BIOTRONIK SE and KG PRIMUS QRIPRIMUS UserMan

© BIOTRONIK GmbH & Co. KG
9
All rights reserved. Specifi cations
subject to modifi cation, revision
and improvement.
2009-D-xx
® BIOTRONIK Home Monitoring and
Entovis are registered trademarks of
BIOTRONIK GmbH & Co. KG
This product conforms with the
directives 90/385/EEC relating to
active implantable medical devices
and 99/5/EC on radio equipment and
telecommunication terminal equip ment. It was approved by independent
Notifi ed Bodies and is therfore
designated with the CE mark. The
product can be used in all European
Union countries as well as in countries
that recognize the above-mentioned
directives.
BIOTRONIK GmbH & Co. KG
Woermannkehre 1
12359 Berlin · Germany
Tel+49 (0) 30 68905–0
Fax+49 (0) 30 6852804
sales@biotronik.com
www.biotronik.com
Evia
Pacemaker with automatic Functions
and BIOTRONIK Home Monitoring
®
Cardiac Rhythm Management
Bradycardia therapy
Technical Manual

Evia DR-T, DR, SR-T, SR
Pacemaker
Bradycardia therapy
Technical manual for the implant
Doc. Id.: 365353-A
Index 365353-ATechnical manual for the implantEvia DR-T, DR, SR-T, SR
sbiotronik

2

3 Table of Contents
Table of Contents
Table of Contents
Product Description. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Intended Medical Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
System Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Implant Variants and NBG Codes. . . . . . . . . . . . . . . . . . . . . . 8
Diagnostic and Therapy Functions . . . . . . . . . . . . . . . . . . . . . 9
Scope of Delivery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
General Safety Instructions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Possible Medical Complications. . . . . . . . . . . . . . . . . . . . . . 13
Possible Technical Complications . . . . . . . . . . . . . . . . . . . . 14
Possible Electromagnetic Complications . . . . . . . . . . . . . . 15
Possible Risks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
Prior to Implantation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Indications and Contraindications . . . . . . . . . . . . . . . . . . . . 17
Ambient Conditions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
Sterility . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Preparing the Implantation. . . . . . . . . . . . . . . . . . . . . . . . . . 20
Implantation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Implanting. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Connecting PM Leads . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
Precautionary Measures while Programming . . . . . . . . . . 24
After Implantation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
Follow-up . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
Notes for the Physician . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28
Replacement Indications. . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
Explantation and Implant Replacement. . . . . . . . . . . . . . . . 31

4 Table of Contents
Parameters. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
Technical Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 47
Pacing Modes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
Timing DR(-T) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
Timing SR(-T) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 37
Pacing and Sensing DR(-T) . . . . . . . . . . . . . . . . . . . . . . . . . 38
Pacing and Sensing SR(-T) . . . . . . . . . . . . . . . . . . . . . . . . . . 40
Rate Adaptation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 41
Preset Programs DR(-T) . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
Preset Programs SR(-T) . . . . . . . . . . . . . . . . . . . . . . . . . . . . 44
Tolerances of Parameter Values . . . . . . . . . . . . . . . . . . . . . 45
Mechanical Characteristics . . . . . . . . . . . . . . . . . . . . . . . . . 47
Electrical Characteristics . . . . . . . . . . . . . . . . . . . . . . . . . . . 48
Battery Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 49
Country-Related Information . . . . . . . . . . . . . . . . . . . . . . . . 51
Legend for the Label . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 52

5
1 Product Description
Product Description1365353-ATechnical manual for the implantEvia DR-T, DR, SR-T, SR
Intended Medical Use
Intended use Evia is a family of implantable pacemakers that may be implanted for all bradycar-
dia arrhythmia indications. The primary objective of the therapy consists of improving patients' symptoms that can be clinically manifested.
The implantation of the pacemaker is a symptomatic therapy with the following
objective:
• Compensation of bradycardia by atrial, ventricular, or AV sequential pacing
Diagnosis and
therapy forms
Required expertise In addition to having basic medical knowledge, the user must be thoroughly famil-
The cardiac rhythm is automatically monitored and bradycardia arrhythmias are
treated. All major therapeutic approaches from the field of cardiology and electrophysiology are unified in the Evia family.
®
BIOTRONIK Home Monitoring
ment any time.
iar with the operation of an implant system. Only qualified medical specialists having the special knowledge required for the proper use of implants are permitted to
use them. If users do not possess this knowledge, they must be trained accordingly.
enables physicians to perform therapy manage-

System Overview
Implant The implant's housing is made of biocompatible titanium, welded from outside and
6 Product Description
Parts The implant system consists of the following parts:
• Implant with connections for unipolar or bipolar sensing and pacing
• Suitable leads and approved accessories
• Programmer
• Current implant programs
thus hermetically sealed. The ellipsoid shape facilitates ingrowth into the pectoral
muscle area.
The housing serves as an antipole in the case of unipolar lead configuration.
BIOTRONIK provides silicone-coated implants to avoid muscle twitching near the
implanted pacemaker in the case of unipolar pacing.
The labeling provides information about the implant type and arrangement of the
connections.
Leads The leads are sheathed with biocompatible silicone. They can be flexibly maneu-
vered, are long-term stable, and are equipped for active or passive fixation. They
are implanted using a lead introducer set. Some leads are coated with polyurethane
to increase the sliding properties of the lead.
The coating of steroid-eluting leads reduces inflammatory processes. The fractal
design of the leads provides for low pacing thresholds, high pacing impedance, and
a low risk of oversensing.
Programmer The transportable programmer is used to transfer the appropriate implant pro-
gram to the implant. In addition to this, the programmer is used for interrogation
and storage of data from the implant. And it acts as an ECG and IEGM monitor with
Miniclinic.
The programmer communicates with the implant via the programming head. The
operation module of the programmer has a TFT touch screen with color display, on
which the ECG, IEGM, marker and functions are shown simultaneously.
The programmer has, among others, the following functions:
• Perform all tests during follow-up
• Display and print real-time and saved IEGMs with annotated markers
• Determine the pacing threshold

7 Product Description
BIOTRONIK
Home Monitoring
Technical manuals The following technical manuals provide information about usage of the implant
In addition to effective pacing therapy, BIOTRONIK provides a complete therapy
®
management system:
• With Home Monitoring, diagnostic and therapeutic information and technical
data are sent via an antenna in the implant header to a mobile or stationary
transmitter. The encrypted data are sent from the transmitter to the
BIOTRONIK Service Center via the cellular phone network.
• The received data are deciphered and evaluated. Each physician can set the
criteria for evaluation to be used for each patient and can configure the time of
notification via fax, SMS or E-mail.
• A clear overview of the analysis results is displayed for the attending physicians
on the protected Internet platform HMSC (Home Monitoring Service Center).
• Data transmission from the implant is performed on a daily basis with the trend
message. Depending on the transmitter used, these data are passed on immediately or, if the data is normal, it is collected for up to 2 weeks. If certain events
occur in the patient's heart or in the implant itself, an event message is sent.
Additionally, patients can send a patient message by applying the magnet.
system:
• Technical manual for the implant
• Technical manual for the programmer
• User manual for the implant program:
— As a help function in the user interface
— As a file on CD
• Technical manual for the leads
8 Product Description
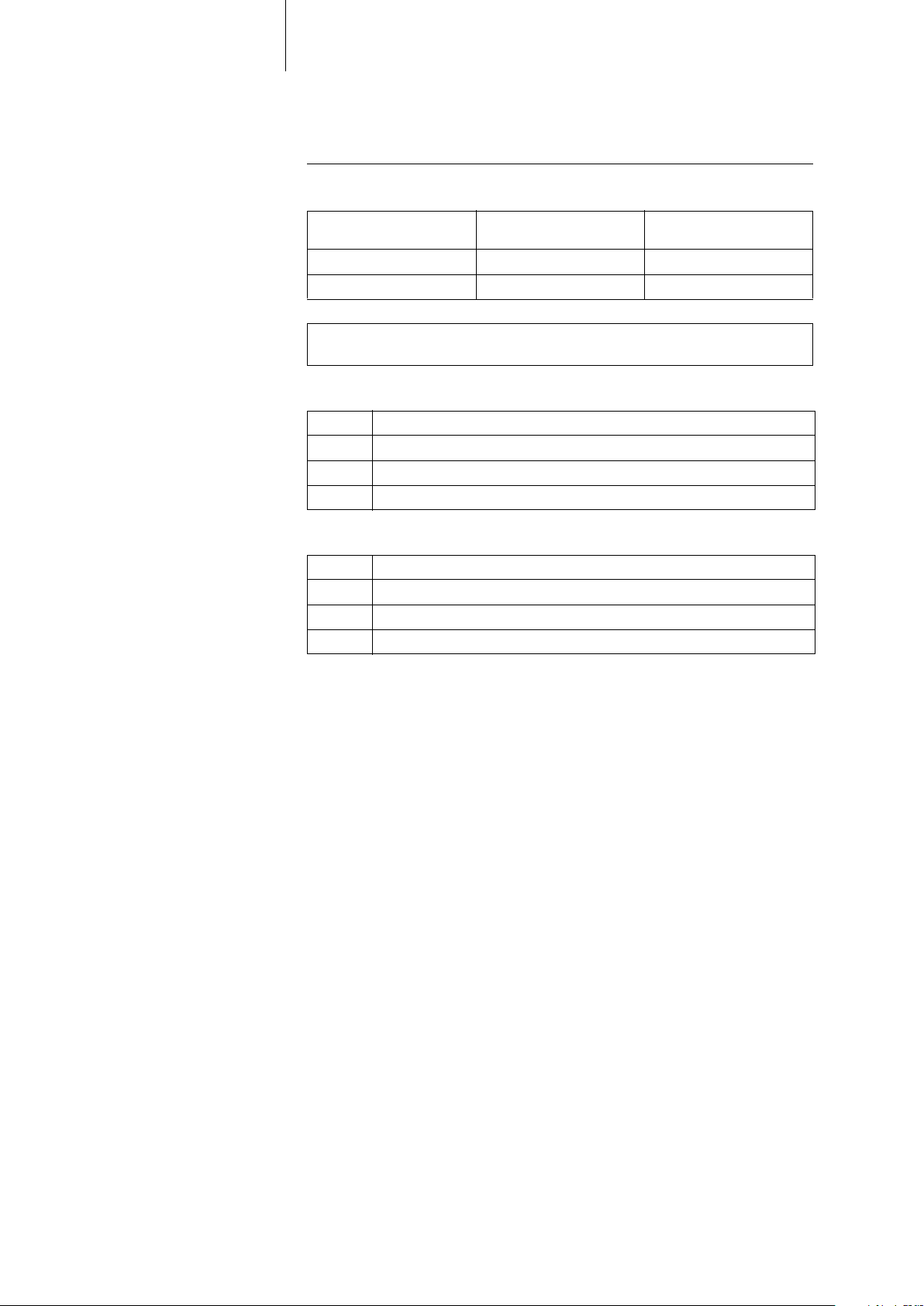
Implant Variants and NBG Codes
Evia family The following implant variants are available:
Implant type Variant with
Home Monitoring
Dual-chamber Evia DR-T Evia DR
Single-chamber Evia SR-T Evia SR
Note: The setting of the pacing mode depends on the individual diagnosis; the
modes are listed in the section pertaining to adjustable parameters.
NBG-Code for Evia DR(-T) The NBG code for dual-chamber implants is DDDR:
D Pacing in both chambers
D Sensing in both chambers
D Pulse inhibition and pulse triggering
R Rate adaptation
NBG-Code for Evia SR(-T) The NBG code for single-chamber implants is AAIR or VVIR:
A/V Pacing in one chamber
A/V Sensing in one chamber
I Pulse inhibition in A/V
R Rate adaptation
Variant without
Home Monitoring

9 Product Description
Diagnostic and Therapy Functions
General overview All the systems have extensive features that allow quick diagnosis and delivery of
safe therapy for bradycardia conditions.
• Automatic functions make it easy and fast to implant, configure, and check the
pacemaker.
• Auto-initialization after implantation: the implant automatically detects the
implanted leads, sets the polarity and activates the automatic functions after
10 min.
Diagnostic functions • Data from the last 10 interrogations and follow-ups are recorded as well as
arrhythmia episodes; they are stored together with other data to assess
patients and the state of the implant at any time.
• Automatic below-threshold impedance measurement is performed in the
implant independent of the pacing pulse in order to check the lead for proper
functioning.
• When performing follow-ups using the programmer, the IEGM is indicated with
markers after applying the programming head during the test procedure.
Antibradycardia pacing • Sensing: the amplitudes of the P and R waves are measured in the implant fully
automatically to record varying amplitudes. The sensitivity for the atrium and
ventricle is adapted automatically on an ongoing basis. The measurement data
are averaged and the trend can be displayed.
• Thresholds: atrial as well as ventricular pacing thresholds are automatically
determined in the implant. Active capture control is used to set the pacing
amplitudes so that pacing is performed with the optimum atrial and ventricular
amplitude for the patients with each change of the pacing threshold.
• Timing: pacing is particularly checked in the atrium by automatic adaptation
of the atrial refractory period to avoid pacemaker-induced tachycardia.
(Auto PVARP function: automatic postal-atrial refractory period)
• Additional, special form of rate adaptation: an increased cardiac output require-
ment is detected using physiological impedance measurement. The measuring
principle is based on contractile changes (ionotropy) of the myocardium
(CLS function: Closed Loop Stimulation). The suitable rate adaptation is automatically initialized and optimized in CLS mode.
• Ventricular pacing suppression: unnecessary ventricular pacing is avoided by
promoting intrinsic conduction (V
adapt itself to conduction changes. In the case of intrinsic conduction, the
implant switches to a mode similar to AAI.
suppression function). The implant can
p

10 Product Description
Home Monitoring The implant automatically sends information to the transmitter once a day. Addi-
tionally, the test messages can be initiated using the programmer. Important medical information include, among others, the following:
• Ongoing atrial and ventricular arrhythmia
• Parameters relevant to leads in the atrium and ventricle: thresholds, sensing
amplitudes, impedances
• Current statistics on bradycardia therapy
• Individually adjustable remote interrogation messages which enhance the
standard message with additional information relevant for follow-up
®
• IEGM online HD
and RV, which each include the intrinsic rhythm and sequences with encouraged sensing and encouraged pacing
• Sending of these IEGM recordings with remote interrogation messages
• Test message triggered by the programmer to immediately check the Home
Monitoring function including notification of the physician
with up to 3 channels in high definition with markers for RA
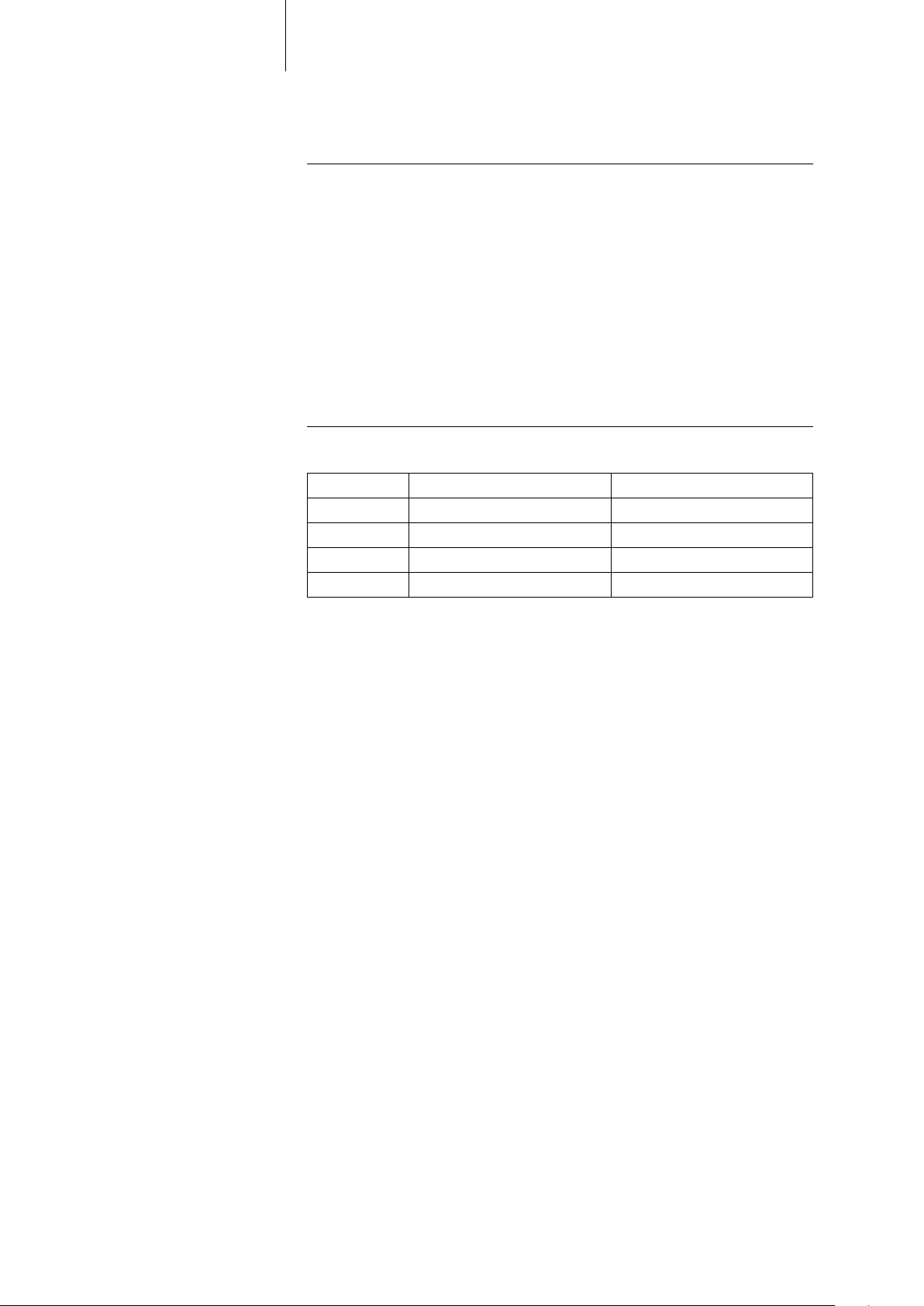
Scope of Delivery
Standard The storage package includes the following:
Order numbers Evia The implants can be obtained as follows:
11 Product Description
• Implant in sterile packaging
• Patient's manual
• Serial number label
• Patient ID card
• Warranty card
• Technical manual
The sterile container contains the following:
• Implant
• Screwdriver
Implant Order number: uncoated Order number: coated
DR-T 359529 359530
DR 359524 359528
SR-T 359533 359534
SR 359531 359532
Accessories All BIOTRONIK products correspond to the requirements of the
EC Directive 90/385/EEC:
• BIOTRONIK leads
• BIOTRONIK programming and monitoring devices
• Permanent magnet
• For Home Monitoring: BIOTRONIK transmitters

12 Product Description

13
2 General Safety Instructions
General Safety Instructions2365353-ATechnical manual for the implantEvia DR-T, DR, SR-T, SR
Possible Medical Complications
General information
on medical complications
Skeletal myopotentials Bipolar sensing and control of sensitivity are adapted by the implant to the rate
Nerve and
muscle stimulation
Complications for patients and implant systems generally recognized among practitioners also apply to BIOTRONIK implants.
• Normal complications may include fluid accumulation within the implant
pocket, infections, or tissue reactions. Primary sources of complication infor-
mation include current scientific and technological knowledge.
• It is impossible to guarantee the efficacy of antitachycardia therapy, even if the
programs have proven successful during tests or subsequent electrophysiological examinations. In rare cases the set parameters may become ineffective. In
particular it cannot be excluded that tachyarrhythmias be induced.
spectrum of intrinsic events so that skeletal myopotentials are usually not sensed.
Skeletal myopotentials can nonetheless be sensed as intrinsic events especially
with a unipolar configuration and, depending on the interference pattern, may
cause inhibition or antiarrhythmia therapy.
An implant system consisting of a unipolar lead and an uncoated implant may
result in undesirable pacing of the diaphragm in the case of an initial or permanent
high setting of the pacing amplitude.
• BIOTRONIK also provides coated implants.

14 General Safety Instructions
Possible Technical Complications
Technical malfunctions Technical implant malfunctions cannot entirely be excluded. Possible causes can
include the following:
• Lead dislocation
• Lead fracture
• Insulation defects
• Implant component failures
• Battery depletion

15 General Safety Instructions
Possible Electromagnetic Complications
Electromagnetic
interference (EMI)
Implant behavior
in case of EMI
Static magnetic fields The Reed contact in the pacemaker closes beginning at a field strength of 1.5 tesla.
Any implant can be sensitive to interference, for example, when external signals
are sensed as intrinsic rhythm or if measurements prevent rate adaptation.
• BIOTRONIK implants have been designed so that their susceptibility to EMI is
minimal.
• Due to the intensity and variety of EMI, there is no guarantee for safety. It is generally assumed that EMI produces only minor symptoms in patients - if any.
• Depending on the pacing mode and the type of interference, sources of interference may lead to pulse inhibition or triggering, an increase in the sensordependent pacing rate or fixed-rate pacing.
• Under unfavorable conditions, for example during diagnostic or therapeutic
procedures, the interference sources may induce such a high level of energy
into the pacing system that the implant or cardiac tissue around the lead tip is
damaged.
Upon exceeding the interference rate, the implant switches to another pacing
mode. Depending on whether the interference occurs in one chamber or both
chambers, the implant switches to the A00(R), V00(R) or D00(R) mode for the dura-
tion of the interference.

Possible Risks
16 General Safety Instructions
Risky diagnostic and
therapeutic procedures
External defibrillation The implant is protected against the energy that is normally induced by external
Contraindicated procedures The following procedures are contraindicated:
If electrical current from an external source is conducted through the body for diagnostic or therapeutic purposes, then the implant can be subjected to interference
and the patient placed at risk. Therefore the following always applies:
• Monitor the patient.
defibrillation. Nevertheless, any implanted device may be damaged by external
defibrillation. Specifically, the current induced in the implanted leads may result in
necrotic tissue formation close to the electrode/tissue interface. As a result, sensing properties and pacing thresholds may change.
• Place adhesive electrodes anterior-posterior or perpendicular to the axis
formed by the implant to the heart at least 10 cm away from the device and from
implanted leads.
• Therapeutic ultrasound and diathermy: damage to the patient via excess warming of body tissue near the implant system
• Transcutaneous electrical nerve stimulation (TENS)
• Lithotripsy
• Electrocautery and high-frequency surgery: damage to the patient via the
induction of arrhythmia or ventricular fibrillation
• Hyperbaric oxygen therapy
• Applied pressures higher than normal pressure
Magnetic resonance
imaging
Therapeutic
ionizing radiation
Magnetic resonance imaging is contraindicated due to the associated magnetic flux
density: damage or destruction of the implant system by strong magnetic interaction and damage to the patient by excessive warming of the body tissue in the area
surrounding the implant system.
• Under certain conditions one can perform special measures with magnetic res-
onance imaging to protect the patient and implant.
Radiation can cause latent damage. This damage cannot be recognized immediately. Therefore, the following applies to X-ray diagnosis and radiation therapy:
• Sufficiently shield implant against radiation.
• After applying radiation, double-check the implant system to make sure it is
functioning properly.
 Loading...
Loading...