Carestream Health DRX1 Users Manual

CARESTREAM DRX-1 System
Safety and Regulatory Information
with
Hardware
User’s Guide
H224_0028HC
Version 2.0
PN 7H8166
22 January 2009
C
a
r
e
s
t
r
e
a
m
C
a
r
e
s
t
r
e
am
D
R
X
1
S
y
s
t
e
m


Table of Contents
1 Safety and Regulatory Information
Document Conventions ....................................................................................................................................1-1
Intended Use and Indications for Use............................................................................................................... 1-1
Safety and Related Information.........................................................................................................................1-2
Medical Equipment Classification...............................................................................................................1-2
Compatibility with Other Manufacturer’s Equipment ..................................................................................1-3
Product Safety Standards............................................................................................................................1-4
EMC Standards for Detector and System.....................................................................................................1-7
Safety .........................................................................................................................................................1-7
Additional Guidance and Manufacturer’s Declaration - Electromagnetic Emissions/Immunity....................1-7
Electromagnetic Compatibility Precautions...........................................................................................1-7
Communications Equipment ................................................................................................................1-8
Replacement of Cables, Accessories, or Transducers ...........................................................................1-8
Other Equipment..................................................................................................................................1-8
Shielded Locations ...............................................................................................................................1-8
DRX-1 System Product Information................................................................................................................1-13
DRX-1 System Detector ............................................................................................................................1-13
DRX-1 System Battery Charger..................................................................................................................1-14
DRX-1 System Battery...............................................................................................................................1-15
DRX-1 System Console .............................................................................................................................1-15
DRX-1 System Interface Box (Internal) ....................................................................................................1-16
DRX-1 System Wireless Access Point ........................................................................................................1-16
DRX-1 System Tether Interface.................................................................................................................1-16
Patient Vicinity .........................................................................................................................................1-17
Mode of Operation...................................................................................................................................1-17
Labels ......................................................................................................................................................1-18
Disposal Information .....................................................................................................................................1-23
Operating Environment ..................................................................................................................................1-23
For European Market Only.......................................................................................................................1-23
General Contact Information ....................................................................................................................1-23
2 Hardware and Operation
Overview ..........................................................................................................................................................2-1
7H8166 1

Table of Contents
CARESTREAM DRX-1 System ............................................................................................................................2-2
Cautions...........................................................................................................................................................2-3
Installing the Hardware....................................................................................................................................2-3
Attaching Accessories.................................................................................................................................2-4
Turning the System On and Off...................................................................................................................2-4
CARESTREAM DRX-1 System Battery................................................................................................................. 2-5
Installing the Battery ..................................................................................................................................2-5
Removing the Battery .................................................................................................................................2-6
Labeling the Detector .......................................................................................................................................2-6
DRX-1 Detector LED.........................................................................................................................................2-7
Positioning the Detector in the Bucky...............................................................................................................2-8
Range of Operation ........................................................................................................................................2-10
Using a Single Detector ............................................................................................................................2-10
Using Two or More Detectors................................................................................................................... 2-10
Using Detectors in Two or More Rooms................................................................................................... 2-11
Wireless Operation.........................................................................................................................................2-11
Tether Operation............................................................................................................................................2-12
Tether Interface Box ................................................................................................................................2-13
Cleaning the Hardware...................................................................................................................................2-13
With Each Occurrence of Patient Contact .......................................................................................................2-14
System Maintenance.......................................................................................................................................2-15
Checking the Equipment Integrity.............................................................................................................2-16
Grid Recommendation ............................................................................................................................. 2-16
Protective Enclosures...............................................................................................................................2-16
2 7H8166

1
Safety and Regulatory Information
The information contained herein is based on the experience and knowledge
relating to the subject matter gained by Carestream Health, Inc. prior to
publication.
No patent license is granted by this information.
Carestream Health reserves the right to change this information without
notice, and makes no warranty, express or implied, with respect to this
information. Carestream Health shall not be liable for any loss or damage,
including consequential or special damages, resulting from any use of this
information, even if loss or damage is caused by Carestream Health’s
negligence or other fault.
Document Conventions
NOTE: Notes provide additional information, such as expanded
explanations, hints, or reminders.
IMPORTANT: Important highlights critical policy information that
affects how you use this manual and this product
CAUTION:
Caution points out procedures that you must follow precisely
to avoid damage to the system or any of its components,
yourself or others, loss of data, or corruption of files in
software applications.
Intended Use and Indications for Use
The CARESTREAM DRX-1 System is intended to capture for display
radiographic images of human anatomy. It is intended for use in general
projection radiographic applications wherever conventional screen-film or
Computed Radiography (CR)systems may be used. Excluded from the
indications for use are mammography, fluoroscopy., tomography, and
angiography applications.
7H8166 1-1
Safety and Regulatory Information
Safety and Related Information
Medical Equipment Classification
CARESTREAM DRX-1 System Detector Medical Electrical
Equipment Classification
Type of protection against electrical shock: Internally powered equipment. Class I
Equipment.
Degree of protection against electrical
shock:
Degree of protection against ingress of water:
Mode of operation: Continuous operation.
Flammable anesthetics: Not suitable for use in the presence of flam-
CARESTREAM DRX-1 System Tether Interface Medical Electrical
Equipment Classification
Type of protection against electrical shock: Class I Equipment.
Degree of protection against electrical
shock:
Degree of protection against ingress of water:
Mode of operation: Continuous operation.
Flammable anesthetics: Not suitable for use in the presence of flam-
Type B Applied Part.
Ordinary protection.
mable anesthetics or a mixture of flammable anesthetics with air or oxygen or
nitrous oxide.
Type B.
Ordinary protection.
mable anesthetics or a mixture of flammable anesthetics with air or oxygen or
nitrous oxide.
1-2 7H8166
Safety and Regulatory Information
• The CARESTREAM DRX-1 System includes the following components:
CARESTREAM DRX-1 System Detector (one or more)
CARESTREAM DRX-1 System Battery (any quantity)
CARESTREAM DRX-1 System Battery Charger
CARESTREAM DRX-1 System Tether Interface
CARESTREAM DRX-1 System Console
CARESTREAM DRX-1 System Access Point
CARESTREAM DRX-1 System Medical Electrical Equipment
Classification
Type of protection against electrical shock: Internally powered equipment. Class I
Equipment.
Degree of protection against electrical
shock:
Degree of protection against ingress of water:
Mode of operation: Continuous operation.
Flammable anesthetics: Not suitable for use in the presence of flam-
Compatibility with Other Manufacturer’s Equipment
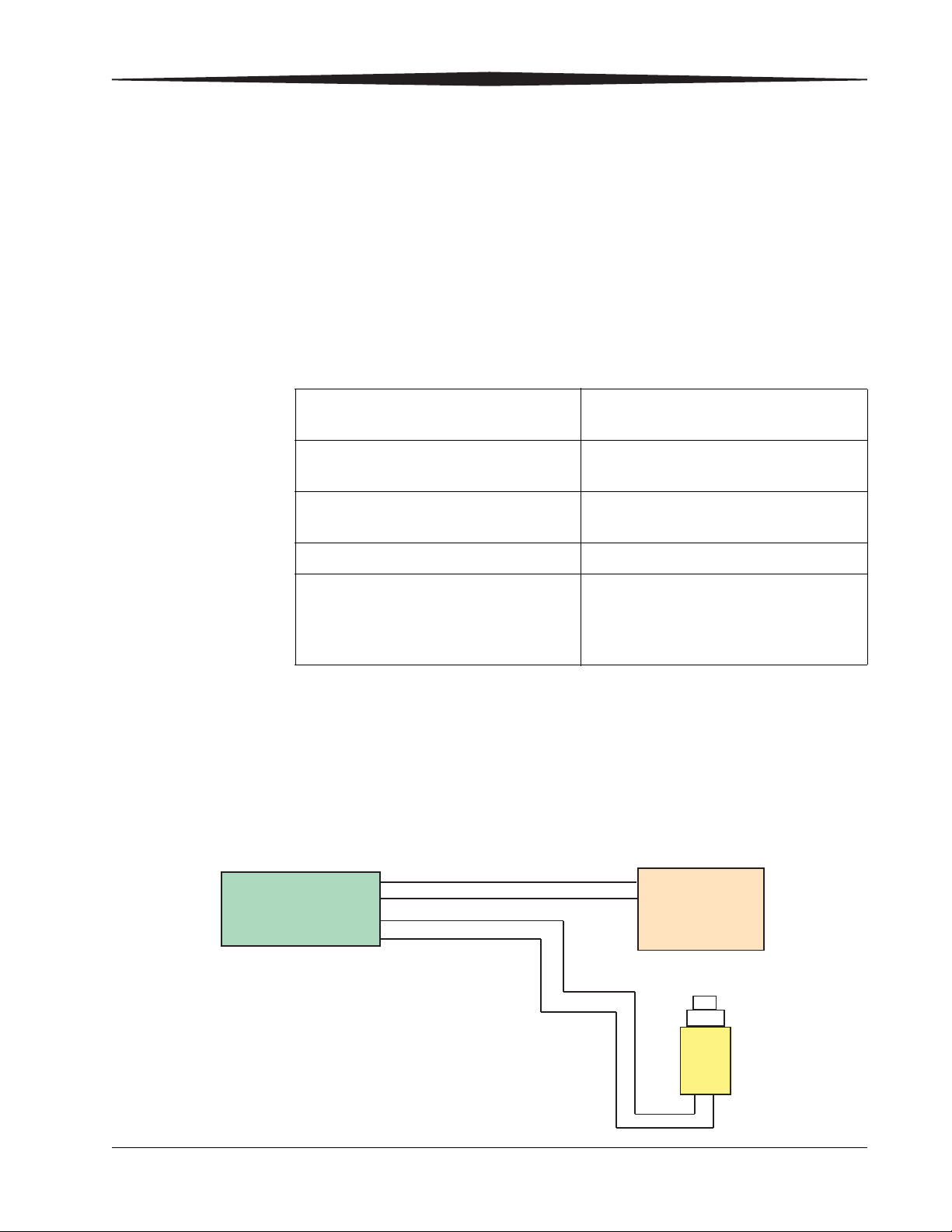
DRX-1 System
Interface
Box
Output
Input
Type B Applied Part.
Ordinary protection.
mable anesthetics or a mixture of flammable anesthetics with air or oxygen or
nitrous oxide.
The CARESTREAM DRX-1 System (DRX-1 System) is a digital X-ray image
capture system. The DRX-1 System connects with existing analog x-ray
equipment using a safety certified electrical isolation device (DRX-1 System
Interface Box). The isolation device is designed to prevent any failures, loss of
power or power surge in the DRX-1 System from affecting the X-ray
equipment.
Prep Start
Expose Start
X-ray Console
H224_9001BA
Prep Request
Expose Request
7H8166 1-3
Exposure
Switch

Safety and Regulatory Information
The DRX-1 System uses an existing exposure switch connector on the X-ray
equipment. No modification to the X-ray equipment is required. The intended
use of the X-ray equipment is not affected and the X-ray equipment remains
certified by the X-ray equipment manufacturer.
Model-specific documentation and cables are provided to allow service
personnel to connect and run functional testing on the DRX-1 System. The
DRX-1 System is compatible with the X-ray equipment listed on the Certificate
of Compatibility available from your local authorized service provider. Contact
your local authorized service provider for further information.
Product Safety Standards
USA UL 60601-1:2003 - Medical Electrical Equipment
Canada CAN/CSA-C22.2 No. 601.1-M90 (R2001) Medical Electrical Equipment
Europe EN 60601-1:1990 + Amendment 1:1993 + Amendment 2:1995 - Medical
The following Product Safety Standards are applicable to:
• CARESTREAM DRX-1 System Detector
• CARESTREAM DRX-1 System Tether Interface
CAN/CSA-C22.2 No. 601.1S1-94 (R1999) - Supplement No. 1-94 to
CAN/CSA-C22.2 No. 601.1-M90
CAN/CSA-C22.2 No. 601.1B-90 (R2002) - Amendment 2 to CAN/CSA-C22.2
No. 601.1-M90
Electrical Equipment
EN 60601-1-1:2001 - Medical Electrical Systems
EN 60601-1-4:1996 + Amendment 1:1999 - Programmable Electrical
Medical Systems
International IEC 60601-1:1988 + Amendment 1:1991 + Amendment 2:1995 - Medical
Electrical Equipment
IEC 60601-1-1:2000 - Medical Electrical Systems
IEC 60601-1-4:1996 + Amendment 1:1999 - Programmable Electrical
Medical Systems
The following Product Safety Standards are applicable to:
• CARESTREAM DRX-1 System. The DRX-1 System includes the following
components:
CARESTREAM DRX-1 System Detector (one or more)
CARESTREAM DRX-1 System Battery (any quantity)
1-4 7H8166

Safety and Regulatory Information
CARESTREAM DRX-1 System Battery Charger
CARESTREAM DRX-1 System Tether Interface
CARESTREAM DRX-1 System Console
CARESTREAM DRX-1 System Wireless Access Point
USA UL 60601-1:2003 - Medical Electrical Equipment, 1st Edition
Canada CAN/CSA-C22.2 No. 601.1-M90 (R2001) Medical Electrical Equipment
CAN/CSA-C22.2 No. 601.1S1-94 (R1999) - Supplement No. 1-94 to
CAN/CSA-C22.2 No. 601.1-M90
CAN/CSA-C22.2 No. 601.1B-90 (R2002) - Amendment 2 to CAN/CSA-C22.2
No. 601.1-M90
Europe EN 60601-1:1990 + Amendment 1:1993 + Amendment 2:1995 - Medical
Electrical Equipment
EN 60601-1-1:2001 - Medical Electrical Systems
EN 60601-1-4:1996 + Amendment 1:1999 - Programmable Electrical
Medical Systems
International IEC 60601-1:1998 + Amendment 1:1991 + Amendment 2:1995 - Medical
Electrical Equipment
IEC 60601-1-1:2000 - Medical Electrical Systems
IEC 60601-1-4:1996 + Amendment 1:1999 - Programmable Electrical
Medical Systems
7H8166 1-5

Safety and Regulatory Information
The following Product Safety Standards are applicable to:
USA UL 60601-1:2003 - Medical Electrical Equipment, 1st Edition
Canada CAN/CSA-C22.2 No. 601.1-M90 (R2001) Medical Electrical Equipment
CAN/CSA-C22.2 No. 601.1S1-94 (R1999) - Supplement No. 1-94 to
CAN/CSA-C22.2 No. 601.1-M90
CAN/CSA-C22.2 No. 601.1B-90 (R2002) - Amendment 2 to CAN/CSA-C22.2
No. 601.1-M90
Europe EN 60601-1:1990 + Amendment 1:1993 + Amendment 2:1995 - Medical
Electrical Equipment
• CARESTREAM DRX-1 System Battery
International IEC 60601-1:1988 + Amendment 1:1991 + Amendment 2:1995 - Medical
Electrical Equipment
The following Product Safety Standards are applicable to:
• CARESTREAM DRX-1 System Battery Charger
• CARESTREAM DRX-1 System Console
• CARESTREAM DRX-1 System Wireless Access Point
USA UL 60950-1, Information Technology Equipment - Safety - Part 1: General
Requirements
Canada CAN/CSA C22.2 No. 60950-1-03, Information Technology Equipment - Safety
- Part 1: General Requirements
Europe EN 60950-1:2001 + A11, Information Technology Equipment - Safety - Part
1: General Requirements
International IEC 60950-1:2001, Information Technology Equipment - Safety - Part 1:
General Requirements
1-6 7H8166

Safety and Regulatory Information
EMC Standards for Detector and System
IEC 60601-1-2:2004 EMC requirements and tests, Medical Electrical
Equipment including CISPR 11:1999+A2:02, Group 1, Class A.
This device complies with part 15 of the FCC Rules. Operation is subject to the
following two conditions:
1. This device may not cause harmful interference.
2. This device must accept any interference received, including interference
that may cause undesired operation.
This equipment has been tested and found to comply with the limits for a Class
A digital device, pursuant to part 15 of the FCC Rules. These limits are
designed to provide reasonable protection against harmful interference when
the equipment is operated in a commercial environment. This equipment
generates, uses and can radiate radio frequency energy and, if not installed
and used in accordance with the instruction manual, may cause harmful
interference to radio communications. Operation of this equipment in a
residential area is likely to cause harmful interference in which case the users
will be required to correct the interference at their own expense.
Changes or modifications not expressly approved by the manufacturer could
void the user’s authority to operate the equipment.
CAUTION:
This is a Class A product. In a domestic environment this
product may cause radio interference, in which case the user
may be required to take adequate measures.
NOTE: For CARESTREAM DRX-1 System Battery Charger or Battery EMC
information and instruction for use, see the CARESTREAM DRX-1
System Battery Charger User’s Guide.
Safety This product complies with 21 CFR 1020.30/31 Performance Standards for
Radiation Safety - Radiographic Equipment.
Additional Guidance
and Manufacturer’s
Declaration Electromagnetic
Emissions/Immunity
Electromagnetic Compatibility Precautions
Medical electrical equipment requires special precautions regarding
electromagnetic compatibility (EMC). Medical equipment must be installed
and put into service according to the EMC information provided in the
following documentation.
7H8166 1-7

Safety and Regulatory Information
Communications Equipment
Portable and mobile radio frequency (RF) communications equipment can
affect medical electrical equipment EMC performance.
The wireless version of the Carestream DRX-1 System Detector operates with
the 802.11n protocol in the 5 GHz frequency band. The radio output power is
50 mW (nominal).
CAUTION:
Replacement of Cables, Accessories, or Transducers
The use of cables, accessories or transducers other than those specified
below with the exception of transducers or cables sold by the manufacturer of
the equipment as replacement parts for internal components, may result in
increased emissions or decreased immunity of the medical equipment.
Other Equipment The CARESTREAM DRX-1 System should not be used adjacent to or stacked
with other equipment. If adjacent or stacked use is necessary, the Carestream
DRX-1 System should be observed to verify normal operation in the
configuration in which it will be used.
Cable, Accessory and Transducer Information for the Carestream DRX-1
System will be available prior to production release of the product.
Shielded Locations The typical location of the CARESTREAM DRX-1 System will be in a shielded
room only because the system functions with sources of X-Ray energy. The
CARESTREAM DRX-1 System is fully compliant with the requirements of IEC
60601-1-2:2004 without being located in a shielded room.
1-8 7H8166
Safety and Regulatory Information
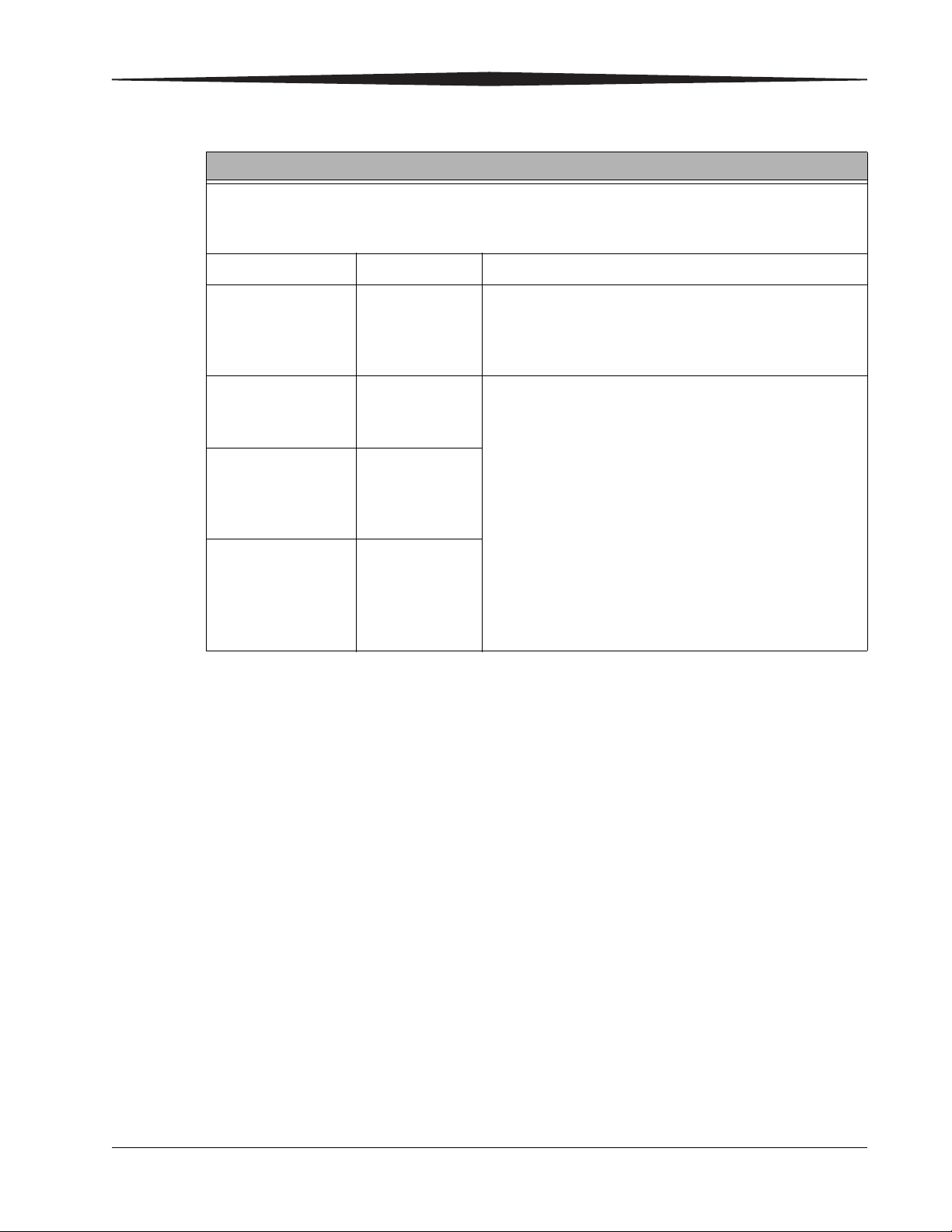
Guidance and Manufacturer’s Declaration - Electromagnetic Emissions
The CARESTREAM DRX-1 System is intended for use in the electromagnetic environment specified
below. The customer or the user of the CARESTREAM DRX-1 System should assure that it is used in
such an environment.
Emissions Test Compliance Electromagnetic Environment - Guidance
RF Emissions
CISPR 11
RF Emissions
CISPR 11
Harmonics
Emissions
IEC 61000-3-2
Voltage
Fluctuations/
Flicker Emissions
IEC 61000-3-3
Group 1 The CARESTREAM DRX-1 System uses RF energy only for its
internal function. Therefore, its RF emissions are very low
and are not likely to cause any interference in nearby
electronic equipment.
Class A The CARESTREAM DRX-1 System is suitable for use in all
establishments other than domestic and those directly
connected to the public low-voltage power supply network
Class A
that supplies buildings used for domestic purposes.
Complies
7H8166 1-9
Safety and Regulatory Information
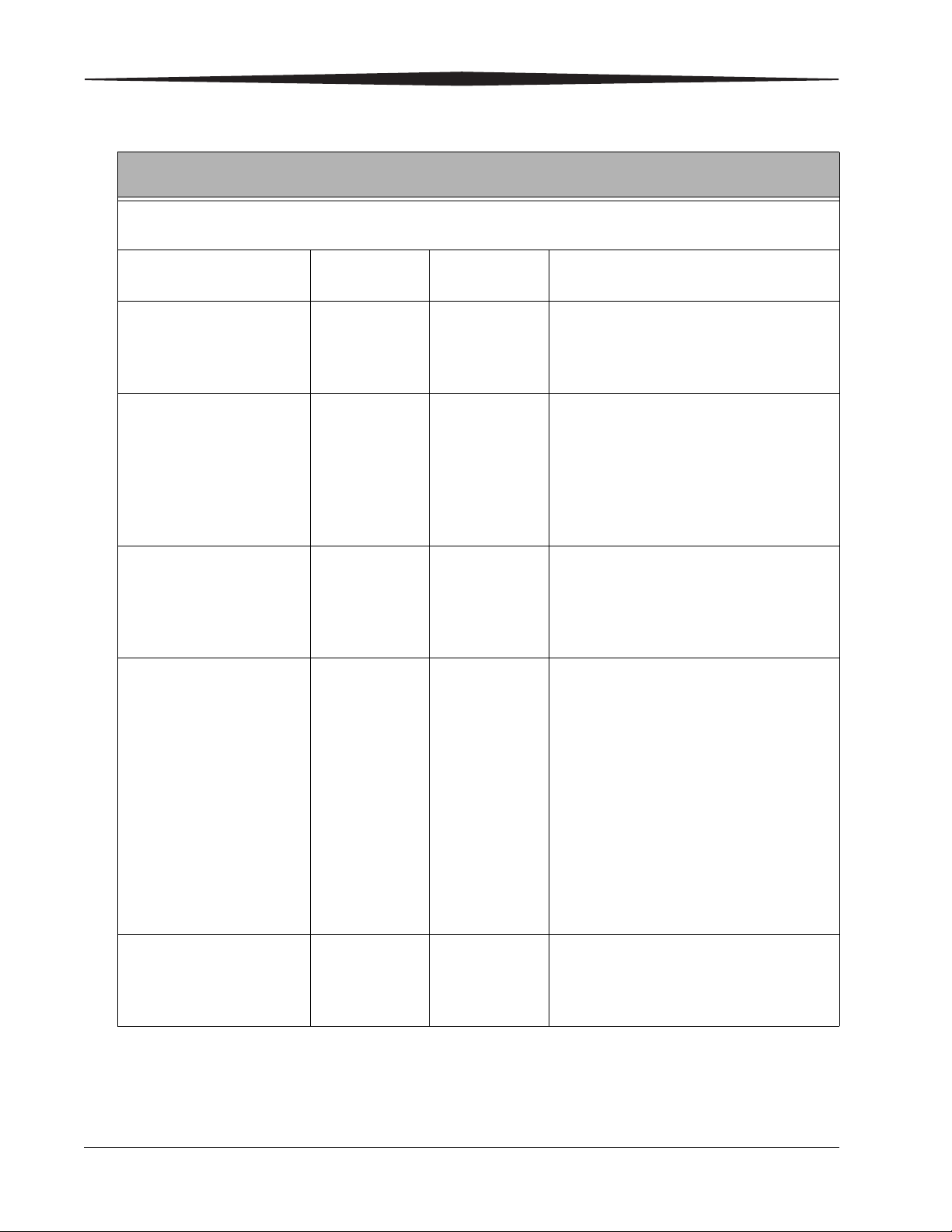
Electromagnetic Immunity for Equipment and Systems Fully Compliant with IEC
60601-1-2:2004
The CARESTREAM DRX-1 System is intended for use in the electromagnetic environment specified below. The
customer or the user of the CARESTREAM DRX-1 System should assure that it is used in such an environment.
Immunity Test IEC 60601
Test Level
Electrostatic Discharge
+/- 6 kV contact
(ESD)
+/- 8 kV air
IEC 61000-4-2
Electrical fast
transient/burst
+/- 2 kV for
power supply
lines
IEC 61000-4-4
+/- 1 kV for
input/output
lines
Surge
+/- 1 kV line to
line
IEC 61000-4-5
+/- 2 kV line to
earth
Voltage dips, short
interruptions and voltage
variations on power supply
lines
IEC 61000-4-11
<5% UT (>95%
dip in UT) for
0.5 cycle
40% UT (60%
dip in UT) for 5
cycles
Compliance
Level
+/- 6 kV contact
+/- 8 kV air
+/- 2 kV for
power supply
lines
+/- 1 kV for
input/output
lines
+/- 1 kV line to
line
+/- 2 kV line to
earth
<5% UT (>95%
dip in UT) for
0.5 cycle
40% UT (60%
dip in UT) for 5
cycles
Electromagnetic EnvironmentGuidance
Floors should be wood, concrete or
ceramic tile. If floors are covered with
synthetic material, the relative humidity
should be at least 30%.
Mains power quality should be that of a
typical commercial or hospital
environment.
Mains power quality should be that of a
typical commercial or hospital environment
Mains power quality should be that of a
typical commercial or hospital
environment.
Note: Most components in the
CARESTREAM DRX-1 System are powered
from an uninterruptible power supply.
Power frequency
70% UT (30%
dip in UT) for25
cycles
<5% UT (>95%
dip in UT) for 5
sec.
3 A/m 3 A/m Power frequency magnetic fields should be
70% UT (30%
dip in UT) for25
cycles
<5% UT (>95%
dip in UT) for 5
sec.
(50/60Hz)magnetic field
IEC 61000-4-8
NOTE: U
1-10 7H8166
is the a.c. mains voltage prior to application of the test level.
T
IEC 61000-4-11 is applicable only to the
CARESTREAM DRX-1 System tether
Interface.
at levels characteristic of a typical location
in a typical commercial or hospital
environment.
Safety and Regulatory Information
Guidance and Manufacturer’s Declaration - Electromagnetic Immunity
The CARESTREAM DRX-1 System is intended for use in the electromagnetic environment specified
below. The customer or the user of the CARESTREAM DRX-1 System should assure that it is used in
such an environment.
Immunity Test IEC 60601
Test Level
Conducted RF
IEC 61000-4-6
3 Vrms
150 kHz to 80
MHz
Radiated RF
IEC 61000-4-3
3 v/m
80 MHz to
2.5GHz
Compliance
Level
3 Vrms
3 v/m
Electromagnetic Environment - Guidance
Portable and mobile RF communications
equipment should be used no closer to any
part of the CARESTREAM DRX-1 System,
including cables, than the recommended
separation distance calculated from the
equation applicable to the frequency of the
transmitter.
Recommended separation distance
d = 1.17 √P
d = 1.17 √P 80 MHz to 800 MHz
d = 2.33 √P 800MHz to 2.5GHz
where P is the maximum output rating of the
transmitter in watts (W) according to the
transmitter manufacture and d is
recommended separation distance in meters
(m).
Field strengths from fixed RF transmitters, as
determined by an electromagnetic site survey
should be less than the compliance level in
each frequency range
b
.
Interference may occur in the vicinity of
equipment marked with the following symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
reflection from structures, objects and people.
7H8166 1-11
a
,
 Loading...
Loading...