BC Biomedical ULT-2000 Owners Manual

ULT-2000 Series Product Overview
Are your TEE and other types of “invasive”
ultrasound transducers safe?
Sonosite TEE Transducer
Courtesy of Sonosite, Inc.
Bothell, WA
www.sonosite.com
Or are you putting your patients
needlessly at risk?
Copyright May, 2007 by BC Group International, Inc.
1 Revision 1 – May 25, 2007
Author: Michael R. Erwine

INDEX
The Next Generation in TEE Transducer Testing is Here...........................................................3
Why Should You Test Your Transducers Anyway?....................................................................3
How Can Ultrasound Transducers Be Easily Tested?................................................................8
Can Non-Technically Oriented Personnel Perform These Tests?............................................10
Can Technically-Oriented Personnel Get Important Measurement Data?................................ 10
Competitive Product Comparison.............................................................................................11
The Difference Starts with the Bath Conductivity Test..............................................................11
Dealing With Real World Parameters.......................................................................................13
Why Test With AC Voltages Instead of DC Voltages?..............................................................14
Can Test Results Be Archived for Legal Liability Purposes?....................................................14
PC Utility Software Makes It Easy............................................................................................14
Ultrasound Transducer Adapter Compatibility..........................................................................14
Available Transducer Adapters.................................................................................................15
Advanced Features for Advanced Users..................................................................................15
Competitive Product Cost Comparison.....................................................................................15
Conclusion................................................................................................................................16
ULT-2000 Series Product Specifications..................................................................................17
Technical References...............................................................................................................18
About the Author.......................................................................................................................19
APPENDICES
APPENDIX A (Siemens DALE 800A Instrument Specifications)..............................................21
APPENDIX B (Fluke Biomedical ULT-800 Instrument Specifications)......................................22
APPENDIX C (Dale Technology DALE800 Instrument Specifications) ....................................23
APPENDIX D (Sonosite TEE Electrical Safety Test Description).............................................24
APPENDIX E (Sonosite TEE Bite Hole Inspection Test – 1st page) .........................................25
APPENDIX F (Sonosite TEE Bite-Hole Inspection Test – 2nd page).........................................26
APPENDIX G (GE Healthcare Simplified Leakage Current Setup) ..........................................27
APPENDIX H (Voltaic Cells).....................................................................................................28
APPENDIX I (Sample Printout of Test Results form ULT-2000)...............................................29
APPENDIX J (History of BC Group International, Inc.).............................................................30
Copyright May, 2007 by BC Group International, Inc.
2 Revision 1 – May 25, 2007
Author: Michael R. Erwine

The Next Generation in TEE Transducer Testing is Here
Until now, commercially available dedicated electrical leakage current testing systems for TEE and other types of
“invasive” diagnostic ultrasound transducers have been limited to red light / green light testing
according to the manufacturer of the test device, a green LED lights up. If the test results in a Failure, a red LED
lights up. So what constitutes this Pass or Fail criteria? You have to dig into the product specifications of the
particular test instrument to find that out, and once you find the information, you will notice that the exact same
test protocol (test voltage, test voltage frequency, lower leakage current limit, and upper leakage current limit) is
used for ALL types of ultrasound transducers tested, totally regardless of clinical application or the level of risk to
the patient.
But what if the user really wants to know if the TEE or other type of diagnostic ultrasound transducer is “safe by a
long shot” or if it passes just marginally? Is it getting worse or staying the same? Is it leaking more electrical
current this time vs. the last time it was tested? Is the insulation barrier holding up or breaking down? Does the
transducer display higher leakage values when flexed or strained in a particular way? Can the test results be
printed out with a time and date stamp for formal documentation purposes? Until now, there was no real way to
get the answers to these important questions with existing commercially available dedicated ultrasound electrical
leakage current testing systems.
The BC Biomedical ULT-2000 Series of Ultrasound Transducer Leakage Current Testers (the ULT-2010 and ULT-2020
instruments)
2
changes all of this and delivers the “next generation” in dedicated test devices for TEE and other diagnostic
ultrasound transducers, with added functionality and flexibility never before seen in a commercially available and
affordable tester.
1
. If the test Passes
Why Should You Test Your Transducers Anyway?
Many types of diagnostic ultrasound transducers come into “intimate” contact with the patient and should be
tested regularly to evaluate the integrity of the insulation barrier between the inner wiring of the transducer and
the outside world. But because of their proximity to the heart during typical clinical application, TEE
(Transesophageal Echocardiography) ultrasound transducers are of paramount concern regarding electrical
safety and the containment of potentially harmful electrical leakage currents. A tiny bite hole from a previous TEE
procedure can leave the next patient at risk to elevated and potentially harmful levels of electrical leakage
currents, with such currents actually being introduced by the TEE transducer into the patient’s thoracic cavity,
within a few centimeters of the heart muscle.
1
This methodology commonly refers to the Dale Technology DALE800, the Fluke Biomedical ULT-800, and the Siemens Medical Solutions DALE800A
product that is purchased under private label agreement with Fluke Biomedical.
2
Commercial availability scheduled for July/August 2007.
3 Revision 1 – May 25, 2007
Copyright May, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
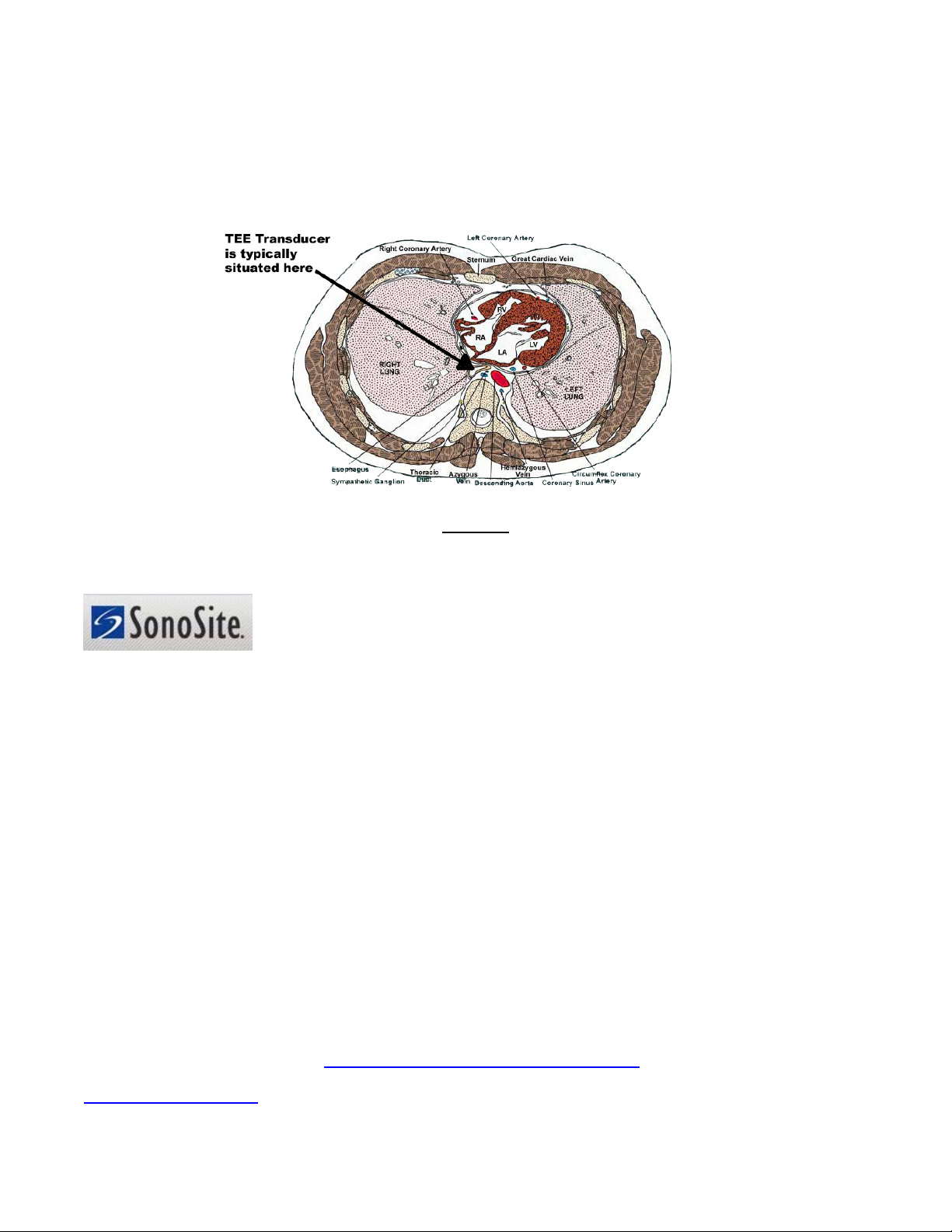
Major diagnostic ultrasound system manufacturers such as GE Healthcare, Philips Medical Systems, Siemens
Medical Solutions, Sonosite, Inc. and Toshiba America Medical Systems highly recommend electrical
leakage current testing of TEE transducers prior to each clinical application (or at least between clinical
applications). TEE transducers such as the Acuson V510B and V705B,
GE Healthcare 6T, Philips Medical
Systems miniMulti, OmniPlane III, S7-3t, MPT- 7-4, Siemens Medical Solutions V5M, V5Ms, V7M, Sonosite
TEE/8-3, and Toshiba America Medical Systems PEF 510SB are just a few examples of transducers that should
be regularly tested for electrical leakage currents per manufacturer recommendations.
Figure 1
for TEE transducers since the introduction of their own TEE transducer. In their TEE Transducer User Guide
(pages 31-33) they highly recommend periodic electrical safety testing. Language from this publication (top of
page 31) is as follows:
Cross-Section of the Human Thoracic Cavity Showing Proximity of a TEE Transducer to the Heart
Sonosite, Inc. (Bothell, WA) has been an advocate of electrical leakage current testing
“The electrical leakage current test should be performed on the TEE transducer after
taking it out of the box and prior to each exam, alternatively, if the bite-hole inspection
test is done prior to each exam, then the electrical leakage current test should be done
yearly at a minimum.”
The “bite hole inspection test” referred to in the Sonosite, Inc. TEE Transducer User Guide publication involves
setting up a water bath with a liquid medium that is electrically conductive to a specified level (water mixed with
50g NaCl/liter of water), using a Digital Multimeter calibrated to NIST traceability, and a copper or aluminum sheet
with an area of at least 25 cm
shaft placed into the liquid up to the 40 cm mark. Readings are taken and compared to desired results. This test
is recommended as a possible alternative to the electrical safety test (described in detail below using the BC
Biomedical ULT-2000 Series), but it would actually be more time consuming than the electrical safety test
described below, and could not be combined with the normal cleaning and disinfection process. Also, because
this test utilizes a simple digital multimeter, it is a DC-only low voltage test, and it will not capture capacitive
electrical leakage currents that may be present during normal use of the ultrasound transducer with the
ultrasound system powered by a customary AC power system at 50 or 60 Hz.
The Sonosite TEE Transducer User Guide publication can be downloaded from the Sonosite, Inc. company
website at the following location:
the page to find it. You can also see Appendices D, E. and F of this document for detailed information, or simply
follow this embedded link to access the file at the Sonosite web site.
4 Revision 1 – May 25, 2007
2
. The TEE transducer is then submersed in the water bath with the endoscopic
http://www.sonosite.com/content/view/55/439/ . Simply scroll to the bottom of
Copyright May, 2007 by BC Group International, Inc.
Author: Michael R. Erwine

Since early 2006, Sonosite, Inc. has purchased and offered for resale to their customers as an accessory item to
their TEE transducers, the Fluke ULT-800 instrument for electrical safety testing.
Siemens Medical Solutions is probably the most pro-active diagnostic ultrasound
manufacturer in the market today when it comes to advocating TEE transducer electrical leakage current testing.
Since April, 1993, Siemens Medical Solutions (Mountain View, CA) has been offering the DALE 800A TEE
Transducer Leakage Current Tester to its TEE transducer customers as an accessory item, under the Siemens
Medical Solutions brand label. In the Siemens DALE 800A TEE Transducer Leakage Current Tester
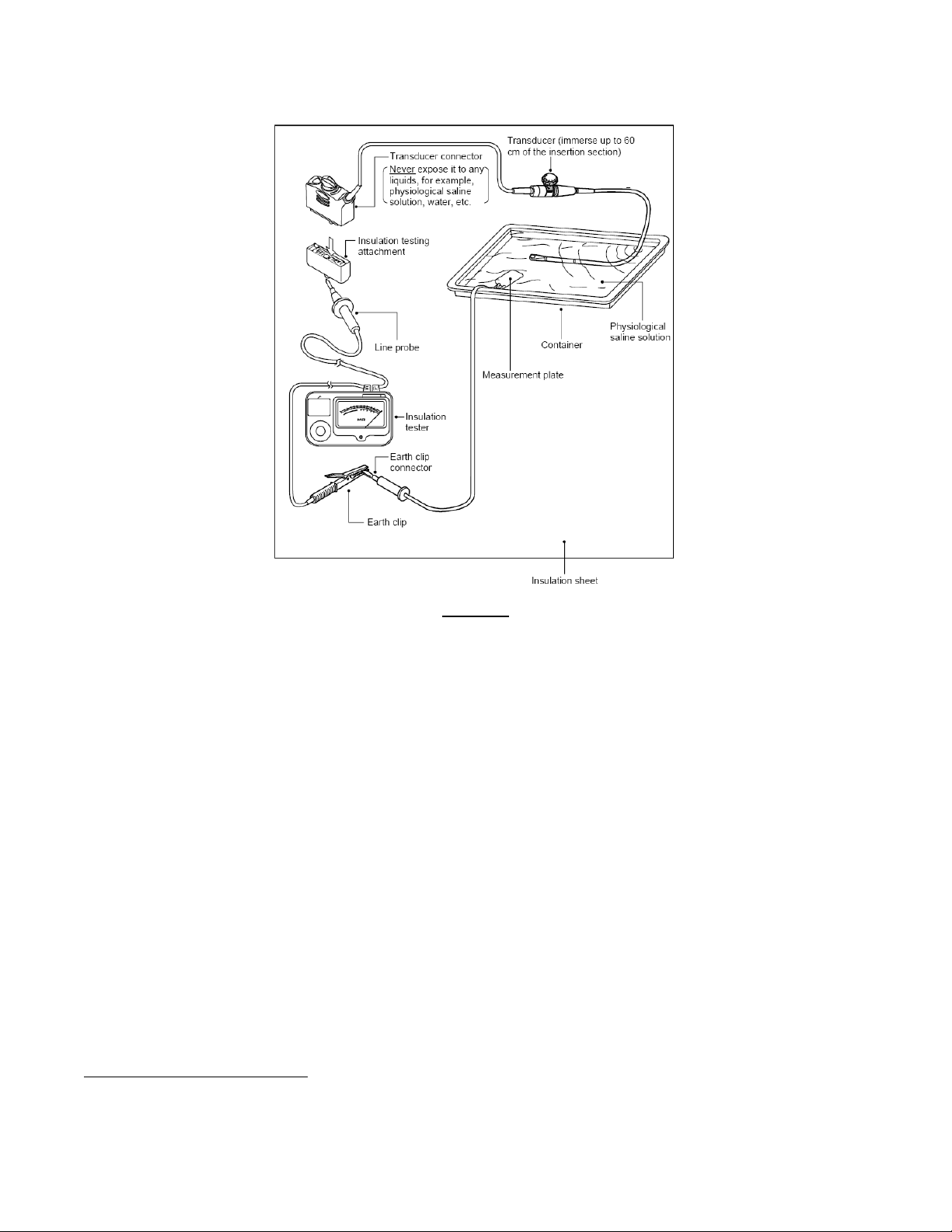
Instruction Manual, the testing method outlined is exactly the same as that shown in Figure 2 below. The
following language appears in the Siemens manual:
“The hand-held battery-operated Tester is designed for use during the routine transducer
cleaning procedure conducted between patient examinations.”
The illustration in Figure 2 below is the recommended test setup that Siemens Medical Solutions gives to their
customers in the DALE 800A TEE Transducer Leakage Current Tester Instruction Manual:
Figure 2
As of the release date of this document, Siemens Medical Solutions markets the DALE800A TEE Transducer
Leakage Current Tester on a worldwide basis and strongly endorses TEE electrical leakage current testing with
its customers, as well as the specific testing methodology outlined in this document. See Appendix A for product
specifications on the Siemens Medical Solutions DALE 800A instrument.
GE Healthcare is another pro-active ultrasound system manufacturer when it comes to advocating
electrical leakage current testing of their TEE transducers. For example, in the GE Service Note # SN76018
(October 2, 2000), they outline an electrical safety testing protocol that is very similar to the one described in
detail in this document. On page 2 of this service note, the following is stated:
5 Revision 1 – May 25, 2007
Recommended TEE Transducer Test Setup Specified by Siemens Medical Solutions
Copyright May, 2007 by BC Group International, Inc.
Author: Michael R. Erwine

“The electrical leakage current in the probe can alternatively be measured in a
simplified test without the access to the ultrasound scanner, by using the procedure
described below. The test described below is not a complete safety test. It is focused on
the most important insulation test for this product”.
The test setup outlined in this GE service note can be seen in Appendix G of this document. In this same service
note, GE Healthcare states the following on page 4 relative to electrical leakage current testing on TEE
transducers in general:
“GE Vingmed Ultrasound AS recommends that leakage current testing be carried out on
a regular basis to obtain the best possible patient safety. Also, a leakage current test
should be conducted prior to the use of the probe in any surgical procedure.”
Philips Medical Systems has a history of recommending routine electrical leakage current
testing of their TEE transducers that dates back to the Hewlett Packard and Agilent days. It was Hewlett Packard
that initially established a working relationship with Dale Technology, Inc. of Thornwood, NY in the late 1990’s
regarding the use and recommendation of the DALE800 to their ultrasound customers. At that time, the DALE800
was the only commercially available dedicated ultrasound transducer electrical leakage current testing solution.
Currently, Philips continues to advocate electrical leakage current testing on their TEE transducers with their
customers, as part of an ultrasound “system” approach. In their TEE Proper Care and Handling Manual
(Publication # 4535 611 90271 Rev B), they have the following comments regarding electrical safety concerns
with TEE transducers:
“Cuts in the transducer cable or cracks in the housing can destroy the electrical safety
features of the transducers.”
“Bites can cause electrical hazards or mechanical malfunction.”
“Cuts in the transducer insulation can result in current leakage and may lead to serious
patient electrical hazards. In addition, fluid that enters the gastroscope via the cut will
cause electrical and mechanical operational problems.”
In some of their latest ultrasound system user manuals (specifically, manuals that are involved with systems that
utilize TEE or other “invasive” types of ultrasound transducers), Philips Medical Systems includes detailed
electrical safety testing instructions that are highly recommended to help determine of there is a hole of any kind
in the transducer outer insulating barrier. Bite holes or cuts in this insulation barrier could lead to elevated levels
of electrical leakage currents, consequently putting the patient at risk. Philips Medical Systems also warns
against conducting transducer electrical safety tests by making simple DC measurements on their transducers,
and further claims that such testing procedures yield inaccurate results regarding electrical leakage currents in
transducers. The prescribed testing method offered by Philips Medical Systems involves the immersion of the
TEE and/or other type of “invasive” ultrasound transducer in a saline solution, typically described within this
document.
Toshiba Medical America is yet another advocate of routine electrical leakage current
testing of their TEE transducers with their customers. In their TEE transducer Operation Manuals, Toshiba
recommends an electrical leakage current test prior to each clinical procedure involving the transducer. They
have an electrical leakage current testing solution in place (the Hioki Model 3451 Digital Megohm Insulation
Copyright May, 2007 by BC Group International, Inc.
6 Revision 1 – May 25, 2007
Author: Michael R. Erwine
HiTester
3
) and they include this tester with each TEE transducer that they sell. A diagram of the recommended
test setup for the Toshiba line of TEE transducers, utilizing the Toshiba supplied tester appears in Figure 3 below.
Figure 3
Recommended TEE Transducer Test Setup for Toshiba TEE Transducers Using the Toshiba Tester
(Hioki Model 3451 Digital Megohm Insulation HiTester)
Toshiba Medical lists the following safety recommendation in their Operation Manual For Multiplane
Transesophageal Transducer Model PET-510MB (2B701-591E*G):
3
“Checks Before Use… Checks before system power ON… Electrical Safety Inspection
(inspection using the safety kit)… Perform electrical safety inspection using the safety kit
to check for damage to the transducer which may not be visible.”
In general, this level concern over transducer electrical leakage currents is slowly but surely expanding to other
types of “invasive” ultrasound transducers such as those used during laparoscopic procedures. Other major
medical device manufacturers in the ultrasound arena are also beginning to make such recommendations to their
customers concerning not only TEE but other types of invasive ultrasound transducers, and the need for electrical
leakage current testing on them. Once again, the typical clinical application of these types of transducers finds
them having been inserted at least several inches into a body cavity or through some type of an incision in the
body, thus violating the human body’s natural protection (the outer layers of otherwise dry skin) from electrical
macroshock and microshock conditions.
The effects of even the smallest levels of electrical leakage currents on the heart muscle and other internal
organs has been studied for over thirty years, and the potential risks are still viewed as critical ones, especially
with such currents in close proximity to the heart, as in the typical application of a TEE transducer.
3
The Hioki 3451 Digital Megohm HiTester is a DC Insulation Tester and it tests the transducer insulation barrier with DC voltage only. It will not pick up
any capacitive electrical leakage currents that may exist in the transducer during actual use on a conventional AC power systems running at 50 or 60 Hz.
Copyright May, 2007 by BC Group International, Inc.
Author: Michael R. Erwine
7 Revision 1 – May 25, 2007
How Can Ultrasound Transducers Be Easily Tested?
TEE and other types of diagnostic ultrasound transducers can be easily tested as part of the routine cleaning and
disinfection process following each clinical procedure
there are no elaborate test setups required. There are two basic approaches that can be taken, depending on the
method commonly used to clean and disinfect ultrasound probes at the medical facility in question.
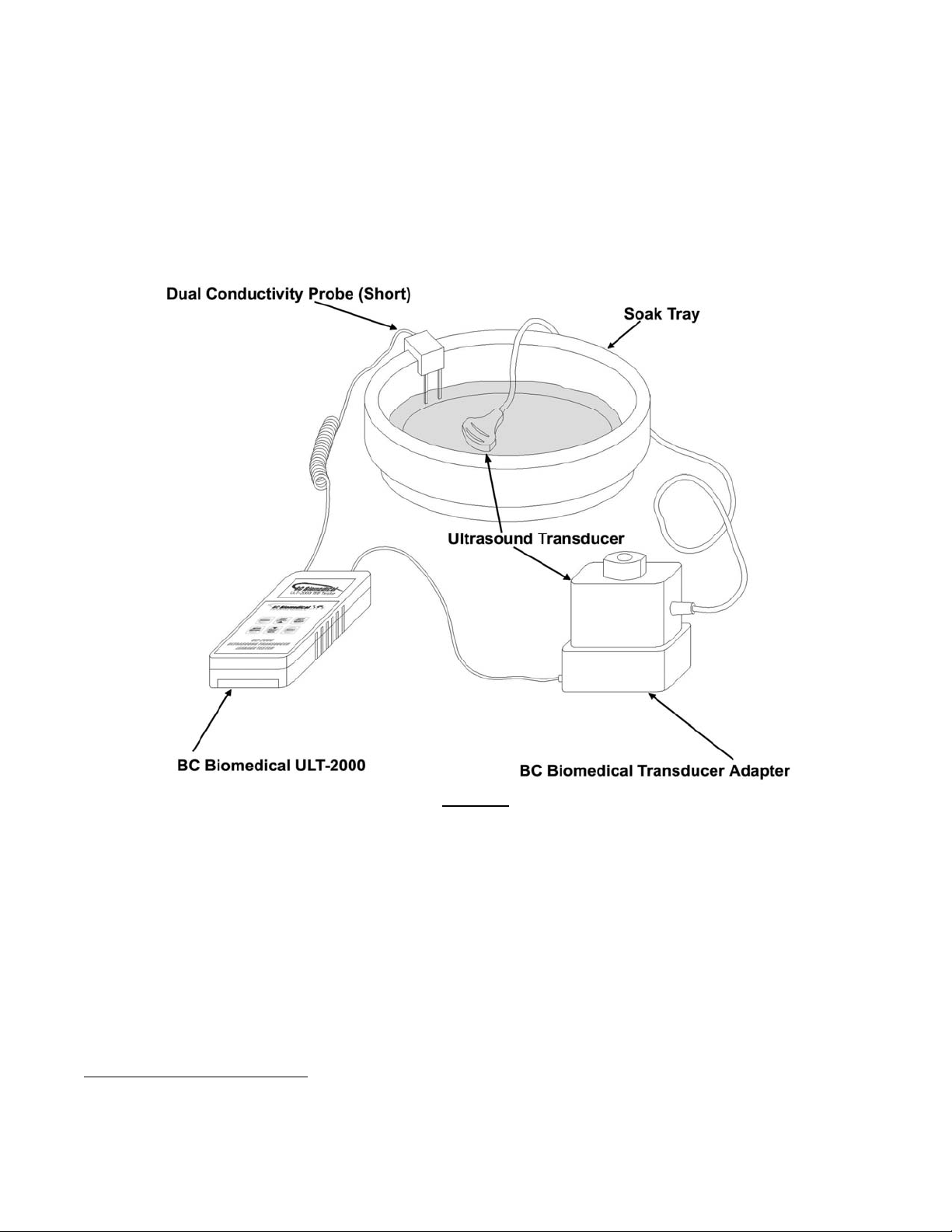
Facilities that utilize a cleaning agent such as Cidex® in a “soak tray” can use this same setup to test the
ultrasound transducer for electrical safety during the cleaning and disinfecting process. A typical test setup is
illustrated in Figure 4 below. The most commonly used soak tray has historically been a Cidex
®
Cidex
2032 compatible tray is available from BC Group International (BC Part # BC20-42200).
4
. There is no need to make a big deal over testing and
®
2032 Tray. A
Figure 4
Cidex® is characteristically an electrically conductive medium that works extremely well for electrical leakage
testing of ultrasound transducers, including TEE transducers. If Cidex
the setup above, a Cidex
This same test setup can be used as a simple test procedure (without the disinfecting function) by using a saline
solution or a mixture of tap water with dissolved table salt. The bath conductivity test function of the BC
Biomedical ULT-200 Series will assist in setting up a bath solution that is suitably conductive to electrical currents
for this test. In such a test, a Cidex
Biomedical #BC20-42210 Generic Soak Tray may be used.
The BC Biomedical # BC20-42100 Dual Conductivity Probe is typically used in the soak tray testing solution as
pictured above. This is the shorter of the two dual conductivity probe types available from BC Group International
for the ULT-2000 Series.
4
The soak tray and commercial cleaning system immersion test procedure outlined in this document should only be conducted on those types of transducers
that are approved by the original medical device manufacturer for immersion in a cleaning agent bath. Consult with the manufacturer of your transducer to
see what transducers are applicable to this procedure.
8 Revision 1 – May 25, 2007
Typical Electrical Leakage Test Setup Using a Soak Tray
®
®
compatible soak tray such as the BC Biomedical # BC20-42200 must be used.
®
compatible soak tray is not required. A less costly tray such as the BC
or another disinfecting agent is used in
Copyright May, 2007 by BC Group International, Inc.
4
Author: Michael R. Erwine
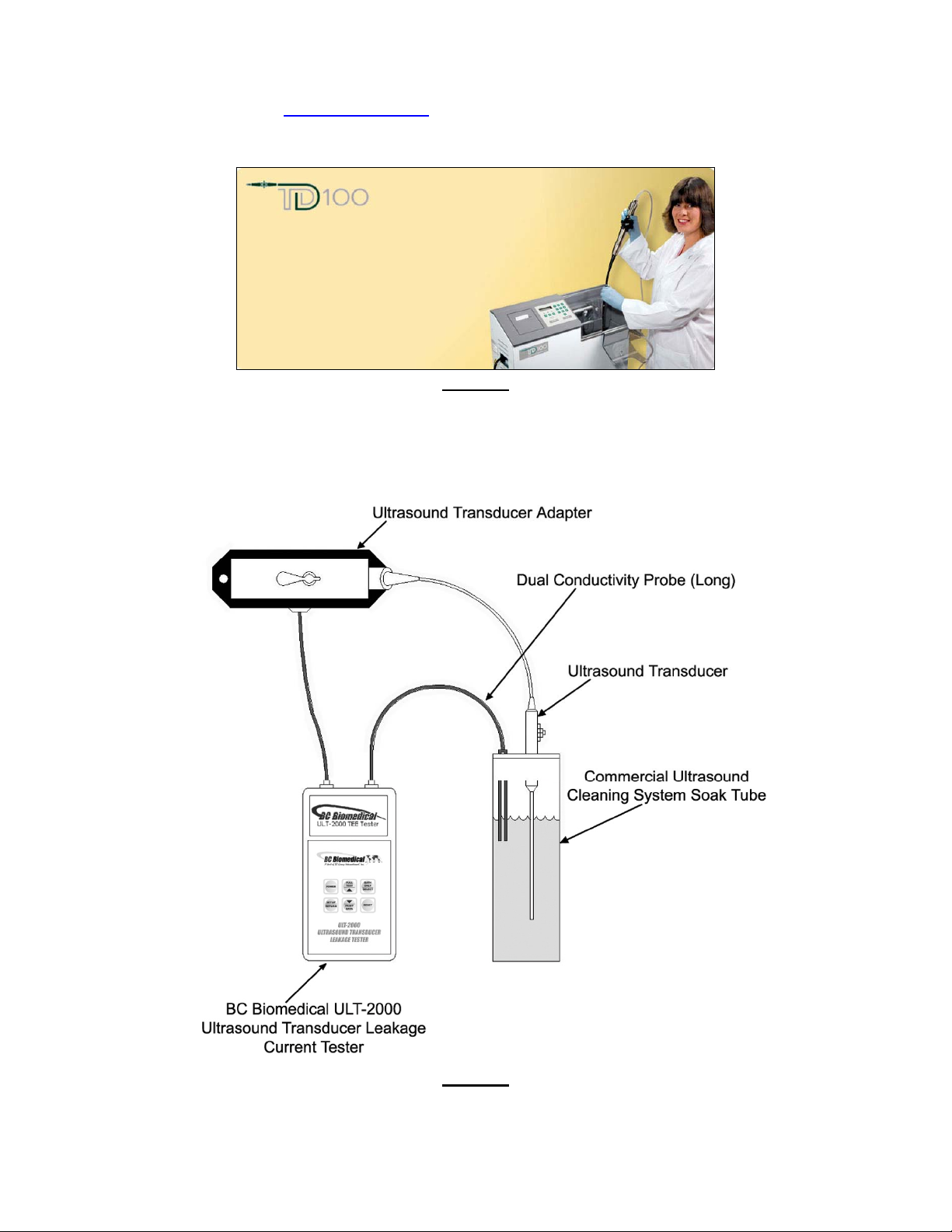
Where a commercially available ultrasound transducer cleaning system such as the TD-100 TEE Probe
Disinfector by PCI Medical (
www.pcimedical.com) is used (shown in Figure 5 below), the BC Biomedical ULT-
2000 Series can also be easily utilized as part of the enzymatic pre-cleaning process, for a combination
cleaning/disinfecting and electrical leakage current testing process with such systems.
Figure 5
The special-purpose BC Biomedical # BC20-42101 Dual Conductivity Probe is typically used in this type of
application. A generic setup using a commercially available disinfecting and cleaning station is illustrated in
Figure 6 below.
PCI Medical TD100 Cleaning System
Figure 6
Generic Functional Diagram of a Typical Electrical Leakage Test Setup Using a Commercial Cleaning Station
Copyright May, 2007 by BC Group International, Inc.
9 Revision 1 – May 25, 2007
Author: Michael R. Erwine
 Loading...
Loading...