
Operation and Service Manual

Limited Warranty for Powerheart AED G3
What is Covered?
Cardiac Science, Inc. (“Cardiac Science”) warrants to the original
purchaser that its products will be free of any defect in material and
workmanship according to the terms and conditions of this Limited
Warranty (“Limited Warranty”). For purposes of this Limited Warranty,
the original purchaser is deemed to be the original end user of the
product purchased. This Limited Warranty is NONTRANSFERABLE and
UNASSIGNABLE.
For How Long?
Five (5) years from the date of the original shipment to the original purchaser for Powerheart AED G3 automated external defibrillators.
Disposable defibrillation electrodes shall be warranted until the expiration date. Lithium batteries have a full operational warranty of Three (3)
years from the date of installation into a Powerheart AED G3. Batteries
must be installed by the installation date as labeled on the battery to be
covered by this warranty. One (1) year from the date of original shipment
to the original purchaser for Cardiac Science AED accessories. The terms
of the Limited Warranty in effect as of the date of original purchase will
apply to any warranty claims.
What You Must Do
Please complete and submit the Warranty Validation Form within 30 days
of original shipment located at http://www.cardiacscience.com/
products/aed_warranty.cfm. If the purchaser does not have internet
access, call (888) 466-8686 or +45 4438 0539.
To obtain warranty service for your product, call us toll free at (888) 4668686 or +45 4438 0539 seven days a week, 24 hours a day. Our
customer service representative will try to resolve your issue over the
phone. If necessary, and at our sole discretion, we will arrange for
service or a replacement of our product.
What We Will Do
If your Cardiac Science product is returned within 30 days of the date it
was purchased, at the direction of a customer service representative, we
will replace it with a new product of equal value at no charge to you,
provided the warranty applies.
If your Cardiac Science product is returned, at the direction of a customer service representative, after 30 days but within the warranty period, Cardiac Science, at its sole discretion, will repair your product or
replace it with a new or reconditioned product of the same or similar
design. The repaired or replacement product will be warranted subject to
the terms and conditions of this Limited Warranty for either (a) 90 days
or (b) the remainder of the original warranty period, whichever is longer,
provided the warranty applies and the warranty period has not expired.
Obligations and Warranty Limits
Limited Warranty Obligation: Exclusive Remedy
THE FOREGOING LIMITED WARRANTY IS IN LIEU OF AND
SPECIFICALLY EXCLUDES AND REPLACES ALL OTHER EXPRESS
OR IMPLIED WARRANTIES. INCLUDING BUT NOT LIMITED TO
THE IMPLIED WARRANTIES OF MERCHANTABILITY AND FITNESS
FOR A PARTICULAR PURPOSE.
Some states do not allow limitations on how long an implied warranty
lasts, so this limitation may not apply to you.
NO PERSON (INCLUDING ANY AGENT, DEALER, OR REPRESENTATIVE OF
CARDIAC SCIENCE) IS AUTHORIZED TO MAKE ANY REPRESENTATION
OR WARRANTY CONCERNING CARDIAC SCIENCE PRODUCTS, EXCEPT
TO REFER PURCHASERS TO THIS LIMITED WARRANTY.
YOUR EXCLUSIVE REMEDY WITH RESPECT TO ANY AND ALL LOSSES
OR DAMAGES RESULTING FROM ANY CAUSE WHATSOEVER SHALL BE
AS SPECIFIED ABOVE. CARDIAC SCIENCE SHALL IN NO EVENT BE
LIABLE FOR ANY CONSEQUENTIAL OR INCIDENTAL DAMAGES OF ANY
KIND, INCLUDING, BUT NOT LIMITED TO, EXEMPLARY DAMAGES,
COMMERCIAL LOSS FROM ANY CAUSE, BUSINESS INTERRUPTION OF
ANY NATURE, LOSS OF PROFITS OR PERSONAL INJURY, EVEN IF
CARDIAC SCIENCE HAS BEEN ADVISED OF THE POSSIBILITIES
OF SUCH DAMAGES, HOWEVER OCCASIONED, WHETHER BY
NEGLIGENCE OR OTHERWISE.
Some states do not allow the exclusion or limitation of incidental or
consequential damages, so the above limitation or exclusion may not
apply to you.
What This Warranty Does Not Cover
This Limited Warranty does not cover defects or damages of any sort
resulting from, but not limited to, accidents, damage while in transit to
our service location, alterations, unauthorized service, unauthorized
product case opening, failure to follow instructions, improper use,
abuse, neglect, fire, flood, war or acts of God. Cardiac Science makes no
warranty claim as to the compatibility with Cardiac Science products
with non Cardiac Science products.
This Limited Warranty is Void if…
• Any Cardiac Science product is serviced or repaired by any person or
entity other than Cardiac Science unless specifically authorized by
Cardiac Science;
• Any Cardiac Science product case is opened by unauthorized
personnel or if a product is used for an unauthorized purpose;
• Any Cardiac Science product is used in conjunction with incompatible
parts or accessories, including but not limited to batteries. Parts and
accessories are not compatible if they are not Cardiac Science
products or the functional equivalent.
If The Warranty Period has Expired…
If your Cardiac Science product is not covered by our Limited Warranty,
call us toll free at (888) 466-8686 or +45 4438 0539 for advice as to
whether we can repair your Cardiac Science product, and for other repair
information, including charges. Charges for non-warranty repairs will be
assessed and are your responsibility. Upon completion of the repair, the
terms and conditions of this Limited Warranty shall apply to such repair
or replacement product for a period of 90 days.
This warranty gives you specific legal rights, and you may also have
other rights, which vary from state to state.
© 2003 Cardiac Science, Inc.
page 1

Limited Warranty for FirstSave AED G3
What is Covered?
Cardiac Science, Inc. (“Cardiac Science”) warrants to the original purchaser
that its products will be free of any defect in material and workmanship
according to the terms and conditions of this Limited Warranty
(“Limited Warranty”). For purposes of this Limited Warranty, the
original purchaser is deemed to be the original end user of the product
purchased. This Limited Warranty is NONTRANSFERABLE and
UNASSIGNABLE.
For How Long?
Five (5) years from the date of the original shipment to the original purchaser for FirstSAve AED G3 automated external defibrillators.
Disposable defibrillation electrodes shall be warranted until the expiration date. Lithium batteries have a full operational warranty of One (1)
year from the date of installation into a FirstSave AED G3. Batteries must
be installed by the installation date as labeled on the battery to be covered by this warranty. One (1) year from the date of original shipment to
the original purchaser for Cardiac Science AED accessories. The terms
of the Limited Warranty in effect as of the date of original purchase will
apply to any warranty claims.
What You Must Do
Please complete and submit the Warranty Validation Form within 30 days
of original shipment located at http://www.cardiacscience.com/
products/aed_warranty.cfm. If the purchaser does not have internet
access, call (888) 466-8686 or +45 4438 0539.
To obtain warranty service for your product, call us toll free at (888) 4668686 or +45 4438 0539 seven days a week, 24 hours a day. Our
customer service representative will try to resolve your issue over the
phone. If necessary, and at our sole discretion, we will arrange for
service or a replacement of our product.
What We Will Do
If your Cardiac Science product is returned within 30 days of the date it
was purchased, at the direction of a customer service representative, we
will replace it with a new product of equal value at no charge to you,
provided the warranty applies.
If your Cardiac Science product is returned, at the direction of a customer service representative, after 30 days but within the warranty period, Cardiac Science, at its sole discretion, will repair your product or
replace it with a new or reconditioned product of the same or similar
design. The repaired or replacement product will be warranted subject to
the terms and conditions of this Limited Warranty for either (a) 90 days
or (b) the remainder of the original warranty period, whichever is longer,
provided the warranty applies and the warranty period has not expired.
Obligations and Warranty Limits
Limited Warranty Obligation: Exclusive Remedy
THE FOREGOING LIMITED WARRANTY IS IN LIEU OF AND
SPECIFICALLY EXCLUDES AND REPLACES ALL OTHER EXPRESS OR
IMPLIED WARRANTIES. INCLUDING BUT NOT LIMITED TO THE
IMPLIED WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A
PARTICULAR PURPOSE.
Some states do not allow limitations on how long an implied warranty
lasts, so this limitation may not apply to you.
NO PERSON (INCLUDING ANY AGENT, DEALER, OR REPRESENTATIVE
OF CARDIAC SCIENCE) IS AUTHORIZED TO MAKE ANY REPRESENTATION OR WARRANTY CONCERNING CARDIAC SCIENCE PRODUCTS,
EXCEPT TO REFER PURCHASERS TO THIS LIMITED WARRANTY.
YOUR EXCLUSIVE REMEDY WITH RESPECT TO ANY AND ALL LOSSES
OR DAMAGES RESULTING FROM ANY CAUSE WHATSOEVER SHALL BE
AS SPECIFIED ABOVE. CARDIAC SCIENCE SHALL IN NO EVENT BE
LIABLE FOR ANY CONSEQUENTIAL OR INCIDENTAL DAMAGES OF ANY
KIND, INCLUDING, BUT NOT LIMITED TO, EXEMPLARY DAMAGES,
COMMERCIAL LOSS FROM ANY CAUSE, BUSINESS INTERRUPTION OF
ANY NATURE, LOSS OF PROFITS OR PERSONAL INJURY, EVEN IF
CARDIAC SCIENCE HAS BEEN ADVISED OF THE POSSIBILITIES
OF SUCH DAMAGES, HOWEVER OCCASIONED, WHETHER BY
NEGLIGENCE OR OTHERWISE.
Some states do not allow the exclusion or limitation of incidental or consequential damages, so the above limitation or exclusion may not apply
to you.
What This Warranty Does Not Cover
This Limited Warranty does not cover defects or damages of any sort
resulting from, but not limited to, accidents, damage while in transit to
our service location, alterations, unauthorized service, unauthorized
product case opening, failure to follow instructions, improper use,
abuse, neglect, fire, flood, war or acts of God. Cardiac Science makes no
warranty claim as to the compatibility with Cardiac Science products
with non Cardiac Science products.
This Limited Warranty is Void if…
· Any Cardiac Science product is serviced or repaired by any person or
entity other than Cardiac Science unless specifically authorized by
Cardiac Science;
· Any Cardiac Science product case is opened by unauthorized
personnel or if a product is used for an unauthorized purpose;
· Any Cardiac Science product is used in conjunction with incompatible
parts or accessories, including but not limited to batteries. Parts and
accessories are not compatible if they are not Cardiac Science products
or the functional equivalent.
If The Warranty Period has Expired…
If your Cardiac Science product is not covered by our Limited Warranty,
call us toll free at (888) 466-8686 or +45 4438 0539 for advice as to
whether we can repair your Cardiac Science product, and for other repair
information, including charges. Charges for non-warranty repairs will be
assessed and are your responsibility. Upon completion of the repair, the
terms and conditions of this Limited Warranty shall apply to such repair
or replacement product for a period of 90 days.
This warranty gives you specific legal rights, and you may also have
other rights, which vary from state to state.
page 2 © 2003 Cardiac Science, Inc.

Cardiac Science AED G3 Operation and Service Manual
CAUTION
Federal law restricts this device to be sold by or on the order of a physician or practitioner licensed by state
law in which he/she practices to use or order the use of the device.
IMPORTANT
Read this Operation and Service Manual carefully. It contains information about your safety and the safety of
others. Become familiar with the controls and how to use the AED properly before operating the product.
Cardiac Science AEDs are manufactured by:
Corporate Headquarters:
Cardiac Science, Inc.
1900 Main Street, Suite 700
Irvine, CA 92614 USA
Web site: www.cardiacscience.com
E-mail: aed@cardiacscience.com
Manufacturing:
Cardiac Science, Inc.
5474 Feltl Road
Minnetonka, MN 55343-7982 USA
Authorized European Representative:
Cardiac Science International
Kirke Vaerloesevej 14
3500 Vaerloese DENMARK
TRADEMARK INFORMATION
FirstSave, Powerheart, MDLink, Saving Minutes Saving Lives, SmartGauge, STAR, IntelliSense, RescueReady,
RescueLink, RHYTHMx and Survivalink are trademarks and registered trademarks of Cardiac Science, Inc.
Microsoft and Windows are registered trademarks of Microsoft Corporation. All other trademarks are the
property of their respective owners.
PATENTS
This device is covered by the following U.S. and foreign patents:
5,792,190, 5,999,493, 5,402,884, 5,579,919, 5,749,902, 5,645,571, 6,029,085, 5,984,102, 5,919,212,
5,891,172, 5,674,266, 5,700,281, 5,891,173, 5,968,080, 6,263,239, 5,797,969, D402,758, D405,754,
5,909,138, 6,173,203, 6,088,616, 5,897,576, 5,955,956, 6,083,246, 6,064,909, 6,038,473, 5,868,794,
6,115,638, 6,366,809, 5,474,574, 6,246,907, 6,289,243, 6,411,846, 6,480,734, EP00756878
Other U.S. and foreign patents pending.
page 3© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

LIMITED WARRANTY
The Cardiac Science AED Operation and Service Manual and any and all information contained herein do not
constitute any warranty as to the Powerheart AED G3, FirstSave AED G3, or any related products in any manner
whatsoever. The “Limited Warranty” is shipped with the AED and serves as the sole and exclusive warranty
provided by Cardiac Science regarding Cardiac Science AED products.
ORDER ENTRY
To order additional Cardiac Science AEDs or accessories:
U.S. and Canada Outside U.S. and Canada
(800) 991-5465 + 45 4438 0500
(949) 851-4418 (Fax) + 45 4438 0501 (Fax)
aedorders@cardiacscience.com sales@cardiacscience.dk
CUSTOMER SERVICE
To receive 24-hour customer support:
U.S. and Canada Outside U.S. and Canada
(888) 466-8686 + 45 4438 0539
(949) 851-4425 (Fax) + 45 4438 0501 (Fax)
aedsupport@cardiacscience.com service@cardiacscience.dk
There is no charge to the customer for a customer support call. Please have the serial and model numbers
available when contacting Customer Service. (The serial and model numbers are located on the underside of
the Cardiac Science AED.)
NOTICE OF RIGHTS
All rights reserved. No part of this documentation may be reproduced or transmitted in any form by any
means without the express written permission of Cardiac Science, Inc. Information in this documentation is
subject to change without notice. Names and data used in the examples are fictitious unless otherwise noted.
DEFIBRILLATOR TRACKING
Defibrillator manufacturers and distributors are required, under the Safe Medical Devices Act of 1990, to track
the location of defibrillators they sell. Please notify Cardiac Science Customer Service in the event that your
defibrillator is sold, donated, lost, stolen, exported, destroyed or if it was not purchased directly from Cardiac
Science, Inc.
page 4 © 2003 Cardiac Science, Inc.112-2025-001 Rev E

Cardiac Science AED G3 Operation and Service Manual
TABLE OF CONTENTS
SECTION 1 - SAFETY
OVERVIEW.......................................................................................................................................7
SAFETY ALERT DEFINITIONS..........................................................................................................7
SAFETY ALERT DESCRIPTIONS......................................................................................................8
SYMBOL DESCRIPTIONS..............................................................................................................11
SECTION 2 - INTRODUCTION
OVERVIEW.....................................................................................................................................15
AED DESCRIPTION........................................................................................................................15
INDICATIONS FOR USE.................................................................................................................15
RHYTHMx AED ECG ANALYSIS ALGORITHM................................................................................16
RESCUE PROTOCOL......................................................................................................................18
STAR BIPHASIC WAVEFORM ........................................................................................................18
STAR BIPHASIC ENERGY PROTOCOLS FOR POWERHEART AED.................................................18
OPERATOR TRAINING REQUIREMENTS.......................................................................................20
SECTION 3 - GETTING STARTED
OVERVIEW.....................................................................................................................................21
UNPACKING AND INSPECTING.....................................................................................................21
AED PARTS....................................................................................................................................22
INTELLISENSE BATTERY...............................................................................................................24
ELECTRODES.................................................................................................................................26
AED INDICATORS..........................................................................................................................27
SETTING THE AED INTERNAL CLOCK ...........................................................................................30
VOICE PROMPTS AND TEXT DISPLAYS .......................................................................................30
SECTION 4 - INSTRUCTIONS FOR USE
OVERVIEW.....................................................................................................................................33
STEP 1: ASSESSMENT AND ELECTRODE PLACEMENT................................................................34
STEP 2: ECG ANALYSIS.................................................................................................................35
STEP 3: SHOCK DELIVERY AND CPR MODE ................................................................................36
STEP 4: POST RESCUE .................................................................................................................37
WARNINGS....................................................................................................................................37
SECTION 5 - DATA MANAGEMENT
OVERVIEW.....................................................................................................................................39
RECORDING RESCUE DATA ..........................................................................................................39
REVIEWING RESCUE DATA...........................................................................................................39
page 5© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

SECTION 6 - MAINTENANCE & TROUBLESHOOTING
OVERVIEW.....................................................................................................................................41
SELF-TESTS...................................................................................................................................41
INDICATOR TROUBLESHOOTING TABLE.......................................................................................42
SCHEDULED MAINTENANCE.........................................................................................................43
AUTHORIZED REPAIR SERVICE....................................................................................................44
FREQUENTLY ASKED QUESTIONS ................................................................................................45
SECTION 7 - TECHNICAL DATA
OVERVIEW.....................................................................................................................................47
PARAMETERS................................................................................................................................47
SAFETY AND PERFORMANCE STANDARDS..................................................................................50
STAR BIPHASIC WAVEFORM.........................................................................................................52
RHYTHMx ECG ANALYSIS PERFORMANCE .................................................................................54
SECTION 8 - ACCESSORIES
OVERVIEW.....................................................................................................................................55
CARDIAC SCIENCE AED G3s .........................................................................................................55
AED ACCESSORIES .......................................................................................................................55
AED DELIVERY SYSTEMS.............................................................................................................56
SOFTWARE ACCESSORIES ...........................................................................................................57
EDUCATION ACCESSORIES...........................................................................................................57
page 6 © 2003 Cardiac Science, Inc.112-2025-001 Rev E

Section 1 - Safety
SECTION 1 - SAFETY
OVERVIEW
This section presents safety information to guard against injury to persons and damage to the Cardiac
Science AED G3.
TOPIC PAGE #
Safety Alert Definitions 7
Safety Alert Descriptions 8
Symbols Descriptions 11
SAFETY ALERT DEFINITIONS
BEFORE OPERATING THE CARDIAC SCIENCE AED G3
Become familiar with the various safety alerts in this section.
Safety alerts identify potential hazards using symbols and words to explain what could potentially harm you,
the patient, or the Cardiac Science AED G3.
SAFETY TERMS AND DEFINITIONS
The triangle attention symbol shown below, left, identifies the potential hazard categories. The definition of
each category is as follows:
DANGER: This alert identifies hazards that will cause serious personal injury or death.
WARNING: This alert identifies hazards that may cause serious personal injury or death.
CAUTION: This alert identifies hazards that may cause minor personal injury, product damage, or
property damage.
PRODUCT REFERENCES
For purposes of retaining simple, clear instructions in this manual, note the product references used.
Features, specifications, operating instructions and maintance common to both the Powerheart AED G3 and
the FirstSave AED G3 will be referred to as “AED.” When necessary to distinguish product differences, the
Powerheart AED G3, model 9300E will be referred to as “Powerheart AED” and the FirstSave AED G3, model
9300C will be referred to as “FirstSave AED.”
Features and specifications vary, so please read this manual carefully. For questions regarding the differing
features among Cardiac Science AEDs, please contact Customer Service at (888)466-8686 in the U.S. and
Canada and +45 4438 0539 outside the U.S. and Canada.
page 7© 2003 Cardiac Science, Inc. 112-2025-001 Rev E
SAFETY ALERT DESCRIPTIONS
The following is a list of Cardiac Science AED safety alerts that appear in this section and throughout this manual.
You must read, understand, and heed these safety alerts before attempting to operate the AED.
DANGER: Fire and Explosion Hazard
Exercise caution when operating the AED close to flammable gases (including concentrated oxygen)
to avoid possible explosion or fire hazard.
WARNING: Shock Hazard
Defibrillation shock current flowing through unwanted pathways is potentially a serious electrical
shock hazard. To avoid this hazard during defibrillation abide by all of the following:
• Do not touch the patient, unless performance of CPR is indicated
• Do not touch metal objects in contact with the patient
• Keep defibrillation electrodes clear of other electrodes or metal parts in contact with patient
• Disconnect all non-defibrillator proof equipment from the patient before defibrillation
WARNING: Shock and Possible Equipment Damage
Disconnect all non-defibrillator proof equipment from the patient before defibrillation to prevent
electrical shock and potential damage to the equipment.
WARNING: Electric Shock and Fire Hazard
Do not connect any telephones or unauthorized connectors to the socket on this equipment.
WARNING: Battery is Not Rechargeable
Do not attempt to recharge the battery. Any attempt to recharge the battery may result in an
explosion or fire hazard.
WARNING: Shock Hazard
Do not disassemble the AED! Failure to observe this warning can result in personal injury or death.
Refer maintenance issues to Cardiac Science authorized service personnel.
CAUTION: Temperature/Humidity/Pressure Extremes
Exposing the AED to extreme environmental conditions outside of its operating parameters may
compromise the ability of the AED to function properly. The RescueReady
impact of extreme environmental conditions on the AED by checking temperature, humidity and
pressure; if the daily self test determines environmental conditions outside of the AED’s operating
parameters for 5 consecutive days, a "SERVICE REQUIRED" alert will be issued to prompt the user
to move the AED to environmental conditions within the acceptable operating parameters at once.
See Section 7 – Technical Data, Parameters, Operation and Standby Conditions on page 48.
CAUTION: Lithium Sulfur Dioxide Battery
Pressurized contents: Never recharge, short circuit, puncture, deform, or expose to temperatures
above 65°C (149°F). Remove the battery when discharged.
page 8 © 2003 Cardiac Science, Inc.112-2025-001 Rev E
®
daily self test verifies the

Section 1 - Safety
CAUTION: Battery Disposal
Recycle or dispose of the lithium battery in accordance with all federal, state and local laws. To
avoid fire and explosion hazard, do not burn or incinerate the battery.
CAUTION: Use only Cardiac Science Approved Equipment
Using batteries, electrodes, cables, or optional equipment other than those approved by Cardiac
Science may cause the AED to function improperly during a rescue.
CAUTION: Possible Improper AED Performance
Using electrodes that are damaged or expired may result in improper AED performance.
CAUTION: Serial Communication Cable
The AED will not function during a rescue when the serial communication cable is connected to its
serial port. When the serial communication cable is connected to the AED during a rescue, the prompt
Remove Cable to Continue Rescue” will be heard until you remove the serial communication cable.
“
CAUTION: Possible Radio Frequency (RF) Susceptibility
RF susceptibility from cellular phones, CB radios and FM 2-way radio may cause incorrect rhythm
recognition and subsequent shock advisory. When attempting a rescue using the AED, do not operate
wireless radiotelephones within 1 meter of the AED – turn power OFF to the radiotelephone and
other like equipment near the incident.
CAUTION: Possible Interference with Implanted Pacemaker
Therapy should not be delayed for patients with implanted pacemakers and a defibrillation attempt
should be made if the patient is unconscious and not breathing. The AED has pacemaker detection
and rejection, however with some pacemakers the AED may not advise a defibrillation shock.
1
Placing Electrodes:
• Do not place the electrodes directly over an implanted device.
• Place the electrode pad at least one inch from any implanted device.
CAUTION: Moving the Patient During a Rescue
During a rescue attempt, excessive jostling or moving of the patient may cause AEDs to improperly
analyze the patient’s cardiac rhythm. Stop all motion or vibration before attempting a rescue.
CAUTION: Serial Communication Cable
The serial communication cable is only for use with the AED; it is not to be used with a telephone.
CAUTION: Systems Statement
Equipment connected to the analog and digital interfaces must be certified to the respective IEC
standards (i.e. IEC 950 for data processing equipment and IEC 601-1 for medical equipment).
Furthermore, all configurations shall comply with the system standard IEC 601-1-1. Anybody who
connects additional equipment to the signal input part or signal output part configures a medical
system, and is therefore, responsible that the system complies with the requirements of the system
standard IEC 601-1-1.
page 9© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

CAUTION: Case Cleaning Solutions
When disinfecting the case, use a non-oxidizing disinfectant, such as ammonium salts or a
glutaraldehyde based cleaning solution, to avoid damage to the metal connectors.
1
Cummins, R., ed., Advanced Cardiac Life Support; AHA (1994): Ch. 4.
page 10 © 2003 Cardiac Science, Inc.112-2025-001 Rev E

Chapter 1 - Setup
0% 100%
SYMBOL DESCRIPTIONS
The following symbols may appear in this manual, on the AED, or on its optional components. Some of the
symbols represent standards and compliances associated with the AED and its use.
Dangerous Voltage: The defibrillator output has high voltage and can present a shock hazard.
Please read and understand all safety alerts in this manual before attempting to operate the AED.
Attention!: Identifies important information in this manual, on the AED, or on its component parts
regarding the safe and proper use of the AED.
Defibrillator Proof Type BF Equipment: The AED, when connected to the patient’s chest
by the electrodes, can withstand the effects of an externally applied defibrillation shock.
CE Mark: This equipment conforms to essential requirements of the Medical Device Directive
93/42/EEC.
IP24
The AED is protected against the effects of splashing water in accordance with IEC 60529.
Classified by ETL Semko with respect to electric shock, fire and mechanical hazards only in
accordance with UL 60601-1, CAN/CSA C22.2 No.601.1-M90, EN60601-1 and EN60601-2-4.
Conforms to UL Standard UL60601-1. Certified to CAN/CSA Standard C22.2 No. 601.1-M90.
International symbol for ON. Open the lid to turn on the AED.
Open the lid to turn ON the AED.
Indicates the AED battery status. The illuminated areas indicate the remaining battery
capacity.
Check electrodes. The electrodes are missing, not connected or have compromised functionality.
Indicates AED requires maintenance by authorized service personnel.
When the
When the
new rescue data in the AED.
SHOCK indicator is lit, push this button to deliver a defibrillation shock.
CONTINUE indicator is lit, push this button to clear the internal memory to allow storage of
© 2003 Cardiac Science, Inc. 112-2025-001 Rev E
page 11
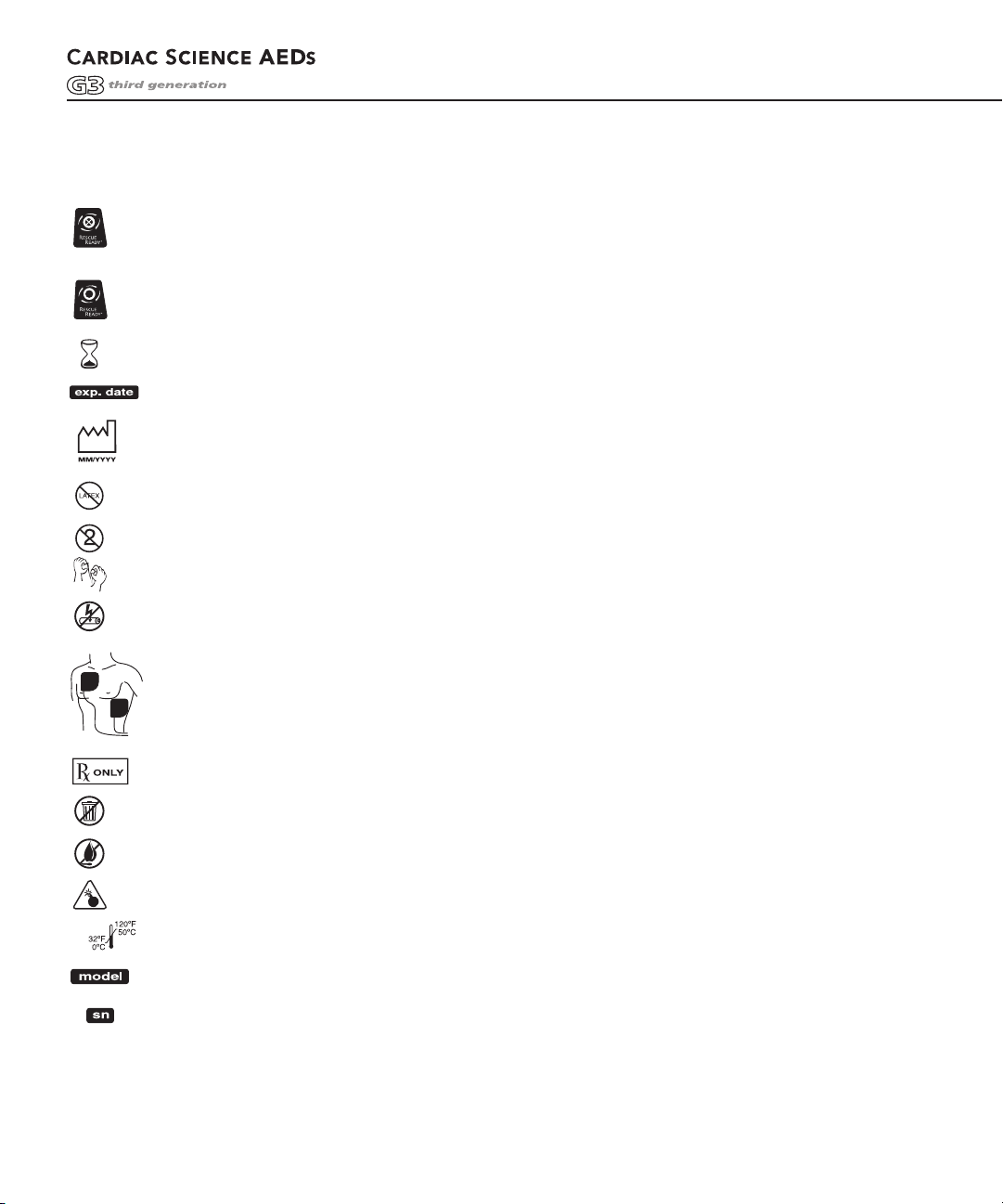
SYMBOL DESCRIPTIONS (CONT.)
A red indicator with a BLACK X means the Powerheart AED requires operator attention or
maintenance, and is not RescueReady. This symbol will be referred to as RED in the remainder
of this manual.
A green indicator without a BLACK X means the Powerheart AED is RescueReady. This symbol
will be referred to as
Use electrodes by this date; install battery by this date.
Expiration Date. Replace by this date.
Date of manufacture.
Latex Free.
Disposable. Single patient use only.
Tear here to open.
Do not recharge battery.
Position of electrodes on the chest of patient.
GREEN in the remainder of this manual.
For use by or on the order of a Physician, or persons licensed by state law.
Dispose of properly in accordance with all state, province, and country regulations.
Do not incinerate or expose to open flame.
Explosion Hazard: Do not use in the presence of a flammable gas, including concentrated oxygen.
Upper and lower temperature limits.
Device Model Number
Serial Number
page 12 © 2003 Cardiac Science, Inc.112-2025-001 Rev E

Lot Number
Option Number
Lithium Sulfur Dioxide
Serial Communication Port
Additional information is provided in the AED Operation and Service Manual.
Points to important information regarding the use of the AED.
Lift Here
Section 1 - Safety
page 13© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

page 14 © 2003 Cardiac Science, Inc.112-2025-001 Rev E

Section 2 - Introduction
SECTION 2 - INTRODUCTION
OVERVIEW
This section presents information about the AED, its use, and the training requirements for
operation.
Topic Page #
AED Description 15
Indications for Use 15
RHYTHMx AED ECG Analysis Algorithm 16
Rescue Protocol 18
STAR Biphasic Waveform 18
STAR Biphasic Energy Protocols for Powerheart AED 18
Operator Training Requirements 20
AED DESCRIPTION
The AED is a self-testing, battery-operated automated external defibrillator (AED). After applying the AED’s
electrodes to the patient’s chest, the AED automatically analyzes the patient’s electrocardiogram (ECG) and
advises the operator to push the button and deliver a shock if needed. The AED uses one button and guides
the operator through the rescue using a combination of voice prompts, audible alerts, and visible indicators.
INDICATIONS FOR USE
The AED with STAR Biphasic Waveform is intended to be used by personnel who have been trained in its operation.
The operator should be qualified by training in basic life support, CPR/AED or other physician-authorized
emergency medical response. The device is indicated for emergency treatment of victims exhibiting symptoms
of sudden cardiac arrest who are unresponsive and not breathing. If the victim is breathing post-resuscitation,
the AED should be left attached to allow for acquisition and detection of the ECG rhythm. If a shockable
ventricular tachyarrythmia recurs, the device will charge automatically and advise the operator to deliver therapy.
When the patient is a child under 8 years of age or weighs less than 55 lbs (25kg), the AED should be used
with the Model 9730 Pediatric Attenuated Defibrillation Electrodes. Therapy should not be delayed to determine
the patient’s exact age or weight.
page 15© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

RHYTHMX®AED ECG ANALYSIS ALGORITHM
The RhythmX AED ECG analysis algorithm provides superior ECG detection capabilities, allowing it to be
placed on patients at risk for sudden cardiac arrest. The features available with the AED include the following:
• Detection Rate
• Asystole Threshold
• Noise Detection
• Non-Committed Shock
• Synchronized Shock
• Pacemaker Pulse Rejection
• SVT Discriminators
• Supraventricular Tachycardia (SVT) Rate
• Continuous Monitoring
DETECTION RATE
All ventricular fibrillation (VF) and ventricular tachycardia (VT) rhythms at or above this rate will be classified
as shockable. All rhythms below this rate will be classified as non-shockable. This rate is programmable
between 120 bpm (beats per minute) and 240 bpm via MDLink Software by the Medical Director. The default
Detection Rate is 160 bpm. The FirstSave AED detection rate is 160 bpm.
ASYSTOLE THRESHOLD
The asystole peak-to-peak threshold is set at 0.08 mV. ECG rhythms at or below 0.08 mV will be classified as
Asystole and will not be shockable.
NOISE DETECTION
The AED will detect noise artifact in the ECG. Noise could be introduced by excessive moving of the patient or
electronic noise from external sources like cellular and radiotelephones. When noise is detected, the AED will
issue the prompt “
then proceed to reanalyze the rhythm and continue with the rescue.
ANALYSIS INTERRUPTED. STOP PATIENT MOTION” to warn the operator. The AED will
NON-COMMITTED SHOCK
After the AED advises a shock, it continues to monitor the patient ECG rhythm. If the patient’s rhythm changes
to a non-shockable rhythm before the actual shock is delivered, the AED will advise that the rhythm has
changed and issue the prompt “
and continue ECG analysis.
page 16 © 2003 Cardiac Science, Inc.112-2025-001 Rev E
RHYTHM CHANGED. SHOCK CANCELLED.” The AED will override the charge
Section 2 - Introduction
SYNCHRONIZED SHOCK
The AED is designed to synchronize shock delivery on the R-wave. The AED will automatically attempt to synchronize
to the R-wave. If delivery cannot be synchronized within one second, a non-synchronized shock will be delivered.
PACEMAKER PULSE DETECTION
The AED contains pacemaker pulse detection circuitry to detect pulses from an implanted pacemaker.
SVT DISCRIMINATORS
Both the Powerheart AED and FirstSave AED are supplied with the SVT Discriminator enabled and with
the default setting "NO THERAPY FOR SVT". With the factory default setting of "NO THERAPY FOR SVT", the
Powerheart AED and FirstSave AED will not shock an SVT rhythm.
SVT Discriminators are sophisticated filters that analyze the morphology of the ECG waveforms and distinguish
VF/VT from SVT and Normal Sinus Rhythms (NSR). The SVT Discriminator will only be applied to rhythms that fall
between the Detection Rate and the SVT Rate. The factory default setting for this feature is "NO THERAPY FOR SVT",
however the Medical Director can enable this feature using MDLink on the Powerheart AED.
The following are optional with the Powerheart AED only.
SVT RATE
All rhythms with rates between the Detection Rate and SVT Rate will be screened through a number of SVT
Discriminators to classify them into VF/VT or SVT. Rhythms classified as SVT between the two set rates are
not shockable. All SVT rhythms above the rates will be classified as shockable. The SVT Rate must be greater
than the Detection Rate and is selectable between 160 and 300 bpm or, “NO THERAPY FOR SVT” can be
selected via MDLink Software by the Medical Director on the Powerheart AED only.
CONTINUOUS MONITORING
Cardiac Science AEDs are supplied with the Continuous Monitoring disabled. This is the factory default setting.
The Powerheart AED can monitor the ECG rhythms continuously throughout the rescue including during
Charge and CPR mode. Continuous Monitoring will interrupt CPR if a shockable rhythm is detected. When
CPR is interrupted, the prompt, “DO NOT TOUCH PATIENT. ANALYZING RHYTHM” will be issued. Only one
false interruption will be allowed during a single rescue cycle. CPR mode will not be interrupted if preceded by
three consecutive shocks. The factory default setting for Continuous Monitoring in CPR is disabled, however
the Medical Director can enable/disable this feature via MDLink software.
page 17© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

RESCUE PROTOCOL
The AED rescue protocol is consistent with the guidelines recommended by the American Heart Association
(AHA)1and the International Liaison Committee on Resuscitation (ILCOR).
Upon detecting a shockable cardiac rhythm, the AED advises the operator to press the SHOCK button to deliver
a series of up to 3 defibrillation shocks followed by performing 1 minute of CPR.
The 3 defibrillation shocks are delivered in a pre-programmed sequence.
Note: The CPR protocol can be modified, for example from 1 to 3 minutes, in increments of 5
seconds, so CPR may be administered if the first analysis is non-shockable or following two
consecutive non-shockable analysis decisions.
STAR BIPHASIC WAVEFORM
The STAR Biphasic Waveform is designed to measure the patient’s impedance and deliver
a customized shock. This allows the delivery of an optimized energy level to each patient.
The energy levels for the Powerheart AED are available in three different defibrillation shock
configurations. See table on next page and page 48 for additional information. The
FirstSave AED is available in Protocol 5 only. See next page.
STAR BIPHASIC ENERGY PROTOCOLS FOR POWERHEART AED
Cardiac Science’s patented STAR®Biphasic defibrillation waveform will deliver variable escalating energy that
is customized to each patient’s needs based upon a patient’s thoracic impedance. This customization adjusts
for the unique physical differences between patients. The range of impedance over which the device will
deliver a shock is 20-180 Ohms. The Powerheart AED comes equipped with five different FDA cleared
biphasic energy protocols.
2
The operator, with guidance, direction and implementation from its designated AED program Medical Director,
may select from one of these five protocols when placing the Powerheart AED into service. The Powerheart
AED’s factory default energy protocol is 200-300-300 Joule (J) escalating Variable Energy (VE). The first
shock is delivered within the range of 140J-250J (200J nominal). Subsequent shocks are delivered within a
range of 190J-360J (300J nominal). The accuracy of the energy for the energy in a 50 Ohm resistor is ±15%.
The FirstSave AED is provided with Protocol 5 (200-200-200J escalating VE) only. See next page.
1
“Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care” American Heart Association; 2000. Suppl.
Circulation 102(8). August 22, 2000
2
The ultra-low current, low current and high current shocks are variable energy. The actual energy is determined by the patient’s impedance.
page 18 © 2003 Cardiac Science, Inc.112-2025-001 Rev E

Section 2 - Introduction
These protocols are selected by using our MDLink software program. The five biphasic energy protocols
available are as follows:
Energy Shock Energy
Protocols Sequence1Level Energy Range (J)
Factory Default 1. 200VE 140J-250J
2. 300VE 190J-360J
3. 300VE 190J-360J
Protocol #2 1. 200VE 140J-250J
2. 200VE 140J-250J
3. 300VE 190J-360J
Protocol #3 1. 150VE 105J-195J
2. 200VE 140J-250J
3. 200VE 140J-250J
Protocol #4 1. 150VE 105J-195J
2. 150VE 105J-195J
3. 200VE 140J-250J
Protocol #5 1. 200VE 140J-250J
2. 200VE 140J-250J
3. 200VE 140J-250J
The FirstSave AED will deliver Protocol 5 only, which is variable energy 200J.
1
The ultra-low current, low current and high current shocks are variable energy. The actual energy is determined by the patient’s impedance.
page 19© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

OPERATOR TRAINING REQUIREMENTS
Persons authorized to operate the AED must have all of the following minimum training.
• Defibrillation training and other training as required by state, province, or country regulations.
• Training on operation and use of the AED.
• Additional training as required by the physician or Medical Director.
• A thorough understanding of the procedures in this manual.
Notes: Keep valid certificates of training and certification as required by state, province, or country
regulations.
page 20 © 2003 Cardiac Science, Inc.112-2025-001 Rev E

Section 3 - Getting Started
SECTION 3 - GETTING STARTED
OVERVIEW
This section presents information on unpacking and setting up the AED
Topic Page #
Unpacking and Inspecting 21
AED Parts 22
IntelliSense Battery 24
Electrodes 26
AED Indicators 27
Setting the AED Internal Clock 30
Voice Prompts and Text Display 30
UNPACKING AND INSPECTING
Every attempt is made to ensure your order is accurate and complete. However, to be sure that your order is
correct, verify the contents of the box against your packing slip.
If you have any questions about your order, contact our Order Entry Department at: (800) 991-5465, or your
local distributor. For customers from countries outside of the United States and Canada, contact your local
distributor or International Operations at +45 4438 0500.
page 21© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

AED PARTS
RescueReady
Status Indicator
(For Powerheart
AED only)
Lid
Latch
(Push in to open)
Battery Compartment
Electrode Expiration
Window
Serial Communication Port
(Behind blue rubber data access cover)
Electrode Holders
Electrode Connector
Speaker
Text Display
Diagnostic Panel
SHOCK/CONTINUE Button
The following drawings show the AED parts and their locations.
page 22 © 2003 Cardiac Science, Inc.112-2025-001 Rev E
(For Powerheart
AED only)

Section 3 - Getting Started
THE AED HAS THREE MODES:
Operating Mode: Defined as having the battery installed and the lid open. This is the mode the AED would be
in during an actual rescue situation.
Standby Mode: When the battery is installed, but the lid is closed. In this mode the AED is not being used in a
rescue. The device will conduct its routine self-tests to ensure proper operation.
Storage Mode: When the battery is removed, such as during shipping or transport. With the battery removed,
the AED is unable to perform self-tests or rescues.
ENVIRONMENTAL OPERATING AND STANDBY CONDITIONS
See Section 7 – Technical Data, Parameters, Environmental Operation and Standby Conditions on page 48.
CAUTION: Temperature/Humidity/Pressure Extremes
Exposing the AED to extreme environmental conditions outside of its operating parameters may
compromise the ability of the AED to function properly. The RescueReady
impact of extreme environmental conditions on the AED by checking temperature, humidity and
pressure; if the daily self test determines environmental conditions outside of the AED’s operating
parameters for 5 consecutive days, a "SERVICE REQUIRED" alert will be issued to prompt the user
to move the AED to environmental conditions within the acceptable operating parameters at once.
See Section 7 – Technical Data, Parameters, Operation and Standby Conditions on page 48.
®
daily self test verifies the
SHIPPING AND TRANSPORT CONDITIONS
(For up to 1 week)
See Section 7 – Technical Data, Safety and Performance Standards/Shipping and Transportation Conditions on
page 52.
page 23© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

INTELLISENSE BATTERY
The Cardiac Science IntelliSense battery technology offers you the most advanced battery capabilities
available for defibrillators. Cardiac Science IntelliSense batteries contain an integrated memory chip that
automatically stores important usage information, enabling the battery to maintain a complete history of its
operating life. The actual battery history can be reviewed using the RescueLink software.
This history includes:
• Battery Identification
• Battery Type
• Original Date of Installation in an AED
• Number of Charges completed
• Time in Operation (hours:minutes)
• Days of Standby Operation
• Battery Capacity Remaining
BATTERY OPERATING LIFE
The battery operating life depends on the type of battery (9142 for Powerheart AED G3 or 9143 for FirstSave
AED G3), actual usage and environmental factors.
The following table represents the expected life of the Powerheart AED when used in Standby Mode.
Estimated Typical
Model Shelf Life Warranty Shocks
9142 Lithium 5 Years 3 Years up to 290 shocks
9143 Lithium 5 Years 1 Year up to 100 shocks
page 24 © 2003 Cardiac Science, Inc.112-2025-001 Rev E

Section 3 - Getting Started
BATTERY SHELF LIFE
The Cardiac Science AED batteries have a shelf-life of five years. Shelf-life is defined as the length of time a
battery can be stored, prior to installation into AED, without degrading its performance.
Note: Storing the battery outside its specific range (0-50°C) will decrease battery life.
BATTERY INSTALLATION
1. With the label on the battery facing the AED battery compartment, insert the battery as
shown in the drawing.
2. Push the latched end of the battery firmly into the AED, as shown in the drawing, until the
battery snaps into place. The exposed side of the battery should be flush with the outside of
the AED case.
3. For the Powerheart AED, open the lid for 5 seconds to initiate self test. If the battery is
installed properly, the
OR
Open the lid for 5 seconds to initiate a self-test. If the battery is installed properly, the
SMARTGAUGE battery indicator LEDs will illuminate; additionally, on the Powerheart AED, the
STATUS INDICATOR will turn GREEN. If service is required, then the SERVICE indicator will
illuminate; call for service.
STATUS INDICATOR will turn GREEN. Close the lid.
Note: Battery part number 9142 is only for use with the Powerheart AED G3. Battery part number
9143 is only for use with the FirstSave AED G3.
WARNING: Battery is Not Rechargeable
Do not attempt to recharge the battery. Any attempt to recharge the battery may result in an
explosion or fire hazard.
CAUTION: Lithium Sulfur Dioxide Battery
Pressurized contents: Never recharge, short circuit, puncture, deform, or expose to temperatures
above 65°C (149°F). Remove the battery when discharged.
CAUTION: Battery Disposal
Recycle or dispose of the lithium battery in accordance with all federal, state and local laws. To
avoid fire and explosion hazard, do not burn or incinerate the battery.
CAUTION: Use only Cardiac Science Approved Equipment
Using batteries, electrodes, cables, or optional equipment other than those approved by
Cardiac Science may cause the Powerheart AED to function improperly during a rescue.
CAUTION: Possible Improper AED Performance
Using electrodes that are damaged or expired may result in improper AED performance.
page 25© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

ELECTRODES
The defibrillation electrodes come in a ready-to-use, sealed package containing
one pair of self-adhesive electrodes with an attached cable and connector. The
electrodes are disposable and should be discarded after each rescue.
The electrodes have a limited shelf-life and should not be used beyond the
expiration date. Keep a fresh, unopened pair of electrodes plugged into the AED
at all times. Refer to the electrode package label for operation temperatures.
An audible alert for the FirstSave AED will be heard after the self-test if the electrodes are missing or
unplugged. On the Powerheart AED, an audible and visual alert will indicate after the self-test if the electrodes
are missing, unplugged or damaged.
CAUTION: Possible Improper AED Performance
Using electrodes that are damaged or expired may result in improper AED performance.
ELECTRODE INSTALLATION
1. Open the lid of the AED.
2. Place the electrode package into the lid so that the expiration label is visible through the
clear window on the lid. The expiration date of the electrodes will then be readable
without opening the lid of the AED.
3. Match the color of the connectors (red to red), then plug the electrode connector into the
AED case as shown in the drawing.
4. Tuck the excess cable length in the bottom holder as shown in the drawing. With the
electrode package completely secured to the AED lid, close the lid.
5. Make sure the expiration date is visible through the clear window of the lid
a. For the Powerheart AED, check to make sure that the
OR
b. For the FirstSave AED, open the lid and check to make sure the electrode indicator
LED is not lit. If the electrodes are not installed properly, the ELECTRODE indicator will
illuminate; call Customer Service for assistance.
CAUTION: Use only Cardiac Science Approved Equipment
Using batteries, electrodes, cables, or optional equipment other than those approved by
Cardiac Science may cause the AED to function improperly during a rescue.
CAUTION: Possible Improper AED Performance
Using electrodes that are damaged or expired may result in improper AED performance.
page 26 © 2003 Cardiac Science, Inc.112-2025-001 Rev E
STATUS INDICATOR is GREEN.

Section 3 - Getting Started
DIRECTIONS FOR USE:
1. Do NOT open until ready to use, short term use only.
2. Ensure the skin site is clean and dry.
3. Separate one electrode from liner.
4. Place one electrode on skin in either direction.
5. Peel and place remaining electrode.
AED INDICATORS
The following indicators are located on the AED.
RESCUEREADY STATUS INDICATOR FOR POWERHEART AED ONLY
The STATUS INDICATOR is located on the Powerheart AED handle. When this indicator
GREEN, the Powerheart AED is RescueReady. This means the Powerheart AED
is
self-tests have verified the following:
• Battery has an adequate charge.
• Electrodes are properly connected to the Powerheart AED and in working order.
• Integrity of the internal circuitry is good.
When the
STATUS INDICATOR is RED, maintenance is required.
AUDIBLE MAINTENANCE INDICATOR
When the daily, weekly or monthly self-test determines service is required, an audible beep is sounded every
30 seconds until the lid is opened or the battery power is depleted. Opening and closing the lid may deactivate
the beep. If the error is not corrected by the next automatic self-test, the beep will be reactivated.
page 27© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

DIAGNOSTIC PANEL
A
B
CD
0% 100%
0% 100%
A SMARTGAUGE BATTERY Indicator
ELECTRODES Indicator
B
C SERVICE Indicator
D SHOCK/CONTINUE Button
SMARTGAUGE™ BATTERY STATUS INDICATOR
The SMARTGAUGE Battery Status Indicator has five (5) LEDs, four (4) green and one (1) red.
The right four green LEDs display the remaining capacity of the battery much like a fuel gauge.
With use, the green LEDs gradually go out, from right to left, as battery capacity decreases.
When the green LEDs go out and the red LED lights up, replace the battery.
Note: When the red LED initially lights up – upon lid opening or at any time during a rescue –
a “BATTERY LOW” prompt will be issued at once. However, the AED is capable of delivering at least 9
more defibrillation shocks after the first “
BATTERY LOW” prompt is issued.
When the AED battery cannot deliver any more shocks, it continuously repeats the “BATTERY LOW” voice
prompt. To continue the rescue, leave the lid open, remove the battery, and replace with a fresh battery. If
battery replacement takes longer than 60 seconds, the first rescue will be terminated and a second rescue will
begin upon opening the lid.
ELECTRODES INDICATOR
The ELECTRODES LED lights up when the electrodes are:
• Not properly connected to the AED
• Not within operational specifications (cold, dried, damaged). This feature applies to the
Powerheart AED only.
• Disconnected from the patient during a rescue.
page 28 © 2003 Cardiac Science, Inc.112-2025-001 Rev E

SERVICE INDICATOR
The SERVICE LED lights up when the AED requires maintenance that can only be
performed by qualified service personnel.
SHOCK/CONTINUE BUTTON
The AED has one button called the SHOCK/CONTINUE button.
This button is located on the diagnostic panel and serves two functions:
• Delivers a defibrillation shock.
• Clears the internal memory of previous rescue data so that new rescue data can be stored.
SHOCK INDICATOR
The word SHOCK and the shock button indicator LED will illuminate red when the
AED is ready to deliver a defibrillation shock to the patient.
Section 3 - Getting Started
CONTINUE INDICATOR
The word CONTINUE will illuminate yellow and the continue button indicator LED will
illuminate red when the previous rescue data has not been cleared from the internal memory.
TEXT DISPLAY FOR POWERHEART AED ONLY
The text display has 2 lines of text. The text display provides the operator with
information regarding system initialization, text prompts and data during a rescue,
and diagnostics.
System initialization occurs when the lid is first opened. The text display shows the operator the identifiers for the
internal code, voice prompts and text prompts versions. The text display also shows the current date and time.
During a rescue, the text display shows the number of shocks delivered and the elapsed time from the
beginning of the rescue (when the lid was first opened). During CPR, a countdown timer will be displayed.
The text version of the voice prompts will also be displayed.
Note: There is a 3 second delay between the time the AED lid is opened and the start of the rescue.
This 3 second delay is not included in the elapsed rescue time.
page 29© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

SETTING THE AED INTERNAL CLOCK
The internal clock is preset at Central Standard Time and should be reset to the correct date and local time.
The AED will automatically adjust itself for daylight savings time. This feature can be turned off using the
MDLink software, (for Powerheart AED only). To set the clock, you will need a Windows 98 or newer PC,
RescueLink software installed, and the AED serial cable connected to the PC.
To set the clock settings:
• Ensure that the PC is set at the correct local time and date.
• Open the lid of the AED and run the RescueLink software on the PC.
• Connect the cable to the serial port on the AED.
• Verify that the voice prompt states “Communications Mode”.
• Click
COMMUNICATIONS on the main menu. Select AED DATE AND TIME.
• Click on the GET button to review the current time in the AED.
• If the time and date is incorrect, click SET to set new time and date. The AED date and time will
automatically be updated to the PC’s time and date.
VOICE PROMPTS AND TEXT DISPLAY
The voice prompts activate when the AED lid is opened and help guide the operator through the rescue. The
Powerheart AED text display provides a visual display of most of the audible voice prompts. The text display
feature is for the Powerheart AED only.
The following table lists the voice and text prompts and a description of when the prompts are issued.
VOICE PROMPT
“Tear Open Electrode Package
and Remove Electrodes.”
TEXT DISPLAY
OPEN PACKAGE AND
REMOVE ELECTRODES
SITUATION
When the lid is opened, this phrase is repeated twice to
initiate the rescue sequence.
“Peel One Electrode from
Plastic Liner.”
“Place One Eectrode on
Bare Upper Chest.”
“Peel Second Electrode and
Place on Bare Lower Chest
as Shown.”
“Do Not Touch Patient!
Analyzing Rhythm.”
“Shock Advised.”
“Charging.”
page 30 © 2003 Cardiac Science, Inc.112-2025-001 Rev E
PEEL ELECTRODE FROM
PLASTIC LINER
PLACE ELECTRODE ON
UPPER CHEST
PLACE ELECTRODE ON
LOWER CHEST
DO NOT TOUCH PATIENT
ANALYZING RHYTHM
SHOCK ADVISED
CHARGING
Repeats until one electrode is peeled off of the liner.
Repeat twice while one electrode is placed.
Repeats until both electrodes are placed on the patient.
When the AED is analyzing the cardiac rhythm of the
patient.
When the AED is preparing to deliver a defibrillation
shock.
Repeated while AED is charging.

Section 3 - Getting Started
VOICE PROMPT
“Stand Clear! Push
Flashing Button to
Deliver Shock.”
“Check for Breathing. If Not
Breathing, Give Patient Two
Breaths.”
“Check for Signs of
Circulation. If No
Circulation Start CPR.”
“Check Electrodes”
“Battery Low”
TEXT DISPLAY
STAND CLEAR
PUSH BUTTON TO SHOCK
IF NOT BREATHING GIVE
TWO BREATHS
IF NO CIRCULATION, START
CPR
CHECK ELECTRODES
BATTERY LOW
SITUATION
After the AED is fully charged and ready to deliver the
defibrillation shock. The RED
and the phrase repeats for 30 seconds or until the
SHOCK button is pushed.
• After the AED delivers 3 consecutive defibrillation
shocks
• After the AED detects a non-shockable cardiac rhythm.
• When 2 1/2 minutes or more has elapsed since CPR
was last administered
• Note: This prompt may be disabled in MDLink, for
Powerheart AED only.
• After previous prompt or if previous prompt is disabled
• After the AED delivers 3 consecutive defibrillation
shocks
• After the AED detects a non-shockable cardiac rhythm
during cardiac rhythm analysis
• When 2 1/2 minutes or more has elapsed since CPR
was last administered
Occurs when patient impedance is too low or too high.
Occurs once when the battery voltage becomes low,
although a rescue can continue for approximately 9
more shocks. When the battery is too low to do a
rescue, the phrase repeats continuously. You must
replace the battery before continuing with the rescue.
If completely depleted, all AED activity will terminate.
SHOCK indicator flashes
“Analysis Interrupted. Stop
Patient Motion.”
“Open Lid to Continue Rescue”
“Rhythm Changed.
Shock Cancelled.”
“Data in memory. Push
Flashing Button to Erase
Data and Perform Rescue.”
“Remove Cable to
Continue Rescue.”
ANALYSIS INTERRUPTED
STOP PATIENT MOTION
OPEN LID TO CONTINUE
RESCUE
RHYTHM CHANGED.
SHOCK CANCELLED
DATA IN MEMORY
PUSH BUTTON TO ERASE
REMOVE CABLE
When the AED detects ECG noise artifact, stop moving
or touching the patient. Remove other electronic
devices within a 5 meter radius.
When the lid is inadvertently closed during a rescue,
this prompt will repeat for 15 seconds.
When the AED detects a change in rhythm – when the
device is prepared to shock.
When there is a previously stored rescue in internal
memory, the yellow
phrase will repeat.
The message can be cleared by pressing the
SHOCK/CONTINUE button to erase the internally stored
rescue data or retrieving the rescue data with
RescueLink and erasing the stored data with
RescueLink. When a serial communication cable is
connected to the AED during a rescue, the phrase
repeats until the cable is disconnected.
CONTINUE button will flash and the
page 31© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

VOICE PROMPT
“Communications Mode”
TEXT DISPLAY
COMMUNICATIONS MODE
SITUATION
When the lid is open and the serial communication
cable is plugged into the AED.
(Beep)
“Continue CPR”
“Service Required”
CONTINUE CPR
SERVICE REQUIRED
“One Beep” occurs in 15-second intervals during CPR
when enabled by the MDLink software program,
“Warble Beep” occurs when the AED requires maintenance.
During CPR mode when enabled, or when a rescue is
resumed in CPR mode after being interrupted by the lid
closing.
Occurs after the self-tests determine that the AED is
not functioning properly. The prompt “
REQUIRED” will be heard when the lid is opened. The
red SERVICE indicator will illuminate and “SERVICE
REQUIRED” will repeat until you close the lid. After
closing the lid, an alarm beep will be heard until the
battery is removed or becomes completely depleted.
SERVICE
page 32 © 2003 Cardiac Science, Inc.112-2025-001 Rev E

Section 4 - Instructions for Use
SECTION 4 – INSTRUCTIONS FOR USE
OVERVIEW
This section presents information about how to use the AED to perform a rescue.
Topic Page #
Step 1: Assessment and Electrode Placement 34
Step 2: ECG Analysis 35
Step 3: Shock Delivery and CPR Mode 36
Step 4: Post Rescue 37
Warnings 37
Text display references are for the Powerheart AED only.
page 33© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

STEP 1: ASSESSMENT AND ELECTRODE PLACEMENT
PREPARATION
Determine that the patient is over 8 years of age or weighs more than 55 pounds (25 kg) and exhibits
the following:
The patient is unresponsive, and
The patient is not breathing.
Remove clothing from the patient’s chest. Ensure the skin site is clean and dry. Dry the patient’s chest and
shave excessive hair if necessary.
Open the AED lid and wait until the LEDs are lit.
Note: When the patient is a child under 8 years of age or weighs less than 55 lbs (25kg), the AED
should be used with the Model 9730 Pediatric Attenuated Defibrillation Electrodes. Therapy
should not be delayed to determine the patient’s exact age or weight. See the directions for use
accompanying pediatric electrodes for procedure on changing adult electrodes to pediatric.
PLACE ELECTRODES
The AED will issue the prompt “Tear Open Electrode Package and Remove Electrodes.” Keep the electrodes
connected to the AED, tear the electrode package along the dotted line and remove the electrodes from the
package. Leave the package attached to the electrode wires.
After the prompt “
pull, carefully peel one electrode away from the release liner.
Then, after the prompt “
electrode with the sticky side of on the patient’s skin on the upper right chest,
placing the top of the electrode on the collarbone. Avoid placing the electrode
directly over the sternum.
Finally, after the prompt “
As Shown
lower left chest, below and left of the breast.
Note: Cardiac Science’s standard defibrillation electrodes are non-polarized and can be placed in
either position as shown on the electrode package. When using pacing/monitoring electrodes, refer
to placement instructions on the pacing/monitoring electrode package.
page 34 © 2003 Cardiac Science, Inc.112-2025-001 Rev E
Peel One Electrode From Plastic Liner,” with a firm, steady
Place One Electrode on Bare Upper Chest,” place the
Peel Second Electrode and Place on Bare Lower Chest
,” pull the second electrode from the release liner and place it on the

Section 4 - Instructions for Use
When the electrodes are placed, the voice prompt will say “Do Not Touch Patient. Analyzing Rhythm.” If the
electrodes are not properly placed or become disconnected at any time during the rescue, the voice prompt
“Check Electrodes” will be heard. When this occurs, ensure that:
Electrodes are firmly placed on clean, dry skin
Electrode cable is securely plugged into the AED
STEP 2: ECG ANALYSIS
As soon as the AED detects proper electrode placement, the voice prompt “Do Not Touch Patient. Analyzing
Rhythm.” will be heard. The AED will begin to analyze the cardiac rhythm of the patient.
If a shock is advised, the voice prompt will say,
AED is ready to deliver a defibrillation shock, the SHOCK button will flash and the prompt,
“Stand Clear. Push Fashing Button to Deliver Shock” will be heard. The tone, flashing
button, and voice prompt will continue until the shock is delivered or change in rhythm is
detected, or 30 seconds elapse.
When the AED is charged, it continues to analyze the patient’s heart rhythm. If the rhythm changes and a
shock is no longer needed, the AED will issue the prompt
and reanalyze.
If no shock is advised, the AED will prompt to start CPR with the prompt “Check for Breathing. If Not
Breathing, Give Patient Two Breaths” (if this prompt is enabled) then “Check for Signs of Circulation. If No
Circulation, Start CPR.”
If noise is detected during analysis, the AED will warn you with the prompt “Analysis Interrupted. Stop Patient
Motion”
electromagnetic emitting electronic device nearby (within 5 meters). Remove the electronic device or stop the
excessive motion when you hear this prompt.
and restart the analysis. This usually occurs if the patient is excessively jostled or there is a strong
“Shock Advised. Charging.” When the
“Rhythm Changed. Shock Cancelled,” disarm
page 35© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

STEP 3: SHOCK DELIVERY AND CPR MODE
When the AED is ready to deliver a defibrillation shock, the SHOCK button will flash and the prompt “Stand
Clear. Push Flashing Button to Deliver Shock” will be heard.
Make sure no one is touching the patient and push the SHOCK button to deliver a
defibrillation shock. If you do not push the
the prompt, the AED will disarm and reanalyze the cardiac rhythm.
After the AED delivers the first defibrillation shock, it reanalyzes the patient’s rhythm to determine if the shock
was successful. If the AED determines that a shockable cardiac rhythm still exists, it will continue to guide
you through additional shocks as needed following the AHA and ILCOR protocols.
Note: During a rescue, the text screen displays voice prompts, elapsed time of rescue and number
of shocks delivered, (for Powerheart AED only).
CPR MODE
After delivery of three consecutive defibrillation shocks or detection of a
non-shockable rhythm, the AED automatically enters CPR mode.
The voice prompt will say, “Check for Breathing. If Not Breathing, Give Patient Two
Breaths” (if this prompt is enabled) then “Check for Signs of Circulation. If No Circulation,
Start CPR.”
Perform CPR if the patient is not responsive and not breathing.
SHOCK button within 30 seconds of hearing
During this time-out for CPR, the Powerheart AED can continue to monitor the patient’s heart rhythm. If the
patient’s condition changes and the Powerheart AED detects a shockable rhythm during the CPR period, the
voice prompt will say, “
in MDLink by the Medical Director, (for Powerheart AED only).
After delivery of three consecutive defibrillation shocks, the AED automatically enters a mandatory CPR time-out
period. During this time-out, the AED will not interrupt the CPR mode if the patient’s condition changes and
the AED detects a shockable rhythm. After the CPR time-out period has expired, the voice prompt “
Touch Patient. Analyzing Rhythm.” will be heard.
Note: During CPR mode, the text screen displays a countdown timer, (for Powerheart AED only).
If the patient is conscious and breathing normally, leave the electrodes on the patient’s chest connected to
the AED. Make the patient as comfortable as possible and wait for Advanced Life Support [ALS] personnel to
arrive. Continue to follow the voice prompts until the ALS personnel arrive, or proceed as recommended by
the Medical Director.
page 36 © 2003 Cardiac Science, Inc.112-2025-001 Rev E
Do Not Touch Patient. Analyzing Rhythm.” This feature is disabled, but can be enabled
Do Not

Section 4 - Instructions for Use
STEP 4: POST RESCUE
After transferring the patient to ALS personnel, prepare the AED for the next rescue:
1. Retrieve the rescue data stored in the internal memory of the AED by using
RescueLink software installed on a PC (see detailed procedure in the Data
Management section).
2. Erase the internal memory of the AED.
3. Connect a new pair of electrodes to the AED.
4. Close the lid.
5. Verify that the
Powerheart AED only).
STATUS INDICATOR on the Powerheart AED handle is GREEN, (for
WARNINGS
The following cautions must be observed to prevent problems during the rescue.
DANGER: Fire and Explosion Hazard
Exercise caution when operating the AED close to flammable gases (including
concentrated oxygen) to avoid possible explosion or fire hazard.
WARNING: Shock Hazard
Defibrillation shock current flowing through unwanted pathways is potentially a serious electrical
shock hazard. To avoid this hazard during defibrillation abide by all of the following:
• Do not touch the patient, unless performance of CPR is indicated
• Do not touch metal objects in contact with the patient
• Keep defibrillation electrodes clear of other electrodes or metal parts in contact with patient
• Disconnect all non-defibrillator proof equipment from the patient before defibrillation
WARNING: Shock and Possible Equipment Damage
Disconnect all non-defibrillator proof equipment from the patient before defibrillation to prevent
electrical shock and potential damage to the equipment.
WARNING: Electric Shock and Fire Hazard
Do not connect any telephones or unauthorized connectors to the socket on this equipment.
CAUTION: Use only Cardiac Science Approved Equipment
Using batteries, electrodes, cables, or optional equipment other than those approved by
Cardiac Science may cause the Powerheart AED to function improperly during a rescue.
CAUTION: Possible Improper AED Performance
Using electrodes that are damaged or expired may result in improper AED performance.
page 37© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

CAUTION: Serial Communication Cable
The AED will not function during a rescue when the serial communication cable is connected to its
serial port. When the serial communication cable is connected to the AED during a rescue, the
prompt “Remove cable to continue rescue” will be heard until you remove the serial communication
cable from the AED
.
CAUTION: Possible Radio Frequency (RF) Susceptibility
RF susceptibility from cellular phones, CB radios and FM 2-way radio may cause incorrect rhythm
recognition and subsequent shock advisory. When attempting a rescue using the AED, do not operate
wireless radiotelephones within 1 meter of the AED – turn power OFF to the radiotelephone and
other like equipment near the incident.
CAUTION: Possible Interference with Implanted Pacemaker
Therapy should not be delayed for patients with implanted pacemakers and a defibrillation attempt
should be made if the patient is unconscious and not breathing. The AED has pacemaker detection
and rejection, however with some pacemakers the AED may not advise a defibrillation shock.
Placing Electrodes:
• Do not place the electrodes directly over an implanted device.
• Place the electrode pad at least on inch from any implanted device.
CAUTION: Moving the Patient During a Rescue
During a rescue attempt, excessive jostling or moving of the patient may cause AEDs to improperly
analyze the patient’s cardiac rhythm. Stop all motion or vibration before attempting a rescue.
page 38 © 2003 Cardiac Science, Inc.112-2025-001 Rev E

Section 5 - Data Management
SECTION 5 – DATA MANAGEMENT
OVERVIEW
The AED is designed for ease of data management and review. The data stored in internal memory can be
displayed on the PC screen using the RescueLink software.
Topic Page #
Recording Rescue Data 39
Review Rescue Data 39
RECORDING RESCUE DATA
RECORDING DATA IN INTERNAL MEMORY
The AED automatically stores up to 34 minutes of rescue data.
If the internal memory contains a previously stored rescue when a rescue is attempted, the voice prompt
“Data in Memory. Push Flashing Button to Erase Data and Perform Rescue” will be heard. Pressing the
CONTINUE button will erase the data and allow the rescue attempt to continue.
Note: Do not press the CONTINUE button unless you are sure you want to erase the internal
memory in the AED.
REVIEWING RESCUE DATA
RETRIEVING DATA FROM MEMORY
1. Open the AED lid.
2. Connect the serial cable to the PC and to the AED’s serial port under the blue rubber data access
cover. The voice prompt will say “
3. Run the RescueLink software program.
4. Select COMMUNICATIONS, GET RESCUE DATA. On the RescueLink software program.
6. Select INTERNAL MEMORY OF AED then select OK.
WARNING: Electric Shock and Fire Hazard
Do not connect any telephones or unauthorized connectors to the socket on this equipment.
CAUTION: Serial Communication Cable
The serial communication cable is only for use with the AED; it is not to be used with a telephone.
Communications Mode.”
page 39© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

page 40 © 2003 Cardiac Science, Inc.112-2025-001 Rev E

Section 6 - Maintenance & Troubleshooting
SECTION 6 – MAINTENANCE & TROUBLESHOOTING
OVERVIEW
This section presents information about the AED diagnostics self-tests, maintenance, and service indications.
Topic Page #
Self-Tests 41
Indicator Troubleshooting Table 42
Scheduled Maintenance 43
Authorized Repair Service 44
Frequently Asked Questions 45
SELF-TESTS
The AED has a comprehensive self-test system that automatically tests the electronics, battery, electrodes,
and high voltage circuitry. Self-tests are also activated every time you open and close the AED lid.
When performing the self-tests, the AED completes the following steps automatically, (for Powerheart AED
only).
• Turns itself ON, and the
• Performs the self-test.
• If successful, the
• Turns itself OFF if the lid is closed.
For the Powerheart AED, there are three types of automatic self-tests. The Daily Self-Test checks the battery,
electrodes, and the electronic components. The Weekly Self-Test completes a partial charge of the high
voltage electronics current in addition to the items tested in the Daily Self-Test. During the Monthly Self-Test,
the high voltage electronics are charged to full energy. On the FirstSave AED, the electrodes are checked only
for presence.
For the Powerheart AED, self-tests will be initiated upon opening the lid and again upon closing the lid. If the
self-test detects an error, the
be issued. The Diagnostic Panel under the lid will indicate the source of the problem according to the
Indicator Troubleshooting Guide Table on the next page.
Self-tests differ for FirstSave AED G3. Please note differences on the following pages.
STATUS INDICATOR changes to RED.
STATUS INDICATOR reverts to GREEN.
STATUS INDICATOR will remain RED. Upon closing the lid, an audible alert will
page 41© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

INDICATOR TROUBLESHOOTING TABLE
0% 100%
The following is a troubleshooting table for the AED indicators.
VIEW SYMPTOM
Red SERVICE indicator (LED) is
lit.
ELECTRODES indicator
Red
(LED) is lit.
The last battery indicator (LED)
is red.
STATUS INDICATOR is RED, (for
Powerheart AED only) and no
other indicators on the
diagnostic panel are lit.
CAUTION: Temperature/Humidity/Pressure Extremes
Exposing the AED to extreme environmental conditions outside of its operating parameters may
compromise the ability of the AED to function properly. The RescueReady
impact of extreme environmental conditions on the AED by checking temperature, humidity and
pressure; if the daily self test determines environmental conditions outside of the AED’s operating
parameters for 5 consecutive days, a "SERVICE REQUIRED" alert will be issued to prompt the user
to move the AED to environmental conditions within the acceptable operating parameters at once.
See Section 7 – Technical Data, Parameters, Operation and Standby Conditions on page 48.
SOLUTION
Maintenance by authorized service personnel is required.
Call Cardiac Science Customer Service (see page 4) or
your local Cardiac Science distributor.
Connect the electrodes or replace with a new pair.
The battery is low. Replace with a new battery.
The battery power is completely depleted. Replace with
a new battery. If
to the Powerheart AED for maintenance.
Call Cardiac Science Customer Service or your local
Cardiac Science distributor.
STATUS INDICATOR remains RED, refer
®
daily self test verifies the
page 42 © 2003 Cardiac Science, Inc.112-2025-001 Rev E

SCHEDULED MAINTENANCE
DAILY MAINTENANCE
For the Powerheart AED, check the STATUS INDICATOR to ensure that it is GREEN. When the
indicator is GREEN, the Powerheart AED is ready for a rescue. If the indicator is RED, refer to
the Troubleshooting Table in this chapter.
MONTHLY MAINTENANCE
1. Open the AED lid.
2. Wait for the AED to indicate status:
a. For the FirstSave AED G3, press the SHOCK/CONTINUE button when the LEDs are illuminating.
Listen for a beep. This indicates that the audible indicator is working.
OR:
b. For the Powerheart AED, observe the change of the STATUS INDICATOR to RED. After less than
5 seconds, verify that the STATUS INDICATOR returns to GREEN.
3. Observe the expiration date on the electrodes.
4. Listen for the voice prompts.
5. Close the lid and confirm that
ANNUAL MAINTENANCE
STATUS INDICATOR remains GREEN, (for Powerheart AED only).
Section 6 - Maintenance & Troubleshooting
Perform the following tests annually to confirm that the diagnostics are functioning properly and to verify the
integrity of the case.
Check the Integrity of the Electrodes and Circuitry
1. Open the AED lid.
2. Remove the electrodes.
3. Close the lid.
4. Confirm that the
5. Open the lid and confirm that the
6. Reconnect the electrodes and close the lid.
7. Make sure the expiration date is visible through the clear window of the lid.
a. For the Powerheart AED G3, check to make sure that the STATUS INDICATOR is
GREEN.
b. For the FirstSave AED G3, open the lid and check to make sure the ELECTRODE
indicator LED is NOT lit. If the electrodes are not installed properly, the ELECTRODE
indicator will illuminate; call Customer Service for assistance.
8. Open the lid and confirm that no diagnostic indicators are lit.
9. Check the expiration date of the electrodes; if expired, replace them.
10. Check the electrode’s packaging integrity.
11. Close the lid.
STATUS INDICATOR turns red, (for Powerheart AED only).
ELECTRODE indicator is lit.
page 43© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

Check the Integrity of the Service Indicator (LED) and Circuitry
1. Immediately after opening the AED lid, press and hold the SHOCK/CONTINUE
button and confirm that the SERVICE LED is lit.
2. Release the SHOCK/CONTINUE button.
3. Close the lid.
4. Verify that the STATUS INDICATOR returns to green, (for Powerheart AED only).
5. Open the lid and confirm that no diagnostic indicators are lit.
6. Close the lid.
Check the Integrity of the Case
Examine the molded case of the AED for any visible signs of stress. If the case shows signs of stress, contact
Cardiac Science Customer Service at one of the following telephone numbers: (888) 466-8686, +45 4438 0539
outside the U.S. and Canada, or contact your local Cardiac Science distributor.
Cleaning the AED Case
Gently clean the surface of the AED case with a damp sponge or with a cloth and mild soap.
CAUTION: Case Cleaning Solutions
When disinfecting the case, use a non-oxidizing disinfectant, such as ammonium salts or a
glutaraldehyde based cleaning solution, to avoid damage to the metal connectors.
AUTHORIZED REPAIR SERVICE
The AED has no user-serviceable internal components. Try to resolve any maintenance issues with the AED by
using the Troubleshooting Table presented in this chapter. If you are unable to resolve the problem, contact
Cardiac Science Customer Service for repair information at one of the following telephone numbers:
(888) 466-8686, +45 4438 0539 outside the U.S. and Canada, or contact your local Cardiac Science distributor.
WARNING: Shock Hazard
Do not disassemble the AED! Failure to observe this warning can result in personal
injury or death. Refer maintenance issues to Cardiac Science authorized service personnel.
Note: The warranty will be void upon unauthorized disassembly or service of the AED.
page 44 © 2003 Cardiac Science, Inc.112-2025-001 Rev E

Section 6 - Maintenance & Troubleshooting
FREQUENTLY ASKED QUESTIONS
QUESTIONS AND ANSWERS
1. Q: Can I give CPR while the AED is analyzing?
A: No. As with all AEDs, the operator should stop CPR compressions during the analysis phase.
2. Q:
3. Q:
4. Q:
5. Q:
6. Q:
Can I transport the victim while the AED is analyzing?
A: No. Vehicle motion may cause noise artifacts that could interfere with proper cardiac rhythm
analysis. Stop the vehicle when cardiac rhythm analysis is necessary.
Do I need to prepare the chest prior to electrode application?
A: Special preparation is not usually necessary. The chest should be as clean, dry, and as oil free as
possible. Follow your Medical Director’s instruction.
What happens if the battery is low when I begin a rescue?
A: When the battery indicator is red, the AED issues a “Battery Low” prompt once; however, the AED is
still capable of delivering approximately 9 more defibrillation shocks.
When the AED is not capable of delivering any more shocks, it continuously repeats the “
Low” prompt. To continue the rescue attempt, leave the lid open and replace the battery. When the
battery replacement takes longer than 60 seconds, the first rescue is terminated and the AED will
begin to record the events from then on as a separate rescue.
How do I set the AED internal clock?
A: Set the clock by using the RescueLink Software Program and a PC. See Setting the AED Internal
Clock in Chapter 3.
What happens if I close the lid in the middle of a rescue attempt?
A: If you close the lid during a rescue, you must re-open the lid within 15 seconds to continue the
rescue. You will hear the prompt, “Open Lid to Continue Rescue.” If the lid remains closed for more
than 15 seconds, a new rescue will initiate when the lid is reopened.
Note: If the lid is closed during a rescue while the electrodes are connected to the patient,
the STATUS INDICATOR may turn RED, (for Powerheart AED only). When the lid is
reopened, however, the rescue may be continued even though the
remains RED.
STATUS INDICATOR
Battery
7. Q: My AED is sounding an audible alert. Why? How do I stop it?
A: The audible alert indicates that the self-test detected a need for maintenance or corrective action.
Determine the maintenance required by using the Troubleshooting Table in this chapter.
Opening and closing the lid may turn OFF the audible alert until the next self-test. However, the
STATUS INDICATOR will remain RED, (for Powerheart AED only).
page 45© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

8. Q: When I open the lid, why do I get the voice prompt “Data in Memory. Push Flashing Button to Erase
Data and Perform Rescue.” How to I get the message to stop occurring?
A: This message occurs when there is a previously stored rescue in the internal memory of the AED.
You can clear the message by:
1. Pressing the SHOCK/CONTINUE button to erase the internally stored rescue data OR
2. Retrieving the rescue data with RescueLink and erasing the stored data with RescueLink.
9. Q:
The AED did not sound an audible alert when I removed the electrodes and closed the lid. Why?
A: The lid-closed electrode self-test only activates the STATUS INDICATOR (on the Powerheart AED).
The AED allows time for replacement of the electrodes – as removing electrodes is a normal
procedure after a rescue - or a battery during the post rescue procedure, however, an audible
maintenance indicator will be triggered after the next Daily Self-Test.
10. Q:
What can I do to keep the AED warm when a rescue is in an isolated area and at subzero temperatures?
A: When travel to a rescue involves exposing the AED to extremely cold temperatures for an extended
period of time, keep the electrodes and the battery warm.
page 46 © 2003 Cardiac Science, Inc.112-2025-001 Rev E

SECTION 7 – TECHNICAL DATA
OVERVIEW
This section presents technical data about the AED.
Topic Page #
Parameters 47
Safety and Performance Standards 50
STAR Biphasic Waveform 52
RHYTHMx ECG analysis performance 54
PARAMETERS
OPERATION
Semi-Automatic (shock advisory)
AUDIBLE ALERTS
Voice Prompt
Maintenance Alert
Section 7 - Technical Data
VISIBLE INDICATORS
Status Indicator
Battery Status Indicator
Service Indicator
Electrodes Indicator
Text Display
page 47© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

RESCUE DATA STORAGE
Storage Capacity
Internal 34 minutes ECG data with event annotation
DIMENSIONS
Measurement Dimension
Height 8 cm (3.3 in)
Width 27 cm (10.6 in)
Depth 31 cm (12.4 in)
WEIGHT
Model Weight with Batteries and Electrodes
9300 3.10 kg (6.6 lb)
ENVIRONMENTAL OPERATION AND STANDBY CONDITIONS
Atmosphere Condition
Temperature 0°C to 50°C (32°F to 122°F)
Humidity 5% to 95% (non-condensing)
Pressure 57kPa (+15,000ft) to 103kPa (-500ft)
SHIPMENT AND TRANSPORT ENVIRONMENTAL CONDITIONS (for up to 1 week)
Atmosphere Condition
Temperature -30°C to 65°C (-22°F to 149°F)
Humidity 5% to 95% (non-condensing)
Atmospheric Pressure 57kPa (+15,000ft) to 103kPa (-500ft)
page 48 © 2003 Cardiac Science, Inc.112-2025-001 Rev E

ELECTRODES
Section 7 - Technical Data
• Self-adhesive, disposable defibrillation electrodes
• Minimum combined surface area: 228cm
• Extended length of lead wire: 1.3m
2
LITHIUM BATTERY SPECIFICATIONS
• Output voltage: 12VDC (max)
• Batteries are non-rechargeable
• Lithium contents: 9.2g (max)
• Check local regulations for disposal information
Estimated Typical
Model Shelf Life Warranty Shocks
9142 Lithium 5 Years 3 Years up to 290 shocks
9143 Lithium 5 Years 1 Year up to 100 shocks
The battery operating life depends on the type of battery (9142 for Powerheart AED G3 or 9143 for FirstSave
AED G3), actual usage and environmental factors.
BATTERIES AND CAPACITOR CHARGE TIMES
A new battery typically takes 10 seconds to charge the AED to maximum energy.
A battery with reduced capacity causes the red LED light to initially turn ON and typically takes 13 seconds to
charge a fully discharged AED to maximum energy.
The maximum time from “Power On” to “Ready to Shock” is 28 seconds for a new rescue.
The maximum time from “Analyze” to “Ready to Shock” is 22 seconds for a new rescue.
DELIVERY OF THREE DEFIBRILLATION SHOCKS
55 seconds (nominal)
page 49© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

AED SELF-TEST SEQUENCE
Frequency of Self-Test
Daily
Weekly
Monthly (every 28 days)
Open Lid (when lid is opened)
Close Lid (when lid is closed)
The FirstSave AED checks for presence of electrodes, not functionality.
What is Tested?
Battery, electrodes, internal electronics, SHOCK/CONTINUE button,
and software (no charge).
Battery, electrodes, internal electronics, SHOCK/CONTINUE button,
and software (partial charge).
Battery under load, electrodes, internal electronics, full-energy charge
SHOCK/CONTINUE button, and software (full charge).
cycle,
Battery, electrodes, internal electronics,
and software.
Battery, electrodes, internal electronics,
and software.
SHOCK/CONTINUE button,
SHOCK/CONTINUE button,
SAFETY AND PERFORMANCE STANDARDS
AED MODELS 9300
The AED has been designed and manufactured to conform to the highest standards of safety and performance
including electromagnetic compatibility (EMC). The Cardiac Science AED Models 9300C/9300E and electrodes
conform to the applicable requirements of the following:
CE
CE Marked by NSAI 0050 per the Medical Device Directive 93/42/EEC of European Nations
ETL
Classified by ETL Semko with respect to electric shock, fire and mechanical hazards only in
accordance with UL 60601-1, CAN/CSA C22.2 No.601.1-M90, EN60601-1 and EN60601-2-4.
Conforms to UL Standard UL60601-1. Certified to CAN/CSA Standard C22.2 No. 601.1-M90.
Electrical, Construction, Safety and Performance
IEC 60601-1 (1998), Amendments 1 (1991) & 2 (1995)
IEC 60601-2-4 (2002)
ANSI/AAMI DF-39 (1993)
Electromagnetic Compatibility (EMC)
IEC 60601-1-2 (2001)
IEC 60601-2-4 Section 36
ANSI/AAMI DF-39(1993) Section 3.3.21
page 50 © 2003 Cardiac Science, Inc.112-2025-001 Rev E

EMISSIONS
Section 7 - Technical Data
Field
E-M
Magnetic
Models
9300
9300
IMMUNITY
Field
E-M
Magnetic
ESD
Models
9300
9300
9300
ENVIRONMENTAL CONDITIONS
Standard or Compliance
IEC 55011/CISPR 11, Group 1, Class B
ANSI/AAMI DF39, <0.5mT on surface, except for within
5cm of the lid magnet and the speaker
Standard or Compliance
IEC 61000-4-3, Level X, (20V/m)
IEC 60601-2-4, Section 36.202.3 (20-V/m)
AAMI DF39, Section 3.3.21.2.1
IEC 61000-4-8 (2001)
IEC 60601-2-4 (2002), Section 36.202.8
AAMI DF39, Section 3.3.21.2.3 80A/m, 47.5Hz – 1,320Hz
IEC 61000-4-2, Level 3
IEC 60601-2-4 (2002), Section 36.202.2
6KV contact discharge, 8KV air gap discharge
Condition
Free Fall Drop
Bump
Vibration (Random)
Vibration (Sine)
Enclosure Protection
Models
9300
9300
9300
9300
9300
Standard or Compliance
IEC 60068-2-32 (1975) AM 2 (1990), 1 meter
IEC 60068-2-29 (1987), 40g and 6000 bumps
2
IEC 60068-2-64 (1993): 10Hz –2KHz, 0.005 – 0.0012 g
IEC 60068-2-6 (1995): 10Hz – 60Hz, 0.15 mm and 60Hz –
150 Hz, 2g
IEC 60529 (2001), IP24
/Hz
page 51© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

SHIPPING AND TRANSPORTATION CONDITIONS
2000.0
1500.0
1000.0
500.0
0.0
-500.0
-1000.0
-1500.0
-1.000 0.000 1.000 2.000 3.000 4.000 5.000 6.000 7.000 8.000 9.000 10.000
TIME (ms)
VOLTS
High Energy Waveform with 50 Ohm Resistive Load
High Variable Energy / 50 Ohm
ISTA Procedure 2A
STAR BIPHASIC WAVEFORM
The waveform generated by the Cardiac Science AED is a BIPHASIC TRUNCATED EXPONENTIAL waveform
that is compliant with ANSI/AAMI DF2 and DF39. The following is a graph of the waveform voltage as a
function of time when the AED is connected to a 50 Ohm resistive load. (See figure A1 and Tables A1
through A3)
FIGURE A1. STAR BIPHASIC WAVEFORM.
Table A1 - Ultra-Low Variable Energy (150 VE) Waveform into Different Resistive Loads (Typical Values)
Patient’s
Impedance
(Ohms)
25
50
75
100
125
150
page 52 © 2003 Cardiac Science, Inc.112-2025-001 Rev E
Phase 1
Delivered
Start Volts
1393V
1420V
1430V
1434V
1437V
1439V
Phase 1
Delivered
End Volts
743V
909V
973V
1007V
1027V
1040V
Phase 1
Duration
(ms)
3.3 ms
4.5 ms
5.8 ms
7.0 ms
8.3 ms
9.5 ms
Phase 2
Delivered
Start Volts
743V
909V
973V
1007V
1027V
1040V
Phase 2
Delivered
End Volts
214V
479V
630V
724V
786V
830V
Phase 2
Duration
(ms)
3.2 ms
3.2 ms
3.2 ms
3.2 ms
3.2 ms
3.2 ms
Total Energy
Delivered
(J)
170J
150J
136J
127J
120J
115J

Section 7 - Technical Data
Table A2 - Low Variable Energy (200VE) Waveform into Different Resistive Loads (Typical Values)
Patient’s
Impedance
(Ohms)
25
50
75
100
125
150
Table A3 - High Variable Energy (270VE) Waveform into Different Resistive Loads (Typical Values)
Patient’s
Impedance
(Ohms)
25
50
75
100
125
150
Phase 1
Delivered
Start Volts
1609V
1640V
1651V
1656V
1660V
1662V
Phase 1
Delivered
Start Volts
1869V
1906V
1918V
1925V
1928V
1931V
Phase 1
Delivered
End Volts
858V
1050V
1124V
1163V
1186V
1201V
Phase 1
Delivered
End Volts
997V
1220V
1306V
1351V
1378V
1396V
Phase 1
Duration
(ms)
3.3 ms
4.5 ms
5.8 ms
7.0 ms
8.3 ms
9.5 ms
Phase 1
Duration
(ms)
3.3ms
4.5ms
5.8ms
7.0ms
8.3ms
9.5ms
Phase 2
Delivered
Start Volts
858V
1050V
1124V
1163V
1186V
1201V
Phase 2
Delivered
Start Volts
997V
1220V
1306V
1351V
1378V
1396V
Phase 2
Delivered
End Volts
247V
553V
728V
835V
907V
959V
Phase 2
Delivered
End Volts
287V
643V
846V
971V
1054V
1114V
Phase 2
Duration
(ms)
3.2 ms
3.2 ms
3.2 ms
3.2 ms
3.2 ms
3.2 ms
Phase 2
Duration
(ms)
3.2 ms
3.2 ms
3.2 ms
3.2 ms
3.2 ms
3.2 ms
Total Energy
Delivered
(J)
226J
200J
182J
169J
160J
153J
Total Energy
Delivered
(J)
305J
270J
246J
229J
216J
207J
ENERGY LEVELS AND PATIENT IMPEDANCE
The Cardiac Science Biphasic Truncated Exponential (BTE) waveform utilizes variable energy. The actual
energy delivered will vary with the patient’s impedance and the device will deliver a shock when impedance
is between 20-180 Ohms. Energy will be delivered at three different levels referred to as ultra-low variable
energy, low variable energy, and high variable energy as shown in the above waveform tables. The accuracy
of the energy for the energy in a 50 Ohm resistor is ±15%, (for Powerheart AED only).
page 53© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

RHYTHMX ECG ANALYSIS PERFORMANCE
The AED RHYTHMx ECG Analysis system analyzes the patient’s ECG and advises you when the AED detects a
shockable or non-shockable rhythm.
This system makes it possible for a person, with no training in the interpretation of ECG rhythms, to offer
defibrillation therapy to victims of sudden cardiac arrest.
CARDIAC RHYTHMS USED TO TEST THE RHYTHM RECOGNITION DETECTION
SYSTEM FOR CARDIAC SCIENCE AED
Rhythm Class Specifications
Shockable Rhythm – VF Meets AAMI DF 39 requirement and AHA recommendation of Sensitivity
of >90%
Shockable Rhythm – VT Meets AAMI DF 39 requirement and AHA recommendation of Sensitivity
of >75%
Non-Shockable Rhythm – NSR Meets AAMI DF 39 requirement (>95%) and AHA recommendation
(>99%) of Specificity
Non-Shockable – Asystole Meets AAMI DF 39 requirement and AHA recommendation of Specificity
of >95%
Non-Shockable Meets AAMI DF 39 requirement and AHA recommendation of Specificity
– all other rhythms of >95%
a
For detailed information contact Cardiac Science for white papers:
P/N 112-2013rD: Pediatric Defibrillation Instructions
P/N 110-0033-001: RhythmX White Paper
P/N 400781: Star Biphasic White Paper
a
Automatic External Defibrillators for Public Access Defibrillation: Recommendations for Specifying and Reporting Arrhythmia Analysis
Algorithm Performance, Incorporating New Waveforms and Enhancing Safety, AHA AED Task Force and approved by the AHA Science
Advisory and Coordinating Committee. Circulation, 1997(95), pp 1677-1682
page 54 © 2003 Cardiac Science, Inc.112-2025-001 Rev E

Section 8 - Accessories
SECTION 8 – ACCESSORIES
OVERVIEW
This section contains a list of parts and software accessories for Cardiac Science AEDs. To place an order, call
Cardiac Science Order Entry at (800) 991-5465 in the U.S. and Canada; +45 4438 0500 elsewhere.
Topic Page #
Cardiac Science AED G3s 55
AED Accessories 55
AED Delivery Systems 56
Software Accessories 57
Education Accessories 57
CARDIAC SCIENCE AED G3s
PART NUMBER DESCRIPTION
AEDs
9300C-001 FirstSave AED G3 (Blue): biphasic waveform, IntelliSense lithium battery
(Model 9143), and internal memory. Includes 1 pair of adult defibrillation electrodes,
Quick Start Guide, serial cable, and CD-ROM containing manual, RescueLink,
MDLink and Training Video.
9300E-001 Powerheart AED G3 (Yellow) with text display: biphasic waveform, IntelliSense
lithium battery (Model 9142), and internal memory. Includes 1 pair of adult
defibrillation electrodes, Quick Start Guide, serial cable, and CD-ROM containing
manual, RescueLink, MDLink and Training Video.
AED ACCESSORIES
9131-001 Defibrillation electrodes (adult) with two-year shelf life
9610-001 Defibrillation/pacing electrodes (adult) with one-year shelf life
9730-002 Pediatric Defibrillation Electrodes with two-year shelf life
9051-002 Electrode adapter to connect Powerheart AED electrodes to Medtronic Physio-
Control defibrillators using QUIK-COMBO
®
connector system
page 55© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

AED ACCESSORIES
PART NUMBER DESCRIPTION
9053-001 Electrode adapter to connect Cardiac Science AED electrodes to Zoll® defibrillators
9055-001 Electrode Cable: reverse adapter for QUIK-COMBO system
9142-001 IntelliSense Lithium battery for Powerheart AEDs, G3 only
9143-001 IntelliSense Lithium battery for FirstSave AEDs, G3 only
AED DELIVERY SYSTEMS
168-6000-001 Soft-sided carrying case for Cardiac Science AED G3
5550-002 Ready Kit: includes nitrile gloves, razor, scissors, towel, 4” gauze, antiseptic
166-0418 Spare battery bag (empty); attaches to AED G3 carrying case
9157-003 Hard-sided, waterproof carrying case for Cardiac Science AED
5500-002 BLS Delivery System Complete: Includes 5510, 5530, 5550, 5560 & 5570
5510-001 Empty soft-sided backpack /carrying case for Delivery System
5530-001 Empty insert to hold basic life support supplies in bag
5560-001 O2 Kit: includes D-size O2 tank, O2 regulator and BVM
5570-001 Basic life support supply kit including: B/P cuff, stethoscope, bandages,
180-2020-101 AED Wall mount storage case
180-2021-101 AED Wall mount storage case with strobe light alarm
5630-001 Heart-Station™ Rescue Case, wall mount, connects to security system
5640-001 Heart-Station™ Rescue Case, recessed wall mount, connects to security system
5501-001 Heart-Station™ Rescue Case replacement lithium battery
170-2145-001 Wall rack
170-2146-101 Wall rack with straps
wipes, one way filter mask
cold packs and other supplies
5650-001 AED Wall mount sign identifying location of AED
page 56 © 2003 Cardiac Science, Inc.112-2025-001 Rev E

Section 8 - Accessories
AED DELIVERY SYSTEMS (CONT.)
PART NUMBER DESCRIPTION
OXYGEN DELIVERY SYSTEMS
Respaide-001 Respaide one-way filter with user instructions
Respaide-002 Respaide one-way filter with mask and mouthpiece and user instructions
Respaide-003 Respaide Kit: includes Respaide filter, mask, mouthpiece, gloves and biohazards bag
and user instructions
SOFTWARE ACCESSORIES
180-0030 Additional Accessory Kit: Quick Start Guide, serial cable, and CD-ROM containing
manual, RescueLink, MDLink and Training Video.
9510-002 1 year extended warranty for AED
9171-001 USB Serial Adapter Cable
EDUCATIONAL ACCESSORIES
180-3011-001 AED Trainer (North American)
180-2080-002 AED Trainer Remote Control
168-1000-003 AED Trainer Carrying Case
9021-002 AED Patient Simulator
9035-003 Adult training electrodes for use with the Cardiac Science AED training device
9725-001 Pediatric training electrodes for use with Cardiac Science training device
400845 AHA Heartsaver AED Text
400846 AHA Heartsaver AED Instructor’s Manual
400847 AHA Heartsaver AED Instructor’s Tool Kit: includes video, presentation,
evaluation forms
page 57© 2003 Cardiac Science, Inc. 112-2025-001 Rev E

page 58 © 2003 Cardiac Science, Inc.112-2025-001 Rev E

 Loading...
Loading...