the owner during the
warranty period if the malfunction of failure is not caused by a defect in material or workmanship or if
the malfunction or failure is caused by unreasonable use, applications in which the product was not
intended or the failure to prove reasonable and necessary maintenance.
To Obtain Service
From Company or the selling dealer under this warranty, the owner must do or abide by the following:
• A written claim must be made within the warranty period to Company or the selling dealer.
• If the claim is made to the Company, the written claim should be sent to:
4717 Adams Rd., P.O. Box 489
Hixson, TN 37343
Phone: (800) 592-7329, outside the US: +1 (423) 870-2281
• The Product must be returned to Company or the selling dealer by the owner.
This warranty gives you specific legal rights and you may also have other rights which vary from state
to state.
The Company does not authorize any person or representative to create for it any other obligation or
liability in connection with the sale of the Product. Any representative or agreement not contained in
the warranty shall be void and of no effect.
60
Page 65

System Utilities
Adjust Screen Contrast / LCD Intensity:
Press and hold the “MAIN MENU/ESCAPE” button. Use the “POWER/INTENCITY up or down arrow
keys to adjust contrast.
Modifying Power-up Preset Parameters
With the exception of output intensity, all power-up preset parameters can be modified using the
following sequence of steps.
1. Select and change the desired parameter setting, then press “ENTER”.
2. Next press and hold the PAUSE button, then press the “ENTER” button.
Interferential Quad Balance Control & Unit Patient Interrupt Switch
The Patient Control Center is configured such that the patient can press either of the buttons (A & B)
to stop a treatment session. It is important to note that when either of these buttons are pressed all
active Channels of stimulation output will be ramped to zero and elapsed treatment times will be
paused.
The Patient Control Center is also used as a balance control for interferential stimulation. When
selected for use, the Quad Balance function will impact the percentage of set amplitude of the active
electrodes.
A. Patient Interrupt Switch. When pressed, this will stop stimulation output in all operating Channels.
B. Patient Interrupt Switch. When pressed, this will stop stimulation output in all operating Channels.
C. Thumbwheel provides fine-tuning of interferential output amplitude balance.
D. Thumbwheel provides fine-tuning of interferential output amplitude balance.
E. Manually position interferential amplitude balance.
Interferential Quandrand Balance functions to change the percent of maximum set amplitude levels
determined by the position of the control lever. Below is a close up schematic of the actual quandrand
control lever and diagram of percentage levels. The percentage change will range from 100% to 60%.
61
Page 66

Maintenance Instructions
To fully maintain compliance with Federal Regulation Title 21 (21 CFR), this unit must be recalibrated
annually. It is recommended that all Chattanooga Group ultrasound products be returned to the factory
or an authorized servicing dealer for repairs or recalibration. It is also recommended after the
replacement or repair of any major component.
The following items should be checked at least monthly to ensure proper operation of this unit:
• Power cord and plug: Check to make sure the cord is not frayed, kinked or does not have torn or
cut insulation.
• Sound head cable: Check to make sure the cable is flexible, free of kinks, not frayed and the
insulation is intact.
• Sound head face: Check to see that there is no build-up of gel or foreign material on the
aluminum face.
• Lead Wires: Check that the cables are not frayed, kinked or do not have torn or cut insulation.
Screen Prompts
The following messages are displayed in the middle of the screen called the “OK Window.” The
screen message is displayed as seen below, the corrective action message presented in this section
will help you to correct the situation.
Overcurrent on Channel #
An excessive change in the current has occurred. This means that the change between current
values has exceeded the Forte preset limit. When this prompt occurs, the Channel (and all
associated Channels on this run) are stopped.
Corrective Action – Check electrodes and leads. Replace if defective.
Ultrasound overcurrent fault!
An overcurrent fault has occurred on the ultrasound. This means that the current has exceeded the
built in limits of the Forte System for that particular sound head size. When this error occurs, the
Channel (and all associated Channels on this run) are stopped.
Corrective Action – Restart unit using lower intensity.
Patient Terminated Treatment.
The patient has pushed the patient safety switch. This immediately stops all active Channels.
Corrective Action – Attend to patient. Check operation of unit. If malfunctioning, return for repair.
62
Page 67

Ultrasound already active!
The user just pressed the US - ultrasound button from the main screen, but the ultrasound is
already running.
Corrective Action – Only one ultrasound treatment at a time is possible.
Sound head not connected.
The user just pressed the US - ultrasound button from the main screen, but there is no ultrasound
sound head currently plugged in.
Corrective Action – Attach correct sound head and fully seat the cable plug in the sound head
connector.
Channels needed for this Mode are Busy
The output Channels needed for this waveform are occupied with another treatment. The user just
pressed a stim button from the main screen but all stim Channels are currently running.
Corrective Action – It is not possible to select Channels which are already selected.
One Wire Device Failure
Not communicating with the ultrasound applicator.
Corrective Action – Check connection of the ultrasound applicator to the unit or the ultrasound
applicator head may need service.
No Head Detected
No ultrasound applicator head is connected or applicator head is damaged.
Corrective Action – Check the connection quality of the ultrasound jack to the unit or ultrasound
applicator head needs service.
HHH displayed on the output LED
The temperature of the ultrasound head has exceeded 140° F. The ultrasound output ceases and
treatment time freezes until the head cools off and START button is pressed.
Corrective Action – Stop ultrasound application and let applicator head cool.
Setting Up Channel Please Wait
Selected electrotherapy session preparing to start.
Corrective Action – None required.
Reading Calibration Data
System is reading the ultrasound applicator head calibration.
Corrective Action – None required.
63
Page 68

Glossary
Amplitude Modulation – The amplitude of one Channel is automatically reduced by a selected amount
(% of set amplitude) and then brought back to the set point in the time designated. This mode is also
called Scan. When two Channels are used they alternate moving from maximum to minimum. The
effect is that the stimulation scans the painful area to be treated.
Beam Non-uniformity Ratio – By nature an ultrasound beam is not homogeneous. The BNR is a ratio of
the highest intensity found in the beam field to the average intensity as indicated on the output
display of the unit. This measure may not exceed 5.0:1. Because of the areas of increased intensity,
the sound head is moved continuously during the treatment.
Beat Frequency – Refers to frequency created when two interferential sine waves cross in the body. It
is equal to the difference between the frequencies of the two original waves.
Burst Frequency – A burst is created when a train of pulses or cycles is interrupted. The burst
frequency is the number of bursts that occur per second.
Carrier Frequency – The original frequency of the interferential sine wave entering the skin. One
Channel will be this value and the other will be equal to the carrier frequency plus the beat frequency.
Combo – This term refers to Combination Therapy. The sound head emits ultrasound and acts as a
moving electrode to simultaneously deliver electrical stimulation to the area. The sound head
completes the electrical circuit with the lead marked with a dot from Channel 2.
Constant Current (CC) – The output waveform maintains its set current amplitude level as prescribed by
the clinician. If resistance increases during a treatment, the unit will automatically increase the voltage
to maintain the current amplitude. The CC setting is commonly used when a goal of the selected
waveform is to elicit a muscle contraction.
Constant Voltage (CV) – If the output waveform encounters resistance, the set voltage remains the
same, causing the current amplitude to decrease. The CV setting is commonly used when a goal of
therapy is traditional pain management or sensory level stimulation.
Continuous Mode (Ultrasound) – The output of the ultrasound is not interrupted during the treatment
time. This mode imparts the most energy to the tissues and is used when a maximal thermal effect is
desired. (See Duty Cycle)
Contract – The time amplitude is held at its maximum in the muscle contraction mode. This is the time
that will be most fatiguing to the muscle.
Coupling Media – An agent used to insure that the ultrasound is transmitted from the sound head to
the tissue to be treated. Gels or lotions labeled for therapeutic ultrasound use is recommended.
Duty Cycle – This is the ratio of the “On” time to “Total” time of the cycle, expressed as a percentage.
The duty cycle describes the burst-on time in the Russian Waveform and the pulsed modes of
ultrasound. The lower the percentage, the lower temporal average intensity. 100% is continuous
ultrasound.
Effective Radiating Area (ERA) – A measure of the ultrasound beam made underwater, 5 mm from the
radiating surface of the sound head. The ERA is always smaller than the geometric area of the sound
head, but should be as close as possible. This measurement is used to calculate the ultrasound
intensity in w/cm
Frequency (Stimulation) – The number of pulses delivered per second (pps) or cycles per second (Hz).
Frequency (Ultrasound) – Selectable to 1 or 3.3 MHz with the 5 cm
frequency, the longer the wavelength, the deeper the penetration of ultrasound.
2
.
2
sound head. The lower the
64
Page 69

High Voltage Pulsed Current (HVPC)– Monophasic pulse type with a double peak shape. High Volt is
designed to deliver
very short duration pulses, which are very low in charge or power output.
Treatment can be administered using either Pads (electrodes) or probes and intensity displays are in
Volts or Peak Current. Twin-Peak High Volt current is available from Channel 2 on the Forte 200 Series
and Channel 2 or 4 on the 400 Series.
Interferential – Two sine waves (2 Channels) of different frequencies are used. Electrodes typically
surround the area of pain in a criss-cross manner. An interference pattern is created where the two
Channels cross, treating the tissue with a third frequency (the difference between the two original
frequencies) called the Beat Frequency.
Interphase Interval – The time in microseconds between the two phases when there is no current flow.
Lead Zirconate Titanate – A synthetic crystal used to create the ultrasound beam by vibrating 1,000,000
(1 MHz) to 3,300,000 (3.3 MHz) times per second. This type of crystal is both durable and efficient in
its func duration is the same.
Microcurrent – A Monophasic square wave whose amplitude is less than 1 mA. Positive (+) or negative
(-) polarity can be used or a mode where the polarity automatically alternates (+/-) to reduce a net DC
effect.
Phase Duration – Time in microseconds for current to flow in one direction. In the VMS Waveform both
phases are symmetrical, thus equal in time. In a Monophasic Waveform, the phase duration and pulse
Polarity – The current flow may be directed in the positive or negative direction in the High Volt and
microcurrent waveforms. In microcurrent, it can also alternate polarity at a rate designated by the
alteration time.
Power – A measure of the intensity of the ultrasound delivered to the patient. Unit of measure is watts
2
(W) or w/cm
.
Premodulated – A single sine wave with modulated amplitude. This waveform is similar to the beat
frequency, the pattern created by the interferential current, but can be delivered through one Channel.
At low frequencies comfortable twitch contractions can be produced.
Presets (Clinical Protocol System) – A library consisting of 100 preset parameters.
Pulsed Mode (Ultrasound) – The output of the ultrasound is automatically interrupted during the treat-
ment time. This limits the amount of energy delivered to the tissues and is used primarily for the
minimizing of the thermal effects of ultrasound (See Duty Cycle).
Ramp Up – The time it takes for the amplitude to go from 0 to set value in the muscle contraction
mode and any time the stimulation is initiated or resumed. This improves the comfort of the
stimulation.
Ramp Down – The time it takes for the amplitude to go from set value to 0.
Rest – The time amplitude is at 0 in the muscle contraction mode. It provides for muscle recovery in
between contractions. An adequate rest time minimizes fatigue. A good “rule of thumb” is three or
four times longer than the contraction time.
Russian – An interrupted sine wave used for maximal motor recruitment. Strong muscle contractions
can be generated with better tolerance due to the parasthesia created by the carrier frequency.
Scan – A term used in the interferential and other pain management waveforms that refers to an
amplitude modulation mode.
Sound Head (Applicator) – The applicator used for delivery of ultrasound to the patient. An aluminum
face contracts the patient’s skin. It covers a transducer mechanism that converts electrical energy to
mechanical energy in the form of a vibrating crystal.
65
Page 70

Sweep – A term used in the interferential and other pain management waveforms that refers to a
frequency modulation mode. The frequency automatically changes or “sweeps” from the HIGH to the
LOW value and back in the time designated.
Ultrasound Intensity – Ultrasound power delivered to the patient expressed in total power as watts (W)
2
or in terms of the sound head’s effective radiating area, watts per centimeter squared (W/cm
).
VMS™ – A symmetrical biphasic square wave that treats the tissue under each electrode equally. Its
single pulses have the same physiologic function as the beat frequency of the interferential current.
Low total current makes this Waveform good for comfortable submaximal muscle contractions.
66
Page 71

Page 72

IISSOO 1133448855 CCEERRTTIIFFIIEEDD
4717 Adams Road
P.O. Box 489
Hixson, TN 37343
1-800-592-7329
1-423-870-2281
www.chattgroup.com
©2004 Encore Medical
78081I
 Loading...
Loading...