Bionet BM3 Service Manual
BM3 www.BIO2NET.com
BM3
Patient Monitor
Service Manual
Rev A
Revision A
- 1 -

BM3 www.BIO2NET.com
Table of Contents
1. How to Reach Us …
2. General Precaution
3. Performance Specification
4. Equipment Overview
5. System Construction
6. System Inspection
7. Trouble Shoot
8. Exploded View
9. Circuit Diagram
Revision A
- 2 -

BM3 www.BIO2NET.com
1. How to Reach Us …
The following are telephone numbers and addresses for contacting various
service, product supplies and sales personnel
Product and Purchase Inquiry
Bionet Co., Ltd.
Address #188-5, KiCOX venture center 501, Guro-dong, Guro-gu, Seoul,
South Korea (ZIP 152-050)
Overseas sales dept. Tel:++82-2-6300-6419
Service call
Tel: ++82-2 –6300 - 6431
Technical support
For any technical questions or problems on the equipment, call;
Tel: ++82-2 –6300 - 6431
Revision A
- 3 -

BM3 www.BIO2NET.com
Web site of Bionet
URL : HTTP:// WWW.BIO2NET.COM
※ In the event of a malfunction or failure, contact Service Dept.Of Bionet
Co., Ltd along with the model name, serial number, date of purchase and
explanation of failure.
Warranty
l This product is manufactured and passed through strict quality
control and thorough inspection.
l Compensation standard concering repair, replacement, refund of
the product complies with “ Consumer’s protection law” noticed by
Economic Planning Dept.
Warranty period is 2 year.
l Warranty repair or replacement will be made by Bionet Service
Center at no charge for warranty period if properly used under
Revision A
- 4 -

BM3 www.BIO2NET.com
normal condition in accordance with the instructions for use.
l Manufacturer or sales agency takes no responsibility for any kind of
damage or breakdown that is caused by misuse and failure to maintain
the equipment.
2. General Precaution
Warning, Caution, Note
l For a special emphasis on agreement, terms are defined as listed below in
operation manual. Users should operate the equipment according to all the
Warning and Caution.
Warning : To inform that it may cause serious injury or
death to the patient, property damage, material losses
Revision A
- 5 -

BM3 www.BIO2NET.com
against the “Warning”sign.
Caution: To inform that it may cause no harm in life but lead to injury
against the “Caution”sign.
Note : To inform that it is not dangerous but important for proper
installation, operation, and maintenance of the equipment.
General Precaution on Environment
l Do not keep or operate the equipment in the environment listed below.
Revision A
- 6 -

BM3 www.BIO2NET.com
Avoid placing in an area
exposed to moist. Do not
touch the equipment with wet
Avoid placing in an area
where there is a high
variation of temperature.
Operating temperature
ranges from 10°C to 40°C.
Operating humidity ranges
from 30% to 85%.
Avoid placing in an area
excessive humidity rise or
Avoid placing in an area
where chemicals are
stored or where there is
danger of gas leakage.
hand .
where there is an
ventilation problem.
Avoid exposure to direct sunlight
Avoid in the vicinity of Electric
heater
Avoid placing in an area where
there is an excessive shock or vibration.
Avoid being inserted dust and
especially metal material into the
equipment
Do not disjoint or
disassemble the equipment.
Bionet Co.Ltd takes no
responsibility for it
Power off when the
equipment is not fully
installed.
Otherwise, equipment could
be damaged.
General Precaution on Electric Safety
Warning: Check the item listed below before operating the equipment.
Revision A
- 7 -
BM3 www.BIO2NET.com
1. Be sure that AC power supply line is appropriate to use.
(AC 100 - 240V)
2.Be sure that the power source is the one supplied from Bionet.
(DC 18V, 2.5A)
3.Be sure that the entire connection cable of the system is properly and firmly
fixed.
4. Be sure that the equipment is completely grounded.(Otherwise, noise
could result.)
5. The equipment should not be placed in the vicnity of electric generator, X-
ray, broadcasting apparatus to eliminate the electric noise during operation.
Otherwise, it may cause incorrect result.
NOTE
The Equipment should be placed far from generator, X-ray equipment, broadcasting
equipment, or transmitting wires, so as to prevent the electrical noises from being
generated during the operation, When these devices are near the Equipment, it can
produce inaccurate measurements. For BM3, both independent circuit and stable
grounding are essentially required. In the event that same power source is shared
with other electronic equipment, it can also produce inaccurate output
Warning
Do not use the System for any monitoring procedure on a patient if the monitor is not
working properly, or of it is mechanically damaged. Contact the hospital biomedical
engineer, or your supplier
Revision A
- 8 -

BM3 www.BIO2NET.com
t in terms of electrical shock prevention. It is not
Medical
NOTE
BM3 is classified as follows. :
- It is a class I type-CF Class equipmen
proper to operate this Equipment around combustible anesthetic or dissolvent.
- The noise level is “B” Class according to the IEC/EN 60601-1 (Safety of Electric
Equipment), and the noise redemption is “B” Level according to the IEC/EN 60601-1-2
(Electromagnetic Compatibility Requirements).
Equipment connection
CAUTION
In the hospital, doctors and patients are expose to dangerous, uncontrollable
compensating currents. These currents are due to the potential differences between
connected equipment and reachable conducting parts often in the medical rooms.
The safety solution to the problem is accomplished with consistent equipment
Maintenance and Cleaning
Using various methods can clean BM3 and its accessories. Please follow the
methods mentioned below to avoid unnecessary damage or contamination to
the Equipment.
In the event that harmful (unauthorized) materials are used for cleaning, the
damaged or contaminated Equipment shall not be serviced without charges
regardless of warranty period.
Caution!
Please check carefully both frame and sensor, after cleaning the Equipment, Do
not use the Equipment that is worn out or damaged.
Revision A
- 9 -

BM3 www.BIO2NET.com
At least once a month, clean and wipe off the frame by using the soft cloth
after wetting it with lukewarm water and alcohol. Do not use lacquer, thinner,
ethylene, or oxides, which could be harmful to the Equipment.
Make sure both cables and accessories are free of dust or contaminants, and
wipe them off with soft cloth wetted with lukewarm water(40℃ / 104℉), and at
least once a week, clean them by using the clinical alcohol.
Do not submerge the accessories under any liquid or detergent. Also, make
sure any liquid not to penetrate into the Equipment or probe.
Caution!
Do not dispose single use probe to any hazard place, Always think about
environmental contamination
Caution!
There is back-up battery on board inside system. When users dispose this
chip. Please waste proper place for environmental protection
CR2032 3.0Volt battery
Warning: Check the electrodes of batteries
before changing them.
3. Performance Specification
Ease of use
• Battery operation
Revision A
- 10 -

BM3 www.BIO2NET.com
• Optional integrated strip chart recorder
• mounting to a roll stand supports bedside availability
Customization
• Tabular and Graphical Trends
• Compatible with Nellcor reusable and disposable SpO2 sensors
Special Features
• Direct DC input for transport needs
• LAN data export interface
• Nurse call alarm capability
Monitor Environmental Specifications
• Operating Temperature: 0°C to 50°C (32°F to 122°F)
• Storage Temperature: - 20°C to 60°C (- 4°F to 140°F)
• Operating/Storage Humidity: 5% to 95% RH, non-condensing
• Operating Altitude: 0 to 3,048 m (0 to 10,000 ft.)
Power
• 18 VDC, 2.5 A max , Adapter
Monitor Performance Specifications
• Display:
– LCD 5.7” color
• Indicators
– Up to 3 waves (ECG, SpO2, Respiration)
– categorized alarms (3 priority levels)
– visual alarm
– heart rate tone
– battery status
– external power LED
• Interfaces
– DC input connector: 10 to 16 V DC, 3A max.
– Defib Sync Output
Signal Level: 0 to 5 V pulse
Revision A
- 11 -

BM3 www.BIO2NET.com
Pulse Width: 100 ±10 ms
Delay from R-wave peak to start of pulse: 35 ms, maximum
Short circuit current: 15 mA Minimum required R-wave
amplitude: 0.5 V
– LAN digital output for transferring data to an external computer
– nurse call
• Battery (standard)
– internal battery: sealed lead-acid
– battery status indicator
– Operating Time: 2.5 hours typical (fully charged battery)
• Thermal Printer (Optional):
– Speeds: 25, 50 mm/sec
– Paper Width: 58 mm
Graphical and Tabular Trends
• Tabular Trends
– Memory Storage: 24 hours
– Data Interval: 1 minute
– Display Interval: 1MIN, 5, 15, 30, 1HR
Tabular Format: One table for all variables
• Graphical Trends
- Display Period: 30MINS, 60, 90, 3HRS, 6, 12
SpO2 Performance Specifications
• % Saturation Range: 0% to 100%
• Pulse Rate Range: 30 to 300 bpm
• SpO2 Accuracy: 70% to 100% ± 2 digits, 0% to 69% unspecified
• Pulse Rate Accuracy: ± 3 bpm
NIBP Performance Specifications
• Technique: oscillometric
• Measurement Modes:
– Manual:Single measurement
- Auto:Automatic intervals of 1MIN,2,3,4,5,10,15,20,30,1HR,2,4,8
• Cuff Pressure Display: 30 to 300 mmHg
• Blood Pressure Measurement Range:
Revision A
- 12 -

BM3 www.BIO2NET.com
– Systolic: 60 to 250 mmHg
– Mean Arterial Pressure: 45 to 235 mmHg
– Diastolic: 40 to 220 mmHg
• Pulse Rate Range: 40 to 200 bpm
ECG Performance Specifications
• Leads: 3 leads
• Heart Rate Range: 30 to 300 bpm
• Heart Rate Accuracy: ± 3 bpm
• Bandwidth: 0.5 Hz to 40 Hz
• Display Sweep Speeds: 25mm/sec
• ECG Size (Sensitivity): 0.5, 1, 2, 4 mV/cm
• Lead-Off Detection with display indicator
• Pacemaker Detection Mode: Indicator on waveform display,
user selectable
• Differential Input Impedance: > 5 Mohm
• Common Mode Rejection Ratio: > 90 dB at 50 or 60 Hz
• Input Dynamic Range: ± 5 mVAC, ± 300 mVDC
• Defibrillator Discharge: <5 s
• Defibrillation Artifact Recovery Time: < 8 s
Respirations Performance Specifications
• Range: 5 to 120 breaths/min
• Accuracy: ± 3 breaths/min
• Display Sweep Speeds: 25mm/sec
Temperature Performance Specifications
• Range: 15°C to 45°C (59°F to 113°F)
• Accuracy: 25°C to 45°C ± 0.2°C, 15 °C to 24°C ± 0.3°C
• Compatible with YSI Series 400 Temperature Probes
Accessories included:
- 3-lead patient cable 1ea
- electrodes 10ea
Revision A
- 13 -

BM3 www.BIO2NET.com
- NIBP tubing, 3 m long 1ea
- Adult cuff, 25-35 cm, reusable 1ea
– SpO2 sensor extension cable(2 m) 1ea
– SpO2 sensor, reusable 1ea
– DC adapter, 18VDC, 2.5A 1ea
- Temperature probe Surface/Skin, reusable(OPTION)
– rolls of paper. (OPTION)
- 5-lead patient cable(OPTION)
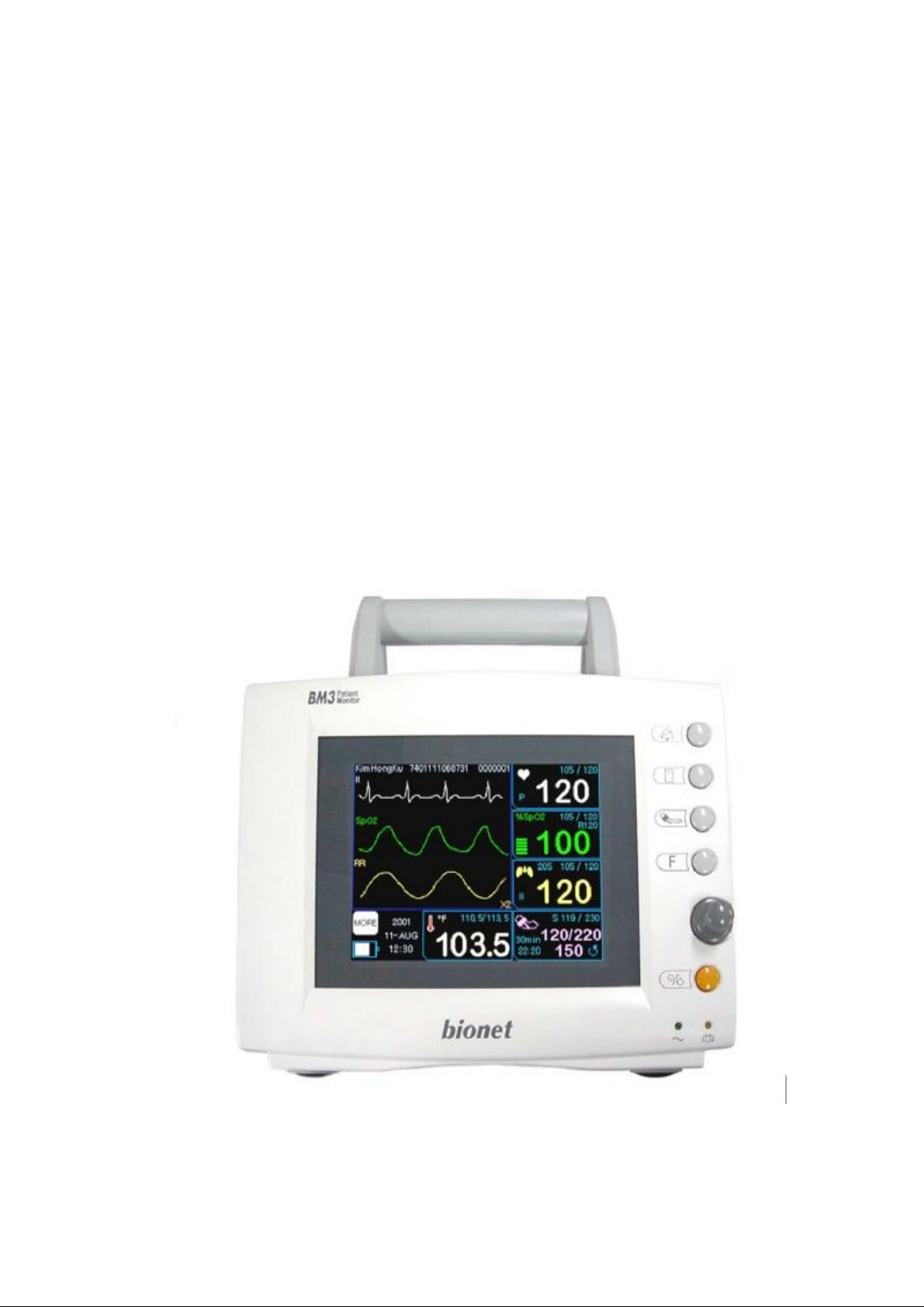
4. Equipment Overview
Overview of the Product
BM3 monitor is a product used for monitoring biological information and
Revision A
- 14 -

BM3 www.BIO2NET.com
occurrence of a patient. Main function
ns of the product include displaying information such as ECG, respiration,
SpO2, NIBP and temperature on its LCD screen and monitoring parameter,
and alarming. It also prints out waves and parameters via a printer.
Features of the Product
BM3 is a small-size multifunctional monitoring equipment for a patient
designed to an easy usage during movement. It features devices for auto
power supply (DC 10V-16V) and DC power supply (DC 18V) as well as
installing its handle to the patient’s bed. The equipment also measures major
parameters such as ECG, SpO2, NIBP, temperature and pulse, displaying it
on a 5.7-inch color LCD screen. It also enables users to check waves and
parameters and other vital signs of a patient via the 58mm thermal printer
and monitor the patient by the remote-controlled alarm system. It also
enables to build a central monitoring system by linking devices used for
separate patients so that one can monitor several patients at a time.
Warning
In order to avoid electrical shock, do not open the cover. Disassembling of the
Equipment have to be done only by the service personnel authorized by
Revision A
- 15 -
BM3 www.BIO2NET.com
BiONET Co. Ltd.
Warning
Users must pay attention on connecting any auxiliary device via LAN port or nurse
calling. Always consider about summation of leakage current, Please check if the
auxiliary device is qualified by IEC 60601-1, or consult your hospital biomedical
engineer
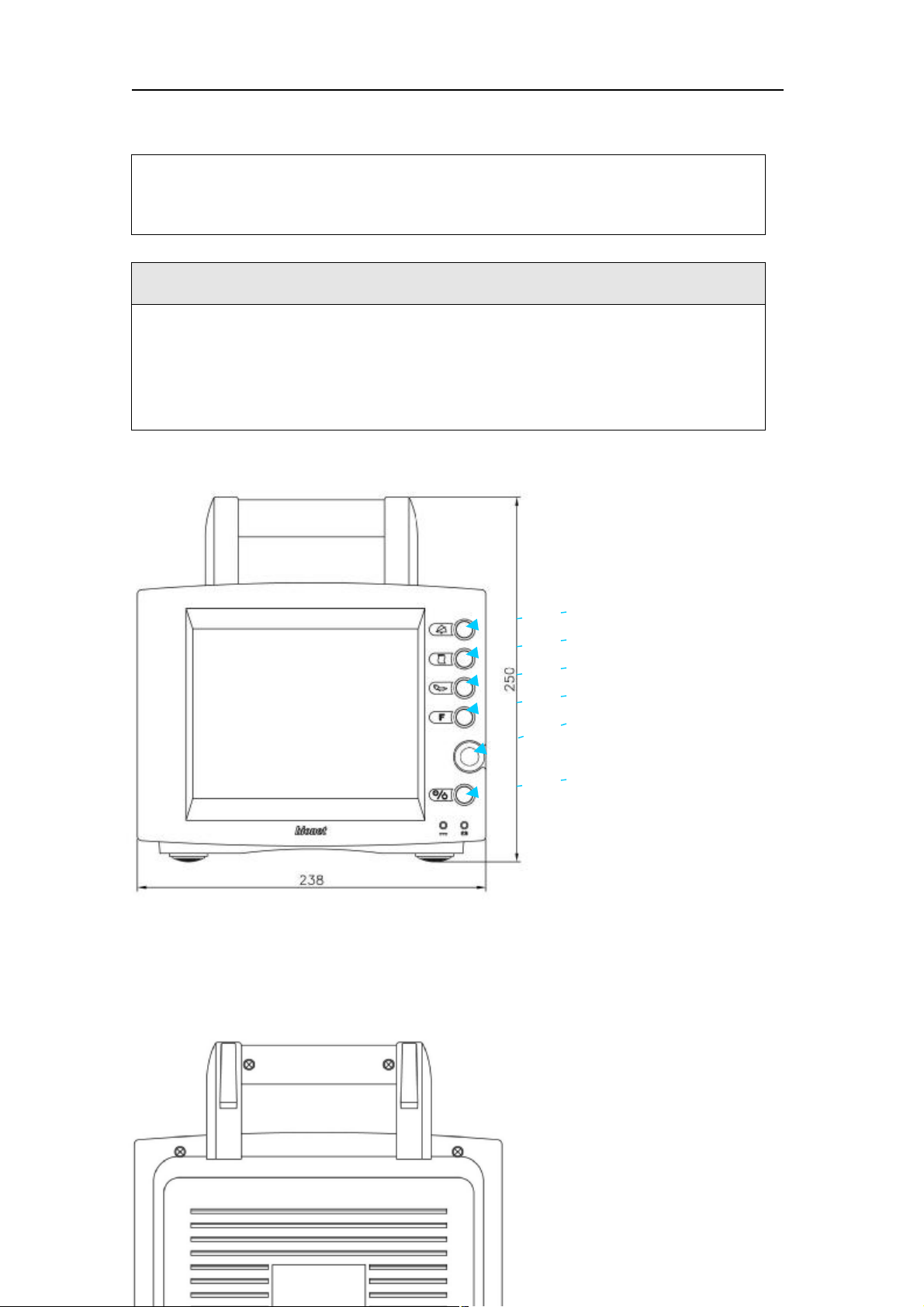
Features of Main Body
SILENCE ALARM KEY
PRINT GO/STOP KEY
NIBP GO/STOP KEY
FUNCTION KEY
TRIM KNOB CONTROL KEY
POWER KEY
Front
Revision A
- 16 -
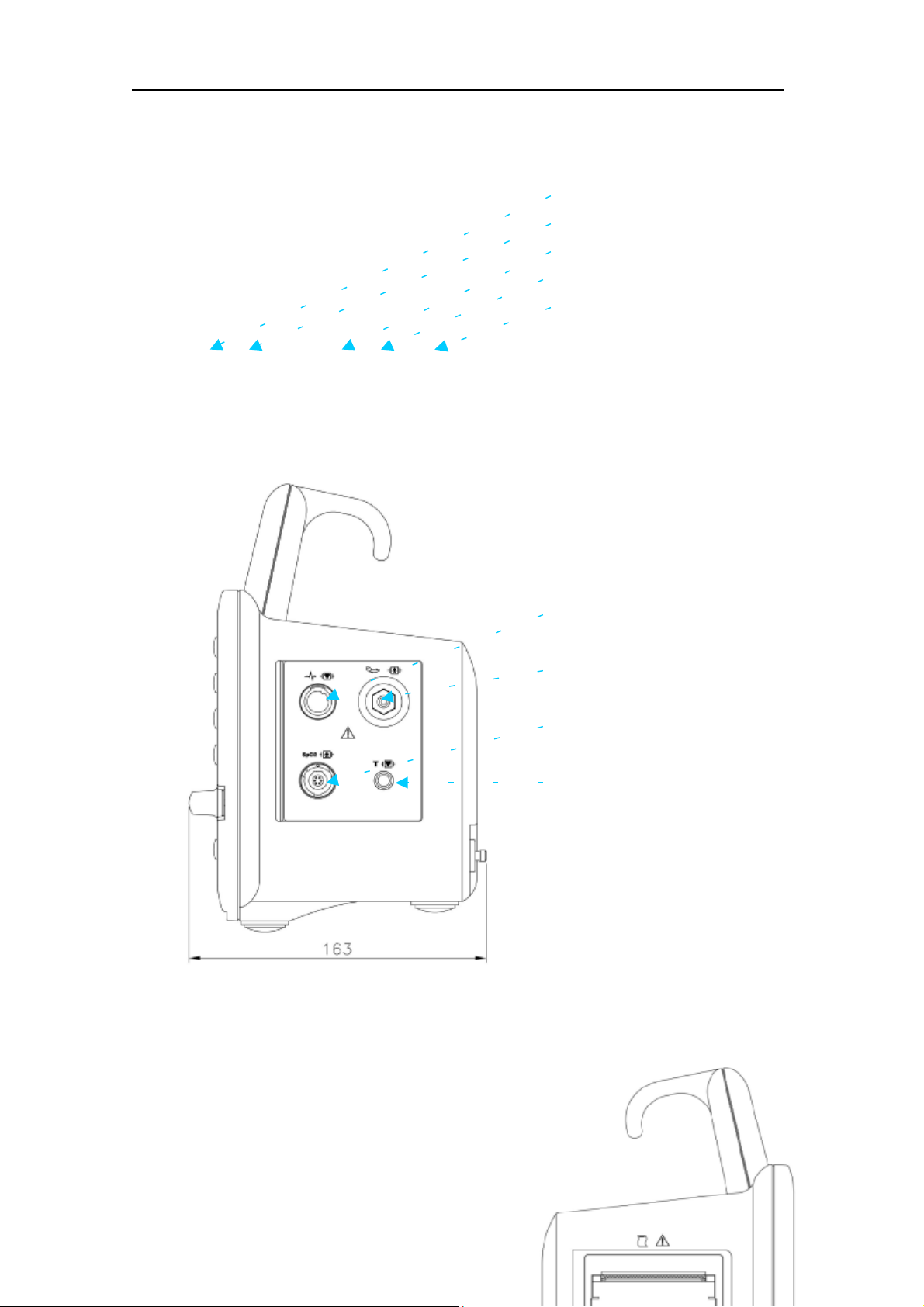
BM3 www.BIO2NET.com
Protective Ground Terminal
DC Power Input Port
Defib Sync
RJ45 LAN Port
RS-232C Serial Port
/Nurse Call
Back
ECG Probe Connecter
NIBP Probe Connecter
SpO2 Probe Connecter
TEMP Probe Connecter
▲ Right side ▼ Left side
Revision A
- 17 -

BM3 www.BIO2NET.com
PRINTER
Accessories
ECG Cable ECG wire
SpO2 Cable SpO2 Extention cable
Revision A
- 18 -
BM3 www.BIO2NET.com
♥
(option) Temperature Cable
NIBP CUFF TEMP(OPTION)
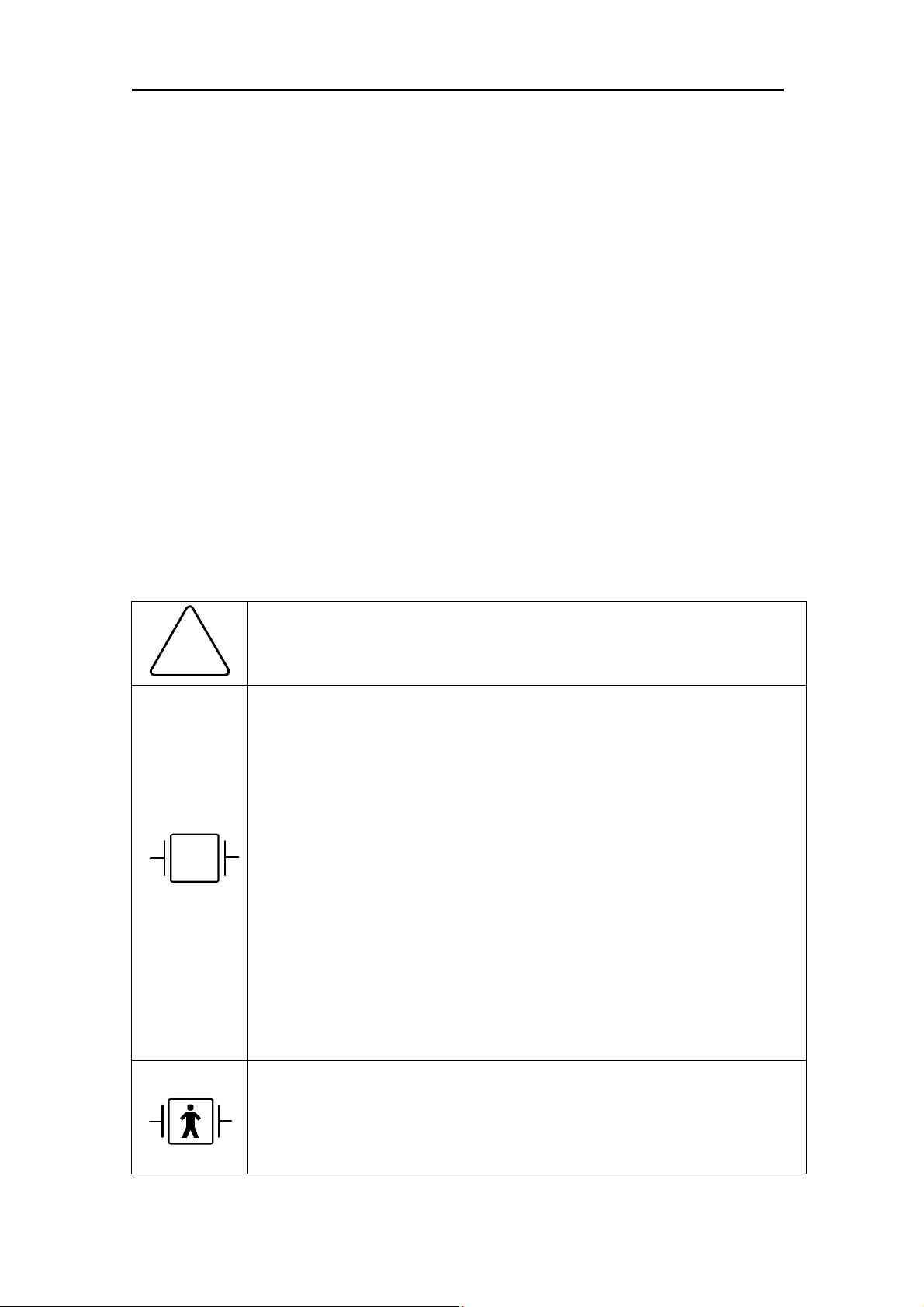
Equipment Symbols
ATTENTION : Consult accompanying documents
!
TYPE CF APPLIED PART : Insulated (floating) applied part
suitable for intentional external and internal application to the
patient including direct cardiac application. “Paddles” outside the
box indicate the applied part is defibrillator proof.
Medical Standard Definition : F-type applied part
(floating/insulated) complying with the specified requirements of
IEC 60601-1/UL 2601-1/CSA 601.1 Medical Standards to provide
a higher degree of protection against electric shock than that
provided by type BF applied parts.
TYPE BF APPLIED PART : I Insulated (floating) applied part
suitable for intentional external and internal application to the
patient excluding direct cardiac application. “Paddles” outside the
box indicate the applied part is defibrillator proof.
Revision A
- 19 -
 Loading...
Loading...