BIOTRONIK SE and KG TACH70 Users Manual

Inventra 7
Iperia 5/7
Itrevia 5/7
VR-T, VR-T DX
DR-T
HF-T, HF-T QP
ICD-Families
Tachyarrhythmia Therapy
Cardiac Resynchronization Therapy
Technical Manual Draft
Subject to completion and modification
1 Product Description
Intended Medical Use
Intended use
Inventra/Iperia/Itrevia are parts of a familiy of implantable cardioverter-defibrillators
(ICDs). Primary objective of the therapy is to prevent sudden cardiac death. Furthermore, the device is capable of treating bradycardia arrhythmias and cardiac resynchronization therapy with multisite ventricular pacing.
The implantation of an ICD is a symptomatic therapy with the following objectives:
•
Termination of spontaneous ventricular fibrillation (VF) through shock delivery
•
Termination of spontaneous ventricular tachycardia (VT) through antitachycardia
pacing (ATP); in case of ineffective ATP or hemodynamically not tolerated VT, with
shock delivery
•
Cardiac resynchronization through multisite ventricular pacing (triple-chamber
devices)
•
Compensation of bradycardia through ventricular (single-chamber devices) or
AV sequential pacing (DX, dual and triple-chamber devices)
Diagnosis and therapy forms
The device monitors the heart rhythm and automatically detects and terminates
cardiac arrest resulting from ventricular tachyarrhythmia. All major therapeutic
approaches from the field of cardiology and electrophysiology are included. BIOTRONIK
Home Monitoring® enables physicians to perform therapy management at any time.
Required expertise
In addition to having basic medical knowledge, the user must be thoroughly familiar
with the operation and the operation conditions of a device system.
•
Only qualified medical specialists having this special knowledge required are
permitted to use implantable devices.
•
If users do not possess this knowledge, they must be trained accordingly.
1

Indications
DF-1
RV
DF-1
SVC
IS-1
RV
DF-1
RV
DF-1
SVC
IS-1
RA
IS-1
RV
DF-1
RV
DF-1
SVC
IS-1
RA
IS-1
RV
DF-1
RV
DF-1
SVC
IS-1
RA
IS-1
RV
IS-1
LV
Inventra/Iperia/Itrevia can treat life-threatening ventricular arrhythmias with antitachycardia pacing and defibrillation.
Generally approved differential diagnostics methods, indications, and recommendations for ICD therapy apply to BIOTRONIK devices. See the guidelines of cardiology
associations for guidance.
We recommend observing the indications published by the German Cardiac Society
(Deutsche Gesellschaft für Kardiologie, Herz- und Kreislaufforschung) and the ESC
(European Society of Cardiology). This also applies to the guidelines published by the
Heart Rhythm Society (HRS), the American College of Cardiology (ACC), the American
Heart Association (AHA), and other national cardiology associations.
Single-chamber and dual-chamber
Single-chamber and dual-chamber ICDs are indicated for patients with the following
risk:
•
Sudden cardiac death caused by ventricular arrhythmias
Triple-chamber
Triple-chamber ICDs are indicated for patients with the following risks:
•
Sudden cardiac death caused by ventricular arrhythmias
•
Congestive heart failure with ventricular asynchrony
Contraindications
Known contraindications:
•
Tachyarrhythmia caused by temporary or reversible irritation, e.g. poisoning, electrolyte imbalance, hypoxia, sepsis or acute myocardial infarction
•
Such frequent VT or VF that the therapies would cause an unacceptably rapid
depletion of the device batteries
•
VT with few or without clinically relevant symptoms
•
VT or VF treatable by surgery
•
Concomitant diseases that would substantially limit a positive prognosis
•
Accelerated idioventricular rhythm
System Overview
Device family
This device families consist of several device types with different lead connections:
DF-1/IS-1, DF4/IS-1 or DF4/IS4/IS-1:
single-chamber: VR-T and VR-T DX (only devices with a DF-1/IS-1 connection)
dual-chamber: DR-T
triple-chamber: HF-T and HF-T QP (only devices with a DF4 connection)
All devices include BIOTRONIK Home Monitoring. Not all device types are available in
every country.
Device
The device's housing is made of biocompatible titanium, welded from outside and thus
hermetically sealed. The ellipsoid shape facilitates implantation in the pectoral muscle
area. The connections for bipolar pacing and sensing (and unipolar connections for the
triple-chamber device) as well as for shock delivery ar e found in the device header. The
housing serves as a potential antipole during shock delivery or in the case of unipolar
lead configuration.
DF-1/IS-1 or DF4/IS-1 or DF4/IS4
BIOTRONIK provides ICDs with headers for different standardized lead connections:
DF-1/IS-1, DF4/IS-1 and DF4/IS4.
Note:
The device type DX can only be connected using a DF-1/IS-1 connector.
The device type HF QP can only be connected using a DF4/IS-1 or DF4/IS4 connector.
DF-1/IS-1 lead connection
The device labeling provides information pertaining to possible lead connections
depending on the device type and pertaining to connection assignment:
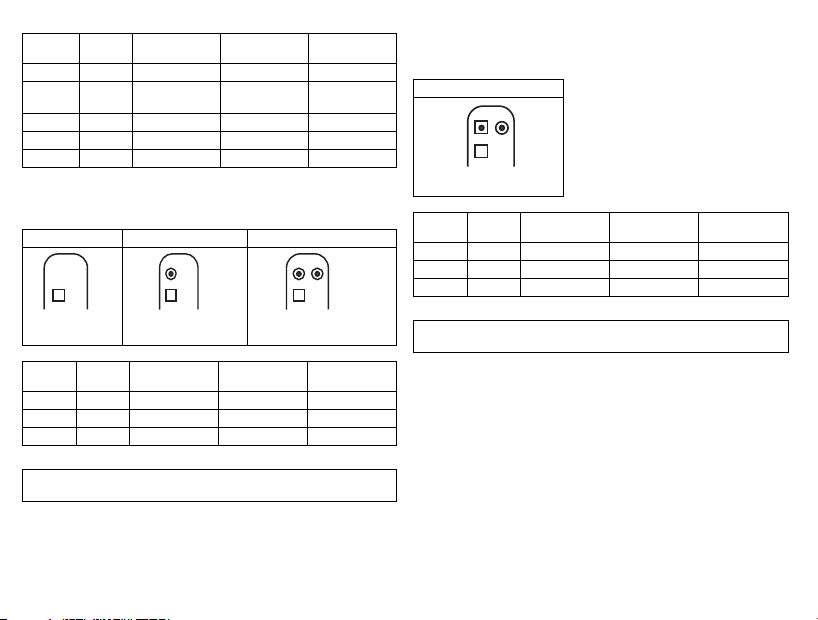
VR DX DR HF
2
Connector
DF4-LLHH
RV
DF4-LLHH
RV
IS-1
RA
DF4-LLHH
RV
IS-1
RA
IS-1
LV
DF4-LLHH
RV
IS4-LLLL
RA
IS-1
LV
Lead
port
Configuration Implantation site Device type
connector
RV DF-1 Shock coil Right ventricle VR, DX, DR, HF
SVC DF-1 Shock coil Superior vena
cava
VR, DX, DR, HF
RA IS-1 Bipolar Atrium DX, DR, HF
(R)V IS-1 Bipolar (Right) ventricle VR, DX, DR, HF
LV IS-1 Unipolar, bipolar Left ventricle HF
DF4/IS-1 lead connection
The device labeling provides information pertaining to possible lead connections
depending on the device type and pertaining to connection assignment:
VR DR HF
Connector
Lead
port
Configuration Implantation site Device type
connector
RA IS-1 Bipolar Atrium DR, HF
LV IS-1 Unipolar, bipolar Left ventricle HF
RV, SVC DF4 Bipolar and shock Right ventricle VR, DR, HF
Note:
The device's DF4 connector port may only be used for connecting leads with a
DF4 connector that conform to ISO 27186.
DF4/IS4/IS-1 lead connection
The device labeling provides information pertaining to possible lead connections
depending on the device type and pertaining to connection assignment:
HF QP
Connector
Lead
port
Configuration Implantation site Device type
connector
RA IS-1 Bipolar Atrium HF QP
LV IS4 Unipolar, bipolar Left ventricle HF QP
RV, SVC DF4 Bipolar and shock Right ventricle HF QP
Note:
The device's DF4/IS4 connector port may only be used for connecting leads w ith
a DF4/IS4 connector that conform to ISO 27186.
Leads
BIOTRONIK leads are sheathed with biocompatible silicone. They can be flexibly
maneuvered, are stable long-term, and are equipped for active or passive fixation. They
are implanted using a lead introducer set. Some leads are coated with polyurethane
which is known to increase the sliding properties for the lead. Leads with steroids
reduce inflammatory processes. The fractal design of the electrodes provides for low
pacing thresholds. BIOTRONIK provides adapters to connect already implanted leads to
new devices.
Telemetry
Telemetric communication between the device and the programmer can be carried out
following initialization either by applying the programming head (PGH) to the device or
by using radio frequency (RF) telemetry in the programmer. BIOTRONIK calls this function SafeSync®.
3

Programmer
Implantation and follow-up are performed with BIOTRONIK's portable programmer:
There is one with integrated RF telemetry and one with a separate SafeSync Module.
The programmer is used during implantation to transfer the current device program to
the device. The pacing thresholds can be determined and all tests can be performed
during in-office follow-up. In addition to this, the programmer is used to set mode and
parameter combinations, as well as for interrogation and saving of data from the
device. Leadless ECG, IEGM, markers and functions are displayed simultaneously on
the color display.
Modes
The mode setting depends on the individual diagnosis:
Device type Modes
VR VVI; VVIR; V00; OFF
DX VDD; VDDR; VDI; VDIR; VVI; VVIR; V00; OFF
DR, HF, QP DDD; DDDR; DDD-ADI; DDDR-ADIR; DDI; DDIR;
NBD and NBG codes
VVE is the NBD code for the antitachycardia mode of the single-chamber, dualchamber, and triple-chamber devices:
V Shock in the ventricle
V Antitachycardia pacing (ATP) in the ventricle
E Detection via IEGM analysis
VDE is the NBD code for the antitachycardia mode of the dual-chamber, and triplechamber devices:
V Shock in the ventricle
D Antitachycardia pacing (ATP) in the atrium and ventricle
E Detection via IEGM analysis
Series 7: VVI-CLS
Series 7: VVI-CLS
VDD; VDDR; VDI; VDIR
VVI; VVIR; AAI; AAIR; V00; D00; OFF
Series 7: VVI-CLS; DDD-CLS
DDDR is the NBG code for the antibradycardia mode of the dual-chamber device:
D Pacing in the atrium and ventricle
D Sensing in the atrium and ventricle
D Pulse inhibition and pulse triggering
R Rate adaptation
DDDRV is the NBG code for the antibradycardia mode of the triple-chamber device:
D Pacing in the atrium and ventricle
D Sensing in the atrium and ventricle
D Pulse inhibition and pulse triggering
R Rate adaptation
V Multisite pacing in both ventricles
VDDR is the NBG code for the antibradycardia mode of the single-chamber DX device:
VVentricular pacing
D Sensing in the atrium and ventricle
D Pulse inhibition and pulse triggering
R Rate adaptation
VVIR is the NBG code for the antibradycardia pacing modes of the single-chamber
device:
VVentricular pacing
V Sensing in the ventricle
I Pulse inhibition in the ventricle
R Rate adaptation
4

BIOTRONIK Home Monitoring
In addition to effective pacing therapy, BIOTRONIK provides a complete therapy
management system:
•
With Home Monitoring, diagnostic and therapeutic information as well as technical
data are automatically sent to a stationary or mobile transmitter via an antenna in
the device header. The data are encrypted and sent from the transmitter to the
BIOTRONIK Service Center via the cellular phone network.
•
The received data are deciphered and evaluated. Each physician can set the criteria
for evaluation to be used for each patient and can configure the time of notification
via E-mail, SMS or fax.
•
A clear overview of the results of this analysis is displayed for the attending physicians on the protected Internet platform Home Monitoring Service Center (HMSC).
•
Data transmission from the device is performed with a daily device message.
•
Device messages which indicate special events in the heart or in the device are
forwarded immediately.
•
A test message can be initiated at any time using the programmer to immediately
check the Home Monitoring function.
®
Order numbers for Iperia with DF-1/IS-1, DF4/IS-1 or DF4/IS4/IS-1 connection
Order numbers for Inventra with DF-1/IS-1, DF4/IS-1 or DF4/IS4/IS-1 connection
Not all device types are available in every country:
Iperia 5 Iperia 7
DF-1/IS-1 DF4/IS-1 DF4/IS4 DF-1/IS-1 DF4/IS-1 DF4/IS4
VR-T 393052 393053 — 393035 3393031 —
VR-T DX 393049 — — 393033 — —
DR-T 392415 392420 — 392410 392424 —
HF-T 393028 393026 — 393008 393010 —
HF-T QP — — 402658 — — 401658
Order numbers for Iperia ProMRI with DF-1/IS-1, DF4/IS-1
or DF4/IS4/IS-1 connection
Not all device types are available in every country:
Iperia 5 ProMRI Iperia 7 ProMRI
DF-1/IS-1 DF4/IS-1 DF4/IS4 DF-1/IS-1 DF4/IS-1 DF4/IS4
VR-T 393034 3393051 — 393050 3393030 —
VR-T DX 393048 — — 393032 — —
DR-T 392418 392419 — 392409 392423 —
HF-T 393027 393025 — 393007 393009 —
HF-T QP — — 402656 — — 401657
5
Order numbers for Itrevia with DF-1/IS-1, DF4/IS-1 or DF4/IS4/IS-1 connection
Not all device types are available in every country:
Itrevia 5 Itrevia 7
DF-1/IS-1 DF4/IS-1 DF4/IS4 DF-1/IS-1 DF4/IS-1 DF4/IS4
VR-T 393058 393059 — 393040 393041 —
VR-T DX 393055 — — 393037 — —
DR-T 392417 392422 — 392412 392426 —
HF-T 393066 393064 — 393014 393016 —
HF-T QP — — 402659 — — 401662
Order numbers for Inventra with DF-1/IS-1, DF4/IS-1 or DF4/IS4/IS-1 connection
Not all device types are available in every country:
Inventra 7
DF-1/IS-1 DF4/IS-1 DF4/IS4
VR-T 399443 399441 —
VR-T DX 399437 — —
DR-T 399431 399429 —
HF-T 399423 399422 —
HF-T QP — — 393012
Order numbers for Itrevia ProMRI with DF-1/IS-1, DF4/IS-1
or DF4/IS4/IS-1 connection
Not all device types are available in every country:
Itrevia 5 ProMRI Itrevia 7 ProMRI
DF-1/IS-1 DF4/IS-1 DF4/IS4 DF-1/IS-1 DF4/IS-1 DF4/IS4
VR-T 393056 393057 — 393038 393039 —
VR-T DX 393054 — — 393036 — —
DR-T 392416 392421 — 392411 392425 —
HF-T 393065 393063 — 393013 393015 —
HF-T QP — — 402657 — — 401661
Order numbers for Inventra ProMRI with DF-1/IS-1, DF4/IS-1
or DF4/IS4/IS-1 connection
Not all device types are available in every country:
Inventra 7 ProMRI
DF-1/IS-1 DF4/IS-1 DF4/IS4
VR-T 399442 399440 —
VR-T DX 399436 — —
DR-T 399430 399428 —
HF-T 399419 393020 —
HF-T QP — — 393011
6

Package contents
The storage package includes the following:
•
Sterile packaging with device
•
Serial number label
•
Patient ID card
•
Warranty booklet
Note:
Technical manuals are available either printed in the storage package or digitally
in the internet.
The sterile container includes the following:
•
Device, blind plug DF-1 (if applicable) and blind plug IS-1 for device type HF
•
Screwdriver
Therapeutic and Diagnostic Functions
Diagnostic functions
•
Data from implantation and the most recent interrogations and follow-ups are
recorded as well as arrhythmia episodes; they are stored together with other data
to assess patients and the state of the device at any time.
•
To check the lead for proper functioning, an automatic impedance measurement
using subthreshold pacing pulses is performed in the device.
•
Leadless ECG function: For all device types, far-field derivation can be measured
without external leads between the right ventricular shock coil and housing, which,
depending on the implantation site, corresponds to ECG derivation II or III
(Einthoven).
•
Once a telemetry connection has been established during a test procedure in an inoffice follow-up, the leadless ECG and the IEGM are displayed with markers.
Antitachycardia pacing
•
The ICD can treat ventricular tachycardia with antitachycardia pacing (ATP); ATP can
also be delivered in the VF zone (ATP One Shot) when the stability criterion indicating
that this will be effective before shock delivery (monomorphic rapid VTs) is met.
•
Arial tachycardia can be treated with antitachycardia pacing (atrial ATP) at stable
heart rhythms and with high frequency bursts (HF burst) at instabil heart rhythms.
•
Depending on the device type, the device program contains not only the ICD functions but also all pacemaker functions for 1, 2, or 3 chambers. The heart rhythm is
continuously monitored; each arrhythmia is classified according to the heart rate
and the adjustable detection criteria. Depending on the preset values, antibradycardia as well as antitachycardia therapy is inhibited or delivered.
Cardioversion, defibrillation
•
The ICD can treat ventricular tachyarrhythmia with cardioversion and/or defibrillation. Shock polarity and energy can be programmed individually. Shock energies
between 2.0 and 45 J are possible. Before delivery of the shock, the ICD can be set
to only deliver a shock when ongoing tachyarrhythmia is confirmed; during this
time period the device can identify spontaneous conversion of the tachyarrhythmia
and cancel the charging process if necessary.
•
The shock paths can be set between the different shock coils (SVC/RV) and/or the
housing.
7

Antibradycardia pacing and CRT
•
Innovative rate hystereses, automatic sensor functions, and a night program
promote the patient's intrinsic rhythm, avoid overdrive pacing, and facilitate adaptation of the device to the individual needs of the patient.
•
Thresholds: atrial as well as ventricular pacing thresholds are automatically determined in the device. Capture control is used to set the pulse amplitudes so that
pacing is performed with the optimum atrial and ventricular amplitude for the
patients with each change of the pacing threshold.
•
Setting an upper tracking rate for the atrium prevents unspecific atrial pacing, thus
reducing the risk of pacemaker-mediated tachycardia.
•
Positive AV hysteresis functions support the intrinsic conduction and thus the
natural contraction sequence. Negative AV hysteresis functions support the cardiac
resynchronization therapy by maintaining pacing in stressful situations.
•
For resynchronization of the ventricles, triple-chamber devices have functions for
multisite ventricular pacing with possible VV delays in either direction.
•
To ensure that no additional surgery is necessary in case of a left-sided increase of
the pacing threshold or undesired phrenic nerve stimulation, different pacing
polarities can be set for the left ventricular lead with a triple-chamber device. With
the HF-T QP device up to 12 vectors are possible.
•
Automatic active capture control is available for the right and left ventricle with
automated tracking of the pacing threshold or automatic threshold monitoring
(ATM) for trend analysis.
•
Series 7: additional, special form of rate adaptation: an increased cardiac output
requirement is detected using physiological impedance measurement. The
measuring principle is based on contractile changes (inotropy) of the myocardium
(CLS function: Closed Loop Stimulation). The suitable rate adaptation is automatically initialized and optimized in CLS mode.
•
Ventricular pacing suppression: unnecessary ventricular pacing is avoided by
promoting intrinsic conduction (Vp suppression function). The device can adapt
itself to conduction changes. In the case of intrinsic conduction, the device switches
to a DDD(R)-ADI(R) mode.
Storing programs
There are two types of therapy programs:
— Default parameters are offered for the most common indications (BradyProgramConsult function).
— Individual settings can be saved in 3 individual therapy programs
Home Monitoring functions
•
The device automatically sends information to the transmitter once a day. It also
sends messages related to events, which are immediately forwarded to the Service
Center. In addition to this, test messages can be initiated using the programmer.
•
Appointments for Home Monitoring-supported follow-ups can be scheduled via the
HMSC.
•
Important medical information in the device messages include the following:
—
Atrial and ventricular arrhythmias
—
Parameters relevant to leads in the atrium and ventricle: pacing thresholds,
sensing amplitudes, impedances
—
Current statistics
—
IEGM online HD® with up to 3 high definition channels
8

2 General Safety Instructions
Operating Conditions
Technical manuals
Folgende Gebrauchsanweisungen informieren über die Anwendung von Implantatsystemen:
— Technical manual for the device
— Technical manual for the HMSC
— Technical manuals for the programmer and the SafeSync Module
— Technical manuals for the user interface
— Technical manuals for cables, adapters and accessories
•
Technical manual s are available either prin ted in the storage package or dig itally in
the internet: https://manuals.biotronik.com/manuals/home
•
Consider all relevant technical manuals.
•
Keep technical manuals for further use.
Care during shipping and storage
•
Devices must not be stored or transported close to magnets or sources of electromagnetic interference.
•
Note the effects of the storage duration; see Battery Data.
Delivery in shipment mode
The device is delivered in shipment mode to protect the battery; capacitor reforming
required during storage could result in controlled extended charge times of the shock
capacitors.
•
The shipment mode is displayed on the programmer after loading the device
program (it is deactivated during implantation on initial measurement of the pacing
impedance).
Temperature
Extremely low and high temperatures affect the service time of the battery in the
device.
•
Temperatures of 5°C to 45°C are permitted for transport, storage, and use.
Sterile delivery
The device and the screwdriver have been gas-sterilized. Sterility is guaranteed only if
the blister and quality control seal have not been damaged.
Sterile packaging
The device and screwdriver are packaged in two separately sealed blisters. The inner
blister is also sterile on the outside so that it can be transferred in a sterile state during
implantation.
Single use only
The device and screwdriver are intended for single use only.
•
Do not use the device if the package is damaged.
•
The device must not be resterilized and reused.
Possible Complications
General information on medical complications
Complications for patients and device systems generally recognized among practitioners also apply to BIOTRONIK devices.
•
Normal complications may include fluid accumulation within the device pocket,
infections, or tissue reactions. Primary sources of complication information include
current scientific and technological knowledge.
•
It is impossible to guarantee the efficacy of antitachycardia therapy, even if the
programs have proven successful during tests or subsequent electrophysiological
examinations. In rare cases the set parameters may become ineffective. It is
possible for therapies to induce or accelerate tachycardia and cause sustained
ventricular flutter or fibrillation.
Skeletal myopotentials
Bipolar sensing and control of sensitivity are adapted by the device to the rate spectrum
of intrinsic events so that skeletal myopotentials are usually not recorded. Skeletal
myopotentials can nonetheless be classified as intrinsic events especially at very high
sensing sensitivity and, depending on the interference, may cause inhibition or antiarrhythmia therapy.
In the case of undesired myopotentials, the device switches to asynchronous pacing if
the interference rate is exceeded.
9
 Loading...
Loading...