BIOTRONIK SE and KG SAFESYNC Users Manual

Cardiac Rhythm Management
External Devices
Gebrauchsanweisung
SafeSync Module
Erweterungsmodul fr Programmergerte zur drahtlosen Kommunkaton
by BIOTRONIK SE & Co. KG
©
Alle Rechte vorbehalten.
Technische Änderungen vorbehalten
Revision: D (2011-04-26)
11-D-xx
380184--D_GA_SafeSyncModule_de_Cover_2011-04-xx.indd 1-2 23.05.2011 12:28:48
BIOTRONIK SE & o K
Woermannkehre 1
12359 Berln ermany
Tel +49 (0) 30 68905-0
Fax +49 (0) 30 6852804
salesbotronkcom
wwwbotronkcom

Table of Contents 3
Table of Contents
Table of Contents
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
About the Device . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
About this Technical Manual. . . . . . . . . . . . . . . . . . . . . . 8
Safety During Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Intended Medical Use . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Required Expertise . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Residual Risk . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
General Safety Instructions. . . . . . . . . . . . . . . . . . . . . . 13
Electromagnetic Interference. . . . . . . . . . . . . . . . . . . . 14
Operating Conditions . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
Maintenance, Care and Disposal . . . . . . . . . . . . . . . . . 18
Startup. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Device Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
Transportation and Setup . . . . . . . . . . . . . . . . . . . . . . . 23
Connections and Cables . . . . . . . . . . . . . . . . . . . . . . . . 24
Unit Handling . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
Appendix . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
Technical Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
Scope of Delivery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 37
Electromagnetic Compatibility in Compliance
with EN 60601-1-2:2007 . . . . . . . . . . . . . . . . . . . . . . . . 38
Legend for the Label . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
Symbols on the Device. . . . . . . . . . . . . . . . . . . . . . . . . . 44
Directories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 45
List of Keywords. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 46

4 Table of Contents
1Introduction
Introduction1380184-DDoc-clas sECM--SafeSync Mod ule
Introduction 5
What's in this
chapter?
This chapter contains the following topics:
Topic Page
About the Device 6
About this Technical Manual 8
6 Introduction
About the Device
General description
The SafeSync Module can be connected to the ICS
3000 and Renamic programmers and permits:
A wandless telemetry connection (SafeSync RF
telemetry) between the programmer and devices
with the BIOTRONIK SafeSync function and
Optional communication with networks via the
cellular phone network or WLAN (depending on the
software version of the programmer).
Devices with the BIOTRONIK SafeSync function are
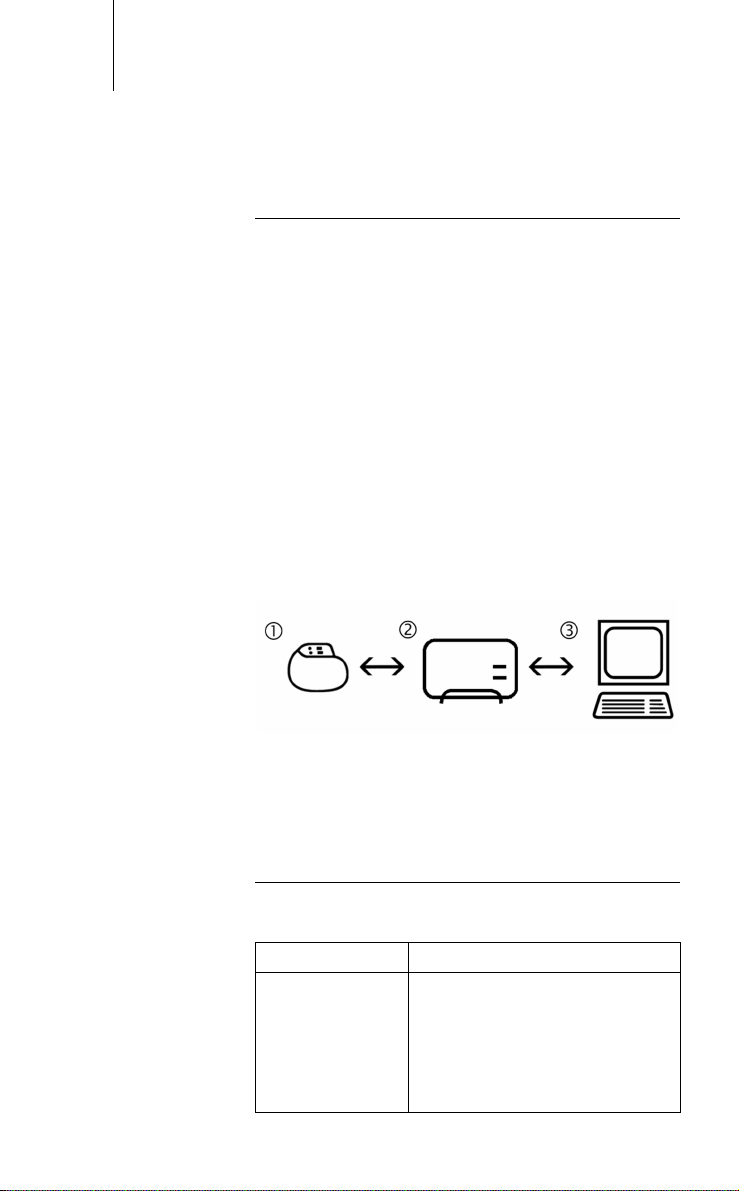
equipped with a special transmitter and receiver (1).
This sends all the relevant information to the
SafeSync Module (2), which then forwards the information to the programmer (3). The device also
receives all information that the programmer
forwards to the SafeSync Module for transmission.
Fig. 1: SafeSync function principle
It is used during the implantation procedure and
follow-up of implantable pacemakers and ICDs
(implantable cardioverter-defibrillators) with the
BIOTRONIK SafeSync function.
Primary function
The device extends the programming devices of
BIOTRONIK to include the following functions:
Function Purpose
BIOTRONIK
SafeSync function
Wandless telemetry connection
(SafeSync RF telemetry) for
interrogating, testing and
programming pacemakers and
ICDs with the BIOTRONIK
SafeSync function
Introduction 7
Other functions
(depending on the
software version of
the programmer)
The device extends the programming devices of
BIOTRONIK to include the following functions:
Function Purpose
Data transfer Exporting the follow-up data in
hospital or private practice
networks
Update function Downloading the latest,
approved software version for
the programmer from
BIOTRONIK
8 Introduction
About this Technical Manual
Objective
Target group
Other technical
manuals
This technical manual provides the user with all the
safety information required to use the device.
The following topics are covered in this manual:
Device startup
This technical manual is intended for physicians and
trained medical personnel who are familiar with the
following:
The use of implantable pulse generators and ICDs
The risks and possible complications associated
with using these systems
Additional requirements include:
Medical knowledge:
- Basic medical knowledge of the therapy applied
- Training in the handling and programming of
implantable pulse generators and ICDs
Technical knowledge:
- Ability to work with a PC
- Ability to use software-controlled medical
devices
To ensure the safe and correct use of the device, you
must follow these additional instructions:
The technical manual for the programmer
Technical software manual for programming the
intended implantable pulse generator / ICD
Technical manual for the intended implantable
pulse generator / ICD
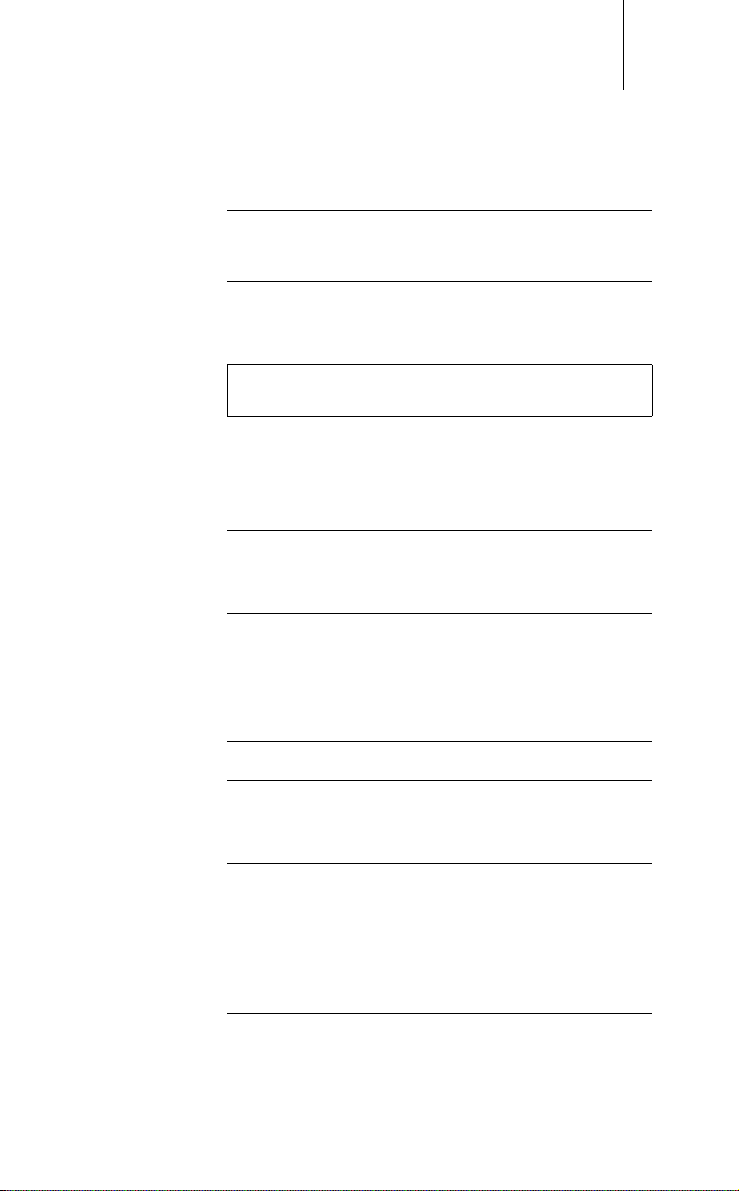
2 Safety During Use
Safety During Use2380184-DDoc- classECM--SafeSyn c Module
Safety During Use 9
What's in this
chapter?
This chapter contains the following topics:
Topic Page
Intended Medical Use 10
Required Expertise 11
Residual Risk 12
General Safety Instructions 13
Electromagnetic Interference 14
Operating Conditions 16
Maintenance, Care and Disposal 18
10 Safety During Use
Intended Medical Use
Intended medical
use
During implantation or follow-up, the SafeSync
Module establishes telemetry between a device with
BIOTRONIK SafeSync function and the ICS 3000 or
Renamic programmer.
Thus the programmer is able to perform the following
without a programming head:
Conduct sensing, pacing threshold and impedance
tests
Interrogate data of the implanted device such as
program parameters, recorded statistical data and
episodes, as well as real-time IEGMs
Display, printout, save and export data of the
implanted device for analysis and reporting
purposes
Transferring parameters to the device

Required Expertise
Safety During Use 11
Required expertise
German medical
device ordinance
The programmer is intended for use by physicians and
trained medical staff. Along with their basic medical
knowledge, a detailed knowledge of cardiac electrotherapy is also required. Only qualified medical
specialists with knowledge of cardiac electrotherapy
can properly operate the device.
This ordinance only applies in the Federal Republic of
Germany. However, we recommend that customers in
other countries comply with this ordinance as well.
According to section 2, § 5, operation and use:
'The user may operate a (...) listed medical product
only after the manufacturer or the authorized agent
who acts on behalf of the manufacturer has
performed the following requirements:
1. Checked the functionality of this medical product
at the location where the device will be used.
2. Trained the staff appointed by the user to
correctly handle, use and operate the medical
product. This training must include handling, using
and operating the product in conjunction with other
medical products, implements and accessories in
accordance with the technical manual, as well as
any applicable safety-related information and
maintenance instructions.
(...)
(3) Proof of a functional test have been performed as
stated in Paragraph 1 Item 1, and the training record
of the staff appointed by the user, discussed in Paragraph 1 Item 2, are to be documented.'

12 Safety During Use
Residual Risk
Risk analysis
The risk analysis carried out by the manufacturer's
Risk Management Team has determined that the
residual risk is as low as reasonably possible.
It is a prerequisite that the programmer has been
serviced and inspected according to the manufacturer's specifications by qualified medical staff and in
compliance with the safety-relevant instructions in
this technical manual.
General Safety Instructions
Safety During Use 13
Technical manual
Risks of improper
handling
Changes not
permitted
Replacement parts
and accessories
Defects
Liquids
Only use the programmer in accordance with this
technical manual.
Disregarding the safety instructions can endanger the
patient, the staff and the equipment.
Note: Failure to observe the safety precautions voids
all damage claims and manufacturer liability.
The following dangers may arise in the event of
improper use:
Failure of important device functions
Danger to persons due to electrical effects
Only the manufacturer or a party expressly authorized
by BIOTRONIK may perform corrective maintenance,
enhancements or modifications to the device.
To ensure safety compliance, use only original
replacement parts and accessories authorized by
BIOTRONIK. Using any other parts voids the manufacturer's liability for any consequences, guarantee and
warranty.
Do not use defective or damaged devices.
Never use a damp or wet device.
Protect the device from the accidental ingression
of fluids (e.g. infusion fluids).
Electrostatic
potentials
Ensure that electrostatic potentials between medical
staff and patients are balanced. Before handling the
device, the electrostatic potential between the doctor
or medical staff and the patient must be balanced by
touching the patient at a point as far away from the
leads as possible.

14 Safety During Use
Electromagnetic Interference
Possible electromagnetic interference
The programmer is protected from disturbances
resulting from electromagnetic irradiation, electrostatic discharges and other sources. Simultaneously,
the emitted interference has been reduced to a
minimum. Thus the programmer conforms to the
requirements of EN 60601-1-2 (in its valid form at the
time of delivery).
However, strong electromagnetic interferences that
occur in the close vicinity of electrical motors, power
cables, PCs, monitors, or other – possibly defective –
electrical devices may compromise the function of the
programmer in certain cases.
This kind of device malfunction should be considered
if the following is observed:
The device switches on by itself.
The unit passes on incorrect intrinsic events, which
are displayed on the ECG, IEGM or marker channel
(artifacts) of the programmer and monitoring
device.
The device displays other inexplicable functions.
Correct operation of the device can be restored with
the following:
Switch off the malfunctioning electronic device.
Remove the source of interference from the device.
Switch the programmer on and off or cut off the
electrical connection between the device and the
source of interference if this is possible without
causing any danger.
If the interference continues, contact BIOTRONIK
immediately.
Note: If accessories other than those specified by
BIOTRONIK are used, increased interference or
lower resistance to interference can be expected.

Safety During Use 15
Note: If accessories specified by BIOTRONIK are
used on other devices, increased interference or
lower resistance to interference can be expected.
Note: Portable radio communication devices can
interfere with the programmer functioning.
EMI test
Telemetry between the SafeSync Module and the
implanted device can be impaired by electromagnetic
interference (EMI). This can be observed when it
becomes difficult or even impossible to interrogate or
program the implanted device. Using the EMI test
(refer to device software help), the source of the electromagnetic interference can be located and then
turned off.
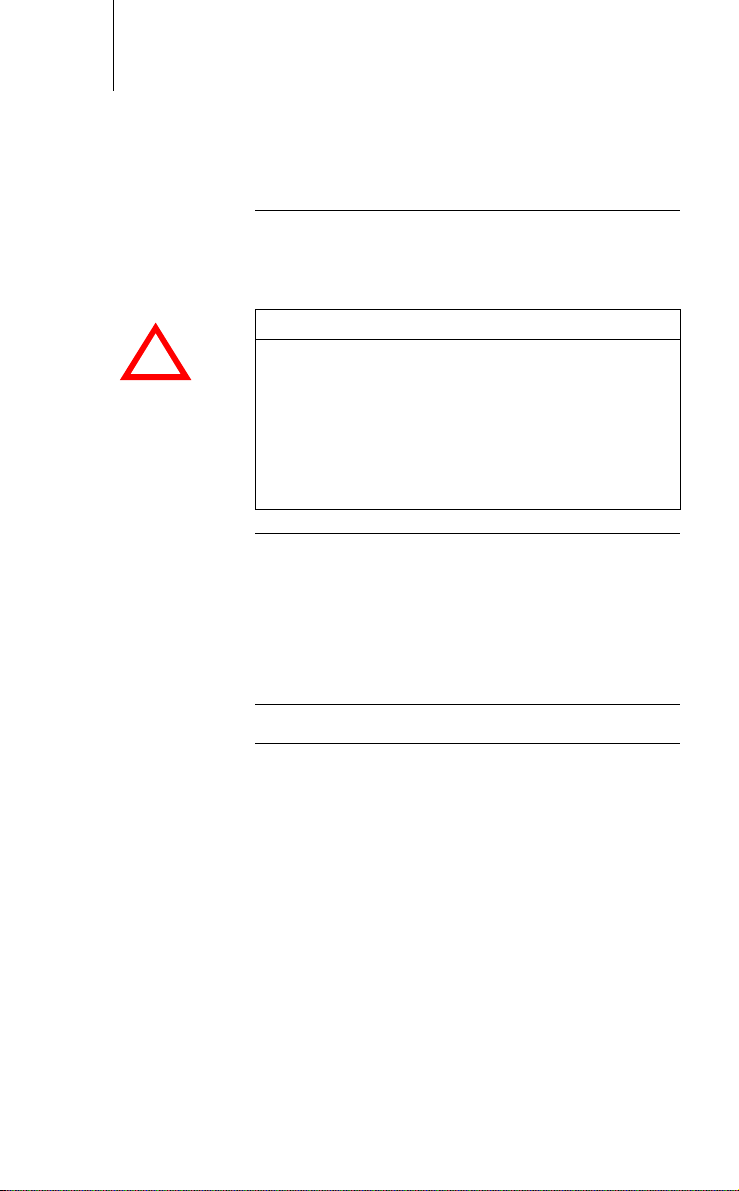
16 Safety During Use
!
!
Operating Conditions
Storage and
transportation
Installation site
Power supply
Cable and plug
connections
If the packaging is damaged, please contact
BIOTRONIK immediately. Do not put the device into
operation.
CAUTION
Functional impairment due to external damage
Mechanical impact, for example dropping the unit even from a height of over 5 cm if unpackaged - can
permanently impair the function of the system.
Do not use the device if it shows visible damage.
Contact BIOTRONIK for testing and, if necessary,
repair of the equipment.
Only operate the device in rooms that fulfill the
following conditions:
No danger of explosion
Suitable for medical purposes
Place the unit on a flat, dry surface so that the patient
cannot touch it. The unit should be placed so that it
cannot slide – even with the cables connected.
The unit is powered via the programmer's USB cable.
Replace any cable that shows even slight damage.
Lay all cables within the measuring apparatus in
such a way that they pose no danger of tripping over
them and that any tensile forces that may occur can
be safely buffered.
As a general rule, cables should only be connected
or disconnected when the unit is switched off,
unless expressly permitted in the corresponding
section of this technical manual.
Ensure that the contacts of all connections and
plugs are clean. Soiled contacts can lead to signal
distortions, and thus to false diagnoses.
Do not touch any connections such as USB ports or
interfaces for modules and the patient at the same
time.
 Loading...
Loading...