BIOTRONIK Ilesto 5 VR-T, ICD, Ilesto 5 VR-T DX, Ilesto 5 HF-T, Ilesto 7 VR-T Technical Manual
...
Ilesto
xxx
xxx
xxx
ICD Familie • Tachyarrhythmietherapie • Kardiale Resynchronisationstherapie
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
Technical Manual
Technická příručka
Brugermanual
Gebrauchsanweisung
Manual técnico
Käyttöohje
Manuel technique
Manuale tecnico di istruzione
Gebruikshandleiding
Instrukcja obsługi
Manual técnico
Bruksanvisning
• en
• cs
• da
• de
• es
•
• fr
• it
• nl
• pl
• pt
• sv
393468--B_GA_Ilesto-II_mul-01xx_Cover.indd 1 27.09.2012 15:44:48
BIOTRONIK SE & Co. KG
Woermannkehre 1
12359 Berlin · Germany
Tel +49 (0) 30 68905-0
Fax +49 (0) 30 6852804
sales@biotronik.com
www.biotronik.com
12-D-xx
Revision: B (2012-xx-xx)
© BIOTRONIK SE & Co. KG
All rights reserved. Specications subject
to modication, revision and improvement.
® BIOTRONIK Home Monitoring, IEGM-Online HD
and SMART Detecton are registered trademarks
of BIOTRONIK SE & Co. KG
0123
0681 2012
393468--B_GA_Ilesto-II_mul-01xx_Cover.indd 2 27.09.2012 15:44:48

1
Ilesto 5/7
VR-T, VR-T DX,
DR-T, HF-T
ICD Family
Tachyarrhythmia Therapy
Cardiac Resynchronization Therapy
Technical Manual for the Device
Doc. Id.: GA-HW_en--mul_393468-B
Index GA-HW_en--mul_393468-BTechn ical[nbsp ]Manual f or the[nbsp ]DeviceIles to 5/7 VR-T, VR-T DX, DR-T, HF-T

2

3 Table of Contents
Table of Contents
Table of Contents
Product Description. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Intended Medical Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
System Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Therapeutic and Diagnostic Functions. . . . . . . . . . . . . . . . . 11
General Safety Instructions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Operating Conditions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Possible Complications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Possible Risks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Implantation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Implantation Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Precautionary Measures while Programming . . . . . . . . . . 21
Magnet Response. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
Follow-up . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Patient Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
Replacement Indications. . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
Explantation and Device Replacement. . . . . . . . . . . . . . . . . 28
Parameters. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
Bradycardia / CRT . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
Tachycardia . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
Sensing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35
Diagnostics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36
Home Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 37
Technical Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 39
Mechanical Characteristics . . . . . . . . . . . . . . . . . . . . . . . . . 39
Electrical Characteristics . . . . . . . . . . . . . . . . . . . . . . . . . . . 40
Battery Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
Legend for the Label . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 45

4 Table of Contents

5 Product Description
1 Product Description
Product Description1GA-HW_en--mul_393468-BTechnical[nbs p ]Manual for the[nbsp ]DeviceI lesto 5/7 VR-T, VR-T DX, DR-T , HF-T
Intended Medical Use
Intended use Ilesto 5/7 is part of a familiy of implantable cardioverter-defibrillators (ICDs).
Primary objective of the therapy is to prevent sudden cardiac death. Furthermore,
the device is capable of treating bradycardia arrhythmias and cardiac resynchronization therapy with multisite ventricular pacing.
The implantation of an ICD is a symptomatic therapy with the following objectives:
• Termination of spontaneous ventricular fibrillation (VF) through shock delivery
• Termination of spontaneous ventricular tachycardia (VT) through antitachycardia pacing (ATP); in case of ineffective ATP or hemodynamically not tolerated VT, with shock delivery
• Cardiac resynchronization through multisite ventricular pacing (triplechamber devices)
• Compensation of bradycardia through ventricular (single-chamber devices) or
AV sequential pacing (DX, dual- and triple-chamber devices)
Diagnosis and therapy
forms
The device monitors the heart rhythm and automatically detects and terminates
cardiac arrest resulting from ventricular tachyarrhythmia. All major therapeutic
approaches from the field of cardiology and electrophysiology are included. BIOTRONIK Home Monitoring
®
enables physicians to perform therapy management at
any time.
Required expertise In addition to having basic medical knowledge, the user must be thoroughly
familiar with the operation and the operation conditions of a device system.
• Only qualified medical specialists having this special knowledge required are
permitted to use implantable devices.
• If users do not possess this knowledge, they must be trained accordingly.

6 Product Description
Indications Ilesto can treat life-threatening ventricular arrhythmias with antitachycardia
pacing and defibrillation.
Generally approved differential diagnostics methods, indications, and recommendations for ICD therapy apply to BIOTRONIK devices. See the guidelines of cardiology associations for guidance.
We recommend observing the indications published by the German Cardiac Society
(Deutsche Gesellschaft für Kardiologie, Herz- und Kreislaufforschung) and the
ESC (European Society of Cardiology). This also applies to the guidelines published
by the Heart Rhythm Society (HRS), the American College of Cardiology (ACC), the
American Heart Association (AHA), and other national cardiology associations.
Single-chamber and dual-
chamber
Single-chamber and dual-chamber ICDs are indicated for patients with the following risk:
• Sudden cardiac death caused by ventricular arrhythmias
Triple-chamber Triple-chamber ICDs are indicated for patients with the following risks:
• Sudden cardiac death caused by ventricular arrhythmias
• Congestive heart failure with ventricular asynchrony
Also indicated for primary prophylaxis in congestive heart failure patients is Ilesto.
Contraindications Known contraindications:
• Tachyarrhythmia caused by temporary or reversible irritation, e.g. poisoning,
electrolyte imbalance, hypoxia, sepsis or acute myocardial infarction
• Such frequent VT or VF that the therapies would cause an unacceptably rapid
depletion of the device batteries
• VT with few or without clinically relevant symptoms
• VT or VF treatable by surgery
• Concomitant diseases that would substantially limit a positive prognosis
• Accelerated idioventricular rhythm

7 Product Description
System Overview
Device family The complete Ilesto 5/7 device familyconsists of several device types with a DF-1/
IS-1 or DF4/IS-1 connection.
Single-chamber: VR-T and VR-T DX (only devices with a DF-1/IS-1 connection);
dual-chamber: DR-T; triple-chamber: HF-T. Not all device types are available in
every country.
Device The device's housing is made of biocompatible titanium, welded from outside and
thus hermetically sealed. The ellipsoid shape facilitates implantation in the pectoral muscle area. The connections for bipolar pacing and sensing (and unipolar
connections for the triple-chamber device) as well as for shock delivery are found
in the device header. The housing serves as a potential antipole during shock
delivery or in the case of unipolar lead configuration.
DF-1/IS-1 or DF4/IS-1 BIOTRONIK provides ICDs with headers for different standardized lead connec-
tions: DF-1/IS-1 and DF4/IS-1.
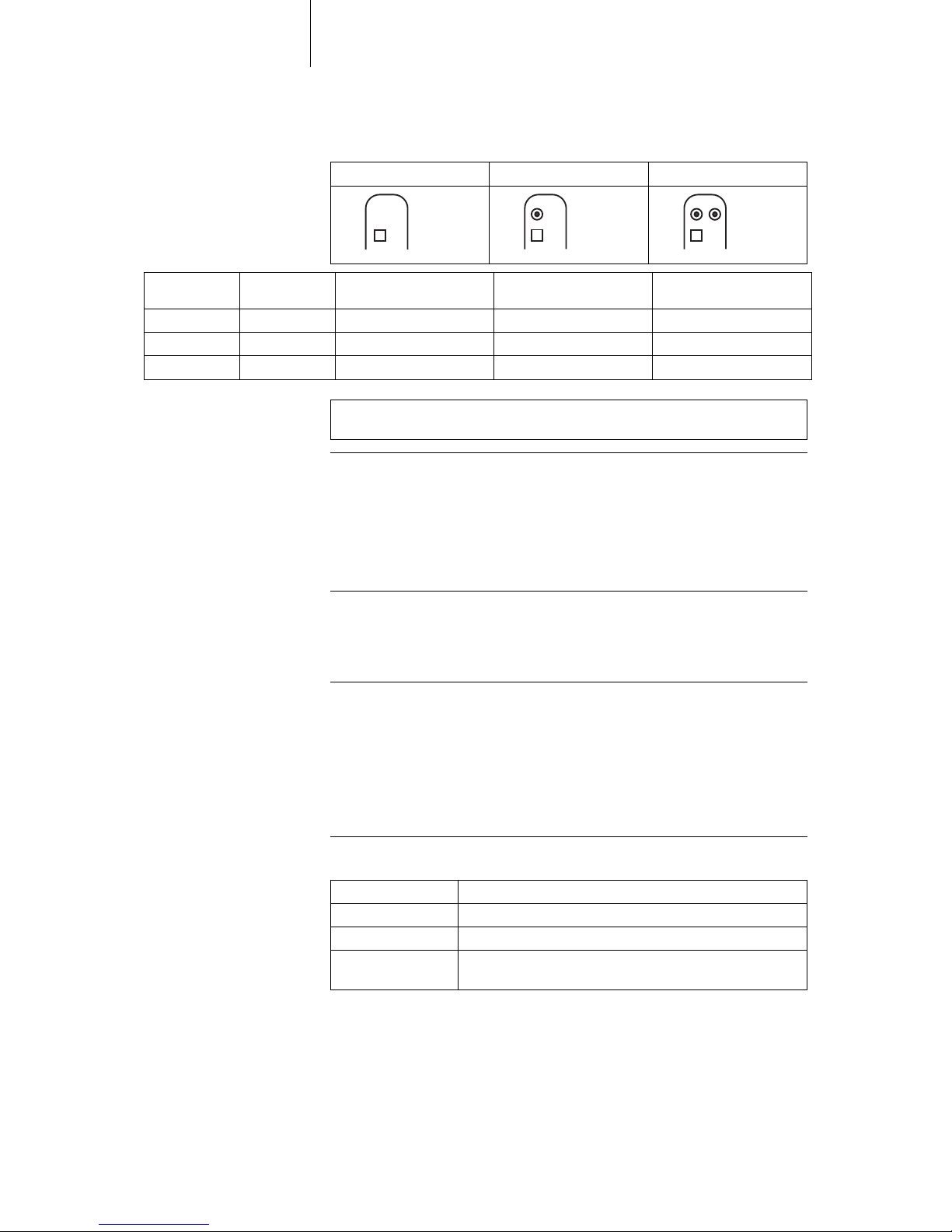
DF-1/IS-1 lead connection The device labeling provides information pertaining to possible lead connections
depending on the device type and pertaining to connection assignment:
Note: The device type DX can only be connected using a DF-1/IS-1 connector.
VR DX DR HF
DF-1
RV
DF-1
SVC
IS-1
RV
DF-1
RV
DF-1
SVC
IS-1
RA
IS-1
RV
DF-1
RV
DF-1
SVC
IS-1
RA
IS-1
RV
DF-1
RV
DF-1
SVC
IS-1
RA
IS-1
RV
IS-1
LV
Connector port Lead con-
nector
Configuration Implantation site Device type
RV DF-1 Shock coil Right ventricle VR, DX, DR, HF
SVC DF-1 Shock coil Superior vena cava VR, DX, DR, HF
RA IS-1 Bipolar Atrium DX, DR, HF
(R)V IS-1 Bipolar (Right) ventricle VR, DX, DR, HF
LV IS-1 Unipolar, Bipolar Left ventricle HF
8 Product Description
DF4/IS-1 lead connection The device labeling provides information pertaining to possible lead connections
depending on the device type and pertaining to connection assignment:
Leads BIOTRONIK leads are sheathed with biocompatible silicone. They can be flexibly
maneuvered, are stable long-term, and are equipped for active or passive fixation.
They are implanted using a lead introducer set. Some leads are coated with polyurethane which is known to increase the sliding properties for the lead. Leads with
steroids reduce inflammatory processes. The fractal design of the electrodes provides for low pacing thresholds. BIOTRONIK provides adapters to connect already
implanted leads to new devices.
Telemetry Telemetric communication between the device and the programmer can be carried
out following initialization either by applying the programming head (PGH) to the
device or by using radio frequency (RF) telemetry in the programmer. BIOTRONIK
calls this function SafeSync
®
.
Programmer Implantation and follow-up are performed with BIOTRONIK's portable pro-
grammer. There is one with integrated RF telemetry and one with a separate
SafeSync Module. The programmer is used during implantation to transfer the
current device program to the device. The pacing thresholds can be determined
and all tests can be performed during in-office follow-up. In addition to this, the
programmer is used to set mode and parameter combinations, as well as for interrogation and saving of data from the device. Leadless ECG, IEGM, markers and
functions are displayed simultaneously on the color display.
Modes The mode setting depends on the individual diagnosis:
VR DR HF
DF4
RV
DF4
RV
IS-1
RA
DF4
RV
IS-1
RA
IS-1
LV
Connector
port
Lead connector
Configuration Implantation site Device type
RA IS-1 Bipolar Atrium DR, HF
LV IS-1 Unipolar, Bipolar Left ventricle HF
RV, SVC DF4 Bipolar and shock Right ventricle VR, DR, HF
Note: The device's DF4 connector port may only be used for connecting leads
with a DF4 connector that conform to ISO 27186.
Device type Modes
VR VVI; VVIR; V00; OFF
DX VDD; VDDR; VDI; VDIR; VVI; VVIR; V00; OFF
DR, HF DDD; DDDR; DDI; DDIR; VDD; VDDR; VDI; VDIR
VVI; VVIR; AAI; AAIR; V00; D00; OFF

9 Product Description
NBD and NBG codes VVE is the NBD code for the antitachycardia mode of the single-chamber, dual-
chamber, and triple-chamber devices:
DDDR is the NBG code for the antibradycardia mode of the dual-chamber device:
DDDRV is the NBG code for the antibradycardia mode of the triple-chamber device:
VDDR is the NBG code for the antibradycardia mode of the single-chamber DX
device:
VVIR is the NBG code for the antibradycardia pacing modes of the single-chamber
device:
V Shock in the ventricle
V Antitachycardia pacing (ATP) in the ventricle
E Detection via IEGM analysis
D Pacing in the atrium and ventricle
D Sensing in the atrium and ventricle
D Pulse inhibition and pulse triggering
R Rate adaptation
D Pacing in the atrium and ventricle
D Sensing in the atrium and ventricle
D Pulse inhibition and pulse triggering
R Rate adaptation
V Multisite pacing in both ventricles
V Ventricular pacing
D Sensing in the atrium and ventricle
D Pulse inhibition and pulse triggering
R Rate adaptation
V Ventricular pacing
V Sensing in the ventricle
I Pulse inhibition in the ventricle
R Rate adaptation

10 Product Description
BIOTRONIK
Home Monitoring
®
In addition to effective pacing therapy, BIOTRONIK provides a complete therapy
management system:
• With Home Monitoring, diagnostic and therapeutic information as well as technical data are automatically sent to a stationary or mobile transmitter via an
antenna in the device header. The data are encrypted and sent from the transmitter to the BIOTRONIK Service Center via the cellular phone network.
• The received data are deciphered and evaluated. Each physician can set the criteria for evaluation to be used for each patient and can configure the time of
notification via E-mail, SMS or fax.
• A clear overview of the results of this analysis is displayed for the attending
physicians on the protected Internet platform Home Monitoring Service Center
(HMSC).
• Data transmission from the device is performed with a daily device message.
• Device messages, which indicate special events in the heart or in the device,
are forwarded immediately.
• A test message can be initiated at any time using the programmer to immediately check the Home Monitoring function.
Technical manuals The following technical manuals provide information about usage of the device sys-
tems:
• Technical manual for the implant
• Technical manual for the HMSC
• Technical manuals for the programmer and the SafeSync Module
• Technical manual for device programs as online help on the user interface and
as a PDF file in the Manual Library at www.BIOTRONIK.com
• Technical manuals for the leads
• Technical manuals for cables, adapters and accessories
Order numbers for
Ilesto with DF-1/IS-1 or
DF4/IS-1 connection
Not all device types are available in all countries:
Scope of delivery The storage package includes the following:
• Sterile container with device
•Serial number label
•Patient ID card
• Warranty booklet
• Technical manual for the implant
The sterile container includes the following:
• Device, blind plug DF-1 (if applicable) and blind plug IS-1 for device type HF
• Screwdriver
Ilesto 5 Ilesto 7
DF-1 DF4 DF-1 DF4
VR-T 383582 383584 383579 383581
VR-T DX 383596 — 390095 —
DR-T 383566 383568 383563 383565
HF-T 383550 383552 383547 383549

11 Product Description
Therapeutic and Diagnostic Functions
Diagnostic functions • Data from implantation and the most recent interrogations and follow-ups are
recorded as well as arrhythmia episodes; they are stored together with other
data to assess patients and the state of the device at any time.
• To check the lead for proper functioning, an automatic impedance measurement using subthreshold pacing pulses is performed in the device.
• Leadless ECG function: For all device types, far-field derivation can be measured without external leads between the right ventricular shock coil and
housing, which, depending on the implantation site, corresponds to ECG
derivation II or III (Einthoven).
• Once a telemetry connection has been established during a test procedure in
an in-office follow-up, the leadless ECG and the IEGM are displayed with
markers.
Antitachycardia pacing • The ICD can treat ventricular tachycardia with antitachycardia pacing (ATP);
ATP can also be delivered in the VF zone (ATP One Shot) when the stability criterion indicating that this will be effective before shock delivery (monomorphic
rapid VTs) is met.
• Depending on the device type, the device program contains not only the ICD
functions but also all pacemaker functions for 1, 2, or 3 chambers. The heart
rhythm is continuously monitored; each arrhythmia is classified according to
the heart rate and the adjustable detection criteria. Depending on the preset
values, antibradycardia as well as antitachycardia therapy is inhibited or delivered.
Cardioversion, defibrilla-
tion
• The ICD can treat ventricular tachyarrhythmia with cardioversion and/or defibrillation. Shock polarity and energy can be programmed individually. Shock
energies between 2.0 and 40 J are possible. Before delivery of the shock, the
ICD can be set to only deliver a shock when ongoing tachyarrhythmia is confirmed; during this time period the device can identify spontaneous conversion
of the tachyarrhythmia and cancel the charging process if necessary.
• The shock paths can be set between the different shock coils (SVC/RV) and/or
the housing.
Antibradycardia pacing and
CRT
• Innovative rate hystereses, automatic sensor functions, and a night program
promote the patient's intrinsic rhythm, avoid overdrive pacing, and facilitate
adaptation of the device to the individual needs of the patient.
• Setting an upper tracking rate for the atrium prevents unspecific atrial pacing,
thus reducing the risk of pacemaker-mediated tachycardia.
• Positive AV hysteresis functions support the intrinsic conduction and thus the
natural contraction sequence. Negative AV hysteresis functions support the
cardiac resynchronization therapy by maintaining pacing in stressful situations.
• For resynchronization of the ventricles, triple-chamber devices have functions
for multisite ventricular pacing with possible VV delays in either direction.
• To ensure that no additional surgery is necessary in case of a left-sided
increase of pacing threshold or undesired phrenic nerve stimulation, different
pacing polarities can be set for the left ventricular lead with a triple-chamber
device.
• Automatic active capture control is available for the right and left ventricle with
automated tracking of the pacing threshold or automatic threshold monitoring
(ATM) for trend analysis.

12 Product Description
Storing programs The parameter settings can be saved in 3 individual therapy programs.
Home Monitoring functions • The device automatically sends information to the transmitter once a day. It
also sends messages related to events, which are immediately forwarded to
the Service Center. In addition to this, test messages can be initiated using the
programmer.
• Appointments for Home Monitoring-supported follow-ups can be scheduled via
the HMSC. This applies to Ilesto 5/7.
• Important medical information in the device messages include the following:
— Atrial and ventricular arrhythmias
— Parameters relevant to leads in the atrium and ventricle: pacing thresh-
olds, sensing amplitudes, impedances
— Current statistics
—IEGM online HD
®
with up to 3 high definition channels

13 General Safety Instructions
2 General Safety Instructions
General Safety Instructions2GA-HW_en--mul_393468-BTechnical[nbsp ]Manual for the[nbsp ]DeviceIlesto5/7 VR-T, VR-T DX, DR-T,HF-T
Operating Conditions
Care during shipping and
storage
• Devices are not to be stored or transported close to magnets or sources of
electromagnetic interference.
• Note the effects of the storage duration; see Battery Data.
Delivery in shipment mode The device is delivered in shipment mode to protect the battery; capacitor
reforming required during storage could result in controlled extended charge
times of the shock capacitors.
• The shipment mode is displayed on the programmer after loading the device
program (it is deactivated during implantation on initial measurement of the
pacing impedance).
Temperature Extremely low and high temperatures affect the service time of the battery in the
device.
• Temperatures of 5°C to 45°C are permitted for transport, storage, and use.
Sterile delivery The device and the screwdriver have been gas-sterilized. Sterility is guaranteed
only if the blister and quality control seal have not been damaged.
Sterile container The device and screwdriver are packaged in two separately sealed blisters. The
inner blister is also sterile on the outside so that it can be transferred in a sterile
state during implantation.
Single use only The device and screwdriver are intended for single use only.
• Do not use the device if the package is damaged.
• The device must not be resterilized and reused.
 Loading...
Loading...