Page 1

EA-200
User’s Guide
E
http://world.casio.com/edu_e/
Page 2
GUIDELINES LAID DOWN BY FCC RULES FOR USE OF THE UNIT IN THE U.S.A.
(not applicable to other areas).
Important!
Please keep your manual and all information handy for future reference.
NOTICE
This equipment has been tested and found to comply with the limits for a Class B
digital device, pursuant to Part 15 of the FCC Rules. These limits are designed to
provide reasonable protection against harmful interference in a residential
installation. This equipment generates, uses and can radiate radio frequency energy
and, if not installed and used in accordance with the instructions, may cause harmful
interference to radio communications. However, there is no guarantee that
interference will not occur in a particular installation. If this equipment does cause
harmful interference to radio or television reception, which can be determined by
turning the equipment off and on, the user is encouraged to try to correct the
interference by one or more of the following measures:
• Reorient or relocate the receiving antenna.
•Increase the separation between the equipment and receiver.
• Connect the equipment into an outlet on a circuit different from that to which the
receiver is connected.
• Consult the dealer or an experienced radio/TV technician for help.
FCC WARNING
Changes or modifications not expressly approved by the party responsible for
compliance could void the user’s authority to operate the equipment.
Proper connectors must be used for connection to host computer and/or peripherals
in order to meet FCC emission limits.
In no event shall CASIO Computer Co., Ltd. be liable to anyone for special, collateral,
incidental, or consequential damages in connection with or arising out of the purchase or
use of these materials. Moreover, CASIO Computer Co., Ltd. shall not be liable for any claim
of any kind whatsoever against the use of these materials by any other party.
• The contents of this manual are subject to change without notice.
• No part of this manual may be reproduced in any form without the express written
consent of the manufacturer.
Connector SB-62 EA-200 to Power Graphic Unit
Declaration of Conformity
Model Number: EA-200
Trade Name: CASIO COMPUTER CO., LTD.
Responsible party: CASIO, INC.
Address: 570 MT. PLEASANT AVENUE, DOVER, NEW JERSEY 07801
Te lephone number: 973-361-5400
This device complies with Part 15 of the FCC Rules. Operation is subject to the
following two conditions: (1) This device may not cause harmful interference, and
(2) this device must accept any interference received, including interference that may
cause undesired operation.
CASIO ELECTRONICS CO., LTD.
Unit 6, 1000 North Circular Road,
London NW2 7JD, U.K.
Page 3

Contents
–– English ––
0-1
English
Handling Precautions ............................................................................ 0-2
Unpacking .............................................................................................0-3
About the EA-200 ................................................................................. 0-3
Before Using the EA-200 for the First Time .......................................... 0-3
Chapter 1
General Guide ......................................................................................1-1
Supported Calculator Models ............................................................... 1-2
Supported Probes ................................................................................. 1-2
Using Commands ................................................................................. 1-3
Using the Voltage Probe, Temperature Probe, Optical Probe,
and Motion Sensor (EA-2) ....................................................................1-3
Using the Built-in Microphone ...............................................................1-4
Using the Built-in Speaker .................................................................... 1-5
Status Request ..................................................................................... 1-5
Auto Setup ............................................................................................ 1-5
Group Link Function ............................................................................. 1-6
Natural Frequency and Sound .......................................................... 2-6-1
Column of Air Resonance and the Velocity of Sound ....................... 2-7-1
Construction of the Musical Scale .................................................... 2-8-1
Direct Current and Transient Phenomena......................................... 2-9-1
AC Circuit ........................................................................................ 2-10-1
Dilute Solution Properties ............................................................... 2-11-1
Exothermic Reaction....................................................................... 2-12-1
Electromotive Force of a Battery..................................................... 2-13-1
Sunlight and Solar Cells ................................................................. 2-14-1
Topographic Conditions and Climate .............................................. 2-15-1
Program Library .................................................................. 2-16-1
Appendix A Command Tables
Command 1 – Channel Setup .......................................................... α-1-1
Command 3 – Sample and Trigger Setup ......................................... α-1-2
Command 4 – Conversion Equation Setup ....................................... α-1-3
Command 5 – Data Range Setup ..................................................... α-1-3
Chapter 2
Examples
Uniformly Accelerated Motion ........................................................... 2-1-1
Period of Pendular Movement ........................................................... 2-2-1
Conservation of Momentum .............................................................. 2-3-1
Charles’ Law ..................................................................................... 2-4-1
Polarization of Light .......................................................................... 2-5-1
Command 6 – System Setup ............................................................ α-1-4
Command 8 – Sampling Start .......................................................... α-1-4
Command 10 – Sensor Warmup ...................................................... α-1-4
Command 11 – Buzzer and LED Operation Commands .................. α-1-4
Command 12 – Data Send Sequence .............................................. α-1-4
Appendix B Specifications ............................................... α-2-1
20020601
Page 4

Handling Precautions
• The EA-200 is made up of precision components. Never try to take it apart.
•Avoid dropping the EA-200 and subjecting it to strong impact.
• Do not store the EA-200 or leave it in areas exposed to high temperatures, humidity, low
temperatures, or large amounts of dust. Low temperatures can shorten battery life.
• Replace the main batteries once every two years regardless of how much the EA-200 is
used during that period. Never leave dead batteries in the battery compartment. They
can leak and damage the unit.
• Keep batteries out of the reach of small children. If swallowed, consult with a physician
immediately.
•Avoid using volatile liquids such as thinner or benzine to clean the unit. Wipe it with a
soft, dry cloth, or with a cloth that has been dipped in a solution of water and a neutral
detergent, and wrung out.
•In no event will the manufacturer and its suppliers be liable to you or any other person
for any damages, expenses, lost profits, lost savings or any other damages arising out of
loss of data and/or formulas arising out of malfunction, repairs, or battery replacement.
The user should prepare physical records of data to protect against such data loss.
• Never dispose of batteries, or other components by burning them.
0-2
English
• Never allow foreign objects to get into connector holes. Doing so can result in
malfunction.
•Always make sure to connect probes only to their correct terminals. Never force the plug
of a probe into a wrong terminal connector.
• Be sure to turn the EA-200 off before connecting or disconnecting probes.
• Make sure that probes are connected securely before using them to take samples.
• Never insert a probe into an electric outlet. Never attempt to measure high voltages or
household AC. Doing so creates the danger of electric shock.
• Never apply more than 15V to analog channels CH1 or CH2, more than 5V to analog
channel CH3, or more than 5.5V to the SONIC, DIG IN, or DIG OUT channels. Doing so
can damage the EA-200.
• The EA-200 is to be used for educational purpose only. It is not appropriate for
industrial, research, medical, or commercial applications.
• Replace batteries as soon as possible after the low battery indicator lamp (Batt) lights.
• Be sure that the power switch is set to OFF when replacing batteries.
• If the EA-200 is exposed to a strong electrostatic charge, its memory contents may be
corrupted or the keys may stop working. In such a case, perform the Reset operation to
clear memory contents and restore normal key operation.
• If you start to experience serious operational problems with the EA-200, use a thin,
pointed object to carefully press the P button on the back of the EA-200. Note, however,
that pressing the P button deletes all data currently in EA-200 memory. Proper operation
does not resume after you press the P button, remove its batteries, and then replace
them correctly in accordance with the instructions on page 0-3 of the User’s Guide.
• Note that strong vibration or impact during program execution can cause execution to
stop or can corrupt EA-200 memory contents.
• Using the EA-200 near a television or radio can cause interference with TV or radio
reception.
• Before assuming malfunction of the EA-200, be sure to carefully reread this User’s
Guide and ensure that the problem is not due to insufficient battery power, programming
or operational errors.
20020601
20020701
Page 5
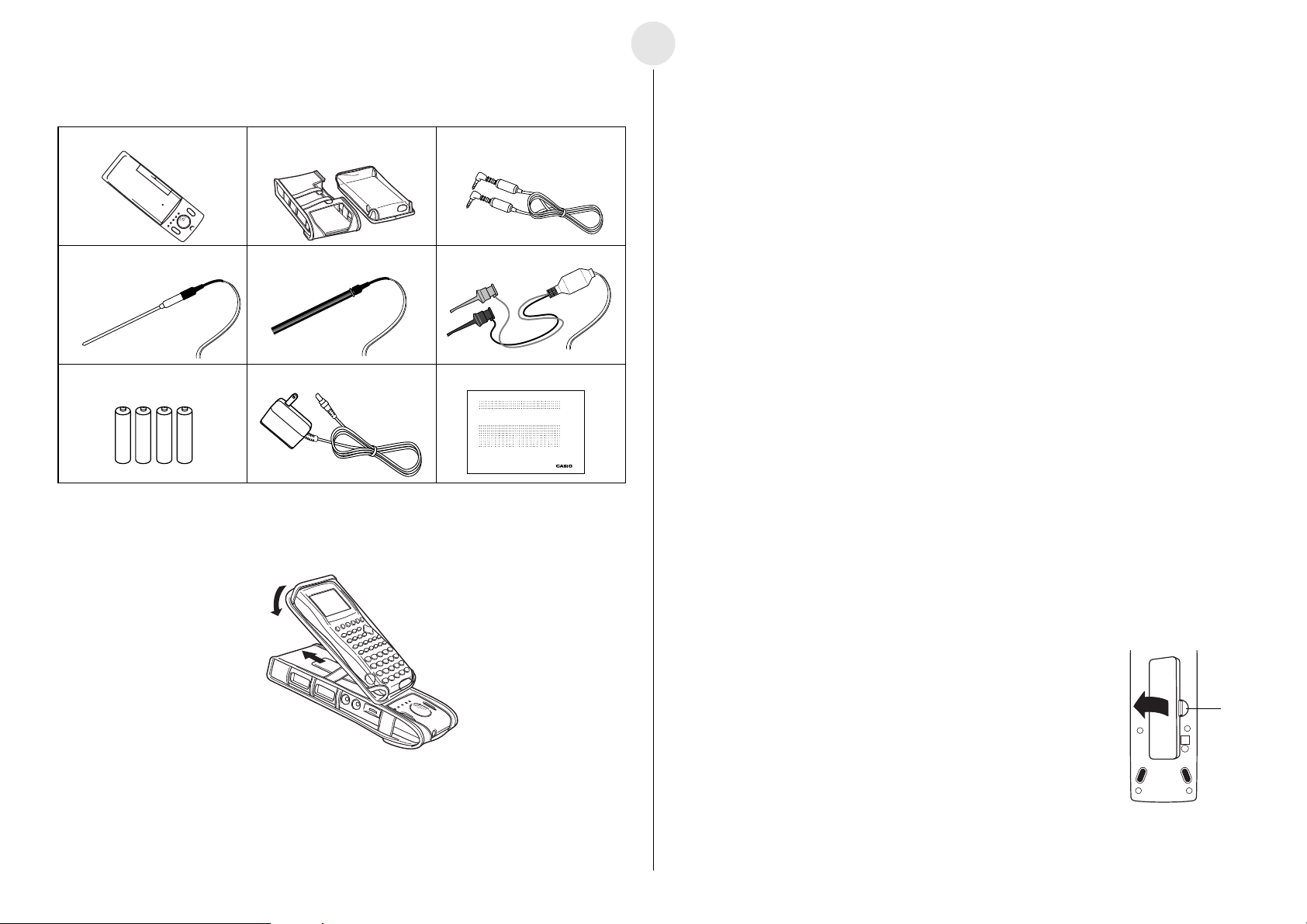
0-3
English
Unpacking
EA-200 Soft case Data communication cable
(SB-62)
Temperature probe Optical probe Voltage probe
Four AA-size alkaline batteries AC adaptor User’s Guide (this manual)
EA-200
User’s Guide
E
Using the Soft Case
2
1
http://world.casio.com/edu_e/
About the EA-200
The EA-200 is a digital device that makes it possible for you to sample data connected with
everyday natural phenomena.
Data You Can Sample with the EA-200
Various different sensors can be used with the EA-200 to sample temperatures, light,
voltage, distance, and other data. The EA-200 supports sampling of up to 120,000 points,
and simultaneous sampling over five channels. Sampled data can be sent to a compatible
Graphic Scientific Calculator, where it can be viewed and graphed.
Before Using the EA-200 for the First
Time
Power Requirements
Your EA-200 requires four AA-size alkaline batteries for power. Battery life depends on the
amount of time the EA-200 is left on, and the amount of current used by the connected
probe(s). A low battery indicator lamp (Batt) lights to let you know when it is time to replace
batteries. To extend battery life, it is a good idea to use the AC adaptor for power whenever
sampling indoors.
When using the EA-200 in combination with the optional “Motion Sensor (EA-2)”, be sure to
power the EA-200 using its bundled AC adaptor (AD-A60024).
Though the EA-200 can normally operate on battery power, separate AC adaptor power is
required while the optional “Motion Sensor (EA-2)” is being used.
Batteries are not loaded in the EA-200 when it is shipped from the factory. Because of this,
use the following procedure to load batteries into the EA-200 before using it for the first time.
To load batteries
1. Remove the battery cover by pulling with your finger at the
point marked 1. If there are batteries in the battery
compartment, remove all four of them.
2. Load four new AA-size batteries. Make sure that the plus and
minus ends of the batteries are facing in the directions shown
by the markings inside the battery case. Replace the battery
cover.
3. Slide the [ON/OFF] switch to turn on the EA-200. To turn off,
slide the [ON/OFF] switch again.
1
20020601
Page 6

0-4
English
Auto Power Off (APO)
To extend battery life, power automatically turns off if you do not perform any operation for
about 30 minutes. APO is disabled automatically whenever the EA-200 is standing by for
sampling (ready state), or while sampling is in progress.
Extended Sampling Mode
The Extended Sampling Mode makes it possible to sample data over an extended period
when operating under battery power. The following are the features of the Extended
Sampling Mode.
•In the Extended Sampling Mode, sampling continues even though the power lamp is not
lit.
• The Extended Sampling Mode is entered automatically whenever the sampling interval
is five minutes or longer.
•To exit the Extended Sampling Mode, press the [START/STOP] key. Note that all other
keys are disabled in the Extended Sampling Mode.
When to Replace Batteries
Replace batteries as soon as possible after the low battery indicator lamp (Batt) lights. The
EA-200 may start to malfunction if you continue to use it while battery power is low.
AC Adaptor
Warning!
• Make sure the voltage of the power supply you are connecting to matches that of the
rating marked on the EA-200. Do not overload extension cords and wall outlets. Failure
to follow these precautions creates the danger of fire and electric shock.
• Never allow the power cord to become damaged, cracked, or broken. Never modify the
power cord in any way, and never subject it to excessive twisting or pulling. Never place
heavy objects on power cord and do not expose it to direct heat. A damaged power cord
creates the danger of electric shock.
• Never touch the AC adaptor while your hands are wet. Doing so creates the danger of
electric shock.
Caution!
•Always grasp the adaptor box and never pull on the power cord when unplugging the AC
adaptor. Doing so runs the risk of damaging the cord and creating the danger of fire and
electric shock.
• Be sure to always unplug the AC adaptor whenever leaving the EA-200 unattended for
long periods.
• Use only the special AC adaptor that is specified for the EA-200.
• Use of any other type of AC adaptor creates the risk of serious problems with and
damage to the EA-200 and/or AC adaptor. Never use another type of AC adaptor. Note
that any damage due to use of the wrong type of adaptor is not covered by your
warranty.
• Be sure to replace the batteries at least once every two years, no matter how much you
use the EA-200 during that time.
• The batteries that come with this EA-200 discharge slightly during shipment and
storage. Because of this, they may require replacement sooner than the normal
expected battery life.
• Before removing the batteries, make sure you make a separate copy of sample data by
transferring it to a Graphic Scientific Calculator or some storage device. Cutting off all
power to the EA-200 by removing its batteries while the AC adaptor is not connected
causes all of the data in memory and all settings to be cleared.
•Whenever performing a sampling operation that requires more than a few minutes, we
recommend that you power the EA-200 using the AC adaptor. This will help to ensure
stable sampling operations.
• Make sure you turn off the EA-200 before connecting the AC adaptor.
• The AC adaptor may become warm if you use it for a long time. This is normal and does
not indicate malfunction.
To connect the AC adaptor to the EA-200
1. Slide the [ON/OFF] switch to turn off the EA-200.
2. Plug the AC adaptor into the port on the lower left of the EA-200.
3. Plug the other end of the AC adaptor into a wall outlet.
4. Slide the [ON/OFF] switch to turn on the EA-200.
20020601
Page 7
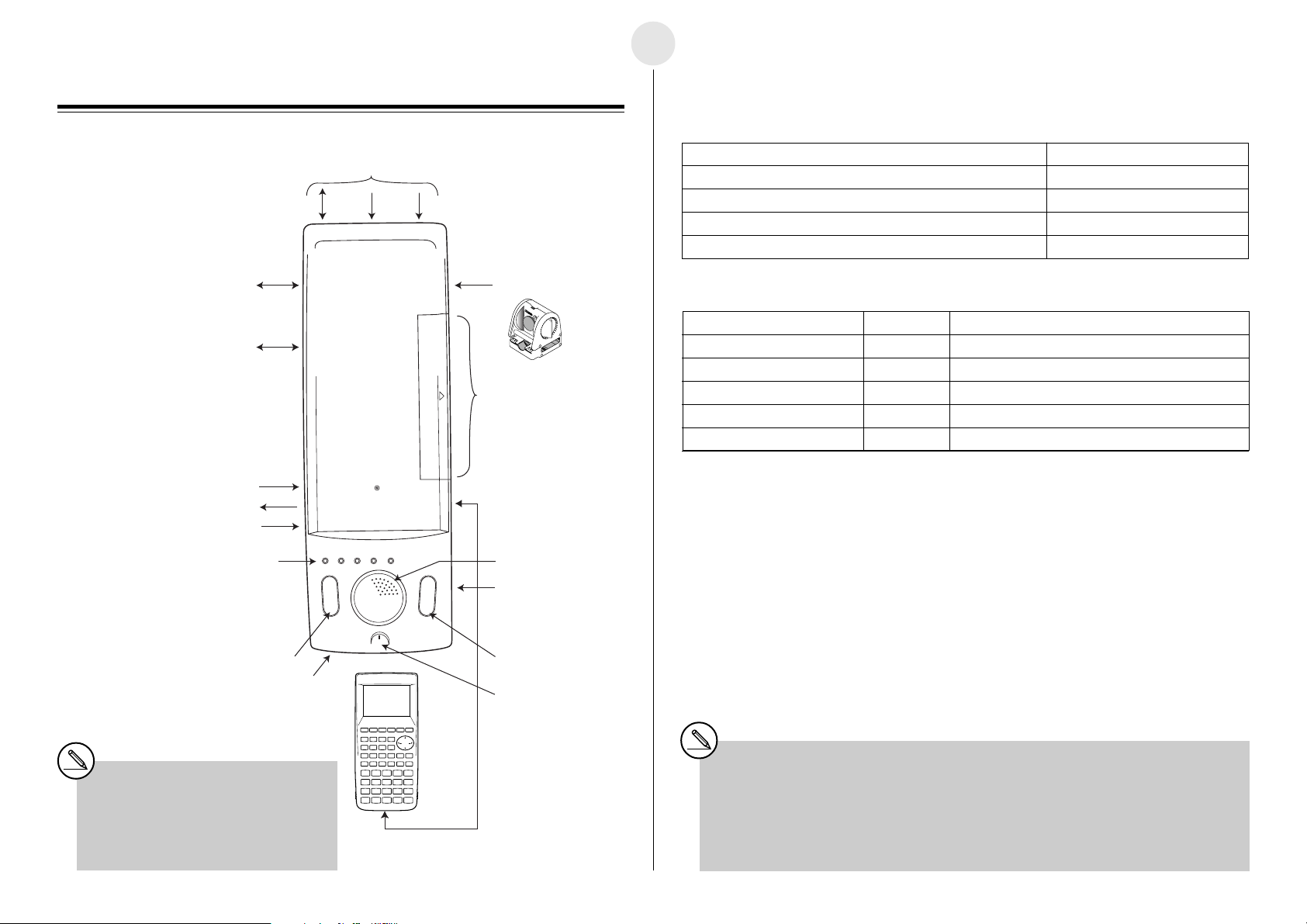
1-1
English
Chapter 1
General Guide
Digital input/output port
Serial 232C (9-pin) port
(for cross cable)
External microphone 2-pin port
(for condenser microphone)
External speaker 2-pin port
Volume
Indicator lamps
Analog Channels × 3
(Analog output for CH3 only)
CH3 CH2 CH1
Ready
Sampling
Error Batt Power
SONIC Channel
EA-2*
0
M
o
t
i
o
n
S
e
n
s
o
0
1
0
8
1
Calculator 3-pin
communication port
(SUB port) × 7
Calculator 3-pin
communication port
(MASTER port)
Built-in speaker
Key Functions and Indicator Lamps
Key Functions
To do this: Press this key:
Start a sampling operation from the ready state [START/STOP]
Stop an ongoing sampling operation [START/STOP]
Use Auto Setup (See “Auto Setup” on page 1-5.) [SET UP]
Cancel the ready state
1
Indicator Lamps
When this indicator lamp: Does this: It means this:
Power (Green) Lights Power is on.
Ready (Green) Lights EA-200 is standing by for data (ready state).
Sampling (Green) Flashes Sampling is in progress.
Error (Red) Lights An error occurred.
Batt (Red) Lights It is time to replace the batteries.
•While the “Ready” lamp is lit, press the [START/STOP] key to start sampling.
•To cancel the ready state, press the [SET UP] key.
•To interrupt a sampling operation, press the [START/STOP] key.
• For details about errors, see “Status Request” on page 1-5.
[SET UP]
SET UP key START/STOP key
AC adaptor port
*1When using the EA-200 in combination
with the optional “Motion Sensor (EA-2)”,
be sure to power the EA-200 using its
bundled AC adaptor (AD-A60024).
SET UP START/STOP
ON/OFF switch
P button (rear side)*
Built-in microphone
Data communication
cable
2
*2If you start to experience serious operational problems with the EA-200, use a thin, pointed
object to carefully press the P button on the back of the EA-200. Note, however, that pressing
the P button deletes all data currently in EA-200 memory. Proper operation does not resume
after you press the P button, remove its batteries, and then replace them correctly in
accordance with the instructions on page 0-3 of the User’s Guide.
20020601
Page 8
1-2
English
Supported Calculator Models
Connection to a supported scientific calculator is essential if you want to get the most out of
your EA-200. A connected Graphic Scientific Calculator sends commands that control the
EA-200 during transfer of sampled data and other operations. Transferred data can be
graphed on the calculator. For details about commands, see “Using Commands” on page
1-3, and “Command Tables” on page α-1-1.
Supported Calculator Models
ALGEBRA FX Series CFX-9850/fx-7400 Series
ALGEBRA FX 2.0 PLUS
ALGEBRA FX 2.0
FX 1.0 PLUS
FX 1.0
Connecting the EA-200 to a Supported Calculator
Use the special data communication cable to connect the EA-200 to a supported Graphic
Scientific Calculator model.
(1) Turn off the EA-200 and the calculator.
(2) Connect one end of the special data communication cable to the scientific calculator.
(3) Connect the other end of the cable to the EA-200’s MASTER port.
• Insert the plugs as far as they will go. If you experience problems when transferring data,
check to make sure that both plugs are fully inserted.
• Be sure to read the user documentation that comes with the scientific calculator you are
connecting.
CFX-9950GB PLUS
CFX-9850GB PLUS
CFX-9850Ga PLUS
CFX-9850G PLUS
CFX-9970G
fx-9750G PLUS
CFX-9950G
CFX-9850G
fx-7400G PLUS
fx-7450G
Supported Probes
A probe is a sensor that connects to the EA-200 for sampling temperature, light, and other
data.
The EA-200 comes with the three probes described below.
• Voltage Probe ................... Measures voltage in the range of –10V to +10V.
CH3 measures in the range of –5V to +5V.
• Optical Probe ................... Measures luminance in the range of 100 to 999.
• Temperature Probe .......... Measures temperature in the range of –20°C to 130°C.
Connecting a Probe to a Channel
A probe connects to an input/output port called a “channel.” The EA-200 has seven
channels: three analog channels (CH1, CH2, CH3), one sonic channel (SONIC), one digital
input/output channel (DIG I/O), a microphone channel, and a speaker channel. You can
connect probes individually, or you can connect multiple probes for simultaneous sampling.
You can use commands to configure the settings for the channel being used for sampling, to
specify how sampled data should be handled, etc.
CH1, CH2, CH3
These channels are for the probes (voltage, temperature, optical) that come bundled with
the EA-200.
SONIC
This channel is for connection of an optional “Motion Sensor (EA-2)”.
DIG I/O
This port is for input and output of an 8-bit binary signal in the range of 0V to 5V.
This could be used, for example, to light an LED.
Built-in Microphone
The microphone can be used to sample sound.
Built-in Speaker
The speaker can be used to output sound samples.
20020601
Page 9

1-3
English
Using Commands
Basically, the EA-200 is controlled by commands from the connected calculator. This section
explains how to send commands from the calculator to the EA-200.
To use a command, you store parameters as List data on the calculator, and then use the
Send command to send the parameters to the EA-200.
EA-200 Operation
The following is an outline of the EA-200 command receive process.
1 Slide the EA-200 [ON/OFF] switch to turn on power.
2 EA-200 receives Command 0 sent from the calculator.
• This initializes the EA-200 setup.
3 EA-200 receives Command 1 sent from the calculator.
• This configures EA-200 channel settings.
4 EA-200 receives Command 3 sent from the calculator.
• This configures EA-200 sampling conditions.
• After sampling conditions are configured, the EA-200 enters the ready state.
5 Press the EA-200 [START/STOP] key.
• This puts the EA-200 into sampling mode.
• Sampling ends in accordance with the sampling conditions configured in step 4.
Sending a Command
Example 1: To initialize the EA-200 setup
{0} → List 1
Send (List 1)
Example 2: To configure channel settings
{1, 1, 2} → List 1
Send (List 1)
__
_ Stores {0} to List 1. 0 is the command number.
__
__
_ Sending List 1 executes Command 0.
__
__
_ Stores {1,1,2} to List 1. The first 1 is the command number, the
__
second 1 is the channel number, and the 2 indicates use of the
voltage probe.
__
_ Sending List 1 executes Command 1.
__
Receive Data Command
As with command execution, all of the operations required to receive data from the EA-200
to the calculator are performed on the calculator.
You can transfer sampled data by executing the calculator's Receive command.
Data is transferred in the sequence shown below.
Record Time → CH1 → CH2 → CH3 → SONIC
Receive (List 1)
__
_ Stores transferred data into List 1 of the calculator.
__
6 Sample data is sent from the EA-200 to the calculator in response to a Receive
command.
Using the Voltage Probe, Temperature
Probe, Optical Probe, and Motion Sensor
(EA-2)
The following examples show how to use the temperature probe, voltage probe, optical
probe, and “Motion Sensor (EA-2)”. Perform the procedures in each example by creating
programs on the calculator.
Taking 60 samples at one-second intervals over one minute
(1) Send Command 0 to initialize the EA-200 setup.
{0} → List 1
Send (List 1)
20020601
__
_ Stores {0} to List 1. 0 is the command number.
__
__
_ Sending List 1 executes Command 0.
__
Page 10
(2) Send Command 1 to configure channel settings.
• Voltage Probe (that is connected to CH1.)
{1, 1, 2} → List 1
Send (List 1)
• Temperature Probe (that is connected to CH1.)
{1, 1, 7} → List 1
Send (List 1)
__
_ The first 1 is the command number, the second 1 is the
__
__
_ channel number, and the 2 indicates use of the voltage
__
probe.
__
_ The first 1 is the command number, the second 1 is the
__
__
_ channel number, and the 7 indicates use of the temperature
__
(Celsius) probe.
1-4
English
Using the Built-in Microphone
To take 255 samples at 50µs intervals
(1) Send Command 0 to initialize the EA-200 setup.
{0} → List 1
Send (List 1)
(2) Send Command 1 to specify microphone as the channel.
{1, 10} → List 1
Send (List 1)
__
_ Stores {0} to List 1. 0 is the command number.
__
__
_ Sending List 1 executes Command 0.
__
__
_ 1 is the command number, and 10 indicates use of
__
__
_ the built-in microphone.
__
• Optical Probe (that is connected to CH1.)
{1, 1, 9} → List 1
Send (List 1)
• Optional “Motion Sensor (EA-2)” (that is connected to SONIC channel.)
{1, 4, 2} → List 1
Send (List 1)
(3) Send Command 3 to configure measurement condition settings.
{3, 1, 60} → List 1
Send (List 1)
At this time, the Ready lamp lights on the EA-200.
Press the EA-200 [START/STOP] key to start sampling.
When sampling is complete, press the calculator’s w key to restart the program.
^^
^ (Disp command) causes processing to stop until you press the w key.
^^
(4) Sample data from the EA-200 is received by the calculator.
Receive (List 1)
Receive (List 2)
__
_ The first 1 is the command number, the second 1 is the
__
__
_ channel number, and the 9 indicates use of the optical probe.
__
__
_ 1 is the command number, 4 is the channel number
__
__
_ (SONIC), and 2 indicates use of the motion sensor (meters).
__
__
_ 3 is the command number, 1 is the sampling interval, and 60
__
is the number of samples.
^^
^ You can change sampling conditions by using different
^^
sampling interval and number of samples values, if you want.
__
_ Record Time data is received and stored in List 1.
__
__
_ Sampling Data of CH1 is received and stored in List 2.
__
(3) Send Command 3 to configure measurement condition settings.
{3, 0.00005, 255} → 3 is the command number, 0.00005 is the sampling interval
__
List 1
_ (50
__
Send (List 1)
At this time, the Ready lamp lights on the EA-200.
Press the EA-200 [START/STOP] key to start sampling.
When sampling is complete, press the calculator’s w key to restart the program.
^^
^ (Disp command) causes processing to stop until you press the w key.
^^
(4) Sample data from the EA-200 is received by the calculator.
Receive (List 1)
Receive (List 2)
•When using the built-in microphone for sampling,
position it so it is about two or three centimeters
from the sound source.
• If the graph shows that the sampled sound is exceeding the sampling range as
shown below, either lower the volume of the sound source or move the microphone
further away from the sound source.
^^
^ You can change sampling conditions by using different
^^
__
_ Record Time data is received and stored in List 1.
__
__
_ Sampling Data of microphone is received and stored in List 2.
__
µs), and 255 is the number of samples.
sampling interval and number of samples values, if you want.
20020601
Page 11
1-5
English
Using the Built-in Speaker
To output sound recorded by the built-in microphone
(1) Send Command 0 to initialize the EA-200 setup.
{0} → List 1
Send (List 1)
(2) Send Command 1 to specify microphone as the channel.
{1, 10} → List 1
Send (List 1)
(3) Send Command 3 to configure measurement condition settings.
{3, 0.00005, 120000} 3 is the command number, 0.00005 is the sampling interval
→ List 1
Send (List 1)
At this time, the Ready lamp lights on the EA-200.
Press the EA-200 [START/STOP] key to start sampling.
When sampling is complete, press the calculator’s w key to restart the program.
^^
^ (Disp command) causes processing to stop until you press the w key.
^^
(4) Send Command 0 to initialize the EA-200 setup.
{0} → List 1
Send (List 1)
(5) After sampling is complete, use Command 1 to configure speaker settings.
{1,12, 5,10} → List 1
Send (List 1)
__
_ Stores {0} to List 1. 0 is the command number.
__
__
_ Sending List 1 executes Command 0.
__
__
_ 1 is the command number, and 10 indicates use of
__
__
_ the built-in microphone.
__
__
_ (50µs), and 120000 is the number of samples.
__
^^
^ You can change sampling conditions by using different
^^
sampling interval and number of samples values, if you want.
__
_ Stores {0} to List 1. 0 is the command number.
__
__
_ Sending List 1 executes Command 0.
__
__
_ 1 is the command number, 12 specifies the speaker as the
__
__
_ channel, 5 is the number of loops, and 10 specifies output of
__
values sampled by the microphone.
Status Request
This function can be used to fetch the current status of the EA-200.
To use Status Request
{7} → List 1
Send (List 1)
Receive (List 1)
Status Error Code Battery Condition OS Version
0: Standby = O: Normal 0 to 999 Version No.
(
No Sample Data in EA-200
1: Ready Integer: Command number < 450:
2: Sampling Decimal Part: Parameter position low battery
3: Standby
(
Sample Data in EA-200
__
_ Sends Command 7.
__
__
_
__
__
_ Receives the status information and stores it in List 1.
__
Line 1 Line 2 Line 3 Line 4
) ≠ O: Error
) Example: 3.2
Second parameter of Command 3.
Sampling interval value error
Auto Setup
Auto Setup detects the Auto-ID of a probe, and configures applicable settings automatically.
The three probes (voltage, temperature, optical) that come bundled with the EA-200 have
Auto-IDs.*
To use Auto Setup
1. Connect a probe to a channel.
2. Slide the [ON/OFF] switch to turn on power.
1
• This causes the Power lamp to light.
(6) Re-configure the sampling condition settings.
{3,0.00005,120000} 3 is the command number, 0.00005 is the sampling interval
→ List 1
Send (List 1)
(7) Press [START/STOP] to output the sound recorded with the microphone.
__
_ (50
__
^^
^ You can change sampling conditions by using different
^^
µs), and 120000 is the number of samples.
sampling interval and number of samples values, if you want.
3. Press the [SET UP] key.
• This causes the Ready lamp to light.
*1The optional “Motion Sensor (EA-2)” does not have an Auto-ID.
20020601
Page 12
4. Press the EA-200 [START/STOP] key to start sampling.
• The Sampling lamp flashes as sampling is performed.
5. Press the [START/STOP] key again to stop sampling.
6. Connect the EA-200 to the calculator, and then use the Receive command to transfer
the sampled data (see the “Receive Data Command” on page 1-3).
• Data is transferred in the following sequence:
Record Time → SONIC →CH1 → CH2 → CH3.
• Any channel that is not being used is skipped automatically.
1-6
English
Example Operation
The example below shows how a teacher can use the EA-200 Group Link function to
distribute a sampling program and sampled data.
1. Students are divided into multiple groups, and each group has its own EA-200. The
teacher uses his or her EA-200 to distribute a sampling program to each of the group
leaders.
Teacher Calculator
Teacher EA-200
Group Leader 1 Calculator
Group Link Function
A single EA-200 can be used to connect one “MASTER” calculator to up to seven other
“SUB” calculators to distribute programs, data, etc.*
the following calculator models connected to the EA-200 at the same time.*
• ALGEBRA FX Series
• CFX-9850/fx-7400 Series*
3
To perform a Group Link operation
1. Use a Link Cable (SB-62) to connect the calculator that contains the data you want to
send to the MASTER port of the EA-200.
2. Use a Link Cable (SB-62) to connect the calculators to which you want to send the
data to the SUB ports of the EA-200).
3. Slide the [ON/OFF] switch of the EA-200 to turn it on.
4. On all of the SUB calculators, use the LINK application to enter the Receiving Mode.
5. On the MASTER calculator, use the LINK application to transmit the data.
6. The data transfer operation is over when the message “Complete” appears on the
displays of the MASTER calculator and all of the SUB calculators.
1
Note, however, that you cannot have
2
Group Leader 2 Calculator
Group Leader 3 Calculator
2. Each group uses the EA-200 to perform sampling using the program that was
distributed to the group leader’s calculator from the teacher’s calculator. After sampling
is complete, the EA-200 Group Link function is used to distribute the sampled data to
the calculator of each group member.
Group Leader Calculator
Group EA-200
Group Member 1 Calculator
Group Member 2 Calculator
Group Member 3 Calculator
Important!
•You can create your own sampling program while referring to the Program Library on
page 2-16-1, or you can download a program at the CASIO Website:
http://world.casio.com/edu_e/
*1Backup data cannot be distributed.
*2See the “Supported Calculator Models” on page 1-2 for more information about compatibility
between various calculator models.
*3fx-7400 Series calculators support Program and List data only.
20020601
Page 13

Chapter 2
Examples
Uniformly Accelerated Motion
Period of Pendular Movement
Conservation of Momentum
Charles’ Law
Polarization of Light
Natural Frequency and Sound
Column of Air Resonance and the Velocity of Sound
Construction of the Musical Scale
Direct Current and Transient Phenomena
AC Circuit
Dilute Solution Properties
Exothermic Reaction
Electromotive Force of a Battery
Sunlight and Solar Cells
Topographic Conditions and Climate
Program Library
Page 14
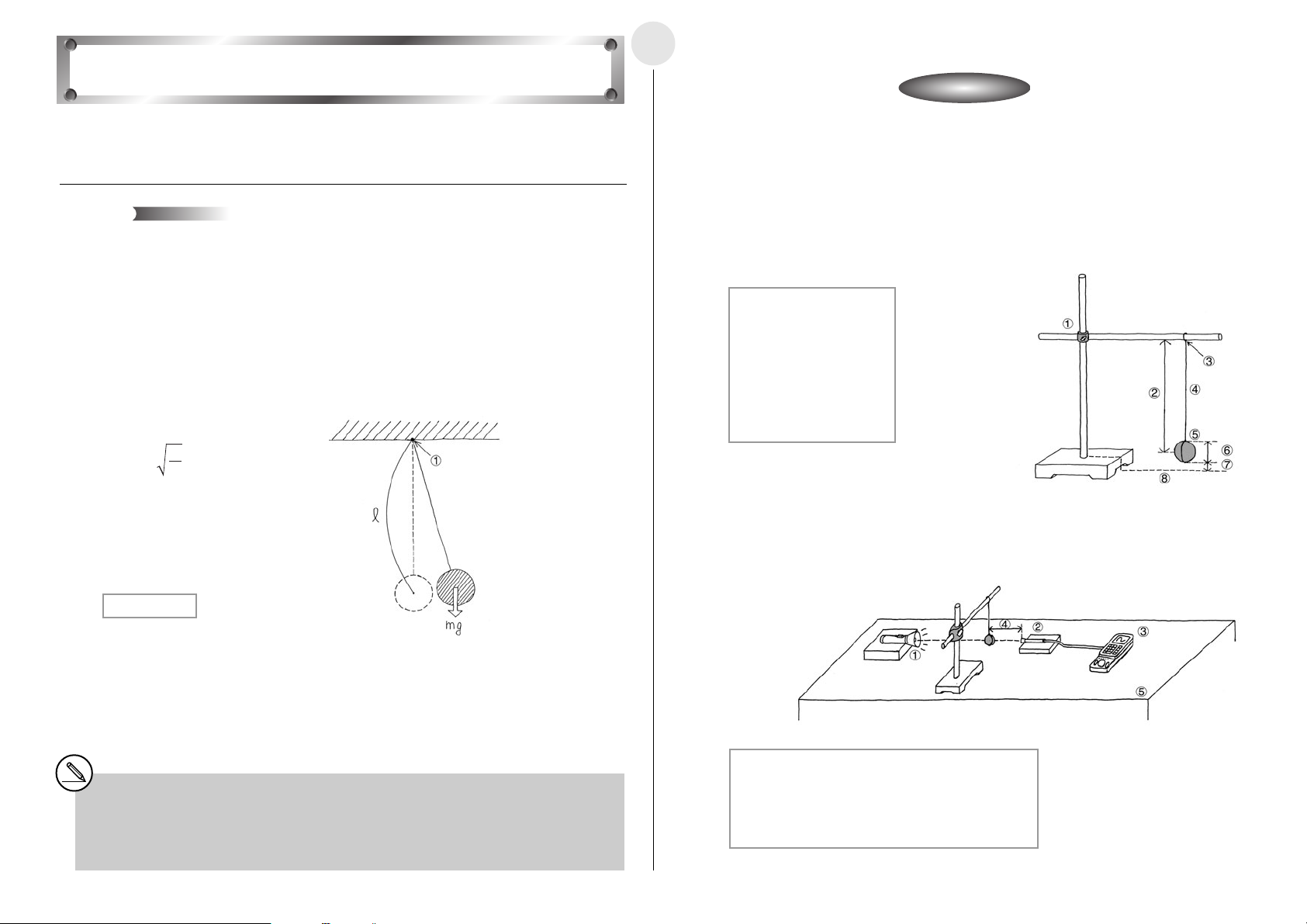
Activity: SetupActivity: Setup
Uniformly Accelerated Motion
This activity observes the movement of a cart down an incline and investigates uniformly
acceleration motion caused by gravity.
2-1-1
English
쐽 Equipment
Cart Ramp Stand Protractor
Distance Measurement Setup (EA-200, graphic scientific calculator, data communication
cable, optional EA-2*
1
)
Theory
A cart placed on an inclined ramp starts to move straight down the ramp. This movement is
due to the force of gravity acting on the cart to pull it down the incline and pulling it against
the ramp. The distribution of these two forces depends on the inclination of the ramp. The
acceleration of the cart is determined by the magnitude of the force that moves the cart.
Movement represented by an acceleration value that does not change over time is called
“uniformly accelerated motion.” The movement of the cart described above is uniformly
accelerated motion. As shown below, the velocity of uniformly accelerated motion is
proportional to time, and the distance traveled is proportional to the time squared. This
means that if you observe the distance covered by the cart over a specific time, you can
determine its acceleration.
F= ma =mg sin
θ
v= at
1
L= at
F(N) : Force Acting on Cart in
a(m/s
m(kg) : Cart Mass
g(m/s
θ
(°):Ramp Angle of Inclination
v(m/s) : Cart Velocity
t(s) : Cart Travel Time
L(m) : Distance Traveled by Cart
2
2
Direction of the Ramp Surface
2
):Cart Acceleration
2
):Gravitational Acceleration
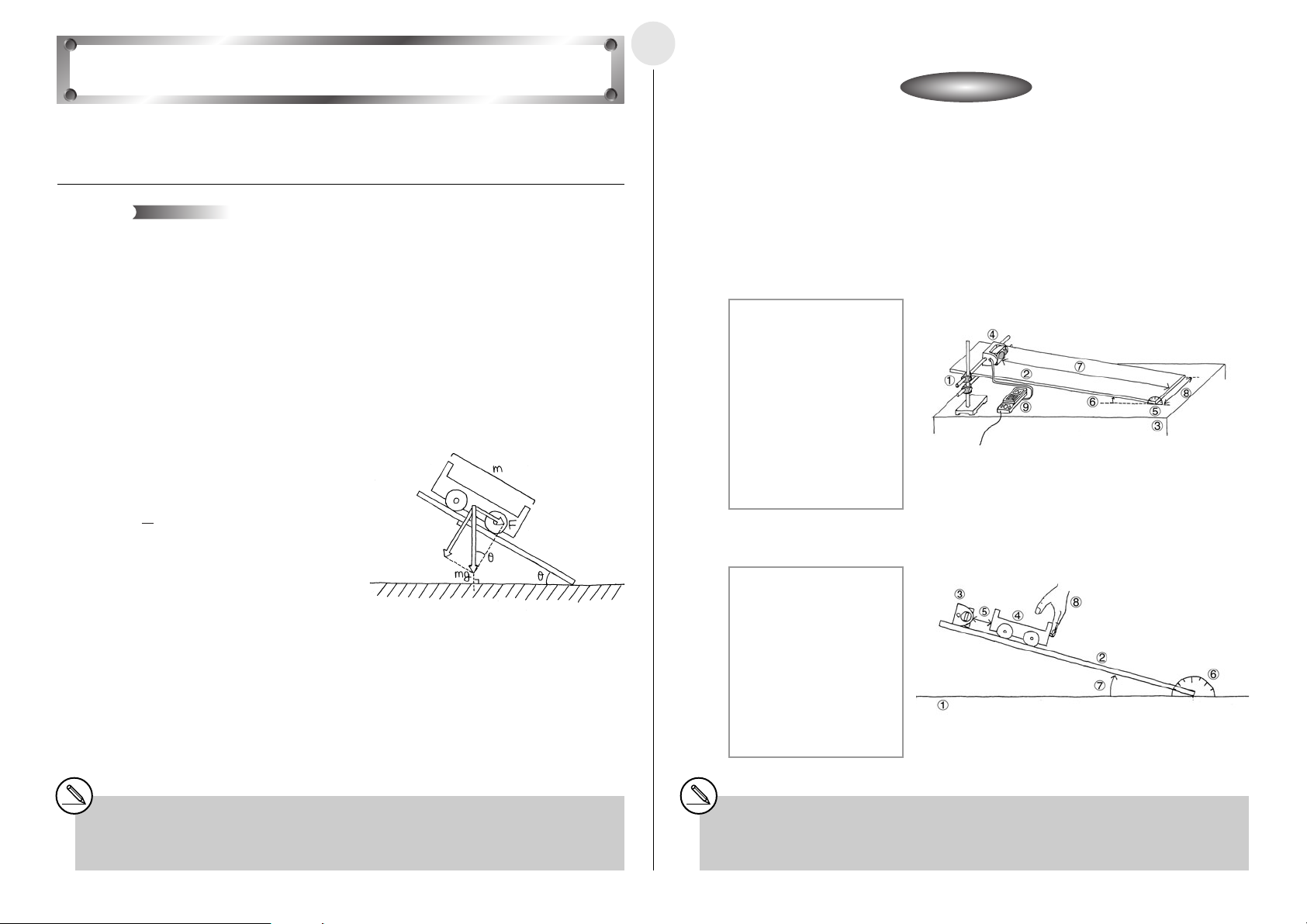
쐽 Setting Up
u Affix the EA-2 to the arm of the stand.
u Measure the incline of the ramp with the protractor, and fix the ramp at an angle of 15
degrees.
1 Stand
2 Ramp
3 Desk
4 EA-2 (SONIC)
5 Protractor
6 Ramp Angle:
15 degrees
7 Distance from EA-2 to
end of ramp: 150cm
8 Ramp Width: 30cm
9 EA-200
u Measure the mass of the cart, place it onto the ramp, and hold it in place with your hand.
1 Desk
2 Ramp
3 EA-2 (SONIC)
4 Cart Mass:
m(kg)
5 Distance Between Cart
and EA-2: 60cm
6 Protractor
7 Ramp Angle: 15 degrees
8 Hand
1
*
Though actual gravitational acceleration depends on latitude and elevation, this activity can be
conducted using the gravitational constant 9.8m/s2.
*1When using the EA-200 in combination with the optional ‘‘Motion Sensor (EA-2) ”, be sure to
power the EA-200 using its bundled AC adaptor (AD-A60024).
20020601
Page 15
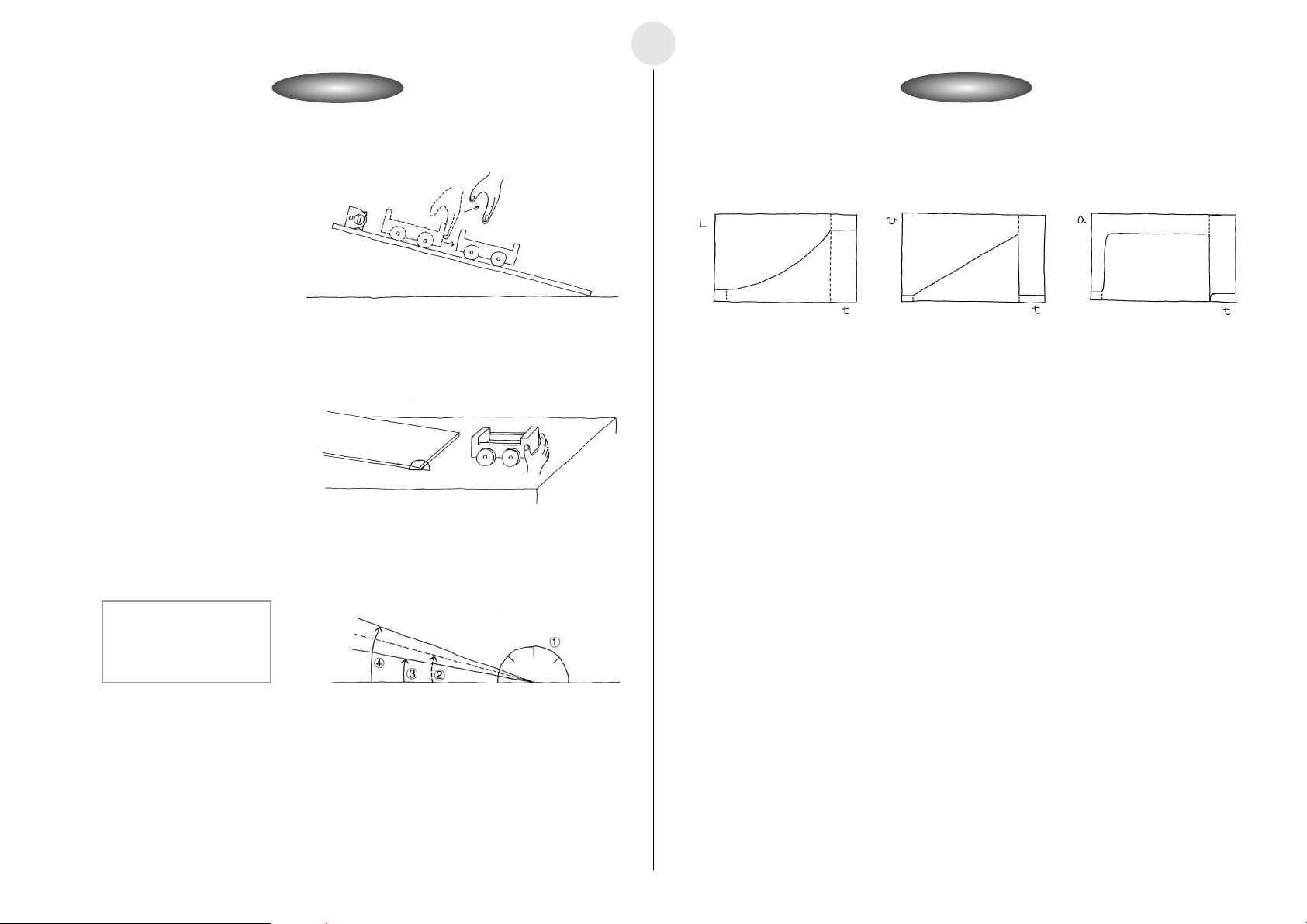
Activity: Operating the EquipmentActivity: Operating the Equipment
MeasurementMeasurement
2-1-2
English
쐽 Measuring Data
u Prepare the Distance Measurement Setup. Immediately after starting the measurement
operation, let the cart go.
u When the cart reaches the desk, stop it.
u Graph the data on the calculator, observe its characteristics, and compare it with
theoretical expectations.
u Change the angle of the ramp 10 degrees and then 20 degrees, and measure again.
u Observe how the graph changes as the ramp angle is changed.
쐽 Calculator Operation
u Find the applicable program in the Program Library (P.2-16-1), input it into your calculator,
and then run it.
u Display graphs for the distance traveled, velocity, and acceleration of the cart.
L(m) : Distance Traveled by Cart
v(m/s):Cart Velocity
2
a(m/s
):Cart Acceleration
t(s):Cart Travel Time
1 Protractor
2 Ramp Angle: 15 degrees
3 Ramp Angle: 10 degrees
4 Ramp Angle: 20 degrees
55555
Other Things To Do
55555555555555555555555
u Study the relationships between the changes of distance traveled, velocity, and
acceleration.
u Consider the reasons why the graphs change as the angle of the ramp is altered.
u Find out how the graph is affected when the mass of the cart is changed by
placing a weight on it.
20020601
55555
5555555555555555555555
Page 16
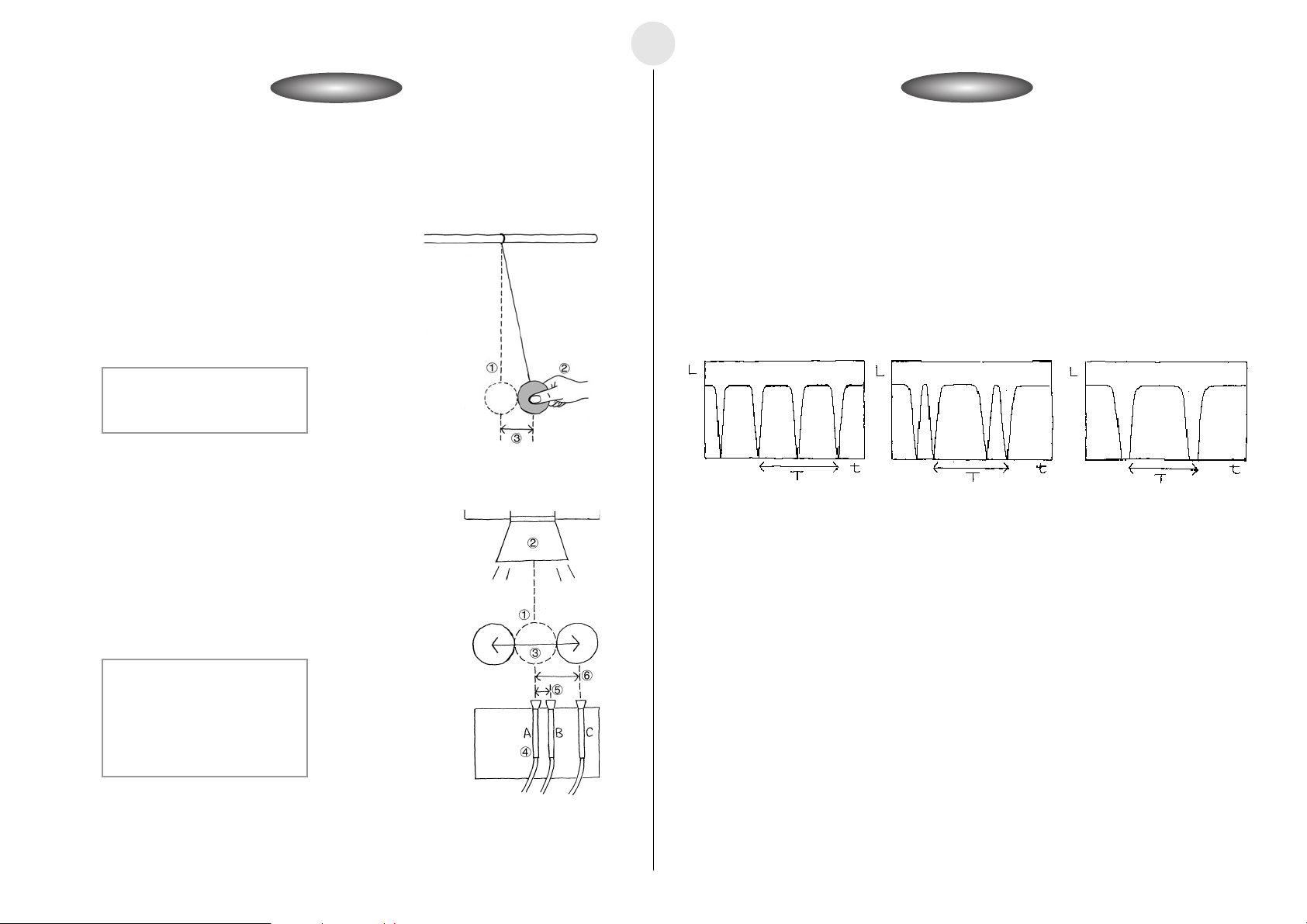
Period of Pendular Movement
In this activity, we create a simple pendulum and then visually check the periodicity of its
movement, while determining its period.
2-2-1
English
Activity: SetupActivity: Setup
쐽 Equipment
String with little elasticity Weight Stand Height adjustment blocks (2)
Optical Measurement Setup (EA-200, graphic scientific calculator, data communication
cable, optical probe)
Theory
A pendulum is a string whose one end is fixed and whose other end has a weight attached
to it. As the weight swings, it keeps returning to the same position at the same velocity over
time. Such motion is called “periodic motion,” and the time it takes for the weight to return to
a particular state is called its “period.” A high-precision pendulum can continue to swing for
a very long time with little change in its period over time. This is why such a pendulum is
often used for timepieces.
A pendulum that moves across a single plane is called a simple pendulum. When the
amplitude of the pendulum is sufficiently short in relation to its length, the period of a simple
pendulum can be expressed as shown below.
T = 2π
R(cm) : Pendulum Length
g(cm/s
m(g) : Mass of Weight
T(s) : Period
1 Fixed Point
All of this means that if gravitational acceleration is a fixed value that depends on the
location where the activity is performed, then the period depends on the length of pendulum
only.
With this activity, a pendulum is setup between a light source and an observer. The shadow
created by the weight makes it possible to observe the periodicity of the pendular motion.
Though actual gravitational acceleration depends on latitude and elevation, this activity can be
conducted using the gravitational constant. When gravitational acceleration is 980cm/s2 and
the length of the pendulum is 25cm, the oscillation period of the pendulum is about one second.
R
g
2
):Gravitational Acceleration
쐽 Building a Pendulum
u Securing the fixed point of the pendulum so it cannot move, assemble the pendulum as
shown in the illustration.
1 Stand
2 Pendulum Length: 30cm
3 Fixed Point
4 String
5 Weight: 100g
6 Weight Size: 3cm
7 Height: 2cm
8 Desk
쐽 Setting Up
u Align the heights of the optical probe and flashlight so the flashlight is shining directly at
the probe.
u Set up the pendulum so the weight blocks the light to the optical probe when it is at rest.
1 Flashlight
2 Optical Probe (Observer) (CH1)
3 EA-200
4 Distance Between Weight and Optical Probe: 10cm
5 Desk
20020601
Page 17
2-2-2
55555555555555555555555
5555555555555555555555
5555555
Other Things To Do
5555555
English
Activity: Operating the EquipmentActivity: Operating the Equipment
쐽 Measuring Data
u Prepare the Optical Measurement Setup.
u Taking care not to allow the string to go slack, move the weight as shown in the illustration
and then gently let it go.
u After the weight swings back and forward a few times, start the measurement operation
on the EA-200.
1 Weight Position of Equilibrium
2 Hand
3 Amplitude: 3cm
u Move the optical probe from position A to position B or C, and then repeat the measure-
ment operation.
MeasurementMeasurement
쐽 Calculator Operation
u Perform the following operation to prepare for light measurement using the optical probe.
Using E-CON
m“E-CON”w1(SETUP)b(Wizard)w
1(CASIO)d(Light) 0.01w255w1(YES)
Using a Calculator Program
Find the applicable program in the Program Library (P.2-16-1), input it into your calculator,
and then run it.)
u Determine the period from graphs of the measurement results obtained at each
measurement position.
L : Light Intensity
t(s) : Time
T(s): Period
1 Weight Position of Equilibrium
2 Flashlight
3 Weight Movement
u Determine the period from the graph of measurement results, and compare this with the
4 Optical Probe
5 Distance Between A and B: 1cm
6 Distance Between A and C: 3cm
calculated value.
u Find out how the period changes when you change the length of the pendulum.
20020601
u Find out how the period changes when you change the mass of the weight.
u Find out how the period changes when you change the size of the weight.
u Find out how the period changes when you change the amplitude.
u Find out what happens when you use an iron or magnetic weight with a magnetic
sheet under the weight.
u Consider why the period changes under the conditions described above.
Page 18
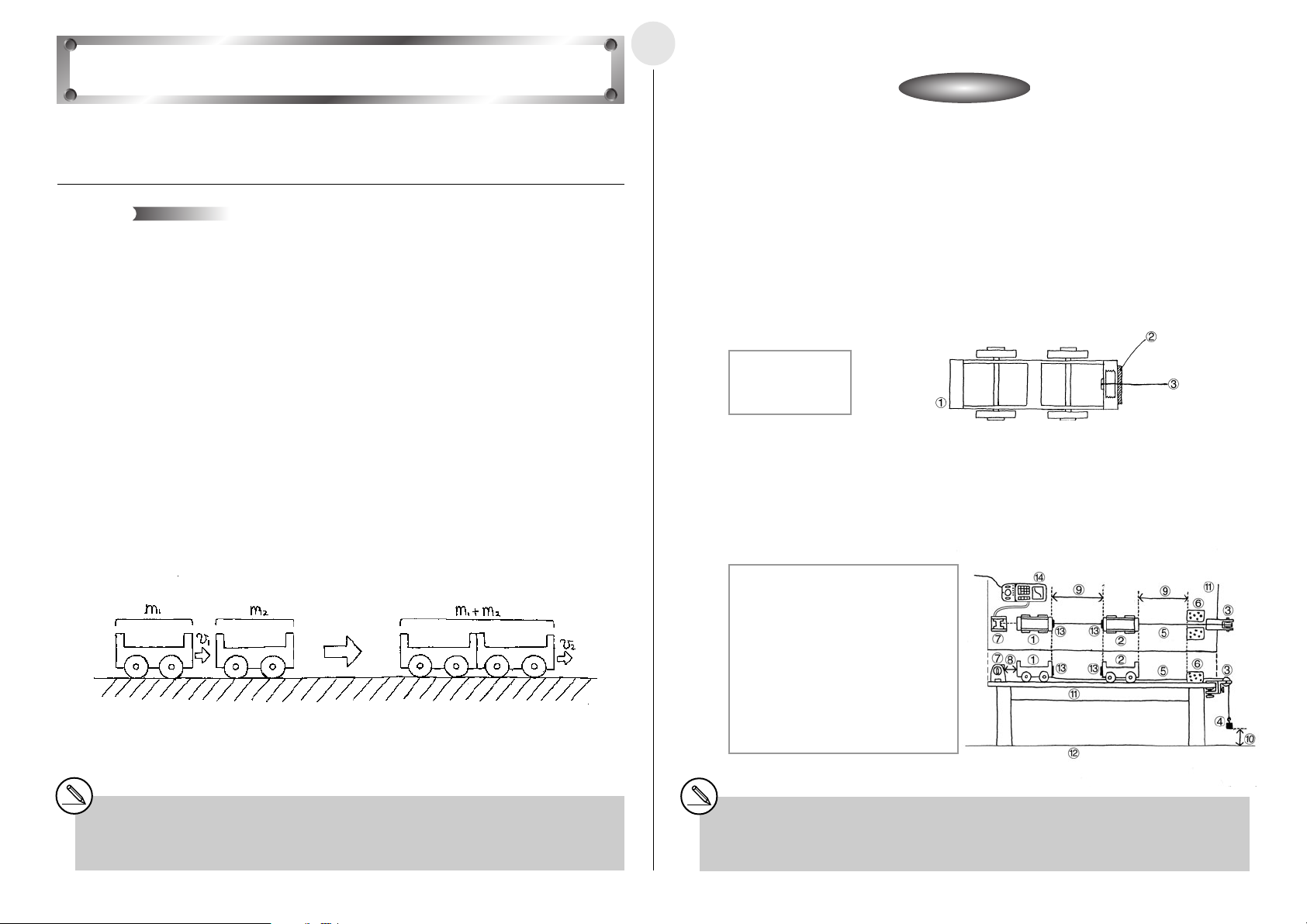
Conservation of Momentum
The purpose of this activity is to investigate the law of conservation of momentum through
the collision of two carts.
Theory
Collisions can take on many different forms, and can involve automobiles, locomotives,
shopping carts, or even two people. The force of the impact when the two objects collide
depends not only on their velocities but also their respective masses (weight), and you can
calculate the momentum of an object by multiplying its mass by its velocity.
Despite the variables involved, one principle always holds true – if external forces such as
friction are ignored, the sum of the momenta of two objects prior to collision is the same as
the sum of the momenta of the objects after collision. This is the principle known as the
“conservation of momentum.” This principle is an excellent tool for understanding the
dynamics of collisions.
The following expresses conservation of momentum in the case of a stationary cart being
struck by another cart, after which the two carts adhere to each other and continue in
motion together.
m1v1 = (m1 + m2)v
m
(kg) : Mass of Cart 1
1
m
(kg) : Mass of Cart 2
2
v
(m/s) : Velocity of Cart 1 Before Collision
1
v
(m/s) : Velocity of Two Carts After Collision
2
This means that when the two carts are of identical mass, the combined velocity of the two
carts after collision is one half that of the velocity of Cart 1 before the collision.
2
2-3-1
English
Activity: SetupActivity: Setup
쐽 Equipment
Two carts of identical mass String Pulley with a bracket to secure it
Ve lcro쑓 500g weight Cushion
Distance Measurement Setup (EA-200, graphic scientific calculator, data communication
cable, optional EA-2*
쐽 Preparing the Carts
u Measure the masses of Cart 1 and Cart 2.
u Affix Velcro쑓 to the impact surfaces of Cart 1 and Cart 2.
u Use tape to securely affix the string to the center of the front end (the same end where
the Velcro쑓 is affixed) of Cart 1.
1 Back of Cart 1
2 String
3 Velcro쑓
쐽 Setting Up
u Align the EA-2, Cart 1, Cart 2 and the pulley in a straight line.
u Run the string under Cart 2 and place it onto the pulley. Attach the weight to the end of
the string.
u Support the weight with your hand so it does not pull Cart 1.
1 Cart 1
2 Cart 2
3 Pulley
4 500g weight
5 String
6 Cushions
7 EA-2 (SONIC)
8 Allow 60cm
between EA-2
and Cart 1.
1
)
9 Distance: 50cm
0 Height of weight
from floor: 30cm
! Desk
@ Floor
# Velcro쑓
$ EA-200
1
*
The above expression can be used to obtain the post-collision velocity of two carts (while they
adhere to each other) of different masses.
*1When using the EA-200 in combination with the optional ‘‘Motion Sensor (EA-2) ”, be sure to
power the EA-200 using its bundled AC adaptor (AD-A60024).
20020601
Page 19

2-3-2
55555555555555555555555
5555555555555555555555
55555
Other Things To Do
55555
English
Activity: Operating the EquipmentActivity: Operating the Equipment
쐽 Measuring Data
u Prepare the Distance Measurement Setup. Immediately after starting the measurement
operation, allow the weight to drop.
1 Hand
2 500g weight
3 Floor
4 String
5 Pulley
u Be ready to catch the carts with your hands if the cushion does not stop them.
u Compare calculated theoretical velocity values with your measured velocity.
MeasurementMeasurement
쐽 Calculator Operation
u Find the applicable program in the Program Library (P.2-16-1), input it into your calculator,
and then run it.
u Display graphs for the distance traveled, velocity, and acceleration of Cart 1.
L(m) : Cart 1 Distance Traveled
v(m/s) : Cart 1 Velocity
2
a(m/s
) : Cart 1 Acceleration
t (s) : Time
20020601
u Think about why Cart 1 Acceleration never registers a value of zero.
u Add weight to Cart 1 and Cart 2 to change their masses and find out how this affects
velocity.
u Think about what would happen if we replaced the Velcro쑓 with a spring.
Page 20

Activity: SetupActivity: Setup
Charles’ Law
This activity is designed to confirm Charles’ law through an actual experiment.
Theory
Increasing the temperature of a gas causes the molecules that make up the gas move
faster. The pressure within the container that holds the gas is determined by the number of
collisions between the molecules and the walls of the container, and by the velocity of the
molecules when they collide with the walls. If pressure remains constant and temperature
increases, the gas expands, which reduces the number of molecular impacts with the
container walls and negates the increase in molecular velocity.
Charles’ Law states that the thermal expansion of rarified gas of constant pressure is
proportional to the increase in temperature, and is represented by the expression shown
below. If the temperature when the volume of gas reaches zero is defined as absolute zero,
absolute zero is –273°C.
0
V
V = (T + 273)
273
3
V(m
):Gas Volume
V
(m3):Gas Volume at 0°C
0
T (°C) : Gas Temperature
2-4-1
English
쐽 Equipment
Syringe (with scale markings) Plastic Container Rubber Tube
Rubber Gasket Clip Mixing Stick
Warm Water, Cold Water, Ice
Temperature Measurement Setup (EA-200, graphic scientific calculator,
data communication cable, temperature probe)
쐽 Assembling the Equipment
u Cut a hole into the side of the plastic container, and affix the rubber gasket around the
hole on the outside of the container.
u Slip the syringe with the rubber tube on its tip into the hole, and pack it with rubber to
make it watertight.
u Affix the clip to the rubber tube to seal the air inside the syringe.
1 Plastic Container
2 Rubber Gasket
3 Rubber Tube
4 Syringe
5 Hole
6 Clip
1 Absolute Zero
쐽 Setting Up
u Fill the plastic container with warm water and wait until the air in the syringe stabilizes.
1 Plastic Container
2 Warm Water: 60°C
3 Mixing Stick
4 Temperature Probe
(CH1)
5 EA-200
20020601
Page 21

Activity: Operating the EquipmentActivity: Operating the Equipment
MeasurementMeasurement
55555555555555555555555
5555555555555555555555
55555
Other Things To Do
55555
2-4-2
English
쐽 Measuring Data
u The water temperature is displayed on the calculator.
u Read the volume of the gas using the markings on the syringe and
read temperature at that time, which is displayed on the calculator.
1 Temperature Probe
2 Mixing Stick
3 Syringe
4 Warm Water
5 Reading Position
u Add a little of the cold water to the water in the container, and mix. After waiting a few
minutes, take readings of the volume and temperature.
u Repeat this step until the temperature of the water in the container approaches the
temperature of the cold water.
1 Cold Water
2 Mixing Stick
u Repeat the above steps, adding ice instead of cold water and taking readings.
1 Ice
2 Mixing Stick
쐽 Calculator Operation
u Use the Temperature Measurement Setup to measure the temperature and then display it.
u Find the applicable program in the Program Library (P.2-16-1), input it into your calculator,
and then run it.
u Graph the readings, which produces a graph that is virtually linear.
u Substitute two of the values on the graph into the expression, and calculate the
temperature where volume becomes zero.
V1 – V0
V = T + V
1
T
Should the plastic container become too full during the above steps, it is all right to remove some
of the water. However, make sure that the syringe remains under the surface of the water.
0
T = –
0T1
V
V1 – V
0
u See if you can approximate a linear graph where a large number of measured
20020601
values (plots) pass the point at –273°C (absolute zero).
u Consider why observed values are scattered around a straight line.
u Try repeating the above steps with the syringe filled with another type of gas
(helium, oxygen, etc.).
Page 22

Activity: SetupActivity: Setup
Polarization of Light
This activity investigates the relationship between reflection, refraction, and polarization of
light.
Theory
Light is electromagnetic radiation that has the properties of transverse waves. Sunlight
includes transverse waves that oscillate in various directions.
A polarizer allows only light vibrating in a specific direction to pass, which means that
sunlight coming out the other side is vibrating in that direction. This is called “polarization of
light.” Stacking together two polarizers with their polarization directions oriented
perpendicular to each other “extinguishes” the light, which means that no light penetrates
the second polarizer.
The expression below represents the change in the amplitude of light passing through the
second polarizer. Since light quantity changes in proportion to the square of its amplitude,
the light passing through the second polarizer is darker than the original light.
A’ = Acos
’
A
A : Amplitude of Light
θ
(°): Angle of Polarization
Most light is polarized when it is reflected or refracted by the boundary surface of material
where electromagnetic fields meet the required boundary conditions.
Especially at an angle called “Brewster’s angle,” polarization is completely linear, and
reflected light and refracted light polarization is orthogonal. The expression shown below
defines the conditions that such an angle needs to satisfy.
α + β
α
(°):Angle of Incidence and
β
(°):Angle of Refraction
θ
: Amplitude of Light
Polarized by Polarizer 2
Polarized by Polarizer 1
Direction of Two Polarizers
= 90°
Angle of Reflection
1 Incident Light
2 Reflected Light
3 Refracted Light
4 Boundary
Surface
2-5-1
English
쐽 Equipment
Polarizers (2) Thick Paper Wood Penlight
Glass Screen Protractor
Optical Measurement Setup (EA-200, graphic scientific calculator, data communication
cable, optical probe)
쐽 Preparing the Polarizers
u Cut holes in three sheets of thick paper, and use the protractor to measure and mark
angles on one of them.
u Mark the polarizing direction on the polarizers, and cut out one as a circle.
u Affix the wood frame and blocks to the thick paper, sandwich the polarizer between the
two sheets of paper as shown in the illustration.
1 Wood Frame
2 Wood Blocks
3 Thick Paper with Hole Cut
Out
4 Arrow Indicating Polarizing
Direction
5 Removable Polarizer
6 Circular Polarizer
7 Angle Markings
쐽 Preparing the Glass Stand
u Fix the screen one centimeter above the glass surface that will be struck by the incident
light.
u Affix the protractor in accordance with the screen position.
1 Glass
2 Screen
3 Protractor
4 Distance Between Screen
and Glass Surface: 1cm
20020601
Page 23

Activity: Operating the EquipmentActivity: Operating the Equipment
MeasurementMeasurement
55555555555555555555555
5555555555555555555555
55555
Other Things To Do
55555
2-5-2
English
쐽 Measuring the Angle of Polarization and the Light Intensity
u Position the optical probe so it is pointing at the penlight and picking
up the maximum light intensity.
u Prepare the Optical Measurement Setup and start the measurement. Rotate the polarizer
90 degrees at a constant speed, every 20 seconds.
1 Penlight
2 Polarizers
3 Optical Probe (CH1)
4 Hand
5 Polarizing Direction
6 Direction of Turn
7 EA-200
4
1
2
5
6
4
3
7
쐽 Measuring Brewster’s Angle
u The light intensity is displayed on the calculator.
u From an angle determined using the protractor, shine the penlight beam onto the glass.
u Position the optical probe so it is pointing at the light beam and picking up the maximum
light intensity.
u Rotate the polarizer until the polarization direction is that where the light intensity is the
greatest.
u Measure the polarizing direction of the reflected light.
u Measure the polarizing direction of the refracted light.
u Determine the angle of the penlight beam that satisfies the condition expression of
Brewster’s angle.
쐽 Calculator Operation
u Prepare for measurement of light intensity using the optical probe, which will let you
determine the angle of polarization.
Using E-CON
m“E-CON”w1(SETUP)b(Wizard)w
1(CASIO)d(Light) 0.1w200w1(YES)
Using a Calculator Program
Find the applicable program in the Program Library (P.2-16-2), input it into your calculator,
and then run it.
u This displays a graph that shows changes in light intensity as the polarizer is rotated.
L : Light Intensity
t(s): Time
u Perform the following operation to measure Brewster’s angle.
u Find the applicable program (Light Multi Meter) in the Program Library (P.2-16-2), input it
into your calculator, and then run it to measure light intensity.
1 Penlight
2 Polarizer for Reflected Light Measurement
3 Optical Probe for Reflected Light
Measurement
4 Polarizer for Refracted Light Measurement
5 Optical Probe for Refracted Light
Measurement
To obtain an accurate picture of changes in polarizer angle and light intensity, it is a good idea to
graph light intensity at various angles.
20020601
u Investigate changes in Brewster’s angle using materials other than glass.
u The 3D effect is possible because of the slight difference between how an object is
viewed by the left and right eyes. Consider how 3D imaging technology uses the
characteristics of light polarization to achieve its effects.
Page 24

Activity: SetupActivity: Setup
Natural Frequency and Sound
This activity investigates sounds produced in accordance with the natural frequencies of
objects we use in everyday life. It also studies the characteristics of frequencies.
2-6-1
English
쐽 Equipment
Box String Bolts (2) Triangular Wood Blocks (2)
Audio Measurement Setup (EA-200, graphic scientific calculator, data communication
cable)
Theory
Hitting, striking, plucking, or otherwise disturbing just about any object will cause it to
vibrate. Dropping a pencil or ruler to the floor, or plucking a banjo string will cause it to
vibrate. The sound produced when you blow over the top of a bottle is the air inside of it
vibrating. The vibration of an object tends to occur at a particular frequency or a particular
set of frequencies, which is the “natural frequency” of the object.
Though the strength of the strike, pluck, or other disturbance applied to an object affects the
frequency of the sound produced, in most cases the sound produced is a louder version of
the natural frequency. Generally, the sound produced by an object is the result of multiple
natural frequency sound waves superimposed on each other.
The expression below provides the natural frequency of a string that is fixed at both ends. In
this case, all of the natural frequencies are integer multiples of f
“fundamental frequency.” The fundamental frequency is the lowest possible frequency at
which an object can vibrate freely.
n
2L
S
ρ
f
=
n
f
(Hz) : String Natural Frequency (n = 1, 2, 3 ...)
n
L (m) : String Length
S(N) : String Tension
ρ
(kg/m) : String Linear Density (per meter)
1, which is called the
쐽 Building a Monochord
u Use tape to affix the bolts at either end of the box, and stretch the string taut between
them.
u Insert a triangular wood block between the string and the box.
1 Box
2 Box Length: 50cm
3 Bolt
4 String
5 Block
쐽 Setting Up
u Insert two wood blocks between the string and box, and set the monochord on a table or
desk.
u Position the Audio Measurement Setup where it can pick up the sound from the
monochord.
1 Desk
2 Monochord
3 EA-200
20020601
Page 25

Activity: Operating the EquipmentActivity: Operating the Equipment
MeasurementMeasurement
55555555555555555555555
5555555555555555555555
55555555555
Other Things To Do
55555555555
2-6-2
English
쐽 Measuring the Sound Frequency
u Position the two wood blocks so there is about 40cm between them, and then lightly pluck
the center of the string to produce a sound.
u Record the sound with the Audio Measurement Setup, perform FFT analysis, and view
the frequency distribution.
1 Monochord
2 Desk
3 Distance Between
Blocks: 40cm
4 Finger
u Position the two wood blocks so there is about 20cm between them, and then lightly pluck
the center of the string to produce a sound.
u Record the sound on the Audio Measurement Setup, perform FFT analysis, and view the
frequency distribution.
1 Monochord
2 Desk
3 Distance Between
Blocks: 20cm
4 Finger
쐽 Calculator Operation
u Prepare the Audio Measurement Setup for recording.
u Find the applicable program in the Program Library (P.2-16-2), input it into your calculator,
and then run it.
u Perform FFT analysis on the sound recorded when the blocks are 40cm apart, and study
the frequency distribution.
1 Waveform
2 Frequency
Distribution
11
3 f
4 f12
u Perform FFT analysis on the sound recorded when the blocks are 20cm apart, and study
the frequency distribution.
1 Waveform
2 Frequency
Distribution
21
3 f
4 f22
S : Sound Volume
t(s) : Time
N(counts) : Number of
Counts
f (Hz) :Frequency
S : Sound Volume
t(s) : Time
N(counts) : Number of
Counts
f (Hz) :Frequency
u Calculate values for f
, f22/f21, f21/f11, and compare them.
12/f11
u Next, note the relationship with each of the above values with the value 2.
u Try changing the distance between the blocks, the location where you pluck the string,
and the strength of the pluck, and see how it affects the frequency.
u Consider the reason why f
12/f11, f22/f21, and f21/f11 are not exactly 2.
u Find out how the natural frequency changes when you use a different type of
string.
u Modify the monochord as shown in the illustration below, and adjust the weight so
it changes the tension of the string. Study how the natural frequency is affected by
changes in the tension of the string.
1 Pulley
2 Weight
u Consider the reason why f12/f11, f22/f21, and f21/f11 are values in the vicinity of 2.
20020601
Page 26

Activity: SetupActivity: Setup
Column of Air Resonance and
v
the Velocity of Sound
This activity uses the resonance of a column of air to measure the velocity of sound.
Theory
Resonance is what occurs when one object vibrating at the same natural frequency of a
second object causes the second object to vibrate. If you have two tuning forks of the same
natural frequency located near each other and strike one of the tuning forks so begins
vibrating, the other tuning fork will also vibrate even if you do not strike it. This is due to
resonance.
This activity uses a fixed-frequency sound source to produce resonance in a vertical
resonance tube. The sound produced by the resonating column of air will sound louder than
the sound produced by the sound source.
The expressions below show the relationships between the length of the column of air and
wavelength, and the velocity of sound and wavelength. The relationship between the
velocity of sound and wavelength is called the basic equation.
2n–1
L
n
= λ –
4
Ln(m) :Air Column Length for Resonance
L(m) :Air Column Open-end Correction
욼
λ
(m) : Wavelength of Sound
v(m/s) : Velocity of Sound
f(Hz) : Frequency of Sound Wave
n (n = 1, 2, 3...)
Point
욼L v
=
f
λ
2-7-1
English
쐽 Equipment
Glass Resonance Tube (Uniform Inside Diameter, With Scale Markings)
Rubber Tube Reservoir Stand
Low Frequency Generator (or Tuning Fork)
Audio Measurement Setup (EA-200, graphic scientific calculator,
data communication cable)
Temperature Measurement Setup (EA-200, graphic scientific calculator,
data communication cable, temperature probe)
쐽 Setting Up
u Set up the equipment as shown in the illustration, and fill with water, taking care it does
not overflow.
u Raise and lower the reservoir and check to make sure that the level of the water changes.
1 Glass Resonance Tube
2 Tube Length: 1 meter
3 Stand
4 Reservoir
5 Rubber Tube
6 Low Frequency Generator:
800Hz
7 Speaker
8 EA-200
Actually, the air around the opening in the resonance tube also behaves like part of the air
column. This is called “open-end correction.” The effects of open-end correction can be
eliminated by measuring the length of the air column at Resonance Point 1 and Resonance
Point 2 and calculating the difference between the two. This can be used in combination
with the wave basic equation to determine the velocity of sound, using the expression
below.
1 Resonance Point 1
2 Resonance Point 2
20020601
Page 27

Activity: Operating the EquipmentActivity: Operating the Equipment
MeasurementMeasurement
55555555555555555555555
5555555555555555555555
5555555555
Other Things To Do
5555555555
2-7-2
English
쐽 Measuring the Resonance Points
u Record the sound on the Audio Measurement Setup,
display the waveform, and observe the amplitude.
u Lower the water level, and find the point of maximum
amplitude.
1 Glass Resonance Tube
2 Resonance Point 1
3 L
1
4 Sound Wave Amplitude
A: Water Level A
B: Water Level B
C: Water Level C
u Lower the water level more, and find the next point of
maximum amplitude.
1 Glass Resonance Tube
2 Resonance Point 1
3 L
1
4 Resonance Point 2
5 L
2
6 Sound Wave Amplitude
D: Water Level D
E: Water Level E
F: Water Level F
u Repeat the measurement three times and calculate
the average of the results.
u Substitute the average values of L
1 and L2 into the
theoretical expression and calculate the velocity of
sound.
쐽 Measuring the Temperature of the Air Column
u Use the Temperature Measurement Setup to measure the temperature and then display it.
u Substitute the measured temperature values into the expression and calculate the
velocity of sound. Next, compare the results with the previously obtained value.
v
= 331.5 + 0.61T
v(m/s) : Velocity of Sound
T(°C) : Air Column Temperature
1 Glass Resonance Tube
2 Temperature Probe (CH1)
3 EA-200
쐽 Calculator Operation
u Use the Audio Measurement Setup to record the sound and display the waveform.
u Find the applicable program in the Program Library (P.2-16-2), input it into your calculator,
and then run it.
1 Waveform
2 Amplitude
S:Sound Volume
t(s):Time
u Confirm that amplitude is at its maximum
for water levels B and E.
u Use the temperature probe to measure the temperature and then display it.
u Find the applicable program (Charles’ Law) in the Program Library (P.2-16-1), input it into
your calculator, and then run it.
u Repeat the experiment using a different frequency, and compare the difference in
resonance points and sound velocity.
u Use FFT analysis to determine the frequency at each water level, and compare the
results with the sound source frequency.
u Substitute the observed velocity into the theoretical formula and calculate the open-
end correction value.
u Investigate what you need to multiply the open-end correction value in order to
obtain the inside diameter of the resonance tube.
u Perform the activity with a glass tube of a different diameter and find out how the
open-end correction value is affected.
Waveform at Water Levels A, C, D, and F
Waveform at Water Levels B and E
20020601
Page 28

Activity: SetupActivity: Setup
Construction of the Musical Scale
The purpose of this activity is to investigate the scale that is most commonly used for
Western-style music, and to listen to some of the consonance it can produce.
2-8-1
English
쐽 Equipment
Piano (Sound Source) Computer (MIDI Sound Source)
Audio Measurement Setup (EA-200, graphic scientific calculator, data communication
cable)
Theory
The pitch of a note is determined by its frequency, and the human ear perceives notes as
differences in frequency ratios, rather than differences in the relative amplitude of the
frequency.
The ratios of the 12-note mean scale used for most Western-style music is governed by a
number of restrictions. First, the frequency ratio of the same note from one octave to the
next is 2:1 (higher note to lower note). Each octave is divided into 12 parts, with the same
frequency ratio between each of the adjoining notes in the octave. The illustration below
shows each of the notes in an octave, on a piano keyboard.
1 1 Octave (2:1 frequency ratio
between notes)
2 Tonic Note (Low Note)
3 Harmonic (High Note)
4 Adjacent Note (Note
Frequency Ratio = 2
The frequencies of this 12-note scale can be expressed as shown below.
n
fn = 2 f
f
f
f
12
0
(Hz) : Tonic Note Frequency
0
(Hz): Harmonic Frequency
12
(Hz) : Frequency of nth Note (n = 1, 2, 3, ....12)
n
(1/12)
)
쐽 Setting Up
u Take care so there is no unwanted noise in the area where you are conducting the activity.
1 Piano
2 Computer (MIDI
Sound Source)
3 EA-200
2
1
3
1 Frequency (Hz)
2 Low Note
3 High Note
Generally, notes consist of sound waves of different frequencies and amplitudes. Producing
two notes of different pitches at the same time sounds pleasing to the human ear, and such
notes are said to be “consonant.” Two notes whose frequency ratio is the ratio of two simple
integers are very consonant.
20020601
Page 29

Activity: Operating the EquipmentActivity: Operating the Equipment
MeasurementMeasurement
2-8-2
English
쐽 Analyzing Do-Re-Mi
u Record the notes Do, Re, and Mi, and then record the peaks of their frequency distribu-
tions. These are called “frequency components.”
u Study the relationship of the frequency components included in the single-note frequency
distribution.
u Study the relationship of the highest peaks of different notes.
1 Waveform
2 Frequency
Distribution
3 Peak
12
3
3
쐽 Octaves
u Record Do in two adjoining octaves, and make a note of its frequency components.
u On the EA-200, double the frequency of the lower Do to synthesize the higher Do, and
then compare the result with the corresponding note played on the piano.
쐽 Consonant Notes
u The sound at a frequency ratio of 1:1 is the original sound, and the sound at 2:1 is called
an “octave.” Two notes such as these are said to possess “absolute consonance.” Play the
same note in two different octaves to see what absolute consonance sounds like.
u Sounds at the frequency ratios 3:2 and 4:3 possess “perfect consonance,” sounds at 5:3
and 5:4 possess “medial consonance,” and sounds at 6:5 and 8:5 possess “imperfect
consonance” Predict the consonance of Do-Re-Mi from their frequencies, and then
actually play the notes on the piano.
u Using the EA-200’s frequency conversion function, create and produce consonant notes.
Next, play the same notes on the piano for comparison.
쐽 Electronic Sound
u Use the EA-200 to record Do played using a piano timbre on the computer MIDI sound
source, and check its frequency components. Next, compare this with the frequency
component of Do played on the acoustic piano.
쐽 Calculator Operation
u Record the sound on the EA-200, perform FFT analysis, and view the frequency.
u Find the applicable program in the Program Library (P.2-16-2), input it into your calculator,
and then run it.
u Use the EA-200 frequency conversion function to create synthesized sounds.
1 Before Conversion
2 After Conversion
f (Hz) :Frequency
N(counts) : Number of Counts
u Find the applicable program (Natural Frequency and Sound) in the Program Library
(P.2-16-2) , input it into your calculator, and then run it to use FFT graph (1
55555
Other Things To Do
55555555555555555555555
u Consider why notes synthesized on the EA-200 are different from those produced
by the piano.
u In many cases, physical properties become evident by studying frequency
components. Consider why this is so.
u Consider what noise is by checking its frequency components.
, 2).
55555
5555555555555555555555
20020601
Page 30
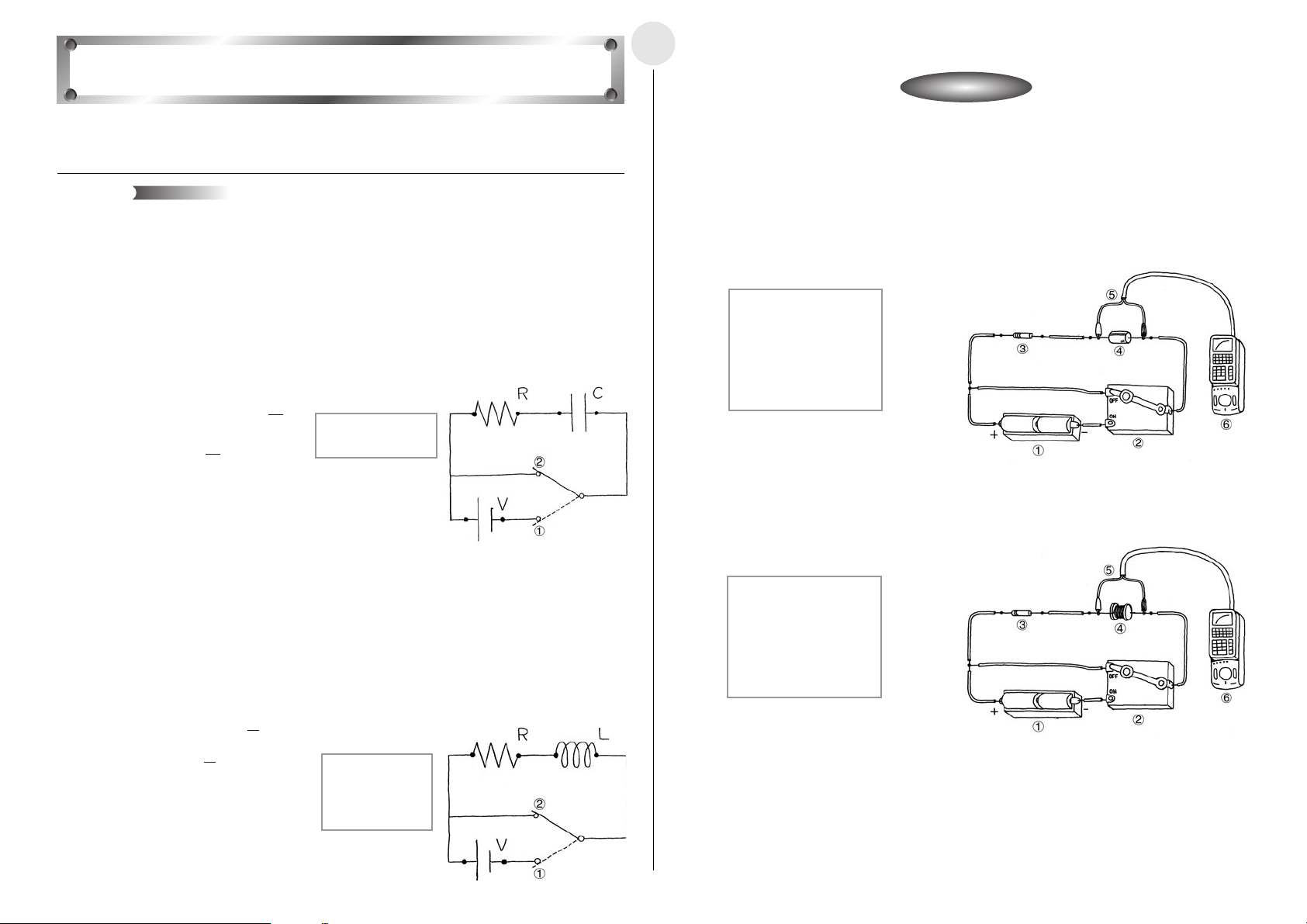
Activity: SetupActivity: Setup
Direct Current and Transient Phenomena
2-9-1
English
This activity investigates transient phenomena when direct current flows through a capacitor
and coil.
Theory
Generally speaking, electrical current is the movement of free electrons within metal. When
electrical current flows through a capacitor, electrons are accumulated, and the capacitor
stores the charge. The accumulation of an electrical charge is called “charging,” while the
loss of the charge by the capacitor is called “discharging.”
Connecting a resistor and capacitor serial circuit to a D.C. power supply causes current to
flow to the capacitor, which charges until it reaches a steady state. Now if the power supply
is removed and the circuit is closed, current flows to the capacitor again, which now
discharges until it reaches a steady state. Current flows to the capacitor in the opposite
direction that it flowed during charging. The change in capacitor voltage during the transient
phase until the capacitor reaches a steady state, while charging and discharging is
represented by the expression shown below.
t
–
VC = V – VR = V 1– e
t
–
C = VR = V
V
V
R (V): Voltage Across the Resistor
V
C(V ): Voltage Across the Capacitor
RC
e
RC
( )
1 Charge Circuit
2 Discharge Circuit
V (V ) : Power Supply Voltage
R (Ω) : Resistance
C (F ) : Capacitance
t (s ) : Time
When the current flowing through a coil changes over time, the coil induces voltage, like an
electrical generator. This is called “self-induced electromotive force,” which acts to oppose
the change in current. When a D.C. power supply is connected in a resistor and coil series
circuit, the effect of the coil’s self-induced electromotive force can be observed until current
reaches a fixed value. Similarly, self-induced electromotive force can also be observed by
cutting off the power supply to a circuit through which steady current is flowing.
The change in coil voltage during these transient phases is represented by the expressions
below.
VL = VR – V = – V
VL = VR = V
V
R (V): Voltage Across the Resistor
V
L(V ): Voltage Across the Coil
V (V ) : Power Supply Voltage
e
–
R
–
t
L
e
R
t
L
1 Circuit With
Power Supply
2 Circuit Without
Power Supply
R (Ω) : Resistance
L (H) : Self-inductance
t (s ) : Time
쐽 Equipment
Battery (D.C. Power Supply) Resistor Capacitor Coil Switch
Voltage Measurement Setup (EA-200, graphic scientific calculator, data communication
cable, voltage probe)
쐽 Building the RC Series Circuit
u The product of the resistance value and the capacitor’s capacitance should be around 1.
1 3V D.C. Power Supply
2 Switch
3 10kΩ Resistor
4 100
µ
F Capacitor
5 Voltage Probe (CH1)
6 EA-200
쐽 Building the RL Series Circuit
u The quotient when the resistance value is divided by the self-inductance of the coil should
be around 1.
1 3V D.C. Power Supply
2 Switch
3 0.1Ω Resistor
4 100mH Coil
5 Voltage Probe (CH1)
6 EA-200
20020601
Page 31

Activity: Operating the EquipmentActivity: Operating the Equipment
MeasurementMeasurement
55555555555555555555555
5555555555555555555555
555555555
Other Things To Do
555555555
2-9-2
English
쐽 Measuring Data
u Prepare the Voltage Measurement Setup. At the same time you start the measurement
operation, move the switch to “ON” to connect the power supply.
u Wait until the circuit achieves a steady state.
1 Switch
2 Power Supply
Connected
3 Power Supply Cut
u Prepare the Voltage Measurement Setup. At the same time you start the measurement
operation, move the switch to “OFF” to disconnect the power supply.
u Wait until the circuit achieves a steady state.
1 Switch
2 Power Supply
Connected
3 Power Supply Cut
u Perform the above operation for the RC series circuit and the RL series circuit.
u Read and check the RC and R/L values from the graph.
쐽 Calculator Operation
u Perform the following operation to prepare for voltage measurement using the voltage
probe.
Using E-CON
m“E-CON”w1(SETUP)b(Wizard)w
1(CASIO)b(Volt) 0.02w255w1(YES)
Using a Calculator Program
Find the applicable program in the Program Library (P.2-16-3), input it into your calculator,
and then run it.
u Graph results for when the power supply is connected and for when it is disconnected.
Voltage Change when Power Is Connected
Voltage Change when Power Is Disconnected
u It appears as if combining the curve during capacitor charging and the curve
20020601
during capacitor discharging will produce a straight line with no change over time.
This, however, is not necessarily so. Consider the reason for this.
u In the case of the coil voltage curves as well, it appears as combining them as
expressions should produce a straight line. However, this also does not happen.
Consider the reason for this.
u Change the resistor, capacitor, and coil and find out how the voltage is affected.
u While the capacitor is charged, cut off the power supply and observe changes in
capacitor voltage with the circuit left open. Compare what you observe with the
change in voltage when the circuit is closed.
Page 32
AC Circuit
This activity investigates the characteristics of a resistor, capacitor, coil (RCL) series circuit,
which is connected to an AC power supply.
Theory
The electrical power sent from power generating stations to homes is alternating current
(AC), not direct current (DC). Though direct current does not alternate with time, alternating
current alternates according to a regular cycle, and this cyclical change can be expressed
as a trigonometric function. When the voltage and frequency of a AC power supply are
defined, the current flowing through the circuit has the same frequency as but a different
phase from the power supply voltage. The phase difference and the magnitude of current
depends on the component parts of the circuit. The voltage and current when a AC power
supply is connected to a series circuit composed of a resistor, capacitor, and coil (RCL
series circuit) can be expressed by the expressions shown below.
V = V0 cos t I = I0 cos( t – ) = 2πf
I0 = tan =
ωω
V0
R2 + (L –
1
ω
C
)
ω
φ
ω
1
φ
(L –
2
R
1
ω
C
)
ω
2-10-1
English
Activity: SetupActivity: Setup
쐽 Equipment
AC Power Supply (Switched) Resistor Capacitor Coil
Voltage Measurement Setup (EA-200, graphic scientific calculator, data communication
cable, voltage probe (3))
쐽 Building the RCL Series Circuit
u Build a circuit for a 3V, 50Hz power supply.
1 3V, 50Hz AC
Power Supply
2 10Ω Resistor
3 100µ F
Capacitor
4 10mH Coil
5 Voltage Probe
6 CH1
7 CH2
8 CH3
9 EA-200
V (V ) : AC Power Supply Voltage R(Ω):Resistance Value
V
0 (V) :
I (A) : AC Current L(H) : Coil Self-inductance
I
0 (A) : Amplitude of AC Current
ω
(rad/s): Angular Frequency t(s) : Time
f (Hz) : AC Frequency
This activity investigates the voltage across the components of a series circuit. When a
current that alternates with time is applied, the voltage across a capacitor and a coil is out of
phase with the power supply voltage by have this characteristic, and so there is no phase lag. All of this means the peaks and
valleys of the waveforms of the voltage across the components will be in different locations.
The voltage across each of the components is expressed as follows.
VR = I0R VC = VL = I0L
We also know that the maximum current value is achieved using a capacitor-coil combination
that satisfies the conditions shown below. A frequency like this is called a “resonant frequency.”
ω
2 =
Amplitude of AC Power Supply Voltage
π/2 and π/2 respectively. The resistor does not
I0
C
ω
ω
1
LC
C(F) : Capacitor Capacitance
φ
(rad) : Current Phase Difference
V
R (V): Voltage Across the Resistor
V
C(V) :Voltage Across the Capacitor
V
L(V) : Voltage Across the Coil
20020601
Page 33

2-10-2
55555555555555555555555
5555555555555555555555
555555
Other Things To Do
555555
English
Activity: Operating the EquipmentActivity: Operating the Equipment
쐽 Measuring Data
u Check the values of the AC power supply, resistor, coil, and capacitor, and then switch on
the power supply.
u Prepare the Voltage Measurement Setup and measure voltage.
u Display and compare the change of voltage across each component.
MeasurementMeasurement
쐽 Calculator Operation
u Perform the following operation to prepare for voltage measurement using the voltage
probe.
Using E-CON
m“E-CON”w1(SETUP)c(Advan)
b(Channel)1(CH1)c1(CASIO)b(Volt)c1(LIST) 2w
c2(CH2)c1(CASIO)b(Volt)c1(LIST) 3w
c3(CH3)c1(CASIO)b(Volt)c1(LIST) 4ww
c(Sample)1(NO)c1(TIMER) 0.001wc1(NUM) 255ww1(START)
Using a Calculator Program
Find the applicable program in the Program Library (P.2-16-3), input it into your calculator,
and then run it.
u Display
VR, VC, and VL.
R(V) : Voltage Across the Resistor (CH1)
V
V
C(V) : Voltage Across the Capacitor (CH2)
V
(V) : Voltage Across the Coil (CH3)
L
t(s) : Time
Make sure that the individual voltage value across the resistor, capacitor, and coil does not
exceed 5V. Consequently, you should make sure that the power supply voltage for the
combination shown here does not exceed 3V.
u Consider what type of combination would be required to keep voltage across all
20020601
parts below 5V.
u Consider combinations that satisfy conditions for resonance.
u Consider what component combinations would satisfy the conditions of this activity
(5V or less across each component, resonance circuit) for other AC frequencies.
u Perform FFT analysis and compare the frequency of each component.
Page 34

Activity: SetupActivity: Setup
Dilute Solution Properties
This activity investigates boiling point elevation and freezing point depression of a dilute
solution.
Theory
Dissolving a small amount of a substance (solute) in a theoretically pure liquid (solvent) to
create a dilute solution causes the boiling point of the dilute solution to become greater than
and the freezing point to become less than that of the solvent. This is because the
proportion of solvent molecules is reduced by the amount of solute molecules mixed in,
which lowers the vapor pressure of the solvent and elevates the boiling point. At the same
time, it also reduces the proportion of solvent molecules that congeal, which suppresses the
freezing point. These changes are determined by the amount of solute molecules, and the
type of solute does not matter, as long as it is non-volatile. Consequently, both boiling point
elevation and freezing point depression are proportional to the solute molality, as shown in
the expressions below.
욼
T
(°C) : Boiling Point Elevation of Solution
욼T1 = K1 m
욼T
= K2 m
2
1 Solvent
2 Solution
3 Boiling Point Elevation
4 Freezing Point Depression
T(°C) : Temperature
P(atm) : Vapor Pressure
t(s) : Time
Here, the proportion coefficient is determined by the solvent type. It is a constant that is not
affected by the solute type. For example, the molal boiling point elevation constant for benzene
is 2.53°C kg/mol, and the boiling point is 80.1°C. The molal freezing point depression constant
is 5.12°C kg/mol, and the freezing point is 5.53°C.
1
욼
T
(°C) : Freezing Point Depression of Solution
2
K
(°C kg/mol) : Molal Boiling Point Elevation Constant
1
K
(°C kg/mol) : Molal Freezing Point Depression
2
Constant
m(mol/kg) : Molality
2-11-1
English
쐽 Equipment
Stand Heater Reflux Condenser Desiccant
Auto Stirrer Beaker Round Bottom Flask (2) Mixing Stick
Ice Water Benzene Naphthalene
Temperature Measurement Setup (EA-200, graphic scientific calculator, data
communication cable, temperature probe)
쐽 Setting Up the Boiling Point Elevation Equipment
u Pour the benzene solution into the flask, and secure it in place as shown in the illustration.
1 Stand
2 Heater
3 Round Bottom Flask
4 Reflux Condenser
5 Desiccant
6 Naphthalene-Benzene
Solution
7 Temperature Probe (CH1)
8 EA-200
9 Water Flow Direction
쐽 Setting Up the Freezing Point Depression Equipment
u Pour the benzene solution into the flask, and secure it in place as shown in the illustration.
1 Stand
2 Auto Stirrer
3 Beaker
4 Round Bottom Flask
5 Mixing Stick
6 Ice Water
7 Temperature Probe (CH1)
8 EA-200
When the solvent is pure water, the molal boiling point elevation constant is 0.515°C kg/mol,
and the boiling point is 100°C. The molal freezing point depression constant is 1.853°C kg/mol,
and the freezing point is 0°C.
20020601
Page 35

Activity: Operating the EquipmentActivity: Operating the Equipment
MeasurementMeasurement
55555555555555555555555
5555555555555555555555
5555555
Other Things To Do
5555555
2-11-2
English
쐽 Measuring Data
u Measure the mass of the beaker used for creating the solution.
u Slowly pour the benzene into the beaker so it flows down the inside surface of the beaker.
u Measure the mass of the small amount of naphthalene being used.
u Dissolve the small amount of naphthalene to create a dilute benzene solution, and then
measure the mass of the solution.
u Calculate the molality of the naphthalene.
u Divide the dilute benzene solution between two flasks, pouring the solution slowly along
the inside surfaces of the flasks.
u Secure one of the flasks in the boiling point elevation system, and the other one in the
freezing point depression system.
1 Benzene
2 Naphthalene
3 Beaker
4 Round Bottom Flask
5 Naphthalene-Benzene
Solution
u As shown in the illustration, force water through the reflux condenser.
1 Reflux Condenser
2 Tap
3 Water Flow Direction
4 Open the tap.
쐽 Calculator Operation
u Perform the following operation to prepare for temperature measurement using the
Temperature Measurement Setup.
Using E-CON
m“E-CON”w1(SETUP)b(Wizard)w
1(CASIO)c(TEMP)b(°C) 5w240w1(YES)
Using a Calculator Program
Find the applicable program in the Program Library (P.2-16-3), input it into your calculator,
and then run it.
u Display a graph of temperature change starting from the point that the solution is first
heated.
T(°C) : Temperature
t(s) : Time
T
(°C): Solution Boiling Point
1
u Display a graph of temperature change starting from the point that the solution is first
cooled.
T(°C) : Temperature
t(s) : Time
T
(°C): Solution Freezing Point
2
u For boiling point elevation, turn on the heater and use the Temperature Measurement
Setup to measure the temperature.
u For freezing point depression, turn on the auto stirrer and use the Temperature
Measurement Setup to measure the temperature.
u Use the measured changes in temperature to determine boiling point elevation and
freezing point depression.
u Compare the measured results with the results calculated from naphthalene molality.
For freezing point depression, use the mixing stick to stir the flask contents.
u Consider how the molecular weight of naphthalene can be determined using the
measurement results.
u Consider why the cooling curve of freezing point depression is not clear.
u Create another dilute solution using a different solute, and determine the boiling
point elevation and the freezing point depression.
u Create another dilute solution using a different solvent, and determine the boiling
20020601
point elevation and the freezing point depression.
Page 36

Activity: SetupActivity: Setup
Exothermic Reaction
This activity uses the neutralization of hydrochloric acid and sodium hydroxide to study heat
that is given off or absorbed by chemical reactions.
Theory
A chemical reaction causes a change in the properties of matter, and always gives off or
absorbs heat. The sum of the heat of reaction when a chemical reaction takes place
depends solely on the condition of the matter at the time of the reaction, and is totally
independent of the reaction pathway and the number of steps between the initial state and
the final state. This is called Hess’s law.
The following illustrates the chemical reaction when sodium chloride (
sodium hydroxide (
s) and hydrochloric acid (aq).
NaOH(s) + aq = NaOH(aq) + 44.5kJ
NaOH(aq) + HCl(aq) = NaCl(aq) + H
NaOH(s) + HCl(aq) = NaCl(aq) + H
1 Reaction Path 1
2 Reaction Path 2
3 Energy
4 Solvent (Water)
s:solid
aq : aqua
O + 56.4kJ
2
O + 100.9kJ
2
aq) is generated from
2-12-1
English
쐽 Equipment
Stand Auto Stirrer Beaker (3)
Hydrochloric Acid (Solution) Sodium Hydroxide (Solid) Distilled Water
Temperature Measurement Setup (EA-200, graphic scientific calculator,
data communication cable, temperature probe)
쐽 Setting Up
u Measure the mass of the hydrochloric acid (aq) and distilled water to be used in the
activity.
u Measure the amount of sodium hydroxide (s) required so the number of its moles is equal
to that of the hydrochloric acid (
u Fix the probe in place at a point between the center of the beaker and the wall of the
beaker, in a location where it does not strike the stirrer’s magnet, at a depth so it is
sufficiently immersed in the solution.
1 Stand
2 Auto Stirrer
3 Beaker
4 Te mperature Probe (CH1)
5 Solution
6 EA-200
aq).
Here, Reaction Path 1 includes the heat of dissolution when sodium hydroxide (
dissolved in distilled water, and the heat of neutralization of sodium hydroxide (
hydrochloric acid (
Reaction Path 2, on the other hand, consists of the heat of neutralization of sodium
hydroxide (
All of this means that the total heat is the same, regardless of whether or not the pathway
includes a process for dissolving the sodium hydroxide (
aq).
s) and hydrochloric acid (aq).
s), as in Reaction Path 1.
s) is
aq) and
20020601
Page 37

Activity: Operating the EquipmentActivity: Operating the Equipment
MeasurementMeasurement
55555555555555555555555
5555555555555555555555
55555
Other Things To Do
55555
2-12-2
English
쐽 Reaction Path 1 Measurement
u Turn on the auto stirrer and start a measurement operation with the calculator.
u Little-by-little, add sodium hydroxide (
temperature changes.
u After all the sodium hydroxide (
determine the heat of dissolution.
1 Beaker
2 Temperature Probe
3 Sodium Hydroxide (s)
u Turn on the auto stirrer and start a measurement operation with the calculator.
u Little-by-little, add the hydrochloric acid (
how the temperature changes.
u After all the hydrochloric acid (
the heat of neutralization.
u Calculate the sum of heat by adding the heat of neutralization to the heat of dissolution.
1 Beaker
2 Temperature Probe
3 Hydrochloric Acid
(aq)
4 Distilled Water
5 Temperature rise due
4 Sodium Hydroxide (aq)
5 Temperature rise due
s) to the distilled water, and observe how the
s) is dissolved and the temperature rise stabilizes,
to heat of dissolution
aq) to the sodium hydroxide (aq), and observe
aq) is added and the temperature rise stabilizes, determine
to heat of neutralization
쐽 Reaction Path 2 Measurement
u Turn on the auto stirrer and start a measurement operation with the calculator.
u Little-by-little, add sodium hydroxide (
temperature changes.
u After all the sodium hydroxide (
determine the heat of neutralization.
u Calculate the heat, and compare it with the total heat you calculated for Reaction Path 1.
s) to the hydrochloric acid (aq), and observe how the
s) is dissolved and the temperature rise stabilizes,
쐽 Calculator Operation
u Use the Temperature Measurement Setup to measure the temperature and then display it.
u Find the applicable program in the Program Library (P.2-16-3), input it into your calculator,
and then run it.
1 Beaker
2 Temperature Probe
3 Sodium Hydroxide
(s)
When converting the temperature rise to joule heat, be sure to take the difference in the volume of
water into consideration. The specific heat of distilled water is 4.2J/g°C.
4 Hydrochloric Acid (aq)
5 Temperature rise due
to heat of neutralization
u Determine whether this activity verifies Hess’s Law. If it does not, consider the
20020601
reason why.
u Compare the theoretical chemical reaction expression and the measurement
results. If they do not match, consider the reason why.
u Find out if Hess’s Law holds true for other chemical reactions.
Page 38

Activity: SetupActivity: Setup
Electromotive Force of a Battery
This activity investigates the changes in the electromotive force of a voltaic battery over
time.
Theory
A voltaic battery is made of a zinc (Zn) plate and a copper (Cu) plate immersed in a dilute
solution of sulfuric acid (H
between the two metals, the zinc plate is the negative electrode (cathode), while the copper
plate is the positive electrode (anode).
Ionization tendency is the tendency of metal to release electrons, which become positively
charged ions. As shown below, zinc and copper are on opposite sides of the hydrogen
molecule.
K Ca Na Mg Al Zn Fe Ni Sn Pb (H2) Cu Hg Ag Pt Au
Exposing zinc, which has a relatively strong ionization tendency, to the hydrogen ions in a
dilute sulfuric acid solution, causes the zinc to dissolve and release electrons, creating a
minus charge. When the battery is connected to a circuit, the released electrons flow
through the circuit to become electric current, and collect on the copper plate, which has a
relatively weak ionization tendency and is not dissolving. The electrons on the surface of the
copper plate are consumed when they attach to hydrogen ions in the dilute sulfuric acid
solution and give off hydrogen gas. As the electrons on the copper plate are consumed, the
zinc plate releases more electrons, which allows the flow of current to continue. The
reaction that occurs at the two electrodes at this time is represented by the chemical
expression shown below.
Zn → Zn
2H
2+
+
+ 2e– (Negative Electrode Reaction)
+ 2e– → H2 (Positive Electrode Reaction)
). Employing the difference in the ionization tendencies
2SO4
2-13-1
English
쐽 Equipment
Stand
Voltaic Battery (Zinc Plate, Copper Plate, Dilute Sulfuric Acid Solution, Cistern)
Flashlight Bulb Hydrogen Peroxide Pipette with a bulb
Voltage Measurement Setup (EA-200, graphic scientific calculator, data communication
cable, voltage probe)
쐽 Setting Up
u Taking care that the two electrodes do not become shorted, setup the equipment as
shown in the illustration.
1 Stand
2 Cistern
3 Dilute Sulfuric Acid Solution
4 Zinc Plate
5 Copper Plate
6 Negative Electrode
7 Positive Electrode
8 Flashlight Bulb
9 Voltage Probe (CH1)
0 EA-200
1 Dilute Sulfuric Acid Solution
2 Flashlight Bulb
3 Negative Electrode (Zinc Plate)
4 Positive Electrode (Copper Plate)
It should be noted here that should too much hydrogen gas foam build up on the copper
plate surface, a phenomenon called “polarization” can cause the hydrogen gas to start to
return to hydrogen ions. Allowing polarization to continue will lead to deterioration of the
electromotive force of the battery. If this happens, hydrogen peroxide can be added to the
area around the copper plate, which reacts with the hydrogen gas and reduces the amount
of foam present. This, in turn, restores the electromotive force of the battery. Repeating this
measure when required will theoretically allow the battery to operate properly until the zinc
plate is completely dissolved. In actual practice, a voltaic battery will reach the end of its
useful life before then.
20020601
Page 39

Activity: Operating the EquipmentActivity: Operating the Equipment
MeasurementMeasurement
55555555555555555555555
5555555555555555555555
55555555
Other Things To Do
55555555
2-13-2
English
쐽 Measuring Data
u The voltage is displayed on the calculator.
u Lower the arm of the stand so the metal plates are submersed in the dilute sulfuric acid
solution, without coming into contact with the cistern.
1 Stand Arm
2 Cistern
u If the variation in voltage becomes small, use the pipette with a bulb to inject a small
amount of hydrogen peroxide into the dilute sulfuric acid around the copper plate.
1 Stand Arm
2 Cistern
3 Copper Plate
4 Pipette with a bulb
5 Hydrogen Peroxide
6 Hydrogen Foam
7 Hand
쐽 Calculator Operation
u The voltage is measured and displayed using the Voltage Measurement Setup.
u Find the applicable program in the Program Library (P.2-16-4), input it into your calculator,
and then run it.
u Repeat this process as you monitor the life of the battery.
1 Hydrogen Peroxide
Added Here
V(V): Voltage
t(s) : Time
20020601
u Compare the initial voltaic battery electromotive force with the theoretical value of
1.1V.
u Try changing the surface area of the two electrodes that is in contact with the dilute
sulfuric acid solution, and note any changes in the electromotive force.
u Find out what you can about the Daniel Cell, which is one type of battery that is not
prone to loss of voltage due to hydrogen build-up on the positive electrode.
u Perform long-term measurement of a commercially available manganese battery
(5V maximum) and determine its useful life.
Page 40

Activity: SetupActivity: Setup
Sunlight and Solar Cells
This activity studies how change in the amount of sunlight available per day affects the
electromotive force of a solar cell, with a view to understanding the characteristics of power
generation by a solar cell.
Theory
Solar cells have been receiving a great deal of attention in recent years as one source of
renewable, environmentally-friendly energy. One problem with solar cells, however, is the
fact that the amount of power that can be generated is unstable, because the quantity of
sunlight hitting the solar cell panel surface is affected by weather and other factors. In order
to maximize power generation efficiency, it is necessary to design a system that is able to
change the orientation of the solar cell panel to suit changes in the angle of the sun due to
the rotation and revolution of the Earth, and seasonal changes. The diagram below shows
the optimum orientation of a solar cell panel in relation to the sun.
1 Sun
2 Morning
3 Afternoon
4 Evening
5 Solar Cell Panel Surface
6 Magnetic Compass
θ
(°): Angle of Inclination
2-14-1
English
쐽 Equipment
Solar Cell Panel (Operating Voltage: 5V max.) Magnetic Compass
Rain Cover Wood Block
Voltage Measurement Setup (EA-200, graphic scientific calculator, data communication
cable, voltage probe)
Optical Measurement Setup (EA-200, graphic scientific calculator, data communication
cable, optical probe)
쐽 Preliminary Setup
u Standing in a location that is exposed to sunlight, hold the equipment in your hands and
point the solar cell panel and optical probe towards the sun.
1 Solar Cell Panel
2 Voltage Probe
(CH1)
3 Optical Probe
(CH2)
4 EA-200
쐽 Setting Up
u Fix the optical probe and solar cell panel so they are pointing in the direction and at the
angle you determined during the preliminary measurement.
In spite of the above, most solar cell panels are pointed in a fixed direction, because it costs
too much to be continually changing their orientation. With this activity, we will investigate
the correlation between the amount of sunlight per day picked up by a fixed solar cell panel
and the electromotive force of a solar cell.
1 Sun
2 Solar Cell Panel
3 Voltage Probe
4 Optical Probe
5 Angle adjustment
block
6 Transparent Rain
Cover
7 CH1
8 CH2
9 EA-200
20020601
Page 41

Activity: Operating the EquipmentActivity: Operating the Equipment
MeasurementMeasurement
55555555555555555555555
5555555555555555555555
5555555555555
Other Things To Do
5555555555555
2-14-2
English
쐽 Performing Preliminary Measurements
u Display the light intensity (or voltage) measurement result on
the calculator.
u Keeping the angle of the solar cell panel and optical probe
fixed, turn around until you find the direction where the
displayed value is at its maximum.
1 Sun
2 Person Holding Equipment
3 Magnetic Compass
4 Direction of Turn
5 Direction Producing Maximum Value
u Fix the solar cell panel and the optical probe so they are
pointed in the direction you determined above.
u Display the light intensity (or voltage) measurement result on
the calculator.
u Starting with the solar cell panel facing the ground, slowly
angle it upwards, until you find the angle that produces the
maximum measured value.
1 Sun
2 Ground
3 Zenith
4 Solar Cell Panel Surface
5 Direction of Turn
6 Angle Producing the Maximum Value
쐽 Performing Main Measurements
u Fix the optical probe and solar cell panel so they are pointing in
the direction and at the angle you determined in the preliminary
step, above.
u Set up the EA-200 for long-term measurement, start
measurement, and then set the rain cover in place.
u After measurements are complete, study the correlation between
light intensity and voltage.
The best time to perform the preliminary steps is noon. If it is hot or if there is the possibility that
the EA-200 might become too hot, place a shade over the EA-200 to block the sun.
쐽 Calculator Operation
u Use the optical probe (or voltage probe) to measure light intensity (or voltage) and display
the results on the calculator.
u Perform the following operation to set up the optical probe and voltage probe for long-
term measurement.
Using E-CON
m“E-CON”w1(SETUP)c(Advan)
b(Channel)1(CH1)c1(CASIO)b(Volt)c1(LIST) 2w
c2(CH2)c1(CASIO)d(Light)c1(LIST) 3ww
c(Sample)1(NO)c1(TIMER) 360wc1(NUM) 241ww1(START)
Using a Calculator Program
Find the applicable program in the Program Library (P.2-16-4), input it into your calculator,
and then run it.
L : Light Intensity
V(V): Voltage
t (s):Time
u Consider why there is such a large change in measured values over a 24-hour
period.
u Consider why there are many changes in the graph over a comparatively short
period of time.
u See if the angle of inclination of the solar cell panel and the light intensity satisfy
the expression shown below.
L = L
L :Light Intensity
L
max
θ
(°):Solar Cell Panel Angle of Inclination
θ
max
u Predict what changes can be expected throughout the year, and take long-term
(24-hour) measurements during different seasons to see what happens.
cos(θ–
max
:Maximum Light Intensity Value
(°):Angle of Inclination Determined by Preliminary Step
θ
)
max
20020601
Page 42
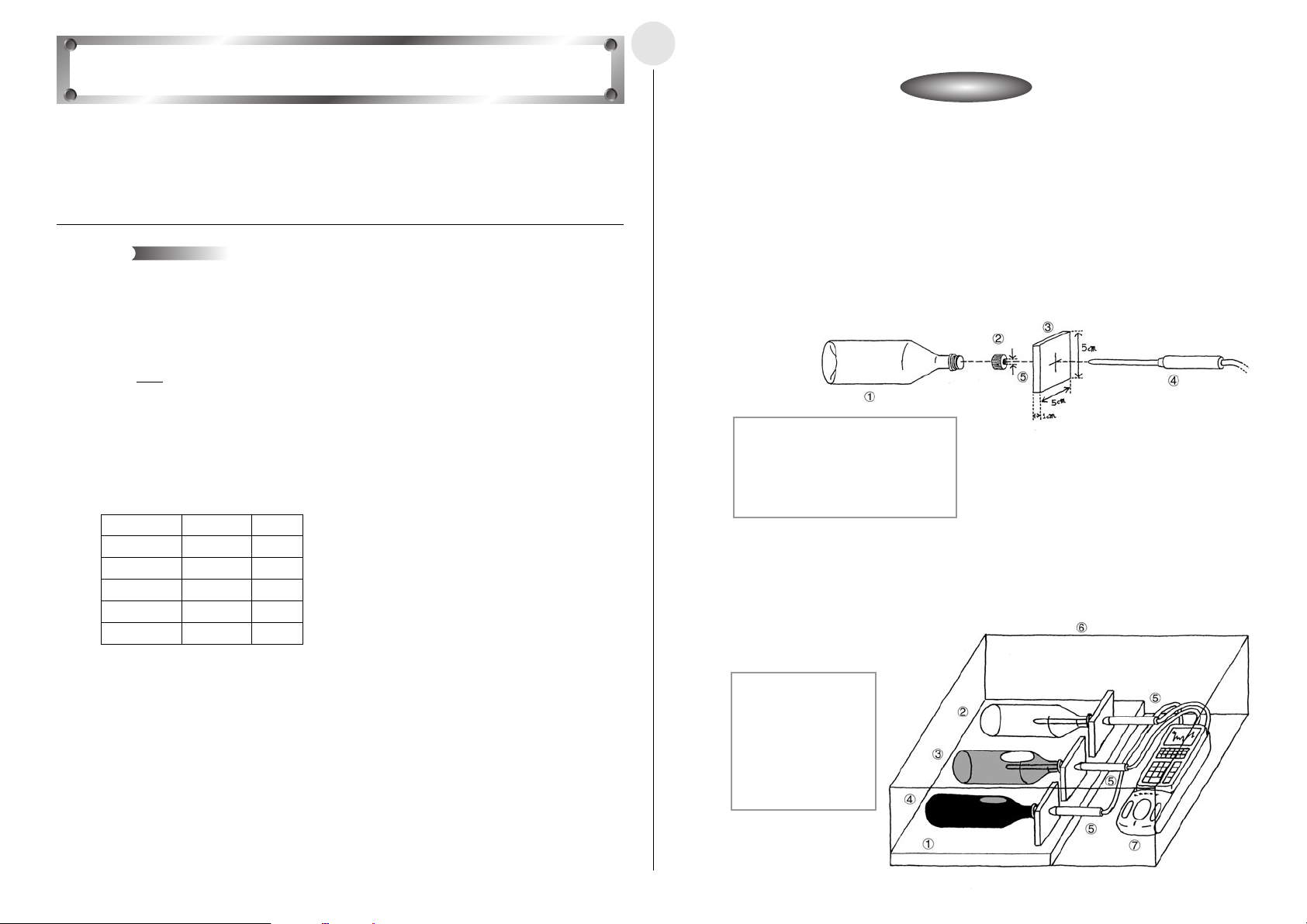
Activity: SetupActivity: Setup
Topographic Conditions and Climate
The difference in the specific heat of landmasses and seawater causes differences between
the climate of coastal areas and inland areas, even when they are located at the same
latitude.
This simple activity investigates these differences.
2-15-1
English
쐽 Equipment
500ml Clear Plastic Bottles (3) Dry Earth (or Sand) Seawater (or Water)
Styrofoam Boards Rain Cover
Te mperature Measurement Setup (EA-200, graphic scientific calculator, data communication
cable, temperature probe (3))
Theory
Specific heat expresses how the temperature of a unit mass of a substance changes when
a quantity of heat is applied. The greater the specific heat of a substance is, the smaller is
its change in temperature. The expression for this change in temperature is shown below.
The table shows the specific heat values for a number of common substances.
욼Q
욼T =
mc
욼T(°C) : Change in Temperature of Substance
욼
Q(J) : Heat Quantity Applied to Substance
m(g) : Mass of Substance
c(J/g°C) : Specific Heat of Substance
T(°C) : Measured Temperature
Substance c(J/g° C) T(°C)
Water 4.2 15
Ice 2.1 0
Seawater 3.9 20
Rock 0.8 20
Wood 1.2 20
The above indicates that seawater is difficult both to heat and cool, while landmasses are
easy to heat and cool. The result is that there tends to be less fluctuation between heat and
cold in marine climates, and greater fluctuation further inland. It should be noted, however,
that there are many different variations in actual climates, due to ocean currents, air
currents, topography, wind patterns, and other factors. This activity investigates changes in
the temperature of common everyday substances as they absorb heat from sunlight. It also
compares the tendency to change temperature with measured values.
쐽 Preparing the Plastic Bottle
u Remove the label from the plastic bottle, wash it out, and let it dry.
u Pierce the center of each of the Styrofoam boards with a temperature probe, attach the
board/probe assembly to a bottle cap, and then seal the bottle.
1 Plastic Bottle
2 Bottle Cap
3 Styrofoam Board 5cm × 5cm × 1cm
4 Temperature Probe
5 Hole Diameter: 6mm
쐽 Setting Up
u Weigh the empty bottle and the other two bottles to determine the mass of the substance
inside each.
u Locate the bottles in an area that is constantly
exposed to sunlight.
1 Styrofoam Board
2 Air
3 Seawater
4 Earth
5 Te mperature Probe
6 Rain Cover
7 EA-200
20020601
Page 43

Activity: Operating the EquipmentActivity: Operating the Equipment
MeasurementMeasurement
55555555555555555555555
5555555555555555555555
555555555
Other Things To Do
555555555
2-15-2
English
쐽 Measuring Data
u Prepare the Temperature Measurement Setup and start temperature measurement.
u Cover the system with the rain cover.
u Remembering that the mass of each of the substances is different, observe the measured
change in temperature and its relationship with the specific heat.
쐽 Calculator Operation
u Perform the following operation to prepare for temperature measurement using the
temperature probe.
Using E-CON
m“E-CON”w1(SETUP)c(Advan)
b(Channel)1(CH1)c1(CASIO)c(TEMP)b(°C)c1(LIST) 2w
c2(CH2)c1(CASIO)c(TEMP)b(°C)c1(LIST) 3w
c3(CH3)c1(CASIO)c(TEMP)b(°C)c1(LIST) 4ww
c(Sample)1(NO)c1(TIMER) 360wc1(NUM) 241ww1(START)
Using a Calculator Program
Find the applicable program in the Program Library (P.2-16-4), input it into your calculator,
and then run it.
u Display the temperature measurements.
1 Air
2 Seawater
3 Earth
T(°C) :Te mperature
t (s) : Time
If there is the possibility that the EA-200 might become too hot, place a shade over the EA-200
to block the sun. Note that you should put a little water into the air bottle and a little air into the
seawater bottle. Doing so reduces the effect of thermal expansion inside each bottle.
u Use the measurement results to estimate differences in air temperature between a
20020601
dry inland area and a coastal area.
u Use the specific heat values provided in the table for seawater and stone to calculate
the quantity of heat absorbed by the seawater and earth in the bottles, and compare
them. Consider why this value is different for each of the bottles.
u Try replacing the substances inside the bottles with other substances.
u Consider why it is impossible to determine the specific heat value from temperature
changes, and to determine the ratio of specific heat between two substances.
u Attach one optical probe to the EA-200, and investigate the relationship between
light intensity and change in temperature.
Page 44

2-16-1
English
Program Library
You can download the programs used in the activities at the CASIO Website: http://world.casio.com/edu_e/
Uniformly Accelerated Motion
Conservation of Momentum Period of Pendular Movement
Charles’ Law
* This program cannot be run on
an fx-7400 Series calculator.
20020601
Page 45

Polarization of Light
2-16-2
English
Natural Ferquency and Sound
Column of Air Resonance and
the Velocity of Sound Construction of the Musical Scale
Light Multi Meter
*This program cannot be run on
an fx-7400 Series calculator.
20020601
Page 46

2-16-3
English
Direct Current and Transient
Phenomena
AC Circuit
Dilute Solution Properties
Exothermic Reaction
20020601
Page 47

2-16-4
English
Electromotive Force of a Battery Sunlight and Solar Cells
*Run the following program after
sampling is complete.
Topographic Conditions and Climate
*Run the following program after
sampling is complete.
20020601
Page 48

Appendix A Command Tables
Command 1 - Channel Setup
*: parameter value marked with asterisk are initial defaults. { 1, Channel, Operation, Post-Processing, FFT Samples }
Channel Operation Post-Processing FFT Samples
0 Clear all channels ––– ––– –––
*1 Channel 1 0 Clear the selected channel. 0 None
2 Channel 2 *1 Auto-ID *1 d/dt
3 Channel 3 2 Voltage (± 10V) 2 d/dt, d2/dt
4 SONIC Channel 0 Clear the SONIC channel. 0 None
5 DIG IN Port 0 Clear the digital input channel.
6 DIG OUT Port Data String Output Loops Data string
1 to 32 Number of output data elements
10 Microphone 0 Clear the Microphone. *0 None
11 Analog Out CH 3 1pin ±3Vout Data String Output Loops Data Output Selection Data string
12 Speaker 1 to 65535 Number of output data elements
• Channel = 1, 2, 3 or 4, Operation = 5, 6, 11
{ 1, Channel, Operation, Pin No, Trigger Threshold, Trigger Edge }
Pin No Trigger Threshold Trigger Edge (Operation = 5, 6)
*2 1pin Vin (± 10V) ± 10 Set input voltage threshold value *0 Rising edge to rising edge
10 6pin Vin-low (0-5V) 0 to 5
(for Voltage probe)
4 Resistance 10 FFT-Real 1 to 14 Samples used
5 Period 11 FFT-Real, Imaginary (*6) 2
6 Frequency
7 Temperature (Celsius)
8 Temperature (Fahrenheit)
9 Light
10 Voltage (0-5V)
11 Absolute Time
*1 Meters *1 d/dt
2 Meters 2 d/dt, d2/dt
3 Feet –––
5 Period
6 Frequency
11 Absolute Time
*1 Active
0 Clear the digital input channel. 0 to 255 Output data element value
(*1)
*1 Active 10 FFT-Real 1 to 14 Samples used
0 Clear the analog out or speaker. *0 Data string ± 1.5 Output data element value
(*1) 1 Channel 1
–10 to +10. 1 Falling edge to falling edge
α-1-1
English
2
2
––– –––
11 FFT-Real, Imaginary (*6) 2
2 Channel 2
3 Channel 3
10 Microphone
2 Rising edge to falling edge
3 Falling edge to rising edge
Trigger Edge (Operation = 11)
*0 Rising edge
1 Falling edge
2 Rising and falling edge
–––
n
(2-16384)
–––
n
(2-16384)
•Record Time for Operations 5, 6, and 11
must be 2, 1, and 1 respectively.
•Trigger Source for Channels 1, 2, 3, and 4
must be 2, 3, 4, and 12 respectively.
•Clock Source must be 10.
20020601
20020701
Page 49

Command 3 - Sample and Trigger Setup
{ 3, Sample Interval, Number of Samples, Record Time, Trigger Source, Trigger Threshold, Trigger Edge, Clock Source }
Sample Interval Number of Samples Record Time
0.00002 Number of seconds 1 to Number of samples 0 Off
to 16000 120000 *1 Absolute time recording
(*0.1) (*100) 2 Relative time recording
Trigger Souce Trigger Threshold Trigger Edge Clock Source
1[START/STOP] key
2 or 5 CH 1 Sampled Values 0 Falling edge 10 Same as Trigger Source
3 or 6 CH 2 • Corrected values when Command 4 *1 Rising edge
4 or 7 CH 3 2 Rising and falling edge
8DIG IN Clock –––
9DIG IN 8bit data 0-255 (D7-D0)
(*1)
10 Microphone Sampled Values ±1.5V 0 Fallig edge
11 SONIC Distance 0 Falling edge
• Unit depends on Command 1. 1 Rising edge
(*0.05) *2 Difference with previous value is below
20 Count down Count Number (sec)
1 to 10 (*10)
–1 Command 8 ––– –––
––– ––– *0Timer (Sample interval)
*1 Rising edge
2Rising and falling edge
3Difference with previous value is above
α-1-2
English
–––
–––
–––
20020601
Page 50

Command 4 – Conversion Equation Setup
{ 4, Equation Number, Equation Type, Number Format, Constants }
Equation Number Equation Type Number Format Constants
* 0 Clear All equations. * 0 Clear equation selected by the equation number parameter. ––– –––
1Equation 1 (Channel 1) 1 Polynomial K0 + K1X + K2X
–m
2Equation 2 (Channel 2) 2 Mixed polynomial KmX
3Equation 3 (Channel 3) 3 Power K0 • X
4 Modified power K0 • K1
+ ...+ K–1X
(K1)
X
5Logarithmic K0 + K1 In(X)
6 Modified logarithmic K0 + K1 In(1 / X)
7 Exponential K0e
8 Modified Exponential K0e
9 Geometric K0X
10 Modified geometric K0X
(K1X)
(K1/X)
(K1X)
(K1/X)
11 Reciprocal logarithmic 1 / {KO + K1 In(K2X)} + K3
12 Steinhart-Hart 1 / {KO + K1(In 1000X) + K2(In 1000X)
4Equation 4
(SONIC channel)
* 0 Clear equation 4
1 Temperature used by distance conversion expression * 0 °C(Celsius) Temperature
1 °F (Fahrenheit) ( *20)
2 °C (Celsius)
3K (kelvin)
4 °R (Rankin)
*1
Polynomial: Input constants in sequence, from n = 0 to 9.
*3
Input of zero for constants can be skipped if all remaining constants are not used.*4Input 0 for constants that are not used.
• When the conversion result of the “conversion equation” selected by Command 4 causes an overflow, the EA-200 sends a result of zero (0) to the calculator.
2
+...+ KnX
–1
+ K2
+ K2
+ K2 K0( , K1, K2, K3) *
+ K2
+ K2
+ K2
*2
Mixed polynomial: Input constants in sequence from m = 4 to 1, and n = 0 to 5.
α-1-3
English
1
n
*
+ KO + K1X +...+ KnX
3
} + K3
* 0 Standard K0( ,K1,...,K9) *
n
2
*
10 Integer part (Decimal part cut off.) K–4( ,..., K–1, K0, K1, ..., K5) *
Unit Temperature
3,4
3,4
3,4
Command 5 - Data Range Setup
{ 5, Channel Select, Data Select, (FFT Samples,) Data Begin, Data End, Step, K }
Channel Select Data Select Data Begin Data End Step K FFT Samples
* 0 Current send channel * 0 Raw data 1 to 120000 1 to 120000 Data Range Steps (*255) 1 to 14 : Samples used
1 Channel 1 1 d/dt (*1) * 0 : Last sample –1: Data range number / K (*6) 2
2 Channel 2 2 d
3 Channel 3 10 FFT-Real
4 SONIC channel 11 FFT-Imaginary
5DIG IN channel
6Recorded time data
10 Microphone
2
/dt2 (*1)
20020601
20020701
n
(2–16384)
Page 51

α-1-4
English
Command 6 - System Setup Command 10 - Sensor Warmup
{ 6, Command, Auto Power Off Time }
Command APO Time(sec)
0 or 2 Abort Sampling
( *0)
3Turns sound off
4Turns sound on
10 APO * 0 1800
(Auto Power Off) 1 10
2360
–––
Command 8 - Sampling Start
{ 8 }
Command 11 Buzzer and LED Operation Commands
Output Select Length (sec) Period (sec)
* 0 Buzzer Operating Time (sec) Period (sec)
2Ready LED
3Sampling LED
4 Error LED
5Batt LED
•An error occurs when fraction data is sent.
•Send commands to the EA-200 in accordance with the command table contents.
•An error occurs when a parameter that does not exist in the command table is sent.
• The EA-200 uses six digits for internal calculations.
–––
{ 10, Warmup Time (sec) }
Warmup Time (sec)
0.1 to 360 Warmup time (sec)
( *0.1)
0Auto
–1 None
–2 Normal warmup
Command 12 - Data Send Sequence
{ 12, Send Sequence }{ 11, Output Select, Length, Period }
Send Sequence
* 0 Non-real Time Format
1Real Time Format
20020601
Page 52

Appendix B Specifications
Model: ............................... CASIO EA-200
Power Supply: ................... Four AA-size alkaline batteries (LR6 (AM3)) or
AC adaptor (AD-A60024)
Power Consumption: ......... 1.5W
Battery Life: ....................... LR6 (AM3): Approximately 50 hours (when is left with
power on) / Approximately one year (when is left with
power off)
Battery life is also affected by the type of probes that
are connected, sampling program setup, etc.
Auto Power Off: ................. Approximately 30 minutes after last key operation. See
page 0-4 for information on conditions under which
Auto Power Off is disabled.
Operating Temperature: .... 0°C to 40°C (EA-200)
The tip of the temperature probe can be used in
temperatures ranging from –20°C to 130°C.
α-2-1
English
Dimensions:....................... 84.0(W) × 246.0(D) × 32.0(H) mm
35/16⬙ (W) ҂ 911/16⬙ (D) ҂ 11/4⬙ (H)
Weight: .............................. 350g (12.3 oz) including batteries
Standard Accessories: ...... Optical Probe (CDAP-01); Temperature Probe (CDAP-
02); Voltage Probe (CDAP-03); four AA-size alkaline
batteries (LR6 (AM3)); AC adaptor (AD-A60024); Data
Communication Cable (SB-62); Soft Case; User’s
Guide
20020601
Page 53

English
MEMO
20020601
Page 54

English
MEMO
20020601
Page 55

CASIO COMPUTER CO., LTD.
6-2, Hon-machi 1-chome
Shibuya-ku, Tokyo 151-8543, Japan
Printed on recycled paper.
SA0208-000102B Printed in Japan
A342984-008V01
 Loading...
Loading...