CareTouch PSW01 User Manual

Manufactured in China for Future Diagnostics LLC
Brooklyn N.Y. 11220
Package Design © 2016
CareTouch is a Trademark of Future Diagnostics LLC
1
CATALOGUE
INTRODUCTION ............................................................... 2
Safety Information
LCD Display Signal
Monitor Components
BEFORE YOU START .......................................................... 5
Installing and Replacing the Batteries
Setting Date, Time and Measurement Unit
MEASUREMENT ............................................................... 9
Tie the Cuff
Start the Measurement
DATA MANAGEMENT 11
Recall the Records
Delete the Records
INFORMATION FOR USER ..................................................... 13
Tips for Measurement
Maintenance
ABOUT BLOOD PRESSURE.................................................... 15
What are systolic pressure and diastolic pressure?
What is the standard blood pressure classification?
Why does my blood pressure fluctuate throughout the day?
Why do I get a different blood pressure at home compared to the hospital?
Is the result the same if measuring on the right wrist?
TROUBLESHOOTING ......................................................... 17
SPECIFICATIONS ............................................................. 18
CONTACT INFORMATION...................................................... 19
COMPLIED STANDARDS LIST...................................................19
FCC STATEMENT ...................................................... 19
..........................................................
General Description
Measurement principle
Indications for use
CATALOGUE
EMC GUIDANCE ...................................................... 20
32
INTRODUCTION INTRODUCTION
The signs below might be in the user manual, labeling or other component.
They are the requirement of standard and using.
Safety Information
Features:
Systolic blood pressure
Diastolic blood pressure
Pulse rate
Historic record of up to 60 measurements
General Description
Thank you for selecting CARE TOUCH
Fully Automatic Wrist Blood Pressure Monitor (PSW01).
The monitor features blood pressure measurement, pulse rate measurement and
the result storage. The design provides you with two years of reliable service.
Readings taken by the PSW01 are equivalent to those obtained by a trained
observer using the cuff and stethoscope auscultation method.
This manual contains important safety and care information, and provides
step by step instructions for using the product.
Read the manual thoroughly before using the product.
Symbol for “THE OPERATION
GUIDE MUST BE READ”
Symbol for “SERIAL NUMBER”
Symbol for “TYPE BF APPLIED
PARTS”
Symbol for “DIRECT CURRENT”
Symbol for “ENVIRONMENT PROTECTION Electrical waste products should not be
disposed of with household waste. Please
recycle where facilities exist. Check with
your local authority or retailer for recycling
advice”
Symbol for “MANUFACTURE
DATE”
CAUTION
Caution: These notes must be observed to
prevent any damage to the device.
SN
This product uses the Oscillometric Measuring Method to detect blood pressure.
Before every measurement, the unit establishes a “zero point” equivalent to the
atmospheric pressure. Then it starts inflating the cuff. Meanwhile, the unit detects
pressure oscillation generated by beat-to-beat pulsatile, which is used to determine
the systolic pressure and diastolic pressure as well as pulse rate.
The device also compares the longest and the shortest intervals of detected pulse
wave to with the average value, and then calculates the standard deviation. The
monitor will light up a warning symbol when the calculated standard deviation is
larger than or equal to 25%.
Measurement Principle
Indications for Use
The Blood Pressure Monitor is digital monitors intended for use in measuring blood
pressure and heartbeat rate with wrist circumference ranging
from 13.5cm to 21.5 cm ( about 5⅓˝-8½˝ ). It is intended for adult indoor use only.
This device is intended for adult use only.
This device is intended for no-invasive measuring and monitoring of arterial blood
pressure. It is not intended for use on extremities other than the wrist or for functions
other than obtaining a blood pressure measurement.
Do not confuse self-monitoring with self-diagnosis. This unit allows you to monitor your
blood pressure.Do not begin or end medical treatment without asking a physician for
treatment advice.
If you are taking medication,consult your physician to determine the most appropriate
time to measure your blood pressure. Never change a prescribed medication without
consulting your physician.
If the cuff pressure exceeds 40 kPa (300 mmHg),the unit will automatically deflate.
Should the cuff not deflate when pressures exceeds 40 kPa (300 mmHg), detach the cuff
from the wrist and press the START/STOP button to stop inflation.
To avoid measurement errors, carefully read this manual before using the product.
The equipment is not AP/APG equipment and not suitable for use in the presence of a
flammable anesthetic mixture with air of with oxygen or nitrous oxide.
The operator shall not touch output of batteries and the patient simultaneously.
Do not use the monitor under the conditions of strong electromagnetic field (e.g. medical
RF equipment) that radiates interference signal or electrical fast transient / burst signal.
The user must check that the equipment functions safely and see that it is in proper
working condition before being used.
Please use ACCESSORIES and detachable partes specified/ authorised by
MANUFACTURE. Otherwise, it may cause damage to the unit or danger to the
user/patients.
Manufacturer will make available on request circuit diagrams, component parts list etc.
This unit is not suitable for continuous monitoring during medical emergencies or
operations. Otherwise, the patient’s wrist and fingers will become anaesthetic, swollen
and even purple due to a lack of blood.
Please use the device under the environment which was provided in the user manual.
Otherwise, the performance and lifetime of the device will be impacted and reduced.
During use, the patient will be in contact with the cuff. The materials of the cuff have
been tested and found to comply with requirements of ISO 10993-5:2009 and ISO
10993-10:2010. It will not cause any potential alergic reaction or contact injury.
The device doesn’t need to be calibrated within the two years of reliable service.
Please dispose of ACCESSORIES, detachable parts, and the ME EQUIPMENT according
to the local guidelines.
When the device was used to measure patients who have common arrhythmias such as
atrial or ventricular premature beats or atrial fibrillation, the best result may occur with
deviation. Please consult your physician about the result.
This device is contraindicated for any female subject who may be suspected of, or is
pregnant. Besides providing inaccurate readings, the effects of this device on the fetus
are unknown.
The device has been evaluated clinically using manual cuff/stethoscope auscultation as
the reference. Blood pressure measurements determined with this device are equivalent
to those obtained by a trained observer using the cuff/stethoscope auscultatory method,
within the limits prescribed by the American National Standard, Manual, electronic, or
automated sphygmomanometers.”
54
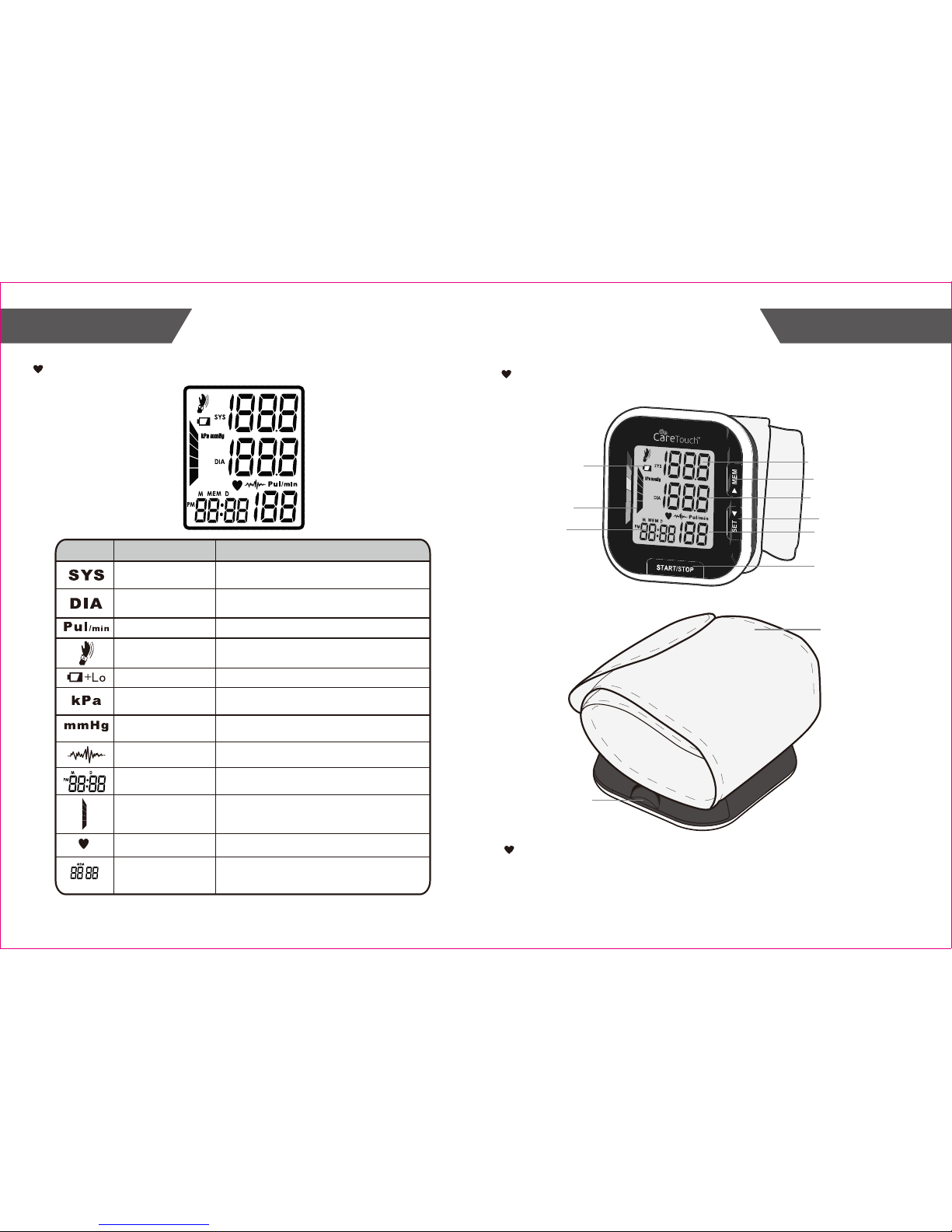
Systolic
blood pressure
High pressure result
Diastolic
blood pressure
Low pressure result
Pulse Pulse/minute; heartbeats/minute
mmHg
Measurement unit the blood pressure
(1mmHg=0.133kPa)
kPa
Measurement unit of the blood pressure
(1kPa=7.5mmHg)
Current time
Shaking
reminding
Shaking will result in inaccurate
Irregular heartbeat Detection
Low battery
Batteries are low and need to be replaced
Grade
The grade of the blood pressure
LCD Display Signal
SYMBOL DESCRIPTION EXPLANATION
Month/Day,Hour/Minute
Heartbeat
Heartbeat detection during the measurement
CUFF
(Type BF applied part)
BATTERY
COMPARTMENT
Monitor Components
Component List:
1. PCBA;
2. Air Pipe;
3. Pump;
4. Valve;
5. Cuff.
List
1) Fully Automatic Wrist Blood Pressure Monitor PSW01
2) 2×AAA batteries
3) User manual
4) Carrying case
INTRODUCTION INTRODUCTION
Memory
Display the serial number of the
measurement
LCD DISPLAY
GRADE
TIME
SYSTOLIC
DIASTOLIC
MEM BUTTON
SET BUTTON
START/STOP BUTTON
PULSE RATE
Irregular heartbeat
 Loading...
Loading...