Page 1

Lumax
Family of Implantable Cardioverter
Defibrillators and Cardiac
Resynchronization Therapy
Defibrillators
• VR ICD
• VR-T ICD
• DR ICD
• DR-T ICD
• HF CRT-D
• HF-T CRT-D
Technical Manual
1-1637
Page 2
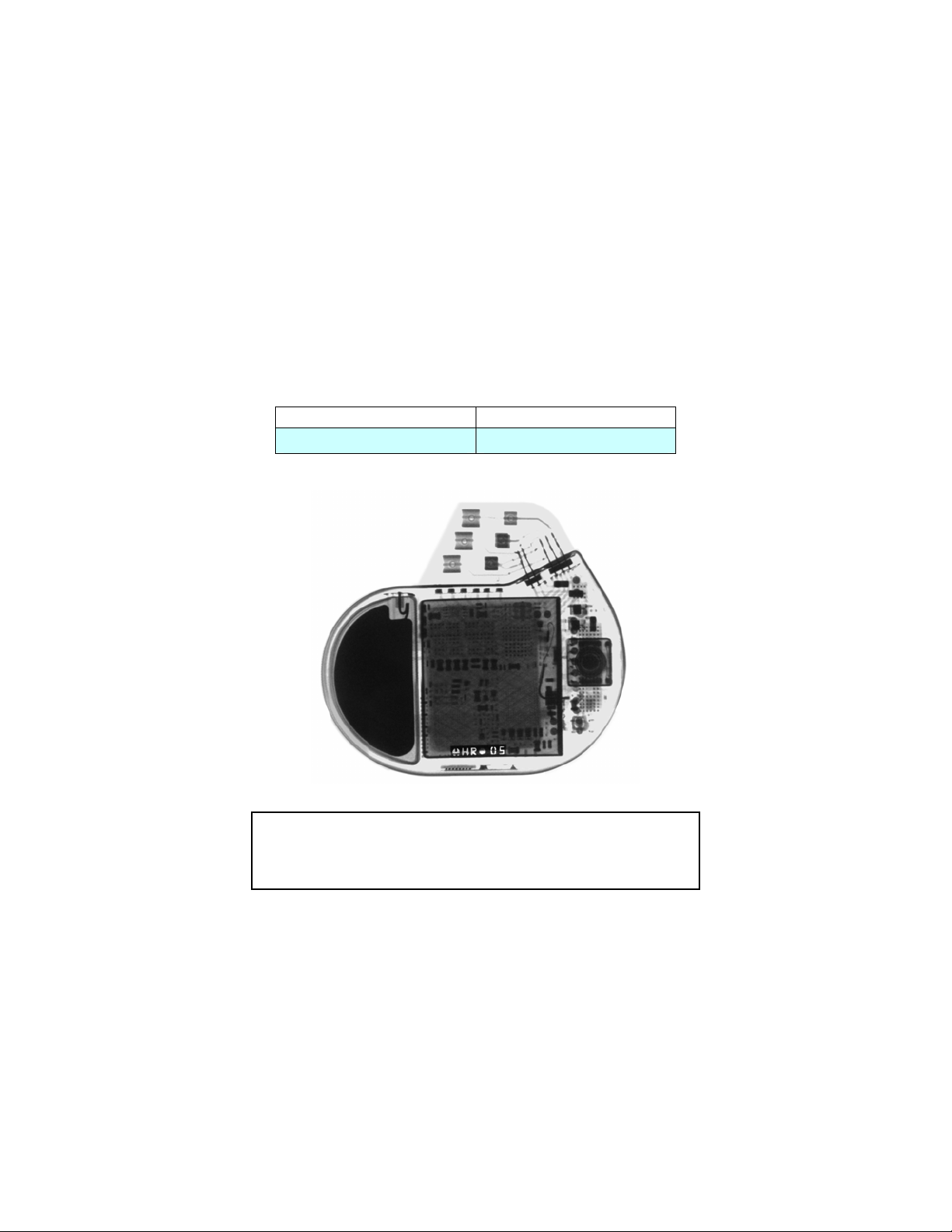
X-ray Identification
Lumax Family
Implantable Cardioverter Defibrillator and
Cardiac Resynchronization Therapy Defibrillators
Inside the housing:
X-Ray identification Year of manufacture
HR nn
CAUTION
Federal (U.S.A.) law restricts this device to sale by, or on the
order of, a physician.
©2006 BIOTRONIK, Inc., all rights reserved.
1-1638
Page 3

Lumax Technical Manual i
Contents
1. General .................................................................................6
1.1 System Description ..........................................................6
1.2 Indications and Usage......................................................8
1.3 Contraindications.............................................................. 9
1.4 Warnings and Precautions ...............................................9
1.4.1 Sterilization, Storage, and Handling ....................... 12
1.4.2 Device Implantation and Programming ..................12
1.4.3 Lead Evaluation and Connection ...........................14
1.4.4 Follow-up Testing ................................................... 16
1.4.5 Pulse Generator Explant and Disposal ..................16
1.4.6 Hospital and Medical Hazards................................ 16
1.4.7 Home and Occupational Hazards ..........................18
1.4.8 Cellular Phones ...................................................... 18
1.4.9 Electronic Article Surveillance (EAS) .....................19
1.4.10 Home Appliances ................................................... 20
1.4.11 Home Monitoring ....................................................20
1.5 Potential and Observed Effects of the Device on Health21
1.5.1 Potential Adverse Events .......................................21
1.5.2 Observed Adverse Events...................................... 22
1.6 Clinical Studies...............................................................32
1.6.1 Kronos LV-T Study ................................................. 32
1.6.2 Tupos LV/ATx Study............................................... 34
1.7 Patient Selection and Treatment....................................54
1.7.1 Individualization of Treatment ................................54
1.7.2 Specific Patient Populations...................................56
1.8 Patient Counseling Information ...................................... 56
1.9 Evaluating Prospective CRT-D/ICD Patients .................57
2. Device Features ................................................................. 58
2.1 Cardiac Resynchronization Therapy (CRT) ................... 58
2.2 Sensing (Automatic Sensitivity Control) ......................... 61
2.2.1 Right Ventricular Sensitivity Settings...................... 61
2.2.2 Minimum Right Ventricular Threshold ....................64
2.2.3 Atrial Sensitivity Settings ........................................ 64
2.2.4 Minimum Atrial Threshold.......................................65
2.2.5 Left Ventricular Sensitivity Settings ........................ 65
2.2.6 Minimum Left Ventricular Threshold....................... 65
1-1639
Page 4

ii Lumax Technical Manual
2.2.7 Far Field Protection ................................................ 65
2.2.8 Additional Sensing Parameters .............................. 66
2.3 Ventricular Tachyarrhythmia Detection........................... 67
2.3.1 VF Classifications ................................................... 68
2.3.2 VT Interval Counters............................................... 69
2.3.3 VT Classification.....................................................69
2.3.4 SMART Detection™ ............................................... 69
2.3.5 Onset ...................................................................... 70
2.3.6 Stability ................................................................... 71
2.3.7 Sustained VT Timer................................................71
2.4 Tachyarrhythmia Redetection.........................................72
2.4.1 VT Redetection.......................................................72
2.4.2 SMART Redetection...............................................72
2.4.3 Forced Termination ................................................73
2.4.4 VF Redetection.......................................................73
2.5 Tachyarrhythmia Termination .........................................73
2.6 Tachyarrhythmia Therapy ............................................... 73
2.6.1 Therapy Options ..................................................... 73
2.6.2 Anti-Tachycardia Pacing (ATP) .............................. 74
2.6.3 Shock Therapy .......................................................76
2.6.4 Progressive Course of Therapy.............................. 81
2.7 Bradycardia Therapy ...................................................... 82
2.7.1 Bradycardia Pacing Modes ....................................82
2.7.2 Basic Rate ..............................................................83
2.7.3 Night Rate............................................................... 83
2.7.4 Rate Hysteresis ...................................................... 84
2.7.5 Dynamic AV Delay.................................................. 87
2.7.6 IOPT Plus ...............................................................90
2.7.7 Upper Tracking Rate ..............................................90
2.7.8 Mode Switching ...................................................... 91
2.7.9 PMT Protection.......................................................93
2.7.10 VES Discrimination after Atrial Sensed Events ...... 93
2.7.11 Rate Adaptive Pacing .............................................94
2.7.12 Pulse Amplitude......................................................95
2.7.13 Pulse Width............................................................. 95
2.7.14 Post Ventricular Atrial Refractory Period................96
2.7.15 PVARP after VES ...................................................96
2.7.16 Auto PVARP ...........................................................96
2.7.17 Noise Response .....................................................96
2.7.18 Post Shock Pacing .................................................96
1-1640
Page 5

Lumax Technical Manual iii
2.8 EP Test Functions...........................................................97
2.8.1 P and R-wave Amplitude Measurements...............97
2.8.2 Pacing Impedance Measurements.........................98
2.8.3 Shock Impedance Measurements..........................98
2.8.4 Testing for Retrograde Conduction ........................98
2.8.5 Pacing Threshold.................................................... 99
2.8.6 Arrhythmia Induction Features .............................100
2.8.7 Manual Shock.......................................................101
2.8.8 Test Shock............................................................101
2.8.9 Manual ATP ..........................................................102
2.8.10 Emergency Shock ................................................ 102
2.9 Special Features...........................................................102
2.9.1 ICD Therapy Status .............................................. 102
2.9.2 Home Monitoring .................................................. 103
2.9.3 Real-time IEGM Transmission .............................110
2.9.4 Capacitor Reforming.............................................110
2.9.5 Patient and Implant Data ...................................... 111
2.9.6 System Status.......................................................111
2.9.7 HF Monitor Statistics ............................................112
2.9.8 Holter Memory ...................................................... 113
2.9.9 Real-time IEGM .................................................... 116
2.9.10 Timing Statistics ................................................... 116
2.9.11 Atrial Arrhythmias .................................................117
2.9.12 Ventricular Arrhythmias ........................................117
2.9.13 Sensor ..................................................................117
2.9.14 Sensing .................................................................117
2.9.15 Impedances .......................................................... 117
3. Sterilization and Storage................................................. 118
4. Implant Procedure ........................................................... 120
4.1 Implant Preparation ...................................................... 120
4.2 Lead System Evaluation............................................... 124
4.3 Opening the Sterile Container......................................124
4.4 Pocket Preparation.......................................................125
4.5 Lead to Device Connection ..........................................126
4.6 Blind Plug Connection .................................................. 128
4.7 Program the ICD/CRT-D ..............................................129
4.8 Implant the ICD/CRT-D ................................................130
5. Follow-up Procedures.....................................................133
5.1 General Considerations................................................133
5.2 Longevity ......................................................................134
1-1641
Page 6
iv Lumax Technical Manual
5.3 Explantation.................................................................. 136
6. Technical Specifications.................................................139
Appendix A .............................................................................149
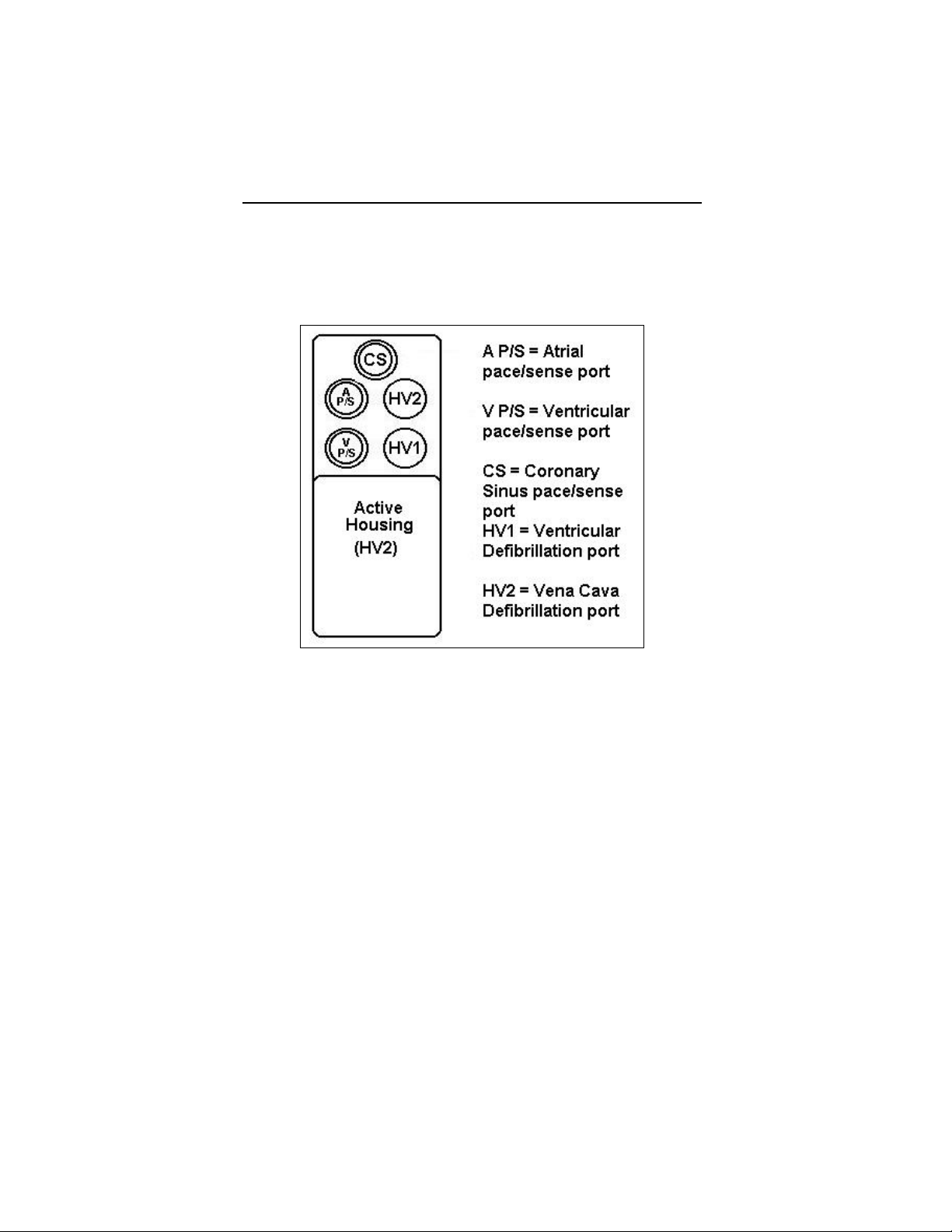
*Lumax VR (-T) and DR (-T) ICDs do not have coronary sinus
pace/sense ports
** Lumax VR (-T) ICDs do not have atrial pace/sense ports
1-1642
Page 7

Lumax Technical Manual v
Lumax Specifications and Description
Battery Voltage: 3.2 Volts
300 models:
Maximum Shock Energy:
340 models:
Maximum Shock Energy:
Defibrillation Lead Ports Two DF-1 (3.2 mm)
Pacing Lead Ports Three IS-1 (3.2 mm)
Dimensions:
Volume:
Mass:
Housing Material: Titanium
Header Material: Epoxy Resin
Sealing Plug Material: Silicone
30 joules programmed
26.6 joules delivered
40 joules programmed
35.7 joules Delivered
(one for Lumax VR (-T)
and two for
Lumax DR (-T)s)
See Technical Details in
Section 6
1-1643
Page 8

6 Lumax Technical Manual
1. General
1.1 System Description
The Lumax family of Implantable Cardioverter Defibrillators
(ICDs) and Cardiac Resynchronization Therapy Defibrillators
(CRT-Ds) detect and treat ventricular tachyarrhythmias and
provide rate adaptive bradycardia pacing support. The HF and
HF-T versions of Lumax provide Cardiac Resynchronization
Therapy (CRT) through biventricular pacing. Both CRT-Ds and
ICDs detect and treat ventricular tachyarrhythmias and provide
rate adaptive bradycardia pacing support. They are designed to
collect diagnostic data to aid aid the physician’s assessment of a
patient’s condition and the performance of the implanted device.
The Lumax family of devices provides therapy for ventricular
tachyarrhythmias with a sophisticated range of programmable
anti-tachycardia pacing (ATP), and/or defibrillation therapy
features. The shock polarity and energy may be programmed to
tailor the therapy to appropriately treat each patient's
tachyarrhythmias. The ICDs/CRT-Ds provide shock therapies
with programmable energies from 5 to 40 joules.
The Lumax family of ICDs/CRT-Ds include the following
members:
• Lumax HF provides three chamber rate adaptive
bradycardia pacing support including biventricular
pacing via a left ventricular pacing lead. The CRT-D
uses right atrial and ventricular sensing/pacing leads to
provide enhanced atrial and ventricular tachyarrhythmia
discrimination through BIOTRONIK’s SMART
Detection
• Lumax HF-T In addition to the functionality found with
HF model Lumax HF-T also has the added functionality
of BIOTRONIK’s Home Monitoring system. The Home
Monitoring System enables automatic exchange of
information about a patient’s cardiac status from the
implant to the physician remotely.
TM
algorithm.
1-1644
Page 9

Lumax Technical Manual 7
• Lumax DR provides dual chamber rate adaptive
bradycardia pacing support. The ICD uses atrial and
ventricular sensing/pacing leads to provide enhanced
atrial and ventricular tachyarrhythmia discrimination
through BIOTRONIK’s SMART Detection
TM
algorithm.
• Lumax DR-T In addition to the functionality found with
the DR model it also has the added functionality of
BIOTRONIK’s Home Monitoring system. The Home
Monitoring System enables automatic exchange of
information about a patient’s cardiac status from the
implant to the physician remotely.
• Lumax VR provides single chamber rate adaptive
bradycardia pacing support as well as tachyarrhythmia
detection and therapy.
• Lumax VR-T In addition to the functionality found with
standard VR model it also has the added functionality of
BIOTRONIK’s Home Monitoring system. The Home
Monitoring System enables automatic exchange of
information about a patient’s cardiac status from the
implant to the physician remotely.
The 300 and 340 reference for each of the above-described
models denote the maximum programmable shock energy of 30
joules and 40 joules, respectively.
All members of the Lumax device family have two DF-1
defibrillation/ cardioversion ports. In addition, the Lumax HF (-T)
models have three IS-1 pacing/sensing header ports. The
Lumax DR (-T) models have two IS-1 pacing/sensing header
ports. The Lumax VR (-T) models have one IS-1 pacing/sensing
header ports. IS-1 refers to the international standard whereby
leads and generators from different manufacturers are assured a
basic fit [Reference ISO 5841-3:1992]. DF-1 refers to the
international standard for defibrillation lead connectors
[Reference ISO 11318:1993].
1-1645
Page 10

8 Lumax Technical Manual
External devices that interact with and test the implantable
devices are also part of the ICD/CRT-D System. These external
devices include the ICS 3000 Programming and Tachyarrhythmia
Monitoring System and the Implant Module System Analyzer for
acute lead testing. This programmer is used to interrogate and
program the ICD/CRT-D.
1.2 Indications and Usage
The Lumax CRT-Ds are indicated for use in patients with all of the
following conditions:
• Indicated for ICD therapy
• Receiving optimized and stable Congestive Heart
Failure (CHF) drug therapy
• Symptomatic CHF (NYHA Class III/IV and LVEF ≤ 35%);
and
• Intraventricular conduction delay (QRS duration
≥130 ms)
The Lumax Implantable Cardioverter Defibrillators (ICDs) and
Cardiac Resynchronization Therapy Defibrillators (CRT-Ds) are
intended to provide ventricular anti-tachycardia pacing and
ventricular defibrillation, for automated treatment of lifethreatening ventricular arrhythmias.
1-1646
Page 11

Lumax Technical Manual 9
1.3 Contraindications
The Lumax devices are contraindicated for use in patients with
the following conditions:
• Patients whose ventricular tachyarrhythmias may have
transient or reversible causes such as:
• Acute myocardial infarction
• Digitalis intoxication
• Drowning
• Electrocution
• Electrolyte imbalance
• Hypoxia
• Sepsis
• Patients with incessant ventricular fibrillation (VF) and
ventricular tachycardia (VT)
• Patients whose only disorder is bradyarrhythmias or
atrial arrhythmias
1.4 Warnings and Precautions
MRI (Magnetic Resonance Imaging) - Do not expose a patient
to MRI device scanning. Strong magnetic fields may damage the
device and cause injury to the patient.
Electrical Isolation - To prevent inadvertent arrhythmia
induction, electrically isolate the patient during the implant
procedure from potentially hazardous leakage currents.
Left Ventricular Lead Systems – BIOTRONIK CRT-Ds maybe
implanted with any legally marketed, compatible LV lead.
Compatibility is defined as:
• IS-1 pacing connector
• Active or passive fixation technology
• Insertion and withdrawal forces as specified by
ISO 5841-3 (IS-1)
The following LV leads were evaluated in the OPTION CRT/ATx
study with BIOTRONIK’s CRT-Ds:
1-1647
Page 12

10 Lumax Technical Manual
• Guidant-Easytrak IS-1
• Guidant-Easytrak LV-1
• Guidant-Easytrak 2
• Guidant-Easytrak 3
• Medtronic-Attain
• St. Jude-Aescula
• St. Jude-Quicksite
• Biomec-Myopore Epicardial
• Medtronic-Epicardial 5071
• Medtronic-CapSure EPI
• Biotronik-ELC 54-UP
The following LV leads were bench tested for compatibility with
BIOTRONIK’s CRT-Ds:
• Guidant EasyTrak 4512 (unipolar)
• Guidant EasyTrak 4513 (bipolar)
• Guidant EasyTrak 3 4525 (bipolar)
• Medtronic Attain OTW 4193 (unipolar)
• Medtronic Attain OTW 4194 (bipolar)
• Medtronic Attain LV 2187 (unipolar)
• St. Jude Medical QuickSite 1056K (unipolar)
• ELA Situs OTW (unipolar)
• Biotronik Corox OTW 75-UP Steroid #346542 (unipolar)
• Biotronik Corox+ LV-H 75-BP #341885 (bipolar)
ICD Lead Systems – BIOTRONIK ICDs/CRT-Ds maybe
implanted with any legally marketed, compatible ICD lead.
Compatibility is defined as:
• IS-1 pacing and sensing connector(s)
• DF-1 shock coil connector(s)
• Integrated or dedicated bipolar pacing and sensing
configuration
• Active or passive fixation technology
1-1648
Page 13

Lumax Technical Manual 11
• Single or dual defibrillation shock coil (s)
• High energy shock accommodation of at least 30 joules
• Insertion and withdrawal forces as specified by
ISO 5841-3 (IS-1) and ISO 11318:1993 (E) DF-1
The following leads were evaluated in a retrospective study with
BIOTRONIK’s ICDs/CRT-Ds:
• Medtronic Sprint 6932
• Medtronic Sprint 6943
• Medtronic Sprint Quattro 6944
• Medtronic Transvene RV 6936
• St. Jude (Ventritex) TVL- ADX 1559
• St. Jude SPL SP02
• Guidant Endotak DSP
• Guidant Endotak Endurance EZ, Endotak Reliance
• Guidant (Intermedics) 497-24.
The following leads were bench tested for compatibility with
BIOTRONIK’s ICDs/CRT-Ds:
• Guidant Endotak Endurance “CPI 0125”
• Guidant Endotak Reliance 0148
• Medtronic Sprint 6932
• Medtronic Sprint 6942
• Medtronic Sprint 6943
• Medtronic Sprint 6945
• Medtronic Sprint Quattro 6944
• St. Jude Riata 1571/65
• St. Jude SPL SPO1
Resuscitation Availability - Do not perform induction testing
unless an alternate source of patient defibrillation such as an
external defibrillator is readily available. In order to implant the
ICD/CRT-D system, it is necessary to induce and convert the
patient’s ventricular tachyarrhythmias.
1-1649
Page 14

12 Lumax Technical Manual
Unwanted Shocks – Always program Therapy status to OFF
prior to handling the device to prevent the delivery of serious
shocks to the patient or the person handling the device during the
implant procedure.
Rate-Adaptive Pacing – Use rate-adaptive pacing with care in
patients unable to tolerate increased pacing rates.
High Output Settings – High ventricular or biventricular pacing
voltage settings may significantly reduce the life expectancy of
the CRT-Ds. Programming of pulse amplitudes, higher than 4.8V,
in combination with long pulse widths and/or high pacing rates
may lead to early activation of replacement indicators.
1.4.1 Sterilization, Storage, and Handling
Device Packaging - Do not use the device if the device’s
packaging is wet, punctured, opened or damaged because the
integrity of the sterile packaging may be compromised. Return
the device to BIOTRONIK.
Re-sterilization - Do not re-sterilize and re-implant explanted
devices.
Storage (temperature) - Store the device between 5° to 55°C
(41° - 131° F) because temperatures outside this range could
damage the device.
Storage (magnets) - To avoid damage to the device, store the
device in a clean area, away from magnets, kits containing
magnets, and sources of electromagnetic interference (EMI).
Temperature Stabilization - Allow the device to reach room
temperature before programming or implanting the device
because temperature extremes may affect initial device function.
Use Before Date - Do not implant the device after the USE
BEFORE DATE because the device may have reduced longevity.
1.4.2 Device Implantation and Programming
Blind Plug - A blind plug must be inserted and firmly connected
into any unused header port to prevent chronic fluid influx and
possible shunting of high energy therapy.
1-1650
Page 15
Lumax Technical Manual 13
Capacitor Reformation - Infrequent charging of the high voltage
capacitors may extend the charge times of the ICD/CRT-D. The
capacitors are reformed automatically at least every 85 days and
may be reformed manually. For further information, please refer
to Section 2.9.4
Connector Compatibility – ICD/CRT-D and lead system
compatibility should be confirmed prior to the implant procedure.
Consult your BIOTRONIK representative regarding lead/pulse
generator compatibility prior to the implantation of an ICD/CRT-D
system. For further information, please refer to Appendix A
ERI (Elective Replacement Indicator) - Upon reaching ERI, the
battery has sufficient energy remaining to continue monitoring for
at least three months and to deliver a minimum of six 30 joule
shocks. After this period, all tachyarrhythmia detection and
therapy is disabled. Bradycardia functions are still active at
programmed values until the battery voltage drops below
3.0 volts.
Magnets - Positioning of a magnet or the programming wand
over the ICD/CRT-D will suspend tachycardia detection and
treatment. The minimum magnet strength required to suspend
tachycardia treatment is 1.8 mT. When the magnet strength
decreases to less than 1 mT, the reed contact is reopened.
, Capacitor Reforming.
.
Programmed Parameters – Program the device parameters to
appropriate values based on the patient’s specific arrhythmias
and condition.
Programmers - Use only BIOTRONIK ICS 3000 programmers to
communicate with the device.
Sealing System - Failure to properly insert the torque wrench
into the perforation at an angle perpendicular to the connector
receptacle may result in damage to the sealing system and its
self-sealing properties.
Defibrillation Threshold - Be aware that the changes in the
patient’s condition, drug regimen, and other factors may change
the defibrillation threshold (DFT) which may result in nonconversion of the arrhythmia post-operatively. Successful
conversion of ventricular fibrillation or ventricular tachycardia
during arrhythmia conversion testing is no assurance that
conversion will occur post-operatively.
1-1651
Page 16

14 Lumax Technical Manual
Manual Shocks – User-commanded shocks may be withheld if
the ICD/CRT-D is already busy processing a manual command or
the Battery Status is low.
Charge Time - When preparing a high energy shock the charge
circuit stops charging the capacitors after 20 seconds, and
delivers the stored energy as shock therapy. After the device
reaches ERI the stored energy may be less than the maximum
programmable energy for each shock.
Shock Therapy Confirmation – Programming CONFIRMATION
to OFF may increase the incidence of the ICD/CRT-D delivering
inappropriate shocks.
Shock Impedance - If the shock impedance is less than twentyfive ohms, reposition the lead system to allow a greater distance
between the electrodes. Never implant the device with a lead
system that has measured shock impedance as less than twentyfive ohms. Damage to the device may result.
Negative AV Delay Hysteresis – This feature insures ventricular
pacing, a technique which has been used in patients with
hypertrophic obstructive cardiomyopathy (HOCM) with normal AV
conduction in order to replace intrinsic ventricular activation. No
clinical study was conducted to evaluate this feature, and there is
conflicting evidence regarding the potential benefit of ventricular
pacing therapy for HOCM patients. In addition, there is evidence
with other patient groups to suggest that inhibiting the intrinsic
ventricular activation sequence by right ventricular pacing may
impair hemodynamic function and/or survival.
1.4.3 Lead Evaluation and Connection
Capping Leads - If a lead is abandoned rather than removed, it
must be capped to ensure that it is not a pathway for currents to
or from the heart.
Gripping Leads - Do not grip the lead with surgical instruments
or use excessive force or surgical instruments to insert a stylet
into a lead.
Kinking Leads - Do not kink leads. This may cause additional
stress on the leads that can result in damage to the lead.
Liquid Immersion - Do not immerse leads in mineral oil, silicone
oil, or any other liquid.
1-1652
Page 17

Lumax Technical Manual 15
Short Circuit - Ensure that none of the lead electrodes are in
contact (a short circuit) during delivery of shock therapy as this
may cause current to bypass the heart or cause damage to the
ICD/CRT-D system.
Far-field sensing of signals from the atrium in the ventricular
channel or ventricular signals in the atrial channel should be
avoided by appropriate lead placement, programming of
pacing/sensing parameters, and maximum sensitivity settings. If
it is necessary to modify the Far Field Blanking parameter, the
parameter should be lengthened only long enough to eliminate
far-field sensing as evidenced on the IEGMs. Extending the
parameter unnecessarily may cause under sensing of actual atrial
or ventricular events.
Suturing Leads - Do not suture directly over the lead body as
this may cause structural damage. Use the appropriate suture
sleeve to immobilize the lead and protect it against damage from
ligatures.
Tricuspid Valve Bioprosthesis - Use ventricular transvenous
leads with caution in patients with a tricuspid valvular
bioprosthesis.
Setscrew Adjustment – Back-off the setscrew(s) prior to
insertion of lead connector(s) as failure to do so may result in
damage to the lead(s), and/or difficulty connecting lead(s).
Cross Threading Setscrew(s) – To prevent cross threading
the setscrew(s), do not back the setscrew(s) completely out of the
threaded hole. Leave the torque wrench in the slot of the
setscrew(s) while the lead is inserted.
Tightening Setscrew(s) – Do not overtighten the setscrew(s).
Use only the BIOTRONIK supplied torque wrench.
Sealing System – Be sure to properly insert the torque
wrench into the perforation at an angle perpendicular to the
connector receptacle. Failure to do so may result in damage to
the plug and its self-sealing properties.
1-1653
Page 18

16 Lumax Technical Manual
1.4.4 Follow-up Testing
Defibrillation Threshold - Be aware that changes in the patient’s
condition, drug regimen, and other factors may change the
defibrillation threshold (DFT), which may result in non-conversion
of the arrhythmia post-operatively. Successful conversion of
ventricular fibrillation or ventricular tachycardia during arrhythmia
conversion testing is no assurance that conversion will occur
post-operatively.
Resuscitation Availability - Ensure that an external defibrillator
and medical personnel skilled in cardiopulmonary resuscitation
(CPR) are present during post-implant device testing should the
patient require external rescue.
Safe Program – Within the EP Test screen, pressing the “Safe
Program” key on the programmer head does not immediately
send the safe program to the ICD/CRT-D. Pressing the “Safe
Program” key activates the emergency function screen, but an
additional screen touch is required to send the safe program to
the ICD/CRT-D.
1.4.5 Pulse Generator Explant and Disposal
Device Incineration – Never incinerate the ICD/CRT-D due to
the potential for explosion. The ICD/CRT-D must be explanted
prior to cremation.
Explanted Devices – Return all explanted devices to
BIOTRONIK.
Unwanted Shocks – Always program Therapy status to
DISABLED prior to handling the device to prevent the delivery of
serious shocks to the patient or the person handling the device
during the implant procedure.
1.4.6 Hospital and Medical Hazards
Electromagnetic interference (EMI) signals present in hospital and
medical environments may affect the function of any ICD/CRT-D
or pacemaker. The ICD/CRT-D is designed to selectively filter
out EMI noise. However, due to the variety of EMI signals,
absolute protection from EMI is not possible with this or any other
ICD/CRT-D.
1-1654
Page 19

Lumax Technical Manual 17
The ICD/CRT-D system should have detection and therapy
disabled prior to performing any of the following medical
procedures. In addition, the ICD/CRT-D should be checked after
the procedures to assure proper programming:
Diathermy - Diathermy therapy is not recommended for
ICD/CRT-D patients due to possible heating effects of the pulse
generator and at the implant site. If diathermy therapy must be
used, it should not be applied in the immediate vicinity of the
pulse generator or lead system.
Electrocautery - Electrosurgical cautery could induce ventricular
arrhythmias and/or fibrillation, or may cause device malfunction or
damage. If use of electrocautery is necessary, the current path
and ground plate should be kept as far away from the pulse
generator and leads as possible (at least 6 inches (15 cm)).
External Defibrillation - The device is protected against energy
normally encountered from external defibrillation. However, any
implanted device may be damaged by external defibrillation
procedures. In addition, external defibrillation may also result in
permanent myocardial damage at the electrode-tissue interface as
well as temporary or permanent elevated pacing thresholds. When
possible, observe the following precautions:
• Position the adhesive electrodes or defibrillation paddles
of the external defibrillator anterior-posterior or along a
line perpendicular to the axis formed by the implanted
device and the heart.
• Set the energy to a level not higher than is required to
achieve defibrillation.
• Place the paddles as far as possible away from the
implanted device and lead system.
• After delivery of an external defibrillation shock,
interrogate the ICD/CRT-D to confirm device status and
proper function.
Lithotripsy - Lithotripsy may damage the ICD/CRT-D. If
lithotripsy must be used, avoid focusing near the ICD/CRT-D
implant site.
1-1655
Page 20

18 Lumax Technical Manual
MRI (Magnetic Resonance Imaging) - Do not expose a patient
to MRI device scanning. Strong magnetic fields may damage the
device and cause injury to the patient.
Radiation - High radiation sources such as cobalt 60 or gamma
radiation should not be directed at the pulse generator. If a
patient requires radiation therapy in the vicinity of the pulse
generator, place lead shielding over the device to prevent
radiation damage and confirm its function after treatment.
Radio Frequency Ablation - Prior to performing an ablation
procedure, deactivate the ICD/CRT-D during the procedure.
Avoid applying ablation energy near the implanted lead system
whenever possible.
1.4.7 Home and Occupational Hazards
Patients should be directed to avoid devices that generate strong
electromagnetic interference (EMI) or magnetic fields. EMI could
cause device malfunction or damage resulting in non-detection or
delivery of unneeded therapy. Moving away from the source or
turning it off will usually allow the ICD/CRT-D to return to its
normal mode of operation.
The following equipment (and similar devices) may affect normal
ICD/CRT-D operation: electric arc or resistance welders, electric
melting furnaces, radio/television and radar transmitters,
power-generating facilities, high-voltage transmission lines, and
electrical ignition systems (of gasoline-powered devices) if
protective hoods, shrouds, etc., are removed.
1.4.8 Cellular Phones
Testing has indicated there may be a potential interaction
between cellular phones and BIOTRONIK ICD/CRT-D systems.
Potential effects may be due to either the cellular phone signal or
the magnet within the telephone and may include inhibition of
therapy when the telephone is within 6 inches (15 centimeters) of
the ICD/CRT-D, when the ICD/CRT-D is programmed to standard
sensitivity.
1-1656
Page 21

Lumax Technical Manual 19
Patients having an implanted BIOTRONIK ICD/CRT-D who
operate a cellular telephone should:
• Maintain a minimum separation of 6 inches
(15 centimeters) between a hand-held personal cellular
telephone and the implanted device.
• Set the telephone to the lowest available power setting, if
possible.
• Patients should hold the phone to the ear opposite the
side of the implanted device. Patients should not carry
the telephone in a breast pocket or on a belt over or
within 6 inches (15 centimeters) of the implanted device
as some telephones emit signals when they are turned
ON, but not in use (i.e., in the listen or stand-by mode).
Store the telephone in a location opposite the side of
implant.
Based on results to date, adverse effects resulting from
interactions between cellular telephones and implanted
ICDs/CRT-Ds have been transitory. The potential adverse effects
could include inhibition or delivery of additional therapies. If
electromagnetic interference (EMI) emitting from a telephone
does adversely affect an implanted ICD/CRT-D, moving the
telephone away from the immediate vicinity of the ICD/CRT-D
should restore normal operation. A recommendation to address
every specific interaction of EMI with implanted ICDs/CRT-Ds is
not possible due to the disparate nature of EMI.
1.4.9 Electronic Article Surveillance (EAS)
Equipment such as retail theft prevention systems may interact
with pulse generators. Patients should be advised to walk directly
through and not to remain near an EAS system longer than
necessary.
1-1657
Page 22

20 Lumax Technical Manual
1.4.10 Home Appliances
Home appliances normally do not affect ICD/CRT-D operation if
the appliances are in proper working condition and correctly
grounded and shielded. There have been reports of the
interaction of electric tools or other external devices (e.g. electric
drills, older models of microwave ovens, electric razors, etc.) with
ICDs/CRT-Ds when they are placed in close proximity to the
device.
1.4.11 Home Monitoring
Patient’s Ability - Use of the Home Monitoring system requires
the patient and/or caregiver to follow the system instructions and
cooperate fully when transmitting data.
If the patient cannot understand or follow the instructions because
of physical or mental challenges, another adult who can follow the
instructions will be necessary for proper transmission.
Use in Cellular Phone Restricted Areas - The mobile patient
device (transmitter/receiver) should not be utilized in areas where
cellular phones are restricted or prohibited (i.e., commercial
aircraft).
Event-Triggered Report – A timely receipt of the event report
cannot be guaranteed. The receipt is also dependent on whether
the patient was physically situated in the required coverage range
of the patient device at the time the event information was sent.
Not for Diagnosis - The data transmitted by Home Monitoring
are not suitable for diagnosis, because not all information
available in the implant is being transmitted.
Follow-Ups - The use of Home Monitoring does not replace
regular follow-up examinations. Therefore, when using Home
Monitoring, the time period between follow-up visits may not be
extended.
1-1658
Page 23

Lumax Technical Manual 21
1.5 Potential and Observed Effects of the
Device on Health
1.5.1 Potential Adverse Events
The following are possible adverse events that may occur relative
to the implant procedure and chronic implant of the CRT-D:
• Air embolism
• Allergic reactions to
contrast media
• Arrhythmias
• Bleeding
• Body rejection
phenomena
• Cardiac tamponade
• Chronic nerve damage
• Damage to heart valves
• Device migration
• Elevated pacing
thresholds
• Extrusion
• Fluid accumulation
• Hematoma
• Infection
• Keloid formation
• Lead dislodgment
• Lead fracture/ insulation
damage
• Lead-related thrombosis
• Local tissue reaction /
fibrotic tissue formation
• Muscle or nerve
stimulation
• Myocardial damage
• Myopotential sensing
• Pacemaker mediated
tachycardia
• Pneumothorax
• Pocket erosion
• Thromboembolism
• Undersensing of intrinsic
signals
• Venous occlusion
• Venous or cardiac
perforation
1-1659
Page 24

22 Lumax Technical Manual
In addition, patients implanted with the ICD/CRT-D system may
have the following risks. These are the same risks relate with
implantation of any ICD/CRT-D system:
Acceleration of arrhythmias
(speeding up heart
rhythm caused by the
CRT-D)
Dependency
Depression
Fear of premature battery
depletion (fear that
battery will stop working
before predicted time)
Fear of shocking while
awake
Fear that shocking ability
may be lost
There may be other risks associated with this device that are not
currently unforeseeable.
Anxiety about the CRT-D
resulting from frequent
shocks
Imagined shock (phantom
shock)
Inappropriate detection of
ventricular arrhythmias
Inappropriate shocks
Potential death due to inability
to defibrillate or pace
Shunting current or insulating
myocardium during
defibrillation with external
or internal paddles
1.5.2 Observed Adverse Events
Reported Adverse Events are classified as either observations or
complications. Complications are defined as clinical events that
require additional invasive intervention to resolve. Observations
are defined as clinical events that do not require additional
invasive intervention to resolve.
1.5.2.1 Kronos LV-T Study
Note:
The Kronos LV-T CRT-D is an earlier generation of
BIOTRONIK devices. The Lumax CRT-Ds are based upon
the Kronos LV-T and other BIOTRONIK CRT-Ds and ICDs
(i.e., Tupos LV/ATx CRT-D, Lexos and Lumos families of
ICDs).
1-1660
Page 25
Lumax Technical Manual 23
s
The HOME-CARE Observational study, conducted outside the
US on the Kronos LV-T cardiac resynchronization defibrillator
(CRT-D) in patients with congestive heart failure (CHF) involved
45 devices implanted with a cumulative implant duration of
202 months (mean implant duration of 4.5 months).
Of the 31 adverse events reported, there have been
26 observations in 23 patients and 5 complications in 3 patients
with a cumulative implant duration of 202 months (16.8 patientyears). 6.7% of the enrolled patients have experienced a
complication with two patients experiencing 2 separate
complications. The rate of complications per patient-year was
0.30. 51% of the enrolled study patients had a reported
observation with 3 patients having more than 1 observation. The
rate of observations per patient-year is 1.54. Complications and
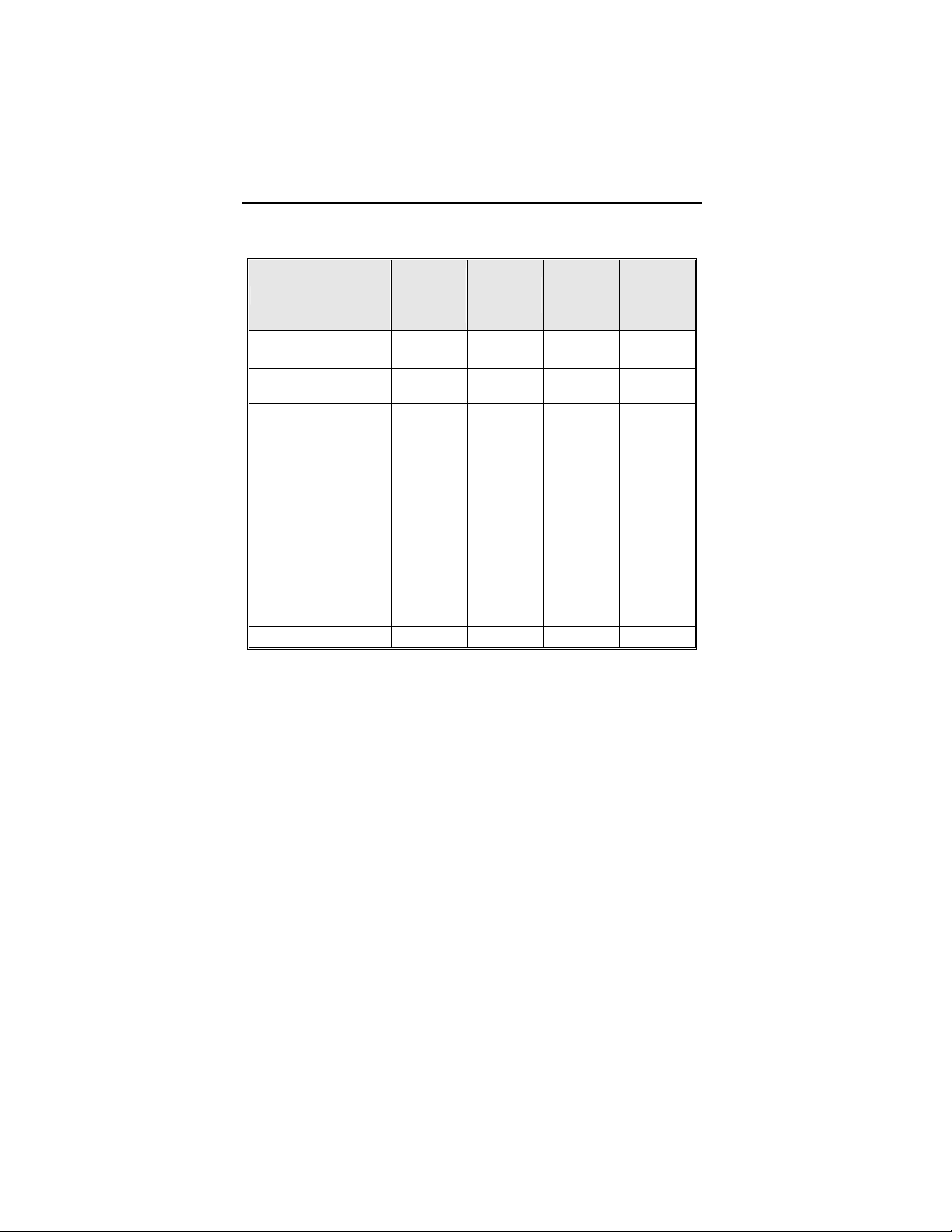
observations for the patient group are summarized in Table 1
Table 2
, respectively.
and
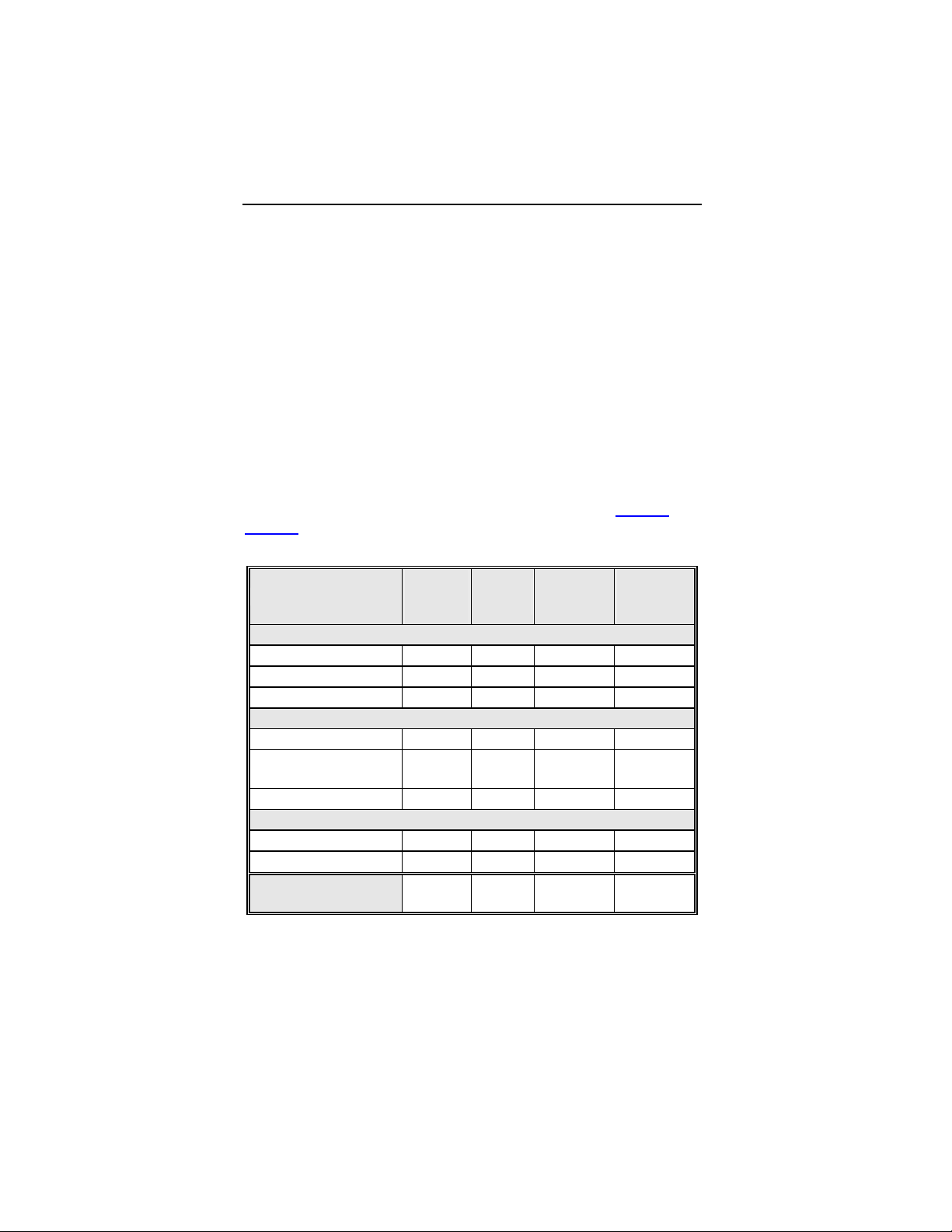
Table 1: Summary of Complications – Kronos LV-T
Category
Number
of
Patients
% of
Patient
Number
Per
patient-
year
Left Ventricular Lead Related
Dislodgement 1 2.2% 1
No Capture 1 2.2% 1
Total 2 4.4% 2
0.06
0.06
0.12
ICD Lead Related
Dislodgement 1 2.2% 1
Elevated Pacing
Threshold
1 2.2% 1
Total 2 4.4% 2
0.06
0.06
0.12
Unrelated to CRT-D or Leads
Hemathorax 1 2.2% 1
Total 1 2.2% 1
Overall
Complication Totals
3 6.7% 5
0.06
0.06
0.30
Number of Patients = 45, Number of Patient-Years = 16.8
1-1661
Page 26
24 Lumax Technical Manual
Table 2: Summary of Observations – Kronos LV-T
Category
Unsuccessful LV
lead implant
Elevated LV pacing
threshold
Phrenic nerve
stimulation
Elevated DFT
measurement
T-wave oversensing
Worsening CHF
Elevated RV pacing
threshold
Hepatitis
Arrhythmias
Cardiac
Decompensation
Number
of
Patients
%of
Patients
Number
8 17.8% 8 0.48
5 11.1% 5 0.30
3 6.7% 3 0.18
2 4.4% 2 0.12
2 4.4% 2 0.12
2 4.4% 2 0.12
1 2.2% 1 0.06
1 2.2% 1 0.06
1 2.2% 1 0.06
1 2.2% 1 0.06
per
patient-
year
All Observations 23 51.1% 26 1.54
Number of Patients = 45, Number of Patient-Years = 16
Two patient deaths were reported during the HOME-CARE
Observational Study. One death resulted from worsening heart
failure and the second death resulted from cardiogenic shock due
to ischemic cardiomyopathy. None of the deaths were related to
the implanted CRT-D system. There were no device explants
during the HOME-CARE Observational Study.
1-1662
Page 27

Lumax Technical Manual 25
1.5.2.2 Tupos LV/ATx Study
NOTE:
The clinical study information included in this section and in
Section 1.6.2
which is an earlier version of the Lumax CRT-D/ICD families.
The clinical study data presented here is applicable because
the Lumax family are downsized versions of the
Tupos LV/ATx CRT-D and Tachos ICD families. The Lumax
family is slightly different as compared to the Tupos LV/ATx
(and Tachos family) in the following areas:
• Reduced size from 50/48 cc to 40/35 cc
• Addition of Home Monitoring functionality
• Addition of triggered pacing for biventricular pacing modes
• True three chamber pacing and sensing capabilities
(CRT-Ds)
The OPTION CRT/ATx study was a prospective, randomized,
multi-center study to demonstrate the safety and effectiveness of
the investigational Tupos LV/ATx Cardiac Resynchronization
Therapy Defibrillator (CRT-D) in patients with congestive heart
failure (CHF) and atrial tachyarrhythmias. All patients enrolled
into the clinical study were randomly assigned to either the study
group or the control group at a 2 to 1 ratio. Patients in the study
group were implanted with the Tupos LV/ATx. Patients in the
control group were implanted with a legally marketed ICD that
provides CRT.
was performed with the Tupos LV/ATx CRT-D,
Of the 278 adverse events reported in the Tupos LV/ATx study
group, there have been 210 observations in 104 patients and 68
complications in 50 patients with a cumulative implant duration of
1240.4 months (101.9 patient-years). 37.6% of the enrolled study
patients have experienced a complication. The rate of
complications per patient-year is 0.67. 78.2% of the enrolled
study patients have a reported observation. The rate of
observations per patient-year is 2.06.
1-1663
Page 28
26 Lumax Technical Manual
s
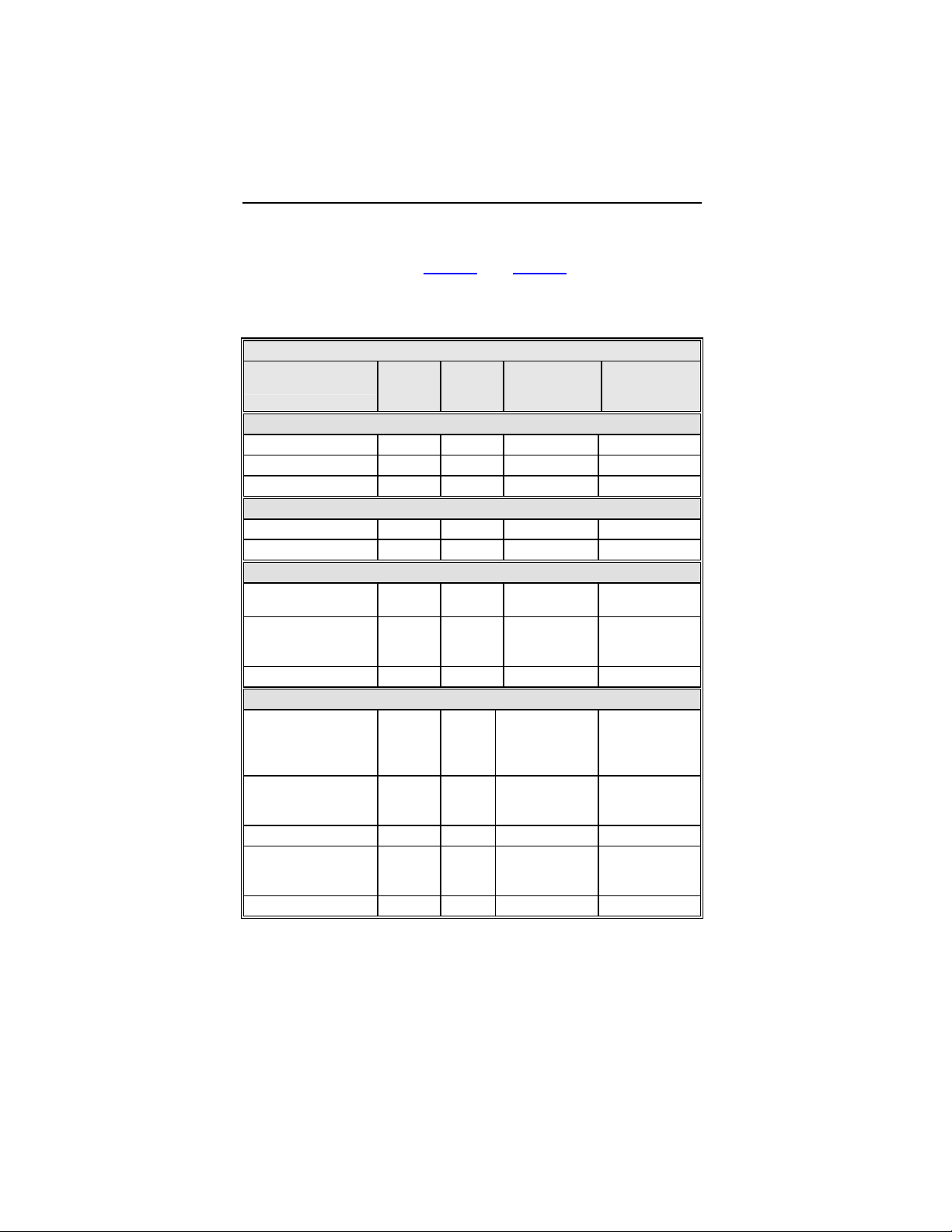
Complications and observations for the Tupos LV/ATx study
group are summarized in Table 3
and Table 4. The total number
of patients may not equal the sum of the number of patients listed
in each category, as an individual patient may have experienced
more than one complication or observation.
Table 3: Summary of Complications – Tupos LV/ATx
Number
Category
Hematoma 4 3.01% 4 0.04
Pneumothorax 2 1.50% 2 0.02
Total 6 4.51% 6 0.06
Dislodgement 3 2.26% 3 0.03
Total 3 2.26% 3 0.03
High threshold/ No
capture
Diaphragmatic/
Intercostal
stimulation (RV)
Total 3 2.26% 3 0.03
High threshold/
Intermittent
biventricular capture/
No capture
Unable to implant
lead via coronary
sinus
Dislodgement 4 3.01% 4 0.04
Diaphragmatic/
Intercostal
stimulation
Total 27 20.30% 29 0.28
of
Patients
Procedure Related
Atrial Lead Related
2 1.50% 2 0.02
1 0.75% 1 0.01
11 8.27% 12 0.12
11 8.27% 11 0.11
1 0.75% 2 0.02
% of
Patients
ICD Lead Related
LV Lead Related
Number of
omplications
Complication
per patient-
year
1-1664
Page 29

Lumax Technical Manual 27
s
Table 3: Summary of Complications – Tupos LV/ATx
Number
Category
Infection 3 2.26% 7 0.07
Device migration 4 3.01% 4 0.04
Elective replacement
indicator reached
Inductions and
conversions
Unable to interrogate
device
Total 12 9.02% 17 0.17
Total Procedure
and Device Related
Non-CHF Cardiac
Symptoms
Ventricular
arrhythmias
Other medical 2 1.50% 2 0.02
Atrial arrhythmia 1 0.75% 1 0.01
Total 9 6.77% 10 0.10
Total – All Patients
and Categories
of
Patients
4 3.01% 4 0.04
1 0.75% 1 0.01
1 0.75% 1 0.01
43 32.33% 58 0.57
Other Medical Related
4 3.01% 4 0.04
2 1.50% 3 0.03
50 37.59% 68 0.67
% of
Patients
Device Related
Number of
omplications
Complication
per patient-
year
Number of Patients = 133, Number of Patient-Years = 101.9
* 1 Unanticipated Adverse Device Effect (UADE) occurred with a
Tupos LV/ATx CRT-D during the OPTION clinical study. The device was
explanted after it was unable to be interrogated with the programmer
software and no pacing output was evident. The analysis showed an
appropriately depleted battery and no anomalies with the IC module. The
battery depletion strongly suggests that the high voltage circuit was
activated over a prolonged period due to a single-bit execution path
failure. The current programmer software with Automatic Battery
Management (ABM) would have prevented the battery from becoming
completely depleted. There were no other instances of this failure
mechanism in Tupos LV/ATx devices.
1-1665
Page 30
28 Lumax Technical Manual
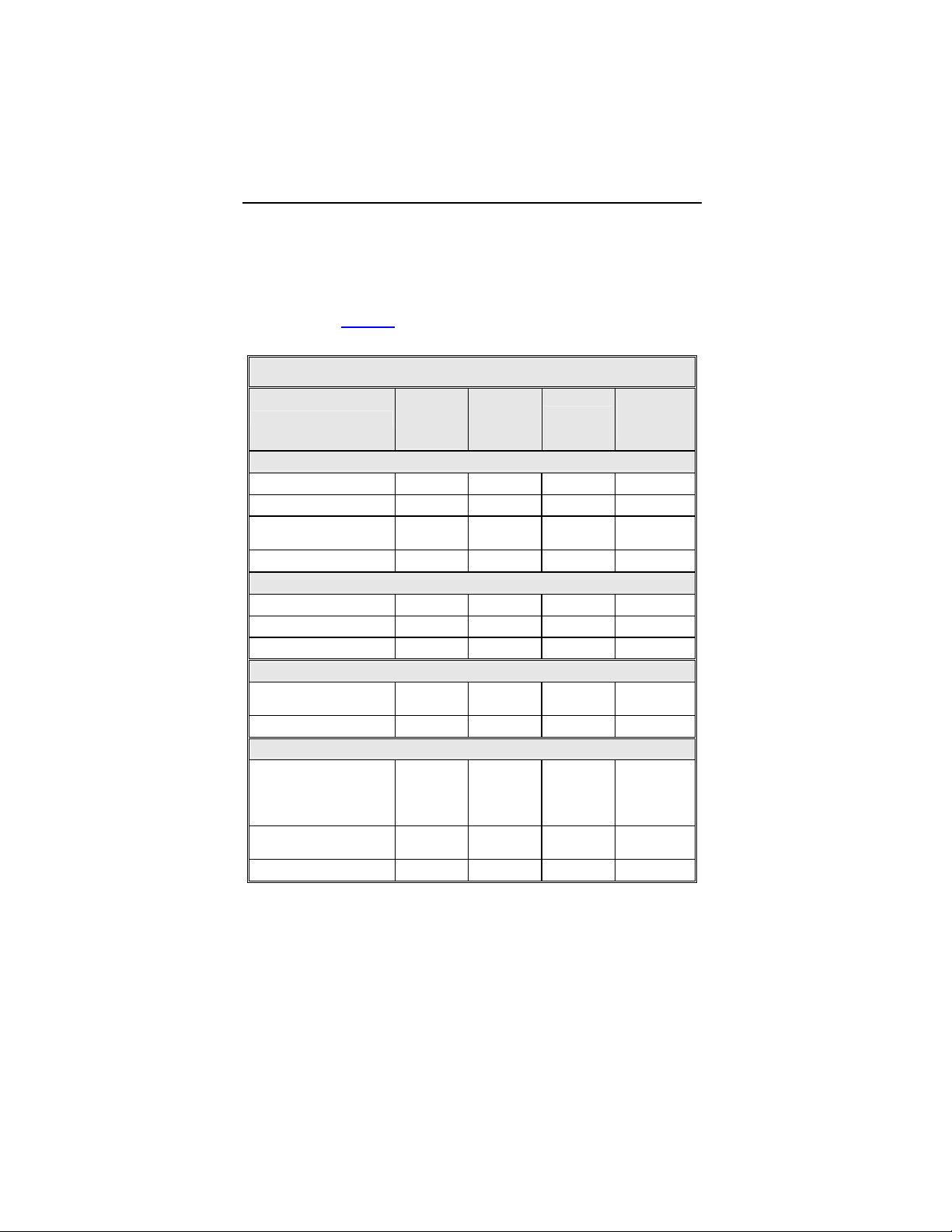
For the Tupos LV/ATx study group, there were 210 observations
in 104 patients with cumulative implant duration of 1240.4 months
(101.9 patient years). 78.2% of the enrolled study patients have a
reported observation. The rate of observations per patient-year
was 2.06. Table 4
summarizes by category each type of
observation for the study group.
Table 4: Summary of Observations – Tupos LV/ATx
Number
Category
Hematoma 10 7.52% 10 0.10
Cardiac arrest 2 1.50% 2 0.02
Unable to implant
system
Total 13 9.77% 13 0.13
Dislodgement 1 0.75% 1 0.01
High threshold 1 0.75% 1 0.01
Total 2 1.50% 2 0.02
High threshold/No
capture
Total 1 0.75% 1 0.01
High threshold/
Intermittent
biventricular capture/
No capture
Diaphragmatic/
Intercostal stimulation
Total 30 22.56% 32 0.31
of
Patients
Procedure Related
1 0.75% 1 0.01
Atrial Lead Related
ICD Lead Related
1 0.75% 1 0.01
LV Lead Related
24 18.05% 24 0.24
8 6.02% 8 0.08
% of
Patients
Number
per patient-
year
1-1666
Page 31

Lumax Technical Manual 29
Table 4: Summary of Observations – Tupos LV/ATx
Number
Category
Infection 1 0.75% 1 0.01
Inductions and
conversions
Inappropriate sensing 20 15.04% 20 0.20
Symptomatic with
biventricular pacing
Total 25 18.80% 29 0.28
Total Procedure,
Lead and Device
Related
Non-CHF Cardiac
Symptoms
Ventricular
arrhythmias
Other medical 26 19.55% 32 0.31
Atrial arrhythmia 14 10.53% 14 0.14
Dizziness 4 3.01% 4 0.04
Medication 5 3.76% 5 0.05
Worsening CHF 46 34.59% 46 0.45
Total 82 61.65% 133 1.31
Total – All Patients
and Categories
Number of Patients = 133 Number of Patient-Years = 101.9
of
Patients
Device Related
6 4.51% 6 0.06
2 1.50% 2 0.02
61 45.86% 77 0.76
Other Medical Related
21 15.79% 21 0.21
11 8.27% 11 0.11
104 78.20% 210 2.06
% of
Patients
Number
per patient-
year
1-1667
Page 32

30 Lumax Technical Manual
There have been 4 patient deaths reported for the control group
(out of 67 total control patients) and 10 patient deaths have been
reported for the study group (out of 133 total study patients).
None of the deaths were related to the implanted CRT-D system.
One patient in the control group died prior to receiving a
biventricular device implant. There is no significant difference
between the number of deaths in the study group versus the
control group (p = 0.777, Fisher's Exact Test, 2 sided). Table 5
provides a summary of reported patient deaths and Table 6
provides survival percentages by follow-up interval during the first
12 months of study participation.
Table 5: Summary of Patient Deaths
Category of
Death
Study
(N = 133)
Control
(N = 67)
Number of Patients Number of Patients
Sudden Cardiac 1 1
Non-Sudden
5 2
Cardiac
Non-Cardiac 4 1
All Causes
10 4
Figure 1 shows the associated Kaplan-Meier survival curves for
the study and control group. The significance level for the
difference between the two study groups based on a Log Rank
test was p = 0.795.
1-1668
Page 33

Lumax Technical Manual 31
Cumulative Survival
1.0
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0.0
Log Rank = 0.795
Control
Study
211815129630
Survival Time (months)
Figure 1: Kaplan-Meier Survival Curves
Table 6 Survival Table
Study Group
(n = 133)
Control Group
(n = 66)
Number % Number %
Enrollment 133 100.00% 67 100.00%
3-month 131 98.50% 63 94.03%
6-month 127 95.49% 63 94.03%
12-month 123 92.48% 63 94.03%
1-1669
Page 34

32 Lumax Technical Manual
1.6 Clinical Studies
The Kronos LV Clinical study (HOME-CARE, Section 1.6.1)
supports the safety of the Lumax CRT-D/ICD device family.
Additionally, because the Tupos LV/ATx and the Lumax CRT-D
devices have identical CRT and ventricular ICD therapy, the
effectiveness results from the OPTION CRT/ATx IDE Clinical
study (Tupos LV/ATx, Section 1.6.2
the Lumax family.
1.6.1 Kronos LV-T Study
The purpose of the HOME-CARE Observational Study is to
demonstrate the safety of the CE-marked Kronos LV-T cardiac
resynchronization defibrillator (CRT-D) in patients with congestive
heart failure (CHF).
1.6.1.1 Methods
The multi-center, non-randomized observational study was
designed to evaluate the safety of the Kronos LV-T through an
analysis of the complication-free rate through three months.
The Home-CARE Observational Study Primary Endpoint was to
evaluate complications (adverse events that require additional
invasive intervention to resolve) related to the implanted CRT
system which includes the Kronos LV-T, the right atrial lead, the
right ventricular ICD lead, and the left ventricular lead
) support the effectiveness of
Inclusion Criteria
To support the objectives of this investigation, patients were
required to meet the following inclusion criteria prior to enrollment:
• Indication for Cardiac Resynchronization Therapy
• Sufficient GSM-network coverage in the patient’s area
• Age greater than or equal to 18 years
1-1670
Page 35

Lumax Technical Manual 33
Exclusion Criteria
To support the objectives of this investigation, the exclusion
criteria at the time of patient enrollment included the following:
• Permanent atrial fibrillation
• Myocardial infarction or unstable angina pectoris within
the last 3 prior to enrollment
• Planned cardio-surgical intervention within 3 months
after enrollment (e.g. PTCA, CABG, HTX)
• Acute myocarditis
• Life expectancy less than 6 months
• Pregnant or breast-feeding woman
• Drug or Alcohol abuse
• The patient is mentally or physically unable to take part
in the observational study
• No signed declaration of consent for the patient
At the enrollment screening, the physician evaluated the patient
to verify that all inclusion/exclusion criteria were met in
accordance to the protocol and the patient signed the informed
consent. After successful enrollment, all patients were implanted
with the Kronos LV-T CRT-D. Evaluations at the One- and Threemonth follow-ups included resting ECG, NYHA classification,
medications, and activation of Home Monitoring.
1.6.1.2 Summary of Clinical Results
The study involved 45 patients (37 males, 82.2%, and 8 females,
17.8%), with a mean age of 64 years (range: 36-79), a left
ventricular ejection fraction of 26 % (range: 15-43), NYHA Class
III (NHYA Class 1 (2.3%), Class II (11.4%), Class III (79.5%),
Class IV (6.8%)) and QRS duration of 154 ms (range: 84-208).
The mean implant duration was 4.5 months with a cumulative
implant duration of 202 months. The patient follow-up compliance
rate was 95.9% out of 221 required follow-ups.
1-1671
Page 36

34 Lumax Technical Manual
Primary Endpoint
The safety of the Kronos LV-T was evaluated based on
complications (adverse events that require additional invasive
intervention to resolve) related to the implanted CRT system
which includes the Kronos LV-T, the right atrial lead, the right
ventricular ICD lead, and the left ventricular lead. 5 complications
were seen in 3 patients with cumulative implant duration of 202
months (16.8 patient-years). 6.7% of the patients had a reported
complication. The rate of complications per patient-year is 0.30.
The freedom from Kronos LV-T system-related complications is
93.3% with a two sided lower 95% confidence bound of 83.8%.
The null hypothesis is rejected, and it is concluded that the
complication-free rate is equivalent to 85% within 10%.
1.6.2 Tupos LV/ATx Study
NOTE:
The clinical study information included in this section was
performed with the Tupos LV/ATx CRT-D, which is an earlier
version of the Lumax CRT-D/ICD families. The clinical study
data presented here is applicable because the Lumax family
are downsized versions of the Tupos LV/ATx CRT-D and
Tachos ICD families. The Lumax family is slightly different as
compared to the Tupos LV/ATx (and Tachos family) in the
following areas:
• Reduced size from 50/48 cc to 40/35 cc
• Addition of Home Monitoring functionality
• Addition of triggered pacing for biventricular pacing modes
• True three chamber pacing and sensing capabilities
(CRT-Ds)
Study Overview
The purpose of the prospective, randomized, multi-center
OPTION CRT/ATx study was to demonstrate the safety and
effectiveness of the investigational Tupos LV/ATx Cardiac
Resynchronization Therapy Defibrillator (CRT-D) in patients with
congestive heart failure (CHF) and atrial tachyarrhythmias.
Patients in the study group were implanted with a BIOTRONIK
Tupos LV/ATx. Patients in the control group were implanted with
1-1672
Page 37

Lumax Technical Manual 35
any legally marketed CRT-D. Patients in both the study and
control groups were implanted with a legally marketed left
ventricular lead.
Methods
Primarily, the study evaluates and compares the functional
benefits of CRT between the two randomized groups using a
composite endpoint consisting of a six-minute walk test (meters
walked) and quality of life measurement (assessed using the
Minnesota Living with Heart Failure Questionnaire). Relevant
measurements were completed twice for each patient: once at the
Baseline evaluation (two-week post implant follow-up) and again
at a six-month follow-up evaluation. The data collected during this
clinical study was used to demonstrate equivalent treatment of
CHF in both the study and control groups. This study also
evaluated other outcomes including: the effectiveness of atrial
therapy to automatically convert atrial tachyarrhythmias, the
percentage of time CRT is delivered, and other measures of CHF
status including NYHA classification, peak oxygen consumption
during metabolic exercise testing, and the rate of hospitalization
for CHF.
1-1673
Page 38

36 Lumax Technical Manual
Inclusion Criteria
To support the objectives of this investigation, patients were
required to meet the following inclusion criteria prior to enrollment:
• Stable, symptomatic CHF status
• NYHA Class III or IV congestive heart failure
• Left ventricular ejection fraction ≤ 35% (measured within
Six-Months prior to enrollment)
• Intraventricular conduction delay (QRS duration greater
than or equal to 130 ms)
• For patients with an existing ICD/CRT-D, optimal and
stable CHF drug regimen including ACE-inhibitors and
beta-blockers unless contraindicated (stable is defined
as changes in dosages less than 50% during the last 30
days)
• Indicated for ICD therapy
• History or significant risk of atrial tachyarrhythmias
• Willing to receive possibly uncomfortable atrial shock therapy
for the treatment of atrial tachyarrhythmias
• Able to understand the nature of the study and give informed
consent
• Ability to tolerate the surgical procedure required for
implantation
• Ability to complete all required testing including the six-minute
walk test and cardiopulmonary exercise testing
• Available for follow-up visits on a regular basis at the
investigational site
• Age greater than or equal to 18 years
1-1674
Page 39

Lumax Technical Manual 37
Exclusion Criteria
To support the objectives of this investigation, the exclusion
criteria at the time of patient enrollment included the following:
• Previously implanted CRT device
• ACC/AHA/NASPE indication for bradycardia pacing (sinus
node dysfunction)
• Six-minute walk test distance greater than 450 meters
• Chronic atrial tachyarrhythmias refractory to cardioversion
shock therapy
• Receiving intermittent, unstable intravenous inotropic drug
therapy (patients on stable doses of positive inotropic
outpatient therapy for at least One-Month are permitted)
• Enrolled in another cardiovascular or pharmacological clinical
investigation
• Expected to receive a heart transplant within 6 months
• Life expectancy less than 6 months
• Presence of another life-threatening, underlying illness
separate from their cardiac disorder
• Acute myocardial infarction, unstable angina or cardiac
revascularization within the last 30 days prior to enrollment
• Conditions that prohibit placement of any of the lead systems
Summary of Clinical Results
A total of 200 patients were enrolled in the OPTION CRT/ATx
clinical study at 25 sites:
There were 133 study patients and 67 active control patients in
this prospective, multi-center, randomized clinical study. For the
study group, there were 129 successful implants (91.4%) of the
Tupos LV/ATx CRT-D system. For the active control group, there
were 64 successful implants (92.2%) of the legally marketed
CRT-D systems.
1-1675
Page 40

38 Lumax Technical Manual
Patient Accountability
After randomization and enrollment, 7 patients (4 in the study
group and 3 in the control group) did not receive an implant. The
reasons for patients not receiving an implant are outlined in
Figure 2
.
Enrolled and Randomized
Patients
Study 133
Control 67
Implant Attempted
Study 130
Control 65
Successful implant
Study 129
Control 64
Patients completed 6-Month
Follow-up
Study 100
Control 49
No implant Attempted
Withdrawal of Consent
Study 2
Control 1
Not Meeting Inclusion Criteria
Study 1
Control 1
Unsuccessful implant
Withdrawal of IC before 2nd Attempt
Study 1
Control 0
Expired before Second Attempt
Study 0
Control 1
6-Month Follow-up Data
Patient Death before 6-Month
Study 7
Control 3
Withdrawal before 6-Month
Study 1
Control 2
Not Reached 6-Month FU
or Data Pending
Study 21
Control 10
Figure 2: Patient Accountability
1-1676
Page 41

Lumax Technical Manual 39
Overall Results
• There were 192 endocardial and 19 epicardial leads
implanted in 193 patients. Investigators were allowed to
choose among any legally marketed LV lead according
to familiarity with the lead and patient anatomy. The
Tupos LV/ATx CRT-D was implanted with 7 endocardial
and 4 epicardial lead models from 6 different
manufacturers. There were no adverse events reported
attributable to lead-generator incompatibility.
• The cumulative implant duration was 1240.4 months
with a mean duration of 9.6 months for the study group.
The cumulative implant duration is 596.5 months with a
mean duration of 9.3 months for the control group.
• For the study group, there have been 278 adverse
events (210 observations in 104 patients and 68
complications in 50 patients). There has been one
unanticipated adverse device effect reported.
• For the control group, there have been 105 adverse
events (81 observations in 44 patients and 24
complications in 19 patients). There have been no
unanticipated adverse device effects reported.
• There have been 10 patient deaths reported in the
study group and 4 patient deaths reported in the control
group. The clinical investigators have determined that
no deaths were related to the study device.
Primary Endpoint 1: Six Minute Walk Test & QOL
(Effectiveness)
The purpose of Primary Endpoint 1 is to evaluate the
effectiveness of the Tupos LV/ATx system in providing CRT as
measured by the average composite rate of improvement in six
minute walk test and QOL.
1-1677
Page 42

40 Lumax Technical Manual
Table 7 presents the average composite rate of improvement in
six minute walk test distance and QOL score, the average 6minute walk test distance and the average QOL score at Baseline
and at the Six-Month follow-up, as well as the average difference
in 6-minute walk test distance and QOL score between Baseline
and the Six-Month follow-up for the Study and Control Groups for
those patients with six minute walk test data and complete QOL
data at both Baseline and the Six-Month follow-up.
1-1678
Page 43

Lumax Technical Manual 41
Table 7: Composite of Six Minute Walk Test and QOL
(Effectiveness)
Category
Distance Walked at
Baseline
Distance Walked at
Six-Months
∆ Distance Walked
QOL Score at
Baseline
QOL Score at Six-
Months
∆ in QOL Score**
Study
Group
(N = 74)
Mean ± SE
310.51 ±
10.89
340.77 ±
12.32
30.26 ±
10.40
17.27% ±
5.59%
44.39 ± 2.78 45.53 ± 4.13 0.817
28.68 ± 2.66 33.95 ± 4.35 0.279
15.72± 2.83
19.08% ±
12.21%
Control
Group
(N = 38)
Mean ± SE
288.76 ±
15.37
301.84 ±
17.02
13.08 ±
13.05
8.71% ±
5.26%
11.58 ± 3.45
-13.42% ±
34.54%
P-value*
0.249
0.067
0.322
0.326
0.376
0.281
Composite Rate***
18.18% ±
7.07%
-2.36% ±
17.73%
0.030
• *The calculated p-values are associated with a Student's t-test
(2-sided) of the equality of means in the two groups, except for
the p-value of the composite rate, which is associated with a
test of equivalence (non-inferiority).
**∆ in QOL Score is calculated as the average of the individual
differences between Baseline and Six-Months for each patient. Negative
values for mean ∆ QOL in percent are possible when positive mean
values for absolute changes in QOL are recorded. In some cases, small,
negative changes in absolute QOL scores resulted in relatively large
percentage changes.
***The Composite Rate (=(∆ Distance Walked (%) + ∆ QOL Score (%)) /
2) is calculated for each patient and then averaged to obtain the
Composite Rates. For all calculations, a positive number represents
improvement from Baseline to Six-Months.
1-1679
Page 44

42 Lumax Technical Manual
Effectiveness Endpoint Analysis and Conclusions
A composite rate of six minute walk test and QOL improvement
from Baseline to the Six-Month follow-up is evaluated as a
measure of CRT effectiveness. For this analysis both six minute
walk test and QOL are equally weighted at 50%.
The mean difference in the composite rate between study and
control group was 20.53% with an associated one-sided, 95%
confidence bound is (-6.10%). The p-value for non-inferiority
within 10% is 0.030. The analysis of the composite rate in six
minute walk test distance and QOL score demonstrates that the
study group is non-inferior to the control group and that the
primary effectiveness endpoint was met (p=0.030).
Primary Endpoint 2: Complication-Free Rate (Safety)
The purpose of Primary Endpoint 2 was to evaluate complications
(adverse events that require additional invasive intervention to
resolve) related to the implanted CRT system which includes the
Tupos LV/ATx, the right atrial lead, the right ventricular ICD lead,
the left ventricular lead, and the implant procedure. The target
complication-free rate at Six-Months is 85%.
Table 8 provides the categorized complication rates at 6-months
for the study and the control group as well as a comparison
between the study and the control group.
1-1680
Page 45

Lumax Technical Manual 43
Table 8: Complications at 6-Month – Study and Control
Study versus Control
Comparison
Delta 95% CI P-value
3.02% [-3.64%,
0.76% [-5.74%,
6.12% [-5.50%,
%
3.78
%
6.94% [-6.46%,
9.21% [-4.96%,
Category
Procedure
Related
Atrial Lead
Related
ICD Lead
Related
LV Lead
Related
Device
Related
Other
Medical
Related
Total
Procedure,
Lead and
Device
Related
Total
Study
N = 133
6 (4.51%) 1
3 (2.26%) 1
3 (2.26%) 0 (0%) 2.26% [-3.03%,
26
(19.55%) 9 (13.43%)
7 (5.26%) 5
9 (6.77%) 2
39
(29.32%)
46
(34.59%)
Control
N = 67
(1.49%)
(1.49%)
(7.46%) -2.20
(2.99%)
15
(22.39%)
17
(25.37%)
8.45%]
5.37%]
6.53%]
16.45%]
[-11.42%,
4.77%]
[-3.82%,
10.13%]
19.17%]
21.99%]
0.428
1.000
0.552
0.329
0.541
0.341
0.317
0.201
Primary Safety Enpoint Analysis and Conclusions
The observed procedure, lead and device related complicationfree rate at 6 months was 70.68%. The 95% confidence interval
for the complication-free rate was [62.16%, 78.25%]. The lower,
one-sided 95% confidence bound for the complication-free rate
was 63.50%. Therefore the procedure, lead and device related
complication-free rate at 6 months did not meet the pre-specified
acceptance criterion for this endpoint.
1-1681
Page 46

44 Lumax Technical Manual
Post-hoc Safety Analysis
BIOTRONIK did not meet the pre-specified objective performance
criteria of 85% within 10% for the safety endpoint. Therefore, a
post-hoc safety analysis was conducted. It was noted that
79.80% (39 out of 49 events) of the complications were right atrial
lead, right ventricular ICD lead, left ventricular lead and procedure
related. The atrial, ICD and LV leads used during this study are
legally marketed devices.
This post-hoc analysis evaluated the LV lead complications that
were “related” or “possibly related” to the Tupos LV/ATx CRT-D,
but excludes the complications that were “not related” to the
Tupos LV/ATx device (see Table 9). There were 11 patients who
had an attempt to implant the LV lead, but the physician was
unsuccessful in either obtaining coronary sinus (CS) access or
unable to find a stable position for the LV lead. Additionally, there
were 4 patients with a documented LV lead dislodgement that has
no direct relationship to the implanted Tupos LV/ATx.
Table 9: Complications at 6-Months (Excluding LV Lead
Related) - Study versus Control
Difference
Study vs
Control
8.36%
11.39%
Category
Procedure Related
Atrial Lead Related
ICD Lead Related
LV Lead Related
Device Related
Other Medical Related
Total Procedure,
Lead and Device
Related
Total
Study
N=133
Control
N=67
6 (4.51%) 2 (2.99%) 1.53%
3 (2.26%) 1 (1.49%) 0.76%
3 (2.26%) 0 (0%) 2.26%
11 (8.27%) 1 (1.49%) 6.78%
7 (5.26%) 5 (7.46%) -2.20%
9 (6.77%) 2 (2.99%) 3.78%
27
(20.30%) 8 (11.94%)
35
(26.32%)
10
(14.93%)
1-1682
Page 47

Lumax Technical Manual 45
The pulse generator related complication rate is higher in the
control group as compared to the study group. The complication
rates for procedure related, atrial lead related, ICD lead related,
LV lead related and other medical related are higher in the study
group as compared to the control group.
Post hoc Safety Analysis Conclusion
There are no clinically substantial differences in the total
complication rate or in the rates for the different complication rate
categories between the study and the control group.
Table 10
compares this post-hoc Safety Endpoint analysis to
previous CRT-D clinical studies:
Table 10 Safety Endpoint Comparisons
CRT-D Study
BIOTRONIK OPTION
(Original Analysis)
BIOTRONIK OPTION
(Post-hoc Analysis)
Estimated
freedom from
Complications
Lower 95%
CI
@ 6mos.
70.68% 63.5% 75%
78.95% 72.29% 75%
95%
lower
bound
criteria
Medtronic Insync ICD 81.1% 77.6% 67%
Guidant Contak CD N/A N/A 70%
St. Jude Medical Epic
HF
93.4% 90.6% 70%
This analysis confirms that the safety profile of the Tupos LV/ATx
is within a similar range determined during trials of other legally
marketed CRT-D devices.
1-1683
Page 48

46 Lumax Technical Manual
Secondary Endpoint Results
1. The purpose of Secondary Endpoint 1 is to evaluate the
overall ability of the Tupos LV/ATx to appropriately convert
spontaneous AT (atrial tachycardia) and AF (atrial fibrillation).
The results from the OPTION study were compared to the
results from BIOTRONIK’s TACT study (P000009/S4, dated
09-09-2002) that demonstrated the effectiveness of these
atrial therapy features in the Tachos DR - Atrial Tx ICD.
Table 11
summarizes success rates for each individual atrial
tachyarrhythmia therapy type and overall success rate from
the OPTION study compared to the TACT study. The
number of episodes and patients receiving any therapy is less
than the total episodes of each therapy type, as episodes
may have included more than one type of therapy.
Table 11 Overall Atrial Conversion Rate
OPTION Study
Patients
Patients Success Episodes
Conversion
rate
ATP 3 3 5 60.0%
HF Burst 17 45 111 40.5%
Shock 12 30 34 88.2%
All
Therapies
25 78 129 60.5%
TACT Study
ATP 29 62 142 43.6 %
HF Burst 49 156 408 38.2 %
Shock 42 84 108 77.8 %
All
Therapies
66 302 542 55.7 %
The overall conversion rate and the conversion rates for each
therapy are comparable to the conversion rates observed in
the TACT study, demonstrating that the Tupos LV/ATx device
has similar atrial conversion capabilities as the legally
marketed Tachos DR – Atrial Tx ICD.
1-1684
Page 49

Lumax Technical Manual 47
2. The purpose of Secondary Endpoint 2 is to evaluate VT
(ventricular tachycardia) and VF (ventricular fibrillation)
detection times of the Tupos LV/ATx. This is a measure of
the ability of the ventricular detection algorithm to detect VT
and VF in an appropriate timeframe. This endpoint was
evaluated based on the review of electrograms following
induced VT/VF episodes. A comparison of data from the
TACT study that utilized the legally marketed Tachos DR –
Atrial Tx ICD (P000009/S4, dated 09-09-2002) to data
collected during the OPTION study for the Tupos LV/ATx was
performed.
Table 12
summarizes and compares the results from these
two clinical studies.
Table 12: Summary of Detection Times
Detection
Time
Individual
Readings
Tachos DR -
Atrial Tx ICD
Mean (SE) / N
2.27 (0.06) / 52
By Patient 2.27 (0.07) / 26
Tupos
LV/ATx Mean
(SE) / N
2.26 (0.06) /
71
2.24 (0.06) /
35
Difference
0.01
0.03
The analysis demonstrates that the average detection times
of the Tupos LV/ATx are comparable to the detection times
observed with the legally marketed Tachos DR - Atrial Tx
ICD. Both devices utilize identical ventricular detection
algorithms and only sense with the right ventricular lead. This
clinical data demonstrates that the ventricular detection times
are similar in both devices.
3. The purpose of Secondary Endpoint 3 is to evaluate the
percentage of ventricular pacing (thus, CRT) as
demonstrated by the device diagnostics at required followups. This data was based on diagnostic data stored by the
Tupos LV/ATx.
Table 13
summarizes the percentage of ventricular pacing
between follow-ups as shown by device diagnostics for
patients in the study group.
1-1685
Page 50

48 Lumax Technical Manual
Table 13: Percentage of Ventricular Pacing – 3-Month and
6-Month Follow-ups
Percentage of
Ventricular
Pacing
3-Months
Patients
(percentage)
6-Months Patients
(percentage)
<80% 9 (7.4%) 4 (4.0%)
81 – 85 % 4 (3.3%) 2 (2.0%)
86 – 90 % 13 (10.7%) 9 (9.1%)
91 – 95 % 19 (15.7%) 20 (20.2%)
96 – 100 % 76 (62.8%) 64 (64.7%)
Totals 121 (100%) 99 (100%)
The majority of the follow-ups (84.9%) show a percentage of
ventricular pacing of 91% or more at Six-Months.
4. The purpose of secondary endpoint 4 is to evaluate
improvement in functional capacity as measured by the six
minute walk test. The six minute walk test is a well-accepted
measure of functional capacity and exercise tolerance. Also,
this test more closely mimics the patient’s day-to-day
activities than maximal exercise testing.
Table 14
summarizes the six minute walk test distance at
Baseline and the Six-Month follow-up for patients in the study
group and the control group.
Table 14: Six Minute Walk Distance
Distance
(meters)
Baseline
N
Mean ± SE
Range
Median
Six-Month
N
Mean ± SE
Range
Median
* Student's t-test, 2-sided
Study Control
127
283.14 ± 9.27
269.43 ± 13.77
23 to 511
302.00
93
329.73 ± 10.82
310.70 ± 15.49
78 to 596
335.00
61
29 to 507
244.00
44
91 to 489
313.00
1-1686
Page 51

Lumax Technical Manual 49
There are no clinically relevant differences in the six minute
walk test results between the study and the control group.
5. The purpose of Secondary Endpoint 5 is to evaluate the
improvement in the patient’s NYHA classification. Table 15
summarizes the average improvement in NYHA from
Baseline to Six-Months for 140 patients that were able to
complete both NYHA classification evaluations.
Table 15: Improvement in NYHA Classification at Six-Months
from Baseline
NYHA Change During OPTION Study
Change in NYHA
Class
Improved 2 classes
Study Patients
(N=97)
(percentage)
Control Patients
(N=43)
(percentage)
10 (10.3%) 2 (4.7%)
Improved 1 class
Total improved
No change
Worsened 1 class
47 (48.5%) 20 (46.5%)
57 (58.8%) 23 (51.2%)
39 (40.2%) 20 (46.5%)
1 (1.0%) 1 (2.3%)
The study and the control group have similar NYHA classes
and similar rates of improvement in NYHA class from
Baseline to the Six-Month follow-up.
1-1687
Page 52

50 Lumax Technical Manual
6. The purpose of Secondary Endpoint 6 is to evaluate the rate
of hospitalization, for CHF and for all other causes. The
occurrence rate and reasons for hospitalization of the study
group were compared to the control group. To be consistent
with other large-scale clinical trials, clinical sites were
instructed to report hospitalizations for CHF using the
following definitions: 1) hospitalization for heart failure
management, 2) outpatient visit in which IV inotropes or
vasoactive infusion are administered continuously for at least
4 hours, or 3) emergency room (ER) visit of at least 12 hours
duration in which intravenous heart failure medications
including diuretics are administered.
Table 16
visits for enrolled patients.
summarizes hospitalization, ER visits and outpatient
1-1688
Page 53

Lumax Technical Manual 51
Table 16: Hospitalization, ER Visits and Outpatient Visits
Medical Visits
Hospital
Admissions
Patients
Hospitalizations
Patients
Hospitalizations
Emergency
Room Visits
Patients
Patients
Outpatient Visits
Patients
Patients
A large percentage of All Cause hospitalizations can be
attributed to pacing lead revisions, device infections, or other
device-related interventions (e.g., pocket revision or device
replacements for ERI or device recall). The CHF
hospitalization rate for both the study and control groups is
clinically acceptable considering the enrollment CHF status of
the patients.
CHF Related:
CHF Related:
Visits
Visits
CHF Related:
Visits
Visits
Study
(N=128)
20 (15.6%)
28
All causes:
68 (53.1%)
76
1 (0.8%)
1
All causes:
13 (10.1%)
16
1 (0.8%)
1
All causes:
5 (3.9%)
5
Control
(N=65)
CHF Related:
5 (7.7%)
9
All causes:
29 (44.6%)
46
CHF Related:
0 (0.0%)
0
All causes:
2 (3.1%)
2
CHF Related:
0 (0.0%)
0
All causes:
2 (3.1%)
2
1-1689
Page 54

52 Lumax Technical Manual
7. The purpose of Secondary Endpoint 7 is to evaluate the
observation rate. Observations are defined as clinical events
that do not require additional invasive intervention to resolve.
For the study group, there were 210 observations in 104
patients with cumulative implant duration of 1240.4 months
(101.9 patient years). 78.2% of the enrolled study patients
have a reported observation. The rate of observations per
patient-year is 2.06. For the control group, there were 81
observations in 44 patients with cumulative implant duration
of 596.5 months (49.0 patient years). 65.7% of the enrolled
control patients had a reported observation. The rate of
observations per patient-year was 1.65.
8. The purpose of Secondary Endpoint 8 is to evaluate peak
VO2 as a measure of effectiveness of the Tupos LV/ATx
system in providing CRT. The core lab was blinded to study
randomization assignments during evaluation of the results of
the cardiopulmonary exercise (CPX) testing in order to
minimize the potential for bias. According to the protocol, to
be included in the analysis, patients were required to attain a
respiratory exchange ratio (RER) of ≥ 1.
Table 17
patients with CPX testing completed at Baseline and the SixMonth follow-up and with an RER of ≥ 1.
provides a summary of peak VO2 results for 42
1-1690
Page 55

Lumax Technical Manual 53
Table 17: Peak VO2 Testing Results – Patients with RER ≥ 1
Results Study Control
Peak VO2
(ml/kg/min)
N=32
Baseline:
Mean:
13.46 ± 0.57
Range:
6.9 to 21.1
Six-Month:
Mean:
13.39 ± 0.53
Range:
7.6 to 20.70
Difference:
Mean:
-0.06 ± 0.42
Range:
-7.9 to 4.9
N=10
Baseline:
Mean:
12.58 ± 0.75
Range:
8.0 to 14.8
Six-Month:
Mean:
12.89 ± 0.94
Range:
7.0 to 17.2
Difference:
Mean:
0.31 ± 0.67
Range:
-2.7 to 4.6
1.6.2.1 Multi-site Poolability and Gender Analysis
The OPTION CRT/ATx clinical report includes data from multiple
centers with centralized coordination, data processing, and
reporting at BIOTRONIK. All of the clinical centers followed the
requirements of an identical clinical protocol, and all of the clinical
centers used the same methods to collect and report the clinical
data. In order to justify pooling of the data from multiple centers,
several analyses were completed. All of the centers were divided
into two groups based on implant volume. Comparisons were
then made between the patient populations based on the results
of each of the endpoints. Additionally, analyses were performed
on the data collected in the OPTION CRT/ATx clinical
investigation in order to compare results between males and
females. The first type of analysis compared enrollment by patient
gender in each of the study and control groups. The second type
of analysis compared effectiveness outcomes in each gender.
1-1691
Page 56

54 Lumax Technical Manual
The results of these analyses demonstrate poolability of the data
between sites. There were no significant differences in the
second primary endpoint or any of the secondary endpoints
between high and low volume implant centers.
The gender distribution in this clinical investigation is consistent
within the study groups and includes a representative proportion
of female participants. There were no significant differences in
any of the primary or secondary endpoints between the male and
female population.
1.6.2.2 Conclusions
The IDE Clinical study (OPTION LV/ATx) demonstrated that the
safety and effectiveness of the Tupos LV/ATx CRT-D device is
equivalent to that of similar legally marketed CRT-D devices.
Although the study missed its primary safety endpoint, additional
post hoc analyses were conducted to reassure that the safety
profile of the device is comparable to other legally marketed
CRT-D devices.
1.7 Patient Selection and Treatment
1.7.1 Individualization of Treatment
• Determine whether the expected device benefits
outweigh the possibility of early device replacement for
patients whose ventricular tachyarrhythmias require
frequent shocks.
• Determine if the device and programmable options are
appropriate for patients with drug-resistant
supraventricular tachyarrhythmias (SVTs), because drugresistant SVTs can initiate unwanted device therapy.
• Direct any questions regarding individualization of patient
therapy to your BIOTRONIK representative or
BIOTRONIK technical services at 1-800-547-0394.
1-1692
Page 57

Lumax Technical Manual 55
The prospective patient’s size and activity level should be
evaluated to determine whether a pectoral or abdominal implant
is suitable. It is strongly recommended that candidates for an
ICD/CRT-D have a complete cardiac evaluation including EP
testing prior to device implant to gather electrophysiologic
information, including the rates and classifications of all the
patient’s cardiac rhythms. When gathering this information,
delineate all clinically significant ventricular and atrial arrhythmias,
whether they occur spontaneously or during EP testing.
If the patient’s condition permits, use exercise stress testing to do
the following:
• Determine the maximum rate of the patient’s normal
rhythm.
• Identify any supraventricular tachyarrhythmias.
• Identify exercise-induced tachyarrhythmias.
The maximum exercise rate or the presence of supraventricular
tachyarrhythmias may influence selection of programmable
parameters. Holter monitoring or other extended ECG monitoring
also may be helpful.
If the patient is being treated with antiarrhythmic or cardiac drugs,
the patient should be on a maintenance drug dose rather than a
loading dose at the time of pulse generator implantation. If
changes to drug therapy are made, repeated arrhythmia
inductions are recommended to verify pulse generator detection
and conversion. The pulse generator also may need to be
reprogrammed.
Changes in a patient’s antiarrhythmic drug or any other
medication that affect the patient’s normal cardiac rate or
conduction can affect the rate of tachyarrhythmias and/or efficacy
of therapy.
If another cardiac surgical procedure is performed prior to
implanting the pulse generator, it may be preferable to implant the
lead system at that time. This may prevent the need for an
additional thoracic operation.
1-1693
Page 58

56 Lumax Technical Manual
1.7.2 Specific Patient Populations
Pregnancy - If there is a need to image the device, care should
be taken to minimize radiation exposure to the fetus and the
mother.
Nursing Mothers - Although appropriate biocompatibility testing
has been conducted for this implant device, there has been no
quantitative assessment of the presence of leachables in breast
milk.
Geriatric Patients - Most (about 71%) of the patients receiving a
CRT-D or ICD in the clinical studies detailed in this manual were
over the age of 60 years (see Clinical Studies).
Handicapped and Disabled Patients - Special care is needed in
using this device for patients using an electrical wheel chair or
other electrical (external or implanted) devices.
1.8 Patient Counseling Information
The pulse generator is subject to random component failure.
Such failure could cause inappropriate shocks, induction of
arrhythmias or inability to sense arrhythmias, and could lead to
the patient’s death.
Persons administering CPR may experience the presence of
voltage on the patient’s body surface (tingling) when the patient’s
CRT-D/ICD system delivers a shock.
A patient manual is available for the patient, patient’s relatives,
and other interested people. Discuss the information in the
manual with concerned individuals both before and after pulse
generator implantation so they are fully familiar with operation of
the device. (For additional copies of the patient manual, contact
the BIOTRONIK at the address listed in this manual.)
1-1694
Page 59

Lumax Technical Manual 57
1.9 Evaluating Prospective CRT-D/ICD
Patients
The prospective ICD/CRT-D implant candidate should undergo a
cardiac evaluation to classify any and all tachyarrhythmias. In
addition, other patient specific cardiac information will help in
selecting the optimal device settings. This evaluation may
include, but is not limited to:
• an evaluation of the specific tachycardia rate(s)
• the confirmation and/or evaluation of any supraventricular
arrhythmias or bradyarrhythmias
• the evaluation of various ATP and cardioversion
therapies
• the presence of any post-shock arrhythmias, and
• an evaluation of the maximum sinus rate during exercise
If a patient’s drug regimen is changed or adjusted while the
CRT-D/ICD is implanted, additional EP testing may be required to
determine if detection or therapy parameter settings are relevant
and appropriate.
Empirical changes to the detection or therapy parameters should
be assessed based on patient safety. Some changes may
necessitate a re-assessment of sensing, pacing, or arrhythmia
conversion treatment. Thorough technical knowledge of
BIOTRONIK CRT-D/ICDs, additional CRT-D/ICD experience, and
individual medical judgment will aid in determining the need for
additional testing and follow-up.
1-1695
Page 60

58 Lumax Technical Manual
2. Device Features
The Lumax family feature set is presented under the following
sub-headings: Tachyarrhythmia Detection, Tachyarrhythmia
Redetection / Acceleration, Tachyarrhythmia Therapy,
Tachyarrhythmia Termination, Bradycardia Therapy, EP Test
Functions and Special Features. The features apply to all
members of the Lumax family except where specifically
referenced differently.
CAUTION
Programmed Parameters – Program the device
parameters to appropriate values based on the patient’s
specific arrhythmias and condition.
2.1 Cardiac Resynchronization Therapy
(CRT)
HF versions only.
For Cardiac Resynchronization Therapy (CRT), a sensing/pacing
lead is placed in the right atrium, while an ICD lead is placed in
the right ventricle. The third lead is positioned to pace the left
ventricle. When connected together, this system provides
coordinated, simultaneous stimulation of the right and left
ventricles. This resynchronization therapy is designed to
coordinate the contraction of both ventricles, which allows the
heart to contract more efficiently. As a result, patients with CHF
and intraventricular conduction delay may have a greater ability to
complete physical activities thus improving their quality of life.
As a result of the device design and header configuration,
ventricular pacing pulses can be delivered between the right / left
ventricular lead tip electrodes simultaneously (cathode) and the
ring of the right ventricular lead (anode). Ventricular sensing
primarily uses the poles of the right ventricular lead tip and ring.
This design avoids sensing of ventricular activity twice during a
single cardiac cycle (double counting) in patients with a wide QRS
complex. However, for diagnostic purposes the Lumax HF
devices can be programmed to sense in the left ventricle.
1-1696
Page 61

Lumax Technical Manual 59
Atrial Channel
The Lumax ICDs/CRT-Ds pace and sense in bipolar
configuration, between the atrial lead’s tip and ring electrodes. A
bipolar atrial lead must be used to ensure reliable sensing of atrial
activity.
Ventricular Channel
The Lumax HF devices can be programmed to pace in both the
right and left ventricle (as well as RV only). The Lumax HF
primarily senses in a bipolar configuration in the right ventricle.
However, for diagnostic purposes the Lumax HF devices can be
programmed to sense in the left ventricle.
Potential Ventricular lead configurations are provided in Table 18
Table 18. Lead Configurations
Configuration Explanation
Sensing
Pacing
Pacing
RV Only
LV Only
RV & LV
Together (BiV)
RV Only
Sensing takes place between
the tip and ring electrodes of
the right ventricular lead.
Sensing takes place between
the tip and ring electrodes
(bipolar) or the tip electrode of
the left ventricular lead and the
CRT-D housing.
Pacing configuration is
programmable between the tip
and ring electrodes of the right
and left ventricular leads.
See Figure 3
Pacing can be programmed to
occur in several combinations
between the tip and ring
electrodes of the right and left
ventricular leads.
.
1-1697
Page 62

60 Lumax Technical Manual
Figure 3 Programmable BiV Pacing Configurations
For CRT to be effective, ventricular pacing must occur.
Therefore, AV delays must be programmed short enough to
override intrinsic ventricular contractions. Additional information
to further optimize AV delays can be obtained with
Echocardiographs.
CRT can be programmed ON or OFF via the programmer using
the [Ventricular Pacing Config.] parameter. Ventricular Pacing
Configuration allows either standard right ventricular [RV] pacing
or Cardiac Resynchronization Therapy [BiV].
The Lumax HF CRT-D can provide triggered biventricular pacing.
This is a functional expansion of the basic ventricular modes
(DDD(R); DDI(R); VDI(R); VDD(R); VVI(R)) used for biventricular
pacing. The “triggering function” was designed to ensure
biventricular pacing therapy is delivered during rapidly conducted
atrial arrhythmias, such as atrial fibrillation. This function triggers
pacing delivery (Vp) in the ventricles after intrinsic sensing in the
right ventricle. The trigger function is only available in the
biventricular pacing configuration and includes a forced
ventricular pace (Vp) after previous sensing (right ventricular
sense event). Triggered pacing can be programmed to react to
only normal RV sensed events or to right ventricular extrasystoles
as well as normal RV sensed events. The maximum Trigger rate
is limited by the programmed UTR.
1-1698
Page 63

Lumax Technical Manual 61
2.2 Sensing (Automatic Sensitivity Control)
The Lumax ICDs/CRT-Ds use Automatic Sensitivity Control
(ASC) to adjust the input stage sensitivity threshold for each
channel to appropriately detect the various cardiac signals. The
characteristics of the sensing circuitry have been optimized to
ensure appropriate sensing during all potential cardiac rhythms.
Cardiac signals vary in amplitude; therefore detection thresholds
cannot be static. With the Automatic Sensitivity Control (ASC)
every sensed event is measured, and the upper and lower
thresholds are re-set accordingly (also known as beat-by-beat
adaptation). The ASC begins by tracking the cardiac signals (R
and P-waves) during the sensed refractory periods. The peak
values measured during this time are used to set the sensing
thresholds during the active detection periods.
2.2.1 Right Ventricular Sensitivity Settings
There are three programmable preset options for setting the
sensitivity of the right ventricular input stage. The sensitivity
selections are designed to adapt the parameters of the input
stage to various signal conditions. The predefined parameter
sets are described in Table 19
.
1-1699
Page 64

62 Lumax Technical Manual
Table 19: Sensitivity Settings
Setting Definition for Use
Standard This setting is recommended for most
patients, especially for those with
measured R-wave amplitude of ≥3 mV.
Enhanced
T Wave
Suppression
This setting offers suppression of T-wave
oversensing. This mode should not to be
used on patients with the following
conditions:
• Sinus rhythms with small signal
amplitudes, R-waves <4 mV
• VF with highly fluctuating signal
amplitudes.
Enhanced
VF Sensitivity
This setting enhances VF detection, in
cases of highly fluctuating signal
amplitudes. It is not to be used for patients
that have sinus rhythms containing large
amplitude T-waves.
Individual This parameter configuration is only
accessible by a special code in the US.
Typically, the upper threshold is reset with each sensed R-wave,
but in order to ensure that pacing does not occur during an
episode of VF, the ASC behaves differently with paced events.
Each paced event is followed by a paced refractory period after
which the ventricular threshold is set to the minimum programmed
value.
For example, the upper threshold is set at 50% of the measured
R-wave for the Standard sensitivity setting following the 100 ms
sensed refractory period. The upper threshold decays 0.125 mV
every 250 ms through the T-wave discrimination period (360 ms).
After the T-wave discrimination period, the threshold is decreased
to the lower threshold. The lower threshold is set to 25% of the
measured peak R-wave. The lower threshold then decreases
0.125 mV every 500 ms until the Minimum Threshold is reach or
until the next sensed (or paced) event.
1-1700
Page 65

Lumax Technical Manual 63
Figure 4 Automatic Sensitivity Control with Standard Stetting
Figure 4
with the sensitivity programmed to Standard. The tracked R –
wave is measured to be 6.0 mV, following the sensed refractory
period, the upper threshold is set to 3.0 mV. After the T-wave
discrimination period, the threshold is further reduced to 1.5 mV.
Both the Upper and Lower Thresholds decay over time, but the
Minimum Threshold is never violated. Nominally, the minimum
threshold is set to 0.8 mV, but it can be adjusted by the user.
The Enhanced VF Sensitivity setting is specifically designed to
improve VF detection when the VF signal is very small. Two
adjustments are made to ASC with this setting:
These adjustments ensure that the threshold reaches the lower
values more quickly in order to assure that all VF signals are
sensed appropriately.
provides an illustration of Automatic Sensitivity Control
• The T-wave discrimination period is decreased to
100 ms, thus eliminating the Upper Threshold.
• The decay rate of the Lower Threshold is increased to
0.125 mV every 250 ms.
1-1701
Page 66

64 Lumax Technical Manual
The Enhanced T-Wave Suppression setting is specifically
designed to avoid double counting of each QRS-T complexes
during normal sinus rhythms. With sensitivity programmed to
Enhanced T-Wave Suppression:
• High pass filtering is increased to reduce low frequency
signal components such as T-waves and respiratory
artifacts.
• The Upper Threshold is increased to 75% of the
measured R-wave.
• The Upper Threshold may not retrigger with each sensed
event, it is only triggered when the new sensed R-wave
crosses the 50% point of the previous measured R-wave.
2.2.2 Minimum Right Ventricular Threshold
This parameter limits the minimum sensitivity of the ICD/CRT-D to
a programmable value. Nominally, the minimum threshold is set
to 0.8 mV, but it can be adjusted from 0.5 to 2.5 mV.
2.2.3 Atrial Sensitivity Settings
DR and HF versions only.
The primary option for setting the sensitivity of the atrial input
stage is “Standard”. When atrial sensing is active, the sensitivity
is set to “Standard” for most patients, which is designed to adapt
the parameters of the input stage to various signal conditions.
The available settings are described in Table 20
Table 20: Atrial Sensitivity Settings
Setting Definition for Use
Standard This setting is recommended for all
patients with a functioning atrial lead.
Inactive This setting deactivates the atrial channel
for sensing, EGM telemetry and Holter
recording and is typically used when no
atrial lead is implanted.
Individual This parameter configuration is only
accessible by a special code in the US.
.
1-1702
Page 67

Lumax Technical Manual 65
Typically, the upper threshold is reset with each sensed P-wave,
but in order to ensure that pacing does not occur during an
episode of AF/VF, the ASC behaves differently with paced
events. Each paced event is followed by a paced refractory
period after which the atrial threshold is set to the minimum
programmed value.
2.2.4 Minimum Atrial Threshold
This parameter limits the minimum sensitivity of the ICD/CRT-D to
a programmable value. Nominally, the minimum threshold is set
to 0.4 mV, but it can be adjusted from 0.2 to 2.0 mV.
2.2.5 Left Ventricular Sensitivity Settings
HF versions only.
The primary option for setting the sensitivity of the left ventricular
input stage is “Standard”. When atrial sensing is active, the
sensitivity is set to “Standard” for most patients, which is designed
to adapt the parameters of the input stage to various signal
conditions. The available settings are described in Table 20
Table 21: Atrial Sensitivity Settings
Setting Definition for Use
Standard This setting is recommended for all
patients with a functioning left ventricular
lead.
Inactive This setting deactivates the left ventricular
channel for sensing, EGM telemetry and
Holter recording and is typically used when
no LV lead is implanted.
Individual This parameter configuration is only
accessible by a special code in the US.
.
2.2.6 Minimum Left Ventricular Threshold
This parameter limits the minimum sensitivity of the CRT-D to a
programmable value. Nominally, the minimum threshold is set to
0.8 mV, but it can be adjusted from 0.5 to 2.5 mV.
2.2.7 Far Field Protection
DR and HF versions only.
1-1703
Page 68

66 Lumax Technical Manual
This parameter blanks the atrial channel of the ICD/CRT-D to the
period before and after each ventricular event. This blanking
period is programmable separately based on whether the
ventricular event is a paced or sensed event and is designed to
prevent sensing of ventricular signals with the atrial leads.
CAUTION
Far-field sensing of signals from the atrium in the
ventricular channel or ventricular signals in the atrial channel
should be avoided by appropriate lead placement,
programming of pacing/sensing parameters, and maximum
sensitivity settings. If it is necessary to modify the Far Field
Blanking parameter, the parameter should be lengthened
only long enough to eliminate far-field sensing as evidenced
on the IEGMs. Extending the parameter unnecessarily may
cause undersensing of actual atrial or ventricular events.
2.2.8 Additional Sensing Parameters
The parameters of the Additional Sensing Parameters menu are
to provide additional flexibility for physicians to non-invasively
correct over/undersensing situations. The ranges and nominal
values are located in table titled Additional Sensing Parameters in
Section 6
.
Upper Threshold (A, RV & LV)- This feature allows the user to
change the upper sensing threshold level (UT in Figure 4
the nominal value of 50% of the sensed R-wave / P-wave
amplitude to either 75% or 87.5% of the R-wave / P-wave value.
This feature is used to eliminate oversensing of large T-waves.
Hold of Upper Threshold (RV & LV) - This parameter
determines when the sensing decrement begins after an event
(small step-down on the 50% threshold before LT in the figure
above). This parameter “holds” the threshold at a constant value
(UT in Figure 4
is programmable from 10 to 600 ms.
) for the programmed time. Maximum Hold Time
) from
1-1704
Page 69

Lumax Technical Manual 67
Lower Threshold (A, RV & LV)- This feature allows the user to
change the lower sensing threshold (labeled LT in Figure 4
the default value of 25% of the sensed R-wave / P-wave
amplitude to either 12.5 or 50% of the measured R-wave/ P-wave
value. This feature is also used to alleviate T-wave oversensing
and/or undersensing of small amplitude events (e.g., fine VF).
Blank after atrial pacing (RV) .- This feature is used to eliminate
sensing of artifacts after atrial paced events. Blank Post Pace is
programmable from 50 to 100 ms. For the left ventricle, this
parameter is equal to the safety window time (100 ms). DR and
HF versions only.
VES Discrimination after As .- This feature is used to correctly
identify and classify ventricular extrasystoles (VES). With each
atrial sensed event a special timing interval is started for the
ventricle, if the subsequent ventricular event does not fall within
the AV delay or the programmed VES discrimination interval, it is
classified as a VES. DR and HF versions only.
LV T-wave Protection - Used to eliminate to avoid unintended
pacing in the vulnerable period of the left ventricle. This feature is
only used when left ventricular sensing is active. HF versions
only.
) from
2.3 Ventricular Tachyarrhythmia Detection
The Lumax ICDs/CRT-Ds detect and measure the rate of sensed
cardiac signals to discriminate ventricular tachyarrhythmias from
supraventricular tachycardias, sinus rhythm or sinus bradycardia.
This is accomplished through programmable rate detection
parameters in the device. When a tachyarrhythmia is present, the
ICD/CRT-D classifies the arrhythmia and delivers the appropriate
therapy. If a tachyarrhythmia continues following the first therapy
attempt, then the ICD/CRT-D will redetect the tachyarrhythmia
and deliver subsequent therapies as necessary.
1-1705
Page 70

68 Lumax Technical Manual
WARNING
Unwanted Shocks – Always program Therapy status to
OFF prior to handling the device to prevent the delivery of
serious shocks to the patient or the person handling the
device during the implant procedure.
Classification of cardiac signals is accomplished primarily by
measuring the cardiac cycle length (R-R, P-R and P-P). In
addition, the ICD/CRT-D can also utilize abrupt changes in rate or
irregularity of the cardiac signal to further differentiate ventricular
tachyarrhythmias. Each detected ventricular tachyarrhythmia is
classified into one of the following zones:
• VT-1 Lower rate ventricular tachycardia
• VT-2 Higher rate ventricular tachycardia
• VF Ventricular fibrillation
Each rhythm class is programmable to a separate rate with the
zone limit defining the lowest rate in each class. The upper rate
limit of each class is equal to the zone limit of the next higher
class, creating a continuous range of rate classes.
2.3.1 VF Classifications
Detection of ventricular fibrillation (VF) utilizes programmable X
out of Y criterion. Both X and Y are programmable. If X number
of intervals within the sliding window (defined by Y) are shorter
than the programmed VF rate interval (>bpm), VF is detected.
After fibrillation is detected, the programmed therapy sequence
for VF is initiated.
Nominal settings for classification of ventricular fibrillation (VF)
are 8 of 12 intervals; meaning that within a sample window of
12 intervals, 8 intervals must meet or exceed the VF zone rate
criteria.
1-1706
Page 71

Lumax Technical Manual 69
2.3.2 VT Interval Counters
The VT Interval Counters are separately programmable for VT-1
and VT-2 rate classifications. The Counter: Detection is the
number of intervals required to declare a tachyarrhythmia as VT.
A tachyarrhythmia must meet both the rate/interval criteria and
the programmed Counter / Detection criteria, in addition to any
other detection enhancements to be declared a tachycardia.
2.3.3 VT Classification
Both VT-1 and VT-2 classification zones utilize separately
programmable detection parameters. Classification of VT-1 or
VT-2 is based on the last interval average preceding declaration
of tachyarrhythmia detection. If this average falls within the VT-1
zone, the programmed VT-1 therapy is delivered. If the average
falls within the VT-2 limits, the programmed VT-2 therapy is
delivered. If additional detection parameters are activated, each
of these supplemental criteria must also be satisfied before a VT
rhythm can be classified and treated.
The ICDs/CRT-Ds may be programmed to use ventricular-only
information, or both atrial and ventricular information for the
discrimination of ventricular tachycardias. With SMART
Detection™ turned ON, the Lumax ICDs/CRT-Ds use atrial and
ventricular signals for discrimination of fast heart rhythms. With
SMART Detection™ turned OFF, only the ventricular rate is used
to discriminate between ventricular rhythm classes. If SMART
Detection™ is enabled, this algorithm evaluates all cardiac signals
within the VT range and increments the VT Sample Count for all
intervals that are deemed VT. A full description of SMART
Detection™ is provided in the following text.
In addition, when the Lumax senses the programmed number of
consecutive intervals (termination count) within the sinus rate
zone, all tachyarrhythmia detection criteria, including the VT
sample counters are reset.
2.3.4 SMART Detection™
DR and HF versions only.
1-1707
Page 72

70 Lumax Technical Manual
This discrimination algorithm enhances VT-1 and VT-2 detection
by applying a series of tests to the sensed cardiac signal.
SMART Detection™ is intended to discriminate VT from a variety
of supraventricular arrhythmias that are conducted to the ventricle
and that would otherwise satisfy VT-1 or VT-2 rate detection
criteria.
First, the average ventricular rate is compared to the average
atrial rate. In the event that the measured ventricular rate is
faster than the atrial rate, the device immediately declares the
rhythm a VT and delivers programmed ventricular therapy for the
detected VT zone.
In the event that an atrial rate is faster compared to the ventricular
rate one of three tests are performed:
Ventricular rhythm stability, (see Stability on page 71
ventricular signal is unstable, then the rhythm is declared a
supraventricular tachyarrhythmia, (SVT) and ventricular therapy is
typically withheld.
If the ventricular signal is stable, and the atrial rate is a multiple of
the ventricle rate, then the rhythm is declared a supraventricular
tachyarrhythmia (SVT) and ventricular therapy is typically
withheld.
If the ventricular rhythm is stable and the atrial rate is not a
multiple of the ventricular rate, then the rhythm is declared a VT
and ventricular tachycardia therapy is delivered.
In the event that both the atrial and ventricular signals are
detected at the same rate, a series of additional discrimination
tests are applied.
) if the
2.3.5 Onset
Another detection enhancement that may be used independently
(VT-1 or VT-2, when SMART Detection is active) or as an adjunct
to the SMART Detection™ algorithm is the Onset parameter.
This parameter measures abrupt changes in ventricular cycle
length to discriminate between sinus tachycardias and ventricular
and atrial tachyarrhythmias, which characteristically begin with an
abrupt change in cardiac rate.
This feature allows therapy to be withheld if a sinus tachycardia
rate crosses into one of the VT zones.
1-1708
Page 73

Lumax Technical Manual 71
2.3.6 Stability
In VT-1 and VT-2 zones, the purpose of STABILITY is to assist in
discriminating between stable ventricular tachyarrhythmias and
supraventricular tachyarrhythmias that conduct irregularly to the
ventricles. STABILITY evaluates sudden changes in the
regularity of cardiac events (R-R and P-P intervals) on a beat by
beat basis. The STABILITY criterion compares the current
measured interval with the three preceding cardiac intervals. If a
difference between the current interval and each of the three
preceding intervals is less than the stability range, then the
current intervals are stable.
The SMART Detection™ algorithm utilizes both atrial and
ventricular STABILITY as integral parts of the discrimination
algorithm. Therefore, when SMART Detection™ is enabled, the
STABILITY parameter must also remain enabled and set to 12%.
2.3.7 Sustained VT Timer
The Sustained VT Timer can be programmed between
30 seconds and 30 minutes (or to OFF). When the timer expires,
therapy is initiated regardless of the detection enhancements.
The Sustained VT parameter is intended to force tachycardia
therapy in cases where a cardiac rhythm meets the VT rate
criteria but does not satisfy one or more detection enhancement
criterion (Onset, SMART Detection, or Stability) for an extended
duration. This “safety” timer is initiated within one of the VT
zones. If the programmed Sustained VT time period expires
without tachycardia detection, redetection is initiated without
utilizing the detection enhancements.
A simple up/down counter is used to initiate the safety timer. The
counter is incremented by one when an interval falls into a VT
zone and decrements by one when an interval falls into the sinus
zone. When the counter reaches a number equal to the
programmed VT detection counter, the safety timer is started.
The timer runs until the programmed time expires and therapy is
delivered or until the timer is reset. The timer is reset with initial
detection or VT termination.
1-1709
Page 74

72 Lumax Technical Manual
The safety timer is not used in redetection. If initial detection was
due to the safety timeout and SMART Redetection is
programmed “ON”, then SMART Detection™ will not be used for
redetection.
2.4 Tachyarrhythmia Redetection
The Lumax ICDs/CRT-Ds offer independently programmable
settings for determining if tachyarrhythmias remain after therapy
has been delivered. The redetection routine allows the
ICDs/CRT-Ds to determine whether further therapy is required
when the initial therapy was unsuccessful in terminating the
arrhythmia.
Tachyarrhythmia redetection criteria are based on cardiac cycle
length and number of intervals. The number of intervals is distinct
and independent of the initial detection criteria.
2.4.1 VT Redetection
The Counter: Redetection parameter may be programmed
separately for each arrhythmia class, independent of the initial
detection parameters:
Redetection of an ongoing tachyarrhythmia is declared when the
Counter: Redetection is satisfied (based on individual cycles). If a
sensed cardiac signal meets any VT rate criteria, following
therapy, that signal is counted and compared to the programmed
Counter: Redetection setting. Tachycardia redetection is
declared when the programmed number of VT samples (Counter:
Redetection) is satisfied.
Redetection functions identically to initial VT detection in regards
to the Stability and Onset detection enhancements and it is based
on individual cycle lengths (not averages).
2.4.2 SMART Redetection
DR and HF versions only.
With SMART Redetection programmed ON, both atrial and
ventricular signals are used for redetection after initial detection
and therapy for a VT. SMART Detection™ will function identically
as in initial VT detection.
1-1710
Page 75

Lumax Technical Manual 73
2.4.3 Forced Termination
DR and HF versions only.
With SMART Redetection programmed ON, this programmed
parameter sets a time after which the SMART Redetection will be
terminated even if the SVT is still ongoing. This forces the device
to terminate the episode and allow detection of a new VT or VF
episode.
2.4.4 VF Redetection
VF redetection uses the same X in Y algorithm as initial detection.
The X and Y values for initial detection are also used for
redetection to ensure consistent classification of VF.
2.5 Tachyarrhythmia Termination
Termination of a ventricular tachyarrhythmia episode is declared
when 12 out of 16 consecutive sensed intervals are longer than
the VT-interval parameter of the lowest VT class (sinus or
bradycardia rhythm).
2.6 Tachyarrhythmia Therapy
The Lumax ICDs/CRT-Ds offer a variety of therapy options that
can be tailored to meet a patient’s specific anti-tachycardia or
defibrillation therapy requirements. Anti-tachycardia pacing (ATP)
therapies can be combined with defibrillation therapies to provide
a broad spectrum of tachyarrhythmia treatment options.
2.6.1 Therapy Options
The Lumax ICDs/CRT-Ds offer multiple therapy options for each
tachyarrhythmia class (VT1, VT2, VF). Therapy options (up to 10
ATP sequences and 8 shocks) are available for the VT1 and VT2
zones, whereas ATPOne Shot and up to 8 shock therapies are
available for the VF class. The specific characteristics of an ATP
and shock therapy are independently programmed for each
VT/VF zone.
The ATP and shock therapy options are discussed in detail in the
following sections.
1-1711
Page 76

74 Lumax Technical Manual
2.6.2 Anti-Tachycardia Pacing (ATP)
Anti-tachycardia pacing therapy (ATP) is available in both VT
detection zones. Available modes of ATP include Burst, Ramp,
and Burst + PES (Programmed Extra Stimuli). In addition, the
Burst and Ramp modes allow interval scanning of the R-S1
Interval, the S1 Decrement, or both. The Attempts parameter
determines the number of burst schemes to be delivered before
the scan parameter is incremented.
Burst – This mode will deliver a series of pacing stimuli with user
defined duration of the burst (Number S1), coupling cycle length
(R-S1) and burst rate (S1-S1). The coupling interval and the start
interval are calculated from the intrinsic R-R average.
Ramp - This mode will deliver a series of pacing stimuli with the
above options including a parameter which decrements each
successive stimuli interval in the burst.
Burst + PES - This mode provides a pulse train followed by one
or more (up to three) additional timed stimuli. The coupling cycle
length of the burst and each extra stimulus (S1-S2 Interval) is
individually programmed either as an adaptive value (as a
percentage) or as an absolute value (expressed in milliseconds).
Ventricular Pacing (CRT-Ds only) - This parameter defines the
type of ventricular pacing performed during delivery of ATP
sequences in CRT-Ds and is programmable to biventricular or
right ventricular only.
Number S1 - This parameter defines the number of stimuli for an
ATP. For Burst + PES, an extra stimulus with a separate
parameter is coupled.
Add S1 - This feature can be programmed with any Burst, Ramp,
or Burst + PES scheme. When “Add S1” is “ON,” the number of
S1 intervals is incremented by one on each successive ATP
therapy. The next S1-S1 interval is dependent on the initial start
interval (S1 decrement) and the programmed Scan Decrement (if
activated).
R-S1 Interval - The R-S1 programmable coupling interval occurs
at the beginning of each ATP. It defines the interval between the
last R-wave signal and the first stimulus (S1). The second
stimulus always follows the first one with the same interval.
1-1712
Page 77

Lumax Technical Manual 75
S1 Decrement - The S1 decrement continuously reduces the
pulse intervals of the ATP from the second pulse onward.
S1-S2 Interval - The S1-S2 programmable coupling interval
occurs between the Burst sequence and the extra stimuli (PES). It
defines the interval between the first stimulus (S1) and the extra
stimuli (S2).
Scan Decrement - The Scan decrement continuously reduces
the starting pulse intervals of each Burst or Scan.
Minimum ATP Interval - The programmed minimum interval
prevents ATPs from being given with stimulation values less than
the minimum interval. When the ATP interval reaches the value of
the minimum interval with the S1 decrement or scan decrement, it
then assumes this value and remains constant.
2.6.2.1 ATP Help
ATP help is a useful tool to assist the physician in choosing and
confirming appropriate ATP programming. The “ATP Help” button
is displayed in each ATP therapy option. When the ATP help
button is pressed, a histogram of the chosen therapy scheme is
shown. The histogram displays the intervals for the programmed
ATP scheme. When rate adaptive intervals are programmed, the
displayed intervals are based on the programmed R-R average.
2.6.2.2 ATP Optimization
In order to optimize future therapies, the ICD will store the
parameter configuration of the last successful ATP attempt in
each the VT1 and VT2 classes. The last successful stored ATP
attempt is then used as the starting point for the next detected
episode of the same arrhythmia class. If the stored parameter
configuration is not successful, it is deleted from the ATP
optimization memory of the respective arrhythmia class and
subsequent therapy sequences will begin with ATP1 for the next
detected episode.
ATP Optimization is programmable ON or OFF for all ATP
therapies and VT zones with one parameter.
1-1713
Page 78

76 Lumax Technical Manual
NOTE:
In VT zones, the ICD/CRT-D stores successful ATP therapies
only. The stored information includes not only the number of
the ATP therapy (e.g., ATP2), but also the successful
configuration in detail (for example: Burst; R-S1 Interval:
320 ms, S1-S1 Interval: 320 ms; etc.).
2.6.2.3 ATP Timeout
ATP Timeout is a timer that decrements after the initial ventricular
ATP is delivered (VT-1 zone) and limits the additional ATP
therapies that may be delivered. Once the timer expires, all
further ATP therapies in the sequence are blocked. If further
therapy is required after the timer has expired, the system
advances to the first programmed shock therapy for the
applicable VT zone. Therapy continues until arrhythmia
termination or all programmed therapy (in the applicable zone)
has been delivered. The ATP Timeout is reset each time an
arrhythmia is terminated.
2.6.2.4 ATP One Shot
ATP One Shot offers the opportunity to treat monomorphic VTs
that are detected in the VF zone with a single ATP sequence
delivered during charging of the high energy capacitors. The
device performs a stability check (same as VT zones) to
determine if the arrhythmia might be a monomorphic VT and if the
rhythm is stable, the programmed ATP sequence is delivered and
the arrhythmia is confirmed prior to shock delivery. If the
arrhythmia is converted by the ATP, the shock is aborted. All
ATP therapy parameters are available for programming ATP One
Shot and it can only be used with shock therapy is programmed
ON.
2.6.3 Shock Therapy
Shock Therapy is developed by internal circuitry that stores
energy across two high energy capacitors. The voltage level for
the charging cycle is based on the programmed energy level.
The energy is then delivered over the connected ICD lead and
through the ICD/CRT-D housing utilizing a biphasic waveform.
The first and second shock energies in each shock module have
independently programmable Shock Energy.
1-1714
Page 79

Lumax Technical Manual 77
A synchronization window is started at the end of the charging
period. During this window, the device will attempt to synchronize
the shock therapy to an R-wave. If no R-wave is detected, the
shock will be delivered asynchronously at the end of the
synchronization period.
2.6.3.1 Confirmation
The Confirmation parameter is used to verify the presence of a
tachyarrhythmia during charging of the shock capacitors. This
function is designed to avoid delivery of shock therapy if a
tachyarrhythmia has spontaneously terminated. The
programmed shock will be delivered unless bradycardia or a
normal sinus rhythm is detected during the Confirmation period.
Confirmation may be programmed ON or OFF for the first and
second shock therapies of each zone and is always OFF for
remaining shock therapies.
Confirmation OFF
When Confirmation is programmed OFF, shock therapy will be
delivered to the patient during the synchronization period
regardless of the detected cardiac signal.
Confirmation ON
If the tachyarrhythmia spontaneously converts to bradycardia or a
normal sinus rhythm during the confirmation period, shock
therapy is aborted. If the device confirms the presence of the
tachyarrhythmia, the device will deliver the programmed shock
therapy.
2.6.3.2 Number of Shocks
The number of shocks defines the total number of shock attempts
per therapy zone (VT-1, VT-2 or VF). Up to 8 shocks are
available in each therapy zone. The first and second shock
energies are independently programmable, while the remaining
shocks are fixed at maximum energy (30 joules for 300 series
devices and 40 joules for 340 series devices) with Confirmation
turned OFF.
1-1715
Page 80

78 Lumax Technical Manual
2.6.3.3 Shock Waveform
Two waveforms of shock therapy are available with the Lumax
ICDs/CRT-Ds, Biphasic and Biphasic 2ms. The following
diagram describes each of the shock waveforms.
Figure 5. Biphasic Waveforms
Both waveforms start at the calculated voltage, based on the
programmed energy level. After an exponential discharge
through the lead system to 40% of the initial charge voltage, both
shock waveforms switch polarity. At the second phase the:
• Biphasic waveform discharges to 20% of the initial
charge voltage before the waveform is truncated
• Biphasic 2ms waveform discharges the remaining
energy for two milliseconds before the waveform is
truncated
Figure 5
waveforms.
BIOTRONIK recommends use of the standard Biphasic shock
waveform for initial defibrillation threshold testing. If testing
demonstrates high defibrillation thresholds, testing with the
Biphasic 2ms waveform is offered as a therapeutic alternative to
the standard Biphasic shock.
provides a pictorial representation of both biphasic
1-1716
Page 81

Lumax Technical Manual 79
2.6.3.4 Shock Energy
The Lumax ICDs/CRT-Ds are designed to charge to the energy
selected on the programmer screen, but similar to all other
commercially available ICDs/CRT-Ds, the actual therapy
delivered is somewhat less depending on several factors
including the shock lead impedance. The first two shock energies
in each therapy class are programmable between 1 joules and
maximum energy for the device type. The energy of the second
shock is always greater than the first shock. The remaining
shock energies will be delivered at maximum programmable
energy.
Actual energy delivered for each programmable shock energy is
approximately equal to the “Energy Delivered” in Table 22.
Table 22 Delivered Shock Energy
Programmed Energy
(joules)
1 0.80
2 1.65
3 2.56
4 3.46
5 4.25
6 5.15
7 6.09
8 6.88
9 7.74
10 8.68
11 9.43
12 10.33
13 11.12
14 12.06
15 12.89
16 13.72
Approximate Delivered
Energy (joules)
1-1717
Page 82

80 Lumax Technical Manual
Programmed Energy
(joules)
18 15.56
20 17.21
22 18.98
24 21.27
26 23.07
28 24.65
30 26.57
32 28.3
34 30.25
36 32.13
38 33.93
40 35.7
Approximate Delivered
Energy (joules)
CAUTION
Shock Impedance - If the shock impedance is less than
twenty-five ohms, reposition the lead system to allow a
greater distance between the electrodes. Never implant the
device with a lead system that has measured shock
impedance as less than twenty-five ohms. Damage to the
device may result.
Defibrillation Threshold - Be aware that changes in the
patient’s condition, drug regimen, and other factors may
change the defibrillation threshold (DFT), which may result in
non-conversion of the arrhythmia post-operatively.
Successful conversion of ventricular fibrillation or ventricular
tachycardia during arrhythmia conversion testing is no
assurance that conversion will occur post-operatively.
Shock Therapy Confirmation – Programming
CONFIRMATION to OFF may increase the incidence of the
ICD/CRT-D delivering inappropriate shocks.
1-1718
Page 83

Lumax Technical Manual 81
2.6.3.5 Shock Polarity
The polarity of the shock therapy may be programmed and
changed non-invasively. The Normal polarity configures the
HV 1 connector port as the negative electrode and the HV 2
connector port and the outer housing of the ICD/CRT-D as the
positive electrode for the first phase of the shock. Reversed
polarity will switch the electrical polarity of the connector ports
and housing. The shock polarity is separately programmable for
each arrhythmia zone.
Alternating polarity delivers the first shock with normal polarity,
the second shock with reversed polarity and alternates the
polarity for all subsequent shocks.
2.6.4 Progressive Course of Therapy
By design, the Lumax ICDs/CRT-Ds will deliver more aggressive
therapy for each successive attempt within a single detected
episode. Therefore, the device will not deliver ATP1 therapy
following ATP2 therapy, and will not deliver ATP therapy following
a high voltage defibrillation shock.
When Progressive Course of Therapy is turned ON, the
ICD/CRT-D will always deliver a maximum energy shock after redetecting in an arrhythmia class with programmed shock energy
less than or equal to the previously delivered therapy. In addition,
the ICD/CRT-D blocks all ATP therapy during the current episode
if a shock has already been delivered during the episode.
Furthermore, the ICD/CRT-D prevents therapies of different
arrhythmia classes from permanently retarding or accelerating a
VT in such a way that the cardiac rhythm fluctuates between the
different arrhythmia classes without achieving termination of the
arrhythmia regardless of the Progressive Course of Therapy
setting.
For example, a 10-joule defibrillation shock is delivered for an
arrhythmia detected in the VT-2 zone and results in a
deceleration of the VT so that it is subsequently redetected in the
VT-1 zone. At that point, the Lumax ICDs/CRT-Ds would
continue with shock therapy, but all shocks programmed at less
than 10 joules would be delivered at 10 joules.
1-1719
Page 84

82 Lumax Technical Manual
If a defibrillation shock is delivered but does not terminate the
arrhythmia, the next shock will always have the same or higher
energy than the last delivered shock. Beginning with the third
shock, all shocks are delivered at maximum energy (30 joules).
2.7 Bradycardia Therapy
The Lumax ICDs/CRT-Ds have independently programmable
single, dual and triple chamber and post-shock pacing functions.
The post-shock bradycardia parameters may be programmed to
higher rates or output values for the period following a delivered
shock, without significantly compromising the longevity of the
ICD/CRT-D for patients who require chronic bradycardia pacing.
The post-shock programmable values are presented in a
separate subsection from the normal bradycardia pacing support
values.
2.7.1 Bradycardia Pacing Modes
The available bradycardia pacing modes for each member of the
Lumax ICD/CRT-D family are listed in Table 23.
Table 23 Lumax Pacing Modes
Mode Lumax HF Lumax DR Lumax VR
DDDR X X N/A
DDIR X X N/A
VDDR X X N/A
VDIR X X N/A
AAIR X X N/A
VVIR X X X
DDD X X N/A
DDI X X N/A
VDD X X N/A
VDI X X N/A
AAI X X N/A
VVI X X X
OFF X X X
1-1720
Page 85

Lumax Technical Manual 83
The basic rate timer is started by a sensed or paced event. A
sensed event outside of the refractory period inhibits pacing and
resets the lower rate time; in the absence of a sensed event, a
pacing pulse will be delivered at the end of the lower rate interval.
The pacing modes with an “R” indicate rate adaptive pacing
controlled by a motion based capacitive sensor. These modes
are functionally the same as the corresponding non-rate-adaptive
modes, except that the pacing rate is increased based on
physical activity.
2.7.2 Basic Rate
The basic rate is the pacing rate in the absence of a patient’s
intrinsic rhythm. This rate may be independently programmed for
normal and post-shock bradycardia pacing.
2.7.3 Night Rate
The night rate is the effective basic rate during the programmed
“sleep” period for the patient. This parameter provides a lower
pacing rate during the patient’s normal sleep time in an attempt to
match the decreased metabolic needs during sleep. When Night
Mode is active, the basic rate automatically decreases to the
programmed NIGHT RATE during the nighttime hours.
At the programmed start time (Begin of Night), the rate gradually
decreases to the night rate. When the internal clock reaches the
programmed end time (End of Night), the pacing rate gradually
changes to the programmed basic rate. The rate changes at the
same rate as the Sensor Gain decrease and increase
parameters.
NOTE:
The Night Mode time is based on the programmer clock.
Therefore, the programmer time should be checked prior to
device programming. If a patient travels across different time
zones, the Night Mode time may require adjustment.
1-1721
Page 86

84 Lumax Technical Manual
2.7.4 Rate Hysteresis
The ability to decrease the effective lower rate through
Hysteresis is intended to preserve a spontaneous rhythm. The
pulse generator operates by waiting for a sensed event throughout
the effective lower rate interval (Hysteresis interval). If no sensed
event occurs, a pacing pulse is emitted following the Hysteresis
interval.
Hysteresis can be programmed OFF or to values as low as
-65 bpm of the basic rate. Hysteresis is initiated by a sensed
event. The resulting Hysteresis rate is always less than the lower
rate. The Hysteresis rate can only be programmed to provide a
basic rate that is 30 bpm or greater.
NOTES:
If rate adaptation is active, the Hysteresis rate is based on the
current sensor-indicated rate and the value of the
programmable parameter.
If Hysteresis is used in the DDI mode, the AV delay must be
programmed shorter than the spontaneous AV conduction
time. Otherwise, stimulation in the absence of spontaneous
activity occurs at the hysteresis rate instead of the lower rate.
Hysteresis is suspended when Night Mode is active.
Programming conflicts arise when the total decrease in rate is
below 30 ppm. Care should be exercised to avoid
programming a Night Mode rate and hysteresis that are
below what is appropriate and may be tolerated by the
individual patient.
2.7.4.1 Repetitive Hysteresis
Repetitive hysteresis is expanded programmability of the
Hysteresis feature. Repetitive hysteresis searches for an intrinsic
cardiac rhythm, which may exist below the programmed lower
rate (or sensor-indicated rate) of the patient. Following 180
consecutive sensed events, this feature allows the intrinsic
rhythm to drop to or below the hysteresis rate. During the time
when the intrinsic rate is at or below the hysteresis rate, pacing
occurs at the hysteresis rate for the programmed number of beats
(up to 10). Should the number of programmed beats be
exceeded, the stimulation rate returns to the lower rate (or
sensor-indicated rate).
1-1722
Page 87

Lumax Technical Manual 85
If an intrinsic cardiac rhythm is detected within the programmed
number of beats between the hysteresis rate and the lower rate,
the intrinsic rhythm is allowed and inhibits the pulse generator.
Figure 6. Repetitive Hysteresis
Repetitive hysteresis has been incorporated to promote
spontaneous cardiac rhythm and may reduce pulse generator
energy consumption.
NOTE:
Repetitive and Scan Hysteresis are not active during the
programmed Night Mode and are only available when
Hysteresis is selected on.
Magnet application (closing of reed switch) suspends 180
consecutive event counter independent of synchronous or
asynchronous magnet effect.
There is one Standard Hysteresis interval which occurs
before the programmable number of Repetitive Hysteresis.
1-1723
Page 88

86 Lumax Technical Manual
2.7.4.2 Scan Hysteresis
Scan hysteresis is expanded programmability of the Hysteresis
feature. Scan hysteresis searches for an intrinsic cardiac rhythm,
which may exist just below the programmed lower rate (or sensorindicated rate). Following 180 consecutive paced events, the
stimulation rate is temporarily decreased to the hysteresis rate for
a programmed number of beats. If a cardiac rhythm is not
detected within the programmed number of beats at the
hysteresis rate, the stimulation rate returns back to the original
lower rate (or sensor-indicated rate). Several programmable beat
intervals are available to allow a greater probability of detecting a
spontaneous rhythm.
If an intrinsic cardiac rhythm is detected within the programmed
number of beats between the hysteresis rate and the lower rate,
the intrinsic rhythm is allowed and the pulse generator inhibits.
Figure 7. Scan Hysteresis
Scan hysteresis has been incorporated to promote intrinsic
cardiac rhythm and may reduce pulse generator energy
consumption.
NOTE:
Magnet application (closing of reed switch) suspends 180
consecutive event counter independent of the magnet effect.
1-1724
Page 89

Lumax Technical Manual 87
2.7.5 Dynamic AV Delay
DR and HF versions only.
The AV Delay defines the interval between an atrial paced or
sensed event and the ventricular pacing pulse. If the pulse
generator is programmed to a dual chamber sensing mode, an
intrinsic ventricular event falling within the AV Delay will inhibit the
ventricular pacing pulse. If not contraindicated, a longer
AV Delay can be selected to preserve intrinsic AV conduction.
Dynamic AV Delay is where the AV Delay is varied depending on
the spontaneous atrial rate. Dynamic AV Delay provides
independent selection of AV Delays from five rate ranges at
preset AV Delay values. In addition, the AV Delay after atrial
pace events can be differentiated from the atrial sense events for
dual chamber pacing modes.
In addition to selecting the preset values (Low, Medium, and
High) with the Dynamic AV Delay window, the Dynamic
AV Delays may be programmed individually (Individual) for each
rate zone or to a fixed AV Delay (Fixed).
The AV Delay feature includes an AV Delay shortening option
(Sense Compensation) for dual chamber pacing modes. When
enabled, the AV Delay is shortened by the programmed value
(20 to 120 ms) from the programmed AV Delay after an intrinsic
atrial sensed event.
The Dynamic AV Delay is intended to mimic physiologicshortening of the AV Delay with increasing heart rate. It also
serves for automatic prevention and termination of “circus
movement” pacemaker mediated tachycardia and for prevention
of reentrant supraventricular tachycardias. Dynamic AV Delay is
only available with the Lumax DR ICD.
1-1725
Page 90

88 Lumax Technical Manual
2.7.5.1 AV Hysteresis
AV Hysteresis allows a user-programmable change in AV delay
that is designed to encourage normal conduction of intrinsic
signals from the atrium into the ventricles. With AV hysteresis
enabled, the AV delay is extended by a defined time value after
sensing a ventricular event (10 … (10) …150 ms). The long AV
interval is used as long as intrinsic ventricular activity is detected.
The programmed short AV delay interval resumes after a
ventricular paced event.
2.7.5.2 AV Repetitive Hysteresis
With AV Repetitive Hysteresis, the AV delay is extended by a
defined hysteresis value after sensing an intrinsic ventricular
event. When a ventricular paced event occurs, a long AV delay is
used for the programmed number of cycles. (1 … 6). If an
intrinsic rhythm occurs during one of the repetitive cycles, the
long duration AV delay interval remains in effect. If an intrinsic
rhythm does not occur during the repetitive cycles, the original AV
delay interval resumes.
2.7.5.3 AV Scan Hysteresis
With AV Scan Hysteresis enabled, after 180 consecutive pacing
cycles, the AV delay is extended for the programmed number of
pacing cycles. (1 … 6). If an intrinsic rhythm is detected within
the extended AV delay and the longer AV delay remains in effect.
If an intrinsic rhythm is not detected within the number of scan
cycles, the original AV delay value resumes.
2.7.5.4 Negative AV Delay Hysteresis
With Negative AV Delay Hysteresis, the AV delay is decreased by
a defined value after a ventricular event is sensed, thereby
promoting ventricular pacing. The Negative AV Delay Hysteresis
value corresponds to the programmed AV delay (Table 24).
1-1726
Page 91

Lumax Technical Manual 89
Table 24: Negative AV Delay Hysteresis Values
AV Delay
(Standard)
100 ms 100 ms
120 ms 100 ms
130 ms 100 ms
140 ms 100 ms
150 ms 100 ms
160 ms 120 ms
170 ms 120 ms
180 ms 130 ms
190 ms 140 ms
200 ms 150 ms
225 ms 170 ms
250 ms 180 ms
300 ms 200 ms
The normal AV delay resumes after the programmed number of
consecutive ventricular paced events (Repetitive Negative AV
Delay Hysteresis) elapses.
(Negative Hysteresis ON)
AV Delay
CAUTION
Negative AV Delay Hysteresis – This feature insures
ventricular pacing, a technique which has been used in
patients with hypertrophic obstructive cardiomyopathy
(HOCM) with normal AV conduction in order to replace
intrinsic ventricular activation. No clinical study was
conducted to evaluate this feature, and there is conflicting
evidence regarding the potential benefit of ventricular pacing
therapy for HOCM patients. In addition, there is evidence with
other patient groups to suggest that inhibiting the intrinsic
ventricular activation sequence by right ventricular pacing may
impair hemodynamic function and/or survival.
1-1727
Page 92

90 Lumax Technical Manual
2.7.6 IOPT Plus
DR versions only.
The IOPT Plus function serves to support the patient’s intrinsic
rhythm and avoid excessive ventricular pacing. This feature
simply activates all of the AV hysteresis parameters with a single
selection. Table 25 details the settings that are preset when
IOPT Plus is turned ON:
Table 25 IOPT Plus Parameters
Parameter IOpt Plus
AV Hysteresis 400 ms
AV Scan Hysteresis 5
Repetitive AV Hysteresis 5
2.7.7 Upper Tracking Rate
DR and HF versions only.
In the atrial tracking modes (DDDR, VDDR, DDD, and VDD)
ventricular pacing tracks atrial pace/sense events. The maximum
tracking rate (ventricular pacing rate) is limited by the Upper Rate
parameter. Furthermore, the upper tracking rate acts as a limit
for the maximum tracking rate in both atrial and ventriculartracked modes.
The UTR response will automatically toggle between 2:1 and
WKB (Wenkebach) depending on the relative programmed values
for upper rate and atrial refractory period.
If the UTR is less than the maximum sensed atrial rate, defined
by the atrial refractory period (60,000/ARP), the WKB response
is utilized. Atrial rates exceeding the selected upper rate will
result in a Wenckebach-type pacing pattern. This is
accomplished by progressively lengthening the AV delay to keep
the ventricular pacing rate at the upper rate. Lengthening of the
AV interval is interrupted as soon as: 1) a P-wave falls within the
atrial blanking period and is not detected; or 2) a succeeding
P-wave is detected before the end of the AV delay previously
started. In the second case, the corresponding ventricular pacing
pulse is suppressed. If the atrial rate is just above the upper rate,
a low degree (i.e. 6:5) block results. Higher atrial rates result in
higher degrees of AV block until the intrinsic atrial cycle length
1-1728
Page 93

Lumax Technical Manual 91
violates the programmed atrial refractory period causing a 2:1 or
greater block.
The 2:1 response is utilized when the rate defined by the atrial
refractory period is less than the upper rate and Automatic Mode
Conversion is OFF. In such a case, the maximum pacing rate is
regulated by the inability to respond to P-waves falling within the
atrial refractory period.
If the resulting length of the spontaneous atrial cycle is shorter
than the atrial refractory period in a rate-adaptive mode, the
resulting pacing rate will depend on whether the 2:1 rate has
been exceeded. If this is the case, the pulse generator will use
the sensor rate as the pacing rate. If the 2:1 rate is not exceeded,
the pulse generator will use a rate that lies between the sensor
rate and the rate determined by the atrial refractory period.
Atrial Upper Rate is designed to prevent pacing in the vulnerable
period after an atrial sensed event during PVARP. It ensures that
the next atrial pace is emitted outside of the patient’s normal
sinus atrial refractory period. Atrial Upper Rate is limited to
240 ms or OFF.
NOTE:
The Lumax DR ICDs and Lumax HF CRT-Ds allow the UTR
to be programmed within the VT-1 zone. This feature is for
patients that are active and have exercise and VT rates that
overlap. This may be desirable in young active patients.
2.7.8 Mode Switching
DR and HF versions only.
Mode switching is designed to avoid tracking of atrial arrhythmias.
In the presence of a high atrial rate, the bradycardia pacing mode
is automatically reprogrammed to a non-atrial tracking mode. The
modes available during mode switching are as shown in
Table 26
pacing period. Mode Switching is only available with the Lumax
DR ICD.
. Mode switching is not available during the post-shock
1-1729
Page 94

92 Lumax Technical Manual
Table 26: Mode Switching Modes
Programmed Mode Converted Mode
DDDR
DDIR
DDI
DDD
DDI
VDIR
DDIR
VDDR
VDI
VDIR
VDD
VDI
Mode switching is initiated in atrial tracking modes when the atrial
rate, defined by the programmable mode switch Intervention
Rate is achieved. However, mode switching will not occur until
the Mode Switch Activation Criteria is also met. The activation
criterion is a programmable X out of 8 high rate intervals as
programmed.
After switching to a non-atrial tracking mode, the ICD/CRT-D
activates a Y out of 8 counter that deactivates mode switching
when Y number of cardiac cycles out of the last 8 are below the
Intervention Rate. When this Deactivation Criteria parameter is
fulfilled, the ICD/CRT-D returns to the normal programmed pacing
mode.
In addition, the ventricular pacing configuration (RV or BiV) and
biventricular pacing parameters (LV T-wave protection and
Triggering) are programmable separately for mode switching
events.
2.7.8.1 Mode Switch Basic Rate
Whenever Mode Switching occurs, the device switches to a nontracking mode and will provide bradycardia pacing support at the
Mode Switch Basic Rate, which is displayed as the Change of
Basic Rate parameter. Once Mode Switching is terminated, the
permanently programmed pacing mode and programmed pacing
rate are restored.
1-1730
Page 95

Lumax Technical Manual 93
2.7.8.2 Post Mode Switch Response
Whenever Mode Switching event terminates, deactivating the
mode switch pacing, the device can be programmed to react with
different basic rate for a specified amount of time. Two
parameters are used to set the Post Mode Switch Response.
Post ModeSw Rate sets the rate difference (from permanent
program) in pacing rate during the programmed Post ModeSw
Duration time period. After the Post ModeSw Duration expires,
the pacing rate is ramped down to the programmed basic rate.
2.7.9 PMT Protection
DR and HF versions only.
PMT protection is a combination of PMT detection and
termination and is programmable ON or OFF. In cases of
Pacemaker Mediated Tachycardia (PMT), the PMT protection
algorithm features provide both detection and termination of
PMTs. In this way, the more hemodynamically favorable AV
synchronization can be quickly re-established. PMT may be
suspected if 16 consecutive cycles of atrial sensing and
ventricular pacing occur and these are within the range of the
PVARP and the PVARP + PVARP extension and are stable.
When a PMT is ongoing, there is coupling between the ventricular
paced (Vp) event and the subsequent atrial sense (As). As such
the VA Criterion is used to detect if a PMT is present.
2.7.10 VES Discrimination after Atrial Sensed
Events
Stratos LV has a special timing interval (VES/As) – VES
discrimination after atrial sense events to identify ventricular
extrasystoles.
With each As and As (PVARP), a VES discrimination interval is
started in the ventricle. If a ventricular sensed event occurs within
the discrimination interval, this event is interpreted as a Vs
(ventricular sensed event), and no PVARP after VES protection
interval is started.
In the factory setting, the VES discrimination after As is set to
350 ms (programmable: OFF, 250…(5)…450 ms). The VES/As
terminates with each ventricular event.
1-1731
Page 96

94 Lumax Technical Manual
If a ventricular event does not fall within the AV delay or the VES
discrimination interval, it is classified as a VES. A ventricular
event that is sensed within the VES discrimination interval, but
outside the AV delay, starts a VA delay after which an atrial
paced is delivered.
2.7.11 Rate Adaptive Pacing
WARNING
Rate-Adaptive Pacing – Use rate-adaptive pacing with care
in patients unable to tolerate increased pacing rates.
Lumax ICD/CRT-D allows the selection of rate-responsive pacing
modes. These modes allow the ICD/CRT-D’s bradycardia
therapy function to adapt the pacing rate to increasing or
decreasing patient physical activity, based on data collected from
a motion based sensor within the ICD/CRT-D. Separately
programmable criteria allow the clinician to control the rate of
increase and decrease of pacing, as well as the sensitivity of the
sensors in response to motion.
2.7.11.1 Sensor Gain and Threshold
The Sensor Gain defines how much the sensor signal is amplified
before it is transformed to a rate change. When the Sensor Gain
is low (e.g., 2), a great deal of exertion is needed to cause a
significant change in sensor output (and an equal change in the
pacing rate). When the Sensor Gain is high (e.g., 18), little
exertion is needed to increase the sensor output. Ideally, the gain
is programmed so the maximum desired pacing rate during
exercise occurs at a maximum exertion level.
The device ignores all activity that occurs below the Sensor
Threshold because the Sensor Threshold defines the lowest
sensor output that initiates a change in the pacing rate. Five
different threshold settings are available including; VERY LOW,
LOW, MEAN, HIGH, and VERY HIGH. When the threshold is
programmed optimally, the basic rate is the effective rate while
the patient is not moving (at rest).
1-1732
Page 97

Lumax Technical Manual 95
2.7.11.2 Rate Increase / Decrease
The Rate Increase and Decrease parameters work with the
Sensor Gain to determine how quickly the pacing rate will
increase or decrease during changes in the sensor output.
2.7.11.3 Maximum Sensor Rate
Regardless of the sensor output, the sensor-driven pacing rate
never exceeds the programmable Max. Sensor Rate. The
maximum sensor rate only limits the pacing rate during sensordriven pacing.
2.7.11.4 Auto Sensor Gain
The Lumax ICDs/CRT-Ds offer Automatic Sensor Gain Auto
Gain settings, which allows the Auto Gain parameter to be
adjusted automatically.
When the Automatic Sensor Gain is activated, the pulse
generator samples the sensor-indicated rate. If, during the
24 hour period beginning at midnight, the total time recorded at
maximum sensor rate exceeds 90 seconds, the sensor gain
setting is reduced by one step. The sensor gain will be increased
by one step after 7 consecutive days during which the time
recorded at maximum sensor rate is less than 90 seconds each
day.
2.7.12 Pulse Amplitude
The Pulse Amplitude parameters, are separately programmable
for atrial and both ventricular channels and they, define the
amplitude in volts of the pacing pulses. The pulse amplitude is
also independently programmable for normal and post-shock
bradycardia pacing.
2.7.13 Pulse Width
The Pulse Width parameters, are separately programmable for
atrial and both ventricular channels and they define the duration
of the pacing pulses. The pulse widths are also independently
programmable for normal and post-shock bradycardia pacing.
1-1733
Page 98

96 Lumax Technical Manual
2.7.14 Post Ventricular Atrial Refractory Period
DR and HF versions only.
Immediately following a each sensed or paced ventricular event,
an atrial refractory period is started, this period is called Post
Ventricular Atrial Refractory Period or PVARP. Atrial signals are
ignored during this time for bradycardia timing purposes to
prevent the ICD/CRT-D from sensing inappropriate signals.
2.7.15 PVARP after VES
DR and HF versions only.
This parameter extends the Post Ventricular Atrial Refractory
Period by the programmed interval, if the ventricular event is not
followed by an atrial sensed event.
2.7.16 Auto PVARP
DR and HF versions only.
This parameter automatically adjusts the Post Ventricular Atrial
Refractory Period (PVARP) and PVARP after VES, if a
pacemaker mediated tachycardia (PMT) has been detected and
terminated to avoid additional PMT events. After seven days,
PVARP and PVARP after VES are reduced to their programmed
values.
2.7.17 Noise Response
The Lumax ICD/CRT-D’s responds to detected noise is to deliver
asynchronous pacing in the affected channel.
2.7.18 Post Shock Pacing
Separately programmable bradycardia pacing support is available
with the ICD/CRT-D following shock therapy delivery. Because a
delay in bradycardia pacing may avoid re-initiation of a
tachyarrhythmia, after a short blanking period (1 second), the
ICD/CRT-D will begin DDI bradycardia therapy at the post shock
pacing rate, amplitude, and pulse width for the programmed Post-
Shock Duration.
1-1734
Page 99

Lumax Technical Manual 97
Separate post shock programming of the following parameters is
available:
• Ventricular Pacing Configuration (RV)
• Basic Rate
• Rate Hysteresis
• AV Delay
If bradycardia pacing is still required after the post shock duration
expires, standard bradycardia pacing parameters are active.
2.8 EP Test Functions
Several EP test functions are available with the Lumax family of
ICD/CRT-Ds including; P and R-wave amplitude, pacing and
shock impedances, retrograde conduction and pacing threshold
measurements. Extensive testing of defibrillation thresholds as
well as the ability to verify the effectiveness of anti-tachycardia
pacing and defibrillation shocks are also available.
2.8.1 P and R-wave Amplitude Measurements
The Lumax ICDs/CRT-Ds provide a P-/R-wave test for measuring
the amplitude of intrinsic events during follow-up examination.
The test determines the amplitudes with a predetermined
temporary pacing mode.
To permit evaluation of the sensing function, the pacing rate must
be lower than the patient's intrinsic rate. In demand pacing, the
proper sensing function can be recognized if the interval between
intrinsic events and the following pacing pulse equals the basic
interval (if no Hysteresis is programmed). The following
parameters are programmable when performing the
measurements:
• Pacing Mode
• Pacing Rate
• Upper Rate (Dual Chamber modes only)
• Pulse Amplitude
• Pulse Width
1-1735
Page 100

98 Lumax Technical Manual
For evaluation of the sensing function, the pulse generator
features an intracardiac electrogram (IEGM) with marker signals
to indicate sensed and paced events.
2.8.2 Pacing Impedance Measurements
The Lumos ICDs/CRT-Ds have the ability to perform automatic
and manual pacing impedance measurements. The devices can
measure the pace impedance in any of the pacing configurations;
RA, RV, LV or BiV. In addition, the LV pacing polarity is also
programmable for impedance measurements (See Section 2.1).
The impedance is measured using a triggered mode with paces
of 2.8 Volts and 0.5 ms. During automatic measurements both
atrial (only with an atrial pacing mode) and ventricular (RV, LV or
BiV) impedances are measured. However, they will not be
measured if the normal pacing output is programmed to a value
greater than the impedance test output (2.8 V).
2.8.3 Shock Impedance Measurements
The Lumos ICDs/CRT-Ds have the ability to perform automatic
and manual painless shock impedance measurements. The
devices can measure the shock impedance by delivering an
undetectable 3 nj shock by applying 1 ma of current. The device
then measures the resulting voltage drop and calculates the
resulting shock impedance. This impedance measurement is tied
to the pacing impedance measurement (they are done
concurrently) and every four days. The results of these
impedance measurements are available through the devices
statistics.
2.8.4 Testing for Retrograde Conduction
Retrograde conduction from the ventricles to the atrium can be
assumed when a 1:1 relationship between the ventricular
stimulation and atrial depolarization has been obtained with a
constant coupling interval during ventricular stimulation. The
ICD/CRT-D features a test for measuring retrograde conduction
time. During operation of this test, the patient is paced (in VDI
mode) at an increased ventricular rate over several cycles while
the retrograde conduction time is measured. Therefore, the
pacing Rate must be programmed at a rate higher than the
patient’s intrinsic rhythm.
1-1736
 Loading...
Loading...