Page 1

ULTRASOUND TRANSDUCER
LEAKAGE TESTER
ULT-2000 SERIES
USER MANUAL
Page 2

Page 3
i
BC BIOMEDICAL
ULT-2000 SERIES
TABLE OF CONTENTS
WARNINGS, CAUTIONS, NOTICES .............................................................................. ii
DESCRIPTION .............................................................................................................. 1
BACKGROUND ............................................................................................................. 4
TEST PROCESS ........................................................................................................... 8
LAYOUT ........................................................................................................................ 9
KEYS ............................................................................................................................ 10
SCREENS ................................................................................................................... 11
SETUP ......................................................................................................................... 18
PC SOFTWARE ........................................................................................................... 21
COMMUNICATION PROTOCOL ................................................................................. 27
MANUAL REVISIONS .................................................................................................. 30
LIMITED WARRANTY ................................................................................................. 30
SPECIFICATIONS ....................................................................................................... 31
NOTES ......................................................................................................................... 33
Page 4
ii
WARNING - USERS
The ULT-2000 is for use by
skilled technical personnel only.
WARNING - USE
The ULT-2000 is intended for testing only and
should never be used in diagnostics, treatment
or any other capacity where it would come in
contact with a patient.
WARNING - CONNECTIONS
All connections to patients must be removed
before connecting the DUT to the ULT-2000. A
serious hazard may occur if the patient is
connected when testing with the ULT-2000.
Do not connect any leads from the patient
directly to the ULT-2000 or DUT.
WARNING – POWER ADAPTER
Remove power before
cleaning the surface of the ULT-2000.
WARNING - LIQUIDS
Do not submerge or spill liquids on the ULT-2000.
Do not operate the ULT-2000 if internal
components may have been exposed to fluid.
WARNING - MODIFICATIONS
The ULT-2000 is intended for use within the
published specifications. Any application
beyond these specifications or any unauthorized
user modifications may result in hazards or
improper operation.
Page 5
iii
WARNING - VOLTAGE
High Voltages are generated by the ULT-2000
when running tests. Do not touch any surface
that is in contact with or connected to the ULT-
2000, including the ultrasound transducer,
adapter, conductivity probe, basin or liquid
medium contained in the basin, as it might be at
an electrical potential of 90 to 275 VAC.
CAUTION - SERVICE
The ULT-2000 is intended to be serviced only by
authorized service personnel. Troubleshooting
and service procedures should only be
performed by qualified technical personnel.
CAUTION - ENVIRONMENT
Exposure to environmental conditions outside
the specifications can adversely affect the
performance of the ULT-2000. Allow ULT-2000 to
acclimate to specified conditions for at least 30
minutes before attempting to operate it.
CAUTION - CLEANING
Do not immerse. The ULT-2000 should be
cleaned by wiping gently with a damp, lint-free
cloth. A mild detergent can be used if desired.
CAUTION - INSPECTION
The ULT-2000 should be inspected before each
use for obvious signs of abuse or wear. The
ULT-2000 should not be used and should be
serviced if any parts are in question.
Page 6
iv
NOTICE – CE
The ULT-2000 Analyzers bear the mark
Based on the following testing standards:
ELECTROMAGNETIC COMPATIBILITY DIRECTIVE
EMC – Directive 89/336/EEC and 2004/108/EC as amended by
92/31/EEC, 93/68/EEC and Directive 91/263/EEC [ TTE/SES ]
EN 61326-1:1997 + A1:1998 + A2:2001 + A3:2003
“Electrical equipment for measurement, control and
laboratory use – EMC requirements”
This equipment has been type tested and compliance was demonstrated
to the above standard to the extent applicable.
EMISSIONS
Radiated and Line Conducted Emissions
EN 61000-3-2 Harmonic Current Emissions
EN 61000-3-3 Voltage Fluctuation and Flicker
IMMUNITY– CLASS C
EN 61000-4-2 Electrostatic Discharge
EN 61000-4-3 Radiated Electric Field Immunity
EN 61000-4-4 Electrical Fast Transients / Bursts
EN 61000-4-5 Surge Voltage
EN 61000-4-6 Conducted Disturbance
EN 61000-4-11 Voltage Dips and Short Interrupts
LOW VOLTAGE DIRECTIVE
EC – Directive 73/23/EC
EN 61010-1:2001
“Safety requirements for electrical equipment for measurement, control, and
laboratory use – General requirements”
This equipment has been type tested and compliance was demonstrated
to the above standard to the extent applicable.
Page 7

v
NOTICE – SYMBOLS
Symbol
Description
Caution
(Consult Manual for Further Information)
Electrical Caution
(Consult Manual for Further Information)
Center Negative
(Refers to Battery Eliminator Connector)
Per European Council Directive 2002/95/EC, do not
dispose of this product as unsorted municipal
waste.
NOTICE – ABBREVIATIONS
ANSI
American National Standards Institute
C
Celsius
°
degree(s)
DC
Direct Current
DUT
Device Under Test
Euro
European
FS
Full Scale
Hz
Hertz
kg
kilogram(s)
µA
microampere(s)
mA
milliampere(s)
mm
millimeter(s)
NEDA
National Electronic Distributors Association
Ω
ohm(s)
PC
Personal Computer
Lbs
pounds
sec
second(s)
TEE
Transesophageal Echocardiography
ULT
Ultrasound (Transducer) Leakage Tester
USA
United States of America
V
Volt(s)
VAC
Volts Alternating Current
VDC
Volts Direct Current
Page 8

vi
NOTICE – DISCLAIMER
BC GROUP INTERNATIONAL, INC. RESERVES THE RIGHT TO
MAKE CHANGES TO ITS PRODUCTS OR SPECIFICATIONS AT
ANY TIME, WITHOUT NOTICE, IN ORDER TO IMPROVE THE
DESIGN OR PERFORMANCE AND TO SUPPLY THE BEST
POSSIBLE PRODUCT. THE INFORMATION IN THIS MANUAL HAS
BEEN CAREFULLY CHECKED AND IS BELIEVED TO BE
ACCURATE. HOWEVER, NO RESPONSIBILITY IS ASSUMED
FOR INACCURACIES.
NOTICE – CONTACT INFORMATION
BC BIOMEDICAL
BC GROUP INTERNATIONAL, INC.
3081 ELM POINT INDUSTRIAL DRIVE
ST. CHARLES, MO 63301
USA
1-800-242-8428
1-314-638-3800
www.bcgroupintl.com
sales@bcgroupintl.com
NOTICE – DISCLAIMER
BC GROUP INTERNATIONAL, INC. WILL NOT BE RESPONSIBLE
FOR ANY INJURIES SUSTAINED DUE TO UNAUTHORIZED
EQUIPMENT MODIFICATIONS OR APPLICATION OF EQUIPMENT
OUTSIDE OF THE PUBLISHED INTENDED USE AND
SPECIFICATIONS.
ULT-2000 Series User Manual Copyright © 2012
www.bcgroupintl.com Made in the USA
10/12 Rev 13
Page 9
1
BC BIOMEDICAL
ULT-2000 SERIES
ULTRASOUND TRANSDUCER LEAKAGE TESTER
The Model ULT-2000 Series is a family of Microprocessor based, Ultrasound Transducer
Leakage Testers. The ULT-2010 measures both the conductivity of the cleaning medium
and the leakage current of the ultrasound transducer. The ULT-2020 offers the same
features of the ULT-2010, plus a Meter mode and Data Logging.
The following are highlights of some of the main features:
ULT-2010 (BASIC FEATURES):
GRAPHICAL LCD DISPLAY WITH CURSOR SELECTION OF OPTIONS
AND SETUP OF PARAMETERS
USER-SELECTABLE SOURCE (CHALLENGE) VOLTAGE
(90 TO 275 VAC) & FREQUENCY (50 OR 60 Hz)
USER-SELECTABLE TEST LIMITS BY ULTRASOUND TRANSDUCER
MANUFACTURER AND MODEL
AUTO RANGING WITH 10, 250, OR 500 A FS RANGES
AUTOMATIC INTERNAL SELF TEST
SINGLE BUTTON PRESS FOR FULL SYSTEM TEST
SIMPLE PASS / FAIL MODE – FOR NON-TECHNICAL USERS
ANALYTICAL MODE – FOR TECHNICAL USERS
DIGITAL CALIBRATION – NO POTS TO TURN
USER-SELECTABLE DISPLAY OPTIONS
BATTERY LIFE DISPLAY (0 to 100%)
PROGRAMMABLE BACKLIGHT TIMER
CONTRAST IS SOFTWARE ADJUSTABLE
FLASH UPGRADEABLE FIRMWARE
RS232 INTERFACE
PC SOFTWARE (LIMIT CONFIGURATION TOOL)
SERIAL PRINTER OUTPUT WITH PROGRAMMABLE USER HEADER
REAL TIME CLOCK ALLOWING TEST RECORDS TO HAVE A TIMESTAMP
COMPATIBLE WITH DALE® TECHNOLOGY DALE800® AND FLUKE®
BIOMEDICAL ULT-800® ULTRASOUND TRANSDUCER ADAPTERS AND
DUAL CONDUCTIVITY PROBES
ULT-2020 (METER, DATALOG)
HAS ALL THE BASIC MODEL FEATURES PLUS:
METER MODE FOR EXTENDED MEASUREMENT PERIODS
PROGRAMMABLE METER SOURCE (CHALLENGE) VOLTAGE AND
FREQUENCY
Page 10
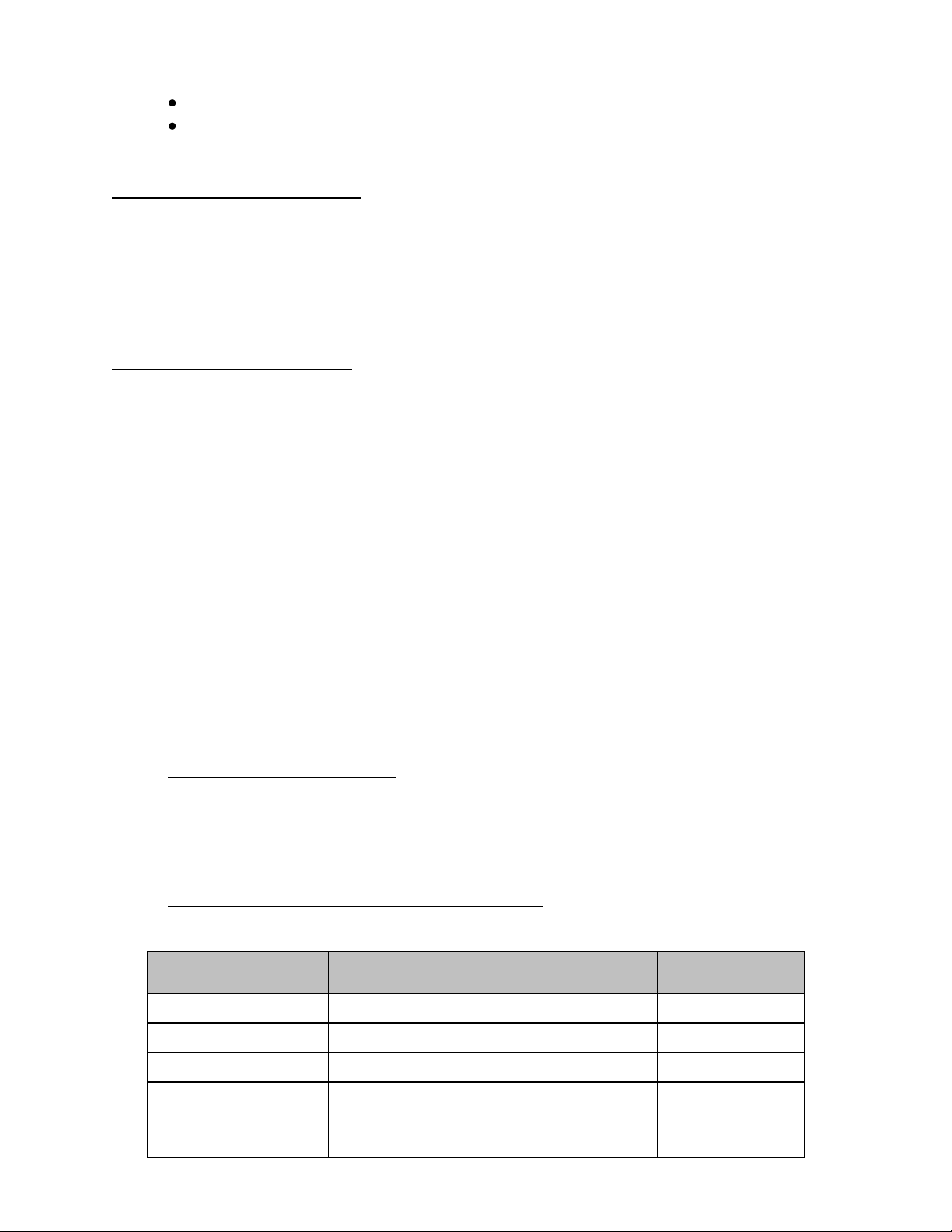
2
PROGRAMMABLE METER TIMER
TRANSDUCER
MANUFACTURER
MODEL
ADAPTER
PART NUMBER
Acuson / Siemens
8v5, 15l8w, V5M, V7M, 3V2c
ULT-PA-10
Acuson / Siemens
ALL 260-pin Transducers
ULT-PA-12
Acuson / Siemens
ALL 156-pin Transducers
ULT-PA-13
Aloka
UST-934N/945BP, ASU-32-3-M,
ASU-32-WSJ, UST-556/5512,
UST-5514DTU
ULT-PA-22
DATALOG WITH STORAGE OF 99 TEST RECORDS
STANDARD ACCESSORIES:
BC20-21103 BATTERY ELIMINATOR (USA Version)
(OR)
BC20-21106 BATTERY ELIMINATOR (Euro Version)
BC20-41357 PC SOFTWARE (LIMIT CONFIGURATION TOOL)
OPTIONAL ACCESSORIES:
BC20-30106 CASE, SMALL SOFT SIDED CARRYING(Instrument only)
BC20-30107 CASE, MEDIUM SOFT SIDED CARRYING(Instrument and printer)
BC20-40614 BATTERY ELIMINATOR, 220V (US Version)
BC20-41337 RS-232 COMMUNICATIONS CABLE (7 Pin Mini-Din to DB-9F)
BC20-41339 USB COMMUNICATION CABLE ADAPTER (USB to DB-9M)
(For use with BC20-41337)
BC20-42200 CIDEX® COMPATIBLE TEST BASIN
BC20-42300 EXTERNAL PRINTER
BC20-42310 PACKAGE OF 5 ROLLS OF PAPER FOR BC20 – 42300
BC20-42322 CABLE, CONDUCTIVITY TEST FIXTURE
(For use with ULT-TF-T2)
BC20-42324 CABLE, BATH TEST FIXTURE (For use with ULT-TF-T2)
BC20-42330 Print Kit (Includes 1: BC20-42300, BC20-42310, BC20-42321,
BC20-30107)
BC20-42331 ULT Kit (Includes 1: BC20-30106, BC20-41337, BC20-41339
ULT-TF-T2 ULT Test Box (Simulates conductivity and leakage current)
CONDUCTIVITY PROBES: (not included, order separately)
ULT-PC-10 DUAL CONDUCTIVITY PROBE (Short)
ULT-PC-15 DUAL CONDUCTIVITY PROBE (Medium)
ULT-PC-20 DUAL CONDUCTIVITY PROBE (Short & Long)
ULTRASOUND TRANSDUCER ADAPTERS: (not included, order separately)
Page 11
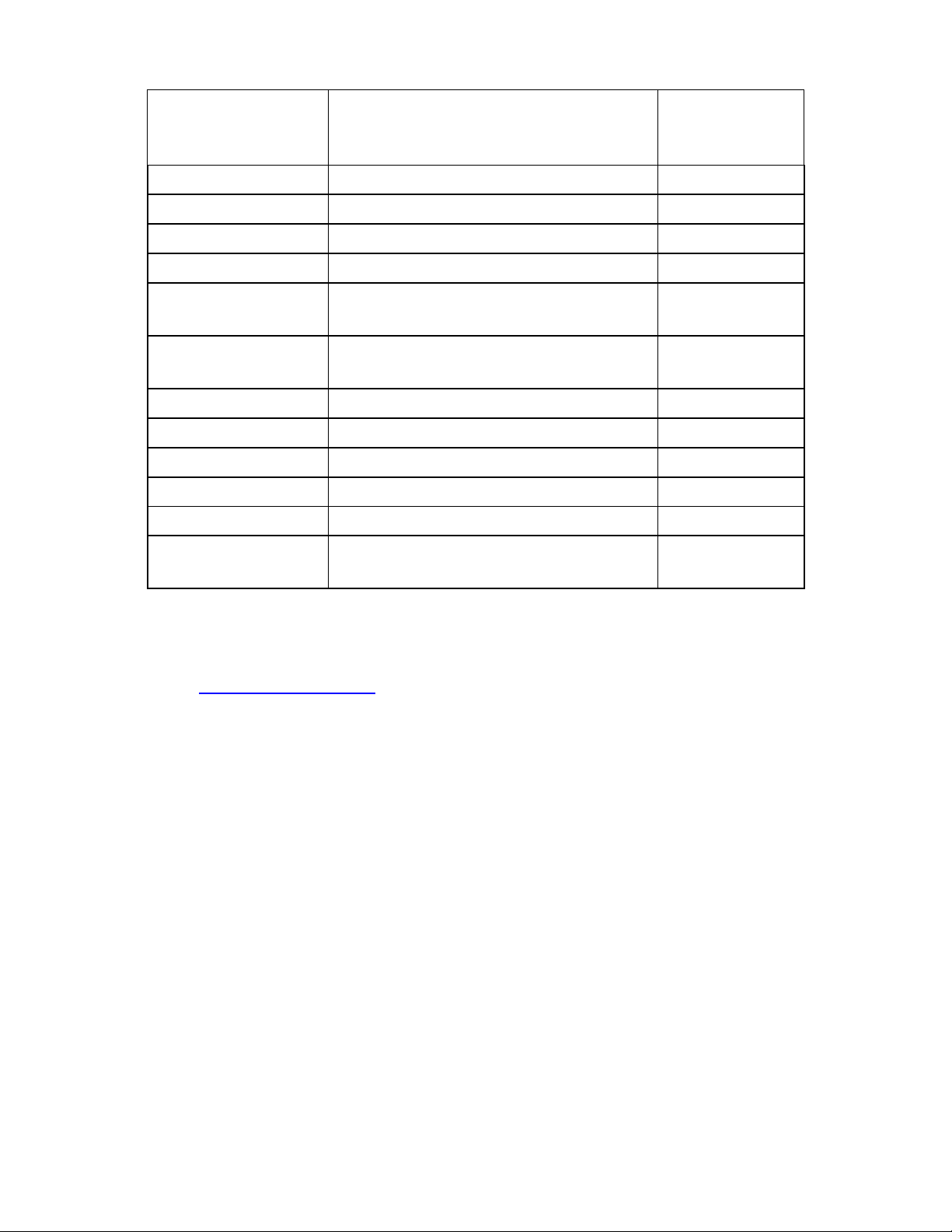
3
ATL / Philips
ATL C9-5 curved array; ATL l7-4
linear array; ATL UM9HDI; ATL HDJ
3000, 3500, 5000; ATL MPT74
ULT-PA-14
ATL / Philips
L7-4 Linear Array
ULT-PA-24
GE
Logiq; Vivid 3, 5, 7, 6T, 9T; P9603AU
ULT-PA-16
GE
Logiqbook, and VIVID I
ULT-PA-25
GE
46-280678P1
ULT-PA-27
Hitachi
Hi Vision 900, 5500, 6500, 8500,
EUB-2000, EUB-525, EUB-405 Plus
ULT-PA-21
Philips
iE33, iU22, S7-2, S7-3t, S3-1, C8-4v,
C9-5
ULT-PA-17
Philips / HP
Philips / HP 4500; Sonos 5500 / 7500
ULT-PA-18
Philips
Cx50 Series
ULT-PA-19
Sonosite
ALL
ULT-PA-11
Toshiba
Aplio XG, Xaria, Nemio XG, Fanio
ULT-PA-20
Zonare
E9-4
ULT-PA-29
All Manufacturers
Universal Pen Style
(For small surface area testing)
ULT-PA-23
For compatibility with specific manufacturer and model ultrasound transducers, please visit our
website at www.bcgroupintl.com . There may be additional adapters available that are not listed
above.
Page 12

4
BACKGROUND
The following is the minimal equipment needed to test the electrical safety of ultrasound
transducers:
1) ULT-2000 Series Ultrasound Transducer Electrical Leakage Tester
2) Dual Conductivity Probe (See list on page 8)
3) Ultrasound Transducer Adapter (See list on page 9)
4) Test Basin (BC20-42200)
The ULT-2000 is designed to test the electrical safety of all types of diagnostic ultrasound
transducers, independent of the ultrasound machines on which they are typically used.
Although the ULT-2000 can be used on virtually any type of ultrasound transducer, it is
especially recommended in the testing of TEE (Transesophageal Echocardiography)
transducers prior to each use, as recommended by many TEE ultrasound manufacturers.
The ULT-2000 tests the integrity of the outer insulation barrier of the transducer and
transducer cable as well as the existing capacitive leakage currents. Due to the proximity
of the TEE transducer to the human heart during a normal procedure, abnormally elevated
electrical leakage currents can be hazardous to the patient. Excessive electrical leakage
could induce microshock, resulting in cardiac fibrillation. It is therefore vital to routinely test
TEE (and other types of) ultrasound transducers prior to their use.
All ultrasound transducers have inherent electrical leakage characteristics, and the
manufacturers of these transducers have carefully tested and documented these
characteristic leakages. These transducers each have characteristic minimum and
maximum leakage currents and associated PASS / FAIL limits as prescribed by the
manufacturer. These are different than the acceptable electrical leakage current limits for
Page 13

5
the actual ultrasound machines. The ULT-2000 is the only battery-operated handheld
tester on the market today that tests according to these established protocols, which have
been adopted by diagnostic ultrasound manufacturers. It tests both the upper and lower
limit thresholds for electrical leakage currents.
Typical electrical safety (leakage) testing of the diagnostic ultrasound transducer should
occur as part of the routine cleaning and disinfecting activity that is performed between
patient ultrasound procedures. The ultrasound transducer (see Item 5 in Figure 1) is
immersed in a basin (see Item 4 in Figure 1) containing conductive liquid (see Item 7 in
Figure 1) suitable for performing electrical safety tests. This liquid can be the routine
cleaning and disinfecting agent used, as long as it is suitably conductive to electrical
current flow. Cidex is an example of a widely used disinfecting agent used for cleaning
ultrasound transducers, and is suitably conductive for performing electrical safety tests on
transducers.
The ultrasound transducer is immersed in this conductive liquid and the electrical
connector of the transducer (see Item 6 in Figure 1) is attached to a suitable adapter (see
Item 3 in Figure 1) for that particular manufacturer and model transducer. This creates one
electrical “pole” for the test. The transducer adapter is then attached to the ULT-2000 as
shown in Figure 1. A special conductive probe (see Item 2 in Figure 1) is then attached to
the ULT-2000. This conductive probe is also immersed in the basin of conductive liquid,
creating the second electrical pole. The setup is now complete for the testing of the
connected transducer.
Page 14

6
During testing, the transducer is subjected to a user selectable source voltage. This
5 – Transducer (DUT)
4 – Test Basin
2 – Conductivity Probe
1 – ULT-2000
3 – Transducer Adapter
6 – Transducer
Electrical Connector
7-Conductive Liquid Medium
voltage is typically set to the normal operating voltage of the transducer’s ultrasound
machine. In North America, the source voltage is typically set to 120 VAC @ 60 Hz. For
countries where the normal operating voltage is 230 VAC, the source voltage can be set to
this level, at either 50 or 60 Hz, as appropriate.
Figure 1
Typical Test Setup for ULT-2000
Page 15

7
NOTICE – TESTING
CONDUCTIVE SURFACES SUCH AS METAL CARTS OR
TABLETOPS CAN CAUSE ERRONEOUS READINGS DUE TO
ALTERNATE LEAKAGE PATHS FROM TRANSDUCER ADAPTERS,
CABLES, ETC. ENSURE TEST IS DONE ON A NON-CONDUCTIVE
SURFACE FOR BEST RESULTS.
Page 16

8
TEST PROCESS
The ULT-2000 series completes four intermediate tests as part of the Full Test to fully
evaluate the integrity of an ultrasound probe. The following is the details of each test step:
Source Voltage Test - The first step is to read the actual source (challenge) voltage that
will be applied during the testing to ensure that it is within range. If it is not, an alarm is
activated and the test is halted.
Self Test - The second step checks the Leakage Measuring Circuitry. A relay switches to
a dummy internal load. The source (challenge) voltage is then applied to this load. The
unit must correctly read the known leakage current. If it is not read correctly, an alarm is
activated and the test is halted.
Bath Conductivity Test – The third step tests the conductivity of the liquid in the test
basin. The ULT-2000 accomplishes this task quite easily and reports a simple PASS /
FAIL, or the actual numerical conductivity of the liquid (depending on the system
configuration). If the conductivity of the liquid is insufficient to perform a valid electrical
leakage current test, the ULT-2000 will report this and will not allow the probe test to be
performed.
Probe Leakage Test – The fourth step tests the electrical leakage of the ultrasound
transducer. The measured leakage current is compared to the selected upper and lower
limits. Again, the results of the test will be reported as a simple PASS / FAIL, or the actual
leakage current values. This step is only included when the user performs a Full Test.
Page 17

9
Locking DUT Connector
– To ultrasound transducer
via Transducer Adapter
RS232
7 PIN Mini-Din
Power
2.1 mm Jack
6 Light Touch Keys for
Selecting Parameters and
Settings:
POWER
- Turns Unit On and Off
FULL TEST / UP
- Tests Conductivity and
Leakage
- Scrolls Through Selected
Parameter Options
SELECT / PRINT DATA
- Selects Next Available
Parameter
- Prints Data
MODE
- Changes Operating Mode
- Enters / Exits Setup Mode
BATH TEST / DOWN
- Tests Conductivity Only
- Scrolls Through Selected
Parameter Options
RESET
- Aborts Any Test
- Returns to Main Screen
9V Battery
Compartment
(Back)
Backlit LCD
Graphical
Display
Locking DUT Connector
– To conductive bath via
Dual Conductivity Probe
LAYOUT
The Locking DUT Connections are Interchangeable
This section looks at the physical layout of the ULT-2000 Series and gives descriptions
of the elements.
Page 18

10
KEYS
Six tactile-touch keys with audio feedback are provided for system operation:
– This key turns the unit on and off. The unit will initiate with the Main Screen.
– At the Main Screen, this key initiates the Full Test, which includes a Source
Voltage Test, a Self Test, a Bath Test, and a transducer Probe Test.
– All other screens, this key scrolls up through the selected
parameter options.
– At the Main Screen, this key initiates a conductivity test of the
conductive liquid medium in the bath.
– All other screens, this key scrolls down through the selected parameter options.
– At the Datalog Screen, this key prints the latest test results to the serial port.
– All other screens, this key selects the next available parameter.
– When not in a SETUP menu, this key resets the system to the main
screen.
– When in a SETUP menu, this key has no function.
– This key toggles the unit through operating modes. Pressing this key toggles
from the Main Screen, to the Meter Screen (ULT-2020 Only), to the Datalog
Screen (ULT-2020 only), and then to the Device Configuration Screen.
– Pressing and holding this key allows entry to the SETUP menus where system
configurations can be viewed and adjusted. When in a SETUP menu, this key
exits the SETUP menu and returns to the previously viewed screen. This also
saves the system settings to the internal EEPROM memory so they are retained
with the power turned off or battery removed.
Page 19

11
DUT Information
Manufacturer: Model
Progress Indicator
Full Test Process:
SOURCE VOLTAGE TEST
- verifies the voltage circuit to be
used during testing.
SELF TEST
- verifies that the ULT-2000
Series unit is working properly
before checking the DUT
BATH CONDUCTIVITY TEST
- ensures that the test fluid has
the proper level of electrical
conductivity needed for a valid
electrical leakage test.
PROBE LEAKAGE TEST
- measures the leakage current
of the DUT (changes to peak
current at the end of this test).
SCREENS
Test Measurements
Numerical values will not
appear if Test Mode is set
to “Pass/Fail”. See
System Configuration in
SETUP.
MAIN SCREEN – The main screen indicates that the ULT-2000 Series unit is initialized
and ready for testing. This screen displays after power-up initialization, and can be
accessed by pressing the key at any time other than during setup mode.
FULL TEST SCREEN – This screen is accessed from the Main Screen by pressing the
button. It displays Full Test information, measurements, and progress.
Page 20

12
BATH TEST SCREEN – This screen is accessed from the Main Screen by pressing the
Bath Test Process:
SOURCE VOLTAGE TEST
- verifies the voltage circuit to be
used during testing.
SELF TEST
- verifies that the ULT-2000
Series unit is working properly
before checking the DUT
BATH CONDUCTIVITY TEST
- ensures that the test fluid has
the proper level of electrical
conductivity needed for a valid
electrical leakage test.
DUT Information
Manufacturer: Model
Progress Indicator
Test Measurements
Numerical values will not
appear if Test Mode is set
to “Pass/Fail”. See
System Configuration in
SETUP.
button. It displays Bath Test information, measurements, and progress.
TEST MESSAGES – Messages appear after each FULL or BATH test is completed.
TEST PASSED – This message indicates a successful test within the selected limits.
TEST FAILED – This message indicates a test failure, and provides failure details. The
example shown below indicates that the probe leakage current is above the selected upper
limit.
Page 21

13
METER MODE SCREEN (ULT-2020 ONLY) – The meter mode screen allows extended
Source Voltage
Selectable from 90
to 275 VAC
Source Frequency
Selectable 50/60 Hz
Output Control
Selectable options:
Off
On
30 Seconds
60 Seconds
90 Seconds
2 Minutes
5 Minutes
10 Minutes
15 Minutes
30 Minutes
leakage current measurements, which can be useful when troubleshooting ultrasound
transducer probes and cables. This screen displays the current leakage current reading
and user-selectable Source Voltage, Source Frequency, and Output Control. This screen
is accessed from the Main Screen by pressing the key. The Source Voltage,
Frequency, and Output Control are selected by pressing the key until the desired
parameter is highlighted. Scroll through the highlighted parameter options using the
keys.
Page 22

14
DATALOG SCREEN (ULT-2020 ONLY) – The Datalog Screen displays the test results of
Test Results
Pass / Fail
Test Limits
1 - 500 A
Probe Information
Manufacturer: Model.
Blank if test was configured
for Fixed or User Test
Limits.
Test Measurements
Measurements taken
during test.
Date / Time Stamp
Date and Time the test
was executed.
Record Number
(1-99)
up to 99 test records. The Datalog Screen includes a Record Number, Date / Time Stamp,
Probe Information, Test Results, Test Measurements, and Test Limits. This screen is
accessed from the Meter Screen by pressing the key. Scroll through the Datalog
Records using the keys.
Page 23

15
Use the key to print the displayed record to the serial printer. Below is a sample
print.
Page 24

16
DEVICE CONFIGURATION SCREEN – The Device Configuration displays the current
Probe Model
For Manufacturer
Default Test Limits or
Custom Test Limits
Test Limit Mode
Fixed
User
Manufacturer Default
Custom (see SETUP)
Probe Manufacturer
For Manufacturer Default
Test Limits or Custom
Test Limits
test configuration. Device Configuration parameters include Test Limit Mode, Probe
Manufacturer, and Probe Model. This screen is accessed from the Datalog Screen by
pressing the key. The parameters are selected by pressing the key until
the desired parameter is highlighted. Scroll through the highlighted parameter options
using the keys.
TEST LIMIT MODES
Fixed – Limits are set by the manufacturer and cannot be adjusted.
User – Limits are user-selectable. Refer to USER TEST CONFIGURATION.
Manufacturer Default – Limits suggested by the probe manufacturer and cannot be
adjusted.
Custom – Manufacturer, Model, and Limits are programmable by the user with the PC
configuration program. Refer to the PC Software section of this manual.
NOTE: If the “Allow User Config” parameter is set to NO, the user will not be able to
modify the test parameters. See SETUP for more details.
Page 25

17
LOW BATTERY – When the battery life is 10% or less, the LOW BATTERY message
box appears and indicates the remaining battery life.
BATTERY ELIMINATOR INPUT – A 2.1 mm receptacle is provided for the 10 VDC
Battery Eliminator (BC20-21103, BC20-21106) that may be used for continuous run
applications. It bypasses the internal battery when plugged in.
NOTE: The unit is shipped with a Red Battery Lock-Out plug installed into the line power
connector as shown below. Its purpose is to prevent the unit from accidentally being
turned on during handling and transport, subsequently depleting the battery. This plug
must be removed before any use.
Page 26

18
SETUP
Two user-selectable setup menus are provided, SYSTEM CONFIGURATION and USER
TEST CONFIGURATION. Enter a setup menu by pressing and holding the key
until the Access Code Screen appears (5 sec). The arrows are then used to enter
the access code.
DEFAULT ACCESS CODES
SYSTEM CONFIGURATION = 1
USER TEST CONFIGURATION = 2
NOTE: The access codes may be changed once you have entered the selected configuration.
See System Configuration and User Configuration for access code details.
When the desired access code is displayed, press the key again to access the
selected setup menu. The configuration parameters are selected by pressing the
key until the desired parameter is highlighted. Scroll through the highlighted
parameter options using the keys.
Exit the setup menu using the key.
SYSTEM CONFIGURATION
Below is the typical System Configuration Screen, followed by a table of the available
parameters and a brief description of each option.
Page 27

19
System Configuration
Parameter
Description
Options
Test Mode
Determines whether the test measurements (actual test
readings) are shown in the test screen, or if the unit will
simply give a PASS or FAIL result. The Default setting
is Numerical.
PASS / FAIL or
Numerical
Test Limits
Determines the settings for the test limits. Fixed limits
are set by the factory and cannot be adjusted. User
limits are programmed by the user through a special
access code. Manufacturer Default limits are suggested
test settings by probe manufacturers. Custom limits are
programmable by the user with the PC configuration
program.
Fixed, User,
Manufacturer
Default, or
Custom
DUT Manuf
Selects the DUT manufacturer.
NOTE: Only available when Test Limit mode is set to
Manufacturer Default or Custom.
User Defined
DUT Model
Selects the DUT Model.
NOTE: Only available when Test Limit mode is set to
Manufacturer Default or Custom.
User Defined
Allow User Config
Selects whether the user is allowed to modify the DUT
Manufacturer/Model in the Device Configuration Screen.
This allows an administrator to “Lock Down” the test
configuration.
yes/no
Setup Clock
Pressing the Up arrow while this parameter is selected
displays the Clock Configuration screen where the Date
and Time are configured.
Press UP
Erase Log
Pressing the Up arrow while this parameter is selected
will erase the test records stored in the Datalog (ULT2020 Only)
Press UP
Battery Life
Displays the current battery life.
0-100%
(Read Only)
Contrast Adjust
Sets the contrast of the display.
0-20
Backlight
Off – Always off
1-30 sec – The elapsed time after which the backlight
will automatically turn off.
ON – Always ON.
The Default setting is 30 seconds.
Off, 1-30 sec, ON
Auto Off Timer
Determines the period of inactivity before the meter is
turned OFF. A timer is started when the meter is turned
ON and is reset each time a key is pressed. When the
timer reaches the value set in this parameter, the power
is automatically turned OFF.
The Default setting is 15 minutes.
(NOTE: Setting this parameter to 0 disables the Auto Off
timer.)
(NOTE: Using a battery eliminator disables the Auto Off
Timer.)
0-30 minutes
Access Code
Sets the required code entry to gain access to the Setup
Menu. If this parameter is set to Zero, the Access Code
feature is disabled and the user will gain direct access
to the Setup Menu.
0-9999
Software
Displays current software program.
(Read Only)
Page 28

20
USER TEST CONFIGURATION
User Test Configuration
Parameter
Description
Range
Test Voltage
The Source Voltage applied during a test when Device
Configuration is set to User Test Limits.
90-275 VAC
Frequency
The Frequency of the Test Voltage when Device
Configuration is set to User Test Limits.
50 or 60 Hz
Lkg Upper Limit
The maximum allowable current leakage during a test
when Device Configuration is set to User Test Limits.
1-500 A
Lkg Lower Limit
The minimum allowable current leakage during a test
when Device Configuration is set to User Test Limits.
1-500 A
Access Code
Sets the required code entry to gain access to the User
Test Configuration Menu. If this parameter is set to
Zero, the Access Code feature is disabled and the user
will gain direct access to this Menu.
0-9999
Below is the typical User Test Configuration Screen, followed by a table of the available
parameters and a brief description of each option.
Page 29

21
PC SOFTWARE
The included PC Software allows the user to create custom limit configurations including
Manufacturer, Model, Leakage Limits, Test Voltage, and Frequency. Install and launch
the PC Software, then connect the PC and ULT-2000 via cable BC20-41337 (See
OPTIONAL ACCESSORIES at the beginning of this manual). Once connected, the
software allows the user to download/read custom configurations to/from the ULT-2000.
Below is a sample PC Software screen.
NOTE: The PC Software only manipulates the “Custom Test Limits” portion of the
“Device Configuration” screen. Refer to the SCREENS section for details.
VIEW THE CURRENT CUSTOM TRANSDUCER CONFIGURATION
Page 30

22
In the “Custom Limit Filename” window, click “Read Configuration from ULT-2000” to view
the custom transducer configuration currently stored on the device. The loaded
Manufacturers and Models are displayed in the respective labeled windows. The user may
alter these transducer settings if desired.
ALTER TRANSDUCER SETTINGS
Select the Manufacturer and Model to be altered. Within the “Settings” window, enter the
desired changes including Manufacturer Name, Model Name, Lower and Upper Leakage
Limits, Test Voltage, and Frequency. To submit the changes to the ULT-2000 series, click
“Download Configuration to ULT-2000” in the “Custom Limit Filename” window.
ADD A MANUFACTURER or MODEL
Page 31

23
Using the “Add” buttons in the “Manufacturer” and “Model” windows, the user may create a
Manufacturer and/or Model entry. If the desired Manufacturer is not currently displayed,
click “Add” in the Manufacturer window. This introduces two “New” entries, one each in the
“Manufacturer” and “Model” windows. See the previous section to alter the “New”
transducer settings.
If the desired Manufacturer currently exists, simply click and highlight the Manufacturer of
the transducer to be added. Click “Add” in the “Model” window to create a “New”
transducer model of the highlighted Manufacturer. See the previous section to alter the
“New” transducer settings.
To submit the changes to the ULT-2000 series, click “Download Configuration to ULT2000” in the “Custom Limit Filename” window.
DELETE A MANUFACTURER or MODEL
Page 32

24
Using the “Delete” buttons in the “Manufacturer” and “Model” windows, the user may
remove a Manufacturer and/or Model entry. To delete an entire Manufacturer, highlight the
Manufacturer’s name and click “Delete” in the “Manufacturer” window. To delete a single
Model from a specific Manufacturer, highlight the Manufacturer, then highlight the Model,
and click “Delete” in the “Model” window. To submit the changes to the ULT-2000 series,
click “Download Configuration to ULT-2000” in the “Custom Limit Filename” window.
SAVE CONFIGURATION
Page 33

25
Using the “Save Configuration to File” button in the “Custom Limit Filename” window, the
user may save the manipulated Custom Limit Configuration. This allows the user to
download the changes at a later time, or to download the changes to several ULT-2000
series devices. Clicking “Save Configuration to File” prompts a save window which allows
the user to name the Custom Limit Configuration. Below is a sample save window.
Page 34

26
OPEN CONFIGURATION
Using the “Open Configuration File” button in the “Custom Limit Filename” window, the
user may open saved Custom Limit Configurations. This allows the user to download the
saved Custom Limit Configuration to several ULT-2000 series devices. Clicking “Open
Configuration File” prompts a load window which allows the user to select the desired
Custom Limit Configuration. Below is a sample load window.
Page 35

27
COMMUNICATION PROTOCOL
The communication protocol provides a means to completely configure and use the ULT2000 from a PC, allowing hands free or automated operation.
Communication Port
The Serial port is configured as 115,200 Baud Rate, 8 Data Bits, 1 Stop Bit, and No Parity.
Command Syntax
The command description is broken into columns; the KEYWORD, the NODE, and the
VALUE.
The KEYWORD provides the name of the command. The actual name of the command
consists of one or more keywords since SCPI commands are based on a hierarchical
structure, also known as a tree system.
In such a system, associated commands are grouped together under a common node in
the hierarchy, analogous to the way leaves at a same level are connected at a common
branch. This, and similar branches are connected to fewer and thicker branches, until they
meet at the root of the tree. The closer to the root, the higher a node is considered in the
hierarchy. To activate a particular command, the full path to it must be specified.
This path is represented in the following table by placing the highest node in the left-most
position. Further nodes are indented one position to the right of the parent node.
The highest level node of a command is called the Keyword, followed by the Node(s), and
then the value. Keywords and Nodes are separated by a colon to create the parent
structure. The parent structure is separated from the value by one space, and the
command is executed with a carriage-return (<cr>). For example, to change the system
display contrast to a value of ten, use “SYSTem:CONtrast 10<cr>”.
Some commands allow for reading and writing data and some commands are Read Only.
To indicate a read function, a question mark (?) is placed at the end of the command path.
For example, to read the system version, use “SYSTem:VERsion?<cr>”. This command
would return the current system version.
Page 36

28
Lowercase letters indicate the long-form of the command (for example,
ULT-2000 Communication Command Summary
Keywords
Nodes
Subnodes
Values
CONFigure
OUTput
VOLTage
90-275
FREQuency
50 hz, 60 hz
TEST
MODE
NUMerical, PASSfail
LIMits
FIXed, DEFaults, CUSTom
DMANufacturer
1-5 (Selects Manufacturer 1-5)
DMODel
1-20 (Selects Model 1-20)
LIMits
CONDuctivity
0 - 500 (uA)
ULEAkage
0 - 500 (uA)
LLEAkage
0 - 500 (uA)
METer
(ULT-2020
ONLY)
VOLTage
90-275
FREQuency
50 hz, 60 hz
OUTput
OFF, ON
INITiate
FULLtest, BATHonly, METrmode
ABORt
SYSTem
TIME
hh,mm (24hr mode only)
TFORmat
AMpm, 24hr
DATE
yy,mm,dd
DFORmat
MMddyy,DDmmyy
BATlife?
[read only]
CONtrast
0-20
BTIMe
OFF, 1-20, ON
AOFF
0-30
VERsion?
[read only]
HEADer
String - Up to 40 Chars
MODEL?
[read only]
DMANufacturer <m>, xxx
<m> = 1-5, xxx = String data up to 10 chars
DMODel <m>, <n>
<m> = 1-5 (Manuf), <n> = 1-20 (model), Model String
DLIMits <m>, <n> , Limits
<m> = 1-5 (Manuf), <n> = 1-20 (model), Limits
DSAVe
Saves DUT tables to Eeprom
KEY
1-6
SENSe
VOLTage?
90 - 275 VAC [read only]
LEAKage?
0 - 500 uA [read only]
CONFigure:OUTput:VOLTage 120<cr>) and can be omitted for simplification.
Uppercase letters indicate the abbreviated, or short-form, of the commands and must be
included (for example, CONF:OUT:VOLT 120<cr>).
NOTE: Commands can be entered in either upper or lowercase or a mixture of the two,
uppercase and lowercase. Commands sent to the device are not case sensitive. Upper
and lower cases are only used when documenting the commands.
Page 37

29
ULT-2000 Communication Command Summary (cont.)
Keywords
Nodes
Values
STATus?
Bit Value
Definition
0 1
Test Running
1 2
Bath Only Test
2 4
Full Test
3 8
4 16
5 32
6 64
Test Failed
7 128
Test Passed
8 256
9 512
10 1024
11 2048
Program Mode
12 4096
Meter Mode
13 8192
14 16384
15 32768
Calibration Mode
FACTory
VTOLerance
0-25 %
FVOLtage
90 - 275 VAC
FCONductivity
0 - 500 uA
FULeakage
0 - 500 uA
FLLeakage
0 - 500 uA
DATAlog
(ULT-
2020)
NUMrecords
1 - 100
ERASelog
[Set to '1' to erase datalog]
RECord <n>?
Returns Record <n> (Omit <n> to read last record)
Page 38

30
MANUAL REVISIONS
LIMITED WARRANTY
WARRANTY: BC GROUP INTERNATIONAL, INC. WARRANTS ITS NEW PRODUCTS TO BE FREE
FROM DEFECTS IN MATERIALS AND WORKMANSHIP UNDER THE SERVICE FOR WHICH THEY
ARE INTENDED. THIS WARRANTY IS EFFECTIVE FOR TWELVE MONTHS FROM THE DATE OF
SHIPMENT.
EXCLUSIONS: THIS WARRANTY IS IN LIEU OF ANY OTHER WARRANTY EXPRESSED OR
IMPLIED, INCLUDING, BUT NOT LIMITED TO ANY IMPLIED WARRANTY OF MERCHANTABILITY
OR FITNESS FOR A PARTICULAR PURPOSE.
BC GROUP INTERNATIONAL, INC. IS NOT LIABLE FOR ANY INCIDENTAL OR CONSEQUENTIAL
DAMAGES.
NO PERSON OTHER THAN AN OFFICER IS AUTHORIZED TO GIVE ANY OTHER WARRANTY OR
ASSUME ANY LIABILITY.
REMEDIES: THE PURCHASER'S SOLE AND EXCLUSIVE REMEDY SHALL BE: (1) THE REPAIR OR
REPLACEMENT OF DEFECTIVE PARTS OR PRODUCTS, WITHOUT CHARGE. (2) AT THE OPTION
OF BC GROUP INTERNATIONAL, INC., THE REFUND OF THE PURCHASE PRICE.
Revision # Program # Revisions Made
Rev 01 DT7331CA Origination
Rev 02 DT7331CA Misc. Edits
Rev 03 DT7331CA Adapter Information Updated
Rev 04 DT7331CB Model Information Updated
Rev 05 DT7331CC Dynamic Device Configuration Screen
Rev 06 DT7331CC Edits to Euro Transformer, Conductivity Probe and
Transducer Adapter List Updated, and Misc. Edits
Rev 07 DT7331CE Address Updated
Rev 08 DT7331CF Transducer Adapter List Update, Accessories List
Updated, Communication Protocol Added, Sample
Data Print Added, PC Software Section Added, Misc.
Edits
Rev 09 DT7331CF Euro Transformer Edited
Rev 10 DT7331CG Specifications Updated, Misc. Edits
Rev 11 DT7331CG Misc. Edits
Rev 12 DT7331CG Format Updated, Printer cable part number updated,
Misc. Edits
Rev 13 DT7331CG Specifications Updated
P:\MANUALS\BCGROUP\…ULT-2000\ULT-2000_UM_Rev13.docx
Page 39

31
SOURCE, LEAKAGE AND CONDUCTIVITY
SOURCE
(CHALLENGE)
VOLTAGE
90 - 275 VAC, ± 1% FS
500 µA Max Load
SOURCE
(CHALLENGE)
FREQUENCY
50 or 60 Hz, ± 0.5 Hz
CURRENT
MEASUREMENT
LEAKAGE
0.50 - 10.00 µA, ± 0.5 µA
10.0 - 250.0 µA, ± 1% Range
250.0 - 500 µA, ± 1% Range
CONDUCTIVITY
0.5 - 500 µA, ± 1% FS
CONNECTIONS
PHYSICAL & ENVIRONMENTAL
DISPLAY
128 X 64 Pixels Graphical LCD,
White LED Backlight
MEMORY
SETUP
EEPROM, All parameters
RETENTION
10 Years Retention w/o Power
CONSTRUCTION
ENCLOSURE
ABS Plastic
OVERLAY
Back-printed Lexan
SIZE
7.27 x 3.97 x 1.80 Inches
(184.7 x 100.8 x 45.7 mm)
WEIGHT
≤ 1.1 Lbs (0.50 kg)
OPERATING
RANGE
15 to 30 °C (59 to 86 °F)
STORAGE RANGE
-40 to 60 °C (-40 to 140 °F)
SPECIFICATIONS
Page 40

32
ELECTRICAL & MISC.
BATTERY
9V Lithium Battery
(ANSI/NEDA 1604LC or equivalent)
BATTERY
ELIMINATOR
10 VDC, 300mA
BC20-21103 (USA Version)
BC20-21106 (Euro Version)
POWER
CONSUMPTION
ON
< 300 mA
OFF
< 250 µA
BATTERY LIFE
CONTINUOUS
> 100 Full Tests
(Note: Backlight set to OFF)
OFF
1 year
RS-232
COMMUNICATIONS
BAUD
115200
DATA BITS
8
START BITS
1
STOP BITS
1
PARITY
none
HANDSHAKING
none
CONNECTIONS
Seven (7) pin Mini-DIN
Pinout:
Page 41

33
NOTES
Page 42

34
NOTES
Page 43

Page 44

BC GROUP INTERNATIONAL, INC.
3081 ELM POINT INDUSTRIAL DRIVE
ST. CHARLES, MO 63301
USA
1-800-242-8428
1-314-638-3800
www.bcgroupintl.com
sales@bcgroupintl.com
ULT-2000 Series User Manual
10/12 – Rev 13
Copyright © 2012
Made in the USA
 Loading...
Loading...