SLEEP&GO
CARDIORESPIRATORY POLYGRAPH
USER’S MANUAL
534-7AB-MU2 • REV. 1.07 • 2014-06
MANUAL
ST
A
RT
SYSTEM
CO
NFIG.
19/06/10
75% 11:15

Sleep&GO Manual
Revision: 534-7AB-MU2 Rev. 1.07
All rights reserved.
Please refer to the device’s Service Manual for additional information.
This manual can be purchased through the After Sales Service.
SIBEL S.A.
Rosselló 500, 08026 Barcelona - Spain
National Sales: Tel. 93 436 00 08 e-mail: comercial@sibelmed.com
International Sales: Tel. +34 93 436 00 07
E-mail: export@sibelmed.com
Technical service: Tel. +34 93 433 54 50
E-mail: sat@sibelmed.com
Fax: +34 93 436 16 11, Website: www.sibelmed.com
SIBEL, S.A. belongs to SIBELGROUP
COPYRIGHT
No part of this publication may be reproduced, transmitted, transcribed, stored in a
back-up system or translated into any language or computer language in any form
or by any means, electronic, mechanical, optical, chemical or manual without the
express written consent from SIBEL S.A.
DISCLAIMER
SIBEL S.A. is responsible for the security, reliability and performance of this
equipment only if:
• The place where the systrem is installed or used meets the requirements for
electrical installations IEC and other applicable regulations.
• All repairs, revisions or modications, both in and out of the warranty period, are
made by technical staff of Meditel Ingeniería Médica S.L. o SIBEL S.A.
• The system is used by qualied staff in accordance with the recommendations
stated in this User’s Manual.
BRANDS
Bitmed is a registered trademark of Sibel, S.A.
534-7AB-MU2 • REV. 1.07

PRODUCT IN COMPLIANCE WITH MEDICAL DEVICE DIRECTIVE
93/42/EEC (CLASS IIa).
Thank you for choosing this product. SLEEP&GO system is designed and
manufactured with the best guarantees of quality.
Applications SLEEP&GO and its related software will open a world of
possibilities in the sleep study.
If you have any possible improvement for this product, we welcome your
suggestions may be directed to Customer Service Department.
Revised Approved
Date: 2014-06 Date: 2014-06
Technical Director Sales Director
534-7AB-MU2 • REV. 1.07
0197

Index
Sleep&Go
User’s Manual
4
534-7AB-MU2 • REV. 1.07
TABLE OF CONTENTS
SAFETY ..............................................................................7
INTENDED USE ..................................................................7
INDICATIONS FOR USE .....................................................8
LIMITATIONS IN USE. CONTRAINDICATION .....................9
WARNING AND PRECAUTIONS ..........................................9
DISPOSAL OF ELECTRICAL AND ELECTRONIC DEVICES BY
DOMESTIC USERS IN THE EUROPEAN UNION .......13
1 INSTRUCTIONS OF INSTALLATION AND USE ...............15
1.1 MODELS ....................................................................15
1.2 PACKING LIST ...........................................................16
1.3 LAYOUT OF CONTROLS, INDICATORS AND
CONNECTORS ..................................................................20
1.3.1 FRONT/TOP/RIGHT PANNEL ..................................20
1.3.2 LEFT PANNEL .......................................................... 20
1.3.3 REAR PANNEL .........................................................21
1.4 INSTALLATION AND START-UP ..................................21
1.4.1 BATTERY PLACEMENT ............................................21
1.4.2 POWER SAVING MODE ............................................22
1.4.3 BLUETOOTH MODULE INSTALLATION .....................22
1.4.4 PLACEMENT OF SENSORS AND ELECTRODES ...........22
1.4.4.1 PLACEMENT OF THE SLEEP&GO POLYGRAPH .......22
1.4.4.2 NASAL CANNULA .................................................23
1.4.4.3 THERMOCOUPLE AIRFLOW SENSOR ..................... 24
1.4.4.4 THORACIC AND ABDOMINAL EFFORT BANDS .......25
1.4.4.5 PULSE OXIMETER ................................................26
1.4.4.6 BODY POSITION AND ACTIVITY ..........................29
1.4.4.7 SNORING SENSOR ...............................................29
1.4.4.8 LIMB MOVEMENT SENSOR ...................................30
1.4.4.9 EKG ELECTRODES ................................................30
1.4.4.10 EMG. EOG AND EEG ELECTRODES .......................31
1.4.4.11 EVENT MARKER ..................................................33
2. OPERATION ................................................................. 34
2.1 WORKING MODES ...................................................... 34

Sleep&Go
User’s Manual
Index
5
534-7AB-MU2 • REV. 1.07
2.2 SYSTEM CONFIGURATION .........................................35
2.3 INITIAL DEVICE SETUP .............................................36
2.3.1 SETTING THE DATE AND TIME ................................ 36
2.3.2 SETTING THE LANGUAGE ........................................ 36
2.3.3 SETTING THE TYPE OF BATTERIES ..........................37
2.3.4 SETTING THE UNITS OF THE CPAP CHANNEL ..........37
2.4 SWITCHING ON/OFF THE BLUETOOTH MODULE ........37
2.5 MAKING POLYGRAPHY TESTS IN ONLINE MODE ........39
2.6 MAKING POLYGRAPHY TESTS IN HOLTER MODE ........39
2.6.1 STARTING AND ENDING A TEST MANUALLY............39
2.6.2 SCHEDULED TESTS .................................................40
2.6.2.1 SCHEDULING A TEST IN THE SLEEP&GO .............. 40
2.6.2.2 DISPLAYING THE SCHEDULED TESTS ...................42
2.6.2.3 CHANNEL CONFIGURATIONS ...............................43
2.7 TRANSFER AND REVIEW OF THE TESTS .....................43
2.8 FIRMWARE UPDATE ...................................................44
2.9 DEVICE OPTIONS UPDATE ......................................... 44
3. TECHNICAL SPECIFICATIONS ...................................... 46
3.1 GENERAL DATA ..........................................................46
3.2 PULSE OXIMETER TECHNICAL SPECIFICATIONS ........50
3.3 CONDITIONS OF OPERATION AND STORAGE OF
ACCESSORIES ..................................................................51
3.4 APPLICABLE STANDARDS .......................................... 51
4. SYMBOLOGY ................................................................ 55
4.1 SYMBOLOGY OF THE SLEEP&GO ................................. 55
4.2 SYMBOLOGY OF THE ACCESSORIES ........................... 56
4.3 VALIDATED ACCESSORIES ......................................... 58
5. CLEANING AND MAINTENANCE ....................................60
5.1 CLEANING .................................................................60
5.2 PREVENTIVE MAINTENANCE ...................................... 60
5.2.1 ACTIONS TO BE TAKEN BY THE USER ......................60
5.2.2 ACTIONS TO BE TAKEN BY QUALIFIED PERSONNEL 61
5.3 CORRECTIVE MAINTENANCE ......................................62
ANNEX 1. ELECTROMAGNETIC COMPATIBILITY ...............63
ANNEX 2. TROUBLESHOOTING GUIDE ............................. 68

Index
Sleep&Go
User’s Manual
6
534-7AB-MU2 • REV. 1.07

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
Declaration of conformity
7
SAFETY
The Sleep&Go cardiorespiratory polygraph has been developed
by the R+D+i Department of SIBEL S.A. with the collaboration
of reference Health Centers and doctors specialized in the area of
Sleep Disorders.
The Sleep&Go cardiorespiratory polygraph is designed and
manufactured in accordance with the Quality Manual of SIBEL S.A.
which is in consistency with the quality standards EN 13485 and
ISO 9001 and European Directive 93/42/EEC concerning medical
devices and 2007/47/EC. According to this directive the equipment
is Class IIa. The Sleep&Go also complies with the EN 60601.1
Electrical Safety and Electromagnetic Compatibility EN 60601.1.2
standards, as specied in the ELECTROMAGNETIC COMPATIBILITY
annex.
The Sleep&Go can transmit data via Bluetooth and therefore follows
the Directive 1999/5/EC on radio equipment and telecommunications
terminal equipment. For other countries complies with FCC rules
(parts 15c) and Canada Industry (IC: 5123A-BGTWT11A).
The Sleep&Go also complies with the following directives and
regulations: Packaging and packaging waste directive 94/62/
EC; Waste Electrical and Electronic Equipment Directive (WEEE)
2002/96/EC; Regulation EC 1272/2008 on classication, labelling
and packaging of substances and mixtures (REACH).
INTENDED USE
Acquisition, storage and display of biomedical signals for the
simplied diagnosis and control of sleep-related breathing disorders
(mainly Sleep Apnea and Hypopnea Syndrome) being anyone or
any combination of the following signals: respiratory airow, snore,
CPAP pressure measurement, thoracic effort, abdominal effort,
SpO2, beats per minute, perfusion wave, body position, body
activity, extremity limb movement, and EEG-EMG-EKG signals.
Next conditions must be taken into account:
• Use in a health center, patient’s home or similar indoor use (not
for outdoor use).
• Not intended for use in moving transport vehicles.

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
Safety
8
• Not intended for monitoring vital signals.
• EKG signal can not be used for heart diagnosis purpose. It’s
only intended for bradycardia and tachycardia detection during
sleep analysis.
INDICATIONS FOR USE
The Sleep&Go has been designed for maximum safety. All of the
operating instructions should been read before proceeding to
operate with the system. Failure to do so may result in injuries to
the user or the patient and damage to the device and/or accessories.
The Sleep&Go has been designed for being used by a doctor or
a technician trained in the acquisition of cardiorespiratory signals
and the transmission of these signals to a PC during polygraphic
tests. The user is allowed to congure the device under these
conditions. However, it is not recommended that the conguration
of the device is changed without understanding the principles of
signal digitalizing.
Minimum age of patients is 5 years, weighing over 15 kg and
a minimum height of 70 cm. The medical staff will instruct the
patient for a correct test execution, to avoid interferences in the
measurement and to replace the sensors in case of movement.
It is therefore important that the patient can understand the
instructions given by medical staff.
The intended environments of use are hospitals, sleep centres
and sleep clinics. Tests may also be carried out at the patient’s
home, with the exception of ExG signals (EEG, EMG, EOG, EKG).
In this case the patient is only authorized to start and stop the
test, and should be adequately instructed by the doctor on this
respect. The Sleep&Go is not designed to be used outdoors, nor in
other conditions or with energy sources not covered in this manual.
Using the Sleep&Go systems does not involve any monitoring or
diagnosis of the patient.

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
Safety
9
LIMITATIONS IN USE. CONTRAINDICATION
The interpretation of the tests and any ensuing treatment must be
carried out by a doctor. The acceptability of a test is the responsibility
of the health personnel.
The symptoms presented by the patient before starting any test
should be considered by the health personnel.
The Sleep&Go should not be used when it is likely that the validity
of the results may be compromised due to external factors (Electro
Magnetic interference – see section EMC).
WARNINGS AND PRECAUTIONS
The Sleep&Go IS NOT CERTIFIED FOR USE IN CONTINUOUS
MONITORING, where a failure in operation may cause injuries or
the death of the patient. This product does not maintain nor does
it help to maintain the life of the patient. The term CONTINUOUS
MONITORING is specied in regulation EN60601-1. The Sleep&Go
is classied as Class IIa in accordance with Directive 93/42/EEC on
medical devices.
The pulse oximeter is not provided with physiological-type alarms.
The pulse oximeter is calibrated to display functional oxygen
saturation and requires no calibration.
The pulse oximeter waveform is not standarized.
The Sleep&Go is not intended for monitoring vital signals.
There are no applicable parts to the patient which produce
stimulation.
The system has no user-serviceable parts. Use only authorized
service and spare parts supplied by the manufacturer.
Contact of liquids with the internal parts of the device and the
connectors must always be avoided.

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
Safety
10
The system is only resistant to moderate splashing and dripping
(Protection level IP22: protection against access to hazardous parts
with a nger; protected against solid objects with a diameter of
12.5 mm and above; protected against water drops falling vertically
with a maximum inclination of 15 degrees of the envelope).
Do not submerge the parts of the device in any liquid. MAY CAUSE
ELECTRIC DISCHARGE.
No parts are allowed for temporary immersion.
The cleaning instructions in this manual and also in the instructions
of use of any sensor supplied but not manufactured by SIBEL S.A.
must be carefully followed.
Keep your device protected from shock and vibration. During
transportation, place all the items in the carrying case. The material
provides enough protection against small accidental impact.
Do not use the system in and MRI environment.
The system is not designed to work in an explosive environment
or in the presence of ammable anesthetics or gases of any kind.
MAY CAUSE EXPLOSION.
This product is intended for indoor use (e.g. at the patient’s home
or hospital) and is not suitable for use during patient transportation.
The polygraph is not intended to be used outdoors or with other
conditions or energy sources that are not covered in this manual.
The Sleep&Go is not protected against debrillation shocks.
Therefore, never use a debrillator on a patient connected to a
Sleep&Go system.
Do not use an electric scalpel or a high frequency surgical device
while the patient is connected to any sensors or electrodes of the
Sleep&Go.
The use of mobile phones, transmitters and similar equipment
generating radio frequency emissions and placed next to the
system is not allowed during the tests. Therefore, do not use the
system in the presence of radio equipment (mobile phones, walkietalkie ...). Follow the recommendations regarding the separation

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
Safety
11
distance specied in the manufacturer’s declaration for EMC in this
manual.
Remember that when multiple devices are connected to a patient
there is a risk of accumulation of leakage current. Minimize the
number of devices.
Do not remove the device cover. The service and repair of the
device should be carried out only by trained personnel.
The Sleep&Go system is prepared to work at room temperature.
Avoid exposing any part of the system to heat sources. Also
avoid direct exposure to sunlight. Temperature changes cause
condensation and moisture. Before using the system, allow the
device to acclimate to ambient temperature. For reference, if the
temperature difference between the system and the environment is
above 10º C a 20 minutes wait time in an intermediate temperature
is recommended.
The polygraph should not be placed adjacent to or stacked with
other equipment.
The system shall be stored and used within the temperature ranges,
pressure and humidity specied in section number 3.
Artefacts in the signal may be produced as a result of ESD. A
trained operator should be able to recognize these artefacts easily.
The operator must be trained to be able to recognize the differences
between a biological signal and signal artifacts caused by patient
movements, RF interference or poor placement of the electrodes or
sensors. The presence of ESD or RF devices will not lead to wrong
conclusions. Unusable data is not considered a risk to the patient
safety.
Cables or sensors should not surround the patient’s neck, especially
when the patient is a child.
The Sleep&Go system does not increase the safety risk for patients
with pacemakers in accordance with the EN50061 standard (Medical
electrical equipment – Safety of implantable cardiac pacemakers).
Before using the system in patients with pacemakers, the operator
should check the documents accompanying the pacemaker with
respect to its certication and requirements of use and, if necessary,

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
Safety
12
contact the manufacturer.
Patients must be insistently warned that they must not open the
Sleep&Go or attempt to adjust it.
Sensors or accessories in bad condition should not be used.
Use only the Sleep&Go with accessories, sensors and electrodes
provided by the manufacturer or dealer, or those that meet the
specications of this manual. The use of other sensors with the
Sleep&Go can cause damage to the device or the quality of the
signals.
Sensors should be handled by their strongest parts, which are
the connectors. Also, they should not get wet or exposed to very
abrupt changes of temperature. To clean the sensors, do not use
abrasive chemicals. Do not apply excessive stress to the sensors.
In particular, avoid bending any part of the sensors. This means
that the material should not bend more than necessary in normal
use.
The polygraph is designed to be used exclusively by medical staff,
who should be supervised and instructed by a physician.
Medical personnel should inform the patient about precautions to
be found in the WARNINGS AND PRECAUTIONS section and to be
taken when using the equipment.
Use the provided carrying case when transporting the device and
its accessories.
Do not reuse single-use accessories as there is risk of infection to
the patient.
In case of doubt or unexpected event contact the manufacturer. You
can nd contact details on page 2 of this manual.
The use of nasal cannulas is not recommended in pregnant women
or children, because they contain phtalates.
Do not leave the batteries inside the device if it won’t be used for
a long period of time.
Ensure to perform the adquisition of signals in an acustic and light
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
13
environment that allows the patient to sleeping normally.
The system cannot be used for DIRECT CARDIAC APPLICATION.
The system is not an electrocardiograph, and therefore can not be
used to generate a separable ELECTROCARDIOGRAM for diagnostic
purposes, electrocardiographic monitoring or for ambulatory
electrocardiography. The EKG signal is only intended for bradycardia
and tachycardia detection during sleep analysis.
As for the electrodes, a proper contact of the skin with the
electrodes must be achieved by using conductive paste. Otherwise,
there is a risk of inaccurate measurements. Avoid contact with
the eyes or mucus membranes of gels, collodion, alcohol, acetone
or any substance used in the placement or removal of electrodes.
Be especially careful in the usage of collodion. Always follow the
recommendations for use provided by your collodion manufacturer.
The conductive part of the electrodes and connectors, including the
ground electrode must not touch other conductive parts, including
the ground.
DISPOSAL OF ELECTRICAL AND ELECTRONIC DEVICES BY
DOMESTIC USERS IN THE EUROPEAN UNION
Never dispose of the Sleep&Go, its accessories and its
batteries in the household trash. It must be disposed
of properly and may need to be recycled in accordance
with the statutory requirements in your country.
• Materials according to the RoHS Directive 2011/65/UE.
Conform from July 22th, 2014.
The device uses a lithium battery and could use an optional
NiMh battery.
For devices commercialised before July 22th, 2014: The
SleepSense sensor for limb movement contain mercury.
All Sleepsense sensors (except the cannulas) contain Pb in the
solderings.
All Sleepsense sensors (except the cannulas) may also contain
PBB and PBDE.
The cables for electrodes (Ref. 08093) contain Pb in the
soldering.

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
14
The interconnection bridge (Ref: 02741) and the cable adapter
(Ref. 01644) contain Cadmium.
• Materials according to the Medical Device Directive: two
of the sensors that may be used in combination with the
device, contains phtalates (Sleepsense cannulas and Pro-Tech
cannulas). The device and all the accesories are latex free.
• Materials according to the REACH regulation: neither the device
nor its accessories use any hazardous substance according to
REACH regulation.
• In the event that the device or its accessories are infected at
the time of recycling, it must be disinfected or disposed by
following the national regulations regarding the disposal of
infected products.
Information on proper disposal is available from your dealer or
from Technical Support at SIBEL S.A.
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
15
1. INSTRUCTIONS OF INSTALLATION AND USE
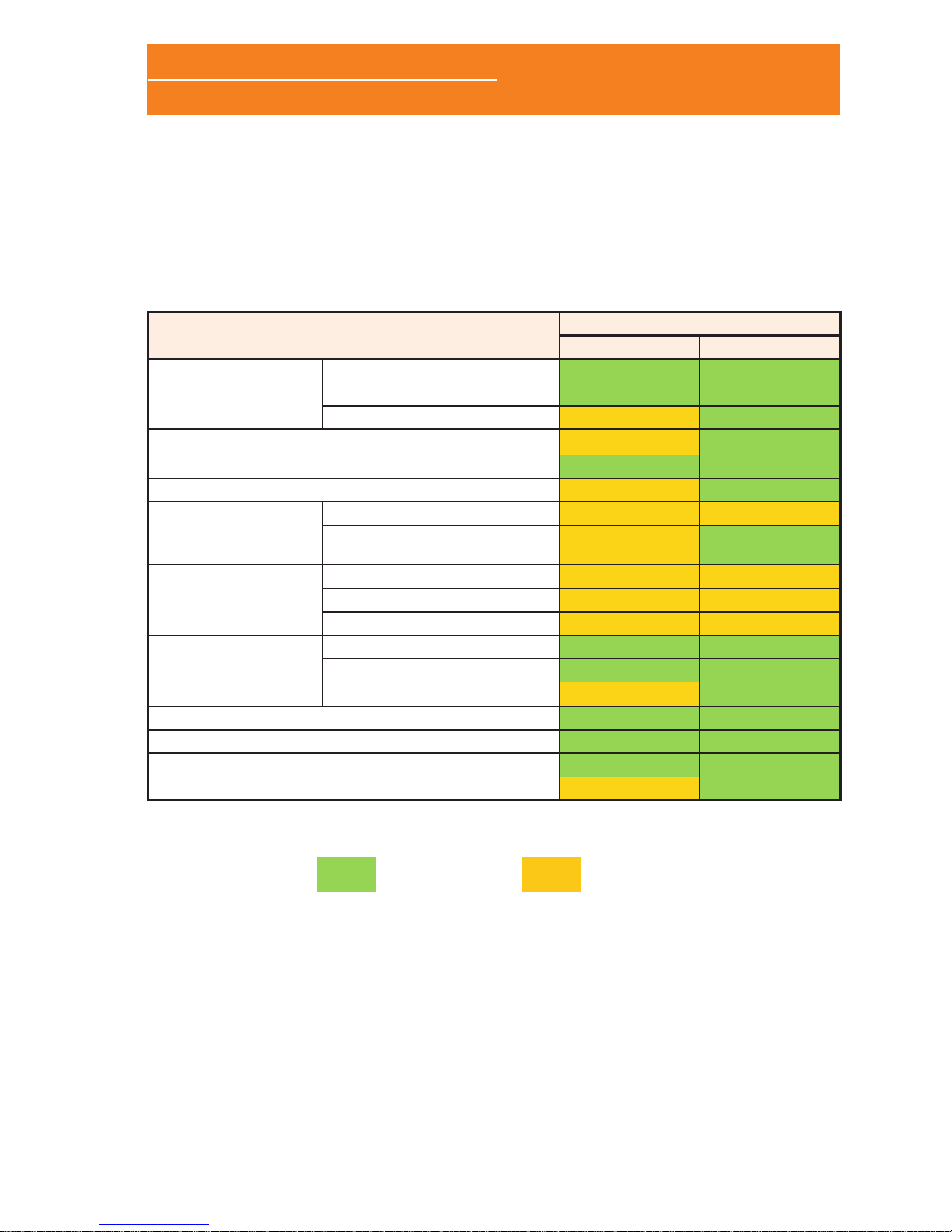
1.1 MODELS
The Sleep&Go cardiorespiratory polygraph is available in two
different models (A and B) with the following features:
Sleep&Go
Channels
A model B model
Nasal cannula
Airow
Snore
CPAP
Thermocouple
Inductive plethysmography band (thoracic)
Inductive plethysmography band (abdominal)
Auxiliary channel
External snore
Limb movements
External EXG
module
EXG 1
EXG 2
EXG 3
External Xpod
SpO
2
BPM
Pulse wave
Position
Activity
Marks
Bluetooth (real time tests)
Default Opon
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
16
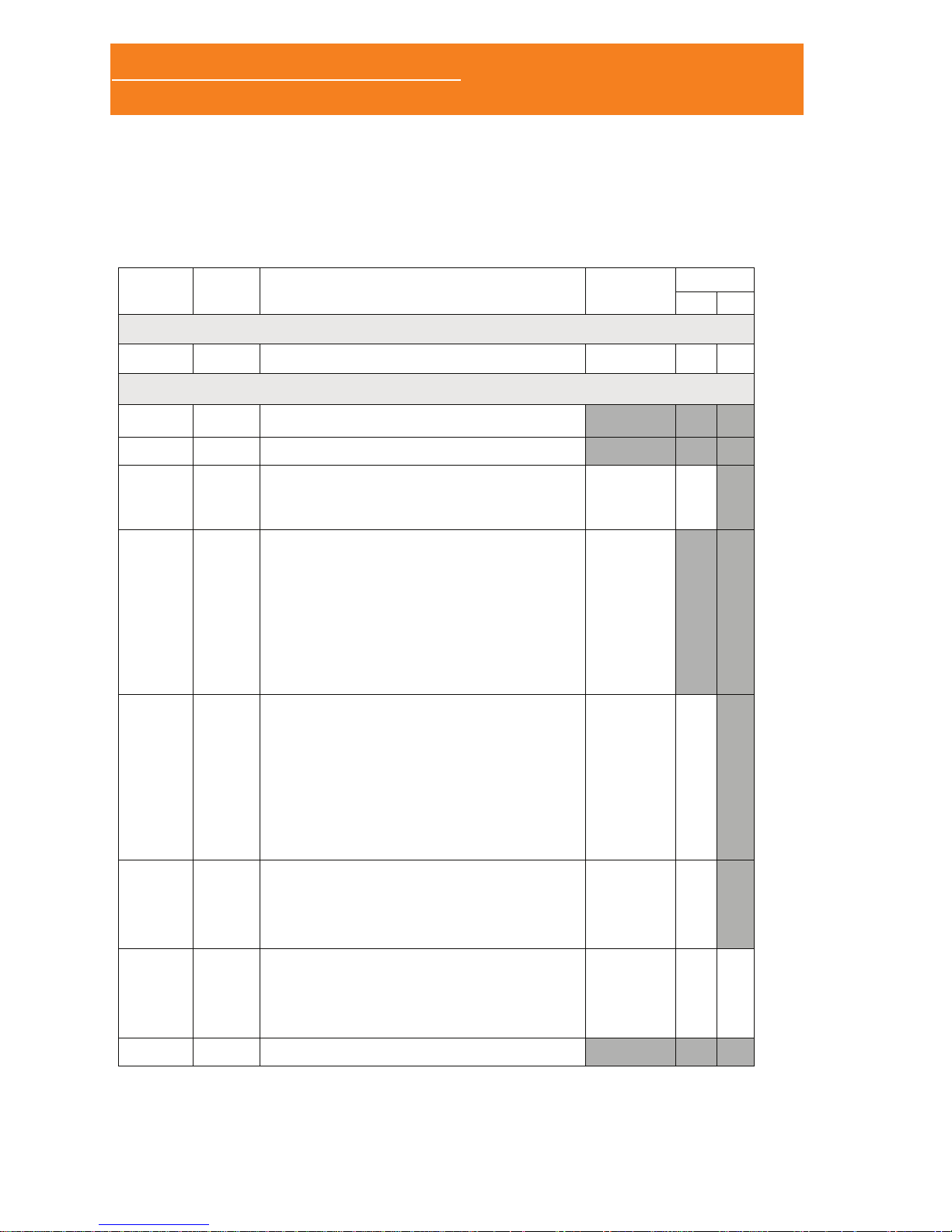
1.2 PACKING LIST
RELACIÓN DE CONTENIDO / PACKING LIST 534-708-120 REV. 4
SCREEN&GO/SLEEP&GO 2014-06
(*)
CUANDO SE ENTREGA LA REFERENCIA 08089 JUNTO CON UN SCREEN/SLEEP&GO NO SE INCLUYE LA REFERENCIA 01420 (PAQUETE DE 2 BANDAS
INDUCTIVAS TAMAÑO XL), PUESTO QUE YA ESTÁ INCLUIDA EN LA REFERENCIA 08088. LA REFERENCIA 01420 SE INCLUIRÁ SÓLO SI EL MÓDULO 08089 SE
SUMINISTRA POR SEPARADO.
WHEN THE REFERENCE 08089 IS SUPPLIED WITH A SCREEN / SLEEP & GO DOES NOT INCLUDE REFERENCE 01420 (PACK OF 2-BAND INDUCTIVE SIZE XL),
SINCE IT IS ALREADY INCLUDED IN THE REFERENCE 08088. THE REFERENCE 01420 IS INCLUDED ONLY IF THE MODULE 08089 IS DELIVERED SEPARATELY.
1/4
MODELOS/ MODELS
CÓDIGO
CODE
CANT.
QTY.
DESCRIPCIÓN
DESCRIPTION
SCREEN&GO
SLEEP&GO
A B
POLÍGRAFO / POLYGRAPH
_______ 1 SCREEN&GO/SLEEP&GO SN: 347 - _______
Accesorios Estándar / Standard Accessories
06312 1
BANDA DE SUJECIÓN TAMAÑO GRANDE (L) /
FASTENING BELT LARGE SIZE (L)
08049 1
CÁNULA DESECHABLE NASAL /
DISPOSABLE NASSAL CANNULA
08087
06309
1
1
MÓDULO TERMOPAR (INCLUYE ACTIVACIÓN DEL
CANAL) / THERMOCOUPLE MODULE (INCLUDES
CHANNEL ACTIVATION)
• SENSOR TERMOPAR / THERMOCOUPLE SENSOR
08088
06314
01420
07678
1
1
1
1
MÓDULO DE ESFUERZO TORÁCICO INDUCTIVO
(INCLUYE ACTIVACIÓN DEL CANAL) /
INDUCTIVE THORACIC EFFORT MODULE (INCLUDES
CHANNEL ACTIVATION)
• INTERFAZ DE AMPLIFICACIÓN BANDA INDUCTIVA
ESFUERZO TORÁCICO / THORAX EFFORT
INDUCTIVE BAND AMPLIFICATION INTERFACE
• BANDA INDUCTIVA ELÁSTICA TAMAÑO EXTRA
GRANDE (XL) / ELASTIC INDUCTIVE BAND XTRA
LARGE SIZE (XL)
• ANILLA SUJECIÓN / FASTENING RING
08089
06308
01420
(*)
07678
1
1
1
(*)
1
MÓDULO DE ESFUERZO ABDOMINAL INDUCTIVO PARA
SLEEP&GO (INCLUYE ACTIVACIÓN DEL CANAL) /
INDUCTIVE ABDOMINAL EFFORT MODULE FOR
SLEEP&GO (INCLUDES CHANNEL ACTIVATION)
• INTERFAZ DE AMPLIFICACIÓN BANDA INDUCTIVA
ESFUERZO ABDOMINAL / ABDOMINAL EFFORT
INDUCTIVE BAND AMPLIFICATION INTERFACE
• BANDA INDUCTIVA ELÁSTICA TAMAÑO EXTRA
GRANDE (XL) / ELASTIC INDUCTIVE BAND XTRA
LARGE SIZE (XL)
• ANILLA DE SUJECIÓN / FASTENING RING
---
08090
06310
1
1
CANAL AUXILIAR (MOVIMIENTO EXTREMIDADES)
PARA SLEEP&GO (INCLUYE ACTIVACIÓN DEL CANAL) /
AUXILIARY CHANNEL (LIMB MOVEMENT) FOR
SLEEP&GO (INCLUDES CHANNEL ACTIVATION)
• KIT SENSOR MOVIMIENTO EXTREMIDADES / LIMB
MOVEMENT SENSOR KIT
---
08091
06346
1
1
CANAL AUXILIAR (RONQUIDO) PARA SLEEP&GO
(INCLUYE ACTIVACIÓN DEL CANAL) /
AUXILIARY CHANNEL (SNORING) FOR SLEEP&GO
(INCLUDES CHANNEL ACTIVATION)
• SENSOR RONQUIDO PIEZOELÉCTRICO /
PIEZOELECTRIC SNORE SENSOR
---
08060 1
TARJETA MEMORIA MICRO SD CON ADAPTADOR SD /
MICROSD MEMORY CARD WITH SD ADAPTER
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
17
RELACIÓN DE CONTENIDO / PACKING LIST 534-708-120 REV. 4
SCREEN&GO/SLEEP&GO 2014-06
2/4
03673 2
PILAS ALCALINAS AA 1.5V /
AA 1.5V ALKALINE BATTERY
08011 1
MALETÍN TRANSPORTE SCREEN&GO
CARRYING CASE SCREEN&GO
--- ---
08010 1
MALETÍN TRANSPORTE SLEEP&GO /
CARRYING CASE SLEEP&GO
---
08070
______
08072
08071
1
1
1
1
CD SOFTWARE BITMEDLAB (CON LICENCIA) /
CD BITMEDLAB SOFTWARE (WITH LICENSE)
• MANUAL DE USO / USER’S MANUAL
(Doc.534-740-MU_)
• MÓDULO BASE DE DATOS / DATABASE MODULE
• MÓDULO ANALISIS AUTOMÁTICO EVENTOS /
AUTOMATIC EVENTS DETECTION MODULE
______ 1
SCREEN&GO MANUAL DE USO /
SCREEN&GO USER’S MANUAL (Doc. 534-70S-MU_)
--- ---
______ 1
SLEEP&GO MANUAL DE USO /
SLEEP&GO USER’S MANUAL (Doc. 534-7AB-MU_)
---
______ 1
GUÍA RÁPIDA SCREEN - SLEEP&GO /
SCREEN-SLEEP&GO QUICK GUIDE (Doc. 534-7AB-GR_)
Accesorios Opcionales / Optional Accessories
08094 1
LECTOR TARJETAS MEMORIA USB PARA PC /
USB MEMORY CARD READER FOR PC
08073 1
MÓDULO BITMED VISION PARA BITMEDLAB /
BITMED VISION MODULE FOR BITMEDLAB
---
06976
08098
08012
07677
1
1
1
1
KIT DE PULSIOXIMETRÍA ADULTOS PARA SCREEN&GOSLEEP&GO (Canales: SPO
2
, BPM) / ADULT PULSE
OXIMETRY KIT FOR SCREEN&GO-SLEEP&GO (Channels:
SpO
2
, BPM)
• MODULO PULSIOXIMETRIA XPOD /
XPOD PULSE OXIMETRY MODULE
• SENSOR PULSIOXIMETRIA SOFT (ADULTO) /
SOFT PULSE OXIMETRY SPO2 SENSOR (ADULT)
• MUÑEQUERA DE SUJECIÓN / WRISTBAND
08069
08098
08013
07677
1
1
1
1
KIT DE PULSIOXIMETRÍA PEDIÁTRICO PARA
SCREEN&GO/SLEEP&GO (Canales: SPO
2
, BPM) /
PEDIATRIC PULSE OXIMETRY KIT FOR
SCREEN&GO/SLEEP&GO(Channels: SpO
2
, BPM)
• MODULO PULSIOXIMETRIA XPOD /
XPOD PULSE OXIMETRY MODULE
• SENSOR PULSIOXIMETRIA SOFT (PEDIÁTRICO)/
SOFT PULSE OXIMETRY SPO2 SENSOR (PEDIATRIC)
•
MUÑEQUERA DE SUJECIÓN / WRISTBAND
08078 1
OPCIÓN FIRMWARE CANAL DE RONQUIDO POR PRESIÓN
/ PRESSURE SNORING CHANNEL FIRMWARE OPTION
08079 1
OPCIÓN FIRMWARE CANAL DE POSICIÓN CORPORAL /
BODY POSITION CHANNEL FIRMWARE OPTION
08080 1
OPCIÓN FIRMWARE CANAL DE ACTIGRAFÍA /
ACTIGRAPHY CHANNEL FIRMWARE OPTION
08081 1
OPCIÓN FIRMWARE CANAL DE MARCAS DEL PACIENTE /
PATIENT’S MARKS CHANNEL FIRMWARE OPTION
08082 1
OPCIÓN FIRMWARE CANAL DE PRESIÓN CPAP /
CPAP PRESSURE CHANNEL FIRMWARE OPTION
---
08083 1
OPCIÓN FIRMWARE CANAL DE ONDA DE PULSO /
PULSE WAVE CHANNEL FIRMWARE OPTION
---
08084 1
OPCIÓN FIRMWARE MÓDULO BLUETOOTH /
BLUETOOTH MODULE FIRMWARE OPTION
---
08022
08251
1
1
MÓDULO DE SENSORES EXG /
EXG SENSOR MODULE
• PIEZA ANTI APERTURA TAPA BATERÍAS /
BATTERY COVER BLOCKING PIECE
---
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
18
RELACIÓN DE CONTENIDO / PACKING LIST 534-708-120 REV. 4
SCREEN&GO/SLEEP&GO 2014-06
3/4
08085
08701
01644
02741
1
1
3
3
KIT DE ELECTRODOS EEG PARA SLEEP&GO / EEG
SENSOR KIT FOR SLEEP&GO
• ELECTRODO CUCHARA ORO EXG (PAQUETE 10U) /
EXG GOLD CUP ELECTRODES (PACKAGE 10PC)
• CABLE ADAPTADOR 1MM A 1.5MM /
CABLE ADAPTER 1MM TO 1.5MM
• PUENTE DE INTERCONEXIÓN /
INTERCONNECTION BRIDGE
---
08086
08093
01027
01644
1
1
1
3
KIT DE ELECTRODOS ECG PARA SLEEP&GO / EKG
SENSOR KIT FOR SLEEP&GO
• CABLES PARA ELECTRODOS DE CORCHETE (PAQUETE
10U) / CABLES FOR BUTTON STUD TYPE ELECTRODES
(PACKAGE 10 PC)
• ELECTRODO DE ECG (PAQUETE 50 uni.
) /
ECG ELECTRODE (PACKAGE 50 units)
• CABLE ADAPTADOR 1MM A 1.5MM /
CABLE ADAPTER 1MM TO 1.5MM
---
06346 1
SENSOR RONQUIDO PIEZOELÉCTRICO / PIEZOELECTRIC
SNORE SENSOR
---
07679 1
CÁNULA NASAL DESECHABLE PROTECH (PAQUETE DE
60u) / DISPOSABLE PROTECH NASAL CANNULA (PACK
OF 60u)
07680 1
CÁNULA ORO-NASAL DESECHABLE PROTECH (PAQUETE
DE 30u) / DISPOSABLE PROTECH ORO-NASAL CANNULA
40cm (PACK OF 30u)
07681 1
CÁNULA ORO-NASAL DESECHABLE SLEEPSENSE
(PAQUETE DE 5u) / DISPOSABLE SLEEPSENSE ORO-
NASAL CANNULA 60cm (PACK OF 5u)
08049 1
CÁNULA NASAL DESECHABLE SLEEPSENSE /
DISPOSABLE SLEEPSENSE NASAL CANNULA
06311 1
BANDA DE SUJECIÓN TAMAÑO PEQUEÑO (S) /
FASTENING BELT SMALL SIZE (S)
06313 1
BANDA DE SUJECIÓN TAMAÑO EXTRA GRANDE (XL) /
FASTENING BELT XTRA LARGE SIZE (XL)
01425 1
BANDA INDUCTIVA ELÁSTICA TAMAÑO PEQUEÑA (S) /
ELASTIC INDUCTIVE BAND SMALL SIZE (S)
01424 1
BANDA INDUCTIVA ELÁSTICA TAMAÑO MEDIANA (M) /
ELASTIC INDUCTIVE BAND MEDIUM SIZE (M)
01421 1
BANDA INDUCTIVA ELÁSTICA TAMAÑO GRANDE (L) /
ELASTIC INDUCTIVE BAND LARGE SIZE (L)
01420 1
BANDA INDUCTIVA ELÁSTICA TAMAÑO EXTRA GRANDE
(XL) / ELASTIC INDUCTIVE BAND XTRA LARGE SIZE (XL)
01417 1
BANDA INDUCTIVA ELÁSTICA TAMAÑO EXTRA EXTRA
GRANDE (XXL) / ELASTIC INDUCTIVE BAND XTRA XTRA
LARGE SIZE (XXL)
08701 1
ELECTRODO CUCHARA ORO EXG (PAQUETE 10u) / EXG
GOLD CUP ELECTRODES (PACK OF 10u)
---
08093 1
CABLES PARA ELECTRODOS DE CORCHETE (PAQUETE DE
10u) / CABLES FOR BUTTON STUD TYPE ELECTRODES
(PACK OF 10u)
---
01027 1
ELECTRODO DE ECG (PAQUETE DE 50u) /
ECG ELECTRODE (PACK OF 50u)
---
ESTÁNDAR
STANDARD
OPCIONAL
OPTIONAL
---
NO DISPONIBLE
NOT AVAILABLE

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
19
RELACIÓN DE CONTENIDO / PACKING LIST 534-708-120 REV. 4
SCREEN&GO/SLEEP&GO 2014-06
4/4
ADVERTENCIA:
• LOS ARTÍCULOS Y CANTIDADES RELACIONADAS ANTERIORMENTE HAN SIDO CUIDADOSAMENTE COMPROBADAS.
EN CASO DE FALTAS O DESPERFECTOS PROCEDAN A COMUNICÁRNOSLO LO MAS PRONTO POSIBLE.
•
SI DETECTA CUALQUIER DAÑO EN EL EMBALAJE, CONTACTE CON SU DISTRIBUIDOR ANTES DE PROCEDER A LA
INSTALACIÓN DEL EQUIPO.
• NO SE DEBE DESPRENDER DE LOS EMBALAJES, BOLSAS, ETC. HASTA QUE VERIFIQUE TOTALMENTE EL CORRECTO
FUNCIONAMIENTO DEL EQUIPO.
• EN CASO DE DEVOLUCIÓN DE MATERIAL O EQUIPO EN DEPOSITO, ROGAMOS NOS LO ENVÍEN EN PERFECTO
ESTADO, COMPLETO DE ACCESORIOS Y DEBIDAMENTE EMBALADO. CUALQUIER DESPERFECTO OCASIONADO
PROVOCARÍA UN CARGO CORRESPONDIENTE EN LA REPARACIÓN O EN LA REPOSICIÓN.
WARNING:
• THE ITEMS AND QUANTITIES RELATED BEFORE HAVE BEEN CAREFULLY CHECKED. IN CASE OF ANY PART IS
MISSING OR IS DAMAGED, NOTIFY US AS QUICKLY AS YOU CAN.
• IF YOU DETECT ANY DAMAGE IN THE PACKAGING, CONTACT WITH YOUR DISTRIBUTOR BEFORE PROCEEDING TO
INSTALL IT.
• DO NOT THROW AWAY THE PACKAGING, BAGS, ETC. UNTIL THE CORRECT FUNCTIONING OF THE DEVICE IS
VERIFIED
• IN THE CASE OF RETURNING THE GOODS, IT WILL BE APPRECIATED THAT YOU SEND THE DEVICE IN PERFECT
ORDER, WITH ALL THE ACCESSORIES AND PROPERLY PACKAGED. ANY DAMAGE SUFFERED WILL MAKE A CHARGE
CORRESPONDING TO REPAIR OR NEW PARTS.
PREPARADO/PREPARED BY: ................................. FECHA/DATE: .............
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
20
1.3 LAYOUT OF CONTROLS, INDICATORS AND
CONNECTORS
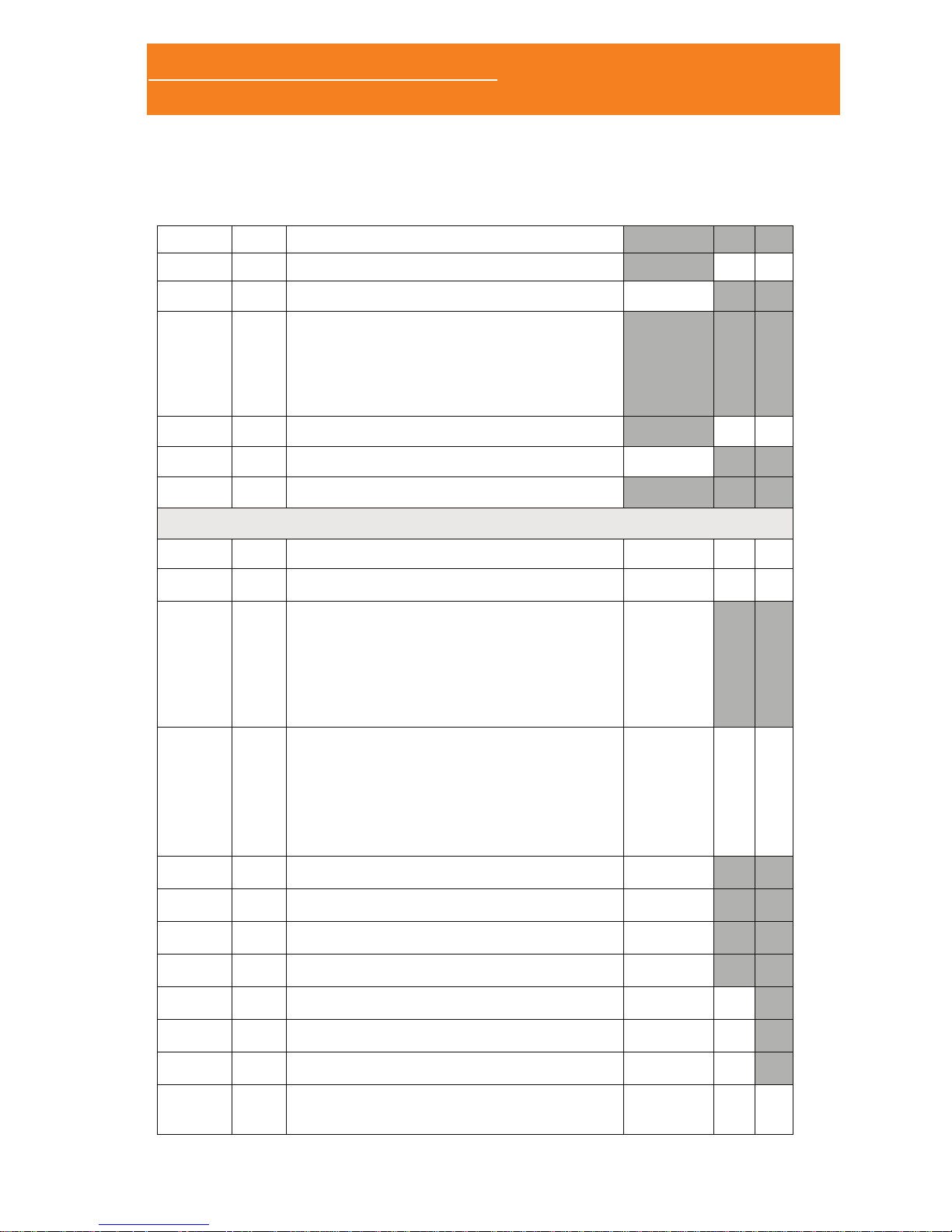
1.3.1 FRONT/TOP/RIGHT PANNEL
SpO2: XPod pulse
oximeter connector
Color LCD
Status LED
Joystick
Ther: thermocouple airow
sensor connector
Tho: thoracic effort band
connector.
Abd: abdominal effort band
connector.
Aux: auxiliary channel
connector (suitable for both
snore and limb movement
sensors)
ExG: ExG module
connector
Nasal: cannula
nasal cannula
connector
1.3.2 LEFT PANNEL
MicroSD memory
card slot
ON/OFF button
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
21
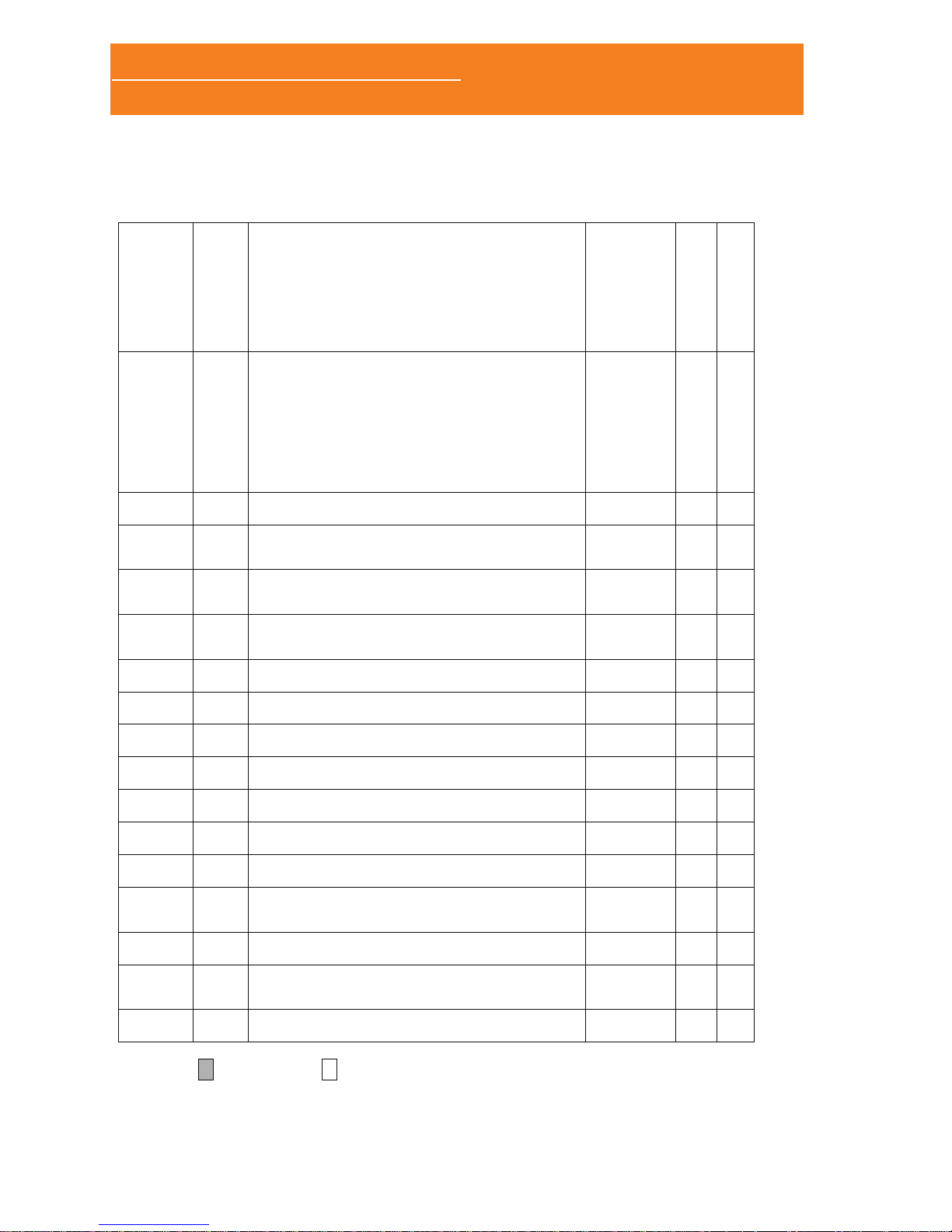
1.3.3 REAR PANNEL
08251 – Battery cover
blocking piece (only with
EXG module).
Battery compartment
(2x AA alkaline or
rechargeable batteries).
1.4 INSTALLATION AND START-UP
This system is made of solid-state professional components
manufactured under stringent quality controls. However,
accidents may occur during the transportation or storage, being
convenient an initial checking of the device and accessories prior
to installation. If you detect a deterioration in the package, please
contact the transport company and your supplier immediately,
prior to installation. Do not dispose the packaging until completely
verifying the proper functioning of the system.
Use only accessories described in this manual. The use of not
recommended accessories could adversely affect both the patient
safety and the equipment.
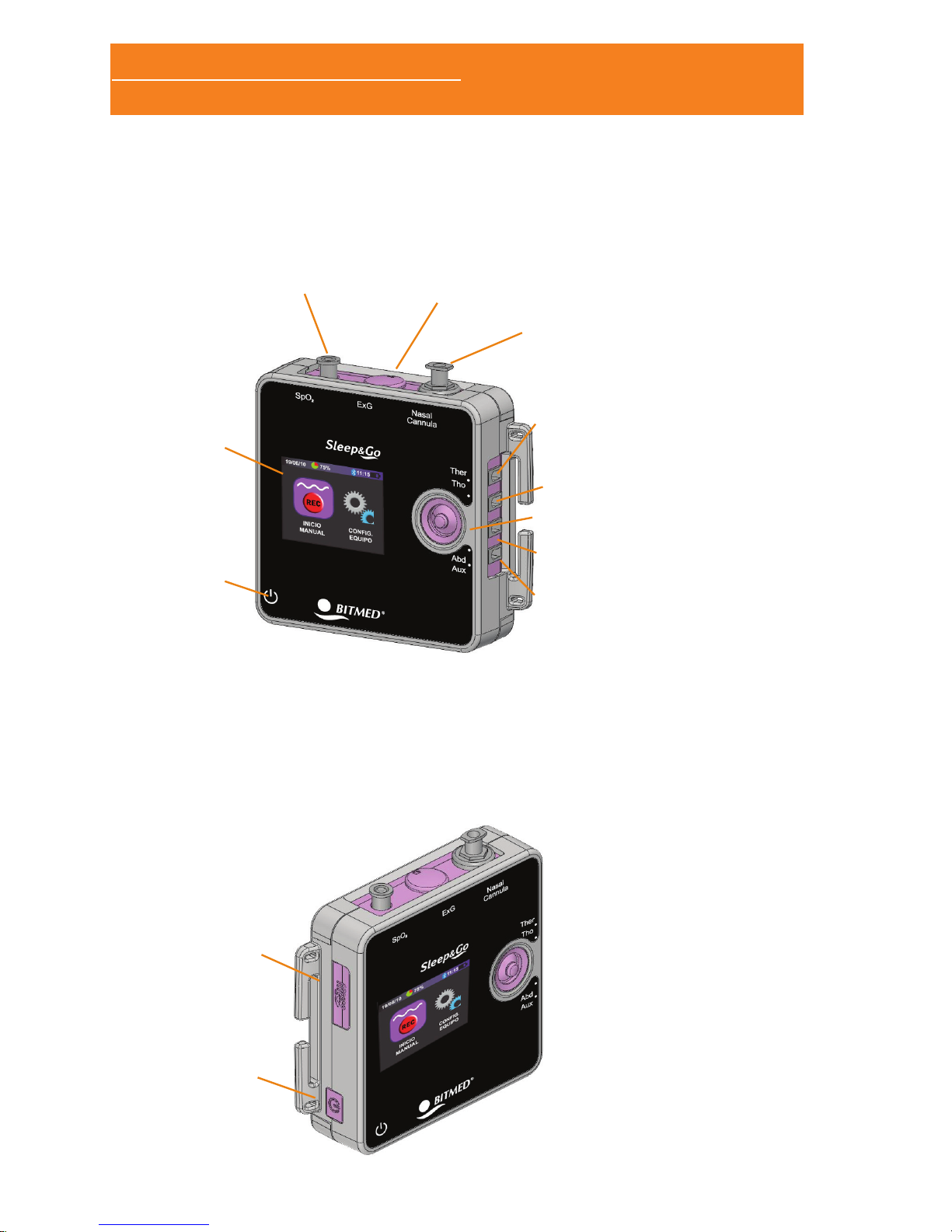
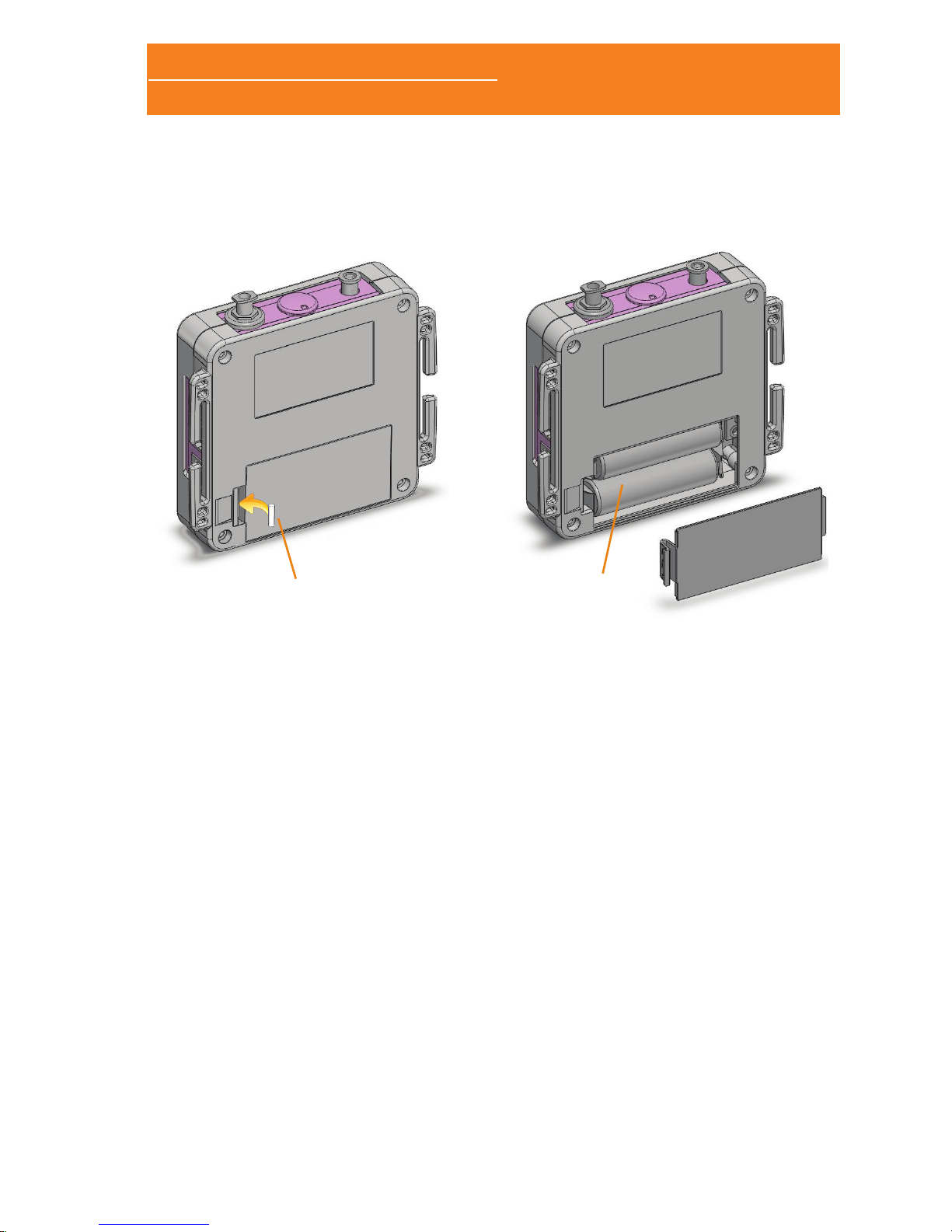
1.4.1 BATTERY PLACEMENT
The Sleep&Go system uses two 1.5V AA batteries. You can use
alkaline or rechargeable batteries with, at least, 2450mA/h. Using
smaller capacity batteries diminishes the autonomy. The autonomy
in normal use is about 12 hours operating in online mode and 24
hours in holter mode.
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
22
The battery compartment is located on the rear panel, protected by
a cap that needs to be removed to proceed to change the batteries
Before opening the baery compartment to replace the baeries
please switch o the Sleep & Go.
1.4.2 POWER SAVING MODE
To save power, the device switches off the display after 50 seconds
of inactivity (even if it is running a test) and the whole device after
5 minutes if the user does not start a test nor interacts with the
joystick.
1.4.3 BLUETOOTH MODULE INSTALLATION
The PC must have enabled and available this type of communication
in order to use the Bluetooth link with the Sleep&Go (optional
module). Install the driver and software for the PC Bluetooth
module, as detailed in the module user’s manual. You must then
search the Sleep&Go from the Bluetooth software in Windows and
pair the PC with the Sleep&Go. The pairing code corresponds to
the last three digits of the Sleep&Go serial number. You will nd
the serial number on the label on the back of the Sleep&Go device.
1.4.4 PLACEMENT OF SENSORS AND ELECTRODES
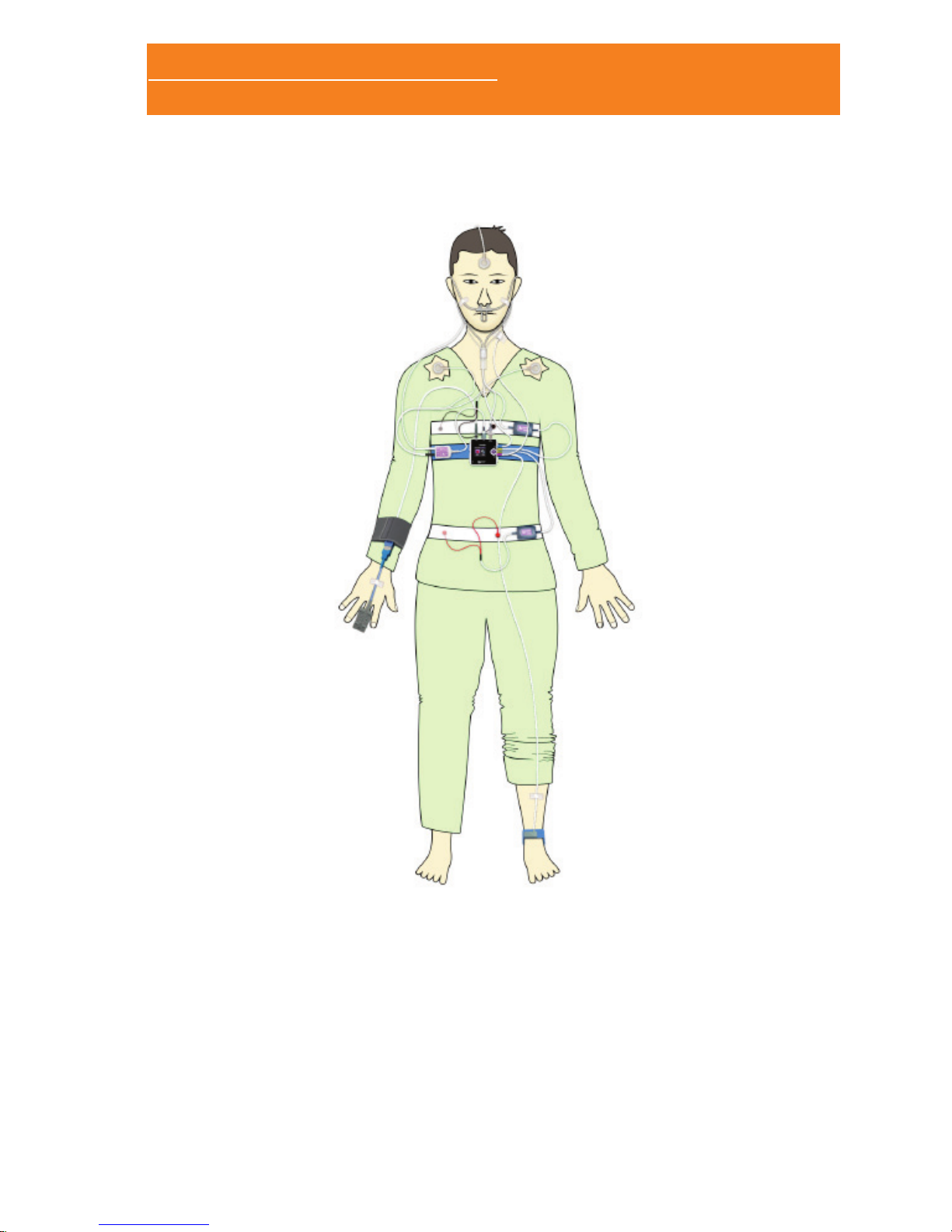
1.4.4.1 PLACEMENT OF THE SLEEP&GO POLYGRAPH
The small size and weight of the Sleep&Go system allows the
patient to wear it comfortably fastened with a strap. Follow these
steps to fasten the device to the patient:
• Insert the strap between the two guides in the rear side of the
Sleep&Go.
• Place the device on the patient’s chest, in a comfortable position
midway between the thoracic effort band (which should be on
top) and the abdominal effort band (which should be below).
• Secure the strap by means of the Velcro so that the device
stands rm.
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
23
General placement schema
1.4.4.2 NASAL CANNULA
The nasal airow cannula (refs. 07679, 07680, 07681, 08049)
is used to acquire the airow, snoring, and CPAP pressure level
signals, by means of the device’s internal pressure transducer.
The placement of the nasal cannula has a signicant impact in
obtaining high quality signals. Follow these steps to place it
properly:

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
24
• Place the cannula over the upper lip. Bend the nasal probes
softly until the tips are exposed to the maximum possible
air ow. The tips should not get in contact with the skin or
penetrate the nasal orice.
• Wrap the tube around the ears and place it under the chin.
Surgical tape can be used to fasten the tubes to the cheeks of
the patient. If necessary, adjust the tubes under the chin.
• Insert the cannula connector in the Luer connector of the
Sleep&Go (labeled as “Nassal cannula”) and turn it until secured.
1.4.4.3 THERMOCOUPLE AIRFLOW SENSOR
In addition to the nasal cannula, the Sleep&Go may also use
the thermocouple sensor (ref. 06309) in order to measure the
respiratory airow. The thermocouple sensor is to be connected to
the connector labeled as “Ther”.
The quality of the registered signal depends on variables such
as the lters selected in the software, the sensor placement, the
patient’s respiration, room temperature and CPAP pressure. Filters
can reduce the noise in the respiratory signal, but they can also
add distortion to the signal if used in excess. Filters are applied in
the BitmedLab software. Read that software’s manual if you need
to modify them.
The placement of the thermocouple sensor has a signicant impact
in obtaining high quality signals. Follow these steps to place it
properly:
• Place the thermocouple on the upper lip. Bend the nasal tips
softly until the ends of the sensor are exposed to the maximum
possible air ow. The tips should not get in contact with the

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
25
skin or penetrate the nasal orice.
• Wrap the cable around the ears and place it under the chin.
Surgical tape can be used to fasten the cables to the cheeks of
the patient. If necessary, adjust the sensor cable on the chin.
• Bend the oral probe softly in its position in front of the mouth,
so that the end of the sensor is exposed to the greatest oral
ow possible, approximately 1 cm from the mouth. The probe
should not touch the skin or the lips, nor penetrate the oral
cavity.
• Insert the sensor connector in the connector of the Sleep&Go
labeled as “Ther”.
The ambient temperature affects the magnitude of the signal. If the
temperature is similar to the patient’s temperature, the signal level
may be very low. On the other side, if the ambient temperature is
very different from the patient’s temperature, the signal level will
be higher.
The placement of a CPAP mask over the thermocouple sensor may
affect the difference between the patient and the room temperature
and may also affect the placement of the thermocouple sensor.
1.4.4.4 THORACIC AND ABDOMINAL EFFORT BANDS
The inductive plethysmography belts (refs. 01425, 01424, 01421,
01420, 01417) supplied with the Sleep&Go are based in the RIP
technology (Respiratory Inductive Plethysmography).
• Strap the sensor bands around the patient’s chest and abdomen,
over any clothing.
• Attach the rst wire to the snap that is on the same side of the

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
26
band as the Velcro patch.
• While holding this end to the left of
the patient’s center line, wrap the
band around the patient’s chest or
abdomen.
• Pull the other end over the rst end
and secure the band on the right side of the center line. The
band should be snug but not too tight.
• Attach the second wire to the exposed snap.
Make the connecon and disconnecon of the snaps with the
interface box disconnected from the computer.
• Make sure the box in the cable that goes to the chest band
is labeled as “Chest” (ref. 06314) and the one going to the
abdominal band is labeled as “Abd.“ (ref. 06308).
• Insert the connector from each the respiratory effort belt in
the corresponding input connector of the Sleep&Go, labeled as
“Thor” for the thoracic belt and “Abd” to the abdominal belt.
• Fix each of the little boxes to the corresponding belt with the
aid of the provided strips.
1.4.4.5 PULSE OXIMETER
The system is able to provide the pulse wave, as well as the
measurement of the pulse rate and the functional oxygen
saturation, based on the technology developed by Nonin Medical
Inc. The external pulse-oximeter (named XPod) (ref. 08098) is to
be connected on the top side of the device. The SpO2 sensor is to
be connected on the free end. Pay special attention to the sensor
being used. Please consult the manufacturer before using non-
recommended sensors.

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
27
To place pulse oximeter sensor, follow these steps:
• Place the wristband provided in the patient’s wrist and insert
the XPod module into the socket.
• Insert a nger (preferably the index nger) in the sensor until
the nger tip reaches the stop. It is not recommended to place
this sensor in the thumb. Keep the ngernail of the patient
pointing toward the top side of the sensor. Make sure that long
ngernails do not interfere with the adequate positioning of the
nger.
• To obtain the best results, fasten the cable to the nger, using
surgical tape, preferably around the base of the nger. Make
sure that the tape fastening the cable does not restrict blood
circulation.
• Take the cable coming out of the wristband over the arm and
connect it to “SpO2” of Sleep&Go.
Furthermore, the following conditions of use should be observed:
• The oximeter must not be used by itself to reach important
medical conclusions. Medical caution must always be taken and
other means must be used, whenever possible, for conrmation.
• Incorrect use or inappropriate handling of the sensor can cause
damage to the sensor or cable. This would lead to incorrect
measurements and readings.
• Remove nail polish or articial nails before applying the sensor,
as they could cause incorrect readings.
• The sensors cannot be sterilized. Do not submerge the sensors
in liquid. The sensors must be disconnected from the Sleep&Go
prior to cleaning or disinfection.
• The operation of the oximeter sensor may be affected by the
presence of strong room lighting. If necessary, the sensor area
must be protected (e.g. with surgical tape).
• The oximeter may not be capable of detecting minimal values
of saturation with the same degree of accuracy and precision as
the maximum values of saturation. It is also possible that it can

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
28
not quantify the degree of existing hyperoxemia.
• Interferences such as movements, abnormal hemoglobins,
intravascular contrasts, states of low perfusion and skin
pigmentation may affect the operation of the pulse oximeter.
• It is unlikely that organisms are transmitted via pulse oximetry.
However, we recommend washing the pulse oximetry sensor for
each change of patient with soapy water or disinfectant solution
as specied by the manufacturer.
• The pulse oximetry module and the pulse oximetry probe is CE
marked. The temperature of the area of the probe in contact
with the nger will not reach temperatures > 42 °.
• See the instructions for use before use. Strictly follow the
manufacturer’s safety instructions, as well as those specied
in this manual.
• The maximum recommended time of application of an pulse
oximeter in the same place is 24 hours. The point of placement
must be checked frequently to determine the position, circulation
and cutaneous sensitivity of the patient. Reaction to the sensors
by the patients may be different depending on their state of
health and skin condition. Adhesive material must not be used
if the patient shows an allergic reaction to such material.
• For long-term measurements, it is recommended to use exible
or disposable sensors.
• The adult sensor is for people over 12 years of age (>40 kg).
The pediatric sensor is for children aged between 5 and 12
years of age (15-40 kg).
• The system can measure the pulse rate and functional oxygen
saturation. A functional meter can not be used to evaluate the
accuracy of a pulse oximetry sensor or a pulse oximetry monitor.
• Do not use blood ow restrictors because they can lead to loss
of signal.

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
29
1.4.4.6 BODY POSITION AND ACTIVITY
The Sleep&Go records information about the patient’s body position
and actigraphy. Both sensors are place inside the Sleep&Go device,
so no external accessory is needed.
1.4.4.7 SNORING SENSOR
Apart from the snoring signal obtained from the nasal cannula
you may also use a piezoelectric snoring sensor (ref. 06346)
to detect the patient’s tracheal sounds during sleep. The
piezoelectric snoring sensor can be plugged into the connector
labeled as “Aux”, if you’re not using the limb movement sensor.
Also, you may connect the sensor in any of the channels provided
by the ExG module (optional).
The snoring sensor is placed on the throat of the patient and it
generates a signal in response to the vibration produced during
snoring. The piezoelectric sensors are based on the movement
produced by the vibrations and not on the sounds. Therefore
they cancel artefacts related to external noise. The snoring signal
is converted into analogical voltage which can be measured.
To place a piezoelectric snoring sensor, follow these steps:
• Tell the patient to imitate a snore while you place your ngers,
slightly off-centre, upon the throat. Try to
select a point where the greatest vibration
related to the snoring sound is produced.
• Do not place the sensor directly over the
larynx.
• Place the sensor with the attest part in
contact with the skin and secure it with
surgical tape.
• Insert the snoring sensor connector into
the corresponding input of the Sleep&Go
(“Aux” connector or any connector in the
ExG module).

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
30
1.4.4.8 LIMB MOVEMENT SENSOR
The limb movement sensor (ref. 06310) is used to detect the
movements of the patient’s limbs during sleep. The limb movement
sensor is to be connected to the “Aux.” connector. However, if you
purchased the ExG module you may also connect the sensor to
any of its channels.
• To place a limb movement sensor, follow these steps: Place the
sensor in the wrist (inner side of the wrist, over the tendons)
or the patient’s ankle (front side of the ankle), in an area that
provides the maximum movement signal, as shown in the gure
below. If you put the sensor in the ankle then x it by using the
provided strap.
• Secure the sensor cable to the patient’s skin with hypoallergenic
tape.
• Insert the movement sensor connector in the corresponding
input of the Sleep&Go (connector labeled as “Aux” or any of the
input channels available in the ExG module).
1.4.4.9 EKG ELECTRODES
We recommend using the electrodes (ref. 02646) provided by the
manufacturer. If you are using other electrodes, carefully read the
instructions before using them and make sure they are compatible
with the Sleep&Go system.
The following steps should be taken to place these electrodes:
• Prepare the area where the electrodes are to be placed. The
skin must be cleaned adequately using abrasive paste. This
allows a good contact impedance (typically less than 10K).

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
31
• Attach the electrode to the patient’s
skin by removing the electrode lm that
protects the adhesive. Place one electrode
on each collar bone.
• Connect the cables (ref. 08093) to the
button stud connectors in the electrodes.
• Use a wire adaptor with 2 1.5mm
TouchProof connectors to 1mm key
connector (ref. 01644) for connecting the
couple of electrodes to the appropriate
channel. In order to acquire the EKG signal
you must have the ExG module (optional,
ref. 08022). Connect the adapter to any
of the three ExG channels included in
the module. Hold the ExG module to the
thoracic effort band.
• Interferences may be reduced by tying together the electrode
wires.
• Place a third electrode on the patient’s forehead and connect it
to the neutral input in the ExG module.
• The EKG signal should not be acquired if the device is used at
patient’s home.
• According to electrical safety standards and due to the use of
electrodes in contact with the patient, the technician must not
touch any internal part of the device that is accessible without
the use of a tool. For this reason, use the piece 08251 (battery
cover blocking piece) on the battery cover when using the
electrodes and the EXG Module.
1.4.4.10 EMG, EOG AND EEG ELECTRODES
We recommend using the cup electrodes (ref. 08701) provided by
the manufacturer. If you are using other electrodes, please carefully
read the instructions before using them and make sure they are
compatible with the Sleep&Go system.
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
32
The following steps should be taken to place these cup electrodes:
• Prepare the area where the electrodes are to be placed. The
skin must be cleaned adequately using abrasive paste. This
allows a good contact impedance (typically less than 10K).
• Fix the electrode to the patient’s skin by lling the cup with
conductive paste and xing it in place with colodion. A hair
dryer can be used to speed up the drying process. When the
test is over the electrodes are easyly removed with acetone.
• Use a wire adaptor with 2 1.5mm TouchProof connectors to
1mm key connector (ref. 01644) for connecting the couple
of electrodes to the appropriate channel. In order to acquire
the EMG, EOG or EEG signals you must have the ExG module
(optional, ref. 08022). Connect the adapter to any of the three
ExG channels included in the module. Hold the ExG module to
the thoracic effort band.
• To use a common reference for several EEG channels, each
electrode must be connected to one of the connectors of the
wire adaptors (ref. 01644). Use one or more interconnection
bridges (ref. 02741) to interconnect the remaining pole of all
referenced channels, so that every channel uses the same
reference.
• Interferences may be reduced by tying together the electrode
wires.
• Place a third electrode on the patient’s forehead and connect it
to the neutral input in the ExG module.
• The EEG, EOG and EMG signals should not be acquired if the
device is used at patient’s home.
• According to electrical safety standards and due to the use of
electrodes in contact with the patient, the technician must not
touch any internal part of the device that is accessible without
the use of a tool. For this reason, use the piece 08251 (battery
cover blocking piece) on the battery cover when using the
electrodes and the EXG Module.
In addition to the aforementioned explanations, the following
precautions and considerations should be taken into account while
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
33
using the electrodes:
The conducve part of the electrodes and connectors, must never touch
other conducve parts, including the earth connecon.
To prevent the risk of burns, all electrodes must be removed from the paent
before using a high frequency (HF) surgical unit.
When several devices are connected to a paent, there is a risk of
accumulang leakage currents, which can become dangerous for the paent.
Reduce to the absolute minimum the number of devices connected to the
paent.
To protect the paent against the eects of electric shock, all electrodes
must be removed from the paent before using a cardiac debrillator.
1.4.4.11 EVENT MARKER
During the test (either online or in holter mode), the patient is
allowed to make a mark by simply pressing the joystick for at least 3
seconds. After these 3 seconds the screen lights on and the mark is
registered. These marks are used to indicate certain events, such as
the patient getting up to the bathroom, taking medication and so on.

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
34
2. OPERATION
The Sleep & Go is tted with a color screen and a joystick to interact
with the options exposed by the device’s software.
The top part of the screen displays an info bar with the device
status at any time:
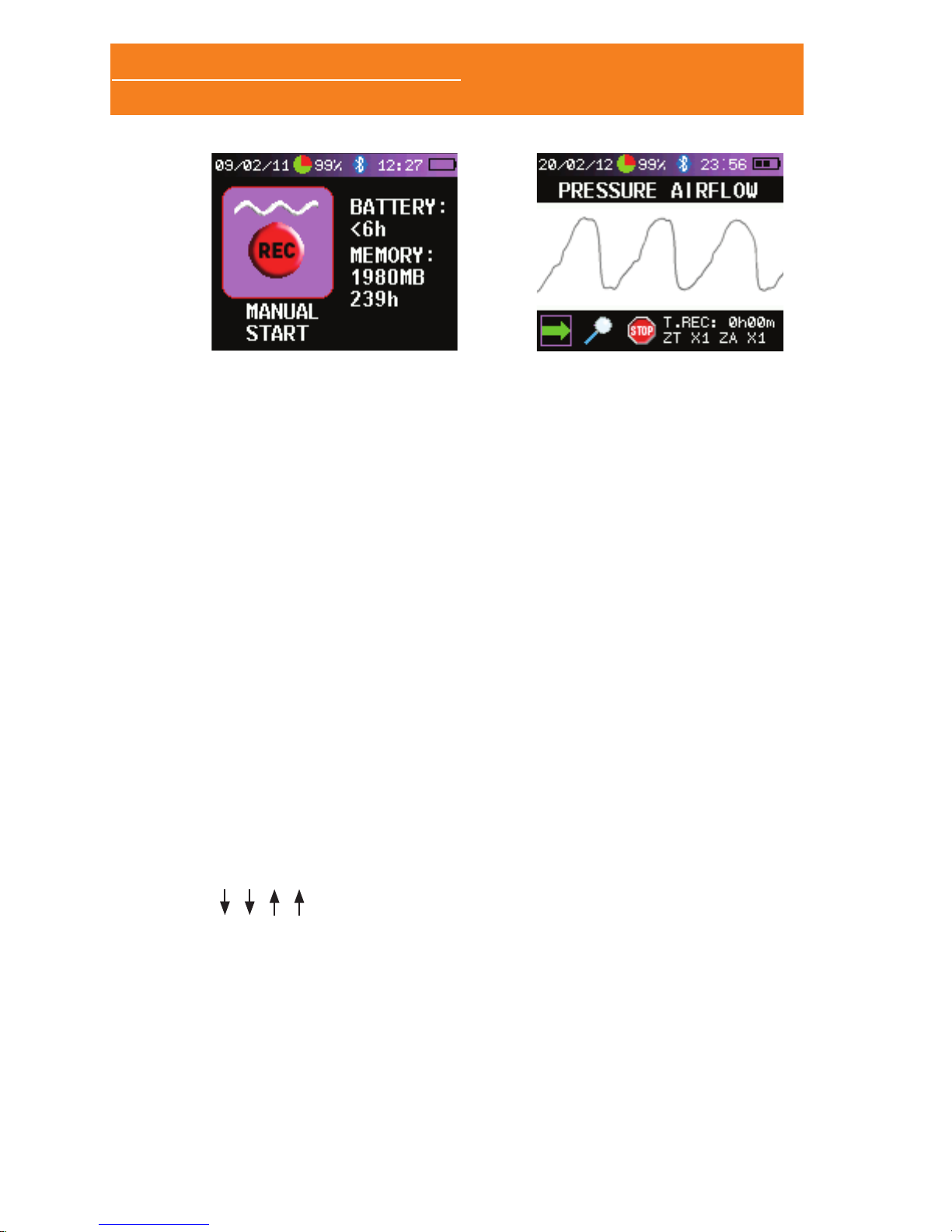
This is the screen displayed when the Sleep&Go is switched on:
This screen displays status information and enables starting a
new test in holter mode. The icon “MANUAL START” is selected by
default. The patient can not modify the device conguration.
Use the joystick to navigate through the different screens that will
be introduced. The joystick supports four directions ( , , , ),
while pressing it is used to click an option.
2.1 WORKING MODES
Two working modes are available, depending on the way tests are
handled:
Holter mode: in this mode the Sleep&Go stores the tests in a
memory card. Later, the tests are transferred to the computer for
evaluation. It is designed for home use or at the hospital or clinic.
There are two ways of starting a test in holter mode:
Free space in
memory card
Battery
status
Date Time

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
35
a. Manual start and stop: the patient starts and stops
manually the recording of the test, through the color
screen and joystick in the Sleep&Go.
b. Automatic start and stop: health personnel program
the Sleep&Go to start and stop automatically the test
at a certain date and time.
The device’s batteries last up to 24 hours in holter mode with a
single charge.
Online mode: in this mode the test data are sent in real time to
the PC via Bluetooth, the transfer from the memory card not being
necessary after the test. This mode is intended for use in the clinic
/ hospital or the sleep lab.
The device’s batteries last up to 12 hours in online mode with a
single charge.
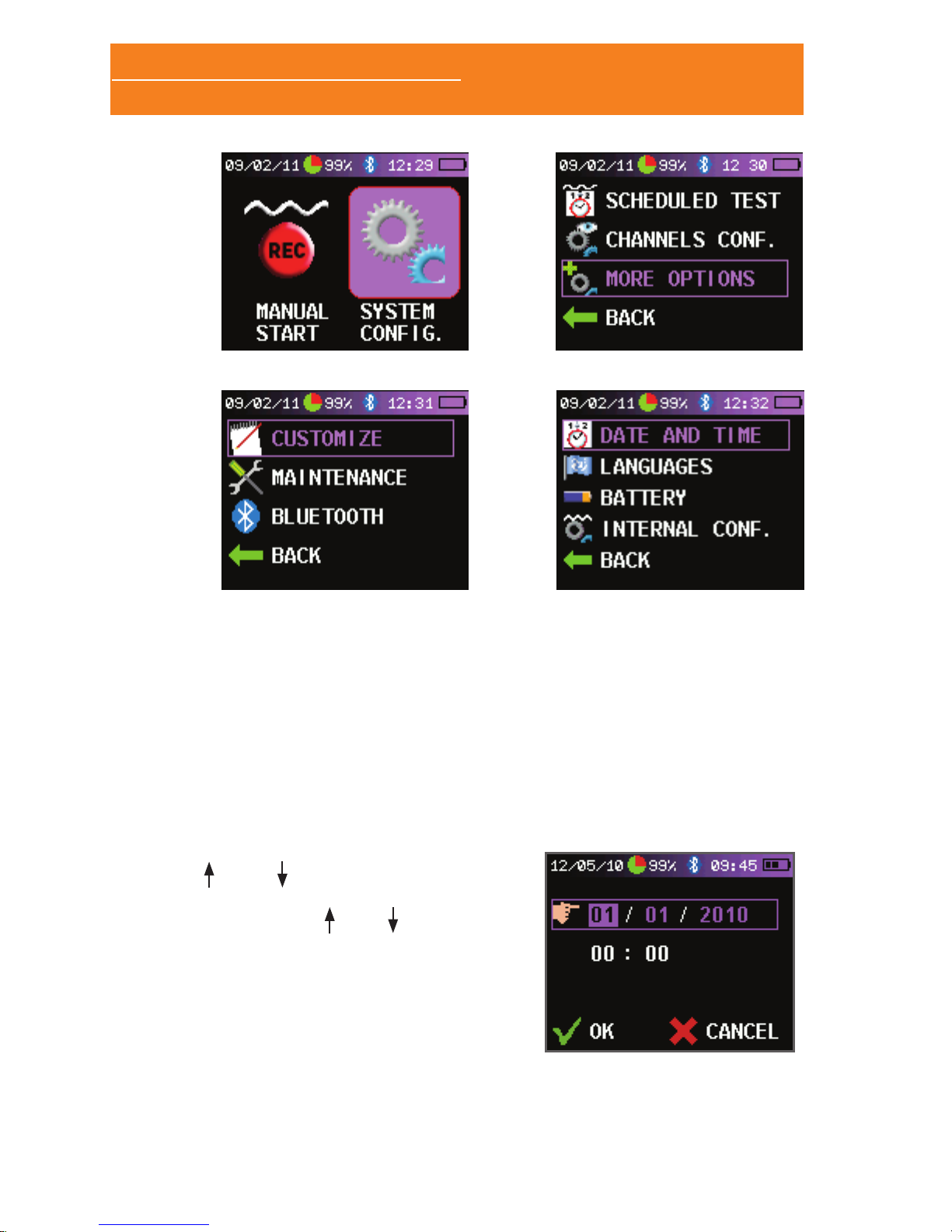
2.2 SYSTEM CONFIGURATION
The setup mode in the Sleep&Go is designed to change the device
conguration (date and time, language, type of batteries used) and
schedule new tests. Only healthcare personnel should access the
device settings. To enter to the setup menu of the Sleep&Go do the
following:
• Switch on the Sleep&Go and make the following keystrokes on
the joystick, one after another and leaving a short interval of
time between each of them: , , , .
• The screen changes and a new icon is displayed: “SYSTEM
CONFIG.” (A). Select the “SYSTEM CONFIG.” icon and press the
joystick to move to the setup screen.
• Select “MORE OPTIONS” (B).
• Select “CUSTOMIZE” (C).
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
36
A B
C D
• If CPAP channel is activated, BATTERY menu changes to OTHER
OPTIONS.
2.3 INITIAL DEVICE SETUP
2.3.1 SETTING THE DATE AND TIME
Once in the “CUSTOMIZE” screen, follow these steps in order to
change the date and time in the Sleep&Go:
• Select “DATE AND TIME”.
• Use
and to access each of the
options to set the date and time in
the device. Use and , to modify
the values in each eld.
• Once you have set the date and
time select “OK. “ To cancel setting
the date and time select “CANCEL”.
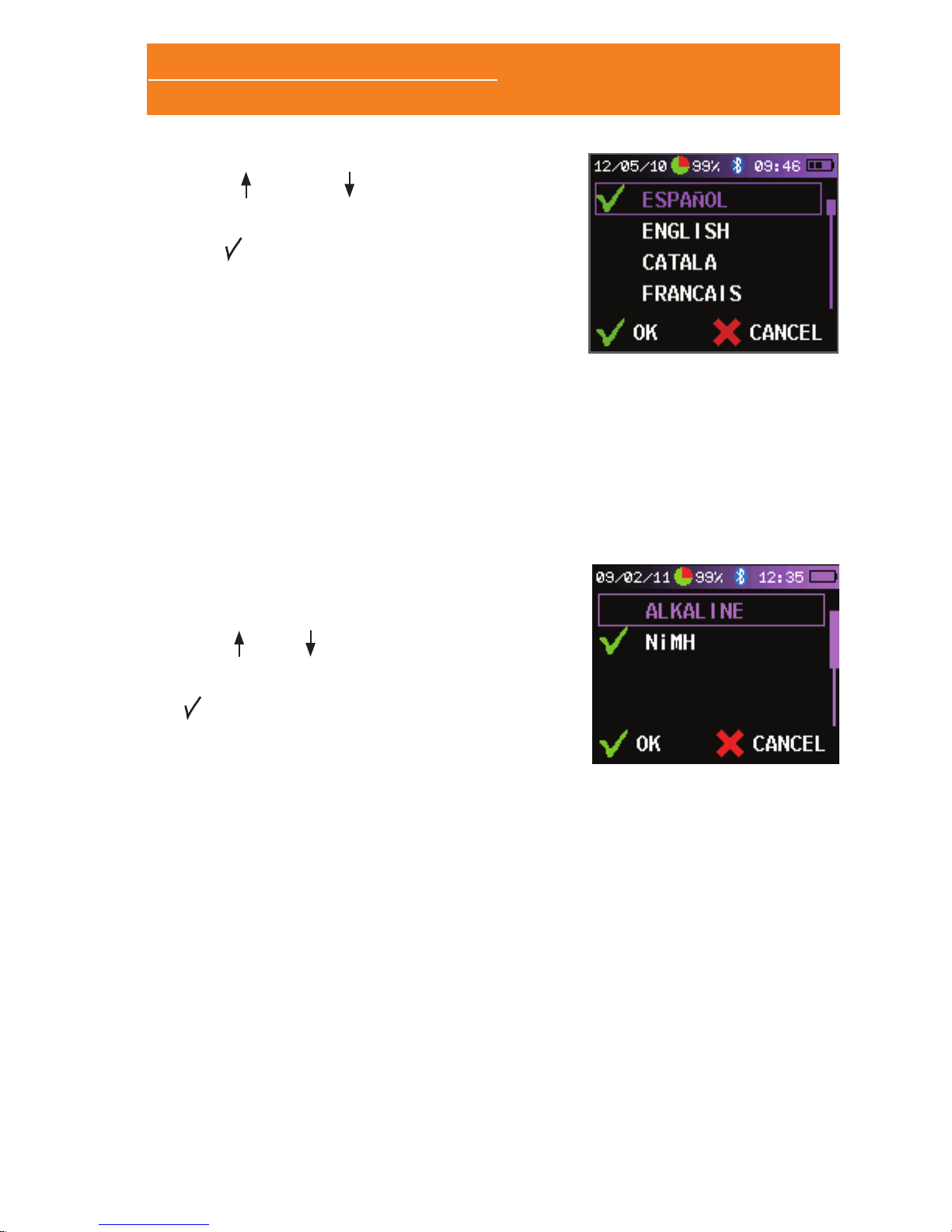
2.3.2 SETTING THE LANGUAGE
Once in the “CUSTOMIZE” screen follow these steps in order to
change the language in the Sleep&Go:
• Select “LANGUAGES”.
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
37
• Use and move along the
different available languages. The
selected language is marked with
the symbol.
• When you have set the desired
language select “OK. “ If you want
to cancel the language settings then
select “CANCEL”.
2.3.3 SETTING THE TYPE OF BATTERIES
It is very important that the type of batteries is correctly congured
for the remaining battery life information to be displayed properly.
Alkaline or rechargeable Nickel-Metal Hydride (minimum 2450mAh)
AA batteries should be used. In the “CUSTOMIZE” screen follow
these steps:
• Select “BATTERY” or “MORE
OPTIONS > BATTERY” in case the
CPAP channel is available.
• Use and to select the type of
batteries used in the device. The
type of batteries is marked with the
mark.
• When nished select “OK”. If you
want to cancel setting the type of
batteries then select “CANCEL”.
2.3.4 SETING THE UNITS OF THE CPAP CHANNEL
• Select “MORE OPTIONS”.
• Select “BATTERY”.
• Select “CPAP UNITS”
• Select cmH2O or hPa and press OK. If you want to cancel the
setting select CANCEL.
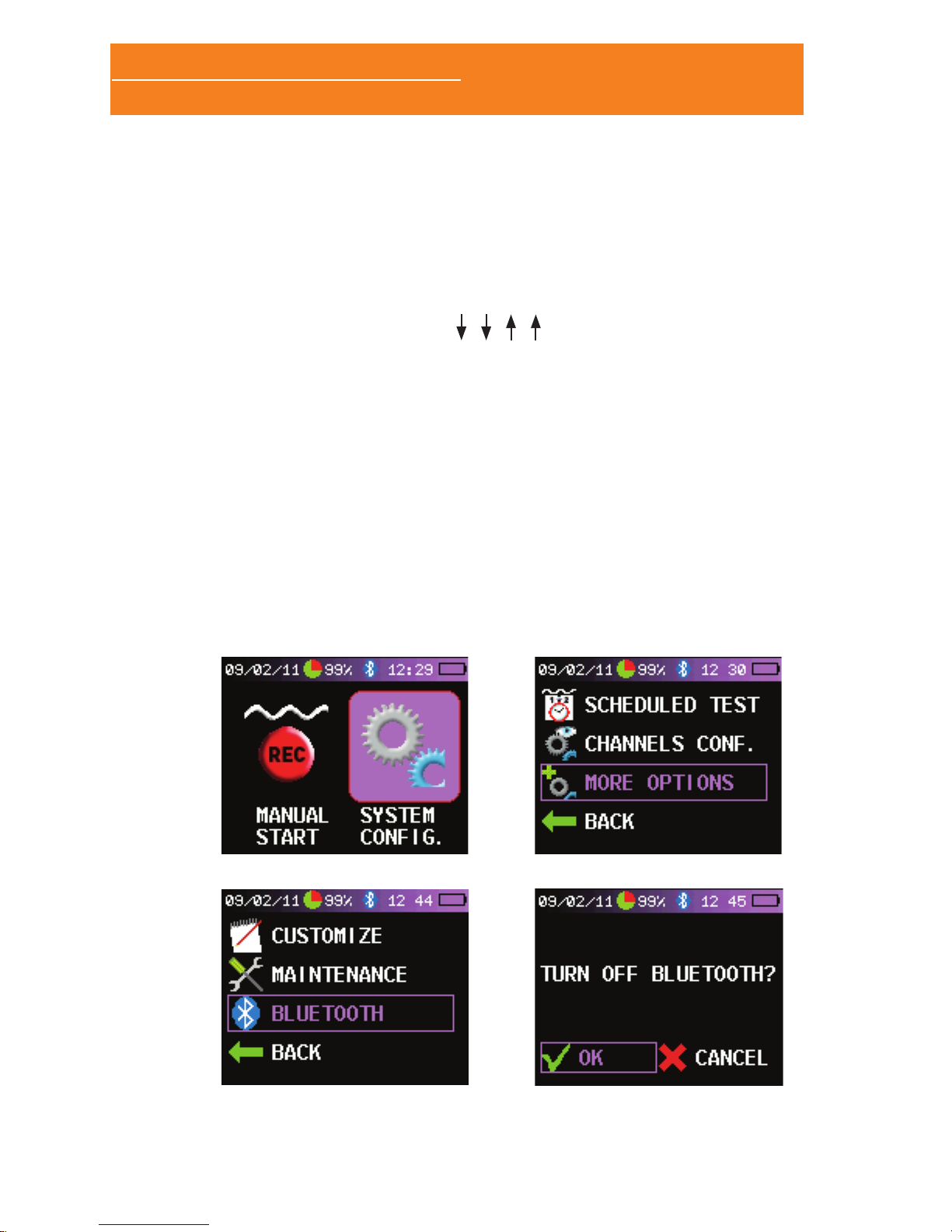
2.4 SWITCHING ON/OFF THE BLUETOOTH MODULE
The Bluetooth module can be turned on and off at any time, in
order to reduce battery consumption and increase the battery life.
You need to switch on the Bluetooth module when making realtime tests (online mode). If you are making holter tests there is no
need of keeping the module powered.
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
38
The Bluetooth module is supplied by default in the Sleep&Go B
Model and is optional in the A Model.
To switch on/off the Bluetooth module follow these steps:
• With the Sleep&Go in the home screen make the following
keystrokes with the joystick: , , , .
• The screen changes and displays a new icon: “SYSTEM CONFIG.”
(A). Select “SYSTEM CONFIG.” and press the joystick to move
to the setup screen.
• Select “MORE OPTIONS” (B).
• Select “BLUETOOTH” (C).
• If the Bluetooth module is switched on you will be prompted to
turn it off. If the module is switched off you will be prompted to
turn it on (D). Select “OK” or “CANCEL “. When the Bluetooth
module is powered a Bluetooth logo is displayed in the status
bar. Otherwhise no icon is displayed. The blue Bluetooth logo
indicates that the module is on, while the green logo means
that data is being transmitted to the PC.
A B
C D

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
39
2.5 MAKING POLYGRAPHY TESTS IN ONLINE MODE
In this mode, the polygraph transmits the real-time data to the PC
via Bluetooth connection, displaying the signals on the computer
screen. The Bluetooth module in the Sleep&Go must be enabled
(optional in the A model, supplied by default in the B model).
Make sure the device is switched on and the Bluetooth
communication is enabled (see above).
All new online tests are to be started from the BitmedLab software.
Please refer to the software’s manual for information on how to
make an online test.
2.6 MAKING POLYGRAPHY TESTS IN HOLTER MODE
When the device operates in holter there are two different ways
to start and stop the tests, either manually or automatically
(scheduled).
2.6.1 STARTING AND ENDING A TEST MANUALLY
In this mode either the patient or the technician will start and end
the test:
• Switch on the device by pressing for 3 seconds the ON/OFF
button.
• Once in the main screen press the joystick (A) to select the
“MANUAL START” icon.
• This starts the recording of the test. If all sensors are properly
connected then the signals are displayed on the screen and
the LED on the front panel starts ashing. If any of the
sensors to be acquired is not properly connected the message
“CHECK SENSOR” is displayed, giving the opportunity to test
the connection. After a few seconds the screen switches off
automatically.
• To stop the test just select the “STOP” option or hold down
the ON/OFF button for 3 seconds (B).
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
40
A B
2.6.2 SCHEDULED TESTS
The Sleep&Go is built with an internal clock suitable for scheduling
new tests in holter mode at certain dates and times, which also end
at the right time as programmed by medical personnel. In this way
the patient does not have to interact at all with the Sleep&Go for
starting or ending the test.
You can schedule up to 5 consecutive tests. These tests may be
scheduled right from the Sleep&Go device or by using the BitmedLab
software in the PC. This manual describes the programming
procedure in the Sleep&Go. Refer to the software manual for
information on the programming process in BitmedLab.
2.6.2.1 SCHEDULING A TEST IN THE SLEEP&GO
• Switch on the Sleep&Go and gain access to the setup mode
by making the following keystrokes on the joystick, one after
another and leaving a short interval of time between each of
them: , , , .
• Select the “SYSTEM CONFIG.” icon and press the joystick to
move to the setup screen.
• Select “SCHEDULED TESTS” (A).
• Select “NEW TEST” (B).
• Select the “DATE” eld and set the starting date for the test.
Select “OK” when nished (C, D).

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
41
• Select the “TIME” eld and set the starting time for the test.
Select “OK” when nished (E).
• Move to the “DURATION” eld and enter the number of hours
and minutes for the test (F).
• Select the option “CHANNELS CONF.”. The device displays all
channel congurations stored on the memory card. A channel
conguration establishes which channels are recorded during
the test, as well as their sampling rates. There may be up to 10
different channel congurations. A detailed explanation on how
to change these congurations is given below. Select which
conguration is more suitable for the type of test you want to
do (G).
• Select “SAVE” once all parameters are congured. You may
switch off now the Sleep&Go.
• The device will start automatically the test at the designated
date and time. The screen will light for 50 seconds, displaying
the signals acquired by the sensors. The front panel LED will
blink during the whole test.
A B
C D

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
42
E F
G
2.6.2.2 DISPLAYING THE SCHEDULED TESTS
You can see the scheduled tests in the Sleep&Go (up to a maximum
of 5 tests):
• Switch on the Sleep&Go and enter the setup mode by making
the following keystrokes with the joystick: , , , .
• Select the “SYSTEM CONFIG.” icon and press the joystick to
move to the setup screen.
• Select “SCHEDULED TEST”.
• Select “SHOW TEST” (A).
• All scheduled tests are displayed. For more info on any scheduled
test select it (B).
• The screen shows the programming data of the test (onset date
and time, duration, channel conguration) (C).
• To view the channel conguration (enabled channels and
sampling frequencies) select “CHANNELS CONF.” (D).

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
43
A B
C D
2.6.2.3 CHANNEL CONFIGURATIONS
he Sleep & Go may hold up to 10 channel congurations. Each
conguration stores which of the available device’s channels will
be acquired, as well as the sampling rates for each of them. The
available sample rates depend on each type of channel. Some
channels, such as pulse oximetry or body position, have a xed
sampling frequency, while in others, such as the respiratory effort
bands or the ExG channels, the sampling rate can be chosen among
several values.
The creation and modication of channel congurations is made in
the BitmedLab software, then they are transferred to the MicroSD
memory card. See the software’s manual for more information on
this point.
2.7 TRANSFER AND REVIEW OF THE TESTS
Tests must be transferred to the PC prior to review. Several tests
may be stored in the memory of the Sleep&Go before transferring

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
44
them to the PC. Transferring the test immediately after it is nished
is not necessary.
In order to transfer the tests to the PC extract the MicroSD memory
card from Sleep & Go and insert it into your PC’s card reader. Most
computers are supplied by default with a memory card reader. If
your computer does not have one you can use an USB card reader.
After inserting the memory card in your PC run the BitmedLab
software and download the tests as explained in the software’s
manual.
The tests are automatically deleted from the memory card when
they are transferred to the PC. Do not delete the les in the memory
card before downloading the tests to the PC.
2.8 FIRMWARE UPDATE
Over time, new versions of the program managing the Sleep&Go
(rmware) might be released, either to improve the operation of
the equipment or to add new features.
• The Technical Service will provide one or several les to be
copied to the MicroSD memory card of the Sleep&Go.
• Insert the memory card in the Sleep&Go and switch it on.
• The rmware update starts automatically. When prompted,
conrm that you want to update the rmware.
• When the update is complete the Sleep&Go switches off
automatically. Your device is already updated.
• In case you are provided with several les the process will be
repeated as many times as needed.
2.9 DEVICE OPTIONS UPDATE
As mentioned in paragraphs 1.2 and 1.3, the Sleep&Go is available
in two different models with increasing features. The available
channels may be expanded, though. In case new features are

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
45
purchased please follow these steps in order to update your device:
• The Technical Service will provide one le to be copied to the
MicroSD memory card of the Sleep&Go.
• Insert the memory card in the Sleep&Go and switch it on.
• The update starts automatically. When prompted, conrm that
you want to update the device’s features.
• When the update is complete the Sleep&Go switches off
automatically. Your device is already updated and the new
channels are available from now on.

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
46
3. TECHNICAL SPECIFICATIONS
3.1 GENERAL DATA
Useful life for Sleep&Go and ExG
module:
7 years
Useful life for Xpod pulseoximeter
module:
5 years
Baery type / baery life:
2x AA alkaline baeries or AA NiMh rechargeable
baeries (2450mAh minimum) / 12h in online
mode, >24h in holter mode.
Enclosure protecon degree: IP22
Memory type: MicroSD
Device classicaon (EN60601-1):
Class: internally powered
Type BF applied parts, not protected against
debrillator
Input impedance: 20 MW
CMRR (common mode rejecon
rao):
>95dB
Signal input connectors:
Pressure transducer (airow, snoring,
CPAP pressure)
Thermocouple, thoracic eort,
abdominal eort, polygraphic
auxiliary, ExG channels (x3)
Pulse oxymeter
Acmetry
Body posion
Event marker
LUER connector
1 mm key connector
Specic 3-pin connector
Internal
Internal
Joysck

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
47
Storage sampling rate (soware
selectable):
Airow (pressure transducer)
Snoring (pressure transducer)
CPAP pressure
Thermocouple
Thoracic eort
Abdominal eort
Polygraphic aux.
ExG channels (x3)
Pulse rate
Oxygen saturaon
Plethysmographic wave
Acmetry
Body posion
Event marker
Selectable: 25, 50, 100 samples/s
Selectable: 50, 100, 250 samples/s
1 sample/s
Selectable: 10, 25 samples/s
Selectable: 10, 25 samples/s
Selectable: 10, 25 samples/s
Selectable: 25, 50, 100 samples/s
Selectable: 100, 250 samples/s
1 sample/s
1 sample/s
75 samples/s
Selectable: 10, 25, 50 samples/s
1 sample/s
1 sample/s
Resoluon:
Airow. (pressure transducer), snore
(pressure transducer), thermocouple,
thoracic eort, abdominal eort,
polygraphic aux., ExG channels (x3),
acmetry
Pulse rate, oxyen saturaon,
plethysmographic wave
Body posion
Event marker
CPAP pressure
12 bits
8 bits
8 bits
8 bits
1cmH
2
0 or 1hPa

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
48
Sample storage size: 16 bits per sample
Input range:
Thermocouple, thoracic eort,
abdominal eort, polygraphic aux,
ExG channels (x3)
Airow (pressure transducer)
CPAP pressure
Snoring (pressure transducer)
Pulse rate
Oxygen saturaon
Plethysmographic wave
Acmetry
Body posion
+/- 5 mVpp
+/- 4 cmH
2
O (3.9 hPa) over the cannula aprox.
0 - 20 cmH
2
O (19,6 hPa)
+/- 0.5 cmH2O (0.49 hPa) over the cannula aprox.
18 – 300 BPM (1/min)
0 – 100 %
0 – 255
aprox. +/- 30 mg (300 m/s
2
)
Supine, prone, le, right, seated
Linearity:
Resp. airow (pressure transducer),
snore (pressure transducer)
Thermocouple, thoracic eort,
abdominal eort, polygraphic aux.,
ExG channels (x3), acmetry
+/- 3%
+/- 3%
Precision:
CPAP pressure
Pulseoximeter
+/- 2cmH
2
O (1,96 hPa)
(See pulseoximeter table)
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
49
Fixed hardware lters:
Thermocouple, thoracic eort,
abdominal eort
Polygraphic aux.
ExG channels (x3)
Airow (pressure transducer)
CPAP pressure
Snoring (pressure transducer)
Pulse rate
Oxygen saturaon
Plethysmographic wave
Acmetry
Body posion
0.05 – 10 Hz
0.05 – 50 Hz
0.05 – 250 Hz
0.05 – 50 Hz
0.02 Hz
30 – 250 Hz
-
-
-
0.5 – 25 Hz
-
PC communicaon interface: Bluetooth (opon)
Environmental condions:
In operaon (*)
Transportaon and
storage (*)
Temperature: 5ºC to 40ºC
Relave humidity: 15-93%
(without condensaon)
Barometric pressure:
700hPa to 1060hPa
(approx. 2950 to -350m)
Temperature:
-20ºC to +60ºC
Relave humidity:
<93% (without
condensaon)
Dimensions: 90 x 90 x 25 mm
Weight: < 200 g
(*) The condions may vary in some of the accessories (like AgCl electrodes Ref. 02646, EXG
silver cup electrodes Ref. 08701 or Cable for buon stud type electrodes Ref. 08093).
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
50
3.2 PULSE OXIMETER TECHNICAL SPECIFICATIONS
The pulse oximeter of the unit is based on the technology of NONIN
MEDICAL, INC.
Range of values / resoluon:
Oxygen saturaon
Pulse rate
0-100% / 1%
18-321 / 1 pulse, 0 = erroneous value
Precision
1
SpO2 (70-100%) (±1DS)
2
Pulse rate
No movement:
Finger clip sensor: adults, paediatric: ±2 digits;
neonates: ±3 digits
Flexible sensor: adults, paediatric, neonates: ±3
digits
Movement:
Finger clip sensor: adults, paediatric: ±2 digits;
neonates: ±3 digits
Flexible sensor: adults, paediatric: ±3 digits;
neonates: ±4 digits
Low perfusion:
All sensors: adults, paediatric: ±2dígitos; neonates:
±3 digits
No movement (18-300 BPM):
Finger clip sensor/exible sensor: adults,
paediatric, neonates: ±3 digits
Movement (40-240 BPM):
Finger clip sensor/exible sensor: adults,
paediatric, neonates: ±5 digits
Low perfusion (40-240 BPM):
Finger clip sensor/exible sensor: adults,
paediatric, neonates: ±3 digits
Measuring wavelength / power Red: 660nm. / 0.8W
Infrared: 910nm. / 1.2mW
Maximum applicaon me Consult the operang instrucons of the pulsioximetric
sensor
Biocompability and toxicity Consult the operang instrucons of the pulsioximetric
sensor
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
51
1. All precision specications were determined from studies of hypoxia
induced in healthy adult volunteers, of both sexes, from Caucasian, Indian
and African ethnic group.
2. Standard Deviation. Due to the statistic distribution of the pulse oximeter
measurements , only about two-thirds of the pulse oximeter measurements
can be expected to be found within ±Arms of the value measured by a COoximeter.
3.3 CONDITIONS OF OPERATION AND STORAGE OF
ACCESSORIES
Although the device and the EXG module stands temperatures
between -25 and 70 ° C, the specied temperature range is lower
(-20 ° C and 60 ° C) in order to ensure the proper storage of most
accessories.
Exceptionally, ECG electrodes (Ref. 02646), silver cup EXG electrodes
(Ref.08701) and the cable for button-stud type electrodes (Ref
08093) support lower storage temperatures. The following table
shows the conditions for these accessories:
Temperature
transportation/
storage
Humidity
transportation/
storage
ECG electrodes (Ref.
02646)
10 to 40ºC
< 93% (without
condensation)
EXG silver cup electrodes
(Ref.08701)
10 to 50 ºC
20 - 80% (without
condensation)
Cable for button stud
type electrodes (Ref
08093)
10 to 50ºC
20 - 80% (without
condensation)
3.4 APPLICABLE STANDARDS
The Sleep&Go has the CE Marking (CE 0197). The CE mark is a
statement that the Sleep&Go fullls the guidelines established by
the EU for medical devices.
The Sleep&Go is manufactured by SIBEL and the system (including
the software) meets the following standards and regulations:

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
52
Recommendations:
• National consensus document on the apnea-hypopnea syndrome
(SAHS). Spanish Sleep Group (GES), September 2005.
• The AASM Manual for the Scoring of Sleep and Associated
Events, 2007
European Directive 93/42 EEC (R.D: 1591/2009):
• EC marking Class II a
Safety:
• EN 60601-1:2006 + AC:2010: Electrical safety (3rd edition).
• EN60601-1:1990+A1:1993+A2:1995+A11:1993+A12:1993
+A13:1996
• EN 60601-2-26:2003 Electromedical Devices. Part 2: Special
Safety Requirements for Electroencephalographs.
• EN 60601-2-40:1998 Safety requirements for Electromyographs.
• EN 60601-1-11:2010 General requirements for basic safety
and essential performance - Collateral standard: Requirements
for medical electrical equipment and medical electrical systems
used in the home healthcare environment.
EMC:
• EN 60601-1-2:2007+AC:2010 EMC in medical devices (No
life support)
See Annex 1. Electromagnetic compatibility
Software:
• EN 62304:2006+AC:2008 Medical device software - Software
life-cycle processes
Usability:
• EN 60601-1-6:2010 Medical Electrical Equipment – Part 1-6
General requirements for basic safety and essential performance
- Collateral standard: Usability
• EN 62366:2008 Medical Devices – Application of usability
engineering to medical devices
Pulse oximetry:
• ISO 80601-2-61:2011 Special requirements for basic safety
and essential operation characteristics of pulse oximeters for
medical use

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
53
Biocompatibility:
• EN ISO 10993-1:2009+AC:2010 Biological evaluation of
medical devices
Vibration and temperature:
• Levels according to EN 60601-1-11:2010 (covers ISO 806012-61)
• Tests according to EN 680068-2-27:1993 and EN 680068-264:1994
Environmental conditions:
• Levels and tests according to EN 60601-1-11:2010
Symbology:
• EN ISO 15223-1:2012 and EN 980:2008 Symbols to be used
with medical device labels, labelling andi nformation to be
suplplied.
Packaging and labelling
• Packaging and packaging waste directive 94/62/EC
• Regulation EC 1272/2008 on classication, labelling and
packaging of substances and mixtures (REACH)
Information supplied by the manufacturer:
• EN 1041:2008 Information supplied by the manufacturer of
medical devices
Electrical and electronic waste:
• Electrical and electronic equipment and waste management.
Transposition of the directive WEEE 2002/96/EC
System quality:
• Manufactured according to the SIBEL S.A. Quality Manual,
which is compliant with EN ISO 13485:2012+AC:2012 and EN
ISO 9001:2008
Risk Management:
• EN ISO 14971:2012
Radio equipment and communication terminals:
• EN 300328 v1.7.1:2006
The user should follow:
• Data protection: Directive 95/46/EC

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
54
In addition, although outside the scope and intended use of the
device, some security aspects from the following standards have
been applied:
EN 60601-2-49:2001
EN 60601-2-27:2006 + CORR: 2006

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
55
4. SYMBOLOGY
4.1 SYMBOLOGY OF THE SLEEP&GO
SERIAL NUMBER
MANUFACTURER (manufacture date, name and address
of the manufacturer)
BATCH NUMBER
REFERENCE
CONSULT INSTRUCTIONS FOR USE
WARNING
START UP (STANDBY)
BF APPLIED PARTS
NO SPO
2
ALARMS
IP22 (see section WARNINGS AND PRECAUTIONS)
DISPOSAL OF WASTE ELECTRICAL / ELECTRONIC
ACORDING TO THE RAEE DIRECTIVE
NEUTRAL CONNECTOR
BATTERIES
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
56
4.2 SYMBOLOGY OF THE ACCESORIES AND PACKAGING
CE MARKING
WARNING
DO NOT REUSE
BATCH NUMBER
REFERENCE
LATEX FREE
PHT (CONTAINS PHTHALATES)
MANUFACTURER (manufacture date, name and address of the
manufacturer)
AUTHORIZED REPRESENTATIVE IN THE EUROPEAN COMMUNITY
DISPOSAL OF WASTE ELECTRICAL / ELECTRONIC ACORDING
TO THE RAEE DIRECTIVE
TEMPERATURE LIMITATION
SERIAL NUMBER
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
57
MANUFACTURING DATE
LATEX FREE
DO NOT STERILIZE
U.S. FEDERAL REGULATION RESTRICTS THIS DEVICE TO SALE
BY ORDER OF PHYSICIAN. MAY ALSO BE APPLICABLE IN OTHER
COUNTRIES
PHT (CONTAINS PHTHALATES), DEHP (CONTAINS DIETILHEXIL
PHTALATE)
HUMIDITY LIMITATION
USE BY
STACKING LIMITATION
THIS WAY UP
FRAGILE
KEEP AWAY FROM WATER
PREASURE LIMITATION

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
58
4.3 VALIDATED ACCESSORIES
The Screen&Go device is used in combination with other medical
accessories manufactured by SIBEL S.A. or by other manufactures.
We only recommend the use of these accessories for proper
operation of the equipment (see section 1.2 PACKING LIST).
The sensors used by other manufacturers are:
Accessory Manufacturer SIBEL
reference
Manufacturer
reference
THERMOCOUPLE SENSOR S.L.P. INC. 06309 1401S-BI
SOFT PULSE OXIMETRY SPO
2
SENSOR
UNIMED MEDICAL
SUPPLIES INC.
08013 U101S-08
SOFT PULSE OXIMETRY SPO
2
SENSOR
UNIMED MEDICAL
SUPPLIES INC.
08012 U401S-08
DISPOSABLE NASSAL CANNULA S.L.P. INC. 08049 15805-2-FT
DISPOSABLE PROTECH NASAL
CANNULA (PACKAGE 60PC)
PRO-TECH 07679 P1328-60
DISPOSABLE PROTECH ORO-NASAL
CANNULA (PACKAGE 30PC)
PRO-TECH 07680 P1343
DISPOSABLE SLEEPSENSE ORO-
NASAL CANNULA (PACKAGE 5PC)
S.L.P. INC. 07681 14802-2-FT
DISPOSABLE SLEEPSENSE NASAL
CANNULAR
S.L.P. INC. 08049 15805-2-FT
THORAX EFFORT INDUCTIVE BAND
AMPLIFICATION INTERFACE
S.L.P. INC. 06314 9102S-BI
ABDOMINAL EFFORT INDUCTIVE
BAND AMPLIFICATION INTERFACE
S.L.P. INC. 06308 9101S-BI
FASTENING BELT SMALL SIZE (S) S.L.P. INC. 06311 1348B
FASTENING BELT LARGE SIZE (L) S.L.P. INC. 06312 1341
FASTENING BELT EXTRA LARGE
SIZE (XL)
S.L.P. INC. 06313 1340
ELASTIC INDUCTIVE BAND SMALL
SIZE (S)
S.L.P. INC. 01425 9002-L40
ELASTIC INDUCTIVE BAND MEDIUM
SIZE (M)
S.L.P. INC. 01424 9002-L60
ELASTIC INDUCTIVE BAND LARGE
SIZE (L)
S.L.P. INC. 01421 9002-L90
ELASTIC INDUCTIVE BAND XTRA
LARGE SIZE (XL)
S.L.P. INC. 01420 9002-L120

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
User’s Manual - Cardiorespiratory polygraph - Sleep&Go
59
Accessory Manufacturer SIBEL
reference
Manufacturer
reference
ELASTIC INDUCTIVE BAND XTRA
XTRA LARGE SIZE (XXL)
S.L.P. INC. 01417 9002-L150
LIMB MOVEMENT SENSOR KIT S.L.P. INC. 06310 1770S-KIT-BI
PIEZOELECTRIC SNORE SENSOR S.L.P. INC. 06346 1250S-BI
EXG GOLD CUP ELECTRODE
(PACKAGE 10PC)
GRASS
TECHNOLOGIES
08701 F-E5GH-30
CABLE ADAPTER 1MM TO 1.5MM PLASTICS ONE, INC. 01644 249497X04010XXX
INTERCONNECTION BRIDGE PLASTICS ONE, INC. 02741 455455X01010XXX
CABLE FOR BUTTON STUD TYPE
ELECTRODES (PACKAGE 10PC)
SPES MEDICA S.R.L. 08093 S2231050261
ECG ELECTRODE (PACKAGE 50PC) TELIC 01027 LF-50

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
Annex 1: Electromagnetic compatibility
60
5. CLEANING AND MAINTENANCE
The Sleep&Go polygraph requires, like any electronic equipment,
a maintenance in order to:
• Ensure the safety of the patient, the operator and its
environment.
• Ensure the reliability and accuracy of the functions for which
the Sleep&Go was developed.
5.1 CLEANING
To clean the unit and its accessories, excluding the sensors and
electrodes, use a damp cloth and mild (hand) soap. To disinfect the
device you can use alcohol (ethyl or isopropil). Do not use other
chemical products or detergents for domestic use. Please read
carefully the SAFETY section.
Properly dispose the disposable sensors and single-use electrodes
immediately after use. Nasal cannulas are single use and must be
replaced with each patient.
For the cleaning and disinfection of reusable sensors and electrodes,
please refer to the information provided by its manufacturer.
The device can not be sterilized. For the cleaning and disinfecon
of sensors and electrodes, please refer to the informaon
provided by its manufacturer.
All sensors should be cleaned properly aer each use and before
use in a new paent.
5.2 PREVENTIVE MAINTENANCE
Preventive maintenance consists of all actions needed to keep the
equipment in good working order.
5.2.1 ACTIONS TO BE TAKEN BY THE USER
Check periodically that all cables, external accessories and other

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
Annex 1: Electromagnetic compatibility
61
items are in perfect condition, not broken and with no external
damage. Pay special attention to the cables and connectors.
It is also advisable from time to time to start a test and check that
the connected sensors respond correctly.
If you detect any problems that you can not solve, please get in
contact with the customer service of SIBEL S.A. or your distributor
for servicing and repair.
You can also check the “Known abnormalities report” (534-701-
MU2) available to the user from SIBEL S.A.
5.2.2 ACTIONS TO BE TAKEN BY QUALIFIED PERSONNEL
The Medical Device Directive 93/42/EEC recommends the regular
servicing and / or calibration to ensure the reliability of the medical
devices and the safety of patients, users and the environment.
The general technical verication of safety systems, settings and
features being part of the device, must be made according to the
Procedure of Verication and Adjustment for the Sleep&Go, of
SIBEL S.A.
These operations must be carried out by service personnel from
the manufacturer or distributor. The latter, must be in possession
of a written authorization from SIBEL S.A., at least during the
warranty period, in order to perform such maintenance.
The manufacturer is not responsible for malfunction or damage to
the equipment resulting from poor maintenance done by personnel
not certied in writing or belonging to SIBEL S.A.
Accessories and spare parts should always be original and requested
to the manufacturer or authorized dealer to ensure the proper
functioning of the polygraph.
Periodic functional and metrological verication is necessary to
ensure the operation of equipment over the life of the product. The
manufacturer recommends annual inspections by an authorized
service center, and in any case not exceeding two years, according
to the verication and adjustment procedures specied by the
manufacturer and applied during the manufacture of the equipment.

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
Annex 1: Electromagnetic compatibility
62
5.3 CORRECTIVE MAINTENANCE
Corrective maintenance is the process of leaving the device in
good conditions of use when it stops working due to malfunction
or misuse.
If you detect a fault in the equipment that prevents normal
operation, please contact the SIBEL S.A. Customer Service
Centre, specifying the type of problem suffered.

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
Annex 1: Electromagnetic compatibility
63
ANNEX 1. ELECTROMAGNETIC COMPATIBILITY
Manufacturer’s guide and statement – electromagnetic emissions
The SCREEN&GO/SLEEP&GO is designed to be used in the electromagnetic environment specied below. The customer
or user must ensure that it is used within this environment.
Emissions Test Compliance level Guide – Electromagnetic environment
Radiated RF Emissions
CISPR 11 (EN 55011)
Group 1
Class B.
The SCREEN&GO/SLEEP&GO uses RF energy
only for internal use. Therefore its emissions are
very low and unlikely to cause any interference to
nearby electronic devices.
Conducted RF Emissions
CISPR 11 (EN 55011)
Not applicable The SCREEN&GO/SLEEP&GO runs on batteries.
Harmonic Emissions
EN-IEC 61000-3-2
Not applicable The SCREEN&GO/SLEEP&GO runs on batteries.
Flicker and tension uctuations
EN-IEC 61000-3-3
Not applicable
The SCREEN&GO/SLEEP&GO runs on batteries.

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
Annex 1: Electromagnetic compatibility
64
Manufacturer’s guide and statement –electromagnetic immunity
The SCREEN&GO/SLEEP&GO is designed to be used in the electromagnetic environment specied below. The
customer or user of the SCREEN&GO/SLEEP&GO must ensure that it is used in this environment.
Immunity Test Test level EN-IEC 60601 Compliance level
Guide Electromagnetic
environment
Electrostatic discharge
(ESD)
EN-IEC 61000-4-2
±6 kV in contact
±8 kV in air
±6 kV in contact
±8 kV in air
The oor must be made of
wood, cement or ceramic.
If the oor is covered with
synthetic material, the
relative humidity must be at
least 30 %.
Electrical fast transient/burst
EN-IEC 61000-4-4
±2 kV feeder cables and earth
lines
±1 kV for input/output lines
Not applicable
Not applicable
The SCREEN&GO /
SLEEP&GO runs on
batteries.
The length of the E/S lines
is less than 3 m.
Surge
EN-IEC 61000-4-5
±1 kV in differential mode
±2 kVin common mode
Not applicable
Not applicable
The SCREEN&GO /
SLEEP&GO runs on
batteries.
Voltage dips, short
interruptions and voltage
variations on power supply
input lines
EN-IEC 61000-4-11
<5 % Ut (>95 % drop of Ut) for
0.5 cycles
40 % Ut (60 % drop of Ut) for
5 cycles
70 % Ut (30 % drop of Ut) for
25 cycles
<95 % Ut (>5 % drop of Ut) for
5 seconds
Not applicable
The SCREEN&GO /
SLEEP&GO runs on
batteries.
Magnetic eld 50 / 60 Hz
EN-IEC 61000-4-8
3 A/m 3 A/m
The magnetic eld in the
room must be low enough
to ensure the performance
of the test.
Note: Ut is the alternating current power tension prior to the application of the test.
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
Annex 2: Troubleshooting guide
65
Manufacturer’s guide and statement –electromagnetic immunity
The SCREEN&GO / SLEEP&GO is designed to be used in the electromagnetic environment specied below. The customer
or user of the SCREEN&GO / SLEEP&GO must ensure that it is used in this environment.
Immunity Test
Test Level EN-IEC
60601
Compliance
level
Guide – Electromagnetic environment
Portable and mobile RF Communications units must
be used no closer to any part of the SCREEN&GO /
SLEEP&GO, including cables, than the recommended
separation distance calculated for the equation
applicable to the transmitter frequency.
Recommended separation distance
Conducted RF
EN-IEC 61000-4-6
3 Vrms
from 150KHz to 80 MHz
3 Vrms
from 80 MHz to 800 MHz
Radiated RF
EN-IEC 61000-4-3
3 V/m
from 80 MHz to 2.5 GHz
3 V/m
from 80 MHz to 800 MHz
from 800 MHz to 2.5 GHz
where P is the maximum power output of the
transmitter in watts (W) according to the manufacturer
of the transmitter and d is the recommended
separation distance in metres (m).
Field intensities from permanent RF transmitters,
determined by an electromagnetic measurement of
the place a, must be less than the level of compliance
in each margin of frequency b.
Interferences may appear in the vicinity of units
marked with this symbol:
Note 1. At 80 MHz and 800 MHz, apply the highest frequency margin.
Note 2. These recommendations may not be applicable in all possible situations. Electromagnetic propagation is affected
by the absorption and reection of structures, objects, and persons.

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
Annex 2: Troubleshooting guide
66
a
The eld intensities emitted by permanent transmitters such as base stations for radiotelephones (mobile-cell phones,
wireless) and mobile radios, amateur radio operators, AM, FM radio and TV transmissions, cannot be theoretically
calculated with any accuracy. To know the electromagnetic environment due to permanent RF transmitters, electromagnetic
measurements of the place of use must be considered. If the eld intensity of the place of use is superior to the level of
compliance, it must be observed whether the behaviour of the SCREEN&GO / SLEEP&GO is normal. Otherwise, additional
measurements such as the reorientation or change in placement of the SCREEN&GO / SLEEP&GO may be necessary.
b
Above the frequency range from 150 kHz to 80 MHz, the eld intensity must be less than 3 V/m.
Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
Annex 2: Troubleshooting guide
67
Recommended separation distances between mobile and portable RF communications units and the
SCREEN&GO/SLEEP&GO
The SCREEN&GO/SLEEP&GO is designed to be used in an electromagnetic environment in which radiated RF disturbances
are controlled. The customer or user of the SCREEN&GO/SLEEP&GO can help to prevent interferences by keeping a
minimum distance between mobile and portable RF communications units (transmitters) and the SCREEN&GO/SLEEP&GO
as recommended below, according to the power output of the communications unit.
Maximum
power
output of the
transmitter
W
Separation distance depending on the transmitter frequency
m
From 150 kHz to 80 MHz
From 80 MHz to 800 MHz From 800 MHz to 2.5 GHz
0.01 0.12 0.12 0.23
0.1 0.37 0.37 0.74
1 1.17 1.17 2.33
10 3.69 3.69 7.38
100 11.67 11.67 23.33
For transmitters with a maximum power output not listed above, the recommended separation distance d in metres (m) can
be estimated using the applicable equation according to the transmitter frequency, where P is the maximum power of the
transmitter in watts (W) according to the manufacturer of the transmitter.
Note 1 At 800 MHz, apply the highest frequency margin
Note 2 These recommendations may not apply in all possible situations. Electromagnetic propagation is affected by the
absorption and reection of structures, objects and persons.

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
Annex 2: Troubleshooting guide
68
ANNEX 2. TROUBLESHOOTING GUIDE
The Sleep&Go does not start
• Make sure the batteries are placed according to the polarity in
the battery compartment.
• Be sure to use AA alkaline or NiMH rechargeable batteries of at
least 2450mAh.
• Make sure you press the power button for at least 3 seconds.
The PC can not communicate with the device
• Make sure you have purchased the Bluetooth option in your
Sleep&Go.
• Make sure the Sleep&Go is switched on and the LED on the
front panel is lighting.
• Verify that the Bluetooth option is activated in the Sleep&Go.
• Verify that the Bluetooth module in the PC is enabled and
working properly.
In a new test the message “CHECK SENSOR” is displayed
on screen
• Check that the erroneous sensor is correctly connected. If
the problem is not solved after 5 minutes, the message will
disappear.
The signals of the electrodes look noisy or distorted
• In the case of the electrodes, make sure that the skin-electrode
impedance is good. Check the PLACEMENT OF SENSORS AND
ELECTRODES section, with suggestions on the placement of the
electrodes.
• Check the connection of the ground electrode. This electrode
plays an important role in signal quality.
• Twist the wires of the different electrodes to reduce the
environmental noise.
• In the BitmedLab software, check that the lters currently
selected are appropriate for the type of signal being acquired.

Sleep&Go
User’s Manual
534-7AB-MU2 • REV. 1.07
Annex 2: Troubleshooting guide
69
Pulse oximetry signals are null
• Make sure the pulse oximeter sensor is correctly connected to
the patient, as indicated the PLACEMENT OF SENSORS AND
ELECTRODES section.
• Make sure the pulse oximetry sensor connector is properly
plugged into the top panel of the Sleep&Go.
• Make sure the red light in the pulse oximetry sensor switches
on when connected to the Sleep&Go.
 Loading...
Loading...