BIOTRONIK SE and KG BM2610 Users Manual

BIOMONITOR III ProMRI
Cardiac Monitor | premounted in the Fast Insert Tool OneStep |
BIOTRONIK Home Monitoring
Technical Manual
Revision: Axx (2018-11-08)
© BIOTRONIK SE & Co. KG
All rights reserved.
Specifications subject to modification, revision and improvement.
® All product names in use may be trademarks or registered trademarks held by
BIOTRONIK or the respective owner.
Index 439151Technical ManualBIOMONITOR III
BIOTRONIK SE & Co. KG
Woermannkehre 1
12359 Berlin · Germany
Tel +49 (0) 30 68905-0
Fax +49 (0) 30 6852804
sales@biotronik.com
www.biotronik.com

2
Table of Contents
Table of Contents
Table of Contents
Product Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Intended Medical Use. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
System Overview. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Diagnostic Functions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
General Safety Instructions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
General Information on Safe Handling of the Device. . . . . . . . 8
Operating conditions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Possible Complications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Possible Risks. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Handling . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Insertion Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Precautionary measures while programming . . . . . . . . . . . . 20
Follow-up . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Patient information. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
Replacement indications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
Explantation and Device Replacement . . . . . . . . . . . . . . . . . . 24
Parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Detection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Sensing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28
Home Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
Saving and Transmitting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
Technical Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
Mechanical Characteristics. . . . . . . . . . . . . . . . . . . . . . . . . . . 31
Electrical Characteristics . . . . . . . . . . . . . . . . . . . . . . . . . . . . 32
Battery Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
Legend for the Label . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
3
Product Description
Intended Medical Use
1 Product Description
Product Description1439151Technical ManualBIOMONITOR III
Intended Medical Use
Intended use
Form of diagnosis
BIOMONITOR III is the product name of an implantable cardiac monitor for monitoring and automatically recording heart rhythms. The primary purpose is to
provide early detection and diagnostics in the following clinical scenarios:
• Patients with clinical syndromes, which lead to
rhythm disturbances
• Patients with temporary clinical symptoms, including dizziness, palpitations,
syncope, or chest
Note: The cardiac monitor does not have any therapeutic function.
BIOMONITOR III is housed in the front part of the FIT OneStep insertion tool
(Fast Insert Tool). The insertion tool is used for forming the device pocket and for
subsequent positioning of the cardiac monitor in the subcutaneous left pectoral
area. The use of this tool ensures an optimal anatomical implantation site, which is
a requirement for recording meaningful subcutaneous ECGs.
The heart rhythm is continuously and automatically recorded and monitored. The
following are possible detection types:
• Atrial tachycardia
• High ventricular rate
• Asystole
• Bradycardia
• Sudden rate drop
Depending on the preset parameters, subcutaneous ECGs, and other data may be
recorded.
The patient can also trigger the recording of subcutaneous ECGs using the
Remote Assistant III accessory if subjectively symptomatic episodes occur.
The recordings can be transmitted to the BIOTRONIK Home Monitoring
Service Center. This enables physicians to perform complete diagnosis management.
pain, which may be the result of a cardiac rhythm disturbance
an increased risk of cardiac
Guidelines of
cardiological societies
It is recommended that the indications published by the DGK (German Cardiac
Society) and the ESC (European Society of Cardiology) are observed. This also
applies to the guidelines published by the HRS (Heart Rhythm Society), the ACC
(American College of Cardiology), the AHA (American Heart Association), and other
national cardiology associations.

4
Product Description
Intended Medical Use
Indications
Contraindications
Generally approved differential diagnostic methods, as well as the indications and
recommendations for the medical use of implantable cardiac monitors apply to
BIOMONITOR III.
The cardiac monitor BIOMONITOR III is an implantable monitoring system that
records subcutaneous ECGs. Recording is activated both automatically and by the
patient. Their use is indicated for the following cases:
• Clinical syndromes or increased risk of cardiac arrhythmias
• Temporary, unexplained symptoms that
• Evaluation of palpitations of unclear etiology
• Recurrent syncope of unclear etiology
• Confirmation or monitoring of atrial fibrillation
e
• Clarification of a cryptog
There are no known contraindications.
However, the particular patient's state of health determines whether a subcutaneous device will be tolerated long-term.
nic cerebrovascular stroke
may indicate cardiac arrhythmia
System Overview
5
Product Description
System Overview
Device
Parts
Incision tool
Insertion tool (with
premounted device)
BIOMONITOR III
This device is not available in all countries.
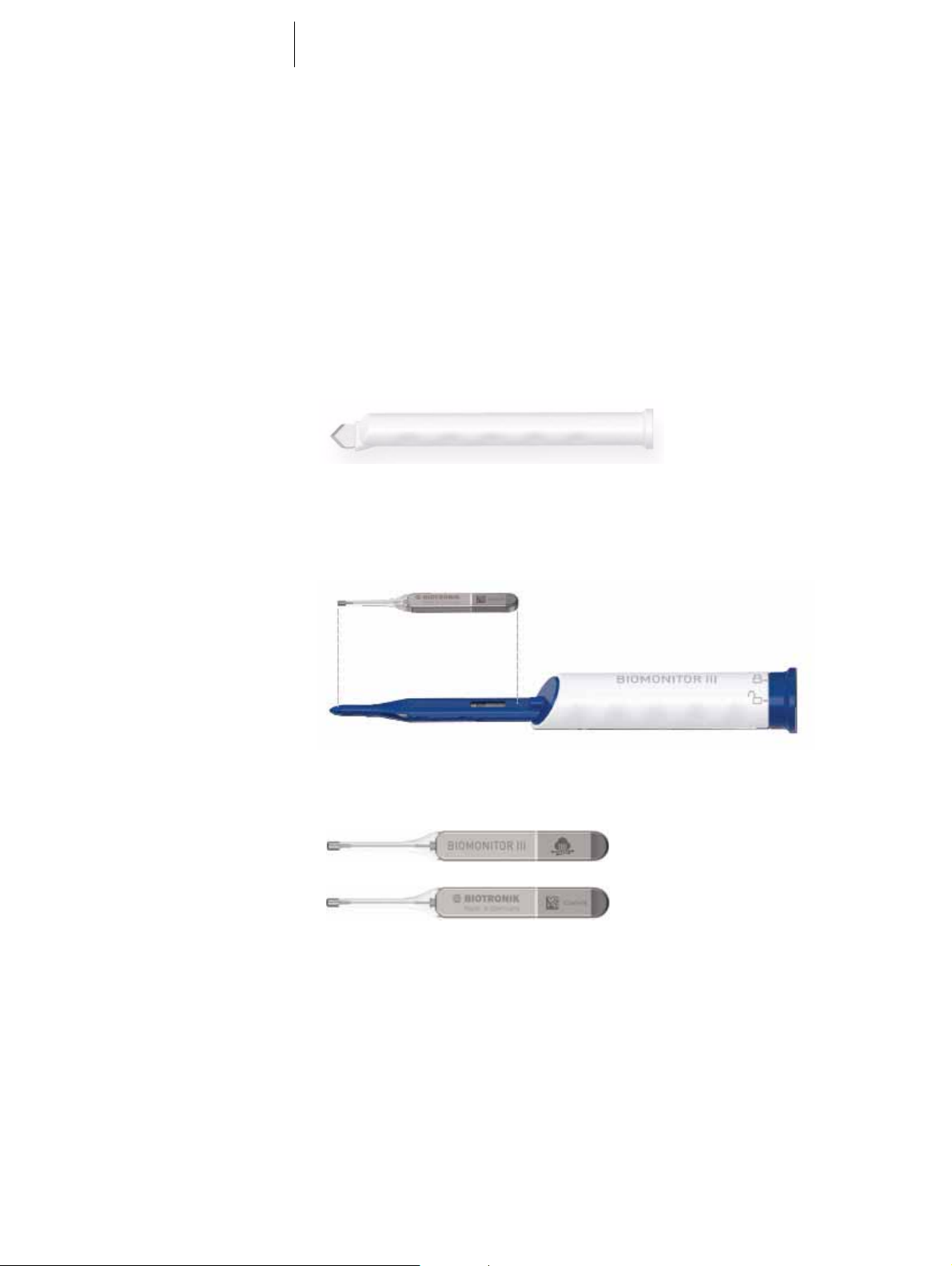
The system consists of the following parts:
• Device with flexible lead body; the device is inside the FIT OneStep insertion tool
• Incision tool
are
• Programmer and current softw
• The Remote Assistant III accessory for triggering recordings by the patient
(optional)
• The incision tool is used for making a surgical cut for the device pocket. The
blade is 13 mm wide and due to its design it can cut a maximum of 10 mm deep.
• The FIT OneStep insertion tool is used for controlled insertion and positioning of
the device. The device itself is sterile and located securely inside the tool, in the
blue tunneling tip in front of the white gripping sleeve; the whole device is not
visible from the outside, only the QR code of the device is visible through a small
window.
version for the device
Cardiac monitor
The device itself is called BIOMONITOR III. It consists of a solid housing and a flexible lead body.
The device's housing is made of hermetically sealed biocompatible titanium coated
in silicone. At the rounded end of the housing there is an opening in the coating, so
that the metal housing forms the antipole to the lead tip.
The flexible lead body is made of silicone and it has a fractally coated electrode on
its tip. The lead’s conductor also serves as an antenna for Home Monitoring.
The device has an overall length of 7.75 cm, which is approximately identical to the
sensing vector and correlates linearly with the sensing amplitude.
6
Product Description
System Overview
Programmer
Telemetry
BIOTRONIK Home Monitoring®The BIOTRONIK cardiac monitors provide a complete diagnosis management
Implantation and follow-up are performed with a portable BIOTRONIK programmer
using the current PSW software version 1901.A or higher.
The standard program is activated in the device on initial programming via the
programmer. The programmer is used to set parameter combinations, as well as
for interrogation and saving of data from the device. Electrocardiogram, subcutaneous ECG, markers, and functions are displayed simultaneously on the color
display.
Note: The programmer's ECG display must not be used for diagnostics, because it
does not meet all the standard requirements for diagnostic ECG devices
60601-2-25).
(IEC
Telemetric communication between the device and the programmer can be carried
out by applying the programming head (PGH).
system:
• With Home Monitoring, diagnostic information as well as device technical data
are automatically and wirelessly sent to a stationary or mobile transmitter via
an antenna in the lead body. The data is encrypted and sent from the transmitter
to the protected internet platform BIOTRONIK Home Monitoring Service Center
(HMSC) through a cellular phone network.
The received data is deciphered and evaluated; the criteria for
•
used for each patient can be set individually and the time of notification via email or fax can be configured.
• A clear overview of the results of this evaluation is displayed on the HMSC.
• Data transmission from the device is performed at a preset time with a daily
e message.
devic
• D
evice messages that indicate special events in the heart or in the device are
also forwarded at the preset time.
test message can be initiated at any time using th
• A
ately check the Home Monitoring function.
• The recording of a subcutaneous ECG in the BIOMONITOR III device can be triggered by the patient using the external Remote Assistant III device.
e programmer to immedi-
evaluation to be
BIOMONITOR III
order number
Package contents
This device is not available in all countries.
Device Order number
BIOMONITOR III 436066
The storage package includes the following:
• Sterile packaging with device premounted in the insertion tool and with incision
tool
• Serial number label
• Patient ID card
• Quick reference guide for in
Note: The technical manu
copy form in the storage package and/or is available in digital form on the internet.
sertion of
al pertaining to the cardiac monitor is included in hard
BIOMONITOR III

7
Diagnostic Functions
Product Description
Diagnostic Functions
General overview
Detection and data storage
Diagnostics
• Automatic functions facilitate quick and simple setting and control of the
BIOMONITOR III cardiac monitor.
• The sensing parameters are combined into one program (SensingConsult) and
can be set individually for each patient:
—Standard
— Sense after large PVCs
— Sense small PVCs
— Sense short intervals
— T-wave suppression
• The signals are automatically recorded and stored if a
and detection occurs.
• Multiple detection types can be activated simultaneously.
• The device can store episodes with subcut
length of 60 min.
•A total of 55 individual episodes with a length of at least 40 s each can be stored
atically. The maximum storage period for an individual episode is 60 s.
autom
A total of 4 recordings triggered by the patient
can be stored. The recording includes 7 min of pre-episode history and 0.5 min
of post-episode history relative to the time of triggering.
• When performing in-office follow-ups using the programmer, the unfiltered
aneous ECG is indicated with markers. The filtered subcutaneous ECG
subcut
can also be displayed if the corresponding settings have been made.
The following functions are available:
•Longest AF episode
•AF burden
•AF details:
—AF trend
—AF time distribution
—AF duration
— Ventricular rate during AF
• Cardiac rhythm disturbances detections:
— Atrial fibrillation
— High ventricular rate
—Bradycardia
— Sudden rate drop
— Asystole
— Patient trigger
• Activity:
— Heart rate variability
— Patient activity
— Heart rate
•Sensing
—R-wave trend
— Noise duration trend
aneous EC
detection type is activated
Gs with a minimum overall
with a duration of at least 7.5 min
Home Monitoring functions
Important medical information includes the following:
• Sustained atrial arrhythmias
• Sustained ventricular arrhythmias
• Current statistics
t
• Periodically recorded subcutaneous ECGs tha
individually adjustable timing interval in addition to the regular device message
This is a necessary condition for performing Home Monitoring-supported
follow-ups.
are transmitted according to an
8
!
!
General Safety Instructions
General Information on Safe Handling of the Device
2 General Safety Instructions
General Safety Instructions2439151Technical ManualBIOMONITOR III
General Information on Safe Handling of the Device
Follow notes and instructions
CAUTION
Risk to patient, risk to doctor, and interferences of device
The operation of electronic devices close to the heart is subject to special conditions. From transport to storage, concerns about sterility and technical complications, requirements for special care or risky therapies, as well as instructions
regarding implantation and care for persons who have an implanted device must
all be adhered to: The device is sensitive and must not be damaged, in order not to
harm patients.
• It is always necessary that all information in this, as well as related technical
manuals, must be observed and followed.
Safety instructions and
warnings in this technical
manual
This technical manual provides safety-relevant information on several topics:
• On the one hand, there are general safety warnings, which are fundamentally
valid. In this technical manual, the main topics are as below:
— General information on the safe handli
— Operating conditions
— Possible technical complications
— Possible medical complications
• On the other hand, there are special and general warnings regarding the insertion of the device that provide education and instructions for safely working with
d perform
an
topics are as below:
— Insertion Procedure
— Precautionary measures while programming
— Patient information
— Disposal
!
!
ing actions related to the device. In this technical manual, the main
Warnings have been particularly indicated in this technical manual
•
with a symbol and a signal word: Non-compliance with the instructions
can caus
e injury to the patient.
ng of the product
9
General Safety Instructions
General Information on Safe Handling of the Device
Technical manuals
Required expertise
The following technical manuals provide information about usage of the device
systems:
— Technical manual for the device
— Technical manual for the Home Monitoring Service Center (HMSC)
— Technical manuals for the programmer and the Remote Assistant III
— Technical manuals for the user interface
• Technical manuals are either included in hard copy form in the product package
or are available in digital form on the internet:
https:\\manuals.biotronik.com
• Follow all relevant technical manuals.
• Keep technical manuals for later use.
Note: Observe the quick reference guide included in the package contents.
In addition to having basic medical knowledge, the user must be thoroughly familiar
with the operating conditions and functionality of an implantable cardiac monitor.
Only qualified medical specialists with specialized knowledge are permitted to
implant the BIOMONITOR III and make diagnoses.

10
!
!
Operating conditions
General Safety Instructions
Operating conditions
CAUTION
Risk to patient and interferences of device
The operation of electronic devices in the proximity of the heart is subject to
special operating conditions. If these are not fulfilled, the functionality of the device
may be impaired; if the functionality of the device is impaired, the patient may be at
risk.
• Please observe the following operating conditions.
Care during
shipping and storage
Temperature
Sterile delivery
Device sterile packaging
Single use only
• Devices must not be stored or transported close to magnets or sources of electromagnetic interference.
• Note the effects of storage period and service time f
Data.
Extremely low and high temperatures affect the service time of the device's battery.
• Permitted for shipping and storage:
-10°C to +45°C (-40°F to 158°F)
The incision tool and the insertion tool as well as the device itself are delivered gassterilized (ethylene oxide). Sterility is guaranteed if the blister itself and the blister's
sealing paper have not been damaged.
Incision tool and insertion tool with premounted device are packaged inside a single
sealed sterile blister pack.
The incision tool and the insertion tool, as well as the device itself, are intended for
single use only.
• Do not use the incision tool or the insertion tool with premounted device if the
sterile packaging is damaged.
• Do not resterilize or reuse the incision tool or the insertion tool, or the device
itself.
or the device. See Battery

11
!
!
Possible Complications
General Safety Instructions
Possible Complications
CAUTION
Risk to patient and interferences of device
Electronic devices close to the heart may be subject to special complications. They
must be considered, so that the functionality of the device is not impaired and as a
result may put the patient at risk.
• Please take all the following safety information carefully into account.
General information on
medical complications
Possible technical failures
Electromagnetic
interference (EMI)
Complications for patients and device systems generally recognized among practitioners also apply to BIOTRONIK devices.
• Known complications are foreign body rejection phenomena, local tissue reactions, migration of the device, accumulation of fluid in the device pocket, transdermal erosions, as well as hemorrhage, and/or infections. Primary sources of
complication information include current scientific and technological knowledge.
Technical failure of a device system cannot be entirely ruled out. Possible causes
can include the following:
• Component failure of the device or an accessory
• Battery depletion
Any device can be sensitive to interference, for example, when external signals are
sensed as intrinsic rhythm.
• BIOTRONIK devices have been designed so that their susceptibility to EMI is
minimal.
• Under unfavorable conditions, especially during diagnostic and therapeutic
procedures,
the device that the data recording can be influenced or the device may be
damaged.
sources of interference may induce such a high level of energy into
 Loading...
Loading...