Page 1

Operator’s Manual
Microplate Spectrophotometer
Epoch
™
Page 2

Page 3

Epoch™
Microplate Spectrophotometer
Operator's Manual
August 2009
© 2009
Part Number 7201000
Revision A
BioTek® Instruments, Inc.
Page 4

ii | Preface
Notices
BioTek® Instruments, Inc.
Highland Park, P.O. Box 998
Winooski, Vermont 05404-0998 USA
All Rights Reserved
© 2009, BioTek® Instruments, Incorporated. No part of this publication may be
reproduced, transcribed, or transmitted in any form, or by any means electronic or
mechanical, including photocopying and recording, for any purpose other than the
purchaser’s use without written permission of BioTek Instruments, Inc.
Trademarks
BioTek® is a registered trademark, and Epoch™, Gen5™, and Take3™ are
trademarks of BioTek Instruments, Inc. BioCell™ is a trademark of BioTek
Instruments and is patented under U.S. patent number 5,963,318.
Microsoft
either registered trademarks or trademarks of Microsoft Corporation in the United
States and/or other countries.
All other trademarks are the property of their respective holders.
®
, Windows®, Windows XP, Windows 2000, and Windows Vista™ are
Restrictions and Liabilities
Information in this document is subject to change and does not represent a
commitment by BioTek Instruments, Inc. Changes made to the information in this
document will be incorporated in new editions of the publication. No
responsibility is assumed by BioTek for the use or reliability of software or
equipment that is not supplied by BioTek or its affiliated dealers.
BioTek® Instruments, Inc.
Page 5

Contents | iii
Contents
Notices ................................................................................................ ii
Contents ..............................................................................................iii
Contacting BioTek Instruments, Inc.......................................................... v
Revision History ................................................................................... vi
Document Conventions..........................................................................vii
Intended Use Statement....................................................................... viii
Quality Control.................................................................................... viii
Warranty and Product Registration ......................................................... viii
Warnings............................................................................................ viii
Hazards and Precautions........................................................................ ix
CE Mark .............................................................................................. xi
Electromagnetic Interference and Susceptibility ........................................xii
User Safety ........................................................................................ xiii
Safety Symbols ................................................................................... xiv
Introduction ...................................................................1
Product Description................................................................................ 2
Package Contents .................................................................................. 2
Optional Accessories .............................................................................. 3
Product Support & Service ...................................................................... 4
Installation.....................................................................5
Product Registration............................................................................... 6
1: Unpack and Inspect the Instrument...................................................... 6
2: Remove the Carrier Shipping Hardware................................................ 7
3: Select an Appropriate Location ........................................................... 8
4: Connect the Power Supply ................................................................. 9
5: Connect the Host Computer ............................................................... 9
6: Install the Software on the Host Computer ........................................... 9
7: Turn on the Reader ........................................................................... 9
Operational/Performance Qualification .................................................... 11
Repackaging and Shipping .................................................................... 11
Controlling Epoch Using Gen5 ......................................15
Overview............................................................................................ 16
Controlling the Reader with Gen5........................................................... 16
Instrument Qualification ..............................................19
Recommendations for Achieving Optimum Performance ............................ 20
Recommended Qualification Schedule..................................................... 21
Installation and Performance Qualification Procedures............................... 22
Absorbance Plate Test .......................................................................... 25
Liquid Testing ..................................................................................... 33
Epoch Operator’s Manual
Page 6

iv | Preface
Preventive Maintenance ...............................................43
Overview............................................................................................ 44
Required Materials ............................................................................... 44
Warnings & Precautions ........................................................................ 45
Routine Cleaning Procedure .................................................................. 45
Decontamination ................................................................................. 46
Specifications ...............................................................49
General Specifications .......................................................................... 50
Read Specifications .............................................................................. 50
Optical Performance............................................................................. 52
Error Codes ..................................................................55
Overview............................................................................................ 56
General Errors..................................................................................... 57
Fatal Errors......................................................................................... 65
BioTek
®
Instruments, Inc.
Page 7

Contacting BioTek Instruments, Inc. | v
Contacting BioTek Instruments, Inc.
BioTek® Instruments, Inc.
Highland Park, P.O. Box 998
Winooski, Vermont 05404-0998 USA
Customer Service and Sales
Internet: www.biotek.com
Phone: 888-451-5171 (toll free in the U.S.)
802-655-4740 (outside the U.S.)
Fax: 802-655-7941
E-Mail: customercare@biotek.com
Service/TAC
Phone: 800-242-4685 (toll free in the U.S.)
802-655-4740 (outside the U.S.)
Fax: 802-654-0638
E-Mail: tac@biotek.com
European Coordination Center/Authorized European Representative
BioTek® Instruments GmbH
Kocherwaldstrasse 34
D-74177 Bad Friedrichshall
Germany
Internet: www.biotek.de
Phone: +49 (0) 7136 9680
Fax: +49 (0) 7136 968 111
E-Mail: info@biotek.de
Epoch Operator’s Manual
Page 8

vi | Preface
Revision History
Revision Date Changes
A 8/2009 Initial release to Production
BioTek® Instruments, Inc.
Page 9
Document Conventions
This manual uses the following typographic conventions:
Example Description
Document Conventions | vii
This icon calls attention to important
Warning!
Caution
Note
A Warning indicates the potential for bodily harm and tells you
how to avoid the problem.
A Caution indicates potential damage to the instrument and tells
you how to avoid the problem.
text is primarily used for emphasis.
Bold
This icon calls attention to important information.
safety notes.
Epoch Operator’s Manual
Page 10
viii | Preface
Intended Use Statement
• The Epoch is a single-channel, automated, benchtop, general-purpose microplate
monochromator that performs optical density measurements of samples in a
microplate format. The user must evaluate this instrument with PC-based
software in conjunction with the specific assay. This evaluation must include the
confirmation that performance characteristics for the specific assay are met.
• This system is designed for use with PC-based software only. BioTek Gen5
software will provide the user with instrument control and data reduction.
• The intended use of this instrument is dependent on the instrument’s labeling. If
there is an IVD label, then the instrument may be used for clinical, research and
development, or other nonclinical purposes. If there is no such label, then the
instrument may only be used for research and development, or for other
nonclinical purposes.
Quality Control
It is considered good laboratory practice to run laboratory samples according to
instructions and specific recommendations included in the package insert or standard
laboratory protocol for the test to be conducted. Failure to conduct Quality Control
checks could result in erroneous test data.
Warranty and Product Registration
Please take a moment to review the warranty information that shipped with your product.
Please also register your product with BioTek to ensure that you receive important
information and updates about the product(s) you have purchased.
You can register online through the Customer Resource Center (CRC) at www.biotek.com
or by calling 888/451-5171 or 802/655-4740.
Warnings
Operate the instrument on a level surface away from excessive humidity.
Bright sunlight or strong incandescent light can reduce the linear
performance range of the instrument.
Measurement values may be affected by extraneous particles (such as
dust) in the microplate wells. A clean work area is necessary to ensure
accurate readings.
When operated in a safe environment according to the instructions in
BioTek® Instruments, Inc.
Page 11
this document, there are no known hazards associated with the
instrument. However, the operator should be aware of certain situations
that could result in serious injury; these may vary depending on the
instrument type. See Hazards and Precautions.
Hazards and Precautions
Hazards
The following hazards are provided to help avoid injury:
Hazards and Precautions | ix
Warning! Power Rating.
The instrument’s power supply must be
connected to a power receptacle that provides voltage and current within the
specified rating for the system. Use of an incompatible power receptacle may
produce electrical shock and fire hazards.
Warning! Electrical Grounding.
Never use a two-prong plug adapter to
connect primary power to the external power supply. Use of a two-prong
adapter disconnects the utility ground, creating a severe shock hazard.
Always connect the power supply directly to an appropriate receptacle with a
functional ground.
Warning! Internal Voltage.
the power supply before cleaning the outer surface of the instrument.
Warning! Potential Biohazards.
Always turn off the power switch and unplug
Some assays or specimens may pose a
biohazard. Adequate safety precautions should be taken as outlined in the
assay’s package insert. Always wear safety glasses and appropriate protective
equipment, such as chemically resistant rubber gloves and apron.
Warning! Liquids.
internal components creates a potential for shock hazard or instrument
Avoid spilling liquids on the reader; fluid seepage into
damage. If a spill occurs while a program is running, abort the program and
turn off the instrument. Wipe up all spills immediately. Do not operate the
instrument if internal components have been exposed to fluid.
Warning! Unspecified Use.
the guidelines and safeguards specified in this manual could result in a
hazardous condition.
Warning! Software Quality Control. The operator must follow the
manufacturer’s assay package insert when modifying software parameters
and establishing reading methods.
checks could result in erroneous test data.
Warning! Reader Data Reduction Protocol. No limits are applied to the
raw absorbance data. All information exported via computer control must be
thoroughly analyzed by the operator.
Epoch Operator’s Manual
Failure to operate this equipment according to
Failure to conduct quality control
Page 12

x | Preface
Precautions
The following precautions are provided to help avoid damage to the instrument:
Caution: Service.
The instrument should be serviced by BioTek authorized
service personnel. Only qualified technical personnel should perform
troubleshooting and service procedures on internal components.
Caution: Environmental Conditions.
temperature extremes. For proper operation, ambient temperatures should
Do not expose the system to
remain within the range listed in Appendix A, Specifications. Performance
may be adversely affected if temperatures fluctuate above or below this range.
Storage temperature limits are broader.
Caution: Sodium Hypochlorite.
to the recommended diluted sodium hypochlorite solution (bleach) for more
Do not expose any part of the instrument
than 20 minutes. Prolonged contact may damage the instrument surfaces. Be
certain to rinse and thoroughly wipe all surfaces.
Caution: External Power Supply.
with the instrument. Operate this power supply within the range of line
voltages listed on it.
Caution: Carrier Shipping Bracket.
bracket must be removed before operating the instrument and reinstalled
Only use the power supply shipped
The microplate carrier shipping
before repackaging the instrument for shipment.
Caution: Disposal.
wiring with lead solder. Dispose of the instrument according to Directive
This instrument contains printed circuit boards and
2002/96/EC, “on waste electrical and electronic equipment (WEEE)” or local
ordinances.
Caution: Warranty.
may
void the warranty. See Chapter 4, Maintenance for preventive
maintenance procedures.
Caution: Electromagnetic Environment.
responsibility to ensure that a compatible electromagnetic environment for
Failure to follow preventive maintenance protocols
Per IEC 61326-2-6 it is the user’s
this instrument is provided and maintained in order that the device will
perform as intended.
Caution: Electromagnetic Compatibility.
proximity to sources of strong electromagnetic radiation (e.g., unshielded
Do not use this device in close
intentional RF sources), because these may interfere with the proper
operation.
BioTek® Instruments, Inc.
Page 13
CE Mark
Based on the testing described below and information contained herein,
this instrument bears the CE mark.
Refer to the Declaration of Conformity for specific details.
DIRECTIVE 2003/108/EC: Electromagnetic Compatibility
Emissions—CLASS A
The system has been type-tested by an independent, accredited testing
laboratory and found to meet the requirements of EN 61326-1 and
EN 61326-2-6: Class A for Radiated Emissions and Line Conducted Emissions.
CE Mark | xi
Verification of compliance was conducted to the limits and methods of
EN 55011—CISPR 11). In a domestic environment it may cause radio
interference, in which case you may need to mitigate the interference.
Immunity
The system has been type-tested by an independent, accredited testing
laboratory and found to meet the requirements of EN 61326-1 and EN 61326-26 for Immunity. Verification of compliance was conducted to the limits and
methods of the following:
EN 61000-4-2, Electrostatic Discharge
EN 61000-4-3, Radiated EM Fields
EN 61000-4-4, Electrical Fast Transient/Burst
EN 61000-4-5, Surge Immunity
EN 61000-4-6, Conducted Disturbances from RFI
EN 61000-4-11, Voltage Dips, Short Interruptions and Variations
Epoch Operator’s Manual
Page 14

xii | Preface
Directive 73/23/EEC Low Voltage (Safety)
The system has been type-tested by an independent testing laboratory and was
found to meet the requirements of EC Directive 73/23/EEC for Low Voltage.
Verification of compliance was conducted to the limits and methods of the
following:
EN 61010-1 (2001) 2
nd
Edition. “Safety requirement for electrical equipment for
measurement, control and laboratory use. Part 1, General requirements.”
Directive 2002/96/EC: Waste Electrical and Electronic Equipment
Disposal Notice: This instrument contains printed circuit boards and wiring
with lead solder. Dispose of the instrument according to Directive
2002/96/EC, “on waste electrical and electronic equipment (WEEE)” or local
ordinances.
Directive 98/79/EC: In Vitro Diagnostics (if labeled for this use)
Product registration with competent authorities.
Traceability to the U.S. National Institute of Standards and Technology
(NIST):
Optical density measurements are traceable to NIST.
Electromagnetic Interference and Susceptibility
USA FCC CLASS A
Changes or modifications to this unit not expressly approved by the
manufacturer could void the user's authority to operate the
equipment.
This equipment has been tested and found to comply with the limits for a Class A
digital device, pursuant to Part 15 of the FCC Rules.
These limits are designed to provide reasonable protection against harmful
interference when the equipment is operated in a commercial environment. Like
all similar equipment, this equipment generates, uses, and can radiate radio
frequency energy and, if not installed and used in accordance with the instruction
manual, may cause harmful interference to radio communications. Operation of
this equipment in a residential area is likely to cause interference, in which case
the user will be required to correct the interference at his own expense.
BioTek® Instruments, Inc.
Page 15
Canadian Department of Communications Class A
This digital apparatus does not exceed Class A limits for radio emissions from
digital apparatus set out in the Radio Interference Regulations of the Canadian
Department of Communications.
Le present appareil numerique n'met pas du bruits radioelectriques depassant les
limites applicables aux appareils numerique de la Class A prescrites dans le
Reglement sur le brouillage radioelectrique edicte par le ministere des
Communications du Canada.
User Safety
This device has been type-tested by an independent laboratory and found to meet the
requirements of the following:
North America
User Safety | xiii
• Underwriters Laboratories UL 61010-1, 2001
“Safety requirements for electrical equipment for measurement, control and
laboratory use; Part 1: general requirements”
• Canadian Standards Association CAN/CSA C22.2 No. 61010-1-04
“Safety Requirements for Electrical Equipment for Measurement, Control
and Laboratory Use; Part 1: General Requirements”
For International User Safety requirements, see CE Mark on page xi.
Epoch Operator’s Manual
Page 16
xiv | Preface
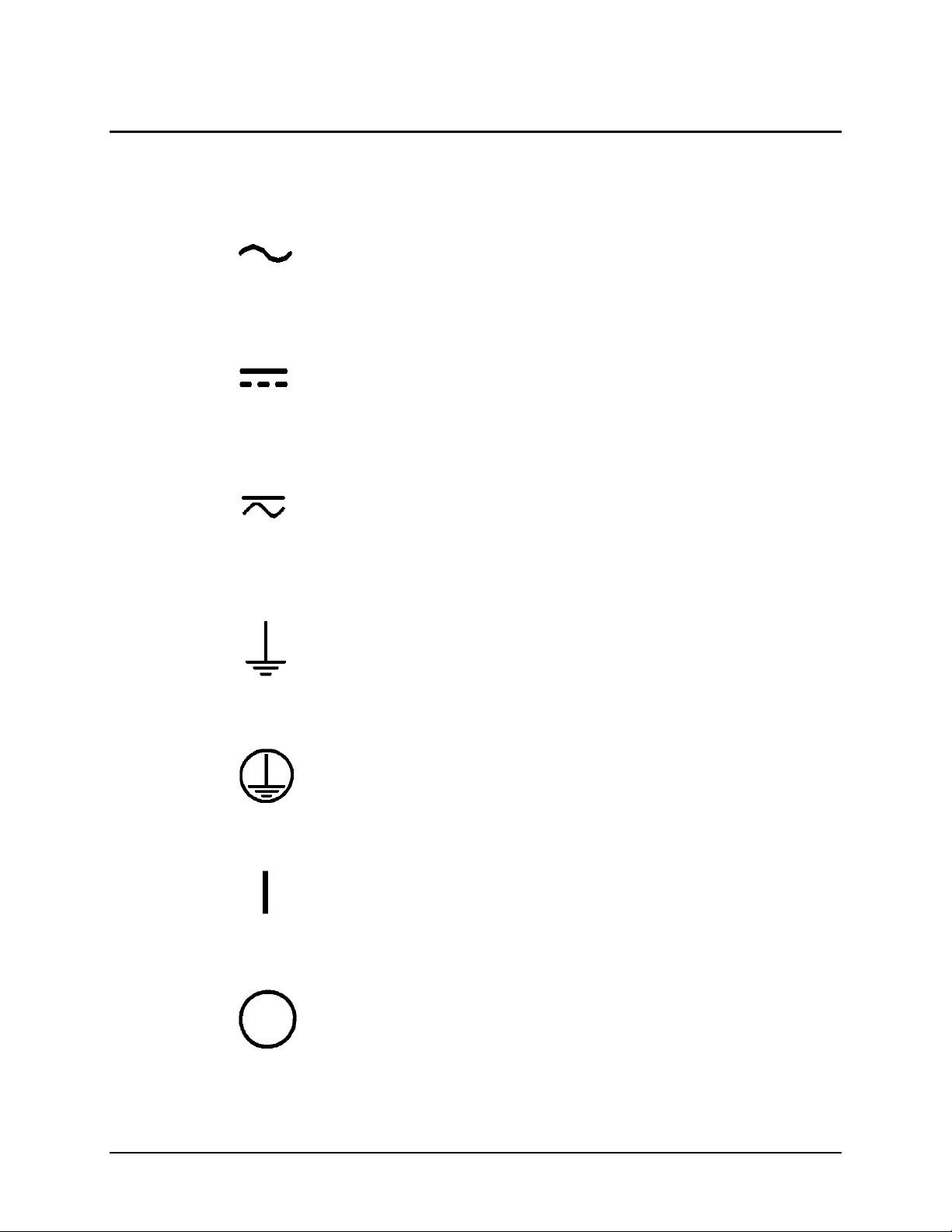
Safety Symbols
Some of the following symbols may appear on the instrument:
Alternating current
Courant alternatif
Wechselstrom
Corriente alterna
Corrente alternata
Direct current
Courant continu
Gleichstrom
Corriente continua
Corrente continua
Both direct and alternating current
Courant continu et courant alternatif
Gleich - und Wechselstrom
Corriente continua y corriente alterna
Corrente continua e corrente alternata
Earth ground terminal
Borne de terre
Erde (Betriebserde)
Borne de tierra
Terra (di funzionamento)
Protective conductor terminal
Borne de terre de protection
Schutzleiteranschluss
Borne de tierra de protección
Terra di protezione
On (Supply)
Marche (alimentation)
Ein (Verbindung mit dem Netz)
Conectado
Chiuso
Off (Supply)
Arrêt (alimentation)
Aus (Trennung vom Netz)
Desconectado
Aperto (sconnessione dalla rete di alimentazione)
BioTek® Instruments, Inc.
Page 17
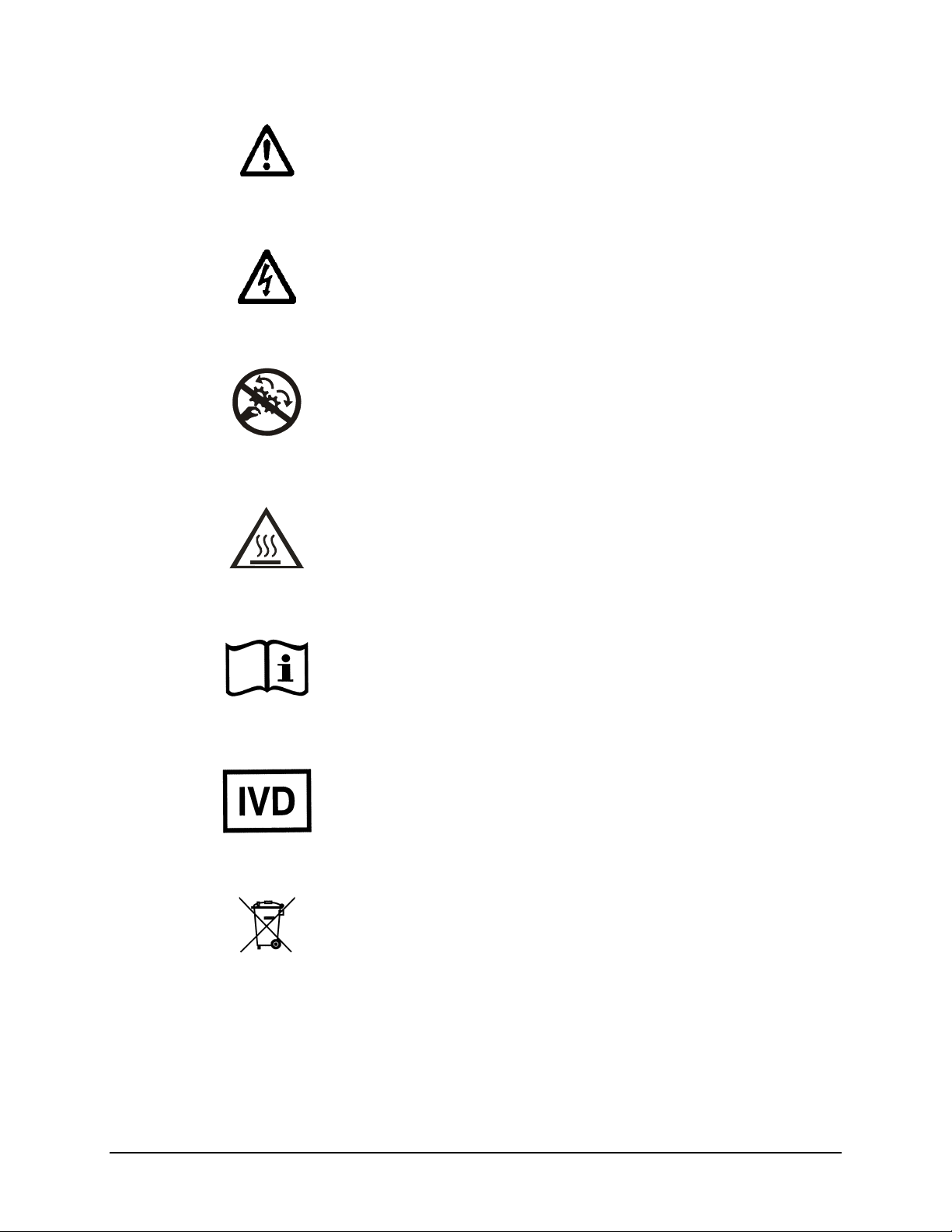
Caution (refer to accompanying documents)
Attention (voir documents d’accompanement)
Achtung siehe Begleitpapiere
Atención (vease los documentos incluidos)
Attenzione, consultare la doc annessa
Warning, risk of electric shock
Attention, risque de choc électrique
Gefährliche elektrische Schlag
Precaución, riesgo de sacudida eléctrica
Attenzione, rischio di scossa elettrica
Warning, risk of crushing or pinching
Attention, risque d’écrasement et pincement
Warnen, Gefahr des Zerquetschens und Klemmen
Precaución, riesgo del machacamiento y sejeción
Attenzione, rischio di schiacciare ed intrappolarsi
Warning, hot surface
Attention, surface chaude
Warnen, heiße Oberfläche
Precaución, superficie caliente
Attenzione, superficie calda
Safety Symbols | xv
Consult instructions for use
Consulter la notice d’emploi
Gebrauchsanweisung beachten
Consultar las instrucciones de uso
Consultare le istruzioni per uso
In vitro diagnostic medical device
Dispositif médical de diagnostic in vitro
Medizinisches In-Vitro-Diagnostikum
Dispositivo médico de diagnóstico in vitro
Dispositivo medico diagnostico in vitro
Separate collection for electrical and electronic
equipment
Les équipements électriques et électroniques font l’objet
d’une collecte sélective
Getrennte Sammlung von Elektro- und Elektronikgeräten
Recogida selectiva de aparatos eléctricos y electrónicos
Raccolta separata delle apparecchiature elettriche ed
elettroniche
Epoch Operator’s Manual
Page 18

xvi | Preface
BioTek
®
Instruments, Inc.
Page 19

Chapter 1
Introduction
This chapter introduces the Epoch and describes its hardware and
software features, and technical specifications. Instructions on how
to contact BioTek for Product Support & Service are included on
page 4.
Product Description.............................................................. 2
Package Contents ................................................................ 2
Optional Accessories ............................................................ 3
Product Support & Service .................................................... 4
Contacting the Technical Assistance Center ........................... 4
Returning Instruments for Service/Repair.............................. 4
Page 20
2 | Chapter 1: Introduction
Product Description
Powerful, versatile, and compact, the Epoch monochromator offers tunable
wavelength selection and wavelength scanning without the need for interference
filters. The single-channel reader is completely computer controlled via BioTek’s
intuitive and user-friendly Gen5 software. Its key features include the following:
• A variety of read modes including endpoint, kinetic, multiwavelength, and
spectral scanning.
• A monochromator for continuous wavelength selection from 200 to 999 nm in
1-nm increments, and a xenon flash lamp for both UV and visible light
absorbance measurements.
• Superior optical specifications, with an extended dynamic range of up to
4.000 OD.
• Ability to read standard microplates from 6- to 384-wells, Terasaki plates (with
adapter), BioTek’s patented BioCell quartz vessel for 1-cm measurements, and
the BioTek Take3 Multi-Volume Plate.
• Two reading speeds: normal and sweep.
Package Contents
Part numbers are subject to change over time. Please contact BioTek
Customer Care with any questions.
Item Part #
Epoch Operator’s Manual 7201000
Power supply 61062
Power cord
USB cable with Virtual COM Driver Software
to country
varies
according
of use
75108
7090204
BioTek® Instruments, Inc.
Page 21

Optional Accessories | 3
Optional Accessories
Part numbers are subject to change over time. Please contact BioTek
Customer Care if you have any questions.
Item Part #
7-filter Absorbance Test Plate for absorbance measurement testing 7260522
Epoch Product Qualification (IQ-OQ-PQ) package 7200515
BioCell quartz vessel for 1-cm wavelength fixed pathlength absorbance
measurements
BioCell adapter plate for containing up to eight BioCells 7270512
Terasaki plate adapter for 60-, 72-, and 96-well Terasaki plates 7220531
Absorbance Liquid Test Solutions:
BioTek Wetting Agent Solution (PN 7773002)
BioTek QC Check Solution No. 1 (25 ml) or
BioTek QC Check Solution No. 1 (125 ml)
Take3 Multi-Volume Plate TAKE3
7272051
7773002
7120779
7120782
Epoch Operator’s Manual
Page 22

4 | Chapter 1: Introduction
Product Support & Service
A superior support staff backs all of BioTek’s products. If your instrument(s) or
software ever fails to function properly, if you have questions about how to use or
maintain it, or if you need to send an instrument to BioTek for service or repair, please
contact our Technical Assistance Center (TAC).
Contacting the Technical Assistance Center
Our Technical Assistance Center is open from 8:30 AM to 5:30 PM (EST), Monday
through Friday, excluding standard U.S. holidays. You can send a fax or an e-mail
any time.
Phone: 800-242-4685 (in the U.S.) or 802-655-4740 (outside the U.S.)
Fax: 802-654-0638
E-Mail: tac@biotek.com
Please be prepared to provide the following information:
• Your name and company information
• A daytime phone or fax number, and/or an e-mail address
• The product name, model, and serial number
• The software part number and basecode version
• For troubleshooting assistance or instruments needing repair, the specific
steps that produce your problem, and any error codes displayed (see also
Appendix C, Error Codes).
Returning Instruments for Service/Repair
If you need to return an instrument to BioTek for service or repair, please contact
the TAC for a Return Materials Authorization (RMA) number before shipping the
instrument. Repackage the instrument properly (see Chapter 2, Installation), write
the RMA number on the shipping box, and ship to this address:
BioTek Instruments, Inc.
ATTN: RMA# xxxxx
100 Tigan Street
Highland Park
Winooski, Vermont 05404 USA
BioTek
®
Instruments, Inc.
Page 23

Chapter 2
Installation
This chapter includes instructions for unpacking and setting up the
Epoch, connecting to a PC, and repackaging the instrument.
Product Registration............................................................. 6
1: Unpack and Inspect the Instrument.................................... 6
2: Remove the Carrier Shipping Hardware.............................. 7
3: Select an Appropriate Location ......................................... 8
4: Connect the Power Supply ............................................... 9
5: Connect the Host Computer ............................................. 9
6: Install the Software on the Host Computer ......................... 9
7: Turn on the Reader ......................................................... 9
Operational/Performance Qualification .................................... 11
Repackaging and Shipping .................................................... 11
Before Repackaging the Instrument...................................... 11
Attach the Carrier Shipping Hardware ................................... 11
Repackage the Instrument .................................................. 12
Page 24
6 | Chapter 2 Installation
Product Registration
If you have not already done so, please register your product(s) with BioTek to ensure that
you receive important information and updates about the product(s) you have purchased.
Register online through BioTek’s Customer Resource Center (CRC) at www.biotek.com or
by contacting BioTek Customer Care.
Once registered, you can log into the Customer Resource Center and:
• Check the status and track your order
• Register your product warranty
• Manage your equipment inventory
• Access documentation on your products
• Download user manuals and software
• Check the status of your instrument’s service
1: Unpack and Inspect the Instrument
Save all packaging materials! If you need to ship the reader to
BioTek for repair or replacement, it must be carefully repackaged
Inspect the shipping box, packaging, instrument, and accessories for signs of damage.
If the reader is damaged, notify the carrier and your manufacturer’s representative.
Keep the shipping cartons and packing material for the carrier’s inspection. The
manufacturer will arrange for repair or replacement of your instrument immediately.
See Repackaging and Shipping at the end of this chapter for complete shipping
instructions.
according to the instructions, starting on page 11, using the original
packing materials. Using other forms of commericially available
packaging materials, or failuire to following the repackaging
instructions, may void your warranty. If the original packaging
materials have been damaged, replacements are available from
BioTek.
BioTek® Instruments, Inc.
Page 25

2: Remove the Carrier Shipping Hardware | 7
2: Remove the Carrier Shipping Hardware
The Epoch is shipped with shipping hardware that must be
1. Using a screwdriver, remove the shipping screw (PN 19502) and o-ring (PN
2. Using your fingers, remove the rubber plug (PN 19610).
3. Install the plug in the hole where the shipping screw was originally located,
The plug prevents light from entering the test chamber during
removed before the reader is used, and saved in case the instrument
needs to be repackaged for shipment. See Figure 1.
19608) assembly.
and insert the screw and o-ring in the hole where the plug was originally
located. See Figure 2
operation.
Shipping screw
and o-ring
Plug
Figure 1: Shipping screw, o-ring, and plug in shipping position
Epoch Operator’s Manual
Page 26

8 | Chapter 2 Installation
Plug
Figure 2: Shipping screw, o-ring, and plug in usage position
3: Select an Appropriate Location
For optimal operation, install the Epoch on a clean, level surface in an area where
ambient temperatures between 18°C (64°F) and 40°C (104°F) can be maintained. The
reader is sensitive to extreme environmental conditions. Conditions to avoid are:
Shipping screw
and o-ring
•
Excessive humidity: Condensation directly on the sensitive electronic
circuits can cause the instrument to fail internal self-checks. The humidity must
be in the range of 10% to 85%, non-condensing.
Excessive ambient light: Bright sunlight or strong incandescent light can
•
reduce the linear performance range and affect the instrument’s readings.
Dust: Optical density readings may be affected by extraneous particles (such
•
as dust) in the microplate wells. A clean work area is necessary to ensure
accurate readings.
Excessive vibration: Do not place the reader on a surface that is shared with
•
machines that cause the surface to vibrate.
BioTek® Instruments, Inc.
Page 27

4: Connect the Power Supply
Power Rating. The instrument’s power supply must be connected to
a power receptacle that provides voltage and current within the
specified rating for the system. Use of an incompatible power
receptacle may produce electrical shock and fire hazards.
Electrical Grounding. Never use a two-prong plug adapter to
connect primary power to the external power supply.
Use of a two-prong adapter disconnects the utility ground, creating a
severe shock hazard. Always connect the power supply directly to an
appropriate receptacle with a functional ground.
1. Connect the power cord to the external 24-volt power supply.
2. Connect the power supply’s outlet plug to the 24-VDC connector on the rear of
the instrument.
3. Tighten the plug barrel to retain the plug.
4: Connect the Power Supply | 9
4. Plug the other end of the power cord into an appropriate power receptacle.
5: Connect the Host Computer
The USB port is located on the rear panel of the reader.
1. Turn the computer off. If the reader is on, turn it off.
2. Using the supplied USB cable, connect one end of the cable to the USB port on
the computer.
3. Connect the other end of the cable to the USB port on the rear of the reader.
6: Install the Software on the Host Computer
Turn on the computer and install Gen5 software on the computer. Refer to the Gen5
Getting Started Guide for software installation (and registration) instructions.
7: Turn on the Reader
1. Locate the power on/off switch on the front of the instrument, next to the
carrier eject button. See Figure 3 below. The power on/off switch has a green,
internal LED lamp that is illuminated when the power is on. The carrier eject
Epoch Operator’s Manual
Page 28

10 | Chapter 2 Installation
button, when pressed, ejects the carrier out of the reader or pulls the carrier
back inside the reader to the carrier home position.
2. Turn on the power. The reader will perform an internal Self-Test and carrier
homing sequence. The carrier will eject outside the reader, then retract to its
home position inside the reader before it ejects again. Ensure that the reader
performs the carrier homing sequence and that the LED light is illuminated
while the power is on.
• If the test is successful, the reader is ready for use.
• If the test fails, note any error codes that are displayed in Gen5, and contact
BioTek. Appendix B, Error Codes, contains a list of error codes that may
appear in Gen5.
On/off switch
Carrier eject button
Figure 3: Power on/off switch and carrier eject button
BioTek® Instruments, Inc.
Page 29

Operational/Performance Qualification | 11
Operational/Performance Qualification
Your Epoch Microplate Spectrophotometer was fully tested at BioTek prior to
shipment and should operate properly following the successful completion of the
installation and setup procedures described throughout this chapter.
If you suspect that problems occurred during shipment, if you received the reader back
from BioTek following service or repair, and/or if regulatory requirements dictate that
Operational/Performance Qualification is necessary, turn to Chapter 4, Instrument
Qualification now to learn about BioTek’s recommended OQ/PQ procedures for the
Epoch.
An Installation/Operational/Performance Qualification (IQ/OQ/PQ)
package for the Epoch is available for purchase (PN 7200515). Contact
your local BioTek dealer for more information.
Repackaging and Shipping
Before Repackaging the Instrument
1. Decontaminate the reader prior to shipping (see Chapter 5, Preventive
Maintenance).
2. Replace the carrier shipping hardware according to the instructions below.
Attach the Carrier Shipping Hardware
The carrier shipping hardware must be re-attached to the carrier
1. If the carrier has not been returned to its home position inside the unit,
2. Turn off the Epoch, and unplug the 24 VDC power supply from the power
before the Epoch can be shipped. See Error! Reference source not
found. on the following page.
press the carrier eject button to retract the carrier.
outlet and from the power supply connector on the back of the reader.
Remove the USB cable from the reader.
3. Using a screwdriver, remove the shipping screw and o-ring from the
reader, and, using your fingers, remove the plug from the reader.
Epoch Operator’s Manual
Page 30

12 | Chapter 2 Installation
4. Insert the plug in its original hole and use the screwdriver to re-install the
shipping screw and o-ring in its original position (see Figure 1).
Repackage the Instrument
Use the instrument’s original shipping container and packaging
material. This shipping system was designed to be used no more
than five times. If the container is damaged and/or has been used
more than five times, contact BioTek for a new set of shipping
materials, and ask for PN 7203003.
The shipping box, accessories box, foam caps, and so on are
included as a whole set under this part number and cannot be
ordered separately.
The instrument’s packaging design is subject to change over time.
If the instructions in this section do not appear to apply to the
packaging materials you are using, please contact BioTek’s
Technical Assistance Center for guidance.
Ensure that the Epoch carrier shipping hardare has been attached to the reader’s
carrier as instructed in the preceding section, Attach the Carrier Shipping
Hardware. Refer to Figure 4 on the following page when performing these steps:
1. Place the foam cap into the bottom of the shipping container. Note the
orientation of the foam cap in the box as illustrated in Figure 4.
2. Place the accessories box back into the shipping container.
3. Place the reader inside the original plastic bag and carefully lower the
reader into the two foam caps in the bottom of the box. Note the orientation
of the reader in the box.
4. Place two foam caps over the reader.
5. Bundle the power cord and place it into the accessories box as shown.
6. Place the power supply and USB cable in the accessories box.
7. Close the top of the box and secure it with shipping tape.
8. A Return Materials Authorization (RMA) number must be obtained
before returning equipment for service. Contact BioTek’s Technical
Assistance Center for this number, then write “
in large, clear letters on the outside of the shipping container.
9. Return the box to BioTek at the address provided in the Product Support &
Service section of Chapter 1.
RMA” and the RMA number
BioTek® Instruments, Inc.
Page 31

Repackaging and Shipping | 13
Figure 4: Packing the Epoch
Epoch Operator’s Manual
Page 32

14 | Chapter 2 Installation
BioTek
®
Instruments, Inc.
Page 33

Chapter 3
Controlling Epoch Using
Gen5
The Epoch can be controlled only using software installed on a PC
connected to the reader via the computer's USB port. This chapter
describes how to configure BioTek Gen5 to control the reader.
Overview............................................................................ 16
Controlling the Reader with Gen5........................................... 16
Setting Up Gen5 ................................................................ 16
Problems .......................................................................... 17
Getting Started with Gen5 .................................................. 17
Page 34

16 | Chapter 3: Controlling Epoch Using Gen5
Overview
The Epoch is a completely computer-controlled instrument. Gen5 is the primary
operating software.
For users requiring a custom interface to the Epoch, there are other methods of
computer-controlling the reader. A typical example of this requirement is the need to
integrate the Epoch into an automated system.
Controlling the Reader with Gen5
Before installing Gen5, verify that your computer meets the
L
minimum system requirements specified in the Gen5 Getting
Started Guide.
Setting Up Gen5
The following instructions briefly show you how to set up Gen5 for operation of
the reader. Refer to the Gen5 Getting Started Guide or Help system for more detailed
instructions.
1. Turn off the computer and the reader. Connect the USB cable (PN 75108)
between the two machines:
2. Turn on both machines.
3. Install Gen5 on the computer’s hard drive and register the software with
BioTek.
4. Refer to the instructions that shipped with the “USB Virtual COM Driver
Software” CD to install the necessary drivers and identify the Com Port
number.
5. Start Gen5.
6. Log in if prompted (Gen5 Secure). The default System Administrator
password is
7. When the “Welcome to Gen5” screen appears, select System Menu.
8. From Gen5’s main screen, select System > Reader Configuration to
open the Reader Configuration dialog.
admin.
9. Click Add to open the Reader Settings dialog.
¾ Gen5 and Gen5 Secure: Up to two readers may be added in Gen5.
10. Use the drop-down list in Reader Type to select the appropriate reader.
11. Enter the appropriate Com Port.
BioTek
®
Instruments, Inc.
Page 35

Controlling the Reader with Gen5 | 17
12. Enter a Baud Rate of 9600.
13. Click Test Comm. Gen5 will attempt to communicate with the reader.
Problems
If Gen5 fails to communicate with the reader, try the following:
• Is the reader connected to the power supply and turned on?
• Is the communication cable firmly attached to both the reader and the
• Did you select the correct Reader Type in step 10?
• Repeat step 11 and choose a different Com Port.
• Did you install the USB driver software?
¾ If you receive “The Reader is communicating!” message, click
then click
OK again to save the settings. Click Close at the Reader
OK, and
Configuration dialog to return to the main screen.
¾ If the test is not successful and you receive an error message, refer to the
Problems section below, or to the Troubleshooting section of the Gen5
Help system for assistance.
¾ Gen5 Secure only: An Audit Trail dialog will appear after exiting Reader
Configuration, whenever you add, modify, or delete a reader. If desired,
enter any comments, then click
Close.
computer?
• Confirm that the reader has passed its Self-Test. The reader will not
communicate if it fails an internal system test.
If the test still fails, refer to the Troubleshooting section in the Gen5 Help system
for further assistance or contact BioTek’s Technical Assistance Center.
Getting Started with Gen5
The following instructions briefly show you how to define and perform a Quick
Read in Gen5 (
because you can perform a reading without having to take the time to create a new
protocol.
If the reading is part of an experiment or assay that you will perform numerous
times, you will need to create a new protocol
Refer to the Gen5 Help system early and often to learn how to create protocols,
assign well identifiers, read plates, print reports, perform data reduction, and
more.
File > New Experiment > Default Protocol). It’s called “Quick”
(File > New Protocol).
Epoch Operator’s Manual
Page 36

18 | Chapter 3: Controlling Epoch Using Gen5
To perform a Quick Read:
1. At the Gen5 Welcome screen, select System Menu > File > New
Experiment
. (Alternative: Select Read a Plate at the Welcome screen,
then proceed to step 4 below.)
2. Click Default Protocol, then click OK. Gen5 opens the Experiment
workspace, which includes the Protocol menu tree and Plate screen.
3. Select Plate > Read or click the Read Plate icon. The Procedure dialog
opens.
¾ Gen5 and Gen5 Secure: If more than one reader was added in Gen5, the
Instrument Selection dialog will appear instead of the Procedure dialog.
4. Select a Plate Type.
The plate the user is using must match the plate defined in Gen5.
Otherwise, the results of the read may be invalid. For example, if a 96well plate is defined for a procedure, a 96-well plate must be used
when running an experiment based on that procedure.
5. Click Read to open the Read Step dialog.
6. Select a Read Type.
7. Select the wavelength(s) at which the plate will be read.
8. Define other reading parameters, as desired. Click Help for assistance.
9. When complete, click OK to return to the Procedure dialog.
¾ Click
Validate if you would like Gen5 to verify the defined parameters.
If all parameters are valid, you will receive confirmation. If any
parameters are invalid, Gen5 provides information for correcting the
problem. Refer also to the Troubleshooting section of the Help system.
10. Click OK again to save and close the Procedure dialog. The Plate Reading
dialog opens.
11. Enter any desired information, place the plate on the carrier, then click
READ to begin the plate read. The Save As dialog opens.
12. Enter a file name, select a file location, and click Save.
13. Click OK when the Load Plate dialog appears. The plate is read.
¾ To view the raw data results, use the Data drop-down arrow in the Plate
screen to select one wavelength. The results are displayed for the
selected wavelength. Repeat, for other wavelengths.
¾ To analyze, manipulate, or print results, define the Protocol parameters.
Refer to the Gen5 Help system for instructions.
Gen5 Reader Control does not support data reduction.
®
BioTek
Instruments, Inc.
Page 37

Chapter 4
Instrument Qualification
This chapter discusses the tasks and procedures necessary for
verifying and qualifying the Epoch’s performance on an ongoing
basis.
Recommendations for Achieving Optimum Performance ............ 20
Recommended Qualification Schedule..................................... 21
Installation and Performance Qualification Procedures............... 22
Routine Procedure ............................................................. 22
System Self-Test ............................................................... 23
Absorbance Plate Test .......................................................... 25
Description ....................................................................... 25
Test Plate Certificates......................................................... 26
Entering the Absorbance Test Plate Data ............................... 27
Running the Absorbance Plate Test....................................... 27
Results............................................................................. 30
Liquid Testing ..................................................................... 33
Stock Solution Formulation.................................................. 34
Liquid Test 1 ..................................................................... 35
Liquid Test 2 ..................................................................... 37
Liquid Test 3 ..................................................................... 40
Page 38

20 | Chapter 4: Instrument Qualification
Recommendations for Achieving Optimum Performance
An Installation/Operational/Performance Qualification (IQ/OQ/PQ)
package (PN 7200515) for the Epoch is available upon request.
Contact your local dealer for more information.
• Microplates should be perfectly clean and free from dust or bottom scratches.
Use new microplates from sealed packages. Do not allow dust to settle on the
surface of the solution; use microplate covers when not reading the plate. Filter
solutions to remove particulates that could cause erroneous readings.
• Although the Epoch supports most flat, U-bottom, and V-bottom microplates,
the reader achieves optimum performance with optically clear, flat-bottomed
wells.
• Non-uniformity in the optical density of the well bottoms can cause loss of
accuracy, especially with U- and V-bottom polyvinyl microplates. Check for
this by reading an empty microplate. Dual wavelength readings can eliminate
this problem, or bring the variation in density readings to within acceptable
limits for most measurements.
• Inaccuracy in pipetting has a large effect on measurements, especially if
smaller volumes of liquid are used. For best results, use at least 100 µL per well
in a 96-well plate and 25 µL in a 384-well plate.
• Dispensing solution into 384-well plates often traps air bubbles in the wells.
Dual wavelength reads will cancel most of these errors; however, for best
results, they should be removed by degassing the plate in a vacuum chamber
prior to reading.
• The inclination of the meniscus can cause loss of accuracy in some solutions,
especially with small volumes. Agitate the microplate before reading to help
bring this problem within acceptable limits. Use Tween 20, if possible (or some
other wetting agent), to normalize the meniscus. Some solutions develop
menisci over a period of several minutes. This effect varies with the brand of
microplate and the solution composition. As the center of the meniscus drops
and shortens the light path, the density readings change. The meniscus shape
will stabilize over time.
BioTek® Instruments, Inc.
Page 39

Recommended Qualification Schedule | 21
Recommended Qualification Schedule
The schedule shown below defines the factory-recommended intervals for
performance testing for an Epoch used for one shift seven days a week.
The risk factors associated with your tests may require that the
Operational and Performance Qualification procedures be performed
more or less frequently than shown below.
IQ OQ PQ
Tasks/Tests
All models:
Unpacking, installation, setup, and
verification
Software Documentation/Verification
Software Wavelengths Table Verification
System Test
“Run Assay” Test
Absorbance Plate Test
Absorbance Liquid Test 1
Absorbance Liquid Test 2*
Absorbance Liquid Test 3 (optional)**
Initially
9
9 9 9
9 9 9
9 9 9
* If you have an Absorbance Test Plate, run Liquid Test 1. If you do not have an Absorbance
Test Plate, run Liquid Test 2.
** Liquid Test 3 is optional; it is provided for sites requiring verification at wavelengths lower
than those attainable with the Absorbance Test Plate.
Initially/
Annually
9 9
9 9
9
9
9
Monthly Quarterly
9
9
9
Epoch Operator’s Manual
Page 40

22 | Chapter 4: Instrument Qualification
Installation and Performance Qualification Procedures
Tests outlined in this section may be utilized to confirm initial and ongoing
performance of the Epoch.
Your reader was fully tested at BioTek prior to shipment and should operate properly
upon initial setup. If it is suspected that problems may have occurred during shipment,
if you reshipped the reader, or if regulatory requirements dictate that Performance
Qualification is necessary, the following tests should be performed. After the initial
confirmation of operation, the Absorbance Plate Test is performed monthly and Liquid
Testing should be performed quarterly. The System Test and Absorbance Plate Test
can be run from Gen5 via
System Self-Test: Verifies proper gains, bulb operation, low electronic noise,
•
and optional incubator functionality.
Absorbance Plate Test: Confirms the mechanical alignment, optical
•
accuracy, linearity, repeatability, and wavelength accuracy of the instrument.
System > Diagnostics.
Liquid Testing: Quantifies performance of the instrument using liquids,
•
which verifies operation in a way that glass test filters cannot.
Routine Procedure
To ensure proper operation of the Epoch on an ongoing basis, the System Self-Test
and the Absorbance Plate Test should be conducted monthly.
Run the Reader System Test to verify that the light levels and electronic noise at all
set wavelengths fall within factory acceptance criteria. The resulting report will
show pass/fail results and the part and version numbers of the basecode software
loaded on the reader.
To run the System Test using Gen5, select
System Test
Run the Absorbance Plate Test to confirm the mechanical alignment,
accuracy/linearity, repeatability, and wavelength accuracy.
To run this test using Gen5, select
In order to perform the wavelength accuracy scan, you must select the Peak
Wavelength feature in Gen5.
.
System > Diagnostics > Run
System > Diagnostics > Test Plates > Run.
BioTek® Instruments, Inc.
Page 41

System Self-Test
The System Self-Test confirms that the light levels and electronic noise at all set
wavelengths fall within factory acceptance criteria, and accomplishes this by
measuring the air and dark readings and evaluating them to ensure they fall
within specified ranges.
The Epoch automatically runs an internal System Test each time it is powered on.
If the carrier shipping hardware has not been removed from the carrier, the poweron System Test will not be initiated and the instrument will beep (see step 2:
Remove the Microplate Carrier Shipping Hardware in Chapter 2).
If the reader fails the Self-Test, note any error messages displayed in Gen5, and
refer to Appendix B for a list of possible error codes.
To obtain a report of the System Test values for either periodic testing
documentation or troubleshooting (see the sample System Test Report in Figure 6
on the following page), follow the instructions in Gen5. The instrument’s System
Test will be conducted and the results reported in a pass/fail format.
Installation and Performance Qualification Procedures | 23
Gen5 System Test Report
Reader: Epoch (Serial Number: 182843)
Basecode: P/N 7200201 (v1.07)
Date and Time: 7/14/2009 12:02:36 PM
User: Administrator
Company: BioTek
Comments:
Test Results
Operator ID:___________________________________________________
Notes:__________________________________________________________
________________________________________________________________
SYSTEM SELF TEST
7200201 Version 1.07 Serial Number 182843
Checksum #1 = CEF3, Checksum #2 = 1D05
VOLTAGE TESTS
24VDC PS V = 24.101
+5VDC PS V = 4.933
Flash 350V = 351
Flash 400V = 400
Flash 450V = 448
Flash 525V = 526
Flash 600V = 600
ABSORBANCE
Epoch Operator’s Manual
Page 42

24 | Chapter 4: Instrument Qualification
Noise Test (Dark Air, Flash On)
Channel: Ref Meas
Noise Max: 5392 16857
Noise Min: 5392 16855
Noise Delta: 0 2
Wavelength 1: 200nm
Cal Data: Rst: 2 Gain: 1.07 Rst/Gain: 1.867
Test Data: Rst: 2 Gain: 1.12 Rst/Gain: 1.781
Channel: Ref Meas
Total Air: 14869 43719
Dark Air (On): 5390 2399
Signal Air: 9479 41320
Wavelength 2: 352nm
Cal Data: Rst: 4 Gain: 1.94 Rst/Gain: 2.062
Test Data: Rst: 4 Gain: 2.08 Rst/Gain: 1.921
Channel: Ref Meas
Total Air: 8944 45824
Dark Air (On): 5391 4411
Signal Air: 3553 41413
Wavelength 3: 620nm
Cal Data: Rst: 2 Gain: 1.97 Rst/Gain: 1.015
Test Data: Rst: 2 Gain: 1.96 Rst/Gain: 1.023
Channel: Ref Meas
Total Air: 9165 45154
Dark Air (On): 5390 4140
Signal Air: 3775 41014
Wavelength 4: 790nm
Cal Data: Rst: 1 Gain: 1.38 Rst/Gain: 0.722
Test Data: Rst: 1 Gain: 1.36 Rst/Gain: 0.734
Channel: Ref Meas
Total Air: 11031 43909
Dark Air (On): 5388 2890
Signal Air: 5643 41019
Wavelength 5: 860nm
Cal Data: Rst: 1 Gain: 1.94 Rst/Gain: 0.515
Test Data: Rst: 1 Gain: 1.94 Rst/Gain: 0.515
Channel: Ref Meas
Total Air: 9394 45433
Dark Air (On): 5389 4098
Signal Air: 4005 41335
Wavelength 6: 962nm
Cal Data: Rst: 1 Gain: 2.12 Rst/Gain: 0.472
Test Data: Rst: 1 Gain: 2.13 Rst/Gain: 0.469
Channel: Ref Meas
Total Air: 9033 45624
Dark Air (On): 5389 4503
Signal Air: 3644 41121
TEST SENSOR
Cal 13869
Self-Test 13868
Delta -1
AUTOCAL ANALYSIS
Upper Left Corner: x = 287 y =15615
Lower Left Corner: x = 279 y =21835
Lower Right Corner: x = 10034 y =21851
Upper Right Corner: x = 10045 y =158630
Delta 1: 287 - 279 = +8
BioTek® Instruments, Inc.
Page 43

Delta 2: 10045 - 10034 = +11
Delta 3: 15630 - 15615 = +15
Delta 4: 21851 - 21835 = +16
MONOCHROMATOR
A = +0.000000 B = -0.000390 C = -0.161696
SYSTEM TEST PASS
0000
Reviewed/Approved By: _______________________________________
Date: ________________
Figure 6: System Self Test report
Absorbance Plate Test
Description
Absorbance Plate Test | 25
This test uses BioTek’s 7-Filter Absorbance Test Plate (PN 7260522) to confirm the
mechanical alignment; optical density accuracy, linearity, and repeatability; and
wavelength accuracy to NIST-traceable values.
An alternate method that may be used to determine
accuracy/linearity, repeatability, and alignment is Liquid Test 2,
described on page 37.
The Absorbance Plate Test confirms the following:
Mechanical Alignment: The Test Plate has several groups of precisely
•
machined holes to confirm the mechanical alignment of different
microplate readers. The amount of light that shines through these holes is
an indication of whether the reader is properly aligned with the absorbance
optical path. A reading of more than 0.015 OD for any of the designated
alignment holes indicates that the light is being “clipped” and the reader
may be out of alignment.
Accuracy/Linearity: The Test Plate contains neutral-density glass filters
•
of known OD values at several wavelengths. Actual measurements are
compared against the expected values provided in the Test Plate’s
Standards Certificate. Since there are several filters with differing OD
values, the accuracy across a range of ODs can be established. Once it is
proven that the reader is accurate at these OD values, the reader is also
considered to be linear.
Repeatability: This test ensures the instrument meets its repeatability
•
specification by reading each neutral-density filter on the Test Plate twice
with the filter in the same location.
Epoch Operator’s Manual
Page 44

26 | Chapter 4: Instrument Qualification
• Wavelength Accuracy: BioTek’s Absorbance Test Plate with the part
number 7260522 contains a glass filter in position C6. This filter is used to
check the wavelength accuracy of the absorbance monochromator. The
filter is scanned across a specified wavelength range in 1-nm increments.
The wavelength of maximum absorbance is compared to the expected peak
wavelength supplied on the Test Plate’s certificate and entered into Gen5.
Test Plate Certificates
To run the Absorbance Plate Test on the Epoch, you will need BioTek’s 7-Filter
Absorbance Test Plate (PN 7260522) with its accompanying certificates.
• The Standards Certificate contains standard OD values for the filters at
several different wavelengths. See the sample Standards Certificate in
Figure 7
• The Peak Wavelength Certificate contains one or more “Peak Wavelength”
values for the glass filter in position C6 on the plate. Each value has a valid
test range associated with it. For example, a Peak Wavelength value may be
586 nm with a test range of 580 to 590 nm
(or tolerance values of –6/ +4).
below.
This test plate can be used for testing the reproducibility, linearity, and alignment of your
BioTek autoreader. The following calibration data has been recorded by a NIST-traceable
spectrophotometer.
WAVELENGTH (nm)
Well 405nm 450nm 490nm 550nm 620nm 630nm 690nm 750nm
C1 0.147 0.140 0.135 0.130 0.136 0.136 0.127 0.134
E2 0.618 0.575 0.574 0.568 0.573 0.568 0.485 0.434
G3 1.133 1.052 1.051 1.040 1.050 1.040 0.881 0.783
H6 1.701 1.578 1.577 1.560 1.575 1.560 1.323 1.179
F5 2.279 2.024 1.976 1.956 1.893 1.865 1.537 1.272
D4 2.945 2.604 2.545 2.513 2.437 2.400 1.972 1.632
Set #2453 Serial # 161259
Figure 7: Sample Standards Certificate, showing OD/Wavelength combinations
for each of six locations on the Absorbance Test Plate
S A M P L E
Before the Absorbance Plate Test can be performed, the standard OD values and
the peak wavelength value(s) must be entered into Gen5. This has to be done only
once.
BioTek® Instruments, Inc.
Page 45

Instructions for entering the Absorbance Test Plate data and for running the test
are provided on the following pages.
The instructions provided below and on the following page are
guidelines. Refer to the Absorbance Plate Test sections of the
Gen5 Help system for detailed instructions when entering the
Absorbance Test Plate data and running the Absorbance Plate Test.
Entering the Absorbance Test Plate Data
Before the Absorbance Plate Test can be performed, the wavelengths settings and
the calibration data for each wavelength selected must be initially entered into
Gen5. Use the Standards Certificate included with the Absorbance Test Plate.
Absorbance Plate Test | 27
In Gen5, select
then click
entering the OD and peak wavelength values.
The wavelengths and corresponding calibration data that have been entered will
now be available in Gen5 each time the Absorbance Plate Test is performed.
System > Diagnostics > Test Plates > Add/Modify Plates,
Add. Click Help for guidance when setting the wavelengths and
Running the Absorbance Plate Test
• Place the Absorbance Test Plate in the carrier so that well A1 is in the right-
rear corner of the carrier.
• To run the test in Gen5, select
Run
. Select the appropriate reader, if the Instrument Selection dialog
appears. Select the appropriate test plate, if the Select Test Plate dialog
appears. Enter the reader serial number and user name, then click
Test
.
• For wavelength accuracy: While performing the steps above, click
Perform peak wavelength test in Gen5.
When the test is complete, print the results. A sample test report is provided in
Figure 8.
System > Diagnostics > Test Plates >
Start
Epoch Operator’s Manual
Page 46

28 | Chapter 4: Instrument Qualification
Absorbance Test Plate Results
Reader: Epoch (Serial Number: 182843)
Basecode: P/N 7200201 (v1.07)
Date and Time: 7/14/2009 8:49:39 AM
Absorbance Plate: 7 Filter Test Plate (P/N 7260522) - S/N 161259
Last Plate Certification: April 2009
Next Plate Certification Due: April 2010
User: Administrator
Comments:
Peak Absorbance Results
Well C6
Reference 586
Tolerance 3
Read 586
Result PASS
Alignment Results
Wells B2 B12 G1 G11
Read 0.000 0.000 0.000 0.000
Tolerance 0.015 0.015 0.015 0.015
Result PASS PASS PASS PASS
Wavelength = 405 nm
Accuracy Results
Wells C1 E2 G3 H6 F5 D4
Reference 0.147 0.618 1.133 1.701 2.279 2.945
Min Limit 0.124 0.586 1.090 1.647 2.168 #N/A
Max Limit 0.170 0.650 1.176 1.755 2.390 #N/A
Read 1 0.143 0.614 1.129 1.693 2.273 2.902
Result PASS PASS PASS PASS PASS #N/A
Repeatability Results
Wells C1 E2 G3 H6 F5 D4
Read 1 0.143 0.614 1.129 1.693 2.273 2.902
Min Limit 0.137 0.603 1.113 1.671 2.200 #N/A
Max Limit 0.150 0.625 1.145 1.715 2.346 #N/A
Read 2 0.143 0.614 1.129 1.694 2.269 2.918
Result PASS PASS PASS PASS PASS #N/A
Wavelength = 450 nm
Accuracy Results
Wells C1 E2 G3 H6 F5 D4
Reference 0.140 0.575 1.052 1.578 2.024 2.604
Min Limit 0.117 0.543 1.011 1.526 1.923 #N/A
Max Limit 0.163 0.606 1.093 1.630 2.125 #N/A
Read 1 0.136 0.571 1.047 1.570 2.016 2.591
Result PASS PASS PASS PASS PASS #N/A
Repeatability Results
Wells C1 E2 G3 H6 F5 D4
Read 1 0.136 0.571 1.047 1.570 2.016 2.591
Min Limit 0.130 0.560 1.031 1.549 1.951 #N/A
Max Limit 0.143 0.581 1.062 1.590 2.081 #N/A
Read 2 0.137 0.571 1.047 1.570 2.017 2.589
Result PASS PASS PASS PASS PASS #N/A
BioTek® Instruments, Inc.
Page 47

Absorbance Plate Test | 29
Wavelength = 490 nm
Accuracy Results
Wells C1 E2 G3 H6 F5 D4
Reference 0.135 0.574 1.051 1.577 1.976 2.545
Min Limit 0.112 0.543 1.010 1.525 1.916 #N/A
Max Limit 0.158 0.605 1.092 1.629 2.036 #N/A
Read 1 0.132 0.571 1.048 1.571 1.973 2.537
Result PASS PASS PASS PASS PASS #N/A
Repeatability Results
Wells C1 E2 G3 H6 F5 D4
Read 1 0.132 0.571 1.048 1.571 1.973 2.537
Min Limit 0.125 0.560 1.033 1.551 1.948 #N/A
Max Limit 0.138 0.582 1.063 1.592 1.998 #N/A
Read 2 0.131 0.571 1.048 1.571 1.974 2.540
Result PASS PASS PASS PASS PASS #N/A
Wavelength = 550 nm
Accuracy Results
Wells C1 E2 G3 H6 F5 D4
Reference 0.130 0.568 1.040 1.560 1.956 2.513
Min Limit 0.107 0.537 0.999 1.509 1.897 #N/A
Max Limit 0.153 0.599 1.081 1.611 2.015 #N/A
Read 1 0.127 0.566 1.038 1.556 1.955 2.510
Result PASS PASS PASS PASS PASS #N/A
Repeatability Results
Wells C1 E2 G3 H6 F5 D4
Read 1 0.127 0.566 1.038 1.556 1.955 2.510
Min Limit 0.121 0.555 1.023 1.535 1.931 #N/A
Max Limit 0.134 0.576 1.053 1.577 1.980 #N/A
Read 2 0.127 0.565 1.038 1.556 1.954 2.505
Result PASS PASS PASS PASS PASS #N/A
Wavelength = 620 nm
Accuracy Results
Wells C1 E2 G3 H6 F5 D4
Reference 0.136 0.573 1.050 1.575 1.893 2.437
Min Limit 0.113 0.542 1.009 1.523 1.835 2.320
Max Limit 0.159 0.604 1.091 1.627 1.951 2.554
Read 1 0.133 0.571 1.049 1.572 1.894 2.430
Result PASS PASS PASS PASS PASS PASS
Repeatability Results
Wells C1 E2 G3 H6 F5 D4
Read 1 0.133 0.571 1.049 1.572 1.894 2.430
Min Limit 0.127 0.560 1.033 1.551 1.870 2.352
Max Limit 0.140 0.582 1.064 1.592 1.918 2.508
Read 2 0.133 0.571 1.049 1.571 1.895 2.430
Result PASS PASS PASS PASS PASS PASS
Wavelength = 630 nm
Accuracy Results
Wells C1 E2 G3 H6 F5 D4
Reference 0.136 0.568 1.040 1.560 1.865 2.400
Min Limit 0.113 0.537 0.999 1.509 1.808 2.284
Max Limit 0.159 0.599 1.081 1.611 1.922 2.516
Read 1 0.134 0.566 1.039 1.557 1.864 2.392
Result PASS PASS PASS PASS PASS PASS
Epoch Operator’s Manual
Page 48

30 | Chapter 4: Instrument Qualification
Repeatability Results
Wells C1 E2 G3 H6 F5 D4
Read 1 0.134 0.566 1.039 1.557 1.864 2.392
Min Limit 0.127 0.555 1.024 1.537 1.840 2.315
Max Limit 0.140 0.577 1.054 1.578 1.888 2.469
Read 2 0.134 0.566 1.039 1.557 1.864 2.390
Result PASS PASS PASS PASS PASS PASS
Wavelength = 690 nm
Accuracy Results
Wells C1 E2 G3 H6 F5 D4
Reference 0.127 0.485 0.881 1.323 1.537 1.972
Min Limit 0.104 0.455 0.843 1.277 1.486 1.913
Max Limit 0.150 0.515 0.919 1.369 1.588 2.031
Read 1 0.125 0.482 0.879 1.319 1.535 1.968
Result PASS PASS PASS PASS PASS PASS
Repeatability Results
Wells C1 E2 G3 H6 F5 D4
Read 1 0.125 0.482 0.879 1.319 1.535 1.968
Min Limit 0.119 0.472 0.865 1.301 1.515 1.943
Max Limit 0.131 0.492 0.893 1.337 1.555 1.992
Read 2 0.125 0.482 0.879 1.318 1.534 1.968
Result PASS PASS PASS PASS PASS PASS
Results
Wavelength = 750 nm
Accuracy Results
Wells C1 E2 G3 H6 F5 D4
Reference 0.134 0.434 0.783 1.179 1.272 1.632
Min Limit 0.111 0.405 0.747 1.135 1.227 1.579
Max Limit 0.157 0.463 0.819 1.223 1.317 1.685
Read 1 0.131 0.432 0.781 1.176 1.273 1.630
Result PASS PASS PASS PASS PASS PASS
Repeatability Results
Wells C1 E2 G3 H6 F5 D4
Read 1 0.131 0.432 0.781 1.176 1.273 1.630
Min Limit 0.125 0.423 0.768 1.159 1.255 1.609
Max Limit 0.138 0.441 0.794 1.193 1.290 1.652
Read 2 0.131 0.432 0.781 1.176 1.273 1.631
Result PASS PASS PASS PASS PASS PASS
Reviewed/Approved By: _______________________________________
Date: ________________
Figure 8: Sample output for the Absorbance Plate Test
• Peak Absorbance: When the test is performed, the C6 filter is scanned at
the test range(s) defined by the user in the Absorbance Test Plate dialog. To
verify wavelength accuracy, the wavelength of the maximum absorbance is
compared with the peak wavelength value entered in the software, which
comes from the Standards Certificate supplied with the Test Plate. The
BioTek® Instruments, Inc.
Page 49

Absorbance Plate Test | 31
accuracy of the wavelength should be ± 3 nm (± 2 nm instrument, ± 1 nm
filter allowance).
For example, if the test range is 580 to 590 nm, the Certificate value is 587 nm,
and the reader reports a peak value of 590 nm, then the reader meets
specifications. If the reader reports 591 nm, then the reader does not meet
specifications.
If the reader fails this test:
¾ Make sure the information entered into Gen5 matches the information
on the Test Plate’s Peak Wavelength Certificate.
¾ Verify that the Test Plate actually has a filter in location C6.
¾ Check the C6 filter to make sure it is clean. If needed, clean it with lens
paper.
Do not remove the filter from the Test Plate, and do not use alcohol or
other cleaning agents.
¾ Make sure the Test Plate is within its calibration certification period. The
calibration sticker is affixed directly to the plate. If it is out of date,
contact BioTek to schedule a recertification.
¾ Check the microplate carrier to ensure it is clear of debris.
•
Alignment: This portion of the test measures the alignment of the
microplate carrier with the optical path. A reading greater than 0.015 OD
represents an out-of-alignment condition. Wells A01, A12, H01, and H12
are the only valid alignment holes for the reader on the PN 7260522 Test
Plate.
If the reader fails this test:
¾ Ensure that the Test Plate is correctly seated in the microplate carrier.
¾ Check the four alignment holes (B2, B12, G1, and G11) to ensure they are
clear of debris.
¾ Check the microplate carrier to ensure it is clear of debris.
•
Accuracy: Accuracy is a measure of the optical density of Test Plate wells
C01, D04, E02, F05, G03, and H06 as compared with known standard values
contained in the Standards Certificate that accompanies each Test Plate.
If the reader fails this test, review the following possible problems and
solutions:
¾ Verify that the filter calibration values entered in Gen5 are the same as
those on the Test Plate’s Standards Certificate.
¾ Check the neutral-density filters on the Test Plate to ensure they are
clean. If necessary, clean them with lens paper.
Epoch Operator’s Manual
Page 50

32 | Chapter 4: Instrument Qualification
Do not remove the filter from the Test Plate, and do not use alcohol or
other cleaning agents.
¾ Verify that the Test Plate is within its calibration certification period. The
calibration sticker is affixed directly to the plate. If it is out of date,
contact BioTek to schedule a recertification.
Repeatability: Repeatability is a measure of the instrument’s ability to
•
read the same well with minimum variation between two reads with the
well in the same location.
If the reader fails this test:
¾ Check the neutral-density filters on the Test Plate to ensure there is no
debris that may have shifted between readings and caused changes.
¾ Check the microplate carrier to ensure it is clear of debris.
Linearity can also be proven by performing a regression analysis on the OD
values in a program such as Microsoft Excel, as illustrated in the following
instructions.
Analyze one wavelength at a time.
1. In Microsoft Excel, open a spreadsheet and label one column Assigned
and the next column
2. Enter the Assigned OD data for each glass filter in the first column from
Observed.
the Standards Certificate provided with the Test Plate.
3. Next, enter the Observed OD values for the same glass filters in the
adjacent column.
4. Click Tools > Data Analysis > Regression. (If the Data Analysis
command is not available on the Tools menu, you may need to install
the Analysis ToolPak in Microsoft Excel. Consult Microsoft Excel Help
for assistance.)
5. Use the Regression Input box to enter the Assigned values as the Input
Y Range
6. Click OK; the Summary Output sheet will be displayed. An R Square
and the Observed OD as the Input X Range.
value of at least 0.99 is expected and the results will often be 0.999 or
better.
BioTek® Instruments, Inc.
Page 51

Liquid Testing
Liquid testing tests the reader in ways that the Absorbance Test Plate cannot. The test
plate will indicate the absolute amount of light absorbed, which will accurately test the
linearity of the electronics. The liquid test will help detect optical defects such as dirt
on the lenses or contamination that can contribute to errant readings.
• If you have the Absorbance Test Plate, you will need to perform only Liquid
Test 1 for routine testing.
• If you do not have the Absorbance Test Plate, you can test the linearity,
repeatability, and alignment of the reader by performing Liquid Test 2.
• BioTek offers a dye solution (PN 7120779, 25 mL; or 7120782, 125 mL) that can
be used in the stock solution formulation for Liquid Tests 1 and 2, or, if you
prefer, you may use the dye solution described in Table 2 on page 35. The
purpose of the formulation is to create a solution that absorbs light in a welldefined manner at ~2.000 OD full strength when dispensed at 200 µL in a flatbottom microplate well.
Liquid Testing | 33
• Alternatively, any solution that gives a stable color will suffice. (This includes
substrates incubated with an enzyme preparation and then stopped with an
acidic or basic solution.) Some enzyme/substrate combinations that may be
used as alternates to the described dye are shown in Table 1 below.
• If you must test the reader’s performance at 340 nm, perform the optional
Liquid Test 3 (see page 19).
Table 1
Typical Enzyme-Substrate Combinations and Stopping Solutions
Enzyme Substrate Stopping Solution
Alkaline Phosphate o-nitrophenyl phosphate 3N sodium hydroxide
beta-Galactosidase o-nitrophenyl -beta-D
galactopyranoside
Peroxidase 2,2'-Azino di-ethylbenzothiazoline-
sulfonic acid (ABTS)
1M sodium carbonate
citrate-phosphate buffer,
pH 2.8
Peroxidase o-phenylenediamine 0.03N sulfuric acid
Epoch Operator’s Manual
Page 52

34 | Chapter 4: Instrument Qualification
Stock Solution Formulation
The stock solution for Liquid Tests 1 and 2 may be formulated from the chemicals
listed below, or by diluting a dye solution available from BioTek. See Procedure A
or Procedure B outlined below and on the following page for details.
Procedure A
Required Materials:
• BioTek QC Check Solution No. 1 (PN 7120779, 25 mL; or 7120782,
125 mL)
• Deionized water
• 5-mL Class A volumetric pipette
• 100-mL volumetric flask
Preparation of Stock Solution:
1.
Pipette a 5-mL aliquot of BioTek QC Check Solution No. 1 into a 100-mL
volumetric flask.
2. Add 95 mL of DI water; cap and shake well. The solution should measure
approximately 2.000 OD when using 200 µL in a flat-bottom microwell.
This should create a solution with an absorbance of about 2.000 OD when
using 200 µL in a flat-bottom microwell. The OD value will be proportional
to the volume in the well and the amount of QC Check Solution No. 1 used.
You can use a larger or smaller well volume, or add more Check Solution or
water to adjust the stock solution.
Too small a well volume may result in increased pipetting-related
Procedure B
Required Materials:
• Deionized water
• FD&C Yellow No. 5 dye powder (typically 90% pure)
errors.
• Tween 20 (polyoxyethylene (20) sorbitan monolaurate) or
agent, PN 7773002
• Precision balance with readability of 0.001 g
• 1-liter volumetric flask
• Weigh boat
BioTek® Instruments, Inc.
BioTek wetting
Page 53

Liquid Testing | 35
Table 2
Stock Solution Formulation for Liquid Test 1 and 2
FD&C Yellow No. 5 powder 0.092 g
Tween 20 0.5 mL
Deionized water to bring volume to: 1000 mL
Preparation of Stock Solution:
1.
Weigh out 0.092 gram of FD&C No. 5 yellow dye powder into a weigh boat.
2. Rinse the contents into a 1-liter volumetric flask.
3. Add 0.5 mL of Tween 20, or 5 mL of BioTek’s wetting agent.
4. Make up to 1 liter with DI water; cap and shake well.
This should create a solution with an absorbance of about 2.000 OD when
using 200 µL in a flat-bottom microwell. The OD value will be proportional
to the volume in the well and the amount of FD&C No. 5 dye used. You can
use a larger or smaller well volume, or add more dye or water to adjust the
solution.
Too small a well volume may result in increased pipetting-related
errors.
Liquid Test 1
A 96-well, flat-bottom microplate is required for this test (Corning
Costar #3590 is recommended). Use a new microplate; any
fingerprints or scratches may cause variations in readings.
This procedure will test for repeatability and alignment, and will reveal any problems
with the system optics.
1. Using a freshly prepared stock solution (see Procedure A on page 34 or
Procedure B on page 34), prepare a 1:2 dilution using deionized water (one
part stock, one part deionized water; the resulting solution is a 1:2
dilution). The concentrated stock solution should have an optical density of
approximately 2.000 OD or lower.
2. Pipette 200 µL of the concentrated solution into column 1.
3. Pipette 200 µL of the diluted solution into column 2
Epoch Operator’s Manual
Page 54

36 | Chapter 4: Instrument Qualification
After pipetting the diluted test solution into the microplate,
4. Read the microplate five times at 405 nm using normal reading mode,
single wavelength, no blanking (“Normal” plate position). Save the data
after each read (“Normal” plate position).
5. Rotate the microplate 180° so that well A1 is now in the H12 position. Read
the plate five more times (“Turnaround” plate position) saving the data
after each read.
The ten sets of raw plate data can be exported to an Excel spreadsheet
using Gen5. The mathematical computations described below may then be
performed and the template kept for future data reduction.
Calculations:
6.
Calculate the mean value for each physical well location in columns 1 and 2
for the five plates read in the Normal position, and then again for the five
plates read in the Turnaround position. This will result in 32 mean values.
we strongly recommend waiting 20 minutes before reading
the plate to allow any air bubbles in the solution to settle and
the meniscus to stabilize.
7. Perform a mathematical comparison of the mean values for each microwell
in its Normal and Turnaround positions (A1/H12, A2/H11, B1/G12,
B2/G11, and so on). In order to pass this test, the differences in the
compared mean values must be within the accuracy specification for the
instrument.
For example:
If the mean value for well A1 in the Normal position is 1.902, where the
specified accuracy is ± 1% ± 0.010 OD, then the expected range for the mean
of the same well in its Turnaround (H12) position is 1.873 to 1.931 OD.
1.902 * 0.01 + 0.010 = 0.029; 1.902 – 0.029 =
Accuracy Specification:
For comparison in this test, the following accuracy specifications are
applied, using Normal reading mode and a 96-well microplate.
± 1% ± 0.010 OD from 0.000 OD to 2.000 OD
± 3% ± 0.010 OD from 2.000 OD to 3.000 OD
1.873; 1.902 + 0.029 = 1.931
BioTek® Instruments, Inc.
Page 55

Liquid Test 2
18 16 14 12 10
2
2 4 6 8 10
The recommended method for testing the instrument’s alignment, repeatability,
and accuracy is to use the Absorbance Test Plate (see page 25). If the Test Plate is
not available, however, Liquid Test 2 can be used for these tests.
Materials
• A new 96-well, clear, flat-bottom microplate (Corning Costar #3590 is
recommended)
• Ten test tubes, numbered consecutively, set up in a rack
• Calibrated hand pipette (Class A volumetric pipette recommended)
• Solution A or B (see the instructions for Liquid Test 1)
• A 0.05% solution of deionized water and Tween 20
Prepare the Dilutions
Liquid Testing | 37
• Create a percentage dilution series, beginning with 100% of the original
concentrated stock solution (A or B) in the first tube, 90% of the original
solution in the second tube, 80% in the third tube, all the way to 10% in the
tenth tube.
• Dilute using the 0.05% solution of deionized water and Tween 20.
Test Tube Dilutions for Liquid Test 2
Tube Number: 1 2 3 4 5 6 7 8 9 10
Volume of Original
Concentrated Solution (mL)
Volume of 0.05% Tween
Solution (mL)
Absorbance expected if original
solution is 2.0 at 200 μL
20
2.0
8 6 4
0
1.8 1.6 1.4 1.2 1.0 0.8 0.6 0.4 0.2
12 14 16 18
The choice of dilutions and the absorbance of the original solution can
be varied. Use this table as a model for calculating the expected
absorbances of a series of dilutions, given a different absorbance of
the original solution.
Prepare the Plate
• Pipette 200 µL of the concentrated solution from Tube 1 into each well of the
first column, A1 to H1, of a new flat-bottom microplate.
Epoch Operator’s Manual
Page 56

38 | Chapter 4: Instrument Qualification
• Pipette 200 µL from each of the remaining tubes into the wells of the
corresponding column of the microplate (Tube 2 into wells A2 to H2, Tube 3
into wells A3 to H3, and so on).
Linearity & Repeatability Tests
1. Using Gen5, read the microplate prepared above five times using Normal
mode, dual wavelength at 450/630 nm. Save the data after each read.
Do not discard the plate; you will use it for the Alignment test.
2. Print out the five sets of Delta OD data, or export them to an Excel
spreadsheet.
3. Calculate the results for Linearity:
• Calculate the mean absorbance for each well, and average the means for
each concentration.
• Perform a regression analysis on the data to determine if there is
adequate linearity. For example, using Microsoft Excel:
¾ In a spreadsheet, create two columns labeled “X” and “Y”. As
displayed in the table
column X, and enter the
¾ Select
Tools > Data Analysis > Regression. Identify column X
earlier, enter the actual absorbance values in
expected absorbance values in column Y.
as the “Input X Range” and column Y as the “Input Y Range.”
¾ Click
OK to perform the analysis, the results of which will be
output in a separate sheet.
If the Data Analysis command is not available on the Tools menu, you
may need to install the Analysis ToolPak in Excel. Consult Excel’s help
system for assistance.
Because it is somewhat difficult to achieve high pipetting accuracy
when conducting linear dilutions, an R Square value of at least 0.99 is
considered adequate.
Calculate the results for Repeatability:
4.
• Calculate the mean and standard deviation for the five readings taken
in Step 1 at each concentration. Only one row of data needs to be
analyzed.
• For each mean below 2.000 OD, calculate the allowed deviation using
the repeatability specification for a 96-well plate of ± 1.0% ± 0.005 OD. If
above 2.000 OD, apply the ± 3.0% ± 0.005 specification.
BioTek® Instruments, Inc.
Page 57

• The standard deviation for each set of readings should be less than the
allowed deviation.
Example: Absorbance readings of 1.950, 1.948, 1.955, 1.952, and 1.950
will result in a mean of 1.951, and a standard deviation of 0.0026. The
mean (1.951) multiplied by 1.0% (1.951 x 0.010) = 0.0195, which, when
added to the 0.005 (0.0195 + 0.005) = 0.0245 OD, which is the allowable
deviation. Since the standard deviation is less than this value, the
reader meets the test criteria.
Repeatability Specification:
± 1.0% ± 0.005 OD from 0.000 to 2.000 OD
± 3.0% ± 0.005 OD from 2.000 OD to 2.500 OD
Alignment Test
1. Using the plate prepared for the Linearity Test on the previous page,
conduct a Turnaround test by reading the plate
well in the H12 position. Save the data after each read.
Liquid Testing | 39
five times with the A1
This test results in values for the four corner wells that can be used to
determine alignment.
2. Calculate the means of the wells A1 and H1 in the Normal plate position
(data from Linearity Test) and in the Turnaround position (from Step 1).
3. Compare the mean reading for well A1 to its mean reading when in the
H12 position. Next, compare the mean values for the H1 well to the same
well in the A12 position. The difference in the values for any two
corresponding wells should be within the accuracy specification for the
instrument.
Example: If the mean of well A1 in the normal position is 1.902, where the
specified accuracy is ± 1.0% ± 0.010 OD, then the expected range for the
mean of the same well in the H12 position is 1.873 to 1.931 OD. (1.902 x
1.0% = 0.019 + 0.010 = 0.029, which is added to and subtracted from 1.902
for the range.)
If the four corner wells are within the accuracy range, the reader is in
alignment.
Epoch Operator’s Manual
Page 58

40 | Chapter 4: Instrument Qualification
Liquid Test 3
This test verifies operation of the reader at 340 nm, and is provided for sites requiring
proof of linearity at wavelengths lower than those attainable with the Absorbance Test
Plate. This test is optional because the reader has good “front end” linearity
throughout its wavelength range.
Materials
• New 96-well, clear, flat-bottom microplate (Corning® Costar #3590
recommended)
Use a new microplate; any fingerprints or scratches may cause
variations in readings.
• Calibrated hand-pipette(s)
• Beakers and graduated cylinder
• Precision balance with readability to 0.01 g
• Buffer solution described below
Buffer Solution
• Deionized water
• Phosphate-Buffered Saline (PBS), pH 7.2–7.6, Sigma tablets, #P4417
(or equivalent)
• β-NADH Powder (β-Nicotinamide Adenine Dinucleotide, Reduced Form)
Sigma bulk catalog number N 8129, or preweighed 10-mg vials, Sigma
number N6785-10VL (or BioTek PN 98233). Store the powder according to
the guidelines on its packaging.
1. Prepare a PBS solution from the Sigma tablets.
2. In a beaker, mix 50 mL of the PBS solution with 10 mg of the β-NADH
powder and mix thoroughly. This is the
3.
(Optional) Read a sample of the solution at 340 nm; it should be within
0.700 to 1.000 OD. If low, adjust up by adding more powder. Do not adjust
if slightly high.
Prepare the Plate
1. Prepare the 75% Test Solution by mixing 15 mL of the 100% Test
Solution with 5 mL of the PBS Solution.
100% Test Solution.
2. Prepare the 50% Test Solution by mixing 10 mL of the 100% Test
Solution with 10 mL of the PBS Solution.
BioTek® Instruments, Inc.
Page 59

Liquid Testing | 41
3. Carefully pipette the three solutions into a new 96-well microplate:
• 150 µL of the
• 150 µL of the
• 150 µL of the
Read the Plate
1. Using Gen5, read the microplate five times using Normal mode, single
wavelength at 340 nm, no blanking. Save the data after each read.
2. Print out the five sets of raw data, or export them to an Excel spreadsheet.
Analyze the Results
1. The plate is read five times at 340 nm. Calculate the Mean OD and Standard
Deviation of those five reads for each well in columns 1–6.
2. For each well in columns 1–6, calculate the allowed deviation using the
repeatability specification for a 96-well plate: ± 1% ± 0.005 OD (Mean x
0.010 + 0.005). For each well, its standard deviation should be less than its
allowed deviation.
100% Test Solution into all wells of columns 1 and 2
75% Test Solution into all wells of columns 3 and 4
50% Test Solution into all wells of column 5 and 6
Example: Five readings in well A1 of 0.802, 0.802, 0.799, 0.798, and 0.801
result in a mean of 0.8004 and a standard deviation of 0.0018. The mean
multiplied by 1.0% (0.8004 * 0.010) equals 0.008, and when added to 0.005
equals 0.013; this is the allowed deviation for well A1. Since the standard
deviation for well A1 is less than 0.013, the well meets the test criteria.
3. Calculate the results for Linearity:
• For each of the three Test Solutions, calculate the average Mean OD for
the wells containing that solution (mean of wells A1 to H2, A3 to H4,
and A5 to H6).
• Perform a regression analysis on the data to determine if there is
adequate linearity. The three average Mean OD values are the “Y”
values. The solution concentrations are the “X” values (1.00, 0.75, 0.50).
Because it is somewhat difficult to achieve high pipetting accuracy when
conducting linear dilutions, an R Square value of at least 0.99 is considered
adequate.
Epoch Operator’s Manual
Page 60

42 | Chapter 4: Instrument Qualification
BioTek
®
Instruments, Inc.
Page 61

Chapter 5
Preventive Maintenance
This chapter contains the procedures for cleaning and
decontaminating the Epoch.
Overview............................................................................ 44
Required Materials ............................................................... 44
Warnings & Precautions ........................................................ 45
Routine Cleaning Procedure .................................................. 45
Purpose............................................................................ 45
Procedure ......................................................................... 46
Decontamination ................................................................. 46
Purpose............................................................................ 46
Procedure ......................................................................... 47
Page 62

44 | Chapter 5: Preventive Maintenance
Overview
A general Preventive Maintenance (PM) regimen for the Epoch includes periodically
cleaning all exposed surfaces and decontaminating the instrument before storage or
shipment. This chapter includes instructions for the following:
• Routine Cleaning Procedure, page 45
• Decontamination, page 46
Required Materials
• Mild detergent
• Deionized or distilled water
• Clean, lint-free cotton cloths
• Sodium hypochlorite (NaClO, or bleach) (decontamination only)
• Safety glasses
• Surgical mask
• Protective gloves
• Lab coat
• Biohazard trash bags
• 125-mL beakers
• Cotton swabs or paper towels
BioTek® Instruments, Inc.
Page 63

Warnings & Precautions
Please read the following before performing any maintenance procedures.
Warning! Internal Voltage. Turn off and disconnect the Epoch
from its power supply for all cleaning and decontamination
operations.
Warning! Wear prophylactic gloves when handling contaminated
instruments. Gloved hands should be considered contaminated at
all times; keep gloved hands away from eyes, mouth, nose, and
ears. Eating and drinking while decontaminating instruments is
not advised.
Warning! Mucous membranes are considered prime entry routes
for infectious agents. Wear eye protection and a surgical mask
when there is a possibility of aerosol contamination. Intact skin is
generally considered an effective barrier against infectious
organisms; however, small abrasions and cuts may not always be
visible. Wear protective gloves when handling contaminated
instruments.
Warnings & Precautions | 45
Important! Do not immerse the instrument, spray it with liquid,
or use a “wet” cloth. Do not allow water or other cleaning solution
to run into the interior of the instrument. If this happens, contact
BioTek’s Technical Assistance Center.
Routine Cleaning Procedure
Turn off the Epoch and disconnect it from the power supply for
Purpose
A regular cleaning regimen is recommended to keep the instrument free from dust
and particulates that can cause erroneous readings. Exposed surfaces may be
cleaned (not decontaminated) with a cloth moistened (not soaked) with water or
water and a mild detergent.
the cleaning procedure.
Epoch Operator’s Manual
Page 64

46 | Chapter 5: Preventive Maintenance
Procedure
1. Turn on the Epoch and press the carrier eject button to eject the microplate
carrier.
2. Turn off and unplug the reader from the power supply.
3. Moisten a clean, lint-free cloth with water, or with water and the mild
detergent.
4. Wipe the plate carrier and all exposed surfaces of the instrument.
5. If detergent was used, wipe all surfaces with a cloth moistened with water.
6. Use a clean, dry lint-free cloth to dry all wet surfaces.
If liquid is spilled inside the reader, call BioTek TAC for cleanup
instructions.
Do not soak the cloth.
Decontamination
Purpose
Any laboratory instrument that has been used for research or clinical analysis is
considered a biohazard and requires decontamination prior to handling.
Decontamination minimizes the risk to all who come in contact with the
instrument during shipping, handling, and servicing. Decontamination is required
by the U.S. Department of Transportation regulations.
Persons performing the decontamination process must be familiar with the basic
setup and operation of the instrument.
BioTek Instruments, Inc., recommends the use of the following
decontamination solutions and methods based on our knowledge
of the instrument and recommendations of the Centers for Disease
Control and Prevention (CDC). Neither BioTek nor the CDC
assumes any liability for the adequacy of these solutions and
methods. Each laboratory must ensure that decontamination
procedures are adequate for the Biohazard(s) they handle.
Internal Voltage. Turn off and unplug the instrument for the
decontamination procedure.
BioTek® Instruments, Inc.
Page 65

Procedure
1. Turn on the Epoch and press the carrier eject button to eject the carrier.
2. Turn off and unplug the reader from the power supply.
3. Prepare an aqueous solution of 0.5% sodium hypochlorite (NaClO, or
bleach).
¾ Be sure to check the percent NaClO of the bleach you are using; this
4. Moisten a clean, lint-free cloth with the bleach solution. Do not soak the
cloth.
Decontamination | 47
The bleach solution is caustic; wear gloves and eye protection
when handling the solution.
information is printed on the side of the bottle. Commercial bleach is
typically 10% NaClO; if this is the case, prepare a 1:20 dilution.
Household bleach is typically 5% NaClO; if this is the case, prepare a
1:10 dilution.
5. Wipe the plate carrier and all exposed surfaces of the instrument.
6. Allow the instrument to dry for 20 minutes for thorough decontamination
by the bleach.
7. Moisten a cloth with deionized or distilled water and wipe all surfaces of
the instrument that have been cleaned with the bleach solution.
8. Use a clean, dry lint-free cloth to dry all wet surfaces.
9. Discard the used gloves and cloths, using a biohazard trash bag and an
approved biohazard container.
Epoch Operator’s Manual
Page 66

48 | Chapter 5: Preventive Maintenance
BioTek
®
Instruments, Inc.
Page 67

Appendix A
Specifications
This appendix contains BioTek’s published specifications for the
Epoch.
General Specifications........................................................... 50
Read Specifications............................................................... 50
Optical Performance ............................................................. 52
Page 68

50 | Appendix A: Specifications
General Specifications
Microplates
The Epoch accommodates standard 6-, 12-, 24-, 48-, 96-, and 384-well microplates
with 128 x 86 mm geometry; Terasaki microplates; up to 8 BioCells; and the BioTek
Take3 Multi-Volume Plate
Hardware & Environmental
Light Source
Dimensions
Weight < 15 lbs. (6.804 kg) (without power supply)
Environment
Humidity 10% to 85% relative humidity (non-condensing)
Power Supply
Power Consumption < 40W maximum
Xenon flash light source, 10W maximum average power, lamp
life 1 billion flashes (not user-changeable)
12.5" D x 12" W x 7.7" H
31.8 cm D x 30.5 cm W x 19.6 cm H
Operational temperature 18ºC to 40°C (65ºF–104ºF)
24-volt external power supply compatible with 100–240 V~;
+/- 10% @50–60 Hz
Read Specifications
The actual plate read time and accuracy are dependent on the method of reading:
•
Normal mode is the slowest of the two available modes. After positioning
the well over the beam, the instrument waits however long the user has
defined the delay (0–2550 ms) before taking the measurement (eight-flash
data collection).
The longer the delay, the more completely the fluids in the wells
settle.
• Sweep mode is the fastest mode. The plate carrier sweeps each row past
the optics channel without stopping and collects data with a single flash at
each well as it goes by.
The following read times are based on a single or dual wavelength measurement.
Actual reading speeds may vary, depending upon the reading wavelength
selected. Each wavelength has a unique location within the monochromator, and
the different locations require varying amounts of time to position.
BioTek Instruments, Inc.
Page 69

Read Specifications | 51
All read speeds are +/– 2 seconds.
96-Well Read Timing Delay 630 nm 630/450 nm
Normal Read Mode Single Dual
Endpoint 100 msec 49 sec 95 sec
Normal Read Mode Single Dual
Endpoint 0 msec 38 sec 75 sec
Sweep Read Mode Single Dual
Endpoint N/A 15 sec 30 sec
Kinetics
Both normal and sweep speeds are available in Kinetics mode. Single Wavelength
reads are limited to the following minimum times:
96-Well Kinetic Read Timing
Normal Read Mode, A1 to A1 100 msec 50 sec
Normal Read Mode, A1 to A1 0 msec 42 sec
Sweep Read Mode, A1 to A1 N/A 13 sec
Delay 630 nm
384-Well Read Timing
Normal Read Mode
Endpoint 100 msec 169 sec 333 sec
Normal Read Mode
Endpoint 0 msec 131 sec 257 sec
Sweep Read Mode
Endpoint N/A 31 sec 56 sec
Kinetics
Delay 630 nm 630/450 nm
Single Dual
Single Dual
Single Dual
Both normal and sweep speeds are available in Kinetics mode. Single Wavelength
reads are limited to the following minimum times:
384-Well Kinetic Read Timing
Normal Read Mode, A1 to A1 100 msec 169 sec
Normal Read Mode, A1 to A1 0 msec 129 sec
Sweep Read Mode, A1 to A1 N/A 30 sec
Epoch Operator’s Manual
Delay 630 nm
Page 70

52 | Appendix A: Specifications
Optical Performance
Accuracy, Linearity, Repeatability
All qualifications were conducted using 96-/384-well, flat-bottom
and round-bottom microplates.
For the performance described here, the Gain on the Optics Test should be equal to or less
than 5.0.
Measurement Range: 0.000 to 4.000 OD Resolution: 0.0001 OD
Accuracy
96-well plate, normal read speed
0.000–2.000 OD +/-1% +/-0.010 OD
2.000–2.500 OD +/-3% +/-0.010 OD
384-well plate, normal read speed
0.000–1.500 OD +/-2% +/-0.010 OD
1.500–2.000 OD +/-5% +/-0.010 OD
96-well and 384-well plate, sweep read speed
0.000–1.000 OD +/-1% +/-0.010 OD
Linearity (by liquid dilution)
96-well plate, normal read speed
0.000–2.000 OD +/-1% +/-0.010 OD
2.000–2.500 OD +/-3% +/-0.010 OD
384-well plate, normal read speed
0.000–1.500 OD +/-2% +/-0.010 OD
1.500–2.000 OD +/-5% +/-0.010 OD
96-well and 384-well plate, sweep read speed
0.000–1.000 OD +/-1% +/-0.010 OD
Repeatability (measured by one standard deviation: 8 measurements/data points)
96-well plate, normal read speed
0.000–2.000 OD +/-1% +/-0.005 OD
2.000–2.500 OD +/-3% +/-0.005 OD
384-well plate, normal read speed
0.000–1.500 OD +/-1% +/-0.005 OD
1.500–2.000 OD +/-3% +/-0.005 OD
96-well and 384-well plate, sweep read speed
0.000–1.000 OD +/-2% +/-0.010 OD
BioTek Instruments, Inc.
Page 71

Optics
Optical Performance | 53
λ range
λ accuracy
λ repeatability
λ bandpass
Detector
200 to 999 nm
± 2 nm
± 0.2 nm
5 nm
Photodiodes (2). Measurements are reference channel-corrected for
light source fluctuation.
Epoch Operator’s Manual
Page 72

54 | Appendix A: Specifications
BioTek Instruments, Inc.
Page 73

Appendix B
Error Codes
This appendix lists and describes Epoch error codes that may
appear in Gen5. If an error is displayed, refer to Product Support
& Service in Chapter 1.
Overview............................................................................ 56
Diagnostics ....................................................................... 56
General Errors..................................................................... 57
Assay Errors—For 2Dnn Error Code ...................................... 64
Fatal Errors......................................................................... 65
Page 74

56 | Appendix B: Error Codes
Overview
An error code is displayed in Gen5 as a four-digit identifier. The first character will be
a number or the letter A.
The letter A indicates an error condition that cannot be resolved by user actions. The
instrument will no longer function, the LED status light will flash, and the beeper will
sound until the unit is depowered. The codes are listed as Fatal Errors on page 64; if an
error of this type is displayed, contact BioTek’s Technical Assistance Center for further
instructions (refer to the Product Support & Service section in Chapter 1 for contact
information).
Error codes marked with a asterisk (*) will stop an assay from running but will
continue to allow the Epoch to communicate with the controlling software and run
system and calibration tests. When these errors are first encountered, the status LED
will flash and the beeper will sound; however, for these nonfatal errors, you can
disable the beeper by pressing the carrier in/out button or by twice requesting the
instrument status from Gen5.
Diagnostics
If any error code is displayed, conduct a System Self-Test for diagnostic purposes.
In Gen5, select
Help system for more detailed instructions.
System > Diagnostics > Run System Test. Refer to the Gen5
BioTek® Instruments, Inc.
Page 75

General Errors
A lowercase letter in the code indicates a variable numeral.
Code Description and Probable Causes
General Errors | 57
*0210
*02x1
The x-axis home opto sensor failed to change as
expected.
A y-axis opto sensor failed to change as expected.
x = 1 for the home sensor.
x = 2 for the test sensor.
Possible causes:
• Dirty axis rail where the bearings are worn and cause
too much friction.
• Defective or broken optical sensors or connection.
• Defective motor, motor controller, or connection.
• Broken or untensioned drive belt.
• Obstruction in read chamber.
• Check that the shipping screw has been removed.
Note: In cases where a sensor is not functioning, the motor
may drive the axis to its mechanical stop and generate
substantial noise but will not cause permanent damage.
*0212
The order sorting filter wheel’s home opto sensor failed
to change as expected.
Probable causes:
• Filter wheel is loose.
• Filter wheel gearing is worn or dirty.
• Defective or broken optical sensor or connection.
• Defective motor, motor controller PCB, or cable.
0233
Epoch Operator’s Manual
Monochromator not able to locate home.
Probable causes:
• Misaligned flash lamp
• Disconnected photodiode cable
Page 76

58 | Appendix B: Error Codes
Code Description and Probable Causes
*0400
*0401
*0402
*0403
Carrier x-axis failed positional verify.
Motor x-axis failed to reach the same position when moved a
known number of steps from the home position and back.
Probable causes:
• Dirty linear bearing.
• Obstruction in read chamber.
Carrier y-axis failed positional verify.
• Dirty linear bearing.
• Obstruction in read chamber
Order sorting filter wheel failed positional verify.
• Filter wheel is loose.
• Filter wheel gearing is worn or dirty.
• Defective or broken optical sensor or connection.
• Defective motor, motor controller PCB, or cable.
Monochromator failed positional verify.
Probable causes:
• Defective measurement diode PCB or connection.
• Defective mechanics or optics
• Defective flash lamp.
• Defective motor/power PCB or connection to it.
• Flash lamp is missing flashes or is not flashing.
• The optic system does not detect the saturation.
• Defective monochromator (low probability).
BioTek® Instruments, Inc.
Page 77

Code Description and Probable Causes
General Errors | 59
*05cr
*0600
A/D input is saturated
This error indicates the light signal level reached full scale A/D
output (65,535) for a particular channel (c = 0 for the
reference channel and c = 1 for the measurement channel)
and for a particular readset r = 1–6 or self-test wavelength r
= 0–5
Probable causes:
• The order sort filter is not at the correct position and
too much light is present.
• The measurement or reference diode PCB or
connection to it is defective.
• The A/D circuitry on the Main PCB is defective.
Gain Calibration error
This error indicates that the measurement channel signal gain
has reached its maximum or the signal itself is greater than
60000 at the wavelength being calibrated. This normally
occurs only during a factor gain calibration procedure.
*0700
*0710
Reference channel failed noise test greater than 20
counts.
Measurement channel failed noise test greater than 20
counts.
This error indicates significant variations in background
electronic noise were detected, when blocking the light and
increasing the gain to maximum.
Probable causes:
• Electrical noise may be penetrating the measurement
chamber. The bottom and top shrouds are part of the
electrical shielding.
• There may be an ambient light leak. Ensure that the
plate carrier door is properly closed.
• Detector PCB or connection failure.
• Internal electronic noise may be caused by a faulty
analog PCB or faulty internal grounding.
Epoch Operator’s Manual
Page 78

60 | Appendix B: Error Codes
Code Description and Probable Causes
*0800
*0810
*09c0
*0901–0906
*0911–0916
Reference channel failed noise offset < 10 and >
32000.
Measurement channel failed noise offset < 10 and >
2000
This error indicates that the background electronic signal
detected is outside of acceptable limits at maximum gain
when blocking the light.
Probable causes:
• Detector PCB or connection failure yielding a noise
reading of zero.
• Detector PCB is too noisy.
The reference (c = 0) or measurement (c = 1) channel
dark range is < 100 or > 32000 during spectral scan.
The reference channel dark range is < 100 during
optical test (readset 1–6).
The measurement channel dark range is < 100 during
optical test (readset 1–6).
The reference channel dark current value has changed since
the last optics test measurement by more than 10%, or the
dark value is less than 100. The last number in the error code
is the lambda table position number used during the failure.
0C00
0D00
Probable causes:
• The photo-detector is more sensitive to temperature
changes.
• Ambient light leakage during the read.
24VDC voltage error
The 24VDC power to the motors was below the level needed
to assure correct performance.
Probable causes:
• Defective power supply, power cable, connection or
Main PCB.
Wavelength calibration data is missing.
The gain/reset data for one or more wavelength is missing.
This data is collected during factory calibration and saved in
nonvolatile memory. An error indicates that the internal
software may be corrupted or the hardware has failed.
BioTek® Instruments, Inc.
Page 79

Code Description and Probable Causes
General Errors | 61
0E01–0E06
0F0r
0F1r
Wavelength not found in table (readset 1–6).
This error indicates that the specified wavelength is not
detected in the instrument’s filter table.
During the read, verify that the lambda table has the
wavelengths loaded into the instrument from the controlling
PC software. Compare the contents of the lambda table with
the software’s filter table.
The reference channel air/dark out of range.
If this occurs while reading absorbance data, it indicates the
correction factor provided by the reference channel is out of
range, presumably because of a defect in the reference
channel optics. r = 0 in spectral scans, readset = r = 1–6 for
all other absorbance reads.
If this occurs while doing self-test or reading blank data, it
indicates the reference channel signal is < 125 for readset = r
= 1–6 (0 during self-test).
The measurement channel air/dark out of range.
If this occurs while doing self-test or reading blank data, it
indicates the measurement channel signal is < 3162 for
readset = r = 1–6 (0 during self-test).
Probable causes:
*110x
• The flash lamp may be out of alignment.
• The monochromator entrance or exit aperture is
defective.
• The order sorting filter is dirty or degraded and does
not allow enough light energy to pass through.
• Dirty or damaged reference channel optics.
• The reference channel photodiode detection circuit is
defective.
Failed configuration checksum test (1 = UI processor, 2
= MC processor).
Probable causes:
• One of the processors on the PCB contains a defective
corrupt flash memory. The basecode software and/or
assays may need to be redownloaded.
• Main PCB failure.
Epoch Operator’s Manual
Page 80

62 | Appendix B: Error Codes
Code Description and Probable Causes
*120x
*1300
*1301
*1302
1600
Carrier Autocalibration data missing.
Probable causes:
x = 0 or x = 1 Main PCB or software is defective.
x = 2 Insufficient light:
• The PCB was changed and the flash memory does not
have the calibration values loaded.
• Flash lamp, optics, measurement diode PCB, or filter
wheel is defective.
• Autocalibration needs to be run.
• Main PCB failure.
Carrier not homed in the x-axis.
Carrier not homed in the y-axis.
Order sorting filter wheel not homed.
This error is usually only seen if an error 0200 is ignored. See
the probable causes for 0200.
PC Command format error.
1700
1900
1F0r
Probable causes:
• The wrong version of Gen5 is being used.
• The USB driver or cable connection is defective.
Interval too short for selected options.
This error indicates that the kinetic interval in the current
assay is too short.
Increase the kinetic interval.
Memory allocation failed.
This error is typically used only for software development
purposes. If it occurs, however, try turning the instrument
off, waiting 30 seconds, and then turning it on again. If the
error persists, contact BioTek TAC.
Overlap error in PC command definition.
This error indicates that the largest wavelength minus the
smallest wavelength is larger than the wavelength interval in
the assay definition command from the PC for readset = r =
1–6.
BioTek® Instruments, Inc.
Page 81

Code Description and Probable Causes
General Errors | 63
21nn
2401
2500
2600
Invalid parameter value selected.
This error indicates that a particular parameter in the assay
definition command from the PC is incorrect. An invalid assay
configuration was sent to the instrument.
Y-axis test sensor position incorrect.
This error occurs at the start of an assay if the carrier fails to
find the test sensor where it is expected. Usually this is
because the carrier was pushed or restricted on its way into
the instrument. Restart the read.
Sweep mode read missed well location.
While attempting to sweep read a row of wells, the X-axis
motor passed the location where it was supposed to read too
soon. This is most likely the result of a main PCB hardware
failure.
Area scan or custom well defined outside valid plate
limits.
The combination of the Gen5 plate definition and the read
mode (area scan or endpoint) being used results in
dimensions that cannot be used by this reader.
2900
2901
20902–6
2907
• Check of nonstandard plate dimensions.
• Reduce the number of data points in an area scan.
• Reduce the well diameter used with an area scan.
The system measures the following voltages to determine if
they are correct. Voltages and limits are listed on the self-test
report.
24V power supply out of range during self-test.
5V power supply out of range during self-test.
Flash lamp reference (lowest to highest) is out of range
during self-test.
Failure indicates there is a defect on the Main PCB assembly.
Flash voltage scale error—done as part of self-test.
Failure indicates the flash module is not connected or there is
a defect on the Main PCB assembly.
Epoch Operator’s Manual
Page 82

64 | Appendix B: Error Codes
Code Description and Probable Causes
2Dnn
2A0x
Assay error.
This error indicates that something about the assay that is
being attempted is wrong. Refer to the list of assay errors
that follows. The most probable cause of these errors is
something in the Gen5 protocol or instrument selection.
Plate jam error.
Motor “x” (0 = X, 1 = Y, 2 = F, 3 = M) has hit something and
lost steps. Usually this is because the carrier was pushed or
restricted on its way into the instrument. Restart the read.
Assay Errors—For 2Dnn Error Code
Error Code Description
00 SAMPLE_START_LATE—missed start of well mode sample
01 INVALID_READSETS—number of readsets defined invalid
05 INVALID_MODE—invalid mode selection received
06 ASSAY_DEF_NOT_SET—validated assay not defined
07 INVALID_ROW_COL—invalid number of rows and columns
0B INVALID_EVENT_COUNT—total events defined invalid
0C INVALID_EVENT_TYPE—invalid event type received
0E PLATE_START_LATE—missed start of plate mode event
12 INVALID_WELL_DATAPTS—number of samples out of range
13 INVALID_WELL_INTERVAL—well mode kinetic interval out of
range
14 INVALID_PLATE_DATAPTS—specified # of plate mode reads
out of range
15 INVALID_PLATE_INTRVAL—plate mode kinetic interval out of
range
16 READ_START_LATE—missed start of read
17 INTERVAL_START_LATE—missed start of kinetic interval setup
43 INVALID_DIRECTION—initial row or column direction is invalid
44 DEBUG_MODE—unit is in debug mode; self-test may not have
been run
BioTek® Instruments, Inc.
Page 83

Fatal Errors
Fatal errors indicate conditions that cannot be resolved by user actions. The
instrument will no longer function. If a fatal error is displayed, contact BioTek’s
Technical Assistance Center for further instructions (refer to the Product Support
& Service section in Chapter 1). {data} indicates a parameter in the second-lowest
digit of the error code. <data> indicates a parameter in the lowest digit of the
error code.
Code Description
Fatal Errors | 65
A100
A200
A300
A400
A500
A600
A700
A800
A900
AA00
Task control block not available.
Dual Processor Code Version Mismatch.
OS <Device> not available.
Flash write timed out.
Read did not match write (test) <chip>.
Data flash write timed out.
Read did not match write (test) <chip>.
<Test type> power supply level error.
Memory allocation heap corrupted.
Absorbance A/D converter never saw the ready
signal.
Probable causes:
• Analog PCB.
• Motor/power PCB.
AB00–ABFF
AC00
AD00
Fatal errors related to internal dual processor
architecture.
External RAM test failure.
Operating System StackCheck error in <processor>
0=UI, 1=Sa, 2=Sb, 3=Sc
Epoch Operator’s Manual
Page 84

66 | Appendix B: Error Codes
BioTek
®
Instruments, Inc.
Page 85

Page 86

 Loading...
Loading...