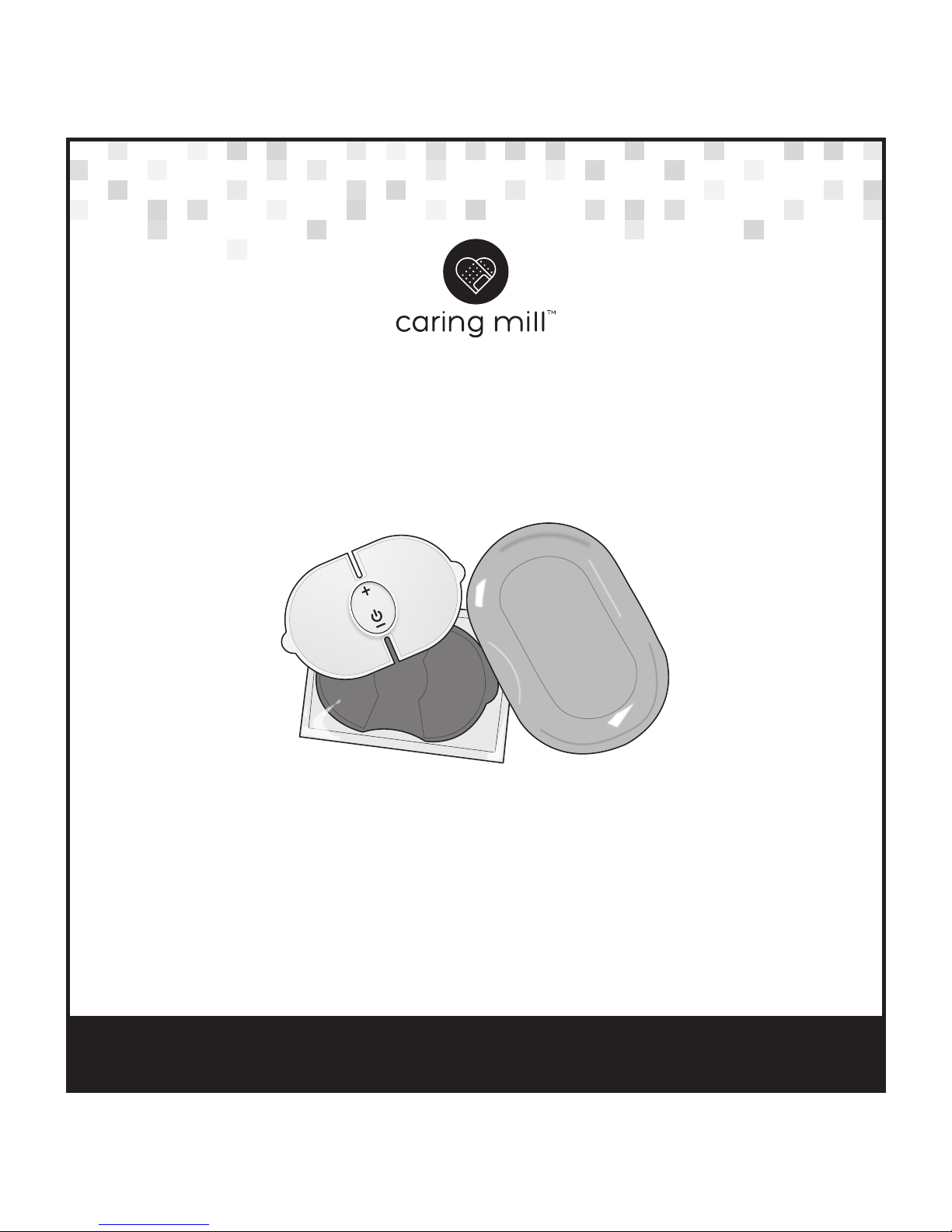
TENS Pain
Management Solution
Model 25517 (VH 56-032)
Intention for Use:
The TENS Pain Management Solution is intended for temporary relief of pain
associated with sore and aching muscles due to strain from exercise or
normal household and work activities.
INSTRUCTION MANUAL
ENGLISH & ESPAÑOL
PLEASE READ THESE INSTRUCTIONS
COMPLETELY BEFORE USING YOUR TENS DEVICE.
2 • ENGLISH
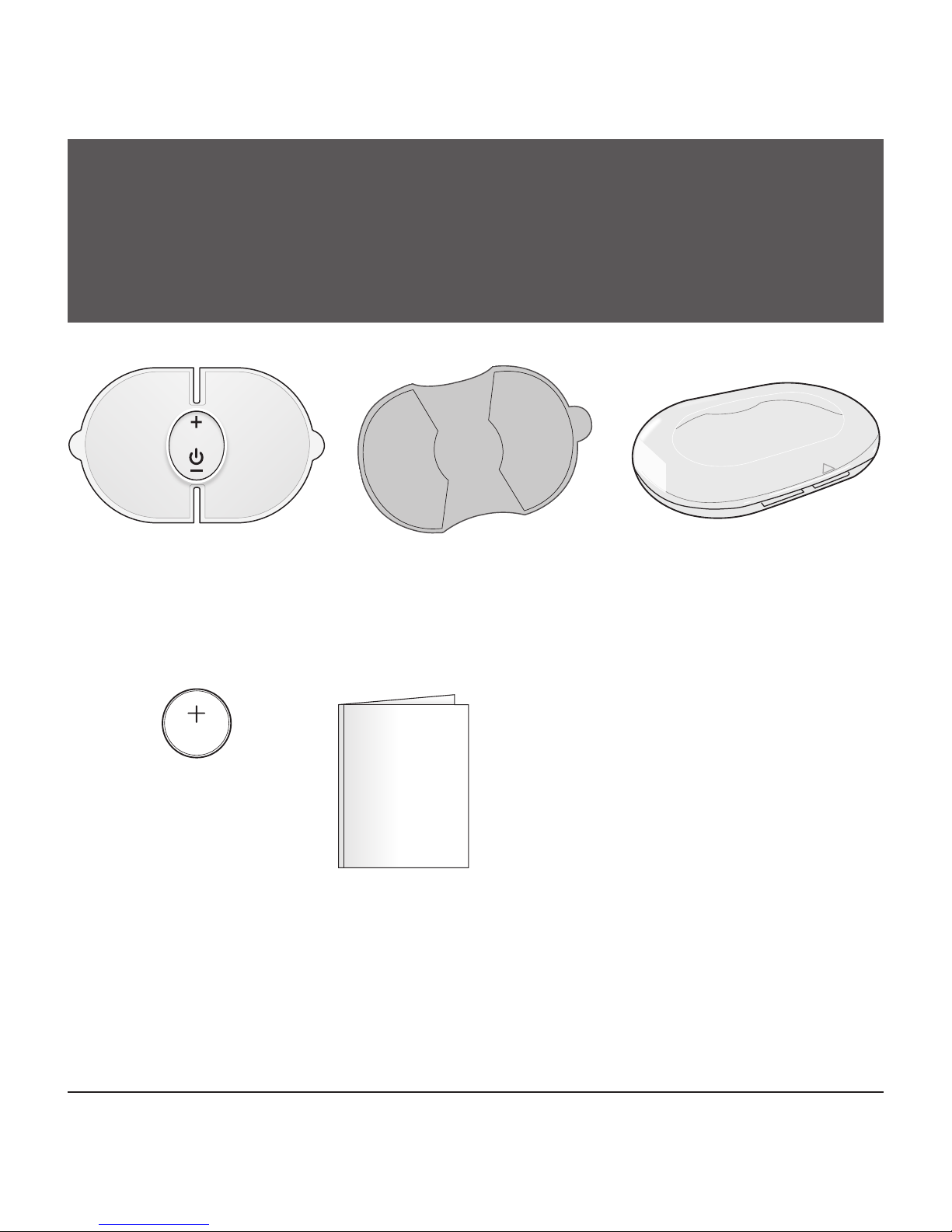
STOP! PLEASE ENSURE YOU HAVE ALL OF THE
FOLLOWING COMPONENTS BEFORE USING YOUR
TENS DEVICE.
IF YOU ARE MISSING ANY PARTS, INCLUDING
INSERTS OR INSTRUCTION MANUALS, DO NOT
RETURN TO PLACE OF PURCHASE.
CONTACT CUSTOMER CARE AT 866-326-1313.
Control Unit Storage Case
Instruction Manual
Replacement Electrode Order Form
One CR2032
Battery
1 Set of Gel
Electrode Pads
ENGLISH • 3
Care and Safety Information .............................................................4-11
Product Features and Symbols .......................................................12-13
Battery Installation / Replacement .......................................................14
Preparing Your Device ..........................................................................15
Placement & Treatment ..................................................................16-17
Instructions for Use ........................................................................18-21
Cleaning and Storage ...........................................................................22
Troubleshooting ...................................................................................23
Device & Label Symbols ......................................................................24
Electromagentic Compatibility ........................................................ 25-28
FCC Statement...............................................................................29-30
Product Specifications .........................................................................31
Warranty Information .....................................................................32-33
Español..........................................................................................35-68
Toll-Free Customer Care Help Line: 1-866-326-1313
Monday – Friday 8:30 a.m. – 4:30 p.m. CST
INDEX
Caring Mill™ 2017
240 W. 37th St.
6th Floor
New York, NY 10018
1-888-372-1450
Made in China
#93-1387 08/17
4 • ENGLISH
IMPORTANT SAFEGUARDS
WARNINGS & CAUTIONS
NOTE: Read all instructions and inserts included with this device
carefully before use. The following basic precautions are needed when
using an electrical product.
CAUTION: Failure to read and observe all precautions could result in
injury or equipment damage.
Improper care or use of your device may result in injury, damage to the
unit or ineffective treatment. Following these instructions will ensure the
device’s efficacy and long life.
CONTRAINDICATIONS :
Do not use this device if you have a cardiac pacemaker, implanted
defibrillator or other implanted metal or electronic device.
Do not use this device to treat undiagnosed chronic pain.
Do not use this device if you have suspected or diagnosed heart prob-
lem.
Do not use this device if you have suspected or diagnosed epilepsy.
Do not use this device if you have a tendency to bleed internally follow-
ing an injury.
Do not use this device if you recently had surgery, or have ever had
surgery on your back.
ENGLISH • 5
IMPORTANT SAFEGUARDS
WARNINGS & CAUTIONS
Do not use this device if areas of skin lack normal sensations, such as
skin that tingles or is numb.
Do not use this device during menstruation or during pregnancy.
GENERAL WARNINGS PRIOR TO USE
• If you are under the care of a Physician, consult with your Physician before using this device
• Long-term effects of this device are not known
• Do not place the device on or close to your heart
• Take care when applying device close to the neck; do not place
this device on the front or sides of the neck. Severe spasm of the
muscles may occur and the contractions may be strong enough to
close the airway or cause difficulty in breathing. Stimulation over
the neck could also affect hearing or blood pressure
• Do not apply stimulation across the chest because the introduction
of electrical current into the chest may cause rhythm disturbances
to the heart
• Do not place the device on or around your head. The effects of
stimulation of the brain are unknown
6 • ENGLISH
IMPORTANT SAFEGUARDS
WARNINGS & CAUTIONS
• Do not use this device while sleeping
• Do not use if you feel numbness
• Do not use this device in or close to water
• Use the only on normal, healthy, clean and dry skin. Do not use on
or close to open wounds or rashes, or over swollen, red, infected
or inflamed skin
• Do not apply stimulation over, or in proximity to, cancerous lesions
• Do not use the device on children or incapacitated persons
• Consult with your physician before using this device, because
the device may cause lethal rhythm disturbances to the heart in
susceptible individuals
GENERAL CAUTIONS PRIOR TO USE
• Read this User Manual before using this device for the first time
• Keep this manual available whenever you use this device
• This device is intended for individual person use only
• This device is not effective for pain associated with Central Pain
Syndromes, such as headaches
ENGLISH • 7
IMPORTANT SAFEGUARDS
WARNINGS & CAUTIONS
• This device is for pain caused by muscle soreness, and should be
placed only around muscles where pain originates
• The pain may indicate that you have some other health problem.
You should know the reason and source of your pain before using
these devices. Do not solely rely on the treatment of this device
for pain
• The safety of using this device during pregnancy or birth has not
been established
• The effectiveness of this device depends greatly on a person’s
individual physical condition. It may not always be effective for
every user
• If you have had medical or physical treatment for your muscle
pain, consult with your treatment provider before using this
device. You should contact your physician prior to using this device
following recent surgical procedures. Stimulation may disrupt the
healing process
8 • ENGLISH
IMPORTANT SAFEGUARDS
WARNINGS & CAUTIONS
OPERATING WARNINGS & CAUTIONS
• CONSULT WITH YOU PHYSICIAN IF YOU HAVE ANY OF THE FOLLOWING:
• If you have suspected or diagnosed heart problem
• If you have suspected or diagnosed epilepsy
• If you have a tendency to bleed internally following an injury
• If you recently had surgery, or have ever had surgery on your back
• If areas of skin lack normal sensations, such as skin that tingles
or is numb
• During menstruation or during pregnancy
• The unit is intended for the temporary relief of pain caused by
muscle soreness. The source or cause of the pain should be addressed by a healthcare professional. Pain could indicate an injury
or condition that requires treatment
• Some people may feel skin irritation or experience a very sensitive
feeling in the skin due to electrical stimulation. If this occurs, stop
using these devices and consult your physician
• If skin under the pad feels irritated after using the stimulator for a
long period of time, use the stimulator for a shorter period of time
ENGLISH • 9
IMPORTANT SAFEGUARDS
WARNINGS & CAUTIONS
• Minor redness at stimulation placement is a normal skin reaction.
It is not considered a skin irritation, and it will normally disappear
within 30 minutes after the electrodes are removed. If the redness
does not disappear after 30 minutes from the removal of electrodes, do not use the stimulator again until after the excessive
redness has disappeared
• Turn off your device if the stimulation feels unpleasant or does not
provide pain relief
• Keep this device out of the reach of children.
• Use this device only with the pads and accessories recommended
by the manufacturer
• Do not use this device when driving, operating machinery or when
swimming
• Before removing the Electrode Pad, be sure to turn it OFF, avoiding
unpleasant stimulation
• TENS is not a substitute for pain medications and other pain
management therapies
• TENS devices have no curative value
10 • ENGLISH
IMPORTANT SAFEGUARDS
WARNINGS & CAUTIONS
• TENS is a symptomatic treatment and, as such, suppresses the
sensation of pain that would otherwise serve as a protective
mechanism
• Do not use the unit while sleeping
• After each use, clean any adhesive residue left on the skin with
soap and water
• The effectiveness of stimulus therapy varies from person to person. It may not be an effective treatment for all users
• The long-term effects of stimulus therapy are unknown
STORAGE WARNINGS & CAUTIONS
• Storage outside of stated storage temperature may result in
measurement error or device malfunction; storage environment
temperature is: 14°F – 140°F (10°C – 60°C); humidity: 30-95%
RH
• Keep the unit out of reach of small children
• Remove the batteries if the unit will not be used for an extended
period of time

ENGLISH • 11
IMPORTANT SAFEGUARDS
WARNINGS & CAUTIONS
• Do not store the device in direct sunlight, dusty or humid environments, or extreme temperatures. Exact figures for appropriate use
and storage can be found in the Product Specifications section of
this manual
CLEANING WARNINGS & CAUTIONS
• Never immerse the unit in water to clean as it may damage the
unit
• Follow the ‘Cleaning and Maintenance’ portion of this manual for
instruction on how to clean and care for your device
12 • ENGLISH
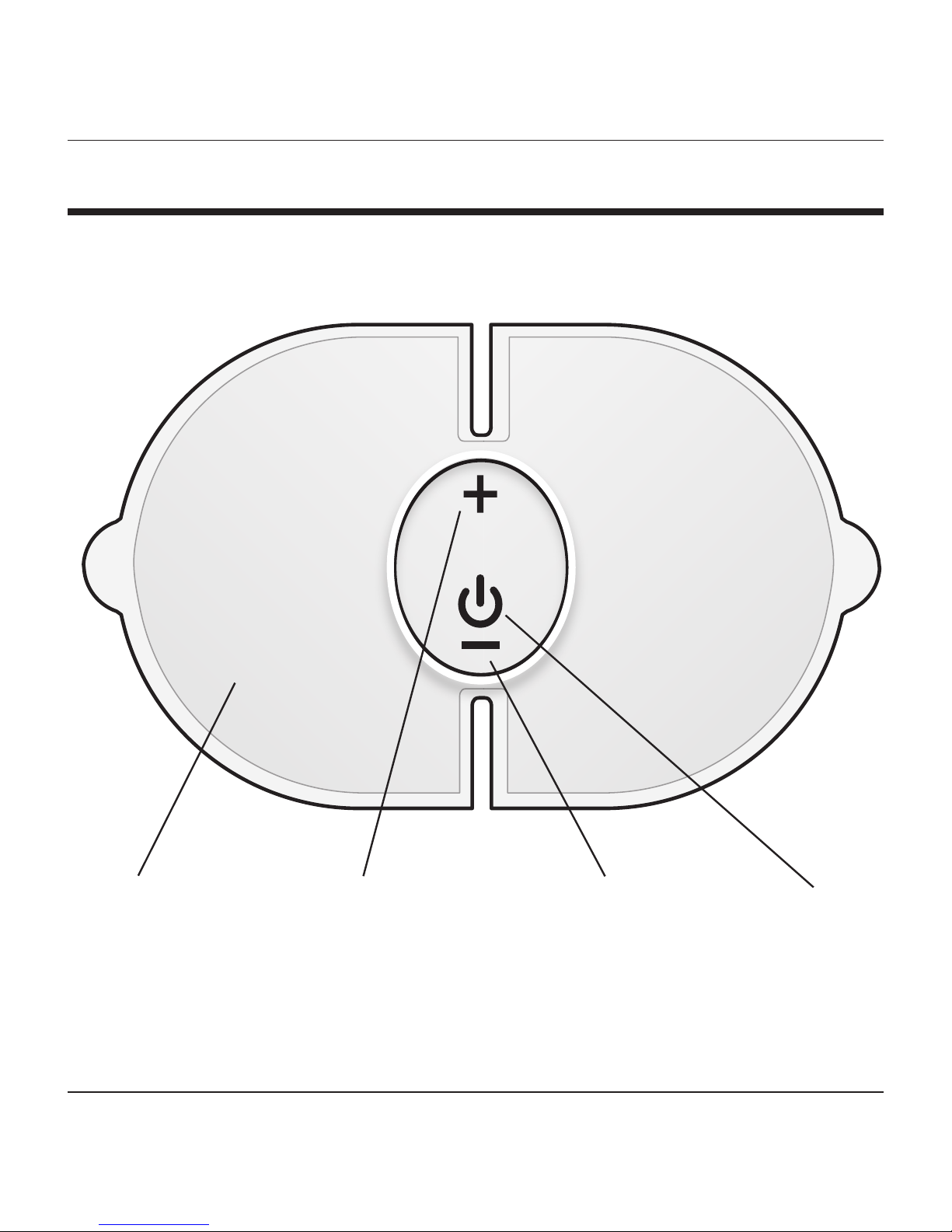
PRODUCT FEATURES
Increase
Intensity
+
Power
Button
Decrease
Intensity
–
TENS
Control Unit
Control Unit
ENGLISH • 13
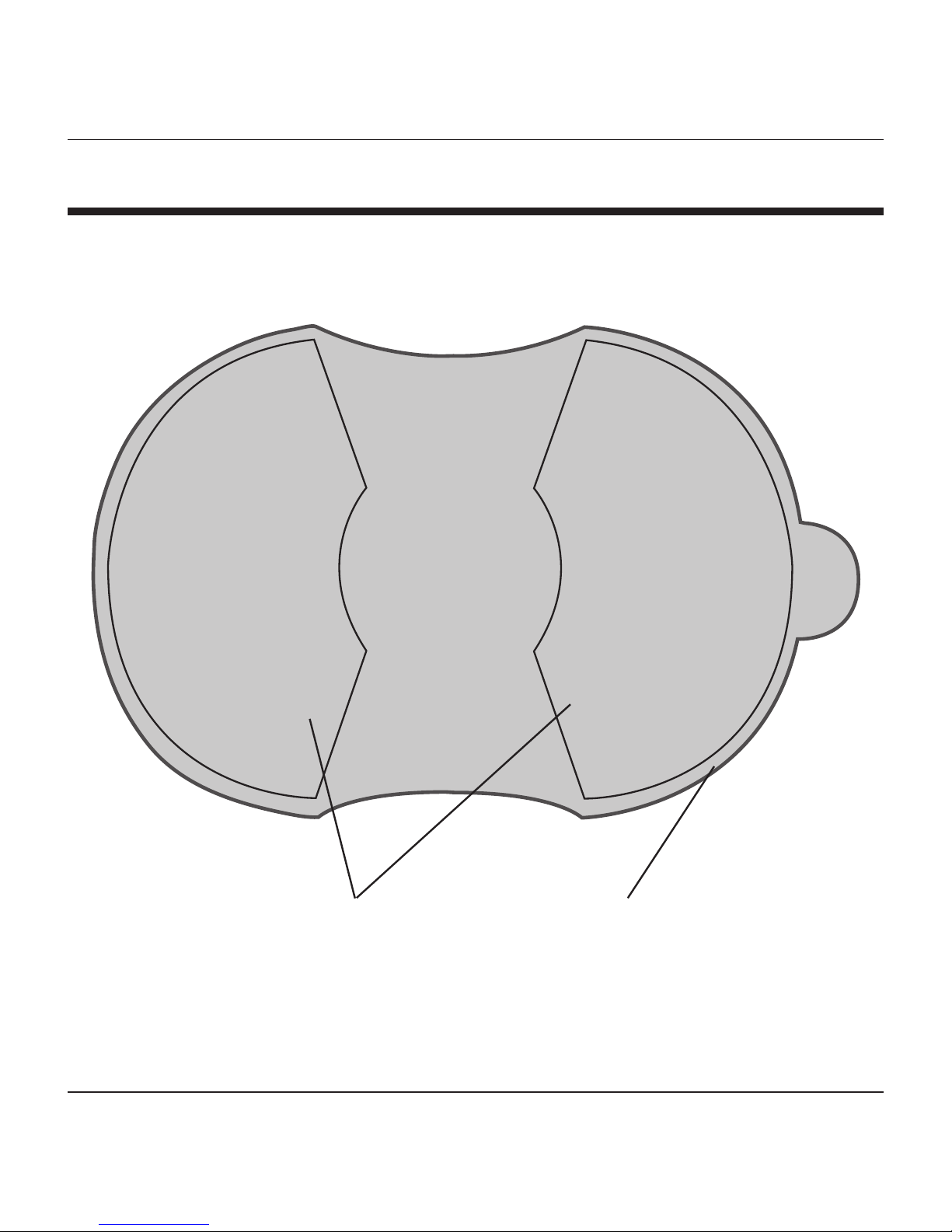
PRODUCT FEATURES
Dual Gel
Pads
Protective
Film
Gel Electrode Pads
14 • ENGLISH
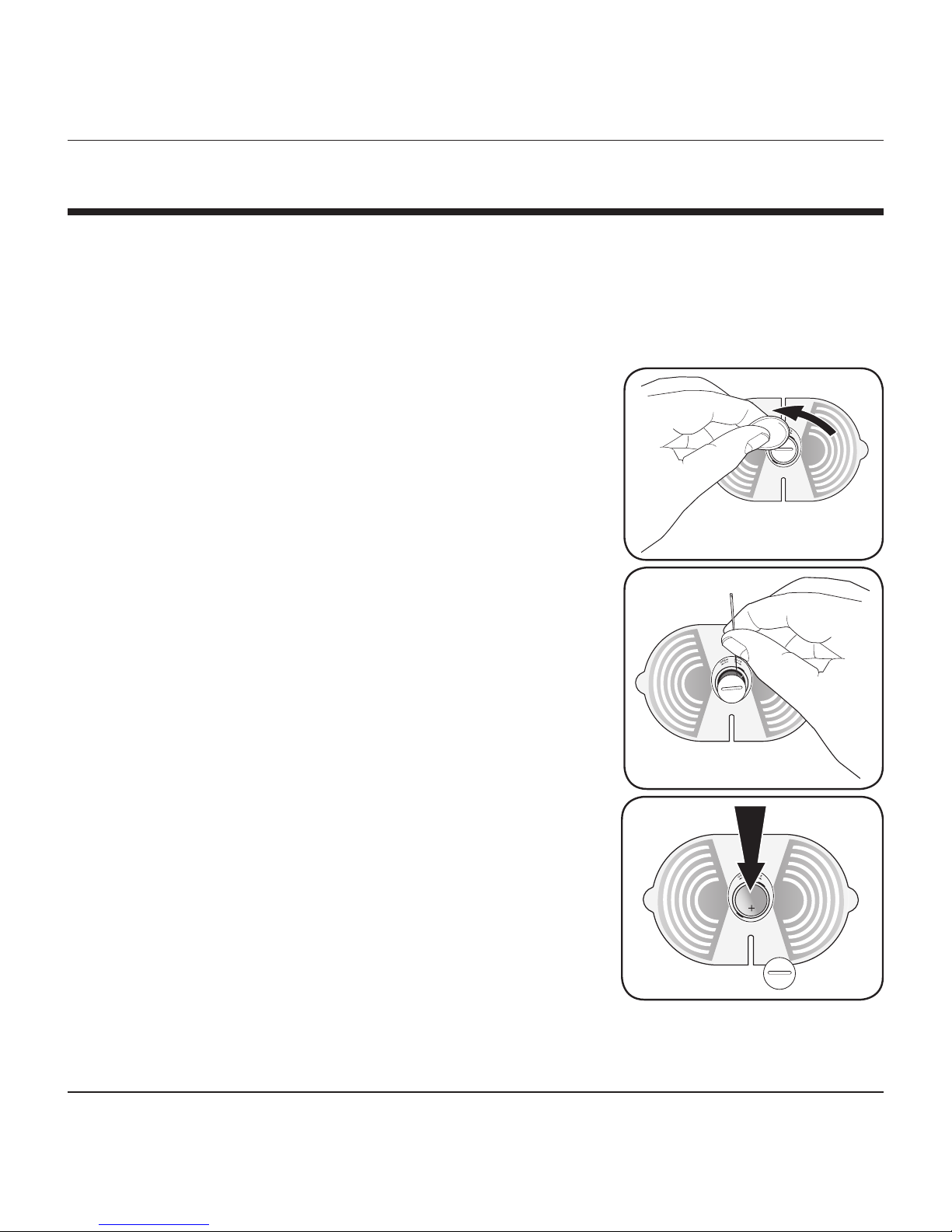
BATTERY INSTALLATION
This unit comes with 1 C2032 battery pre-installed. To begin
using, carefully pull the protective tab from the battery compartment; the
device is now ready for use.
To replace the batteries:
1. Use the included ‘Battery Compartment
Opener’ or a coin to open the battery cover.
2. Follow the indicators on the battery cap—
left/open; right/lock—to open the battery
cap. If necessary, use a paper clip to pop
off the cap.
3. Remove the expired battery; you may need
a pin or paper clip to pop out the battery.
4. Replace C2032 battery into the battery
compartment, with the plus (+) side facing
up. Always use a new battery.
5. Replace the battery cover.
6. Dispose of batteries according to local
disposal and recycling regulations.
It is recommended to remove the battery if the
unit will not be used for an extended period of
time.
ENGLISH • 15
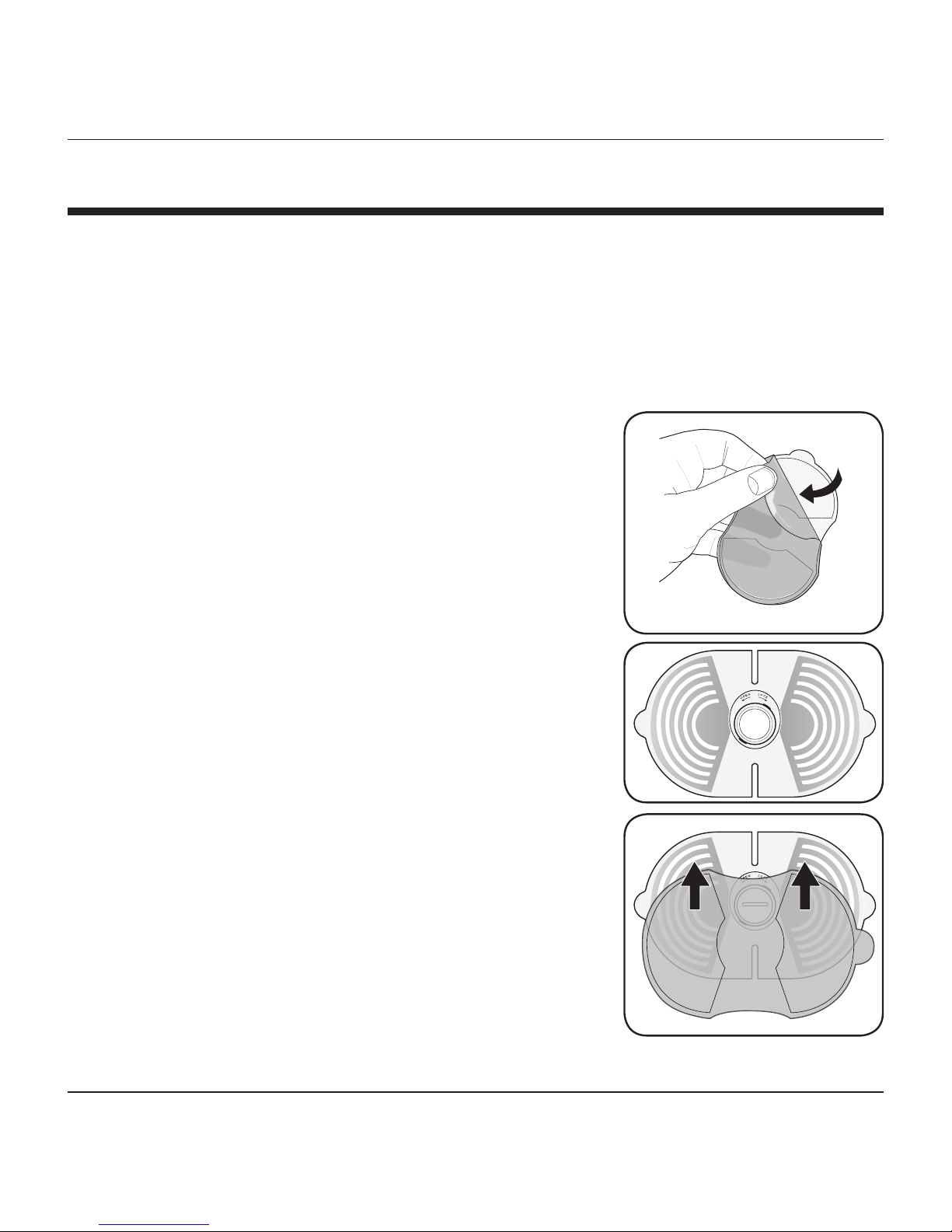
PREPARING YOUR DEVICE
It is necessary to prepare and assemble your device prior to treatment.
NOTE: Save the large plastic film for storage after treatment to
protect the gel pads from dirt and debris.
1. Peel off the one side of the blue plastic
from the gel pads.
2. The gel pads are applied to the back of
the TENS Control Unit along the curved
electrode ridges.
3. While the device is OFF (the GREEN light
is NOT illuminated), carefully align the gel
pads on the back side as shown and press
firmly to ensure the pads are completely
adhered to the electrode ridges.
4. Carefully remove the plastic film from the
electrode pads.
5. You are ready to apply the pad to the
affected treatment area.
Do not turn the power ON until the device
is in place on the user’s skin as indicated
in the ‘Instructions for Use’ section of this
manual.

16 • ENGLISH
PLACEMENT & TREATMENT
Read all warnings and cautions completely before use.
The electrode pads are applied directly to the skin. Clothing may be
worn over the device; clothing should not constrict the device and should
allow access to the control unit buttons.
Targeted treatment areas
may include:
• shoulder
• elbow
• thigh
• knee
• calf
• ankle
• back of neck
• upper back
• lower back
Never place device on
over the heart, on the
front or sides of neck or
the face.
ENGLISH • 17
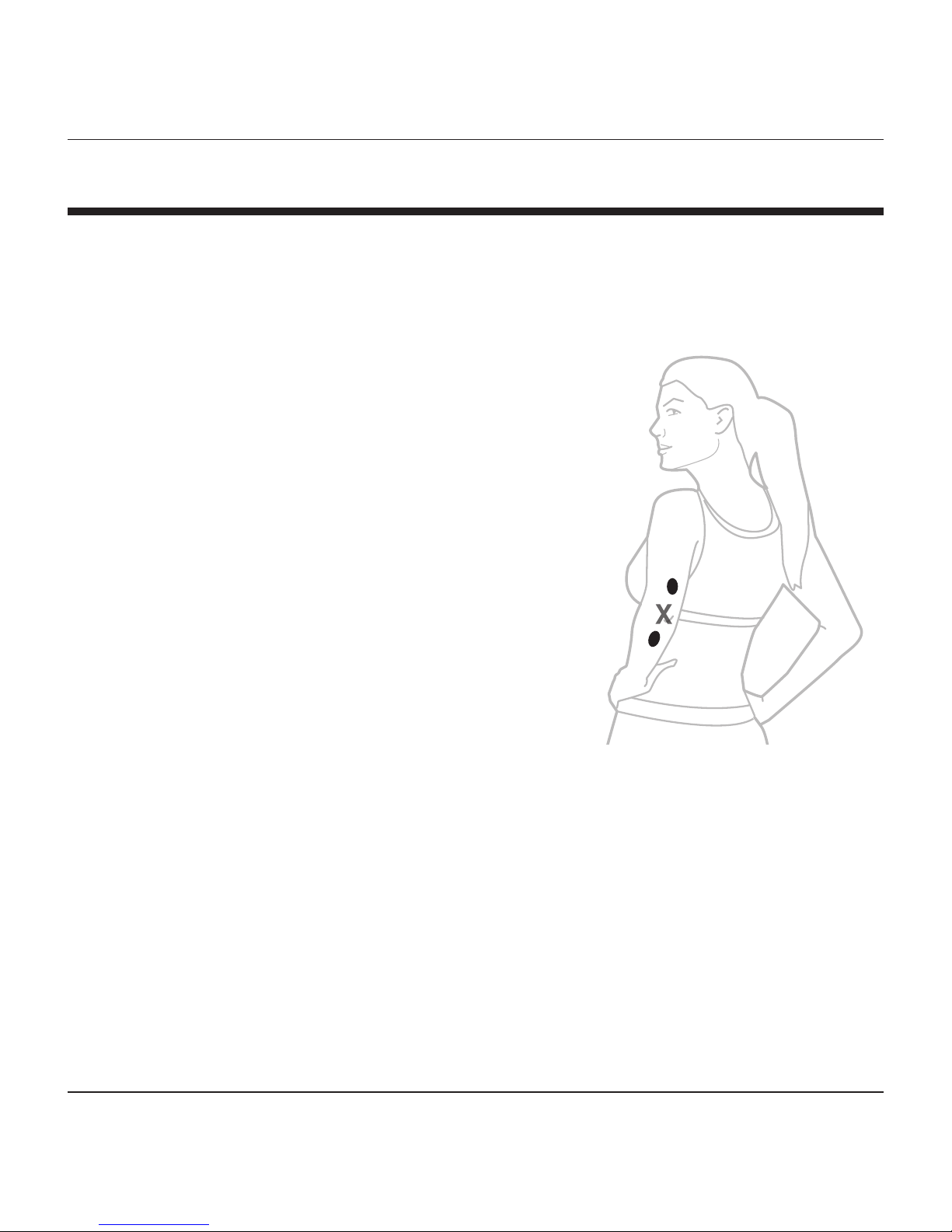
PLACEMENT & TREATMENT
The TENS device may be placed directly on the treatment site where you
are experiencing pain.
An alternative therapy is to apply the
TENS device on alternating sides of the
location of the pain.
For example, if the treatment site is the
elbow, place the TENS unit above the pain
site for the 20 minute cycle, and then
place below the pain site for 20 minutes,
surrounding the location.
X
18 • ENGLISH
Read all warnings and cautions completely before use.
Treatment time is 20-minutes. Discontinue use immediately if
treatment is uncomfortable or painful.
1. Treatment area should be clean and dry before applying the control unit. Note that excessive skin lotion or soap residue may affect
the adhesiveness of the pad. Only use on HEALTHY skin areas with
no cuts, sores or wounds.
2. Apply the control unit and pad to the desired treatment area.
3. Ensure the electrode pads are smoothly applied and the gel adhesive is holding the pad firmly in place on the skin.
4. Press the POWER symbol button for 3 seconds. A GREEN led light
will illuminate to indicate the power is on.
5. The TENS unit will begin to operate at
the lowest level setting. The default
lowest level mode is a rotation of 4
pulse types at level 1 — scraping,
massage, acupuncture and tapping.
6. Press and release the ‘+’ button to
find your desired therapy sensation
level. This device offers 15 intensity
levels. Press and release the ‘–’
button to reduce the intensity level.
INSTRUCTIONS FOR USE
POWER
On/Off
+ Increase
Intensity
Level
– Decrease
Intensity Level

ENGLISH • 19
As you press and release the button, the green light will
illuminate once for each intensity level adjustment.
7. To change the mode, while the unit is on/operating, press and
hold the ‘+’ for 3 seconds; the mode will switch the next in the
sequence of pulse types — scraping, massage, acupuncture,
tapping or combination (all 4 types in sequence/default mode). As
you press and hold the button, the green light will illuminate two
times to indicate the change in mode type.
8. The sensation should be strong but comfortable; press the + or –
button at any time during the treatment to change the intensity as
desired.
9. The device will pulse for several seconds, pause and resume
repeatedly until the treatment is finished.
10. The device will automatically shut off after 20 minutes of use. To
discontinue use prior to the end of treatment time, press and hold
the POWER button. The green light will flash three times and the
device will turn off. Do NOT attempt to remove the device during
operation.
INSTRUCTIONS FOR USE
Until you are familiar with use of the unit, begin with the lower
intensity levels and gradually progress to more advanced levels
as you feel comfortable.

20 • ENGLISH
11. Be sure the device is OFF (no sensation is felt) before removing the
device from your skin. Take care during removal to best protect the
adhesive and preserve the life of the electrode pad.
12. Place the plastic cover over the gel pads to prevent them from
drying out. Store the device in the included storage case.
13. To conserve the battery and prevent shocks, the device will automatically power off when not in use. The green light will illuminate
for three seconds, then flash three times before shutting off.
14. It is recommended to remove the batteries if the unit will not be
used for an extended period of time
INSTRUCTIONS FOR USE

ENGLISH • 21
INSTRUCTIONS FOR USE
Gel electrode pads
• The electrode pads can be reused as long as they retain their
stickiness. Cover the pads with the included film pieces when not
in use to prevent them from drying out
• Properly cared for, the electrode pads may last up to 50 uses; the
life of the electrode pad is largely dependent upon proper care and
the condition of the skin as applied for use
• To help prolong the life of the electrode pad, between uses, moisten the gel side of the pad with a drop of water and allow to dry;
replace the film for storage
• If you notice that the pads have become dry, no longer adhere
well to the skin, or begin to irritate the skin, they are in need of
replacement.
Replacement pads are available through FSAstore.com.
Please use model number 25518.
Note:
You may wish to clean the treatment site after use to remove any adhesive
residue from the electrode pad.

22 • ENGLISH
CLEANING AND STORAGE
Cleaning
• Remove the battery before cleaning the device
• The Control Unit can be cleaned with a soft, dry cloth
• Never use cleaning agents or excessive water to clean any part of
the unit. Do not submerge any part of the unit or let it get wet
• Never disassemble the unit or attempt to repair it (other than
replacing the adhesive pads and batteries)
Storage
• The gel pads must remain on the control unit between uses; place
the film cover over the electrode pads to prevent drying out or
getting dirt and debris on them
• Protect the unit from mechanical shock or heavy impact
• Avoid exposure to extreme temperatures
• Remove the batteries during extended storage

ENGLISH • 23
TROUBLESHOOTING
LED does not illuminate /
Control unit will not turn on
LED illumination is weak
Intensity output is low / no
stimulation is occurring
Device turns off prematurely /
before the 20-minute time is up
Check the polarity of the battery.
Replace the battery.
Battery power is low, replace the battery.
Electrode pad may be dirty, clean and attempt
again.
Confirm that the control unit is firmly attached
to the electrode pads and attempt again.
Battery power is low, replace the battery.
Electrode pad may be dirty, clean and attempt
again
Device may not be applied properly.
Problem Solution

24 • ENGLISH
DEVICE & LABEL SYMBOLS
These symbols may appear on your device, instructions or packaging
and may vary by make and model.
Read This Manual/Consult
Instructions Before Use
Caution/consult
accompanying documents
before use
Use by Date
Date of Manufacture
Batch Code
Catalogue Number
Serial Number
Symbol Meaning
Symbol Meaning
Manufacturer
Temperature Limitation
Humidity Limitation
Non-sterile
Fragile, Handle with Care
Keep Device Dry
Product Packaging is
Recyclable

ENGLISH • 25
ELECTROMAGNETIC COMPATIBILITY
Table 1
For all ME EQUIPMENT and ME SYSTEMS
Guidance and manufacture’s declaration - electromagnetic emissions
This monitor is intended for use in the electromagnetic environment specied below.
The customer or the user of this monitor should assure that it is used in such an environment.
Emmission Test Compliance Electromagnetic Environment Guidance
Group 1
Class B
Class A
Complies
This monitor uses RF energy only for its internal function. Therefore, its RF
emissions are very low and are not likely to cause any interference in nearby
electronic equipment.
This monitor is suitable for use in all establishments, including domestic
establishments and those directly connected to the public low-voltage power
supply network that supplies buildings used for domestic purposes.
RF emissions
CISPR 11
RF emissions
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage uctuations/
icker emissions IEC
61000-3-3

26 • ENGLISH
Table 2
For all ME EQUIPMENT and ME SYSTEMS
Guidance and manufacture’s declaration - electromagnetic immunity
This monitor is intended for use in the electromagnetic environment specied below.
The customer or the user of this monitor should assure that it is used in such an environment.
Electrostatic discharge
(ESD)
IEC 61000-4-2
Electrical fast
transient/burst
IEC 61000-4-4
Surge
IEC 61000-4-5
Voltage dips, short
interruptions and
voltage variations
on power supply
input lines
IEC 61000-4-11
Power frequency
(50/60 Hz)
magnetic eld
IEC 61000-4-8
± 6 kV contact
± 8 kV air
± 2 kV for power supply lines
± 1 kV for input/output lines
± 1 kV line(s) to line(s)
± 2 kV line(s) to earth
<5 % UT
(>95 % dip in UT)
for 0.5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
for 25 cycles
<5 % UT
(>95 % dip in UT)
for 5 s
3 A/m
± 6 kV contact
± 8 kV air
± 2 kV for power supply lines
± 1 kV line(s) to line(s)
± 2 kV line(s) to earth
<5 % UT
(>95 % dip in UT)
for 0.5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
for 25 cycles
<5 % UT
(>95 % dip in UT)
for 5 s
3 A/m
Floors should be wood, concrete or ceramic tile. If
oors are covered with synthetic material, the relative
humidity should be at least 30 %.
Mains power quality should be that of a typical
commercial or hospital environment.
Mains power quality should be that of a typical
commercial or hospital environment.
Mains power quality should be that of a typical
commercial or hospital environment. If the user of
this monitor requires continued operation during
power mains interruptions, it is recommended that
this monitor be powered from an uninterruptible
power supply or a battery.
Power frequency magnetic elds should be at
levels characteristic of a typical location in a typical
commercial or hospital environment.
Immunity Test IEC 60601 Test Level Compliance Level Electromagnetic Environment Guidance
ELECTROMAGNETIC COMPATIBILITY

ENGLISH • 27
ELECTROMAGNETIC COMPATIBILITY
Table 3
For ME EQUIPMENT and ME SYSTEMS that are not LIFE-SUPPORTING
Guidance and manufacturer’s declaration - electromagnetic immunity
This monitor is intended for use in the electromagnetic environment specied below.
The customer or the user of this monitor should assure that it is used in such an environment.
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reection from structures,
objects and people.
* Field strengths from xed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio,
AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to
xed RF transmitters, an electromagnetic site survey should be considered. If the measured eld strength in the location in which this monitor is
used exceeds the applicable RF compliance level above, this monitor should be observed to verify normal operation. If abnormal performance is
observed, additional measures may be necessary, such as re-orienting or relocating this monitor.
** Over the frequency range 150 kHz to 80 MHz, eld strengths should be less than 3V/m.
3 Vrms 150 kHz to 80 MHz
3 V/m 80 MHz to 2,5 GHz
3 Vrms
3 V/m
Portable and mobile RF communications equipment
should be used no closer to any part of this monitor,
including cables, than the recommended separation
distance calculated from the equation applicable to the
frequency of the transmitter.
Recommended separation distance:
80 MHz to 800 MHz
800 MHz to 2,5 GHz
Where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter
manufacturer and d is the recommended separation
distance in meters (m).
Field strengths from xed RF transmitters, as determined
by an electromagnetic site survey,* should be less than
the compliance level in each frequency range.**
Interference may occur in the vicinity of equipment
marked with the following symbol:
Immunity Test IEC 60601 Test Level Compliance Level Electromagnetic Environment Guidance
1
d = 2.
1
d = 2.
d = 2.3

28 • ENGLISH
ELECTROMAGNETIC COMPATIBILITY
Table 4
For ME EQUIPMENT and ME SYSTEMS that are not LIFE-SUPPORTING
Recommended separation distances between portable and mobile RF communications equipment and this monitor
This monitor is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of this monitor can help prevent electromagnetic interference by maintaining a minimum distance between
portable and mobile RF communications equipment (transmitters) and this monitor as recommended below, according to the
maximum output power of the communications equipment.
0,01
0,1
1
10
100
0,12
0,38
1,2
3,8
12
0,12
0,38
1,2
3,8
12
0,23
0,73
2,3
7,3
23
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be determined
using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W)
according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reection from structures,
objects and people.
Separation distance according to frequency of transmitter
m
150 kHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2,5 GHz
1
d = 2.
1
d = 2.
d = 2.3
Rated maximum
output
power of
transmitter
W

ENGLISH • 29
DECLARACIÓN DE LA FCC
NOTA:
POTENCIAL DE INTERFERENCIA PARA RADIO/TELEVISIÓN
(sólo para EE.UU.)
Este producto ha sido probado y se comprobó que cumple con los
límites para un dispositivo digital Clase B, de acuerdo con la parte 15 de
las disposiciones de la FCC (Comisión Federal de Comunicaciones).
Estos límites están diseñados para proporcionar una protección
razonable contra la interferencia dañina de una instalación residencial.
El producto genera, usa y puede irradiar energía de radiofrecuencia y, si
no se instala y usa de acuerdo con las instrucciones, podría ocasionar
una interferencia dañina para las comunicaciones de radio. No obstante,
no hay garantía alguna de que la interferencia no ocurrirá en una
instalación en particular. Si el producto ocasiona interferencia dañina
para la recepción de radio o televisión, la cual se puede determinar al
encender y apagar el producto, se exhorta al usuario a que trate de
corregir la interferencia mediante una o más de las medidas siguientes:
• Reoriente o cambie de lugar la antena receptora
• Aumente la separación entre el producto y el receptor.
• Conecte el producto a un tomacorriente en un circuito diferente al que
está conectado el receptor.
• Consulte al distribuidor o a un técnico de radio/TV experimentado para

30 • ENGLISH
DECLARACIÓN DE LA FCC
obtener ayuda
POTENCIAL DE INTERFERENCIA PARA RADIO/TELEVISIÓN (sólo para
Canadá)
Este aparato digital no excede los límites de Clase B para emisiones
de ruido de radio para un aparato digital, como se estipula en la norma
sobre equipos causantes de interferencia titulada “Aparato digital”,
ICES-003 del Departamento Canadiense de Comunicaciones.
Cet appareil numérique respecte les limites de bruits radioeléctriques
applicables aux appareils numériques de Clase B prescrites dans la
norme sur le materiel brouilleur: “Appareils Numériques”, ICES-003
édictée par le minister des communications.
Los cambios o modiUcaciones no aprobados expresamente por la parte
responsable del cumplimiento podrían anular la autoridad del usuario
para operar el equipo.

ENGLISH • 31
NAME Tens Pain Management Solution
MODEL NUMBER 25517 (VH 56-032)
CHANNEL Single Channel
OUTPUT VOLTAGE 0~34V(vp) +/- 20% (at 500 Ohm load)
PULSE INTENSITY 0~60mA +/- 20%; 0~15 stages Adjustable.
PULSE WIDTH 50~100 uS
PULSE RATE 0 ~ 200Hz
AUTOMATIC SHUT-OFF After 20 minutes of operation upon
completion of therapy treatment
POWER SOURCE 3V CR2032
OPERATION ENVIRONMENT Temperature 50 F - 104 F (10 C - 40C);
Humidity 40% - 90%
STORAGE ENVIRONMENT Temperature 14 F - 140 F (-10 C - 60 C);
Humidity 30% - 95%
DEVICE DIMENSIONS 2-3/4” x 4-1/2”x1/3”
WEIGHT Controller weight 6.7 oz (with battery)
INCLUDED Control unit, 1 electrode gel pads set,
battery compartment opener, detailed
guidebook, replacement electrodes order
form, one CR2032 battery
AVAILABLE SEPARATELY Replacement electrodes 3-pack,
Model 25518 (VH 22-037)
Specifications are subject to change without notice
PRODUCT SPECIFICATIONS

32 • ENGLISH
Congratulations on your purchase of the Tens Pain Management Solution. Your
Tens Pain Management Solution is covered by the following limited warranty
commencing upon the date of purchase, and subject to the following terms and
conditions:
The Warrantor warrants that its Verde Tens Pain Management Solution will be
free from defects in materials and workmanship under normal consumer usage
for a period of one year for the original purchaser of the product.
Periodic maintenance, repair and replacement of parts due to normal wear
and tear are excluded from coverage. Defects or damage that result from: (a)
improper operation, storage, misuse or abuse, accident or neglect, such as
physical damage (cracks, scratches, etc.) to the surface of the product resulting
from misuse; (b) contact with liquid, water, rain, extreme humidity or heavy
perspiration, sand, dirt or the like, extreme heat, or food; (c) use of the Tens Pain
Management System for commercial purposes or subjecting the Tens Pain Management System to abnormal usage or conditions; or (d) other acts which are not
the fault of the Warrantor, are excluded from coverage. This warranty does not
cover batteries or other power sources that may be provided with, or used with
the Tens Pain Management Solution.
If the Tens Pain Management Solution fails to conform to this limited warranty,
return the Tens Pain Management Solution postage prepaid to: Attn: Repair De-
partment, 1175 Lakeside Drive, Gurnee, IL, 60031. When returning a product,
please also include: (i) a copy of your receipt, bill of sale or other comparable
proof of purchase; (ii) a written description of the problem; and (iii) your name,
address and telephone number. Carefully package the product to avoid any
1-YEAR LIMITED WARRANTY

ENGLISH • 33
damage that may occur while in transit; shipping insurance with returned receipt
is recommended. At our option, the Warrantor will repair or replace the unit found
to be defective in materials or workmanship under normal consumer usage. The
purchaser will be notified of any additional repairs required prior to completing
the repair, and will be responsible for parts charges, if any, and repair charges
not covered by this limited warranty.
EXCEPT AS PROVIDED FOR IN THIS LIMITED WARRANTY, ALL EXPRESS AND
IMPLIED WARRANTIES AND CONDITIONS ARE DISCLAIMED, INCLUDING WITHOUT
LIMITATION THE IMPLIED WARRANTIES OF MERCHANTABILITY AND FITNESS FOR
A PARTICULAR PURPOSE. THE REPAIR OR REPLACEMENT AS PROVIDED UNDER
THIS LIMITED WARRANTY IS THE EXCLUSIVE REMEDY OF THE CONSUMER, AND
IS PROVIDED IN LIEU OF ALL OTHER WARRANTIES, EXPRESS OR IMPLIED. IN NO
EVENT SHALL THE WARRANTOR BE LIABLE, WHETHER IN CONTRACT OR TORT
(INCLUDING NEGLIGENCE) FOR DAMAGES IN EXCESS OF THE PURCHASE PRICE
OF THE PRODUCT, OR FOR ANY INDIRECT, INCIDENTAL, SPECIAL OR CONSEQUENTIAL DAMAGES OF ANY KIND, OR FOR DAMAGES TO, OR LOSS OF, OTHER
PROPERTY OR EQUIPMENT OR PERSONAL INJURIES TO THE FULL EXTENT THESE
DAMAGES MAY BE DISCLAIMED BY LAW.
Some states and jurisdictions do not allow the limitation or exclusion of incidental or consequential damages, or limitation on the length of an implied warranty,
so the above limitations or exclusions may not apply to you. This warranty gives
you specific legal rights, and you may also have other rights that vary from state
to state or from one jurisdiction to another.
1-YEAR LIMITED WARRANTY


TENS Solución de
Administración de Dolor
Modelo 25517 (VH 56-032)
Intención de uso:
La solución de gestión de dolor de decenas está indicada para el alivio temporal del dolor
asociado con los músculos adoloridos en las parte baja de la espalda debido a la tensión
de ejercicio o normal del hogar y las actividades laborales.
MANUAL DE INSTRUCCIONES
ESPAÑOL
LEA TODAS LAS INSTRUCCIONES ANTES DE
COMENZAR A USAR EL DISPOSITIVO TENS.

36 • ESPAÑOL
¡ALTO! ASEGÚRESE DE TENER TODOS LOS COMPONENTES QUE SE INDICAN A CONTINUACIÓN ANTES
DE UTILIZAR SU DISPOSITIVO TENS.
SI LE FALTA ALGUNA PARTE, INCLUIDOS LOS FOLLETOS O MANUALES DE INSTRUCCIONES, NO LO DEVUELVA AL LUGAR DONDE
LO COMPRÓ. COMUNÍQUESE CON EL SERVICIO DE ATENCIÓN AL
CLIENTE AL 866-326-1313
Unidad de
control
Caja de
Almacenamiento
Manual de instrucciones
Formulario de pedido de electrodo
de repuesto
Una batería
CR2032
Un Pista Gel
del Electrodo

ESPAÑOL • 37
Medidas importantes de seguridad ................................................ 38-45
Características del producto ..........................................................46-47
Colocación de las baterías ..................................................................48
Preparación de su unidad ...................................................................49
Colocación y tratamiento ...............................................................50-51
Instrucciones de uso ......................................................................52-55
Mantenimiento, limpieza y almacenaje ................................................56
Solución de problemas ........................................................................57
Símbolos del dispositivo y las etiquetas ...............................................58
Compatibilidad electromagnética ................................................... 59-62
Declaración de la FCC .................................................................... 63-64
Especificaciones del producto ..............................................................65
Garantía .........................................................................................66-67
Línea de ayuda gratuita de asistencia al cliente: 1-866-326-1313
De lunes a viernes de 8:30 a 16:30 (hora central del Este)
ÍNDICE
Caring Mill™ 2017
240 W. 37th St.
6th Floor
New York, NY 10018
1-888-372-1450
Hecho en China
#93-1387 08/17

38 • ESPAÑOL
MEDIDAS IMPORTANTES DE
SEGURIDAD ADVERTENCIAS
Y PRECAUCIONES
NOTA: Antes de su uso, lea detenidamente todas las instrucciones y los
folletos que se incluyen con este dispositivo. Cuando se usa un producto
eléctrico se deben tener en cuenta las siguientes precauciones básicas.
PRECAUCIÓN: Si no lee ni presta atención a todas las precauciones, puede resultar lesionado o dañar el equipo.
El cuidado o uso inadecuado de su dispositivo puede ocasionarle
lesiones, dañar la unidad o hacer que el tratamiento no sea efectivo. Si
sigue estas instrucciones, se garantizará la precisión y vida útil prolongada del dispositivo.
CONTRAINDICACIONES:
No use este dispositivo si tiene un marcapasos cardíaco, un desfibrilador implantable o cualquier otro dispositivo de metal o electrónico
implantado
No use este dispositivo para el tratamiento del dolor crónico no diagnosticado
No use este dispositivo si le han diagnosticado problemas cardíacos o
existen sospechas de que los padece
No use este dispositivo si le han diagnosticado epilepsia o existen
sospechas de que la padece
No use este dispositivo si tiene tendencia a sangrar internamente luego
de una herida

ESPAÑOL • 39
MEDIDAS IMPORTANTES DE
SEGURIDAD ADVERTENCIAS
Y PRECAUCIONES
No use este dispositivo si ha tenido una operación en la espalda o un
procedimiento quirúrgico reciente
No use este dispositivo si tiene anomalías en la sensibilidad de la piel,
por ejemplo hormigueo o adormecimiento
No use este dispositivo durante la menstruación o el embarazo
PRECAUCIONES GENERALES ANTES DEL USO
• Si usted está bajo el cuidado de un médico, consulte con su médico
antes de usar este dispositivo
• No se conocen los efectos a largo plazo • de este dispositivo
• No coloque el dispositivo sobre o cerca de tu corazón
• Tenga cuidado al aplicar el dispositivo cerca del cuello; No coloque
este aparato sobre la frente o los lados del cuello. Severo espasmo
de los músculos puede ocurrir y las contracciones pueden ser lo suficientemente fuertes como para cerrar las vías respiratorias o causar
dificultad en la respiración. Estimulación sobre el cuello también
podría afectar audiencia o la presión arterial
• No aplique la estimulación en el pecho debido a la introducción de
corriente eléctrica en el pecho puede provocar alteraciones del ritmo
del corazón

40 • ESPAÑOL
• No coloque el dispositivo sobre o alrededor de su cabeza. Se desconocen los efectos de la estimulación del cerebro
• No utilice este aparato mientras duerme
• No utilice si usted siente entumecimiento
• No utilizar estos dispositivos en o cerca del agua
• Utilice el único en normal, sano, limpie y seque la piel. No utilice en
o cerca de heridas abiertas o erupciones o sobre la piel hinchada,
enrojecida, infectada o inflamada
• No aplicar estimulación sobre o cerca de lesiones cancerosas
• No utilice el aparato en niños o incapacitados personas
• Consulte con su médico antes de usar este dispositivo, porque el
dispositivo puede causar alteraciones del ritmo letal al corazón en
personas susceptibles
PRECAUCIONES GENERALES ANTES DEL USO
• Lea este Manual del usuario antes de utilizar este aparato por
primera vez
• Guarde este manual disponible cuando se utiliza este dispositivo
• Este dispositivo está diseñado para un uso individual de la persona
MEDIDAS IMPORTANTES DE
SEGURIDAD ADVERTENCIAS
Y PRECAUCIONES

ESPAÑOL • 41
• Este dispositivo no es eficaz para el dolor asociado con síndromes
de dolor Central, tales como dolores de cabeza
• Este dispositivo es para el dolor causado por dolores musculares y
debe ser colocado solamente alrededor de los músculos donde se
origina el dolor
• El dolor puede indicar que usted tiene algún otro problema de salud. Usted debe saber la razón y la fuente del dolor antes de utilizar
estos dispositivos. No confíe únicamente en el tratamiento de este
dispositivo para el dolor
• La seguridad de la utilización de este producto durante el embarazo o el parto no se ha establecido
• La eficacia de este dispositivo depende enormemente de la condición física individual de la persona. No siempre puede ser eficaz
para todos los usuarios
• Si ha tenido tratamiento médico o físico para el dolor de músculo,
consulte a su proveedor de tratamiento antes de usar este dispositivo. Usted debe contactar a su médico antes de usar este dispositivo siguiendo procedimientos quirúrgicos recientes. Estimulación
puede interrumpir el proceso de curación
MEDIDAS IMPORTANTES DE
SEGURIDAD ADVERTENCIAS
Y PRECAUCIONES

42 • ESPAÑOL
ADVERTENCIAS Y PRECAUCIONES DE OPERACIÓN
• CONSULTE CON EL MÉDICO SI USTED TIENE CUALQUIERA DE LOS
SIGUIENTES:
• Si tiene sospecha o diagnóstico de problema cardíaco
• Si tiene sospecha o epilepsia diagnosticada
• Si usted tiene una tendencia a sangrar internamente después de
una lesión
• Si usted recientemente operaron, o alguna vez ha había tenido
cirugía en la espalda
• Si las áreas de piel carecen de sensaciones normales, como
hormigueo o entumecimiento en la piel
• Durante la menstruación o durante el embarazo
• La unidad está diseñado para el alivio temporal del dolor causado
por el dolor muscular. La fuente o la causa del dolor debe
abordarse por un profesional de la salud. El dolor podría indicar
una lesión o condición que requiere tratamiento
• Algunas personas pueden sentir irritación de la piel o experimentar
una sensación muy sensible en la piel debido a la estimulación
eléctrica. Si esto ocurre, deje de usar estos dispositivos y consulte
a su médico
MEDIDAS IMPORTANTES DE
SEGURIDAD ADVERTENCIAS
Y PRECAUCIONES

ESPAÑOL • 43
• Si la piel debajo de la almohadilla se siente irritada después de
usar el aparato durante un largo periodo de tiempo, utilice el
estimulador para un período más corto de tiempo
• Leve enrojecimiento en la colocación de estimulación es una
reacción normal de la piel. No se considera una irritación de la
piel, y normalmente desaparecerá dentro de 30 minutos después
de desconectar los electrodos. Si el enrojecimiento no desaparece
después de 30 minutos con la remoción de los electrodos, no
utilice el estimulador nuevamente hasta después de la irritación
excesiva ha desaparecido
• Apagar el dispositivo si la estimulación se siente desagradable o
no proporciona alivio del dolor
• Mantenga el aparato fuera del alcance de los niños
• Utilice este aparato sólo con los pads y los accesorios recomendados por el fabricante
• No use este dispositivo al conducir, operar maquinaria o al nadar
• Antes de retirar el pista del electrodo, no olvide de apagarlo,
evitando la estimulación desagradable
• Si el dolor no mejora, (!) o si continúa durante cuatro a seis días,
deje de usar este dispositivo y consulte a su médico
MEDIDAS IMPORTANTES DE
SEGURIDAD ADVERTENCIAS
Y PRECAUCIONES

44 • ESPAÑOL
• TENS no es un sustituto de medicamentos para el dolor y otras
terapias de manejo de dolor
• DECENAS dispositivos no tienen ningún valor curativo
• TENS es un tratamiento sintomático y, como tal, suprime la
sensación de dolor que de lo contrario serviría como un mecanismo de protección
• No utilice la unidad mientras duerme
• Después de cada uso, limpie cualquier residuo de adhesivo dejar
sobre la piel con agua y jabón
• La eficacia de la terapia de estímulo varía de persona a persona.
No puede ser un tratamiento eficaz para todos los usuarios
• Se desconocen los efectos a largo plazo de la terapia de estímulo
ADVERTENCIAS Y PRECAUCIONES
DE ALMACENAMIENTO
• Almacenamiento fuera de la temperatura de almacenamiento
indicado puede resultar en falla de error o dispositivo de medición;
temperatura ambiente de almacenamiento es: 14° F a 140° F (10°
C a 60° C); humedad: 30-95% RH
MEDIDAS IMPORTANTES DE
SEGURIDAD ADVERTENCIAS
Y PRECAUCIONES

ESPAÑOL • 45
• Mantenga la unidad fuera del alcance de niños pequeños
• Retire las baterías si no se utilizará la unidad por un período
prolongado de tiempo
• No guarde el dispositivo en la luz sol directa, ambientes polvorientos o húmedos o temperaturas extremas. Cifras exactas para
el uso apropiado y el almacenamiento de información se pueden
encontrar en la sección de especificaciones de este manual
ADVERTENCIAS Y PRECAUCIONES DE LIMPIEZA
• Nunca sumerja la unidad en agua para limpiar ya que puede dañar
la unidad
• Siga la porción “Limpieza y mantenimiento” de este manual para
instrucciones sobre cómo limpiar y cuidar de su dispositivo
MEDIDAS IMPORTANTES DE
SEGURIDAD ADVERTENCIAS
Y PRECAUCIONES

46 • ESPAÑOL
FUNCIONES DEL PRODUCTO
Unidad de Control
Aumentar la
intensidad
+
Botón de
encendido
Reduzca la
intensidad
–
Unidad de
control

ESPAÑOL • 47
FUNCIONES DEL PRODUCTO
Electrodos de Gel
Almohadillas
de Gel Dual
Cubierta
Protectora

48 • ESPAÑOL
COLOCACIÓN DE LA BATERÍA
Esta unidad viene con 1 batería C2032 preinstalada. Para
comenzar a utilizar, cuidadosamente tire de la lengüeta protectora del
compartimiento de la batería; el dispositivo está listo para use.
Cómo reemplazar la batería:
1. Utilice el incluido ‘abridor de compartimiento de batería’ o una moneda para abrir la
cubierta de la batería.
2. Siga los indicadores en la tapa de la batería
— izquierda/abierto; derecho/cerradura—
para abrir la tapa de la batería. Si es necesario, utilice un clip para estallar la tapa.
3. Retire la batería expirada; puede que
necesites un alfiler o un clip para sacar la
batería.
4. Reemplace C2032 batería en el compartimiento de la batería, con el lado (+) hacia
arriba. Siempre use una batería nueva.
5. Vuelva a colocar la tapa.
6. Deseche las baterías de conformidad con los
reglamentos locales de desecho y reciclaje.
Se recomienda retirar las baterías si la unidad no está en uso durante un
periodo prolongado.

ESPAÑOL • 49
PREPARACIÓN DEL DISPOSITIVO
Es necesario preparar y armar el equipo antes del tratamiento.
NOTA: Conserve el la película plástica grande rectangular que
está del lado blanco de los electrodos para guardarlo después
del tratamiento.
1. Pele un lado de la azul de plástico de las
almohadillas de gel.
2. Las almohadillas de gel se aplican a la parte
posterior de la unidad de Control de decenas a
lo largo de las crestas del electrodo curvado.
3. Mientras el dispositivo está apagado (no
se enciende la luz LED VERDE), alinee las
almohadillas de gel en la parte posterior como
se indica y presione firmemente para asegurar
completamente que las almohadillas se atiene
a las crestas del electrodo.
4. Retire con cuidado la película plástica de los
cojines del electrodo.
5. Usted está listo para aplicar la almohadilla en
la zona afectada tratamiento.
No ponga el dispositivo en ON (ENCENDIDO) hasta que no esté
en la piel del usuario tal como lo indica la sección ‘Instrucciones
de Uso’ de este manual.

50 • ESPAÑOL
COLOCACIÓN Y TRATAMIENTO
Lea todas las advertencias y precauciones antes de usar. Los
electrodos se aplican directamente sobre la piel. Ropa puede ser usado
sobre el dispositivo; la ropa no debe restringir el dispositivo y debe
permitir el acceso a los botones de la unidad de control.
Áreas de tratamiento específicas pueden incluir:
• hombro
• codo
• muslo
• rodilla
• becerro
• tobillo
• parte posterior
del cuello
• espalda
superior
• zona lumbar
Nunca coloque el aparato sobre el corazón, en la
frente o los lados de la
cara o el cuello.

ESPAÑOL • 51
COLOCACIÓN Y TRATAMIENTO
Las decenas dispositivo puede ser colocado directamente en el sitio de
tratamiento donde usted está experimentando dolor..
Una terapia alternativa consiste en aplicar
las decenas dispositivo alternando lados
de la localización del dolor.
Por ejemplo, si el lugar de tratamiento es
el codo, coloque las decenas la unidad
sobre el sitio del dolor para el ciclo de 20
minutos y luego lugar debajo del sitio de
dolor durante 20 minutos, alrededor de la
ubicación.
X

52 • ESPAÑOL
INSTRUCCIONES DE USO
Lea todas las advertencias y precauciones antes de usar. El
tiempo de tratamiento está a 20 minutos. Deje de utilizar inmediatamente si el tratamiento es doloroso o incómodo.
1. Área de tratamiento debe estar limpio y seco antes de aplicar la
unidad de control. Tenga en cuenta que ese residuo de jabón o
loción excesiva de la piel puede afectar la adherencia de la almohadilla. Use solamente en las áreas de piel sana con sin cortes,
llagas o heridas.
2. Aplique la unidad de control y la almohadilla en el área de tratamiento deseado.
3. Asegúrese de que los electrodos se aplican suavemente y el gel
adhesivo está sosteniendo la almohadilla firmemente en su lugar
en la piel.
4. Pulse el botón símbolo durante 3 segundos. Luz led VERDE se encenderá
para indicar que está conectada la
alimentación.
5. Las decenas unidad comenzará a
funcionar en el ajuste del nivel más
bajo. El modo predeterminado de nivel
más bajo es un tipo de rotación 4
pulso en el nivel 1— raspado masaje,
acupuntura y golpeando.
Botón de
encendido
+ Aumentar
el nivel de
intensidad
– Disminuir el nivel
de intensidad

ESPAÑOL • 53
INSTRUCCIONES DE USO
6. Presione y suelte el ‘ +‘ botón para encontrar tu nivel de sensación
de terapia deseada. Este dispositivo ofrece 15 niveles de
intensidad. Presione y suelte el ‘ –’ botón para reducir el nivel de
intensidad. Presione y suelte el botón, la luz verde se encenderá
una vez para cada ajuste del nivel de intensidad.
7. Para cambiar el modo, mientras la unidad está en/funcionamiento,
mantenga pulsado el ‘ +‘ durante 3 segundos; el modo cambiará
el siguiente en la secuencia de pulso tipos — raspado, masaje,
acupuntura, golpeando o combinación (todos 4 tipos en modo
secuencia/por defecto). Presione y mantenga presionado el botón,
se encenderá la luz roja dos veces para indicar el cambio en el tipo
de modo.
8. La sensación debe ser fuerte pero cómodo; Presione la o el botón
en cualquier momento durante el tratamiento para cambiar la
intensidad deseada.
9. El dispositivo será de pulso durante varios segundos, pausar y
reanudar repetidamente hasta que se termine el tratamiento.
Hasta que usted está familiarizado con el uso de la unidad, comienza
con los bajos niveles de intensidad y poco a poco avance a niveles más
avanzados como usted se sienta cómodo

54 • ESPAÑOL
INSTRUCCIONES DE USO
10. El dispositivo se apagará automáticamente después de 20 minutos
de uso. Para poder usar antes del final del tiempo de tratamiento,
mantenga pulsado el botón de encendido. La luz verde parpadeará
tres veces y el dispositivo se apagará. No intente quitar el dispositivo durante el funcionamiento.
11. Asegúrese de que el dispositivo está apagado (sensación no es
fieltro) antes de retirar el dispositivo de su piel. Tenga cuidado
durante el desmontaje para proteger mejor el adhesivo y preservar
la vida del cojín del electrodo.
12. Coloque la tapa de plástico sobre las almohadillas de gel para
evitar que se sequen. Guarde el aparato en el estuche incluido.
13. Para conservar la batería y evitar choques, el dispositivo se
apagará automáticamente cuando no esté en uso. La luz verde se
encenderá durante tres segundos, luego flash tres veces antes de
apagarse.
14. Se recomienda retirar las pilas Si la unidad no se utilizará durante
periodos prolongados de tiempo.

ESPAÑOL • 55
INSTRUCCIONES DE USO
Almohadillas de electrodos
• Las almohadillas de electrodos se pueden volver a usar siempre y
cuando conserven la pegajosidad. Cuando no está en uso, cubra
los lados adhesivos de las almohadillas con el film que se incluye
para evitar que se sequen.
• Con los cuidados adecuados, una almohadilla de electrodos
se puede usar hasta 20 veces; la vida útil de la almohadilla de
electrodos depende en gran medida de un buen cuidado y de la
condición de la piel sobre la que se lo va a usar.
• Para prolongar la vida útil de la almohadilla de electrodos, entre
usos humedezca el lado con gel de la almohadilla con una gota de
agua y deje secar; cambie el film para guardarla.
• Si nota que las almohadillas están secas, ya no se adhieren bien a
la piel o comienzan a irritarla, entonces hay que cambiarlas
Las almohadillas de repuesto están disponibles
a través de FSAstore.com. Utilice el número de modelo 25518.
Nota:
Después del uso puede limpiar la zona de tratamiento para eliminar cualquier residuo de adhesivo proveniente de las almohadillas de electrodos.

56 • ESPAÑOL
LIMPIEZA Y ALMACENAMIENTO
Limpieza
• Quite la batería antes de limpiar el dispositivo
• La unidad de control se puede limpiar con un paño suave y seco
• No use nunca agentes de limpieza o agua en exceso para limpiar
cualquiera de las partes de la unidad. No sumerja ni deje que se
mojen ninguna de las partes de la unidad
• Nunca desarme ni intente reparar la unidad (salvo reemplazar las
almohadillas adhesivas y las baterías)
Almacenamiento
• La almohadilla de electrodos puede permanecer unido a la unidad
de control entre usos; coloque las cubiertas de film sobre los
lados adhesivos de la almohadilla de electrodos para evitar que
se sequen
• Proteja la unidad de golpes mecánicos o de impactos fuertes.
• Evite la exposición a temperaturas extremas
• Quite la batería cuando no la use durante un tiempo prolongado

ESPAÑOL • 57
SOLUCIÓN DE PROBLEMAS
El LED no se ilumina / La unidad
de control no se encenderá
La iluminación del LED es débil
La intensidad es baja / no se
produce la estimulación
El dispositivo se apaga antes de
lo previsto / antes de terminar
los 20 minutos
Asegúrese de haber quitado el film del
parche.
Verifique la polaridad de la batería.
Cambie la batería.
La batería está baja, cámbiela.
Puede ser que la almohadilla de electrodos
esté sucia; límpiela e intente de nuevo
Confirme que la unidad de control está firmemente adherida a la almohadilla de electrodos
e intente de nuevo
La batería está baja, cámbiela.
Puede ser que la almohadilla de electrodos
esté sucia; límpiela e intente de nuevo
El dispositivo no se puede aplicar correctamente
Problema Solución

58 • ESPAÑOL
SÍMBOLOS DISPOSITIVO/ETIQUETAS
Estos símbolos pueden aparecer en su aparato, instrucciones o
embalaje y pueden variar según la marca y el modelo.
Lea este manual/Consulte las
instrucciones antes del uso
Precaución/consulte los
documentos adjuntos antes
del uso
Uso por fecha
Fecha de fabricación
Código de lote
Número de catálogo
Número de serie
Símbolo Signicado
Símbolo Signicado
Fabricante
Límite de temperatura
Límite de humedad
No estéril
Frágil, trate con cuidado
Mantenga el dispositivo
seco
El embalaje del producto
es reciclable

ESPAÑOL • 59
COMPATIBILIDAD ELECTROMÁGNETICA
Tabla 1
Para todos los EQUIPOS ME y SISTEMAS ME
Guía y declaración del fabricante – emisiones electromagnéticas
Este monitor tiene la nalidad de usarse en el ambiente electromagnético que se especica a continuación.
El cliente o usuario de este monitor debe asegurarse de que se utilice en dicho entorno.
Prueba de emisiones Cumplimiento Guía sobre el ambiente electromagnético
Grupo 1
Clase B
Clase A
Cumple
Este monitor usa energía de RF únicamente para su funcionamiento interno. Por
lo tanto, sus emisiones de radiofrecuencia son muy bajas y no es probable que
causen ninguna interferencia en los equipos electrónicos cercanos.
Este monitor es apto para usarse en todo tipo de establecimiento, inclusive en
ámbitos domésticos y en aquellos conectados directamente a la red pública de
baja tensión que alimenta a los edicios destinados a vivienda.
Emisiones de radiofrecuencia CISPR 11
Emisiones de radiofrecuencia CISPR 11
Emisiones armónicas
CEI 61000-3-2
Fluctuaciones y parpadeo
de tensión CEI 61000-3-3

60 • ESPAÑOL
COMPATIBILIDAD ELECTROMÁGNETICA
Tabla 2
Para todos los EQUIPOS ME y SISTEMAS ME
Guía y declaración del fabricante – inmunidad electromagnética
Este monitor tiene la nalidad de usarse en el ambiente electromagnético que se especica a continuación.
El cliente o usuario de este monitor debe asegurarse de que se utilice en dicho entorno.
Descarga
electrostática
CEI 61000-4-2
Transitorios
eléctricos
rápidos/en ráfagas
CEI 61000-4-4
Sobretensión
CEI 61000-4-5
Bajas de tensión,
interrupciones
breves y variaciones
de tensión en las
líneas de entrada de
suministro eléctrico
CEI 61000-4-11
Campos magnéticos
de frecuencia de
energía (50/60 Hz)
CEI 61000-4-8
± 6 kV por contacto
± 8 kV por aire
± 2 kV para líneas de
alimentación eléctrica
± 1 kV para líneas de
entrada/salida
± 1 kV línea(s) a línea(s)
± 2 kV línea(s) a tierra
<5 % UT (baja de >95 % en
UT) durante 0.5 ciclos
40 % UT (baja de 60% en UT)
durante 5 ciclos
70 % UT (baja de 30% en UT)
durante 25 ciclos
<5 % UT (baja de >95 % en
UT) durante 5 seg.
3 A/m
± 6 kV por contacto
± 8 kV por aire
± 2 kV para líneas de
alimentación eléctrica
± 1 kV línea(s) a línea(s)
± 2 kV línea(s) a tierra
<5 % UT (baja de >95 % en
UT) durante 0.5 ciclos
40 % UT (baja de 60 % en
UT) durante 5 ciclos
70 % UT (baja de 30 % en
UT) durante 25 ciclos
<5 % UT (baja de >95 % en
UT) durante 5 seg.
3 A/m
Los pisos deben ser de madera, concreto o
baldosas de cerámica. Si el piso está recubierto
con material sintético, la humedad relativa debe
ser por lo menos del 30%.
La calidad de la corriente suministrada por la
red de distribución de energía debe ser la de un
entorno comercial u hospitalario típico.
La calidad de la corriente suministrada por la
red de distribución de energía debe ser la de un
entorno comercial u hospitalario típico.
La calidad de la corriente suministrada por la
red de distribución de energía debe ser la de
un entorno comercial u hospitalario típico. Si el
usuario de este monitor requiere que éste continúe
funcionando aún durante interrupciones en el
suministro de energía, se recomienda alimentar
el monitor desde una fuente de alimentación
ininterrumpida o con una batería.
Los campos magnéticos de frecuencia de
energía deben tener los niveles característicos
de un lugar típico en un ambiente comercial u
hospitalario típico.
Prueba de Nivel de prueba CEI Nivel de Guía sobre el ambiente
Inmunidad 60601 cumplimiento electromagnético

ESPAÑOL • 61
COMPATIBILIDAD ELECTROMÁGNETICA
Tabla 3
Para los EQUIPOS ME y SISTEMAS ME que no son
Sistemas de Soporte Vital
Guía y declaración del fabricante – Inmunidad electromagnética
Este monitor tiene la nalidad de usarse en el ambiente electromagnético que se especica a continuación.
El cliente o usuario de este monitor debe asegurarse de que se utilice en dicho entorno.
RF conducida
CEI 61000-4-6
RF irradiada
CEI 61000-4-3
NOTE 1 A 80 MHz y 800 MHz, se aplica la gama de frecuencias más alta.
NOTE 2 Estas guías pueden no aplicarse en todas las situaciones. La absorción y la reexión provocadas por estructuras, objetos y personas afectan
la propagación electromagnética.
** Las intensidades de campo creadas por los transmisores jos, como por ejemplo los de estaciones base para telefonía de radio (celular/inalámbrica) y radios
móviles terrenas, de radio amateur, emisoras de radio AM y FM y emisoras de televisión, no se pueden predecir con precisión en forma teórica. Para evaluar el entorno
electromagnético provocado por transmisores de RF jos, se debe considerar la posibilidad de realizar una prueba electromagnética en el lugar. Si la intensidad de campo
medida en el lugar donde se usará el monitor excede el nivel de conformidad de RF indicado anteriormente, se debe vigilar el monitor con el n de vericar su buen
funcionamiento. En caso de detectarse un funcionamiento anormal, puede que sea necesario tomar medidas adicionales, como la reorientación o reubicación del monitor.
3 Vrms 150 kHz to 80 MHz
3 V/m 80 MHz to 2,5 GHz
3 Vrms
3 V/m
Los equipos de comunicaciones por RF portátiles y
móviles no deben utilizarse a una distancia inferior
de la distancia de separación recomendada, respecto
de cualquier parte del monitor (incluso los cables). La
distancia de separación recomendada se calcula a partir
de la ecuación aplicable a la frecuencia del transmisor.
Recommended separation distance:
80 MHz to 800 MHz
800 MHz to 2,5 GHz
donde P es la potencia máxima de salida del transmisor
en vatios (W) según el fabricante del transmisor, y d es
la distancia de separación recomendada en metros (m).
Las intensidades de campo de transmisores jos de RF,
determinadas en base a un estudio electromagnético del
lugar* deben ser menores que el nivel de conformidad
en cada gama de frecuencias.**
Puede haber interferencias cerca de equipos marcados
con el siguiente símbolo:
1
d = 2.
1
d = 2.
d = 2.3
Prueba de Nivel de prueba CEI Nivel de Guía sobre el ambiente
Inmunidad 60601 cumplimiento electromagnético

62 • ESPAÑOL
COMPATIBILIDAD ELECTROMÁGNETICA
Tabla 4
Para los EQUIPOS ME y SISTEMAS ME que no son
Sistemas de Soporte Vital
Distancias de separación recomendadas entre los equipos de comunicaciones por
RF portátiles y móviles y este monitor
Este monitor ha sido diseñado para utilizarse en un entorno electromagnético en el cual las perturbaciones por emisiones
de RF irradiada están bajo control. El cliente o usuario del monitor puede contribuir a que no ocurran interferencias
electromagnéticas manteniendo una distancia mínima entre el monitor y los equipos de comunicaciones por RF portátiles y
móviles (transmisores), según lo recomendado a continuación, de acuerdo con la potencia máxima de salida del equipo de
comunicaciones.
0,01
0,1
1
10
100
0,12
0,38
1,2
3,8
12
0,12
0,38
1,2
3,8
12
0,23
0,73
2,3
7,3
23
En el caso de los transmisores cuya potencia máxima de salida no gura en la lista anterior, la distancia de separación recomendada d en
metros (m) puede determinarse por medio de la ecuación aplicable a la frecuencia del transmisor, donde P es la potencia máxima de salida
del transmisor en vatios (W), conforme lo declarado por su fabricante.
NOTA 1: A 80 MHz y 800 MHz, se aplica la distancia de separación para la gama de frecuencias más alta.
NOTA 2: Estas guías pueden no aplicarse en todas las situaciones. La absorción y la reexión provocadas por estructuras, objetos y personas
afectan la propagación electromagnética.
Distancia de separación según la frecuencia del transmisor
m
150 kHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2,5 GHz
1
d = 2.
1
d = 2.
d = 2.3
Potencia máxi-
ma de salida del
transmisor
W

ESPAÑOL • 63
DECLARACIÓN DE LA FCC
NOTA:
POTENCIAL DE INTERFERENCIA PARA RADIO/TELEVISIÓN
(sólo para EE.UU.)
Este producto ha sido probado y se comprobó que cumple
con los límites para un dispositivo digital Clase B, de acuerdo
con la parte 15 de las disposiciones de la FCC (Comisión
Federal de Comunicaciones).
Estos límites están diseñados para proporcionar una
protección razonable contra la interferencia dañina de una
instalación residencial. El producto genera, usa y puede
irradiar energía de radiofrecuencia y, si no se instala y usa
de acuerdo con las instrucciones, podría ocasionar una
interferencia dañina para las comunicaciones de radio. No
obstante, no hay garantía alguna de que la interferencia
no ocurrirá en una instalación en particular. Si el producto
ocasiona interferencia dañina para la recepción de radio o
televisión, la cual se puede determinar al encender y apagar
el producto, se exhorta al usuario a que trate de corregir la
interferencia mediante una o más de las medidas siguientes:
• Reoriente o cambie de lugar la antena receptora
• Aumente la separación entre el producto y el receptor.
• Conecte el producto a un tomacorriente en un circuito
diferente al que está conectado el receptor.

64 • ESPAÑOL
• Consulte al distribuidor o a un técnico de radio/TV
experimentado para obtener ayuda
POTENCIAL DE INTERFERENCIA PARA RADIO/TELEVISIÓN
(sólo para Canadá)
Este aparato digital no excede los límites de Clase B para
emisiones de ruido de radio para un aparato digital, como se
estipula en la norma sobre equipos causantes de interferencia
titulada “Aparato digital”, ICES-003 del Departamento
Canadiense de Comunicaciones.
Cet appareil numérique respecte les limites de bruits
radioeléctriques applicables aux appareils numériques de
Clase B prescrites dans la norme sur le materiel brouilleur:
“Appareils Numériques”, ICES-003 édictée par le minister
des communications.
Los cambios o modiUcaciones no aprobados expresamente
por la parte responsable del cumplimiento podrían anular la
autoridad del usuario para operar el equipo.
DECLARACIÓN DE LA FCC

ESPAÑOL • 65
NOMBRE Tens Soluci
ón de Administración de Dolor
NÚMERO DEL MODELO 25517 (VH 56-032)
CANAL Solo Canal
VOLTAJE DE LA SALIDA 0~34V(vp) +/- 20% (a 500 Ohm de carga)
INTENSIDAD DEL PULSO 0~60mA +/- 20%; 0~15 etapas Ajustable
ANCHURA DE PULSO 50~100 uS
PULSO 0 ~ 200Hz
APAGADO AUTOMÁTICO Después de 20 minutos de la operación
POWER SOURCE 3V CR2032
AMBIENTE OPERATIVO Temperatura 50ºF - 104ºF (10ºC - 40ºC);
Humedad 40% - 90%
AMBIENTE ENVIRONMENT Temperatura 14ºF - 140ºF (-10ºC - 60ºC);
Humedad 30% - 95%
CONTROLADOR DIMENSIONES 2-3/4” x 4-1/2”x1/3”
PESO Peso del regulador 6.7 oz (con batería)
INCLUDE Unidad de Control, 1 almohadillas gel para
electrodos, batería abridor compartimento,
guía detallada, electrodos de repuesto
formulario de pedido, una batería CR2032
ACCESORIOS Electrodos de repuesto 2-pack,
Modelo 25518 (VH 22-037)
Las especificaciones están sujetas a cambios sin previo aviso
ESPECIFICACIONES DEL PRODUCTO

66 • ESPAÑOL
Felicitaciones por su compra de un TENS. Su TENS está cubierto por la siguiente
garantía limitada a partir de la fecha de compra y está sujeto a los siguientes
términos y condiciones:
El warrantor garantiza que su TENS estará libre de defectos en materiales y
mano de obra bajo el uso normal del consumidor por el tiempo que el comprador
original sea propietario del producto.
Se excluyen de la cobertura el mantenimiento periódico, las reparaciones y el
reemplazo de partes debidos al desgaste normal. Los defectos o daños que
resulten de: (a) la operación incorrecta, el almacenamiento incorrecto, el uso
inadecuado o abuso, accidente o negligencia, como el daño físico (grietas,
raspones, etc.) en la superficie del producto resultado del uso inadecuado; (b) el
contacto con líquidos, agua, lluvia, humedad extrema o transpiración abundante,
arena, polvo o suciedad en general, calor extremo, o alimentos; (c) el uso del
TENS con propósitos comerciales o someter al TENS a un uso o condiciones
anormales; u (d) otros actos que no son culpa de warrantor, se excluyen de la
cobertura. Esta garantía no cubre baterías ni otras fuentes de energía que se
puedan suministrar o usar con el Producto.
Si la TENS Solución de Administración de Dolor no se ajuste a esta garantía
limitada, devuelva el franqueo TENS Solución de Administración de Dolor no se
ajuste a esta garantía limitada, devuelva el franqueo prepago a: Attn: Repair
Department, 1175 Lakeside Drive, Gurnee, IL, 60031. Cuando devuelva un
producto, por favor incluya además: (i) una copia de su recibo, factura u otro
comprobante de compra; (ii) una descripción por escrito del problema; y (iii) su
nombre, dirección y número telefónico. Embale cuidadosamente el producto
para evitar daños mientras está en tránsito; se recomienda contratar un seguro
GARANTÍA LIMITADA DE UN AÑO

ESPAÑOL • 67
de envío con acuse de recibo. Según lo que elija, el warrantor reparará o reemplazará la unidad que se considere defectuosa en materiales o mano de obra
bajo el uso normal del consumidor. Al comprador se le notificará cualquier reparación adicional requerida antes de completar la reparación, y será responsable
de pagar el cargo por las piezas, si lo hubiese, y los cargos de reparación que no
estén cubiertos por esta garantía limitada.
EXCEPTO COMO LO DISPONE ESTA GARANTÍA LIMITADA, NO SE ACEPTA
RESPONSABILIDAD ALGUNA POR TODAS LAS GARANTÍAS Y CONDICIONES
EXPRESAS E IMPLÍCITAS, INCLUIDAS EN FORMA NO RESTRICTIVA, LAS
GARANTÍAS IMPLÍCITAS DE COMERCIABILIDAD Y APTITUD PARA UN PROPÓSITO
EN PARTICULAR. LA REPARACIÓN O REEMPLAZO, COMO SE ESTIPULA EN ESTA
GARANTÍA LIMITADA, ES EL ÚNICO RECURSO EXCLUSIVO DEL CONSUMIDOR Y SE
PROPORCIONA EN LUGAR DE TODAS LAS DEMÁS GARANTÍAS, EXPRESAS O IMPLÍCITAS. EN NINGÚN CASO EL WARRANTOR SERÁ RESPONSABLE, CON BASE EN
OBLIGACIONES CONTRACTUALES O CULPA EXTRACONTRACTUAL (INCLUIDA LA
NEGLIGENCIA), POR DAÑOS Y PERJUICIOS QUE SUPEREN EL PRECIO DE COMPRA
DEL PRODUCTO, O POR CUALQUIER DAÑO INDIRECTO, INCIDENTAL, ESPECIAL
O CONSECUENTE DE CUALQUIER TIPO, O POR DAÑOS A OTRA PROPIEDAD O
EQUIPO, O PÉRDIDA DE PROPIEDAD O EQUIPO O LESIONES PERSONALES, EN
LAS MÁS AMPLIAS EXTENSIÓN EN QUE LA LEY PERMITA EL DESCARGO DE LA
RESPONSABILIDAD POR DICHOS DAÑOS.
Algunos estados y jurisdicciones no permiten la limitación o exclusión de daños
incidentales o consecuentes, o la limitación en la duración de una garantía
implícita, de modo tal que las limitaciones o exclusiones podrían no aplicarse
a usted. Esta garantía le otorga derechos legales específicos y usted también
puede tener otros derechos, que varían dependiendo del estado o de una
jurisdicción a otra.
GARANTÍA LIMITADA DE UN AÑO

PLEASE FILL-IN INFORMATION FOR FUTURE REFERENCE AND ATTACH
YOUR RECEIPT BELOW.
This information is necessary should you need to contact Customer Care
in the future.
COMPLETE TODA LA INFORMACIÓN PARA REFERENCIA FUTURA Y
ADJUNTE ABAJO SU COMPROBANTE.
Esta información es necesaria para el caso que en el futuro usted necesite
comunicarse con Atención al Cliente.
Model / Modelo: 25517 (VH 56-032)
Name / Nombre: TENS Pain Management System
Date Purchased / La Fecha Compró: _____________________________
Store Name / Nombre del Almacén: _____________________________
Lot No. (located on the back of the controller)/
Lot No. (localizado en la parte inferior del monitor) ______________
ATTACH RECEIPT HERE
ADJUNTE AQUÍ EL RECIBO
 Loading...
Loading...