Page 1

DR. HARVEV
UNIVERSITY
DEPARTMENT
9500 GIU^AN DRIVE
J,
KARTEN,
OF
CALIFORNIA,
OF
NEUROSCIENCES, 0608
M.D.
SAN
DIEGO
LA JOLLA, CA 92093-0608 February 1990
AXOCLAMP-2A MICROELECTRODE CLAMP
THEORY AND OPERATION
Written for Axon Instruments, Inc.
by Alan Finkel, Ph.D.
Copyright 1988, 1990 Axon Instruments, Inc.
No part of this manual may be reproduced, stored in a retrieval system, or transmitted, in any
form or by any means, electronic, mechanical, photocopying, microfilming, recording, or
otherwise, without written permission from Axon Instruments, Inc.
QUESTIONS? Call (415) 571-9400
Part Number 2500-000 REV B PriMcd in
U.S.A.
Page 2
OUT i(r..;.,) • • St^f 4<i-.^^
Page 3

Ill
COPYRIGHT
THE CIRCUITS AND INFORMATION IN THIS MANUAL ARE COPYRIGHTED AND
MUST NOT BE REPRODUCED IN ANY FORM WHATSOEVER WITHOUT WRITTEN
PERMISSION FROM AXON INSTRUMENTS, INC.
VERIFICATION
THIS INSTRUMENT IS EXTENSIVELY TESTED AND THOROUGHLY CALIBRATED
BEFORE LEAVING THE FACTORY. NEVERTHELESS, RESEARCHERS SHOULD
INDEPENDENTLY VERIFY THE BASIC ACCURACY OF THE CONTROLS USING
RESISTOR/CAPACITOR MODELS OF THEIR ELECTRODES AND CELL MEMBRANES.
DISCLAIMER
THIS EQUIPMENT IS NOT INTENDED TO BE USED AND SHOULD NOT BE USED IN
HUMAN EXPERIMENTATION OR APPLIED TO HUMANS IN ANY WAY.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 4

IV
Illustrations of the rear-panel view of the
AX0CLAMP-2A are shown on the fold-out page at the
rear of the manual.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 5

9500 GILMAN DR^VE '"'''^''' °^°^
LA JOLLA, CA 92093-0608
TABLE OF CONTENTS
Page
INTRODUCTION 1
FEATURES 3
FEATURES ..3
GLOSSARY 9
QUICK GUIDE TO OPERATIONS 11
DETAILED GUIDE TO OPERATIONS 15
ANTI-ALIAS FILTER 15
BATH PROBE i, 16
Bath Potential Measurement 16
Grounding 16
BLANKING 16
BRIDGE MODE 17
Description 17
Suggested Use 17
Intracellular Balancing 18
BUZZ 20
Remote Buzz 20
CALIBRATION SIGNAL 21
CAPACITANCE NEUTRALIZATION AND INPUT CAPACITANCE 21
Primary 21
Secondary 21
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 6

VI
Page
CLEAR 22
COMMAND GENERATORS 22
Step Conunand Generator 22
DC Command Generators 23
Extemal Conunand Inputs 23
Mixing Conunands. 23
CURRENT MEASUREMENT 25
DCC MODE 25
GROUNDING AND HUM 31
HEADSTAGES 32
The Meaning Of H 32
Which Headstage To Use .32
Capacitance Neutralization Range 34
7 Headstage Connectors 34
Tip Potentials - Detection 36
Tip Potentials - Prevention 37
Interchangeability 37
• Cleaning 37
Input Leakage Current And How To Trim It To Zero 37
Warning 38
DC Removal 38
Input Resistance ; 39
HOLDERS : .39
Features 39
Parts 40
Use 40
T
IONOPHORESIS
42
A
LINK-UP
AXOCLAMP-2A THEORY & OPERATION. COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
42
Page 7

Vll
Page
MICROELECTRODES FOR FAST SETTLING 43
Microelectrode Capacitance 43
Microelectrode Resistance 44
Filling Solutions.... 44
Recommended Reading 44
MODEL CELLS 45
The CLAMP-1 Model CeU 46
MONITOR
48
NOISE IN DCC AND dSEVC MODES 49
OFFSET CONTROLS 50
OUTPUT
FILTER 50
High-Order Lowpass Filters For Low-Noise Recordings 51
Rise Time Of High-Order Filters 51
Note On Ultimate Rise Time ;.... 51
OUTPUT IMPEDANCE AND PROTECTION 51
PANEL METERS 51
V„(mV) 51
V2(mV) 52
I(nA) 52
PHASE • 52
POWER-SUPPLY GLITCHES 53
POWER SUPPLY VOLTAGE SELECTION & FUSE CHANGING 54
Supply Voltage 54
Changing The Fuse 54
REMOTE 55
RMP BALANCE 57
AXOCLAMP-2A THEORY & OPERATION,
COPYRIGHT
FEBRUARY
1990,
AXON
INSTRUMENTS.
INC.
Page 8

Vlll
Page
SERIES RESISTANCE 57
Origin 57
Problem 57
Solutions 57
What is Uie True Membrane Potential Time Course? 58
SEVC MODE - CONTINUOUS.... 60
Important Note - Anti-Alias Filter 60
Suggested Use 60
cSEVC Compared WiUi Whole-Cell Patch Clamp 62
SEVC MODE - DISCONTINUOUS .....64
Description 64
Suggested Use 67
Important Note 70
Which SEVC to use witii a Suction Electrode 70
Minimum Sampling Rate and Maximum Gain 74
Clamp Error 74
Gain.... 74
SPACE CLAMP 75
TEN-TURN POTENTIOMETERS 75
TEVC MODE ; 75
Description 75
Suggested Use 76
Extremely Important Note - Coupling Capacitance 76
Saturation During The Capacitance Transient 79
Choosing the Microelectrode Resistances 79
TRIGGERED CLAMPING 79
TROUBLE SHOOTING 80
UNITY-GAIN RECORDING - THIRD POINT 80
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 9

Page
IX
VIRTUAL-GROUND CURRENT MEASUREMENT
10.V„
AND I^
SPECIFICATIONS
REFERENCES
WARRANTY
RMA FORM
B-1
C-1
POLICY STATEMENT
SERVICE
D-l
COMMENT FORM
OUTPUTS
81
82
A-1
D-l
E-1
80
FRONT AND REAR PANEL -
f^ir^yf'
iZ^^f ^Iffff^^P
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY
1990,
AXON INSTRUMENTS,
INC.
Page 10

CThis page ia intentionally left blank)
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 11

iNTRODUcrroN Page 1
INTRODUCTION
!
The AXOCLAMP-2A Microelectrode Clamp can be used as a dual channel microelectrode probe, or as a
microelectrode voltage clamp.
Voltage clar; ping is a powerful technique for the control of membrane potential and for the investigation of
processes alfecting membrane conductance. Voltage clamping has traditionally been performed using two
intracellular microelectrodes and the AXOCLAMP-2A can be used for this purpose.
The AXOCLAMP-2A can also be used for discontinuous single-electrode voltage clamping (dSEVC) and for
continuous single-electrode voltage clamping (cSEVC). A single-electrode voltage clamp (SEVC) is more
convenient to use than a two-electrode voltage clamp (TEVC) in very small cells and cells which cannot be
visualized. A particular advantage of a dSEVC is that the voltage drop due to current flow through the
series component of cell membrane resistance (Rg) is not clamped. In addition, for both types of SEVC
instabilities due to coupling capacitance and coupling resistance between two microelectrodes do not arise.
The disadvantages of a dSEVC compared with a TEVC are that the response speed is slower, the maximum
achievable gain is lower, and the noise in the current and voltage records is greater. The design of the
AX0CLAMP-2A reduces these disadvantages towards their theoretical minimums, thereby allowing singleelectrode voltage clamping to be performed in the many situations where conventional voltage clamping is
not suitable.
A cSEVC i.s as low in noise as a TEVC but has a severe disadvantage in that the voltage drop across the
microelectrode is clamped unless compensation is made. Since the required compensation is never
perfect, tha rSF,V(;^ y^in nnly he. psfid wh^-ij thg e|ectrode resistance is very small compared with the cell
input resistance. These favorable conditions can often be achieved by the whole-cell patch technique.
Because of the AXOCLAMP-2A's advanced design, it itself does not limit the achievable performance.
Instead, the dominant factor affecting SEVC performance is the microelectrode. Users of the
AXOCLAMP-2A in eidier of the SEVC modes should be quick to question, then adjust, the microelectrode
and its placement.
TTie AXOCLAMP-2A is a sophisticated instrument. Even experienced researchers are advised to read this
manual thoroughly and to familiarize themselves widi the instrument using model electrodes (i.e. resistors)
and cells (e.g. parallel RC) before attempting experiments with real microelectrodes and cells.
We will be pleased to answer any questions regarding the theory and use of the AX0CLAMP-2A. Any
conmients and suggestions on the use and design ofthe AX0CLAMP-2A will be much appreciated.
We would be most grateful for reprints of papers describing work performed with the AX0CLAMP-2A.
Keeping abreast of research performed helps us to design our instruments to be of maximum usefulness to
you who use them.
Axon Instruments, Inc.
AX0CLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 12

Page 2 iNTRODUcnoN
(This page is intentionally left blank)
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 13

FEATURES
Page 3
FEATURES
The AXOCLAMP-2A is a complete microelectrode current and voltage clamp for intracdiular investigations. It
combines state-of-the-art single-electrode voltage clamping, two-electrode voltage clamping, and two complete
bridge amplifiers into one instrument. Precision command voltages, meters, filters, offsets and many other
features are built in to give you unprecedented flexibility.
4 discontinuous single-electrode voltage clamping
4 continuous single-electrode voltage clanqiing
4 two-electrode voltages clamping
4 discontinuous current clamping
4 two complete bridge amplifiers
4 high-speed headstages
4 low-noise low-hum operation
4 push-button selection of operating mode
4 computer selection of operating mode
4 two digital meters for voltage display
4 digital counter for display of sample rate
4 3-input digital meter for current display
4 separate current-measurement circuits for
each microelectrode
4 virtual-ground current measurement
VOLTAGE CLAMPING Voltage clamp with one or two microelectrodes — your choice is dictated
by the needs of your investigation; the AXOCLAMP-2A does both.
Discontinuous Single-Electrode Voltage Clamping (dSEVC) is based on
the technique of sampling the membrane potential while zero current flows
and then retaining this sampled value while current is injected into the
cell. This procedure is rapidly repeated to produce a smooth response.
Continuous Single-Electrode Voltage Clamping uses a low resistance
electrode to continuously record membrane potential and inject current.
The error caused by voltage drop across the electrode resistance can be
partially reduced by series resistance compensation. With Two-Electrode
Voltage Clamping (TEVC) one microelectrode is used to continuously
record membrane potential while the other is used to inject current. ~^
4 bath potential measurement and compensation
4 intemally generated precision command voltages
4 automatic clamping at resting membrane potential
4 offset compensation
4 rejection of stimulus artifacts
4 output bandwidth selection
4 calbration signal on outputs
4 electrode buzz
4 electrode clear
4 hands-free operation of buzz and clear
4 anti-alias filter
4 phase control
4 sampling clock synchronization
4 model cell
Gain of the voltage-clamp amplifier is quickly set on a smooth-acting
nonlinear control. The phase response of the amplifier is altered from
lead to lag by a Phase Shift potentiometer with a Center Frequency
switch to select the range.
A unique variable Anti-Alias Filter helps reduce noise towards the
theoretical minimum during dSEVC by slowing the response of the
sampling circuit to suit the sample rate and the microelectrode response.
The Sample Rate can be continuously altered from a low value of 500 Hz
to a high of
in noise and response times occurring when faster sampling rates are used.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
SO
kHz. This enables you to take advantage of the decrease
Page 14

Page 4
FEATURES
The sample clocks of two AXOCLAMP-2A's can be synchronized in a
'Master-Slave' configuration. This is useful in experiments in which two
cells in the same preparations are independently voltage clamped using
dSEVC. Linking the two clocks prevents the generation of spurious
signals which would otherwise appear at harmonics of the difference in the
two clocks firequencies.
Output compliance in TEVC mode is ±30 V. This reduces the chance of
saturation while the membrane capacitance is charging after a step change
in voltage. To further minimize the chance of saturation during TEVC a
relay-switched headstage (HS-4) is available to automatically bypass the
current-sensing resistor inside the headstage. The HS-4 headstage must
therefore be used in conjunction with a virtual-ground current monitor
(VG-2).
The HS-4 headstage is recommended only when large, ultra-fast
voltage steps in big cells must be established.
Another unique control is a Resting Membrane Potential (RMP) Balance
Indicator which enables you to preset the clamp offset so that when you
switch into voltage-clamp mode the cell membrane will automatically be
clamped at its resting value, irrespective of the clamp gain.
A remarkable "BLANK" facility can be used to force the voltage clamp
system to ignore stimulus artifacts that would otherwise be picked up by
the voltage-recording circuit and result in large current artifacts which
could damage the cell under clamp.
A "Monitor" output enables the input to the sampling circuit to be
observed. It is essential to observe this signal during dSEVC to ensure
that the microelectrode voltage due to current passing has time to
adequately decay at the end of each cycle. An oscilloscope trigger signal
at the sample rate is provided for use with the Monitor signal.
The AX0CLAMP-2A allows very fast discontinuous single-electrode
voltage clamping. In a test cell (see specifications) the 10% to 90% rise
time is only 100 /ts. In a real setup the response speed is limited by the
microelectrode characteristics, but membrane potential rise times (without
overshoot) of less than 1 ms have been regularly achieved in a variety of
cell types. Two-electrode voltage clamping is much faster.
CURRENT CLAMPING Two controls for each microelectrode are devoted to clearing blocked
microelectrode tips and assisting cell penetration. One is a "Clear"
switch which can be used to force large hyperpolarizing or depolarizing
currents through the microelectrode. The other is a "BUZZ" switch
which causes the mocroelectrode voltage to oscillate. Depending on the
microelectrode and the preparation, one of these two methods will often
succeed in lowering the resistance of blocked microelectrode tips. When
used while the tip of the microelectrode is pressing against the membrane,
Buzz and Clear may also cause the microelectrode to penetrate the cell.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 15

FEATURES
Page 5
HEADSTAGES
Unity-voltage-gain HS-2 headstages are available in several current gains.
These cover the range of cell input impedances from less than 1 MO to
greater than 1 GO. Ultrahigh-input impedance versions are also available
for ion-sensitive electrodes.
High speed and low noise are achieved by using bootstrapped power
supplies for the input circuit of each headstage. These bootstrapped
power supplies are derived from special high-voltage circuits so that the
headstages will not be saturated by the large voltages that may occur
during the passage of ciurent through high-resistance microelectrodes.
Capacitance Neutralization is also derived from high-voltage circuits so
that fast responses are not degraded during large input signals.
Current in each microelectrode is continuously measured during both
voltage clamp and current clamp. This measurement does not include
currents from sources other than the microelectrode (e.g. hum,
ionophoresis, the other microelectrode) and indicates zero if the
microelectrode blocks.
Headstages have a gold-plated 2 mm (0.08") input socket to directly
accept standard microelectrode holders. 2 mm plugs are supplied with the
headstages to connect wire leads, if used.
COMMAND GENERATORS
In any mode, level and step commands can be generated intemally.
Level Commands (one for voltage clamp and one for each microelectrode
for a total of 3) are set on precision ten-tum potentiometers. The Step
Command is set on a 3'/i-digit thumbwheel switch and can be directed to
either one of the microelectrodes or to the voltage clamp. An indicator
light for each microelectrode illuminates during current commands.
Extemal command sources can be used simultaneously with the intemal
command sources.
OUTPUTS Two dedicated Digital Voltmeters continuously display the
microelectrode voltages while a third displays the currmt in the selected
microelectrode or in a virtual-ground circuit, if used. Front-panel
controls for each microelectrode and the virtual ground set the scaling of
the current meter to suit the gain of your headstage.
A Digital Counter lets you know precisely what sampling rate you are
using during single-electrode voltage clamp or discontinuous current
clamp.
Offset Controls are provided for each microelectrode, and a variable
Lowpass Filter is provided for the microelectrode used in single-electrode
voltage clamping. As well, an intemally generated Calibration Signal
can be superimposed onto each of the outputs. Hence, the output signals
in many cases can be wholly conditioned within the AXOCLAMP-2A to
suit your recording apparatus.
AXOCLAMP;2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 16

Page 6
FEATURES
Six outputs are conveniently located at the front panel for connectmg to
your oscilloscope. These outputs are repeated at the rear panel, where
the other outputs, the inputs and the headstage connectors are also
located.
REMOTE CONTROL
Hands-free operation of Buzz is possible using the footswitches supplied
with every AXOCLAMP-2A. Selection of the operating mode can be made
remotely for computer sequencing of experiments.
All AXOCLAMP-2AS have a Buzz oscillator to assist in cell penetration.
The duration of the Buzz oscillation is normally equal to the time that the
front-panel switch is pressed. Practically, the shortest duration that this
switch can be pressed is about 100 ms. For small cells, 100 ms Buzz
oscillation sometimes damages the cells immediately after penetration.
The Remote Buzz Duration Control supplied with the AX0CLAMP-2A is
a hand held control that contains a trigger switch to buzz either electrode,
and a duration control for setting the Buzz duration in the range 1-50 ms.
An appropriate duration can be fotmd for most cells that is sufficiently
long to allow penetration of the membrane but short enough that the cell is
not damaged after penetration.
MODEL CELL Every AXOCLAMP-2A is supplied with a CLAMP-1 model cell. This
model cell plugs directly into the input sockets of the headstages. A
switch allows the
—
two 50 MQ electrodes to ground, or (b) CELL mode — two electrodes
CLAMP-1
model cell to be configured as (a) BATH mode
connected to a 50 MO // 500 pF cell.
The CLAMP-1 model cell can be used to test and practice using bridge
current clamp, discontinuous current clamp, single-electrode voltage
clamp and two-electrode voltage clamp. It is a useful tool to use while
leaming the operation of the AX0CLAMP-2A and subsequently to verify
the correct operation of the AXOCLAMP-2A and the recording pathway.
GENERAL A third HS-2 headstage can be used extracellulariy to record bath
potential. The bath potential is then subtracted from the potentials
recorded by the two intracellular microelectrodes to compensate for shifts
in bath potential due to changing of solutions or temperature.
A VG-2 Virtual-Ground headstage may be used to measure total bath
current. Generally, the built-in current monitors are more useful since
they yield the microelectrode currents separately without any interfering
currents (e.g. from ionophoresis). Since both microelectrode amplifiers
are complete, one microelectrode can be used for ionophoresis while the
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 17

FEATURES Page?
other is used intracellularly. Internally generated hum due to the built-in
power supply has been prevented by using a specially constmcted lowradiation transformer, by placing the supply well away from the rest of
the circuitry, and by using intemal shielding. The incoming power is
filtered to remove radio-frequency interference (RFI).
QUALITY The excellence of the components and constmction will be obvious to you
from the high quality of the cabinet and controls. Precision ten-tura
potentiometers and reliable switches abound. But the high qualify is more
than "skin deep' gold plated connectors ar^ used throughout, ultralowdrift operational amplifiers are used in all critical positions, I.C.s are
socketed for easy maintenance, and the circuit designs and operation have
been well tested in laboratories throughout the world. All this adds up to
low-noise, low-drift, reliable and accurate operation. And the
excellence does not stop with the hardware. We also provide a detailed
operator's manual that serves as a handbook of procedures for
microelectrode users. A separate service manual is also supplied.
FURTHER INFORMATION
AND ORDERING
The AXOCLAMP specification sheet contains complete technical details
and ordering information. Please call the factory for answers to any
questions you may have.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 18

Pages
FEATURES
Crhis page is intentionally left blank)
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 19

GLOSSARY
Page 9
GLOSSARY
AXOCLAMP and AX0CLAMP-2A are used interchangeably.
Cia Total input capacitance of the headstage due mainly to the microelectrode and any
connecting cable
Cm Input capacitance of cell
cSEVC Continuous single-electrode voltage clamp
DCC Discontinuous current clamp
dSEVC Discontinuous single-electrode voltage clamp
fg Sampling rate; rate for switching from current passing to voltage recording in DCC
and dSEVC modes
G The average gain during dSEVC
GT
The instantaneous gain of the controlled current source during dSEVC
H Headstage current gain
11 Continuous current flow in microelectrode 1
12 Current flow in microelectrode 2
Im Membrane current flow
Lag High-frequency cut
Lead High-frequency boost
MEI Microelectrode 1
ME2 Microelectrode 2
Re Electrode resistance
Rg Resistance in series with membrane
RMP Resting membrane potential
Rm.Rin Input resistance of cell membrane
SEVC Single-electrode voltage clamp
TEVC Two-electrode voltage clamp
Vl Continuous voltage recorded by microelectrode 1
Vj Voltage recorded by microelectrode 2
VC Voltage Clamp
VG Virtual-ground output attenuation
Vm Membrane potential recorded by microelectrode 1
Vmon Voltage at the input of the sample-and-hold amplifier (SHI)
AX0CLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 20

Page 10 GLOSSARY
Crtiis page is intentionally left blank)
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 21

QUICK
GUIDE TO OPERATIONS Page 11
QUICK GUIDE TO OPERATIONS
The controls and operation of die AXOCLAMP-2A are very briefly described in this section. Detailed
explanations are given in the alphabetically organized Section E of this manual.
Dl.
HEADSTAGES
(1) HS-2 Series
HS-2 series headstages are standard. Two supplied with AX0CLAMP-2A.
All HS-2 headstages record voltage at unity gain.
Z'
i .^. K [J> CC ^^^-M- j
Available in several headstage current gains (H). Front-panel controls read direcdy in indicated units
when H = xl. All H values are powers of 10. Small H values used widi high-resistance cells and
electrodes. Large H values used to pass large curretits.
H= xlO, xl, xO.l, xO.Ol for recording and clamping. H = 0.0001 for ion-sensitive electrodes.
Headstages normally supplied in L version (low-noise, low capacitance-neutralization range). M version
can be supplied to compensate large capacitance of grounded shield.
Red connector: Microelectrode input
Gold Connector: Driven shield; case
Yellow connector: Ground output
(2) HS-4 Series
Optional for current-passing electrode (ME2) in two-electrode voltage clamp. (Requires VG-2 for current
measurement.) Bypasses internal current-setting resistor during two-electrode voltage clamp so output
voltage appliad directly to electrode.
Supplied in L or M versions only.
When AX0CLAMP-2A is not in two-electrode voltage clamp mode HS-4 operates same as HS-2.
(3) VG-2 Series
Optional virtual-ground headstage measures total badi current. Not required for normal operation.
Required in two-electrode voltage clamp if HS-4 headstage used. Virtual Ground output attenuation (VG)
specifies the sensitivity. Smaller VG is more sensitive; used for low currents.
AXOCLAMP-2A
THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 22

Page 12
D2.
MODE GROUP
QUICK GUEIE TO OPERATIONS
Illuminated pushbuttons reconfigure AXOCLAMP-2A for different operating modes.
BRIDGE: Two conventional microelectrode amplifiers.
DCC:
Discontinuous current clamp on microelectrode 1.
SEVC: Single-electrode voltage clamp on microelectrode 1.
Discontinuous SEVC (dSEVC) uses time-sharing technique (electrode switches repetitively
from voltage recording to current-passing).
Continuous SEVC (cSEVC) is .analogous to whole-cell patch clamp (electrode
simultaneously does voltage recording and current passing).
TEVC: Two-electrode voltage clamp. Microelectrode 1 does voltage recording. Microelectrode
^2 does current passing. -——- - . - •
Cont./Discont.: Switch and lamps operate only in SEVC mode.
D3.
MICROELECTRODE 1 (MEI) GROUP
Complete intracellular/extracellular electrometer.
Capacitance Neutralization:
Neutralizes electrode input capacitance. Clockwise rotation reduces
effective input capacitance and speeds response. Overutilization oscillates
headstage.
Buzz:
Deliberate overutilization of capacitance neutralization. Oscillation helps
cell penetration. Footswitches supplied as standard accessories.
Bridge:
Compensates electrode voltage drop during current passing. Resistance
(scaled by H) read on ten-tum dial. Range automatically reduced tenfold
during cSEVC.
Input Offset:
Adds ±500 mV DC to electrode voltage at early stage,
electrode voltage while extracellular.
DC Current Command:
For injection of j^nsjant current. Magnitude set on ten-tum dial.
Polarity set on switch. LED indicates when current injection activated.
Clear:
Passes large hyperpolarizing and depolarizing current to clear blocked
electrodes or help cell impalement.
Voltmeter:
Indicates membrane potential (Vm) in mV.
Use to zero
D4.
MICROELECTRODE 2 (ME2) GROUP
An independent intracellular/extracellular electrometer similar to MEI. Differences are:
Potential is labelled V2.
Output offset adds ±500 mV to electrode voltage in output stage.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 23

QUICK GUIDE TO OPERATIONS
D5.
VOLTAGE-CLAMP GROUP
Page 13
Gain:
Sets open-loop gain during voltage clamp. In SEVC modes output is
current source. Therefore gain is nA/mV. In TEVC mode ou^ut is
voltage source. Therefore gain is V/V.
Holding Position:
RMP Balance Lamps:
Sets holding potential during voltage clamp. Range ±200 mV.
Null during Bridge or DCC so that when activated, voltage clamp will be
at resting membrane potential.
Phase shift:
Modifies frequency response of voltage-clamp amplifier. Compensates for
nonideal phase shifts of membrane. Potentiometer adds phase advance
Oead) or phase delay Gag). Switch selects range.
Anti-Alias Filter:
D6.
STEP-COMMAND GROUP
Used in DCC or dSEVC modes to reduce noise of electrodes that have fast
and slow setding characteristics.
Uses D/A converter to generate precision command voltage.
Destination Switch:
Selects voltage clamp or either microelectrode as target for command.
Commands are mV or nA respectively.
Thumbwheel Switch:
Sets magnitude widi 0.05% resolution.
Ext./Cont./Off Switch:
Cont. position activates step command. Ext. position thumbwheel switch
is off unless logic level HIGH applied to rear-panel Step Activate input.
Off position overrides logic input.
Indication:
When destination is a microelectrode and step command is activated, lamp
in microelectrode DC Current Command Section illuminates.
D7.
RATE GROUP
Counter indicates sampling rate (cycling rate) in DCC and dSEVC modes.
Potentiometer adjusts rate from 500 Hz to 50 kHz.
AXOCLAMP-2A THEORY St OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
•v7
Page 24

Page
14
D8.
INPUTS
QUICK GUIDE TO OPERATIONS
AND
OUTPUTS
Vm, Im Output Bandwiddi switch selects
Current
used
All BNC inputs
Im
11
12
G)
voltmeter displays
to
select meter input. Decimal point
and
outputs located
DC
output: Membrane current recorded
Cont. Output:
MEI
current (equals Im
output: ME2 current.
-3 dB
frequency
of
single-pole lowpass
current from eidier microelectrode
set on Hi, H2 or
on
rear panel. Frequendy used outputs repeated
VG switches.
by MEI.
in
Bridge, cSEVC
IviRT output: Virtual-ground current.
10.Vm output: Membrane potential recorded
Vl Cont. output: Instantaneous
Monitor output: Input
of
MEI
potential.
sample-and-hold amplifier. Should
oscilloscope during DCC
by MEI;
No
Bridge Balance.
and
dSEVC modes.
gain
V2 output: ME2 potential. Includes Bridge Balance.
Sample Clock output: Logic-level pulses
VBATH
output: Potential recorded
Cal. Activate input:. Logic HIGH
at the
sample rate; used
by
bath electrode.
on
this input puts calibration voltage proportional
on
Im
and
10.
Vm outputs.
or
virtual ground
and
TEVC modes).
if
used. Switch
on
front panel.
of 10.
be
observed
to
trigger monitor oscilloscope.
on
to
thumbwheel
second
setting onto voltage
and
current outputs.
Step Activate input: Logic HIGH activates Step Command.
Blank Activate input: Logic HIGH activates Blank. During Blank,
Thus stimulus artifacts
Ext.
VC
Command input: Voltage
Ext.
MEI
Command input: Voltage
Ext. ME2 Command input: Voltage
R» Comp. input: Used
to
normally required.
VBATH
IN
input: Bath potential recorded
connected
D9.
REMOTE
Allows certain functions
to be
remotely activated
on
this input converted into voltage-clamp command.
on
this input converted into
on
this input converted into ME2 current command.
compensate voltage drop across membrane
to
this input.
are
See
service manual
by
by
computer
rejected.
MEI
current command.
for
suggested circuit.
other equipment subtracted from
or
switches. These
Clear.
DIG. CLOCK LINK-UP
Allows sampling clocks from
talk when
two
AXOCLAMP-2As
two
AX0CLAMP-2AS
in
dSEVC mode used
to be
synchronized. This eliminates electrode cross-
to
clamp
two
cells
LU-1 link-up cable.
Vm
prevented from updating.
R,
during TEVC.
Not
Vi and V2 if
are
Mode, Buzz
in
same preparation. Requires
and
AXOCI-AMP-2A
THEORY & OPERATION, COPYRIGHT FEBRUARY
1990,
AXON INSTRUMENTS,
INC.
Page 25

DETAILED GUIDE TO OPERATIONS Page 15
DETAILED GUIDE TO OPERATIONS
ANTI-ALIAS FILTER
A property of all digital sampling systems is that noise in the input signal at frequencies greater than 0.5 of
die sample rate (fg) is folded down to appear as extra noise in the bandwidth from zero to 0.5 of fg (see
section on noise). This phenomenon is known as aliasing.
Aliasing can be overcome by filtering die input signal before sampling, thereby reducing die highfrequency noise content. However, this filtering procedure degrades the dynamic response of the input
signal and when used with an ideal microelectrode worsens the clamp performance.
The voltage across a real microelectrode often has a two-phase decay after the end of a current pulse,
either because of redistribution of ions in the tip, or because of the distributed nature of the capacitance
through the wall of the microelectrode (see Fig. 1). The final stages of the decay may often be so slow
that additional delay introduced by a filter us^ to prevent aliasing (an Anti-Alias Filter) causes
insignificant worsening of the dynamic response. The Anti-Alias Filter can be used by the experimenter
to trade off the noise recorded in DCC and dSEVC modes against the dynamic response. That is,
increasing the Anti-Alias Filter setting decreases the noise but can lead to instability in dSEVC and can
make it more difficult in DCC to balance the response to a current step.
The Anti-Alias Filter also has an effect in the continuous modes. It acts as a lowpass filter on the voltage
recorded by MEI. Thus the effects during TEVC and cSEVC are the same as those due to a slow voltagerecording microelectrode. Using the Anti-Alias Filter in these modes is not recommended.
Rotating the Anti-Alias Filter control clockwise logarithmically increases die amount of filtering. In the
fully counterclockwise position the filter time constant is 0.2 ;xs and the discontinuous clamp responses are
unaffected. In the fiiUy clockwise position the filter time constant is 100 /iS. There is a maximal
reduction in noise but the maximum sampling rate which can be achieved is severely limited (to about 1
kHz or less).
FIGURE 1 - TWO-PHASE MICROELECTRODE DECAY
AXOCI-AMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 26

Page 16 DETAILED GUIDE TO OPERATIONS
BATH PROBE
Bath Potential Measurement
In certain experimental circumstances it is desirable to make all voltage measurements relative to a
reference point in the bathing solution radier than relative to ground. (These conditions may include
precision measurements during changes of temperature or ion content of the saline, or cases of restricted
access from the extracellular space to the grounding point.)
All measurements are normally made relative to the system ground. However, if an HS-2 headstage is
plugged into the rear-panel Bath Probe connector, measurements by both MEI and ME2 are automatically
made relative to the potential recorded by this headstage. For optimum voltage-clamp performance, the
bandwidth of die bath potential is limited to 300 Hz before it is subtracted from the potentials recorded by
MEI and ME2 (see Finkel & Gage, 1985). The bath microelectrode cannot be used for current passing.
The fiill-bandwiddi voltage recorded by die badi microelectrode is available at the VBATH OUT connector.
If there is no HS-2 headstage plugged into the Bath Probe connector, a reference potential from an external
amplifier can be subtracted by connecting a reference source to the VBATH IN connector.
Grounding
It is quite uncommon to measure the bath potential. Irrespective of whether or not the bath potential is
measured, the preparation bath should be grounded by direcdy connecting it to the yellow ground
connector on the back ofthe MEI headstage (or to a virtual-ground headstage if used).
BLANKING
A common problem when using stimulating electrodes is that some of the stimulus is direcdy coupled into
the recording microelectrode. This can saturate subsequent high-gain amplifiers and die coupling
capacitors of AC circuits. The saturation effects may take tens or hundreds of milliseconds to subside.
The best way to minimize or even eliminate this artifact is at the source, by using small stimuli, isolated
stimiilators, placing an grounded shield between the stimulating electrodes and the microelectrodes, etc.
Often, though, it is not possible to reduce the artifact to manageable levels.
The AXOCLAMP-2A can circumvent the effects of the stimulus artifact by Blanking. At the moment the
logic level of the Blank Activate input goes HIGH the value of
Vm
is sampled and saved. For the duration
ofthe HIGH signal, this saved value is used instead ofthe actual potential.
In voltage-clamp modes the voltage-clamp current during the Blanking period will be held at the level
which existed at the start of the period. A small deviation from the command potential may develop
during the Blanking period as a result of comparing the command to the sampled value of
the instantaneous value of
Vm.
This deviation will only be seen when the Blanking period ends. Usually
Vm
instead of
this deviation is preferable to the situation that can occur if Blanking is not^used. If Blanking is not used
the artifact pick^ up by MEI is treated by the voltage-clamp circuit as an attempt by the cell to change its
potential. Therefore, the voltage-clamp circuit causes a current to be passed into the cell to clamp this
presumed membrane potential change. If
the
stimulus artifact is large, the consequent current artifact can
be large enough to damage the cell.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRITMENTS, INC.
Page 27

DETAILED GUIDE TO OPERATIONS Page 17
The width of the Blanking period should be no longer than the minimum width required to cover the
period of the stimulus artifact. It is important not to Blank for longer than necessary since during
Blanking no updating of
minimize the artifact at the source.
BRIDGE MODE
Description
In Bridge mode the microelectrode voltages are monitored continuously, and continuous currents can be
injected down MEI or ME2.
Associated with the current flow (I) in a microelectrode is a voltage drop across the microelectrode which
depends on the product of the current and the microelectrode resistance (Re)- This unwanted IR voltage
drop adds to the recorded potential. The Bridge Balance control can be used to balance out this voltage
drop so that only membrane potential is recorded. The term "Bridge" refers to the original Wheatstone
Bridge circuit used to balance the IR voltage drop and is retained by convention even though die circuitry
has been r^laced by operational amplifier techniques.
Vm
is allowed. Even when Blanking is used, attempts should still be made to
The particular setting required to balance the Bridge is aJmeasure^Qhe-microelectrodTiresistance. j =• /\
In cSEVC mode the Bridge potentiometer compensates electrode IR voltage drop at one-tenth sensitivity.
Suggested Use
Set die Destination switch to ME 1/2 and externally trigger die Step Command generator so that ^uls^ of
current are repetitively injected into MEl/2. (Altematively, derive the command for injecting current
pulses by connecting a signal source to the Ext. ME 1/2 Command input.) Start with the Bridge Balance
control set to zero. Advance the dial until the fast voltage steps seen at die start and finish of the current
step are just eliminated. The Bridge is correcdv balanc^. The residual transient at the start and finish
of the current step is due to the finite response speed of'the ihicroelectrode. No attempt is made to
balance this transient since it covers a very brief period only and it is a useful indication of the frequency
response of the microelectrode. The transient can be minimized by correcdy setting the Capacitance
Neutralization.
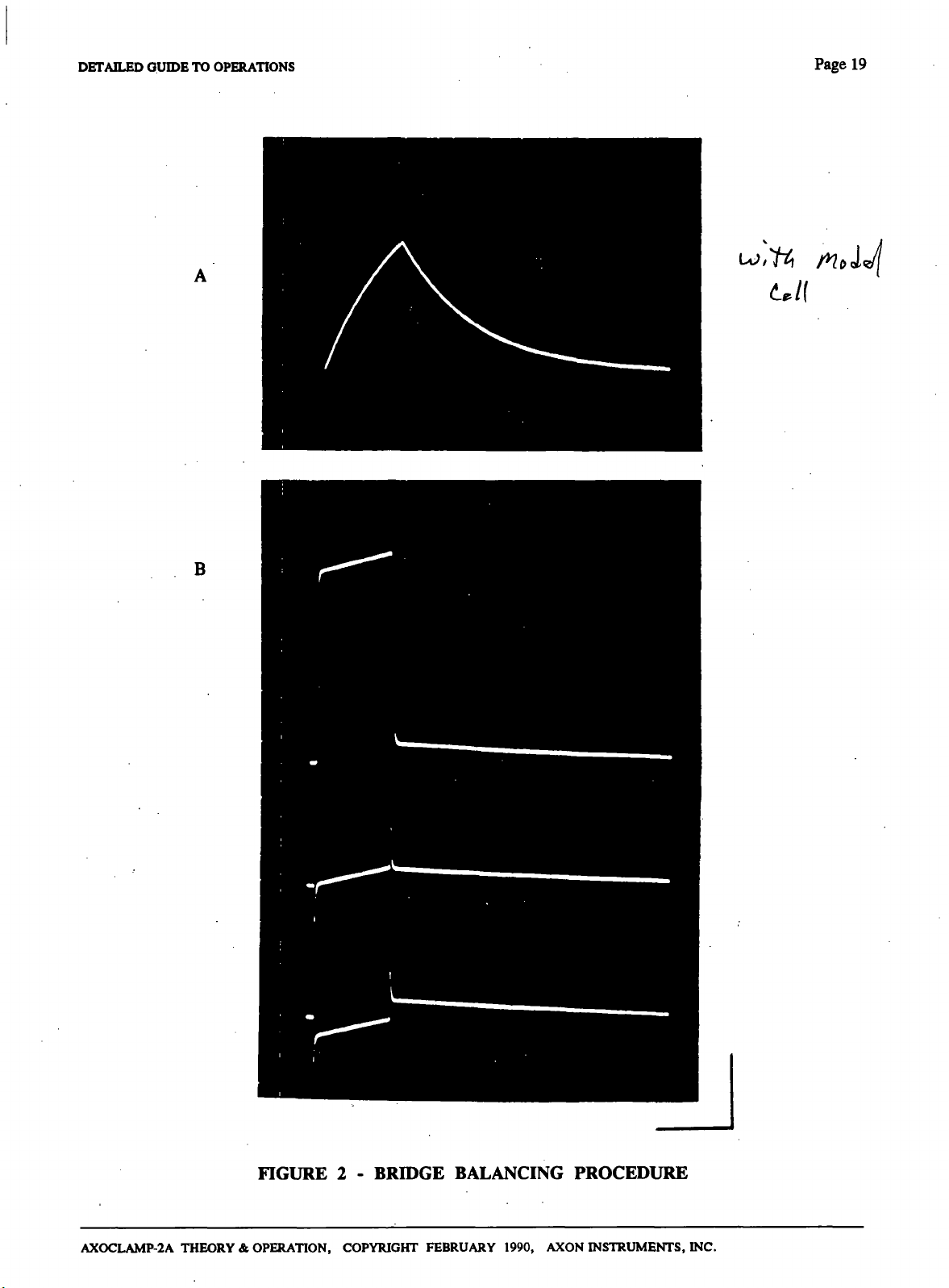
The Bridge balancing procedure is illustrated in Fig. 2. The trace in A was recorded in a_model cell when
the Bridge Balance control was correctly set. In response to a positive current pulse the membrane
potential began to charge up. Before the membrane potential reached its final value die current pulse was
terminated and the membrane potential exponentially decayed to its
Hie traces in B were recorded at a sweep speed which was fast compared with the membrane time
constant, hence the membrane responses look like straight lines. The top trace shows the voltage
recorded when no Bridge Balance was used. The response was dominated by the IR voltage drop across
the electrode. In the middle trace the Bridge Balance was optimum and in the bottom trace it was slighdy
overused.
final
value.
7
When the Bridge is correctly balanced the resistance of the microelectrode can be read directly from the
dial. The sensitivity is 10 -^ H
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
MO
per turn.
Page 28

Page 18
DETAILED GUIDE TO OPERATIONS
The Bridge Balance controls operate on die 10.Vm output and on the
V2
output. On the 10.Vm output the
Bridge Balance control satiirates when the IR voltage drop exceeds ±600 mV referred to the input.
Intracellular Balancing
The traces in Fig 2. were all recorded with the electrode inside the cell. Since the electrode response and
the oscilloscope swe^ speed were fast compared with the membrane time constant (as in Fig. 2B), the
correct Bridge Balance setting was easy to see, even through die electrode was inside the cell.
It is sometimes usefiil to inject a brief small current pulse at the start of each oscilloscope sweep in order to
continually check the Bridge Balance setting during the course of an experiment.
Figure 2
Illustration of Bridge balancing technique. All traces were recorded from the 10.Vm output. The model
cell was 10 M0//1 nF.
R*
was 10 MO.
Recording bandwiddi: 30 kHz.
Vertical calibration: 20 mV referred to Vm-
A. Response.to.+5.nA.10.nis current pulse,. Bridge correctly balanced. Trace is membrane
response only"!
Cal. bar: 20 ms.
B.
Response to -t-5 nA 1 ms pulse.
Cal. bar:
1
ms.
Top trace: No Bridge balance used.' Fast voltagesteps at start and finish of the current
pulse are the electrode IR voltage drop.
Middle
trace:
Bridge correcdy balanced. Trace is membrane response only. Transient
electrode response remains.
Bottom trace: Bridge balance overused. Negative going step is introduced by the Bridge
Balance circuit.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 29
DETAILED OinDE TO OPERATIONS
Page 19
B
FIGURE 2 - BRIDGE BALANCING PROCEDURE
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 30

Page 20
DETAILED GUIDE TO OPERATIONS
BUZZ
When the Buzz switch or the footswitch is depressed, the amount of Capacitance Neutralization is
increased. If the Capacitance Neutralization control is within a few tums of optimuni, this extra
compensation causes the headstage to go into high-frequency oscillation. If this is done while the tip of
the microelectrode is pressing against the cell membrane the oscillation will often help the microelectrode
impale the cell. The exact mechanism is unknown, but it may involve attraction between the charge at
the tip of
the
microelectrode and bound charges on the inside of
the
membrane.
To use the FS-3 footswitches, plug them into the 4 mm jacks on the back panel. The red jack labelled
"+5 V" is shared by the two footswitches. There is one violet jack for each of the two footswitches.
Precise control of the duration of Buzz can be achieved by connecting a pulse generator to pin 15 of the
Remote connector (see Remote Section). For some small cells a long duration Buzz can be deadly. In
this case it may be helpful to use an external pulse generator connected to pin 15 of the Remote connector
to gate the Buzz oscillation so that it is on for just a few milliseconds. The hand-held Remote Buzz
generator (see next page) is designed to allow you to conveniently generate Buzz durations between 1 and
50 ms.
It is difficult to interpret the operation of Buzz by watching die 10.Vm trace. This is because the xlO gain
and lowpass filter on the 10.Vm output strongly affect the amount of headstage oscillation seen. As a
quick guide, if the 10.Vm trace is unaffected dien Buzz did not succeed (so increase die Capacitance
Neutralization setting). If
the
10.Vm trace jumps then Buzz was successful.
The Buzz oscillation can be clearly observed on the Vi Cont. output.
If a grounded shield adds a lot of capacitance to ME2 the Capacitance Neutralization range may be
insufficient when an HS-2L headstage is used. In this case it will be necessary to use an HS-2M headstage
(see Headstage Section).
Ranote Buzz
Installation: Plug the Buzz control into the rear-panel ' remote' connector of the Axoclamp.
If you want to use some of the pins on the rear-panel remote connector to remotely select
the operating mode or activate the Clear currents, you will have to remove the cover from
the plug on the Remote Buzz unit and solder your inputs to the appropriate spare pins on
this plug.
Use:
Set the desired Buzz duration on the Duration control of die Remote Buzz unit. Press the
button corresponding to the electrode you want to buzz. Note that the Duration control is
shared by the two electrodes.
For Buzz durations greater dian 50 ms, use the buttons on the front panel of the
Axoclamp. Neither the buttons on the front panel of the Axoclamp nor the footswitches
use the duration set on the Remote Buzz unit.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 31

DETAILED
GUIDE TO OPERATIONS Page 21
CALIBRATION SIGNAL
A calibration signal can be simultaneously superimposed on all of the voltage and current outputs (except
IVIRT) for die duration of a HIGH signal on the Cal. Activate input. —
For voltage outputs, die_magnitude ofthe Cal. signal is direcdy equal to the setting of die Step Command
thumbwheel switch. For example, +123A will put -(-123.4 mV on the voltage outputs.
For current outputs, the magnitude of die Cal. Signal is lOx the setting of the Step Command thumbwheel
switch. For example, -019.6 will put -196 mV on the current outputs. The equivalent current depends
on H. In this example, the Cal. signal of -196 mV would correspond to -19.6 nA for H = xl, -1.96 nA
forH = x0.1 etc.
Suggested Use
At the start of a recording sequence, briefly activate Cal. After a short interval, activate the Step
Command. The Cal signal will be a permanent record of the command voltage or current.
CAPACITANCE NEUTRALIZATION AND INPUT CAPACITANCE
The Capacitance (Cin) at die input of die headstage amplifier is due to the capacitance of the amplifier input
itself (Cini) plus the capacitance to ground of the microelectrode and any connecting lead (Cin2)- Cin
combined with the microelectrode resistance (Re) acts as a lowpass filter for signals recorded at the tip of
the microelectrode. Two techniques may be used to increase the recording bandwidth.
Primary
A special technique is used in the headstages to keep the contribution to Cm from the input amplifier as
small as possible. This consists of adding the input signal voltage to the power-supply voltages used by
the input stages. This technique, known as bootstrapping, fixes the voltage drop across Cini to a
constant value thereby preventing current flow through
Cini •
The effective value of
Cini
is thus reduced to
well below its real value.
Secondary
A conunonly used technique known as capacitance neutralization is used to negate Cin2 and the effective
remnant of Qni. The capacitance neutralization circuit attempts to inject into the headstage input a current
which it anticipates will be required to charge aind discharge Cin during signal changes. To use the
cqiacitance neutralization circuit the voltage response to a current step should be observed on an
oscilloscope. Advance the capacitance neutralization control as far as is possible without introducing
overshoot in the step response. This setting is optimal for current passing and is also optimal for
recording potentials at die tip of the microelectrode.
It is important to recognize that the capacitance neutralization circuit is not more than 90% effective even
for ideal microelectrodes. This is because of the finite frequency responses of the headstage amplifiers
AXOCLAMP-2A
THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 32

Page 22 DETAILED GUIDE TO OPERATIONS
and the capacitance neutralization circuit, and also because Ci„ does not behave ideally as a linear lumped
capacitor. Consequently, die amount of Cin that the circuit must neutralize should be kept as small as
possible. To this end, avoid using long lengths of shielded cable to connect the microelectrode to the
input. If possible, plug the microelectrode holder directly Into the input. Use shallow bathing
solutions. Avoid having grounded objects near the electrode. Do not ground the headstage case.
If metal objects (such as the microscope) must be very near the electrode, they may be disconnected from
ground and connected to the gold shield socket in the headstage. This technique can improve the
microelectrode response speed. However, it may be that in DCC and dSEVC modes there will be an
increase in the amount of switching noise picked up by independent recording electrodes, if used.
See also the section tided Microelectrodes for Fast Settling.
CLEAR
There is one Clear switch for each microelectrode. It is used to pass up to ±600 x H nA down the
microelectrode. " + " and "-" correspond to depolarizing and hyperpolarizing currents respectively. The
Clear switch is used for two purposes:
(1) When die microelectrode tip resistance goes high diis condition can often be cleared by rapidly
toggling the Clear switch from + to -. Because of the large current passed this should only be
done extracellulariy.
(2) Sometimes microelectrode tips press against the cell membrane but fail to penetrate. A quick flick
of the Clear switch will often force the microelectrode to penetrate. Whether to use a
hyperpolarizing or depolarizing current depends on the preparation and must be determined by trial
and error. Like Buzz, the mechanism for impalement is unknown.
COMMAND GENERATORS
Command levels for voltage clamp or current clamp can be obtained from the internal step command
generator, from the internal DC command generators, and from external sources.
Step Command Generator
The Step Command generator can be used either as a current-clamp or voltage-clamp command depending
on the position of the Destination switch. If the Destination switch is used to select VC then the
magnitude on the thumbwheel switch represents voltage-clamp potential in mV's irrespective of the
headstage current gain (H). If the Destination switch is used to select MEI or ME2 then the magnitude on
the thumbwheel switch represents the number of nA of current to be injected down MEI or ME2
respectively. The current range is scaled by the H. The maximum magnitude on the thumbwheel switch
is 199.9.
"
+ ' corresponds to depolarizing voltage shifts and currents. "-" corresponds to
hyperpolarizing voltage shifts and currents.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 33

DETAILED GUIDE TO OPERATIONS Page 23
The duration for which the Step Command is activated can be made continuous by switching the
Ext./Cont./Off toggle to "Cont." or externally determined by a logic HIGH level on the rear-panel .Slai
Activate input. When rotating the diumbwheel switch in continuous mode, be decisive. If the switch is
rotated slowly the output will momentarily fall to zero because the switching contacts will pass through an
open-circuit state. ^
DC Command Generators
Separate DC command generators are provided for VC, MEl and ME2.
The DC command for VC is called "Holding Position." It allows the membrane potential holding position
during voltage clamp to be shifted to a value in the range ±200 mV. It is always operative during voltage
clamp. Before the voltage clamp mode is selected, the Holding Position potentiometer is used to set'the
RMP Balance (see the RMP Balance section). The Holding Position potentiometer is deliberately not
calibrated because the exact setting depends on the adequacy of the clamp gain. Instead, the holding
position should be read direcdy from the digital voltmeter displaying Vm- A ten-tum locking dial is used
so that once set, the Holding Position potentiometer can be locked to prevent accidental changes.
The MEI and ME2 DC commands are called "DC Current Command." Each is controlled by a precision
ten-tum dial and can be switched by a toggle switch ft-om depolarizing (+) to hyperpolarizing (-) or off
(OFF).
An LED illuminates whenever the toggle switch is in the -t- or - position. It also illuminates if
the Destination switch is tumed to the microelectrode in question and the Step Command generator is
activated eidier by die Ext./Cont. switch or by a logic HIGH level on die Step Activate input. The current
is scaled by the H. If the Step Command and the DC Current Command are used simultaneously, the
total command is their sum.
External Command Inputs
Three extemal command inputs are provided. These are for setting the voltage-clamp command (Ext. VC
Command), the ciirrent-clamp command in MEI (Ext. MEI Command), and the current-clamp command
in ME2 (Ext. ME2 Command). These inputs are active simultaneously widi the intemal command
generators and do not depend on the position of the Destination switch. The sensitivity of Ext. VC
Command is 20 mV/V. The sensitivity of die Ext. ME1/ME2 Command is 10 x H nA/V.
The external command inputs are DC connected. ' Therefore, when using the Ext. MEI and ME2
Command inputs any deviation from zero volts of the external signal source while it is in its "ofT state
will cause a DC current to flow in the electrode.
This can be avoided by using:
(1) A very high-quality extemal source which puts out a true zero voltage level in its off state or
which can be trimmed to do so.
(2) An isolated extemal source.
Mixing Commands
Complex command waveforms can be generated by appropriately mixing the Step Command, the DC
Command and the Ext. Command. For example, die command waveform in Fig. 3 can be used to
establish the current injected into MEI by setting the Destination switch to the MEI position and using the
MEI DC Command and the Ext. MEI Command input.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRIJARY 1990, AXON INSTRUMENTS, INC.
Page 34

Page 24
DETAILED GUIDE TO OPERATIONS
EXT.
COMMAND
(external sine wave
in this example)
DC CURRENT COMMAND
(set
on pot.)
HGURE 3 - SUMMATION OF COMMANDS
STEP COMMAND
(set
on
thumbwheel)
This figure shows the command potential that would result if all command sources were switched on one at
a time and left on.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 35

DETAILED GUIDE TO OPERATIONS Page 25
CURRENT MEASUREMENT
The current injected down each microelectrode is independendy measured. The measurement is true.
Thus if the electrode blocks the measured current falls to zero even though a current command may exist.
Two current ou^uts apply to MEl. Im is the membrane current while Ii Cont. is the instantaneous current
in MEI. In continuous modes (Bridge, cSEVC and even TEVC) Im and Ii Cont. are identical. However,
in discontinuous modes (i.e. DCC and dSEVC) Im and Ii Cont. are different. Ii Cont. switches firom zero
to some finite value at the sample rate. This is because for 30% of each period MEl is used for passing
current while for the remaining 70% of each period no current is passeid and the IR drop due to the
previous current is allowed to passively decay (see DCC and cSEVC sections). On the other hand, Im is
the true membrane current. It is recovered from the instantaneous electrode current by a circuit which
samples the current pulses, retains the samples during the passive-decay period, then scales the samples
to yield the average current for the whole period. The Im output is smoothed by the output filter (see the
Output Filter section).
The current in ME2 is labelled I2.
The gain of the current measurement circuits depends on the headstage current gain (H). It is 10 -^ H
mV/nA.
The whole current into the bath can be separately measured using a virtual-ground headstage. (See die
Virtual Ground section.)
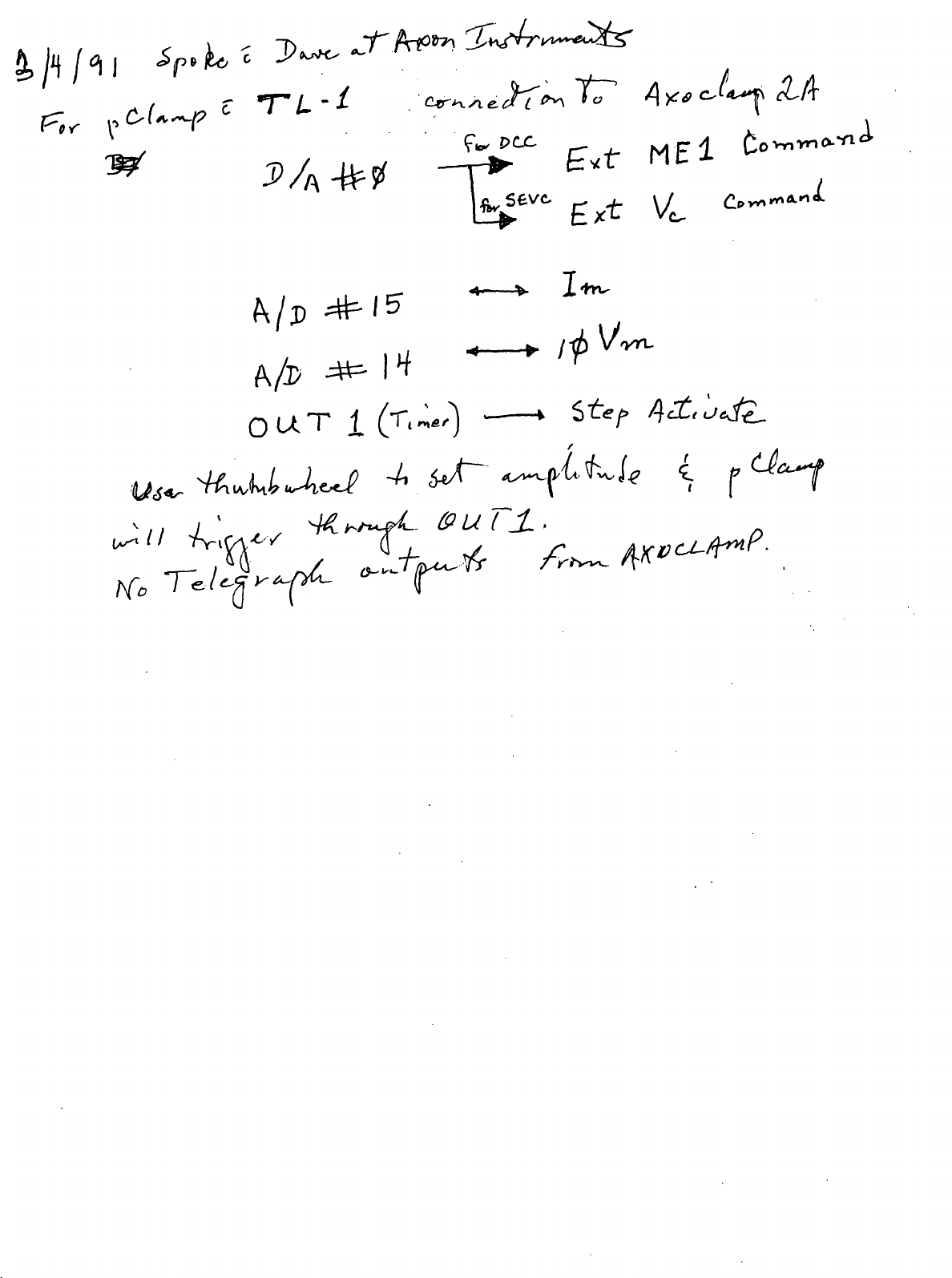
DCC MODE
Description
In Discontinuous Current Clamp (DCC) mode MEl is cyclically used to pass current. The voltage
recorded at the tip of MEl is memorized by a sample-and-hold circuit inbetween each current-passing
period after all transient voltages due to current passing have decayed. Thus the membrane potential can
be recorded independendy of IR voltage drops across the electrode. The advantage of DCC mode
compared with Bridge mode is that it is tolerant of small changes in microelectrode resistance. The
disadvantage is that DCC mode is noisier than Bridge mode. During DCC mode ME2 can be used for
continuous current passing.
The principles of operation are oudined in the block diagram and timing diagram of Fig. 4, and in the
following discussion.
The voltage recorded by the microelectrode (Vi) is buffered by a unity-gain head stage (Al). To begin the
discussion assume that Vt is exactly equal to the instantaneous membrane potential (Vm). Switch S2
briefly closes thereby enabling the voltage on die holding capacitor
S2 opens again after the "sample" period and Vm is held by
CH-
(CH)
to charge up to the value of
A buffer amplifier (A2) interfaces
Vm-
CH
to
the recording apparatus. This switch, capacitor and buffer amplifier arrangement constitute an analog
memory known as a sample-and-hold amplifier.
AXOCI-AMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 36

Page 26 DETAILED GUIDE TO OPERATIONS
Immediately after the sample period, the current injection period begins when switch SI changes over
ft-om the 0 volts position to the current-command voltage (Vc) position. This connects Vc to a differential
amplifier (A4) arranged so that its output is Vi -I- Vc. The voltage appearing across Ro is exacdy equal to
Vc diereby forcing the current (lo) into the microelectrode to be equal to Vc/Ro. Amplifiers A4 and Al
and resistor Ro constitute a controlled-current source (CCS) which injects a current into the microelectrode
direcdy proportional to the voltage at the input of the CCS irrespective of the resistance of the
microelectrode or die voltage at its tip.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 37

DETAILED GUIDE TO OPERATIONS
Inside
headstage,-
Page 27
• CRO
S1
'm
sample
-Vr
0 volts
sample
FIGURE 4 - DCC MODE BLOCK DIAGRAM AND TIMING DIAGRAM
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 38

Page 28 DETAILED GinOE TO OPERATIONS
During the current-injection period a square pulse of current proportional to Vc is injected into the
electrode. Because of this current Vi rises. The rate of rise of Vi is limited by die parasitic effects of
capacitance through the wall of the glass microelectrode to the solution, and capacitance at the input of the
buffer amplifier. The final value of V] reached consists mosdy of the IR voltage drop across the microelectrode resistance. Only a tiny fraction of Vi consists of the membrane potential recorded at the tip.
After 30% of one cycle has elapsed, the voltage-recording period begins when SI changes over to the
0 volts position. Passive decay occurs because the input of the CCS is 0 volts and thus its output current
is zero. Sufficient time must be allowed during the voltage-recording period for Vi to decay to widiin a
millivolt or less of Vm. At the end of the passive decay period S2 is again briefly closed and a new
sample of
Vm
is taken to begin a new cycle.
The actual voltage used for recording purposes is the sampled voltage. The sampled membrane potential
is connected to the 10.Vm output. The Vi Cont. output is the instantaneous electrode voltage.
The instantaneous current into die microelectrode is monitored by a differentia] amplifier (A3). The
output of
A3
is taken to an averager (not shown) which samples, smoodis and scales the current pulses and
connects the average value to die Im output.
During DCC mode die input to the CCS and the output of the MEl current monitor are automatically scaled
so that they r^resent the true membrane current even though the instantaneous current flows for only 30%
of the time.
The cycling (sampling) rate must be chosen so that there are ten or more cycles per membrane time
constant. This enables the membrane capacitance to smooth the membrane voltage response to the current
pulses.
Suggested Use
Turn the Anti-Alias Filter to the minimum value and switch to DCC mode. Set the Destination switch to
MEl and set up a repetitive square current command. Observe Im and 10.Vm on the main oscilloscope.
Observe the voltage at the Monitor output on a second oscilloscope (which need not be a high quality type)
with the gain at 100 mV/div (= 10 mV/div input referred). Trigger this oscilloscope from the Sample
Clock output on the rear panel.
Proceed to adjust the Capacitance Neutralization in one of two 1
A. For acceptable but not optimum Capacitance Neutralization, advance the Capacitance Neutralization
control until the square step at the leading edge of the
B.
For optimum Capacitance Neutralization, switch the Step Command generator to continuous.
10.
Vm response is first eliminated.
Advance the Capacitance Neutralization control until the Monitor waveform decays most rapidly to a
horizontal baseline, but without any overshoot.
These techniques are illustrated in Fig. 5. The traces in Fig. 5A show that poorly adjusted Capacitance
Neutralization during DCC mode is similar to poorly adjusted Bridge Balance during Bridge mode.
If the square step cannot be eliminated (without overshoot on the Monitor waveform), decrease the sample
rate (fg).
Set die Output Bandwidth to 1/5 or less of fg.
Reduce the noise on the 10.Vm and Im traces either by advancing the Anti-Alias Filter or by increasing fg,
adjusting the capacitance neutralization where necessary.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 39

DETAILED GUIDE TO OPERATIONS
Page 29
Figure 5
Illustration of Capacitance Neutralization adjustment during DCC. All traces were recorded with a model
cell
10 M0//1 nF. Re was 10 MO. Cycling rate was 25 kHz.
A. 10.Vm ou^ut. Response to a 10 nA 1 ms current pulse.
Vertical calibration: 20 mV referred to VmHorizontal calibration: 1 ms.
B.
Vmon output during the 10 nA current pulse.
Vertical calibration: 40 mV referred to Vm.
Horizontal calibration: 10 /is.
There are diree pairs of corresponding traces.
Traces 1: Capacitance neutralization underutilized. There was a fast step in Vm at the start and
finish of the current pulse because Vmon decayed too slowly to reach its final value.
Traces 2: Capacitance neutralization optimum. Vm shows the membrane response only. Vmon
decay was fast widi no overshoot and easily reached the final value.
Traces 3: Capacitance neutralization overutilized. The fast steps in Vm reappeared, this time
because of overshoot and ringing in Vmon- Note that unlike a Bridge circuit, the effect of
two much compensation can put either a positive or a negative step on Vm (positive in this
example) depending on which cycle ofthe ringing in Vmon is sampled.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 40

Page 30
DETAILED GUIDE TO OPERATIONS
B
FIGURE 5 - HOW TO SET THE CAPACITANCE NEUTRALIZATION DURING DCC MODE
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 41

DETAILED GUIDE TO OPERATIONS Page 31
GROUNDING AND HUM
A perennial bane of electrophysiology is line-firequency pickup (noise), often referred to as hum. Hum
can occur not only at the mains frequency but also at multiples of it.
The AX0CLAMP-2A has inherendy low hum levels (\ess than 20 /iV peak-to-peak). To take advantage of
these low levels great care must be taken when integrating the AX0CLAMP-2A into a complete recording
system. The following procedures should be followed.
(1) Only ground the preparation bath by directly connecting it to the yellow ground connector on
the back of the MEl headstage (or to a virtual-ground headstage if used).
(2) Place the AXOCLAMP-2A in a position in the rack where transformers in adjacent equipment are
unlikely to radiate into its electronics. The most sensitive part of the electronics is the right hand
side looking ftom die front. A diick sheet of steel placed between the AX0CLAMP-2A and die
radiating equipment can effectively reduce the induced hum.
(3) Initially make only one connection to the AX0CLAMP-2A. This should be to the oscilloscope
ft-om the Vl or lO.Vm outputs. Ground die MEl headstage input dirough a 1 MO resistor to die
yellow ground connector. After verifying that the hum levels are low, start increasing the
complexity of the connections one lead at a time. Leads should not be draped near transformers
which are inside other equipment. In desperate circumstances the continuity of the shield on an
offending coaxial cable can be broken.
(4) Try grounding auxiliary equipment ftom a ground distribution buss. This buss should be
connected to the AXOCLAMP-2A via the yellow 0.16 inch (4 mm) socket on the rear panel. This
socket is connected to the AX0CLAMP-2A's isignal ground (i.e. the outer conductors of all the BNC
connectors). The signal ground in the AX0CLAMP-2A is isolated ftom the chassis and power
ground.
(5) If more than one headstage is used, all the headstage cables should run ftom the AXOCLAMP-2A
to the preparation in a bundle. The bundle can be formed either by gendy twisting the cables
together or by loosely tying them together.
(6) Experiment. While hum can be explained in theory (e.g. direct pickup, earth loops), in practice
the ultimate theory is die end result. Following the rules above is the best start. The final hum
level can often be kept to less than 100 /zV peak-to-peak referred to Vm. One technique that
should not be used to reduce the hum is die delicate placement of cables so that a number of
competing hum sources cancel out. Such a procedure is too prone, to accidental alteration.
DR. HARVEY J. KARTEN, M-D-
UNIVERSITY OF CALIFORNIA, SAN DIEGO
DEPARTMENT OF NEUROSCIENCES, 0608
9500 GILMAN DRIVE
LA JOLLA, CA 92093-0608
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 42

Page 32
DETAILED GUIDE TO OPERATIONS
HEADSTAGES
The HS-2 unity gain headstage buffers the high impedance of the microelectrode, making the potential
recorded by the microelectrode available to the rest of the circuitry. It also provides the means for
injecting current into the microelectrode and for neutralizing the input capacitance.
The Meaning Of H
A precision resistor (Ro in Fig.4) inside the headstage sets the headstage current gain (H). Choosing the
H depends on the cell to be clamped (see below). The particular value of H used affects the Bridge
Balance range, the sensitivity to current commands, the sensitivity of the current monitors and the gain in
SEVC mode. The effects are clearly marked on the front and rear panels, and since they always appear in
multiples of 10 they are easy to calculate. For your convenience, Table 1 summarizes these effects.
Note that voltage commands during voltage clamp are not affected.
Which Headstage To Use
The H value required depends on the typical input resistances (Rin) of your cells. The recommended
values are in Table 2.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY
1990,
AXON INSTRUMENTS, INC.
Page 43

DETAILED GUIDE TO OPERATIONS
TABLE 1
How H affects control and measuremait ranges
Page 33
HO)
Ro
Max. Bridge Balance
Max. Step Command
Max. DC Current Command
Ext. Command
Max Total Current^^)
I OuQiut
Max. Gain in dSEVC
Max. Gain in cSEVC
Max. Gain in TEVC
xlO
IMQ
10 MO
±1999nA
±1000 nA
100 nA
6000 nA
ImV/nA
1000 nA/mV
10000 nA/mV
10000
xl
10 MQ
100 MO
± 199.9 nA
±100nA
10 nA/V
600 nA
10 mV/nA
100 nA/mV
1000 nA/mV
10000
(1) For H = xO.Ol replace MO by GO, nA by pA in xlO column
For H = xO.OOOl replace MO by GO, nA by pA in
For H.= xlOO replace MO by kQ, tiA by /tA in
xO. 1 colunm
xO. 1 colunm
(2) Measured with electrode resistance Re = Ro
TABLE 2
ell input resistances
xO.l
100 MQ
1000 MO
± 19.99 nA
±10nA
InA/V
60 nA
lOOmV/nA
10 nA/mV
lOOnA/mV
10000
H
H
H
H
H
xlO
xl
xO.l
xO.Ol
xO.OOOl
for 300 kO < Rin
for 3M0 < Rin
for 30 MO < Rin
for Rin
for
ion-sensitive electrodes
<
<
<
>
3 MO
30 MO
300 MQ
300 MO
Some overlap in these recommendations is allowable. The guiding principles are these:
(1) For maximum sampling rates in dSEVC and DCC modes use the largest feasible H value. (This is
because the current-passing response is best with low values of Ro.)
(2) A limitation on using large H values is that as Ro becomes smaller the input leakage current of the
headstage becomes more prone to increase with time and temperature (see Input Leakage Current
discussion later in this section).
(3) A ftirther limitation on using large H values is that if Ro (see Table 1) is less than the
microelectrode resistance (Re) the high-frequency noise is worse.
(4) The H sets the current-passing sensitivity in Bridge and DCC modes and the Gain in SEVC modes.
Hence it should be chosen for sensitivities suitable for your cell. These sensitivities are listed in
Table 1 above.
(5) If
Re
> > Rin a smaller H value should be favored.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 44

Page 34 DETAILED GUIDE TO OPERATIONS
Capacitance Neutralization Range
HS-2 Series headstages are available with L or M suffixes representing low and medium ranges respectively
of Capacitance Neutralization (see Table 3). The increased Capacitance Neutralization range is a trade-off
against microelectrode noise. The HS-2L has die lowest noise close to the theoretically predicted thermal
noise of the electrode. The HS-2M has about 25% extra noise.
TABLE 3
HS-2L HS-2M
Cap Neut Range:
in MEl Slot -1 to 4 pF -2 to 12 pF
in ME2 Slot -1 to 11 pF -2 to 35 pF
Headstage Connectors
There are three teflon-insulated 2 mm (0.08 inch) sockets in the headstage (see diagram). These are
standard-diameter sockets.
1.
Microelectrode Input Connector
The red socket is the microelectrode input. The connection between the microelectrode and this socket
should be kept as short as possible. Some excellent methods are:
(i) Solder a silver/silver-chloride wire direcdy to one of the 2 mm plugs supplied. Use the
-y'^^-j'^-''^^'^^^^..?^fi-iffl'^'^'^^^^yr9i^^ which can be supported on a separate mounting.
(ii) For greater mechanical stability, use an HL-2 series microelectrode holder from Axon
Instruments.
(iii) Plug a standard microelectrode holder (2 mm plug) direcdy into the input socket. The
teflon input socket should allow enough clearance for most standard holders.
(iv) Use a BNC-type microelectrode holder. This requires an HLB-2 adaptor from Axon
Instruments.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 45

DETAILED GUIDE TO OPERATIONS
INPUT
(white)
SHIELD
(gold)
{Connected to case)
AXON INSTRUMENTS
MODEL:
GAIN:
SERIAL:
o
Leakage current
trim access
Mounting
rod
Page
35
Notes
"Model"
"Gain"
HS-2 and HS-4
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
may be
refers
HS-2L, HS-2M
to
headstage current gain
or
HS-4M
(H)
HEADSTAGE CONNECTION DIAGRAM
Page 46

Page 36 DETAILED GUIDE TO
2.
Shield Drive Connector
OPERATIONS
The Shield drive is connected to die gold-plated guard socket and to the case of die HS-2 xlL, xO.lL,
xO.OlM and xO.OOOlM headstages. This drive is protected against continuous short circuits, however for
best frequency response the case must not be grounded. In general, this necessitates using an insulated
mounting for the headstage (such as die rod provided).
The shield connection is provided primarily for driving the shield of microelectrodes pr^ared for deep
immersion (see notes in Microelectrodes for Fast Setding section). It may also be used for driving metal
objects near the input, or even the hutch in which the preparation is housed. It can be used for driving
the shield of a coaxial cable used to connect the microelecfrode to the input, although it is not
recommended that the microelectrode be connected in this way. If not used, the shield socket is simply
left unconnected.
There are two reasons why we do not recommend using shielded cable to connect the microelectrode to
the headstage. (1) The leakage resistance of shielded cable can degrade the input resistance when used
with ion-sensitive and other high-impedance elecfrodes. If shielded cable is used it should have teflon as
the insulating material separating the shield and the inner conductor. . (2) Shielded cables add signiflcant
input capacitance. The shield drive circuit mosdy removes the effect of this capacitance on electrode
response speed. However, from a noise point of view the capacitance remains and causes an increase in
high-frequency electrode noise.
To optimize the response speed of low and medium impedance electrodes (up to approx. 300 MO) when a
driven shield is us^, the shield of headstages with H = xO.l and larger is driven from the capacitance
neutralization circuit. To optimize the headstage input resistance when a driven shield is used, die shield
of headstages with H = xO.Ol and smaller is driven from the output of the unity gain buffer inside the
headstage.
If a shielded cable is being used and unusual electrode responses are observed, try disconnecting the
shield.
No shield drive is provided on the HS-2 xlMG, xlOMG and the HS-4 xlMG. On these headstages die case
is grounded. This is because they are primarily used for current passing in a two-electrode voltage clamp
(TEVC). In TEVC, it is essential to minimize the amount of coupling capacitance between the
voltage-recording electrode and the current-passing electrode. This, coupling can be minimized most
conveniendy if the case of the current-passing headstage is grounded.
3.
Ground Output Connector
The yellow ground socket of the MEl headstage is used for grounding the preparation. Using this
connection as the preparation ground minimizes hum.
Tip Potentials - Detection
During the passage of current the tip potentials of many electrodes change. Changes in tip potential are
indistinguishable from the membrane potential and can therefore represent a serious source of error. To
prevent this error the following checks should be made.
(1) While the microelectrode is outside the cell, set the offset to zero. In bridge or DCC mode pass a
constant current into die bath for about 10 seconds. The current magnitude should be die same as
the maximum sustained current likely to be passed during the experiment. When the current is
switched off the recorded potential should return to zero within a few milliseconds at most. Some
electrodes either retum very slowly to zero potential, or not at all. These electrodes should be
discarded.
AXOCLAMP-2A THEORY &
OPERATION,
COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 47

DETAn.ED GUIDE TO OPERATIONS Page 37
(2) Once the experiment is in progress occasionally check the resistance of the microelectrode.
Changes in tip potential are usually accompanied by changes in electrode resistance.
Note that the tip potential changes described in this section are h^pening widi a slower time course
than the ones described in the Anti-Aliasing section. The causes of these slow changes in tip
potential are unknown.
Tip Potentials - Prevention
Not much can be done to prevent tip potentials from changing but the following may be helpful.
(1) Sometimes the slow changes in tip potentials are worse when standard microelectrode holders with
an embedded AgCI pellet are used instead of an Ag/AgCI wire. Some holders are all right while
other ostensibly identical holders are not. Therefore holders should be tested and selected.
The variability of the tip potentials may in some way be related to pressure developed when the
microelectrode is pressed into the holder. A narrow hole drilled into the side of the holder to
relieve pressure might help.
(2) Using filling solutions with low pH, or adding small concentrations of polyvalent cations like
Th^+, may reduce the size of the tip potential (Purves, 1981) and therefore the magnitude of any
changes.
Interchangeability
Any unity-gain headstage in the HS-2 series can be used for MEl or ME2. The equipment will not be
damaged if headstages are exchanged while the AXOCLAMP-2A is switched on.
Cleaning
To clean salt spills from die input connectors wipe with a damp cloth. Avoid spilling liquids on the
headstage.
Input Leakage Current And How To Trim It To Zero
All DC-connected systems suffer from die problem of drift. With changes in temperature and the passage
of time the DC transfer functions of all semiconductor devices can drift by many millivolts away from their
initial values. The major worry in a microelecfrode system is that the cumulative effects of drift in
various parts of the circuit may lead to the development of a DC offset across the resistor (Ro) used to set
die H. As a result, an undesirable DC leakage current is injected into die microelecfrode.
Careful consideration to this problem has been applied diroughout the design of the AXOCLAMP-2A and
die overall DC offset has been made as insensitive as possible to the drift in die integrated circuits. As
well, special low-drift integrated circuits have been used in all critical positions. The magnitude of the
DC leakage current increases with increases in H. This normally introduces no greater error in the DC
offset voltage developed across the microelecfrode or the cell membrane because larger Hs are usually used
with lower-resistance cells and microelecfrodes.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON WSTRUMENTS, INC.
Page 48

Page 38 DETAILED GUIDE TO OPERATIONS
Before leaving die factory, die DC offset voltage of each HS-2 headstage is trinuned so diat die input
leakage current is no more than:
100
10
1
1
10
Tliese input current levels are very low and cause negligible shifts in the cell membrane potential when the
headstages are used with the recommended ranges of cell input resistances (see Table 2). (The shift in Vm
is calculated from input current x Rin.)
If you ever suspect that the input current has grown to a level where Vm is significandy affected, it can be
re-adjusted by the following procedure.
(1) Switch off all current commands and disconnect any external current commands.
(2) Remove the plastic cap from the access hole in the headstage cover.
(3) Ground the headstage input via a resistor equal to Ro -^ 10 (where Ro is given in Table 1).
(4) Now ground the headstage input via a resistor equal to Ro^'^ in Table 1. Observe the shift
(5) Rq)etitively swap from grounding via Ro -^ 10 to grounding via
Note 1. For values of 1 GO or more it is important to clean the surface of the resistor thoroughly to
pA
pA
pA
pA
fA
On an oscilloscope at 2 mV/div observe the lOVm output through die filter set to 100 Hz.
Use the Offset control to center the trace on the screen.
of the oscilloscope frace.
inside the headstage until there is no shift.
remove leakage pathways.
for
for
for
for
for
= xlO
H
= xl
H
= xO.l
H
= xO.Ol
H
= xO.OOOl
H
R©.
Adjust the ttim pot
Depending on the reason for a trim being necessary, the frim procedure may have to be repeated if die
headstage is changed.
Warning
If an extemal source is connected to the Ext. MEl and ME2 Command input, any time the source is non-
zero a proportional current will flow in the microelectrode. Many external sources do not put out a tme
zero voltage when in the "off state, thus diere may be an unwanted electrode current due to the fact that
an extemal source is connected. To avoid this, use an extemal source in which you can adjust the offstate voltage, or use an isolated extemal source.
DC Removal
One potential source of a small but variable input leakage current is due to DC current flow through the
dielecfric of die capacitor (Co) used for capacitance neutralization. For example, die elecfrode potential
might be 200 mV (diough the experimenter does not see diis potential because of die offset compensation).
To compensate several pF of input capacitance die gain of the capacitance neutralization circuit might be 2.
Thus 4<)0 mV would be fed back to Cn resulting in 200 mV across it. If the dielecfric resistance of Co
were 10" 0 (the guaranteed minimum of high-quality capacitors) there would be 2 pA flowing through the
capacitor.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 49

DETAILED GUIDE TO OPERATIONS Page 39
To eliminate this source of leakage current a DC removal circuit removes the DC voltage from across Cn.
The DC removal circuit operates widi a 1 s or 10 s time constant. There may be a fransient shift in die
elecfrode voltage while the Capacitance Neufralization confrol is being adjusted. The DC voltage is also
removed from the shield drive.
Input Resistance
The input resistance of die headstages is predornkj^dy related to Ro. A circuit inside the AXOCLAMP
V^,
3j»
•
called a constant current source (CCS) controls the voltage across Ro. Ideally, the voltage across Ro is / a ^
independent of the elecfrode voltage. The accuracy of die CCS in controlling the voltage across Ro is
preset at the factory. Extremely stable components are used in the CCS so that the accuracy will not
deteriorate widi time. In general the CCS is effective to one part in 10^ so that the input resistance is
lO^Ro.
Other possible factors which would decrease the input resistance are minimized. For example, the field
effect fransistor (FET) input of the headstage is referenced to the input voltage rather than to ground. This
technique is known as bootsfrapping. Thus the effective resistance of the input is much greater than die
already high resistance of the FET. Leakage current and resistive loading through the insulation of the
input socket are minimized by using Teflon insulation and by driving the case with the DC input voltage.
HOLDERS
Features
The HL-2 series holders have been designed for low-noise mechanically stable microelectrode recordings
with or without suction. The body of the holders are made out of polycarbonate for lowest noise and easy
cleaning. Maintenance is simple because the holder can be fully disassembled for cleaning and parts
replacement.
Mechanical stability of the elecfrode is assured several ways. For example, as the elecfrode cap is
closed, the 'O' ring is forced into a special recess and pulls the elecfrode fimdy back into the holder so
diat its end presses tighdy against the electrode seat. The holder mates fimdy with the special teflon
connectors on the HS-2, HS-4 and VG-2 series headstages. A 2 mm diameter pin is used for die elecfrical
connection.
The holders are designed to emerge along the long axis of the headstage. A right-angle adapter can be
purchased if it is necessary for the holder to emerge at 90° from die headstage. A BNC-to-Axon adaptor
(HLB-2) can be purchased if you wish to use third-pary BNC-style holders.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 50

Page 40 DETAILED GUroE TO OPHIATIONS
Parts
The various parts of the holders are shown in the exploded view:
-Ag/AgCI '0" RING BARREL SUCTION 90' BEND ^PIN
PELLET / / / TUBE
(1.25mm)
-C^:^
ELECTRODE
CAP RECESS SEAT SEAT PIERCED SEAL PIN CAP
ELECTRODE
PIN
Five spare 'O' rings and one spare pierced seal are provided widi each holder. Additional 'O' rings,
pierced seals, pins and Ag/AgCl pellet assemblies can be purchased from Axon Instmments.
HL-2-12 holders use a plain Ag wire and 'O' rings widi a 1.2 mm hole. HL-2-17 holders use a Ag/AgCl
pellet assembly and 'O' rings with a 1.7 mm hole.
To replace the silver wire, insert the nonchlorided end through the hole of the pierced seal and bend the
last 1 mm over to an angle of 90°. Press the pierced seal and die wire into the pin seat. Push die large
end of the pin down onto the bent-over wire and into the pin seat. This assures good elecfrical contact.
Screw the pin cap down firmly but without excessive force.
Use
Insertion of electrode
Make sure the electrode cap is loosened so diat pressure on the 'O' ring is relieved, but do not remove the
elecfrode c^. Push die back end of die elecfrode through the electrode cap and 'O' ring until it presses
against the elecfrode seat. Gendy tighten die electrode cap so diat the electrode is gripped firmly.
To minimize cutting of the 'O' ring by the sharp back end of the electrode, you can smooth the electrode
edges by rotating the back end of the electrode in a bunsen bumer flame.
Qeaning
For lowest noise, keep the holder clean. Frequendy rinse the holder with distilled water. If heavier
cleaning is required, briefly wash in ethanol or mild soapy water. Never use methanol or strong
solvents.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 51

DETAILED GUIDE
1X3
OPERATIONS page 41
Filling electrodes
Only the taper and a few millimeters of the shaft of the elecfrode should be filled with solution. The
chlorided tip of the wire should be inserted into this solution. Avoid wetting the holder since this wUl
increase the noise.
Silver Chloriding
The HL-2-17 holders are supplied with a Ag/AgCI pellet that should give you many months of DC-stable
recordings. The silver wire is surrounded by a Sylgard-sealed teflon tube. This ensures that the
elecfrode solution only contacts die Ag/AgCl pellet.
Ag/AgCI SYLGARD _ TEFLON
PELLET / / TUBING
Ag WIRE
It is not practical to make a pellet small enough to fit inside the shaft of the narrow glass elecfrodes used in
die and HL-2-12 holders, therefore these holders are supplied widi a piece of 0.25 mm silver wire. It is
up to you to chloride the end of this wire as required. Chloriding procedures are contained in many
elecfrophysiology texts (e.g. Purves, 1981). Typically the chlorided wire will need to be replaced every
few weeks.
Heat smoothing the back end of the electrode extends the life of the chloride coating by minimizing the
amount of scratch damage. Anodier way to protect die AgCI coating is to slip a perforated teflon tube
over the chlorided region.
The chlorided region should be long enough so that die electrode solution does not come in contact widi
the bare silver wire.
(Hass Dimensions
Use the HL-2-12 holders for glass from 1.0 to 1.2 mm outside diameter (OD). The optimal dimensions are
1.15 mm OD and > 0.5 mm ID.
Use the HL-2-17 holders for glass from 1.5 to 1.7 mm outside diameter (OD). The optimal dimensions are
1.65 mm OD and > 1.1 mm ID.
For other glass dimensions you can drill out the bore of the HL-2-12 holder.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 52

Page 42 DETAILED
GuroETX)
OPERATIONS
I
IONOPHORESIS
A
When ME2 is not used for intracellular penefrations it can be used for ionophoresis. To set the retaining
and pulse currents:
(1) Set the desired retaining current on die ME2 DC Current Command control.
(2) Switch the Destination switch to ME2. Set the Step Command equal to die desired pulse
cunent minus the retaining cunent.
or Connect a pulse generator to the Ext. ME2 Command input to set the desired pulse current
nunus the retaining current.
e.g. For retaining current = -5 nA, ejection current = 40 nA.
Set ME2 DC Current Conunand = -5 nA, Step Command (or Ext. ME2 Conunand) = 45 nA.
Use a headstage widi the appropriate H. xl is generally useful.
LINK-UP
When the AXOCLAMP-2A is used in dSEVC and DCC modes the voltage across the microelecfrode rapidly
switches up and down. To an extent which depends on proximity, a second microelecfrode used in the
same pr^aration will pick up some switching noise.
If die second elecfrode is used in a continuous mode the picked up noise can usually be removed by a
lowpass filter.
If the second elecfrode is also used in a discontinuous mode (e.g. when two interconnected cells in the
same preparation are placed under dSEVC) the pick-up from one to the other can become a problem. The
two switching signals mix and a beat frequency signal appears at the difference frequency. When both
elecfrodes are switched at similar frequencies the beat frequency signal appears at a low frequency which
cannot be filtered out. Worse, in an effort to clamp out the beat signal the clamping circuit passes beatfrequency currents into the cell.
There are two ways to avoid this problem.
(1) Place an extensive grounded shield between the two electrodes. This method has disadvantages.
The shield may be physically difficult to arrange, and it may introduce sufflcient capacitance at the
headstage inputs to worsen the electrode performances.
(2) Use the Clock Link-Up facility provided widi each AXOCLAMP-2A to synchronize dieir sampling
clocks. A 15-pin connector on the rear panel enables the sampling clock circuits of two
AXOCLAMP-2AS to be linked by a cable. One AX0CLAMP-2A becomes the Master and die odier
the Slave (which is which is determined by die orientation of the cable).
After Link-up, whenever bodi AXOCLAMP-ZAs are in DCC or dSEVC modes, die Slave's
sampling clock is overridden by die Master's. In all odier combinations of operating modes die
two AXOCLAMP-2AS remain fiilly independent. For example, if the Slave is in DCC or dSEVC
modes but the Master is in neidier, the Slave's sampling clock is re-enabled.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 53

•t
DETAILED GUIDE TO OPERATIONS Page 43
By forcing both AXOCLAMP-2AS to sample synchronously the beat frequency problem is
eliminated. At the instant that both AX0CLAMP-2AS sample dieir elecfrode voltages there will be
no pick-up from one elecfrode to the odier because the voltages across both elecfrodes must have
decayed to near zero in order for the clamps to operate.
Clock Link-Up only affects the sampling clocks. All odier functions of the two AXOCLAMP-2AS
remain fully indqiendent.
MICROELECTRODES FOR FAST SETTUNG
The key to discontinuous voltage and current clamping with a single nucroelecfrode is the character of the
microelectrode
microelecfrode must be able to pass current without large changes in resistance.
Microelectrode Capacitance
To get fast setding it is essential to minimize the transmuraL-capacitance^(Ct) from the inside of the
microelecfrode to the extemal solution. Ct is usually 1-2 pF per mm of immersion. Two applications
requiring different appiroaches are discussed here.
itself.
The microelectrode voltage must settle rapidly after a current pulse, and the
Target Cell Near Surface Of Solution.
In an isolated pr^aration, Ct can be reduced, by lowering.the.surfacjejcrf_the_so.lution,aSi.,faL3s.pp.ssibJe^(see
note below). Precautions must be taken to prevent surface tension effects from drawing a thin layer of
solution up the outer wall of die microelectrode. If this film of saline is allowed to develop, Ct will be
much worse that otherwise. Because die film of saline has axial resistance the contribution to Ct will be
very nonlinear, and the voltage decay after a current pulse will eidier be biphasic (as in Fig. 1), or if it is
monophasic it will not be very fast even when capacitance neufralization is used. To prevent the saline
film ft'om developing, the electrode should be coated with a hydrophobic material. This can be-donejust^
before use by dipping the filled microejecfrodeinto a fluid such as silicpnejoil^^orjmMraTod. Another
method is to coat die elecfrode with Sylgafd'^Hamill et al., 1981). • • ~ - .
Sylgard or Q-dope (airplane glue) can also be used to build up the wall diickness of the elecfrode thereby
reducing Ct. The selected material should be painted onto the elecfrode to widiin 100 /im ofthe tip.
Note: For a long slender microelectrode we regard 2(X) fim or less as a low solution level. 500 /im is
tolerable. 1 mm or more is regarded as deep. For a microelecfrode which tapers steeply (i.e.
a stubby microelectrode) deeper solutions can be used with less loss of performance. When
working with very low solution levels there is a risk of evaporation exposing the cells to the air
unless a continuous flow of solution is provided across or through the preparation. If
evaporation is a problem one way to overcome it is to float a layer of mineral oil on the surface
of die solution. If used, this layer of oil will have the additional advantage of automatically
coating the electrode as it is lowered into the solution.
.L
..._
AXOCLAMP-2A THEORY
&.
OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 54

Page 44 DETAILED GIHDE TO OPERATIONS
Target Cell Deep In Solution.
In some pr^arations, e.g., in vivo CNS, the target cell is several millimeters below the surface of the
solution. In this case the more difflcult procedure of guarding the electrodes may have to be used. This
involves coating the outside of the microelecfrode with a metal layer and connecting this layer to the case
socket of the unity-gain headstage. Depending upon H the case socket is either connected to the
capacitance neutralization circuit or to the unity-gain output. The guarding procedure does not reduce Ct.
Instead, it reduces the effect of Ct by confrolling the voltage across it. The metal guard layer must be
insulated from the preparation solution. For different i^proaches to this method see Schwartz & House
(1970),
Shielding the elecfrode introduces high-frequency noise therefore it should only be done when absolutely
necessary. The amount of added noise is proportional to the amount of shield c^acitance, so only the
minimum necessary length of microelectrode should be shielded.
Because of the disfributed nature of the axial resistance of the microelectrode, of the axial resistance of the
metal layer, and of Ct, the shielding technique is not perfect. In practice, the effect of these nonidealities
is to cause the step response of the microelectrode to overshoot even when the Capacitance Neutralization
gain is unity. For this reason, the Capacitance Neutralization circuit has a minimum less than unity.
Suzuki, Rohli?ek & Frbmter (1978), Sachs & McGarrigle (1980) and Finkel & Redman (1983).
I
Microelectrode Resistance
Another important aspect of the microelectrode is die tip resistance (Re). This should be as low as possible
consistent with good impalements of the cell. There are two advantages associated with low values of Re:
Settling Time
The decay time constant for the microelectrode voltage after a current pulse depends sfrongly on Re.
Hence, lower Re values produce faster settling times. As well, high Re values are sometimes associate
with a slow final decay even after Ct has been eliminated.
Stability
Re of most microelecfrodes changes with time and with current passing. Re is affected not only by the
magnitude of the current but also by its polarity. In general, microelectrodes of lower resistance are
more stable during current passing than microelectrodes of higher resistance.
Filling Solutions
The best filling solution to use depends on the preparation under investigation and the experience of the
investigator. Although KCI gives one of the lowest tip resistances for a given tip diameter it is not
necessarily the fastest to setde after a current pulse. K-cjtrate is sometiniesJiastec.
It is important to be aware that during current-passing large amounts of ions from inside the microelectrode
can be ionophoresed into the cell. For example, if current is passed by the flow of ion species A from
the microelectrode into the cell, then after 50 seconds of current at 1 nA (or 1 second of current at 50 nA)
the change in concentration of A inside a cell 100 /zm in diameter is 1 mM. If A is an impermeant ion,
-the cell may swell due to the inflow of water to balance the osmotic pressure.
'V,6
Recommended Reading
A small book by Purves (1981) serves as an excellent general reference for microelectrode techniques.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMEI4TS, INC.
Page 55

DETAILED GUIDE TO OPERATIONS
Page 45
MODEL CELLS
We recommend diat you practice using the AXOCLAMP-2A on an RC cell model. The resistor provided
with each headstage can be conveniendy used to simulate die microelecfrode and the RC cell model can be
soldered direcdy to the free end (see Fig. 6). If two-elecfrode voltage clamping is being practiced it is
important to place a grounded shield between the model elecfrodes and between the headstages.
Notes:
1.
Rei and Re2 are resistors to simulate the microelectrodes.
2.
Rm and
Cm
are a resistor and capacitor to simulate die cell.
FIGURE 6A - SUGGESTED CELL MODEL
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 56

Page 46 DETAILED GUIDE
TO
OPERATIONS
The CLAMP-1 Model Cell
If you do not need to model your cell exacdy, the CLAMP-1 Model Cell shipped with your
AX0CLAMP-2A is a convenient model to work with. The cell and elecfrode components simulate a
small-to-medium sized cell having an input resistance of 50 MQ, a membrane time constant of 25 ms and
electrode resistances of 50 MO. See Figure 6B. The case of the model cell is connected to ground.
Shielding between the two elecfrode resistors is effected by the body ofthe switch.
Install the model cell by plugging it into one or both of your headstages. Connect the gold-plated ground
jack to the yellow jack on the back of the MEl headstage using the cable provided. Do not make any
connection to the gold-plated jack on the front of the HS-2 headstage - this is connected to die headstage
case which is driven to the electrode potential.
When the switch is in the BATH position, both elecfrode resistors are connected to ground. This is a
convenient position for practicmg^idge balancing techniques and offset adjustment. -^ p $D
When the switch is in the CELL position, both electrode resistors are effectively intracellular. In Bridge
or DCC mode you should see exponential voltage responses to steps of current. In dSEVC mode you
should be able to clamp the cell at gains of up to 0.8 nA/mV using an HS-2 xO.l headstage, at sampling
rates up to 8 kHz. In TEVC mode, use one of the following electrode combinations: 1) two xO.l
headstages, two xl headstages, or a xl headstage for ME2 and a xO.l headstage for MEl. The elecfrode
resistances in this model cell are too large for you to practice cSEVC.
AX0CLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 57

DETAILED GUIDE TO OPERATIONS
Page 47
CELL
BATH
m
.CONNECT
TD ME2 O
50M
—W-
50M S
50M CONNECT
-^W O TD MEl
470P
_ CONNECT TD MEl
"^ HEADSTAGE GROUND
rti
FIGURE 6B-CLAMP-I MODEL CELL
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 58

Page 48
DETAILED GUIDE TO OPERATIONS
MONITOR
The Monitor ou^ut is used to check the setding characteristics of the voltage at the input to the sampleand-hold device. This is advisable during DCC and dSEVC and the notes on these two modes should be
consulted for details.
The Monitor signal is derived from Vi (see Fig. 7). After amplification by 10, Vj is filtered by die
Anti-Alias Filter. The output of the Anti-Alias Filter is the input of the sample-and-hold device and the
signal provided to the Monitor output.
A baseline correction circuit compensates for shifts in W\ so that Vmon always decays to zero. This
prevents Vmon from moving off the oscilloscope screen when the holding potential is shifted during voltage
clamp.
BASELINE
CORRECTION
Monitor
output
To rest
of circuit
nGURE 7 - ANTI-ALIAS FILTER & MONITOR CIRCUIT
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 59

DETAILED GUIDE TO OPERATIONS Page 49.
NOISE IN DCC AND dSEVC MODES
The noise inherent in discontinuous microelecfrode clamps (discontinuous current clamp or discontinuous
single-elecfrode voltage clamp) is four or more times worse than the noise in continuous microelecfrode
clamps (bridge current clamp or two-elecfrode voltage clamp) when the discontinuous microelecfrode
clamps are adjusted for the same dynamic response and accuracy as the continuous microelecfrode clamps.
There are two major reasons for this inherent deterioration in noise performance.
The first is to do with c^acitance neufralization. A fundamental property of all capacitance neutralization
circuits is that they introduce noise in excess of what is contributed by the thermal noise of die recording
microelecfrode and the input noise of the buffer amplifier. The excess noise becomes progressively larger
as the microelecfrode time constant is reduced. In discontinuous systems the microelectrode time constant
must be reduced more than in continuous systems so that after a current pulse the microelecfrode voltage
will decay to Vm within the time allotted for passive recording. The excess noise due to optimizing the
capacitance neutralization can vary from a factor of about two in a system where primary efforts have been
taken to keep the input capacitance low, to much larger factors in systems where large amounts of
c^acitance-to-earth and capacitance-to-shield are tolerated.
The second major reason for die deterioration in noise performance of discontinuous microelecfrode clamps
has to do with the sampling process. As discussed in the section on the Anti-Alias Filter, sampling
processes alias the noise in die input signal spectmm into a larger-magnitude spectmm confined to a
bandwidth equal to half of the sampling rate (fg). The normal procedure used in digitizing systems to
avoid aliasing is to reduce the bandwidth of the input signal to fs/2 or below. This is not possible in
discontinuous microelecfrode clamping because reducing the bandwidth of the microelectrode increases the
time constant and therefore prevents adequate settling. The amount of aliased noise depends in part on the
current duty cycle used in the discontinuous clamp. The 30% duty cycle used in the AXOCLAMP-2A has
been chosen to give a good compromise between aliased noise and dynamic performance (Finkel &
Redman, 1984b). With this duty cycle the increase in noise due to aliasing is a factor of about two.
The two confributions to noise discussed above lead to a factor of four or more deterioration in noise. To
keep the deterioration as small as this the experimenter should aim to do the following.
(1) Keep die real value of
Cin
as small as possible so diat only minimal capacitance neufralization must
be used. (Avoid using coaxial cable to connect the microelectrode to the headstage.)
(2) Either increase die Anti-Alias Filter setting at a given cycle rate, or increase the cycle rate at a
given setting of
Finally, the amount of noise recorded can be reduced to some extent by using as much output
the
Anti-Alias Filter, so that the amount of aliased noise is minimized.
filtering
as
possible. However, the output filtering should neyer be increased to the extent that dynamic information
(e.g. rise time) is lost. Usually, output filtering at fs/10 is a good compromise. The best way of
reducing noise in the records is by averaging repetitive signals. This well-known procedure reduces the
noise by the square root of
the
number of averages without affecting the time course of
the
signal.
Notwithstanding the comparatively poor noise performance of discontinuous single-electrode voltage
clamps compared with two-electrode voltage clamps, the single-electrode technique is extremely rewarding
because it allows voltage-clamps to be performed in preparations where two-electrode voltage clamping is
just not feasible. As well, the signal-to-noise ratio in many preparations during discontinuous single-
electrode voltage clamp is, despite the above considerations, adequate for data to be analyzed without
averaging.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 60

Page 50 DETAILED GUIDE TO OPERATIONS
OFFSET CONTROLS
The Offset confrols compensate for
die
junction potentials in the experimental setup.
The offset compensation for the V2 output works by adding a DC voltage to the output. Therefore, it is
called die •Output" Offset control.
The offset compensation for the lO.Vm and Vj outputs is performed in the first stage of die recording
circuit. This is necessary so that after amplification of the input signal the full range of the sample-andhold circuitry can be utilized. The MEl offset compensation should not be altered during voltage clamp
because the voltage-clamp circuitry will interpret the change in the offset setting as a change in Vm. To
remind you of
this
important characteristic the control is called the "Input" Offset.
For both confrols, the compensation range is ±500 mV. The no-compensation point is in the middle of
the range of the multi-tum dials. Each tum of the dials is approximately 100 mV. The dials can be
locked after setting. The dial markings are not meaningfiil. Calibrated dials are used for these controls
because they have brakes to prevent accidental movement.
The normal procedure for using the Ofiset controls is to zero the voltmeter readings when the
microelecfrode is outside the cell. All subsequent readings are dien with respect to the potential of the
exfracellular solution.
OUTPUT FILTER
Built-in filters are provided to smooth the lO.Vm and Im outputs. These are single-pole lowpass filters.
Six -3 dB
frequencies
(fO can be selected.
As well as reducing the noise, a filter also slows the rise time of the filtered signal. A single-pole filter
converts a st^ into an exponential. There is no overshoot. The time constant of the exponenti<d is
T = (2irfL)-i
The 10% -
90%
rise
time
of
the
tr = 2.2T.
exponential is
The six available ft's and the corresponding r's and tr's are given in Table 3.
TABLE 3
fL(kHz)
TQIS)
tr(MS)
0.1
1600
3500
0.3
1 3 10 30
530
160 53 16 5.3
1200
350 120 35 12
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 61

DETAILED GUIDE TO OPERATIONS Page 51
High-Order Lowpass Filters For Low-Noise Recordings
The "order" of a filter refers to die number of poles (RC sections). For example, a third-order fdter has
three poles. Each pole attenuates the high-frequency noise at 20 db/decade.
During TEVC the current noise increases at -1-20 db/decade above a frequency determined by the
membrane time constant (Finkel & Gage, 1984). To adequately limit this noise the filter used for data
display and storage should be at least
2™*
order and preferably
3"*
or 4* order.
Rise Time Of High-Order Filters
As a mle of diumb it can be noted that for lowpass multiple-pole filters having less than 10% overshoot,
the 10-90% rise time is within a few percent of t in a single-pole filter having the same -3 dB frequency.
However, the frequency specified for many multiple-pole lowpass filters is the -3 dB frequency of the
component lower-order filters instead of being the -3 dB
frequency
of the complete filter. Before using
these filters it is advisable to check the 10-90% rise time of a step signal applied to the input.
Note On Ultimate Rise Time
When a signal with 10-90% rise time ti is passed through a filter widi 10-90% rise time t2 the rise time of
the output signal is approximately
tr = V (tl2 -»• t22)
OUTPUT IMPEDANCE AND PROTECTION
All outputs are protected by 560 0 output resistors.
All outputs can withstand a continuous short circuit to ground or any voltage in the ±15 V range.
However, in keeping with normal practice, such short circuits should be avoided.
PANEL METERS
Three digital panel meters (DPMs) are provided to continuously display the DC level of some of the
important outputs. These displays are.
V^CmV)
This DPM indicates the membrane potential in all modes. It is derived from the 10.Vm output. The
maximum displayed value is approximately ±600 mV, which is the value which will typically be seen
when the MEl headstage input is open circuit.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 62

Page
52
DETAILED GUIDE
V2(mV)
TO
OPERATIONS
This DPM indicates
are indicated
by a
V2 in all
modes.
The
partially blanked display,
maximum displayed value
and + or - to
indicate polarity.
is ±
KnA)
This
DPM can
chosen using
Three small switches
in
nA for the
Im position;
To use,
—
tum the
—^ f^ti- o.\y
display
the
one of die
I-Display Select switch.
are
used
to
headstage being used.
the
HCGi switch
switch
to the
is
gain
following currents:
change
active when I2
the
decimal point location
The
HCGi switch
of
your headstage.
is
Im, I2 or IVIRT. The
so
that
is
selected;
active when
the
VG switch
the
. ^ , , •
PHASE
A voltage-clamp
Ideally this phase shift
significandy less than
The frequency response
clamp circuit
can
is a
negative-feedback circuit
is
90°
diereby
supplied
phase shift
of
the voltage-clamp circuit
be
by the
adjusted
capacitance
(see
discussion
to
compensate
and as
can be
for the
such
it
requires
of
the membrane.
by
Finkel & Gage, 1984).
modified
a 90°
In
by die
nonideal phase response
1999
mV.
Out-of-range signals,
current
the
display
I-Display Select switch
is
active when
phase shift within
to be
can be
IVIRT is
displayed
read direcdy
is in the
selected.
the
circuit.
practice, membranes introduce
Phase controls.
of
The
voltage-
real membranes.
is
The controls
are in two
parts; a potentiometer
time constant.
Phase lead boosts
used
to
sharpen
debit side,
reduce
the
use of
risk
the phase lead.
of 2, achieved when
Phase
lag
cuts
but
at die
same time
control circuit introduces pure
The action
of
combination
of phase lead
extreme
increased
the
net
lag
by
change
the
high-frequency gain
the
step response
and
phase lead increases
of
oscillations
The
added phase
the
the
high-frequency gain
it
the
phase-control circuit
lag
potentiometer control
slows
die
response
lag.
the Phase Shift potentiometer
of
phase lead
is
gradually reduced
position.
and lag is
At the
by
same time
counterclockwise rotation
to the
frequency characteristics ofthe voltage-clamp circuit
The Time Constant switch changes
some pr^arations
switched
With
to the Off
an
RC cell model
no
phase
position.
the
lag or
best voltage-clamp will
to
shift from lead
of the
improve
the
restricts
of
the voltage-clamp circuit. This
and
introduced such that
voltage-clamp circuit.
the
voltage clamping
noise
and can
is
arranged
die
maximum increase
is
tumed
to the
introduces ringing.
can be
summarized
the
counterclockwise rotation
the
the
lead
of the
potentiometer.
maximum
is
required.
amount
of
lag and
If
be
achieved when
to lag, and a
In
of
fast conductance changes.
4-position switch
some preparations this
also cause high-frequency oscillations.
to
always introduce some phase
in the
high-frequency gain
extreme lead position.
can be
In die
as
follows.
extreme
In the
high-frequency gain
of
the potentiometer
phase
lag
cutting
At the
center position
used
to
reduce
lag
position
extreme lead position
is
doubled.
and
falls
the
high-frequency gain
of the
is nil.
lead values
this
is so, the
as
listed
in the
Specifications.
Time Constant switch should
no
Phase shift
is
used.
to set the
can be
On the
To
lag
with
to a
factor
the
noise
the
phase-
a
The
amount
to
zero
at the
is
potentiometer
In
be
AXOCLAMP-2A
THEORY & OPERATION,
COPYRIGHT FEBRUARY
1990,
AXON INSTRUMENTS,
INC.
Page 63

DETAILED GUIDE TO OPERATIONS Page 53
Use .
The Phase confrols can be used during voltage clamp to compensate for die frequency characteristics of
membranes which are not well modeled by a parallel resistance and c^acitance. Both the membrane
voltage and current stq) responses should be improved by using the Phase controls. If only the membrane
voltage step response is improved it is likely that there is a resistance (Rg) in series with the membrane.
See the Series Resistance Section for a discussion of
this
problem.
In some cases using some phase lag wUl reduce die current noise during voltage clamp. See the Sections
on each type of
voltage
clamp for more details.
POWER-SUPPLY GLITCHES
The AXOCLAMP-2A has been designed to minimize the effects of power supply transients (glitches). This
is achieved by:
1) taking the incoming power through a radio
frequency
interference (RFI)
filter
and
2) capacitively isolating the transformer primaries and secondaries.
Nevertheless, some power-supply glitches do get through. These can cause
fransients
to
^pear on the voltage and current outputs which may cormpt high-sensitivity recordings
(for example, during
fluctuation
analysis).
The only completely effective way to gain immunity from mains glitches is to eliminate them at the source.
Most glitches are due to the switching on and off pf odier equipment and lights on the same power-supply
circuit. Precautions to be taken include:
(1) Avoid switching equipment and lights on or off while recordings are being made.
(2) Water baths, heaters, coolers etc. should operate from zero-crossing relays.
(3) RFI
filters
should be installed in glitch-producing equipment.
In most circumstances occasional transients on the outputs are inconsequential aiid
therefore no precautions have to be taken.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 64

Page 54 DETAILED GUroE TO OPERATIONS
POV^^R SUPPLY VOLTAGE SELECTION & FUSE CHANGING
Supply Voltage
The AXOCLAMP-2A can work from all international supply voltages. The two input ranges are:
(1) 115V: For 100 Vac to 125 Vac operation.
(2) 230 V: For 200 Vac to 250 Vac operation.
To change the supply voltage setting:
(1) Disconnect the power cord
(2) Remove the top cover
(3) Locate the slide switch labeled "S2" at the back of the power supply board. The power supply
board is the small horizontal board in the left side of the instmment.
(4) For 115 V operation slide S2 to die left towards die label " 115".
For 230 V operation slide S2 to
die right towards die label "230".
(5) Replace the top cover.
(6) Re-connect the power cord.
(7) Mark the new operating voltage on the identification plate on the rear of the instmment.
Changing The Fuse
The AXOCLAMP-2A uses a 0.5 A 250 V slow acting 5 x 20 mm fuse on bodi voltage ranges. Before
changing the fiise investigate the reason for its failure.
To change the fiise:
(1) Disconnect the power cord.
(2) Use a screwdriver or something similar to lever out the fiise holder.
(3) Discard the fiise from the active slot, i.e. the slot which places the fuse closest to the inside of die
instmment.
(4) Shift the spare fiise from the spare slot (i.e. the slot which places the fuse towards the outside of
the instmment) to the active slot.
(5) Re-connect the power cord.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 65

DETAILED GUHJE TO OPERATIONS Page 55
REMOTE
Some of the front-panel functions can be activated via the Remote connector at the rear of the Clamp.
These are Mode selection. Buzz, and Clear. Possible uses of this facdity include using a computer to
select the ModK, and using hand-operated or foot-operated switches for Buzz and Clear so that these
hinctibns can ^ used by the experimenter without moving from the nucroscope.
The selected functions are activated by HIGH logic levels applied to the ^propriate pin. New Modes are
selected and kept after a HIGH level of 1 /is or more in duration. Buzz and Clear are activated for the
duration of
the
HIGH level. Using the Remote facility does not disable the
front-panel
switches.
The pin connections for the Remote connector are as follows:
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
DIGITAL Ground
-1-5 V output
BRIDGE mode
DCC mode
SEVC mode
TEVC mode
CLEAR MEl "-I-"
CLEAR MEl "-"
Not used
Not used
Not used
CLEAR ME2"-!-"
CLEAR ME2 "-"
BUZZ ME2
BUZZ MEl
To use the Remote controls, the extemal control signals can be wired to a 15-pin D-type connector which
can then be plugged into the Remote connector on the rear panel.
-1-5 V is provided for wiring up any remote switches you may use. Do not short circuit this supply.
The Mode-Select inputs (pins
7
kO
input resistances.
3H5)
have 50 kO input resistances; the other inputs (pins 7, 8, 12-15) have
The FS-3 footswitches provided with the AX0CLAMP-2A consist of a pair of normally open switches for
activating Buzz of each elecfrode. If footswitches are not convenient you can easily connect your
preferred switches by following the wiring diagram below.
For remote operation of microelectrode 1 Buzz and microelectrode 2 Buzz.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRIftlENTS, INC.
Page 66

Page
56
Buzz
+5
V
1
DETAILED GUIDE
15
TO
OPERATIONS
•14
Buzz
2 /
'
10
foot
(3m)
EXTERNAL SWITCH WIRING DIAGRAM
cable
"Remote"
15-pin connector
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY
1990,
AXON INSTRUMENTS,
INC.
Page 67

DETAILED GWDE TO OPERATIONS Page 57
RMP BALANCE
The two indicator lights for monitoring resting membrane potential (RMP) are used in two ways.
Before switching into a voltage-clamp mode the Holding Position potentiometer is adjusted until the two
lamps are equally dim (nulled). This ensures that when a voltage-clamp mode is selected the membrane
potential will be held within a few millivolts of
RMP.
When adjusting the Holding Position control before
voltage clamping the sensitivity of the null point is affected by the Gain.
During voltage clamp the RMP Balance lights provide a quick indication of when the cell is being held at
its resting level. That is, the RMP Balance lights are nulled at diis point.
SERIES RESISTANCE
Origin
A resistance (Rg) in series with the membrane can arise a number of different ways. In cSEVC, Rg would
mainly be due to the resistance of die suction electrode. In dSEVC, Rg would be due to a slow
microelecfrode response. In TEVC, R, would be due to the tissue, the bathing solution and the
grounding elecfrode.
Problem
The voltage-recording microelectrode (MEi) records the voltage across Rg and Rm, thus the recorded
membrane potential is in error due to the IR voltage drop across Rg. In addition, Rg limits the maximum
rate at which die membrane capacitance can be charged.
Solutions
There are no perfect solutions for these two problems. As always, the best solution is to take steps to
minimize Ri in the first place. These include:
(1) In cSEVC arrange for die elecfrode resistance (Re) to be extremely small, since Rg » Re.
(2) In dSEVC eliminate Rg altogether by watching the Monitor output to make sure the transient decays
completely before the next sample is taken.
(3) In TEVC keep the resistance of the grounding padi low. This includes the solution resistance, the
grounding electrode, and a virtual ground if used. Usually R. is only a problem in TEVC if very
large currents are passed.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 68

Page 58 DETAILED GUIDE TO OPERATIONS
Secondary solutions are the following:
(1) In cSEVC elecfronically subfract from the conunand voltage a voltage equal to the product of the
membrane current and the presumed series resistance (see cSEVC section). This technique can
begin to cope widi both die error and the limited charging rate. Unfortunately, die compensation
can rarely exceed 70% before introducing instabilities.
(2) The high-frequency current noise is proportional to the gain, but the clamp speed is limited by Rg.
Since the membrane potential stqi response time is slow anyway, it tums out that using some
phase lag can significandy lessen die current noise without worsening the response speed. This is
illustrated in Fig. 8. Note that even though the recorded potential is made faster by using the
Phase controls, the tme membrane potential and current are not speeded up.
(3) In TEVC elecfronically subfract from the command voltage a voltage equal to the product of the
membrane current and the presumed series resistance. To do this you would need to use an
extemal potentiometer to find a fraction of h, and feed it into the rear-panel R. COMP input.
What is the True Membrane Potential-Time Course?
For an isopotential cell, the time course of the tme membrane potential is the same as that of the recorded
membrane current. The recorded potential, which includes the voltage drop across Rg, may be much
faster. See Fig. 8 for an illusfration of this effect.
In a non-isopotential cell, for example a neuron with an axon and dendrites, the tme membrane potential
recorded at the tip of the voltage-recording electrode will in fact settle faster in response to a step voltage
command than will the membrane current. In this situation the presence of a series resistance will
exaggerate the difference in time courses.
Fig. 8
Membrane potential and current during TEVC.
Cell model was Rm = 10 MQ, Cm = 1 nF, Rg = 300 kO, Rei = R* = 10 MO. For all fraces
recording bandwidth was 30 kHz. Gain was 700 WfV. Vertical calibration: 10 inV/div. Horizontal
calibration: 1 ms/div.
Upper trace is tme membrane potential recorded by a third indqiendent electrode.
Middle frace is membrane potential recorded by MEl and clamped by the voltage-clamp circuit.
Bottom
trace,
is
the membrane current.
A. No added phase shift.
B.
Phase control set to Center Frequency time constant of 0.2 ms. Phase shift on -4. The tme
membrane potential response is unaffected, but the membrane current noise is gready reduced, to
a level consistent with the slow membrane potential response.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 69

DETAILED GOTDE TO OPERATIONS
Page 59
B
nOURE 8 - HOW SERIES RESISTANCE (Rg) AFFECTS VOLTAGE CLAMP PERFORMANCE
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 70

Page 60 DETAILED GUnJE TO OPERATIONS
SEVC MODE - CONTINUOUS
Continuous single-elecfrode voltage clamping (cSEVC) is one of two single-elecfrode voltage clamp modes.
In cSEVC current pasising and voltage recording are performed simultaneously as shown in die block
diagram of Fig. 9. The voltage (Vi) recorded by the microelecfrode buffer (Al) is compared in a highgain differential amplifier (A2) to a command potential (Vc). The output of
A2
acts to keep the difference
at its input (e\) very small. Hence, Vi is clamped equal to Vc.
The circuit clamps the voltage across the microelecfrode (MEl) as well as the membrane potential (Vm).
The voltage across MEl is non-zero because of the current (Im) which flows through it. This voltage drop
is equal to the product of
Im
and the resistance (Rei) of MEl.
To keep the error due to ImRei small it is necessary that Rei be much smaller than the membrane resistance
(Rm).
Thus a 3 MQ microelectrode would be appropriate for use widi a
3(X)
MQ cell.
The voltage across MEl can be partially compensated by using the Bridge potentiometer. Note that the
range of die Bridge potentiometer is ten times less in cSEVC mode than in Bridge mode. The reduced
range is indicated by a small LED. It is not normally possible to compensate more than about 70% of the
elecfrode resistance without introducing oscillations.
During cSEVC, Rei has the nature of a series resistance (Rg). Rg is discussed in the Series Resistance
Section.
Important Note - Anti-Alias Filter
The Anti-Alias Filter is not recommended for use in cSEVC mode. The reasons why are the same as those
given in the
TEVC
Section.
Suggested Use
In Bridge mode set the Capacitance Neutralization control for the best step response.
Set die Gain and Anti-Alias Filter to minimum values. Switch the Phase control off. Switch off all
current commands.
Use the Holding Position control to yield equal brightness in each of the two RMP Balance lights. At this
setting the command potential during voltage clamp will be equal to the resting membrane potential (RMP).
Lock the Holding Position control if desired.
Switch into cSEVC mode. Set up a repetitive step command. Monitor 10.Vm and !„• For maximum
stabUity switch the Phase Time Constant to 20 or 200 ms. Increase the Gain for the best response on bodi
Vm
and
Im-
Sometimes lower current noise can be achieved for the same step response with the Phase Time
Constant on 0.2 or 2 ms. Before switching to these values reduce the Gain since the stability margin is
lower for smaller values of the Time Constant. Advance the Bridge potentiometer to speed
up.
the
current
and voltage setding times.
An example of a cSEVC set up in a cell model is shown in Fig. 10. The cell model was 300 MQ//33 pF
and the elecfrode was modeled by a 3 MQ resistor. Because of Re there was a limit to how fast the
membrane c^acitance could be charged. This can be seen from the duration of
the
capacitance
fransient
in
the upper trace. The clamping electrode (MEl) records die tme membrane potential as well as the IR drop
across
itself,
thus the step response of the recorded voltage (middle trace) is faster than the tme membrane
potential (lower trace) recorded by an independent elecfrode. As discussed in the Series Resistance section,
the time course of the tme membrane potential corresponds to the time course of
AXOCLAMP-2A THEORY
&.
OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
the
membrane current.
Page 71

DETAILED GUIDE TO OPERATIONS
Page 61
- V
m.
FIGURE 9 - SIMPLinED SCHEMATIC OF cSEVC
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 72

Page 62
DETAILED GUIDE TO OPERATIONS
cSEVC Compared With Whole-Cell Patch Clamp
The simplified schematic in Fig. 9 shows that cSEVC is similar to whole-cell clamping using the patch-
clamp technique. However the implementation is very different.
In the patch-clamp technique die voltage-clamp is established by a specialized headstage containing a
virtual-ground circuit. In the cSEVC technique the headstage is a general-purpose unity-gain buffer and
the voltage-clamp circuit is located in the main unit.
This difference is significant. In the dedicated virtual-ground headstage much less circuitry is involved
and thus nonidealities of the electronics have much less effect. Thus for fast events the patch-clamp
technique is considerably better than cSEVC. On the other hand for slow and moderate events the
techniques become comparable.
Fig. 10
Current and potential recorded during cSEVC. Cell model was 300 MO//33 pf. Electrode was 3 MQ.
Bandwidth was 3 kHz for all fraces. H of HS-2L headstage was xO.l. Clamp gain was 3.3 nA/mV.
Voltage command was a 10 mV step. Phase Time Constant was 0.2 ms. Phase Shift was full lag. A-A
Filter was off. Capacitance Neutralization was minimum.
Upper frace: Membrane current. Charging time was limited by Re.
Middle frace: Potential recorded by clamping elecfrode (MEl) and available at the 10.Vm ou^ut.
Includes IR drop across MEl.
Lower trace: Tme membrane potential recorded by an independent electrode. Time course is the same
as diat of die membrane current.
Noise: The current noise in the 3 kHz bandwidth was 55 pA peak-to-peak. If the gain was
reduced so that the capacitance transient took 1.5 ms to setde the current noise fell to
12 pA peak-to-peak. The noise looks worse in the photo due to blooming of the
photographed oscilloscope frace.
AXOCLAMP-2A THEORY
St.
OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 73

DETAILED GUIDE TO OPERATIONS
Page 63
3nA
10 mV
1 ms
FIGURE 10-CURRENT AND POTENTIAL RECORDING DURING cSEVC IN A CELL MODEL
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 74

Page 64 DETAILED GUIDE
TO
OPERATIONS
SEVC MODE - DISCONTINUOUS
Description
In discontinuous single-electrode voltage clamp (dSEVC) mode the tasks of voltage-recording and current-
passing are allocated to the same elecfrode. Time-sharing techniques are used to prevent interactions
between the two tasks. The principles of operation have been published (Brenneke & Lindeniannn, 1974;
Wilson & Goldner, 1975; Finkel & Redman, 1984) and are oudined in the block diagram and timing
diagram of
Fig.
11, and in the following discussion.
A single microelecfrode (MEl) penefrates the cell and the voltage recorded (Vi) is buffered by a unity-gain
headstage (Al). To begin the discussion assume diat at this moment Vi is exacdy equal to the
instantaneous membrane potential (Vm). A sample-and-hold circuit (SHl) samples Vm and holds it for the
rest of the cycle.
The sampled membrane potential is compared with a command voltage (Vc) in a differential amplifier (A2).
The output of this amplifier becomes die input of a controlled-current source (CCS) if the switch SI is in
the current-passing position. The gain of the
CCS
is Gr.
The CCS injects a current into the microelectrode which is directly proportional to the voltage at die input
of
the CCS
irrespective of
die
resistance of the microelecfrode.
The period of current injection is illusfrated at the start of the timing waveform. SI is shown in the
current-passing position during which a square pulse of current is injected into the microelecfrode.
Because of
this
current Vi rises.
The rate of rise is limited by the parasitic effects of die capacitance dirough the wall of the glass
microelecfrode to the solution, and the capacitance at the input of the buffer amplifier. The final value of
Vl mosdy consists of die IR voltage drop across the microelectrode due to the passage of current lo
through the microelecfrode resistance Re. Only a tiny fraction of Vi consists of the membrane potential
recorded at the tip.
SI then switches to the voltage-recording position. When the input of die CCS is 0 volts, its output
current is zero and Vi passively decays. During the voltage-recording period Vi decays asymptotically
towards Vm. Sufficient time must be allowed for V] to reach within a millivolt or less of Vm. This
requires a period of up to 9 electrodes time constants (re). At die end of the voltage-recording period a
new sample of
Vm
is taken and a new cycle begins.
The actual voltage used for recording purposes is the sampled voltage. As illustrated in the bottom timing
waveform the sampled value of Vm moves in small increments about the average value. The difference
between Vm(ave) and Vc is the steady-state error (ci) of the clamp which arises because the gain (Gr) of
the
CCS
is finite. The error becomes progressively smaller as
GT
is increased.
The duty cycle used in dSEVC is current passing for 30% of each cycle, and voltage recording for 70% of
each cycle.
The cycling rate (sample rate) must be chosen so that there are ten or more cycles per membrane time
constant. This enables the membrane capacitance to smooth the membrane voltage response to the current
pulses.
When optimally adjusted, the circuit enables the first steady-state measurement of voltage to be taken 1 to
2 cycle periods after the onset of a membrane conductance change or a change in the command voltage.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 75

DETAILED GUTOE TO OPERATIONS Page 65
Two controls not shown in die Figure are the Anti-Alias Filter and the Phase control. The Anti-Alias
Filter is a single-pole filter between the output of die unity-gain headstage (Al) and SHI (see Fig. 7). It
can be used to reduce noise at a given sampling frequency. The output of die Anti-Alias Fdter can be
observed on the Monitor output. In practice it is this voltage, not Vi, which has to decay to Vm before a
sample is taken. The Phase confrol ^ters the frequency response of the differential amplifier (A2). It can
be used to compensate for the complicated frequency characteristics of a real cell.
The Gain confrol alters
GT-
Refer to the Specifications for its operating range.
While MEl is occupied by the dSEVC it is still possible to independendy use ME2. For example, ME2
could be used for recording from and stimulating other cells which make connections to the cell being
voltage-clamped.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 76

Page 66
DETAILED GUIDE TO OPERATIONS
vohoge recording
0 volts
S2
current
clamp clomp
51
current passing
voltage recording
17*=
Vc
"m (ova)
FIGURE 11 - SEVC BLOCK DIAGRAM AND TIMING WAVEFORMS
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 77

DETAILED GUIDE TO OPERATIONS Page 67
Suggested Use
Use two oscilloscopes. To the main one connect the 10.Vm and Im outputs. Trigger this oscdloscope
from the source used to time the command signals. To the second one (which need not be a high-quality
type) connect the Monitor ou^ut. Set the gain to 100 mV/div (= 10 mV/div input referred). Trigger
this oscilloscope from the SAMPLE CLOCK output on the rear panel.
Set up a repetitive current pulse in Bridge Mode. Balance the elecfrode voltage drop as shown in Fig. 2
in the Bridge Section.
Set Gain and Anti-Alias to minimum. Switch Phase Shift off
(i.e.
set Center Frequency to OFF).
Switch to DCC mode.
Proceed to optimally set the Ci4}acitance Neufralization as described in the DCC Section, method B, and
illusfrated in Fig. 5.
Set the Output Bandwidth to 1/5 or less of fg.
Switch off the current pulse. Use the Holding Position control to achieve equal brightness in each of the
two
RMP
Balance lights.
Switch to SEVC.
Set up a repetitive 10 mV step command.
Increase the Gain as far as possible without causing overshoot or instability in.the step response. Reduce
the Gain slighdy below the maximum value to get a safety margin.
Introduce Phase lag or lead if by doing so the step response of both the current and the voltage can be
improved.
Increase the Anti-Alias Filter while checking the settling characteristics on the monitor waveform. The
noise on
Vm
and
Im
may be reduced by this procedure. Only use as much Anti-Alias as is consistent with
stability.
Set the Anti-Alias Filter back to minimum before using a new electrode.
An example of a correcdy set up dSEVC is shown in Fig. 12.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 78

Page
68
DETAILED
Fig.
12
An example
100
MQ.
command
of a
correcdy
Gain
was 1
was a 10 mV st^. No
Recording bandwidth
A.
Top
set up
dSEVC
in a
cell model. Rm
nA/mV. HS-2L headstage;
Phase Shift
in A was 1 kHz.
frace: Membrane current.
was 1(X) MO. Cm was 33 pF. Rei was
H = xO.l.
or A-A
Filter. Capacitance Neufralization
Cal: 4 nA, 1 ms
Sampling rate
GumE
TO
was 7 kHz.
was
OPERATIONS
Voltage
optimum.
Note that
Middle trace: Sampled membrane potential (available
Cal:
10
Lower frace:
Cal: 10 mV, 1
the
two voltage records
B.
Multiple sweeps
rest.
The
The important feature
samples
are
Cal:
mV, 1
Tme
are
of the
current pulses vary from sweep
taken (arrow) even
20
mV, 40/iS.
ms.
membrane potential recorded
ms.
identical because
the
Monitor waveform. This photo
is
that
the
voltage transients decay completely
for the
largest transients.
at the
10.Vm output).
by an
independent elecfrode.
Capacitance Neufralization
was
taken with
to
sweep because
of
the sampled voltage noise.
was
correcdy
the
cell held
by the
set.
time
at
the
AXOCLAMP-2A
THEORY & OPERATION,
COPYRIGHT FEBRUARY
1990,
AXON INSTRUMENTS,
INC.
Page 79

DETAn.£D GUIDE TO OPERATIONS
Page 69
B
FIGURE 12 - CORRECTLY SET UP dSEVC IN A CELL MODEL
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
4nA
10 mV
20 mV
1 ms
40/iS
Page 80

Page 70 DETAILED GUIDE TO OPERATIONS
Important Note
If
the
Phase controls are used it is possible to find false settings of Capacitance Neufralization (or the AntiAlias Filter) and Phase which together give a seemingly fast step response to Vm whereas in fact the step
response in the cell is much slower.
This situation arises by undemtilizing the Capacitance Neutralization (or ovemtilizing the Anti-Alias
Filter) so that the Monitor waveform fails to decay adequately when the voltage sample is taken. The
elecfrode voltage sampled has the nature of an IR drop across a series resistance (Rg; see Series Resistance
Section). Normally this would make the clamp unstable, but by introducing phase lag stability can be reimparted although without any reduction of die voltage error.
This false condition only arises if the Capacitance Neufralization setting is altered after the Phase control
has been switched in. There are two ways to guarantee diat this false condition will not occur.
1.
Don't use the Phase controls.
2.
If the Phase controls are used be sure to conscientiously observe the Monitor waveform to make
sure that the decay to a horizontal baseline is complete at the end of each cycle.
An example of a false clamp is shown in Fig. 13.
The recorded value of
Im
is always a tme measure of the membrane current even during diis false setting.
Only the Vm record is erroneous. The danger of this false condition is diat most of the presumed
membrane potential is in fact voltage drop across the microelectrode.
Which SEVC to use with a Suction Electrode
In the previous section we discussed how a continuous SEVC can be implemented by taking advantage of
the low resistance of a suction electrode.
The problem with the cSEVC technique is the error introduced by Re which can only be partially overcome
by series resistance compensation. This problem can be completely avoided by using the dSEVC mode.
It turns out that the conditions when a suction electrode is used are ideal for dSEVC. That is , because Re
is very small the electrode time constant is fast. In addition, the magnitude of the voltage transient across
the electrode for a given current is proportional to Re and dierefore small when Re is small. This double
advantage of low Re values means that die dSEVC can be cycled very rapidly widiout introducing a
sampling error.
Fig. 14 shows the result of a dSEVC in exacdy the same cell model that was used in the cSEVC shown in
Fig. 0. The most significant difference in the set-up besides the clamping mode used was the fact that no
phase shift was used in the dSEVC.
Since die IR drop across the electrode was not sampled, die recorded potential during dSEVC had the same
time course as the membrane current and the tme membrane potential recorded by an independent
elecfrode (not shown).
The disadvantage of the dSEVC mode was the additional current noise.
AXOCLAMP-2A THEORY & OPERATION. COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 81

DETAILED GUHJE TO OPERATIONS
Page 71
Fig. 13
An example of an incorrecdy set up dSEVC (i.e. a "false" clamp) in a cell model. Rm, Cm, Rei, Gain,
H, sampling rate, recording bandwiddi and A-A Filter were the same as in Fig. 12. Phase Shift was at
maximum lag. Time Constant was 2 ms. Capacitance Neufralization was under-utilized.
A. Top frace: Membrane current.
Cal: 1 nA, 4 ms.
Note that this membrane current is much smaller and slower than the one in Fig. 12.
Middle frace: Sampled membrane potential (available at the
10.
Vm output).
Cal: 10 mV, 4 ms.
Lower trace: Tme membrane potential recorded by an independent electrode.
Cal: 10 mV, 4 ms.
Note that the two voltage records are not die same. The sampled membrane potential includes a large
error due to the voltage across the microelectrode at the sampling time (see B below).
B.
Multiple sweeps of the Monitor waveform. This photo was taken with the cell held at
.-1-50 mV 'from rest. (This was done because when the cell was held at rest with the
considerable amount of phase lag used the noise current pulses were too small to allow the
adequacy of the decay to be seen.) The voltage transients did not decay to a horizontal
baseline at the times the samples were taken (arrow), therefore the samples included some
of the IR voltage drop across the microelectrode.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 82

Page 72
DETAILED GUIDE TO OPERATIONS
B
1 nA
10 mV
20 mV
4 ms
40/iS
FIGURE 13 - INCORRECTLY SET UP dSEVC (i. e. "FALSE" CLAMP) IN A CELL MODEL
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 83

DETAILS) GUIDE TO OPERATIONS
Page 73
Fig. 14
Current and potential during dSEVC using the same suction elecfrode model used in Fig. 10.
Differences were: Gain was 0.7 nA/mV. Phase Shift and A-A Filter were both off. Sampling rate was
50 kHz.
Upper frace: Membrane current
Lower frace: Sampled membrane potential recorded from the 10.Vm output.
Noise: Hie current noise in the 3 kHz bandwiddi was 80 pA peak-to-peak.
1 nA
10 mV
1 ms
FIGURE 14 - CURRENT AND POTENTIAL RECORDING DURING dSEVC USING A
SUCTION ELECTRODE MODEL
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 84

Page 74 DETAILED GUIDE TO OPERATIONS
Minimum Sampling Rate and Maximum Gain
If
the
sampling rate is too slow the dSEVC will become unstable. This is because the long current-passing
period allows the the membrane potential to charge right through and past the desired potential before the
clamp has an opportunity to take a new sample of potential and adjust die current accordingly. The larger
the cell membrane capacitance (Cm) die slower the sampling rate (fg) which can be used for a given average
gain (G). The stability criterion is (see Brenneke & Lindemann, 1974; Finkel & Redman, 1984)
G
0 < < 2
»^m tg
For critical damping we require
= 1
t.'m Ig
Thus for a given G, if
As an example, if G = 1 nA/mV and Cm =
Cm
is small fg must be large.
iOO
pF, dien fg must be 10 kHz for critical damping. If
fg
is less than 10 kHz in this example, the step response will overshoot and at 5 kHz the clamp will oscillate
destmctively.
If the sampling rate in this example cannot be as great as 10 kHz because the microelecfrode response is
too slow, then a lower value of G will have to be used to maintain stability.
Clamp Error
With finite gains in die voltage clamp circuit
Vm
does not quite follow Vc. The error is
£1 = Vc-Vm
Similarly, if Vc is constant and the cell membrane conductance changes there is an error in the
measurement of the current underlying the conductance change. This error is similar in percentage to the
voltage error.
Usually the gain of the voltage clamp circuit can be increased so that ei is 10% or less. The percentage
error d^ends on the frequency of the command signal or of the conductance change. It is smallest for
slow signals and DC, and largest for the fastest signals. Thus very fast transients (such as the rising
phase of synaptic currents) will be clamped less well than slower transients (such as the decay phase of
synaptic currents).
Gain
The clamp gain during dSEVC mode is given in nA/mV. This refers to how many nanoamps the output
current will change by for each millivolt of difference between Vm (die membrane potential) and Vc (the
command potential). The value indicated on die front panel is the average value. The average value
depends upon the instantaneous gain during the current-passing period and upon the duty-cycle.
AXOCLAMP-2A THEORY
SL
OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 85

DETAILED GUIDE TO OPERATIONS Page 75
SPACE CLAMP
When interpreting the current measured during voltage clamp, due consideration should be given to the
adequacy of the spatial extent of the membrane voltage confrol.
In general, measurements of currents generated more than 0.1 elecfrotonic lengths from the point of the
voltage clamp elecfrode(s) will be subject to significant error. This problem is discussed in detail by
Johnston and Brown (1983).
TEN-TURN POTENTIOMETERS
The ten-tum potentiometers used in die AXOCLAMP-2A are high-quality wirewound types.
An inherent problem of wirewound potentiometers is that the wire elements tend to oxidize. This
condition is curable.
If a potentiometer becomes noisy, the potentiometer manufacturer recommends rapidly spinning the knob
20-30 times between full clockwise and fiill counterclockwise. This clears the oxide off die element and
restores noise-free operation.
TEVC MODE
Description
In TEVC (Two-Elecfrode Voltage Clamp) mode the AXOCLAMP-2A acts as a conventional voltage clampi.
MEl is the voltage-recording elecfrode and ME2 is the current-passing electrode.
The ou^ut of die clamp is a voltage source (in contrast to SEVC modes in which the clamp ou^ut is a
current source) which is connected to ME2. The voltage-clamp gain control is marked in units of V/V.
This refers to how many volts the output will change by for each volt of difference between Vm (the
membrane potential) and Vc (the command potential). For example, when the gain is at its maximum
value of 10,000 V/V, a 100 yiV difference between Vm and Vc would cause the output to shift by 1 V. If
die resistance of
ME2
was 10 MO diere would be a current of 100 nA.
The best settings of the voltage-clamp parameters are found by setting up the best possible response to a
step change in Vc. Usually, the abUity of the voltage clamp to follow a.step change in command is
identical to the ability of the voltage clamp to follow a step change in membrane conductance (Finkel &
Gage, 1985).
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS. INC.
Page 86

Page 76 DETAILED GUIDE TO OPERATIONS
Factors affecting the voltage-clamp response are these:
The Gain control determines the steady-state accuracy and the response speed.
The Phase control introduces a combination of phase lag and phase lead (a zero) in the voltageclamp amplifier.
Hie Holding Position control shifts the clamped membrane potential.
Hie Capacitance Neufralization setting of MEl affects the voltage-clamp response. The
Capacitance Neufralization setting of ME2 affects the current monitoring circuit at high frequencies
and also has a small effect on the voltage-clamp response.
The Anti-Alais filter slows the microelecfrode response and should not be used in TEVC mode.
Suggested Use
In Bridge mode set the Capacitance Neutralization of each microelectrode for the best step responses.
This is important but not critical, and in order to be tolerant of changes in the microelecfrodes' resistances
which might occur during TEVC it is suggested that Capacitance Neutralization should be slighdy
undemtilized.
Use a second-order or better lowpass filter to remove the high-frequency noise from I2 (see Ou^ut Filter
section).
Set the Gain and Anti-Alias Filter to minimum values. Switch the Phase control off. Switch off all
current commands.
Use the Holding Position control to yield equal brightness in each of the two RMP Balance lamps. At this
setting the command potential during voltage clamp will be equal to the resting membrane potential (RMP).
Lock the Holding Position control if desired.
Switch into TEVC mode. Set up a repetitive step command. Monitor both lO.Vm and I2. Increase the
Gain as far as possible without causing overshoot in the st^ response. In cells whose membranes do not
cause the same phase shift (90°) as a parallel RC cell model, the Phase control can be used to increase the
maximum gain achievable. The Capacitance Neutralization setting of MEl should not be altered during
voltage clamp unless there is reason to believe the resistance of MEl has altered.
Elxtremely Important Note - Coupling Capacitance
The most significant factor in achieving a good two-electrode voltage clamp is adequate prevention of
interactions between the two electrodes. Coupling capacitance as low as 0.01 pF can destablize the
response at high gain settings. f?
To minimize the coupling capacitance it is essential that a grounded shield be placed between the two
microelectrodes and their headstages to prevent signals in ME2 being picked up by MEl. It should extend
between the two elecfrodes to within a millimeter of the surface of the solution. It is possible to coat ME2
with a conductive paint which is then grounded. This procedure works well but has a minor disadvantage
in that it vasdy increases the capacitance at the input of the ME2 headstage, which may affect the highfrequency measurement of h unless the capacitance neutralization of
ME2
is properly set.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 87

DETAn.ED
GinDE TO OPERATIONS
Page 77
Fig. IS shows the defrimental effects of only a small amount of coupling capacitance. The fraces in Fig.
ISA show the membrane current and voltage responses in a cell model when an extensive grounded shield
was placed between the two elecfrodes. In Fig 15B a 2-3 mm wide gap in the shield caused highfrequency oscillations and noise. The back end of the electrodes were 40-50 mm ^art. When the gap
size was increased further the clamp went unstable.
Figure 15
The destabilizing effects of coupling capacitance. Traces were recorded in a cell model; Rm = 10 MO,
Cm = 1 nF, Rei = Re2 = 10 MO, Gain = 1000 V/V, recording bandwiddi = 10 kHz, Phase Shift and
Anti-Alias Filter both off. 10 mV st^ conunand. Upper fraces are I2 (the membrane current). Lower
fraces are Vm. Elecfrodes were 40-50 mm apart.
A. Extensive grounded shielding between the two elecfrodes.
B.
2-3 mm gap in the grounded shield caused high-frequency oscillations.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 88

Page 78
DETAn,ED GUIDE TO OPERATIONS
B
100
200 nA
10 mV
MS
FIGURE 15 - AN EXAMPLE OF THE DESTABLIZING EFFECTS OF COUPLING
CAPACITANCE
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 89

DETAILED GUIDE TO OPERATIONS Page 79
Saturation During The Capacitance Transient
The output voltage of the AXOCLAMP-2A main unit during TEVC is ±30 V. This is usually sufficient to
drive the current through ME2 required to charge the membrane capacitance during a step voltage change.
However, for large stqis in some cells the output niay saturate and the time required to establish the step
change will be longer than necessary.
Part of die reason for the saturation is that not all of the ±30 V appears across the microelectrode. Some
of it appears across the current-sensing resistor (Ro; see Fig. 4) in the headstage. If
appears across the microelectrode, but if
Re
< Ro the voltage across the microelectrode is even less.
Re
= Ro then ±
15
V
To overcome this a headstage (designated HS-4) is available which has a relay inside it to automatically
link out Ro whenever TEVC mode is selected. There are two advantages to using the HS-4 headstage.
The first is that even in the linear operating region the time to establish a step voltage change is quicker,
and die second is that larger step changes can be established widiout entering the nonlinear (i.e. saturating)
region. The disadvantage is that the HS-4 headstage must be used in conjunction with a virtual-ground
current-measurement headstage. This is because the normal built-in current monitors need Ro in order to
operate.
Because it requires a virtual-ground headstage as well, we do not normally recommend the HS-4 headstage
unless the experimental circumstances demand it. Contact Axon Instmments for more details.
Choosing the Microelectrode Resistances
If large currents must be passed, such as may occur during large depolarizations of excitable cells, then
the resistance of
ME2
should be as low as possible. If low-noise recordings are required, which would be
necessary for resolving small transmitter-activated currents from the background noise, dien the resistance
of MEl should be as low as possible.
If two headstages with different Hs are used, the one with the larger H (and therefore greater currentpassing ability) should be used with ME2.
TRIGGERED CLAMPING
In some experiments it is desirable to switch into voltage clamp only when a specific event threshold is
reached. For examjple, it may be desirable to switch into voltage clamp when the undamped action
potential goes above a predetermined level.
To do this an extemal device must be used to detect the event and signal its occurrence by putting out a
logic HIGH. The logic HIGH is then applied to pin 5 or 6 of die Remote connector on the rear panel of
die AXOCLAMP-2A. The AXOCLAMP-2A will then remain in voltage clamp mode until die logic HIGH is
removed from pin 5 or 6 and a separate logic HIGH applied to pin 3 or 4 of die Remote connector.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 90

Page 80 DETAILED GUIDE TO OPERATIONS
TROUBLE SHOOTING
It has been our experience at Axon Instmments that the majority of froubles rq>orted to us have been
caused by faulty equipment connected to our instmments.
If you have a problem, please disconnect all instmments connected to the AXOCLAMP excqit for the
oscilloscope and one headstage. Ground the headstage through the original test resistor supplied by Axon
Instmments. If the problem persists, please call us for assistance.
UNITY-GAIN RECORDING - THIRD POINT
In normal operation both MEl and ME2 can be used for unity-gain recording and current-passing. A diird
point in the preparation can be recorded from if virtual-ground current measurement is not being used.
To do so, a unity-gain headstage (HS-2) is plugged into the Virtual-Ground connector on the rear panel.
The voltage recorded spears on the rviRT output. No current can be passed via the HS-2 headstage used
in the Virtual-Ground connector. When plugged into the Virtual-Ground connector the input capacitance
of the unity-gain headstage is 4 pF.
VIRTUAI^GROUND CURRENT MEASUREMENT
A Virtual-Ground headstage can be used to ground the preparation badi. All of the current flowing into
the Virtual-Ground input is measured and a voltage proportional to the current is provided at the IVIRT
output. The output gain is 10 mV/nA when the virtual-ground output attenuation (VG) is xl, 1 mV/nA
when VG is xlO, and 100 mV/nA when VG is xO.l
A Virtual-Ground headstage is not required for normal use of the AX0CLAMP-2A because built-in currentmeasurement circuits are provided for each microelectrode. However, in TEVC mode the current output of
the Virtual-Ground headstage has slighdy less high-frequency noise than die output of the built-in currentmeasurement circuit.
The Virtual-Ground circuit measures all currents into the preparation bath, hence special care must be
taken to ensure that conducting connections to the preparation bath do not act as antennae which pick up
hum. Saline-filled tubes act as excellent antennae. To prevent them carrying hum, long saline-filled
tubes should have the saline pathway broken by an air-filled drip near the preparation.
More complete explanations and instmctions are provided with the VG series of headstages.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 91

DETAn.ED GUIDE TO OPERATIONS Page 81
10.Vn, AND In^ OUTPUTS
The 10.Vm output is proportional to ten times die membrane potential (Vm)- It is derived from the
potential (Vl) recorded by MEl. Initially Vi is amplified, then depending on the operating mode, one of
two techniques is used to derive the 10.Vm signal from the amplified Vi signal. In Bridge mode, the
Bridge Balance technique is used to counter the effect of voltage drop (IR voltage drop) across MEI during
current passing so that only the membrane potential measured at the tip is passed to the lO.Vm output. In
DCC or dSEVC mode samples of the amplified Vi signal are taken after the IR voltage drop across MEl
due to the previous current pulse has completely decayed. Only the sampled values are passed to the
lO.Vm output.
The maximum recording range of the 10.Vm output is ±600 mV referred to the input. This range is
centered on the zero value set by use of the Input Offset control. In Bridge mode this range includes the
IR drop even though the IR drop may not be seen because the Bridge Balance is correcdy set. The full
±600 mV input-referred range is available in DCC and dSEVC modes irrespective of die current.
The Im output is proportional to the membrane current. In Bridge, cSEVC and TEVC modes it is the
continuous electrode current. In DCC and dSEVC modes, Im is found by sampling the current during the
current-passing period and multiplying by the duty cycle.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 92

SPECIFICATIONS
MODES
Page 82
SPECIFICATIONS
Five main opanting modes selectable by color-coded illuminated push buttons,
These are:
1.
Bridge
2.
3.
4.
5.
DCC:
dSEVC:
cSEVC:
TEVC:
Discontinuous Current Clamp
Discontinuous Single-Electrode Voltage Clamp
Continuous Single-Electrode Voltage Clamp
Two-Elecfrode Voltage Clamp
MICROELECTRODE AMPLIFIERS (Two Channels)
Unity-Gain Headstages:
Standard is the HS-2L type. HS-2M types arie die same except:
1) the noise is greater by about 20%
2) the capacitance neutralization range is extended.
HS-2MG types are similar to die HS-2M types except that the case is
grounded instead of driven.
Hum (line-frequency pickup):
Headstage Current Gain (H):
Less than 10 nW peak-to-peak, grounded input.
Available in 5 values (specify two widi order). Select on basis of
cell input resistance (Rin) and maximum current capacity (Imax)-
H = xO.OOOl for ion-sensitive electrodes
H = xO.Ol for Rin greater dian about 300 MO
H = xO.l for Rin about 30-300 MO
H = X 1 (standard) for Rin about 3-30 MQ
H = xlO for Rin about 300 kO to 3 MQ
These ranges are suggested for optimum performance. Some
overlap is allowable.
or remotely.
Maximum Current:
Noise with grounded input:
Imax = 1000 X H nA.
5 fiW rms measured with a 10 kHz single-pole filter in the
measurement circuit.
51 (47) (iW rms measured with a 10(100) MQ source resistance and
Noise with a source resistance:
c^acitance neutralization adjusted for a 10(1) kHz bandwidth and
with a 10 (1) kHz single-pole- filter in the measurement circuit.
Values are for H = xl (xO.l), HS-2L headstage.
16(54) /ts for a voltage step applied to the input via a 10(100) Mfl
1%
Settling Time:
low-capacitance resistor and 16(60) /is for a current step into the
same resistor. Capacitance neutralization adjusted for zero
overshoot. Values are for H = xl (xO.l).
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 93

SPECmCATIONS
Page 83
Working Input Voltage Range: ±
13
V for transients and steady state, protected to ±30 V.
Input Resistance: lO'-'-lO'* 0, H = x
10»3
0, H = X .01
0.0001
(see note)*
10'2 0, H = x0.1
10"
0, H = X 1
lO'O 0, H = X 10
*Note: For the xO.OOOl headstage, the input resistance of each headstage is measured individually. The
unique test results are supplied with each xO.OOOl headstage.
Input Capacitance:
Input Leakage Current:
Not relevant. See
Adjustable to zero.
Input Leakage Current vs. Temperature:
Offset Neutralization Range:
±500 mV. Ten-tum potentiometers.
10
100
1
10
1 %
setding time and noise specifications.
fA/
fA/
pA/
pA/
H = xO.OOOl
°c.
"C,
H = xO.Ol, xO.l
H = xl
-c.
"C,
H = xlO
Capacitance Neutralization Range:'^
HS-2L: -1 to 7 pF
HS-2M: -2to20pF
HS-2MG:
-4 to
18
pF
Buzz:
Buzz Duration:.
Clear:
Bridge Balance Range:
Digital Voltmeters:
These values apply when headstage is used with microelecfrode
1 amplifier. With microelectrode 2 amplifier the maximum values
are doubled.
Instandy increases capacitance neutralization to cause oscUlation.
Operatol by spring-loaded pushbutton switch, footswitch or by
Remote Buzz Duration control. The latter allows the Buzz
duration to be set in the range 1-SO ms.
1-50 ms when activated by the remote buzz confrol.
Forces ±Imax through the microelectrode. Spring-loaded toggle
switch.
10-^H MO/turn in Bridge mode.
1+H MO/tura in cSEVC mode.
Ten-tum potentiometers.
Voltage Displays:
Current Displays:
±1999mV.
± 19.99 pA,
± 1.999 nA,
± 19.99 nA,
±199.9 nA,
±1.999 mA,
Scaling is set by miniature panel switches
II,
hand Ivin.
Sq)arate meters for Vi and V2
H = X 0.0001
H = X .01
H = x.0.1
H = X 1
H = X 10
Display selections are
Currents exceeding the digital display range can be measured on
the
BNC
outputs.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990. AXON mSTRUMENTS. INC.
Page 94

Page 84
SPECmCATIONS
Outputs: 10-Vm and Im are membrane voltage (gain = 10) and current recorded by
microelecfrode 1.
Vl and II are the continuous microelecfrode 1 voltage and current.
V2 and I2 are microelecfrode 2 voltage and current.
MONITOR is the output of the anti-alias filter (equals the input of the
sampling device). Gain = 10. Baseline correction circuit automatically
references Monitor trace to zero volts.
Gain of current outputs is 10
H-
H mV/nA. Maximum ou^ut level is ± 13V.
Ciurent outputs indicate the true dectrode current.
Output Lowpass Filter
Cutoff:
0.1,
0.3, 1, 3, 10, 30 kHz.
Operates on Vm and Im. Single-pole filter.
Output impedances are 500 0. ± 10%
VOLTAGE CLAMP
10%
- 90% Rise lime:
The following values were measured using 10 MO and 1 nF in parallel to
model the cell, 10 MO resistors to model the microelecfrodes, and a
10 mV step command.
Rise Time in dSEVC mode = 100 us.
Gain:
Rise Tme in
Maximum in dSEVC mode is 100 x H nA/mV.
TEVC
mode = 30 fis.
Maximum in cSEVC mode is 1000 x H nA/mV.
Maximum in TEVC mode is 10,000 mV/mV.
Range is
300:1,
logarithmic scale.
Output compliance:
Phase Shift:
Anti-Alias Filter:
RMP Balance Indicators:
±25 V.
Time Constant (ms)
Lead range (ms)
Lag range (ms)
OFF
0
0
0.02
0-0.04
0-0.02
0.2
0-0.4
(W).2
2
0-4
0-2
20
0-40
0-20
Time constant range 0.2-100 /is
Equal brightness indicates voltage clamping will be at resting membrane
potential.
Blank:
Stops clamp from responding to new inputs for the duration of a HIGH
control signal on the BLANK ACTIVATE input. Used to reject stimulus
artifacts.
Series Resistance Compensation:
Operates in cSEVC mode. Value set on Bridge potentiometer. Extemal
input at 100 mV/V can be used in TEVC mode.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
200
0-400
0-200
Page 95

SPECmCATIONS
SAMPLING CIRCUIT
Page 85
Rate:
Counter:
Sample Clodc
Sample Acquisition Time:
500 Hz to 50 kHz. Operates in
3Hdigit display to 99.9 kHz max. Blanked in continuous modes.
Logic-level frigger output at the sampling rate.
l/«(10Vstepto0.1%)
INTERNAL COMMANDS
Note: Commands from all sources sum Uneaiiy.
Voltage Clamp Step Command:
± 199.9 mV. Set on diumbwheel switch. Activated by a HIGH
control signal on the STEP ACTIVATE input or by a front-panel
switch.
Voltage Clamp Holding Position:
Range ±200 mV transmembrane potential,
potentiometer.
Current Clamp Step Command:
DC Current Command:
± 199.9 X H nA. Set on thumbwheel as above.
±
100 X
H nA. Ten-tum potentiometers.
DCC
and dSEVC modes only.
Ten-tum
EXTERNAL COMMANDS
Sensitivities:
Ext.
VC
conunand: 20 mV/V
Series resistance compensation: 100 mV/V
Ext. ME 1 (microelecfrode 1) command: 10 x H nA/V
Ext.
ME
2 (microelectrode 2) command: 10 x H nA/V
Input Impedance: 22 kO
Max. Input Voltages:
±30 V for voltage-clamp conunands
±60 V for current-clamp commands
CALIBRA-nON SIGNAL
A pulse equal in magnitude to the setting on the thumbwheel switch is superimposed on the voltage and
current ou^juts for the duration of a HIGH control signal on the CAL ACTIVATE input.
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 96

Page 86 SPECIFICATIONS
BATH POTENTIAL COMPENSATION
Signal recorded by badi headstage or by an extemal amplifier is automatically subfracted from die
intracellular measurements. If bath potential is not measured the system automatically reverts to using
O V as the reference potential. Standard headstages (HS-2) work as badi headstages when plugged into
the bath headstage connector.
VIRTUAL-GROUND CURRENT MEASUREMENT
A VG-2 virtual-ground headstage can be plugged into the connector provided. Tlie current measured is
the sum of all currents into the preparation. The correct operation of the AXOCLAMP is not affected by
the use or nonuse of virtual-ground current measurement.
REMOTE
Logic HIGH control signals activate BUZZ and CLEAR of each microelectrode, and select between
BRIDGE, DCC, SEVC and TEVC modes. 15-pin connector.
MODEL CELL
A model cell is provided widi die AXOCLAMP-2A. Electrodes are 50 MQ. The cell is 50 MO // 500 pF.
A switch grounds the electrodes direcdy (BATH mode) or through the cell (CELL mode). Special plugs
connect directly to the headstages.
GROUNDING
Signal ground is isolated from the chassis and power ground.
CONTROL INPUTS
Above 3 V is accepted as logic HIGH. Below 2 V is accepted as logic LOW. Inputs are protected to
±15 V.
HEADSTAGE DIMENSIONS
Case is 2.25 x 1.14 x 0.87" (57.2 x 29.0 x 22.1 mm). Mounting rod is 4" (102 mm) long. Available
mounting rod diameters are 1/4, 5/16 or 3/8" (6.3, 7.0 or 9.5 mm). Specify required mounting rod
diameter with order. Input sockets for die microelectrode, shield and ground are 0.08" (2 mm) diameter.
Cable lengdi is 10 feet (3 m).
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 97

SPECffiCATiONS Page 87
CASE DIMENSIONS
T {Vll mm) high, 19" (483 mm) wide, 12.5" (317 mm) deep. Mounts in standard 19" rack. Handles
are included. N^ weight 18 lbs (8 kgs).
SUPPLY REQUIREMENTS
Une voltage: 100-125 Vac or 200-250 Vac. User selectable by an intemal switch.
Line Frequency: 50-60 Hz.
Power: 20 W.
Fuse:
0.5 A slow. S x 20 mm.
Line Filter: RFI filter is included.
Line Cord: Shielded line cord is provided.
ACCESSORIES PROVIDED
Operator's & Service Manuals
2 mm plugs for use with headstages
Low-capadtance test resistor for each headstage.
Spare globes for Mode switches
Spare fuse
Footswitches to operate Buzz of bodi elecfrodes
Clamp-1 Model Cell
Remote Buzz Duration hand-held control
OPTIONAL ACCESSORIES (not required for normal operation)
HS-4 Relay-Switched Headstage.
Miniature relay inside headstage automatically bypasses the current-measuring resistor during twoelecfrode voltage clamp mode. In all odier modes HS-4 headstage behaves like an HS-2MG headstage widi
H = xl. Must be used in conjunction with a VG-2 virtual-ground headstage.
VG-2 virtual-ground headstage.
Measures total bath current. The virtual-ground output attenuation (VG) is available in three values
(specify with order): x 0.1, xl (standard), and xlO. The output (Ivin) is 10 -;- VG mV/nA.
ORDERING INFORMATION
When ordering please specify:
1.
Current gain (H) of two headstages provided
2.
Gain and type of any extra headstages
3.
Diameter (D) of headstage mounting rods.
Unless you specify otherwise the AXOCLAMP-2A will be supplied with one HS-2L xl and one
HS-2L X 0.1 headstage, each witii D= ^k^" (7.9 mm).
AXOCLAMP-2A THEORY
St.
OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 98

Page 88 sPEcmcATioNS
CThis page is intentionally left blank)
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 99

REFERENCES
A-1
REFERENCES
Brennecke, R. & Lindemann, B. (1974). Theory of a membrane voltage clamp with discontinuous
feedback dirough a pulsed current clamp. Rev. Sci. Instmm. 45, 184-188.
Finkel, A. S. & Gage, P. W. (1985). Conventional voltage clamping with two infracellular
microelectrodes. In Voltage Clamping widi Microelectrodes, ed. T. G. Smidi, et al, Williams &
Wilkins: Baltimore.
Finkel A. S. & Redman, S. J. (1985). Optimal voltage clamping widi a single microelectrode. In
Voltage Clamping with Microelecfrodes, ed. T. G. Smith, et al, Williams & Wilkins: Baltimore.
Finkel, A. S. & Redman S. J. (1983). A shielded microelectrode suitable for single-elecfrode voltage
clampingof neurons in the CNS. J. Neurosci. Meths. 9, 23-29.
Hamill, O.P., Marty, A., Sakmann, B. & Sigworth, F. J. (1981). Improved patch-clamp techniques
for high-resolution current recording from cells and cell-free membranes patches. Pflugers Arch. 391,
85-100.
Johnston, D. & Brown, T. H. (1983). Interpretation of voltage-clamp measurements in hippocampal
neurons.
J. Neurophysiol. SO, 464^86.
j^Purves,
Academic Press. -^ =^ ^
Sachs,
3,
Schwartz, T. I & House, Randall C. (1970). A small-tipped microelectrode designed to minimize
c^acitive artifacts during the passage of current through die badi. Rev. Sci. Inst. 41, 515-517.
Suzuki, K., Rohligek, V. & Frbmter, E. (1978). A quasi-totally shielded, low-capacitance glassmicroelectrode with suitable amplifiers for high-frequency intracellular potential and impedance
measurements. Pflugers Arch. 378, 141-148.
R.D. (1981). Microelectrode Methods for Intracellular Recording and Ionophoresis. London: J, s^
F. & McGarrigle, R. (1980). An almost completely shielded microelecfrode. J. Neurosci. Meths.
151-157.
Wilson, W. A. & Goldner, M. M. (1975). Voltage clamping with a single microelectrode. J. Neurobiol.
6,411-422
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
Page 100

A-2 REFERENCES
CThis page is intentionally left blank)
AXOCLAMP-2A THEORY & OPERATION, COPYRIGHT FEBRUARY 1990, AXON INSTRUMENTS, INC.
 Loading...
Loading...