baseclick ClickTech Oligo Link Kit, Size S, S (Amount Range of Oligonucleotide per Reaction: From 70 pmol to 22 nmol) User guide

Version 1.1
User Manual
ClickTech Oligo Link Kit
ClickTech Oligo Link Kit
2
Ordering information
(for detailed kit content see Table 1)
ClickTech Oligo Link Kit:
Product Number
Product Name
Amount Range of Oligonucleotide per Reaction
1
BCK-OL-S
ClickTech Oligo Link Kit S
From 70 pmol to 22 nmol
BCK-OL-L
ClickTech Oligo Link Kit L
From 1 nmol to 90 nmol
1 = These are the minimal and maximal amounts of reacted oligonucleotide that can be achieved using the
kits. The actual feasible amounts are also dependent on the available concentrations of the reaction
partners.
For References, FAQs and ordering please see online or contact us:
online: www.baseclick.eu
orders: orders@baseclick.eu
support: support@baseclick.eu
phone: +49 89 9699 3401
fax: +49 89 9699 4696

ClickTech Oligo Link Kit
3
ClickTech Oligo Link Kit
For click chemistry with 70 pmol to 90 nmol of modified oligonucleotide
Introduction and product description:
This kit was especially optimized to link an oligonucleotide containing a terminal alkyne to an azidemodified oligonucleotide without the need for splint oligos. Labeling of alkyne- or azide-modified
oligonucleotides using label-azides or label-alkynes, respectively, is also possible using this kit.
For research use only.
Information in this document is subject to change without notice. baseclick GmbH assumes no
responsibility for any errors that may appear in this document.
baseclick GmbH disclaims all warranties with respect to this document, expressed or implied, including but
not limited to those of merchantability or fitness for a particular purpose. In no event shall baseclick GmbH
be liable, whether in contract, tort, warranty, or under any statute or on any other basis for special,
incidental, indirect, punitive, multiple or consequential damages in connection with or arising from this
document, including but not limited to the use thereof.
Please read the material safety data sheets (MSDS) provided for each product/component available online
at www.baseclick.eu.
Literature Citation: When describing a procedure for publication using this product, please refer to it as
baseclick ClickTech Oligo Link Kit.
1. Materials and Storage Conditions
Table 1: Contents of the ClickTech Oligo Link Kits and storage conditions.
Color code
Quantity
Component
Storage
yellow
50 µL (Kit S)
----------------------150 µL (Kit L)
10x Activator²
RT °C
green
10x
Reactor 25 (Kit S)
-----------------------------
Reactor 100 (Kit L)
RT
blue
1 mL
DMSO (solvent)
RT

ClickTech Oligo Link Kit
4
2. Required Material and Equipment – not provided with this kit
• Alkyne-modified oligonucleotide and azide-modified oligonucleotide from enzymatic or
commercial source, preferably dissolved in water (read also section 3 and 11).
• In case of labeling reactions: label-azides or label-alkynes (depending on oligonucleotide
functionality)
• baseclick will be glad to provide you with high quality oligonucleotides as well as labels; please
check our website or inquire via email at quotes@baseclick.eu for an official quote.
• Microcentrifuge tubes
• Thermomixer, thermocycler or water bath
• Polyacrylamide or agarose gel electrophoresis
• PCR or gel purification kit (optional, e.g. from Qiagen)
• HPLC/IEC system for analyses (optional)
3. General Considerations
• The “Reactor” contains a stable heterogeneous catalyst, which won’t be dissolved during the
reaction.
• The DMSO component is not needed for the standard protocol, but is included in the kit to dissolve
label azides/alkynes or as additive (see section 11).
• The click reaction can be performed with 10-100 µM DNA oligonucleotide solutions using the basic
click protocol. For more concentrated samples a “preparative click” protocol might be needed. For
RNA oligonucleotides check extra section.
• The click reaction is optimized for 1 h at 45 °C. For low concentrations (up to 20 µM) DNA
decomposition in this reaction environment can start after 2 h at 45 °C. For optimal results it might
be necessary to adjust the incubation time.
• Low reaction temperatures (e.g. 20 °C) can be applied as well in combination with longer reaction
time.
• Only terminal alkynes can react with azides using the kit reaction conditions.
• It is not feasible to use azide and alkyne functional groups within the same molecule when reaction
to a second reaction partner is desired.
• Read section 11 (Troubleshooting) before getting started.
ClickTech Oligo Link Kit
5
4. Basic Label-Oligo Click Protocol
4.1. Dissolve/Dilute your label azide/label alkyne in DMSO or water to 0.2 - 10 mM to use it as a stock
solution.
4.2. Add the appropriate amount of 10x Activator2 to the Reactor, e.g. 2.5 µL 10x Activator2 are added
to Reactor 25 to be used at a total reaction volume of 25 µL. Depending on Reactor amount and
final volume, this needs to be adjusted (see Table 2).
4.3. Add the alkyne/azide modified DNA oligonucleotide to the vial to a final concentration of
10 - 100 µM.
Note: For reactions of more concentrated solutions, see Preparative Label-Oligo Click Protocol on
page 7.
4.4. Add 2 equivalents of label azide/alkyne per equivalent of alkyne/azide in the oligonucleotide. For
example, a 10 µM solution of a singly alkyne-modified oligonucleotide is mixed with 20 µM of a
label azide for the click reaction.
4.5. Close the vial and incubate the mixture at 45 °C, 600 rpm for 1 h in a thermomixer. Alternatively,
a water bath can be used. When using fluorophores, protect the vial from light. Make sure that
the Reactor is within the reaction solution during the reaction. Spin down the solution if needed.
4.6. Spin down the Reactor. Transfer the supernatant with the clicked oligonucleotide to a new vial.
Note: For long-term storage, reacted samples (without Reactor) should be kept at – 20 °C.
4.7. Analyze the reaction mixture by gel electrophoresis, HPLC or ion exchange chromatography (IEC).
Purifications using column-based kits for oligonucleotide purification (e.g. PCR purification kit
from Qiagen) give good results. Make sure the length of the oligonucleotide is compatible with
the purification kit. Alternatively, purification can be done by HPLC, IEC or ethanol precipitation
(product loss likely).
Exemplary Label-Oligo Click
This guide will help you decide which stock solution concentration of the label azide/alkyne should be
prepared. All concentrations within the table refer to the stock solution concentrations for exemplary
setups using two equivalents of label azide/alkyne for a singly modified oligonucleotide alkyne/azide.
Table 2: Exemplary volumes needed for reaction setups of “basic” label-oligonucleotide click reactions.
Reactor
V (Activator²)
c (Oligo)
V (Oligo)
c (Label)
V (Label)
V (H2O)
Oligo (n)
25
2.5 µL
10 µM
20.0 µL
200 µM
2.0 µL
0.5 µL
200 pmol
25
2.5 µL
50 µM
20.0 µL
1 mM
2.0 µL
0.5 µL
1.0 nmol
25
2.5 µL
100 µM
20.0 µL
2 mM
2.0 µL
0.5 µL
2.0 nmol
100
10.0 µL
10 µM
80.0 µL
1 mM
1.6 µL
8.4 µL
0.8 nmol
100
10.0 µL
50 µM
80.0 µL
2 mM
4.0 µL
6.0 µL
4.0 nmol
100
10.0 µL
100 µM
80.0 µL
10 mM
1.6 µL
8.4 µL
8.0 nmol
ClickTech Oligo Link Kit
6
5. Basic Oligo Link Click Protocol (Oligo – Oligo Connection)
5.1. Add the appropriate amount of 10x Activator² to the Reactor, e.g. 2.5 µL Activator are added to
Reactor 25 to be used with at a total reaction volume of 25 µL. Depending on Reactor and final
volume, this needs to be adjusted (see Table 3).
5.2. Add an equimolar mixture of singly modified alkyne- and azide-modified oligonucleotide (same
final concentration of each oligo) to dilute the Activator to a final 1x concentration.
Note: To achieve optimal reaction progress, do not increase or decrease the final activator
amount. Oligo concentrations of 100 µM will give best yields; down to 10 µM good yields are
achieved. For reaction of more concentrated solutions see “preparative clicks” on page 8.
5.3. Close the vial and incubate the mixture at 45 °C, 600 rpm for 1 h in a thermomixer. Alternatively,
a water bath can be used. Make sure that the Reactor is within the reaction solution during the
reaction. Spin down the solution if needed.
5.4. Spin down the Reactor. Transfer the supernatant with the product to a new vial.
Note: For long-term storage, reacted samples (without Reactor) should be kept at – 20 °C.
5.5. Analyze the reaction mixture by gel electrophoresis, HPLC or ion exchange chromatography (IEC).
Purifications using column-based kits for oligonucleotide purification (e.g. PCR purification kit
from Qiagen) give good results. Make sure the length of the oligonucleotide is compatible with
the purification kit. Alternatively, purification can be done by HPLC, IEC or ethanol precipitation
(product loss likely).
Reaction Volumes and Amounts
This guide will help you to choose the Reactor and calculate the amounts needed.
Table 3: Reaction volumes, final concentrations and molar amounts in “basic” click reactions between oligonucleotides.
Reactor
10x Activator²
Oligo solution1 V
Total V
Final oligo (c)
Oligo (n)
25
2.5 µL
22.5 µL
25 µL
10 - 100* µM
0.25 - 2.5 nmol
100
10.0 µL
90.0 µL
100 µL
10 - 100* µM
1.0 - 10 nmol
1
Note: this “oligo solution” volume is referring to the final volume of the equimolar mixture of the singly-
labeled azide and alkyne oligonucleotide to be used in the reaction.
* Note: In order to achieve a final oligo concentration of 100 µM, at least a 220 µM stock solution of each
oligo is needed; for 10 µM, 22 µM is required.
ClickTech Oligo Link Kit
7
6. Preparative Label-Oligo Click Protocol
For click reactions using more concentrated oligo stock solutions (>100 µM final concentration) the
protocol needs some adaption.
6.1. Dissolve/Dilute your label azide/label alkyne in DMSO or water to 10 - 50 mM to use it as a stock
solution.
6.2. Add the appropriate amount of 10x Activator2 to the Reactor, e.g. 10 µL Activator² are added to
Reactor 100 to be used with a total reaction volume of 100 µL. Depending on Reactor and final
volume, this needs to be adjusted (see Table 4).
6.3. Add the alkyne/azide modified oligonucleotide to the vial to a final concentration of 100 - 700 µM.
6.4. Add 2 - 4 equivalents of label azide/alkyne per equivalent of alkyne/azide in the oligonucleotide.
For example a 200 µM solution of a singly alkyne-modified oligonucleotide is mixed with
400 - 800 µM of a label azide for the click reaction.
6.5. Incubate the mixture at 45 °C, 600 rpm for 2 h in a thermomixer. Alternatively, a water bath can
be used. Make sure that the Reactor is within the reaction solution during the reaction. Spin down
the solution if needed.
6.6. Spin down the Reactor. Transfer the supernatant with the product to a new vial. Wash the
remaining pellet with half the reaction volume of H2O to get all reaction mix to the new vial.
Note: For long-term storage, reacted samples (without Reactor) should be kept at – 20 °C.
6.7. Analyze the reaction mixture by gel electrophoresis, HPLC or ion exchange chromatography (IEC).
Purifications using column-based kits for oligonucleotide purification (e.g. PCR purification kit
from Qiagen) give good results. Make sure the length of the oligonucleotide is compatible with
the purification kit. Alternatively, purification can be done by HPLC, IEC or ethanol precipitation
(product loss likely).
Exemplary Preparative Label-Oligo Click Setups
Table 4: Exemplary volumes needed for reaction setups of “preparative” label-oligonucleotide clicks.
Reactor
V (Activator²)
C1 (Oligo)
V(Oligo)
C (Label)
V2 (Label)
V (H2O)
Oligo (n)
25
2.5 µL
200 µM
20 µL
10 mM
1.2 µL
1.3 µL
4.0 nmol
25
2.5 µL
500 µM
20 µL
20 mM
1.5 µL
1.0 µL
10 nmol
25
2.5 µL
1.0 mM
20 µL
50 mM
1.2 µL
1.3 µL
20 nmol
100
10 µL
200 µM
80 µL
10 mM
4.8 µL
5.2 µL
16 nmol
100
10 µL
500 µM
80 µL
20 mM
6.0 µL
4.0 µL
40 nmol
100
10 µL
1.0 mM
80 µL
50 mM
4.8 µL
5.2 µL
80 nmol
1
Concentration of the oligonucleotide stock solution.
2
Note: The volume of label azide/alkyne in the table has been calculated using 3 equivalents for the
reaction.

ClickTech Oligo Link Kit
8
7. Preparative Oligo Link Click Protocol (Oligo – Oligo Connection)
For click reactions between a singly modified alkyne oligonucleotide and a singly modified azide
oligonucleotide, using more concentrated oligo stock solutions (>200 µM stock, >100 µM final
concentration) the protocol needs some adaption compared to the basic protocol described on page 6.
7.1. Dissolve/Dilute your oligonucleotide azide/alkyne in water to 200 µM - 2.0 mM to use it as a stock
solution. The same stock solution concentration of each alkyne and azide oligo is recommended
to allow convenient reaction setup.
Note: The very common TE buffer, which is commonly used for DNA, is not suitable for click
reactions involving this kit, since the EDTA content will interfere with reaction catalysis (see also
section 11).
7.2. Add the appropriate amount of 10x Activator² to the Reactor, e.g. 10 µL Activator² are added to
Reactor 100 to be used with a total reaction volume of 100 µL. Depending on Reactor and final
volume, this needs to be adjusted (see Table 5).
7.3. Add the alkyne/azide modified oligonucleotide to the vial to a final concentration of 100 - 900 µM.
Equimolar amounts of each reaction partner are recommended, i.e. singly modified
oligonucleotides with a stock solution of the same concentration will be applied at same volumes
(see Table 5 for examples). 90% of the total reaction volume can be used for the reaction
partners, dH2O can be used to reach the total volume if necessary. Do not exceed or decrease the
total reaction volume to obtain good reaction progress.
7.4. Incubate the mixture at 45 °C, 600 rpm for 2 h in a thermomixer. Alternatively, a water bath can
be used. Make sure that the Reactor is within the reaction solution during the reaction. Spin down
the solution if needed.
7.5. Spin down the Reactor. Transfer the supernatant to a new vial. Wash the remaining pellet with
half the reaction volume of H2O to get all reaction mix to the new vial.
Note: For long-term storage, reacted samples (without Reactor) should be kept at – 20 °C.
7.6. Analyze the reaction mixture by gel electrophoresis, HPLC or ion exchange chromatography (IEC).
Purifications using column-based kits for oligonucleotide purification (e.g. PCR purification kit
from Qiagen) give good results. Make sure the length of the oligonucleotide is compatible with
the purification kit. Alternatively, purification can be done by HPLC, IEC or ethanol precipitation
(product loss likely).

ClickTech Oligo Link Kit
9
Exemplary Preparative Oligo Link Click Setups
Table 5: Exemplary volumes needed for reaction setups of “preparative” oligonucleotide-oligonucleotide clicks.
Reactor
V (Activator²)
c (Oligo1)
V (Oligo1)
c (Oligo2)
V (Oligo2)
c (final)
Oligo (n)
25
2.5 µL
500 µM
11.2 µL
500 µM
11.2 µL
224 µM
5.6 nmol
25
2.5 µL
1.0 mM
11.2 µL
1.0 mM
11.2 µL
448 µM
11 nmol
25
2.5 µL
2.0 mM
11.2 µL
2.0 mM
11.2 µL
896 µM
22 nmol
100
10 µL
500 µM
45 µL
500 µM
45 µL
225 µM
23 nmol
100
10 µL
1.0 mM
45 µL
1.0 mM
45 µL
450 µM
45 nmol
100
10 µL
2.0 mM
45 µL
2.0 mM
45 µL
900 µM
90 nmol
oligo1 = e.g. singly alkyne modified oligonucleotide
oligo2 = e.g. singly azide modified oligonucleotide.
8. Special Protocols
8.1. Smallest Possible Click Reaction
In order to minimize the amounts needed for a single click reaction, it is possible to adjust the click protocol
for smallest scale reactions. When e.g. using 100 µM final oligonucleotide concentrations, only 700 pmol
oligonucleotide is needed for a single click. Down to 70 pmol oligonucleotide per reaction can be used
without a major decrease in yield.
8.1.1. A single Reactor pellet (0.6 - 0.8 mm diameter) is transferred to a 200 µL vial.
8.1.2. Add 0.7 µL of 10x Activator² to the Reactor pellet.
8.1.3. A volume of 6.3 µL can be used for the reaction partner mix, e.g. alkyne containing
oligonucleotide and fluorophore azide.
8.1.4. Briefly spin down the reaction mix and control if the Reactor pellet is in solution.
8.1.5. Incubate the reaction mix at 45 °C for 45 min without shaking. Spin down the Reactor.
Transfer the supernatant to a new vial.
Note: For long-term storage, reacted samples (without Reactor) should be kept at – 20 °C.
8.1.6. Analyze the reaction mixture by gel electrophoresis, HPLC or ion exchange chromatography
(IEC). Purifications using column-based kits for oligonucleotide purification (e.g. PCR
purification kit from Qiagen) give good results. Make sure the length of the oligonucleotide
is compatible with the purification kit. Alternatively, purification can be done by HPLC, IEC
or ethanol precipitation (product loss likely).

ClickTech Oligo Link Kit
10
8.2. RNA Oligonucleotide Click Reactions
Click reactions involving alkyne and azide modified RNA oligonucleotides have been performed
successfully using this ClickTech Oligo Link Kit protocol. RNA is more prone to chemical and enzymatic
degradation than DNA, please handle with precautions. We recommend to use RNase free components
for the reactions and to keep the RNA on ice apart from the reaction incubation step. The provided
reagents in this kit are not tested to be RNase free.
8.3. Small Molecule Click Reactions
The ClickTech Oligo Link Kit S protocol has been successfully used to join azide modified molecules with
terminal alkyne containing small molecules. For example, 1.1 mM of an amino alkyne linker (BCL-091) was
clicked to 1.1 mM 6-FAM azide (BCFA-001) in 2 hours at 45 °C. Complete conversion of the 6-FAM azide
was observed by analytical HPLC. Using the same click conditions, reactions of amino alkyne linker with 3Azido-7-hydroxycoumarin (BCFA-047), Eterneon-Red 645 Azide (BCFA-201) and 5/6-Sulforhodamine 101
PEG3-Azide (BCFA-044) proceeded with at least 91% conversion yield.
Please note that these reaction examples are no guarantee that every reactant combination between azide
and alkyne containing small molecules will perform well using the ClickTech Oligo Link Kit. It might be a
possible option for reaction catalysis. Experimental optimization of reaction time and temperature for
each system should be considered.
9. Alternative Reaction Temperatures
In case that 45 °C incubation is not possible, alternative reaction temperatures can be used. This will
require adjustment of the incubation time. Here are our recommendations for some alternative reaction
temperatures and the standard condition in the basic protocol (10 - 100 µM oligo) for standard click
ligation reactions described herein. If more complex (bio)molecules should be clicked to oligonucleotides
tests on finding the optimal conditions need to be performed using these recommendations as a starting
point.
Temperature
Duration
20 °C
4 - 5 h
30 °C
90 min
45 °C
60 min
60 °C
50 min
ClickTech Oligo Link Kit
11
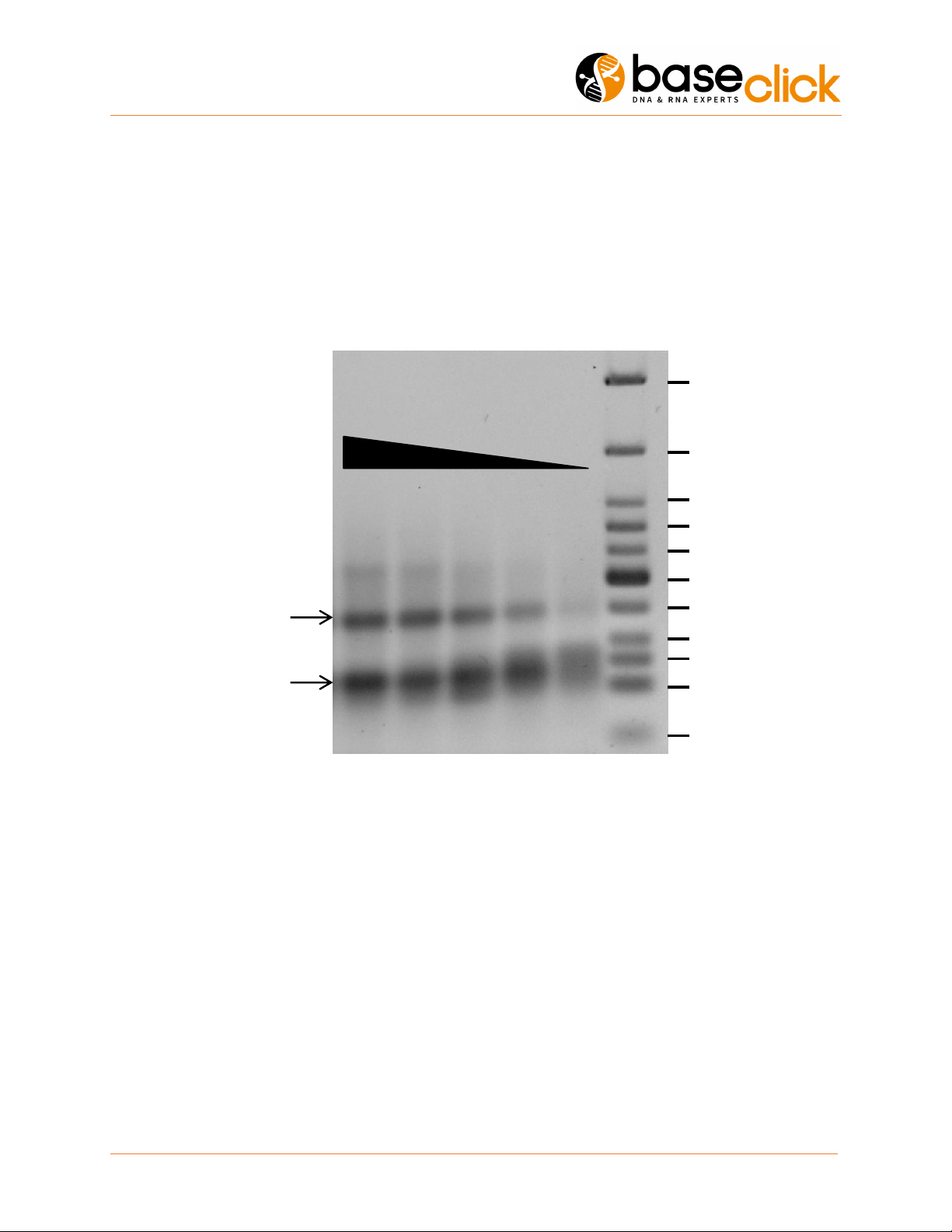
10. Exemplary Gel Image from Oligo link Clicks
Here is an example where the kit has been used to click ligate a 5’-azide oligo (59 mer) to a 5’-alkyne oligo
(59 mer) using varying oligonucleotide amounts. Very low oligonucleotide amounts were studied in order
to determine the lower working range for the kit. No splint oligonucleotides were used for preorganization
of the strands.
Figure 1. Ethidium bromide stained 3% agarose gel from oligo-oligo click reactions using different oligo amounts. For the
analysis the same amount of oligonucleotide was loaded onto each lane of the gel. The sample volumes were adjusted
accordingly. Yield means the densidometric ratio between product band and the sum of all bands in the corresponding lane.
oligo amount in pmol
350 175 87 43 25
product
25
50
75
100
150
200
766
500
350
250
300
27 30 24 18 11 Myield [%]
starting
material

ClickTech Oligo Link Kit
12
11. Trouble Shooting
Some standard commercial buffer components can decrease the click reaction efficiency or even impair
reaction process. For example, TE buffer contains EDTA, which can chelate CuII ions and decrease the
reaction rate. Thiol groups from reducing agents like β-mercaptoethanol or dithiothreitol (DTT) can stop
the click reaction.
Oligonucleotides (DNA and RNA) can have folded structures, which hinder accessibility of the functional
groups that are needed for the reaction. By adding some DMSO (5 - 10% (v/v) final) these folded structures
are destabilized and improved reaction progress can be observed.
Some (dye) labels show low solubility in the final reaction mixture, when used at higher concentrations
and amounts, especially in the preparative label-oligo click protocol. This can impair reaction progress and
decrease the yield. Try to use additional or alternative co-solvents like methanol or tetrahydrofuran if
possible.
When slow reaction progress is observed, increasing the reaction temperature, e.g. to 60 °C might help to
improve the reaction (see 9) and solubility of the reaction partners.
Dissolved oxygen from air in stock solutions can change the reaction kinetic. Much oxygen decreases, less
oxygen increases the reaction progress. As cold solutions can keep more dissolved gas, cold stock solutions
contribute the most oxygen. A main source for the change of dissolved oxygen are freezing and thawing
cycles. By freshly dissolving dry components in degassed or room temperature stored water the reaction
progress can increase. Saturation of solutions with inert gas (e.g. N2 or argon) can also increase the reaction
progress, if available. Be aware that this will not only decrease the reaction time, but might also decrease
the time from which oligonucleotide degradation starts.
 Loading...
Loading...