Page 1
INSTRUCTION 11-9026
FYRITE® Gas Analyzer
CO2 and O2 Indicators
Operation/Maintenance
Rev. 8 – September 2005
20
16
%CO
2
12
8
4
0
®
Bacharach, Inc.
621 Hunt Valley Circle, New Kensington, PA 15068
Phone: 724-334-5000 • Fax: 724-334-5001
Web: www.bacharach-inc.com
Printed in U.S.A. ® Registered Trademarks
Page 2
WARRANTY
Bacharach, Inc. warrants to Buyer that at the time of delivery this Product will
be free from defects in material and manufacture and will conform substantially
to Bacharach Inc.’s applicable specifications. Bacharach’s liability and Buyer’s
remedy under this warranty are limited to the repair or replacement, at
Bacharach’s option, of this Product or parts thereof returned to Seller at the
factory of manufacture and shown to Bacharach Inc.’s reasonable satisfaction to
have been defective; provided that written notice of the defect shall have been
given by Buyer to Bacharach Inc. within one (1) year after the date of delivery of
this Product by Bacharach, Inc.
Bacharach, Inc. warrants to Buyer that it will convey good title to this Product.
Bacharach’s liability and Buyer’s remedy under this warranty of title are limited
to the removal of any title defects or, at the election of Bacharach, to the
replacement of this Product or parts thereof that are defective in title.
The warranty set forth in paragraph 1 does not apply to parts the Operating
Instructions designate as having a limited shelf-life or as being expended in
normal use.
THE FOREGOING WARRANTIES ARE EXCLUSIVE AND ARE GIVEN AND
ACCEPTED IN LIEU OF (I) ANY AND ALL OTHER WARRANTIES, EXPRESS
OR IMPLIED, INCLUDING WITHOUT LIMITATION THE IMPLIED
WARANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR
PURPOSE: AND (II) ANY OBLIGATION, LIABILITY, RIGHT, CLAIM OR
REMEDY IN CONTACT OR TORT, WHETHER OR NOT ARISING FROM
BACHARACH’S NEGLIGENCE, ACTUAL OR IMPLIED. The remedies of the
Buyer shall be limited to those provided herein to the exclusion of any and all
other remedies including, without limitation incidental or consequential damages. No agreement varying or extending the foregoing warranties, remedies or
this limitation will be binding upon Bacharach, Inc. unless in writing, signed by
a duly authorized officer of Bacharach.
NOTE: Gaskets are considered consumable items and are excluded
from the terms of this warranty.
Register Your W arranty by Visiting
www.bacharach-inc.com
CAUTION
Corrosive fluid is contained inside this unit.
These instructions should be thoroughly read
before operation is attempted.
Copyright © 1998-2005 Bacharach, Inc., all rights reserved.
A
Page 3
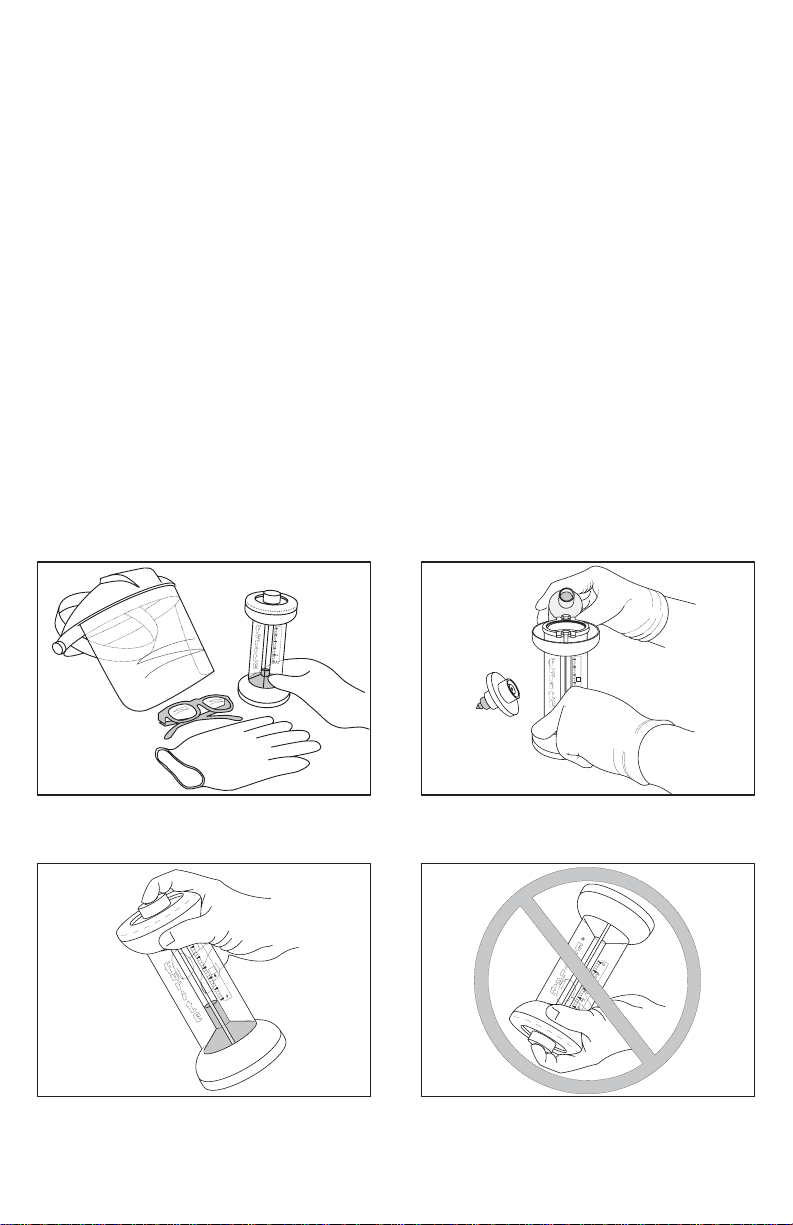
FYRITE SAFETY PRECAUTIONS
The operator (s) of this instrument should thoroughly familiarize themselves with the
applicable safety precautions before handling or using the FYRITE for gas analysis.
Review Figures 1a,1b, 1c and 1d on this page. Make certain to follow the steps
outlined below and read the fluid handling precautions for your personal safety.
1) Always use protective equipment such as safety goggles, gloves, and protective
clothing as illustrated in Figure 1a.
2) Read the instruction manual thoroughly. When filling the FYRITE with the
applicable fluid charge, use safety equipment and perform the operation in the
vicinity of running tap water (Figure 1b).
3) The FYRITE fluid should always be in the bottom reservoir before and during
sample introduction to the FYRITE. When venting the FYRITE to atmosphere,
hold the instrument in the vertical position and at a location away from your face
(Figure 1c).
4) NEVER vent FYRITE in the inverted position (with the plunger facing downward). This will cause fluid to spill, which is corrosive and contains harmful
elements (Figure 1d).
NOTE: In the event of fluid spill - refer to Section 2.2 (FYRITE Handling
Precautions). For Maintenance - refer to Section 6.0.
20
16
12
8
%CO
2
®
20
16
12
8
4
%CO
2
0
®
Figure 1a. Protective equipment
required before attempting gas
analysis.
20
16
12
2
%CO
4
®
Figure 1c. Venting FYRITE to
atomsphere at 45° angle.
Figure 1b. Preparing to fill FYRITE
using recommended protective
safety equipment.
®
0
4
%CO
2
8
12
16
20
Figure 1d. NEVER vent FYRITE in
the inverted position, which will
cause fluid to spill.
i
Page 4

TABLE OF CONTENTS
1.0 DESCRIPTION ............................................................................................................................................. 1
2.0 FYRITE INSPECTION BEFORE AND DURING TEST............................................................................ 1
2.1 Pre-Operational Check .................................................................................................................... 1
2.2 FYRITE FLUID HANDLING PRECAUTIONS ............................................................................ 2
3.0 BASIC FYRITE OPERATION PERCENT CO2 AND 02............................................................................ 3
3.1 Determining CO2 and O2 FYRITE Fluid Strength ......................................................................... 7
3.2 Operating Precautions to Ensure Maximum Accuracy..................................................................7
3.2.1 Draining Fluid Droplets .................................................................................................. 8
3.3 FYRITE Temperature ...................................................................................................................... 8
3.3.1 Temperature of Gas Sample............................................................................................ 8
4.0 PRINCIPLE OF OPERATION ...................................................................................................................... 9
5.0 APPLICATION INFORMATION ............................................................................................................... 10
5.1 FYRITE Absorbing Fluid .............................................................................................................. 10
5.1.1 FYRITE Refill Fluid (Storage Life, etc.) ..................................................................... 10
5.2 FYRITE Operating Temperature Range ....................................................................................... 11
5.3 Typical FYRITE Applications....................................................................................................... 11
5.4 CO2 FYRITE Only Combustion Testing....................................................................................... 12
5.4.1 Percent CO2 Shows Volume of Excess Air................................................................... 12
5.4.2 Too Much Excess Air Means Inefficient Combustion .................................................13
5.4.3 What is Proper CO2 Value? ........................................................................................... 13
5.4.4 How To Calculate Combustion Efficiency .................................................................. 13
5.5 O2 FYRITE Only Combustion Testing ......................................................................................... 13
5.5.1 Percent O2 Shows Volume of Excess Combustion Air ................................................ 13
5.5.2 Too Much Excess Air Means Inefficient Combustion .................................................13
5.5.3 What is Proper O2 Value? .............................................................................................. 14
5.5.4 Measuring O2 in Addition to CO2................................................................................ 14
5.5.5 Where is Percent CO2 or O2 Measured? ....................................................................... 14
5.6 Background Gases Affecting FYRITE Readings ......................................................................... 16
5.7 General FYRITE Applications ...................................................................................................... 17
5.8 Altitude Correction Table .............................................................................................................. 18
6.0 FYRITE MAINTENANCE ........................................................................................................................ 18
6.1 Increasing FYRITE Fluid To Proper Level ...................................................................................18
6.1.1 Decreasing FYRITE Fluid to Proper Level ..................................................................19
6.2 Checking FYRITE Fluid Strength ................................................................................................ 19
6.2.1 CO2 FYRITE Fluid Only............................................................................................... 19
6.2.2 O2 FYRITE Fluid Only ................................................................................................. 21
6.3 Replacing FYRITE Fluid............................................................................................................... 21
6.4 Inspection of FYRITE for Fluid Leakage ..................................................................................... 22
6.5 Cleaning FYRITE .......................................................................................................................... 24
6.6 Replacing FYRITE Plastic and Rubber Parts............................................................................... 24
6.7 Aspirator Bulb - Sampling Assembly ........................................................................................... 26
6.8 Storing FYRITE and Refill Fluid.................................................................................................. 26
6.9 Replacing Saturator Filter Packing Part #11-0121 ...................................................................... 26
7.0 ILLUSTRATED PARTS LIST FYRITE CO2/O2........................................................................................ 29
7.1 FYRITE Illustrated Parts ............................................................................................................... 30
7.2 FYRITE Sampling Assemblies...................................................................................................... 31
ii
Page 5

INSTRUCTIONS 11-9026
OPERATION/MAINTENANCE
BACHARACH FYRITE MODELS
CO2 and O2 INDICATORS
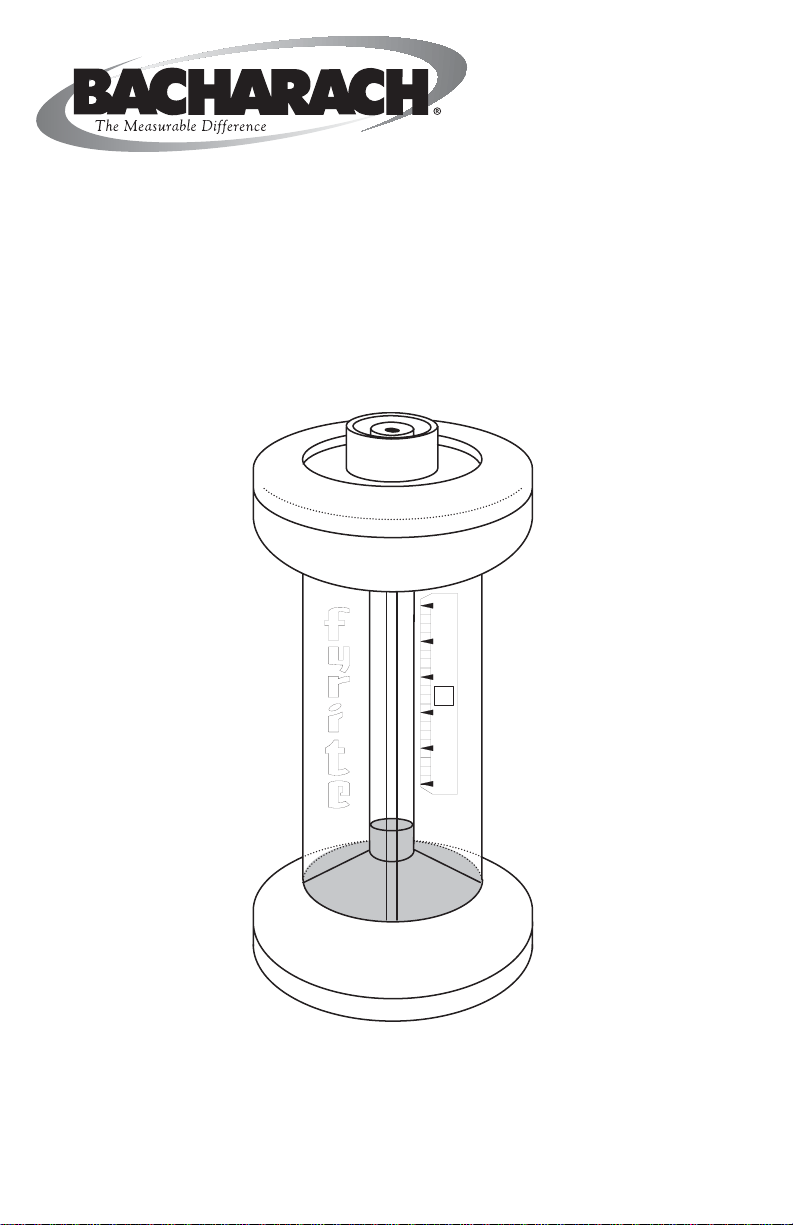
1.0 DESCRIPTION (Refer to Figure 1)
The FYRITE employs the well-known “Orsat” method of volumetric analysis involving chemical absorption of a sample gas, such as carbon dioxide or oxygen. The
reagent used to absorb carbon dioxide (CO2) is potassium hydroxide (dyed red), and
chromous chloride (blue) is the absorbent for oxygen (O2). The unique feature of the
FYRITE is that the absorbing fluid is also used as the indicating fluid so that one
vessel takes the place of both measuring burette and absorption pipette.
The body of the FYRITE is molded of clear high-strength plastic, comprises top and
bottom reservoirs and a center tube connecting the two reservoirs. The bottom of the
lower reservoir is sealed off by a synthetic rubber diaphragm that rests on a perforated metal plate. The upper reservoir is covered by a molded plastic cap that
contains a double-seated plunger valve. A spring holds this valve against a carefully
finished seat in the top cap providing a perfect seal which makes the instrument
spill-proof in any position. When the valve is partially depressed, the entire instrument is open to the atmosphere and the instrument is, of course, is no longer spillproof.
The bottom reservoir is filled with the absorbing fluid, which extends about 1/4 inch
into the bore of the center tube when the instrument is held upright. The scale
position, mounted to one side of the center tube, is adjustable.
2.0 FYRITE INSPECTION BEFORE AND DURING TEST
CAUTION
Corrosive fluid is contained inside unit, instructions should be
thoroughly read before operation is attempted.
2.1 Pre-Operational Check
To ensure proper FYRITE operation and reliable results, check the following:
(a) With FYRITE vented and in a vertical position, it should be possible to adjust
scale zero to the top of the fluid column. Refer to FYRITE Operation for proper
setup (Section 3.0 Steps 1 through 5). If this is not possible, add or remove a
small amount of fluid per Section 6.1.
Instruction 11-9026 Page 1
Page 6

(b) FYRITE Fluid Strength
To check fluid strength.
NOTE: When repeating procedures as outlined in Section 3.0
Steps 7 through 9 (absorbing and reading percent O
absorbing and reading percent CO2) and before venting
and
2
FYRITE to atmosphere for next sample, if the reading increases by more than 1/2 percent for either CO2 or O2, replace
the fluid.
It is often desirable to check fluid strength before taking the FYRITE to a location
where it will be used. It can be tested on sample atmospheric air (which contains
20.9% O
maximum accuracy. (See Section 6.9)
O
2
samples containing 10% O2.
CO
approximately 350 samples containing 10% CO
). Be certain to wet Filter Saturator when checking atmospheric air for
2
(Oxygen) Fluid - Fresh FYRITE fluid will absorb all O2 from approximately 100
(Carbon Dioxide) Fluid - Fresh FYRITE Fluid will absorb all CO2 from
2
.
2
(c) FYRITE Sampling Assembly - Always check sampling assembly for proper
(leak-free) operation as outlined in Section 6.7 before proceeding with test.
(d) Make certain wool in Filter Saturator Tube is moistened with water if using
either O2 or CO2 FYRITE in a noncombustible application where the gas sample
is not fully saturated with water vapor. Examples where filter-saturator must
be wetted include measuring CO
samples. Refer to Section 6.9 when replacing or moistening saturator (wool)
or O2 in ambient air or from compressed gas
2
filter is required.
FYRITE OPERATION PRECAUTION: To prevent fluid
spill, never depress Plunger Valve to vent FYRITE when unit
is in the inverted or any other non-upright position. When
depressing Plunger Valve in the upright position to vent
FYRITE, hold at a slight angle away from the operator’s face.
2.2 FYRITE FLUID HANDLING PRECAUTIONS
CAUTION
FYRITE fluids used in the CO
and contain poisonous elements which must not be taken
internally. In the event of a spill or accidental body contact
with FYRITE fluid, read the following carefully.
Page 2 Instruction 11-9026
and O2 analyzers are corrosive
2
Page 7

HAZARDS
Corrosive liquid causes burns. May cause blindness if splashed in eyes. Vapors are
irritating and may be harmful.
PRECAUTIONS
Prevent contact with eyes, skin and clothing. Wear eye protection and gloves. Do not
vent instrument until fluid has drained from top well. Do not vent instrument
(FYRITE) in inverted position.
FIRST AID
For contact with eyes: Immediately flush eyes with water 20 minutes. Get immediate medical attention.
For contact with skin: Immediately flush skin with water 20 minutes. Get immediate medical attention.
For inhalation overexposure: If irritation develops, move victim to fresh air.
For swallowing: Do not induce vomiting. Give two glasses of water or milk if conscious and not convulsing. Get immediate medical attention.
Refer to the material safety data sheet for further information.
3.0 BASIC FYRITE OPERATION PERCENT CO2 AND 0
2
Four basic steps are required when making an analysis, many being common to both
the CO2 and O2 FYRITE.
FYRITE MODEL CO
(Red Fluid) (Blue Fluid)
2
FYRITE MODEL O
2
(a) Vent and adjust scale zero. (a) Vent and adjust scale zero.
(b) Pump sample into FYRITE. (b) Pump sample into FYRITE.
(c) Absorb CO2 from sample. (c) Absorb O2 from sample.
(d) Read % CO2 on scale. (d) Read % O2 on scale.
NOTE: To prevent warming of FYRITE fluid during analysis,
hold FYRITE by the fins only.
Instruction 11-9026 Page 3
Page 8
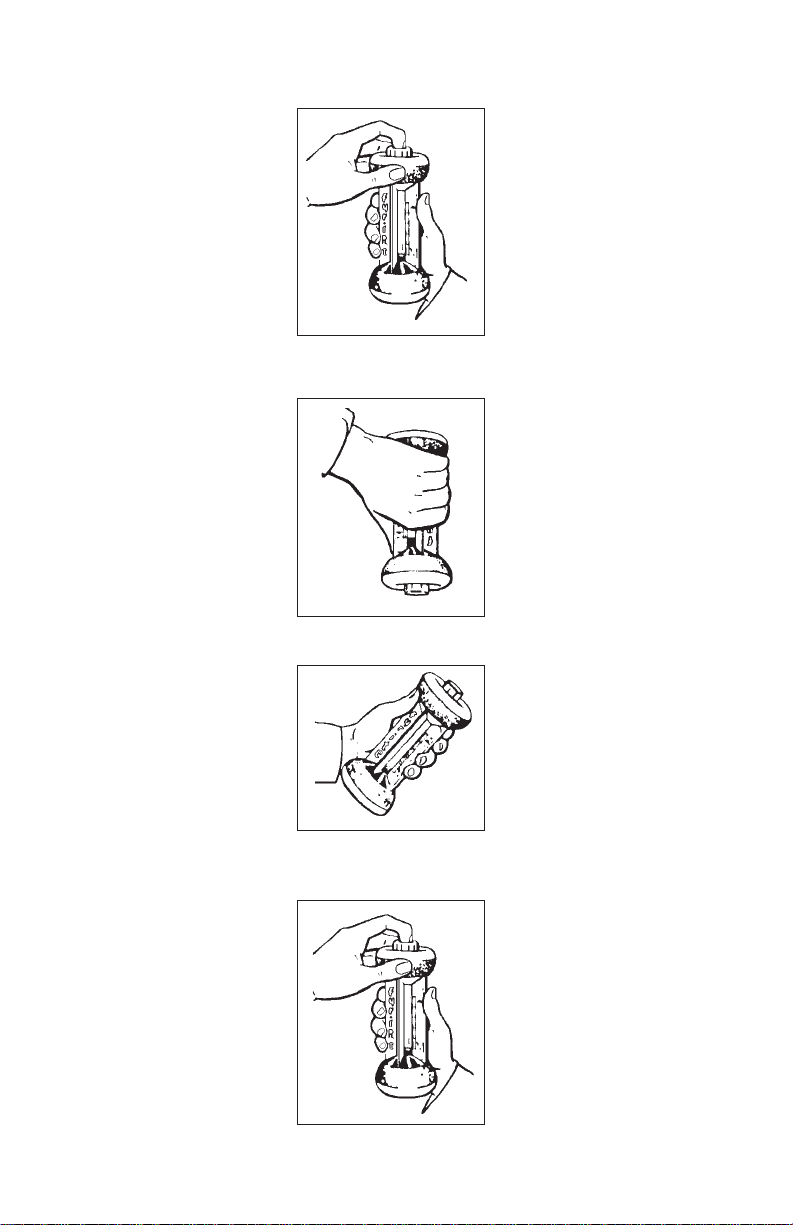
CO2 FYRITE OPERATION O2 FYRITE OPERATION
(RED FLUID) (BLUE FLUID)
1. Hold upright (Fig. 1) and
away from face. Depress
Plunger Valve (momentarily) to vent FYRITE,
and release.
2. Invert FYRITE (Fig. 2).
Hold at slight angle to
drain fluid into top
reservoir.
3. Turn upright. Hold
FYRITE at 45° angle
(Fig. 3) momentarily to
allow fluid droplet
drainage into bottom
reservoir.
Figure 1
Figure 2
Figure 3
1. Hold upright (Fig. 1) and
away from face. Depress
Plunger Valve (momentarily) to vent FYRITE,
and release.
2. Invert FYRITE (Fig. 2) to
absorb O2 drawn into
FYRITE whenever
Plunger Valve is depressed). Hold at slight
angle to drain fluid into
top reservoir.
3. Turn upright. Hold
FYRITE at 45° angle to
drain fluid into bottom
reservoir. Turn upright.
Repeat sequence of Steps
1, 2, and 3 twice until
fluid level does not drop
more that 1/2% when
Plunger Valve is depressed. Turn upright.
Hold FYRITE at 45°
angle (Fig. 3) momentarily until excess fluid
droplets have been
drained from inside
surfaces.
4. Hold FYRITE in upright
(Fig. 4) position and away
from face. Depress
Plunger Valve (momentarily) and release.
4. Hold FYRITE in upright
(Fig. 4) position and away
from face. Note fluid level
in column. Depress
Plunger Valve (momentarily), and release.
Figure 4
Page 4 Instruction 11-9026
Page 9
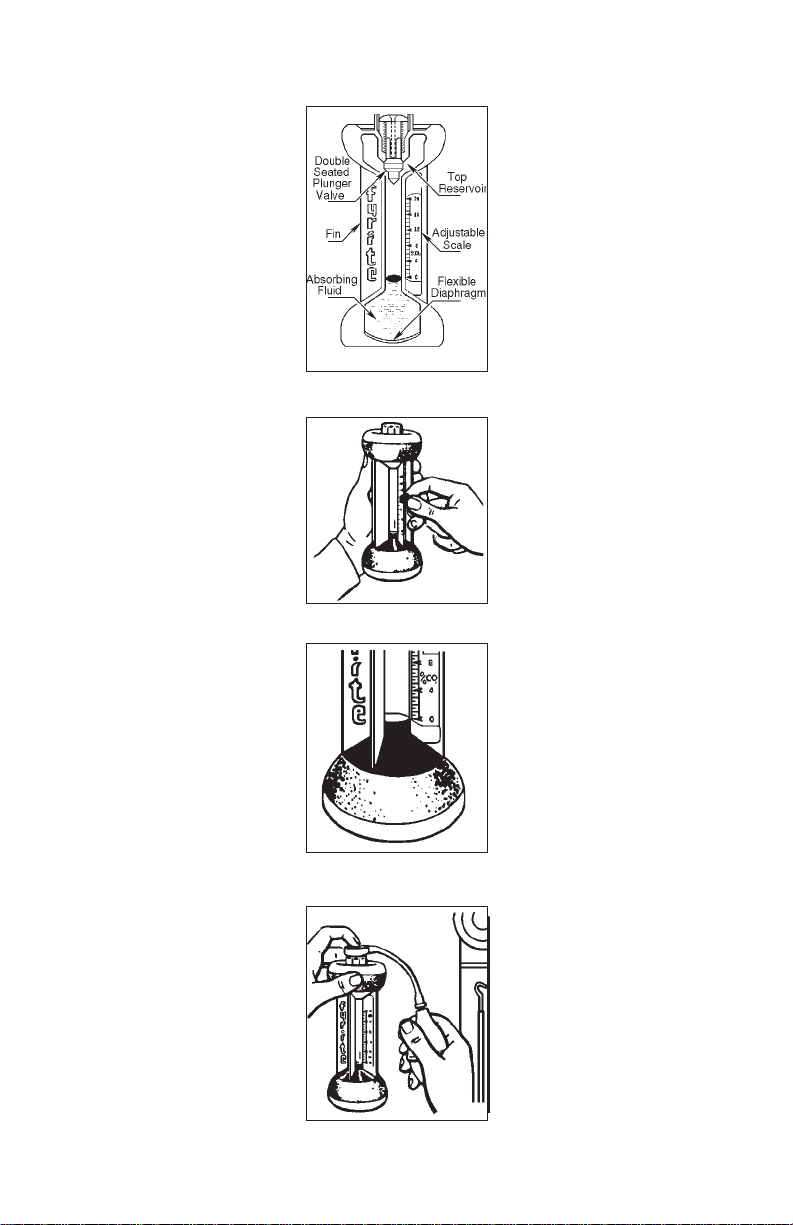
CO2 FYRITE OPERATION O2 FYRITE OPERATION
(RED FLUID) (BLUE FLUID)
5. Holding FYRITE upright
(Fig. 5), loosen locknut at
rear of scale. Slide scale
(Fig. 5a) until top of fluid
column lines up with zero
line on scale (Fig. 5b).
Tighten scale locknut.
NOTE: When setting
scale zero, hold FYRITE
vertically as shown and
level with eyes while
sighting across scale to
top of fluid column.
6. To pump gas sample into
FYRITE (Fig. 6), insert
open end of metal
sampling tube into area
bearing gas for analysis.
Hold FYRITE in upright
position and place
sampling assembly
rubber connector tip over
the Plunger Valve.
Depress Plunger Valve
firmly with connector tip.
Figure 5
Figure 5a
Figure 5b
5. Holding FYRITE upright
(Fig. 5), loosen locknut at
rear of scale. Slide scale
(Fig. 5a) until top of fluid
column lines up with zero
line on scale (Fig. 5b).
Tighten scale locknut.
NOTE: When setting
scale zero, hold FYRITE
vertically as shown and
level with eyes while
sighting across scale to
top of fluid column.
6. To pump gas sample into
FYRITE (Fig. 6), insert
open end of metal
sampling tube into area
bearing gas for analysis.
Hold FYRITE in upright
position and place
sampling assembly
rubber connector tip over
the Plunger Valve.
Depress Plunger Valve
firmly with connector tip.
Pump sample by squeezing and releasing
aspirator bulb 18 times.
During 18th bulb squeeze
(with bulb held deflated)
release connector tip and
Plunger Valve.
Pump sample by squeezing and releasing
aspirator bulb 18 times.
During 18th bulb squeeze
(with bulb held deflated)
release connector tip and
Plunger Valve.
Figure 6
Instruction 11-9026 Page 5
Page 10
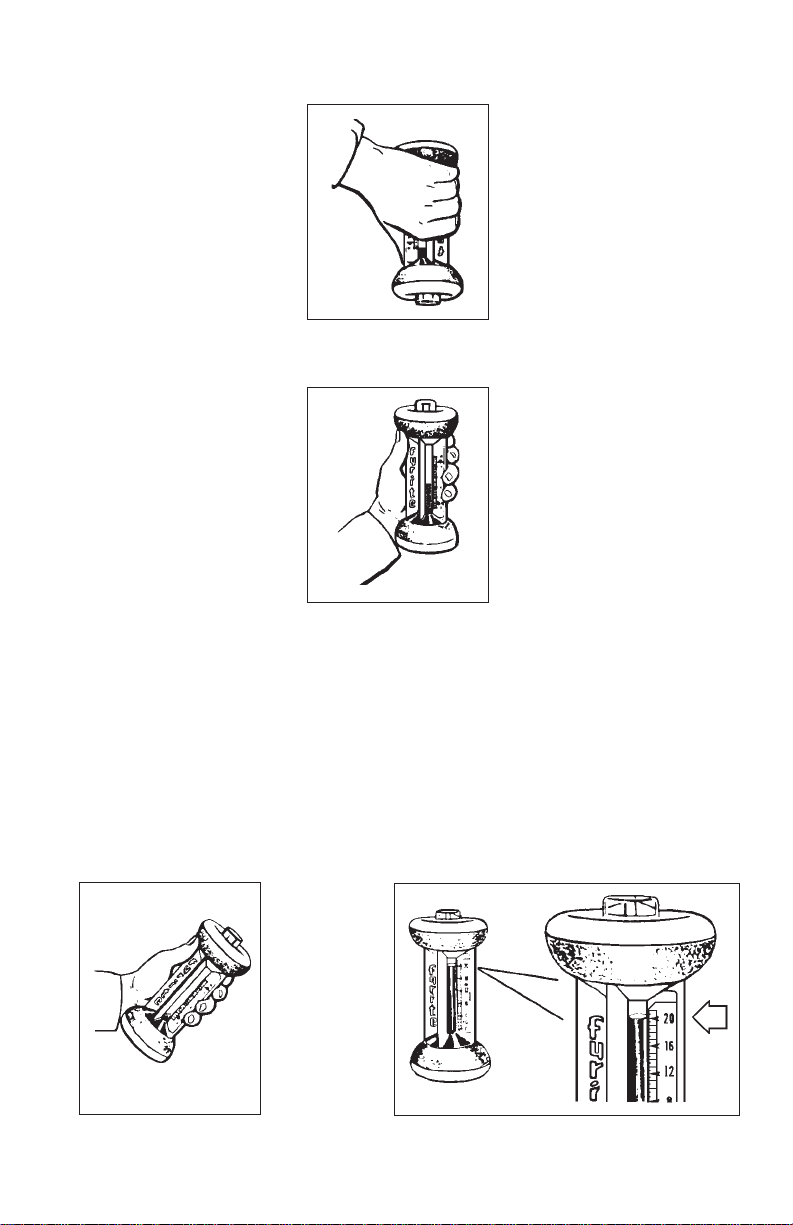
CO
FYRITE OPERATION O2 FYRITE OPERATION
2
(RED FLUID) (BLUE FLUID)
7. Absorb sample gas into
FYRITE by inverting
until fluid drains into top
reservoir (Fig. 7). Then
turn upright (Fig. 8) to
drain fluid into bottom
reservoir. Repeat this
step once.
8. Hold FYRITE at 45°
angle (Fig. 9) momentarily to allow fluid
droplets to drain into the
bottom reservoir.
9. With FYRITE held
upright (Fig. 10), permit
fluid in column to
stabilize a few seconds,
then immediately read %
carbon dioxide on scale at
the point corresponding
to top of the fluid column.
7. Absorb sample gas into
FYRITE by inverting
until fluid drains into top
reservoir (Fig. 7). Then
turn upright (Fig. 8) to
drain fluid into bottom
reservoir. Repeat this
step three (3) more times
(four complete inversions
total).
Figure 7
8. Hold FYRITE at 45°
angle (Fig. 9) momentarily to allow fluid
droplets to drain into the
bottom reservoir.
Figure 8
9. With FYRITE held
upright (Fig. 10), permit
fluid in column to
stabilize a few seconds,
then immediately read %
oxygen on scale at the
point corresponding to
top of the fluid column.
Figure 9
Page 6 Instruction 11-9026
Figure 10
Page 11

10. This step completes CO2 or O2 FYRITE gas sample analysis. A few points to
remember when reading the FYRITE:
(a) FYRITE accuracy is within ±1/2% CO
or O2 compared to actual value.
2
(b) Always handle FYRITE by fins to ensure body heat is not absorbed by fluid.
(c) A delay in reading of 5 or 10 seconds may decrease accuracy of reading
slightly but longer delays may cause substantial error.
(d) The FYRITE is calibrated to indicate on a “dry” basis for flue gas samples,
which are normally fully saturated with moisture. Failure to artificially
moisten wool packing in Filter Saturator Tube when actual sample is not
fully saturated will cause FYRITE to read slightly low.
Therefore, make certain wool packing in Filter Saturator Tube is sufficiently moistened with water for accurate results on non-flue gases. Avoid
excessive moisture which can be drawn into the Aspirator Bulb and forced
into the FYRITE during sampling.
CAUTION
Never depress plunger valve to vent FYRITE in the inverted
position. This will cause fluid to spill, which is corrosive and
contains poisonous elements. In the event of a spill, read Instructions in Section 2.2 FYRITE fluid handling precautions.
3.1 Determining CO2 and O2 FYRITE Fluid Strength
FYRITE Fluid strength can be conveniently checked immediately after the first
sample reading. Without venting, repeat the absorbing operations (Steps 7 through
9) by inverting FYRITE again and positioning upright until all fluid drains to the
bottom reservoir. Observe scale reading. An increase of more than 1/2% CO
the second reading as compared to the first indicates a need for fluid replacement.
or O2 in
2
3.2 Operating Precautions to Ensure Maximum Accuracy
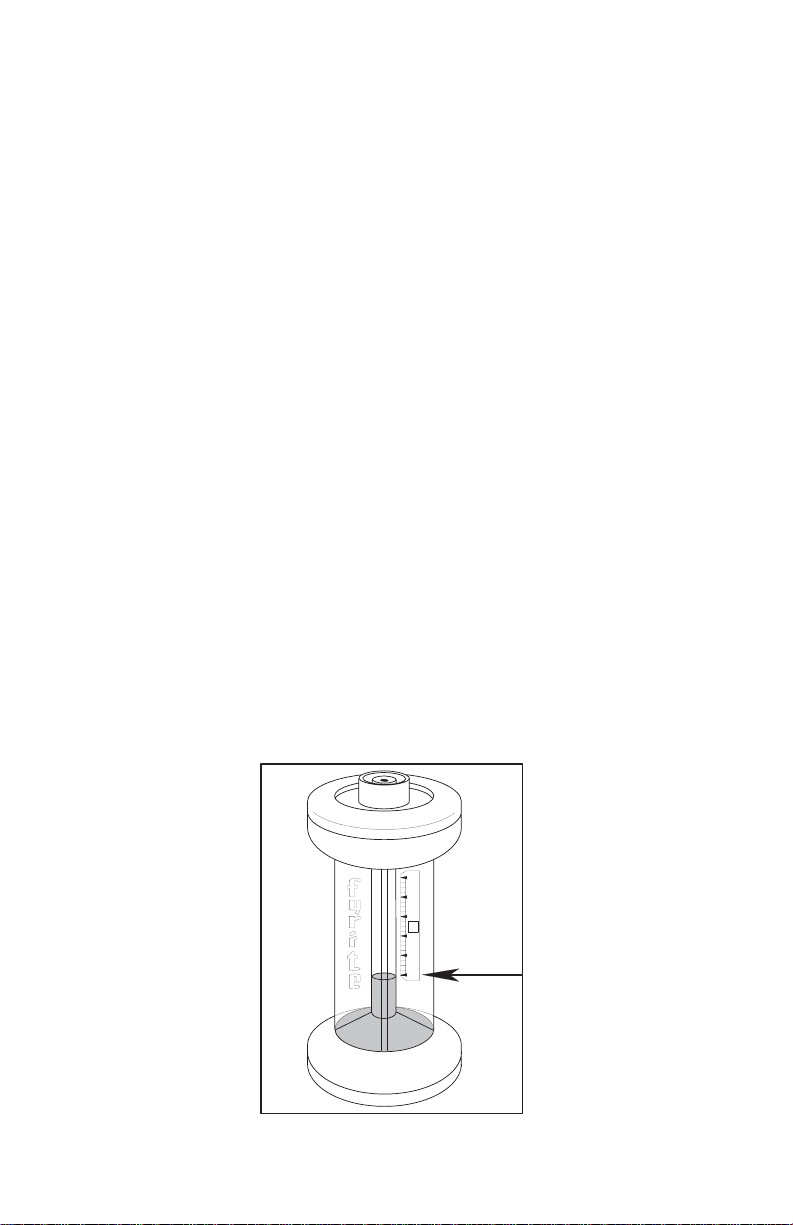
Locate top of fluid column (refer to Fig. 11). The surface at the top of the fluid
column (meniscus) in the small, center bore will be dish-shaped as shown in Figure 11. Either the high or low point of this dish-shaped surface may be used to
locate top of fluid column providing the same point is always used both for setting
scale zero and reading percent CO
operation and low point for the other will cause an error.
Best practice is to use high point of this fluid surface just at the small center bore
wall. In setting scale zero or reading percent CO
level with eyes and sight across scale to top of fluid column.
Instruction 11-9026 Page 7
or O2. Obviously, using high point for one
2
or 02, hold FYRITE vertically and
2
Page 12
3.2.1 Draining Fluid Droplets
For maximum accuracy, it is important to form the habit of following a standard
procedure in this operation and to use the same procedure both before adjusting
scale zero and before reading percent CO2 or O2 .
3.3 FYRITE Temperature
The FYRITE temperature should be at or close to the temperature of the working
environment where the analysis is being made and should not be subject to sudden
temperature changes. If FYRITE is carried from cooler to warmer location or vice
versa, expose FYRITE to new temperature condition to permit uniform temperature
stabilization. Usually 15 or 20 minutes will suffice, except when extreme temperature variations are involved. Temperature stabilization can also be accelerated by
inverting FYRITE frequently.
3.3.1 Temperature of Gas Sample
The gas sample as it enters FYRITE should be cooled (or warmed if sampling cool
gases) to same (ambient) temperature as the FYRITE.
Capacity of Standard Aspirator Bulb and Sampling Assembly (Part # 11-7029) to
cool the gas sample is sufficient if the temperature of gas being sampled does not
exceed 850°F (454°C), even where a large number of consecutive samples are
required if there is an interval of not less than 5 minutes between samples.
FYRITE indicators operate over a wide temperature range. They may be exposed to
ambient temperatures from –30° to 150°F (–34° to 66° C). Gases up to 850° F
(454° C) may be tested with Standard Aspirator Sampling Assembly (11-7029).
Special sampling equipment for high temperatures and unsaturated gas samples are
listed in Section 7.2. FYRITE Sampling Assemblies.
20
16
12
8
%CO2
4
0
®
Top of
Fluid
Column
Figure 11. Locating Top of Fluid Column.
Page 8 Instruction 11-9026
Page 13

4.0 PRINCIPLE OF OPERATION
(Refer to Figures 12, 13, and 14)
Figure 12. When plunger
valve is depressed, a gas
sample is pumped through
top reservoir with center
bore is sealed off.
Figure 14. Absorption of
gas sample by fluid creates
suction, which causes
diaphragm to flex up and
fluid to rise in center bore
to replace gas absorbed.
Figure 13. When plunger
valve is released, the gas
sample is locked into
FYRITE and the top
reservoir is opened to
center bore so that gas
sample can pass through
absorbing fluid.
Instruction 11-9026 Page 9
Page 14

To make a test with the FYRITE, the metal Sampling Tube at one end of Sampling
Assembly Hose is inserted into the gas to be analyzed. The rubber Connector Plug at
the other end of the Rubber Hose is then firmly pressed down on the spring-loaded
Valve of the instrument (See Figure 12). This simultaneously opens a passage into
the Top Reservoir and seals off the center bore.
Next, a sample of the gas is pumped into the Top Reservoir by squeezing the rubber
Aspirator Bulb. At least 18 bulb squeezes are required to assure that the rubber
Sampling Hose and the Top Reservoir of the FYRITE are thoroughly purged of the
previously analyzed sample. During the 18th Aspirator Bulb squeeze and immediately before releasing Bulb, the finger is lifted from the Connector Plug, which
automatically returns the Plunger Valve to upper position against its top seat.
Refer to Figure 13. With the Valve in this position, the final 60 cc of the gas sample
is locked into the FYRITE and the Top Reservoir is opened to the center bore so that
the gas sample can pass to the absorbing fluid. The FYRITE is then inverted, forcing
the gas sample to bubble through the absorbing solution which absorbs either CO
or O2 depending on the type FYRITE in use.
2
Refer to Figure 14. The instrument is then turned and held upright. Absorption of
the CO
at the bottom to flex upward permitting the level of the absorbing fluid to rise in the
or O2 by the absorbing fluid creates a suction, which causes the Diaphragm
2
center tube an amount proportional to the gas absorbed. The scale indication
corresponding to the top of the fluid column is then read as a percentage of gas
absorbed by the FYRITE fluid.
5.0 APPLICATION INFORMATION
FYRITE Analyzers are available for either Carbon Dioxide or Oxygen analysis, and
each of these models is made in the three scale ranges shown in Section 5.3. All six
instruments are similar in appearance and size, but they differ in important construction details, as well as in absorbing fluids.
5.1 FYRITE Absorbing Fluid
FYRITE absorbing fluid is selective in the chemical absorption of carbon dioxide or
oxygen respectively. Therefore, the FYRITE’s accuracy is well within the range
required for industrial and professional applications, and does not depend upon
complicated sequential test procedures. Furthermore, the FYRITE readings are
unaffected by the presence of most background gases in the sample.
The appearance of a dark red fluid floating on top of CO
entirely normal. The darker fluid consists of a small amount of material added to
prevent excessive foaming at the meniscus and improves readability.
5.1.1 FYRITE Refill Fluid (Storage Life, etc.)
FYRITE refill bottles should be stored at room temperature (70° F [21° C]) in the
carton provided, and placed in use within one year.
NOTE: Before opening refill bottle, read the label and make
certain to observe all precautions.
Page 10 Instruction 11-9026
FYRITE solution is
2
Page 15

FYRITE CO2 refill fluid may develop a white insoluble precipitate upon aging. The
precipitate does not affect fluid performance in any way, but if present, should be
filtered out to prevent adherence to internal surfaces of the FYRITE.
To filter, place a clean piece of thin cloth or netting loosely over the open CO
FYRITE and slowly pour refill contents into top reservoir. Before disposing of cloth,
2
rinse thoroughly with water. FYRITE fluid is corrosive!
FYRITE O
can be prevented by purging the FYRITE body with Nitrogen, natural gas or other
refill fluid reacts very rapidly with oxygen in air. Oxidation of absorbent
2
oxygen-free gas, just before filling, and by maintaining a stream of this gas around
mouth of the bottle (see Figure 21) while its contents are emptied into the FYRITE.
Another method for refilling the O
absorbent) is to invert the unit and place it over the 60 cc bottle of fluid. Then invert
both so that the FYRITE is now upright and the O
(see Figure 21a). Immediately install plastic Top Cap Ring without delay. This
FYRITE (and still limiting the oxidation of the
2
fluid is filling the instrument
2
method helps to minimize oxidation and improve the life of the fluid.
5.2 FYRITE Operating Temperature Range
The FYRITE indicators operate over a wide temperature range, and may be exposed
to ambient temperatures from –30° to 150° F. (–34° to 66°C). Gases up to 850° F
(454° C) may be tested with standard aspirator sampling equipment (11-7029).
Special sampling equipment to reduce high combustion gas temperatures (2000° F
[1093° C]) to usable FYRITE temperature (ambient) and assemblies with extra large
saturators for dry gas sampling are listed in Section 7.2, FYRITE Sampling Assemblies.
NOTE: Remember, for most accurate results, the FYRITE
must be at temperature equilibrium with its surroundings
(ambient temperature) and the incoming gas sample at the
same temperature as the FYRITE.
5.3 Typical FYRITE Applications
Listed below are six models of FYRITE Indicators and two Aspirator Sampling
Assemblies with part numbers identifying their application.
Bacharach Part Numbers
Aspirator Complete
For Scale Sampling Kit with
Testing Range FYRITE Assy. Fluid Case
Carbon 0 - 7.6% 11-7042 11-7029 10-5100 10-5053
Dioxide 0 - 20% 11-7032 11-7029 10-5057 10-5000
0 - 60% 11-7034 11-7029 10-5057 10-5032
0 - 7.6% 11-7044 11-7039 10-5103 10-5054
Oxygen 0 - 21% 11-7036 11-7029 10-5060 10-5011
0 - 60% 11-7038 11-7029 10-5060 10-5046
Instruction 11-9026 Page 11
Page 16

TYPICAL APPLICATIONS WITH FULL SIZE
SECTIONS OF FYRITE SCALES
0-7.6% CO2 or O
2
CO2 tests of controlled atmospheres in fruit,
vegetable, and meat storage rooms. Oxygen
determination in flammable gases. Oxygen tests
to check atmospheres made inert with nitrogen
(silos, fuel tanks, etc.)
0-20% CO
or 0-21% O
2
2
Flue gas combustion tests, oxygen deficiency tests,
and CO
Checking oxygen concentrations in hydrogen-cooled
tests of heat treating atmospheres.
2
generators and oil-sealed inert-gas transformers.
0-60% CO2 or O
2
Checking CO2 in inert gas blankets in tankers and
barges carrying gasoline and other combustibles.
CO
tests on lime kilns. Checking CO2 in sewage
2
plant digesters. Oxygen tests in connection with
oxygen and gas anesthesiology.
5.4 CO2 FYRITE Only Combustion Testing
5.4.1 Percent CO2 Shows Volume of Excess Air (Refer to Figure 15)
For any given fuel, theoretical maximum percent CO
produced when exactly enough air (but no excess air whatsoever) is supplied to burn
all of the fuel. As increasing amounts of excess air are supplied, percentage CO
naturally decreases from theoretical Ultimate CO2 due to the diluting effect of
excess air.
Thus, measuring percent CO
enough is supplied to permit clean combustion without adding so much that combus-
is also a method of indicating excess air to ensure that
2
tion efficiency is unnecessarily decreased.
Page 12 Instruction 11-9026
(Ultimate CO2 ) would be
2
2
Page 17

5.4.2 Too Much Excess Air Means Inefficient Combustion
Excess combustion air is heated and carries some of this heat to the flue where it is
wasted. The FYRITE CO
minimum (maximum CO
Analyzer is used to adjust combustion excess air to a
2
), which will permit clean efficient combustion.
2
Calculation of combustion efficiency is possible (assuming complete combustion) if
percentage of CO
and net temperature of combustion products are known.
2
5.4.3 What is Proper CO2 Value?
Proper CO
margin for variations in fuel, draft, atmospheric conditions, and mechanical wear.
is that which will ensure complete, clean combustion with some safety
2
Consult manufacturer of heating equipment for specific recommendations. Generally
accepted values for good combustion practice in residential furnaces and boilers
when firing the following fuels are:
Natural Gas ...... 8 to 9.5% CO
No.2 Oil ............ 10 to 12.5% CO
2
2
These are only guidelines, however, and in all cases recommendations of the equipment or fuel supplier would also include recommendations for allowable smoke (oil
firing) or combustibles (gas firing), which are undesirable combustion by-products.
Testing for smoke, CO, or combustibles is important since in extreme fuel rich
firing, high CO
values also occur (see Figure 16).
2
5.4.4 How To Calculate Combustion Efficiency
Measure percent CO
ture with a suitable thermometer (e.g., TEMPOINT) at the same sampling point.
in flue gases with FYRITE. Then measure flue gas tempera-
2
Deduct temperature of basement or combustion air supplied from measured flue gas
temperature to obtain net flue gas temperature. Use a Bacharach FIRE EFFICIENCY FINDER to calculate combustion efficiency. Instructions for using the
FIRE EFFICIENCY FINDER are printed on the face of this slide rule calculator.
5.5 O2 FYRITE Only Combustion Testing
5.5.1 Percent O2 Shows Volume of Excess Combustion Air (Refer to Fig. 15)
All fuels require some excess air (in addition to air theoretically required to burn the
fuel) to ensure clean, complete combustion. As the amount of this excess air increases, the percentage of O2 in combustion products increase.
5.5.2 Too Much Excess Air Means Inefficient Combustion
Excess combustion air is heated and carries some of this heat to the flue where it is
wasted. The FYRITE O2 analyzer is used when adjusting combustion air to minimum excess air (minimum O
Instruction 11-9026 Page 13
) which will permit clean, efficient combustion.
2
Page 18

5.5.3 What is Proper O2 Value?
Proper O
clean combustion with adequate safety margin for variations in fuel, draft, atmo-
content for any fuel fired is the lowest O2 value that will ensure complete,
2
spheric conditions, and mechanical wear. Consult manufacturer of heating equipment or local authorities for specific recommendations. Figure 15 illustrates the
relationship between CO
, excess air and oxygen for gas and oil.
2
For guidance, it should be added that modern boilers are capable of 80% or greater
combustion efficiency. Rough guidelines for setting conventional power burners are
10-15% excess air for natural gas and 15-20% excess air for oil, with little or no
smoke and carbon monoxide formation.
5.5.4 Measuring O
in Addition to CO
2
2
Figure 16 illustrates why it is good practice to measure oxygen, in addition to carbon
dioxide, when firing gaseous fuels. Note that the same CO
on both sides of the stoichiometric (or perfect) mixture. Carbon dioxide alone cannot
reading can be obtained
2
define proper excess air operation for gaseous fuels.
A check must also be made for the presence of oxygen, which confirms operation
with excess air. Gaseous fuels should also be checked for presence of carbon monoxide (CO) in the flue gases and adjusted for either “no CO” or to conform to applicable
safety regulations.
5.5.5 Where is Percent CO
For residential and most commercial and industrial combustion units, percent CO
or O2 is measured by analyzing gas sample obtained through a 1/4-inch diameter
or O2 Measured? (Refer to Figure 17)
2
2
hole located between the flue outlet (breeching) and any mechanical opening in the
flue such as the barometric draft regulator or draft diverter. Best practice is to
locate sampling hole at least six inches upstream from such flue openings. The
metal FYRITE Sampling Tube should extend at least 2-1/2 inches into the flue
gases.
For larger installations, consult manufacturer of combustion equipment for special
instructions regarding sampling point, special sampling tube required, or advice on
averaging readings.
Residential gas furnaces with built-in draft diverter (gas designed) will require the
FYRITE metal Sampling Tube to be inserted as illustrated in Figure 17 to avoid
dilution of flue gas sample.
Additional information on residential furnace combustion testing may be obtained
from the following Bacharach publication:
Bulletin 4097 - Technical Combustion Brochure
Page 14 Instruction 11-9026
Page 19

PERCENT EXCESS AIR
Figure 15. Relation between Oxygen, CO2 and excess
air in flue gases for Natural Gas and Fuel Oil.
THEORETICAL EXCESS AIR CURVE
STOICHIOMETRIC MIX
Figure 16. CO
combustion air setting when firing gaseous fuels.
Instruction 11-9026 Page 15
measurements alone do not determine
2
Page 20

5.6 Background Gases Affecting FYRITE Readings
As a rule of thumb, background gases/vapors may be present in concentrations up to
1/2% by volume (5000 ppm) before they present a significant interference problem to
the CO
An exception exists with the action of ammonia on Oxygen FYRITE Fluid. Ammonia
neutralizes the acidic solution of chromous chloride, and therefore use of the O
FYRITE with even trace amounts of ammonia in the backgrounds is not advised
unless suitable filters are used.
Inert gases, even in concentrations approaching 100% by volume, do not interfere
with either the CO
(chemical reaction initiated) or a mechanical nature, with resultant error, but are
also given up by the solution when the sample no longer contains the interfering
substance. They can therefore be compensated for by saturating the FYRITE
solution with samples containing the mechanical interferant as long as the background stays reasonably constant. Usually 3 or 4 tests are sufficient to saturate
FYRITE solution with the mechanical interferant in question.
or O2 FYRITE (20/21% ranges).
2
or O2 FYRITE. In general, interferences can be of a chemical
2
TYPICAL TYPE OF APPROXIMATE
INTERFERENCE INTERFERENCE EFFECT
(CO
FYRITE)
2
All Acid Gases Chemical 1:1
(SO
, H2S, Hydro-
2
cynanic Acid,
Cyanogen, etc.).
2
TYPICAL TYPE OF APPROXIMATE
INTERFERENCE INTERFERENCE EFFECT
FYRITE)
(O
2
Acetone Chemical 1:1
Acetylene Chemical 1:1
and other
unsaturated
hydrocarbons
CO
2
Mechanical, 20% CO
background can be to approximately 30%
2
Can be zeroed out up
zeroed out in 3 to 4 CO2 background.
tests if background
remains constant.
Ammonia Chemical Ammonia neutralizes
FYRITE solution
O
2
and instrument use on
this background is not
advised unless suitable filters are used.
Nitrous Oxide (N
Page 16 Instruction 11-9026
O) Mechanical - - -
2
Page 21

Figure 17. Checking CO2 of gas
designed furnace (sampling tube
inserted through draft diverter;
flue gas temperature test can
be made at same point).
5.7 General FYRITE Applications
It is only possible to specify a few general rules for such applications. Where possible, sample should be obtained at a point where the gases are well mixed to a
uniform composition. Where this is impossible; it will be necessary to average a
number of measurements taken at different locations to obtain representative
average. Where gases being sampled are not saturated with moisture (many noncombustion applications), it is very important to maintain the wool packing of Filter
Saturator Tube in a moist condition.
NOTE: The FYRITE is calibrated to analyze gases which are
normally saturated with moisture. Failure to moisten wool
packing in filter saturator tube with water will cause FYRITE
to indicate slightly lower than actual gas concentration.
Where special length sampling lines are required, add one more bulb squeeze to the
18 required with standard Sampling Assembly for each additional 3 cubic inches
(50cc) volume of special sampling line.
EXAMPLE: For each 9 ft. of 3/1 6 inch (I.D.) additional
length of sampling line, add one more bulb squeeze.
If it is necessary to trap a sample over water for later analysis, a minimum of 900 cc
of sample will be required. If sample is trapped over water, it is good practice to use
10 to 15% NaCl (table salt) in the water to minimize absorption of CO
water.
or O2 by
2
Trapping a sample over mercury will eliminate any possible error due to absorption
of CO
or O2.
2
Instruction 11-9026 Page 17
Page 22

5.8 Altitude Correction Table
The gas concentration read on the FYRITE is directly dependent upon the mass of
air in the sample. The aspirator bulb used in the FYRITE is a constant-volume
pump, not a constant-mass pump. Altitude, therefore, affects the FYRITE reading
due to the air’s density changing with altitude, thus requiring higher CO
concentrations to reach the same mark on the scale.
Use the following table to find the altitude correction (e.g., add the correction to the
reading to get the correct concentration).
TABLE 5-1. ALTITUDE CORRECTION TABLE
Altitude Correction %
Ft. (Meters) 20% CO
2
21% O
2
7% O2 or CO
1000 (305) 0.0 0.0 0.0
2000 (610) 0.0 0.0 0.0
3000 (914) 0.1 0.1 0.0
4000 (1575) 0.1 0.2 0.1
5000 (1219) 0.2 0.2 0.2
6000 (1829) 0.2 0.3 0.2
7000 (2134) 0.3 0.3 0.3
8000 (2438) 0.4 0.4 0.3
9000 (2743) 0.4 0.5 0.4
10,000 (3048) 0.5 0.6 0.5
11,000 (3353) 0.6 0.7 0.5
12,000 (3658) 0.7 0.8 0.6
13,000 (3962) 0.7 0.8 0.6
14,000 (4267) 0.8 0.9 0.7
or O
2
2
2
6.0 FYRITE MAINTENANCE
NOTE: With FYRITE vented and in a vertical position, it
should be possible to adjust scale zero to the top of the fluid
column. Refer to FYRITE Operation (Section 3.0) Steps 1
through 4 (for CO2) or Steps 1 through 5 (for O2) for proper
setup. If this is not possible, add or remove a small amount of
fluid as described below.
6.1 Increasing FYRITE Fluid To Proper Level
Refer to Figure 18. Hold FYRITE upright and cover hole in center of Plunger Valve
with finger. Add clean water, a few drops at a time (dripping water faucet is convenient) into space around Plunger Valve. Work Plunger Valve up and down several
times. Repeat process until fluid is at proper level.
Page 18 Instruction 11-9026
Page 23

6.1.1 Decreasing FYRITE Fluid to Proper Level
Refer to Figure 19. To remove excess fluid, insert small diameter glass tube into
FYRITE fluid through the small center FYRITE bore (with Top Cap Assembly
removed). Seal open end of glass tube with finger and dip out fluid with glass tube
until FYRITE fluid is at proper level. Avoid unnecessary exposure of O
since it will rapidly absorb O2 and become exhausted.
fluid to air
2
6.2 Checking FYRITE Fluid Strength
6.2.1 CO2 FYRITE Fluid Only
Fresh FYRITE fluid will absorb all CO
CO
. Where it is desirable to check fluid strength before taking FYRITE to the test
2
location to be used, or when a questionable (low) FYRITE reading is obtained the
instrument may be tested on any sample of CO
proximately 4% CO2) is often most convenient.
from more than 350 samples containing 10%
2
. Exhaled breath (containing ap-
2
Disconnect the rubber bushing and Sampling Hose Assembly from the Filter
Saturator Tube. Place the rubber connector tip over the Plunger Valve and depress
Plunger Valve down firmly with rubber connector tip. Take a deep breath, hold for 3
or 4 seconds and exhale at a steady rate into the rubber bushing end of the Sampling Hose Assembly. Simultaneously squeeze the Aspirator Bulb several times
while exhaling breath. Release rubber connector tip from Plunger Valve while still
exhaling breath. Perform steps outlined below to absorb exhaled breath into
FYRITE fluid.
1. Absorb sample gas into FYRITE by inverting until fluid drains into top reservoir, then turn upright to drain fluid into bottom reservoir. Repeat this step
once more (two complete inversions total).
2 Hold FYRITE at 45° angle momentarily to allow fluid droplets to drain into
bottom reservoir.
3. With FYRITE held upright, permit fluid in column to stabilize a few seconds,
then immediately read % carbon dioxide on scale at point corresponding to top
of fluid column.
To check CO
gas by inverting and turning upright. Repeat this step once more (two complete
inversions total). If reading increases by more than 1/2 percent CO
initial reading; replace fluid.
FYRITE fluid strength - DO NOT VENT FYRITE but reabsorb sample
2
as compared to
2
Formation of a frothy, persistent foam on FYRITE fluid also indicates need for fluid
replacement. A few “beads” or small air bubbles floating near the small center bore
wall is not considered foaming. FYRITE fluid may be colored for ease in readings.
Color has no bearing on fluid strength or ability to absorb CO
Cloudy fluid or small flakes of solid material in fluid do not necessarily mean CO
fluid is exhausted. Precipitate may form in CO2 Refill Bottles due to extended
.
2
2
storage, and can be filtered through cheese cloth or other screen like material when
refilling FYRITE.
Instruction 11-9026 Page 19
Page 24

20
16
12
8
%CO
2
4
0
®
20
16
12
8
%CO
2
4
0
®
Figure 18. Increasing FYRITE
fluid to proper level.
TOP GASKET
20
16
12
8
%CO
2
4
0
®
TOP CAP/
PLUNGER
VALVE
ASSEMBLY
OVAL HEAD
SCREWS
TOP CAP RING
WITH GASKET
Figure 19. Decreasing FYRITE
fluid to proper level
20
16
12
8
%O
2
4
0
®
Figure 20. Removing top gasket. Figure 21. Flushing FYRITE with
a stream of O
see Fig. 21a for alternate method).
Page 20 Instruction 11-9026
free, inert gas (Also
2
Page 25

NOTE: If compressed gas standards are used to check
FYRITE accuracy don’t forget to saturate filter saturator wool
packing as such gas standards are usually supplied “bone
dry”.
6.2.2 O
Fresh FYRITE fluid will absorb all O
10% O
FYRITE Fluid strength can be checked by performing steps outlined below:
FYRITE Fluid Only
2
from approximately 100 samples containing
. After completing test (as outlined in Section 3.0 Steps 1 through 9) O
2
2
2
1. DO NOT VENT FYRITE, but reabsorb sample gas into FYRITE by inverting
until fluid drains into top reservoir, then turn upright to drain fluid into bottom
reservoir. Repeat this step three (3) more times (four complete inversions total).
2. Hold FYRITE at 45° angle momentarily to allow fluid droplets to drain into
bottom reservoir.
3. With FYRITE held upright, permit fluid in column to stabilize a few seconds,
then immediately read % oxygen on scale at the point corresponding to top of
fluid column. If reading increases by more than 1/2 percent O2 as compared to
the initial reading, replace fluid.
When it is desirable to check fluid strength before taking the FYRITE to a location
where it will be used, it can be conveniently tested on a sample of atmospheric air
which contains 20.9% O2.
NOTE: Make certain wool in filter saturator tube is moistened
with water when using FYRITE in a non-combustible application where the gas sample is not fully saturated with water
vapor.
Formation of a frothy, persistent foam on FYRITE fluid also indicates need for fluid
replacement. A few “beads” or small air bubbles floating near the small center bore
wall is not considered foaming. Color of FYRITE O
ability to absorb oxygen although towards the end of fluid life, a greenish cast can be
(blue) fluid is not an index of
2
detected.
NOTE: If compressed gas standards are used to check
FYRITE accuracy, don’t forget to saturate filter saturator wool
packing as such gas standards are usually supplied “bone
dry”.
6.3 Replacing FYRITE Fluid (Refer to Figs 20, 21, and 22)
CAUTION
Because of the corrosive effect of the FYRITE fluid, always
change FYRITE fluid in immediate vicinity of a sink with
running water available as shown in Figure 18.
Instruction 11-9026 Page 21
Page 26

Remove 4 screws, metal Top Cap Ring, plastic Top Cap Assembly and Top Gasket.
Drain old fluid from FYRITE and rinse all parts in clean, lukewarm water.
NOTE: FYRITE fluid is corrosive to skin, clothing, some
metals, and painted or lacquered surfaces. Dispose of these
fluids in accordance with Local, State and Federal Laws. If
draining into a porcelain sink is permitted, keep water faucet
turned on while draining and flush for at least 1/2 minute
afterwards.
NOTE: Examine top gasket for warpage, if distorted as shown
in Figure 22, replace gasket with part # 11-0143 before
proceeding.
Drain all parts of excess water. Stand FYRITE Body upright to center replacement
Top Gasket in recess provided in top flange of body.
O2 FYRITE Only: Before adding fluid, flush FYRITE with a stream of O2 - free,
inert gas (Figure 21) or be prompt in reassembling Top Cap to prevent excessive
fluid contact with air.
Uncap FYRITE fluid bottle, invert FYRITE and place over the bottle. Invert both
FYRITE and bottle (Figure 21a) so that FYRITE comes to an upright position and
the fluid is filling the unit. Immediately install plastic Top Cap Assembly and metal
Top Cap Ring without delay.
CO
FYRITE Only: Uncap CO2 Refill Bottle and pour entire contents into FYRITE.
2
Reinstall plastic Top Cap Assembly and metal Top Cap Ring.
CO
/ O2 FYRITEs: Make certain that Top Cap Ring Gasket (11-0109, Figure 33) is
2
in place between metal Top Cap Ring and plastic Top Cap. Draw the 4 machine
screws down with light screwdriver force.
Then, going from one screw to the next, draw down 1/4 turn until all are firmly
tightened. Avoid excessive force in tightening which may damage plastic parts.
6.4 Inspection of FYRITE for Fluid Leakage
Gradual loss of fluid, moisture or dried encrustation around Plunger Valve does not
indicate fluid leakage. Gas enters and leaves unit during sampling with considerable velocity and some fluid vapor is carried out during normal sampling. To check
Top Assembly for fluid leakage in upright position, depress Plunger Valve and
release.
Note the scale reading at top of fluid column, and then stand FYRITE upside down
overnight in glass or porcelain test dish (See Figure 23). Then, return FYRITE to
upright position and, after allowing 5 minutes for drainage, depress and release
Plunger Valve. Leakage will be evidenced by lower scale reading. Extreme leakage
will be apparent by presence of fluid in test dish.
Page 22 Instruction 11-9026
Page 27

®
0
4
2
%CO
8
12
16
20
20
16
12
8
2
%CO
4
0
Figure 21a. Alternate filling
method. Invert FYRITE (with
bottle in place) to upright position.
®
0
4
%CO
2
8
12
16
20
Figure 23. FYRITE inverted in test
disk (overnight) for leakage test.
Figure 22. Examining top gasket
for warpage.
TOP CAP
ASSEMBLY
GASKET
20
16
12
8
%CO
2
4
Top Gasket used in 7%,
0
®
20% & 21% Models only
BODY
Figure 24. Top gasket properly
centered in top flange of body.
Instruction 11-9026 Page 23
Page 28

Follow the same procedure to check fluid leakage from Bottom Cap Assembly with
FYRITE stood overnight in the upright position.
When leakage is proven, examine plastic and rubber parts for deterioration to
determine replacement parts necessary for repair, or drain instrument and return if
factory service or repair is desired.
6.5 Cleaning FYRITE
Use only soapy lukewarm water if cleaning is required (lukewarm water is usually
sufficient).
NOTE: Use of gasoline, naptha, carbon tetrachloride or any other
organic solvent or oil will destroy plastic and rubber parts.
6.6 Replacing FYRITE Plastic and Rubber Parts (Refer to Illustrated
Parts List FYRITE CO
Replace plastic parts when cracked or crazed in location exposed to fluid and rubber
parts when badly swollen, warped or showing other evidence of deterioration.
In replacing Top Gasket, make certain it is properly centered in the recess provided
in top flange of FYRITE Body (See Figure 24). When installing plastic Top Cap
Assembly, be sure that assembly is centered on Top Gasket.
To remove Top Cap Assembly or replace Top Cap, Plunger Valve, or Plunger Tip
Gasket, use the procedure as outlined in Section 6.3.
[Section 7.0 and Fig. 33])
2/O2
When replacing Plunger Tip Gasket, simply depress Plunger Valve against its
spring limit and strip old Tip Gasket from the end of Plunger Valve.
Before assembling new Gasket, wet inside surface of Tip Gasket, then force it over
the end of Plunger Valve (after depressing Plunger Valve against the spring limit).
Make certain that new Tip Gasket is seated uniformly against the mating surface in
plastic Top Cap.
To replace Diaphragm, stand FYRITE upside down, remove 4 screws and metal
Bottom Cap.
Refer to Figure 25a. Remove old Diaphragm and center new replacement with the
lettering facing you, so that after the FYRITE Bottom Cap is installed the letters will
face up into its recess. Center Bottom Cap Assembly in Body Recess and reinstall 4
screws, observing same precautions in tightening as outlined in Section 6.3.
To replace Body, remove the 4 hex head Bezel screws and 4 Bezels. Install Bezels on
new Body.
NOTE: Make certain the rubber bezel gaskets are properly
seated and clamped between bezel and body as shown in
Figure 25.
Page 24 Instruction 11-9026
Page 29

LETTERING
®
0
4
%CO2
8
12
16
20
BOTTOM CAP
(RECESS)
Figure 25a. Replacement Diaphragm
properly installed.
Figure 25. Locating Bezel
Rubber Gaskets.
Figure 26. FYRITE Sampling Assembly;
Locating Inlet/Outlet Check Valves.
Instruction 11-9026 Page 25
Page 30

6.7 Aspirator Bulb - Sampling Assembly (Refer to Figs. 26, 27, & 28)
Defective Check Valves or a leaking Sampling Assembly can result in sample loss, or
sample dilution with resultant loss of accuracy.
To inspect Sampling Assembly, seal hole in the center of rubber Connector Tip
firmly with finger and squeeze Aspirator Bulb (Figure 27). Bulb should remain firm.
If Bulb collapses, check Bulb and Hose to Connector Tip for cracks or other source of
leakage. Replace defective parts. If there are no apparent leaks in the Sampling
Assembly, replace Inlet Check Valve. Small hole end of Inlet Check Valve fits in
Hose and large hole end fits in the Aspirator Bulb (Figure 26).
Now seal end of metal Sampling Tube with finger, and collapse Aspirator Bulb.
(Figure 28). If the Bulb returns to original shape in less than 15 seconds, inspect
Hose, Filter, Saturator Tube and Metal Sampling Tube for leaks. If none are found,
replace the Outlet Check Valve. Small hole end of Outlet Check Valve fits in
Aspirator Bulb and large hole end fits in the Hose (Figure 26).
Discard Filter Packing when it becomes dirty or clogged. Push old packing out of,
and new packing into, Filter Saturator Tube with a pencil or small rod (See Section 6.9).
6.8 Storing FYRITE and Refill Fluid
When FYRITE is not used over periods between tests, store it and its Sampling
Assembly in a cool, dry place. Prolonged storage, for example, at high temperatures
reached in an automobile trunk over the Summer is not recommended. When stored
unused over the Summer, it is good practice to drain old fluid and replace with fresh
charge (fluid) when needed in the Fall.
FYRITE Refill Fluid should also be stored in a cool, dry location in the carton
provided. A recommended practice is to accumulate stock only sufficient for one year
requirement and to use oldest stock first. Fluid can be tested for performance
according to Section 6.2.
Use only Bacharach CO
below contain three Refill Bottles each.
or O2 Fluid Refills for the range FYRITE selected. The Kits
2
7% CO2 range ............................. Refill Kit 10-5100
20% and 60% CO2 range ........... Refill Kit 10-5057
7% O2 range ............................... Refill Kit 10-5103
21% and 60% O2 range .............. Refill Kit 10-5060
6.9 Replacing Saturator Filter Packing Part #11-0121
(Refer to Figs 29 thru 32)
Remove the filter tube nipple and bushing (See Figure 29) from each end of the tube.
Use a pencil or similar object to remove packing when it becomes dirty or clogged.
Wrap replacement packing around the hand (See Figure 30). If testing unsaturated
gases (most non-combustion applications), saturate packing in water as shown in
Figure 3, and squeeze out excess moisture.
Insert saturated packing using a pencil or small rod into filter tube (See Figure 32);
then reassemble nipple and bushing.
Page 26 Instruction 11-9026
Page 31

RUBBER CONNECTOR TIP
(HOLD FINGER HERE)
ASPIRATOR BULB SHOULD
REMAIN FIRM WHEN SQUEEZED
Figure 27. Testing Sampling Assembly (outlet side) for leaks.
CHECK FOR BULB INFLATION
AFTER SQUEEZING
SAMPLING TUBE END
(HOLD FINGER HERE)
Figure 28. Testing Sampling Assembly (inlet side) for leaks.
Figure 29. Removing End Plugs from Saturator Filter Tube.
Instruction 11-9026 Page 27
Page 32

Figure 30. Wrapping Replacement Filter Material.
Figure 31. Wet Filter Material then squeeze out excess water.
Figure 32. Installing wetted Filter Material into Saturator Tube.
Page 28 Instruction 11-9026
Page 33

7.0 ILLUSTRATED PARTS LIST FYRITE CO2/O
2
(Refer to Figure 33 FYRITE Parts Breakout)
Part # Description Qty-Rq’d
11-0102 Bezel 4
01-0661 Oval Head Screw 8
11-0110 Bezel Screw 4
11-0109 Top Cap Ring Gasket (Optional, Part of 11-0136) 1
11-0105 Scale Screw 1
02-3690 Scale Screw Nut 1
11-0021 Diaphragm 1
11-0126 Bottom Cap 1
11-0132 Top Cap 1
11-0019 Valve Plunger 1
11-0026 Valve Plunger Spring 1
11-0020 Valve Plunger Gasket 1
11-0136 Top Cap Ring with Gasket 1
11-0143 Top Gasket (7%, 20% & 21% Models only) 1
11-0140 FYRITE Body (All Models except 7.0% CO2 / O2)1
11-0154 FYRITE Body (7% Models only) 1
11-0062 Filler 60% FYRITE (Adapter Plug) 1
05-5134 Bezel Gasket “O” Ring 2
05-5169 O-Ring Filler (Outer 60%) 1
05-5155 O-Ring Filler (Inner 60%) 1
11-0144 CO2 Scale 20% 1
11-0145 O
11-0147 CO
11-0150 O
11-0155 CO
11-0157 O
10-5057 Carton of FYRITE CO
10-5060 Carton of FYRITE O
10-5100 Carton of FYRITE CO
10-5103 Carton of FYRITE O
Scale 21% 1
2
Scale 60% 1
2
Scale 60% 1
2
Scale 7% 1
2
Scale 7% 1
2
Fluid (3 Bottles) 20/60% range Models 1
2
Fluid (3 Bottles) 21/60% range Models 1
2
Fluid (3 Bottles) 7% range Models 1
2
Fluid (3 Bottles 7% range Models 1
2
Instruction 11-9026 Page 29
Page 34

7.1 FYRITE ILLUSTRATED PARTS
Figure 33. FYRITE parts breakout.
Page 30 Instruction 11-9026
Page 35

7.2 PARTS LIST FOR FYRITE SAMPLING ASSEMBLIES
STANDARD SAMPLING ASSEMBLY
Part No. 11-7029
Gases saturated with water
vapor (combustion products).
Dry gases when filter material
is wetted.
OPTIONAL INDUSTRIAL SAMPLING ASSEMBLY Part No. 11-7046
High temperature combustion applications where heat radiation and high
localized ambients are factors. Optional ceramic High Temperature Sampling
Tube for sample gases over 1400° F.
OPTIONAL SAMPLING ASSEMBLY Part No. 11-7045
Combined FYRITE O2, CO2, and Monoxor CO tests on
combustion products.
OPTIONAL SAMPLING ASSEMBLY Part No. 11-7039
Large Filter-Saturator Assembly provides artificial sample saturation for noncombustion FYRITE applications. Bacharach Indicator Tubes may be used with
this Assembly.
Instruction 11-9026 Page 31
Page 36

Current Former
Part # List # Description # Req'd
11-0152 10-0019 Connector Tip with Tube 1
11-0156 10-0029 Rubber Tubing, 10' Length 1
11-0118 10-0020 Rubber Tubing, 6" Length 2
11-0165 10-0030 Filter Tube, Aluminum 1
19-5004 19-9004 Gas Collecting Bladder with Orifice 1
11-0120 10-0022 Aspirator Bulb 1
11-0138 10-0023 Inlet/Outlet Valve (Red) 2
11-0119 10-0024 Rubber Tubing, 3' length 1
11-0180 10-0025 Filter Nipple with Bushing 2
11-0130 10-0026 Filter Tube, Plastic 1
11-0106 10-0027 Sampling Tube 1
11-0161 10-0040 Condenser Coil 1
11-0151 10-0041 Moisture Trap Assembly 1
11-0179 10-0042 Rubber Tubing, 15' length 1
11-0121 10-0033 Envelope of Filtering Material (10 per pack)
11-0164 10-0047 Ceramic High Temperature Gas
Sampling Probe, 18" length (optional)
11-0181 10-0032 Filter Saturator (Aluminum) Assembly
Comprising 11-0180, 11-0122, and 11-0165
Page 32 Instruction 11-9026
Page 37

Instruction 11-9026 Page 33
Page 38

Page 34 Instruction 11-9026
 Loading...
Loading...