Bard Poly Per-Q-Cath Instructions For Use Manual

with Safety Microintroducer
Instructions For Use
Bard Access Systems
3
Triple Lumen PICC
Bard Access Systems 1
Product Description
The Poly Per-Q-Cath
*
3
Triple Lumen PICC is a triple lumen peripherally placed central catheter. Each Poly
Per-Q-Cath
*
3
Triple Lumen PICC is made from specially formulated and processed polyurethane and other
medical grade materials. The Poly Per-Q-Cath
*
3
Triple Lumen PICC catheters feature a thin wall design
with one large and two smaller lumens. Catheters are packaged in a tray with accessories for reliable long
(greater than 30 days) or short (less than 30 days) term vascular access. This microintroducer kit is an
introducer system designed for access of peripheral veins using a minimal insertion techniques for the
placement of Bard Access Systems Peripherally Inserted Central Catheters and Midline Catheters.
New Important Information:
• Warning: When using alcohol or alcohol containing antiseptics with polyurethane PICCs, care should be
taken to avoid prolonged or excessive contact. Solutions should be allowed to completely dry before applying an occlusive dressing. Chlordexidine gluconate and/or povidone iodine are the suggested antiseptics to
use.
• Warning: Alcohol should not be used to soak or declot polyurethane PICCs because alcohol is known to
degrade polyurethane catheters over time with repeated and prolonged exposure.
• The
Poly Per-Q-Cath
*
3
Triple Lumen PICC catheter features a reverse-taper catheter design.
Caution:
Placement of larger catheters at or below antecubital fossa may result in an increased incidence of phlebitis.
Placement of
Poly Per-Q-Cath
*
3
Triple Lumen PICC
above antecubital fossa is recommended.
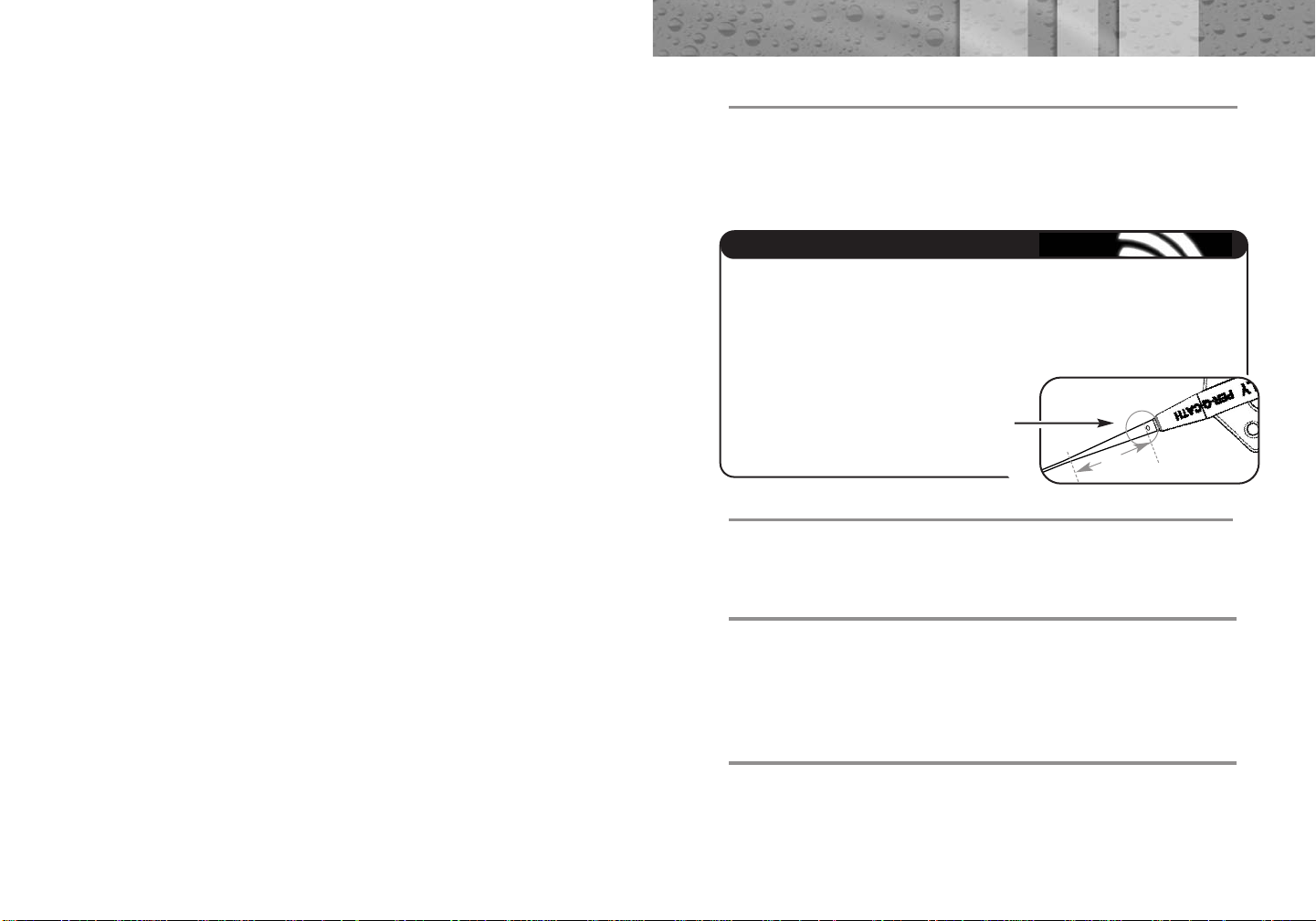
• For Superior Vena Cava (SVC) placement, measure from
the planned insertion site to the right clavicular head,
then down to the third intercostal space. Use the zero
mark as reference for point of insertion.
• Catheter does not require “s” curve for dressing and
securement.
Indications
The Poly Per-Q-Cath
*
3
Triple Lumen PICC is indicated for short or long term peripheral access to the central venous system for intravenous therapy and blood sampling. For blood therapy, it is recommended that a
4 french or larger catheter be used.
Contraindications:
The device is contraindicated whenever:
• The presence of device-related infection, bacteremia, or septicemia is known or suspected.
• The patient’s body size is insufficient to accommodate the size of the implanted device.
• The patient is known or is suspected to be allergic to materials contained in the device.
• Past irradiation of prospective insertion site.
• Previous episodes of venous thrombosis or vascular surgical procedures at the prospective placement site.
• Local tissue factors will prevent proper device stabilization and/or access.
Warnings:
• When using alcohol or alcohol containing antiseptics with polyurethane PICCs, care should be taken to
avoid prolonged or excessive contact. Solutions should be allowed to completely dry before applying an
occlusive dressing. Chlordexidine gluconate and/or povidone iodine are the suggested antiseptics to use.
• Alcohol should not be used to soak or declot polyurethane PICCs because alcohol is known to degrade
polyurethane catheters over time with repeated and prolonged exposure.
• Acetone and polyethylene glycol containing ointments should not be used with polyurethane catheters, as
these may cause failure of the device.
Reverse
Taper

Bard Access Systems 3
Poly Per-Q-Cath
*
3
Triple Lumen PICC 2
• Avoid accidental device contact with sharp instruments and mechanical damage to the catheter material.
Use only smooth-edged atraumatic clamps or forceps.
• Avoid perforating, tearing, or fracturing the catheter when using a stylet.
• Do not use the catheter if there is any evidence of mechanical damage or leaking.
• Avoid placement or securement of the catheter where kinking may occur, to minimize stress on the
catheter, patency problems or patient discomfort.
• Avoid sharp or acute angles during implantation which could compromise the patency of the catheter
lumen(s).
• Do not suture around the catheter as sutures may damage the catheter or compromise catheter
patency.
• Do not cut the stylet.
• Do not use the device if there is any evidence of mechanical damage or leaking. Damage to the
catheter may lead to rupture, fragmentation and possible embolism and surgical removal.
• Accessories and components used in conjunction with this device should incorporate luer lock connec-
tions.
• If signs of extravasation exist, discontinue injections. Begin appropriate medical intervention immedi-
ately.
• Infusion pressure greater than 25 psi (172 kPa) may damage blood vessels and viscus and is not rec-
ommended. DO NOT USE ASYRINGE SMALLER THAN 10 ml.
• For further information or questions, please call 800-443-3385 or 801-595-0700.
Possible Complications
The potential exists for serious complications including the following:
• Air Embolism • Exit Site Infection • Phlebitis
• Bleeding • Exit Site Necrosis • Spontaneous Catheter
• Brachial Plexus Injury • Extravasation Tip Malposition or Retraction
• Cardiac Arrhythmia • Fibrin Sheath Formation • Thromboembolism
• Cardiac Tamponade • Hematoma • Venous Thrombosis
• Catheter Erosion • Intolerance Reaction to • Ventricular Thrombosis
Through the Skin Implanted Device • Vessel Erosion
• Catheter Embolism • Laceration of Vessels or • Risks Normally Associated with
• Catheter Occlusion Viscus Local or General Anesthesia,
• Catheter-related • Myocardial Erosion Surgery and Post Operative
Sepsis • Perforation of Vessels Recovery
• Endocarditis or Viscus
III. After placement, observe the following precautions to avoid
device damage and/or patient injury:
II. To avert device damage and/or patient injury during placement.
• This is not a right atrium catheter. Avoid positioning the catheter tip in the right atrium. Placement or
migration of the catheter tip into the right atrium may cause cardiac arrhythmia, myocardial erosion or
cardiac tamponade.
• Intended for Single Patient Use. DO NOT REUSE. Bard Access Systems products are single use
devices and should never be reimplanted. Reuse carries with it the attendant concern of cross-infection regardless of the cleaning or sterilization method. Resterilization of incompletely cleaned devices
may not be effective. Any device that has been contaminated by blood should not be reused or resterilized.
• After use, this product may be a potential biohazard. Handle and discard in accordance with accepted
medical practice and applicable local, state and federal laws and regulations.
ChloraPrep
*
One-Step Applicator
• Flammable, keep away from fire or flame.
• Do not use with electrocautery procedures.
• For external use only.
• When using this product keep out of eyes, ears, and mouth. May cause serious or permanent injury if
permitted to enter and remain. If contact occurs, rinse with cold water right away and contact a physician.
• Stop use and ask a doctor if irritation, sensitization, or allergic reaction occurs. These may be signs of
a serious condition.
• Keep out of reach of children. If swallowed, get medical help or contact a poison control center right
away.
Precautions:
• Carefully read and follow all instructions prior to use.
• Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
• Only qualified healthcare practitioners should insert, manipulate and remove these devices.
•
The
Poly Per-Q-Cath
*
3
Triple Lumen PICC catheter features a reverse-taper catheter design.
Placement of larger catheters at or below antecubital fossa may result in an increased incidence of
phlebitis. Placement of the Poly Per-Q-Cath
*
3
Triple Lumen PICC above antecubital fossa is recom-
mended.
• Never use force to remove the stylet. Resistance can damage the catheter.
If resistance or bunching of the catheter is observed, stop stylet withdrawal and allow the catheter to
return to normal shape. Withdraw both the catheter and stylet together approximately 2 cm and reattempt stylet removal. Repeat this procedure until the stylet is easily removed. Once the stylet is out,
advance the catheter into the desired position (zero mark).
• To minimize the risk of catheter breakage and embolization, the catheter must be secured in place.
• Do not cut stylet.
• Follow Universal Precautions when inserting and maintaining the catheter.
• Follow all contraindications, warnings, cautions, precautions and instructions for all infusates as speci-
fied by their manufacturer.
• Use aseptic techniques whenever the catheter lumen is opened or connected to other devices.
• The fluid level in the catheter will drop if the catheter connector is held above the level of the patient’s
heart and opened to air. To help prevent a drop in the fluid level (and thus air entry) while changing
injection caps, hold the connector below the level of the patient’s heart before removing the injection
cap.
• Examine the package carefully before opening to confirm its integrity and that the expiration date has
not passed. The device is supplied in a sterile package and is non-pyrogenic. Do not use if package is
damaged, opened or the expiration date has passed. Sterilized by ethylene oxide. Do not resterilize.
•
Inspect kit for inclusion of all components.
• Flush the catheter with sterile normal saline or heparinized saline prior to use. Catheter stylet must be
wetted prior to stylet repositioning or withdrawal.
I. Prior to beginning placement procedure, do the following:
 Loading...
Loading...