Barkey Plasmatherm Use manual
Manufactured by:
Barkey GmbH & Co. KG
Gewerbestrasse 8
33818 Leopoldshoehe
Germany
Instructions for use
Barkey plasmatherm
Version 1 EN
Issued 2009-12-22
for SN 1107500 onwards

Contents
1 Introduction
1.1 Pictograms, signal words and symbols
1.2 Target group
1.3 What you must bear in mind at all times
1.4 Conformities
1.5 Copyright
2 Description of the device
2.1 Components of the Barkey plasmatherm
2.2 Technology description
2.3 Symbols
2.3.1 Operating panel symbols
2.3.2 Symbols used on labels
2.4 Intended purpose
2.5 Contraindication
2.6 Overtemperature protection
2.7 Safety features
3 Safety advice
3.1 Safety advice on the use of the device
3.2 Safety advice on handling the device
3.3 Safety advice on environmental influences
3.4 Electromagnetic properties / safety distances
3.4.1 Electromagnetic emission
3.4.2 Electromagnetic immunity
3.4.3 Recommended safety distance
4 Operation
4.1 Putting into service
4.1.1 Siting the device
4.1.2 Connecting the power cord, printer and barcode scanner
4.1.3 Switching on
4.1.4 Switching on if the fill level is too low
4.1.5 Filling
4.2 Opening the heating chamber
4.3 Loading
4.4 Selecting and starting a program with the menu system
4.4.1 Selecting and starting the "PLASMA" program
4.4.2 Changing the remaining time
4.4.3 Messages at the start of a program
4.4.4 Messages while a program is running
4.4.5 Messages after the end of a program
4.4.6 Presetting the heating duration of the "PLASMA" program
4.4.7 Guideline values for the heating duration when thawing FFP
4.4.8 Presetting the heating duration and starting the "BLOOD" program
4.4.9 Presetting the heating duration and starting the "HPC" program
4.4.10 Presetting the heating duration and starting the "USER" program
4.4.11 Heating in continuous operation
4.5 Function button assignment
4.6 Fill level display
4.7 Barcode scanner
5 Cleaning and care
5.1 Cleaning
5.2 Dry paper
5.3 Changing the water
5.3.1 Draining off the water
5.3.2 Filling with water
6 Maintenance
6.1 Technical safety inspection (TSI)
6.2 Replacing the battery
7 Error messages
7.1 Moisture sensors
7.2 Overtemperature
7.3 Paddle blocked
7.4 Tank is empty
7.5 Device errors
7.6 Error numbers
8 Warranty and disclaimer
9 Customer service
10 Factory settings of the programs
11 Specifications
Declaration of Conformity
ISO Certificate
Barkey plasmatherm device master data sheet
Introduction
WARNING
If disregarded: danger to persons.
CAUTION
If disregarded: danger to property,
the device or basic device functions.
NOTE
Additional useful advice and information.
(the "i" stands for "information".)
►
Instruction step.
Carry out this step as indicated.
1 Introduction
Congratulations on your decision to use the Barkey plasmatherm for the
timed heating of whole blood and blood products
thawing and timed heating of fresh frozen plasma (FFP)
thawing and timed heating of HPC (stem cells)
heating and maintaining the warmth of non-denaturable infusion solutions and other
materials in continuous mode.
You have opted for a high-quality product that will give reliable service for many years.
In these instructions you will find all the information you require about the functions, op-
eration and use of the Barkey plasmatherm.
1.1 Pictograms, words and symbols
These instructions use the following pictograms, symbols and words to highlight warnings and special advice:
You will find the following symbol in instructions about the use and maintenance of the
device:
NOTE
The symbols representing the controls and device displays, and the symbols used
on device labels, are listed and described in the Chapter Symbols in these instructions.
1.2 Target group
These instructions are intended for use by:
Medical specialists in hospitals who hold a recognised vocational qualification in
human medicine,
CAUTION
Only persons who meet this criterion may use the device.
1.3 What you must bear in mind at all times
You must follow the conditions of use and safety advice contained in this instruction
manual at all times when using the device. This will ensure that the device is handled
properly and that patients and users cannot be put at risk and equipment cannot be damaged.
Barkey GmbH & Co. KG can accept no liability for damage caused as a result of failure
to follow these instructions.
Introduction
WARNING
These instructions for use are an integral part of the product. They must be retained
throughout the life of the product and handed to any subsequent owner or user.
Please ensure that any supplementary instructions which may be issued are kept together with the original instructions.
Carefully read through these instructions before using the device.
Please follow the advice about the intended use of the device in the Chapter In-
tended purpose and the safety information provided in the Chapter Safety advice.
For a better understanding of these chapters you should familiarise yourself with
the basic functions of the device as described in the Chapter Description of the device.
You should also comply with the requirements for the training and skills of persons
using the device, as indicated in the Chapter Target group.
Medical electrical devices are subject to special safety measures in regard to EMC
(electromagnetic compatibility), and you should therefore always ensure that the
device is installed and operated in accordance with the EMC advice contained in
these instructions.
1.4 Conformities
Please read the declaration of conformity in the Appendix to these instructions.

Introduction
1.5 Copyright
These instructions for use and all illustrations they contain are protected by copyright.
Translation, duplication, reprinting, extraction of images, reproduction using photographic technology and storage and processing in electronic systems, even only excerpts,
and any alterations shall require the written authorisation of Barkey GmbH & Co. KG.
Any further usage which goes beyond the use of the contents described in connection
with the product purchased is not permitted.
Third party products, protected trademarks etc. are always stated without reference to the
registration or copyright status. Existing industrial property rights and registered trademarks are explicitly acknowledged.
We reserve the right to make typographical errors and mistakes, also changes in the interest of technical progress, or which are necessary due to changes in regulations.
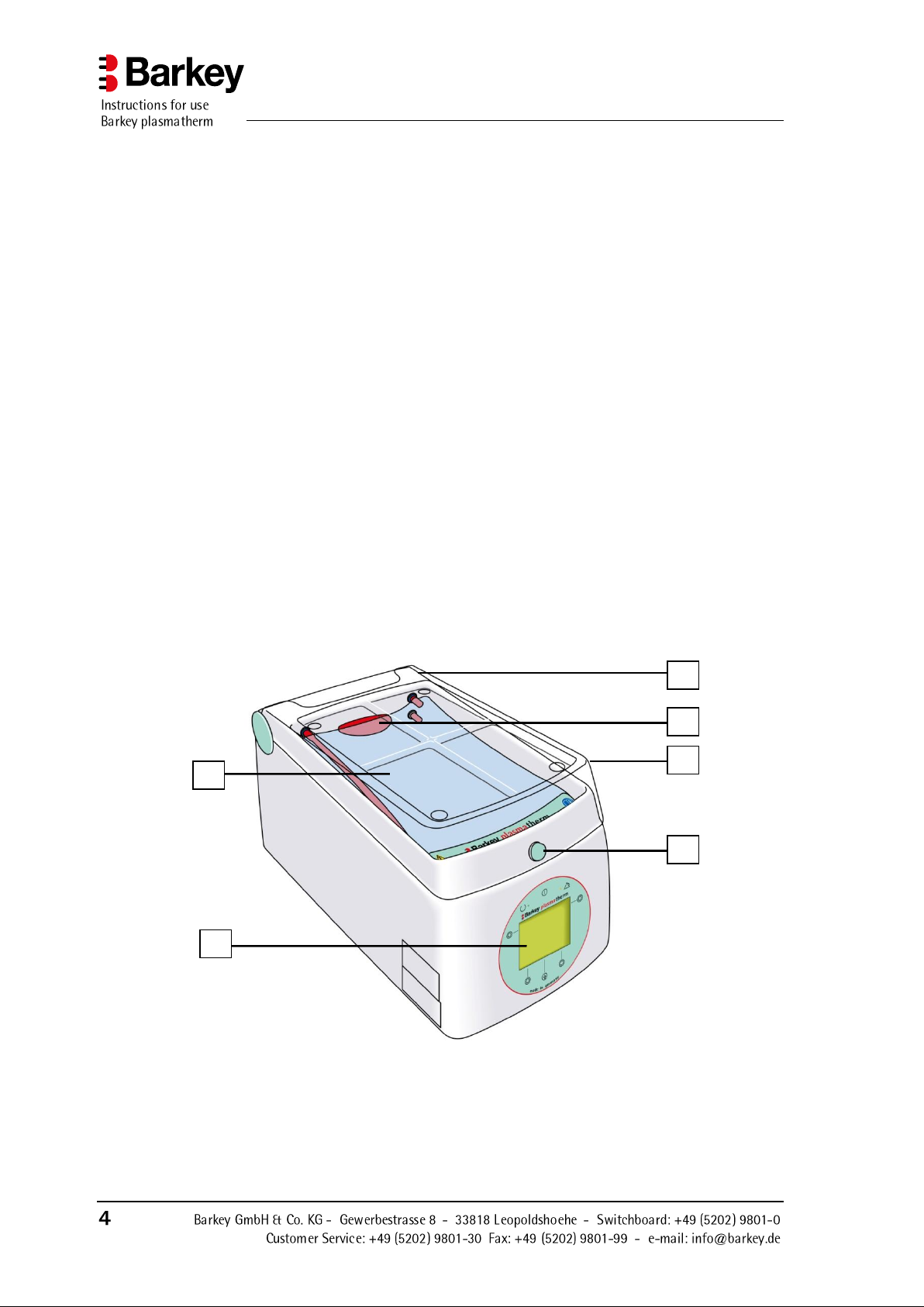
2 Description of the device
1.
Heating chamber
cover
Covers the heating chamber while heating or
thawing is in progress
2.
Filler opening
The filler opening is used to fill the device with
heat transfer fluid
3.
Paddle
Gently agitates FFP´s during the heating process
4.
Heating cushion
Heat transfer fluid flows through the heating
cushions. The cushions heat the materials placed
in the device and keep them warm.
5.
Cover locking/release
button
The locking/release button is used to open and
close the heating chamber cover
6.
Operating panel
The Barkey plasmatherm has an operating panel
on the front of the device with a multi-line display, 6 buttons and 2 lamps (LEDs).
2 3 1 5 4
6
The Barkey plasmatherm is used primarily for thawing and heating fluids contained in
bags or bottles and which are intended for medical transfusion or infusion in living organisms. Typically these fluids are whole blood, blood products, blood preparations and infusion solutions.
2.1 Components of the Barkey plasmatherm
Description of the device
Figure 1: Barkey plasmatherm
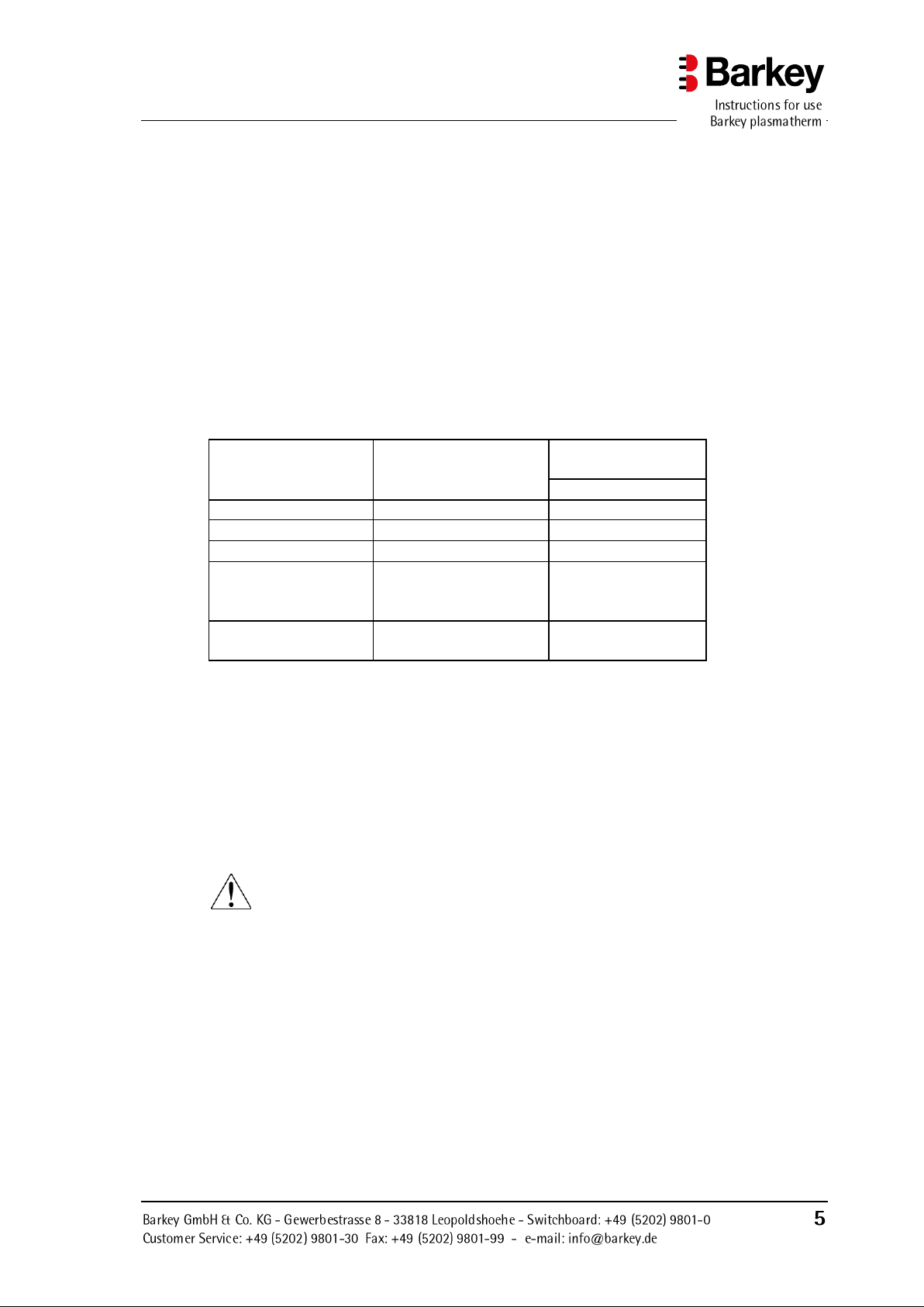
Description of the device
Program name
User preselectable
Acting special func-
tions
Undulation**
BLOOD
Heating time
no
PLASMA*
Heating time
yes
HPC***
Heating time
yes
USER
various
parameters can be
set as required
adjustable or select-
able
CONTINUOUS
OPERATION
no
no
2.2 Technical description
The Barkey plasmatherm is configured as an electronically regulated dry heating device
with an enclosed heating chamber. Bags of fresh frozen plasma (FFP), blood and erythrocyte concentrates (EC), cryoconserved preparations, cryoconserved stem cells (HPC =
haematopoietic progenitor cells) or infusion solutions are placed between soft heating
cushions made from a flexible synthetic material. A heat transfer fluid (distilled or demineralised water) flows through the heating cushions which heat up the materials placed
in the device and keep them warm.
All heating is controlled by heating programs. To heat a particular preparation, the user
selects the appropriate program on the operating panel using the display and buttons. Different functions of the device act on the preparation depending on the program selected.
A number of preferences can also be set. The following table provides an overview of
these:
* The setpoint temperature can be increased to +45°C to accelerate the thawing of fro-
zen plasma conserves. The process now operates at a temperature of +45°C and is
monitored and timed by the program.
** An undulation function which agitates the heated materials is provided for mixing
certain materials such as plasma (FFP, fresh frozen plasma).
*** HPC Haematopoietic progenitor cells (stem cells)
CAUTION
With plasma, thorough mixing of the bag's contents is essential as all protein precipitates (cryoproteins) must be dissolved before the plasma can be used.
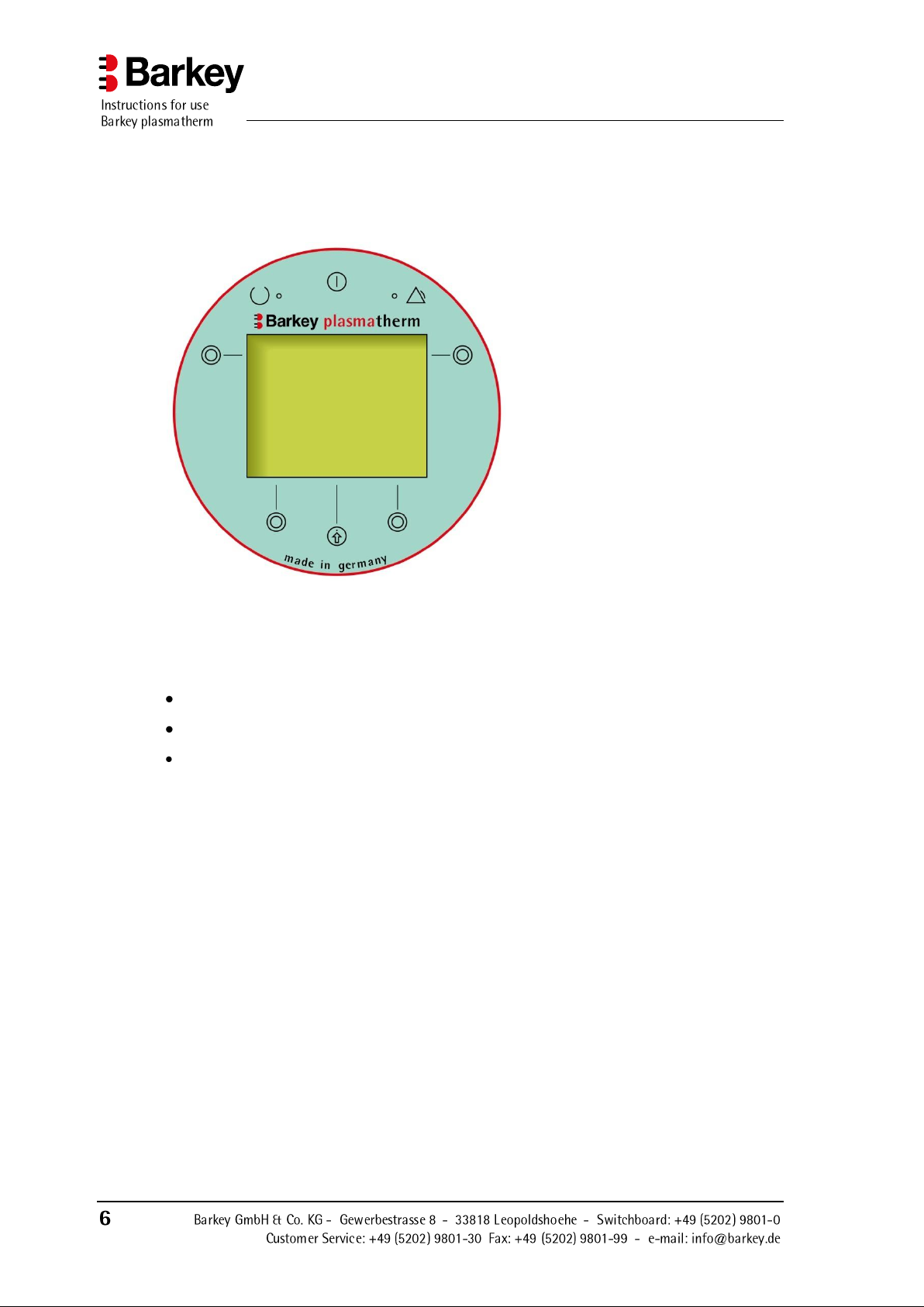
2.3 Symbols
2.3.1 Operating panel symbols
Description of the device
Figure 2: Operating panel
The Barkey plasmatherm has an operating panel on the front of the device with a multiline display, 6 buttons and 2 lamps (LEDs).
The display
displays the menu system for the operation of the device,
displays information about the currently running program and its status, and
outputs warning and error messages.
An audible signal draws your attention to the fact that a program has finished, warns of
operator error or indicates that an error has occurred. A message is additionally shown in
the display in the event of errors.
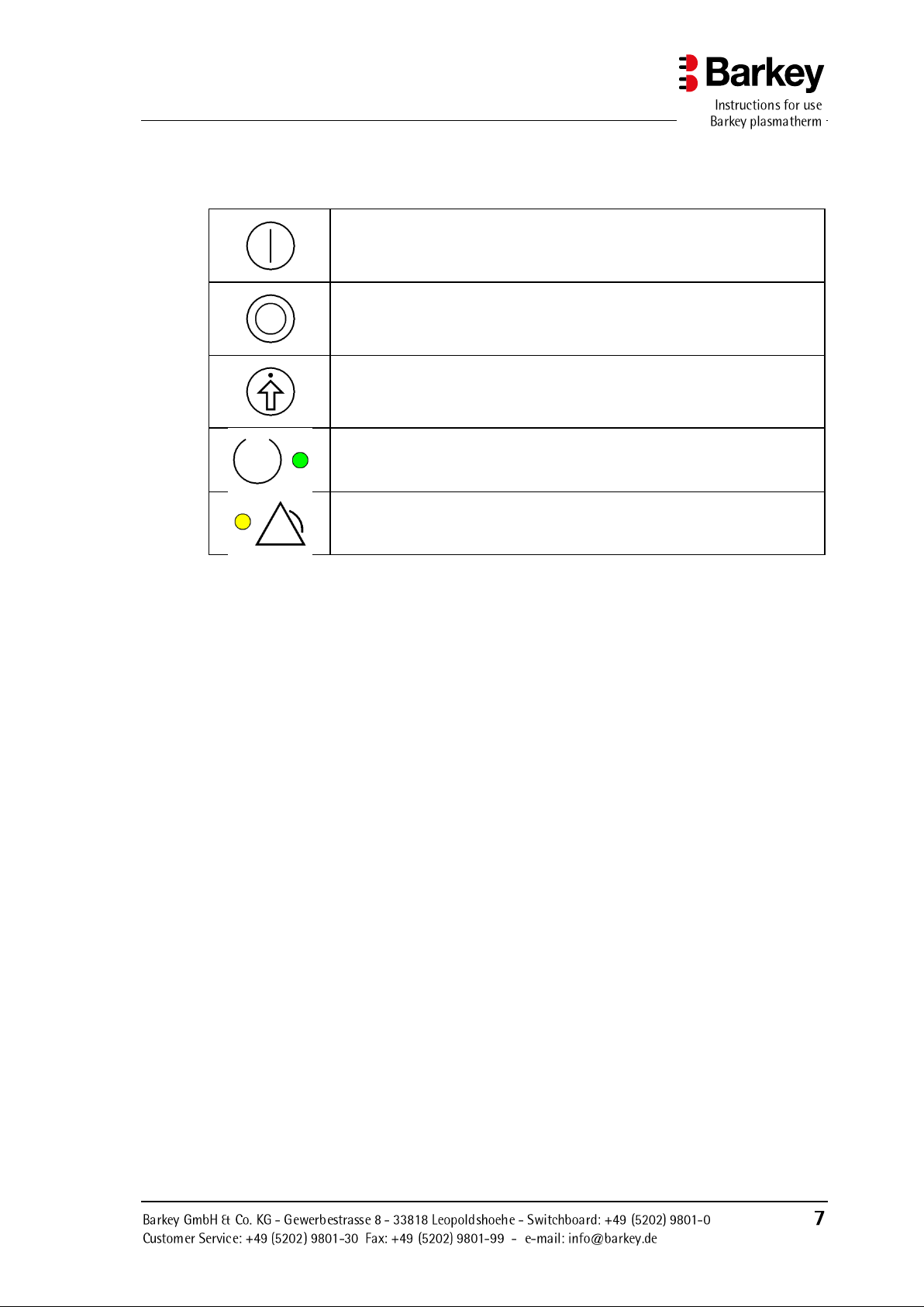
Description of the device
Device On/Off button.
Function button or selection button for menu navigation.
The button's particular function is indicated in the display.
Confirm button for menu navigation.
This button is used to acknowledge / confirm the function which currently appears in the display.
The green light (LED) shows that the device has been switched on.
The yellow light (LED) indicates a device malfunction.
The display elements and controls are identified by symbols as described in the following
table:
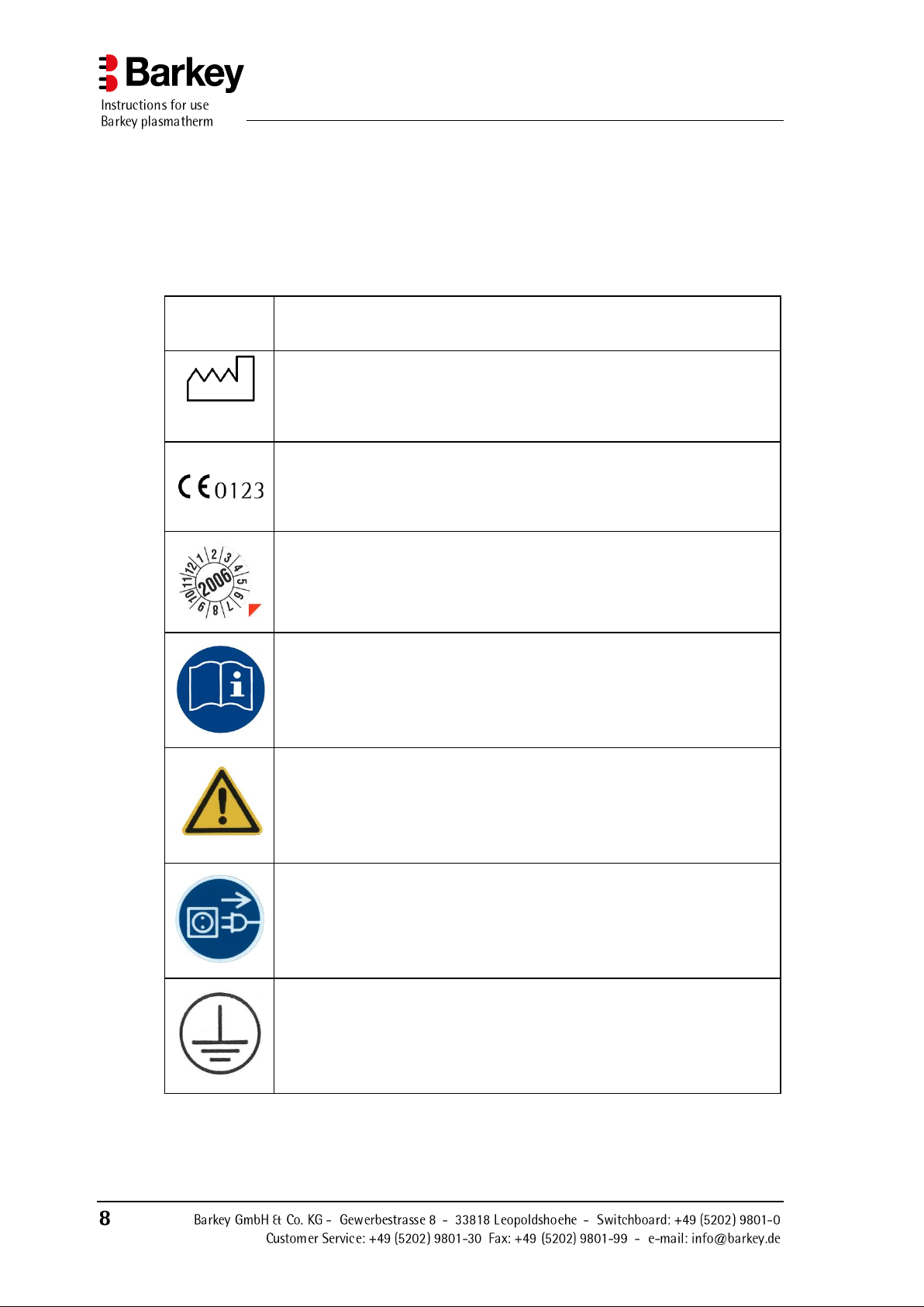
2.3.2 Symbols used on labels
IPX 1
This symbol means that the device is protected against vertically dripping water according to IEC 601-1 in its intended operation conditions.
2010
This symbol shows the year of manufacture as a four-digit number.
This symbol declares that the device conforms to Medical Device Directive 93/42/EEC of 14 June 1993. The four-digit number indicates the
appointed body (TÜV SUED Product Service GmbH) which supervises
the manufacturer's quality assurance system.
This symbol indicates the month and year in which the next safety inspection is due.
This symbol advises you that you must read the instruction manual supplied thoroughly.
This hazard symbol advises you that failure to follow the instructions
contained in the instruction manual can result in hazards to patients, the
device user or the device itself.
This symbol advises you that the device must be disconnected from the
mains supply before the device housing can be opened by removing the
device screws.
This symbol (on a label inside the device) informs you of the earth connection.
Labels showing printed symbols are affixed to the device. These have the following
meaning:
Description of the device

Description of the device
2.4 Intended purpose
The Barkey plasmatherm is a thawing and heating device intended for the following applications:
timed heating of whole blood and blood products
thawing and timed heating of frozen plasma conserves
thawing and timed heating of HPC (haematopoietic progenitor cells)
heating and maintaining the warmth of non-denaturable infusion solutions and other
materials in continuous operation.
The Barkey plasmatherm can be used whenever it is desirable to prevent the cooling of
patients as a result of transfusions, infusion solutions or other materials.
These summarised statements on the intended use of the device are supplemented in this
instruction manual by specific descriptions of the various different applications and of the
handling of the device. You will find these descriptions in the Chapters Safety advice to
Operation of this instruction manual. Please use these chapters to find specific information about usage of the device in individual cases.
2.5 Contraindication
The device must not be used to heat or keep warm animals or to thaw, heat or keep warm
objects or fluids of any kind except those as described under „Intended purpose‟.
There are no known contraindications when thawing and/or heating blood and blood
products.
2.6 Overtemperature protection
Independent overtemperature protection systems monitor the temperature of the device.
In the event of a fault or if an overtemperature limit is reached, the device's heating is
switched off, the yellow LED in the display and operating panel lights up and a continuous alarm tone sounds.
Should this occur, switch off the device or disconnect the mains plug and wait for the device to cool down. This may take several minutes. Then switch on the device again, however the fault will return if the cause of the problem has not been rectified.
WARNING
If the overtemperature alarm sounds, any preparations that are in the device must
be removed and checked before being transferred to the patient.
The Barkey plasmatherm must not be used if it has a fault. The device should be
examined by Barkey GmbH & Co. KG or authorised personnel.

2.7 Safety features
Safe, gentle thawing and heating conditions for the Blood, Plasma and HPC programs
are ensured by a dual overtemperature protection which switches off the device in the
event of overtemperature
Proven not to destroy important and sensitive biological components of blood and
blood products as a result of excessive temperatures or violent mechanical agitation
Automatic detection of possible leaks by moisture sensors in the heating chamber
The device uses a dry heating process that prevents the contents of damaged con-
serves (hairline cracks) being contaminated by the heat transfer fluid
The heating procedure can be monitored. Fluid leakage is easy to detect through the
use of transparent heating cushions, the light colours used in the heating chamber and
white dry-paper (filter paper) on the heating chamber floor
Plain text displays in the local language
Clearly arranged and labelled displays and controls
The device is designed for continuous operation
Synthetic enclosure is corrosion free and saves energy
Description of the device
The device is stable, designed not to tip over, and has non-slip feet
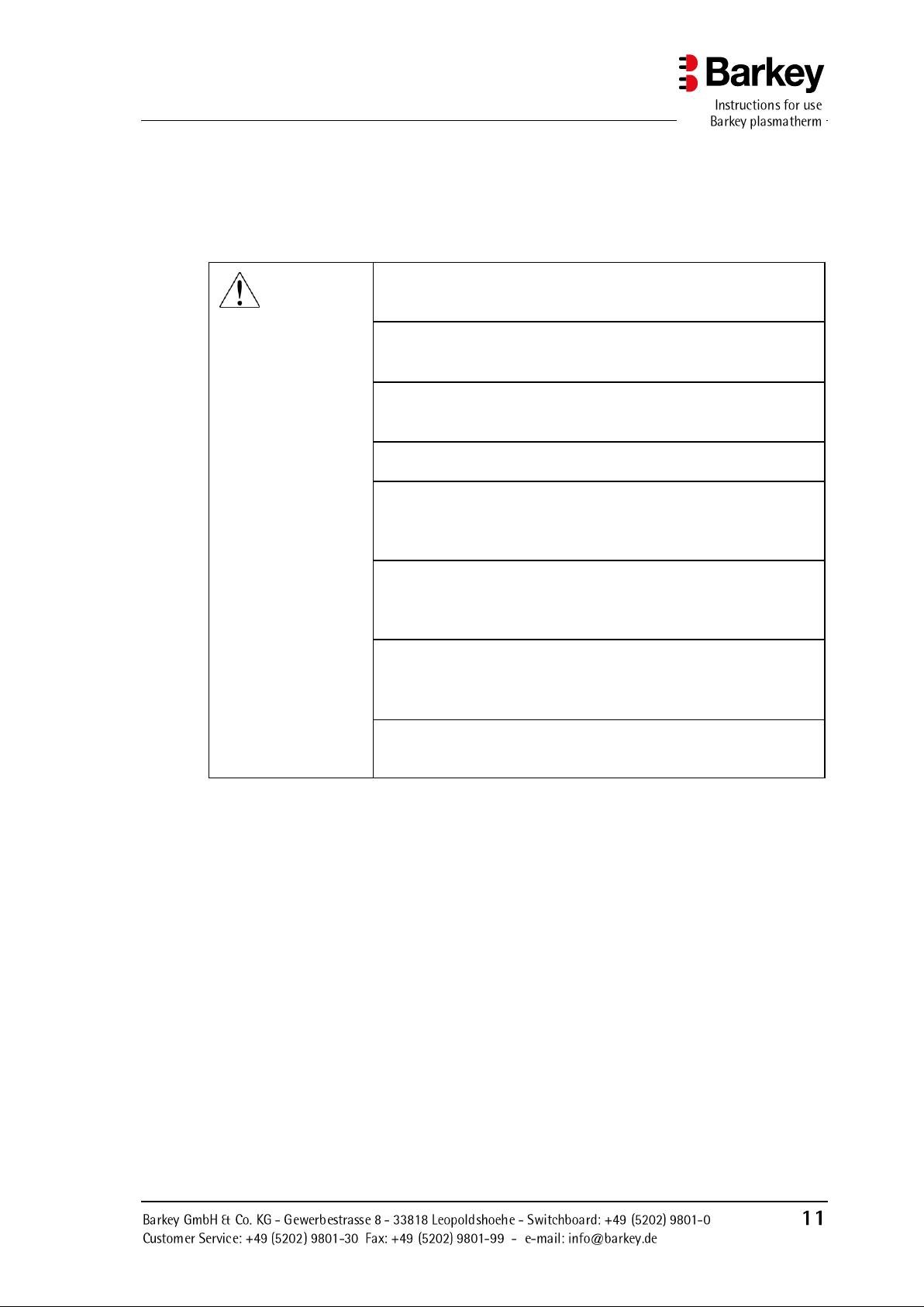
Safety advice
WARNING
Before using the device, carefully read and familiarise yourself
with these instructions and the user documentation for the optional accessories.
Only use the device in accordance with the regulations as described previously in this chapter and in accordance with the
processes described in this instruction manual.
When heating blood and blood products, always ensure that the
operating temperature and time limit are not exceeded. Remove and transfuse immediately when signal sounds!
The blood products may only be heated and/or thawed with the
programs specifically intended for them.
If infusion solutions or medications are heated in the Barkey
plasmatherm, you must ensure that their efficacy is maintained
during heating and that the timed heating is approved by the
manufacturer of the medication.
If preparations leak, this is due to previously damaged conserve bags (e.g. hairline cracks, damage in transit). The sensors
in the Barkey plasmatherm detect leaking moisture and stop
the heating process.
The undulation function is only activated in the Plasma and
HPC programs. Do not use the undulation function for blood
conserves due to possible mechanical damage and agglutination of erythrocytes.
The device must not be used if it has a fault. The device should
be examined by Barkey GmbH & Co. KG or authorised personnel.
3 Safety advice
3.1 Safety advice on the use of the device
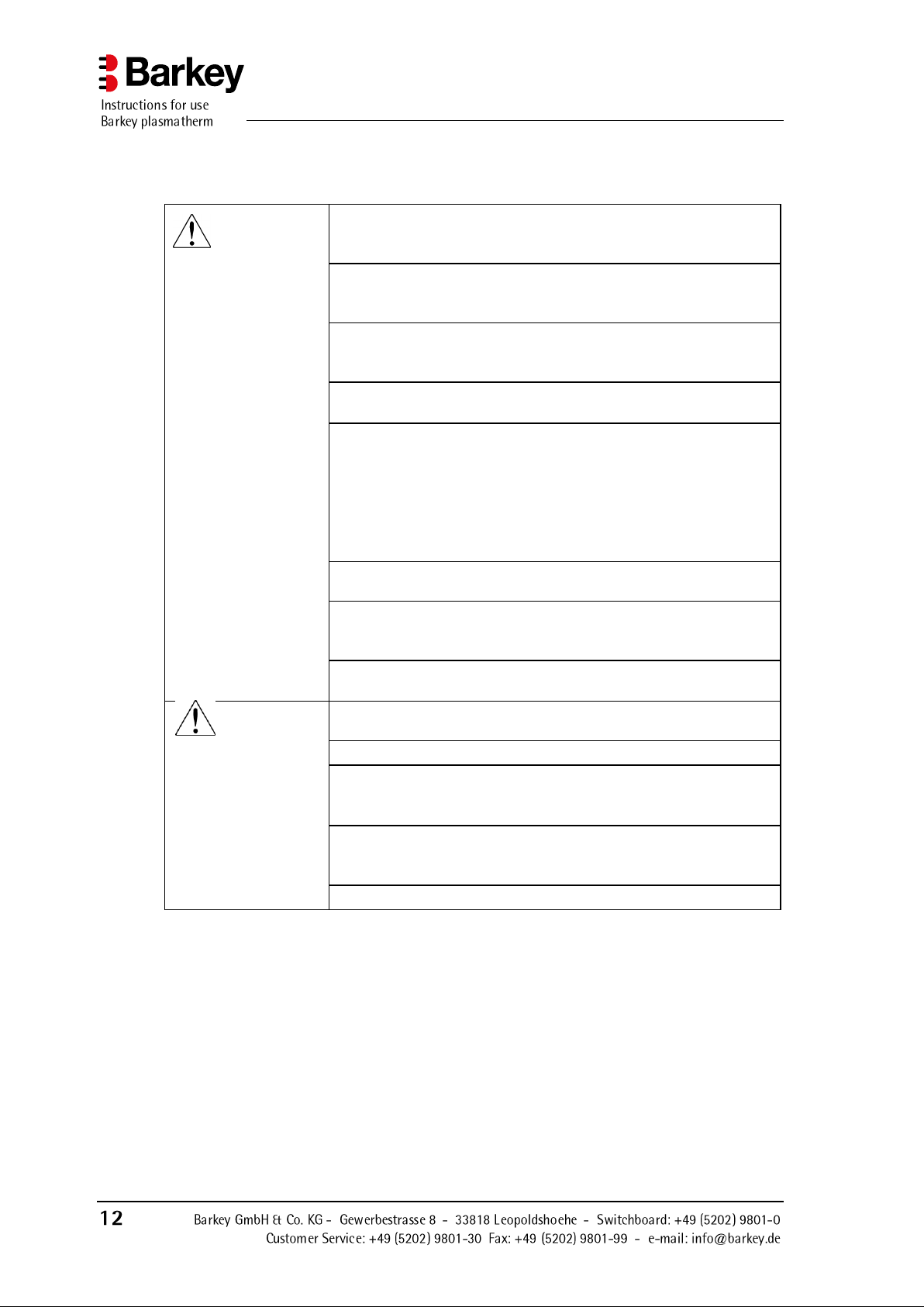
3.2 Safety advice on handling the device
WARNING
Before using the device, carefully read and familiarise yourself
with these instructions and the user documentation for the optional accessories.
All electrical installations must comply with the relevant applicable regulations and standards in addition to the specifications
stated by the manufacturer.
Only power supply connections supplied by Barkey GmbH &
Co. KG which are designed for the device's rated voltage may
be used.
The mains plug must be removed from the mains socket to
ensure safe isolation of the device from the power supply.
The device contains no parts which can be repaired by the user.
Do not attempt to repair the device yourself. You should contact the manufacturer or your medical service technician who
can request information about repairs from the manufacturer if
necessary. Repairs and modifications to the device may only
be carried out by Barkey GmbH & Co. KG or authorised personnel.
The heating cushions of the device must not be allowed to
come into contact with sharp-edged objects.
The heating chamber and the heating cushions must be cleaned
and disinfected at least once per week! The filter paper must be
replaced after each cleaning.
An annual safety inspection must be carried out by qualified
service technicians or employees of Barkey GmbH & Co. KG.
CAUTION
The water must be changed once a year! You should always
add two micropur tablets when refilling.
Do not tilt the device when it is switched on!
The battery (lithium battery CR 1225, 3 V) must be replaced
every three years by qualified service personnel or employees
of Barkey GmbH & Co. KG.
Repairs and modifications to the device may only be carried
out by qualified service technicians or by employees of Barkey
GmbH & Co. KG.
The device's rating plate is on the left-hand side of the housing.
Safety advice
Safety advice
WARNING
The influence of strong electromagnetic fields (e.g. through the
use of HF therapy or surgical devices) can lead to malfunctions
in the Barkey plasmatherm. If interference of this type occurs,
increase the distance between the Barkey plasmatherm and the
device causing the interference, or operate the devices at different times. The Barkey plasmatherm works perfectly within
the limit values set in the EN 60601-1-2 standard. The device
can be influenced outside the limit values set by EN 60601-1-
2.
Portable and mobile HF communication equipment such as
mobile phones can affect the device.
Do not use the device in the immediate vicinity of
flammable materials (e.g. gases, liquids),
flammable mixtures of anaesthetic substances with air,
flammable mixtures of anaesthetic substances with oxygen
or nitrous oxide
whose flashpoint is below 50°C. It is imperative that the device
is not used in areas in which alcohol disinfectants and anaesthetising substances are being used simultaneously.
The device may not be set up or operated in the immediate
vicinity of devices with a high heat output.
The device must be positioned so as to ensure an unrestricted
flow of air around its base.
3.3 Safety advice on environmental influences
3.4 Electromagnetic properties / safety distances
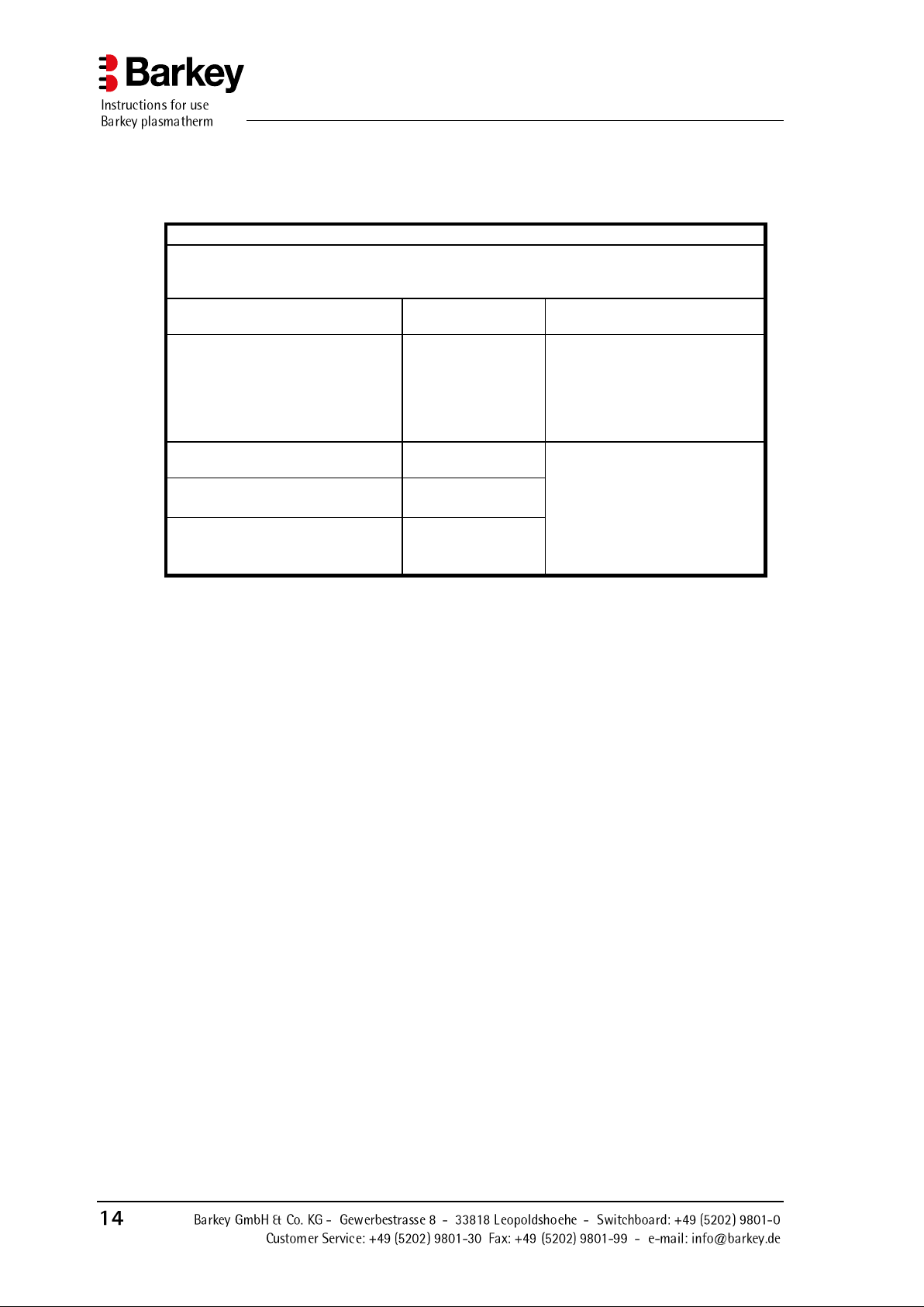
Guidelines and manufacturers declaration - Electromagnetic emission
The Barkey plasmatherm is intended for operation in one of the environments listed below.
The customer or user of the Barkey plasmatherm must ensure that it is operated in one of these
environments.
Radiated EMI measurements
Compliance
Electromagnetic environment guidelines
HF outputs in accordance with
CISPR 11
Group 1
The Barkey plasmatherm uses
high-frequency energy for internal functions only. This means
that HF emission is very low, and
neighbouring electronic devices
are unlikely to be affected.
HF outputs in accordance with
CISPR 11
Class B
The Barkey plasmatherm is suitable for use in buildings other
than residential and those which
are directly connected to a public
supply network which is also
used to supply buildings used for
residential purposes.
Harmonic output in accordance
with IEC 61000-3-2
Class A
Emission of voltage fluctuations/
flicker according to IEC 61000-3-3
Complies
3.4.1 Electromagnetic emission
Safety advice
Safety advice
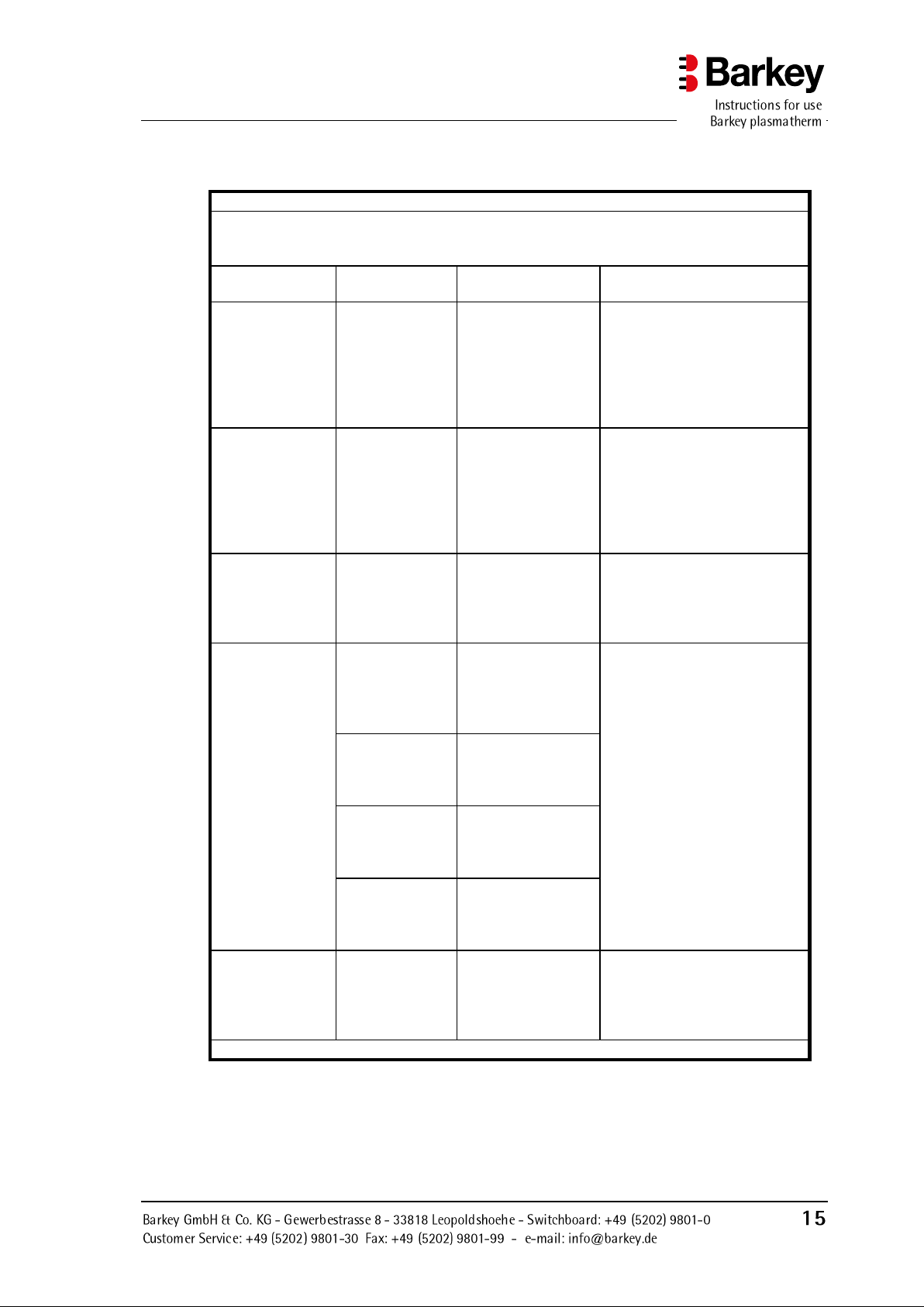
Guidelines and manufacturers declaration - Electromagnetic immunity
The Barkey plasmatherm is intended for operation in one of the electromagnetic environments
listed below. The customer or user of the Barkey plasmatherm must ensure that it is operated
in one of these environments.
Immunity test
IEC 60601 test
level
Compliance level
Electromagnetic environment
- Guideline
Static discharge
(ESD) according
to
IEC 61000-4-2
± 6 kV
contact discharge
± 8 kV air discharge
± 6 kV
contact
discharge
± 8 kV air discharge
The floor should be constructed
in wood or concrete or be covered with ceramic tiles. If the
floor is covered with synthetic
material, the relative humidity
must be at least 30%.
Rapid transient
electrical noise/
bursts according
to
IEC 61000-4-4
± 2 kV for power
cords
± 1 kV for input
and output cords
± 2 kV for power
cords
± 1 kV for input and
output cords
The quality of the supply voltage should comply with a typical business or hospital environment.
Surges according
to IEC 61000-4-5
± 1kV voltage
phase-to-phase
± 2kV voltage
phase-to-earth
± 1kV voltage
phase-to-phase
± 2kV voltage
phase-to-earth
The quality of the supply voltage should comply with a typical business or hospital environment.
Voltage dips,
short-time interruptions and fluctuations in supply
voltage according
to
IEC 61000-4-11
< 5 % UT
(> 95 % drop
of UT )
for 1/2 period
< 5 % UT
(> 95 % drop of UT )
for 1/2 period
The quality of the supply voltage should comply with a typical business or hospital environment. If the user of the
Barkey plasmatherm requires
continued use even if the power
supply is interrupted, we recommend that the Barkey plasmatherm is connected to an
uninterruptible power supply or
battery.
40 % UT
(60 % drop in
UT ) for 5 periods
40 % UT
(60 % drop in UT )
for 5 periods
70 % UT
(30 % drop in
UT ) for 25 periods
70 % UT
(30 % drop in UT )
for 25 periods
< 5 % UT
(> 95 % drop of
UT )
for 5 s
< 5 % UT
(> 95 % drop of UT )
for 5 s
Magnetic field at
supply frequency
(50/60 Hz) according to IEC
61000-4-8
3 A/m
3 A/m
Magnetic fields at mains frequency should comply with the
typical values as found in business and hospital environments.
NOTE: UT is the mains AC supply before applying the test rule
3.4.2 Electromagnetic immunity
Safety advice
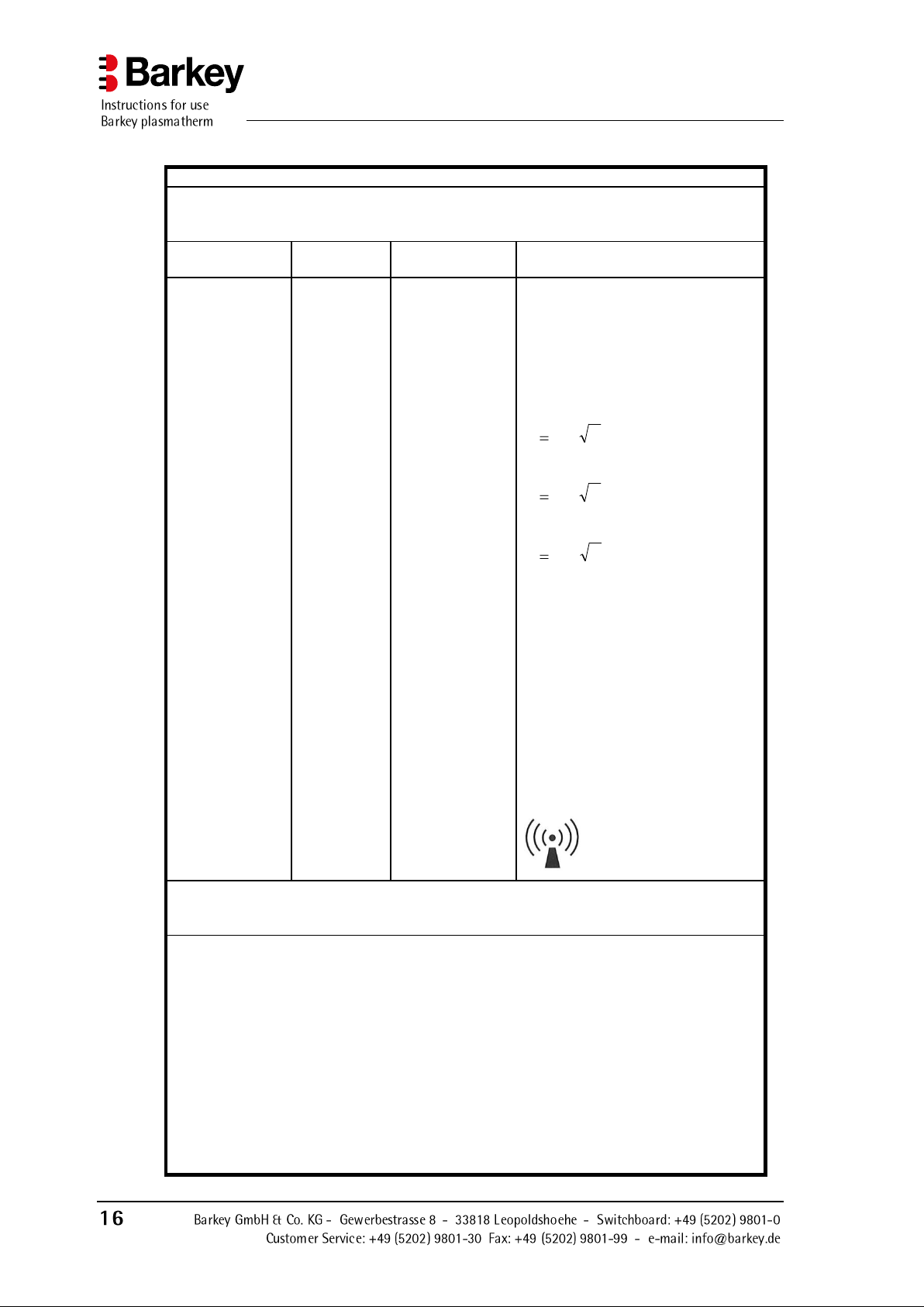
Guidelines and manufacturers declaration - Electromagnetic immunity
The Barkey plasmatherm is intended for operation in one of the electromagnetic environments listed below. The customer or user of the Barkey plasmatherm must ensure that it is
operated in one of these environments.
Immunity test
IEC 60601
test level
Compliance level
Electromagnetic environment guideline
Conducted HF
interference according to IEC
61000-4-6
Radiated HF interference according to IEC 610004-3
3 V
eff
150 kHz to
80 MHz
3 V/m
80 MHz to
2.5 GHz
3 V
eff
150 kHz to 80
Mhz
3 V/m
80 MHz to 2.5
GHz
Portable and mobile radio sets should
not be used within a distance from the
Barkey plasmatherm, including cords,
that is less than the recommended
safety distance as calculated by the
relevant equation for the transmit
frequency.
Recommended safety distance
GHz 2.5 to MHz800for
33.2
MHz800 to MHz80for
17.1
MHz80 tokHz 150for
17.1
Pd
Pd
Pd
where P is the nominal power of the
transmitter in Watts (W) as stated by
the transmitter manufacturer, and d is
the recommended safety distance in
metres (m).
The field strength of stationary
transmitters should always be less
than the compliance level b at all
frequencies in accordance with an onsite investigationa.
Interference is possible near devices
that display the following symbol.
NOTE 1 At 80 MHz and 800 MHz the higher frequency range applies.
NOTE 2 These guidelines may not apply in all cases. The propagation of electromagnetic
variables is affected by absorption and reflection of buildings, objects and people.
a
The field strength of static transmitters, such as base stations of radio telephones and
mobile land radios, amateur radios, AM and FM radio and television transmissions cannot be
accurately theoretically determined in advance. You should consider carrying out a site survey
to determine the electromagnetic environment with regard to static transmitters. If the measured field strength at the location where the Barkey plasmatherm is being used exceeds the
above compliance levels, the Barkey plasmatherm should be monitored to ensure that the
device is functioning as intended. If unusual performance characteristics are observed, additional measures such as changing the alignment or location of the Barkey plasmatherm may be
necessary.
b
The field strength should be less than 3 V/m in the frequency range 150 kHz to 80 MHz.

Safety advice
Recommended safety distances between portable and mobile
HF telecommunications devices and the Barkey plasmatherm
The Barkey plasmatherm is intended for operation in an electromagnetic environment in
which HF interference is controlled. The customer or user of the Barkey plasmatherm can help
avoid electromagnetic interference by observing the minimum distance between portable and
mobile HF communications devices (transmitters) and the Barkey plasmatherm as stated below, depending on the output power of the communication device.
Nominal
transmitter
power W
Safety distance depending on transmitter frequency m
150 kHz to 80 MHz
Pd 17,1
80 MHz to 800 MHz
Pd 17,1
800 MHz to 2.5 GHz
Pd 33,2
0.01
0.12
0.12
0.23
0.1
0.37
0.37
0.74
1
1.17
1,17
2.33
10
3.69
3.69
7.38
100
11.67
11.67
23.33
For transmitters whose maximum nominal output is not given in the above table, the recommended safety distance d in metres (m) can be determined using the equation belonging to the
appropriate column, where P is the maximum nominal output of the transmitter in Watts (W)
as stated by the transmitter manufacturer.
NOTE 1 The higher frequency level applies at 80 MHz and 800 MHz.
NOTE 2 These guidelines may not apply in all cases. The propagation of electromagnetic
variables is affected by absorption and reflection of buildings, objects and people.
3.4.3 Recommended safety distance
4 Operation
4.1 Putting into service
If you are putting a new or repaired device into service, you should first
select a suitable location for it
connect the necessary cords
and fill the device with heat transfer fluid.
CAUTION
You must disinfect the heating cushions and heating chamber before using the device. This procedure is described in the Chapter Cleaning of these instructions.
4.1.1 Siting the device
The Barkey plasmatherm is designed for use as a fixed installation inside buildings. It is
not intended for mobile use.
The Barkey plasmatherm must be set up on a stable, hard and level surface. Unobstructed
access to the device from above and in front must be guaranteed.
Operation
CAUTION
The device must be lifted by a minimum of 2 people. When carrying, grip the device by the bottom edge only. Recesses are provided in the base for safe carrying.
CAUTION
The device is ventilated from below. It should therefore not be placed on a soft sur-
face into which its feet could sink. There must be a minimum distance of 50 mm on
3 sides of the Barkey plasmatherm between it and walls, cabinets or other devices.
CAUTION
When selecting the location for the device, it is imperative that you maintain the
distances stated in the Chapter of this
instruction manual from other devices with electromagnetic emission.
 Loading...
Loading...