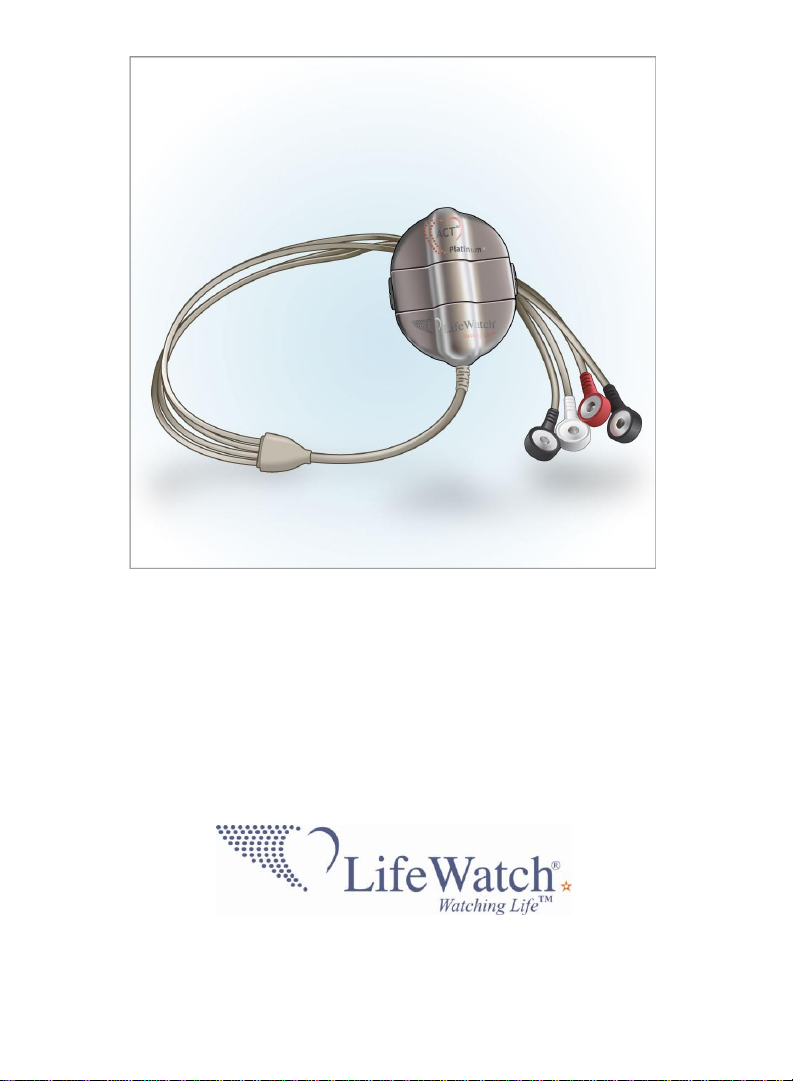
ACT I and ACT III
Patient User Guide
PLEASE CALL
1.800.517.6330
FOR 24/7 CUSTOMER SUPPORT
SUP362, ACT Patient User Guide, Rev C, DCR 11-023
Federal Law (USA) restricts this device to sale by or
on the order of a practitioner licensed by the law of the
State in which he/she practices to use or order the use
of the device.
CAUTION:
This manual should always accompany the unit.
All personnel utilizing the ACT system must have read
and be familiar with the contents of this manual.
First time use – You must call LifeWatch to receive
instructions on how to proceed for the first time use.
The first time the ACT monitoring system is activated
and is attached to you, it will display screens that are
not seen in regular use. These screens are calibration
procedures the ACT monitoring system needs to
perform to adjust its operation for first time use. Please
read the “First Time Activation” section, for more details.
ACT I and ACT III Patient User Guide
This user guide includes information and instructions about the
ACT (Continuous ECG Monitor and Arrhythmia Detector) monitoring
system. Please read it carefully before you begin testing.
If you have any questions regarding the ACT monitoring system
please contact LifeWatch at 1-800-517-6330.
Page 2

Europe
Obelis s.a
Boulevard Général
Wahis 53
1030 Brussels,
BELGIUM
Tel: + (32) 2. 732.59.54
Fax: + (32) 2.732.60.03
E-mail: mail@obelis.net
USA
LifeWatch, Inc.
O’Hare International Center
10255 West Higgins Road
Suite 120
Rosemont, IL 60018
Tel: 847-720-2295
Fax: 847-720-1995
Toll Free: 800-633-3361
Fax: 800-954-2375
Email:
webmaster@lifewatch.com
Israel
Card Guard Scientific
Survival, Ltd.
2 Pekeris St.
Rabin Science Park
Rehovot 76100
Israel
Tel: 972 8 9484000
Fax: 972 8 9484044
Email:
users@cardguard.com
Authorized representatives:
Copyright Declaration
Copyright © 2011 LifeWatch Services, Inc. All rights reserved.
LifeWatch, Watching Life, and LifeStar ACT are trademarks of
LifeWatch Services, Inc. LifeWatch reserves the right to change
specifications at any time without notice.
Microsoft, ActiveSync, MSN, Outlook, Windows, Windows Media, Win
CE, Windows NT, and the Windows logo are either registered
trademarks or trademarks of Microsoft Corporation in the United
States and/or other countries.
Microsoft products are licensed to OEMs by Microsoft Licensing, Inc.,
a wholly owned subsidiary of Microsoft Corporation.
The Bluetooth trademarks are owned by Bluetooth SIG, Inc., U.S.A.
and licensed to Taiyo Yuden Co., Ltd.
Card Guard, Card Guard logo, Instromedix, Instromedix logo,
CG-6108, ACT, ACT-III and PMP4® are trademarks or registered
trademarks of the LifeWatch® Group of Companies.
All other brand names and product names used in this document are
trade names, service marks, trademarks, or registered trademarks of
their respective owners.
Page 3

Illustrations included in this manual are general
representations only and are not meant to comply with
specific regulatory requirements.
The information and screens provided in this manual are subject to
change without notice.
Card Guard Scientific Survival Ltd. SHALL NOT BE LIABLE FOR
TECHNICAL OR EDITORIAL ERRORS OR OMISSIONS
CONTAINED HEREIN; NOR FOR INCIDENTAL OR
CONSEQUENTIAL DAMAGES RESULTING FROM THE
FURNISHING, PERFORMANCE, OR USE OF THIS MATERIAL.
Page 4

Table of Contents
1. Introduction............................................................... 8
Intended Use ................................................................. 8
Important Symbols ....................................................... 12
Warnings and Cautions ............................................... 13
Symbols on Equipment and Labeling .......................... 22
Glossary....................................................................... 24
2. General Description ............................................... 26
3. System Description ................................................ 28
4. The ACT Kit ............................................................. 29
Contents ...................................................................... 29
The ACT III 3-lead Description ..................................... 31
The ACT III 3-lead Description
with Connectable Patient Lead Wires .......................... 32
Cell Phone Monitor Information ................................... 33
Important Information Before Use ................................ 35
General ........................................................................ 35
Starting/Stopping the ACT Sensor ............................... 36
No Connection with Cell Phone Monitor ...................... 36
Sensor Sound Prompts ................................................ 36
5. Using the ACT Monitoring System ........................ 37
Before Starting ............................................................. 37
Electrode Information ................................................... 38
Electrode Placement .................................................... 39
Changing Electrodes ................................................... 42
Sensor Battery Insertion / Replacement ...................... 43
Page 5

Cell Phone Monitor Recharging Procedure .................. 50
First Time Activation .................................................... 53
Monitoring Period ......................................................... 57
Manual Event Recording .............................................. 61
6. Cell Phone Monitor Messages ............................... 67
Sensor Battery Low ...................................................... 68
Cell Phone Monitor Battery Low ................................... 69
Sensor (Bluetooth) Disconnection ................................ 70
Cell Phone Monitor Transmission Problem .................. 71
Electrode Connectivity ................................................. 72
Code Messages ........................................................... 76
7. Maintenance ............................................................ 77
Conditions of Use ......................................................... 77
Caring for your ACT ..................................................... 77
Environment ................................................................. 78
Preventive Maintenance .............................................. 78
8. Troubleshooting ..................................................... 79
9. Technical Specifications ........................................ 85
ACT I Sensor Technical Specifications ........................ 86
ACT III Sensor Technical Specifications ...................... 88
10. Appendix A Monitor (Cellular Phone) Warnings 90
Using Your Phone Near Other Electronic Devices ....... 90
Implantable Medical Devices ....................................... 90
Hearing Aid Compatibility with Mobile Phones ............. 90
Other Medical Devices ................................................. 92
Children Using Wireless Phones .................................. 92
Page 6

Body-worn Operation ................................................... 93
11. Appendix B Message Codes ................................ 94
12. Limited Warranty .................................................. 96
13. Software End User License Agreement .............. 98
Table of Figures
Figure 1. Front view of ACT I sensor ........................................... 30
Figure 2. Rear view of ACT I sensor ............................................ 30
Figure 3. Front view of ACT III sensor (slide open battery cover) . 31
Figure 4. Rear view of ACT III sensor .......................................... 31
Figure 5. Front view of ACT III sensor (flip open battery cover) ... 32
Figure 6. Rear view of ACT III sensor .......................................... 32
Figure 7. ACT I Electrode Placement ........................................... 40
Figure 8. ACT III Electrode Placement ......................................... 41
Figure 9. Removing Battery Cover ............................................... 44
Figure 10. Replacing Battery ................................................... 45
Figure 11. Replacing Battery Cover ........................................ 46
Figure 14. HTC Ozone monitor power button and socket ........ 51
Figure 15. SGH-i617 monitor power button and socket ........... 51
Figure 16. SGH-i637 monitor power button and socket ........... 52
Figure 17. Sensor Manual Recording ...................................... 61
Page 7

Sensor
Cell Phone Monitor
Sensor
Cell Phone Monitor
1. Introduction
Intended Use
ACT I – CG 6108
The ACT I Continuous ECG Monitor and Arrhythmia Detector is
intended for use by patients who experience transient symptoms that
may suggest cardiac arrhythmia. The device continuously monitors a
one lead ECG, automatically generates an alarm triggered by an
arrhythmia detection algorithm, or generates an alarm manually
triggered by the patient, and transmits the recorded data trans
telephonically to a monitoring center. The monitoring center provides
the ECG data to the medical practitioner for evaluation.
ACT III – CG 6108-3L
The ACT III Continuous ECG Monitor and Arrhythmia Detector is
intended for use by patients who experience transient symptoms that
may suggest cardiac arrhythmia. The device continuously monitors
patient ECG, automatically generates an alarm triggered by an
arrhythmia detection algorithm, or generates an alarm manually
triggered by the patient, and transmits the recorded data trans
Page 8

telephonically to a monitoring center. The monitoring center provides
the ECG data to the medical practitioner for evaluation.
The ACT monitoring system is intended to be prescribed for patients
who have demonstrated a need for cardiac monitoring and are at low
risk of developing life-threatening arrhythmias. Conditions where the
system should not be used include patients likely to experience
primary Ventricular Fibrillation or Ventricular Tachycardia and patients
who have other co-morbid cardiovascular conditions where an
arrhythmia could be potentially life threatening.
The ACT monitoring system is intended to be used in conjunction with
a monitoring service that reviews the recorded transmissions and
provides that information to the physician for his/her final diagnostic
interpretation. The monitoring system is not intended for use as an
emergency response system for patients who may experience lifethreatening arrhythmias.
The following list represents patient populations for whom use of the
ACT monitoring system is most appropriate. This list should be used
in conjunction with Medicare and other payor medical necessity
guidelines:
Patients with dizziness or lightheadedness
Patients with palpitations
Patients with syncope of unknown etiology
Patients who require monitoring for non-life-threatening
arrhythmias, such as Atrial Fibrillation, Supra-ventricular
Arrhythmias, evaluation of various Bradyarrhythmias. This
includes post-operative monitoring for these rhythms.
Patients recovering from coronary artery bypass graft (CABG)
surgery who require monitoring for arrhythmias
Patients requiring monitoring for arrhythmias-including co-morbid
conditions such as hyperthyroidism or chronic lung disease
Patients with obstructive sleep apnea to evaluate possible
Page 9

nocturnal arrhythmias
Patients requiring arrhythmia evaluation for etiology of stroke or
transient cerebral ischemia, possibly secondary to Atrial
Fibrillation
To use the ACT monitoring system, the user or primary care provider
must be able to perform all of the following:
Understand the principle of operation and system messages
described in this manual
Place the sensor and electrodes on the chest
Operate a handheld device (cell phone monitor)
Operate simple push-buttons
The ACT monitoring system is safe for use by patients wearing an
oxygen mask for breathing.
The ACT monitoring system is not water resistant and must not get
wet. Do not use or store the ACT monitoring system where liquids of
any nature may come into contact with it. Raindrops, water spray,
juice, coffee, steam, perspiration, perfume, deodorant, etc. may also
affect the performance of the monitoring system and cause a possible
malfunction. While bathing or showering, the system should be placed
in a dry environment, outside of the bathroom. The electrode patches
may be worn in the shower or bath as long as they are disconnected
from the sensor.
The function of the ACT monitoring system is dependent on cellular
phone service and Bluetooth technology. Limitations in data
transmission may occur if there is limited cellular service in the area.
A landline modem can be provided for locations with limited cellular
service coverage and/or if interference with the wireless Bluetooth
connection is experienced.
Page 10

You may occasionally experience a delay in the ability to send
recorded events due to unexpected cellular limitations. If this occurs,
contact LifeWatch as soon as possible. Any technical difficulties
should be reported as quickly as possible so as to resolve the issue
with minimal service interruption.
As with all standard cell phones, charge the cell phone monitor
whenever possible, and at least every night. The battery in the sensor
should be changed as instructed by the low battery messages. The
performance of the Cell Phone Monitor and Sensor, including data
recording and transmission, may be adversely impacted if not
adequately charged.
Page 11
Warning
Symbol indicates a potentially hazardous situation,
which, if not avoided, could result in death or serious
injury to the user.
Caution
Symbol indicates a situation that the user must take
into consideration to ensure the safe and effective
operation of the equipment and associated
accessories.
Notes
Symbol indicates important general information for
using the system successfully.
Important Symbols
A number of symbols are used throughout this manual in order to
draw attention to safety items and other important information.
The following symbols are used:
Page 12
Warning
The ACT monitoring system is intended to be
used in conjunction with a monitoring service that
reviews the recorded transmissions and provides
that information to the physician for his/her final
diagnostic interpretation.
The ACT monitoring system is not intended for
use as an emergency medical response system
and should not be used by patients at risk for
serious or life-threatening cardiac arrhythmias,
such as ventricular tachycardia and ventricular
fibrillation. Refer to the Physician Manual
Specification for the types of arrhythmias detected
by the ACT monitoring system.
The ACT monitoring system is not intended for
use in the diagnosis of myocardial infarction or for
chest pain monitoring.
Due to the risk of ignition or fire, the ACT
monitoring system is not intended for use in a
hyperbaric chamber, within an oxygen tent or in
the presence of flammable anesthetics / medical
gases.
Warnings and Cautions
The following section contains a complete list of the major warnings
and cautions relevant to the ACT monitoring system. These warnings
and cautions are also repeated, as appropriate, in sections of this
manual. Your prescribing physician is responsible for reading and
understanding all warnings and cautions prior to prescribing the ACT
monitoring system.
Page 13
Warning
To prevent fire or shock hazard, do not expose the
ACT monitoring system to moisture, liquids or
condensation.
To prevent an allergic reaction, do not use the
ACT monitoring system or accessories if you have
a known allergy to nickel or other metals.
The ACT monitoring system is not defibrillation-
proof. Exposure to defibrillation may damage the
ACT monitoring system, or the ACT monitoring
system may interfere with the operation of the
defibrillator. The ACT monitoring systems MUST
be removed prior to defibrillation as it contains
metals that could cause the defibrillator to arc.
Use of conductive, connected devices and patient
lead wires/electrodes like the ACT monitoring
system in MRI procedures may result in serious
burns.
If you should come into possession of your ECG
recording do not take any actions of a medical
nature based on your understanding UNLESS you
are a medical professional.
Page 14
Warning
The ACT monitoring system is not intended
for use on persons with an Implantable
Cardioverter Defibrillator (ICD).
Use with Implanted Conventional Pacemakers (not including ICDs)
If you have an implanted pacemaker, the
manufacturer may recommend certain precautions
when using a cellular phone. Since the ACT cell
phone monitor is also a cellular phone, you should
take the same precautions when carrying and
using the cell phone monitor. In general, most
manufacturers recommend the following:
Keep a distance of at least six inches (15 cm)
between the cell phone monitor and a
pacemaker.
Carry the cell phone monitor on the opposite
side of the body from the pacemaker.
Don’t carry a cell phone in a breast pocket or
on a belt if that would place the phone within
six inches (15 cm) of the pacemaker.
Refer to the manufacturer's information for
guidance regarding the pacemaker and
interference issues.
Page 15
Caution
The ACT monitoring system is intended to
be worn during normal daily activities. If
vigorous physical activity or exercise is part
of your normal daily activity, the associated
perspiration and lead wire movement can
loosen the electrodes. Contact LifeWatch
to obtain special electrodes for these
situations.
Disposable electrodes must be changed
according to instructions provided in this
manual to assure optimal recording quality
and limited skin irritation.
The ACT monitoring system generates,
uses, and can radiate radio frequency
energy and, if not installed and used in
accordance with the instruction manual,
may cause harmful interference to radio
communications.
The ACT monitoring system employs
Bluetooth and cellular technology. The
location of the ACT monitoring system and
the associated environment, including
cellular phone coverage in the particular
area, may cause transmission interruption
or delay.
Do not open or attempt to repair the sensor.
Only authorized service personnel may
repair the system components.
Over-the-counter batteries should never be
used as they can seriously damage the
sensor. Only use the specialized batteries
included in the kit. If more batteries are
needed, contact LifeWatch.
Page 16
Caution
To avoid damage to the system, the
system and accessories should be kept
away from extreme heat including
placement of the ACT monitoring system
on the dashboard of a car or near a
heater.
The system should not be subjected to
severe impact or bending force. Exposure
to these types of stresses can damage
the system components.
Charge the cell phone monitor every night
(irrespective of indicator status), making
sure that it is within 10 feet (3 meter) of
the sensor. In addition, charge the cell
phone monitor whenever possible during
the day.
The cell phone monitor energy
consumption may be high during the first
few days of monitoring (up to 72 hours).
Keep the cell phone monitor charged at
all times.
Return the used and unused sensor
batteries to LifeWatch for proper disposal.
Do not discard the batteries in or near a
fire.
If the sensor battery is replaced when the
sensor is out of Bluetooth range from the
cell phone monitor, the sensor will not be
able to connect with the cell phone
monitor and will not be able to record until
it reconnects with the cell phone monitor.
Page 17
Caution
If the sensor (ACT I firmware version 0.1g
and ACT III firmware version 1.0.4) is not
connected to the cell phone monitor (via
Bluetooth) for more than 2 hours (ACT I) or 6
hours (ACT III) and the sensor's battery is
replaced, the sensor (on reconnection) will
start with new data and not download the
data stored, thus overriding the data stored
when it was not connected.
Electrode disconnection might cause a faulty
ECG analysis and/or false events due to
noise created by the electrode
disconnection.
The impedance test (occurring every two
minutes) overrides ECG recording, which
means the ECG will lack 1 second (ACT I) or
0.5 second (ACT III) of recording every two
minutes.
Do not turn the cell phone monitor sound off
or reduce the volume so that it is inaudible.
After exiting the ACT (monitor) application, it
will take up to 3 minutes for all the processes
to end. This means you must wait 3 minutes
before starting the ACT (monitor) application
again.
Take the charged cell phone monitor with
you and wear the sensor at all times (except
when showering or bathing) during the
monitoring period.
Page 18
Caution
A Bluetooth disconnection between the cell
phone monitor and the sensor might occur due
to electromagnetic interference. In this case
the sensor will search for the cell phone
monitor device every 3 minutes.
If the cell phone monitor Bluetooth
communication is not active for 60 minutes, the
ACT (monitor) application will automatically
restart the Bluetooth communication.
Always change the sensor battery when
connected (Bluetooth) to the cell phone
monitor, a low sensor battery message
indicates there are up to three hours before the
battery fails.
First time use – The patient must call
LifeWatch to receive instructions on how to
proceed for the first time use. The first time the
ACT monitoring system is activated and is
attached, certain screens are displayed that
are not seen during normal operation. These
screens are used for calibration. Please refer
to “First Time Activation” section of the manual
for more details.
Page 19
Caution
Please refer to the user manual of the
manufacturer of the cell phone monitor for
Health and Safety Information pertaining to the
use and operation of the cell phone monitor.
The cell phone monitor manual can be
downloaded from the Internet.
Do not use the cell phone monitor for any
reason outside of the designated monitoring
function.
Keep kit contents away from children.
Page 20
FCC Note
Important Safeguard in the Medical Environment
Note
Modifications not expressly approved by the
manufacturer could void the user authority to operate
the equipment under FCC Rules.
THE MANUFACTURER IS NOT RESPONSIBLE FOR
ANY RADIO OR TV INTERFERENCE CAUSED BY
UNAUTHORIZED MODIFICATIONS TO THIS
EQUIPMENT. SUCH MODIFICATIONS COULD VOID
THE USER'S AUTHORITY TO OPERATE THE
EQUIPMENT.
This equipment has been tested and found to comply with the limits
for a Class B digital device, pursuant to Part 15 of the FCC Rules.
These limits are designed to provide reasonable protection against
harmful interference in a residential installation. This equipment
generates, uses and can radiate radio frequency energy and, if not
installed and used in accordance with the instructions, may cause
harmful interference to radio communications. However, there is no
guarantee that interference will not occur in a particular installation. If
this equipment does cause harmful interference to radio or television
reception, which can be determined by turning the equipment off and
on, the user is encouraged to try to correct the interference by one or
more of the following measures:
Reorient or relocate the receiving antenna
Increase the separation between the equipment and receiver
Connect the equipment into an outlet on a circuit different from
that to which the receiver is connected
Consult the dealer or an experienced radio/TV technician for help
Page 21
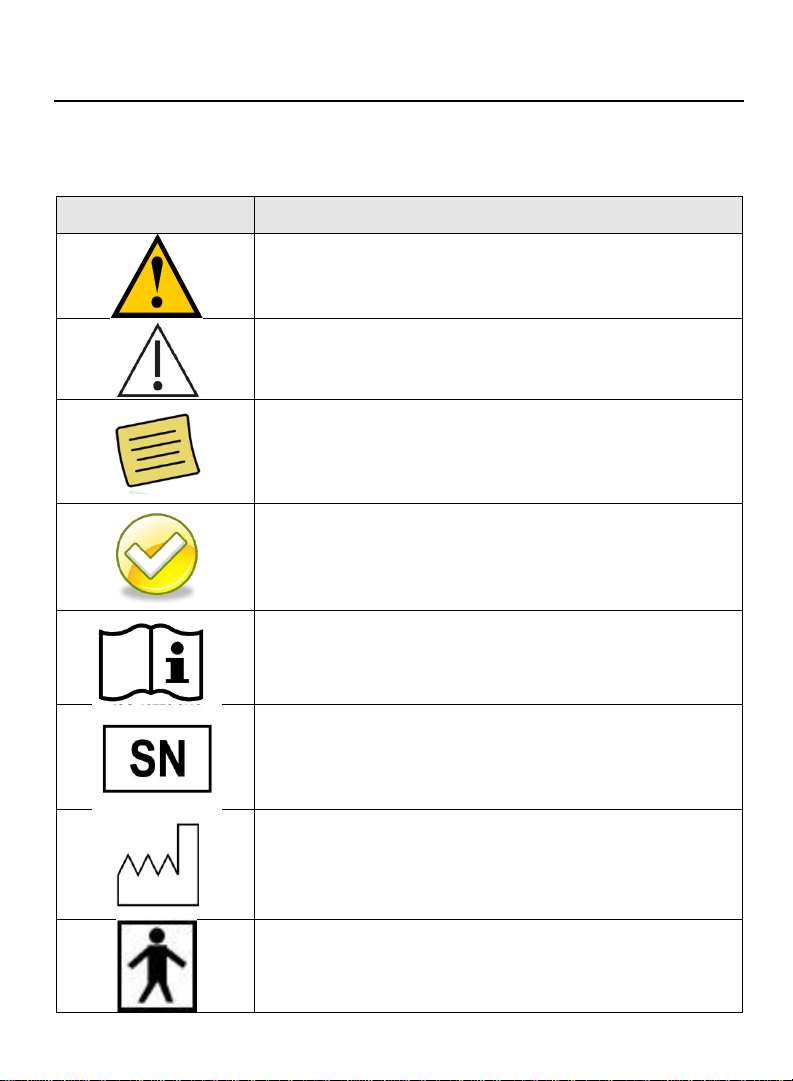
Label
Description
Warning, consult accompanying text or
documents
Caution, consult accompanying text or
documents
Notes, indicates important general information
for using the system successfully.
Tips, indicates important tips on using the
system.
Consult instructions for use
Serial Number
Date of Manufacture
Type BF Applied Part
Symbols on Equipment and Labeling
The following section contains a complete description of all symbols
that may be located on either the equipment or labeling of ACT device
and accessories.
Page 22
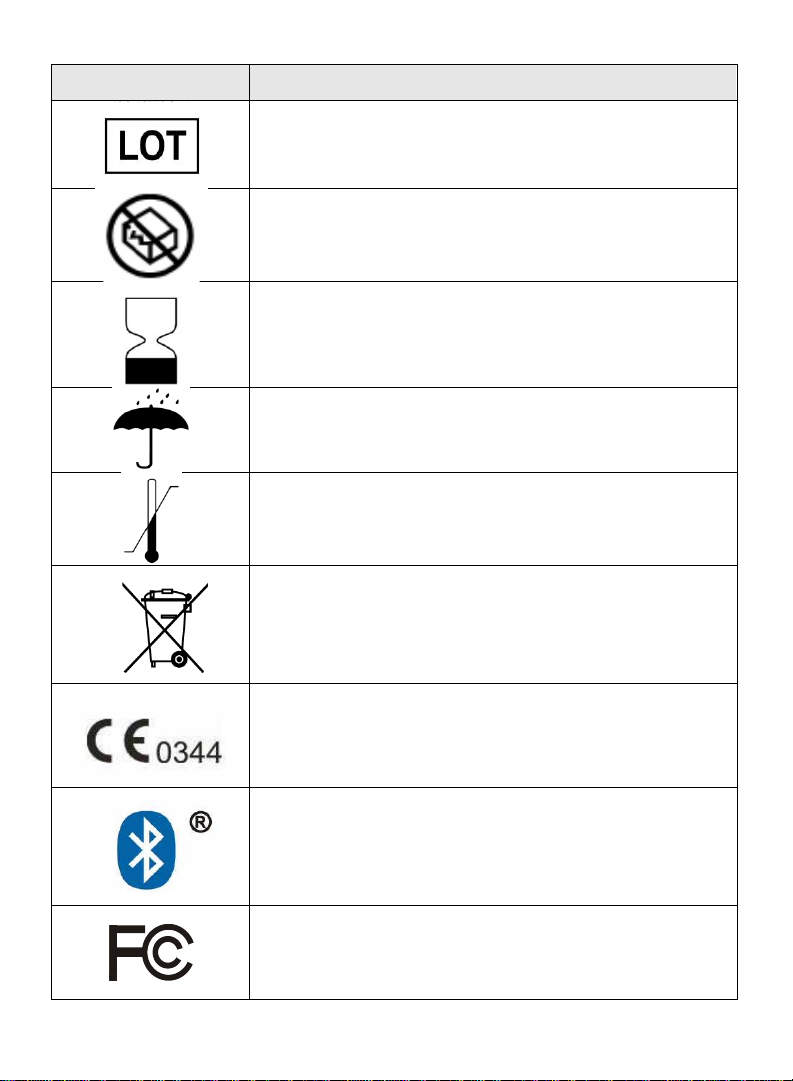
Label
Description
Batch code
Do not use if package is damaged
Use by
Keep dry
Store at specified temperatures
Electrical and Electronic Equipment
MDD (Medical Device Directive certification)
Bluetooth trademark indication conformity to
specifications.
Compliant with FCC Part 15
Page 23

ECG
Electrocardiogram; a representation of the
heart's electrical activity recorded from
electrodes on the body.
ACT
Ambulatory Cardiac Telemetry; Continuous
ECG Monitor and Arrhythmia Detector (sensor
and monitor)
ACT Ex
The service of collecting and analyzing
recorded ECG data (usually 24 hours) using the
ACT device.
Sensor
ACT device attached to patient
Monitor
Hand held device/cellular phone using ACT
monitoring software
Heart Rate
Number of heart beats per minute, measured
as bpm (beats per minute).
Bluetooth (BT)
Wireless communication protocol.
Monitoring
Center
Monitoring center responsible for reviewing
clinical data transmissions, and providing them
to the physician.
Charger
Power supply for recharging cell phone monitor
Disposable
electrode
(electrode patch)
Adhesive connector that connects the lead wire
to the body.
Glossary
Page 24

Arrhythmia
Irregular heartbeat
Manual event
Event manually recorded by a patient when
he/she feels it is necessary
Page 25

2. General Description
The ACT is an automatically activated cardiac monitoring
system that requires no patient intervention to capture or
transmit an arrhythmia when it occurs. When an arrhythmia is
detected, the ACT monitoring system utilizes an integrated cell
phone monitor to transmit the data to the Monitoring Center for
analysis.
Sensor
The sensor records and transmits the data to the cell phone
monitor. It can hold up to 6 hours (ACT III) or 2 hours (ACT I) of
data in its memory. This means that if a patient is away from the
cell phone monitor and wearing the sensor, the data is still being
recorded. Once the patient is in range (within 30 feet /10
meters) of the cell phone monitor, the data will be transmitted.
Cell Phone Monitor
The cell phone monitor receives ECG data from the sensor via
Bluetooth and can store up to 30 days of data. The cell phone
monitor has a special application that converts the raw ECG
data and sends it using a cellular network to the monitoring
center for interpretation or, in the case of no cellular coverage,
data can be sent using a landline and a modem. The cell phone
monitor should be carried in the supplied pouch whenever
possible.
Batteries
The sensor batteries are special batteries. DO NOT use over
the counter AA batteries. DO NOT dispose of the batteries –
they should be returned to LifeWatch when your monitoring
session is completed.
Page 26

About Bluetooth
Bluetooth is a wireless technology that enables the sensor and
cell phone monitor to communicate with each other. The ACT
system uses Bluetooth technology to transmit ECG data from
the sensor to the cell phone monitor. The cell phone monitor
internal Bluetooth component is on and running continuously
(24 hours a day) for the entire monitoring period; therefore, the
cell phone monitor must be ON at all times. Bluetooth operates
like a radio and is susceptible to interference. If the sensor and
cell phone monitor are more than 30 feet (10 meter) apart and a
Bluetooth connection cannot be made, no data loss will occur as
long as the sensor and cell phone monitor are reconnected
within 6 hours (ACT III) or 2 hours (ACT I). When the sensor
and cell phone monitor are within 30 feet (10 meter) of one
another, the Bluetooth will automatically re-connect. Charge the
phone every night for the whole night and whenever possible
throughout the day.
For optimal system performance, the recommended distances
between the cell phone monitor and sensor during the
monitoring period should be as follows:
Normal operation - within 20 inches (50 cm).
During cell phone monitor charging - within10 feet (3
meter)
Page 27

Note
The cell phone monitor automatically transmits the
detected ECG events to the monitoring center and the
user has the ability to send manually recorded events.
The data is saved on the storage card and the event
data is deleted after a successful transmission to the
monitoring center.
3. System Description
The ACT Continuous ECG Monitor and Arrhythmia Detector is
designed for self-testing by patients at home and for analysis by
trained technicians at a remote Monitoring Center.
The sensor is used for the acquisition and transmission of the ECG
signal. The sensor is equipped with three (ACT I) or four (ACT III)
electrodes on a harness and works in conjunction with a cell phone
monitor.
The sensor houses a 3.6V AA lithium-thionyl chloride battery, an ECG
channel circuit, an impedance measurement circuit, a pacemaker
detection circuit, a flash buffer memory, a Bluetooth transceiver and a
buzzer. The ECG signals are received, filtered and amplified in the
input circuit, stored in the flash memory buffer and transmitted via
Bluetooth to a cell phone monitor. The cell phone monitor runs a
proprietary application that is configured to process and transmit the
ECG recordings via a cellular network while storing them and the
detected physiological events on a micro-SD memory card. When a
physiological event is detected, the cell phone monitor transmits the
recorded ECG automatically via cellular link, to a Monitoring Center
for professional analysis. If the patient is out of the cellular network
coverage area, the cell phone monitor will send all events that were
stored when the cellular link is re-established. The cell phone monitor
can also transmit ECG alarms via landline telephone with an
additional POTS Bluetooth modem.
The sensor loops up to 2 hours (ACT I) or 6 hours (ACT III) cyclic
buffer of ECG data in the internal flash memory in order to preserve
the ECG in cases when the Bluetooth connection to the cell phone
monitor is down.
Page 28

1. ACT sensor with integrated or
detachable lead wires
2. ACT sensor batteries
(3.6 V AA lithium-thionyl chloride)
3. Disposable electrodes
4. User Guide
5. Carrying pouch
6. ACT monitor (cell phone)
7. Monitor charger
8. PSTN modem (optional)
9. Pre-paid return envelope
4. The ACT Kit
The ACT Kit provided may contain either the ACT 1-lead or ACT 3-lead
sensor, one cell phone monitor and all the accessories needed for use of
the system.
Contents
Page 29

Lead wires for
connecting the
electrodes.
1
2
1, 2 - Buttons for
manual recording
of an event.
The ACT I 1-lead Description
Figure 1. Front view of ACT I sensor
Battery cover closed Battery cover open
Figure 2. Rear view of ACT I sensor
Page 30

Lead wires for
connecting the
electrodes.
1
2
1, 2 - Buttons for
manual recording
of an event.
The ACT III 3-lead Description
Sliding Battery Cover
Figure 3. Front view of ACT III sensor (slide open battery cover)
Battery cover closed Battery cover open
Figure 4. Rear view of ACT III sensor
Page 31

Lead wires for connecting
the electrodes.
1
2
1, 2 - Buttons for
manual recording
of an event.
The ACT III 3-lead Description with Connectable Patient
Lead Wires
Flip Open Battery Cover
Figure 5. Front view of ACT III sensor (flip open battery cover)
Battery cover closed Battery cover open
Figure 6. Rear view of ACT III sensor
Page 32

HTC Ozone mobile
phone
HTC Ozone monitor
keys and buttons
Samsung SGH-i617
mobile phone
SGH-i617 monitor keys
and buttons
Navigation
key
Left soft
key
Right soft
key
Power
key
Cell Phone Monitor Information
One of the following cell phone monitors will be provided with the kit.
Page 33

Samsung SGH-i637
mobile phone
SGH-i637 monitor keys
and buttons
CAUTION
Please refer to the User Manual of the manufacturer of the
cell phone monitor for Health and Safety Information
pertaining to the use and operation of the cellular phone.
The cell phone monitor manual can be downloaded from
the internet.
CAUTION
Do not change the cell phone monitor settings.
Do not turn the cell phone monitor sound off.
Do not mute the volume so that it is inaudible.
Do not use the cell phone monitor for any reason
outside of the designated monitoring function
The ACT employs Bluetooth and cellular
technology. The location of the device and the
associated environment, including cellular phone
coverage in the particular area may cause
transmission loss or delay.
Page 34

Caution
Do not use the provided cell phone monitor for any
reason other than the designated monitoring function.
Note
First time use – Please call LifeWatch to receive
instructions on how to use for the first time, see “First
Time Activation” section.
Important Information Before Use
General
The recommended ambient temperature for use of the ACT System
sensor is between 50°F (10°C) and 104°F (40°C).
Remove the lead wires and the sensor before bathing.
The cell phone monitor and the sensor are not to be exposed to direct
water contact. The sensor and the cell phone monitor should not be in
the bathroom while bathing or showering.
Please consult your doctor or LifeWatch regarding the end of the
service.
You must take the fully charged cell phone monitor with you and wear
the sensor at all times (except when showering or bathing).
Page 35

Sensor Sound
Description:
1 beep, one-time
Sensor activated
1 long beep then short beep, every
ten minutes
Low battery level warning
1 long beep then short beep,
repetitive
Critical battery level error,
sensor stops recording
3 beeps, repetitive
Critical error, system stops
Continuous sensor sound
Manual event buttons pressed
Starting/Stopping the ACT Sensor
The insertion of the battery starts the sensor and connection to the cell
phone monitor via Bluetooth.
Removing the battery will stop the sensor and end the connection to the
cell phone monitor.
The ECG recording will start only after connection is established with the
cell phone monitor.
No Connection with Cell Phone Monitor
When the sensor disconnects from the cell phone monitor (Bluetooth
disconnection), the sensor will continue to record and store the ECG data
(2 hours for ACT I; 6 hours for ACT III).
In case the disconnection period is longer than the maximum recording
time, the sensor will store the LAST time period (2 hours for ACT I; 6
hours for ACT III) of the disconnection period.
Sensor Sound Prompts
The sensor will signal its status (through beeps), i.e., normal
operation, battery level, system errors, manual events.
Page 36

Note
Contact LifeWatch to receive instructions on using
the system for the first time as shown in “First Time
Activation”, section.
5. Using the ACT Monitoring System
Before Starting
Make sure you have all items needed to initiate the service:
ACT chest-worn sensor with battery and electrodes
ACT cell phone monitor with compatible application
installed.
Follow instructions for electrode placement.
(For ACT III with flip open battery cover) Make sure that the
sensor lead wire is well connected to the sensor.
See Troubleshooting, “Sensor lead wire disconnected”, for
instructions on reconnecting the lead wire.
Page 37

Note
There are physiological conditions that may affect the
cell phone monitor from detecting the electrode
connection and can last for several hours. Even
though the electrodes seem to be in good contact
with the body, the message will continue to appear.
Some examples are very dry skin or right after
attaching the electrodes. If the condition does not
resolve, contact LifeWatch.
Electrode Information
The ACT monitoring system comes with ECG lead wires that have
standard snap connectors for quick and easy connection/removal to
ECG electrodes. To ensure maximum safety and performance of the
ACT monitoring system, use only the ECG electrodes supplied with
the ACT monitoring system kit.
If electrodes irritate your skin, please contact LifeWatch for alternative
electrodes.
Do not apply electrodes to skin that is broken or irritated.
Hypoallergenic electrodes are available for patients with a history of
sensitivity and/allergy to adhesives. Hypoallergenic electrodes can be
requested by calling LifeWatch.
Skin Preparation
Wash the skin on your chest and abdomen using warm water
and gentle soap.
Remove excess hair by carefully clipping with scissors. Avoid
shaving in order to minimize irritation.
Make sure that your skin is clean and fully dry before
proceeding.
Do not apply electrodes to skin that is broken or irritated.
If you have any concerns contact LifeWatch.
Page 38

Snap each lead wire onto an
electrode.
Sensor with connected
electrodes.
Remove the electrodes from the
backing.
Electrode Placement
1. Place the electrodes as shown in Figures 7 (ACT I) and 8
(ACT III).
2. The electrodes should be replaced periodically, according to
the electrode's manufacturer instructions as described below.
Preparing Electrodes (three for ACT 1 and four for ACT III)
Page 39

Attach the white lead wire with electrode under the second right
rib. (3 fingers below the collarbone) towards the breastbone
(center).
Attach the red lead wire with electrode on the lower right portion
of your chest.
Attach the black lead wire with electrode to the lower left portion
of your chest, between the fifth and sixth ribs.
ACT I Electrode Placement
Figure 7. ACT I Electrode Placement
Page 40

Attach the white lead wire with electrode under the second right
rib. (3 fingers below the collarbone) towards the breastbone
(center).
Place the red lead wire with electrode in a direct line from the
end of the breastbone that intersects with the imaginary line that
extends down from the midpoint of the collarbone, as shown in
ACT III Electrode Placement figure.
Place the black lead wire with electrode at the point where a
horizontal line from the red electrode intersects with the middle
armpit line.
Note: For the red and black lead wires, if necessary lift the left breast to
place the electrode properly.
Place the green lead wire with electrode on the left side of the
abdomen.
ACT III Electrode Placement
Figure 8. ACT III Electrode Placement
Page 41

Changing Electrodes
Replace electrodes (sticky patches) every three days or when
the adhesive is not firmly attached to the skin.
Check the electrodes daily by pulling gently to verify that the
adhesive is firmly attached to your skin. If an electrode is not
firmly attached, change all the electrodes per the instructions.
Disconnect the lead wire from the electrode.
Gently remove electrodes, by grasping the edge/tab of the
electrode and peeling back in a slow continuous motion. You
may want to remove the electrodes in the shower or by using a
wet, warm washcloth. Do not rapidly remove the electrode to
avoid skin damage.
Remove any excess adhesive or dead skin between electrode
changes, using a wet, warm washcloth or gauze pad.
Completely dry the skin before attaching new electrodes.
If electrodes become dislodged/loose prior to 3 days, replace
all electrode(s) per instructions above.
Minor skin irritation can occur with electrode use. If skin
irritation occurs, do not re-apply electrode to the immediate
area. Apply in close proximity to that location on healthy skin.
If irritation worsens or becomes severe, contact LifeWatch.
Page 42

CAUTION:
1) Use only batteries supplied by LifeWatch for the sensor.
2) Replace the sensor battery only when the sensor and the
cell phone monitor are within 20 inches (50 cm) of each
other, so that a connection can be established. A low
sensor battery message begins when there are up to
three hours before the battery fails. As soon as you
replace the sensor battery, the sensor and cell phone
monitor will connect and the sensor will download stored
ECG data to the cell phone monitor. After the download is
completed, the sensor will start recording again. If for
some reason connection is not established within 5
minutes, please call LifeWatch.
3) If the sensor battery is replaced when the sensor is out of
Bluetooth range from the cell phone monitor, the sensor
will not be able to record (including manual events) until
the sensor is within Bluetooth range of the cell phone
monitor. Once in range, the connection will be renewed
after a few minutes.
4) If the sensor battery has been changed while
disconnected from the cell phone monitor and within the
maximum recording time of the sensor (ACT I up to 2
hours; ACT III up to 6 hours) then upon reconnection to
the cell phone monitor the sensor will download the stored
data to the cell phone monitor and will not record until the
download is finished.
5) If the sensor battery (ACT I firmware version 0.1g and
ACT III firmware version 1.0.4) has been changed, while
disconnected from the cell phone monitor and after the
maximum recording time of the sensor (ACT I up to 2
hours; ACT III up to 6 hours) then upon reconnection to
the cell phone monitor the sensor will start a new
recording that will immediately overwrite the stored data
(stored data not downloaded).
Sensor Battery Insertion / Replacement
Page 43

6) Place any used and unused batteries in the return
envelope provided in the kit and return to LifeWatch at the
end of your monitoring period.
Note
For a visually or hearing impaired patient, replace the
sensor battery EVERY 48 HOURS.
Figure 9.
Removing Battery
Cover
ACT with Slide-off Cover
1. Change the sensor battery when the following occurs:
1. The sensor beeps two times, 1 long beep then 1 short beep.
2. A “Low sensor battery” message is displayed on the cell
phone screen
2. Place sensor within 20 inches (50 cm) of the cell phone monitor,
place the battery in the sensor:
a. Place your thumb on the depression of the battery cover and
gently push the battery cover backwards, until the battery
cover is free.
b. Set the battery cover aside.
Page 44

c. Remove a new battery from the plastic bag, place the new
battery in the battery compartment (if a battery is already in
place, remove the existing battery).
d. Pay careful attention to the battery polarity marks inside the
battery compartment (plus sign on the neck strap side, minus
sign on the sensor lead wire side). The sensor should beep
once within 10 seconds of placing a new battery. If no sound is
heard after ten seconds, verify that the battery has been
placed in the correct position. If the position is correct, try
another battery. If the second battery fails, contact LifeWatch.
Figure 10. Replacing Battery
Page 45

Figure 11.
Replacing Battery
Cover
Note
Before changing the sensor battery, make sure
the cell phone monitor is within range 20 inches
(50 cm).
If you are missing batteries, contact LifeWatch.
e. Place the battery cover back on the sensor as it was before it
was removed. Put your thumb on the depression of the battery
cover and gently push the battery cover towards the neck strap
until you hear a click indicating the battery cover is now in
place (see Figure 11).
f. It may take up to 5 minutes for the cell phone monitor to
display the default recording screen after inserting a new
battery.
3. Connect the sensor to the neck strap (if it was not attached).
Page 46

Figure 12.
Opening Battery
Cover
ACT III with Flip Open Battery Cover
1. Place sensor within 20 inches (50 cm) from the cell phone monitor,
place the battery in the sensor:
a. Hold the ACT sensor with the battery compartment facing up.
Press the orange button on the upper part of the ACT sensor
to release the battery compartment cover.
NOTE: Do not attempt to remove the battery cover. If the battery
cover becomes separated from the device please refer to the
troubleshooting section for replacing the battery cover.
b. Remove a new battery from the plastic bag, place the new
battery in the battery compartment (if a battery is already in
place, remove the existing battery using the battery strap).
Page 47

c. Pay careful attention to the battery polarity marks inside the
battery compartment (plus sign on the neck strap side, minus
sign on the sensor lead wire side). The sensor should beep
once within 10 seconds of inserting a new battery. If no sound
is heard after ten seconds, verify that the battery has been
place in the correct position. If the position is correct, try
another battery. If the second battery fails, contact LifeWatch.
Figure 13. Replacing Battery
d. Gently push the battery cover down towards the neck strap
(battery strap should be under the battery cover) until you hear
a click indicating the battery cover is in place.
e. It will take up to 5 minutes for the cell phone monitor to display
the default recording screen.
2. Connect the sensor to the neck strap (if it was not attached).
Page 48

Cell Phone Monitor Placement
For optimal system performance, the recommended distances
between the cell phone monitor and sensor should be as
follows:
Within 20 inches (50 cm) for normal operation
Within 10 feet (3 meters) during cell phone monitor charging
Within 30 feet (10 meters) maximum distance
Page 49

Note
The cell phone monitor battery energy consumption
in the first few days (up to 72 hours) of monitoring
may be high. Always have a charged cell phone
monitor and the charger with you during this time.
Cell Phone Monitor Recharging Procedure
Charge the cell phone monitor whenever possible during the day. In
addition, charge the monitor every night (regardless of battery
indicator status), making sure that it is within 10 feet (3 meters) of the
sensor.
1. Plug the cell phone monitor charger power cord into the power socket
on the cell phone monitor (see Figures 14 to 16 for location).
2. Plug the supplied cell phone monitor charger unit into a standard wall
outlet.
A red or yellow light (color depends on cell phone monitor type)
indicating charging is needed will appear on the cell phone monitor
when the charger is properly connected. When charging is complete,
the light will turn green.
3. Disconnect the cell phone monitor charger from the wall outlet and
then from the cell phone monitor.
Page 50

HTC Ozone mobile
phone
Connection for power
supply
Power button
Samsung
SGH-i617 mobile
phone
Connection for
power supply
Power button
Figure 14. HTC Ozone monitor power button and socket
Figure 15. SGH-i617 monitor power button and socket
Page 51

Samsung
SGH-i637 mobile
phone
Connection for
power supply
Power button
Figure 16. SGH-i637 monitor power button and socket
Page 52

After a few seconds the
screen on the cell phone
monitor will display the ACT
opening page.
After a few seconds the
application will start
automatically.
First Time Activation
The first time the ACT monitoring system is activated, it performs a
baseline capture and calibration procedure. The screens you see
during this process will only be seen once when the cell phone
monitor is turned on for the first time.
1. Connect the sensor electrodes (as shown in Figures 7 and 8).
2. Turn on the cell phone monitor by pressing the POWER button of
the cell phone monitor for 5 seconds until the screen lights up.
Page 53

Note
ACT III is used as an example for this section.
3. When the welcome screen comes up, press Ok using the right soft
key to continue or Exit to close the program.”
4. Place the battery in the sensor.
5. The cell phone monitor will display the following screen after the
first connection is established (which can take up to five minutes).
A picture of a beating heart will appear and will continue to appear
as long as the ACT application is running. It is not a representation
of your actual heartbeat.
Page 54

Note
Step 1 and Step 2 may be performed in parallel by
the system and the Step 2 screen may not be
displayed.
6. Step 1 – Data collection and sending baseline recording. Remain
relatively still, without moving or exerting yourself until the baseline
recording is sent.
7. When Step 1 is finished the monitoring screen will be displayed for
a few seconds, then Step 2 will be displayed.
Page 55

Note
If you have any questions on the use of the ACT
Monitoring System, please contact LifeWatch.
CAUTION
Take a charged cell phone monitor with you and
wear the sensor at all times during the monitoring
period (except when showering or bathing).
8. Step 2 – Analyzing electrode contact. The electrode contact is
being checked and will be used for calibration. This process may
take up to ten minutes.
9. At the end of Step 2 the success message will be displayed and
the service will begin automatically.
Page 56

After a few seconds the
screen on the cell phone
monitor will display the ACT
opening page.
Monitoring Period
This section describes the steps that you need to know if you exit the
ACT application after first time activation and additional information
about the monitoring period.
The recommended distance between the cell phone monitor and
sensor is within 20 inches (50 cm) (see Cell Phone Monitor Placement
section).
NOTE: verify the sensor lead wire is connected to the sensor, as
described in the Troubleshooting section (for ACT III with Flip Open
Battery Cover).
1. Connect the sensor electrodes (as shown in Figures 7 and 8).
2. Turn on the cell phone monitor, press and hold the POWER button
of the cell phone monitor for 5 seconds until the screen lights up.
3. After a few seconds the screen on the cell phone monitor will
display the ACT opening pages.
Page 57

After a few seconds the
application will start
automatically.
4. The following screens will be displayed.
ACT III
ACT I
Page 58

Caution
The ACT application should not be closed during
the monitoring period.
5. Please place the battery in the sensor (as shown in Sensor Battery
Insertion / Replacement section).
After the connection is established between cell phone monitor and
sensor (which can take up to five minutes), the heart icon will be
pulsing indicating the system is running (does not represent
patient’s actual pulse).
If the application needs to be restarted, press the Start button to
display the cell phone monitor desktop to manually start the
application using the icon.
Page 59

Note
ACT Ex icon may be
displayed during the service
period.
Note
If there is a problem with the cellular communication
or CG-3800BT modem (if in use), the device will
continue to function and store the recordings for
sending later when the problem is resolved.
REMEMBER: The events that are recorded automatically will also
be transmitted automatically while the application is running.
Page 60

CAUTION:
The manual event recording is not available during the
baseline process, even though pressing the manual event
buttons will initiate the manual event recording beep.
After sensor battery insertion and before connection to the
cell phone monitor or during the process of downloading
data from the sensor (reconnection after battery
replacement) manual event recording is not available.
Press the sensor side
buttons simultaneously,
then release the buttons
when there is a beep.
Figure 17. Sensor Manual Recording
Manual Event Recording
If you experience dizziness, fatigue, chest pain or any other symptom,
record a manual event.
There are two options for recording a manual event, by pressing the
sensor side buttons or by pressing the cell phone monitor manual
event button.
When you record a manual event, the ACT application will display a
series of screens and a “doorbell” sound will be heard on the cell
phone monitor. The screen will return to the normal display when
finished.
First Option Recording a Manual Event with the Sensor
The cell phone monitor’s manual event screen will be displayed after
a few seconds (when connected to the cell phone monitor).
Page 61

Note
When there is a disconnection between the sensor and
the cell phone monitor the manual event will be
transmitted when there is reconnection to the cell phone
monitor.
Select and mark the applicable symptom(s) then press the Send
button to send the form. If your symptom is not on the list, select and
mark “Other” and send the form. If you don’t have any symptom,
select and mark “No Symptoms” and send the form.
Scroll
If “Send” is not pressed the manual event will be automatically sent at
the end of the countdown without the form. The cell phone monitor
screen will return to the normal operation screen.
Page 62

Press the cell phone monitor right
soft key to initiate a Manual Event
recording.
Second Option Recording a Manual Event with the cell
phone monitor
If you have pressed the cell phone monitor’s Manual Event button the
following confirmation screen will be displayed on the cell phone
monitor. To send the manual event press Yes or press No to cancel
the manual event (will resume to normal operation).
This screen disappears after a few seconds if no button was pressed
and the event will not be sent.
Page 63

When Yes has been pressed, the manual event form will be
displayed. Select and mark the applicable symptom(s) then press the
Send button to send the form. If your symptom is not on the list, select
and mark “Other” and send the form. If you don’t have any symptom,
select and mark “No Symptoms” and send the form.
Scroll
If “Send” is not pressed the manual event will be automatically sent at
the end of the countdown. The cell phone monitor screen will return to
the normal operation screen.
Page 64

Note
Situations when it is not possible to record a manual
event with the cell phone monitor.
During the process of collecting
manual event data it is not
possible to initiate a new manual
event.
When the sensor and the cell
phone monitor are disconnected
for any reason, the manual event
can be recorded with the sensor.
It will be transmitted when the
connection to the cell phone
monitor is reestablished.
Disconnections may occur if the
sensor and cell phone monitor
are out of range as described in
the Cell Phone Monitor
Placement section or
immediately following sensor
battery replacement.
Page 65

During the process of
downloading data from the
sensor (reconnection after
battery replacement), manual
event recording is not available.
Page 66

Sensor battery low level
Cell phone monitor battery low level
Sensor (Bluetooth) disconnection
Cell phone monitor transmission problem
Electrode connectivity
Code Messages
Note - It is possible for
multiple messages to be
displayed on the cell phone
monitor screen. The message
will not be displayed when the
correction has been
registered.
6. Cell Phone Monitor Messages
Messages inform you about a problem that might occur during
operation of the ACT monitoring system. The message may include
steps to resolve the problem (cellular coverage may resolve by itself).
A message screen may be closed (by pressing OK), but if the
underlying problem has not been resolved it will be indicated on the
cell phone monitor screen.
The message will be accompanied by a sound prompt and cell phone
monitor vibration (optional). It might take a few minutes to remove a
message after a correction has been resolved.
Message Types
Page 67

“Sensor battery needs to be
replaced” screen, replace
battery and press OK to
continue.
After inserting the new
battery it will take up to five
minutes for the system to
register the change and
display the default recording
screen.
This screen is displayed
until the change is
registered.
Sensor Battery Low
The low battery message appears when the sensor battery needs to
be replaced. Please refer to “Sensor Battery Insertion / Replacement”,
section, for information on how to replace the battery.
Be aware that the sensor alerts its low battery status with 1 long beep
then a short beep from the sensor.
Page 68

“Cell phone monitor low
battery” message, press OK
and charge the cell phone
monitor.
Screen displayed until cell
phone monitor is connected
to charger and inserted in
the wall outlet
Cell phone monitor low
battery message that can be
initiated from the cell phone
monitor operating system.
Press OK and charge the
cell phone monitor.
Cell Phone Monitor Battery Low
The cell phone monitor low battery message appears when the cell
phone monitor needs to be charged. Please refer to “Cell Phone
Monitor Charging Procedure” section for information on how to charge
the cell phone monitor.
Page 69

“Connection lost between
the sensor and the cell
phone monitor” screen,
make sure the cell phone
monitor and sensor are
within 20 inches (50 cm)
and then press OK to
continue.
Screen displayed until
connection is
re-established.
Sensor (Bluetooth) Disconnection
The sensor disconnection message appears when there is Bluetooth
disconnection from the cell phone monitor.
Be aware that a disconnection can be caused by the sensor being in
low battery mode (accompanied by 1 long beep then a short beep
from the sensor), please refer to “Sensor Battery Low” section.
Page 70

Make sure you have
good cellular coverage
by checking the cellular
signal strength in the
upper right corner and
press OK to continue.
This screen will be
displayed until the
communication issue
has been resolved.
Cell Phone Monitor Transmission Problem
The cell phone monitor transmission message appears when there
are transmission problems that could be caused by poor cellular
coverage, cellular network problems or communication problems with
the CG-3800BT modem (if modem is in use).
Page 71

Electrode connectivity message
Screen after pressing OK.
The screen will return to normal
operation after problem is solved.
Note - There are physiological conditions that may affect
the cell phone monitor from detecting the electrode
connection and can last for several hours. Even though
the electrodes seem to be in good contact with the body,
the message will continue to appear. Some examples
are very dry skin or right after attaching the electrodes. If
the condition does not resolve, contact LifeWatch.
Electrode Connectivity
The electrode connection message appears when there are problems
with the contact of one or more electrodes.
Please check the electrode connections when you see these screens.
When the electrodes are reconnected, it will take up to 3 minutes to
update the screen (assuming that the Bluetooth link is up).
Electrode connectivity message screen examples:
Please follow these instructions to ensure proper contact of the
electrodes:
1) Make sure that the sensor lead wires are properly connected to the
electrodes.
2) Make sure the electrodes are properly attached.
Please refer to “Electrode Placement” section for further information.
Page 72

Black electrode problem
White electrode problem
Red (or all) electrode(s) problem
Correct the problem and press
OK.
Electrode Messages for ACT I
Page 73

White electrode problem
Black electrode problem
Red electrode problem
Electrode Messages for ACT III
Page 74

Red and black electrodes
problem
Note
When two electrodes (white, red or black) have
problems there will be a message indicating which two
electrodes need to be checked.
Green (or all) electrode(s)
disconnected or lead wire is
disconnected from sensor (ACT
III sensor with flip-open cover)
Correct the problem and press
OK.
Page 75

Code Messages
Code messages are displayed on the cell phone monitor screen
whenever an unexpected situation occurs. Please follow the
instructions displayed in the screen. See Appendix B for a list of the
message codes.
Code Message example
Page 76

CAUTION
Do not open or attempt to repair the sensor or cell phone
monitor yourself. Only authorized service personnel may
repair the product.
Do not drop your sensor or cell phone monitor or subject
them to severe impact.
Do not bend the sensor or the sensor lead wire.
Do not use extreme force when pressing the sensor
buttons.
Do not use solvents to clean your ACT sensor or cell
phone monitor.
Do not spray perfume or other substances on the sensor.
The ACT sensor is not waterproof. Do not use it or store
it where fluids such as water can splash onto it.
Raindrops, water spray, juice, coffee, steam,
perspiration, etc. may also cause a malfunction.
Do not expose the sensor batteries or battery contacts to
fluids such as water, raindrops, water spray, juice,
coffee, steam, perspiration, etc.
Do not shower, bathe or swim with the cell phone
monitor or the sensor. Keep the cell phone monitor and
the sensor out of the bathroom when showering or
bathing.
7. Maintenance
Conditions of Use
Your ACT system conforms to international standards as long as it is
used under normal conditions and in accordance with the following
instructions.
Caring for your ACT
Page 77

Environment
Keep the ACT monitoring system away from extreme heat and
cold. Do not leave any part on the dashboard of a car or near a
heater.
Do not leave any part of the ACT monitoring system in any
place that is wet, damp or dusty.
Preventive Maintenance
The following simple preventive maintenance tasks should be performed
monthly to ensure continued performance of the device at maximum
capacity, and to reduce the possibility of a failure.
Mechanical Inspection
Check for splits, cracks or other related flaws in the ACT monitoring
system. If you have any questions or doubts, call LifeWatch.
Cleaning
To clean the outside of the ACT monitoring system use a lint-free cloth
lightly moistened with isopropyl alcohol.
Never use abrasives such as wire wool or metal polish.
During cleaning, make sure you do not expose the device to
temperatures in excess of 113°F (45°C).
Page 78

Problem
Possible Cause(s)
Solution
No beep when
the sensor is
activated (up to
10 seconds after
battery
inserted).
Battery not placed
properly/reversed
polarity.
The battery is very
low or depleted.
1) Check the battery
placement and
polarity.
2) Sensor battery needs
to be changed, refer to
“Sensor Battery
Insertion/Replacement
” section, for
information on how to
replace the battery.
Displays an
electrode
problem
(message).
Electrode connection
does not provide
proper contact
(loose, disconnected
or bad contact with
skin).
1) Make sure that the
sensor lead wire is
properly connected to
the electrodes and
sensor (for attachable
leads).
2) Make sure the
electrodes are
properly attached to
the skin.
3) There are
physiological
conditions that may
affect the cell phone
monitor from detecting
the electrode
connection and can
last for several hours.
8. Troubleshooting
Please follow the steps listed below in order to resolve the problem.
If this does not solve the problem contact LifeWatch.
Page 79

Problem
Possible Cause(s)
Solution
Even though the
electrodes seem to be
in good contact with
the body, the message
will continue to
appear.
Some examples are
very dry skin or right
after attaching the
electrodes.
4) Please refer to
“Electrode Placement”
section for further
information.
Sensor
disconnection
message.
Lack of
communication
between sensor and
cell phone monitor.
Sensor low battery
can cause
disconnection
(accompanied sound
prompt of 1 long
beep then a short
beep from the
sensor).
1) Make sure the sensor
and cell phone monitor
are within 20 inches
(50 cm).
2) Sensor battery needs
to be changed, please
refer to “Sensor
Battery
Insertion/Replacement
” section for
information on how to
replace the sensor
battery.
3) Cell phone monitor
needs to be
recharged, please
refer to “Cell Phone
Monitor Recharging
Procedure” section for
Page 80

Problem
Possible Cause(s)
Solution
information on how to
recharge the cell
phone monitor.
Cell phone
monitor low
battery
message.
The cell phone
monitor low battery
message appears
when the cell phone
monitor needs to be
recharged.
The cell phone monitor
needs to be recharged,
please refer to “Cell
Phone Monitor
Recharging Procedure”
section for information
on how to recharge the
cell phone monitor.
Sensor beeps 1
time.
Sensor activated.
Normal operation
Cell phone
monitor
communication
message.
The cell phone
monitor transmission
message appears
when there are
transmission
problems that could
be caused by poor
cellular coverage,
cellular network
problems or
communication
problems with the
CG-3800BT modem
(if modem is in use).
1) Make sure you have
good cellular
coverage.
2) Make sure you are
within 30 feet (10 m )
of the cell phone
monitor and CG3800BT modem (if in
use).
3) Cell phone monitor
needs to be charged,
please refer to “Cell
Phone Monitor
Charging Procedure”
section for information
on how to charge the
cell phone monitor.
Sensor beeps 3
times, repetitive.
Critical system error.
1) Remove sensor
battery for 30
Page 81

Problem
Possible Cause(s)
Solution
seconds; then reinsert battery. Allow up
to 5 minutes for
sensor and cell phone
monitor to reconnect.
2) Make sure that the
sensor lead wire is
properly connected to
the sensor (for
attachable leads).
Sensor beeps
1 long beep
then short beep.
Sensor battery low or
critical.
Sensor battery needs to
be replaced, please refer
to “Sensor Battery
Insertion/Replacement”
section, for information
on how to replace the
battery.
Page 82

Problem
Possible
Cause(s)
Solution
Battery cover
separates from
device
Note: Relevant
to ACT III Flip
Open Battery
Cover ONLY
Replace
battery
cover
1) Place battery cover on device in
the proper place (see picture A
and B)
2) Press down firmly on battery
cover at point (1) as shown in
picture C until you hear a click.
4) Press down firmly on battery
cover at point (2) in picture D
until you hear a click.
Picture A
Picture C
Picture B
Picture D
Battery Cover Properly Placed
1
2
Page 83

Problem
Possible Cause(s)
Solution
Sensor lead
wire
disconnected
NOTE: for ACT
III with Flip
Open Battery
Cover
Sensor lead wire
detached from
sensor
Verify the sensor lead
wire is connected to the
ACT sensor socket by
first aligning the arrow
on the lead wire (1) with
the arrow on the ACT
sensor (2) and then push
firmly. This will make the
proper connection.
2
1
Page 84

9. Technical Specifications
IMPORTANT
This device complies with Part 15 of the FCC Rules. Operation is
subject to the following two conditions:
(1) This device may not cause harmful interference, and
(2) This device must accept any interference received, including
interference that may cause undesired operation.
Declaration of Conformity
Conformance to Standards – non-clinical testing demonstrated
conformance to voluntary Safety standard IEC 60601-1:1998
(with Amendments 1 & 2)
and to EMC standard IEC 60601-1-2:2001 Class B
Card Guard’s Quality System conforms to ISO-9001:2008,
ISO 13485:2003, and complies with CE MDD requirements
Tested for compliance with FCC 47 CFR Part 15,
subpart B and subpart C
Page 85

Parameter
Min
Max
Typical
Units
Input Impedance
19.5
20.5
20
MΩ
Input dynamic range
+/- 5
mV
Average current
consumption
4
12
6.5
mA
Peak current
Consumption
N/A
80
75
mA
CMRR
75
dB
ADC sample rate
250
250
250
samples/sec
DC offset correction
150
|mV|
LPF cutoff frequency
61.8
90.45
76
Hz
HPF cutoff frequency
.043
.065
.005
Hz
Impedance
measurement range
0
793
KΩ
Arrhythmia algorithm
detection voltage range
0.4 5
|mV|
System noise
32
μV
Pacemaker pulse width
marking
0.2 2
msec
Pacemaker pulse
amplitude marking
2
250
mV
ECG data buffer
32
MBit
ECG buffering time
2:19
Hrs
Manual ECG event
triggering
Yes
PM detection
Yes
ACT I Sensor Technical Specifications
Page 86

Parameter
Min
Max
Typical
Units
Bluetooth transmission
range - open space
N/A
20
10
meters
Bluetooth protocol
SPP profile, Sniff mode, Autoconnection mode
Battery type
3.6V lithium-thionyl chloride AA
Battery life (dependent
upon Bluetooth
connectivity)
2 4
Days
MTBF (hours)
36,305
Hrs
Operating temperature
10
(50)
40
(104)
°C (°F)
Transport & storage
temperature
-20
(-4)
65
(149)
°C (°F)
Relative humidity
(non-condensing)
30
85 %
Dimensions (max.)
75 x 58
x 23
mm
Net weight (w/o battery)
42.2
gr.
Page 87

Parameter
Min
Max
Typical
Units
Input operating DC
voltage
3.0
3.6
3.5
V
Input Impedance
19.5
20.5
20
MΩ
Input dynamic range
+/-
4.5
+/-
5.5
+/- 5
mV
Average current
consumption
5
25
15
mA
Peak current
consumption
N/A
80
75
mA
CMRR
60
N/A
75
dB
ADC sample Rate
246
254
250
samples/sec
DC offset correction
0
+/150
+/-115
mV
LPF cutoff frequency
99.6
157.9
115.7
Hz
HPF cutoff frequency
.035
.055
.041
Hz
Impedance
measurement range
0
793
N/A
KΩ
Arrhythmia algorithm
detection voltage
range
0.4 5 N/A
mV
System noise
0
50
35
μV
Pacemaker pulse width
marking
0.2 2 N/A
msec
Pacemaker pulse
amplitude marking
2
250
N/A
mV
ECG data buffer
(storage memory)
32
32
32
MB
ACT III Sensor Technical Specifications
Page 88

Parameter
Min
Max
Typical
Units
ECG buffering time
6:13
N/A
6:19
Hours:Minutes
Manual ECG event
triggering
N/A
N/A
Yes
None
PM detection
N/A
N/A
Yes
None
Bluetooth
Transmission range open space
N/A
20
10
meters
Bluetooth protocol
SPP profile, Sniff mode,
Auto-connection mode
None
Battery type / output
lithium-thionyl chloride
AA
N/A
Battery life (dependent
upon Bluetooth
connectivity)
2
3
Use
dependent
Days
MTBF (hours)
N/A
N/A
26,058
Hours
Operating temperature
10
(50)
40
(104)
N/A
°C (°F)
Transport & storage
temperature
-20
(-4)
65
(149)
N/A
°C (°F)
Relative humidity
(non-condensing)
30
85
N/A
%
Dimensions (max.)
N/A
N/A
75 x 58 x
23
mm
Net weight (w/o
battery)
55
60
57
gr.
Page 89

10. Appendix A Monitor (Cellular Phone)
Warnings
Using Your Phone Near Other Electronic Devices
Most modern electronic equipment is shielded from radio frequency
(RF) signals.
However, certain electronic equipment may not be shielded against
the RF signals from your wireless phone. Consult the manufacturer to
discuss alternatives.
Implantable Medical Devices
A minimum separation of six (6) inches (15 cm) should be maintained
between a handheld wireless phone and an implantable medical
device, such as a pacemaker, to avoid potential interference with the
device.
Persons who have such devices:
• Should ALWAYS keep the phone more than six (6) inches (15 cm)
from their implantable medical device when the phone is turned ON;
• Should not carry the phone in a breast pocket;
• Should use the ear opposite the implantable medical device to
minimize the potential for interference;
• Should turn the phone OFF immediately if there is any reason to
suspect that interference is taking place.
• Should read and follow the directions from the manufacturer of your
implantable medical device. If you have any questions about using
your wireless phone with such a device, consult your health care
provider.
Hearing Aid Compatibility with Mobile Phones
FCC Hearing-Aid Compatibility (HAC) Regulations for Wireless
Devices
On July 10, 2003, the U.S. Federal Communications Commission
(FCC) Report and Order in WT Docket 01-309 modified the exception
Page 90

of wireless phones under the Hearing Aid Compatibility Act of 1988
(HAC Act) to require digital wireless phones be compatible with
hearing-aids. The intent of the HAC Act is to ensure reasonable
access to telecommunications services for persons with hearing
disabilities.
While some wireless phones are used near some hearing devices
(hearing aids and cochlear implants), users may detect a buzzing,
humming, or whining noise. Some hearing devices are more immune
than others to this interference noise, and phones also vary in the
amount of interference they generate.
The wireless telephone industry has developed a rating system for
wireless phones, to assist hearing device users find phones that may
be compatible with their hearing devices. Not all phones have been
rated. Phones that are rated have the rating on their box or a label
located on the box.
The ratings are not guarantees. Results will vary depending on the
user’s hearing device and hearing loss. If your hearing device
happens to be vulnerable to interference, you may not be able to use
a rated phone successfully. Trying out the phone with your hearing
device is the best way to evaluate it for your personal needs.
M-Ratings: Phones rated M3 or M4 meet FCC requirements and are
likely to generate less interference to hearing devices than phones
that are not labeled. M4 is the better/higher of the two ratings.
T-Ratings: Phones rated T3 or T4 meet FCC requirements and are
likely to be more usable with a hearing aid’s telecoil than phones that
are not rated. T4 is the better/higher of the two ratings.
Hearing devices may also be rated. Your hearing device manufacturer
or hearing health professional may help you find this rating. Higher
ratings mean that the hearing device is relatively immune to
interference noise. The hearing aid and wireless phone rating values
are then added together. A sum of 5 is considered acceptable for
normal use. A sum of 6 is considered for best use.
In the above example, if a hearing aid meets the M2 level rating and
the wireless phone meets the M3 level rating, the sum of the two
values equal M5. This should provide the hearing aid user with
“normal usage” while using their hearing aid with the particular
Page 91

wireless phone. “Normal usage” in this context is defined as a signal
quality that is acceptable for normal operation.
The M mark is intended to be synonymous with the U mark. The T
mark is intended to be synonymous with the UT mark. The M and T
marks are recommended by the Alliance for Telecommunications
Industries Solutions (ATIS). The U and UT marks are referenced in
Section 20.19 of the FCC Rules. The HAC rating and measurement
procedure are described in the American National Standards Institute
(ANSI) C63.19 standard.
Some digital wireless phones may interfere with some hearing aids. In
the event of such interference, you may wish to consult your hearing
aid manufacturer to discuss alternatives.
Other Medical Devices
If you use any other personal medical devices, consult the
manufacturer of your device to determine if it is adequately shielded
from external RF energy. Your physician may be able to assist you in
obtaining this information. Switch your phone off in health care
facilities when any regulations posted in these areas instruct you to do
so. Hospitals or health care facilities may be using equipment that
could be sensitive to external RF energy.
Children Using Wireless Phones
The scientific evidence does not show a danger to users of wireless
phones, including children and teenagers. If you want to take steps to
lower exposure to radio frequency energy (RF), the measures
described above would apply to children and teenagers using wireless
phones. Reducing the time of wireless phone use and increasing the
distance between the user and the RF source will reduce RF
exposure.
Some groups sponsored by other national governments have advised
that children be discouraged from using wireless phones at all. For
example, the government in the United Kingdom distributed leaflets
containing such a recommendation in December 2000. They noted
Page 92

that no evidence exists that using a wireless phone causes brain
tumors or other ill effects. Their recommendation to limit wireless
phone use by children was strictly precautionary; it was not based on
scientific evidence that any health hazard exists.
Body-worn Operation
To comply with RF exposure requirements, a minimum separation
distance of 0.50 inch (1.5 cm) must be maintained between the user’s
body and the handset, including the antenna. Third-party belt-clips,
holsters, and similar accessories used by this device should not
contain any metallic components. Body-worn accessories that do not
meet these requirements may not comply with RF exposure
requirements and should be avoided.
Use only the supplied or an approved antenna. Unauthorized
antennas, modifications, or attachments could impair call quality,
damage the phone, or result in violation of regulations. Do not use the
phone with a damaged antenna. If a damaged antenna comes into
contact with the skin, a minor burn may result. Please contact your
local dealer for replacement antenna.
Page 93

Message displayed
CODE 201
Please call LifeWatch at 1-800-517-6330
CODE 211
To resolve: Turn phone OFF and then ON
If not resolved call LifeWatch at 1-800-517-6330
CODE 212
Monitoring has not started
Please call LifeWatch at 1-800-517-6330 to begin monitoring
CODE 213
To resolve: Turn phone OFF and then ON
If not resolved call LifeWatch at 1-800-517-6330
CODE 220
Please call LifeWatch at 1-800-517-6330
CODE 221
Please call LifeWatch at 1-800-517-6330
CODE 222
Please call LifeWatch at 1-800-517-6330
CODE 223
Please call LifeWatch at 1-800-517-6330
CODE 230
Please call LifeWatch at 1-800-517-6330
CODE 240
To resolve: Turn phone OFF and then ON
If not resolved call LifeWatch at 1-800-517-6330
CODE 241
To resolve: Turn phone OFF and then ON
If not resolved call LifeWatch at 1-800-517-6330
CODE 242
11. Appendix B Message Codes
Page 94

To resolve: Turn phone OFF and then ON
If not resolved call LifeWatch at 1-800-517-6330
CODE 250
Please call LifeWatch at 1-800-517-6330
CODE 260
Please call LifeWatch at 1-800-517-6330
CODE 270
Please call LifeWatch at 1-800-517-6330
CODE 280
Please call LifeWatch at 1-800-517-6330
CODE 290
Please call LifeWatch at 1-800-517-6330
CODE 401
ACT Monitor will restart
If this re-occurs please call LifeWatch at 1-800-517-6330
CODE 403
Please remove and then re-insert battery into sensor
If not resolved call LifeWatch at 1-800-517-6330
CODE 700
Please remove and then re-insert battery into sensor
If this re-occurs please call LifeWatch at 1-800-517-6330
CODE 701
Please remove and then re-insert battery into sensor
If this re-occurs please call LifeWatch at 1-800-517-6330
CODE 901
Please call LifeWatch at 1-800-517-6330
Page 95

12. Limited Warranty
1. This Card Guard® Device ("CG Device") is warranted against defective material
and workmanship for a warranty term of 1 year following shipment from Card
Guard facility of this product to the customer ("Warranty Term"). If this product or
any part thereof, in the judgment of Card Guard, is proven to be defective in
material or workmanship within the warranty term, Card Guard will at its sole
discretion either repair the item or replace it with a similar one (refurbished
device), to enable the designated use of the Card Guard Device (the “Hardware
Services”) free of charge for parts or labor.
2. The Hardware Services shall not include any of the following:
i. Replacement of consumable items, supplies or accessories (such as
printer consumables, disks, paper, disposable electrodes, disposable
mouthpieces etc.);
ii. Services required as a result of failure of electrical power, air conditioning,
dust or humidity control;
iii. Services required as a result of the use of attachments or any other
devices which are not compatible with the CG Device or with the system in
which they are installed and do not meet Card Guard's specifications or
standards;
iv. Services required as a result of fire, lightning, flood, wind, accident, theft,
abuse, negligence, misuse, vandalism, corrosion, natural disaster, or any
causes other than ordinary use for which the CG Device was designed;
v. CG Device which has been damaged by accident or which has been
misused, abused, altered or repaired by anyone other than Card Guard;
vi. Customer abuse, such as, marks, scratches, broken parts corrosion etc.;
and
vii. Electrical work the necessity of which is not related to the regular function
of the CG Device. Card Guard holds the sole discretion to decide which of
the CG Devices will not validated under this warranty.
3. The warranty period for the repaired / refurbished CG Device is for a three (3)
month period following the Hardware Services’ delivery to Card Guard.
4. Without derogating from any other provisions of this warranty, the customer shall
only use attachments or any other devices which, at Card Guard’s discretion
and as approved by Card Guard in writing, are compatible to the CG Device in
Page 96

which they are installed or to which they are connected; this Section 4 pertains
to the importance of liability for Card Guard Products;
5. CARD GUARD SHALL NOT BE LIABLE TO ANY PERSON FOR ANY
SPECIAL, CONSEQUENTIAL OR INDIRECT DAMAGES, INCLUDING, BUT
NOT LIMITED TO, DAMAGES TO OR LOSS OF PROPERTY OR
EQUIPMENT, LOSS OF PROFIT, LOSS OF USE OF DATA, LOSS OF
REVENUES OR DAMAGES TO BUSINESS OR REPUTATION ARISING
FROM ANY CAUSE WHATSOEVER ARISING FROM OR IN ANY WAY
CONNECTED WITH THE MANUFACTURE, SALE, HANDLING, REPAIRS
MAINTENANCE OR USE OF THE CG DEVICE, WHETHER OR NOT CARD
GUARD SHALL HAVE BEEN MADE AWARE OF THE POSSIBILITY OF SUCH
LOSS PROVIDED THAT IT IS NOT OTHERWISE REGULATED BY THE
APPLICABLE LAW. NOTWITHSTANDING ANY OF THE FOREGOING, CARD
GUARD'S LIABILITY FOR ANY CLAIMS ARISING OUT OF OR IN
CONNECTION WITH THIS WARRANTY, SHALL IN THE AGGREGATE, BE
LIMITED TO THE TOTAL PRICE PAID FOR THIS PRODUCT, IF SUCH
LIABILITY DOES NOT ARISE FROM THE GROSS NEGLIGENCE OR FAULT
OF CARD GUARD.
6. This warranty is in lieu of all other warranties expressed or implied, including any
implied warranty of merchantability or fitness for a particular purpose, and no
person is authorized to assume for Card Guard any other liability in connection
with the sale of this product.
7. To obtain factory service, this product should be shipped to Card Guard. The
customer shall bear the cost of shipment to Card Guard, and similarly, Card
Guard shall bear the cost of shipment from Card Guard.
8. All repaired or replaced parts will be released by Card Guard within 30 days
from receiving.
9. The customer may, 60 days prior to the end of the Warranty Term, notice Card
Guard of its request to receive from Card Guard, post warranty services
according to Card Guard's policies and upon signing a Post Warranty Services
Agreement.
Page 97

13. Software End User License Agreement
Using any part of the enclosed Card Guard Scientific Survival Ltd software (the “Software”)
indicates that you agree to the terms and conditions set forth below. If you do not agree to the
terms of this Software License Agreement (the “License”), you are not allowed to use the
Software.
GRANT OF LICENSE: Card Guard Scientific Survival Ltd grants you a non-exclusive, nontransferable (except as noted below) license to use the Software on a single computer or
workstation. The Software may be executed from different workstations or computers only if you
have licensed one copy of Software for each workstation or computer executing the Software.
You may not sublicense, sell, lend, or lease any portion of the Software without Card Guard
Scientific Survival Ltd's approval, or export the Software in any form from the country where the
Software was originally furnished.
COPYRIGHT: The Software is owned by Card Guard Scientific Survival Ltd or its suppliers and
is protected by United States and international copyright laws and international treaty provisions.
Any rights not expressly granted to you herein are retained by Card Guard Scientific Survival
Ltd. You may create one copy of the Software solely for backup purposes. You may not copy
the accompanying documentation (the documentation).
NO ASSIGNMENT; NO REVERSE ENGINEERING: You may not transfer the Software and this
License to another party unless the other party agrees in writing to accept the terms and
conditions of this License and you have received Card Guard Scientific Survival Ltd's prior
written consent. Upon such transfer you must at the same time either destroy or transfer to the
same party any copy of the Software and documentation. You may not modify, reverse
engineer, reverse compile, disassemble or otherwise reduce the Software to a humanperceivable form.
TERM: This License is effective until terminated. You may terminate it at any time by
destroying the Software and documentation, together with any copy. This License automatically
terminates if any terms or conditions are breached. Upon such termination you agree to destroy
the Software and documentation, together with any copies.
LIMITED WARRANTY: Card Guard Scientific Survival Ltd warrants that the Software will
perform substantially in accordance with the documentation and will be free from defects in
materials and workmanship under normal use and service for a period of ninety (90) days from
the date of receipt. This Limited Warranty is void if failure of the Software resulted from
accident, abuse, or misapplication or if the Software was modified by any person other than
Card Guard Scientific Survival Ltd. Card Guard Scientific Survival Ltd MAKES NO OTHER
EXPRESS OR IMPLIED WARRANTIES AND SPECIFICALLY DISCLAIMS THE IMPLIED
WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE AND
NONINFRINGEMENT OF THIRD PARTY RIGHTS. No representation or statement not
expressly contained in this agreement shall be binding upon Card Guard Scientific Survival Ltd
as a warranty or otherwise. If such disclaimer is not permitted by law, the duration of any such
implied warranties is limited to the duration of the express warranty. Some jurisdictions do not
allow the exclusion of implied warranties or limitations on the duration of an implied warranty, or
the exclusion or limitation of incidental or consequential damages, so the above exclusions or
limitations may not apply to you. This Limited Warranty gives you specific legal rights. You may
have other rights which vary from jurisdiction to jurisdiction.
Page 98

CUSTOMER REMEDIES: Card Guard Scientific Survival Ltd's entire liability and your sole
remedy shall be repair or replacement of the Software. Replacement Software will be warranted
for the remainder of the original warranty period or thirty (30) days, whichever is longer.
NO LIABILITY FOR CONSEQUENTIAL DAMAGES: IN NO EVENT SHALL Card Guard
Scientific Survival Ltd OR ITS SUPPLIERS BE LIABLE FOR ANY CONSEQUENTIAL (including
but not limited for any lost revenue, profit, or data, or punitive damages), SPECIAL,
INCIDENTAL OR INDIRECT DAMAGES OF ANY KIND ARISING OUT OF THE USE OF THE
SOFTWARE, EVEN IF Card Guard Scientific Survival Ltd HAS BEEN ADVISED OF THE
POSSIBILITY OF SUCH DAMAGES. IN NO EVENT WILL Card Guard Scientific Survival Ltd'S
LIABILITY FOR ANY CLAIM, WHETHER IN CONTRACT, TORT OR ANY OTHER THEORY OF
LIABILITY, EXCEED THE LICENSE FEE PAID BY YOU.
U.S. GOVERNMENT RESTRICTED RIGHTS: Any part of the Software and documentation that
is delivered to or acquired by or on behalf of the United States Government shall be considered
“computer software” or “computer software documentation” and/or provided with restricted rights
so that, in the absence of a written agreement to the contrary, the United States Government's
rights with respect to the use, duplication or disclosure of the Software by the United States
Government are limited by the terms of this License pursuant, and otherwise subject, to the
restrictions as set forth in the Rights in Technical Data provisions of 48 CFR §252.227-7013 or
as set forth in the Commercial Computer Software Restricted Rights provisions at 48 CFR
§52.227-19(c)(1)-(2).
GOVERNING LAW: This License is governed by the laws of the State of New York and by the
laws of the United States, excluding their conflicts of laws principles. The United Nations
Convention on Contracts for the International Sale of Goods (1980) is specifically disclaimed.
For more information about Card Guard Scientific Survival Ltd's licensing policies, please call
Card Guard Scientific Survival Ltd Customer Service at +972-8-9484000 or write to: Card Guard
Scientific Survival Ltd Customer Service, 2 Pekeris St, Rehovot, Israel 76100.
SEVERABILITY: In the event of the invalidity of any provision of this License, such invalidity
shall not affect the validity of the remaining portions of this License.
ENTIRE AGREEMENT: This License constitutes the entire agreement between you and Card
Guard Scientific Survival Ltd, supersedes all prior agreements, whether written or oral, with
respect to the Software, and may be amended only in writing signed by both parties.
UG-00066-06 2011-02
Page 99

Manufacturer:
Card Guard Scientific Survival Ltd.
2 Pekeris St., P.O. Box 527
Rehovot, 76100
Israel
Telephone: 972-8-948-4000
Fax: 972-8-948-4044
www.cardguard.com
Distributor & Service:
LifeWatch Corp.
O’Hare International Center II
10255 W. Higgins Road ,Suite 100
Rosemont, IL , 60018
Telephone: 1-877-774-9846
Fax: 847-720-2013
www.lifewatch.com
Page 100
 Loading...
Loading...