CDC Laboratory Assessment of Antibiotic User Manual

Laboratory Assessment of Antibiotic
Resistance Testing Capacity
User’s Guide and Questionnaire
VERSION 2.0
AUGUST 2020
CS320242-A
Division of Healthcare Quality Promotion

LAARC User’s Guide and Questionnaire
ersion 2.0
V
August 2020
The Laboratory Assessment of Antibiotic Resistance Testing Capacity is a publication of the Division of
Healthcare Quality Promotion in the National Center for Emerging and Zoonotic Infectious Diseases within the
U.S. Centers for Disease Control and Prevention (CDC).
U.S. Centers for Disease Control and Prevention
Robert Redfield, MD, Director
National Center for Emerging and Zoonotic Infectious Diseases
Rima Khabbaz, MD, Director
Division of Healthcare Quality Promotion
Denise Cardo, MD, Director
Photo Credit: Daniella Coker
Cover page photo features (l-r) Dr. Hein Bui of CDC, Vietnam; Mr. Truong Nguyen, a healthcare informatics
consultant in Vietnam; and Dr. Mai Van Tuan, a clinical microbiologist in Hue, Vietnam. They are examining a
lidded and sealed non-infectious petri dish in an anteroom of a non-CDC laboratory in Vietnam.
Suggested citation:
Centers for Disease Control and Prevention. Laboratory Assessment of Antibiotic Resistance Testing Capacity.
Atlanta, GA: U.S. Department of Health and Human Services, CDC; 2020. Available at:
https://www.cdc.gov/drugresistance/intl-activities/laarc.html.
Version 2 | August 2020 Page 1 of 90

LAARC User’s Guide and Questionnaire
ACKNOWLEDGEMENTS
Susan Bollinger (International Infection Control Program, Division of Healthcare Quality Promotion, U.S. Centers
for Disease Control and Prevention, Atlanta, Georgia, USA) led the overall development of the LAARC
questionnaire and coordinated the piloting and revision of the tool in collaboration with internal and external
stakeholders. She also provided expert subject matter input to the development of the Excel scoring tool. Sonya
Arundar and Joyce Thomas (Division of Healthcare Quality Promotion, CDC) provided professional editing (plain
language and usability) assistance.
Antoine Pierson (Integrated Quality Laboratory Services, IQLS, Lyon, France) led the development of the Excel
scoring tool and provided expert subject matter input on the LAARC content to optimize the use of the scoring
tool. Additional support was provided by Abdoulaye Nikièma (IQLS).
The following experts participated in technical consultations to guide the development and provided technical
review of the tool: Rachel Smith, Ulzii Luvsansharav, Nora Chea, Michael Omondi, T.J. McKinney (Division of
Healthcare Quality Promotion, CDC), Michele Parsons (Division of Global Health Protection, CDC).
The following experts piloted the tool in resource-limited settings and provided technical expertise and
feedback: Nino Macharashvili, Lan Nguyen, Hien Bui, Valan Siromany, Wangeci Gatei, Molly Freeman, Pawan
Angra (Division of Global Health Protection, CDC). Lynee Galley, Emma Muir, Martin Evans, John TarBush, John
Aldom, Abdul Chagla, Vlademir Cantarelli, Victor Silva, American Society for Microbiology (ASM); Mona ElShokry,
Dana Itani, Walaa Khater, the World Health Organization (WHO); and Lindsey Shields, Rogers Kisame, Moctar
Mouiche, (FHI360).
Funding for the development of the Excel scoring tool was provided by the Division of Global Health Protection
in the Center for Global Health through a Cooperative Agreement.
DISCLAIMERS
All rights reserved. Publication of the Centers for Disease Control and Prevention is available on the U.S. CDC
website Lab Assessment of Antibiotic Resistance Testing Capacity (LAARC)
(https://www.cdc.gov/drugresistance/intl-activities/laarc.html) or can be obtained from Centers for Disease
Control and Prevention, 1600 Clifton Rd., Atlanta, GA, 30329, USA (email:
The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed
or recommended by the Centers for Disease Control and Prevention in preference to others of a similar nature
who are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by
initial capital letters.
The contents of the LAARC are solely the responsibility of the authors and do not necessarily represent the official
views of the U.S. Centers for Disease Control and Prevention. All reasonable precautions have been taken to
verify the information contained in this publication. However, the published material is being distributed without
warranty of any kind, either expressed or implied. The responsibility for the interpretation and uses of the
material lies with the reader. In no event shall the Centers for Disease Control and Prevention or IQLS be liable for
damages arising from its use.
IICP@cdc.gov).
Version 2 | August 2020 Page 2 of 90

LAARC User’s Guide and Questionnaire
TABLE OF CONTENTS
ACKNOWLEDGEMENTS ..............................................................................................................................................2
DISCLAIMERS ..............................................................................................................................................................2
TABLES AND FIGURES .................................................................................................................................................4
ACRONYMS .................................................................................................................................................................5
EXECUTIVE SUMMARY ................................................................................................................................................7
1. INTRODUCTION .................................................................................................................................................7
1.1. Rationale .................................................................................................................................................7
1.2. Purpose ...................................................................................................................................................7
1.3. Scope .......................................................................................................................................................8
2. ASSESSMENT PLANNING and PREPARATION ................................................................................................ 10
2.1. Assessment Team ................................................................................................................................ 10
2.2. Team Preparation ................................................................................................................................ 10
2.3. Laboratory Preparation ........................................................................................................................ 11
2.4. Assessment Process ............................................................................................................................. 11
2.4.1. Take GPS Location ...................................................................................................................... 11
2.4.2. Meet with staff ........................................................................................................................... 11
2.4.3. Tour the laboratory .................................................................................................................... 12
2.4.4. Review Documents and Fill the Questionnaire .......................................................................... 12
2.4.5. Professionalism .......................................................................................................................... 12
3. LAARC TOOL STRUCTURE ............................................................................................................................... 13
3.1. Files ...................................................................................................................................................... 13
3.2. Excel File Organization ......................................................................................................................... 13
3.2.1. Yellow: ....................................................................................................................................... 13
3.2.2. Blue: Questionnaire (15 tabs) .................................................................................................... 13
3.2.3. Questionnaire Organization and Structure ................................................................................ 14
4. Red: ........................................................................................................................................... 15
3.2.
4. ENTERING DATA INTO THE EXCEL TOOL ........................................................................................................ 16
4.1. Generate a Unique Filename ............................................................................................................... 16
4.2. Select Language ................................................................................................................................... 16
4.3. Answer Questions ................................................................................................................................ 16
4.3.1. Drop-down boxes ....................................................................................................................... 16
4.3.2. Free-text cells and Comment cells ............................................................................................. 17
4.3.3. Color Coding ............................................................................................................................... 17
5. SCORING SYSTEM........................................................................................................................................... 18
5.1. Questions ............................................................................................................................................. 18
Version 2 | August 2020 Page 3 of 90

LAARC User’s Guide and Questionnaire
5.2. Indicators and Modules ....................................................................................................................... 18
5.3. Flags ..................................................................................................................................................... 19
6. RESULTS: SUMMARY, FLAGS, CONCLUSION and PHOTOS ............................................................................ 20
6.1. Summary tab ........................................................................................................................................ 20
6.2. Flag tab ................................................................................................................................................. 20
6.3. Conclusion tab ..................................................................................................................................... 21
6.4. Photograph tab .................................................................................................................................... 21
7. INTERPRETING RESULTS and DEVELOPING A WORK PLAN ........................................................................... 21
8. EXPORTING DATA .......................................................................................................................................... 22
9. REFERENCES ................................................................................................................................................... 24
Appendix 1: Sample Letter ...................................................................................................................................... 25
Appendix 2: Recommended resources .................................................................................................................... 27
Culture and Identification .............................................................................................................................. 27
AST/AMR ........................................................................................................................................................ 27
Quality Control ............................................................................................................................................... 27
Laboratory Quality Management Systems (QMS) ......................................................................................... 27
Laboratory Biosafety ...................................................................................................................................... 28
Appendix 3: LAARC Questionnaire .......................................................................................................................... 29
TABLES AND FIGURES
Table 1: GLASS priority specimens and pathogens for surveillance of AR .................................................................8
Table 2: GLASS priority pathogen-antimicrobial combinations for surveillance of AR by priority pathogen ............9
Table 3: Sample agenda........................................................................................................................................... 11
Table 4: LAARC Questionnaire Modules .................................................................................................................. 13
Table 5: Description of Module Structure ............................................................................................................... 15
Figure 1: Module architecture and organization ................................
Figure 2: Numeric Responses .................................................................................................................................. 17
Figure 3: Example of a clarifying comment ............................................................................................................. 17
Figure 4: Color coding in the Module tabs .............................................................................................................. 17
..................................................................... 15
Figure 5: Module and Indicator score examples ..................................................................................................... 18
Fi
gure 6: Question, Indicator, and Module Scoring ................................................................................................ 19
Figure 7: Flag Examples ........................................................................................................................................... 20
Figure 8: Color coded heat map for the Module: Safety Appendix and its four Indicators .................................... 20
Figure 9: Flag Tab ..................................................................................................................................................... 21
Figure 10 : Geographical representation of indicators ........................................................................................... 23
Version 2 | August 2020 Page 4 of 90
LAARC User’s Guide and Questionnaire
Abbreviation
Term
AMR/AR
Antimicrobial Resistance
AST
Antibiotic Susceptibility Testing
ATCC
American Type Culture Collection
BMD
Broth microdilution
BSL
Biosafety Level
CAP
College of American Pathologists
CDC
U.S. Centers for Disease Control and Prevention (Atlanta)
CIP
Collection de l'Institut Pasteur
CLSI
Clinical & Laboratory Standards Institute
CRE
Carbapenem-Resistant Enterobacteriaceae
CSF
Cerebrospinal Fluid
CSV
Comma Separated Value
EQA
External Quality Assessment
ESBL
Extended-spectrum beta-lactamase
EUCAST
European Committee on Antimicrobial Susceptibility Testing
GHSA
Global Health Security Agenda
GLASS
Global Antimicrobial Resistance Surveillance System
GPS
Global Positioning System
HIS
Hospital Information System
ICR
Inducible Clindamycin Resistance
ID
Identification
ILAC
International Laboratory Accreditation Cooperation
IQLS
Integrated Quality Laboratory Services
ISO
International Standardization Organization
LAARC
Laboratory Assessment of Antimicrobial Resistance Testing Capacity
LIS
Laboratory Information System
LQSI
Laboratory Quality Stepwise Implementation
MALDI
Matrix Assisted Laser Desorption Ionization
MCIM
Modified carbapenem inactivation method
MIC
Minimal Inhibitory Concentration
MRSA
Methicillin-Resistant Staphylococcus aureus
NA
Not Applicable
NCTC
National Collection of Type Cultures
NLF
Non-Lactose Fermenting
NRL
National Reference/Referral Laboratory
PCR
Polymerase Chain Reaction
PPE
Personal Protective Equipment
PT
Proficiency Testing
QA
Quality Assurance
QC
Quality Control
QMS
Quality Management Systems
SLIPTA
Stepwise Laboratory Quality Improvement Process Towards Accreditation
SOP
Standard Operating Procedure
ACRONYMS
Version 2 | August 2020 Page 5 of 90

LAARC User’s Guide and Questionnaire
Abbreviation
Term
STD
Sexually Transmitted Disease
TB
Tuberculosis
VISA
Vancomycin-Intermediate Staphylococcus aureus
VRE
Vancomycin-Resistant Enterococci
VRSA
Vancomycin-Resistant Staphylococcus aureus
WHO
World Health Organization
Version 2 | August 2020 Page 6 of 90
LAARC User’s Guide and Questionnaire
Questionnaire content is based on internationally
EXECUTIVE SUMMARY
The Laboratory Assessment of Antibiotic Resistance Testing Capacity (LAARC) was designed for use in resource
limited settings to:
• Evaluate the technical skill and expertise of clinical bacteriology laboratories
• Evaluate the quality management practices related to bacterial identification and antibiotic susceptibility
testing (AST)
• Generate numerical indicators of quality and capacity in fifteen domains of laboratory practice
• Aid development of workplans for improvement
• Monitor the status of laboratory improvements over time
The specimen types, organisms and antibiotics
addressed by the tool are based on the priorities set
by WHO Global Antimicrobial Resistance Surveillance
System (GLASS) in 2015.
Assessments using the LAARC require two full days to
complete. Due to the technical nature of the
questions, assessments must be carried out by
bacteriologists with ample AST experience and strong
familiarity with clinical bacteriology laboratory
requirements and standards, which may differ from
research, industrial, environmental or veterinary laboratory standards.
accepted standards of clinical laboratory practice
including:
• International Organization for Standardization
(ISO)
• European Committee on Antimicrobial
Susceptibility Testing (EUCAST)
• Clinical & Laboratory Standards Institute (CLSI)
• The World Health Organization (WHO)
e LAARC scoring tool is a Microsoft (MS) Excel® file. It does not contain macros, thus, it can be used on any
Th
computer and works independently from operating system type and language. The tool is currently available in
English, French, Spanish, and Portuguese. Additional languages may be added to the translation table by the
end-user, including non-Latin alphabets.
1. INTRODUCTION
Control of antibiotic resistance (AR) is a global public health priority. Strong AR laboratory networks are
critical to inform policy and control efforts. Such networks often obtain AR data from clinical laboratories;
thus, the usefulness of the aggregate data largely depends on the ability of the laboratories to produce
accurate and reliable bacterial identification (ID) and antibiotic susceptibility testing (AST) results.
1.1 Rationale
Many existing laboratory assessment tools are designed to evaluate the quality management system
(QMS) requirements described by international laboratory standards organizations (e.g., ISO and
CLSI). These tools are inadequate to detect deficiencies in bench-level testing because they lack
technical depth and granularity. The LAARC assessment tool is designed to fill that technical gap and
is specifically adapted for laboratories in low- and middle- income countries which have not yet
established comprehensive laboratory regulations and/or accreditation requirements. The tool
contains extensive Quality Control (QC) and Quality Assurance (QA) questions, but it is primarily
technical in nature and does not provide a comprehensive QMS assessment.
1.2 Purpose
The purpose of the LAARC is to objectively evaluate technical proficiency in the bacteriologic
techniques and related quality processes that are required for accurate, reliable AR detection. Results
Version 2 | August 2020 Page 7 of 90

LAARC User’s Guide and Questionnaire
Priority Specimens
Priority pathogens for surveillance
Blood
Escherichia coli
Urine
Escherichia coli
Urethral and cervical swabs
Neisseria gonorrhoeae
provide a clear pathway toward improvement. The LAARC was designed for use in hospital-based
laboratories that receive and process clinical specimens for the purposes of routine patient care.
National reference laboratories (NRLs) and other public health laboratories will benefit from the
technical assessment, but may find certain sections, such as Specimen Collection, less relevant.
Other areas of importance to public health laboratories and institutions are not addressed by this
tool, such as:
• Molecular testing capacity and other advanced techniques (PCR, sequencing, MALDI)
• Packaging, shipping, transport, receiving, and storage following testing
• Participation in laboratory-based surveillance systems (e.g., STDs, TB, enterics, vaccine escape, AR)
• Funding and budget
• Non-laboratory personnel: epidemiologists, data managers and analysts, administrative support staff
• Public health roles: Notifiable Diseases, Outbreak response, provider of EQA/PT
1
A survey
addressing several of these topics was developed by WHO and is publicly available for use
in conjunction with the LAARC to comprehensively assess the AR capacity of NRLs. The LAARC does
not assess the readiness of the national health system to implement AR surveillance. Multiple WHO
2,3, 4,5
tools
are available to assess national health systems.
1.3 Scope
The LAARC was built around the WHO priority AR specimen types, pathogens and antibiotics included in
their Global Antimicrobial Resistance Surveillance System (GLASS) initiative of 2015; see Tables 1 and 2.
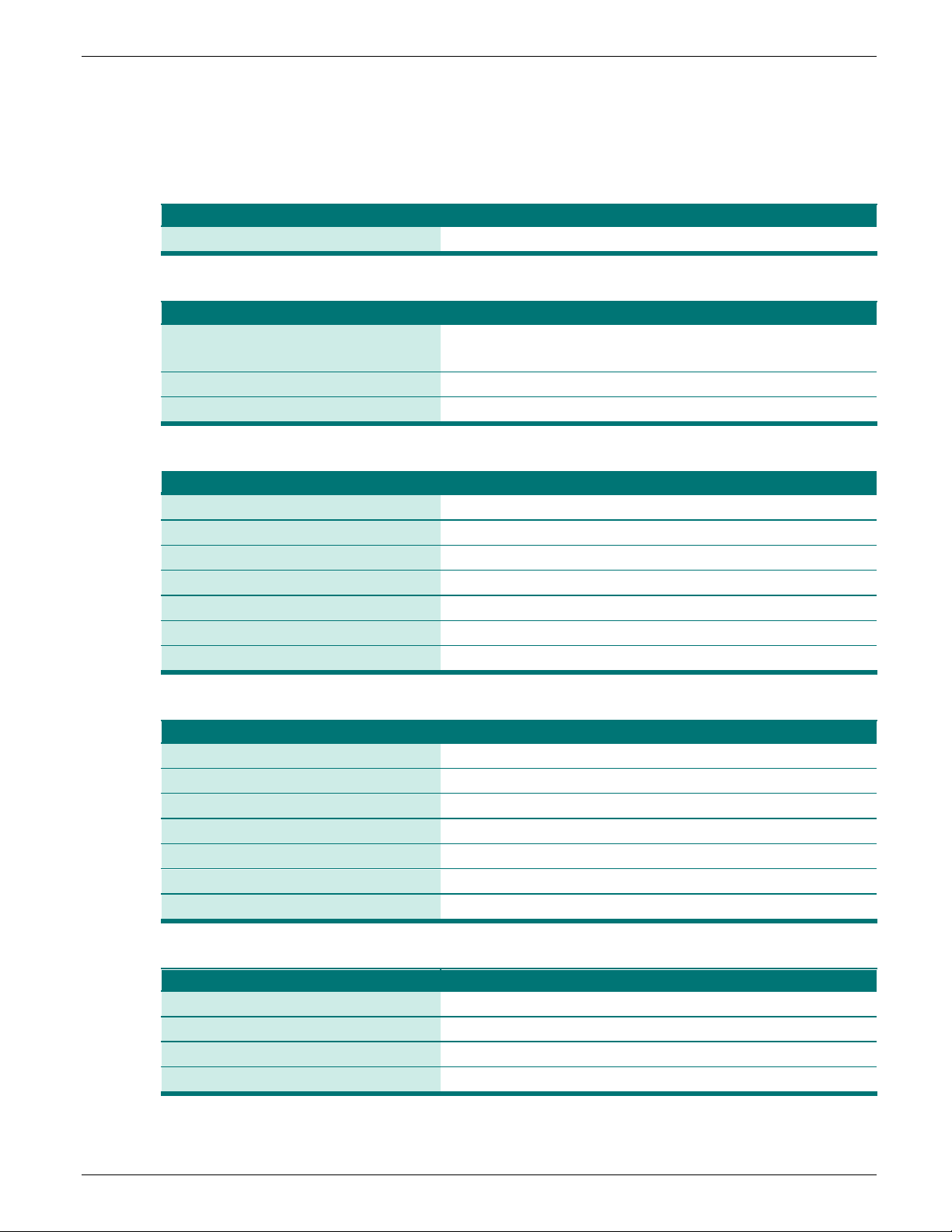
Table 1: GLASS priority specimens and pathogens for surveillance of AR
Klebsiella pneumoniae
Acinetobacter baumannii
taphylococcus aureus
S
Streptococcus pneumoniae
Salmonella spp.
Klebsiella pneumoniae
Feces/Stool
Salmonella spp.
Shigella spp.
*
Many labs are unable to definitively differentiate Acinetobacter calcoaceticus from A. baumannii, so in practice this refers to
Acinetobacter calcoaceticus-baumannii complex
†
N. gonorrhoeae was excluded from this tool due to the complexities involved with routine culture and recovery, identification and
AST, and the existence of other surveillance networks and STD clinics dedicated exclusively to this pathogen.
*
†
1
Laboratory Assessment Questionnaire for Antimicrobial Resistance Testing (https://extranet.who.int/dataform/549586?lang=en)
2
WHO AR Surveillance Questionnaire for Assessment of National Networks [PDF - 24 pages] (https://www.who.int/antimicrobial-
resistance/whocdscsrrmd20031.pdf)
3
WHO Laboratory Assessment Tool / System Questionnaire [PDF - 42 pages] (https://www.who.int/ihr/publications/Annex1_LAT.pdf)
4
WHO GLASS Implementation Questionnaire [PDF - 6 pages] (http://apps.who.int/iris/bitstream/10665/251558/1/WHO-DGO-AR-
2016.10-eng.pdf)
5
WHO GLASS Core Components Checklist [PDF - 35 pages] (https://apps.who.int/iris/bitstream/handle/10665/251552/WHO-DGO-AMR-
2016.5-eng.pdf)
Version 2 | August 2020 Page 8 of 90
LAARC User’s Guide and Questionnaire
Antibacterial class
Antibacterial agents
Penicillinase-stable beta-lactams
Cefoxitin
Antibacterial class
Antibacterial agents
Penicillin G
Sulfonamides and Trimethoprim
Co-trimoxazole
Third-generation cephalosporins
Ceftriaxone or cefotaxime
Antibacterial class
Antibacterial agents
Penicillins
Ampicillin
Third-generation cephalosporins
Ceftriaxone or Cefotaxime + Ceftazidime
Fourth-generation cephalosporin
Cefepime
Carbapenems
Imipenem, Meropenem, Ertapenem, Doripenem
Fluoroquinolones
Ciprofloxacin or Levofloxacin
Sulfonamides and Trimethoprim
Co-trimoxazole
Polymyxins
Colistin
Antibacterial class
Antibacterial agents
Penicillins
Ampicillin
Third-generation cephalosporins
Ceftriaxone or Cefotaxime + Ceftazidime
Fourth-generation cephalosporin
Cefepime
Fluoroquinolones
Ciprofloxacin or Levofloxacin
Sulfonamides and Trimethoprim
Co-trimoxazole
Polymyxins
Colistin
Antibacterial class
Antibacterial agents
Aminoglycosides
Gentamicin and Amikacin
Carbapenems
Imipenem, Meropenem, Doripenem
Tetracyclines
Tigecycline or Minocycline
Polymyxins
Colistin
Table 2: GLASS priority pathogen-antimicrobial combinations for surveillance of AR
by priority pathogen
The antibiotics listed below are important for AR surveillance purposes. However, they may not be
first-line options for testing or for treatment and should not be interpreted as such.
Staphylococcus aureus
Streptococcus pneumoniae
Penicillins Oxacillin (as a screen for Penicillin resistance)
Escherichia coli
Klebsiella pneumoniae
Carbapenems Imipenem, Meropenem, Ertapenem, Doripenem
Acinetobacter baumannii
Version 2 | August 2020 Page 9 of 90
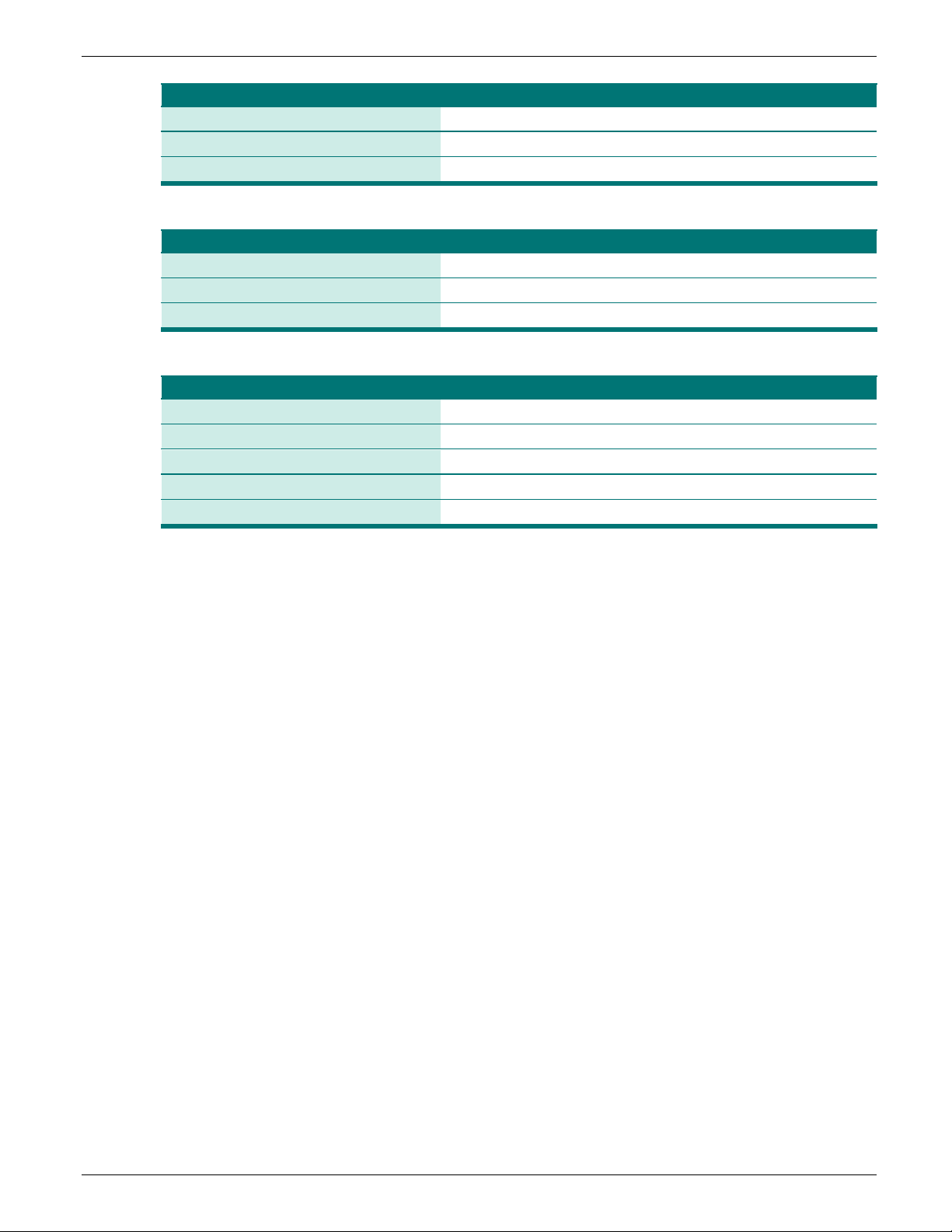
LAARC User’s Guide and Questionnaire
Antibacterial class
Antibacterial agents
Third-generation cephalosporins
Ceftriaxone or Cefotaxime + Ceftazidime
Fluoroquinolones
Ciprofloxacin or Levofloxacin
Antibacterial class
Antibacterial agents
Fluoroquinolones
Ciprofloxacin or Levofloxacin
Macrolides
Azithromycin
Antibacterial class
Antibacterial agents
Aminoglycosides
Gentamicin
Fluoroquinolones
Ciprofloxacin
Macrolide
Azithromycin
Third-generation cephalosporins
Cefixime and Ceftriaxone
Salmonella spp.
Carbapenems Imipenem, Meropenem, Ertapenem, Doripenem
Shigella spp.
Third-generation cephalosporins Ceftriaxone or Cefotaxime + Ceftazidime
Neisseria gonorrhoeae
Aminocyclitols Spectinomycin
Additional culture types, pathogens and antibiotics may be assessed pursuant to national priorities;
however, the current iteration of this tool focuses only those listed in Tables 1 and 2. Users cannot
edit or modify.
2. ASSESSMENT PLANNING and PREPARATION
2.1 Assessment Team
Due to the highly technical nature of the questions, assessments are most effective when carried out
in conjunction with a clinical bacteriologist. This person should be well experienced in the full range
of clinical bacteriology laboratory practices, from specimen collection to AST, and the standard
quality practices associated with each step.
Ideally, all team members, including translators, would have a background in bacteriology laboratory
practices and the general operations of hospitals and clinical laboratories. Preferably, assessors will
also have previous experience with performing laboratory assessments. Persons with expertise that is
primarily research-based or that is grounded in other areas of microbiology (e.g., parasitology,
virology) are not ideal.
2.2 Team Preparation
Read the User’s Guide in full and review all LAARC questions in advance to establish familiarity with
the contents. Seek clarification if needed, decide how to allocate the work, and prepare a translation
if necessary. The questionnaire is provided in both PDF (Appendix 3) and Excel formats. Print the PDF
(approximately 70-80 pages depending on the language) for paper data collection and subsequent
data entry into the Excel tool. Or, enter answers directly into the Excel tool during the assessment.
Allow two full days to complete each assessment. The assessment must be carried out during
laboratory operating hours to observe staff at work. A sample agenda follows:
Version 2 | August 2020 Page 10 of 90
LAARC User’s Guide and Questionnaire
Table 3: Sample agenda
Day 1
8:00 – 8:30 am
• Introductions: Laboratory leadership, other
laboratory staff, and the assessment team
• Review purpose of the evaluation and
expected timeline
8:30 – 9:30 am
• Tour laboratory
• Begin review of pre-assembled documents,
begin filling tool
9:30 – 10:00 am
• Break for tea
10:00 am – Noon
• Continue filling assessment
Noon– 1:00 pm
• Break for lunch
1:00 – 4:30 pm
• Continue filling assessment
7:30 – 09:30 am
• Observe laboratory staff at the bench
• Continue filling assessment
9:30 – 10:00 am
• Break for tea
10:00 am – Noon
• Continue filling assessment
Noon – 1:00 pm
• Break for lunch
1:00 – 2:30 pm
• Complete assessment
2:30 – 3:30 pm
• Summation/exit meeting with laboratory
leadership, other relevant staff
Day 2
Useful (but not required) items include:
• Digital camera: obtain permission before taking photos; avoid capturing patient identifiers
• GPS device: for GPS positioning (can also be performed using a smartphone application)
• Small infrared thermometer: to rapidly check temperatures of refrigerators, rooms, incubators
• Video projector: to share final results with the team, if a projector is not available at the facility
2.3 Laboratory Preparation
At least one week in advance of the assessment, share an agenda with the laboratory so that they
know what to expect and can plan accordingly. Request that the laboratory preassemble key
documents and manuals for review; doing so will save a significant amount of time during the actual
assessment. There is a sample letter containing an agenda and a list of key documents in Appendix A;
send this letter to the laboratory at least one week in advance of the assessment.
2.4 Assessment Process
2.4.1. Take GPS Location
GPS coordinates are not essential but may be useful, especially if performing multiple assessments
throughout a country. Record GPS position in digital degrees, using a GPS device or a smartphone
application. It is also possible to get latitude and longitude from Google Maps®, (but not altitude):
1. Right-click the location on the map
2. Select “What’s here?”
3. A card with latitude (first position) and longitude (second position) will display at the bottom
• If using Maps in “Lite mode,” you’ll see a lightning bolt at the bottom and you won’t be able
to get the coordinates.
4. Record digital degrees to 5 decimals and the + or – sign, if present.
• For example: latitude 41.40338, longitude -2.17403
2.4.2. Meet with staff
Meet briefly with facility and laboratory leadership and staff. Review the agenda and explain the
assessment purpose, process, and expected outcome. Point out that this is not a “regulatory
Version 2 | August 2020 Page 11 of 90

LAARC User’s Guide and Questionnaire
inspection” intended to penalize the laboratory, but a way to discover areas for improvement and
develop a workplan to achieve it. This meeting is also an opportunity to ask about the laboratory
organizational structure, the population served, and any known management issues (staffing,
procurement, equipment, financing, etc.).
2.4.3 Tour the laboratory
After the preliminary meeting, tour the laboratory in the direction described below. Following this
“sample path” provides insight into the general workflow and is an opportunity to ask questions and
observe general cleanliness, organization, and staff adherence to biosafety practices.
• Patient reception/sampling rooms (if the laboratory collects specimens)
• Specimen receiving area (observe specimen storage, data processes, generation of specimen ID)
• Specimen culture plating area (BSC, incubators, blood culture instruments, direct Gram stain
preparation)
• Culture reading and workup area (Gram staining, benches, reagent refrigerators/freezers)
• ID/AST instruments
• Temporary storage and final disposal of culture plates
• Support rooms (e.g., media preparation room, autoclave room, stock room, glassware washroom,
waste management areas)
2.4.4 Review Documents and Fill the Questionnaire
Upon completion of the tour, begin filling out the LAARC questionnaire. Direct questions to the
laboratory manager, quality manager, and both senior and junior bench technologists.
Documentation is key. Confirm answers whenever possible by reviewing the supporting
documentation. Many questions are intentionally designed to require confirmatory documentation.
For example, the question “Do QC records demonstrate that XXX practice is in place?” demands that
the assessor review the pertinent QC records to determine if they meet the defined criteria. This is a
core tenant of quality systems. Without confirmatory documentation, the answer to the question
must be “no,” even if the laboratory claims that the practice is in place.
Partial credit. Some questions have “partial” responses available, but most are either “yes” or “no” for
simplicity of scoring. It may be tempting to mark a question as “yes” when a laboratory partially meets the
criteria, but if the criteria are not fully met and “partial” is not available, then the answer must be “no.”
Marking the response as “no” creates an opportunity for the laboratory to make the changes needed to
become fully compliant. Marking it as “yes” eliminates this opportunity to improve, which is a disservice
to the laboratory. Use the Comment boxes next to each question to add clarifying information.
Research specimens. Many laboratories have equipment, reagents and SOPs that are used for
research specimens but are not used for routine patient specimens. The questions in the LAARC
questionnaire refer only to the equipment, reagents and SOPs that are used with cultures submitted
for clinical patient management in the routine course of care.
2.4.5 Professionalism
Establishing a good relationship with laboratory personnel is vital if recommendations are to be
received well. Give recommendations and advice in a friendly and supportive manner. If there are
findings that may be embarrassing or upsetting for the laboratory, discuss them in private with the
laboratory manager and those in charge. Always obtain permission prior to taking photographs.
Version 2 | August 2020 Page 12 of 90

LAARC User’s Guide and Questionnaire
Modules
# of
Indicators
# of
Questions
0 General
accreditation
6
85
1 Facility
9
135
2 Laboratory Information System (LIS)
3. LAARC TOOL STRUCTURE
3.1 Files
The tool is a combination of three files:
• PDF file containing the User’s Guide and the LAARC questionnaire for printing (available in each
language: English, French, Spanish, Portuguese)
• Multilingual MS Excel tool for data entry and scoring
• (Optional) MS Excel “export reception” file to consolidate output from multiple assessments for
further analysis by statistical software; available in English only
3.2 Excel File Organization
The MS Excel tool has 25 worksheets (or “tabs”) organized into three groups: yellow, blue, and red.
• Yellow tabs contain introductory information
• Blue tabs contain the LAARC questionnaire
• Red tabs contain the assessment results
• Worksheet tabs are titled in English only
3.2.1 Yellow (5 tabs)
• Cover: Cover page
• Introduction: Contextual information about the development and intended use of the tool.
• Language: Language table and mechanism for selecting desired language. New languages may be
added to column F.
Registration: Information about optional registration and link to registration webpage.
•
• Assessor’s guide: Helpful reference materials required to answer specific assessment questions,
including select CLSI and EUCAST breakpoint tables.
3.2.2 Blue: Questionnaire (15 tabs)
The LAARC questionnaire is organized into 15 modules; each module contains 3 to 10 indicators. Each
indicator contains several closed questions.
Table 4: LAARC Questionnaire Modules
Facility demographics, test menu and workload, staffing,
Laboratory conditions, equipment availability, calibration and
maintenance, temperature monitoring, autoclave and inventory
management
Detailed questions about data field configuration and connective
Version 2 | August 2020 Page 13 of 90
capability of electronic data management systems
6 46

LAARC User’s Guide and Questionnaire
Modules
# of
Indicators
# of
Questions
3 Data management
7
73
4 QA
4
45
5 Media QC
6 ID QC
4
113
7 AST QC
systems
5
49
8 Specimen
general specimen management and rejection
5
59
9 Processing
cultures
4
30
10 Identification
methods, kits and automated methods; identification flowcharts
10
185
11 Basic AST
reading and interpreting results and breakpoints standards
6
66
12 AST expert rules
10
107
13 AST policy
antibiograms and stewardship
3
33
Safety
and documentation
4
28
Total
89
1,117
Patient and specimen identification, request forms, culture and
AST data reporting, data backup and sharing
QMS basics, staff competency assessments, troubleshooting
mechanisms and EQA
Routine and specialized media preparation and QC, including
Muller Hinton blood culture bottles and distilled water
Quality Control of bacterial identification systems, including Gram
stain, manual biochemical methods, enteric serologies,
commercial kits and automated identification systems
Quality Control of AST methods including reference strain
maintenance, disc diffusion, gradient strips and automated
6 63
Collection and transport of blood, urine and stool specifically, plus
Plating and inoculation of blood cultures, urine cultures and stool
Quality of SOPs for conventional biochemical identification
Maintenance of discs and strips, inoculum preparation, incubation,
Detailed questions to determine if CLSI and/or EUCAST
breakpoints and AST expert rules for the priority pathogens are
properly applied by the laboratory
Selection and application of routine antibiotic panels, cumulative
Biosafety equipment, safety behaviors, PPE and biosafety training
3.2.3 Questionnaire Organization and Structure
Module structure and components are described in the graphic and table below.
Version 2 | August 2020 Page 14 of 90

LAARC User’s Guide and Questionnaire
Number
Description
1
Module Name – Module names are in blue; each module is numbered
2
Module Score – Module scores are blue; explained in section 5 of the User’s Guide
3
Comments – Empty cells where you may enter free text comments for each question, if
4
Indicator Name – Indicators have a black background with white letters
5
Indicator Score – Indicator scores are color coded; explained in section 5
6
Question Numbers – Leading number corresponds to the Module, the second is sequential
Response Cells – Grey cells contain drop-down boxes with the optional responses to each
question
Question Scores
9
Flags – Responses to some questions generate “flags”; explained in section 5
Table 5: Description of Module Structure
necessary
7
8
– Question scores are color coded; explained in section 5
3.2.4. Red (5 tabs)
• Summary: Summary of module and indicator scores, workload statistics, equipment summaries,
summary of biochemical identification reagents; four pages when printed
• Flags: Summary of all “Flagged” questions and answers; five pages when printed
• Conclusions: Includes an embedded Microsoft Word document where the assessor may insert their
conclusions in narrative form (recommended); number of pages depends on length of narrative
• Photos: Tab for inserting relevant photographs of the laboratory if desired (six positions); two pages
• Export: Compiles all scores and other select assessment data for optional export into GIS or
statistical analysis software. English only. Must be used in conjunction with Export Reception file
Version 2 | August 2020 Page 15 of 90
LAARC User’s Guide and Questionnaire
4. ENTERING DATA INTO THE EXCEL TOOL
4.1 Generate a Unique Filename
Before entering any data, save the file using a new filename. This is particularly important when
multiple laboratories are being assessed in order to keep the files distinct. Open the master file, click
“File, Save As.” Select an appropriate location to save the file and assign a new name. The
recommended naming convention is: LAARC_[Name of Laboratory]_[Month & Year of assessment].
Example: “LAARC_CDC Hospital _March 2020.xls.”
4.2 Select Language
The LAARC Excel file contains four language options: English, French, Spanish, and Portuguese. To
select the desired language, go to the Language tab and click the drop-down menu in cell A3, then
select the appropriate number:
• 1 = English
• 2 = French
• 3 = Spanish
• 4 = Portuguese
The entire tool will convert to your selected language, with two exceptions:
• The drop-down menus for responding to each question remain in English and cannot be translated
into other languages.
• The tab labels will remain in English.
Users may add new language translations to column F of the Language tab. The tool will accept Chinese,
Russian, or other left-to-right languages, but it is not well designed to accept Arabic or Persian
languages.
4.3 Answer Questions
4.3.1 Drop-down boxes
Most of the data is entered using drop-down boxes. If you
try to type a value into the drop-down box, an error
message will appear.
Click the response box, then click the small arrow at the right side of the cell to open a box containing
the authorized values. The answers to most questions are limited to “yes,” “no,” or “NA” (not
applicable). Select NA if the question doesn’t apply to the laboratory.
For example, if the laboratory does not perform stool cultures, select NA for questions pertaining to
stool cultures. Note: “NA” is not available for all questions, for some it is compulsory to select an
answer. In case of doubts about the appropriate answer, systematically select “no”.
Some questions have a numbered response system (see Figure 2). The corresponding response key is
located below the question; keys are translated into all languages.
A simple rule:
Fill in all the grey cells! Do not
enter values in any other cells,
except comment cells.
Version 2 | August 2020 Page 16 of 90

LAARC User’s Guide and Questionnaire
4.3.2 Fee-text cells and Comment cells
In the 0-General module, many of the grey cells are free-text, meaning there is no drop-down box.
Answers must be typed into these cells:
• Name of laboratory and key staff
• Assessor names and affiliations
• Number of equipment
• Number of tests performed daily
• Number of technicians
Comment boxes are found next to each indicator and question on all 15 blue modules. Transcribe notes
taken during the assessment directly into a comment box, so they are not lost. See example below.
4.3.3 Color Coding
As each question is answered, a score between 0% and 100% displays in column G. Scores are color
coded as the following:
• Below 50%: Red
• Between 50 - 79%: Yellow
• 80% or more: Green
Unanswered questions and questions answered “NA” display an apostrophe in column G, indicating no
score. Question scores are automatically averaged together to generate indicator scores, which follow
the same color-coding scheme, and module scores, which are always blue.
Version 2 | August 2020 Page 17 of 90
LAARC User’s Guide and Questionnaire
5 SCORING SYSTEM
Scoring occurs automatically as questions are answered, and
scores display simultaneously on the Module tabs and on the
Summary tab. Four levels of scores are generated: Questions ->
Indicators -> Modules -> Overall. Indicator scores are an
average of the question scores comprising that indicator.
Module scores are calculated by averaging all questions in the
module, not by averaging the indicator scores making up the
module. The overall score is calculated by averaging the module
scores.
5.1 Questions
Most questions have three possible answers: Yes, No, or NA (not applicable); some offer partial
responses.
• “Correct” answers score 100%
• “Incorrect” answers score 0%
• Partial responses vary in value: 25%, 50%, 75%
• “NA” and unanswered questions have no value and are excluded from score calculations
Note: The overall score excludes the
LIS Module score, since the laboratory
is not directly responsible for
deficiencies in the LIS.
5.2 Indicators and Modules
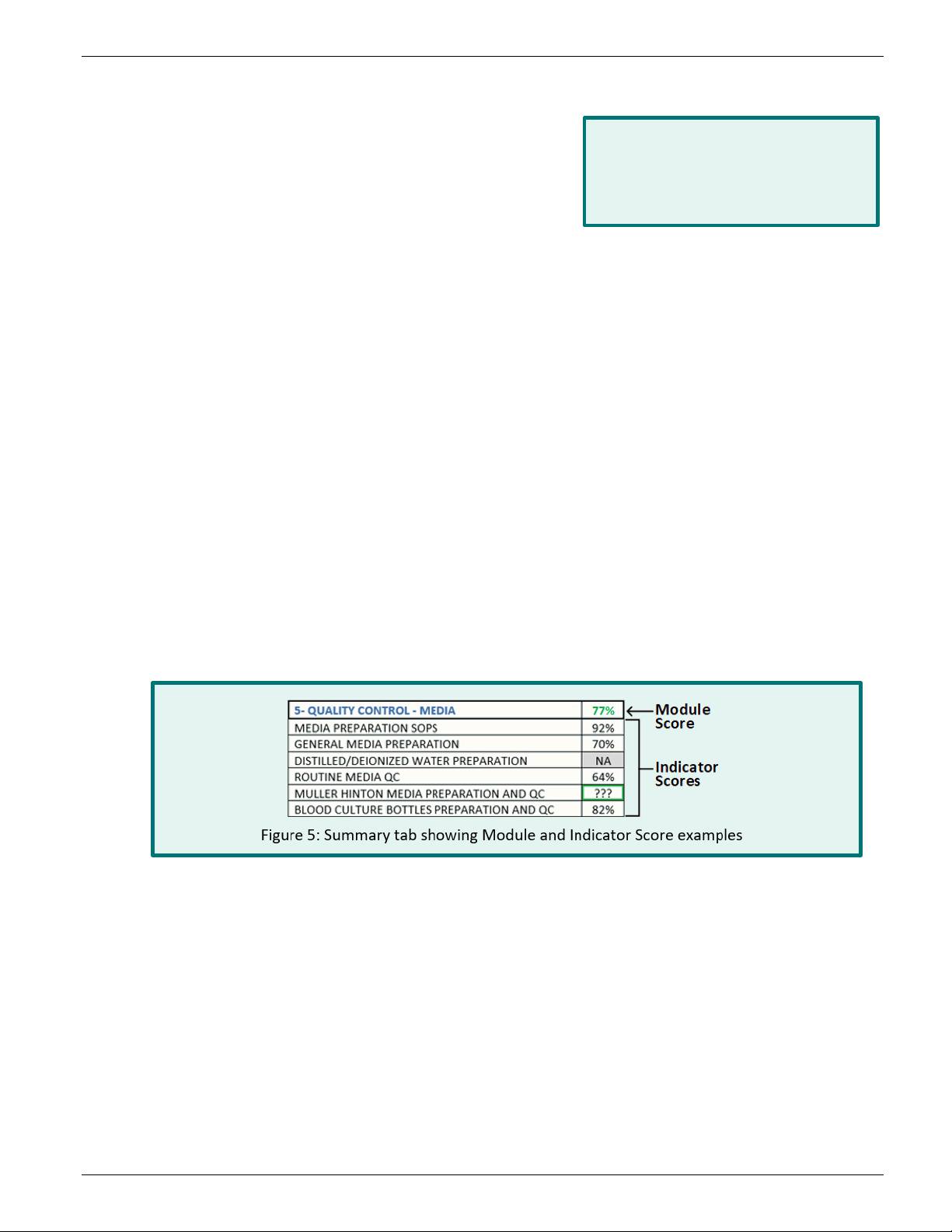
Indicator scores display as percentages. When an indicator displays “NA” instead of a percentage, it
means none of the questions in that section were applicable to the laboratory being assessed. When
an indicator displays “???”, it means the questions within that section were left unanswered. Review
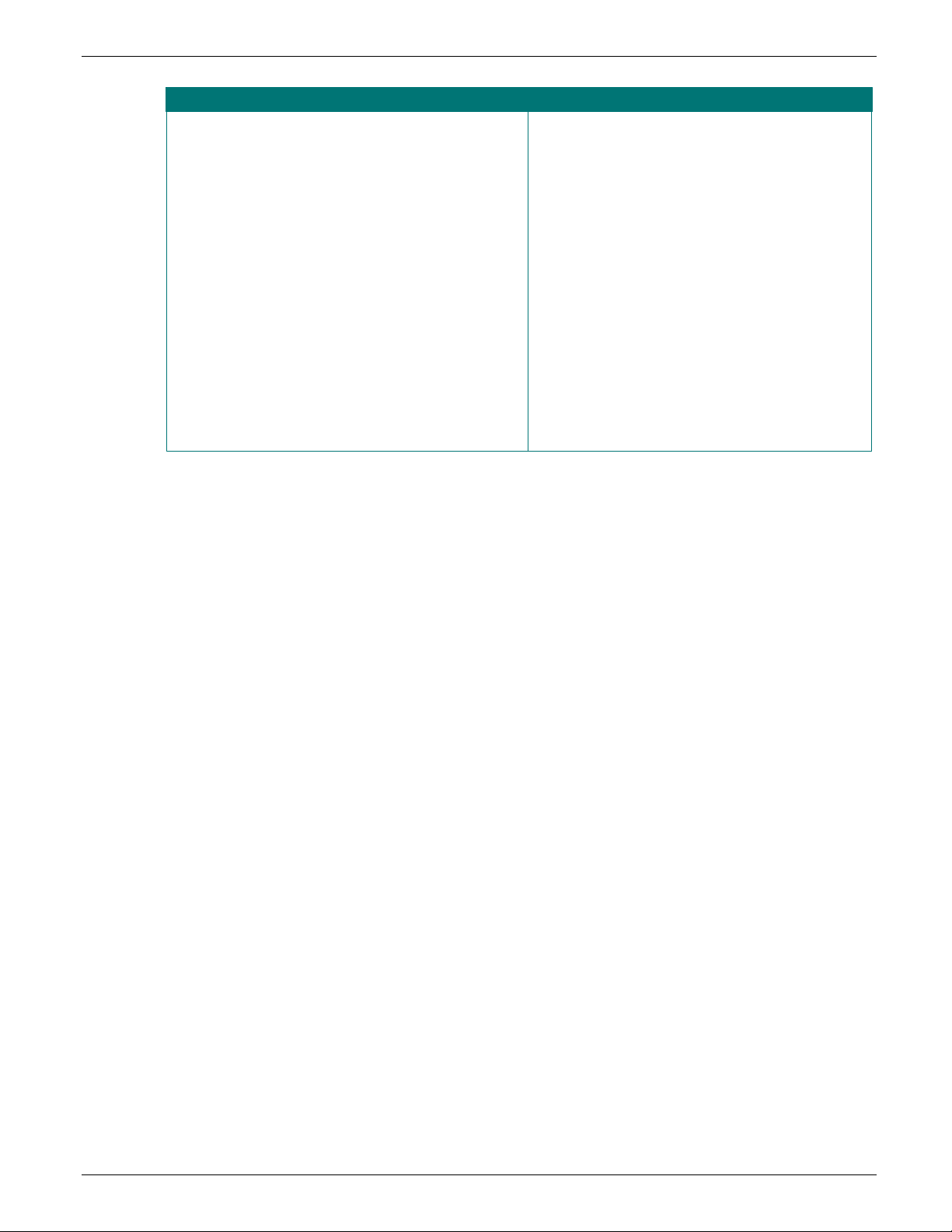
the section and answer the questions if possible. Refer to Figure 5 for examples.
e example in Figure 6, below, shows a portion of the Quality Assurance Module (blue lettering) and
Th
two of the module’s indicators (black background).
Version 2 | August 2020 Page 18 of 90

LAARC User’s Guide and Questionnaire
first indicator score is the average of questions 4.1 – 4.11, which is 68% (750/11). The second
The
indicator score is the average of questions 4.12 – 4.16, which is 100% (400/4). Note that the answer
to question 4.16 is NA, so the question is excluded from the denominator of the calculation. The
module score is not the average of the two indicator scores, which would be 84% (100+68/2). The
module score is the average of all questions, 4.1 – 4.16, excluding NA responses, which is 77%
(1150/15). The rationale for this method of calculation is that it gives equivalent weight to each
question and does not assign greater importance to any indicator.
5.3 Flags
Some questions generate “flags” that appear next to the score. Flags do not impact the score, but
they are useful for prioritizing corrective actions.
• Red Flags represent practices that may put patients or laboratory staff at risk. The laboratory should
correct these items immediately. There are 101 possible red flags
• Training Opportunity Flags highlight areas where sufficient training is commonly lacking. There are
10 possible training opportunities
• System Flags highlight problems for which the solution is often found at the level of the hospital or
national system. Laboratory leadership may need to reach out to hospital, regional, or national
leadership for assistance with correcting these issues. There are 24 possible System Flags
Version 2 | August 2020 Page 19 of 90

LAARC User’s Guide and Questionnaire
6. RESULTS: SUMMARY, FLAGS, CONCLUSION and PHOTOS
These four tabs summarize the results of the evaluation.
6.1 Summary tab
The Summary tab includes eight parts, four pages when printed:
• Laboratory Identification and Date of Assessment
• Test Menus and Annual Workload data
• Staffing Level
• Module Score Summary and Number of Flags
• Indicator Score Summary
• QC & SOP Scores for Biochemical Identification Reagents
• Equipment Availability Summary
• QMS Mentoring and Laboratory Accreditation Summary
Scores for each Module and Indicator are summarized and displayed in a heat map, shown in Figure 8.
Figure 8:
Color coded heat map for the Module: Safety Appendix and its four Indicators
6.2 Flag tab
The Flag tab populates after the questionnaire is completed. This tab will:
• Display all potential flags and the laboratory’s response to each
• Highlight all flags generated
• Show where the flagged questions are located within the tool
Version 2 | August 2020 Page 20 of 90

LAARC User’s Guide and Questionnaire
6.3 Conclusion tab
The conclusion tab contains an embedded Microsoft Word file where the assessor may summarize
their main findings and recommendations in a narrative format. Embedding the Microsoft Word
document within the Excel file allows the narrative findings and calculated scores to always remain
together in a single file. Double click on the document and a Microsoft Word file will open, which can
be saved or printed. To exit the Word document, click anywhere in the Excel grid.
6.4 Photograph tab
Insert up to six photographs (less than 500KB
each) here, allowing all materials to exist
together in a single file.
1. Click on Insert at the top of the page
2. Click Illustrations
3. Click Pictures
4. Select a file from your computer
Note: Inserting large photos will make the file
difficult to share by email.
Before inserting, resize photos to less than
500KB/2MP (size “Medium”) to keep the final
Excel file small.
Always ask permission before photographing anything, particularly individuals. If photographing
laboratory documents, obscure any personally identifying information (PII). Example: covering
patient names with a piece of paper.
7. INTERPRETING RESULTS and DEVELOPING A WORK PLAN
Some general recommendations follow for interpreting the LAARC findings and developing an improvement
plan for the laboratory.
1. Review the data
Review the Summary and Flag tabs in detail with the laboratory staff. Check for errors and make any
necessary corrections before sharing with a wider audience.
2. Develop a work plan
Work plans are at the discretion of the assessor. These are brief suggestions for how to approach a
workplan:
• Develop lists of needed equipment, reagents, supplies, and service contracts
• Prioritize correction of Red Flags, since these highlight practices that may put patients or staff at risk.
If rapid correction is not possible due to lack of funding, the immediate action should be to request
the necessary funding from hospital administrators or others as appropriate.
• Use Training Flags to request specific training for staff
Version 2 | August 2020 Page 21 of 90

LAARC User’s Guide and Questionnaire
• Use System Flags to request high-level meetings with administrators to discuss remediation
• Review all questions to identify corrections that can be made immediately and/or with very few
resources. This may include developing or updating SOPs, QC forms or job aids, implementing
temperature monitoring
• Review module and indicator scores to prioritize areas for improvement. Note that the areas with the
lowest scores may not be the most urgent for correction
• Develop a timeline for improvements based on resources available and resources needed
3. Summarize findings
Use the Word document in the Conclusions tab to write brief narrative summaries of the findings in
each module, noting both strengths and weaknesses.
4. Explain findings and recommendations
Use a video projector, if possible, to display the results on a large screen or a blank wall. This will enable
more people to attend and view the results.
5. Leave paper and electronic copies of the file with the laboratory
We recommend leaving an electronic copy of the Excel file with relevant members of the laboratory
leadership team so that they can revisit each question as a basis for laboratory improvement. They can
also use it to monitor improvements over time by changing the answers as deficiencies are corrected.
8. EXPORTING DATA
In some cases, it may be useful to compile data from multiple laboratory assessments for comparison
purposes. For example, comparing assessment results from multiple laboratories to one another, or
comparing the results of one laboratory to itself over time. For this purpose, there is an Export tab
embedded in the file. This tab captures all data from the General, Summary and Flag tabs, as well as answers
to select questions from many of the Module tabs. Data from the Export tab may be copied and pasted into
another Microsoft Excel spreadsheet that has been developed for this purpose called the “Reception file.”
Data from the reception file may then be exported into analysis software.
Directions for copying and pasting into the Reception file are as follows:
1. Open both the LAARC Data file and the LAARC Data Reception file.
2. In the LAARC Data file, make sure all questions are answered. Unanswered questions will display as zeros
in the export.
3. Go to the Export tab.
• Select row 6 entirely by clicking on the number “6” at the left edge of the table
• Copy the selected data to the clipboard
• Go to the Export Reception file and select row number 8 entirely by clicking on the number “8” at the
left edge of the table. Row 8 should be blank
• Select “Paste Special,” then click “Values”
• NOTE: A “regular/simple” paste will not allow you to export the data correctly, you must “paste
special” as described above
4. Repeat steps 1-3 for each laboratory using the same Data Reception file. Each additional line of data will
be pasted on the next available blank line: 9, then 10, etc.
5. Once complete, save the Export Reception file
Version 2 | August 2020 Page 22 of 90
LAARC User’s Guide and Questionnaire
6. Save the file a second time, this time as a .csv (comma separated value)
• Go to “File” select “Save as”
• Keep the same file name, but in “File type,” select “CSV” (Comma Separated value” (*.csv)” in the drop-
down list
7. Save the file
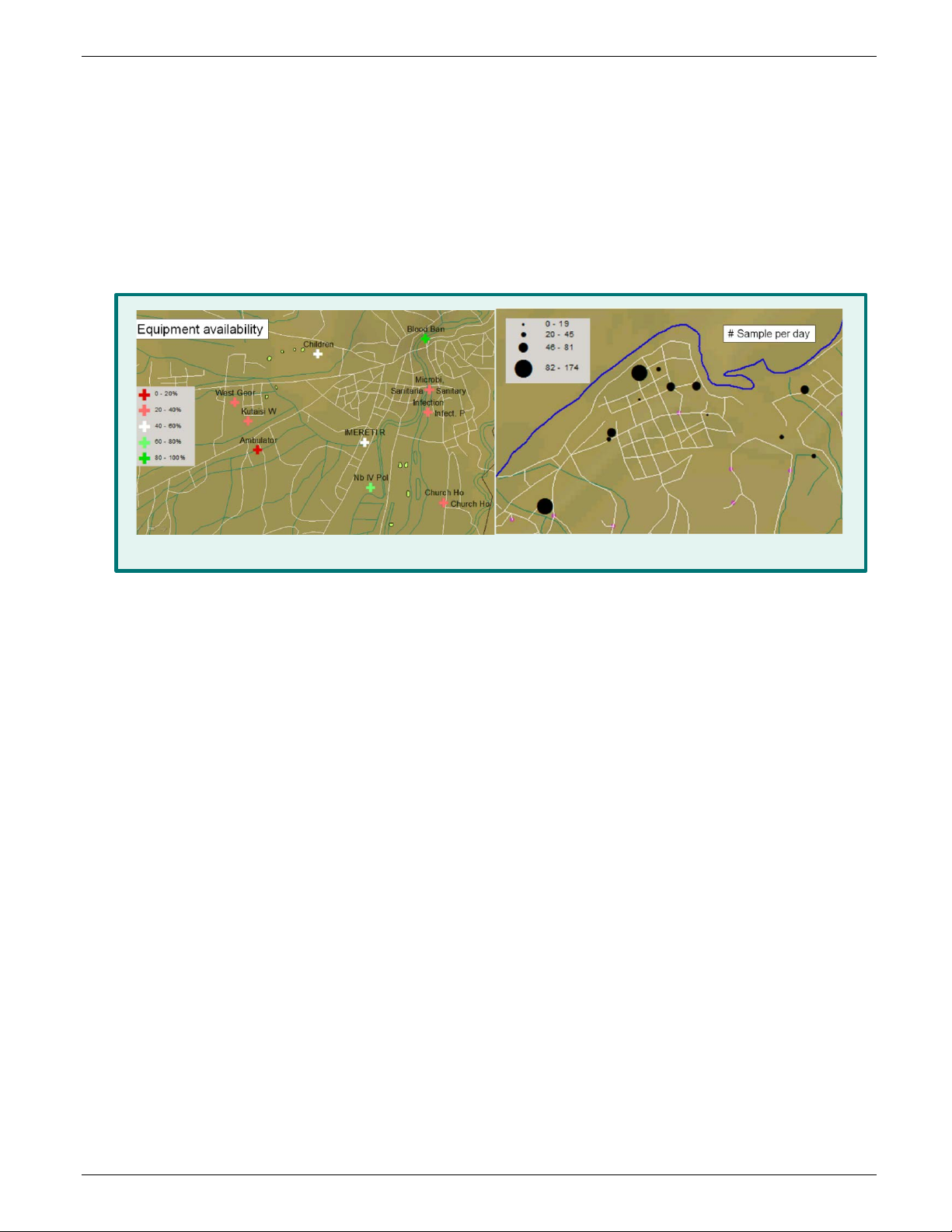
The .csv file can be opened by any database or GIS software. If you have shapefiles of the country or region,
you’ll be able to graphically represent indicators and data on maps. The figure below displays examples of GIS
mapping of equipment and sample volumes from another assessment tool (not LAARC).
Figure 10 : Geographical representation of indicators
Version 2 | August 2020 Page 23 of 90

LAARC User’s Guide and Questionnaire
9. REFERENCES
1. Clinical & Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility
Testing. 30
Suite 2500, Wayne, Pennsylvania 19087 USA, 2020.
2. College of American Pathologists Laboratory Accreditation Program Checklists. Laboratory General
Checklist and Microbiology Checklist. Northfield, Illinois, College of American Pathologists, 2017.
th
ed. CLSI supplement M100. Clinical & Laboratory Standards Institute, 950 West Valley Road,
3. The European Committee on Antimicrobial Susceptibility Testing
(https://eucast.org/). Breakpoint tables
for interpretation of MICs and zone diameters. Version 10.0, 2020.
4. ISO 15189:2012. Medical laboratories – Particular requirements for quality and competence.
International Standardization Organization. 2012.
5. Laboratory Assessment Tool. World Health Organization. 2012.
6. Laboratory Checklist. American Society for Microbiology. 2013.
Version 2 | August 2020 Page 24 of 90
LAARC User’s Guide and Questionnaire Appendix 1: Sample Letter
Appendix 1: Sample Letter
ar Sir/Madam,
De
The Ministry of Health of [COUNTRY] is developing a surveillance system for antimicrobial resistance (AR) of
priority bacterial pathogens. [LABORATORY NAME] may serve as a sentinel site for the surveillance system. As
such, an evaluation of the baseline capacity of the laboratory to perform basic bacteriology including isolation,
identification and antibiotic susceptibility testing (AST) has been proposed. The evaluation will be carried out
using the Laboratory Assessment of AR Testing Capacity (LAARC) developed by the International Infection
Control Program at the U.S. Centers for Disease Control and Prevention. The purpose of the evaluation is to
identify gaps in capacity and aid in development of plans for improvement prior to initiating surveillance.
The laboratory assessment may take up to two full days to complete. A proposed schedule is included below:
Day 1
8:00 – 8:30 am
• Introductions: Laboratory leadership and
other laboratory staff, assessment team
• Review purpose of the evaluation, and
expected timeline
8:30 – 9:30 am
• Tour laboratory
9:30 – 10:00 am
• Break for tea
10:00 – Noon
• Review pre-assembled documents and
begin filling questionnaire
Noon – 1:00 pm
• Break for lunch
1:00 – 4:30 pm
• Continue filling questionnaire
• Evening – transfer paper responses into
Excel tool
7:30 – 9:30 am
• Observe laboratory staff at the bench
• Continue filling assessment
9:30 – 10:00 am
• Break for tea
10:00 – Noon
• Continue filling assessment
Noon – 1:00 pm
• Break for lunch
1:00 – 2:30 pm
• Complete assessment
2:30 – 3:30 pm
• Summation/exit meeting with laboratory
leadership, other relevant staff
Day 2
The assessment will be carried out by an experienced clinical bacteriologist, [NAME, TITLE, AND AFFILIATION OF
ASSESSOR if available], a representative from the Ministry of Health, and [ANY ADDITIONAL PERSONNEL].
We will perform the assessment during regular business hours, on days when staffing levels will be adequate to
permit the assessors to interact with the bacteriology technologists without disrupting their workflow. We
request that Bacteriology section heads, supervisors, and quality managers are present during the assessment
and that their schedules are clear of meetings or other obligations to the extent possible.
The following documents and information will require review by the assessors. To the extent these can be
assembled in advance into a single clean room for the team, the time required for the evaluation will be greatly
reduced:
• Names, job titles, and email addresses of relevant bacteriology laboratory leadership (e.g., Director,
Manager, Supervisor, Section Head, Quality Officer, etc.)
• Copies of any recent assessments by a third party
• Annual test volume for each specimen type
Version 2 | August 2020 Page 25 of 90

LAARC User’s Guide and Questionnaire
• Records of staff qualifications, training and experience
• Accreditation and/or Certification documents
• Equipment inventory
• Equipment calibration and maintenance records
• Contingency plans in the event of an emergency or extended downtime
• Specimen requisition form
• Bacteriology logbooks or Laboratory Information System records
• Standard form used for reporting ID/AST results to clinicians
• Quality Manual
• Records of the last three AST EQA/PT performance results, and associated discrepancy
investigations
• Quality Control records for temperatures, media, reagents, and AST
• Specimen collection guidelines or SOPs
• SOPs for specimen processing, reagents, ID and AST test systems
• Copies of any recent safety audits
• Reserve a room with a video projector, if possible, for the final summation meeting
All findings and recommendations shall be discussed with the bacteriology supervisor in private prior to the final
summation. Please reach out to [Assessment Team Lead] with any questions.
The following dates have been proposed [dd/mm/yyyy – dd/mm/yyyy]. Please contact [Ministry Official] to
accept or reschedule your assessment dates.
Sincerely,
[Assessment Team Lead]
Version 2 | August 2020 Page 26 of 90

LAARC User’s Guide and Questionnaire Appendix 2: Recommended Resources
Appendix 2: Recommended Resources
The following documents are useful resources for clinical bacteriology laboratories. Many are free, others may
be obtained for a fee.
Culture and Identification
• CLSI M35: Abbreviated Identification of Bacteria and Yeast
• CLSI M47: Principles and Procedures for Blood Cultures
• CLSI M54: Principles and Procedures for Detection of Fungi in Clinical Specimens-Direct Examination and
Culture
• CLSI M56: Principles and Procedures for Detection of Anaerobes in Clinical Specimens
• CLSI M58: Methods for the ID of Cultured Microorganisms using MALDI-TOF Mass Spectrometry
AST/AMR
• CLSI M02: Performance Standards for Antimicrobial Disk Susceptibility Tests
• CLSI M02QG: Disk Diffusion Reading Guide
• CLSI M07: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically
• CLSI M39: Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data
• CLSI M45: Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or
Fastidious Bacteria
• CLSI M100 Performance Standards for Antimicrobial Susceptibility Testing
• ETEST Reading Guide [PDF - 2 pages]
• EUCAST Breakpoint Tables
• EUCAST Disk Test Reading Guide
• EUCAST reading guide for broth microdilution
• EUCAST Manual Disk Test
• EUCAST Preparation of agar plates and broth for EUCAST AST
• EUCAST Intrinsic Resistance and Unusual Phenotypes
• EUCAST Expert Rules for Enterobacterales, Staphylococcus, and other species
(http://www.eucast.org/expert_rules_and_intrinsic_resistance/)
• EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or
epidemiological importance
(http://www.ilexmedical.com/files/ETEST_RG.pdf)
Quality Control
• CLSI M22: Quality Control for Commercially Prepared Microbiological Culture Media
• CLSI M40: Quality Control of Microbiological Transport Systems
• CLSI M50: Quality Control for Commercial Microbial Identification Systems
• CLSI M52: Verification of Commercial Microbial ID and AST Systems
• EUCAST QC tables
Laboratory Quality Management Systems (QMS)
• WHO Laboratory Quality Stepwise Implementation Tool
• CLSI QMS01: A QMS Model for Laboratory Services
• CLSI QMS01CL: Gap Analysis Checklists
• CLSI QMS02: QMS: Development and Management of Laboratory Documents
• CLSI QMS03: Training and Competence Assessment
• CLSI QMS04: Laboratory Design
• CLSI QMS05: QMS: Qualifying, Selecting and Evaluating a Referral Laboratory
Version 2 | August 2020 Page 27 of 90
 Loading...
Loading...