BEWELL CONNECT BW TSX Users manual
Manufacture:Nexmed Technology Co., LTD.
Address:2nd Floor of No.1 building, Jia An Technological Industrial
Park 67 District, Bao An 518101 Shenzhen - China BW-TSX
Web site: bewell-connect.com
All Rights Reserved.Rev.V1.0 © 2015, 20150805
1 1
BewellConnect - BW-TSX - User Manual - 072015 BewellConnect - BW-TSX - User Manual - 072015
0197
BW-TSX

This device complies with all the applicable standards and regulations related to expo-
The device complies with all the standards relating to Class ll electrical medical devices and to
devices which use electrical stimulation for use at home.
Electromagnetic compatibility (EMC) : The device complies with safety standards EMC
ISO 60601-1-2. It is designed to be used at home .
Dear customer,
Thank you for buying the Mytens device. We hope you get the best use out of it, and we
recommend that you read these instructions carefully so that you can use it as
device, please follow the
treatment instructions in this user manual when using the device.
CONTENTS
1. WARNINGS . . . .. . . .. . . . . . . . . .. . . .. . . .. . . .. . . .. . . .. . . . . . .. . . . . .. . . .. . . .. . . .. . .... 1
2. TRANSCUTANEOUS NEUROSTIMULATION INFORMATION . . . . . . .. . . . . . . . . .. . . . . . . 4
3. CHARACTERISTICS . . .. . . .. . ..................................................... 5
4. USE ............................................................................ 5
4.1 DESCRIPTION. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
4.2. PRECAUTIONS BEFORE USE .................................................... 7
4.3 PRESENTATION OF THE PROGRAMMES .. . . .. . . .. . . ............................. 7
4.3.1. FORMS OF WAVES . . . . . . . . ................................................... 7
4.3.2. TENS PROGRAMMES . . . . . . . . .. . . . . . . . . .. . . . . . . . .. . . . . .. . . . .. . . . ............. 9
....................
4.4. INDICATIONS BEFORE USE . . . .. . . . . . .. . . .. . . . . . .. . . ...........................
4.5. DOWNLOADING THE APPLICATION . .. . . .. . . .. . . .. . . ...........................15
4.6. STARTING AND USING . . .. . . .. . . ...................................... ........ 16
5. STORAGE AND MAINTENANCE . . . .. . . . . . .. . . .. . . .. . . .. . . ........................19
5.1. STORAGE OF THE ELECTRODES . . . .. . . .. . . .. . . .. . . .. . . .. . ......................19
5.2. CLEANING . . . . . . . . . .. . . . . . . . .. . . . . .. . . . . . . . .. . . . . .. . . . . . . . .. ..................19
6. TROUBLESHOOTING GUIDE . . . . .. . . .............................................20
7. AVOIDING MALFUNCTIONS . . . . .. . . .............................................20
8. TECHNICAL CHARACTERISTICS .. . . .. . . .. . . .. . . .. . . .. . . .. ........................21
9. PLACING THE ELECTRODES . . . .. . . .. . . .. . . .. . . .. . . . . . .. . . .. . . . . . .. . . . . . ..........22
10. SAFETY PRECAUTIONS
11. DISPOSAL . . . . .
...................................................... .... 26
................................................. .............. 26
12. PLACING THE ELECTRODES .. . . .. . . .. . . .. . . .. . . .. . . .. . . .. . . .. . . .. .............. 27
12
.................... .....
............. ..............29
13.
WARRANTY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .... ..............30
without prior notice.
1. WARNINGS
- Please read this manual carefully and always follow the treatment instructions.
- Only use this device for its intended purpose as described in these instructions.
- This device may be used for personal use at home.
- Use this device in an ambient temperature range of 5 to 40°C.
- Do not expose this device to extreme temperature conditions > 50°C or < -10°C.
- Do not use this device at a relative humidity of over 93%.
- This device must always be placed in a clean and dry place.
- Do not expose this device to lint,dust, sunlight or water.
- Do not expose this device to electric shocks.
11
14
- Never drop the device.
- Do not attempt to open the device. In case of problems, contact your dealer.
- This is a medical device. Keep out of the reach of children to avoid inhalation or swallowing of
small parts..
- Discontinue use of the device in case of anomalies or malfunction.
- Electronic medical equipment requires special precautions regarding electromechanical compatibility. It must be installed and used in accord with EMC (ElectroMagnetic Compatibility)
information.
- This device must not be used in an environment of strong electromagnetic interference: Near a TV,
a microwave oven or a mobile phone in use, etc.
- This device is not designed to be used by persons (including children) whose physical, sensory
or mental capabilities are reduced, or persons without experience or knowledge, unless they
surveillance or prior instructions concerning use of the device. It is possible that they will not
be able to use it in accordance with the instructions of this user manual and be disturbed by
27
the treatment.
- This product is not designed for use on children.
- Do not modify the device or the electrodes without authorization of the manufacturer.
This could cause a malfunction.
- This device is designed for use by a single person and one person only.
- Do not maintain or service the device while the device is in use.
1
2

-Pads should not touch each other when placed onto your skin.
-The materials (e.g. ABS) of expect contact with patient had passed the ISO 10993-5 and ISO
10993-10 standards test, no toxicity, allergy and irritation reaction. However, based on the
current science and technology, other potential allergic reactions are unknown.If you have
allergic reaction to materials, please stop treatment immediately and consult your physician.
-Keep the device out of the reach of children and pets to avoid inhalation or swallowing of small
parts. Do not allow children to take their temperatures unattended. Children may not be able
to use the device according to the instructions in this user manual. It is not a toy.
-TENS is not eective for pain of central origin, including headache;
-TENS is not a substitute for pain medications and other pain management therapies;
-TENS devices have no curative value;
-TENS is a symptomatic treatment and, as such, suppresses the sensation of pain that would
otherwise serve as a protective mechanism;
-Eectiveness is highly dependent upon patient selection by a practitioner qualied in the
management of pain patients;
-Use this device only with the self-adhesive hydrogel electrodes,the adapter and USB cord
supplied with the device,which proved by manufacturer.
-This device complies with Part 15 of the FCC Rules. Operation is subject to the following two
conditions: (1) this device may not cause harmful interference, and (2) this device must accept
any interference received, including interference that may cause undesired operation.
Do not treat the the following parts or areas :
-If you are in the care of a physician, consult with your physician before using this device;
-If you have had medical or physical treatment for your pain, consult with your
physician before using this device;
-If your pain does not improve, becomes more than mild, or continues for more than
ve days, stop using the device and consult with your physician;
-The long-term eects of electrical stimulation are unknown;
- D
o not apply stimulation on your torso. Indeed, the introduction of electric current on this area
can cause heart rhythm disturbances, with a risk of death.
-
be avoided.
-
on your blood pressure or your heart rhythm.
-Do not apply stimulation over your neck because this could cause severe muscle spasms
resulting in closure of your airway, diculty in breathing, or adverse eects on heart rhythm
or blood pressure;
-Do not apply stimulation over, or in proximity to, cancerous lesions;
- Do not position the electrodes on broken or injured skin, or which is dirty or unhealthy. Skin
with irritation, sores or other lesions can lead to the injection of too much current on the area,
which can cause burns.
- Do not place the electrodes near cancerous lesions because this may have a negative impact
on these injuries.
- Do not place the electrodes on skin areas whose sensations are not normal. You may burn
yourself due to a lack of feeling of the high intensity of the current.
-
(eg. phlebitis, thrombophlebitis and varicose veins). Stimulation should not be performed on
areas of thrombosis or thrombophlebitis because it can promote the circulation and lead to a
greater risk of embolism.
- Do not put the electrodes on redness or open wounds. Open wounds may lead to applying too
much current on the zone, causing burns. They can also favour the penetration of substances
from the electrode into the skin.
- Do not place the electrodes on the inside of body cavities, such as in the mouth. Indeed, this
device is only designed for external application.
-
Do not make sudden movements during a session. This could cause a dysfunction of the device.
-
-
Do not place the electrodes directly on the eyes,chest and the upper back or crossing over the
heart.
Do not use the device in the following conditions :
- Do not use the device if you are connected to high frequency surgical equipment. This could
lead to burns on the skin under the electrodes and damage the device.
- Do not use the electro-stimulator if you are monitored by a doctor and you have not consulted
him before using it.
- In the case of internal bleeding due to impacts or injury, do not use the device.
- To contract a muscle, do not use the electrical muscle stimulation in case of risk of muscle
contraction that can disrupt the healing process. If the tendon or the muscle is torn, a muscle
contraction can aggravate the wear, like a voluntary contraction. After recent surgery, after an
acute trauma or fracture, this situation can also happen. In case of occurrence of a tendonitis, a
muscle contraction can also aggravate the symptoms.
- Do not use the device while driving, operating the machines or any other activity during which
the electrical stimulation may lead to a risk of injury.
- Do not use the appliance if you are subject to falling asleep during the session, as this may
cause you to feel pain too late. If using at the time of goign to bed, set the timer so that the
- Never use the device in co nt ac t with water (in the bathroom, in the shower or in the pool, etc. )
because this increases the risk of an electric shock and skin burns.
3
4

2. TRANSCUTANEOUS NEUROSTIMULATION INFORMATION
The device works on the principle of transcutaneous electrical neurostimulation (TENS),
which enables you to relieve pain and soothe muscle tension. This device also helps to promote
venous return and to strengthen the muscle mass. The device has been specially designed to be
used at home. Transcutaneous electrical neurostimulation (TENS) is a non-analgesic drug therapy
the nerves. This stimulation brings the body to produce and disseminate endogenous analgesicpainkilling substances (endorphins, enkephaline) whose function is to anesthetize the pain.
In case of pain or muscle tension, mini-electrical impulses in the nervous tissue may block the
transmission of pain signals to the nervous system and trigger the release of endorphins. You
can choose your programme among 11 preset TENS protocols on theapplication.
For the strengthening of your muscle mass, you can select a programme among 8 protocols
of electrical muscle stimulation (EMS). The electrical stimulator sends, via the electrodes placed
on the area to stimulate, electrical pulses causing a muscle contraction which strengthens the
muscle. It is controlled from the application. A diagram of the human body with treatment
zones helps you to position the electrodes correctly (see paragraph 10).
The application (Mytens) is available on the App Store or Google play. The app operates on IOS,
Android platforms (IOS 8.0, Android 5.0).
Intended use
The device designed to be used for temporary relief of pain associated with sore and aching
muscles in the shoulder, waist, back, neck, upper extremities (arm), and lower extremities (leg)
due to strain from exercise or normal household work activities. It should be applied to normal,
healthy, dry and clean skin of adult patients. And is to be used for stimulate healthy muscles in
order to improve and facilitate muscle performance.
Contraindication
Do not use this device with the following medical devices:
-Implanted electronic medical devices, such as pacemakers. This may cause electric shock, burns,
or death.
-Electronic life support equipment, such as respirators.
-Electronic medical devices worn on the body, such as electrocardiographs.
If you use this device together with other electronic medical devices, these devices may not work
correctly.
Counter indications
Do not use the device :
- In case of heart disease
- If you have epilepsy
-
example a drug administration system). In these conditions, use could cause an electric shock,
interference, burns or even death.
-
-
tus are not yet known. During pregnancy, do not use the appliance on the uterus or abdomen
to avoid triggering of contra
ctions. Always consult your doctor or midwife if you are pregnant
and intend to use the device.
-
cal muscle stimulation (EMS) device could in this case cause a blood clot.
- In case of cognitive impairment
- - If your pains have not been diagnosed, with the exception of the positive opinion of your doctor to use this device
-if stimulation is applied over the menstruating or pregnant uterus;
-if stimulation is applied over areas of skin that lack normal sensation
FCC
Caution: The user is cautioned that changes or modications not expressly approved by the party
responsible for compliance could void the user's authority to operate the equipment.
This device complies with Part 15 of the FCC Rules. Operation is subject to the following two conditions:
(1) this device may not cause harmful interference, and (2) this device must accept any interference
received, including interference that may cause undesired operation.
NOTE: This equipment has been tested and found to comply with the limits for a Class B digital device,
pursuant to Part 15 of the FCC Rules. These limits are designed to provide reasonable protection
against harmful interference in a residential installation. This equipment generates, uses and can
radiate radio frequency energy and, if not installed and used in accordance with the instructions, may
cause harmful interference to radio communications. However, there is no guarantee that interference
will not occur in a particular installation.
If this equipment does cause harmful interference to radio or television reception, which can be
determined by turning the equipment o and on, the user is encouraged to try to correct the
interference by one or more of the following measures:
-- Reorient or relocate the receiving antenna.
-- Increase the separation between the equipment and receiver.
-- Connect the equipment into an outlet on a circuit dierent from that to which the receiver is
connected.
-- Consult the dealer or an experienced radio/TV technician for help.
FCC Radiation Exposure Statement:
This equipment complies with FCC radiation exposure limits set forth for an uncontrolled environment.
This transmitter must not be co-located or operating in conjunction with any other antenna or
transmitter.
.
5
6
- During the EMS session or shortly after, redness of the skin may occur at the level of the electrodes (around or below). They usually disappear within two hours of the session. In case of
persistence of the redness for more than 24h, please consult your doctor.
- An electrical muscle stimulation (EMS) session can cause muscle pain for some time.
Reactions
-
fects may occur in the form of :
- Nausea or fainting
- Burns at the location of the electrodes
- Painful sensations, headache
- Irritation of the skin in the place of the electrodes, even if the gel present on the electrodes
does not present any known risk of allergic reactions to this day.
-You should stop using the device and should consult with your physician if you experience
adverse reactions from the device
3. CHARACTERISTICS
- Pain relief and healing of muscle tension (TENS : 11 preset programmes)
- Strengthening of the muscular mass (EMS : 8 standard programmes)
- Stimulation of the venous return (TENS)
- Easy to use
- Data transfer onto a phone or a Bluetooth 4.0 tablet
4. USE
4.1 DE
SCRIPTION
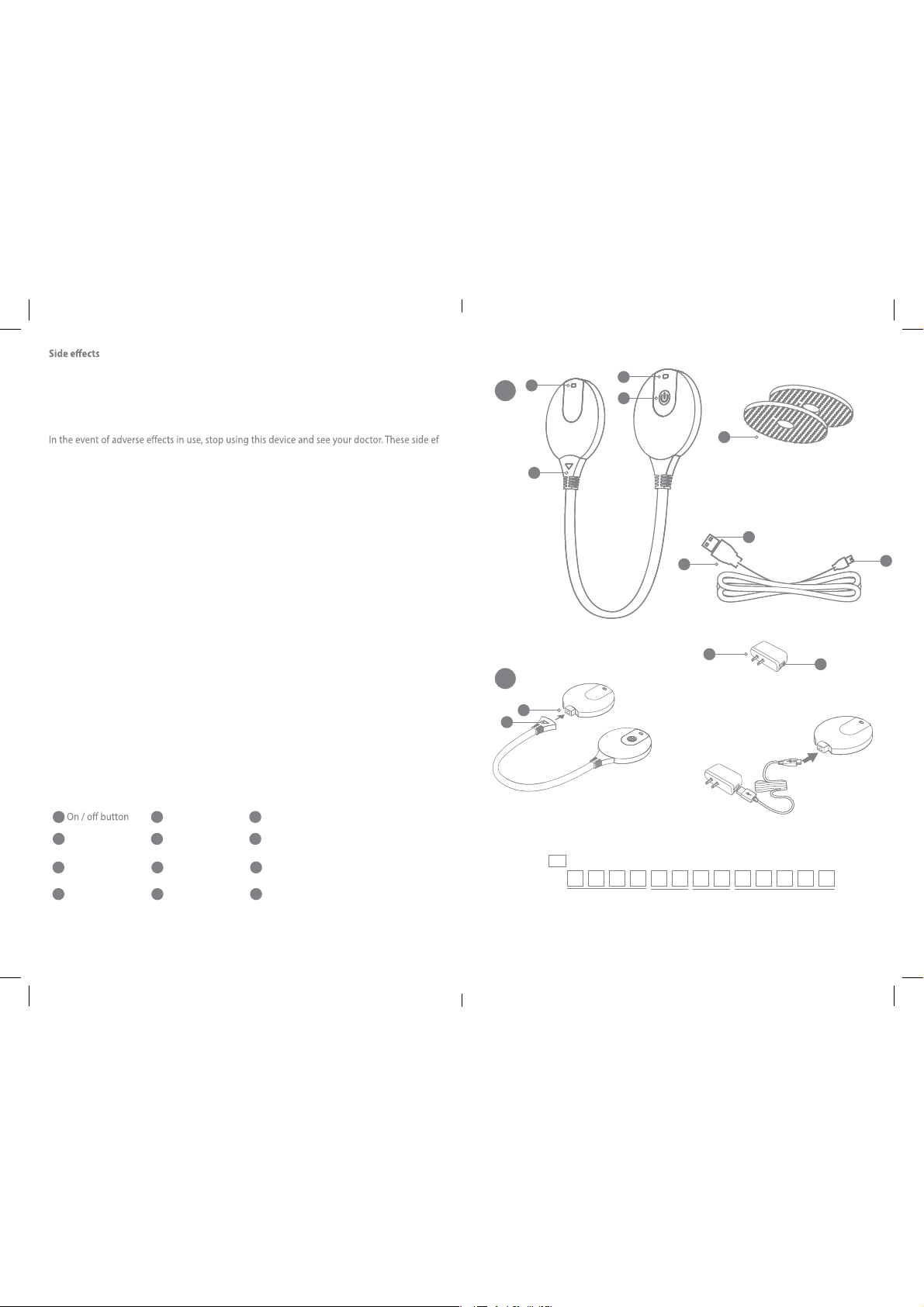
The gift box contains the follwing conponents:
Standard Parts:
NO. Description Quantity
1 BW_TSX 1PCS
2 Electrode pad(50*56mm) 2pcs
3 Adaptor 1PCS
4 Micro-USB cable 1PCS
5 Instruction manual 1PCS
See schema in page 8
1
4
Micro-USB socket
of the device
7
Adaptor
10
Standard USB plug
2
Status indicator
5
ctrodes
Ele
8
Battery indicator
11 12
Micro-USB plug Detachable connector
3
Removable connector
6
Micro-USB cable
9
Socket for standard USB plug
See schema A page 6
8
A
4
Micro-USB socket
of the device
5
3
B
4
12
Serial number
SN
SN:
Year
2
5
Status indicat
2
1
Electrodes
Month
5
10
6
7
Day
Serial number
9
11
87
4.2. PRECAUTIONS BEFORE USE
g
- Use the transcutaneous electrical neurostimulation programmes in bursts (TENS Burst) at rest.
Indeed, they can cause a muscle contraction in the treatment area.
- In case of recent surgery, consult a doctor before using the device. The use of this device may
- It is necessary to complete the treatment in the
of the device before removing the electrodes. If you do not complete the treatment and
remove the electrodes or the device, and if you touch the connectors, it is possible to feel pain
- Before any use of electrodes, check their expiration date on the packaging. If the date has
expired, do not use them.
- Use new electrodes when the electrodes are damaged, dirty, less adhesive or if you begin to
feel discomfort during stimulation (uncomfortable pinching, tingling).
- This product must only be used with the adapter, the cable and accessories recommended by
the manufacturer.
- Before use, check that the device and the electrodes are not damaged. In case of any damage,
do not use the device or one of the electrodes.
- Usable indoors and outdoors, the device is not designed to withstand all weather conditions.
- The device is not waterproof. Do not wet it and never use it in a damp environment.
- Do not use plaster or tape to attach the electrodes to the skin.
- For storing the electrodes, please follow the instructions in section 6.1 “Storage of electrodes”.
-
In case of abnormal operation of the device in
accordance with this user manual, discontinue
use and contact the after-sales department. Consult section 7 “Troubleshooting Guide” for in
formation concerning potential malfunctions of the device.
-
The patients can operate the equipment by themselves. The patient can safely use the all the
functions of the device. They can charge the device, cleaning the device and accessories by
themselves.
The patient should contact the MANUFACTURER or the MANUFACTURER'S representative:
For assistance, if needed, in setting up, using or maintaining the ME EQUIPMENT
or ME SYSTEM; or
-
To report unexpected operation or events.
4.3 PRESENTATION OF THE PROGRAMMES
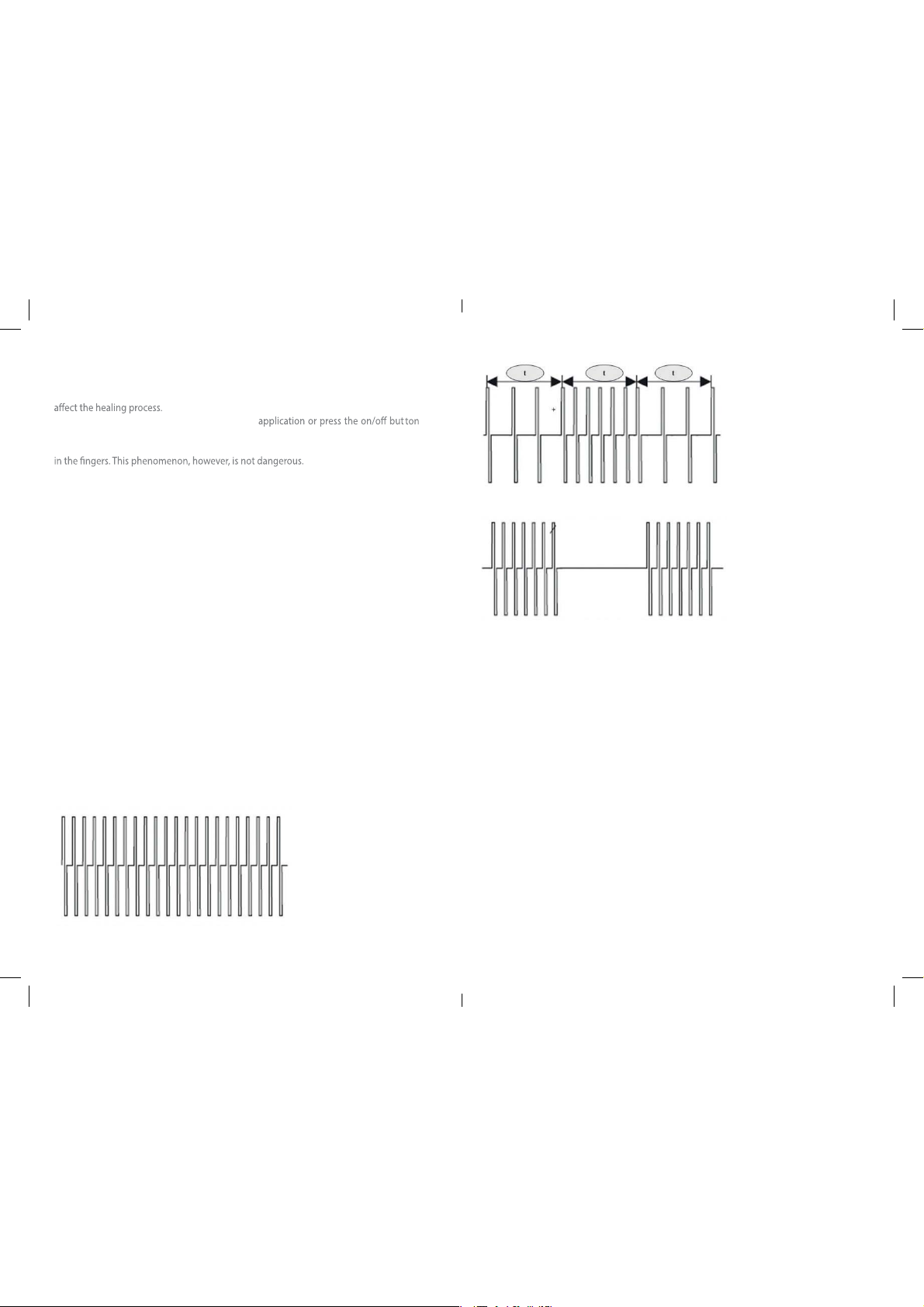
4.3.1. FORMS OF WAVES
CONT : Form of continuous wave
HANS : Form of alternating wave
BURST : Wave form deviation
9 10
 Loading...
Loading...