CAE Healthcare Fidelis Lucina User Manual

Maternal Fetal Simulator
User Guide

©2015 CAE Healthcare
905K470052 v2.1

End-User License Agreement
CAE HEALTHCARE END-USER LICENSE
AGREEMENT
END-USER LICENSE FOR CAE HEALTHCARE MEDICAL EDUCATION PRODUCTS (THE “PRODUCT(S)”)
THIS IS A LEGAL AGREEMENT. PLEASE READ THIS DOCUMENT CAREFULLY.
The software you are about to access is provided to you pursuant to the purchase of the Product by the legal
entity which employs you, or which you represent (the “Licensee” or You”) from, if the Product is labeled as
designed and manufactured in Canada, CAE Healthcare Inc. a Canadian corporation having its principal place of
in Montreal, Qc, Canada, otherwise from CAE Healthcare USA Inc., a United States company having a place of
business in San Jose, California, USA. This purchase of the Product is subject to the CAE Healthcare Medical
Education Products General Terms and Conditions (the “MEPGTC”) and this End-User License agreement
(“License”).
This License governs the grant of licenses for the software, in object code only, embedded in the Product (the
“Embedded Software”), as well as all related Product documentation and information (the “Data”) supplied by
CAE Healthcare either with or separately from the Product, which items as indicated in the MEPGTC are not sold
but licensed.
Acceptance of these terms and conditions must be without modification of any of the terms, conditions and
notices contained herein.
Consequently, please be sure to read the terms of this License carefully.
If you do not accept these terms, conditions and other provisions in their entirety, without modification of any
sort, your access to the Software and Data is prohibited.
1. DEFINITIONS AND INTERPRETATION
1.1 The preamble forms an integral part of this License.
1.2 Terms with a capital letter defined in the Preamble have the meaning indicated in the Preamble. Whenever
used in this License, the following terms have the meaning set out below:
(a) “Confidential Information" means any and all scientific and technical information which is in the possession of,
or belonging to, CAE Healthcare and relating to the Product, including without limitation, all Data, Embedded
Software, trade secrets, know-how, processes, methodologies, samples, components, analyses, compilations,
guides and other information or documents prepared by CAE Healthcare, its subsidiaries and affiliates and/or
their officers, servants, agents, representatives, employees or advisers which contain or are otherwise generated
from or reflect any CAE Healthcare proprietary information, whether or not covered by intellectual property rights
or explicitly designated as confidential or proprietary, which is disclosed by any means in written, oral, electronic,
or any other form.
(b) “Purpose” means the use of the Embedded Software and the Data solely for the operation and maintenance of
the Product, and the use of the Product solely as an educational tool.
i

End-User License Agreement
2. LICENSE
2.1 In consideration of Licensee’s agreement and compliance with the terms and conditions contained in the
MEPGTC and in this License, CAE Healthcare grants to Licensee, and Licensee accepts, a personal, non-exclusive,
non-transferable license to use the Embedded Software and Data exclusively with the Product, and with the
computer on which this License appears.
2.2 Except for the License granted herein, CAE Healthcare grants no express or implied right under any patent,
copyright, mask work right, trademark, know how or other intellectual property rights. Without limiting the
foregoing, the Licensee shall not obtain any rights to CAE Healthcare’s property, or any part thereof, by
implication, estoppel or otherwise. Title to and full ownership of any trade secrets and other intellectual property
rights related to the Product and components thereof shall remain with CAE Healthcare and, if applicable, its
suppliers. For clarification, Licensee agrees that the source code for the Embedded Software is a trade secret of
CAE Healthcare and only CAE Healthcare shall have the right to alter, maintain, enhance or otherwise modify the
Embedded Software.
2.3 Without limiting the foregoing or any other terms in this License, Licensee shall, and shall ensure that any
person authorized to access the Product, which are limited to Licensee’s employees, agents, representatives,
medical staff and students (“Authorized Users”):
(a) not copy (save and except for normal back up and disaster recovery purposes provided such copy shall include
CAE Healthcare’s copyright and any other proprietary notices indicated on the Embedded Software and Data),
ghost, export or produce any derivative works from the Product, or any part thereof, not network the Product
without CAE Healthcare’s prior written approval, or make it available for concurrent use;
(b) not sell, attempt to sell or transfer (unless in compliance with the MEPGTC), sublicense, encumber the
Embedded Software or Data;
(c) not modify the Product in any way, combine with other programs, or reverse engineer, screen scratch,
decompile or disassemble any Embedded Software nor otherwise attempt to create or derive the source code
related thereto;
(d) not deface or remove any copyright or proprietary notices;
(e) not use the Product without the Key, if provided with the Product, or attempt to develop or develop any
means or technology which would enable Licensee to bypass the use of the Key to operate the Product;
(f) prevent anyone other than Authorized Users from accessing or using the Product;
(g) not incorporate the Product, in whole or in part, to any product or service that Licensee would make available
to a third party, on a commercial basis or not.
2.4 Notwithstanding anything else contained in this License, in no event shall Licensee use the Product and/or
Confidential Information to enable, support, or otherwise aid Licensee or a third party to develop any product,
software or service competitive with any of CAE Healthcare’s products.
2.5 Licensee agrees to grant CAE Healthcare, its agents and representatives, at any time during Licensee’s normal
business hours and upon reasonable prior notice, the right to access to Licensee’s premises, to ensure that the use
of the Product is done at all times in compliance with the terms and conditions of this License.
ii

End-User License Agreement
2.6 CAE Healthcare reserves the right to embed a software security mechanism within the Product to monitor
usage of the Product to verify Licensee’s compliance with this Agreement, as well as to control access to the
Software through use of: a) a hardware lock device and/or b) a license administration software and/or c) a license
authorization key (collectively, the “Key”).
2.7 Some Products may provide Licensee with the option of saving and reproducing the images created by such
Products (“Work”) during their use. In this regard, Licensee hereby recognizes that the entire rights, title and
interests in and to such Work remain the exclusive property of CAE Healthcare.
Licensee shall not modify such Work in any way whatsoever and shall not remove or alter any CAE Healthcare
notices. However, Licensee is permitted to produce and reproduce such Work only for non-commercial
educational purposes.
3. FEEDBACK
Licensee agrees to provide CAE Healthcare, from time to time, with comments, suggestions, data, information or
feedback (“Feedback”) on the Product. Licensee acknowledges and agrees that such Feedback may be freely used
by CAE Healthcare, at its sole discretion, for the design, development, improvement, marketing and
commercialization of its products and services, without any restrictions based on confidentiality or intellectual
property rights.
4. TERM AND TERMINATION
4.1 This License shall become effective as of the date of your execution of this License and shall remain in effect
until terminated as provided hereafter.
4.2 This License terminates immediately upon termination of the MEPGTC.
4.3 CAE Healthcare may terminate this License immediately, upon written notice, should Licensee:
(a) fail to comply with any of the terms and conditions of this License;
(b) terminate or suspend its business; make an assignment for the benefit of creditors, or any proceedings are
instituted by any party or against it seeking to declare it bankrupt or insolvent, or seeking liquidation, windingup, reorganization, arrangement, adjustment, protection, relief or composition of its debts under any law relating
to bankruptcy, insolvency, reorganization or relief of debtors, or seeking the entry of an order for relief or the
appointment of a receiver, trustee or other similar official for it or for any substantial part of its property;
4.4 Upon termination of this License, Licensee agrees to immediately discontinue use of the Confidential
Information and the Product, and to return same to CAE Healthcare as well as any copies, summaries or extracts
thereof, with any associated CD ROM(s), DVD, keys, dongles or other devices as may be directed by CAE
Healthcare. At CAE Healthcare’s request, Licensee shall promptly provide a written certificate signed by an officer
of Licensee confirming that such items have been returned to CAE Healthcare or destroyed as so directed by CAE
Healthcare.
4.5 The following shall survive and continue in full force and effect notwithstanding any termination of this
License: the obligations of Licensee under Sections 2 (License), 5 (Non-Disclosure); as well as any other clauses
which by their nature and context are intended to survive.
iii

End-User License Agreement
5. NON-DISCLOSURE
5.1 Licensee agrees to keep this License and all Confidential Information obtained hereunder in strict confidence,
and shall only disclose same a) to Authorized Users solely for the Purpose and provided such access to the
Product conforms, at all times, to the terms and conditions governing the use of the Product contained herein, or
b) if required to be disclosed by law, and only to the extent of such disclosure and limited to the purpose
requested, with prior notice to CAE Healthcare to permit it to seek an appropriate remedy to prevent the
disclosure, or alternatively to agree to the terms of such disclosure.
5.2 The obligations of confidentiality, use and non-disclosure referred to in this Section 5 shall not apply to
information which: (i) is or becomes publicly available through no fault of Licensee; (ii) was already in the rightful
possession of Licensee prior to its receipt from CAE Healthcare; (iii) is independently developed by Licensee,
provided it is not, in whole or in part, related to the Product; and (iv) is obtained by Licensee in good faith and on
a non-confidential basis and without a use restriction from a third party who lawfully obtained and disclosed such
information. However, Confidential Information does not come within the foregoing exceptions merely because
features of it may be found separately or within a general disclosure in the public domain.
5.3 Licensee agrees to be responsible for enforcing the terms of this Section 5 and to take such action, legal or
otherwise, to the extent necessary to cause anyone having access to the Confidential Information to comply with
the terms and conditions set forth herein (including all actions that Licensee would take to protect its own trade
secrets and confidential information but with not less than reasonable care). Licensee shall be responsible and
indemnify, defend and hold harmless CAE Healthcare for any default caused by any such persons.
6. IRREPARABLE HARM
6.1 Licensee acknowledges that the Embedded Software and Data constitute a special, irreplaceable asset of
great value to CAE Healthcare, and that a breach, in any way, of any of Licensee’s obligations under Sections 2
(License), and 5 (Non-Disclosure) hereof would cause serious and irreparable harm to CAE Healthcare which may
not be adequately compensated for in damages. If the Licensee breaches any of such provisions, Licensee
consents to an injunction being issued against it restraining it from any further breach of such provision, without
derogation from any other remedy which CAE Healthcare may have in the event of such a breach.
7. WARRANTY, LIMITATION OF LIABILITY
7.1 THE SOLE WARRANTIES PROVIDED BY CAE HEALTHCARE ARE LIMITED TO THE WARRANTIES PROVIDED IN THE
MEPGTC. ANY WARRANTIES PROVIDED ARE PERSONAL AND NOT TRANSFERABLE.
7.2 CAE HEALTHCARE’S LIABILITY SHALL IN NO CIRCUMSTANCES EXCEED THE LIMITATION OF LIABILITY
INDICATED IN THE SPTGC. LIABILITY, IF ANY, SHALL BE SOLELY FOR DIRECT DAMAGES, NOT TO EXCEED ON A
CUMULATIVE BASIS THE AMOUNT PAID BY LICENSEE FOR THE PRODUCT.
iv

End-User License Agreement
8. GOVERNING LAW
8.1 Save and except for Products designed and manufactured in Canada, this License, is governed by the internal
substantive laws of the State of California applicable to agreements to be made and to be performed solely within
California, without giving effect to any conflicts or choice of laws principles that otherwise might be applicable.
For those Products designed and manufactured in Canada, the substantive laws of the Province of Ontario
(excluding its conflict of law rules) and the laws of Canada applicable therein, shall apply. In all cases, the parties
expressly exclude and waive the application of the United Nations Convention on Commercial Agreements for
the International Sale of Goods (1980) (Vienna Sales Convention) as amended.
8.2 Save and except for Products designed and manufactured in Canada, all disputes arising from or related to this
Agreement shall be litigated in Santa Clara County in the State of California. For those Products designed and
manufactured in Canada, the venue shall be Toronto, Canada. Each party hereby waives any right that it might
otherwise have to object to such venue or seek dismissal of the action on the basis of forum non-conveniens.
EACH PARTY HERETO IRREVOCABLY WAIVES, TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW, ANY AND
ALL RIGHT TO TRIAL BY JURY IN ANY LEGAL PROCEEDING ARISING OUT OF OR RELATING TO THIS AGREEMENT.
8.3 Notwithstanding the foregoing, if a party seeks injunctive proceedings to preserve confidentiality obligations
or intellectual property rights, then it is entitled to seek relief before the competent court/body of any
jurisdiction.
9. MISCELLANEOUS
9.1 United States Government Customer: If Licensee is the United States Government (“U.S. Government”) or a
unit or agency of the U.S. Government, the Software and Data are deemed to be “commercial computer software”
and “commercial computer software documentation”, respectively, pursuant to DFAR Section 227.7202 and FAR
Section 12.212 b) as applicable. Any use, modification, reproduction, release, performance, display, or disclosure
of the Embedded Software and/or Data by the U. S. Government, or any of its units or agencies shall be governed
solely by the terms of this License and the MEPGTC. Any technical data provided by CAE Healthcare with the
Product that is not covered by the above provisions is deemed to be "technical data-commercial items" pursuant
to DFAR Section 252.227.7015(a).
9.2 Amendment. This License may only be amended by the duly authorized representatives of CAE Healthcare.
9.3 No Waiver: The failure of CAE Healthcare to enforce at any time any of the provisions of this License, or to
require at any time the performance by Licensee of any of the provisions hereof, shall not be construed to be a
waiver of such provisions, nor in any way affect the validity of this License or any part thereof, or the right of CAE
Healthcare thereafter to enforce any such provision.
9.4 No third-party beneficiaries. Nothing in this Agreement shall be construed as creating or giving rise to any
rights for any third parties or any persons other than the parties to this Agreement.
v

End-User License Agreement
9.5 Notices: Notices or communications pertaining to this Agreement must be given in writing and delivered to
the addressee as indicated in the MEPGTC.
9.6 Preamble/Headings. The preamble forms an integral part of this Agreement. The division of this Agreement
into Clauses, Articles, sections, subsections and other subdivisions and the insertion of headings are for
convenience of reference only and will not affect the construction or interpretation of this Agreement.
9.7 Severability. If any one or more of the provisions of this License shall be held to be invalid, illegal or
unenforceable, the validity, legality or enforceability of the remaining provisions of this Agreement shall not in
any way be affected or impaired thereby.
PROPRIETARY NOTICE: This document, including the information contained herein is confidential and/or
proprietary to CAE Healthcare Inc., and shall not be reproduced or disclosed in whole or in part, or used for any
purpose whatsoever unless authorized in writing by CAE Healthcare Inc. Information on copyright and
trademarks.
vi

System Requirements
SYSTEM REQUIREMENTS
If you are not using a TouchPro computer or Wireless Remote provided by CAE Healthcare, please be
sure to utilize a computer with wireless capability. When operating the TouchPro or Müse software, a
computer with a Macintosh® or Microsoft Windows® operating system may be used.
To run the TouchPro or Müse software, the computer used must meet the following minimum
requirements:
Macintosh® Operating System
Mac OS X10.6x (minimum)
Firefox® 24 ESR (minimum) Adobe Flash
Player® 16.x.x.x (minimum) Adobe
Reader 11.x (or higher)
Windows® Operating System
Windows 7 or Windows 8
Firefox
Adobe Flash Player
Adobe Reader 11.x (or higher)
®
24 ESR, Internet Explorer® 9 (minimum)
®
Windows Hardware
Intel Core 2 Duo, 2.0 GHz (minimum)
4 GB DDR3 RAM (minimum)
32 GB Hard Drive space available
1366x768 screen resolution (minimum)
USB 2.0
Wireless 802.11b/g/n Ethernet card
100BASE-T Ethernet Adapter
16.x.x.x (minimum)
Standard DVD+/-R Drive
vii

System Requirements
Macintosh Hardware
Intel Core 2 Duo, 2.0 GHz (minimum)
2 GB DDR3 RAM (minimum)
8 GB Hard Drive space available
1024x768 screen resolution (minimum)
USB 2.0
Wireless 802.11b/g/n Ethernet card
100BASE-T Ethernet Adapter
Standard DVD+/-R Drive
NOTE: Macintosh, Quicktime are registered trademarks of Apple Inc. Windows Media and Internet
Explorer
countries. Firefox is a registered trademark of the Mozilla Foundation. Adobe® Flash Player is a
trademark of Adobe Systems Inc.
are registered trademarks of the Microsoft Corporation in the United States and/or other
viii

Specifications
SPECIFICATIONS
All hardware and software needed for the operation of the simulator are supplied. If you wish to extend
the Instructor Workstation to other computers, contact CAE Healthcare.
Size
Mannequin/Simulator:
Fetus/Simulator: 19” H x 6” W x 4.5.” D (48cm x 15cm x 11.5cm)
Instructor Workstation
69” H x 22” W x 15” D (175cm x 56cm x 38cm
: .95” H x 12.78” W x 8.94” D (2.4cm x 32.5cm x 22.7cm)
Weight
Mannequin/Simulator: 111lbs (50 kg)
Fetus/Simulator: 5.5 lbs (2.5 kg)
Instructor Workstation: 4.5lbs (2.1kg)
Environmental Requirements
Ambient Temperature Range
Mannequin/Simulator
Operation: 40°F to 104°F (4°C to 40°C)
Storage: 40°F to 122°F (4°C to 50°C)
Relative Humidity: 0% to 90% noncondensing
Instructor Workstation
)
Operation: 50°F to 95°F (10°C to 35°C)
Storage: -13°F to 113°F (-24°C to 45°C)
Relative Humidity: 0% to 90% noncondensing
Maximum Altitude
Instructor Workstation
Maximum operating altitude: 10,000 ft
Maximum storage altitude: 15,000 ft
Maximum shipping altitude: 35,000 ft
ix

Specifications
Power
Mannequin/Simulator
Fetus/Simulator
Instructor Workstation
AC Input: AC 100 – 240VAC, 50/60Hz
Consumption: Maximum 150W (Charging), 100W (charged), Typical 40W
Internal Batteries: 14.4V 90-watt-hour lithium-ion, rechargeable
Run Time: 4 hours (Typical)
Run Time: 7 hours (Typical)
AC Input: AC 100 – 240VAC, 50/60Hz
Consumption: Maximum 85W (Charging)
Internal Battery: 10.8V 60-watt-hour lithium-polymer, rechargeable
Run Time: 2 to 4 hours (Typical)
TouchPro Computer
Please see the product’s user guide for power specifications.
Communications
Simulator Network
Wired: 10/100 Ethernet or
Wireless: IEEE 802.11g
Wireless Voice
537 MHz to 819MHz (Country Specific)
Electrotherapy
Defibrillation: 20 to 360 joules (Monophasic, Biphasic)
Pacing: 20ma to 180ma
Air Supply
To properly regulate psi, the optional wall air kit must be used in conjunction with the facility supply
source and facility wall adapter.
x

Cautions and Warnings
CAUTIONS AND WARNINGS
Please read and understand these cautions and warnings before you begin using the CAE Fidelis™
Maternal Fetal Simulator system.
USE OF THIS EQUIPMENT IN AN UNSPECIFIED MANNER, MAY IMPAIR DESIGNED
PROTECTION.
Your safety is in your hands. Be sure to follow the instructions on the proper setup,
breakdown and use of the Fidelis system.
SHOCK HAZARD
Electrical Safety
• This product must be connected to an electrical outlet that is properly
grounded. Precautions should be taken so that grounding or polarization is
not defeated.
• Do not place defibrillator paddles on or adjacent to the ECG patient
electrodes. Contact between defibrillator paddles and the electrodes may
cause injury to the user and damage to the equipment.
• Always use the supplied power cords. Do not substitute.
• Operate the system from a power source with the following rating:
º 115VAC, 50/60 hertz (cycles per second) (e.g., North America, Japan)
º 230VAC, 50/60 hertz (cycles per second) (e.g., Europe)
• Do not allow excess fluids to flow on or into electronic parts.
• Do not attempt to disassemble the simulator or service any of the electrical
components other than changing the batteries.
• Always use the supplied power adapter to charge or run simulator from AC.
Latex Warning
CAE Healthcare simulators incorporate latex into their design. When performing certain maintenance
procedures, the latex can become exposed. Users with latex sensitivity should take necessary
precautions when handling the simulator while performing those procedures.
xi

Cautions and Warnings
General Use Warnings
Please observe the following warnings when using the Fidelis Lucina simulator.
Electrical System
• Operate the system from a power source with the following rating:
115VAC, 50/60 hertz (cycles per second) (e.g. North America, Japan), and
230VAC, 50/60 hertz (cycles per second) (e.g. Europe)
• Do not operate the MFS system in rain. Apply water to the mannequin only
in accordance with the supported clinical procedures identified in this User
Guide.
• Do not allow excess fluids to flow on or into electronic parts.
Bleeding System
• DO NOT modify the tank or any assembly component.
• ALWAYS protect eyes, skin and clothing against accidental exposure.
• ALWAYS read and follow instructions for creating trauma fluids (e.g. blood).
NEVER fill the tank with more than 2 liters (0.5 gallons) of fluid.
•
Transport
• Prior to using the stretcher packed with the shipping container, the
• CAE is not responsible for damage to the mannequin skin if the mannequin
After use, ALWAYS drain the tank. DO NOT store liquids in the tank.
mannequin must be wrapped in a sheet. Failure to wrap the mannequin in
a sheet may result in permanent damage to the mannequin skin.
is not wrapped in a sheet while using the stretcher.
xii

Table Of Contents
TABLE OF CONTENTS
CAE Healthcare End-User License Agreement ..................................................................... i
System Requirements ................................................................................................... vii
Specifications ................................................................................................................ ix
Size ....................................................................................................................................ix
Weight ...............................................................................................................................ix
Environmental Requirements ..............................................................................................ix
Ambient Temperature Range..................................................................................................................ix
Maximum Altitude..................................................................................................................................ix
Power..................................................................................................................................x
Communications ..................................................................................................................x
Electrotherapy .....................................................................................................................x
Air Supply ............................................................................................................................x
Cautions and Warnings................................................................................................... xi
Electrical Safety...................................................................................................................xi
Latex Warning.........................................................................................................................................xi
General Use Warnings............................................................................................................................xii
Table of Contents......................................................................................................... xiii
Introduction................................................................................................................... 1
Contained in this User Guide ................................................................................................ 2
Equipment Overview....................................................................................................... 3
Standard Equipment ........................................................................................................... 3
Full-Body Wireless Mannequin ............................................................................................................... 3
Laptop Instructor Workstation ................................................................................................................ 3
TouchPro Workstation............................................................................................................................. 4
Power Adapters and Cords (2)................................................................................................................. 4
Gown ...................................................................................................................................................... 4
Prepartum/Early Labor Kit....................................................................................................................... 4
Vaginal Delivery Kit................................................................................................................................. 5
Postpartum Kit........................................................................................................................................ 5
xiii

Table Of Contents
Accessories.............................................................................................................................................. 5
Optional Equipment................................................................................................................................ 6
Supplemental Static Cervices Kit ............................................................................................................. 6
Operation Supplemental Accessories ...................................................................................................... 6
Additional Equipment.......................................................................................................... 7
Setup ............................................................................................................................. 9
Before Beginning Setup....................................................................................................... 9
Step 1: Place Mannequin in the Work Area ...........................................................................10
Step 2: Power on the Maternal Mannequin ..........................................................................11
Step 3: Power on the Fetus..................................................................................................13
Step 4: Power on the Instructor Workstation ........................................................................14
Step 5a: Connect to the Wireless Network - Laptop Instructor Workstation (Macintosh) ..........15
Step 5b: Connect to the Wireless Network - Laptop or Tablet Instructor Workstation (Windows) . 16
Step 6: Connect a TouchPro™ Workstation to the Wireless Network (Optional)........................17
Configuring The Mannequin .......................................................................................... 19
Prepartum.................................................................................................................... 20
Removing the Rotation Ring ...............................................................................................20
Installing the Static Cervix ..................................................................................................22
Prepartum and Latent Cervix Installation............................................................................................. 22
Installing the Backplate......................................................................................................24
Prepartum and Latent Backplate Installation........................................................................................ 24
Installing the Support Tubs .................................................................................................25
Loading the Fetus - Prepartum ............................................................................................25
Prepartum and Latent........................................................................................................................... 25
Active Phase.......................................................................................................................................... 26
Delivery ....................................................................................................................... 26
Installing the Rotation Ring................................................................................................26
Installing the Uterine Funnel with Dynamic Cervix ...............................................................28
Loading the Fetus - Delivery................................................................................................29
Vaginal Delivery - Breech ...................................................................................................................... 29
Vaginal Delivery - Vertex....................................................................................................................... 32
xiv

Table Of Contents
Installing the Placenta .......................................................................................................35
Vaginal Delivery Placenta Pouch Placement ......................................................................................... 37
Installing the Abdomen ......................................................................................................38
Delivery Abdomen Installation.............................................................................................................. 38
Non-Gravid (Non-Pregnant) Abdomen ................................................................................................. 41
Disconnecting the Umbilical Cord ........................................................................................43
Postpartum .................................................................................................................. 44
Filling the Blood Tank.........................................................................................................44
Internal Filling....................................................................................................................................... 44
External Filling ..................................................................................................................................... 45
Installing the Blood Tank....................................................................................................46
Installing the Boggy/Contracted Uterus With Bag.................................................................48
Installing the Postpartum Invertible Uterus.........................................................................54
Postpartum Backplate Installation........................................................................................................ 59
Using Müse................................................................................................................... 61
Starting the Application......................................................................................................61
Navigating the Home Page .................................................................................................63
The SCE Selection Panel ......................................................................................................64
The SCE Operating Mode Icon................................................................................................................ 67
The SCE Summary Panel........................................................................................................................ 68
Running an SCE ..................................................................................................................69
SCE Information .................................................................................................................................... 72
Using the Patient Status Display..........................................................................................73
The Event Logs ...................................................................................................................................... 75
Displaying Patient Records.................................................................................................................... 75
NOTE: Only one patient record can be displayed at a time..................................................................... 77
Adding a Scenario to a Running SCE...................................................................................................... 78
Changing Physiology and Controlling Delivery...................................................................................... 79
Selecting CTG Monitor Options.............................................................................................................. 97
SCE Time Controls.................................................................................................................................. 98
The Battery Status Icon ......................................................................................................................... 99
xv

Table Of Contents
Traction Feedback ............................................................................................................................... 100
The CPR Monitor ................................................................................................................................. 101
Using the Event Recorder to Save States ............................................................................................. 102
Creating a New Patient ....................................................................................................................... 104
Resetting a Patient.............................................................................................................................. 107
The Medication Monitor...................................................................................................................... 108
Returning to the Home Page............................................................................................................... 109
Stopping the SCE................................................................................................................................. 109
Developing SCEs ...............................................................................................................111
Creating a New SCE ............................................................................................................................. 111
The SCE Editor ..................................................................................................................................... 113
Editing a Patient’s Profile.................................................................................................................... 114
Creating a New Scenario ..................................................................................................................... 123
Editing a Scenario ............................................................................................................................... 124
Modifying Scenario States................................................................................................................... 128
Deleting Scenario States ..................................................................................................................... 133
Deleting Parameters and Transitions .................................................................................................. 133
Saving the Scenario............................................................................................................................. 134
Emptying the Trash............................................................................................................................. 135
Saving States to the State Library ....................................................................................................... 136
Administrative Tools......................................................................................................... 138
History ................................................................................................................................................ 138
System Administration........................................................................................................................ 139
Account Profile.................................................................................................................................... 157
Using The TouchPro Patient Monitor ............................................................................ 163
Accessing the TouchPro Patient Monitor Software ..............................................................163
Modifying the TouchPro Patient Monitor Display................................................................165
Selecting a Preconfigured Layout........................................................................................................ 165
xvi
Changing a Waveform or Numeric Display .......................................................................................... 167
Adding a Waveform ............................................................................................................................ 168
Adding a Numeric Display ................................................................................................................... 170
Moving a Waveform or Numeric Display ............................................................................................. 171

Table Of Contents
Saving a Layout................................................................................................................................... 172
Sounds.............................................................................................................................173
12-Lead ECG .....................................................................................................................174
NIBP Cycling and Manual NIBP ..........................................................................................177
Patients...........................................................................................................................179
Configuring the TouchPro Software ...................................................................................180
Changing the TouchPro Language .....................................................................................181
Exiting the TouchPro Software ..........................................................................................182
Using the TouchPro CTG Monitor Software.................................................................... 183
Accessing the TouchPro CTG Software ................................................................................ 183
Configuring CTG Alarms.....................................................................................................185
Setting CTG Alarm Thresholds ............................................................................................................. 185
Setting CTG Alarm Suspension Time.................................................................................................... 186
Adjusting Audio Settings..................................................................................................................... 188
Muting CTG Alarms ........................................................................................................... 190
MNIBP Cycling and Manual MNIBP .....................................................................................190
Resetting Tocography Noise..............................................................................................192
Viewing the CTG Strip ....................................................................................................... 193
Changing the CTG Monitor Language .................................................................................195
Exiting the CTG Monitor ....................................................................................................197
Using Lucina ............................................................................................................... 199
Neurological Features....................................................................................................... 200
Eyes.................................................................................................................................202
Seizures............................................................................................................................................... 203
Respiratory Features ........................................................................................................ 204
Realistic Upper Airway ........................................................................................................................ 207
Cardiovascular Features....................................................................................................208
Pulses..............................................................................................................................212
4-Lead ECG.......................................................................................................................................... 214
Manual Blood Pressure........................................................................................................................ 214
Korotkoff Sounds................................................................................................................................. 216
Defibrillation and Cardioversion.......................................................................................................... 217
xvii

Table Of Contents
Cardiac Pacing..................................................................................................................................... 219
Fluids ..............................................................................................................................219
Vaginal Bleeding................................................................................................................................. 221
Hematology Model ...........................................................................................................223
The Genitourinary System................................................................................................................... 224
Medication and Fluid Administration.................................................................................................. 225
Priming the Epidural Site .................................................................................................................... 228
Administering Suppositories............................................................................................................... 228
Sounds.............................................................................................................................229
Patient Speech .................................................................................................................................... 229
Speech Sounds.................................................................................................................................... 229
Breath Sounds..................................................................................................................................... 231
Heart Sounds....................................................................................................................................... 232
Audible Breathing Sounds................................................................................................................... 233
UA Synchronized Vocal Clip................................................................................................................. 234
Neonate Cry Selection ......................................................................................................................... 235
Fetal Heart Sound Location ................................................................................................................. 236
Bowel Sounds (Non-Gravid Only)........................................................................................................ 237
Care and Maintenance................................................................................................. 239
CAE Healthcare Assurance Programs ..................................................................................239
General Information............................................................................................................................ 239
Units Out of Plan ................................................................................................................................. 239
Plan Period.......................................................................................................................................... 239
Limitations of Plan.............................................................................................................................. 239
Return Materials Authorization (RMA) ................................................................................................ 240
Training for LifeTM.............................................................................................................................. 240
System Software Upgrade Support ....................................................................................241
Time and Materials ............................................................................................................................. 241
How to Contact Customer Service.......................................................................................241
For customer service, please contact CAE Healthcare. ......................................................................... 241
Breakdown ......................................................................................................................243
Step 1: Clean the Simulator and Fluid Systems.................................................................................... 243
xviii

Table Of Contents
Step 2: Shut Down the Software ......................................................................................................... 243
Step 3: Power Off the Simulator.......................................................................................................... 244
Step 4: Power Off the Fetus................................................................................................................. 244
Maintenance Advice ......................................................................................................... 245
General Simulator Care ....................................................................................................................... 245
Storing the Simulator.......................................................................................................................... 245
Caring for Electronic Equipment.......................................................................................................... 246
Inspecting the Airway ......................................................................................................................... 246
Charging the Batteries ........................................................................................................................ 246
Charging the Fetus Battery.................................................................................................................. 249
Cleaning the Internal Systems............................................................................................................. 250
Draining Condensation from the Simulator......................................................................................... 256
Replacing the Standard Birth Canal..................................................................................................... 258
Respiratory: Desaturation ................................................................................................................... 271
Cardiovascular: Blood Pressure............................................................................................................ 272
Cardiovascular: Heart Rate .................................................................................................................. 273
Respiratory: Respiratory Rate.............................................................................................................. 274
Müse Parameter Descriptions ...................................................................................... 275
Neurological - Parameters.................................................................................................275
Eyes: Pupil Control .............................................................................................................................. 275
Eyes: Blinking...................................................................................................................................... 276
Eyes: Blink Speed ................................................................................................................................ 276
Reactive Pupils.................................................................................................................................... 276
Light Reactivity Speed......................................................................................................................... 276
Seizures............................................................................................................................................... 276
Intracranial Pressure (ICP)................................................................................................................... 277
Neuromuscular Blockade (NMB) ......................................................................................................... 277
Temperature: Body ............................................................................................................................. 277
Temperature: Blood ............................................................................................................................ 277
Respiratory – Basic Parameters.........................................................................................278
Bronchial Occlusion (Left and Right) ................................................................................................... 278
Respiratory Rate.................................................................................................................................. 278
xix

Table Of Contents
Respiratory Rate Factor ....................................................................................................................... 279
Shunt Fraction..................................................................................................................................... 279
SpO2 ................................................................................................................................................... 279
Neuromuscular Blockade (NMB) ......................................................................................................... 280
Tidal Volume....................................................................................................................................... 280
Intrapleural Volume (Vol): (Left and Right)......................................................................................... 280
Fraction of Inspired O2 (FiO2).............................................................................................................. 281
Respiratory – Additional Parameters.................................................................................281
Respiratory Rate.................................................................................................................................. 282
Tidal Volume....................................................................................................................................... 283
Tidal Volume Factor ............................................................................................................................ 283
pH Shift............................................................................................................................................... 283
Positive End Expiratory Pressure (PEEP) .............................................................................................. 284
O2 Consumption ................................................................................................................................. 284
CO2 Production Factor......................................................................................................................... 284
PaCO2 Set-point.................................................................................................................................. 284
PaO2 Set-point.................................................................................................................................... 285
I to E Ratio (1:X) .................................................................................................................................. 285
PetCO2 - PaCO2 Factor ........................................................................................................................ 285
Respiratory Gain Factor ....................................................................................................................... 286
Respiratory Quotient........................................................................................................................... 286
Volume/Rate Control Factor................................................................................................................ 286
Chest Wall Capacity............................................................................................................................. 286
Chest Wall Compliance Factor ............................................................................................................. 287
Distended Chest Wall Compliance Factor ............................................................................................ 287
Functional Residual Capacity............................................................................................................... 287
Lung Compliance Factor: (Left and Right) ........................................................................................... 287
Venous CO2 Shift................................................................................................................................. 288
Bronchial Resistance Factor (Left and Right)....................................................................................... 288
Alveolar Enflurane............................................................................................................................... 288
Fraction of Inspired Enflurane ............................................................................................................. 289
Alveolar Halothane ............................................................................................................................. 289
xx

Table Of Contents
Fraction of Inspired Halothane............................................................................................................ 289
Alveolar Isoflurane .............................................................................................................................. 290
Fraction of Inspired Isoflurane............................................................................................................. 290
Alveolar Nitrous Oxide......................................................................................................................... 290
Fraction of Inspired Nitrous Oxide ....................................................................................................... 291
Alveolar Sevoflurane ........................................................................................................................... 291
Fraction of Inspired Sevoflurane.......................................................................................................... 291
Cardiovascular – Basic Parameters .................................................................................... 292
Blood Pressure .................................................................................................................................... 292
Heart Rate........................................................................................................................................... 292
Heart Rate Factor ................................................................................................................................ 293
Cardiac Rhythm................................................................................................................................... 293
Arterial Catheter.................................................................................................................................. 295
Central Venous Catheter...................................................................................................................... 295
Pulmonary Artery (PA) Catheter.......................................................................................................... 296
Pulmonary Artery (PA) Balloon ........................................................................................................... 296
Defibrillation (Defib) ........................................................................................................................... 296
Pacing Current .................................................................................................................................... 297
Pacing Rate ......................................................................................................................................... 297
Pacing Capture Threshold ................................................................................................................... 297
Cold Fluid Inject .................................................................................................................................. 297
Cardiovascular – Additional Parameters ............................................................................ 298
Baroreceptor Maximum Pressure ........................................................................................................ 298
Baroreceptor Minimum Pressure......................................................................................................... 299
Left Ventricle Contractility Factor........................................................................................................ 299
Right Ventricle Contractility Factor...................................................................................................... 300
Systemic Vascular Resistance Factor.................................................................................................... 300
Venous Capacity Factor ....................................................................................................................... 300
Systemic Arteries Compliance Factor................................................................................................... 301
Pulmonary Arteries Compliance Factor ............................................................................................... 301
Pulmonary Vasculature Resistance Factor ........................................................................................... 301
Venous Return Resistance Factor ........................................................................................................ 302
xxi

Table Of Contents
Baroreceptor Gain (Overall) Factor ...................................................................................................... 302
Baroreceptor Gain (Cardiac) Factor...................................................................................................... 302
Baroreceptor Gain (Peripheral) Factor................................................................................................. 303
Chest Compression Efficacy ................................................................................................................. 303
Tamponade Volume............................................................................................................................ 303
Ischemic Index Sensitivity ................................................................................................................... 304
Ischemic Index Averaging ................................................................................................................... 304
Aortic Valve Resistance Factor............................................................................................................. 305
Mitral Valve Resistance Factor............................................................................................................. 305
Pulmonic Valve Resistance Factor ....................................................................................................... 305
Fetal and Labor - Basic Parameters ....................................................................................306
Rate of Descent ................................................................................................................................... 306
Fetal State........................................................................................................................................... 306
Contraction Frequency ........................................................................................................................ 307
Contraction Amplitude........................................................................................................................ 307
Contraction Duration........................................................................................................................... 307
Patient Pushing................................................................................................................................... 307
Early Deceleration Magnitude............................................................................................................. 307
Late Deceleration Amplification Factor ............................................................................................... 308
Shoulder Dystocia ............................................................................................................................... 308
Shoulder Dystocia Resolution.............................................................................................................. 308
Extraction of Posterior Arm ................................................................................................................. 309
Arrested Labor .................................................................................................................................... 309
Arrested Labor Trigger Station ............................................................................................................ 310
Arrested Labor Resolved by Traction ................................................................................................... 310
Traction Force Required to Resolve Arrested Labor ............................................................................. 310
Manual Release of the Baby ................................................................................................................ 310
Fetal and Labor - Additional Parameters ............................................................................311
xxii
Maternal Position................................................................................................................................ 311
Contraction Resting Tone .................................................................................................................... 312
Contraction Frequency Variability ....................................................................................................... 312
Contraction Amplitude Variability....................................................................................................... 312

Table Of Contents
UA Noise.............................................................................................................................................. 312
TOCO Amplitude Gain.......................................................................................................................... 312
FHR Baseline ....................................................................................................................................... 312
FHR Variability Coefficient................................................................................................................... 313
Umbilical Cord Compressibility Factor ................................................................................................. 313
FHR Acceleration Amplitude ............................................................................................................... 313
FHR Acceleration Interval.................................................................................................................... 313
FHR Acceleration Duration .................................................................................................................. 313
Placental Perfusion Factor................................................................................................................... 314
Umbilical Flow Factor.......................................................................................................................... 314
Cardiotocograph (CTG) Configuration - Parameters .............................................................315
FHR Probe ........................................................................................................................................... 316
UA Probe............................................................................................................................................. 316
MHR Probe .......................................................................................................................................... 317
MSPO2 Probe ...................................................................................................................................... 317
MNIBP Probe....................................................................................................................................... 318
Temp Probe......................................................................................................................................... 318
FHR Signal Loss ................................................................................................................................... 319
UA Signal Loss..................................................................................................................................... 319
TOCO Offset ......................................................................................................................................... 319
MHR Graph.......................................................................................................................................... 319
MHR Overrides FHR ............................................................................................................................. 319
MSPO2 Printing Frequency.................................................................................................................. 319
MNIBP Printing Frequency .................................................................................................................. 320
Patient Baseline - Parameters...........................................................................................320
Operating Mode .................................................................................................................................. 320
Delivery Paused on Run....................................................................................................................... 321
Postpartum Included........................................................................................................................... 321
Cervix .................................................................................................................................................. 322
Presentation ....................................................................................................................................... 322
Initial Station ...................................................................................................................................... 322
Vertex Rotation Type........................................................................................................................... 323
xxiii

Table Of Contents
Breech Initial Position ......................................................................................................................... 323
Cord Prolapse ...................................................................................................................................... 323
Nuchal Cord......................................................................................................................................... 323
Placenta Condition.............................................................................................................................. 324
Postpartum Initial Uterine State ......................................................................................................... 324
Postpartum - Basic Parameters .........................................................................................325
Uterine Massage ................................................................................................................................. 325
Resolve Boggy Uterus.......................................................................................................................... 326
Inverted Uterus Can Be Reverted......................................................................................................... 326
Postpartum - Additional Parameters .................................................................................326
Maternal Position................................................................................................................................ 326
Parameter Display Definitions ..................................................................................... 327
Müse Patient Status Display..............................................................................................327
Waveform Widget Displays................................................................................................................. 327
Numeric Widget Displays .................................................................................................................... 328
Volume Widget Displays ..................................................................................................................... 330
Other Widget Displays......................................................................................................................... 330
CPR Monitor .....................................................................................................................330
Traction Feedback ............................................................................................................332
TouchPro Patient Monitor and TouchPro CTG......................................................................332
Waveform Widget Displays................................................................................................................. 332
Numeric Widget Displays .................................................................................................................... 333
12-Lead ECG Report Parameters.......................................................................................................... 334
Recommended Clinical Supply Sizes ............................................................................. 335
The Ischemic Index (Death Spiral) ................................................................................ 335
Wireless Voice Link...................................................................................................... 337
Cautions and Warnings .....................................................................................................337
What’s Included ...............................................................................................................338
How It Works.................................................................................................................... 338
Recommendations for Use ................................................................................................ 338
Wireless Voice Link Devices ............................................................................................... 339
xxiv

Table Of Contents
Physical Features .............................................................................................................340
Preparing the Base Station in the Simulator ......................................................................341
Preparing the Handset for Use...........................................................................................342
Selecting the Radio Frequency Channel..............................................................................342
Powering Up the WVL Pair.................................................................................................343
Using the iPhone/Standalone Microphone .........................................................................343
Special Handset Settings ..................................................................................................344
Battery Capacity Indicator.................................................................................................345
Troubleshooting............................................................................................................... 345
Power Problems.................................................................................................................................. 345
Audio Problems................................................................................................................................... 345
RF Channel Initial Operating Frequencies........................................................................... 347
Fidelis Lucina Medication Information ......................................................................... 349
Müse Mannequin Setup Screens................................................................................... 351
Mannequin Setup Screen for a Prepartum SCE ....................................................................352
Mannequin Setup Screen for an Active Phase Breech SCE..................................................... 353
Mannequin Setup Screen for an Active Phase Vertex SCE .....................................................354
Mannequin Setup Screen for a Vaginal Delivery Breech Left Sacrum SCE............................... 355
Mannequin Setup Screen for a Vaginal Delivery Breech Right Sacrum SCE.............................356
Mannequin Setup Screen for a Vaginal Delivery Vertex SCE..................................................357
Mannequin Setup Screen for a Cesarean Breech SCE............................................................358
Mannequin Setup Screen for a Cesarean Vertex SCE ............................................................ 359
Mannequin Setup Screen for a Non-Gravid SCE....................................................................360
xxv

Table Of Contents
This page intentionally blank
xxvi
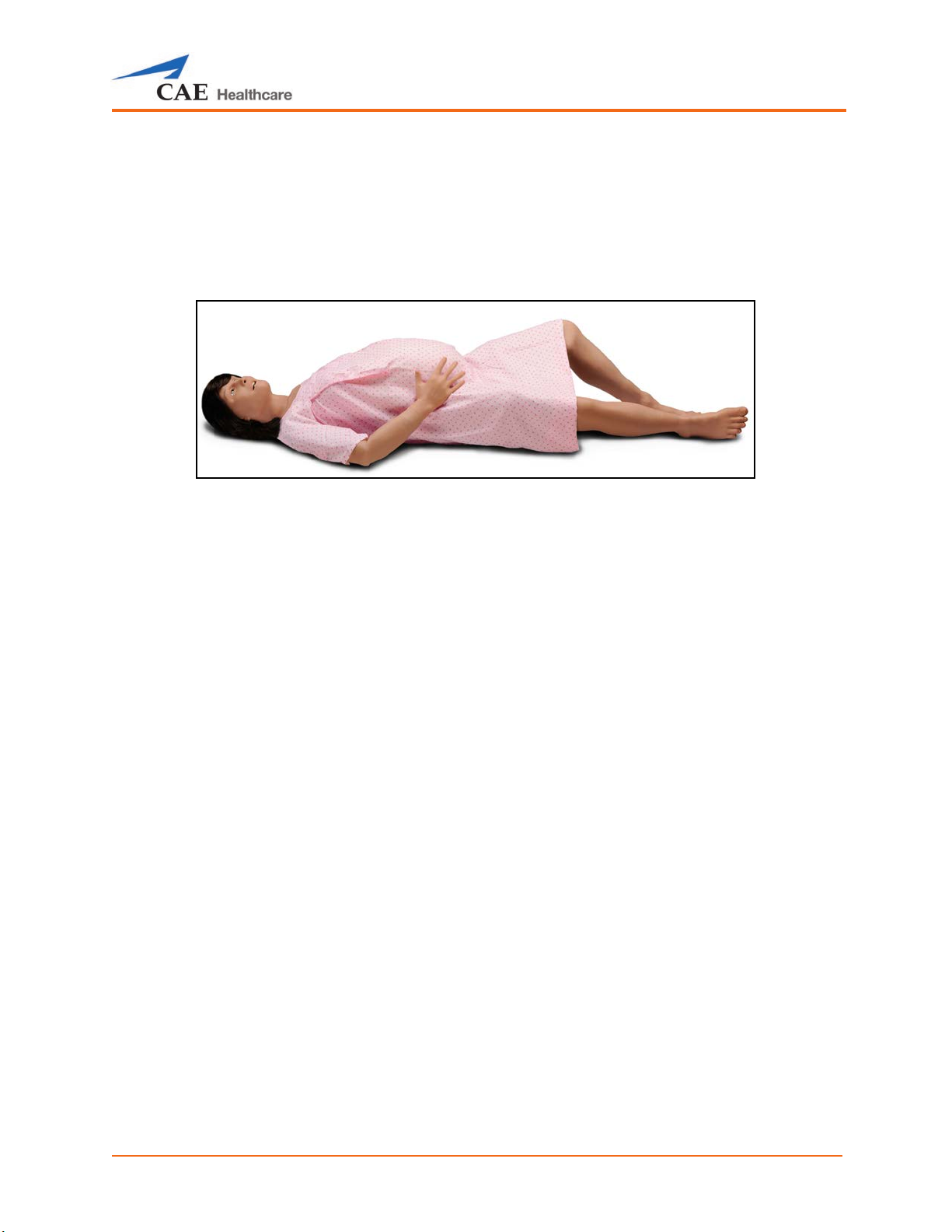
Introduction
INTRODUCTION
Welcome to the CAE FidelisTM Lucina Maternal Fetal Simulator user guide. This guide provides complete
instructions on how to use and maintain your simulator. The Fidelis Lucina simulator has been designed
to provide instructors and learners with advanced tools for obstetrical training.
The CAE Fidelis Lucina Maternal Fetal Simulator - Maternal Mannequin
The autonomous simulator reacts to medical interventions with appropriate physiological responses
and wirelessly communicates with the Instructor Workstation, creating an authentic experience for the
learner and keeping the instructor informed as the scenario progresses. The Instructor Workstation
provides the instructor with scenario development tools and programmable patient physiology to
create an immersive, realistic training environment.
The Fidelis Lucina simulator provides all the components necessary for prepartum care, normal and
complicated vaginal deliveries and postpartum care. The simulator supports cephalic and breech
delivery, placenta delivery, shoulder dystocia, nuchal cord, neonate crying, postpartum vaginal
bleeding, and boggy and inverted uterus. In addition, the following medical interventions can be
performed:
• ECG and electrical therapy
• Epidural administration
• Forceps delivery
• Intubation
• Leopold’s maneuvers
• McRoberts maneuver
• Medication and fluid administration
• Mechanical ventilation support
• Neonate suctioning
• Rotational maneuvers
• Suppository administration
• Uterine massage and bimanual compression
• Vacuum delivery
• Zavanelli maneuver
1

Introduction
Contained in this User Guide
This User Guide has been designed for quick access to information on how to use and maintain the CAE
Fidelis™ Maternal Fetal Simulator. Please be sure to read and follow the Cautions and Warnings on the
pages preceding the Table of Contents. This is for the safety of users as well as for the protection of the
simulator.
The Equipment Overview outlines the items that come standard with the purchase of a Fidelis. Before
using the system, follow the step-by-step instructions included in the Setup section.
The Configuring the Mannequin section shows users how to configure the internal components (i.e.,
cervix, uterus, fetus) of the Fidelis for the different birthing options.
The Using Müse section describes the different features and functions in the Müse software.
The Using TouchPro Patient Monitor and Using the TouchPro CTG sections describe how to setup
and utilize the TouchPro Patient Monitor and TouchPro CTG software.
The Using the Maternal Fetal Simulator section provides instructions on the use of the various
parameters and hardware features integrated into the simulated experiences. This section also includes
information on how the simulator and software components work and the functionality that each
supports.
The Care and Maintenance section contains warranty details and cleanup and care instructions that
must be followed to ensure optimal functioning of the Fidelis.
The user guide also includes information on:
• Condition Guidelines for Programming Patients in Müse
• Müse Parameter Descriptions
• Parameter Display Definitions
• Recommended Clinical Supply Sizes
• Ischemic Index Conditions
• Wireless Voice Link Instructions
• Maternal Fetal Simulator Medication Information
• Müse Mannequin Setup Screens
2
 Loading...
Loading...