CardiacScience AED G3+ Service manual

OPERATOR AND SERVICE MANUAL
112-2025-108 A
Revised December 2007
Notice of Rights
© 2008 Cardiac Science Corporation. All rights reserved. No part of this documentation may
be reproduced or transmitted in any form by any means without the express written
permission of Cardiac Science Corporation. Information in this documentation is subject to
change without notice. Names and data used in the examples are fictitious unless otherwise
noted.
Trademark Information
FirstSave, Powerheart, MasterTrak, MDLink, STAR, IntelliSense, RescueReady,
RescueCoach, RescueLink, RHYTHMx, and Survivalink are trademarks and registered
trademarks of Cardiac Science Corporation. Microsoft and Windows are registered
trademarks of Microsoft Corporation. All other trademarks are the property of their
respective owners.
PATENT S
This device is covered by the following U.S. and foreign patents:
5,792,190; 5,999,493; 5,402,884; 5,579,919; 5,749,902; 5,645,571; 6,029,085; 5,984,102;
5,919,212; 5,891,172; 5,674,266; 5,700,281; 5,891,173; 5,968,080; 6,263,239; 5,797,969;
D402,758; D405,754; 5,909,138; 6,173,203; 6,088,616; 5,897,576; 5,955,956; 6,083,246;
6,064,909; 6,038,473; 5,868,794; 6,115,638; 6,366,809; 5,474,574; 6,246,907; 6,289,243;
6,411,846; 6,480,734; 6,658,290; EP00756878
Other U.S. and foreign patents pending.
Cardiac Science Corporation
3303 Monte Villa Parkway
Bothell, WA 98021, USA
+1.800.426.0337 (U.S.A. and Canada)
+1.425.402.2000
techsupport@cardiacscience.com
www.cardiacscience.com
ii
(&
5(3
MDSS GmbH
Schiffgraben 41
D-30175 Hannover
Germany
Tel: +49 511 62 62 86 30
Fax: +49 511 62 62 86 33
112-2025-108 A

Contents
Limited Warranty
Chapter 1: Product Information and Safety
Contact Information .................................................................. 1-1
Defibrillator Tracking ................................................................ 1-2
Product Models.......................................................................... 1-2
Product References..................................................................... 1-2
Warranty Information................................................................ 1-2
Safety Terms and Definitions ..................................................... 1-3
Safety Alert Descriptions............................................................ 1-3
Symbol Descriptions .................................................................. 1-6
Chapter 2: Introduction
AED Description........................................................................ 2-1
Indications for use...................................................................... 2-1
RHYTHMx AED ECG Analysis Algorithm ............................... 2-2
Detection Rate ..................................................................... 2-2
Asystole Threshold............................................................... 2-2
Noise Detection.................................................................... 2-3
Non-Committed Shock ........................................................ 2-3
Synchronized Shock ............................................................. 2-3
Pacemaker Pulse Detection................................................... 2-3
SVT Discriminators.............................................................. 2-3
SVT Rate.............................................................................. 2-4
Rescue Protocol ......................................................................... 2-4
STAR Biphasic Waveform.......................................................... 2-4
STAR Biphasic Energy Protocols for Powerheart G3 AEDs ....... 2-5
Operator Training Requirements ............................................... 2-6
112-2025-108 A Contents iii

Chapter 3: Getting Started
Unpacking and Inspecting .......................................................... 3-1
AED Parts.................................................................................. 3-1
AED Modes ............................................................................... 3-2
Environmental Operating and Standby Conditions .............. 3-3
Shipping and Transport Conditions ..................................... 3-3
IntelliSense Battery..................................................................... 3-3
Battery Operating Life ......................................................... 3-5
Battery Shelf Life.................................................................. 3-5
Battery Installation............................................................... 3-5
Pads ........................................................................................... 3-6
Pad Installation .................................................................... 3-7
Directions for Use ................................................................ 3-8
AED Indicators .......................................................................... 3-8
RescueReady Status Indicator .............................................. 3-8
Audible Maintenance Indicator............................................ 3-9
Diagnostic Panel .................................................................. 3-9
SmartGauge Battery Status Indicator ....................................... 3-10
Pads Indicator.......................................................................... 3-10
Service Indicator ...................................................................... 3-11
Shock Indicator........................................................................ 3-11
Text Display ............................................................................ 3-11
Setting the AED Internal Clock................................................ 3-12
Voice Prompts and Text Display.............................................. 3-12
Chapter 4: Instructions For Use
Warnings and Cautions.............................................................. 4-1
Step 1: Patient Preparation......................................................... 4-3
Step 2: Place Pads....................................................................... 4-3
Step 3: ECG Analysis ................................................................. 4-5
Step 4: Shock Delivery ............................................................... 4-5
Step 5: CPR Mode ..................................................................... 4-6
Step 6: Post Rescue .................................................................... 4-7
Contents Version 112-2025-008 Biv

Chapter 5: Data Management
Recording Rescue Data .............................................................. 5-1
Reviewing Rescue Data.............................................................. 5-1
Chapter 6: Maintenance and Troubleshooting
Self-Tests.................................................................................... 6-1
Indicator Troubleshooting Table................................................ 6-2
Scheduled Maintenance.............................................................. 6-3
Daily Maintenance ............................................................... 6-3
Monthly Maintenance.......................................................... 6-3
Annual Maintenance............................................................ 6-3
Authorized Repair Service.......................................................... 6-5
Frequently Asked Questions....................................................... 6-5
Chapter 7: Technical Data
Parameters ................................................................................. 7-1
Star Biphasic Waveform............................................................. 7-8
112-2025-008 B Contents v

Contents Version 112-2025-008 Bvi

Limited Warranty
Limited Warranty Cardiac Science Corporation (“Cardiac Science”)
warrants to the original purchaser that its AEDs and stated battery operating
life will be free of any defect in material and workmanship according to the
terms and conditions of this Limited Warranty (“Limited Warranty”). For
purposes of this Limited Warranty, the original purchaser is deemed to be the
original end user of the product purchased. This Limited Warranty is
NONTRANSFERABLE and UNASSIGNABLE.
For How Long? This Limited Warranty covers the following products or
parts for the following time periods:
◆ Seven (7) years from the date of the original shipment to the original
purchaser for Powerheart AED automated external defibrillators with
AED battery P/N (9146). Warranty duration for the pads, batteries and
accessories are covered below.
◆ Disposable defibrillation pads shall be warranted until the expiration
date.
◆ Lithium batteries P/N (9146) have a full operational replacement
warranty of four (4) years from the date of installation into a Powerheart
AED.
◆ One (1) year from the date of original shipment to the original purchaser
for Powerheart AED accessories. The terms of the Limited Warranty in
effect as of the date of original purchase will apply to any warranty
claims.
What You Must Do: Please complete and submit the Warranty Validation
Form within 30 days of original shipment. You will find the Warranty
Validation Form enclosed in your original package, or you can fill it out and
submit it online at http://www.cardiacscience.com/products/aed_
warranty.cfm. Or, complete and mail the warranty validation card enclosed
in your original package.
To obtain warranty service for your product, call us toll free at
888.466.8686 seven days a week, 24 hours a day. Our customer service
representative will try to resolve your issue over the phone. If necessary, and
at our sole discretion, we will arrange for service or a replacement of our
product.
Limited Warranty 112-2025-108 Avii

What We Will Do: If your Cardiac Science product is returned within 30
days of the date it was purchased, at the direction of a customer service
representative, we will repair or replace it with a new product of equal value
at no charge to you or offer a full refund of the purchase price, provided the
warranty applies. Cardiac Science retains the exclusive right to repair or
replace the product or offer a full refund of the purchase price at its sold
discretion. SUCH REMEDY SHALL BE YOUR SOLE AND EXCLUSIVE
REMEDY FOR ANY BREACH OF WARRANTY.
If your Cardiac Science product is returned, at the direction of a customer
service representative, after 30 days but within the warranty period, Cardiac
Science, at its sole discretion, will repair your product or replace it. The
repaired or replacement product will be warranted subject to the terms and
conditions of this Limited Warranty for either (a) 90 days or (b) the
remainder of the original warranty period, whichever is longer, provided the
warranty applies and the warranty period has not expired.
Obligations and Warranty Limits
Limited Warranty Obligation: Exclusive Remedy
THE FOREGOING LIMITED WARRANTY IS IN LIEU OF AND
SPECIFICALLY EXCLUDES AND REPLACES ALL OTHER EXPRESSED
OR IMPLIED WARRANTIES INCLUDING BUT NOT LIMITED TO THE
IMPLIED WARRANTIES OF MERCHANTABILITY AND FITNESS FOR
A PARTICULAR PURPOSE.
Some states do not allow limitations on how long an implied warranty lasts,
so this limitation may not apply to you.
NO PERSON (INCLUDING ANY AGENT, DEALER, OR
REPRESENTATIVE OF CARDIAC SCIENCE) IS AUTHORIZED TO
MAKE ANY REPRESENTATION OR WARRANTY CONCERNING
CARDIAC SCIENCE PRODUCTS, EXCEPT TO REFER PURCHASERS TO
THIS LIMITED WARRANTY.
YOUR EXCLUSIVE REMEDY WITH RESPECT TO ANY AND ALL
LOSSES OR DAMAGES RESULTING FROM ANY CAUSE
WHATSOEVER SHALL BE AS SPECIFIED ABOVE. CARDIAC SCIENCE
SHALL IN NO EVENT BE LIABLE FOR ANY SPECIAL, PUNITIVE,
INDIRECT, CONSEQUENTIAL OR INCIDENTAL DAMAGES OF ANY
KIND, INCLUDING, BUT NOT LIMITED TO, EXEMPLARY DAMAGES,
112-2025-108 A Limited Warranty viii

COMMERCIAL LOSS FROM ANY CAUSE, BUSINESS INTERRUPTION
OF ANY NATURE, LOSS OF PROFITS OR PERSONAL INJURY OR
DEATH, EVEN IF CARDIAC SCIENCE HAS BEEN ADVISED OF THE
POSSIBILITIES OF SUCH DAMAGES, HOWEVER OCCASIONED,
WHETHER BY NEGLIGENCE OR OTHERWISE.
Some states do not allow the exclusion or limitation of incidental or
consequential damages, so the above limitation or exclusion may not apply
to you.
What This Warranty Does Not Cover: This Limited Warranty does not cover
defects or damages of any sort resulting from, but not limited to, accidents,
damage while in transit to our service location, product tampering,
unauthorized product alterations, unauthorized service, unauthorized
product case opening, failure to follow instructions, improper use, abuse,
neglect, fire, flood, war or acts of God. Cardiac Science makes no warranty
claim as to the compatibility of Cardiac Science products with any nonCardiac Science products, parts or accessories.
This Limited Warranty is Void if:
◆ Any Cardiac Science product is serviced or repaired by any person or
entity other than Cardiac Science unless specifically authorized by
Cardiac Science.
◆ Any Cardiac Science product case is opened by unauthorized personnel
or if a product is used for an unauthorized purpose.
◆ Any Cardiac Science product is used in conjunction with incompatible
products, parts or accessories, including but not limited to batteries.
Products, parts and accessories are not compatible if they are not Cardiac
Science products intended for use with the Powerheart AED.
If The Warranty Period has Expired: If your Cardiac Science product is not
covered by our Limited Warranty, call us toll free at 888.466.8686 for advice
as to whether we can repair your Powerheart AED, and for other repair
information, including charges. Charges for non-warranty repairs will be
assessed and are your responsibility. Upon completion of the repair, the terms
and conditions of this Limited Warranty shall apply to such repair or
replacement product for a period of 90 days.
This warranty gives you specific legal rights, and you may also have other
rights, which vary from state to state.
Limited Warranty 112-2025-108 Aix

112-2025-108 A Limited Warranty x

1 Product Information and
Safety
What’s in this chapter
◆ Contact Information
◆ Product Models
◆ Product References
◆ Safety Terms and Definitions
◆ Safety Alert Descriptions
◆ Symbol Descriptions
Before Operating the Powerheart G3 AED:
Become familiar with the various safety alerts in this section.
Safety alerts identify potential hazards using symbols and words to explain
what could potentially harm you, the patient, or the Powerheart G3 AED.
Contact Information
To order additional Powerheart G3 AEDs or accessories worldwide:
◆ Toll Free (USA and Canada): +1.800.991.5465
◆ Telephone: +1.425.402.2690
◆ Fax: +1.425.402.2001
◆ Email: customerservice@cardiacscience.com
To receive 24-hour customer support:
There is no charge to the customer for a customer support call. Please have
the serial and model numbers available when contacting Customer Service.
(The serial and model numbers are located on the underside of the AED.)
◆ Toll Free (USA and Canada): +1.888.466.8686
◆ Telephone: +1.425.402.2691
112-2025-108 A Product Information and Safety 1-1

◆ Email: techsupport@cardiacscience.com
◆ Website: www.cardiacscience.com
Defibrillator Tracking
Defibrillator manufacturers and distributors are required, under the Safe
Medical Devices Act of 1990, to track the location of defibrillators they sell.
Please notify Cardiac Science Customer Service in the event that your
defibrillator is sold, donated, lost, stolen, exported, destroyed or if it was not
purchased directly from Cardiac Science or an authorized dealer.
Product Models
This manual is for Powerheart G3 Plus model 9390E and Powerheart G3
Plus Automatic 9390A AED models. They share a basic set of features and
differences are noted throughout the manual.
Product References
For purposes of retaining simple, clear instructions in this manual, note the
product references used. Features, specifications, operating instructions and
maintenance common to product models will be referred to as:
“Powerheart G3 AED”, “AED”, or “device” refers to both Powerheart G3
model 9390E and Powerheart G3 Automatic model 9390A AEDs unless
otherwise noted.
Warranty Information
The Powerheart G3 AED Operation and Service Manual and any and all
information contained herein (except for the Limited Warranty chapter) do
not constitute any warranty as to the Powerheart G3, Powerheart G3
Automatic or any related products in any manner whatsoever. The Limited
Warranty chapter in this manual serves as the sole and exclusive warranty
provided by Cardiac Science regarding Powerheart G3 AED products.
Defibrillator Tracking 112-2025-108 A1-2
Safety Terms and Definitions
The symbols shown below identify potential hazard categories. The
definition of each category is as follows:
DANGER
!
!
!
This alert identifies hazards that will cause serious personal injury or death.
WARNING
This alert identifies hazards that may cause serious personal injury or death.
CAUTION
This alert identifies hazards that may cause minor personal injury, product
damage, or property damage.
Safety Alert Descriptions
The following is a list of Powerheart G3 AED safety alerts that appear in this
section and throughout this manual.
Read and understand these safety alerts before operating the AED.
DANGER: Fire and Explosion Hazard
!
!
!
112-2025-108 A Product Information and Safety 1-3
Do not use in the presence of flammable gasses (including concentrated oxygen)
to avoid possible explosion or fire hazard.
WARNING: Shock Hazard
Defibrillation shock current flowing through unwanted pathways is potentially a
serious electrical shock hazard. To avoid this hazard during defibrillation abide by
all of the following:
- Do not use in standing water or rain. Move patient to dry area
- Do not touch the patient, unless performance of CPR is indicated
- Do not touch metal objects in contact with the patient
- Keep defibrillation pads clear of other pads or metal parts in contact with patient
- Disconnect all non-defibrillator proof equipment from the patient before
defibrillation
WARNING: Shock and Possible Equipment Damage.
Disconnect all non-defibrillator proof equipment from the patient before
defibrillation to prevent electrical shock and potential damage to the equipment.
WARNING: Electric Shock and Fire Hazard.
!
!
!
!
!
Do not connect any telephones or unauthorized connectors to the socket on this
equipment.
WARNING: Battery is Not Rechargeable.
Do not attempt to recharge the battery. Any attempt to recharge the battery may
result in an explosion or fire hazard.
WARNING: Shock Hazard.
Do not disassemble the AED. Failure to observe this warning can result in
personal injury or death. Refer maintenance issues to Cardiac Science authorized
service personnel.
WARNING: Possible Radio Frequency (RF) Susceptibility.
RF susceptibility from cellular phones, CB radios, and FM 2-way radio may cause
incorrect rhythm recognition and subsequent shock advisory. When attempting a
rescue using the AED, do not operate wireless radiotelephones within 1 meter of
the AED – turn power OFF to the radiotelephone and other like equipment near
the incident.
WARNING: Possible Interference with Implanted Pacemaker.
Therapy should not be delayed for patients with implanted pacemakers and a
defibrillation attempt should be made if the patient is unconscious and not
breathing. The AED has pacemaker detection and rejection, however with some
pacemakers the AED may not advise a defibrillation shock. (Cummins, R., ed.,
Advanced Cardiac Life Support; AHA (1994): Ch. 4)
When placing Pads:
- Do not place the pads directly over an implanted device.
- Place the pad at least one inch from any implanted device.
WARNING: Electromagnetic Compatibility.
!
!
Use of accessories or cables other than those specified, with the exception of
accessories and cables sold by Cardiac Science Corporation as replacement parts
for internal components, may result in increased emissions or decreased
immunity of the AED.
WARNING: Improper Equipment Placement.
Position the AED away from other equipment. If it is necessary to use the AED
adjacent to or stacked with other equipment, then observe the AED to verify
normal operations.
Safety Alert Descriptions 112-2025-108 A1-4
CAUTION: Restricted Use.
!
!
!
!
Federal law restricts this device to be sold by or on the order of a physician or
practitioner licensed by state law in which he/she practices to use or order the
use of the device.
CAUTION: Read this Operation and Service Manual carefully.
It contains information about your safety and the safety of others. Become
familiar with the controls and how to use the AED properly before operating the
product.
CAUTION: Temperature Extremes.
Exposing the AED to extreme environmental conditions outside of its operating
parameters may compromise the ability of the AED to function properly. The
RescueReady
conditions on the AED. If the daily self-test determines environmental conditions
outside of the AED’s operating parameters, a “Service Required” alert will be
issued to prompt the user to move the AED to environmental conditions within
the acceptable operating parameters at once. See Chapter 7, Technical Data.
CAUTION: Lithium Sulfur Dioxide Battery.
Pressurized contents: never recharge, short circuit, puncture, deform, or expose
to temperatures above 65°C (149°F). Remove the battery when discharged.
®
daily self-test verifies the impact of extreme environmental
CAUTION: Battery Disposal.
!
!
!
!
112-2025-108 A Product Information and Safety 1-5
Recycle or dispose of the lithium battery in accordance with all federal, state and
local laws. To avoid fire and explosion hazard, do not burn or incinerate the
battery.
CAUTION: Use only Cardiac Science Approved Equipment.
Using batteries, pads, cables, or optional equipment other than those approved
by Cardiac Science may cause the AED to function improperly during a rescue.
CAUTION: Possible Improper AED Performance.
Using pads that are damaged or expired may result in improper AED
performance.
CAUTION: Serial Communication Cable.
The AED will not function during a rescue when the serial communication cable is
connected to its serial port. When the serial communication cable is connected to
the AED during a rescue, the prompt “Remove Cable to Continue Rescue” will
be heard until you remove the serial communication cable.
CAUTION: Moving the Patient During a Rescue.
!
!
!
!
During a rescue attempt, excessive jostling or moving of the patient may cause
AEDs to improperly analyze the patient’s cardiac rhythm. Stop all motion or
vibration before attempting a rescue.
CAUTION: Serial Communication Cable.
The serial communication cable is only for use with the AED; it is not to be used
with a telephone.
CAUTION: Systems Statement.
Equipment connected to the analog and digital interfaces must be certified to the
respective IEC standards (i.e. IEC 60950 for data processing equipment and
IEC 60601-1 for medical equipment).
Furthermore, all configurations shall comply with the system standard
IEC 60601-1-1. Anybody who connects additional equipment to the signal input
part or signal output part configures a medical system, and is therefore,
responsible that the system complies with the requirements of the system
standard IEC 60601-1-1.
CAUTION: Case Cleaning Solutions.
When disinfecting the case, use a non-oxidizing disinfectant, such as ammonium
salts or a glutaraldehyde based cleaning solution, to avoid damage to the metal
connectors.
CAUTION: Equipment Malfunction.
!
!
Portable and RF communications equipment may affect the AED. Always observe
the recommended separation distances as defined in the EMC declaration tables.
CAUTION: Equipment Malfunction.
The AED requires special precautions regarding EMC. Use the AED according to
the guidelines of the EMC declaration tables.
Safety Alert Descriptions 112-2025-108 A1-6
Symbol Descriptions
The following symbols may appear in this manual, on the AED, or on its
optional components. Some of the symbols represent standards and
compliances associated with the AED and its use.
Symbol Description
Caution. Consult accompanying documentation.
!
Dangerous Voltage: The defibrillator output has high voltage and
can present a shock hazard.
Please read and understand all safety alerts in this manual before
attempting to operate the AED.
Defibrillator Proof Type BF Equipment: The AED, when connected
to the patient’s chest by the pads, can withstand the effects of an
externally applied defibrillation shock.
CE Mark: This equipment conforms to essential requirements of the
Medical Device Directive 93/42/EEC.
IP24
112-2025-108 A Product Information and Safety 1-7
The AED is protected against the effects of splashing water in
accordance with IEC 60529.
Classified by ETL Semko with respect to electric shock, fire and
mechanical hazards only in accordance with UL 60601-1, CAN/CSA
C22.2 No.601.1-M90, EN60601-1 and EN60601-2-4.Conforms to
UL Standard UL60601-1. Certified to CAN/CSA Standard C22.2 No.
601.1-M90.
International symbol for ON. Open the lid to turn on the AED.
Symbol Description
Indicates the AED battery status. The illuminated areas indicate the
0% 100%
remaining battery capacity.
Check pads. The pads are missing, not connected or have
compromised functionality.
Indicates AED requires maintenance by authorized service
personnel.
When the SHOCK indicator is lit, press this button to deliver a
defibrillation shock.
A red indicator with a BLACK X means the AED requires operator
attention or maintenance, and is not RescueReady.
A green indicator without a BLACK X means the AED is
RescueReady.
Use pads by this date.
Date of manufacture, year and month.
Symbol Descriptions 112-2025-108 A1-8

Symbol Description
Date of factory recertification (R).
2
R
Latex free.
Disposable. Single patient use only.
Tear here to open.
Do not recharge battery.
Position of pads on the chest of patient.
For use by or on the order of a Physician, or persons licensed by
state law.
Dispose of properly in accordance with all state, province, and
country regulations.
112-2025-108 A Product Information and Safety 1-9
Symbol Description
Do not incinerate or expose to open flame.
Explosion hazard: Do not use in the presence of a flammable gas,
including concentrated oxygen.
Upper and lower temperature limits.
Serial Number.
MODEL
OPTION
Symbol Descriptions 112-2025-108 A1-10
Device model number, battery model number.
Lot number
LOT
Option number
Lithium sulfur dioxide
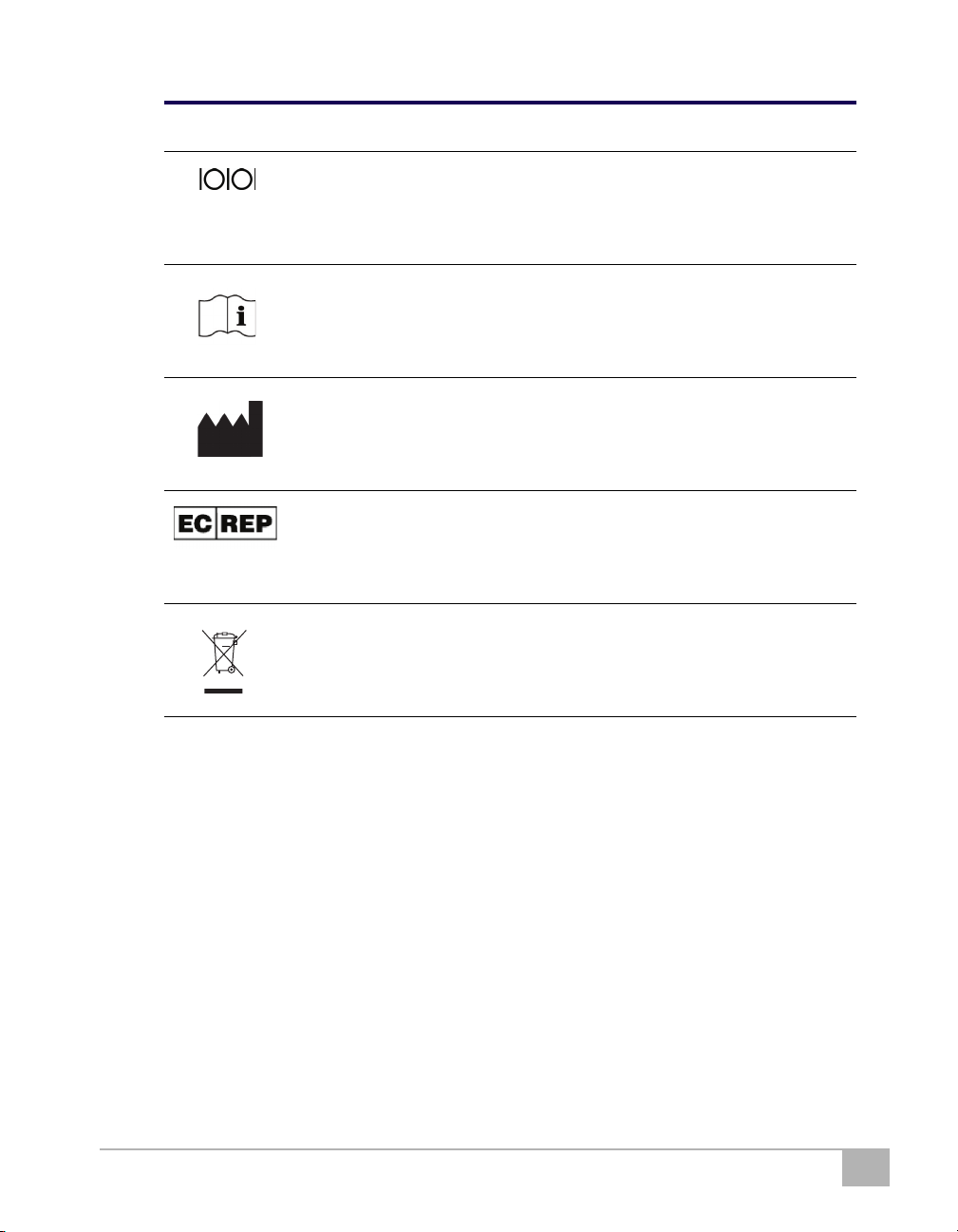
Symbol Description
Serial communication port
Additional information is provided in the AED Operation and
Service Manual.
Manufacturer
Authorized representative in the European Community
Waste Electronic Electrical Equipment (WEEE). Separate collection
for waste electrical and electronic equipment.
112-2025-108 A Product Information and Safety 1-11

Electromagnetic Emissions Standards Compliance
Guidance and manufacturer’s declaration—electromagnetic
emissions
The AED is intended for use in the electromagnetic environment specified
below. The customer or the user of the AED should assure that it is used in
such an environment.
Electromagnetic environment—
Emissions test Compliance
guidance
RF emissions
CISPR 11
RF emissions
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations/flicker
emissions
IEC 61000-3-3
Group 1 The AED uses RF energy only for its internal
function. Therefore its RF emissions are very
low and are not likely to cause any
interference in nearby electronic
equipment.
Class B The AED is suitable for use in all
establishments, including domestic
establishments and those directly
connected to the public low-voltage power
Not applicable
Not applicable
supply network that supplies buildings used
for domestic purposes.
Electromagnetic Emissions Standards Compliance 112-2025-108 A1-12

Guidance and manufacturer’s declaration—electromagnetic
immunity
The AED is intended for use in the electromagnetic environment specified
below. The customer or the user of the AED should assure that it is used in
such an environment.
Immunity test
Electrostatic
discharge (ESD)
IEC 61000-4-2
Electrical fast
transient/burst
IEC 61000-4-4
Surge
IEC 61000-4-5
Voltage dips, short
interruptions and
voltage variations
on power supply
input lines
61000-4-11
IEC 60601 test
level Compliance level
±6 kV contact
±8 kV air
±2 kV for power
supply lines
±1 kV for input/
output lines
±1 kV differential
mode
±2 kV common mode
<5% UT
(>95% dip in UT) for
0.5 cycle
40% UT
(60% dip in UT)
for 5 cycles
±6 kV contact
±8 kV air
Not applicable
Not applicable
Not applicable
Electromagnetic environment—
guidance
Floors should be wood, concrete or
ceramic tile. If floors are covered
with synthetic material, the relative
humidity should be at least 30%
70% UT
(30% dip in UT)
for 25 cycles
<5% UT
(>95% dip in UT)
for 5 sec.
112-2025-108 A Product Information and Safety 1-13

Immunity test
IEC 60601 test
level Compliance level
Electromagnetic environment—
guidance
Power frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
Note: UT is the a.c. mains voltage prior to application of the test level.
3 A/m 80 A/m Power frequency magnetic fields
should be at levels no higher than
those characteristic of a typical
location in typical heavy industrial
and power plants and the control
rooms of H.V. sub-stations.
Guidance and manufacturer’s declaration—electromagnetic
immunity
The AED is intended for use in the electromagnetic environment specified
below. The customer or the user of the AED should assure that it is used in
such an environment.
Compliance
Immunity test IEC 60601 test level
level Electromagnetic environment—guidance
Portable and mobile RF communications
equipment should be used no closer to any
part of the AED, including cables, than the
recommended separation distance calculated
from the equation applicable to the frequency
of the transmitter.
Conducted RF 3 Vrms Not
Applicable
IEC 61000-4-6 150 kHz to 80 MHz
outside ISM bands
10 Vrms
150 kHz to 80 MHz in
ISM bands
Electromagnetic Emissions Standards Compliance 112-2025-108 A1-14
a
a
Not
Applicable
Recommended separation distance
 Loading...
Loading...