Page 1

Cytation™ 5
Cell Imaging Multi-Mode Reader
Instructions for Use
BioTek® Instruments, Inc.
January 2015
© 2015
PN 1321022
Revision A
Page 2

ii | Preface
Notices
BioTek® Instruments, Inc.
Highland Park, P.O. Box 998
Winooski, Vermont 05404-0998 USA
All Rights Reserved
®
© 2015, BioTek
transcribed, or transmitted in any form, or by any means electronic or mechanical, including
photocopying and recording, for any purpose other than the purchaser’s use without written
permission of BioTek Instruments, Inc.
Trademarks
Instruments, Incorporated. No part of this publication may be reproduced,
BioTek
®
is a registered trademark, and Cytation™, Gen5™, BioStack™, and Take3™ are
trademarks of BioTek Instruments, Inc. Glowell™ is a trademark of LUX Biotechnology, Ltd.
Harta™ is a trademark of Harta Instruments.
Microsoft®, Windows®, and Excel® are either registered trademarks or trademarks of Microsoft
Corporation in the United States and/or other countries.
All other trademarks are the property of their respective holders.
Restrictions and Liabilities
Information in this document is subject to change and does not represent a commitment by
BioTek Instruments, Inc. Changes made to the information in this document will be incorporated
in new editions of the publication. No responsibility is assumed by BioTek for the use or
reliability of software or equipment that is not supplied by BioTek or its affiliated dealers.
BioTek Instruments, Inc.
Page 3
Contact Information| iii
Internet:
www.biotek.com
Phone:
888-451-5171 (toll-free in the U.S.)
802-655-4740 (outside the U.S.)
Fax:
802-655-7941
Email:
customercare@biotek.com
BioTek US—World Headquarters
BioTek China—Beijing
Phone: +86 (10) 85865569
BioTek China—Shanghai
BioTek France
Phone: +33 (3) 89206329
BioTek Germany—European
Coordination Center
BioTek India
Phone: +49 (0) 71369680
Phone: +91 (22) 66870046
Website: www.biotek.in
BioTek Japan
BioTek Singapore
Phone: +81(0)3 5812 8109
Website: www.biotek.com/ja
Phone: +65 65922100
Website: www.biotek.com
Contact Information
Customer Service and Sales
Global Service and Support
BioTek instrument service and repair is available worldwide at one of BioTek's
International Service Centers and in the field at your location. For technical
assistance, contact the Technical Assistance Center (TAC) at BioTek US—World
Headquarters. To arrange for service or repair of your instrument, contact the office
nearest you.
Phone: (802)655-4740
Toll-Free: (888)451-5171
Service Toll-Free: (800)242-4685
Email: CustomerCare@biotek.com
Service Email: TAC@biotek.com
www.biotek.com
Phone: +86 (021)50435800
Email: infochina@biotek.com
Website: www.biotekchina.com.cn
Email: info@biotek.de
Website: www.biotek.de
Email: infojapan@biotek.com
Email: infochina@biotek.com
Website: www.biotekchina.com.cn
Email: info@biotek.fr
Website: www.biotek.fr
Fax: +91 (22) 28759944
Email: biotek@biotek.in
Email: singapore@biotek.com
Cytation 5
Page 4
iv | Preface
BioTek South Korea
BioTek Switzerland
Phone: +82 (0) 2 5624740
www.biotekinstruments.co.kr
Phone: +41 (41) 2504060
BioTek Taiwan
BioTek United Kingdom (UK)
Phone: +886(2)26277725
Website: www.biotek.com
Email: CustomerCare@biotek.com
Email: korea@biotek.com
Website:
Email: infotaiwan@biotek.com
Email: info@biotek.ch
Website: www.biotek.ch
Website: www.biotek.uk.com
Instructions for Use Requirements
This document fulfills the basic needs of persons operating this device, according to
the requirements of the In Vitro Diagnostic Directive for "Instructions for Use." Some
of the device's higher-level functions and features, as well as certain detailed
maintenance and qualification routines, are described in the Cytation 5 Operator's
Manual.
Intended Use Statement
The Cytation 5 is a hybrid multi-mode microplate reader. The performance
characteristics of the data reduction software have not been established with any
laboratory diagnostic assay. The user must evaluate this instrument and PC-based
software in conjunction with their specific assay(s). This evaluation must include the
confirmation that performance characteristics for the specific assay(s) are met.
• If the instrument has an "IVD" label, it may be used for clinical and non-clinical
purposes, including research and development. If there is no such label, the
instrument may be used only for research and development or other nonclinical purposes.
Quality Control
It is considered good laboratory practice to run laboratory samples according to
instructions and specific recommendations included in the assay package insert for
the test to be conducted. Failure to conduct Quality Control checks could result in
erroneous test data.
BioTek Instruments, Inc.
Page 5

Warnings
Operate the instrument on a level, stable surface away from excessive
Bright sunlight or strong incandescent light can reduce the linear
performance range of the instrument.
Measurement values may be affected by extraneous particles (such as dust)
readings.
When operated in a safe environment according to the instructions in this
However, the operator should be aware of certain situations that could result
Hazards and Precautions.
Warning! Power Rating. The instrument’s power supply or power
hazards.
Warning! Electrical Grounding. Never use a plug adapter to connect
functional ground.
Warning! Service. Only qualified technical personnel should perform
service procedures on internal components.
Warning! Accessories. Only accessories that meet the
manufacturer's specifications shall be used with the instrument.
Warning! Lubricants. Do not apply lubricants to the microplate
particles, which may obstruct the carrier path and cause the instrument
to produce an error.
Warning! The instrument with all available modules weighs up to 80
instrument.
Warning! Liquids. Avoid spilling liquids on the instrument; fluid
rogram is running, abort the program and turn
BioTek TAC for assistance.
humidity.
in the microplate wells. A clean work area is necessary to ensure accurate
document, there are no known hazards associated with the instrument.
in serious injury; these may vary depending on the instrument model. See
Hazards
Warnings| v
The following hazards are provided to help avoid injury:
cord must be connected to a power receptacle that provides voltage
and current within the specified rating for the system. Use of an
incompatible power receptacle may produce electrical shock and fire
primary power to the external power supply. Use of an adapter
disconnects the utility ground, creating a severe shock hazard. Always
connect the power cord directly to an appropriate receptacle with a
carrier or carrier track. Lubricant on the carrier mechanism or
components in the carrier compartment will attract dust and other
lbs. (36.3 kg). Use two people when lifting and carrying the
seepage into internal components creates a potential for shock hazard.
If a spill occurs while a p
off the instrument. Wipe up all spills immediately. Do not operate the
instrument if internal components have been exposed to fluid. Contact
Cytation 5
Page 6
vi | Preface
Warning! Unspecified Use. Failure to operate the equipment
could result in a hazardous condition.
Warning! Software Quality Control. The operator must follow the
quality control checks could result in erroneous test data.
Warning! Reader Data Reduction Protocol. No limits are applied to
control must be thoroughly analyzed by the operator.
Warning! Internal Voltage. Always turn off the power switch and
Warning! Potential Biohazards. Some assays or specimens may
equipment, such as chemical-resistant rubber gloves and apron.
Warning! LED Lights. Serious eye injury may occur if you stare
directly at the LED during operation of the light. This hazard is noted by
Warning! Pinch Hazard. Some areas of the dispense module can
present pinch hazards when the instrument is operating. The module is
marked with the symbol shown here. Keep hands/fingers clear of these
areas when the instrument is operating.
Caution: Service. The instrument should be serviced by BioTek-
Caution: Spare Parts. Only approved spare parts should be used for
or cause damage to the instrument.
Caution: Environmental Conditions. Do not expose the system to
temperature extremes. For proper operation, ambient temperatures should
range. Storage temperature limits are broader.
according to the guidelines and safeguards specified in this manual
manufacturer’s assay package insert when modifying software
parameters and establishing reading methods. Failure to conduct
the raw measurement data. All information exported via computer
unplug the power supply before cleaning the outer surface of the
instrument or removing its top case.
pose a biohazard. This hazard is noted by the symbol shown here.
Adequate safety precautions should be taken as outlined in the assay’s
package insert. Always wear safety glasses and appropriate protective
the symbol shown here.
Precautions
The following precautions are provided to help avoid damage to the instrument:
authorized service personnel. Only qualified technical personnel should
perform service procedures on internal components.
maintenance. The use of unapproved spare parts and accessories may
result in a loss of warranty and potentially impair instrument performance
remain within the range listed in the Specifications chapter. Performance
may be adversely affected if temperatures fluctuate above or below this
BioTek Instruments, Inc.
Page 7

| vii
Caution: Sodium Hypochlorite. Do not expose any part of the
instrument surfaces. Be certain to rinse and thoroughly wipe all surfaces.
Caution: Power Supply. Use only the power supply shipped with the
listed on it.
Caution: Disposal. Dispose of the instrument according to Directive
local ordinances.
Caution: Warranty. Failure to follow preventive maintenance protocols
may void the warranty. See the Maintenance chapter.
Caution: Shipping Hardware. The shipping brackets must be removed
the instrument. See the Installation chapter.
Caution: Electromagnetic Environment. Per IEC 61326-2-6 it is the
the device will perform as intended.
Caution: Electromagnetic Compatibility. Do not use this device in
proper operation.
instrument to the recommended diluted sodium hypochlorite solution
(bleach) for more than 20 minutes. Prolonged contact may damage the
instrument. Operate this power supply within the range of line voltages
2012/19/EC, “on waste electrical and electronic equipment (WEEE)” or
before operating the instrument. They must be reinstalled before shipping
user’s responsibility to ensure that a compatible electromagnetic
environment for this instrument is provided and maintained in order that
close proximity to sources of strong electromagnetic radiation (e.g.,
unshielded intentional RF sources), because these may interfere with the
Cytation 5
Page 8
viii | Preface
CE Mark
Based on the testing described below and information
contained herein, this instrument bears the CE mark
Refer to the Declaration of Conformity for specific details.
Directive 2014/30/EU: Electromagnetic Compatibility
Emissions—Class A
The system has been type-tested by an independent, accredited testing laboratory
and found to meet the requirements of EN 61326-1: Class A for Radiated Emissions
and Line Conducted Emissions.
Verification of compliance was conducted to the limits and methods of EN 55011 –
(CISPR 11) Class A. In a domestic environment it may cause radio interference, in
which case you may need to mitigate the interference.
Immunity
The system has been type-tested by an independent, accredited testing laboratory
and found to meet the requirements of EN 61326-1 and EN 61326-2-6 for Immunity.
Verification of compliance was conducted to the limits and methods of the following:
EN 61000-4-2, Electrostatic Discharge
EN 61000-4-3, Radiated EM Fields
EN 61000-4-4, Electrical Fast Transient/Burst
EN 61000-4-5, Surge Immunity
EN 61000-4-6, Conducted Disturbances from RFI
EN 61000-4-8, Power Frequency Magnetic Field Immunity Test
EN 61000-4-11, Voltage Dips, Short Interruptions and Variations
Directive 2014/35/EU Low Voltage (Safety)
The system has been type-tested by an independent testing laboratory and was
found to meet the requirements of this Directive. Verification of compliance was
conducted to the limits and methods of the following:
EN 61010-1. "Safety requirement for electrical equipment for measurement, control
and laboratory use. Part 1, General requirements."
EN 61010-2-081. “Particular requirements for automatic and semi-automatic
laboratory equipment for analysis and other purposes.”
EN 61010-2-010. “Particular requirements for laboratory equipment for the heating of
materials.“
BioTek Instruments, Inc.
Page 9

Electromagnetic Interference and Susceptibility| ix
EN 60825-1, "Safety of laser products. Part 1: Equipment classification and
requirements."
Directive 2012/19/EU: Waste Electrical and Electronic
Equipment
Disposal Notice: Dispose of the instrument according to Directive 2002/96/EC, “on
waste electrical and electronic equipment (WEEE)” or local ordinances.
Directive 98/79/EC: In Vitro Diagnostics (if labeled for this
use)
• Product registration with competent authorities
• EN 61010-2-101. “Particular requirements for in vitro diagnostic (IVD) medical
equipment.”
• Traceability to the U.S. National Institute of Standards and Technology (NIST).
Electromagnetic Interference and Susceptibility
USA FCC CLASS A
RADIO AND TELEVISION INTERFERENCE
NOTE: This equipment has been tested and found to comply with the limits for a
Class A digital device, pursuant to Part 15 of the FCC Rules. These limits are
designed to provide reasonable protection against harmful interference when the
equipment is operated in a commercial environment. This equipment generates, uses,
and can radiate radio frequency energy and, if not installed and used in accordance
with the instruction manual, may cause harmful interference to radio
communications. Operation of this equipment in a residential area is likely to cause
harmful interference, in which case the user will be required to correct the
interference at their own expense.
In order to maintain compliance with FCC regulations, shielded cables must be used
with this equipment. Operation with non-approved equipment or unshielded cables
is likely to result in interference to radio and television reception.
Canadian Department of Communications Class A
This digital apparatus does not exceed Class A limits for radio emissions from digital
apparatus set out in the Radio Interference Regulations of the Canadians Department
of Communications.
Le present appareil numerique n'emet pas du bruits radioelectriques depassant les limites
applicables aux appareils numerique de la Class A prescrites dans le Reglement sur le
brouillage radioelectrique edicte par le ministere des Communications du Canada.
Cytation 5
Page 10

x | Preface
User Safety
This device has been type-tested by an independent laboratory and found to meet the
requirements of the following:
• Underwriters Laboratories UL 61010-1, “Safety requirements for electrical
equipment for measurement, control and laboratory use; Part 1: General
requirements.”
• Canadian Standards Association CAN/CSA C22.2 No. 61010-1, “Safety
requirements for electrical equipment for measurement, control and laboratory
use; Part 1: General requirements.”
• EN 61010 Standards, see CE Mark starting on page viii.
BioTek Instruments, Inc.
Page 11
Safety Symbols| xi
intrappolarsi
Corrente continua e corrente
fascio
Safety Symbols
Some of the following symbols may appear on the instrument or accessories:
Alternating current
Courant alternatif
Wechselstrom
Corriente alterna
Corrente alternata
Warning, risk of crushing or
pinching
Attention, risque d'écrasement et
pincement
Warnen, Gefahr des Zerquetschens
und Klemmen
Precaución, riesgo del
machacamiento y sejeción
Attenzione, rischio di schiacciare ed
Direct current
Courant continu
Gleichstrom
Corriente continua
Corrente continua
Both direct and alternating
current
Courant continu et courant
alternatif
Gleich - und Wechselstrom
Corriente continua y
corriente alterna
alternata
Earth ground terminal
Borne de terre
Erde (Betriebserde)
Borne de tierra
Terra (di funzionamento)
Warning, hot surface
Attention, surface chaude
Vorsicht, heiße Oberfläche
Precaución, superficie caliente
Attenzione, superfice calda
Laser radiation: Do not stare into
beam
Rayonnement laser: Ne pas
regarder dans le faisceau
Laserstrahlung: nicht in den strahl
blicken
Radiación de laser: No mire
fijamente al rayo
Radiazione di laser: Non stare nel
Warning, potential biohazards
Attention, risques biologiques
potentiels
Warnung! Moegliche biologische
Giftsoffe
Atención, riesgos biológicos
Attenziones, rischio biologico
Protective conductor
terminal
Borne de terre de protection
Schultzleiteranschluss
Borne de tierra de
protección
Terra di protezione
Cytation 5
Caution (refer to accompanying
documents)
Attention (voir documents
d'accompanement)
Achtung siehe Begleitpapiere
Atención (vease los documentos
incluidos)
Attenzione, consultare la doc
annessa
Page 12
xii | Preface
Chiuso
On (Supply)
Marche (alimentation)
Ein (Verbindung mit dem
Netz)
Conectado
Off (Supply)
Arrêt (alimentation)
Aus (Trennung vom Netz)
Desconectado
Aperto (sconnessione dalla
rete di alimentazione)
Consult instructions for use
Consulter la notice d'emploi
Gebrauchsanweisung beachten
Consultar las instrucciones de uso
Consultare le istruzioni per uso
In vitro diagnostic medical device
Dispositif médical de diagnostic in
vitro
Medizinisches In-Vitro
Diagnostikum
Dispositivo médico de diagnóstico
in vitro
Dispositivo medico diagnostico in
vitro
Warning, risk of electric
shock
Attention, risque de choc
électrique
Gefährliche elektrische
schlag
Precaución, riesgo de
sacudida eléctrica
Attenzione, rischio di scossa
elettrica
Separate collection for electrical
and electronic equipment
Les équipements électriques et
électroniques font l'objet d'une
collecte sélective
Getrennte Sammlung von Elektround Elektronikgeräten
Recogida selectiva de aparatos
eléctricos y electrónicos
Raccolta separata delle
apparecchiature elettriche ed
elettroniche
BioTek Instruments, Inc.
Page 13

Installation
Page 14

2 | Installation
Item
Part #
Cytation 5 Operator's Manual (delivered on USB flash
drive)
#2 Phillips screwdriver
01188
9/64" hex wrench
01623
Models with the imaging module:
FireWire cable
1220538
Microplate slide holder
1220548
Isolation table
1220521
Objective adapter collar wrench
1222187
Objective setup plate
1222531
3/32" hex wrench
48570
Models with an external dispense module (packed separately), with the following
accessories:
Injector
8040541
Inlet tubes (2) from supply bottles to syringe drives
7082121
250-µL syringes (2)
7083000
Package Contents
1321000
Power cord set (specific to installation environment):
Europe (Schuko)
USA/International
United Kingdom
Australia/New Zealand
USB cable 75108
FireWire desktop interface
OR
FireWire laptop card and power supply
75010
75011
75012
75013
01604
1220535
Power supply only:
01062
BioTek Instruments, Inc.
Page 15

1: Unpack and Inspect the Reader | 3
Item
Part #
Syringe thumbscrews
19511
Priming plate
8042202
Injector tip priming trough
8042068
Dispense module communication cable
75107
Dispense module front cover
8042197
Dispense module box
8040534
Supply bottles (2, 30 mL)
7122609
Supply bottle holders (2)
8042193
Injector tip cleaning stylus and plastic storage bag
2872304
Strap reagent racks (6)
7212035
Models with the gas controller ("G" models)(packed separately):
Gas controller unit, CO2/O2 control
1210500
Shipping accessories, CO2/O2 control
1210010
Gas Controller Unit, CO2 only
1210504
Shipping accessories, CO2 only
1210009
1: Unpack and Inspect the Reader
The Cytation 5 should be removed from the box by two people. The instrument
with all available modules weighs up to 80 pounds (36.6 kg).
Save all packaging materials. If you need to ship the reader to BioTek for repair
or replacement, you must use the original materials. Using other forms of
commercially available packaging, or failing to follow the repackaging
instructions, may void your warranty.
During the unpacking process, inspect the packaging, reader, and accessories
for shipping damage. If the reader is damaged, notify the carrier and your
BioTek representative. Keep the shipping boxes and the packaging materials
for the carrier's inspection. BioTek will arrange for repair or replacement
immediately.
1. Open the shipping box, remove the instrument from the box, and place it on
a level, stable surface.
2. Place the packaging materials back into the shipping box for reuse if the
instrument needs to be shipped again.
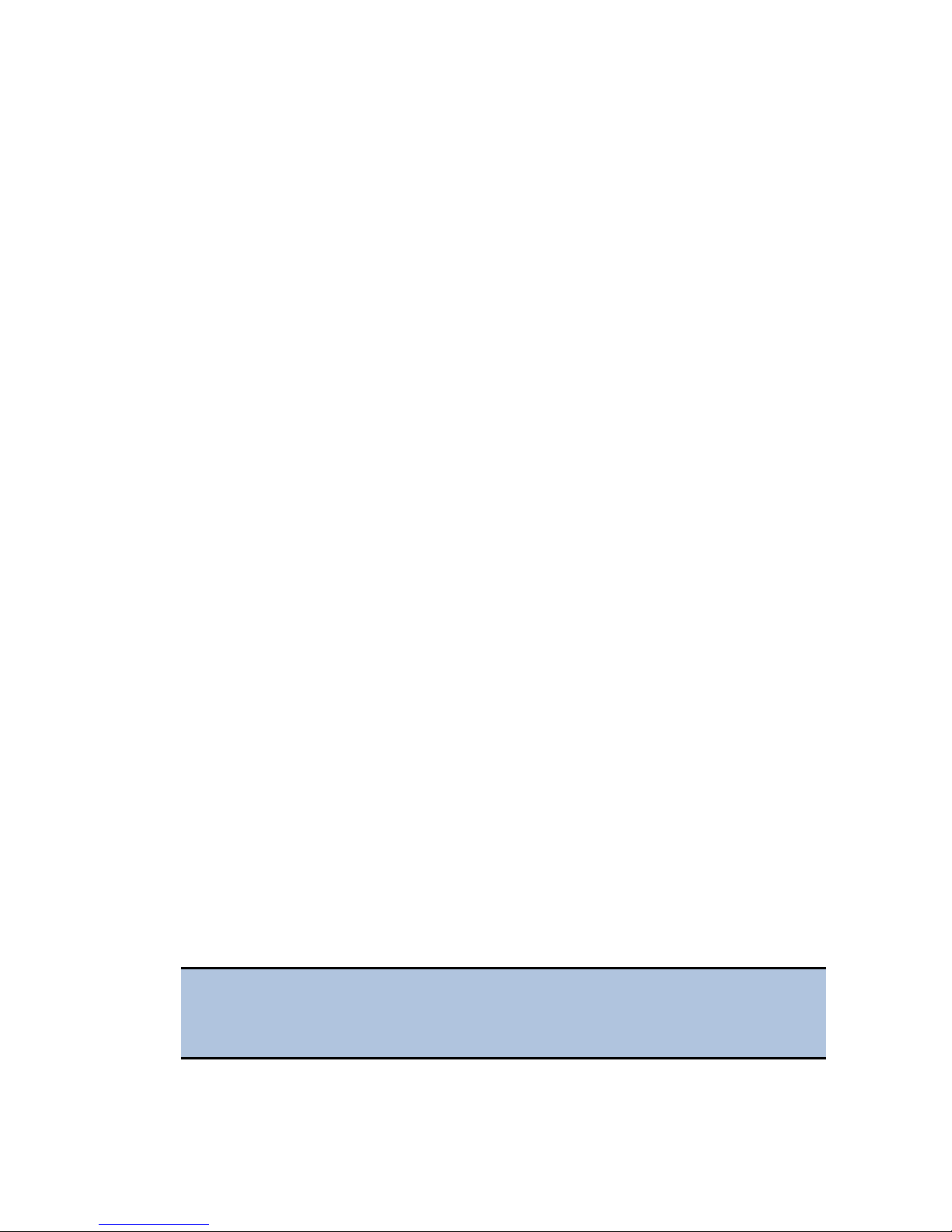
3. For the instruments with the imaging module: Open the accessories box, and
remove the isolation table.
Cytation 5
Page 16
4 | Installation
2: Unpack and Inspect the Dispenser
If applicable:
1. Open the shipping box. Remove the accessories box and foam insert that
contains the injector tubing and bottle holders.
2. Lift out the dispenser and place it on a level surface.
3. Open the accessories box and remove its contents.
4. Place all packaging materials into the shipping box for reuse if the dispenser
needs to be shipped.
3: Unpack and Inspect the Gas Controller
If applicable:
1. Open the shipping box. Remove the accessories, and set them aside.
2. Lift out the gas controller, and place it on a level surface.
3. Place all packaging materials into the shipping box for reuse if the gas
controller needs to be shipped.
4: Select an Appropriate Location
Install the reader on a level, stable surface in an area where ambient temperatures
between 18°C (64°F) and 30°C (86°F) can be maintained.
Leave at least six inches of space between the instrument’s rear panel and any other
object. This space ensures proper air flow in and out of the instrument.
The reader is sensitive to extreme environmental conditions. Avoid the following:
• Excessive humidity. Condensation directly on the sensitive electronic circuits
can cause the instrument to fail internal self-checks. The humidity must be in
the range of 10–85%, non-condensing.
• Excessive ambient light. Bright light may affect the reader’s optics and
readings, reducing its linear range.
• Dust. Readings may be affected by extraneous particles (such as dust) in the
microplate wells. A clean work area is necessary to ensure accurate readings.
• Vibration. The instrument should be installed in a vibration-free environment.
Be sure to position the instrument away from other devices that could
potentially create vibration during the read process.
If you are installing a BioStack for operation with the Cytation 5, you may
wish to seat the instruments in their alignment plates now. Refer to the
stacker's operator's manual for more information.
BioTek Instruments, Inc.
Page 17
4: Select an Appropriate Location | 5
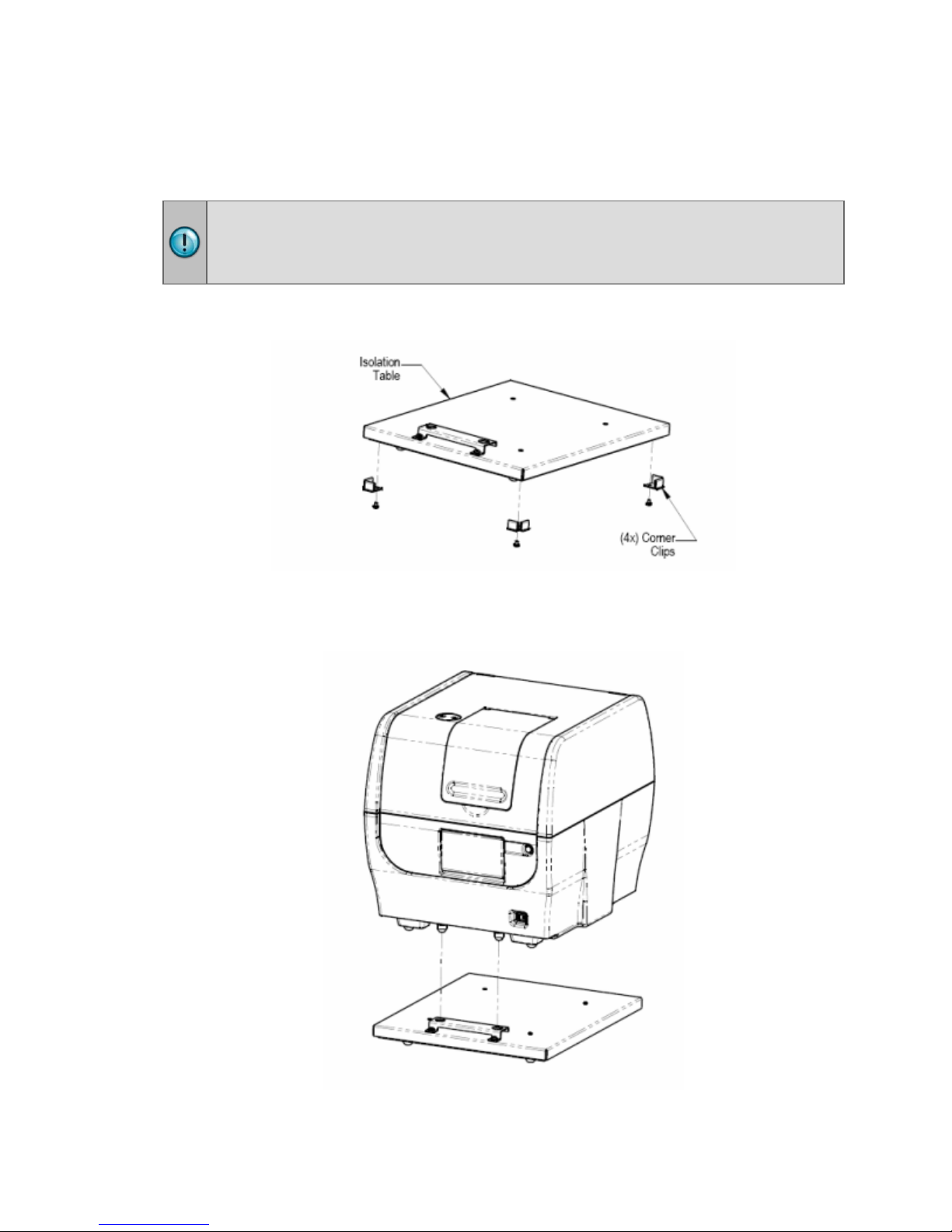
Installing Instruments with the Isolation Table
Cytation 5 models with the imaging module can be used with an isolation table,
which helps to eliminate vibration during image reads.
Do not use the isolation table when operating the Cytation 5 with a microplate
stacker. Store the isolation table in a clean, dry location.
1. Remove the four corner clips from the isolation table.
2. Place the isolation table in the selected installation location.
3. Place the instrument on the table as shown next:
Cytation 5
Page 18
6 | Installation
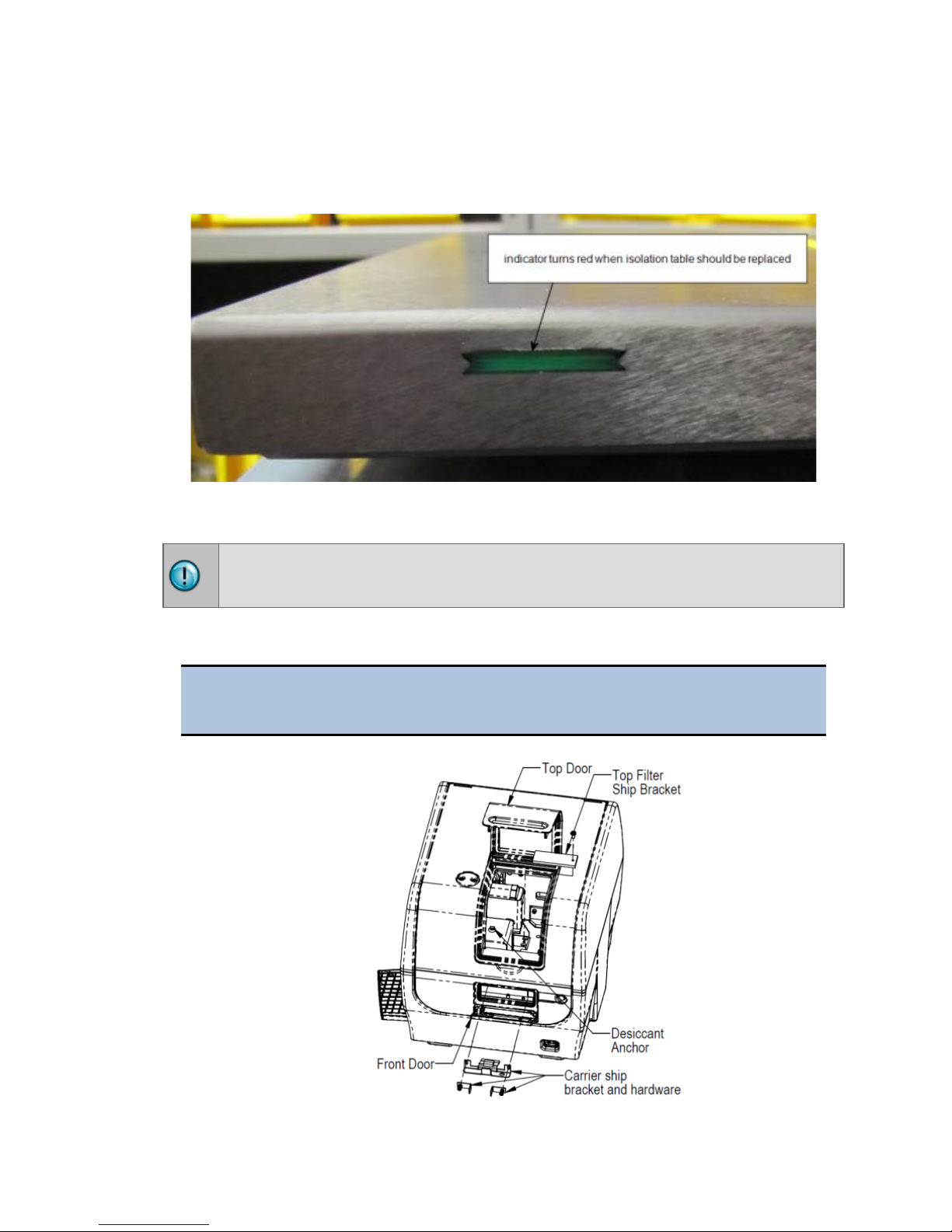
The isolation table contains material that dampens vibration. Over time, this material
becomes compressed and can lose effectiveness. The isolation table has a color
indicator that turns from green to red to show when the table should be replaced
because the dampening material has been compressed.
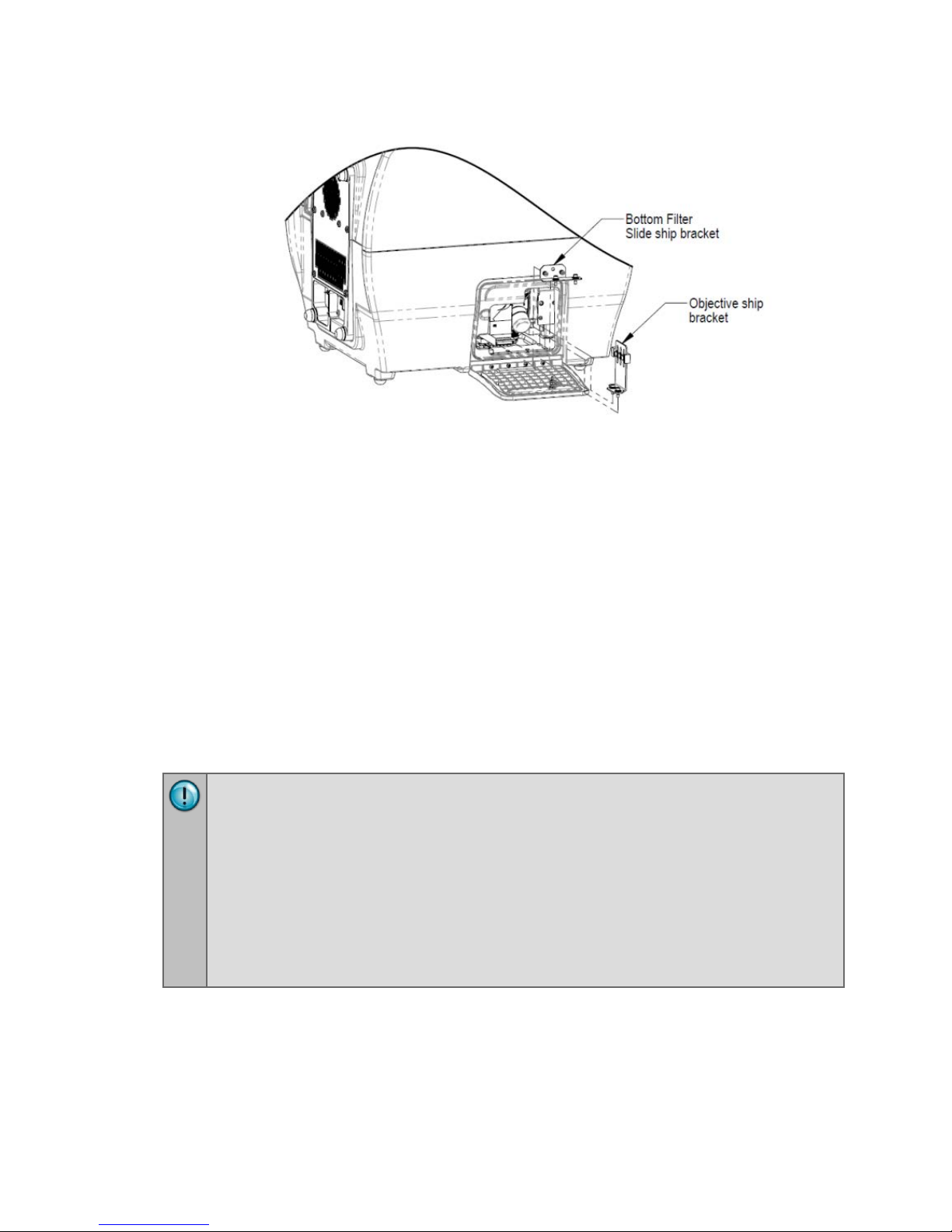
5: Remove the Shipping Hardware
Remove all shipping hardware before you turn on the reader.
1. Locate the shipping hardware.
The figures below depict a Cytation 5 with the filter module and imaging
module.
BioTek Instruments, Inc.
Page 19
6: Install the Power Supply | 7
2. Open the top door, and remove the reusable zip tie and desiccant packet
from the desiccant anchor.
3. If equipped, use the supplied screwdriver to remove the top filter shipping
bracket.
4. Open the front door, and using the supplied screwdriver, remove the carrier
shipping bracket.
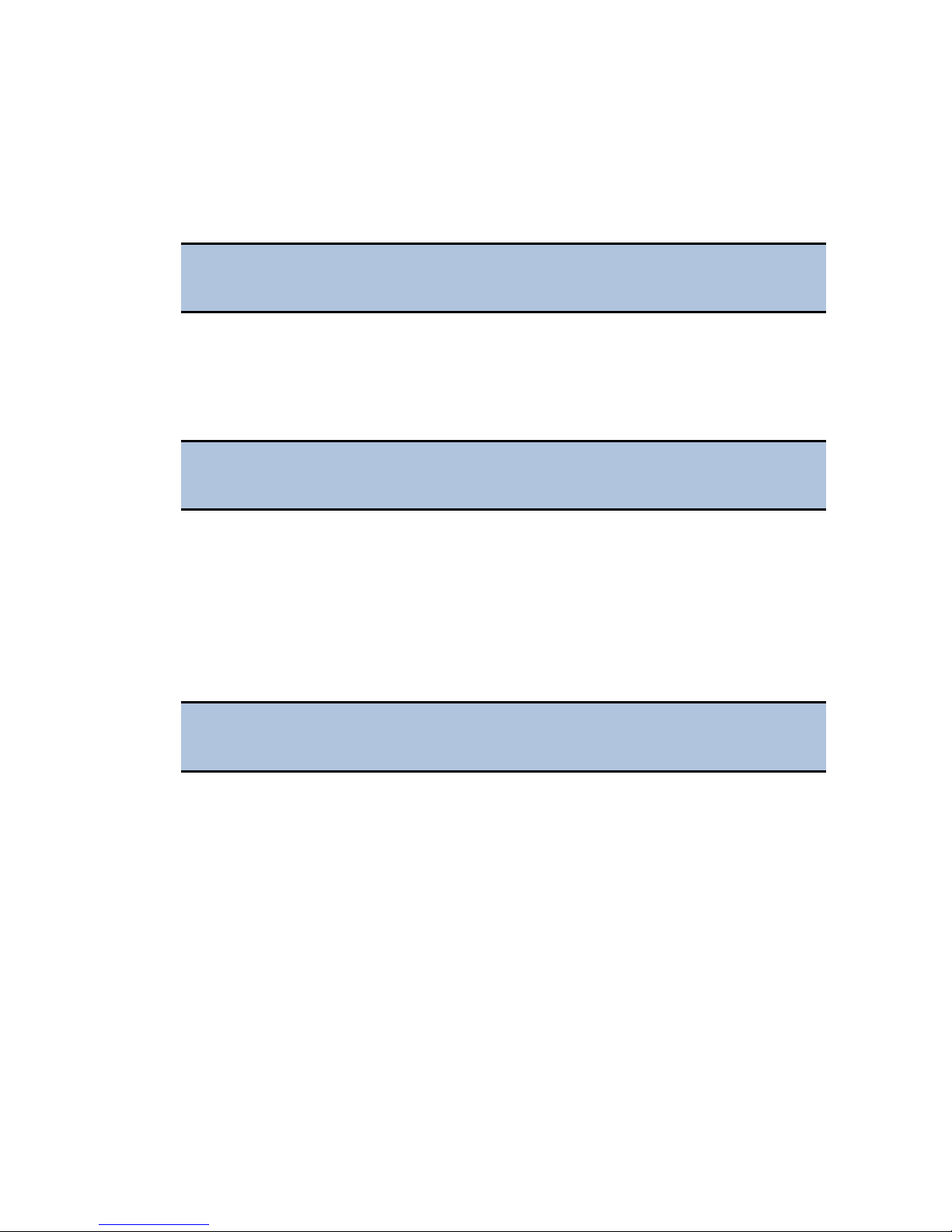
5. If equipped with the imaging module, use a 9/64" hex wrench to remove the
bottom filter slide ship bracket
6. Push the filter slide back, and remove the two-piece objective ship bracket.
7. Store the shipping hardware in a safe location, in case the instrument needs
to be shipped again.
6: Install the Power Supply
Power Rating. The instrument must be connected to a power receptacle that
provides voltage and current within the specified rating for the system. Use of
an incompatible power receptacle may produce electrical shock and fire
hazards.
Electrical Grounding. Never use a plug adapter to connect primary power to
the instrument. Use of an adapter disconnects the utility ground, creating a
severe shock hazard. Always connect the system power cord directly to an
appropriate receptacle with a functional ground.
1. Locate the power inlet on the back of the reader.
2. Examine the power supply's plug. It has a small groove that lines up with a
Cytation 5
tab inside the power inlet.
3. Insert the plug into the power inlet and plug the power supply's cord into an
appropriate power receptacle.
Page 20

8 | Installation
Do not plug the power supply into a power receptacle until after the
power supply is connected to the instrument.
7: Install the Gas Controller (if applicable)
The gas controller is an external module that enables the user to control CO2 and O2
concentrations inside the attached instrument’s reading chamber. If you purchased
the module for operation with the Cytation 5, refer to the Gas Controller User Guide
for installation instructions.
8: Unpack and Install the Joystick (if
applicable)
If applicable:
1. Open the shipping box, lift out the joystick, and place it on a level surface.
2. Place all packaging materials into the shipping box for reuse if the joystick
needs to be shipped.
3. Locate the joystick cable. Plug one end into the port on the back of the
joystick. Plug the other end into the joystick port on the rear of the reader.
9: Install the Dispenser
Place the dispense module on top of the reader or on top of the gas
controller (if equipped). Do not place the dispenser next to the reader.
BioTek Instruments, Inc.
Page 21
9: Install the Dispenser | 9
1. Open the plastic bag containing the injector tube and tips. Remove the clear
plastic shrouds from the tubes.
2. Remove the two inlet tubes from their plastic canisters.
3. Identify the two syringe valves on the dispense module. Each is labeled with
a left-pointing arrow.
When installing the inlet and outlet tubes, do not use any tools. Fingertighten only!
4. Screw the fitting of one inlet tube into the right side of the Syringe 1 valve.
5. Identify the #1 outlet tube, and screw it into the left side of the Syringe 1
valve.
6. Repeat these steps to attach the inlet and outlet tubing for Syringe 2.
It is critical that the tubing is installed in the correct ports. Otherwise,
injected fluid may miss the intended well.
7. Remove the round tubing feed-through cover from the top of the reader (2
screws). Store the cover and screws with the shipping hardware in case the
reader needs to be shipped again.
8. Thread the injector tip holder, with outlet tubing connected to both ports,
through the hole in the top of the reader.
9. Open the reader's top door, and, holding the injector tip holder by the tab,
insert the injector tips into the appropriate holes inside the reader.
A magnet located between the injector tips helps to guide the tips into
place and secures them in the reader.
10. Place the tubing feed-through cover over the hole in the top of the reader and
finger-tighten the thumbscrews to secure it.
11. Remove the two syringes from their protective boxes. They are identical and
interchangeable.
12. Install both syringes.
• Hold the syringe vertically with the threaded end at the top.
• Screw the top of the syringe into the bottom of the syringe valve. Finger-
tighten only.
• Carefully pull down the bottom of the syringe until it rests inside the hole
in the bracket.
• Pass a thumbscrew up through this hole and thread it into the bottom of
the syringe. Hold the syringe to prevent it from rotating while tightening
the thumbscrew. Finger-tighten only.
Cytation 5
Page 22
10 | Installation
The Cytation 5 is controlled by Gen5 software running on a host computer.
provided in Gen5 Getting Started Guide to install the software.
13. Locate the dispenser cable. Plug one end into the port on the left side of the
dispenser. Plug the other end into the “Dispenser Port” on the rear of the
reader.
14. Locate the injector tip-cleaning stylus, packaged in a small cylinder. Attach
the cylinder to the back of the dispenser for storage.
10: Connect the Host Computer
The Cytation 5 is equipped with a USB port for connection to the host computer.
Connect the supplied USB cable between the USB port on the back of the reader and
an available USB port on the computer.
11: Install Gen5
There is a certain sequence of events that must be followed to ensure that the
software is properly installed and configured. Please follow the instructions
12: Turn on the Reader
If you have not already done so, turn on the reader. The reader’s power switch is
located on the lower-right corner of the front panel. The reader performs a system
test. When the test is completed, the reader extends the microplate carrier.
The carrier eject button, located above to the reader’s power switch, can
be used to extend/retract the microplate carrier.
13: Establish Communications
Before performing this step, refer to the instructions that shipped with
the USB Driver Software on the Gen5 software media to install the
necessary drivers.
1. If not already done, start Gen5 and log in if prompted. The default System
Administrator password is admin.
2. From the Gen5 main screen, select
and click
3. Set the Reader Type to
4. Select
Add Reader.
Cytation 5, and click OK to continue.
Plug & Play.
System > Instrument Configuration
BioTek Instruments, Inc.
Page 23
14: Install the Imager Module | 11
A Cytation 5 must be connected via USB to the computer and turned
on to appear in the Available Plug & Play Readers list.
5. To test that Gen5 can communicate with the instrument, click Test
Communications
displays a success message. Return to Gen5’s main screen.
. If the communication attempt is successful, Gen5
Communication Errors
If the communication attempt is not successful, try the following:
• Is the reader connected to the power supply and turned on?
• Is the communication cable firmly attached to both the reader and the
computer?
• Did you select the correct Reader Type in Gen5?
• Did you install the USB driver software?
If you remain unable to get Gen5 and the reader to communicate with
each other, contact BioTek’s Technical Assistance Center.
14: Install the Imager Module
Several steps are required to install the imager module.
1. Install the FireWire card and driver
2. Set up Gen5 for imaging
3. Install the objectives, LED cubes, and imaging filter cubes, and run Auto
Calibration
Tools:
• Screwdriver: Desktop computer users typically need a screwdriver to install the
FireWire card.
• 3/32" hex (or Allen) wrench
1. FireWire Video Card: Computer and Camera Setup
BioTek supplies the required FireWire card (PCI Express Card-IEEE-1394b) for either
a desktop computer or a laptop. Follow the applicable instructions for your
workplace, then install the FireWire software driver on your computer.
Cytation 5
Page 24
12 | Installation
Desktop Computer: Install the FireWire Card
To avoid electrostatic discharge and damage to internal components,
ground yourself by using wrist grounding straps or by touching a metal
surface on the computer's chassis.
The following directions provide general steps for installing a PCI Express
card. Talk to your company's IT representative for assistance with these
steps. For more detailed help, contact BioTek TAC.
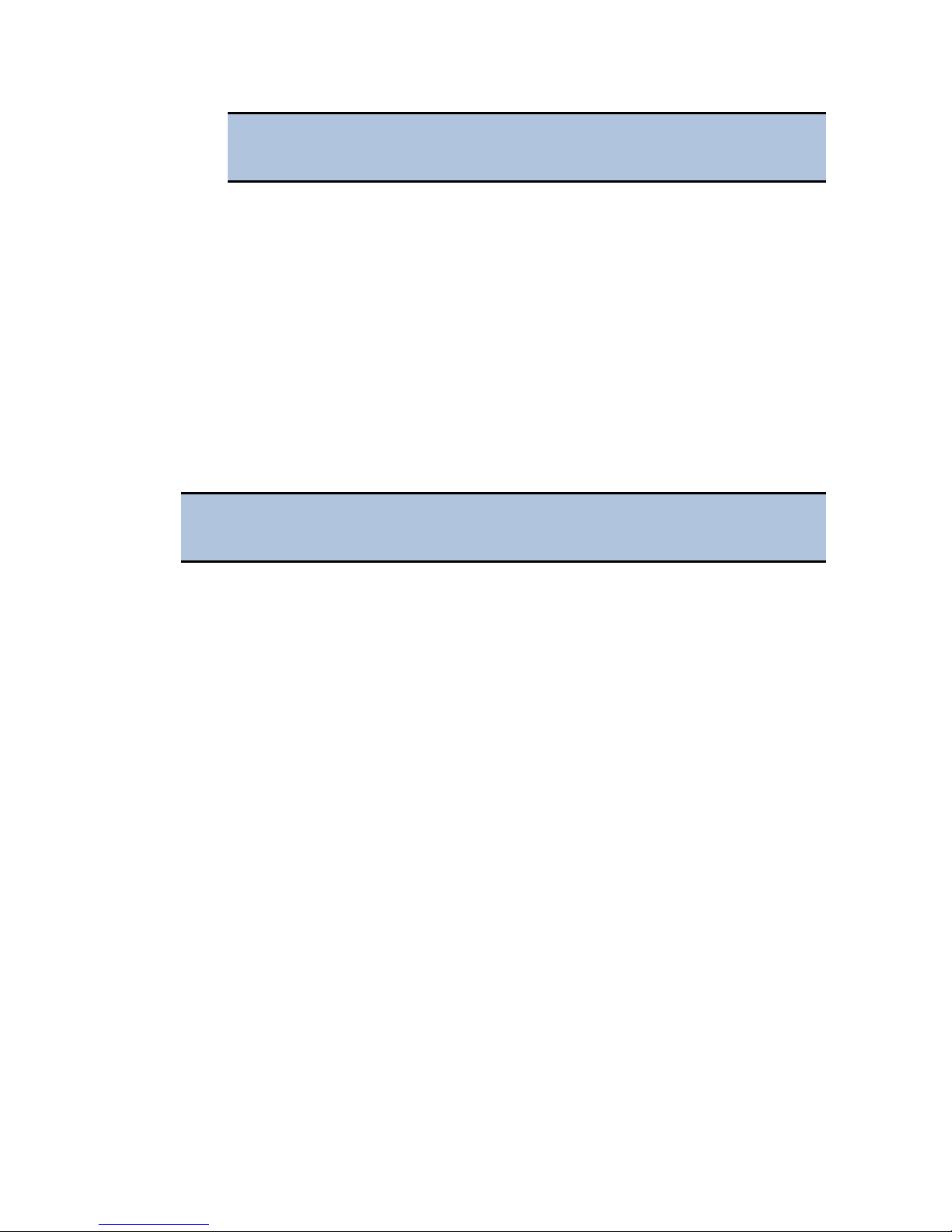
1. Turn off your computer, then remove the outside case. Depending on the
computer, remove either one side of the tower or the entire cover.
2. Locate the PCI Express slot. Open the card retainer, and remove any existing
graphics card (if necessary) or blank port located in the PCI Express slot.
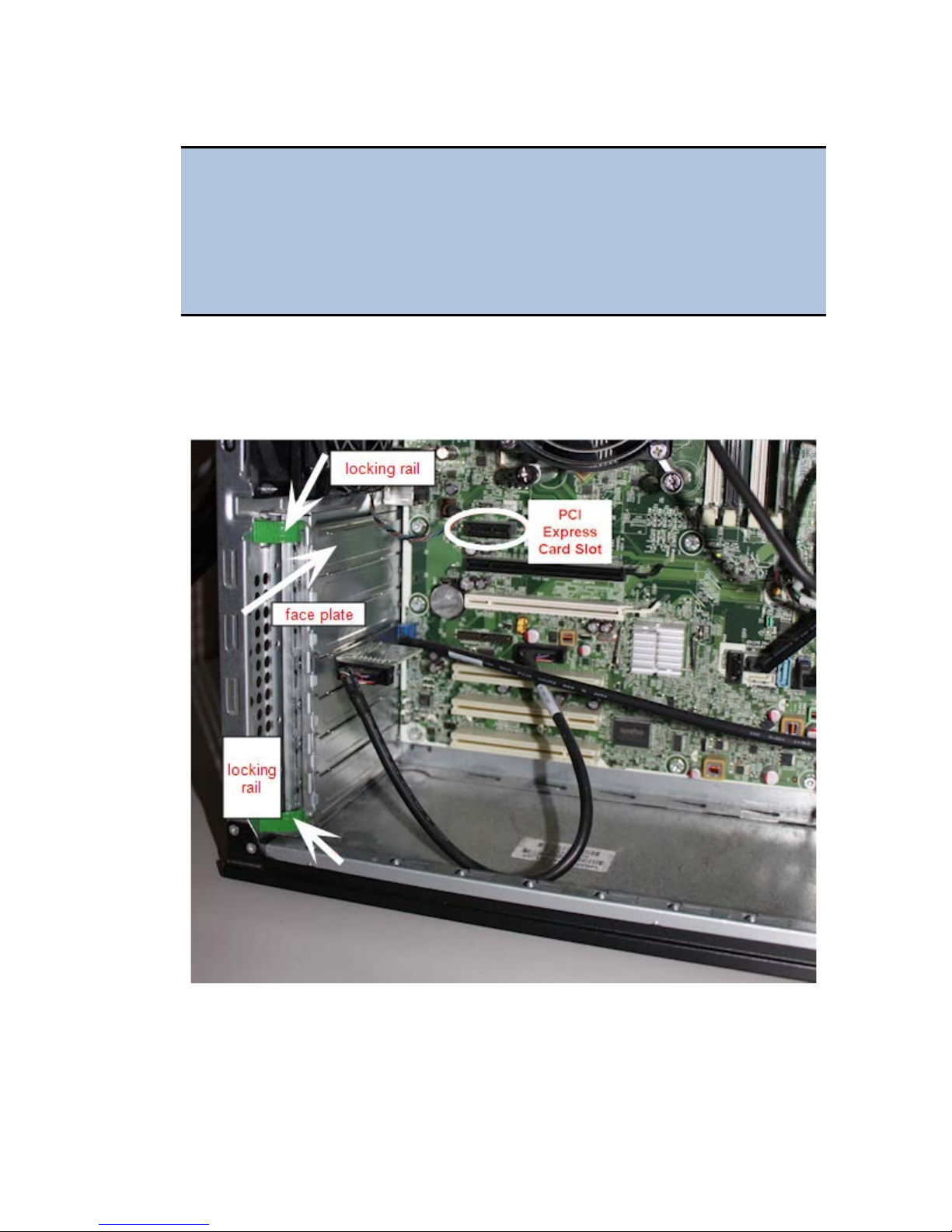
3. Insert the PCI Express card, aligning it with its slot.
4. Press the card firmly into place, and secure the card with a locking rail or
screws.
5. Replace your computer's outside case, then power on the computer.
BioTek Instruments, Inc.
Page 25
14: Install the Imager Module | 13
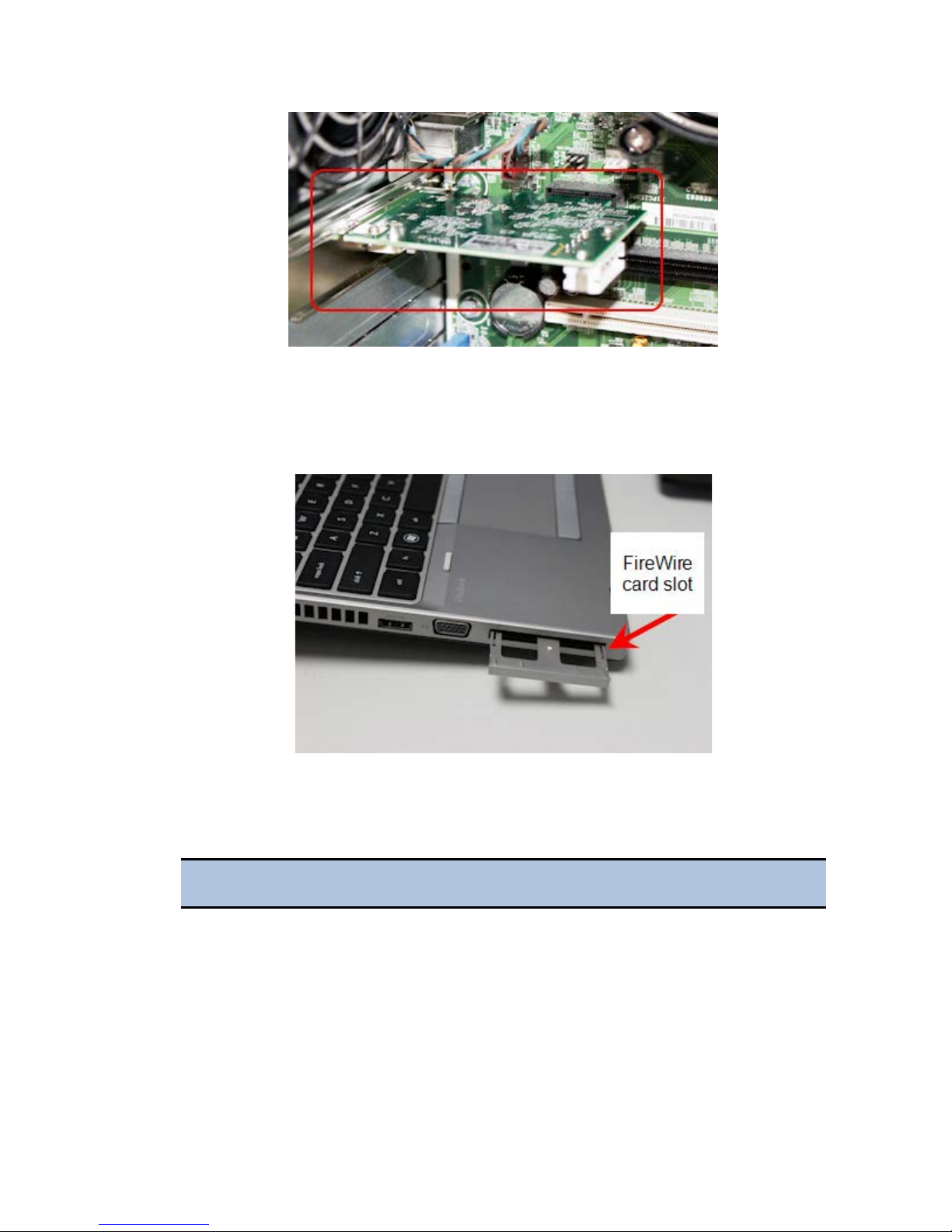
Laptop Computer: Install the FireWire Card
1. Remove the PCI Express port cover or any existing PCI Express card from
your laptop.
2. Touch a metal object to discharge any static electricity.
3. Remove the PCI Express card from the packaging.
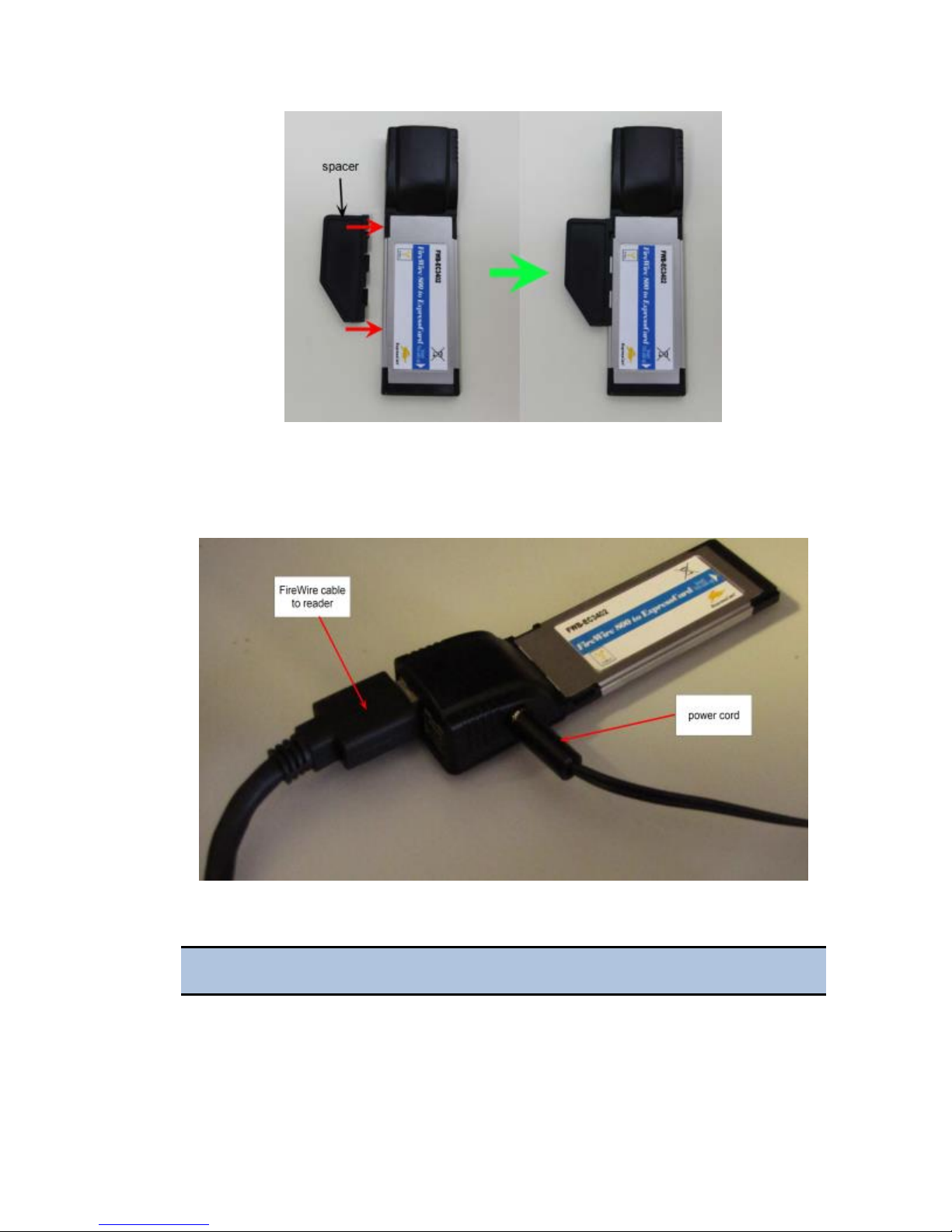
4. Attach the spacer, if required, to the FireWire PCI Express card.
The spacer does not fit tightly. Ensure that it does not fall off.
Cytation 5
Page 26
14 | Installation
5. Insert the FireWire Express card, with the label facing up, into the
appropriate slot on your laptop.
6. Plug the power cord into the card, then plug the cord into a power outlet.
Install the FireWire Driver
You must install the PCI Express card before performing this step.
1. Navigate to the Gen5 program files on your computer, for example,
C:\Program Files\BioTek\Gen5 2.07.
BioTek Instruments, Inc.
Page 27

14: Install the Imager Module | 15
2. Open the Firewire Drivers folder, and then open the folder appropriate for
your computer: Windows_32 (for Windows 32-bit) or Windows_64 (for
Windows 64-bit).
3. In Windows 7 and higher, right-click
as Administrator
to run the driver installer. When the installer is finished,
InstallPGRDriver.bat and select Run
a message appears: "SUCCESS: Installed package <path to package>". If you
do not see this message, contact BioTek TAC.
4. After installing the FireWire driver, restart your computer.
5. When the reboot process is complete, insert one end of the FireWire cable
into the back of the reader, and insert the other end into the new port (in the
card you installed) on your computer.
Establish Communication with the Camera
1. From the Gen5 main screen, select System > Instrument Configuration,
select
Cytation 5, and then click View/Modify.
2. Click
Camera Information. If communication is successful, Gen5 displays
information about the camera.
Troubleshooting Communication with the Camera
• Have you established communication with the instrument first?
• Did you install the FireWire software driver?
• Is the FireWire cable installed? Is your laptop card plugged into an electrical
outlet?
• Is the LED light on the back of the reader lit up?
• Red: Power to the camera from the FireWire card
• Half-Red/Half-Green: Ready state
• Green: Communicating/activity
Cytation 5
Page 28
16 | Installation
2. Set Up Gen5 for Imaging
Configuration information must be set before any optical components are installed.
Use Gen5 to update the instrument's onboard settings. The LED cubes and imaging
filter cubes must be installed with the reader off. Follow these installation steps in
the order in which they are given.
1. In Gen5, select
View/Modify > Setup
System > Instrument Configuration > Cytation 5 >
.
2. On the Imaging Configuration tab, input the objective and LED cube and
imaging filter cube configurations (see 3a. Setting the Objective, LED Cube,
and Imaging Filter Cube Configuration on page 17), then click
Values
.
Send
Click Access next to an objective position in the Objective
Configuration area to lower the focusing system into the desired
access position and to rotate the objective turret to the selected
objective's installation position.
3. Install the objectives (see 3b. Install the Objectives on page 17) in their
defined locations.
4. Power off the instrument, and install the LED cubes and imaging filter cubes
(see 3c. Install the LED Cubes and Imaging Filter Cubes on page 17) in their
defined locations. Close the side access door.
Leave Gen5 open with the Reader Setup dialog onscreen while
performing the next steps.
5. Power on the instrument. After the self-test, the instrument will beep and the
eject button LED will be red, indicating that the objectives must be
calibrated.
6. Press the carrier eject button to stop the beeping.
7. Click
Auto Calibration. Note that this process can take up to 15 minutes on
an instrument with six objective installed.
Phase contrast components are calibrated in the factory before shipment.
You do not need to run the Phase Ring Configuration and Calibration
routines at this time.
BioTek Instruments, Inc.
Page 29
14: Install the Imager Module | 17
3a. Setting the Objective, LED Cube, and Imaging Filter Cube
Configuration
When you install an LED cube, an imaging filter cube, or an objective, you must set
the cubes' and objectives' configuration before physically changing or installing
them.
If you physically install an LED cube, an imaging filter cube, or an
objective before setting the new configuration, the instrument may fail its
self-test.
1. From the main Gen5 screen, click System > Instrument Configuration.
2. Select the
Imaging Configuration tab.
3. In the Objective Configuration area, select the objective for the position or
positions you want to define, or select
objective from the instrument. Click
4. In the LED and Imaging Filter Cube Configuration area, select the filter cube
for the position you want to define, or select
filter cube from the instrument. The corresponding LED cube part number is
filled in automatically.
5. Click
6. For LED cube and imaging filter cube changes, follow the on-screen
procedure.
Cytation 5, click View/Modify > Setup, and select the
None if you are removing an
Access.
None if you are removing the
Send Values.
3b. Install the Objectives
Before installing a 20X, 40X, or 60X objective, either phase or standard,
set its correction collar to match your plate type. See the Cytation 5
Operator’s Manual for more information and instructions.
After defining the objectives and their locations in Gen5:
1. Turn the knob on the side access door to release the latch, and open the door.
Cytation 5
Page 30

18 | Installation
If you are installing an objective with a correction collar (i.e., a 20X, 40X, or
60X objective), be sure to grasp the objective by the adapter, not by the
correction collar, to avoid changing the correction collar settings.
2. Screw each objective into its defined position. Do not overtighten the
objectives.
3c. Install the LED Cubes and Imaging Filter Cubes
1. With the instrument powered off, slide the filter slide out of the instrument.
2. Place the new LED cube in the appropriate position (Position 1, 2, 3, or 4) on
the filter slide.
3. Use the 3/32" hex wrench to screw the LED cube onto the filter slide.
4. Place the imaging filter cube on top of the LED cube you installed, and use
the hex wrench to screw it to the LED cube.
5. Insert the LED cube’s wire clip into the socket on the carrier.
BioTek Instruments, Inc.
Page 31

14: Install the Imager Module | 19
Cytation 5
Page 32

20 | Installation
6. Slide the filter slide back into the instrument.
7. Close the side access door.
Run Auto Calibration
After you have physically installed the LED cubes, imaging filter cubes, and
objectives, you must run Auto Calibration before you can use the imaging module.
This process can take up to 15 minutes on an instrument with six objectives installed.
1. Turn on your instrument, and allow the self-test to run. The instrument will
beep, indicating that the self-test has failed.
2. Press the carrier eject button to stop the beeping.
3. If the Reader Setup dialog is not already visible on your screen, go to
> Instrument Configuration
> Setup
.
4. On the Instrument Configuration tab, click
, select Cytation 5, then click View/Modify
Auto Calibration.
5. If you have a 40X or 60X objective installed, Gen5 prompts you to place the
objective setup plate (PN 1222531) on the carrier. After you place the setup
plate on the carrier, click
OK.
System
After the calibration procedure is finished, the instrument is ready to use.
Troubleshooting Auto Calibration
If error messages indicate a problem with the imaging Auto Calibration process:
1. In Gen5, click
View/Modify > Setup
System > Instrument Configuration > Cytation 5 >
.
BioTek Instruments, Inc.
Page 33

15: Configure the Dispensers in Gen5 | 21
2. On the Imaging Configuration tab, set the value of all objectives to none, and
then click
Send Values.
3. Set the correct values for all objectives, and then click
4. Click
Auto Calibration.
Send Values.
If problems persist, contact BioTek TAC.
15: Configure the Dispensers in Gen5
Before you use the dispenser with a BioTek reader, you must set the calibration
values in Gen5.
1. In Gen5, go to
instrument, and click
2. Click
Setup, and then select the Dispenser 1 tab.
3. On the keyboard, press CTRL+SHIFT+M to enter maintenance mode for the
Dispenser 1 window.
4. Enter the syringe calibration values from the label on the rear of the
dispenser box. (See Install the Dispenser.)
5. Click
Send Volumes, and then click Get Volumes to verify that the
entered values were sent to the instrument.
6. Select the
System > Instrument Configuration, select your
View/Modify.
Dispenser 2 tab, and repeat steps 3 through 5 for Dispenser 2.
Cytation 5
Page 34

22 | Installation
16: Test the Dispenser
1. If necessary, press the carrier eject button to extend the microplate carrier.
2. Place the tip priming trough in the rear pocket of the carrier.
3. Place the priming plate on the carrier.
BioTek Instruments, Inc.
Page 35

17: Run a System Test | 23
4. Fill the two reagent bottles with distilled or deionized water. Place the bottles
in their holders, and place the holders directly in front of the syringes. Insert
the inlet tubes into the bottles.
5. From the Gen5 main screen, select
Cytation 5
6. Click the
7. With Dispenser set to
.
Prime tab.
1, set the Volume to 5000 µL and click Prime.
System > Instrument Control >
The syringe should move down and up repeatedly, drawing fluid from the
bottle. The fluid should pump through the tubing and dispense into the
priming plate. Examine the fittings; no leaks should be detected. If leaks are
detected, tighten all fittings and repeat the prime. If leaks are still detected,
contact BioTek’s Technical Assistance Center.
8. When the prime finishes, set Volume to
2000 µL and click Purge to clear
the fluid lines.
9. Set Dispenser to
2 and repeat steps 7 and 8.
10. When finished, remove and empty the priming plate.
11. Return to the Gen5 main screen.
17: Run a System Test
Running a system test will confirm that the reader is set up and running properly, or
will provide an error code if a problem is detected.
1. Turn on the incubator:
• From the Gen5 main screen, select
Cytation 5
• Click the
.
Pre-Heating tab.
• Enter a Requested temperature of at least 37°C and click
Wait until the incubator temperature reaches the set point before
continuing.
2. Return to Gen5's main view and select System > Diagnostics > Run
System Test
OK.
. If prompted to select a reader, select Cytation 5 and click
3. When the test is completed, a dialog requesting additional information
appears. Enter the information and click
System > Instrument Control >
On.
OK.
If a message appears that a pending system test is waiting from the
initial power-up self-test, view the pending system test and repeat steps
2 and 3.
Cytation 5
Page 36

24 | Installation
4. The results report appears. Scroll down toward the bottom; the text should
read “SYSTEM TEST PASS.”
• You may wish to print the report and store it with your records.
• The Gen5 software stores system test information in its database; you can
retrieve it at any time.
5. Turn off the incubator:
• Select
• Click the
System > Instrument Control > Cytation 5.
Pre-Heating tab and click Off.
Repackaging and Shipping Instructions
Refer to the Cytation 5 Operator’s Manual for complete instructions for
repackaging and shipping the instrument and accessories.
If the reader and/or dispenser has been exposed to potentially hazardous
material, decontaminate it to minimize the risk to all who come in contact
with the reader during shipping, handling, and servicing. Decontamination
prior to shipping is required by the U.S Department of Transportation
regulations. See the Maintenance chapter for decontamination instructions.
Remove the microplate and tip prime trough (if equipped) from the carrier
before shipment. Spilled fluids can contaminate the optics and damage the
instrument.
The instrument with all available modules weighs up to 80 lbs (36.3 kg).
Use two people when lifting and carrying the instrument.
The instrument's packaging design is subject to change. If the instructions in
this section do not appear to apply to the packaging materials you are using,
please contact BioTek's Technical Assistance Center for guidance.
Replace the shipping hardware before repackaging the reader. Please
contact BioTek if you have misplaced any of these items.
• Carrier ship bracket (PN 1220510)
• Carrier ship bracket screws (PN 1032190)
• Top filter shipping bracket (PN 8042187)
• Bottom filter slide ship bracket (PN 1380501)
• Objective ship bracket (PN 1380503)
If you need to ship the Cytation 5 and/or the dispense module to BioTek for
service or repair, be sure to use the original packaging materials. Other
forms of commercially available packaging are not recommended and can
void the warranty. The shipping materials are designed to be used no more
than five times. If the original materials have been damaged, lost, or used
more than five times, contact BioTek to order replacements.
BioTek Instruments, Inc.
Page 37

Getting Started
Page 38

26 | Getting Started
1
The entry for the dispense outlet tubes and injectors (if equipped)
2
Filter cube access door
3
The microplate carrier eject button
4
The light-blocking microplate carrier access door
5
The power switch
6
LED cubes, imaging filter cubes, and objectives access door
Overview
The Cytation 5 is a hybrid multi-mode microplate reader. Depending on the model,
Cytation 5 detection modes include fluorescence intensity (FI), fluorescence
polarization (FP), time-resolved fluorescence (TRF), luminescence, UV-visible
absorbance, imaging, and alpha. The instrument is modular, and upgrade options are
available; contact BioTek Customer Care for more information.
The reader is computer-controlled using Gen5 software for all operations, including
data reduction and analysis. The Cytation 5 is robot accessible and compatible with
the BioStack 3 and BioStack 4. Gen5 supports OLE automation to facilitate the
Cytation 5’s integration into an automated system.
Refer to the Gen5 Help system for operational and data analysis
instructions.
The Cytation 5 Operator's Manual contains recommendations for ensuring
optimum performance.
External Components
BioTek Instruments, Inc.
Page 39

Internal Components | 27
1
Dispenser port
2
Joystick port
3
USB port
4
Power inlet
5
Gas controller hookup
6
FireWire port
Component
Description
Applies to models with the dispense module. The syringes may
cleaning at regular intervals.
Applies to models with the imaging module. The imaging system,
images in live mode.
Internal Components
The filter cube can contain excitation and emission filters, mirrors,
and polarizing filters. Preconfigured cubes are available from BioTek,
Filter Cube
Injector
System
or you can change the filters and mirrors yourself.
Note: These cubes are not to be confused with the imaging
LED cubes and filter cubes.
require replacement over time. The tubing and injectors require
Imaging
System
Cytation 5
comprising a CCD camera, objectives, LED cubes, and filter cubes,
allows you to run experiments with imaging reads as well as view
Page 40

28 | Getting Started
Filter Cube
The Cytation 5 is equipped with a filter cube that contains excitation and emission
filters, mirrors, and, if required, polarizing filters. Each filter cube contains two filter
sets, each of which contains one excitation filter, one mirror, and one emission filter.
The filter cube is accessed through a hinged door on the front of the instrument.
Synergy H1 filter cubes are interchangeable with the filter cubes for the
Cytation 5.
Do not open the door to access the filter cube during instrument
operation. Doing so may result in invalid data.
Define the Filters
Gen5 keeps track of each cube's contents and communicates this information to the
instrument during operation. You must define characteristics for your filter cube(s) in
the Gen5 Filter Cube Table (
System > Instrument Configuration > Setup).
• Select
Band Pass, Long Pass, or Short Pass, as appropriate for each filter
type.
• Band Pass, a standard interference filter with a defined central wavelength
and bandwidth.
• Long Pass, cutoff filters that transmit longer wavelengths and block shorter
wavelengths.
• Short Pass, cutoff filters that transmit shorter wavelengths and block longer
wavelengths.
• Select
• Select
Configuring the System for Luminescence Measurements
PLUG to indicate the presence of a plug.
HOLE to indicate an empty location.
If your tests require that light emitted from the samples remain unfiltered, the
Emission filter position in the filter cube should be empty.
1. Click
System > Optics Library > Filter Cubes.
2. Select the checkbox in the On Site column for the filter cubes you have
available.
Injector System
The tubing and injectors should be cleaned at least every three months.
Inspect the injector system daily for leaks, preferably immediately after
priming and whenever tubing changes have been made.
BioTek Instruments, Inc.
Page 41

Internal Components | 29
1
Two 250 µL syringes draw fluid from the supply bottles
2
Inlet tubes transport fluid from the supply bottles to the
one end and threaded fittings on the other end.
3
Solenoid valves allow the fluid drawn from the supply
tubes.
4
Outlet tubes transport fluid from the syringes into the
threaded fittings on each end.
If a syringe is leaking, it may need to be replaced.
Dispense Module
The dispense module sits on top of the reader or gas controller and pumps fluid from
the reagent bottles to injector located inside the instrument. Fluid is injected into one
well at a time. The injectors support plate types from 6- to 384-well plates.
syringes. These tubes are short pieces of opaque PTFE
(Teflon) tubing connected to stainless-steel probes on
bottles by the syringe pumps to flow into the outlet
instrument, through the tubing ports on the Cytation 5's
top cover. The outlet tubes are opaque PTFE tubes with
Dispense Module Components and Materials Composition
Continuous contact with harsh chemicals is not recommended. Always rinse the
fluid path with deionized water after contact with any strong acid, base, or solvent.
Cytation 5
Page 42

30 | Getting Started
Components
Material Composition
Tubing, syringe fittings
PTFE (polytetrafluoroethylene)
Injector tips
315 stainless steel
Injector body
PVC (polyvinyl chloride)
Priming plate and trough
Polyproylene
Valve diaphragms
Ethylene propylene (EPDM)
Valve body
PEEK (polyether ether ketone)
Syringe barrel
Borosilicate glass
Priming the Injector System
Before running a dispense assay, prime the system with the reagent or dispensing
fluid. In addition, tip priming can be performed at the start of an assay and,
sometimes, just before each dispense to a well. The tip prime compensates for any
fluid loss at the injector tip due to evaporation since the last dispense. All priming
activities are controlled via Gen5.
If the injector system is not primed adequately, air bubbles can get
trapped in the system and affect injection volumes. Air bubbles in the
system can also result in fluid spraying or scattering inside the reader.
Both types of primes require a fluid reservoir to be present on the microplate carrier.
• The priming plate is placed on the microplate carrier for a Prime operation (to
prime the dispense system with fluid).
• The tip priming trough is placed in the rear pocket of the carrier, and is used for
performing the Tip Prime before dispensing. The trough holds up to 1.5 mL of
liquid and must be periodically emptied and cleaned by the user.
Do not perform tip priming when using tall plates. Generally, plates
with fewer than 96 wells are too tall for error-free tip priming; and, tip
priming is rarely required for these larger-volume plates.
The priming tray should be empty before priming and contain fluid after
priming.
Imaging System
Instruments with the imaging module can perform image reads, view and capture
images in manual mode, and save the images for later analysis. The imaging module
comprises up to four LED cubes and four imaging filter cubes, up to six objectives,
and a CCD digital camera, which captures images directly through the selected
objective and filter cube assembly. The imaging module supports two modes,
manual mode and experiment mode.
BioTek Instruments, Inc.
Page 43

Internal Components | 31
Camera
Gen5 controls the CCD camera via FireWire. Using Gen5, you can focus the camera,
determine exposure settings, and capture images.
LED Cubes and Imaging Filter Cubes
The LED cubes and imaging filter cubes are located behind a door on the left side of
the instrument and are user-changeable.
Objectives
The objectives are located next to the LED cubes and imaging filter cubes in an
objective turret. Gen5 supports the installation of up to six user-changeable
objectives.
Imaging Modes
The Gen5 imaging module provides two modes of use: manual and experiment.
• Manual mode allows you to view, capture, and analyze images outside of a
protocol or experiment. Images are displayed in real time. You can also retrieve
previously captured and saved images to analyze in manual mode.
• In experiment mode, you can perform an image read step as an endpoint read
or include it in a kinetic block in your procedures and experiments. An imaging
procedure can include additional steps (as supported by the reader), such as
dispense, shake, incubate, and different detection modes.
Change the Virtual Memory Settings
You may find that you need more hard disk space than is allocated by default. For
example, running a multiplate experiment, imaging all 96 wells in a 4x4 montage in
three colors will require an increase of virtual memory. Without this change, the
message, “This procedure may require that you increase the size of the virtual
memory in Windows,” may appear. In this case, please consult with your IT group to
increase virtual memory, using the following instructions to prevent errors:
1. From the Windows Start menu, go to
2. In the left pane, select
Advanced system settings.
Control Panel and select System.
3. In the System Properties dialog, on the Advanced tab in the Performance area,
click
Settings.
4. In the Performance Options dialog, on the Advanced tab, in the Virtual Memory
area, click
5. Clear
6. Select
then click
• Initial size: 10 GB
• Maximum size: 20 GB
Change.
Automatically manage paging file size for all drives, if it is selected.
Custom Size, enter the following minimum and maximum values, and
OK:
Cytation 5
Page 44

32 | Getting Started
You may need to restart your computer for the change to take effect.
Shaking System
Three shake modes are available for selection in Gen5: Linear, Orbital, and Double
Orbital. A linear shake simply moves the carrier's y-axis back and forth in a line. An
orbital shake moves both carrier axes to scribe a circle. With double orbital, the
carrier scribes a figure-eight pattern.
For any mode, a slider bar in the software allows you to adjust the shake frequency
from "Slower" to "Faster." With each adjustment, the corresponding cycles per minute
(CPM) is displayed.
For either orbital mode, you can further expand the frequency options by clicking a
Slow or Fast button.
BioTek Instruments, Inc.
Page 45

Carrier Shake Definitions
Displacement
(mm/steps)
Period
(msecs)
Frequency
(Hz)
Ramp
Profile
Linear
1/6
54.8
18.3
1096
13
2/12
82.0
12.2
731
14
3/18
105.8
9.5
567
15 4/24
121.6
8.2
493
16
5/30
146.3
6.8
410
17
6/36
166.9
6.0
360
18
Slow
orbital
2/12
164.4
6.1
365
20
3/18
212.5
4.7
282
21
4/24
253.5
3.9
237
22
5/30
292.1
3.4
205
23 6/36
334.2
3.0
180
24
Fast orbital
1/6
74.3
13.4
807
25
2/12
109.6
9.1
548
26
3/18
141.3
7.1
425
27
4/24
169.0
5.9
355
28
5/30
195.4
5.1
307
29
6/36
222.8
4.5
269
30
Sample
Volume
Orbital
Slow
Orbital
Fast
Double Orbital
Slow
Double Orbital
Fast
6-well Microplate
1–6
mm
1–6
mm
Shaking System | 33
Shake Type
CPM
1/6 107.3 9.3 559 19
Maximum Shaking Amplitude Based on Assay Volume and
Plate Type
If the wells of a microplate are almost full, vigorous shaking can result in spillage
inside the instrument. The following table is designed to help avoid this issue. Find
your microplate type and sample volume to ascertain the acceptable shake amplitude
on your instrument.
Linear
0–3 mL
3–4 mL
Cytation 5
1–6 mm 1–6 mm 1–6 mm 1–6 mm
1–6 mm 1–6 mm 1–6 mm 1 mm
Page 46

34 | Getting Started
Sample
Volume
Orbital
Slow
Orbital
Fast
Double Orbital
Slow
Double Orbital
Fast
4–5 mL
1 mm
1–6 mm
1–6 mm
1–6 mm
1 mm
5–7 mL
1 mm
1–6 mm
1–3 mm
1 mm
No
7–8 mL
1 mm
No
1–3 mm
No
No
9 mL
1 mm
No
No
No
No
12-well Microplate
1–6
mm
1–2
mm
1–2
mm
3–4 mL
No
1–6 mm
No
No
No
24-well Microplate
1–6
mm
0.75–1 mL
1 mm
1–6 mm
1–6 mm
1–6 mm
1–6 mm
1–1.5 mL
1 mm
1–6 mm
1–6 mm
1–6 mm
No
1.5–2 mL
1 mm
1–6 mm
No
1–6 mm
No
48-well Microplate
1–6
mm
0.5–1 mL
1 mm
1–6 mm
1–6 mm
1–6 mm
1–6 mm
1–1.3 mL
No
1–6 mm
1–6 mm
1–6 mm
No
96-well Microplate
1–6
mm
Linear
0–1.5 mL
1.5–2.5 mL
2.5–3 mL
0–0.75 mL
0–0.5 mL
1–6 mm 1–6 mm 1–6 mm 1–6 mm
1–6 mm 1–6 mm 1–6 mm 1 mm
1–6 mm No No 1 mm
1–6 mm 1–6 mm 1–6 mm 1–6 mm
1–6 mm 1–6 mm 1–6 mm 1–6 mm
0–0.25 mL
Gen5 Software
BioTek Gen5 software supports all Cytation 5 reader models. Use Gen5 to control the
reader, the imaging module (if equipped), the dispense module (if equipped), and
the stacker (if equipped); perform data reduction and analysis on the measurement
values; print or export results; and more. This section provides brief instructions for
working with Gen5 to create protocols and experiments and read plates. Refer to the
Gen5 Help system for more information and to learn about the various license levels
available.
1–6 mm 1–6 mm 1–6 mm 1–6 mm
BioTek Instruments, Inc.
Page 47

Gen5 Software | 35
Define the Fluorescence Filter Cube
For "F" models (filter fluorescence), the filter cube's characteristics must be entered
into Gen5 and downloaded to the reader. Perform these steps before using the reader
for the first time, and again if you change the filter cube's contents or switch to a
different cube.
1. Select
and click
2. Click
System > Instrument Configuration. Highlight the Cytation 5,
View/Modify.
Setup, and then click the Filter Cube tab.
3. Enter a name for the filter cube.
4. Select
Fluorescence Polarization Cube, if applicable.
5. Enter a filter set name for Filter Set 1, and define the excitation, mirror, and
emission settings:
• Band Pass, a standard interference filter with a defined central wavelength
and bandwidth.
• Long Pass, cutoff filters that transmit longer wavelengths and block
shorter wavelengths.
• Short Pass, cutoff filters that transmit shorter wavelengths and block
longer wavelengths.
• Plug indicates the presence of a plug.
• Hole indicates an empty location.
6. Repeat step 5 for Filter Set 2.
Protocols and Experiments
In Gen5, a protocol contains instructions for controlling the reader and (optionally)
instructions for analyzing the data retrieved from the reader. At a minimum, a
protocol must specify the procedure for the assay you wish to run. After creating a
protocol, create an experiment that references the protocol. You’ll run the experiment
to read plates and analyze the data.
These instructions briefly describe how to create a protocol in Gen5. See the Gen5
Help system for complete instructions.
1. Open a new protocol.
2. Open the Procedure dialog. If prompted to select a reader, select the
Cytation 5 and click OK.
3. Select a Plate Type.
Gen5 stores measurements and other characteristics for individual plate
types in a database. It is essential that you select (or define) the plate
type to match the assay plate. Otherwise, results may be invalid.
Cytation 5
Page 48

36 | Getting Started
For imaging reads, you must also define the Bottom Elevation parameter
for the plate or slide. If the relevant measurements are not defined, Gen5
will not allow the objective to move closer than 1 mm from the bottom of
the carrier, thus disabling Cytation 5’s focus capabilities.
4. Add steps to the procedure for shaking or heating the plate, dispensing fluid,
reading the plate, and more. Click
supports the defined steps, and then click
Validate to verify that the reader
OK.
Optionally, perform the next steps to analyze and report the results:
5. Open the Plate Layout dialog and assign blanks, samples, controls, and/or
standards to the plate.
6. Open the Data Reduction dialog to add data reduction steps. Categories
include Transformation, Well Analysis, Curve Analysis, Cutoff, and
Validation.
7. Create a report or export template via the Report/Export Builders.
8. Select
File > Save and give the file an identifying name.
These instructions briefly describe how to create an experiment and then read a plate
in Gen5. See the Gen5 Help system for complete instructions.
1. Open a new experiment.
2. Select the desired protocol and click
OK.
3. Select a plate in the menu tree and read it.
4. When the read is complete, measurement values appear in Gen5. Select the
desired data set from the Data list.
5. Select
File > Save and give the file an identifying name.
Imaging Module: Manual Mode
Applies only to models with the imaging module.
The following sections briefly describe how to use the Gen5 imaging module in
manual mode. See the Gen5 Help for more complete instructions and descriptions of
these features.
Focusing the Camera
1. From the Task Manager, select Read Now > Run Imager Manual Mode.
Alternatively, from the main Gen5 screen, click
2. Click
Find Image. Gen5 automatically sets the exposure and focuses on the
image. If the displayed image requires additional fine-tuning:
• Click
Auto Expose, or manually adjust the Exposure settings until you
see an image.
.
BioTek Instruments, Inc.
Page 49

Gen5 Software | 37
1
Select a color channel
4
Set to neutral settings (similar to the default settings)
6
Reset to default settings
7
Select a color channel
10
Adjust the shift using the arrows
• Click Auto Focus, or click the arrow buttons until the image is in focus.
• Repeat these two steps until the image is exposed and focused to your
liking.
Capture and Save an Image
When you have a satisfactory image, you can capture and save it for later analysis.
1. When an image in the Capture dialog is in focus, click
2. To save the captured images, click Review/Save.
3. Click
to adjust the brightness and contrast adjustments or to
manually align overlaid images to compensate for pixel shift, then click
Save.
# Description
to capture it.
2 Use the sliders or +/– buttons to adjust brightness and contrast
3 Have Gen5 automatically adjust brightness and contrast
5 If selected, changes to brightness and contrast will apply to all color
channels of the image
8 Gen5 displays the number of pixels the channel is shifted
9 Reset to default settings
Cytation 5
Page 50

38 | Getting Started
Adjustments made using the B&C (brightness and contrast) and Channel
Shift dialogs are for display purposes only; the changes do not affect the
data measurements from the images.
Analyze Captured and Saved Images
1. With your captured or saved images displayed in the left panel of the
Capture dialog, click
Review/Save.
2. If necessary, adjust the brightness and contrast of the image.
3. Click
Analyze.
4. In the Analyze Tool, select which type of analysis you want to perform and
define the parameters for the analysis, then click
Start. The results are
displayed in the right pane.
Imaging Module: Experiment Mode
Applies only to models with the imaging module.
The following sections briefly describe how to use the Gen5 imaging module in
experiment mode. See the Gen5 Help for more complete instructions and
descriptions of these features.
Perform an Image Read
You can create an Image Read step to be used alone or in combination with the
monochromator- or filter-based optics of the Cytation 5.
1. Create a new protocol and select the Plate Type.
2. Create a read step, and select
Image as the Read Method.
3. (Optional) Enter a step label or unique name for this step. Data sets of the
reading results will use the label in online views, reports, and export files.
4. Select the objective.
5. Define whether to read a full plate or specific wells.
6. Define up to four channels to read:
a. Defining the color filter.
b. Select whether exposure is automatic or defined.
c. Define which wells are used for auto-exposure for each channel, if
applicable.
d. Define focus and exposure options, if needed.
7. Define the horizontal and vertical offset from the center of the well.
8. Select whether to create a single image for each well, an image montage, or
an image Z-stack.
9. Define advanced options, if needed.
BioTek Instruments, Inc.
Page 51

Gen5 Software | 39
After you have defined and saved the protocol, you can create an experiment and
read the plate:
1. Create an experiment using an existing protocol.
2. Select the protocol you created with the imaging read step, and click
OK.
3. Select a plate in the menu tree and read it.
4. When prompted, give the file an identifying name.
5. When the read is complete, images and measurement values appear in Gen5.
Select the desired data set from the Data list.
Using the Slide Holder
Manual Mode
1. When prompted upon entering manual mode, select one of the slide holders
as your plate type.
2. The slide holder plate map contains two well positions that correspond with
the two slide locations on the slide holder. Select the well position that
contains the slide you want to image.
3. By default, the carrier moves so that the middle of the selected slide position
is below the objective. However, the sample on your slide may be in a
different location on the slide. To find the sample:
a. Select your lowest-power objective.
b. Select either
move the slide around until you find your sample. The cross in the slide
display to the right of the arrow buttons shows the general position of the
image in relation to the slide. The back arrow to the left of the slide display
shows the direction that the slide enters the read chamber.
Small step or Large step, then use the arrow buttons to
4. After you find your sample, you can change to a higher-power objective, if
desired, or define a Read step in a protocol using the x- and y-coordinates of
your sample, which are displayed below the Well button.
Experiment Mode
1. In an experiment, select one of the slide holders as the plate type, and create
an Image read step.
2. Clear the
Auto box to turn off Auto Exposure, then click to enter
manual mode.
3. Follow steps 2 and 3 in the manual mode procedure (above) to find your
samples, then click
Cytation 5
Save settings. Gen5 imports your exposure settings
Page 52

40 | Getting Started
and the values for Horizontal and Vertical offset from the center of the well
to your read step.
4. Select
Auto to turn Auto Exposure back on. Gen5 will auto-expose your
image, retaining the well offset imported from manual mode.
You can also enter the values for Horizontal and Vertical offset from
center of well if you had determined them during previous testing.
Dispense Module Control
This section applies only to models with injectors.
Gen5 is used to perform several dispense functions, such as initialize, dispense,
prime, and purge. The Prime and Purge functions are introduced here. See the Gen5
Help system for more information.
Priming and purging routines are used to clean the fluid paths.
Prime
Before running an experiment with a dispense step, prime the system with the fluid
to be used.
1. Place the priming plate on the carrier.
2. Fill the supply bottle with a sufficient volume of the fluid to be used for the
prime and the assay. Insert the appropriate inlet tube into the bottle.
3. In Gen5, select
Prime tab.
the
System > Instrument Control > Cytation 5 and click
4. Select the Dispenser number (1 or 2) associated with the supply bottle.
5. Enter the Volume to be used for the prime. The minimum recommended
prime volume is 2000 µL.
6. Select a prime Rate, in µL/second.
7. Click
Prime to start the process.
8. When finished, carefully remove the priming plate from the carrier and
empty it.
If the priming plate is empty, the prime volume was too low.
Purge
To save reagent, Gen5 provides the option to purge fluid from the system back into
the supply bottle.
BioTek Instruments, Inc.
Page 53

Gen5 Software | 41
1. In Gen5, select System > Instrument Control > Cytation 5 and click
the
Prime tab.
2. Select the Dispenser number (1 or 2) associated with the supply bottle.
3. Enter the desired purge Volume in µL (e.g., 2000).
4. Select a prime Rate in µL/second.
5. Click
Purge to start the process.
Cytation 5
Page 54

42 | Getting Started
BioTek Instruments, Inc.
Page 55

Maintenance
Page 56

44 | Maintenance
Important! Do not immerse the instrument, spray it with liquid, or use a
Assistance Center.
Important! Do not apply lubricants to the microplate carrier or carrier
carrier path and cause errors.
Warning!
keep gloved hands away from eyes, mouth, nose, and ears.
Preventive Maintenance
A general preventive maintenance regimen for all Cytation 5 models includes
periodically cleaning all exposed surfaces and inspecting/cleaning the objectives,
emission and excitation filters, and mirrors (if used).
For models with the external dispense module, additional tasks include
flushing/purging the fluid path and cleaning the tip prime trough, priming plate,
supply bottles, dispense tubing, and injectors.
Daily Cleaning for the Dispense Module
To ensure accurate performance and a long life for the dispense module and
injectors, flush and purge the fluid lines with deionized (DI) water every day or after
completing an assay run, whichever is more frequent. Some reagents may crystallize
or harden after use and clog the fluid passageways. Take special care when using
molecules that are active at very low concentrations (e.g., enzymes, inhibitors).
Remove any residual reagent in the dispense lines using a suitable cleaning solution
(review the reagent’s package insert for specific recommendations).
Flushing the tubing at the end of each day, letting the DI water soak overnight, and
then purging the lines at the beginning of each day ensures optimal performance of
the dispense system. BioTek recommends performing a visual inspection of the
dispense accuracy before running an assay protocol that includes dispense steps.
Models with injectors: Accumulated algae, fungi, or mold may require
decontamination. See the Cytation 5 Operator’s Manual for complete
decontamination instructions.
Warnings and Precautions
Read the following before performing any maintenance procedures:
Warning! Internal Voltage. Turn off and unplug the instrument for all
maintenance and repair operations.
“wet” cloth on it. Do not allow water or other cleaning solution to run into
the interior of the instrument. If this happens, contact BioTek’s Technical
track. Lubricant attracts dust and other particles, which may obstruct the
Wear protective gloves when handling contaminated
instruments. Gloved hands should be considered contaminated at all times;
BioTek Instruments, Inc.
Page 57

Clean Exposed Surfaces | 45
Warning!
contaminated instruments.
Caution!
Test records before and after maintenance so that changes can be noted.
Warning!
80 lbs.
(36.3 kg). Use two people when lifting and carrying the instrument.
Mucous membranes are considered prime entry routes for
infectious agents. Wear eye protection and a surgical mask when there is a
possibility of aerosol contamination. Intact skin is generally considered an
effective barrier against infectious organisms; however, small abrasions and
cuts may not always be visible. Wear protective gloves when handling
The buildup of deposits left by the evaporation of spilled fluids
within the read chamber can impact measurements. Be sure to keep System
The instrument with all available modules weighs up to
Clean Exposed Surfaces
Exposed surfaces may be cleaned (not decontaminated) with a cloth moistened (not
soaked) with water or water and a mild detergent. You’ll need:
• Deionized or distilled water
• Clean, lint-free cotton cloths
• Mild detergent (optional)
• Canned air
1. Turn off and unplug the instrument.
2. Moisten a clean cotton cloth with water, or with water and mild detergent.
Do not soak the cloth.
3. Wipe the plate carrier and all exposed surfaces of the instrument.
4. Instruments with imaging capability: Used canned air to blow debris from
the aperture on the carrier. Do not wipe with liquid, which can seep inside
aperture’s glass plates and impact imaging reads.
Cytation 5
Page 58

46 | Maintenance
5. Wipe all exposed surfaces of the dispense module (if used).
6. Wipe all exposed surfaces of the gas controller module (if used).
7. If detergent was used, wipe all surfaces with a cloth moistened with water.
8. Use a clean, dry cloth to dry all wet surfaces.
Models with a dispenser: If the Tip Priming Trough overflows or other
spills occur inside the instrument, wipe the carrier and the surface
beneath the carrier with a dry cotton cloth. The internal chamber and
probes are not customer-accessible. If overflow is significant, contact
BioTek’s Technical Assistance Center with any questions about your
particular model.
Inspect/Clean Mirrors
Applies only to Cytation 5 models with filter-based fluorescence capabilities.
We recommend inspecting/cleaning the mirrors and polarizing filters (if equipped)
annually, especially if the filter cube has been opened or changed.
These optical elements are delicate and should be handled as carefully as possible.
The glass and anti-reflective (AR) coated surfaces will be damaged by any contact,
especially by abrasive particles. In most cases, it is best to leave minor debris on the
surface. However, if performance indicators or obvious defects in the mirrors or
filters suggest cleaning them, here are some guidelines:
• Use of oil-free dry air or nitrogen under moderate pressure is the best method
for removing excessive debris from an optical surface. If the contamination is
not dislodged by the flow of gas, please follow the cleaning instructions below.
BioTek Instruments, Inc.
Page 59

Inspect/Clean Mirrors | 47
• The purpose of the cleaning solvent is only to dissolve any adhesive
contamination that is holding debris on the surface. The towel needs to absorb
both the excessive solvent and entrap the debris so that it can be removed from
the surface. Surface coatings on dichroics are typically less hard than the
substrate. It is reasonable to expect that any cleaning will degrade the surface at
an atomic level. Consideration should be given as to whether the contamination
in question is more significant to the application than the damage that may
result from cleaning the surface. In many cases, the AR coatings that are
provided to give maximum light transmission amplify the appearance of
contamination on the surface.
Materials
• 7/64" hex key
• Linen or cloth gloves
• Anhydrous reagent-grade ethanol
• Kimwipes
• Magnifying glass
• 100% pure cotton balls (for the polarizing filters)
Procedure
1. Open the access door on the front of the instrument and slide the filter cube
straight out of its compartment.
2. Set the filter cube on the work surface. Using a 7/64" hex key, remove the
screw and washer located between the emission filter positions.
3. Carefully lift the filter cube top from the cube.
4. Wearing linen or cloth gloves, grasp the mirror by its edges and lift it out of
the cube.
The mirrors are seated on a shelf in the bottom of the cube and are not
secured in place.
Cytation 5
Page 60

48 | Maintenance
5. Wet absorbent towels such as Kimwipes, not lens paper, with anhydrous
reagent-grade ethanol. Wear gloves or use enough toweling so that solvents
do not dissolve oils from your hands that can seep through the toweling onto
the coated surface.
6. Drag the trailing edge of the ethanol-soaked Kimwipe across the surface of
the mirror, moving in a single direction. A minimal amount of pressure can
be applied while wiping. However, too much pressure will damage the
mirror.
7. Use the magnifying glass to inspect the surface; if debris is still visible, repeat
with a new Kimwipe.
8. To replace the mirror, hold it by its edges, turn it so that its label is face-up
and readable, and place it on the shelf in the filter cube.
9. Place the filter cube top back onto the cube and replace the screw and
washer.
10. When finished, reinstall the filter cube in the reader.
Inspect/Clean Excitation and Emission Filters
Applies only to Cytation 5 models with filter-based fluorescence capabilities.
Laboratory air is used to cool the flash bulb, and the filter cubes can become dusty as
a result. Filters should be inspected and cleaned at least every three months. You’ll
need:
• Isopropyl, ethyl, or methyl alcohol
• 100% pure cotton balls or high-quality lens-cleaning tissue
• Cloth gloves
• Magnifying glass
BioTek Instruments, Inc.
Page 61

Clean the Objectives | 49
Non-recommended Cleaning
Solvents
HyperClean (hexamethyldisoloxan and ethanol),
available from Olympus
Isopropyl alcohol, 70%/30% with deionized water
Dimethyl ketone (acetone)
Methyl alcohol, 70%/30% with deionized water
Ethyl alcohol, 70%/30% with deionized water
Do not touch the filters with your bare fingers!
1. Open the access door on the front of the instrument. Slide the filter cube out
of its compartment.
2. Inspect the glass filters for speckled surfaces or a “halo” effect. This may
indicate deterioration due to moisture exposure over a long period of time.
If you have any concerns about the quality of the filters, contact your
BioTek representative.
3. Using cotton balls or lens-cleaning tissue moistened with a small amount of
high-quality alcohol, clean each filter by lightly stroking its surface in one
direction.
4. Use a magnifying glass to inspect the surface; remove any loose threads left
from the cotton ball.
5. Replace the filter cube and close the door.
Clean the Objectives
Applies only to models with the imaging module.
The objectives used in the Cytation 5 should be cleaned when necessary using
optical-grade swabs or lens paper moistened with lens cleaning solution or deionized
water. Do not rub the lens.
Materials
• Air puffer
• Tweezers
• Magnifying glass
• Lens cleaning tissue
• Optical-grade swabs
• Cleaning solvent
Recommended Cleaning Solvents
Methyl ethyl ketone (MEK)
Cytation 5
Page 62

50 | Maintenance
1. From the Gen5 main screen, go to System > Instrument Configuration,
select
Cytation 5, click View/Modify > Setup.
2. In the Objective Configuration area in the Imaging Configuration tab, click
Access next to the desired objective to rotate the objective turret to the
access position for that objective.
3. Open the side door of the instrument. Grasp one of the objectives, unscrew it
from the objective turret, and remove it from the instrument.
4. Inspect the lens, using a magnifying glass if necessary, to determine if there
is dirt or dust present. If so, use a blower or a small paintbrush to remove
any dirt and dust.
Any dirt or dust on the surface of the lens can cause extensive damage if
dragged across the surface.
5. Soak either an optical-grade swab or a piece of lens cleaning tissue wrapped
around tweezers in lens cleaning solvent or deionized water.
6. Hold the swab or tissue-wrapped tweezers still and rotate the objective's lens
around it.
7. Dry the lens immediately with a clean lens tissue.
8. Replace the objective in the objective turret, and screw it in to secure it.
9. Repeat these steps to clean the second objective, if necessary.
10. In the Imaging Configuration tab on Reader Setup dialog (which you opened
in step 1), click
Auto Calibration to calibrate the objectives.
When the calibration is finished, the instrument is ready to use.
Tips
• Do not allow the lens to air dry.
• Always use an unused portion of the lens tissue when wiping the lens.
• If smears are still present after performing these steps, repeat the procedure.
Flush/Purge the Fluid Path
Applies only to Cytation 5 models with a dispenser.
At the end of each day that the dispense module is in use, flush the fluid path using
the Gen5 priming utility. Leave the fluid to soak overnight or over a weekend, and
then purge the fluid before using the instrument again.
BioTek Instruments, Inc.
Page 63

Empty/Clean the Tip Priming Trough | 51
This flushing and purging routine is also recommended before
disconnecting the outlet tubes from the reader, and before
decontamination to remove any assay residue prior to applying isopropyl
alcohol or sodium hypochlorite.
To flush the fluid path:
1. Fill two supply bottles with deionized or distilled water. Insert the supply
(inlet) tubes into the bottles.
2. Place the priming plate on the carrier.
3. From the Gen5 main screen, select
Cytation 5
4. Click the
5. Set the Volume to
6. Click
.
Prime tab and select Dispenser 1.
5000 µL. Keep the default prime rate.
Prime to start the process. When the process is complete, carefully
System > Instrument Control >
remove the priming plate from the carrier and empty it.
7. Repeat the process for Dispenser 2.
Leave the water in the system overnight or until the instrument will be used again.
Purge the fluid from the system (see below) and then prime with the dispense
reagent before running an assay.
To purge the fluid from the system:
1. Place the inlet tubes in empty supply bottles or a beaker.
2. Select
3. Click the
4. Set the Volume to
5. Click
System > Instrument Control > Cytation 5.
Prime tab and select Dispenser 1.
2000 µL.
Purge to start the process.
6. When the purge is complete, repeat the process for Dispenser 2.
After purging the system, you may wish to run a quick Dispense protocol
to visually verify the dispense accuracy.
Empty/Clean the Tip Priming Trough
Applies only to Cytation 5 models with a dispenser.
The tip priming trough is a removable cup located in the rear pocket of the
microplate carrier, used for performing the Tip Prime. The trough holds about 1.5 mL
of liquid and must be periodically emptied and cleaned by the user. Gen5 will
instruct you to do this at the start of an experiment that requires dispensing.
1. Extend the microplate carrier and carefully remove the tip priming trough
from the carrier.
Cytation 5
Page 64

52 | Maintenance
2. Wash the trough in hot, soapy water. Use a small brush to clean in the
corners.
3. Rinse the trough thoroughly and allow it to dry completely.
4. Replace the trough in the microplate carrier.
Clean the Priming Plate
Applies only to Cytation 5 models with a dispenser.
Clean the priming plate regularly to prevent bacteria growth and residue buildup.
Wash the plate in hot, soapy water, using a small brush to clean in the corners. Rinse
thoroughly and allow it to dry completely.
Clean the Dispense Tubes and Injectors
Applies only to Cytation 5 models with a dispenser.
The Cytation 5’s dispense tubes and injectors require routine cleaning, at least
quarterly and possibly more frequently depending on the type of fluids dispensed.
Required Materials
• Protective gloves
• Safety glasses
• Mild detergent
• Clean, lint-free cotton cloths
• Deionized or distilled water
• Stylus (stored in a plastic cylinder affixed to the rear of the dispense module or
reader) (PN 2872304)
Remove the Dispense Tubes and Injector Holders
BioTek Instruments, Inc.
Page 65

Clean the Dispense Tubes and Injectors | 53
1. Open the door on the front of the reader.
2. Grasp the injector tip holder by the tab and pull it up out of its socket.
3. Using your fingers, remove the thumbscrews securing the light shield to the
top of the reader and slide the shield up the outlets tubes.
4. Slide the injector tip holder through the hole in the top of the reader.
5. Turn each tube’s thumbscrew counterclockwise and gently pull each tube
from its injector tip.
6. On the dispense module, turn each outlet tube’s thumbscrew
counterclockwise to disconnect it from the injector.
Clean the Dispense Tubes and Injectors
Some reagents can crystallize and clog the tubing and injectors. Daily flushing and
purging can help to prevent this, but more rigorous cleaning may be necessary if
reagent has dried in the tubing or injectors.
To clean the dispense tubes, soak them in hot, soapy water to soften and dissolve any
hardened particles. Flush each tube by holding it vertically under a stream of water.
To clean the injectors:
1. Gently insert the stylus into each injector tip to clear any blockages. (The
stylus is stored in a plastic cylinder affixed to the rear of the dispense
module.)
2. Stream water through the pipe to be sure it is clean. If the water does not
stream out, try soaking in hot, soapy water and then reinserting the stylus.
Be careful not to damage the injector tips. A damaged tip might not
dispense accurately.
Cytation 5
Page 66

54 | Maintenance
BioTek Instruments, Inc.
Page 67

Instrument Testing
Page 68

56 | Instrument Testing
System Test
Each time the Cytation 5 is turned on, it automatically performs a series of tests on
the reader’s motors, lamp(s), the PMT(s), and various subsystems. If all tests pass, the
microplate carrier is ejected and the green LED on the carrier switch remains on.
If any test results do not meet the internally coded Failure Mode Effects Analysis
(FMEA) criteria established by BioTek, the reader beeps repeatedly and the red LED
on the carrier switch flashes. If this occurs, press the carrier eject button to stop the
beeping. If necessary, initiate another system test using Gen5 to try to retrieve an
error code from the reader.
1. Turn on the reader and launch Gen5.
2. If your assays use incubation, we recommend enabling Temperature Control
and allowing the incubator to reach its set point before running the system
test. To access this feature, from the Gen5 main screen, select
Instrument Control
3. Select
4. When the test is complete, a dialog appears, requesting additional
information. Enter your user name and other information (if desired) and
then click
5. The results report appears. It shows either “SYSTEM TEST PASS” or
“SYSTEM TEST FAIL *** ERROR (error code) DETECTED.”
6. Print the report if desired.
7. If the test failed, look up the error code in the Cytation 5 Operator’s Manual to
determine its cause. If the cause is something you can fix, turn off the reader,
fix the problem, and then turn the reader back on and retry the test. If the test
continues to fail, or if the cause is not something you can fix, contact BioTek’s
Technical Assistance Center.
System > Diagnostics > Run System Test.
OK.
and click the Pre-Heating tab.
System >
Absorbance Plate Test
This test uses BioTek’s Absorbance Test Plate (PN 7260522) to confirm mechanical
alignment; optical density accuracy, linearity, and repeatability; and wavelength
accuracy. The Absorbance Plate Test compares the reader’s optical density and
wavelength measurements to NIST-traceable values. The Absorbance OD Standards
section contains NIST-traceable standard OD values for the filters at several different
wavelengths. We recommend testing at six wavelengths—those at or close to the
wavelengths used in your assays.
Setup
1. Obtain the certificates that came with the Test Plate.
2. Start Gen5 and from the main screen select
Plates > Add/Modify Plates
System > Diagnostics > Test
.
BioTek Instruments, Inc.
Page 69

Absorbance Plate Test | 57
2.4 nm Spectral Band Pass
5.0 nm Spectral Band Pass
Expected Peak
Test Range
Expected Peak
Test Range
241
-5+5
242
-5+5
278
-6+4
279
-6+4
287
-4+6
288
-4+6
334
-5+5
334
-5+5
360
-5+5
362
-5+5
417
-5+5
417
-5+5
484
-5+5
485
-5+5
537
-5+5
538
-5+5
642
-5+5
643
-5+5
3. Click Add. The Absorbance Test Plate dialog appears.
4. Select the appropriate Plate Type and enter the plate’s serial number.
5. Enter the Last Certification and Next Certification dates from the calibration
sticker on the Test Plate.
6. If the wavelength values in the top row of the grid in Gen5 are appropriate
for your tests, enter the OD values from the data sheet into the grid. Make
sure you enter the correct value for each well/wavelength combination.
7. Select the number of Peak Wavelength tests to run (up to 4), based on the
number of expected peak wavelength values provided on the certificate.
8. Enter the Expected Peak value(s) from the certificate and set the Test Range –
and + values.
• If the C6 filter is Holmium or Erbium glass, the certificate contains two
Spectral Band Pass tables. The Cytation 5 has a band pass wider than 5
nm for wavelengths greater than 285 nm and less than 4 nm for 230–285
nm. As a results, we recommend you use the expected peak values in
the 5.0 nm table for your tests.
For the Erbium glass, any peak can be used.
For the Holmium glass, use the expected peak values closest to 242,
279, 362, 417, and 538 nm. For example, if your certificate looks like
the one below, you might choose to run the test at four of the five
highlighted Expected Peak/Test Range combinations:
• If your C6 filter is Didymium glass, a single peak wavelength value is
provided. Enter this value and set the Test Range – and + values so the
range displayed in parentheses is 580 to 590, as shown here:
Cytation 5
Page 70

58 | Instrument Testing
9. Review all the values you entered. Click OK to save the data.
The information you just entered is available on Gen5 each time the Absorbance Plate
Test is performed. It may need to be modified after the annual recertification of your
test plate.
Procedure
1. From the Gen5 main screen, select System > Diagnostics > Test Plates
> Run
2. When the Absorbance Test Plate Options dialog appears, select
Peak Wavelength Test
3. Highlight the wavelength(s) to be included in this test.
. If prompted, select the desired Test Plate and click OK.
Perform
if it is not already selected.
Select only those wavelengths most appropriate for your use of the
reader.
4. (Optional) Enter any Comments.
5. Click
Start Test.
6. Place the Test Plate in the microplate carrier so that well A1 is in the rightrear corner of the carrier.
7. Click
OK to run the test.
8. When the test is completed, the results report appears. Scroll through the
report; every result should show “PASS”.
Imaging Tests
These procedures test the level position of the carrier and the reliability of the Auto
Focus feature.
Required Materials
• Imaging qualification plate, PN 1222520
• 10X or lower objective
• DAPI 377/447 cube (PN 1225100)
• Gen5 protocols described beginning on page 61
• Cytation 5 <x>X_CarrierLevel.prt
• Cytation 5 AF_Reliability_<x>X.prt
BioTek Instruments, Inc.
Page 71

Imaging Tests | 59
Carrier Level Test
This test determines how level the carrier is in relation to the imaging system. This
test may be run with any of the following objectives: Meiji 2.5X, Zeiss 2.5X, 4X, or
10X.
1. Place the imaging qualification plate on the carrier with well A1 facing up in
the right-rear corner of the carrier.
2. If you have not already done so, create the protocol described on page 61.
3. Create a new experiment in Gen5 using the <x>X_CarrierLevel.prt protocol,
with <x> representing the lowest-power objective you have available.
4. Click
5. When prompted by Gen5, rotate the plate 180 degrees in the carrier.
6. When the read is finished, analyze the results as described below.
Analyze the Results
1. Calculate the Mean focus height for each set of ten reads in the "Normal"
2. Calculate the Mean focus height for each set of ten reads in the "Turnaround"
3. Compare the Mean values for each well in its Normal and Turnaround
a. –1 * (A1 Normal Mean – H12 Turnaround Mean)
b. (H12 Normal Mean – A1 Turnaround Mean)
c. –1 * (A12 Normal Mean – H1 Turnaround Mean)
d. (H1 Normal Mean – A12 Turnaround Mean)
4. For A1/H12:
• Calculate the Mean Delta (µm): (step a results + step b result) / 2
• Calculate the Carrier Tilt: Mean Delta / 25400 µm
• The Carrier Tilt must be less than 0.004" to Pass.
5. For A12/H1:
• Calculate the Mean Delta (µm): (step c results + step d result) / 2
• Calculate the Carrier Tilt: Mean Delta / 25400 µm
• The Carrier Tilt must be less than 0.004" to Pass.
Plate > Read Plate, save the experiment, then click OK.
position in wells A1, A12, H1, and H12.
position in wells A1, A12, H1, and H12.
positions by performing these calculations:
AutoFocus Test
The AutoFocus test confirms the imaging system's ability to repeatedly focus on a
known target.
1. Place the imaging qualification plate on the carrier with well A1 facing up in
the right-rear corner of the carrier.
Cytation 5
Page 72

60 | Instrument Testing
Depth of Field
Objective
Magnification
NA
e (µm)
λ (nm)
DOF (µm)
Limit (µm)
10X
10
0.3
6.45
377
6.3
12.7
4X 4 0.13
6.45
377
34.7
69.4
Zeiss 2.5X
2.74
0.12
6.45
377
45.8
91.6
Meiji 2.5X
2.25
0.07
6.45
377
117.9
235.8
Objective
FM Ratio must be
Meiji 2.5X
> 1.4
Zeiss 2.5X
> 1.4
4X
> 25
10X
> 50
2. If you have not already done so, create the protocol described starting on
page 61.
3. Create a new experiment in Gen5 using the AF_Reliability_<x>.prt protocol,
with <x> representing the objective you are using for each test.
4. Click
Plate > Read Plate, save the experiment, then click OK.
5. When the read is finished, analyze the results as described below.
Analyze the Results
1. For the set of 100 data points in well F7, calculate the Mean, Slope,
and Intercept. (If using Microsoft Excel, use the SLOPE and INTERCEPT
functions.)
2. For each data point, calculate the Residual:
[value]–((Slope*[data point sequence #])+Intercept)
Example: 325–((0*1)+325.9)=–0.9 Residual for data point #1
3. For each data point, compare the absolute value of the Residual with the
objective's Limit (µm) value in the chart below (e.g., 95.4 for 2.5X). If the
absolute value of the Residual is greater than the Limit, note "1" for that data
point; otherwise, note "0".
4. After conducting this comparison for all 100 data points, add up the
notations and divide the sum by 100; this is the Measured Autofocus Failure
Rate. The Measured Autofocus Failure Rate must be ≤ 1.0% to Pass.
5. In the Gen5 plate matrix, select the
FM Ratio [0:DAPI 377,447] data set.
Refer to the chart below for passing criteria for the value displayed in well
F7.
BioTek Instruments, Inc.
Page 73

Imaging Tests | 61
Parameter
Default Setting
Detection Method:
Imaging
Read Type:
Endpoint
Read Step 1
Process Mode:
Well Mode
Run Time: 18:00
Reads: 10
Objective:
Meiji 2.5X/Zeiss 2.5X/4X/10X
Color Channel:
Bright Field
Troubleshooting
If any of the imaging tests fail:
• Ensure the imaging qualification plate is clean. You can use canned air to blow
any debris from the surface of the plate.
• Ensure your objectives are clean.
• If your imaging tests still fail after cleaning the imaging qualification plate and
the objectives, contact BioTek TAC.
Imaging Protocols
The information in the following tables represents the recommended reading
parameters. It is possible that your tests will require modifications to some of these
parameters, such as the Plate Type.
The Plate Type setting in each Gen5 protocol should match the plate you
are actually using.
Carrier Level Tests
Cytation 5 Meiji 2.5X_CarrierLevel.prt, Cytation 5 Zeiss
2.5X_CarrierLevel.prt, Cytation 5 4X_CarrierLevel.prt, Cytation 5
10X_CarrierLevel.prt
Plate Type: 1222520 (imaging qualification plate)
Kinetic Step:
Read Wells:
Interval: 2 seconds
A1, A12, H1, H12
Cytation 5
Page 74

62 | Instrument Testing
Parameter
Default Setting
LED: 5
Gain: 0
Focus method: Auto focus with optional scan
Offset from bottom of well: 0
Horizontal offset from center of well:
0
Vertical offset from center of well:
0
Single image per well:
Selected
Delay after plate movement:
300 msec
After Read Step 1
Plate Out, In
"Please rotate the plate 180 degrees"
Read Step 2
Process Mode:
Well Mode
Run Time: 18:00
Reads: 10
Objective:
Meiji 2.5X/Zeiss 2.5X/4X/10X
Color Channel:
Bright Field
LED: 5
Gain: 0
Focus method: Auto focus with optional scan
Offset from bottom of well: 0
Exposure:
Auto Focus Options:
Vibration Detection Options:
Integration time: 50
Minimum focus metric ratio: 3
Scan distance: 600
Scan increment: 30
Minimum focus delta: 8
% of capture exposure for focus: 75
CV Threshold: 0.01
Images to average: 1
Kinetic Step:
Read Wells:
Exposure:
Auto Focus Options:
Interval: 2 seconds
A1, A12, H1, H12
Integration time: 50
Minimum focus metric ratio: 3
Scan distance: 600
Scan increment: 30
Minimum focus delta: 8
% of capture exposure for focus: 75
BioTek Instruments, Inc.
Page 75

Imaging Tests | 63
Parameter
Default Setting
Horizontal offset from center of well:
0
Vertical offset from center of well:
0
Single image per well:
Selected
Delay after plate movement:
300 msec
Parameter
Default Setting
Detection Method:
Imaging
Read Type:
Endpoint
Run Time: 19:59, Interval: 12 seconds, Reads:
100
Objective:
Meiji 2.5X/Zeiss 2.5X
Read Wells:
F7
Color Channel:
DAPI 377, 447
Exposure:
Auto
Focus method: Auto focus with optional scan
Offset from bottom of well: -50
Target exposure %: 75
Integration threshold: 100
CV Threshold: 0.01
Images to average: 5
Horizontal offset from center of
well:
Vertical offset from center of well:
0
Vibration Detection Options:
CV Threshold: 0.01
Images to average: 1
AutoFocus Reliability Tests
Cytation 5 AF_Reliability_Meiji 2.5X.prt, Cytation 5 AF_Reliability_Zeiss
2.5X.prt
Plate Type: 1222520_AF (imaging qualification plate)
Kinetic Step:
Auto-Focus Options:
Auto-Exposure Options:
Vibration Detection Options:
Minimum focus metric ratio: 3
Scan distance: 600
Scan increment: 30
Minimum focus delta: 8
% of capture exposure for focus: 75
Skip %: 0.1
0
Cytation 5
Page 76

64 | Instrument Testing
Parameter
Default Setting
Single image per well
Enabled
Delay after plate movement:
0 msec
Parameter
Default Setting
Detection Method:
Imaging
Read Type:
Endpoint
Run Time: 19:59, Interval: 12 seconds, Reads:
100
Objective:
4X
Read Wells:
F7
Color Channel:
DAPI 377, 447
Exposure:
Auto
Focus method: Auto focus with optional scan
Offset from bottom of well: -50
Target exposure %: 75
Integration threshold: 100
CV Threshold: 0.01
Images to average: 5
Horizontal offset from center of
well:
Vertical offset from center of well:
0
Single image per well
Enabled
Delay after plate movement:
0 msec
Parameter
Default Setting
Detection Method:
Imaging
Read Type:
Endpoint
Cytation 5 AF_Reliability_4X.prt
Plate Type:
Kinetic Step:
Auto-Focus Options:
Auto-Exposure Options:
Vibration Detection Options:
1222520_AF (imaging qualification plate)
Minimum focus metric ratio: 25
Scan distance: 600
Scan increment: 30
Minimum focus delta: 8
% of capture exposure for focus: 75
Skip %: 0.1
Cytation 5 AF_Reliability_10X.prt
0
BioTek Instruments, Inc.
Page 77

Absorbance Liquid Test | 65
Parameter
Default Setting
Run Time: 19:59, Interval: 12 seconds, Reads:
100
Objective:
10X
Read Wells:
F7
Color Channel:
DAPI 377, 447
Exposure:
Auto
Focus method: Auto focus with optional scan
Offset from bottom of well: -50
Target exposure %: 75
Integration threshold: 100
CV Threshold: 0.01
Images to average: 5
Horizontal offset from center of
well:
Vertical offset from center of well:
0
Single image per well
Enabled
Delay after plate movement:
0 msec
Plate Type: 1222520_AF (imaging qualification plate)
Kinetic Step:
Minimum focus metric ratio: 50
Scan distance: 600
Auto-Focus Options:
Scan increment: 30
Minimum focus delta: 8
% of capture exposure for focus: 75
Auto-Exposure Options:
Vibration Detection Options:
Skip %: 0.1
0
Absorbance Liquid Test
This test confirms the reader’s ability to perform to specification with liquid samples.
If the test passes, then the lens placement and optical system cleanliness are proven.
Materials
• New 96-well, clear, flat-bottom microplate (Corning Costar #3590
recommended)
• Stock Solution A or B, which may be formulated by diluting a dye solution
available from BioTek (A) or from the ingredients listed below (B).
Solution A
• BioTek QC Check Solution No. 1 (PN 7120779, 25 mL; PN 7120782, 125 mL)
• Deionized water
Cytation 5
Page 78

66 | Instrument Testing
• 5-mL Class A volumetric pipette
• 100-mL volumetric flask
1. Pipette a 5-mL aliquot of BioTek QC Check Solution No. 1 into a 100-mL
volumetric flask.
2. Add 95 mL of DI water; cap and shake well. The solution should measure
approximately 2.000 OD when using 200 µL in a flat-bottom microwell.
Solution B
• Deionized water
• FD&C Yellow No. 5 dye powder (typically 90% pure)
• Tween 20 (polyoxyethylene (20) sorbitan monolaurate) or BioTek wetting agent
(PN 7773002) (a 10% Tween solution)
• Precision balance with capacity of 100 g minimum and readability of 0.001 g
• Weigh boat
• 1-liter volumetric flask
1. Weigh out 0.092 g of FD&C Yellow No. 5 dye powder into a weigh boat.
2. Rinse the contents into a 1-liter volumetric flask.
3. Add 0.5 mL of Tween 20, or 5 mL of BioTek’s wetting agent.
4. Fill to 1 liter with DI water; cap and shake well. The solution should measure
approximately 2.000 OD when using 200 µL in a flat-bottom microwell.
Procedure
1. Using freshly prepared stock solution (Solution A or B), prepare a 1:2
dilution using deionized water (one part stock, one part deionized water; the
resulting solution is a 1:2 dilution).
2. Pipette 200 µL of the concentrated solution (A or B) into the first column of
wells in the microplate.
3. Pipette 200 µL of the diluted solution into the second column of wells.
4. Using Gen5, read the plate five times at 405 nm using the Normal read mode,
single wavelength, no blanking. Save the data after each read (“Normal plate
position”).
5. Without delay, rotate the plate 180 degrees so that well A1 is in the H12
position. Read the plate five more times, saving the data after each read
(“Turnaround plate position”).
6. Print ten sets of raw data, or export them to an Excel spreadsheet.
BioTek Instruments, Inc.
Page 79

Fluorescence Liquid Tests | 67
For the Filter-Based Fluorescence System
Corners, Sensitivity, and Linearity tests, using the top
optics
Cytation 5_FP.prt
Fluorescence Polarization test
Cytation 5_TRF.prt
Time-Resolved Fluorescence test
Analyze the Results
• The plate is read five times in “Normal” position at 405 nm. Calculate the
Mean OD and Standard Deviation of those five reads for each well in
columns 1 and 2.
• For each well in columns 1 and 2, calculate the Allowed Deviation using the
repeatability specification for a 96-well plate: ± 1% ± 0.005 OD from 0.000 to
2.000 OD (Mean * 0.010 + 0.005). For each well, its standard deviation should
be less than its allowed deviation.
• The plate is read five times in the “Turnaround” position at 405 nm.
Calculate the Mean OD of those reads for each well in columns 11 and 12.
• Perform a mathematical comparison of the Mean values for each microwell in
its Normal and Turnaround positions (that is, compare A1 to H12, A2 to H11,
B1 to G12,… H2 to A11). To pass the test, the differences in the compared
mean values must be within the accuracy specification for a 96-well
microplate: ± 1.0% ± 0.010 OD from 0.000 to 2.000 OD.
Repeatability Specification
± 1% ±0.005 OD from 0.000 to 2.000 OD
±3.0% ±0.010 OD from 2.000 OD to 2.500 OD
Fluorescence Liquid Tests
Required Materials
All Tests:
• Deionized or distilled water
• Various beakers, graduated cylinders, and pipettes
• 95% ethanol (for cleaning clear-bottom plates)
• Aluminum foil
• (Optional, but recommended) 0.45-micron filter
• (Optional) Black polyethylene bag(s) to temporarily store plate(s)
• Gen5 protocols listed below and described on page 77
Cytation 5_FI_T_SF.prt
Cytation 5
Page 80

68 | Instrument Testing
For the Monochromator-Based Fluorescence System
Cytation
5_M_FI_T_SF.prt
Corners, Sensitivity, and Linearity tests, using the Top
optics
Cytation
5_M_FI_B_SF.prt
Corners, Sensitivity, and Linearity tests, using the Bottom
optics
Filter Set Setup
Before using the filter-based fluorescence test protocols, create the applicable filter
sets shown below in Gen5 ("Green" is used for sodium fluorescein tests, "Blue" for
MUB).
Corners/Sensitivity/Linearity Tests
• Buffer:
NIST-traceable Sodium Borate Reference Standard (pH 9.18) (e.g., Fisher-
Scientific 1 L Sodium Borate Mfr. #159532, or equivalent), or
Phosphate-Buffered Saline (PBS), pH 7.2–7.6 (e.g., Sigma tablets, Mfr.
#P4417, or equivalent) and pH meter or pH indicator strips with pH range 4
to 10
• Sodium Fluorescein Powder (1-mg vial, BioTek PN 98155)
BioTek Instruments, Inc.
Page 81

Fluorescence Liquid Tests | 69
• If testing both Top and Bottom optics (mono-based fluorescence only): A
new, clean 96-well glass-bottom Greiner SensoPlate (Mfr. #655892); or a clean
Hellma Quartz 96-well titration plate (Mfr. #730.009.QG); or equivalent
• If testing the Top optics only: A new, clean 96-well solid black microplate, such
as Corning Costar #3915, or equivalent
• Excitation filter 485/20 nm installed
• Emission filter 528/20 nm installed
• 510-nm dichroic mirror installed
Fluorescence Polarization (FP) Test
• A new, clean, 96-well solid black microplate, such as Corning Costar #3915. A
Greiner SensoPlate can also be used.
The FP Test can be performed in conjunction with the top
Corners/Sensitivity/Linearity Tests, in the same microplate.
• The recommended test solutions are available from Invitrogen Corporation in
their “FP One-Step Reference Kit” (PN P3088) or from BioTek (PN 7160014).
This kit includes:
(Green) Polarization Reference Buffer, 15 mL
Green Low Polarization Reference, 4 mL
Green High Polarization Reference, 4 mL
The kit also includes two red polarization solutions; these are not used.
• Excitation filter 485/20 nm installed
• Emission filter 528/20 nm installed
• 510-nm dichroic mirror and polarizers installed
Time-Resolved Fluorescence (TRF) Test
• 15-mL conical-bottom, polypropylene sample tube
• Excitation filter 360/40 nm installed
• Emission filter 620/40 nm installed
• 400-nm dichroic mirror installed
• A new, clean 96-well solid white microplate, such as Corning Costar #3912
• The recommended test solution (FluoSpheres carboxylate-modified
microspheres, 0.2 µm europium luminescent, 2 μL) is available from Invitrogen
Corporation (PN F20881) or from BioTek (PN 7160011)
Cytation 5
Page 82

70 | Instrument Testing
With
Buffer:
To
Make:
0.53 mL of 1.3288 mM stock
solution
110 µL of 50.2 μM SF
13.89 mL
400 nM
3.5 mL of 400 nM SF
10.5 mL
100 nM
0.46 mL of 100 nM SF
13.54 mL
3.3 nM
Corners Test
Sensitivity/Linearity
Tests
Test Solutions
Corners/Sensitivity/Linearity Tests
If using BioTek’s sodium fluorescein powder (PN 98155), be sure to hold the
vial upright and open it carefully; the material may be concentrated at the top.
If a centrifuge is available, spin down the tube before opening.
When diluting the sodium fluorescein powder in buffer, it takes time for the
powder to completely dissolve. Allow the solution to dissolve for five minutes,
with intermittent vortexing, before preparing the titration dyes.
Wrap the vial containing the stock solution in foil to prevent exposure to light.
Discard unused solution after seven days. Discard any open, unused buffer
solution after seven days.
1. The Sodium Borate solution does not require further preparation; proceed to
step 2. If you are using PBS, prepare the solution:
• (Optional, but recommended) Using a 0.45-micron filter, filter 200 mL of
deionized or distilled water.
• Follow the manufacturer’s instructions on the PBS packaging to create 200
mL, dissolving the necessary amount of PBS into the filtered water.
• Stir the solution (preferably using a stir table) until the PBS is completely
dissolved.
• Check the pH; it should be between 7.2 and 7.6 at 25°C.
2. Prepare the sodium fluorescein stock solution:
• Add 2.0 mL of the buffer solution to the 1 mg Sodium Fluorescein (SF)
vial. This yields a 1.3288 mM stock solution.
• Ensure that the dye has completely dissolved and is well mixed.
3. Carefully prepare the dilutions. Label each with “SF” and the concentration:
Mix This SF Solution:
4.24 mL of 3.3 nM SF 9.76 mL 1 nM
13.47 mL 50.2 μM
BioTek Instruments, Inc.
Page 83

Fluorescence Liquid Tests | 71
Fluorescence Polarization (FP) Test
The recommended test solutions are available from Invitrogen Corporation or from
BioTek. They do not require additional preparation.
Time-Resolved Fluorescence (TRF) Test
The recommended test solutions are available from Invitrogen Corporation or from
BioTek.
• Shake the FluoSpheres container vigorously for 30 seconds prior to pipetting.
Alternatively, sonicate or vortex the container.
• Mix 10 µL of FluoSpheres with 10 mL of deionized water, in a 15 mL conical-
bottom, polypropylene sample tube. This yields a 20 nM equivalent suspension.
• Shake the vial vigorously for 30 seconds prior to pipetting. Alternatively,
sonicate or vortex the container.
• Mix 10 µL of 20 nM suspension with 10 mL of deionized water, in a 15 mL
conical-bottom, polypropylene sample tube. This yields a 20 pM equivalent
suspension.
• Refrigerate any unused portions of the FluoSpheres. The temperature must be
between +2°C to +6°C.
The prepared TRF plate can be kept for a maximum of seven days, if
covered and stored in the dark between +2°C to +6°C.
Allow the plate to sit at room temperature for approximately 15 minutes
prior to use.
Procedure
1. If you have not already done so, create the Gen5 protocols described starting
on page 77.
2. If you have not already done so, prepare the solutions for the tests you plan
to perform. See Fluorescence Test Solutions on page 70.
Refer to the pipette maps, next, for the remaining steps.
3. Perform the Corners/Sensitivity/Linearity tests using the Top optics of the
filter-based fluorescence system:
• Pipette the test solutions into a clean 96-well microplate.
• Create an experiment based on the Cytation 5_FI_T_SF.prt protocol. Read
the plate, and then save the experiment.
Cytation 5
Page 84

72 | Instrument Testing
4. To test Fluorescence Polarization capability:
• Pipette the solutions for the “FP” test into the same plate as used in step 3.
• Create an experiment based on the Cytation 5_FP.prt protocol. Read the
plate, and then save the experiment.
5. Perform the Corners/Sensitivity/Linearity tests for the monochromatorbased fluorescence system:
• Create experiments based on the Cytation 5_M_FI_T_SF.prt (for the top
optics) and Cytation 5_M_FI_B_SF.prt (for the bottom optics)
• Read the plate and then save the experiment.
6. To test the Time-Resolved Fluorescence capability:
• Pipette the solutions for the “TRF” test into a new 96-well solid white
plate.
• Create an experiment based on the Cytation 5_TRF.prt protocol. Read the
plate and then save the experiment.
Pipette Maps
Seal the plates with foil or store them in black polyethylene bags until
use. When using a clear-bottom plate, if the base of the plate is touched,
clean the entire base with alcohol (95% ethanol) and then wipe with a
lint-free cloth. Before placing the plate in the instrument, blow the
bottom of the plate with an aerosol duster.
Perform these steps carefully, and refer to the grid on the next page.
For the Corners test (light gray wells):
• Pipette 200 μL of the 3.3 nM SF solution into wells A1–A3, A10–A12, H1–H3,
and H10–H12.
• If using a Hellma plate: Pipette 200 μL of buffer into the wells surrounding the 3.3
nM wells ("CBUF" in the grid).
For the Sensitivity test (dark gray wells):
• Pipette 200 μL of the 1 nM SF solution into well D7.
• Pipette 200 μL of the buffer solution into wells C9, D9, and E9.
For the Linearity test (wells C1–F5):
• Use a multichannel pipette with just four tips installed.
• Pipette 150 μL of buffer solution into wells C2–F5. Discard the tips.
• Pipette 150 μL of the 1 nM SF solution into wells C1–F1.
• Pipette 150 μL of the 1 nM SF solution into wells C2–F2. Mix the wells using the
pipette. Do not discard the tips.
BioTek Instruments, Inc.
Page 85

Fluorescence Liquid Tests | 73
1 2 3 4 5 6 7 8 9 10
11
12
A
BUF
HPR
LPR B
BUF
HPR
LPR C
BUF
LPR D
BUF
LPR E
BUF
LPR F
BUF
LPR
G
BUF
LPR
H
BUF
LPR
• Aspirate 150 μL from wells C2–F2, and dispense into wells C3–F3. Mix the wells
using the pipette. Do not discard the tips.
• Aspirate 150 μL from wells C3–F3, and dispense into wells C4–F4. Mix the wells
using the pipette. Do not discard the tips.
• Aspirate 150 μL from wells C4–F4, and dispense into wells C5–F5. Mix the wells
using the pipette. Do not discard the tips.
• Aspirate 150 μL from wells C5–F5, and discard the tips.
Fluorescence Polarization (FP) Test
• Pipette 200 µL of the (green) polarization buffer (BUF) into wells A6–H6.
• Pipette 200 µL of the green high polarization reference (HPR) into wells A7–B7.
• Pipette 200 µL of the green low polarization reference (LPR) into wells A8–H8.
Cytation 5
Page 86

74 | Instrument Testing
1 2 3 4 5 6 7 8 9 10
11
12
A
B
DI
C
DI
D
E
F
G
H
Time-Resolved Fluorescence (TRF) Test
• Pipette 200 µL of deionized water into wells A6–C6.
• If you have not already done so, shake the vial of 20 pM europium suspension
vigorously for 30 seconds prior to pipetting. Alternatively, sonicate or vortex
the vial.
• Pipette 200 µL of the 20 pM europium suspension (Eu) into well A8.
DI Eu
Results Analysis
Corners Test
1. Calculate the Mean of the 12 wells containing the 3.3 nM SF test solution
(A1–A3, A10–A12, H1–H3, and H10–H12).
2. Calculate the Standard Deviation for the same 12 wells.
3. Calculate the %CV:
The %CV must be < 3.0 to pass.
Sensitivity Test
1. Calculate the Mean and Standard Deviation of the 16 reads for each of the
buffer wells (C9, D9, E9).
2. Among the three buffer wells, find the Median Standard Deviation and
corresponding Mean.
3. Calculate the Mean for the 16 reads of the SF Concentration well (D7).
4. Calculate the Signal-to-Noise Ratio (SNR) using the Mean SF Concentration,
Buffer Median STD with its corresponding Buffer Mean:
(SF Mean – Buffer Mean)/(3 * Buffer STD)
5. Calculate the Detection Limit, in pM, using the known concentration value of
SF and the Calculated
(Standard Deviation / Mean) * 100
SNR: 1000/SNR
BioTek Instruments, Inc.
Page 87

Fluorescence Liquid Tests | 75
Filter-Based Fluorescence System
To pass, the Detection Limit
must be less than or equal to:
Top
10 pM
Monochromator-Based Fluorescence System
To pass, the Detection Limit
must be less than or equal to:
EX 485 nm,
EM 528 nm
Filter- and Monochromator-Based
Fluorescence System
x
y
1000
Mean of the 1000 pM wells
500
Mean of the 500 pM wells
250
Mean of the 250 pM wells
125
Mean of the 125 pM wells
62.5
Mean of the 62.5 pM wells
Optic Probe
Optic Probe
Top/Bottom: 20 pM
Linearity Test
1. Calculate the Mean of the four wells for each concentration in columns 1–5.
2. Perform linear regression using these values as inputs:
3. Calculate the R-Square value; it must be >= 0.950 to pass.
Fluorescence Polarization (FP) Test
1. Using the raw data from the Parallel read:
• Calculate the Mean Blank (wells A6–H6).
• Calculate the Signal for each HPR well: Subtract the Mean Blank from its
measurement value.
• Calculate the Signal for each LPR well: Subtract the Mean Blank from its
measurement value.
2. Using the raw data from the Perpendicular read:
• Calculate the Mean Blank (wells A6–H6)
• Calculate the Signal for each HPR well: Subtract the Mean Blank from its
measurement value.
• Calculate the Signal for each LPR well: Subtract the Mean Blank from its
measurement value.
Cytation 5
Page 88

76 | Instrument Testing
To pass, the Mean PHPR
must be greater than:
Top, with 510 nm dichroic mirror
340 mP
To pass, the Standard Deviation
of the PLPR must be less than:
Top, with 510 nm dichroic mirror
5 mP
To pass, the Detection Limit
must be less than or equal to:
Top, with 400 nm dichroic mirror
250 fM
3. Calculate the G-Factor for each LPR well:
(Parallel LPR Sign * (1–0.02)) / (Perpendicular LPR Signal * (1+0.02))
4. Calculate the Mean G-Factor.
5. Calculate the Polarization value in mP for each HPR well (“PLPR”):
Parallel HPR Signal – Mean G-Factor * Perpendicular HPR Signal * 1000
Parallel HPR Signal + Mean G-Factor * Perpendicular HPR Signal
6. Calculate the Mean PHPR, in mP.
Optic Probe
7. Calculate the Polarization value in mP for each LPR well (“PLPR”):
Parallel LPR Signal – Mean G-Factor * Perpendicular LPR Signal * 1000
Parallel LPR Signal + Mean G-Factor * Perpendicular LPR Signal
8. Calculate the Standard Deviation of the “PLPR,” in mP.
Optic Probe
Time-Resolved Fluorescence (TRF) Test
1. Calculate the Mean and Standard Deviation of the 16 reads for each of the
buffer wells (A6, B6, C6).
2. Among the three buffer wells, find the Median Standard Deviation and
corresponding Mean.
3. Calculate the Mean for the 16 reads of the Eu Concentration well (A8).
4. Calculate the Signal-to-Noise Ratio (SNR) using the Mean Eu Concentration
and Buffer Median STD with its corresponding Buffer Mean:
(Eu Mean – Buffer Mean)/(3 * Buffer STD)
5. Calculate the Detection Limit, in fM:
20000 / ((Mean Eu – Mean DI water) / (3 * Standard Deviation DI water)
Optic Probe
Troubleshooting
If any tests fail, please try the following suggestions. If the test(s) continue to fail,
print the results and contact BioTek’s Technical Assistance Center.
BioTek Instruments, Inc.
Page 89

Fluorescence Liquid Tests | 77
• Are the solutions fresh? Discard the plate and any open, unused test solutions
after seven days.
• Are the excitation/emission filters clean? Are they in the proper locations and
in the proper orientation in the filter cube?
• If the Corners Test continues to fail, the hardware may be misaligned. Contact
BioTek TAC.
• Are you using new/clean plates? If the base of a clear-bottom plate is touched,
clean the entire base with alcohol (95% ethanol) and then wipe with a lint-free
cloth. Before placing the plate in the instrument, blow the bottom of the plate
with an aerosol duster. If the test fails again, the optical probe(s) may need to be
cleaned. Contact BioTek’s Technical Assistance Center for instructions.
• Review the pipetting instructions to verify the plate was correctly prepared.
• Does the Plate Type setting in the Gen5 protocol match the plate you used?
• For injector models, spilled fluid inside the reader may be fluorescing, which
can corrupt your test results. If you suspect this is a problem, contact BioTek
TAC.
• When testing Fluorescence Polarization capability using a solid black plastic
microplate, if the standard deviation for the buffer wells is too high, try moving
the buffer wells to another column. With some black plastic plates, the wells in
the center of the plate may be slightly distorted due to the plate molding
process, and this can affect the standard deviation.
• The Read steps in the protocols use the Gen5 Automatic Gain Adjustment
feature to determine optimum sensitivity values for the plate. If an Auto Gain
Result value is outside the range of 50–200, this may indicate a problem.
If the value is less than 50:
The stock solution/dilution concentrations may be too high. Try creating
fresh solutions/dilutions, and rerun the test using a new, clean plate.
If all of the tests are passing but the Gain value is low, a PMT in your reader
may just be very sensitive. Contact BioTek’s Technical Assistance Center to
confirm that this may be the case.
If the value is greater than 200:
The stock solution/dilution concentrations may be too low. Try creating
fresh solutions/dilutions, and rerun the test using a new, clean plate.
For injector models, spilled fluid inside the reader may be fluorescing, which
can corrupt your test results. If you suspect this is a problem, contact BioTek
TAC.
The PMTs or optical path(s) may be deteriorating, or the optics or other
hardware may be misaligned. Contact BioTek’s Technical Assistance Center.
Gen5 Protocol Reading Parameters
The information in the following tables represents the recommended reading
parameters. It is possible that your tests will require modifications to some of these
parameters, such as the Plate Type (see Troubleshooting Tips on page 76).
Cytation 5
Page 90

78 | Instrument Testing
Parameter
Default Setting
Read Step 1
Detection Method:
Fluorescence
Read Type:
Endpoint
Run Time: 00:00:45
Reads: 16
Step Label:
"Sensitivity Read"
Read Well:
D7
Filter Set:
1 (Green)
Filters:
EX 485/20 nm, EM 528/20 nm
Optics Position:
Top 510 nm
Gain:
Auto, Scale to High Wells, D7, 30000
Normal
Dynamic Range: Standard
Read Height:
7.00 mm
Read Step 2
Detection Method:
Fluorescence
Read Type:
Endpoint
Run Time: 00:01:35
Reads: 16
Step Label:
"Sensitivity Read Buffer"
Read Wells:
C9..E9
Filter Set:
1 (Green)
The Plate Type setting in each Gen5 protocol should match the plate you
are actually using.
Cytation 5_FI_T_SF.prt
This procedure contains two Read steps using filters to test the top
optics: one for the Corners Test and one for the Sensitivity/Linearity Test.
Plate Type: Costar 96 black opaque (#3915)
Kinetic Loop
Read Speed:
Interval: 00:00:03
Delay after plate movement: 350 msec
Measurements per data point: 50
Lamp Energy: Low
Kinetic Loop
Interval: 00:00:06
BioTek Instruments, Inc.
Page 91

Parameter
Default Setting
Filters:
EX 485/20 nm, EM 528/20 nm
Optics Position:
Top 510 nm
Gain:
Auto, Use first filter set gain from FIRST Read Step
Read Speed:
Normal
Dynamic Range: Standard
Read Height:
7.00 mm
Read Step 3
Detection Method:
Fluorescence
Read Type:
Endpoint
Step Label:
"Corners Read"
Read Wells:
A1..A3, A10..A12, H1..H3, H10..H12
Filter Set:
1 (Green)
Filters:
EX 485/20 nm, EM 528/20 nm
Optics Position:
Top 510 nm
Gain:
Auto, Scale to High Wells, A3, 30000
Normal
Dynamic Range: Standard
Read Height:
7.00 mm
Read Step 4
Detection Method:
Fluorescence
Read Type:
Endpoint
Step Label:
"Linearity Read"
Read Wells:
C1..F5
Filter Set:
1 (Green)
Filters:
EX 485/20 nm, EM 528/20 nm
Optics Position:
Top 510 nm
Gain:
Auto, Scale to High Wells, C1, 30000
Fluorescence Liquid Tests | 79
Delay after plate movement: 350 msec
Measurements per data point: 50
Lamp Energy: Low
Read Speed:
Delay after plate movement: 350 msec
Measurements per data point: 50
Lamp Energy: Low
Cytation 5
Page 92

80 | Instrument Testing
Parameter
Default Setting
Normal
Dynamic Range: Standard
Read Height:
7.00 mm
Parameter
Default Setting
Detection Method:
Fluorescence polarization
Read Type:
Endpoint
Plate Type:
Costar 96 black opaque (#3915)
Shake Step:
Linear for 15 seconds
Delay Step:
5 seconds after shake
Synchronized Mode:
Plate Mode with Timing Control
Read Wells:
A6–H8
Filters:
EX 485/20 nm, EM 528/20 nm
Optics Position:
Top 510 nm
Gain:
Auto, Scale to high Wells, A8, 10000
Normal
Lamp Energy: Low
Read Height:
7.0 mm
Parameter
Default Setting
Plate Type:
Costar 96-well white opaque
Delay Step:
3 minutes
Read Step 1
Detection Method:
Time-resolved fluorescence
Delay after plate movement: 350 msec
Read Speed:
Cytation 5_FP.prt
Measurements per data point: 50
Lamp Energy: Low
This procedure contains one Read step using filters with Fluorescence
Polarization enabled, inside a Plate Mode block.
Filter Sets: 1 (filter cube)
Read Speed:
Cytation 5_TRF.prt
Delay after plate movement: 350 msec
Measurements per data point: 60
BioTek Instruments, Inc.
Page 93

Fluorescence Liquid Tests | 81
Parameter
Default Setting
Read Type:
Endpoint
Run Time: 00:00:15
Reads: 16
Step Label:
"Sensitivity Read"
Read Well:
A8
Filters:
EX 360/40 nm, 620/40 nm
Optics Position:
Top 400 nm
Gain:
Auto, Scale to High Wells, A8, 30000
Normal
Lamp Energy: Low
Read Height:
7.00 mm
Read Step 2
Detection Method:
Time-resolved fluorescence
Read Type:
Endpoint
Run Time: 00:00:45
Reads: 16
Step Label:
"Sensitivity Read Buffer"
Read Well:
A6–C6
Filters:
EX 360/40 nm, 620/40 nm
Optics Position:
Top 400 nm
Gain:
Auto, Use first filter set gain from FIRST Read Step
Normal
Lamp Energy: Low
Read Height:
7.00 mm
Kinetic Loop
Interval: 00:00:01
Filter Sets: 1 (filter cube)
Read Speed:
Delay after plate movement: 100 msec
Measurements per data point: 50
Kinetic Loop
Interval: 00:00:03
Filter Sets: 1 (filter cube)
Read Speed:
Delay after plate movement: 100 msec
Measurements per data point: 50
Cytation 5
Page 94
82 | Instrument Testing
Parameter
Default Setting
Top: Costar 96 black opaque
Bottom: Greiner SensoPlate
Read Step 1
Detection Method:
Fluorescence
Read Type:
Endpoint
Run Time: 00:00:45
Reads: 16
Step Label:
"Sensitivity Read"
Wavelength:
1, EX 485/14, EM 528/14 nm
Optics Position:
Top/Bottom
Normal
Dynamic Range: Standard
Read Height (for top optics):
7.00 mm
Read Step 2
Detection Method:
Fluorescence
Read Type:
Endpoint
Run Time: 00:01:35
Reads: 16
Step Label:
"Sensitivity Read Buffer"
Wavelength:
1, EX 485/14, EM 528/14 nm
Optics Position:
Top/Bottom
Cytation 5_M_FI_T_SF.prt and Cytation 5_M_FI_B_SF.prt
Plate Type:
Kinetic Loop
Interval: 00:00:3
Read Well: D7
Gain
Auto, Scale to High Wells, D7, 30000
Delay after plate movement: 100 msec
Read Speed:
Measurements per data point: 50
Lamp Energy: Low
Kinetic Loop
Read Well: C9..E9
Gain
Interval: 00:00:6
Auto, Use first filter set gain from FIRST Read Step
BioTek Instruments, Inc.
Page 95

Fluorescence Liquid Tests | 83
Parameter
Default Setting
Normal
Dynamic Range: Standard
Read Height (for top optics):
7.00 mm
Read Step 3
Detection Method:
Fluorescence
Read Type:
Endpoint
Step Label:
"Corners Read"
Wavelength:
1, EX 485/14, EM 528/14 nm
Optics Position:
Top/Bottom
Normal
Dynamic Range: Standard
Read Height (for top optics):
7.00 mm
Read Step 4
Detection Method:
Fluorescence
Read Type:
Endpoint
Step Label:
"Linearity Read"
Wavelength:
1, EX 485/14, EM 528/14 nm
Optics Position:
Top/Bottom
Delay after plate movement: 100 msec
Read Speed:
Measurements per data point: 50
Lamp Energy: Low
Read Wells: A1..A3, A10..A12, H1..H3, H10..H12
Gain Auto, Scale to High Wells, A3, 30000
Delay after plate movement: 100 msec
Read Speed:
Measurements per data point: 50
Lamp Energy: Low
Read Wells: C1..F5
Gain
Auto, Scale to High Wells, C1, 30000
Cytation 5
Page 96

84 | Instrument Testing
Parameter
Default Setting
Normal
Dynamic Range: Standard
Read Height (for top optics):
7.00 mm
Delay after plate movement: 100 msec
Read Speed:
Measurements per data point: 50
Lamp Energy: Low
Luminescence Test
BioTek provides two methods for verifying the performance of luminescence reads.
One method measures a Harta Luminometer Reference Microplate, which is an LEDbased test plate. Contact BioTek to purchase a plate, or go to
www.hartainstruments.com for more information. The other method measures a
LUX Biotechnology, Ltd., Glowell unit, which is a small, sealed cylinder with a
gaseous tritium light source.
Before using the Cytation 5 F-LumTest_Harta.prt or Cytation 5 F-
LumTest_Glowell.prt protocols described in this section, create the filter set shown
below.
Harta Plate Test
Materials
• Harta Luminometer Reference Microplate, PN 8030015
• Harta Plate Adapter, PN 1222205
• Filter cube PN 8040553, LUM Filter Block or a filter cube with a plug in
EX position 1 or 2 and a hole in the corresponding EM position
• Gen5 protocol described started on page 88
BioTek Instruments, Inc.
Page 97

Luminescence Test | 85
1 2 3 4 5 6 7 8 9 10
11
12
A2
meas
B
C
D
BUF
BUF
BUF
BUF
E
BUF
BUF
BUF
BUF
F
BUF
BUF
BUF
BUF
G
BUF
BUF
BUF
BUF
H
Procedure
1. Turn on the Harta reference plate using the I/O switch on the back of the
plate.
2. Check the plate’s battery by pressing the test button on the back of the plate
and ensuring that the test light turns on.
The test light may be difficult to see in bright light. Change your angle of
view or move to a darker environment if you cannot see it.
3. Place the Harta plate adapter on the reader’s carrier, then place the test plate
on top of the adapter.
4. Create an experiment based on the Cytation 5 F-LumTest_Harta.prt or
Cytation 5 M-LumTest_Harta.prt protocol and read the plate.
5. Calculate and evaluate the results as described on page 86.
Be sure to turn off the Harta plate when you are finished with the test to
preserve battery life.
Plate Layout
Cytation 5 F-LumTest_Harta.prt
A
battery
check
battery
check
Cytation 5
Page 98

86 | Instrument Testing
1 2 3 4 5 6 7 8 9 10
11
12
A2
meas
B
C
D
E
F
BUF
BUF
BUF
BUF
BUF
BUF
BUF
BUF
BUF
BUF
BUF
BUF
G
BUF
BUF
BUF
BUF
BUF
BUF
BUF
BUF
BUF
BUF
BUF
BUF
H
Cytation 5 M-LumTest_Harta.prt
A
battery
check
battery
check
Harta Plate Results Analysis
Through a manual correlation process, it was found that the system
requires approximately 35 photons per attomole of ATP, thus a
conversion factor of 0.02884 attomole/photon was applied to determine
ATP concentration from the NIST data in photons/s.
1. On the Harta plate’s Calibration Certificate, locate the NIST measurement for
the A2 position and convert it to attomoles:
(A2 NIST measurement * 0.02884)
2. Determine if the plate’s battery is still functioning properly:
If A8 > (0.2 * A7), the battery is good. Otherwise, it requires replacement.
A replacement battery is included with each Harta plate. A new
spare battery will be supplied when the plate is recertified.
3. Calculate the signal-to-noise ratio:
(A2 – Mean of the buffer cells)/(3 * Standard deviation of buffer cells)
4. Calculate the detection limit:
A2 NIST measurement in attomoles/signal-to-noise ratio
If the reader is equipped with the low-noise PMT, the detection limit must
be <= 75 amol to pass.
If the reader is equipped with the red-shifted PMT, the detection limit
must be <= 500 amol to pass.
To determine which PMT is installed, check the label on the back of
the reader. #49984 = low-noise PMT; #49721 = red-shifted PMT.
BioTek Instruments, Inc.
Page 99

Luminescence Test | 87
1 2 3 4 5 6 7 8 9 10
11
12
A
BUF
BUF
BUF
B
BUF
BUF
BUF
C
BUF
BUF
BUF
D
BUF
BUF
BUF
E
BUF
BUF
BUF
F
BUF
BUF
BUF
G
BUF
BUF
BUF
H
BUF
BUF
BUF
Glowell Test
Materials
• Glowell, PN GLO-466, formerly available from LUX BioTechology, Ltd.
(www.luxbiotech.com)
• Glowell Adapter Plate, available from BioTek, PN 7160006
• Filter cube PN 8040553, LUM Filter Block, or a filter cube with a plug in
EX position 1 or 2 and a hole in the corresponding EM position.
• Gen5 protocols described starting on page 88
Procedure
1. If you have not already done so, insert the Glowell (“window” side up) into
well D8 of the Adapter Plate.
2. Create an experiment based on the Cytation 5 F-Lum-Test_Glowell.prt or
Cytation 5 M-LumTest_Glowell.prt protocol, and read the plate.
3. Calculate and evaluate the results as described on page 87.
Plate Layout
Glowell
Glowell Results Analysis
1. Locate these items on the Glowell’s Calibration Certificate: Calibration Date,
Radiant Flux (pW), Measurement Uncertainty of the Radiant Flux.
2. Calculate the number of days between the Calibration Date and the date the
test was performed.
3. Correct the Glowell’s Radiant Flux value for deterioration over time:
Radiant Flux * (1 – 0.000333 * number of days since calibration)
4. Convert the Corrected Radiant Flux value to attomoles:
(Corrected Radiant Flux / 0.021) * 1800
Cytation 5
Page 100

88 | Instrument Testing
Parameter
Default Setting
Plate Type:
8030015 Harta - w/o 8032028 adapter
Delay Step:
3 minutes
Read Step 1
Detection Method:
Luminescence
Read Type:
Endpoint
Step Label:
"Reference well A2"
Filter Sets:
1 (open)
Excitation:
Plug
Emission:
Hole
Integration Time:
0:10.00 MM:SS.ss
Delay after plate movement:
350 msec
Dynamic Range:
Standard
Read Height:
7.00 mm
5. Calculate an error factor for the Corrected Radiant Flux (amol):
(Corrected Radiant Flux in amol * Measurement Uncertainty) / 100
6. Calculate the min/max criteria for the Corrected Radiant Flux (amol):
MIN: Corrected Radiant Flux in amol – Error Factor
MAX: Corrected Radiant Flux in amol + Error Factor
7. Calculate the Signal-to-Noise Ratio:
Measurement value of the Glowell – Mean of Column 9
3 x Standard Deviation of Column 9
8. Calculate the Detection Limit:
Corrected Radiant Flux in amol/Signal-to-Noise Ratio
9. Calculate the min/max criteria for the Detection Limit:
MIN: MIN for Corrected Radiant Flux in amol/Signal-to-Noise Ratio
MAX: MAX for Corrected Radiant Flux in amol/Signal-to-Noise Ratio
If the reader is equipped with the low-noise PMT, the detection limit must
be <= 75 amol to pass.
If the reader is equipped with the red-shifted PMT, the detection limit
must be <= 500 amol to pass.
Gen5 Protocol Reading Parameters
Cytation 5 F-LumTest_Harta.prt
Read Wells: A2
Gain: 135
BioTek Instruments, Inc.
 Loading...
Loading...