Page 1

Ilesto
xxx
xxx
xxx
ICD Familie • Tachyarrhythmietherapie • Kardiale Resynchronisationstherapie
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
Technical Manual
Technická příručka
Brugermanual
Gebrauchsanweisung
Manual técnico
Käyttöohje
Manuel technique
Manuale tecnico di istruzione
Gebruikshandleiding
Instrukcja obsługi
Manual técnico
Bruksanvisning
• en
• cs
• da
• de
• es
•
• fr
• it
• nl
• pl
• pt
• sv
Page 2
0123
© BIOTRONIK SE & Co. KG
All rights reserved. Specications subject
to modication, revision and improvement.
® BIOTRONIK Home Monitoring, IEGM-Online HD
and SMART Detecton are registered trademarks
of BIOTRONIK SE & Co. KG
0681 2012
12-D-xx
Revision: B (2012-xx-xx)
BIOTRONIK SE & Co. KG
Woermannkehre 1
12359 Berlin · Germany
Tel +49 (0) 30 68905-0
Fax +49 (0) 30 6852804
sales@biotronik.com
www.biotronik.com
Page 3

1
Ilesto 5/7
VR-T, VR-T DX,
DR-T, HF-T
ICD Family
Tachyarrhythmia Therapy
Cardiac Resynchronization Therapy
Technical Manual for the Device
Doc. Id.: GA-HW_en--mul_393468-B
Index GA-HW_en--mul_393468-BTec hnical[nbsp ]Manual f or the[nbsp ]Device Ilesto 5/7 VR-T, VR-T D X, DR-T, HF-T
Page 4

2
Page 5

3 Table of Contents
Table of Contents
Table of Contents
Product Description. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Intended Medical Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
System Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Therapeutic and Diagnostic Functions. . . . . . . . . . . . . . . . . 11
General Safety Instructions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Operating Conditions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Possible Complications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Possible Risks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Implantation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Implantation Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Precautionary Measures while Programming . . . . . . . . . . 21
Magnet Response. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
Follow-up . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Patient Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
Replacement Indications. . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
Explantation and Device Replacement. . . . . . . . . . . . . . . . . 28
Parameters. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
Bradycardia / CRT . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
Tachycardia . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
Sensing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35
Diagnostics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36
Home Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 37
Technical Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 39
Mechanical Characteristics . . . . . . . . . . . . . . . . . . . . . . . . . 39
Electrical Characteristics . . . . . . . . . . . . . . . . . . . . . . . . . . . 40
Battery Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
Legend for the Label . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 45
Page 6

4 Table of Contents
Page 7

5 Product Description
1 Product Description
Product Description1GA-HW_en--mul_393468-BTechnic al[nbsp ]Manual for the[nbsp ]Device Ilesto 5/7 VR-T, VR-T DX, DR -T, HF-T
Intended Medical Use
Intended use Ilesto 5/7 is part of a familiy of implantable cardioverter-defibrillators (ICDs).
Primary objective of the therapy is to prevent sudden cardiac death. Furthermore,
the device is capable of treating bradycardia arrhythmias and cardiac resynchronization therapy with multisite ventricular pacing.
The implantation of an ICD is a symptomatic therapy with the following objectives:
• Termination of spontaneous ventricular fibrillation (VF) through shock delivery
• Termination of spontaneous ventricular tachycardia (VT) through antitachycardia pacing (ATP); in case of ineffective ATP or hemodynamically not tolerated VT, with shock delivery
• Cardiac resynchronization through multisite ventricular pacing (triplechamber devices)
• Compensation of bradycardia through ventricular (single-chamber devices) or
AV sequential pacing (DX, dual- and triple-chamber devices)
Diagnosis and therapy
forms
Required expertise In addition to having basic medical knowledge, the user must be thoroughly
The device monitors the heart rhythm and automatically detects and terminates
cardiac arrest resulting from ventricular tachyarrhythmia. All major therapeutic
approaches from the field of cardiology and electrophysiology are included. BIOTRONIK Home Monitoring
any time.
familiar with the operation and the operation conditions of a device system.
• Only qualified medical specialists having this special knowledge required are
permitted to use implantable devices.
• If users do not possess this knowledge, they must be trained accordingly.
®
enables physicians to perform therapy management at
Page 8

6 Product Description
Indications Ilesto can treat life-threatening ventricular arrhythmias with antitachycardia
pacing and defibrillation.
Generally approved differential diagnostics methods, indications, and recommendations for ICD therapy apply to BIOTRONIK devices. See the guidelines of cardiology associations for guidance.
We recommend observing the indications published by the German Cardiac Society
(Deutsche Gesellschaft für Kardiologie, Herz- und Kreislaufforschung) and the
ESC (European Society of Cardiology). This also applies to the guidelines published
by the Heart Rhythm Society (HRS), the American College of Cardiology (ACC), the
American Heart Association (AHA), and other national cardiology associations.
Single-chamber and dual-
chamber
Triple-chamber Triple-chamber ICDs are indicated for patients with the following risks:
Contraindications Known contraindications:
Single-chamber and dual-chamber ICDs are indicated for patients with the following risk:
• Sudden cardiac death caused by ventricular arrhythmias
• Sudden cardiac death caused by ventricular arrhythmias
• Congestive heart failure with ventricular asynchrony
Also indicated for primary prophylaxis in congestive heart failure patients is Ilesto.
• Tachyarrhythmia caused by temporary or reversible irritation, e.g. poisoning,
electrolyte imbalance, hypoxia, sepsis or acute myocardial infarction
• Such frequent VT or VF that the therapies would cause an unacceptably rapid
depletion of the device batteries
• VT with few or without clinically relevant symptoms
• VT or VF treatable by surgery
• Concomitant diseases that would substantially limit a positive prognosis
• Accelerated idioventricular rhythm
Page 9
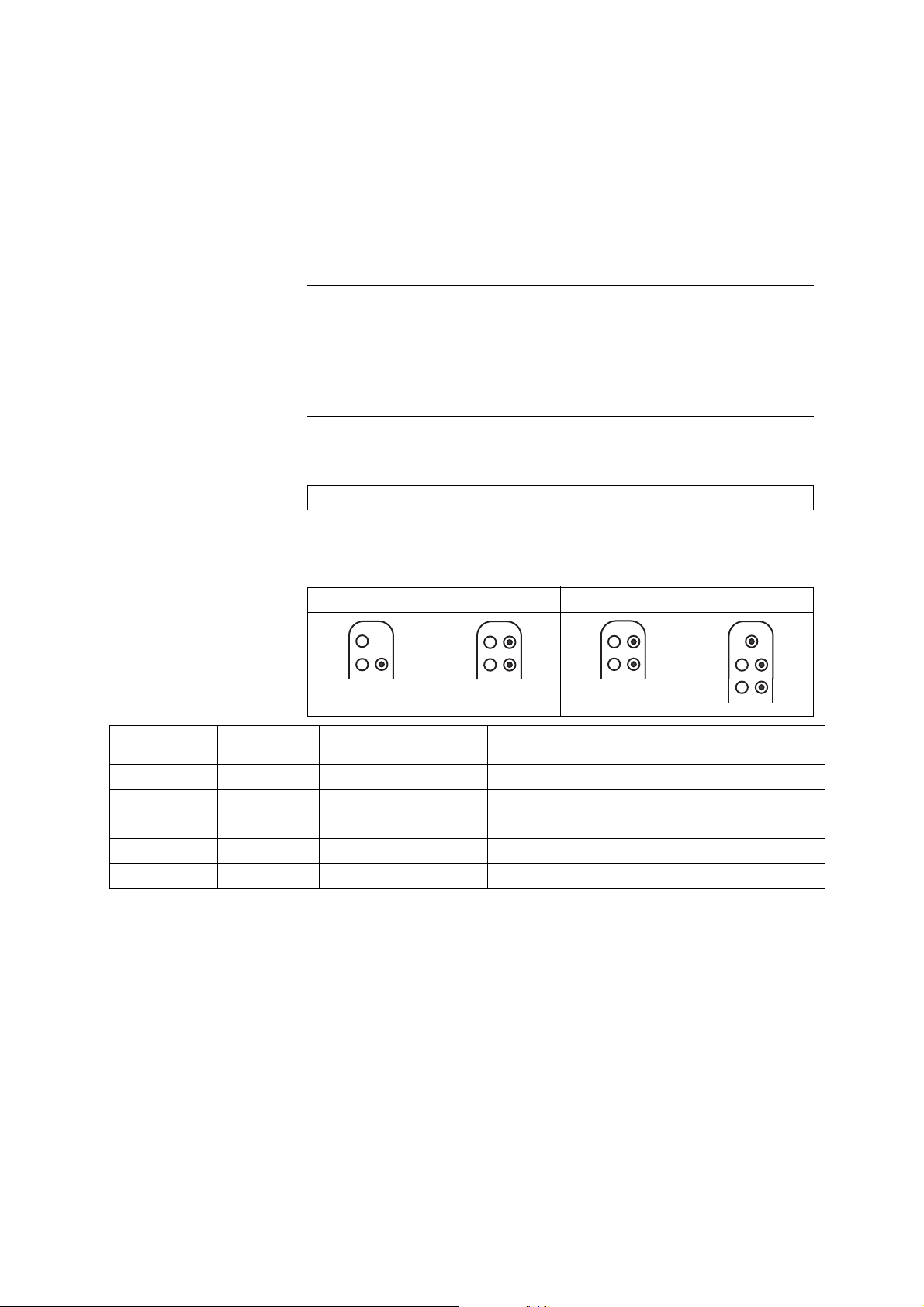
7 Product Description
DF-1
RV
DF-1
SVC
IS-1
RV
DF-1
RV
DF-1
SVC
IS-1
RA
IS-1
RV
DF-1
RV
DF-1
SVC
IS-1
RA
IS-1
RV
DF-1
RV
DF-1
SVC
IS-1
RA
IS-1
RV
IS-1
LV
System Overview
Device family The complete Ilesto 5/7 device familyconsists of several device types with a DF-1/
IS-1 or DF4/IS-1 connection.
Single-chamber: VR-T and VR-T DX (only devices with a DF-1/IS-1 connection);
dual-chamber: DR-T; triple-chamber: HF-T. Not all device types are available in
every country.
Device The device's housing is made of biocompatible titanium, welded from outside and
thus hermetically sealed. The ellipsoid shape facilitates implantation in the pectoral muscle area. The connections for bipolar pacing and sensing (and unipolar
connections for the triple-chamber device) as well as for shock delivery are found
in the device header. The housing serves as a potential antipole during shock
delivery or in the case of unipolar lead configuration.
DF-1/IS-1 or DF4/IS-1 BIOTRONIK provides ICDs with headers for different standardized lead connec-
tions: DF-1/IS-1 and DF4/IS-1.
Note: The device type DX can only be connected using a DF-1/IS-1 connector.
DF-1/IS-1 lead connection The device labeling provides information pertaining to possible lead connections
depending on the device type and pertaining to connection assignment:
VR DX DR HF
Connector port Lead con-
Configuration Implantation site Device type
nector
RV DF-1 Shock coil Right ventricle VR, DX, DR, HF
SVC DF-1 Shock coil Superior vena cava VR, DX, DR, HF
RA IS-1 Bipolar Atrium DX, DR, HF
(R)V IS-1 Bipolar (Right) ventricle VR, DX, DR, HF
LV IS-1 Unipolar, Bipolar Left ventricle HF
Page 10
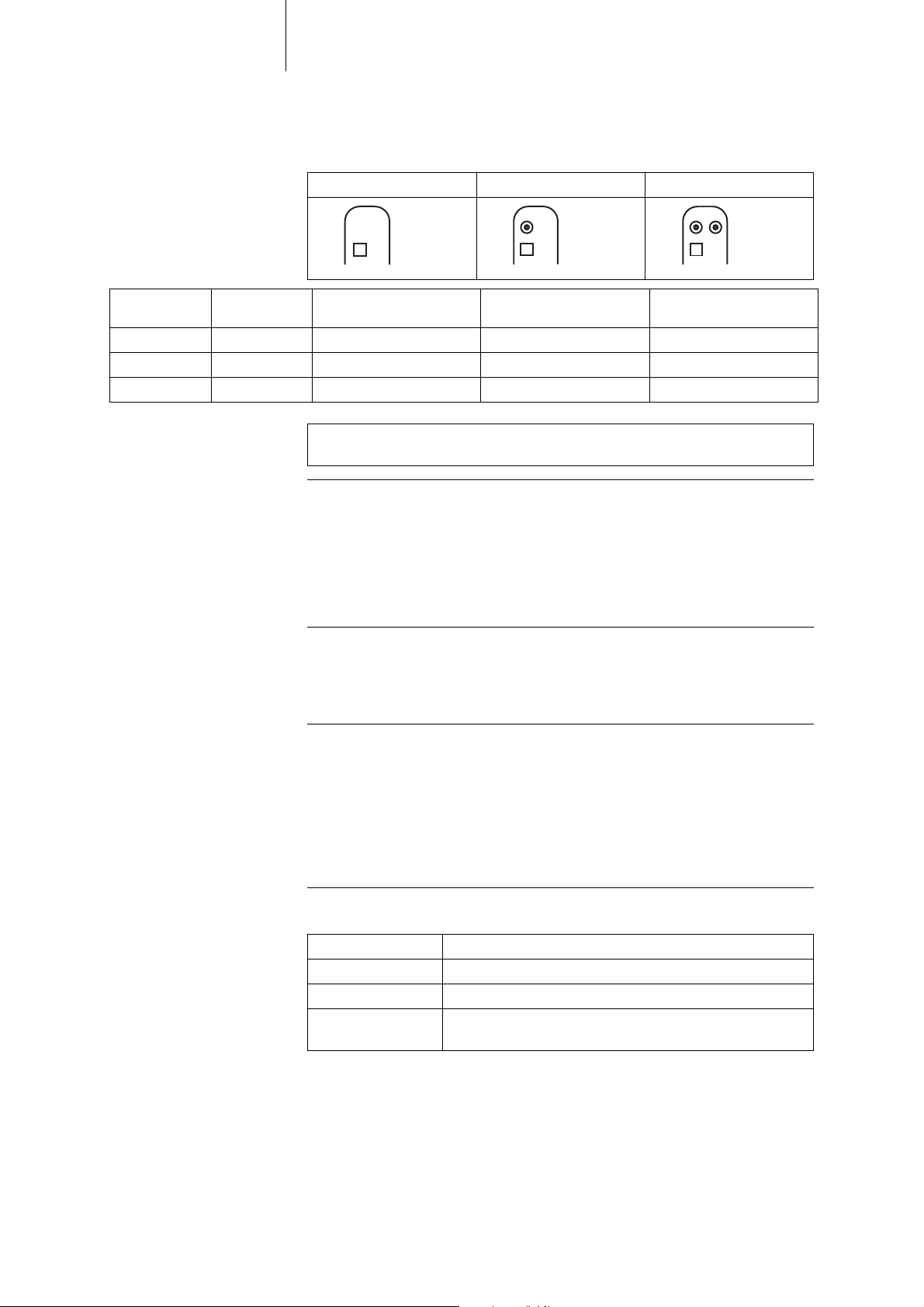
8 Product Description
DF4
RV
DF4
RV
IS-1
RA
DF4
RV
IS-1
RA
IS-1
LV
DF4/IS-1 lead connection The device labeling provides information pertaining to possible lead connections
depending on the device type and pertaining to connection assignment:
VR DR HF
Connector
port
Lead connector
Configuration Implantation site Device type
RA IS-1 Bipolar Atrium DR, HF
LV IS-1 Unipolar, Bipolar Left ventricle HF
RV, SVC DF4 Bipolar and shock Right ventricle VR, DR, HF
Note: The device's DF4 connector port may only be used for connecting leads
with a DF4 connector that conform to ISO 27186.
Leads BIOTRONIK leads are sheathed with biocompatible silicone. They can be flexibly
maneuvered, are stable long-term, and are equipped for active or passive fixation.
They are implanted using a lead introducer set. Some leads are coated with polyurethane which is known to increase the sliding properties for the lead. Leads with
steroids reduce inflammatory processes. The fractal design of the electrodes provides for low pacing thresholds. BIOTRONIK provides adapters to connect already
implanted leads to new devices.
Telemetry Telemetric communication between the device and the programmer can be carried
out following initialization either by applying the programming head (PGH) to the
device or by using radio frequency (RF) telemetry in the programmer. BIOTRONIK
®
calls this function SafeSync
.
Programmer Implantation and follow-up are performed with BIOTRONIK's portable pro-
grammer. There is one with integrated RF telemetry and one with a separate
SafeSync Module. The programmer is used during implantation to transfer the
current device program to the device. The pacing thresholds can be determined
and all tests can be performed during in-office follow-up. In addition to this, the
programmer is used to set mode and parameter combinations, as well as for interrogation and saving of data from the device. Leadless ECG, IEGM, markers and
functions are displayed simultaneously on the color display.
Modes The mode setting depends on the individual diagnosis:
Device type Modes
VR VVI; VVIR; V00; OFF
DX VDD; VDDR; VDI; VDIR; VVI; VVIR; V00; OFF
DR, HF DDD; DDDR; DDI; DDIR; VDD; VDDR; VDI; VDIR
VVI; VVIR; AAI; AAIR; V00; D00; OFF
Page 11

9 Product Description
NBD and NBG codes VVE is the NBD code for the antitachycardia mode of the single-chamber, dual-
chamber, and triple-chamber devices:
V Shock in the ventricle
V Antitachycardia pacing (ATP) in the ventricle
E Detection via IEGM analysis
DDDR is the NBG code for the antibradycardia mode of the dual-chamber device:
D Pacing in the atrium and ventricle
D Sensing in the atrium and ventricle
D Pulse inhibition and pulse triggering
R Rate adaptation
DDDRV is the NBG code for the antibradycardia mode of the triple-chamber device:
D Pacing in the atrium and ventricle
D Sensing in the atrium and ventricle
D Pulse inhibition and pulse triggering
R Rate adaptation
V Multisite pacing in both ventricles
VDDR is the NBG code for the antibradycardia mode of the single-chamber DX
device:
V Ventricular pacing
D Sensing in the atrium and ventricle
D Pulse inhibition and pulse triggering
R Rate adaptation
VVIR is the NBG code for the antibradycardia pacing modes of the single-chamber
device:
V Ventricular pacing
V Sensing in the ventricle
I Pulse inhibition in the ventricle
R Rate adaptation
Page 12
10 Product Description
BIOTRONIK
Home Monitoring
Technical manuals The following technical manuals provide information about usage of the device sys-
In addition to effective pacing therapy, BIOTRONIK provides a complete therapy
®
management system:
• With Home Monitoring, diagnostic and therapeutic information as well as technical data are automatically sent to a stationary or mobile transmitter via an
antenna in the device header. The data are encrypted and sent from the transmitter to the BIOTRONIK Service Center via the cellular phone network.
• The received data are deciphered and evaluated. Each physician can set the criteria for evaluation to be used for each patient and can configure the time of
notification via E-mail, SMS or fax.
• A clear overview of the results of this analysis is displayed for the attending
physicians on the protected Internet platform Home Monitoring Service Center
(HMSC).
• Data transmission from the device is performed with a daily device message.
• Device messages, which indicate special events in the heart or in the device,
are forwarded immediately.
• A test message can be initiated at any time using the programmer to immediately check the Home Monitoring function.
tems:
• Technical manual for the implant
• Technical manual for the HMSC
• Technical manuals for the programmer and the SafeSync Module
• Technical manual for device programs as online help on the user interface and
as a PDF file in the Manual Library at www.BIOTRONIK.com
• Technical manuals for the leads
• Technical manuals for cables, adapters and accessories
Order numbers for
Ilesto with DF-1/IS-1 or
DF4/IS-1 connection
Scope of delivery The storage package includes the following:
Not all device types are available in all countries:
Ilesto 5 Ilesto 7
DF-1 DF4 DF-1 DF4
VR-T 383582 383584 383579 383581
VR-T DX 383596 — 390095 —
DR-T 383566 383568 383563 383565
HF-T 383550 383552 383547 383549
• Sterile container with device
•Serial number label
•Patient ID card
• Warranty booklet
• Technical manual for the implant
The sterile container includes the following:
• Device, blind plug DF-1 (if applicable) and blind plug IS-1 for device type HF
• Screwdriver
Page 13

11 Product Description
Therapeutic and Diagnostic Functions
Diagnostic functions • Data from implantation and the most recent interrogations and follow-ups are
recorded as well as arrhythmia episodes; they are stored together with other
data to assess patients and the state of the device at any time.
• To check the lead for proper functioning, an automatic impedance measurement using subthreshold pacing pulses is performed in the device.
• Leadless ECG function: For all device types, far-field derivation can be measured without external leads between the right ventricular shock coil and
housing, which, depending on the implantation site, corresponds to ECG
derivation II or III (Einthoven).
• Once a telemetry connection has been established during a test procedure in
an in-office follow-up, the leadless ECG and the IEGM are displayed with
markers.
Antitachycardia pacing • The ICD can treat ventricular tachycardia with antitachycardia pacing (ATP);
ATP can also be delivered in the VF zone (ATP One Shot) when the stability criterion indicating that this will be effective before shock delivery (monomorphic
rapid VTs) is met.
• Depending on the device type, the device program contains not only the ICD
functions but also all pacemaker functions for 1, 2, or 3 chambers. The heart
rhythm is continuously monitored; each arrhythmia is classified according to
the heart rate and the adjustable detection criteria. Depending on the preset
values, antibradycardia as well as antitachycardia therapy is inhibited or delivered.
Cardioversion, defibrilla-
tion
Antibradycardia pacing and
CRT
• The ICD can treat ventricular tachyarrhythmia with cardioversion and/or defibrillation. Shock polarity and energy can be programmed individually. Shock
energies between 2.0 and 40 J are possible. Before delivery of the shock, the
ICD can be set to only deliver a shock when ongoing tachyarrhythmia is confirmed; during this time period the device can identify spontaneous conversion
of the tachyarrhythmia and cancel the charging process if necessary.
• The shock paths can be set between the different shock coils (SVC/RV) and/or
the housing.
• Innovative rate hystereses, automatic sensor functions, and a night program
promote the patient's intrinsic rhythm, avoid overdrive pacing, and facilitate
adaptation of the device to the individual needs of the patient.
• Setting an upper tracking rate for the atrium prevents unspecific atrial pacing,
thus reducing the risk of pacemaker-mediated tachycardia.
• Positive AV hysteresis functions support the intrinsic conduction and thus the
natural contraction sequence. Negative AV hysteresis functions support the
cardiac resynchronization therapy by maintaining pacing in stressful situations.
• For resynchronization of the ventricles, triple-chamber devices have functions
for multisite ventricular pacing with possible VV delays in either direction.
• To ensure that no additional surgery is necessary in case of a left-sided
increase of pacing threshold or undesired phrenic nerve stimulation, different
pacing polarities can be set for the left ventricular lead with a triple-chamber
device.
• Automatic active capture control is available for the right and left ventricle with
automated tracking of the pacing threshold or automatic threshold monitoring
(ATM) for trend analysis.
Page 14

12 Product Description
Storing programs The parameter settings can be saved in 3 individual therapy programs.
Home Monitoring functions • The device automatically sends information to the transmitter once a day. It
also sends messages related to events, which are immediately forwarded to
the Service Center. In addition to this, test messages can be initiated using the
programmer.
• Appointments for Home Monitoring-supported follow-ups can be scheduled via
the HMSC. This applies to Ilesto 5/7.
• Important medical information in the device messages include the following:
— Atrial and ventricular arrhythmias
— Parameters relevant to leads in the atrium and ventricle: pacing thresh-
olds, sensing amplitudes, impedances
— Current statistics
—IEGM online HD
®
with up to 3 high definition channels
Page 15

13 General Safety Instructions
2 General Safety Instructions
General Safety Instructions2GA-HW_en--mul_393468-BTechnical[nbsp ]Manual for the[nbsp ]DeviceIlesto5/7 VR-T, VR-TDX, DR-T, HF-T
Operating Conditions
Care during shipping and
storage
Delivery in shipment mode The device is delivered in shipment mode to protect the battery; capacitor
Temperature Extremely low and high temperatures affect the service time of the battery in the
Sterile delivery The device and the screwdriver have been gas-sterilized. Sterility is guaranteed
Sterile container The device and screwdriver are packaged in two separately sealed blisters. The
• Devices are not to be stored or transported close to magnets or sources of
electromagnetic interference.
• Note the effects of the storage duration; see Battery Data.
reforming required during storage could result in controlled extended charge
times of the shock capacitors.
• The shipment mode is displayed on the programmer after loading the device
program (it is deactivated during implantation on initial measurement of the
pacing impedance).
device.
• Temperatures of 5°C to 45°C are permitted for transport, storage, and use.
only if the blister and quality control seal have not been damaged.
inner blister is also sterile on the outside so that it can be transferred in a sterile
state during implantation.
Single use only The device and screwdriver are intended for single use only.
• Do not use the device if the package is damaged.
• The device must not be resterilized and reused.
Page 16

14 General Safety Instructions
Possible Complications
General information on
medical complications
Skeletal myopotentials Bipolar sensing and control of sensitivity are adapted by the device to the rate
Possible technical failures Technical failure of a device system cannot be entirely ruled out. Possible causes
Complications for patients and device systems generally recognized among practitioners also apply to BIOTRONIK devices.
• Normal complications may include fluid accumulation within the device pocket,
infections, or tissue reactions. Primary sources of complication information
include current scientific and technological knowledge.
• It is impossible to guarantee the efficacy of antitachycardia therapy, even if the
programs have proven successful during tests or subsequent electrophysiological examinations. In rare cases the set parameters may become ineffective.
It is possible for therapies to induce or accelerate tachycardia and cause sustained ventricular flutter or fibrillation.
spectrum of intrinsic events so that skeletal myopotentials are usually not
recorded. Skeletal myopotentials can nonetheless be classified as intrinsic events
especially at very high sensing sensitivity and, depending on the interference, may
cause inhibition or antiarrhythmia therapy.
In the case of undesired myopotentials, the device switches to asynchronous
pacing if the interference rate is exceeded.
can include the following:
• Lead dislodgement, lead fracture
• Insulation defects
• Device component failures
• Battery depletion
• Interrupted telemetry
Electromagnetic interfer-
ence (EMI)
Device behavior in case of
Static magnetic fields The reed switch in the device closes starting at a field strength of 1.8 mT. The reed
Any device can be sensitive to interference if external signals are sensed as
intrinsic rhythm or if measurements prevent rate adaptation.
• BIOTRONIK devices have been designed so that their susceptibility to EMI is
minimal.
• Due to the intensity and variety of EMI, there is no guarantee for safety. It is
generally assumed that EMI produces only minor symptoms, if any, in patients.
• Depending on the pacing mode and the type of interference, sources of interference may lead to pulse inhibition or triggering, an increase in the sensordependent pacing rate or asynchronous pacing.
• Under unfavorable conditions, for example during therapeutic or diagnostic
procedures, interference sources may induce such a high level of energy into
the pacing system that the cardiac tissue surrounding the lead tip is damaged.
In case of electromagnetic interference, the device switches to asynchronous
EMI
pacing for as long as the interference rate is exceeded.
switch opens if the magnetic field falls below 1 mT.
Page 17

Possible Risks
15 General Safety Instructions
Contraindicated proce-
dures
Risky therapeutic and diag-
nostic procedures
The following procedures are contraindicated as they may cause harm to the
patient or damage the device and, as a result, put the system functionality at risk:
• Therapeutic ultrasound: Harm to the patient via excess warming of body tissue
near the device system
• Transcutaneous electrical nerve stimulation
• Hyperbaric oxygen therapy
• Applied pressures higher than normal pressure
If electrical current from an external source is conducted through the body for
diagnostic or therapeutic purposes, then the device can be subjected to interference, which can place the patient at risk.
Arrhythmia or ventricular fibrillation can be induced during diathermic procedures
such as electrocautery, HF ablation or HF surgery. For example, damaging heat
can result during lithotripsy. Influences on the device are not always immediately
clear.
If risky procedures cannot be avoided, the following should be observed at all
times:
• Electrically insulate the patient.
• Switch off the ICD's detection function. The pacemaker function can remain
active. The device may need to be switched to asynchronous modes for this.
• Do not introduce energy near the device system.
• Additionally check the peripheral pulse of the patient.
• Monitor the patient during and after every intervention.
External defibrillation The device is protected against the energy that is normally induced by external defi-
brillation. Nevertheless, any implanted device may be damaged by external defibrillation. Specifically, the current induced in the implanted leads may result in
necrotic tissue formation close to the electrode/tissue interface. As a result,
sensing properties and pacing thresholds may change.
• Place adhesive electrodes anterior-posterior or perpendicular to the axis
formed by the device to the heart at least 10 cm away from the device and from
implanted leads.
Radiation therapy The use of radiation therapy is contraindicated due to possible damage to the
device and the resulting impaired functional safety. If this type of therapy is to be
used anyway, prior risk/benefit analysis is absolutely necessary. The complexity of
influencing factors such as different sources of radiation, a variety of devices and
therapy conditions makes it impossible to issue directives that guarantee radiation
therapy without an impact on the device. The EN 45502 standard pertaining to
active implantable medical devices requires the following measures during the
administration of therapeutic ionizing radiation:
• Adhere to instructions for risky therapy and diagnosis procedures.
• Shield device against radiation.
• After applying radiation, double-check the device system to make sure it is
functioning properly.
Note: Please contact BIOTRONIK with questions during the risk/benefit analysis.
Page 18

16 General Safety Instructions
Magnetic resonance
imaging
Magnetic resonance imaging (MRI) is contraindicated due to the high frequency
fields and the associated magnetic flux density: damage or destruction of the
device system by strong magnetic interaction and damage to the patient by excessive warming of the body tissue in the area surrounding the device system.
• Under certain conditions one can perform special measures with magnetic resonance imaging to protect the patient and device.
Page 19

17 Implantation
!
!
3 Implantation
Implantation3GA-HW_en--mul_393468-BTechnical[nbsp ]Manual for the[nbsp ]DeviceIlesto5/7 VR-T, VR-T DX, DR-T, HF-T
Implantation Procedure
Having parts ready The following parts that correspond to the requirements of the EC Directive 90/
385/EEC are required:
• BIOTRONIK device with blind plug and screwdriver
• BIOTRONIK leads and lead introducer set
— Single-chamber device: One bipolar ICD lead with 1 or 2 shock coils for the
ventricle
— Dual-chamber device: One bipolar lead for the atrium and one bipolar ICD
lead for the ventricle with 1 or 2 shock coils
— Triple-chamber device: an additional unipolar or bipolar LV lead
• DF-1, DF4 and IS-1 connections are approved. For leads with a different connection or leads from other manufacturers, use adapters approved by BIOTRONIK only.
• BIOTRONIK programmer (with integrated SafeSync RF telemetry or with separate SafeSync Module) and approved cable
• External multi-channel ECG device
• Keep spare parts for all sterile components.
Keeping an external defi-
brillator ready
Unpacking the device
In order to be able to respond to unforeseeable emergencies or possible technical
failures of the device:
• Keep an external defibrillator and paddles or patch electrodes ready.
WARNING
Inadequate therapy due to defective device
If an unpacked device is dropped on a hard surface during handling, electronic
parts could be damaged.
• Use a replacement device.
• Return the damaged device to BIOTRONIK.
• Peel the sealing paper off of the outer blister at the marked position in the
direction indicated by the arrow. The inner blister may not come into contact
with persons who have not sterilized their hands or gloves, nor with non-sterile
instruments.
• Take hold of the inner blister by the gripping tab and take it out of the outer
blister.
• Peel the sealing paper off of the sterile inner blister at the marked position in
the direction indicated by the arrow.
Page 20

18 Implantation
!
!
!
!
Checking parts Damage to any of the parts can result in complications or technical failures.
• Check for damage before and after unpacking all parts.
• Replace damaged parts.
• The ICD is shipped with tachyarrhythmia therapy deactivated and is only to be
connected and implanted in this state.
• Leads may not be shortened.
Implantation site • Depending on lead configuration and the patient's anatomy, the ICD is generally
implanted subpectorally on the left side.
Preventing leakage
currents
Preventing unintentional
shock delivery
Avoiding damage to the
header
Leakage currents between the tools and the device must be prevented during
implantation.
• Electrically insulate the patient.
WARNING
Shock delivery with activated ICD
There is a risk of unintended shock delivery when handling an activated ICD.
• Deactivate ICD therapy before touching the device during implantation,
device replacement and explantation.
There is a blind plug for DF-1 and IS-1 connections in the header. The provided set
screws must be carefully loosened or tightened.
• Loosen set screws with the supplied screwdriver. Use only BIOTRONIK screwdrivers with torque control!
•Do not forcibly pull out the blind plug!
• If lead repositioning is necessary, re-order sterile screwdrivers from BIOTRONIK.
Preventing short circuits in
the header
Ensure that connections are
clean
WARNING
Short circuit due to open lead connector ports
Connector ports in the header which are open and thus not electrolyte-proof may
cause undesired current flows to the body and penetration of body fluid into the
device.
• Either leave unused ports closed with the premounted blind plugs, or close
them using the supplied blind plugs.
In case of contamination during implantation:
• Clean lead connectors with a sterile cloth.
• Rinse connection only with sterile water.
Page 21

19 Implantation
!
!
Connecting the lead
connector to the device
1 Disconnect stylets and stylet guides.
2 DF-1/IS-1 connection:
• Connect the DF-1 connector for the right-ventricular shock coil to RV.
• Connect the DF-1 connector for the supraventricular shock coil to SVC.
Or connect a subcutaneous array to SVC.
DF4/IS-1 connection:
• Connect the DF4 connector to RV
3 DF-1/IS-1 connection:
• Connect the bipolar IS-1 lead connector for the atrium to RA.
• Connect the IS-1 lead connector for the right ventricle to RV.
• Connect the unipolar or the bipolar IS-1 lead connector for the left ventricle to LV.
DF4/IS-1 connection:
• Connect the bipolar IS-1 lead connector for the atrium to RA.
• Connect the unipolar or the bipolar IS-1 lead connector for the left ventricle to LV.
4 Push the lead connector into the header without twisting or bending the
connector or conductor until the connector tip (on the DF-1 connector) or
the insertion indicator (on the DF4 connector) becomes visible behind the
set screw block. This indicator can vary depending on the manufacturer of
the lead used.
5 If you cannot easily plug the lead connector into the connection:
• Use only sterile water as lubricant.
6 If the lead connector cannot be inserted completely, the set screw may be
protruding into the drill hole of the set screw block.
• Use the screwdriver to perpendicularly pierce through the slitted point
in the center of the silicone plug until it reaches the set screw.
• Carefully loosen the set screw without completely unscrewing it, so that
it does not become tilted upon retightening.
7 Turn the set screw clockwise until torque control starts (you will hear a
clicking sound).
8 Carefully withdraw the screwdriver without retracting the set screw.
• In case of IS-1 connections with two set screws, tighten both screws!
• When the screwdriver is withdrawn, the silicone plug automatically
safely seals the lead connector port.
Keeping distance between
leads
WARNING
Inadequate therapy
When leads are not spaced sufficiently apart or are positioned inappropriately,
this can lead to far-field sensing or insufficient defibrillation.
• The distance between 2 shock coils must be greater than 6 cm.
• Tip and ring electrodes must not have contact with each other.
Page 22

20 Implantation
Implanting
1 Prepare the vein.
2 Implant the leads, perform the measurements, and fixate the leads.
3 Form the device pocket.
4 Connect the lead connector to the device.
5 Insert the device.
6 Guide the fixation suture through the opening in the header and fixate the
device in the prepared device pocket.
7 Close the device pocket.
8 Check the device with standard tests.
Applying the programming
head
Establishing telemetry
contact
Activating ICD therapy • Load the device program that is suitable for the device type in the programmer.
The programming head (PGH) features a diagram of the device. This is used to
assist in positioning the head to ensure proper telemetry.
• Make sure the PGH is positioned correctly.
The programmer (or the SafeSync Module) can be no more than 3 m from the
device; ideally there should be no hindrances between the patient and the programmer.
• Switch on RF telemetry on the programmer.
• Apply the programming head for about 2 s until successful initialization is displayed on the programmer:
The SafeSync symbol is displayed in the navigator and the signal
strength is displayed in the status line.
•Remove the programming head.
• Activate ICD therapy.
• Shipment mode is permanently deactivated once the leads have been connected and initial measurement of the pacing impedance has been performed.
The device data are saved.
• Take precautionary measures while programming.
• If the device induces tachycardia while programming ATPs or does not deliver
adequate therapy in the DFT test: use emergency shock or an external defibrillator.
Page 23

21 Implantation
Precautionary Measures while Programming
Performing standard tests and monitoring the patient
Cancelling telemetry Programmer interference or interrupted telemetry during performance of tempo-
Avoiding critical parameter
settings
Critical conditions can occur for the patient even during standard tests due to inadequate parameter settings or interrupted telemetry.
• Ensure sufficient patient care even during tests.
• After the threshold test, check to determine whether the threshold is clinically
and technically justifiable.
• Continuously monitor the ECG and the patient's condition.
• Cancel testing if necessary.
rary programs (follow-up tests) can result in inadequate pacing of the patient. This
is the case if the programmer can no longer be operated due to a program error or
a defective touch screen and therefore the temporary program cannot be terminated. Under these circumstances, it is helpful to cancel telemetry, in which case
the device automatically switches to the permanent program.
• In the case of telemetry with programming head: lift the PGH by at least 30 cm.
• In the case of RF telemetry: switch off and reposition the programmer.
• Turn off possible sources of interference.
No modes and parameter combinations that pose a risk to the patient should be
set.
• Prior to setting rate adaptation, determine the patient's capacity for strain.
• Check compatibility and effectiveness of parameter combinations after making
settings.
Check for leads suitable for
shock path
Monitoring the patient
when setting asynchronous
modes
Setting sensing Manually set parameters can be unsafe. For example, unsuitable far-field protec-
Preventing device-induced
complications
Three shock paths can be set, two of which form an electrical path to the device
housing.
• A second shock coil (dual shock coil) must be available for the shock path RV >SVC.
The asynchronous modes V00 and D00 can only be set if tachyarrhythmia sensing
is deactivated. This would leave the patient without sensing and therefore without
ICD therapy.
• Continually monitor the patient.
• Keep an external defibrillator ready.
tion may impede sensing of intrinsic pulses.
• Note automatic sensitivity control.
BIOTRONIK devices feature several functions to prevent device-induced complications to the greatest extent possible:
• Measure the retrograde conduction time.
• Set PMT protection.
• Set the VA criterion.
Page 24

22 Implantation
Preventing conduction of
atrial tachycardia
Observing the shock imped-
ance limit
Preventing recurrence
after therapy shock
BIOTRONIK devices feature several functions to prevent conduction of atrial tachycardia to the ventricle(s):
• Set mode switching for indicated patients.
• Set the upper rate and the refractory periods to prevent abrupt ventricular rate
switching.
• Give preference to Wenckebach response and avoid 2:1 behavior.
• Set all parameters so as to prevent constant changing between atrial and ventricular-controlled modes.
The implanted device could be damaged if the shock impedance is too low.
• The shock impedance must be > 25 Ω.
After a therapy shock, pacing can be performed with a post-shock program if there
is no intrinsic rhythm.
Permanent program Post-shock program
DDD, DDI, AAI DDI
VDD, VDI VDI
VVI and OFF VVI
• The following post-shock program parameters can be adjusted: post-shock
duration, basic rate, rate hysteresis, ventricular pacing, LV-T-wave protection,
triggering, AV delay (fixed, not dynamic).
• The default settings for the post-shock program are as follows:
A and RV: 7.5 V, 1.5 ms
LV: settings from the permanent program
Phrenic nerve stimulation
that cannot be terminated
Avoiding risks in the case of
exclusive LV pacing
Recognizing lead failure Automatic impedance measurement is always switched on.
In rare cases, chronic phrenic nerve stimulation cannot be terminated by reprogramming of the available left ventricular pacing configurations or by other measures.
• As the case may be, set a right ventricular mode both in the permanent
program as well as the ATP, in the post-shock program and for
mode switching.
Lead dislodgement in the case of exclusive left ventricular pacing could pose the
following risks: loss of ventricular pacing and ATP therapy, induction of atrial
arrhythmias.
• Consider sensing and pacing parameters with reference to loss of therapy.
• Exclusive LV pacing is not recommended for patients who depend on the device.
• Take non-availability of automatic active capture control into consideration.
• In the case of follow-ups and threshold tests, take loss of synchronized ventricular pacing into consideration.
• Mode switching and post-shock do not allow for exclusive LV pacing. Also take
the effects into account when setting the mode switching and post-shock
parameters.
• Impedance values that indicate technical failure of a lead are documented in
the event list.
Page 25

23 Implantation
Considering power
consumption and service
time
RF telemetry requires somewhat more power: Consumption during implantation
corresponds to approximately 10 days of service time and consumption during a
20-minute follow-up corresponds to approximately 3 days.
• Do not establish unnecessary RF telemetry.
• After 5 minutes without input, SafeSync switches to the economy mode.
• Check the battery capacity of the device at regular intervals.
Page 26

24 Implantation
Magnet Response
Application of the program-
ming head when ICD
therapy is set
Programming head applica-
tion
Application of a permanent
magnet
If a connected programming head is applied and is communicating with the programmer and ICD therapy is permanently set, detection and therapy remain intact
except during the diagnostic tests. If ICD therapy is not set as permanent, no
therapy is delivered when the programming head is applied.
When the programming head is applied, time remains for device interrogation and
for manual activation or deactivation of the therapy before the device switches back
to the previously set permanent therapy mode. The same applies to programming
head application to establish RF telemetry contact.
Applying a permanent magnet interrupts detection and therapy of tachycardia
events. After 8 hours of this type of deactivation, the device automatically reactivates the therapy functions to prevent accidental permanent deactivation.
• If detection interruptions of longer than 8 hours are required, the magnet has
to be briefly removed from the device. The 8 hour countdown restarts when the
magnet is applied again.
• Use BIOTRONIK magnets: type M-50 permanent magnets.
Page 27

Follow-up
Follow-up intervals Follow-ups must be performed at regular, agreed intervals.
25 Implantation
• The first follow-up should be carried out by the physician using the programmer (in-office follow-up) approximately 3 months after implantation following the lead ingrowth phase.
• The next in-office follow-up should be carried out once a year and no later than
12 months after the last in-office follow-up.
Follow-up with BIOTRONIK
Home Monitoring
Follow-up with the
programmer
Monitoring using the Home Monitoring function does not serve to replace regular
®
in-office appointments with the physician required for other medical reasons.
Follow-up supported by Home Monitoring can be used to functionally replace inoffice follow-up under the following conditions:
• The patient was informed that the physician must be contacted despite use of
the Home Monitoring function if symptoms worsen or if new symptoms arise.
• Device messages are transmitted regularly.
• The physician decides whether the data transmitted via Home Monitoring with
regard to the patient's clinical condition as well as the technical state of the
device system are sufficient. If not, an in-office follow-up has to be carried out.
Possible early detection due to information gained via Home Monitoring may
necessitate an additional in-office follow-up. For example, the data may indicate at
an early stage lead problems or a foreseeable end of service time (ERI). Furthermore, the data could provide indications of previously unrecognized arrhythmias or
modification of the therapy by reprogramming the device.
Use the following procedure for in-office follow-up:
1 Record and evaluate the ECG.
2 Interrogate the device.
3 Evaluate the status and automatically measured follow-up data.
4 Check the sensing and pacing functions.
5 Possibly evaluate statistics and IEGM recording.
6 Manually perform standard tests if necessary.
7 Possibly customize program functions and parameters.
8 Transmit the program permanently to the device.
9 Print and document follow-up data (print report).
10 Finish the follow-up for this patient.
Page 28

26 Implantation
Patient Information
Patient ID card A patient ID card is included in delivery.
Prohibitory signs Places with prohibitory signs must be avoided.
• Provide the patient with the patient ID.
• Request that patients contact the physician in case of uncertainties.
• Draw the patient's attention to prohibitory signs.
Possible sources of inter-
ference
Electromagnetic interference should be avoided in daily activities. Sources of
interference should not be brought into close proximity with the device.
• Draw the patient's attention to special household appliances, security checkpoints, anti-theft alarm systems, strong electromagnetic fields, cell phones,
and transmitters among other things.
• Request patients to do the following:
— Use cell phones on the side of their body that is opposite of the device.
— Keep the cell phone at least 15 cm away from the device both during use
and when stowing.
Page 29

27 Implantation
!
!
!
!
Replacement Indications
Possible battery levels • BOS: Beginning of Service: > 70% charge
• MOS 1: Middle of Service: 70% to 40% residual charge
• MOS 2: Middle of Service: < 40% residual charge
• ERI: Elective Replacement Indication, (i.e. RRT: Recommended Replacement
Time)
• EOS: End of Service
Elective Replacement Indi-
cation (ERI)
EOS replacement indication End of Service can be detected by Home Monitoring.
Elective Replacement Indication can be detected by Home Monitoring.
CAUTION
Temporally limited therapy
If ERI occurs shortly after follow-up and is only detected during the subsequent
follow-up, then the remaining service time can be much less than 3 months.
• Replace device soon.
• The device can monitor the heart rhythm for at least 3 more months.
• At least 6 maximum energy shocks can be delivered until EOS occurs.
• The selected parameters in the device program do not change.
WARNING
Patient at risk of death
If EOS replacement indication occurs before replacement of the device, then the
patient is without therapy.
• Replace device immediately.
• Monitor patient constantly until immediate replacement of the device!
• VT and VF detection and all therapies are deactivated!
• The antibradycardia function remains active in the VVI mode:
— Ventricular pacing: RV; basic rate 50 bpm; without special pacemaker func-
tions such as hysteresis, etc.
— Pulse amplitude of 6 V; pulse width of 1.5 ms
— Time of transmission for Home Monitoring: 90 days
Page 30

28 Implantation
Explantation and Device Replacement
Explantation • Interrogate the device status.
• Deactivate VT and VF therapies prior to explantation.
• Remove the leads from the header. Do not simply cut them loose.
• Use state-of-the-art techniques to remove the device and, if necessary, the
leads.
Note: Normal oxidation processes may cause ICD housing discolorations. This is
neither a device defect nor does it influence device functionality.
• Explants are biologically contaminated and must be disposed of safely due to
risk of infection.
Device replacement If, upon replacing the device, already implanted leads are no longer used but left in
the patient, then an additional uncontrolled current path to the heart can result.
• Deactivate VT and VF therapies prior to device replacement.
• Insulate connections that are not used.
Basic principles:
• The device must not be resterilized and reused.
Cremation Devices should not be cremated.
• Explant the device before the cremation of a deceased patient.
Disposal BIOTRONIK takes back used products for the purpose of environmentally safe dis-
posal.
• Clean the explant with an at least 1% sodium hypochlorite solution.
• Rinse off with water.
Page 31

29 Parameters
4 Parameters
Parameters4GA-HW_en--mul_393468-BTechnical[nbsp ]Manual for the[nbsp ]DeviceIlesto5/7 VR-T,VR-T DX, DR-T,H F-T
Bradycardia / CRT
General ICD therapy
Parameter Range of values Standard
ICD therapy OFF; ON ON xxxx
Programs Display standard program; Display
safe program; Display first interrogated program; Individual 1,2,3
Timing: Basic rate day/
night and rate hystereses
Parameter Range of values Standard
Basic rate 30 ... (5) ... 100 ... (10) ... 160 bpm 40 bpm x x
Night rate OFF; 30 ... (5) ... 100bpm OFF xxxx
Begin of night 00:00 ... (00:01) ...23:59hh:mm 06:00 hh:mmxxxx
End of night 22:00 hh:mm
Rate hysteresis OFF
-5 ... (-5) ... -25 ... (-20) ... -65 bpm
Scan/repetitive OFF; ON ON xxxx
– xxxx
60 bpm x x
OFF xxxx
VR
VR
DX
DX
DR
DR
HF
HF
Page 32

30 Parameters
Timing: AV delay
Parameter Range of values Standard
AV dynamics Low; Medium; High; Fixed; (Individual) Low x x x
AV delay (1 or 2) after:
– Pacing 15; 40 ... (5) ... 350 ms – x x x
– Sensing Either automatic: AV delay after pacing
+ sense compensation
Or: 40 ... (5) ... 350 ms
– At rate 1 50 ... (10) ... 130 bpm 60 bpm
– At rate 2 60 ... (10) ... 140 bpm 130 bpm
Sense compensation OFF
-5 ... (-5) ... -120 ms
AV hysteresis mode OFF
Positive; Negative; IRSplus
OFF; Positive; Negative OFF x
AV hysteresis (positive) 70; 110; 150; 200 ms 70 ms x x x
AV hysteresis (negative) 10 ... (10) ... 150 ms 50 ms x x x
AV scan and repetitive (positive) OFF; ON ON x x x
Timing: Post-shock pacing
Parameter Range of values Standard
Post shock duration OFF
10s; 30s; 1min; 2min; 5min; 10min
Post-shock basic rate 30 ... (5) ... 100 ... (10) ...160bpm 60bpm xxxx
AV delay post-shock 50 ... (10) ... 350 ms 140 ms x x
Ventricular post-shock pacing RV; BiV RV x
–
-40 ms x x
OFF x x
10s xxxx
VR
VR
DX
DX
DR
DR
HF
HF
Timing: Upper rate
Parameter Range of values Standard
Upper rate 90 ... (10) ... 160 bpm 130 bpm x x x
Atrial upper rate OFF
175; 200; 240 bpm
200 bpm x x
VR
DX
DR
HF
Page 33

31 Parameters
Timing: Mode switching
Parameter Range of values Standard
Intervention rate OFF; 120 ... (10) ... 200 bpm 160 bpm x x x
Onset criterion 3 ... (1) ... 8 (out of 8) 5 x x x
Resolution criterion
Modification of basic rate OFF; 5 ... (5) ... 30 bpm 10 bpm x x x
Mode VDI(R); VDD(R) VDI x x x
DDI(R); DDD(R) DDI x x
After mode switching:
– Rate OFF; 5 ... (5) ... 50 bpm 10 bpm x x x
– Duration 1 ... (1) ... 30 min 1 min
Timing: Ventricular pacing
Parameter Range of values Standard
Permanent RV; BiV; LV BiV x
Triggering OFF; RVs; RVs+PVC RVs x
LV T-wave protection OFF; ON ON x
Maximum trigger rate:
– DDD(R) and VDD(R) UTR + 20;
90 ... (10) ... 160 bpm
– DDI(R), VDI(R) and VVI(R) 90 ... (10) ... 160 bpm 130 bpm
Initially paced chamber RV; LV LV x
VV delay after Vp 0 ... (5) ... 100 ms 5 ms x
UTR + 20 x
VR
VR
DX
DX
DR
DR
HF
HF
Timing: Refractory periods
and blanking periods
Parameter Range of values Standard
PVARP AUTO; 175 ... (20) ... 600 ms 225 ms x x x
Blanking after atrial pacing 50 ... (10) ... 100 ms 50 ms x x
LV blanking after RV pacing 80 ms x
RV blanking after LV pacing
Far-field protection after Vs OFF; 25 ... (25) ... 225 ms 75 ms x x x
Far-field protection after Vp 50 ... (25) ... 225 ms 75 ms x x x
Timing: PMT protection
Parameter Range of values Standard
PMT detection/termination OFF; ON ON x x x
VA criterion 250 ... (10) ... 500 ms 350 ms x x x
VR
VR
DX
DX
DR
DR
HF
HF
Page 34

32 Parameters
Timing: Rate adaptation via
accelerometer
Parameter Range of values Standard
Maximum sensor rate 80 ... (10) ... 160bpm 160bpm xxxx
Sensor gain AUTO
Very low; Low; Medium; High;
Very high
Sensor threshold Very low; Low; Medium; High;
Very high
Rate increase 1; 2; 4; 8bpm/cycle 2bpm xxxx
Rate decrease 0.1; 0.2; 0.5; 1.0bpm/cycle 0.5bpm xxxx
Pacing: Pulse amplitude
and pulse width
Parameter Range of values Standard
Pulse amplitude A 0.5 ... (0.25) ... 4.0 ... (0.5) ... 6.0; 7.5 V 2.5 V x x
Pulse amplitude V/RV xxxx
Pulse amplitude LV x
Pulse width A 0.4; 0.5 ... (0.25) ... 1.5 ms 0.4 ms x x
Pulse width V/RV xxxx
Pulse width LV 0.5 ms x
Medium xxxx
Medium xxxx
VR
VR
DX
DX
DR
DR
HF
HF
Pacing: Ventricular capture
control
Parameter Range of values Standard
Capture control OFF; ATM; ON ATM xxxx
Threshold test start 2.5 ... (0.5) ... 5.0 V ATM: 2.5 V
Minimum amplitude 1.0 ... (0.25) ... 4.0 V 1.0 V xxxx
Safety margin 1.0; 1.2 V 1.0 V xxxx
Pacing: atrial capture
control
Parameter Range of values Standard
Capture control OFF; ATM ATM x x
LV lead configuration
Parameter Range of values Standard
LV pacing polarity LV tip -> LV ring;
LV sensing polarity UNIP; BIPL UNIP x
VR
DX
DR
xxxx
ON: 3.5 V
VR
DX
DR
VR
DX
DR
LV tip -> RV ring;
LV ring -> LV tip;
LV ring -> RV ring;
UNIP
LV tip -> RV
ring
x
HF
HF
HF
Page 35

Tachycardia
33 Parameters
Detection
Parameter Range of values Standard
Interval AT/AF 240 ... 600 ms 300 ms x x x
Interval VT1 OFF; 270 ... (10) ... 600ms OFF xxxx
Interval VT2 OFF; 270 ... (10) ... 500 ms
Interval VF OFF; 240 ... (10) ... 400 ms 300 ms
Detection counter VT1 10 ... (2) ... 60 26 xxxx
Detection counter VT2 10 ... (2) ... 40 16
Detection counter VF 6 out of 8; 8 out of 12; 10 out of 12;
12 out of 16; 14 out of 16; 16 out of 20;
18 out of 24; 20 out of 24; 20 out of 24;
22 out of 24; 24 out of 30; 28 out of 30
Redetection counter VT1 10 ... (2) ... 30 20 xxxx
Redetection counter VT2 14
SMART detection VT1/VT2 OFF; ON ON x x x
SMART detection ON:
– Onset VT1/VT2 4 ... (4) ... 32% 20% x x x
– Stability VT1/VT2 8 ... (4) ... 48% 12%
SMART detection OFF:
– Onset VT1/VT2 OFF; 4 ... (4) ... 32% 20% xxxx
– Stability VT1/VT2 OFF; 8 ... (4) ... 48 ms 24 ms
SustainedVT OFF; 1; 2; 3; 5; 10; 20; 30min OFF xxxx
Forced termination OFF; 1 ... (1) ... 10 min 1 min x x x
8 out of 12
VR
DX
DR
HF
Therapy: ATP
Parameter Range of values Standard
ATP type for VT1/VT2 Burst; Ramp OFF xxxx
ATP type for VF OFF; Burst; Ramp Burst xxxx
ATP optimization OFF; ON OFF xxxx
Attempts OFF; 1 ... (1) ... 10 OFF xxxx
Number S1 for VT1/VT2 1 ... (1) ... 10 5 xxxx
Number S1 for VF 8
S1 decrement forVT1/VT2 and forVF 5 ... (5) ... 40ms 10ms xxxx
Scan decrement OFF; 5 ... (5) ... 40ms OFF xxxx
Additional S1 forVT1/VT2 OFF; ON ON xxxx
Ventricular pacing for VT1/VT2 RV; LV; BiV RV x
Ventricular pacing for VF RV
R-S1 intervalfor VT1/VT2 70 ... (5) ... 95% 80% xxxx
R-S1 interval for VF 85%
VR
DX
DR
HF
Page 36

34 Parameters
Therapy: Shock
Parameter Range of values Standard
Number of shocks VT1/VT2 0; 1; 2; 6; 8 8 xxxx
Number of shocks VF 6; 8 8 xxxx
1. Shock for VT1/VT2 OFF
2 ... (2) ... 20 ... (5) ... 40 J
2. Shock for VT1/VT2 OFF
4 ... (2) ... 20 ... (5) ... 40 J
3rd-nth shock forVT1/VT2 4*40J; 6*40J 6*40J xxxx
1. Shock for VF OFF
2 ... (2) ... 20 ... (5) ... 40 J
2. Shock for VF OFF
4 ... (2) ... 20 ... (5) ... 40 J
3rd-nth Shock forVF 4*40J; 6*40J 6*40J xxxx
For shock in VT1/VT2 and VF:
Confirmation OFF; ON ON xxxx
Polarity Normal; Reverse; Alternating Normal
Waveform Biphasic; Biphasic 2 Biphasig
Shock path RV -> ICD+SVC
RV -> ICD
RV -> SVC
40J xxxx
40J xxxx
40J xxxx
40J xxxx
RV->
ICD+SVC
RV -> ICD x
VR
DX
DR
HF
xxx
Page 37

35 Parameters
Sensing
Sensitivity and thresholds
Parameter Range of values Standard
Sensing A STD; OFF; IND STD x x x
Sensing RV STD; TWS; VFS; IND STD xxxx
Sensing LV STD; OFF; IND STD x
Upper thresholdRV 50; 75% 50% xxxx
Upper threshold LV 50; 75 % 50% x
Upper threshold duration
after detection
Upper threshold duration after pacing 400 ms
Lower threshold RV 25; 50% 25% xxxx
T-wave suppression afterpacing OFF; ON OFF xxxx
Minimum threshold A 0.2 ... (0.1) ... 2.0 mv 0.4 mv x x x
Minimum threshold RV 0.5 ... (0.1) ... 2.5mv 0.8mv xxxx
Minimum threshold LV 0.5 ... (0.1) ... 2.5 ... (0.5) ... 5.0 mv 1.6 mv x
110; 150 ... (50) ... 500 ms
VFS: 110 ms
350ms xxxx
VR
DX
DR
HF
Page 38

Diagnostics
36 Parameters
The following can be set:
Parameter Range of values
For AT/AF OFF; ON
For Ilesto 7: Extended ON
For SVT OFF; ON xxxON
Periodic recording When Home Monitoring OFF:
OFF; 30 ... (30) ... 180 days
IEGM configuration RA, RV, LV
RA, RV, FF
FF; RV; LV
The following can additionally be set for Ilesto 7:
Parameter Range of values
Start resting period 0:00 ... (1:00) ... 23:00hh:mm xxxx2:00 hh:mm
Duration of resting period 0.5 ... (0.5) ... 12h xxxx4h
AV delay modification in sensing test OFF; 300 ms xxx300ms
Thoracic impedance (TI) OFF, ON xxxxOFF
VR
DX
xxxON
xxxx90days
VR
DX
Standard
DR
HF
xRA, RV, LV
Standard
DR
HF
Page 39

Home Monitoring
37 Parameters
Parameter Range of values Standard
HomeMonitoring OFF; ON OFF xxxx
Time of transmission STD; 00:00 ... (01:00) ... 23:00hh:mm STD xxxx
IEGM for:
– Therapy episodes OFF; ON ON xxxx
– Monitoring episodes
Ongoing atrial episode OFF; 6, 12, 18 h 12 h x x x
VR
DX
DR
HF
Page 40

38 Parameters
Page 41

39 Technical Data
5 Technical Data
Technical Data5GA-HW_en--mul_393468-BTechnical[nbsp ]Manual for the[nbsp ]DeviceIlesto5/7 VR-T, VR-T DX, DR-T, HF-T
Mechanical Characteristics
Housing Devices with a DF-1/IS-1 header:
Materials in contact with
body tissue
X-ray identification NT
Type Connection W x H x D in mm Volume in
ccm
VR, DX, DR DF-1 65 x 55 x 11 31 80
HF DF-1 65 x 58.5 x 11 33 80
Devices with a DF4/IS-1 header:
Type Connection W x H x D in mm Volume in
ccm
VR DF4 65 x 52 x 11 29.9 80
DR DF4 65 x 56 x 11 31.5 80
HF DF4 65 x 56 x 11 32.5 80
• Housing: Titanium
• Header: Epoxy resin
• Blind plug and silicone plug: Silopren or Silastik; DF4 seal: Silastik
Mass in g
Mass g
Page 42

40 Technical Data
Electrical Characteristics
Standards The specifications are made according to EN 45502-2-2:2008.
Measuring conditions If not indicated otherwise, all specifications refer to the following conditions:
• Ambient temperature: 37 ºC ± 2 °C
• Pacing/sensing: 500 Ω ± 1%
• Shock: 50 Ω ±1%
Factory settings • Arrhythmia zones VT1, VT2, VF: OFF
• Antibradycardia pacing: OFF
• Home Monitoring: OFF
Telemetry data • Nominal carrier frequency: 403.6 MHz
• Maximum power of transmission: < 25 µW (-16 dBm)
International radio certifi-
cation
Pulse form The pacing pulse has the following form:
Devices with BIOTRONIK Home Monitoring® are equipped with an antenna for
wireless communication.
Telemetry data for Canada and the USA:
This device must neither interfere with meteorological and earth resources technology satellites nor with meteorological stations working in the 400,150 to 406,000
MHZ band, and it must accept any interference received, including interference
that may cause undesired operation.
• This device will be registered with Industry Canada under the following
number:
IC: 4708A-TACHNXT
The code IC in front of the certification/ registration number only indicates that
the technical requirements for Industry Canada are met.
• This device will be registered with Federal Communications Commission under
the following number:
FCC ID: QRITACHNXT
Telemetry data for Japan:
In accordance with Japanese law, this device has been assigned an identification
number under the "Ordinance concerning certification of conformity with technical
regulations etc. of specified radio equipment", Article 2-1-8.
• R: 202-SMA026
The pulse amplitude reaches its maximum value at
the beginning of the pulse (Ua). With increasing
pacing duration (tb), the pulse amplitude is
reduced dependent on the pacing impedance.
Page 43

41 Technical Data
Resistance to interference • Note on device type DX (only devices with a DF-1/IS-1 connection): The EMC
requirements are met as long as atrial sensitivity is set to 1.0 mV (factory settings) or values ≥ 1.0 mV. Measures must be taken to assure interference-free
therapy if more sensitive values are set.
• Note on device type HF: In the case of unipolar sensing, the requirement for
interference voltages of ≤ 0.3 mV (tip to tip) is met.
Common mode rejection
Rate Common mode rejection ratio
Atrium: DX* Atrium: DR, HFV right: VR, DR, HFV left: HF
16.6 Hz 58 dB 53 dB 64 dB 66 dB
50 Hz 55 dB 55 dB 64 dB 66 dB
60 Hz 56 dB 56 dB 64 dB 68 dB
* only devices with a DF-1/IS-1 connection.
ATP amplitude A burst was measured at 500 Ω, an amplitude of 7.5 V (tolerance ±1.5 V), pulse
width of 1.5 ms, R-S1 interval of 300 ms and an S1 count of 5:
ATP amplitude Measured
RV 7.67 V 7.67 V 5.00 V
LV 7.67 V 7.67 V 4.99 V
Automatic sensitivity
setting
Shock energy / peak voltage With shock path: RV to housing + SVC
Measurement of actual values and test signal wave shape: standard triangle. For
the device type DX, the programmed atrial sensitivity is intensified by a factor of 4.
Sensitivity Value Tolerance Measured value
A: positive 0.2 mV 0.2 ... 0.5 0.24 mV
A: negative 0.24 mV
DX: A: positive 0.2 mV 0.2 ... 0.52
DX:A:negative 0.05mV
RV: positive 0.5 mV 0.3 ... 0.7 0.48 mV
RV: negative 0.40 mV
LV: positive 0.5 mV 0.3 ... 0.7 0.48 mV
LV: negative 0.56 mV
Shock energy (Tolerance)
1 J (0.7 ... 1.18) 90 ... 120 V 0.84 J 100 V
20 J (16.9 ... 20.9) 440 ... 480 V 18.1 J 469 V
40 J (33.8 ... 41.4) 620 ... 690 V 36.9 J 667 V
minimum
Tolerance peak
voltage
Measured
maximum
(0.05 to 0.13)
Measured
value
Shock energy
Mean value
0.05 mV
Measured
value
Peak voltage
Page 44

42 Technical Data
Battery Data
Battery characteristics The following data is provided by the manufacturers:
Manufacturer GREATBATCH, INC.
Battery type GB 2992 LiS 3410 RA
Battery ID number shown on
the programmer
Device type VR, (DX), DR, HF
Battery voltage at ERI 2.5 V 2.85 V
Charge time at BOS 8 s 8 s
Charge time at ERI 10 s 10 s
Usable capacity until ERI Ilesto 5: 1390 mAh
Usable capacity until EOS 1730 mAh 1520 mAh
Storage period The storage period affects the battery service time.
• Devices should be implanted within 19 months between the date of manufacture and the use by date (indicated on the package).
• If the ICD is implanted shortly before the use by date, the expected service time
may be reduced by up to 16 months.
Calculation of service times • The services times have been calculated as follows – in all chambers
depending on the device type:
— Pulse amplitude: 2.5 V
— Pulse width: 0.4 ms
— Pacing impedance: 500 Ω
—Basic rate: 60bpm
— Home Monitoring: ON, 1 device message each day and 12 transmissions of
an IEGM online HD per year
— Diagnostic functions and recordings: permanently set
• Capacitor reforming is performed 4 times per year and therefore at least
4 maximum charges for shocks have to be assumed per year even if less than 4
are delivered.
Clarence, NY 14031
34
Ilesto 7: 1600 mAh
LITRONIK GmbH & Co
01796 Pirna,
Germany
1390 mAh
Calculation of the number
of shocks
Ilesto5VR-T Service times with GB 2992 or LiS 3410 RA battery:
Calculation of the number of shocks: Longevity [in years] x number of shocks per
year
Stimulation
0% 10.42 8.39 7.01 6.03 5.28
15% 10.14 8.20 6.89 5.93 5.21
50% 9.55 7.81 6.60 5.72 5.05
100% 8.81 7.31 6.24 5.45 4.83
Longevity [in years] at number of shocks per year
4 8 12 16 20
Page 45

43 Technical Data
Ilesto 5 VR-T DX Service times with GB 2992 or LiS 3410 RA battery:
Stimulation
0% 9.48 7.76 6.57 5.70 5.03
15% 9.24 7.61 6.46 5.61 4.96
50% 8.75 7.26 6.21 5.42 4.81
100% 8.12 6.83 5.89 5.17 4.62
Ilesto 5 DR-T Service times with GB 2992 or LiS 3410 RA battery:
Stimulation
0% 9.48 7.76 6.57 5.70 5.03
15% 9.02 7.45 6.35 5.53 4.89
50% 8.10 6.81 5.88 5.17 4.61
100% 7.08 6.07 5.32 4.73 4.26
Ilesto5HF-T Service times with GB 2992 or LiS 3410 RA battery:
Stimulation
0% 8.78 7.29 6.23 5.44 4.82
15% 8.21 6.89 5.94 5.21 4.65
50% 7.14 6.12 5.35 4.76 4.28
100% 6.01 5.27 4.69 4.23 3.85
Longevity [in years] at number of shocks per year
4 8 12 16 20
Longevity [in years] at number of shocks per year
4 8 12 16 20
Longevity [in years] at number of shocks per year
4 8 12 16 20
Ilesto7VR-T Service times with GB 2992 battery:
Stimulation
0% 11.78 9.52 7.98 6.87 6.03
15% 11.48 9.32 7.84 6.76 5.95
50% 10.81 8.87 7.52 6.53 5.76
100% 9.99 8.31 7.11 6.21 5.52
Ilesto 7 VR-T DX Service times with GB 2992 battery:
Stimulation
0% 10.73 8.82 7.48 6.50 5.74
15% 10.48 8.65 7.36 6.40 5.66
50% 9.92 8.26 7.08 6.19 5.50
100% 9.22 7.77 6.71 5.91 5.27
Longevity [in years] at number of shocks per year
4 8 12 16 20
Longevity [in years] at number of shocks per year
4 8 12 16 20
Page 46

44 Technical Data
Ilesto 7 DR-T Service times with GB 2992 battery:
Stimulation
0% 10.73 8.82 7.48 6.50 5.74
15% 10.22 8.47 7.23 6.31 5.59
50% 9.20 7.76 6.70 5.90 5.27
100% 8.05 6.92 6.07 5.40 4.87
Ilesto7HF-T Service times with GB 2992 battery:
Stimulation
0% 9.96 8.29 7.10 6.20 5.51
15% 9.33 7.85 6.77 5.95 5.31
50% 8.12 6.97 6.11 5.43 4.89
100% 6.85 6.01 5.36 4.83 4.40
Longevity [in years] at number of shocks per year
4 8 12 16 20
Longevity [in years] at number of shocks per year
4 8 12 16 20
Page 47

45 Technical Data
STERILIZE
2
NON
STERILE
Legend for the Label
Label on the package The label icons symbolize the following:
Manufacturing date Use by
Temperature limit Order number
Serial number Product identification
Dangerous voltages! CE mark
Contents Follow the instructions
number
for use
Sterilized with ethylene oxide
Do not resterilize Do not reuse
Do not use if packaging is
Non-sterile
damaged
Transmitter with non-ionizing radiation at designated frequency
Device: NBG code and compatible
leads
Example
Factory settings for therapy: OFF
Example
Screwdriver
Example of DF-1/IS-1 header
Examples of DF-1/IS-1 or DF4/IS-1 header
Page 48

46 Technical Data
Bipolar IS-1 connector
Unipolar IS-1 connector
Unipolar DF-1 connector
DF4 connector
 Loading...
Loading...