auris Monarch Platform User Manual

MONARCH™ PLATFORM
User Manual
300-002547-00 rev7

300-002547-00 rev7

Monarch™ Platform
User Manual
Date of Last Revision: 2018-05-08
300-002547-00 rev7

Auris Health, Inc. Headquarters and Manufacturing
150 Shoreline Drive
Redwood City, CA 94065
Tel: +1.650.264.6000
Manufacturer Responsible for Placing Products on the Market
Auris Health, Inc.
150 Shoreline Drive
Redwood City, CA 94065
Tel: +1.650.264.6000
Customer Support
Only qualified service personnel should service or maintain hardware components. If you
feel that the Monarch Platform hardware components or associated features or functions
do not perform as expected, or they provide results that are inconsistent with your
established clinical and research protocols, contact Auris Customer Care at
+1.800.434.0032 (toll-free within the United States) or +1.650.264.6000 (Worldwide).
You can also email CustomerCare@AurisHealth.com.
Copyright © 2016-2018 Auris Health, Inc. All rights reserved.
This document, the software and products to which this document refers, and any other
related materials are the copyrighted and proprietary information of Auris Health, Inc.
and may not be used or distributed without written authorization of Auris Health, Inc. No
part of this document may be photocopied, reproduced, or translated into another
language without written permission from Auris Health, Inc.
AURIS, MONARCH, MONARCH READY, and MONARCH ELEVATE are trademarks
and/or registered trademarks of Auris Health, Inc. in the United States and in other
countries and may not be used or distributed without written authorization from Auris
Health, Inc. Use of Auris Health, Inc.’s trademarks require written authorization from
Auris Health, Inc. The following logos are trademarks of Auris Health, Inc.:
ii 300-002547-00 rev7

All other trademarks and product names used herein are the property of their respective
owners. Monarch™ Pre-Op Planning Application and Monarch™ Navigation Application
are distributed on Auris Health, Inc. hardware.
Auris Health, Inc. provides this guide "as is" and "as available" without any warranties,
including, without limitation, warranties of merchantability, fitness for any particular
purpose, title and noninfringement. Auris Health, Inc. expressly excludes and disclaims
such warranties.
To the maximum extent permitted by law, Auris, its suppliers, affiliates, officers,
directors, employees, agents, and assigns will not be liable: (a) for costs of procurement
of substitute goods, technology, or services, lost profits or business opportunities or any
other special, indirect, incidental, consequential or reliance damages arising from any
claim related to this manual or use of Auris’s products discussed herein, however
caused and under any theory of liability, whether based in contract, tort (including
negligence and strict liability) or otherwise; (b) for content or data that is transmitted over
or through Auris’s networks, equipment or systems; (c) for any unavailability, defect,
error, interruption, delay, downtime, loss, or attenuation of services caused by or
resulting from any act or omission by the user of Auris’s products and services; or (d) for
injury, death, or harm of any nature (whether direct or indirect, and whether or not
foreseeable) resulting from (i) materials, equipment or accessories not furnished by
Auris, (ii) lost components, parts, and accessories, (iii) any product which has been
modified, altered, repaired, serviced, or reinstalled by anyone other than Auris or its
authorized representative, (iv) any product stored, used, or handled in any manner
inconsistent with Auris’s trainings and written instructions, or by individuals who have not
been adequately trained on the proper use of the product, (v) any product damaged due
to accident, negligence, misuse, or abnormal use, (vi) any product damaged through no
fault of Auris during shipment, or (vii) damage or failure of a product for which Auris
provided an update or replacement and the damage or failure would not have occurred
but for user’s failure to install such update or replacement (failure to install includes
failure to allow Auris to remotely install security patches and provide preventive and
remedial diagnostic services by connecting the Monarch System to Auris’s proprietary
cloud platform over the internet).
Unauthorized modifications of any Auris products may void any and all warranties. Auris
Health, Inc. does not assume any responsibility or liability with respect to unauthorized
modification or substitution of subsystems or components.
This manual applies to the Monarch™ Platform, Monarch™ Pre-Op Planning Application
2.1.1, and Monarch™ Navigation Application 2.1.1.
Expected Life Statement
With proper care and maintenance, the expected service life of the system is 7 years.
300-002547-00 rev7 iii

Introduction
This manual provides information specific to the Monarch™ Platform, also known as the
Monarch™ Platform. The operating instructions and feature descriptions herein are
specific to the software versions listed on page iii.
NOTE: Anyone who operates, services, maintains, or is otherwise associated with the
Monarch Platform must read, understand, and be thoroughly familiar with the
information in this manual, and take precautions to protect themselves, their
associates, patients, and the equipment. At each step in the installation, specific
warnings and cautions are given for specific actions.
Auris Health, Inc. reserves the right to revise this publication and to make changes in
content from time to time without obligation on the part of Auris Health, Inc. to provide
notification of such revision or change.
Intended Use/Indications for Use
The Monarch™ Endoscopy Platform (Monarch Platform) and its accessories are
intended to provide bronchoscopic visualization of and access to patient airways for
diagnostic and therapeutic procedures.
Contraindications
Contraindications include but are not limited to:
Patient whose general health or respiratory function or both are compromised to the
point that the patient would not tolerate flexible bronchoscopy.
Absence of a trained bronchoscopist to perform or closely and directly supervise the
procedure, as well as manage complications common to flexible bronchoscopy.
Use of the system in patients with electrically or magnetically activated implanted
medical devices.
Warnings
A thorough understanding of the technical principles, clinical applications, and risks
associated with pulmonary bronchoscopy is necessary before using this device.
Additional warnings are detailed throughout this document to describe actions or
conditions that could result in injury or death.
Adverse Effects
Complications from bronchoscopy are rare and most often minor, but if they occur, may
include breathing difficulty, vocal cord spasm, hoarseness, slight fever, vomiting,
dizziness, bronchial spasm, infection, low blood oxygen, bleeding from biopsied site, or
an allergic reaction to medications. Only rarely do patients experience other more
iv 300-002547-00 rev7

serious complications (for example, collapsed lung, respiratory failure, heart attack
and/or cardiac arrhythmia).
Prescription Device Statement
CAUTION: Federal law restricts this device to sale by or on the order of a physician.
Safety Precautions and Warnings
Safe operation of the Monarch Platform requires careful attention to the serious hazards
associated with use of the device and ways to avoid or minimize the hazards, and
familiarity with emergency procedures. Untrained or careless operation of the Monarch
Platform can damage the system, its components or other property; cause poor
performance; or lead to serious bodily injury and possibly death.
Auris Health, Inc. strongly recommends that personnel be trained by Auris on the
Monarch Platform prior to use for research or clinical purposes. Only physicians having
adequate training and experience with endoscopic techniques should perform
endoscopic procedures.
Users must follow all instructions for use supplied with the system, its components,
instruments and accessories, including any instructions for use (IFUs) provided with
instruments or accessories. The IFU provided are the primary sources for detailed safety
information.
Under the conditions described in the declaration of immunity section, the system may
fault in an immobilized state. Follow the directions indicated to return to normal
operation.
System Error Messages
Under the error conditions described in Appendix B, all robotic functions are disabled.
Follow the directions indicated to return to normal operation.
Notations
This manual uses the special notations below to bring your attention to important
information.
WARNING: Describes actions or conditions that could result in injury or death.
CAUTION: Describes actions or conditions that could result in damage to the
equipment or minor harm to the user or patient.
NOTE: Provides more information about a subject.
300-002547-00 rev7 v
Disposal
When an Auris product reaches the end of its useful life and your facility desires to
remove the device, contact Auris Customer Care at +1.800.434.0032 (toll-free within the
United States) or +1.650.264.6000 (Worldwide) to uninstall and appropriately dispose of
the components.
When disposing of instruments, accessories, or any of their components, follow all
applicable national and local laws and guidelines.
Regulatory Compliance with Directives and Standards
The Monarch Platform complies with the regulatory requirements of Directive 2017/745,
the Medical Device Directive of the European Economic Community (EEC).
The Monarch Platform and accessories have been tested for compliance to the following
standards:
Standard Number Standard Title
AAMI/ANSI ES60601-1:2005 (Third
Edition)
EN 60601-1-2:2007 (Third Edition)
IEC 60601-1-6:2010 (Third Edition) Medical electrical equipment – Part 1-6: General
IEC 60601-2-18:2009 (Third Edition)
IEC 62366:2007
IEC 62366-1:2015 Medical devices -- Part 1: Application of usability
ISO 15223-1:2016
ISO 14971:2007 Medical devices -- Application of risk management to
Medical electrical equipment - Part 1: General
requirements for basic safety and essential performance
Medical electrical equipment - Part 1-2: General
requirements for basic safety and essential performance –
Collateral standard: Electromagnetic compatibility –
Requirements and tests
requirements for basic safety and essential performance –
Collateral standard: Usability
Medical electrical equipment - Part 2-18: Particular
requirements for the basic safety and essential
performance of endoscopic equipment
Medical devices -- Application of usability engineering to
medical devices
engineering to medical devices
Medical devices -- Symbols to be used with medical device
labels, labelling and information to be supplied -- Part 1:
General requirements
medical devices
ISO 11135:2014
ISO 11607-1:2006 Packaging for terminally sterilized medical devices – Part
vi 300-002547-00 rev7
Sterilization of health-care products – Ethylene Oxide –
Requirements for the development, validation, and routine
control of a sterilization process for medical devices
1: Requirements for materials, sterile barrier systems and
packaging systems
Standard Number Standard Title
ISO 11607-2:2006 Packaging for terminally sterilized medical devices – Part
2: Validation requirements for forming, sealing and
assembly processes
ISO 10993-5:2009
ISO 10993-10:2010 Biological evaluation of medical devices -- Part 10: Tests
ISO 10993-11:2006 Biological evaluation of medical devices – Part 11: Tests
ISO 8600-1:2015
ISO 8600-3:1997 Optics and optical instruments -- Medical endoscopes and
ISO 8600-4:2014
BS EN 1041:2008 Information supplied by a manufacturer of medical devices
BS EN 556-1:2001 Sterilization of medical devices - Requirements for medical
ANSI/AAMI/ISO 14937:2009 Sterilization of health care products -- General
Biological evaluation of medical devices -- Part 5: Tests for
in vitro cytotoxicity (Biocompatibility)
for irritation and skin sensitization (Biocompatibility)
for systemic toxicity (Biocompatibility)
Endoscopes -- Medical endoscopes and endotherapy
devices -- Part 1: General requirements
endoscopic accessories -- Part 3: Determination of field of
view and direction of view of endoscopes with optics
Endoscopes -- Medical endoscopes and endotherapy
devices -- Part 4: Determination of maximum width of
insertion portion
devices to be designated ""STERILE"" – Part 1:
Requirements for terminally sterilized medical devices"
requirements for characterization of a sterilizing agent and
the development, validation and routine control of a
sterilization process for medical devices
CSA 22.2 NO 60601-1:14 Medical Electrical Equipment, Part 1: General
requirements for basic safety and essential performance
FCC ID: 2AOXMMonarch
NOTE: This equipment has been tested and found to comply with the limits for a Class
A digital device, pursuant to part 15 of the FCC Rules. These limits are designed to
provide reasonable protection against harmful interference when the equipment is
operated in a commercial environment. This equipment generates, uses, and can
radiate radio frequency energy and, if not installed and used in accordance with the
instruction manual, may cause harmful interference to radio communications.
Operation of this equipment in a residential area is likely to cause harmful interference
in which case the user will be required to correct the interference at his own expense.
300-002547-00 rev7 vii

viii 300-002547-00 rev7

300-002547-00 rev7 ix

Contents
Monarch Platform Device Overview ............................................................................ 2
System Overview ........................................................................................................ 3
Pre-Procedural Planning ....................................................................................... 3
Monarch Cart ......................................................................................................... 3
Monarch Tower ..................................................................................................... 7
Monarch Bronchoscope System ........................................................................... 9
Working Channel Instruments ............................................................................. 10
Accessories ......................................................................................................... 10
Classifications ........................................................................................................... 11
Monarch Platform Labels .......................................................................................... 12
Label Locations ................................................................................................... 15
............................................................. 1
Technical Specifications ............................................................................................ 16
Physical Dimensions, Weight, and Power Requirements .................................... 16
Declaration of Emissions ........................................................................................... 17
Declaration of Immunity ............................................................................................. 19
Original Documentation ............................................................................................. 22
................................................................................................ 23
Recommended CT Scan and Reconstruction Parameters ....................................... 24
Monarch Pre-Op Planning Application User Interface ............................................... 26
Patient List ........................................................................................................... 26
Planning .............................................................................................................. 29
Manual Path ........................................................................................................ 32
Before You Can Plan ................................................................................................. 33
Create a Plan ............................................................................................................ 33
Identify a Target .................................................................................................. 35
Export a Patient Case ............................................................................................... 37
Import a Patient Case ................................................................................................ 38
x 300-002547-00 rev7

Perform an Unplanned Case ..................................................................................... 38
............................................................................................................ 39
Software Information ................................................................................................. 40
Suggested Bronchoscopy Suite Configuration .......................................................... 40
Prepare the Bronchoscopy Suite ............................................................................... 41
Prepare the Patient ................................................................................................... 41
Prepare the Monarch Bronchoscope System ............................................................ 43
Monarch Tower System Setup Guidance .................................................................. 44
Monarch Controller .............................................................................................. 44
Fluidics System ................................................................................................... 45
Camera ................................................................................................................ 46
Navigation ........................................................................................................... 46
Monarch Cart System Setup Guidance ..................................................................... 48
Unstow Cart ......................................................................................................... 48
Load the Monarch Bronchoscope System ........................................................... 51
............................................................................................ 53
Overview ................................................................................................................... 54
Functional Descriptions of Monarch Components ..................................................... 54
Monarch Bronchoscope and Monarch Bronchoscope Sheath ............................ 54
Monarch Controller .............................................................................................. 55
Working Channel Instruments ................................................................................... 57
Monarch Navigation Application User Interface ........................................................ 58
Top Navigation Bar .............................................................................................. 60
Primary Area ....................................................................................................... 60
Scrolling Area ...................................................................................................... 61
CT Area ............................................................................................................... 62
Full Screen Mode ................................................................................................ 63
Menu System ...................................................................................................... 63
Navigation Views ................................................................................................. 65
Configuring View Screens ................................................................................... 75
Quick Action Button ............................................................................................. 77
300-002547-00 rev7 xi

Procedural Steps or Actions ...................................................................................... 78
Scope Insertion ................................................................................................... 78
Navigation Initialization ........................................................................................ 79
Monarch Bronchoscope Advancement ............................................................... 79
Perform Biopsy .......................................................................................................... 80
Monarch Controller .............................................................................................. 80
Post-procedure .......................................................................................................... 81
Retraction ............................................................................................................ 81
........................................................................ 83
Prepare the Patient for Discharge ............................................................................. 84
Remove the Monarch Bronchoscope System ..................................................... 84
Remove Monarch Navigation Patient Sensors .................................................... 84
Remove the Bronchoscope Patient Introducer .................................................... 84
Remove and Clean Equipment ................................................................................. 84
Disassemble and Clean the Monarch Bronchoscope System ............................. 84
Clean Monarch Navigation Patient Sensors ........................................................ 85
Disassemble and Clean the Monarch Navigation Field Generator ...................... 86
Disassemble and Clean Bronchoscope Patient Introducer Mount ...................... 86
Clean and Store the Monarch Cart ...................................................................... 86
Clean the Monarch Tower and Store the Monarch Platform ............................... 86
Servicing .................................................................................................................... 87
Preventative Maintenance ................................................................................... 87
Configuration ....................................................................................................... 87
Maintenance ........................................................................................................ 87
............................................................................... 91
User Administration ................................................................................................... 92
User Types .......................................................................................................... 92
Add New User ..................................................................................................... 92
Change User Password ...................................................................................... 93
Disable Existing User .......................................................................................... 94
xii 300-002547-00 rev7

System Security ........................................................................................................ 95
Virus Protection ................................................................................................... 95
Patient Data Security ........................................................................................... 95
Networking ................................................................................................................ 95
Firewall ................................................................................................................ 95
Internet Connectivity ............................................................................................ 95
...................................................................................................... 97
Fault Messages and Troubleshooting ....................................................................... 98
Emergency Removal ........................................................................................... 98
E-Stop Recovery ................................................................................................. 98
Joystick Articulations Not Matching Directionally ................................................ 98
Controller Does Not Appear Functional ............................................................... 98
Field Generator or Patient Introducer Mount Clamps Do Not Tighten ................. 99
Monarch Platform Will Not Turn On..................................................................... 99
Tower and Cart Do Not Turn On Simultaneously ................................................ 99
Video (Start Screen) Does Not Appear on Tower and Cart Monitor After System
Turns On ........................................................................................................... 100
Loss of Video on Tower or Cart Touchscreen (During Procedure) ................... 100
Navigation Patient Sensors Not Detected During Setup ................................... 100
CT Scan or Pre-Planned Case Cannot be Uploaded to Tower ......................... 101
Keyboard on Planning Laptop Is Unresponsive ................................................ 101
Planning Laptop Will Not Turn on ...................................................................... 101
Unable to log on to Physician User Account on Tower or Planning Laptop ...... 101
Unable to log on to Admin User Account on Tower or Planning Laptop ........... 101
................................................................................................................................ 103
Errors and Messages ................................................................................................................ 103
System Messages ................................................................................................... 104
300-002547-00 rev7 xiii

xiv 300-002547-00 rev7

Safety Information and Technical
Description
This chapter provides information that is essential for the safe operation, transport, and
storage of the Monarch Platform.
Monarch Platform Device Overview ............................................................................ 2
System Overview ........................................................................................................ 3
Classifications ........................................................................................................... 11
Monarch Platform Labels .......................................................................................... 12
Technical Specifications ............................................................................................ 16
Declaration of Emissions ........................................................................................... 17
Declaration of Immunity ............................................................................................. 19
Original Documentation ............................................................................................. 22
CAUTION: Do not modify this equipment. Modification of this equipment may result in
injury to users or patients and may void product warranties.
Installation of this system is to be performed only by an authorized Auris representative.
There are no user serviceable components. Contact Auris Customer Care at
+1.800.434.0032 (toll-free within the United States) or +1.650.264.6000 (Worldwide) to
schedule installation or service.
300-002547-00 rev7
Monarch Platform Device Overview
This user manual refers to the Monarch Platform and the associated components
used with it. Refer to the following table for a list of products.
Official Product Name Shortened Name
Monarch™ Platform
Monarch™ Pre-Op Planning Application Planning Application
Monarch™ Pre-Op Planning System Planning System
Monarch™ Tower Tower
Monarch™ Cart Cart
Monarch™ Controller Controller
Monarch™ Touchscreen Touchscreen
Monarch™ Navigation Application Navigation App
Monarch™ Bronchoscope System Bronchoscope System (consists of both the
Bronchoscope and Sheath)
Monarch™ Bronchoscope Bronchoscope
Monarch™ Bronchoscope Sheath Sheath
Aspirating Biopsy Needle Needle
Bronchoscope Patient Introducer Mount Patient Introducer Mount
Bronchoscope Patient Introducer Patient Introducer
Monarch™ Navigation Field Generator Field Generator
Monarch™ Navigation Field Generator Mount Field Generator Mount
Monarch™ Navigation Patient Sensors Patient Sensors
Navigation Patient Patches Patient Patches
Bronchoscope Swivel Adapter Swivel Adapter
Bronchoscope Fluidics Tubing Fluidics Tubing
Bronchoscope Sheath Valve Sheath Valve
Instrument Device Manipulators IDM
2 300-002547-00 rev7

System Overview
The Monarch Platform enables electro-mechanical articulation and precise
control of a flexible endoscope (bronchoscope) under continuous and direct
control by a physician operator. The Monarch Platform includes fused navigation
that integrates a pre-operative computed tomography scan into an intra-operative
interface displaying endoscope tip location relative to the pre-operative scan
anatomy.
Pre-Procedural Planning
Prior to the procedure, the Monarch Pre-Op Planning Application running on the
Monarch Pre-Op Planning System or Monarch Tower Touchscreen enables a
physician to review a pre-procedural CT scan and plan a pathway through the
airways for a physician-controlled bronchoscope to navigate towards the target of
interest. During the procedure, the software integrates a pre-procedural CT scan
into a user interface.
The Monarch Pre-Op Planning Application provides the following features:
• CT viewing capabilities similar to standard CT DICOM viewer software.
• Airway segmentation: segmentation of the trachea and main airways to aid
• Target identification: ability to select one or more targets of interest.
• Path planning: computer generated path to the target based on segmented
• Manual path planning: ability for user to identify airways and modify the path
visualization of the airways and path planning.
airways.
on the CT scan to finalize a pathway to the target as necessary.
Monarch Pre-Op Planning Application is accessible from both the Monarch
Tower Touchscreen and the Monarch Pre-Op Planning System.
Monarch Cart
The Monarch Cart is a maneuverable piece of capital equipment that can be
transported in and out of the bronchoscopy suite, stowed away when not in use,
and positioned relative to the patient table as needed for a given procedure. It is
used to transmit physician controls to the Monarch Bronchoscope System
(insertion, retraction, and articulation). It contains a Touchscreen monitor, two
robotic arms, and mating robotic Instrument Device Manipulators (IDM) which are
mountable, robotic interfaces for the bronchoscope.
300-002547-00 rev7 3

The following image shows the Monarch Cart with the robotic arms in the stow
position (left) and load Bronchoscope System position (right):
The Monarch Cart is a carrier for the robot arms. It includes two robotic arms
which contain rotary pulleys to actuate the drive cables in the Monarch
Bronchoscope System. The cart houses the electronic systems required to power
and operate the robot arms.
4 300-002547-00 rev7
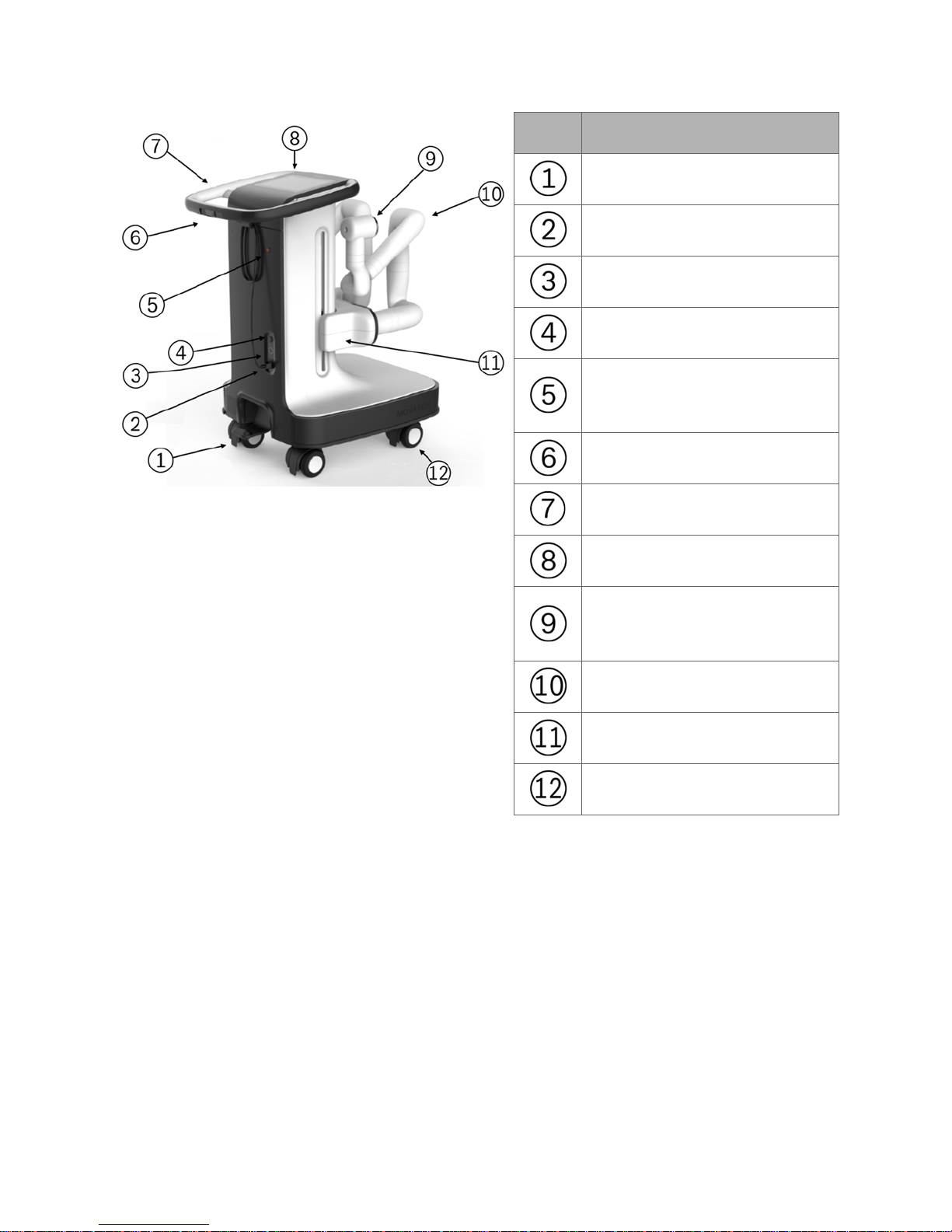
Item Description
Foot pedal
Power cable
Umbilical port
Power button
Emergency stop button
(E-stop)
Cart directional lock switch
Cart handle
Cart Touchscreen
Instrument Device Manipulator
(IDM)
Robotic arm
Vertical lift
Immobilization feet (not shown)
300-002547-00 rev7 5
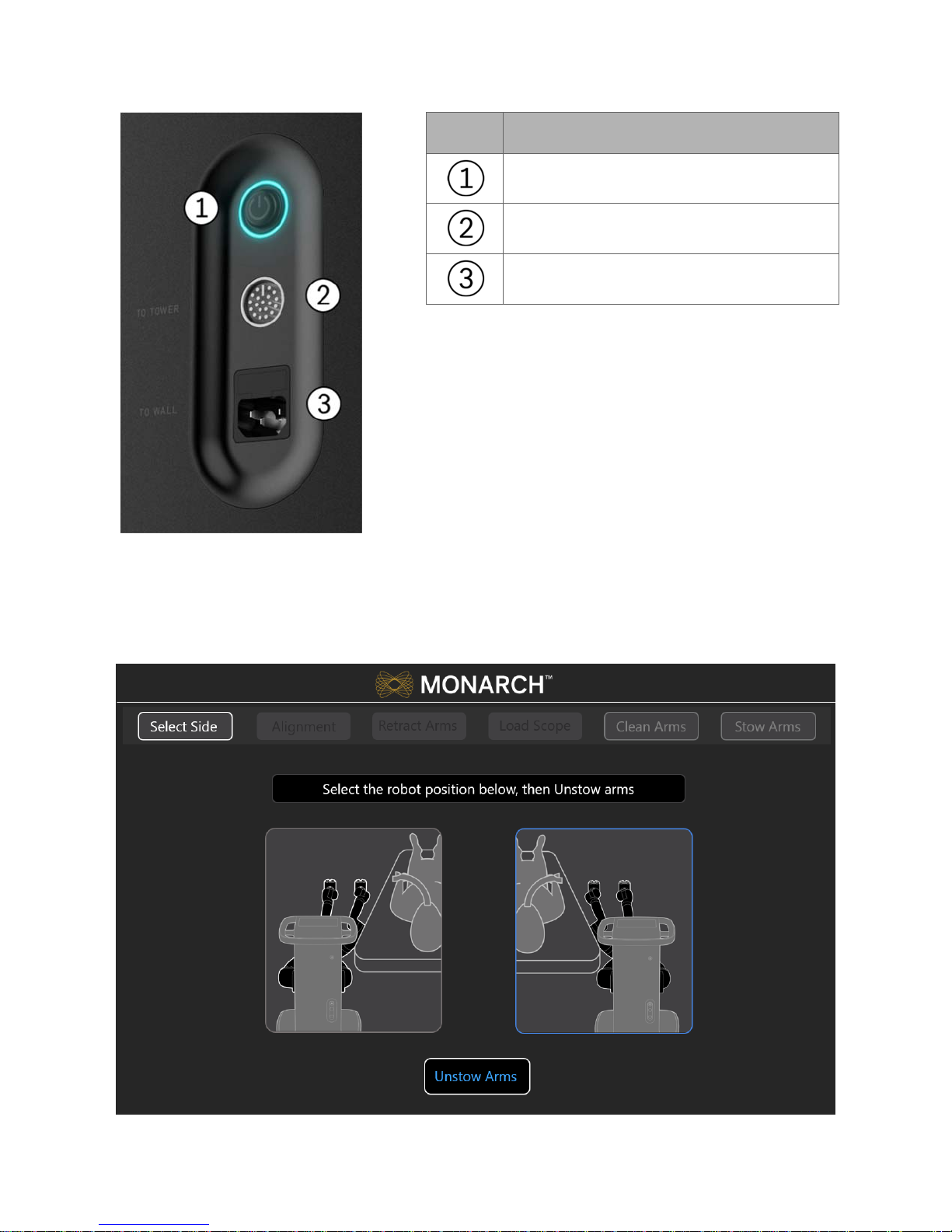
Item Description
Power button
Umbilical port to the Monarch Tower
Power cord plug
Automated lift controls will raise and lower the height of the robotic arms based
on your control. The cart handle allows the cart to be directionally locked for ease
of maneuverability. An embedded Touchscreen on the cart handle provides
system setup instructions and allows user input, as shown in the following image.
6 300-002547-00 rev7
Monarch Tower
The Monarch Tower is a maneuverable piece of capital equipment that can be
transported in and out of the bronchoscopy suite and stowed away when not in
use. It is the primary physician procedural display interface. It contains a monitor
for physician viewing and computers running the Monarch Bronchoscopy Tower
Software, the Monarch Navigation Application, which displays real time video
captured from the Monarch Bronchoscope camera overlaid with information on
the status of the Monarch Platform and the Monarch Pre-Op Planning
Application. The tower also provides connectivity for the Monarch Bronchoscope
camera and lighting, as well as the fluidics system. The fluidics system has an
integrated pump and pinch valve to control irrigation and aspiration respectively.
The monitor is Touchscreen for physician input during setup, planning, and
procedure.
The following images shows the tower:
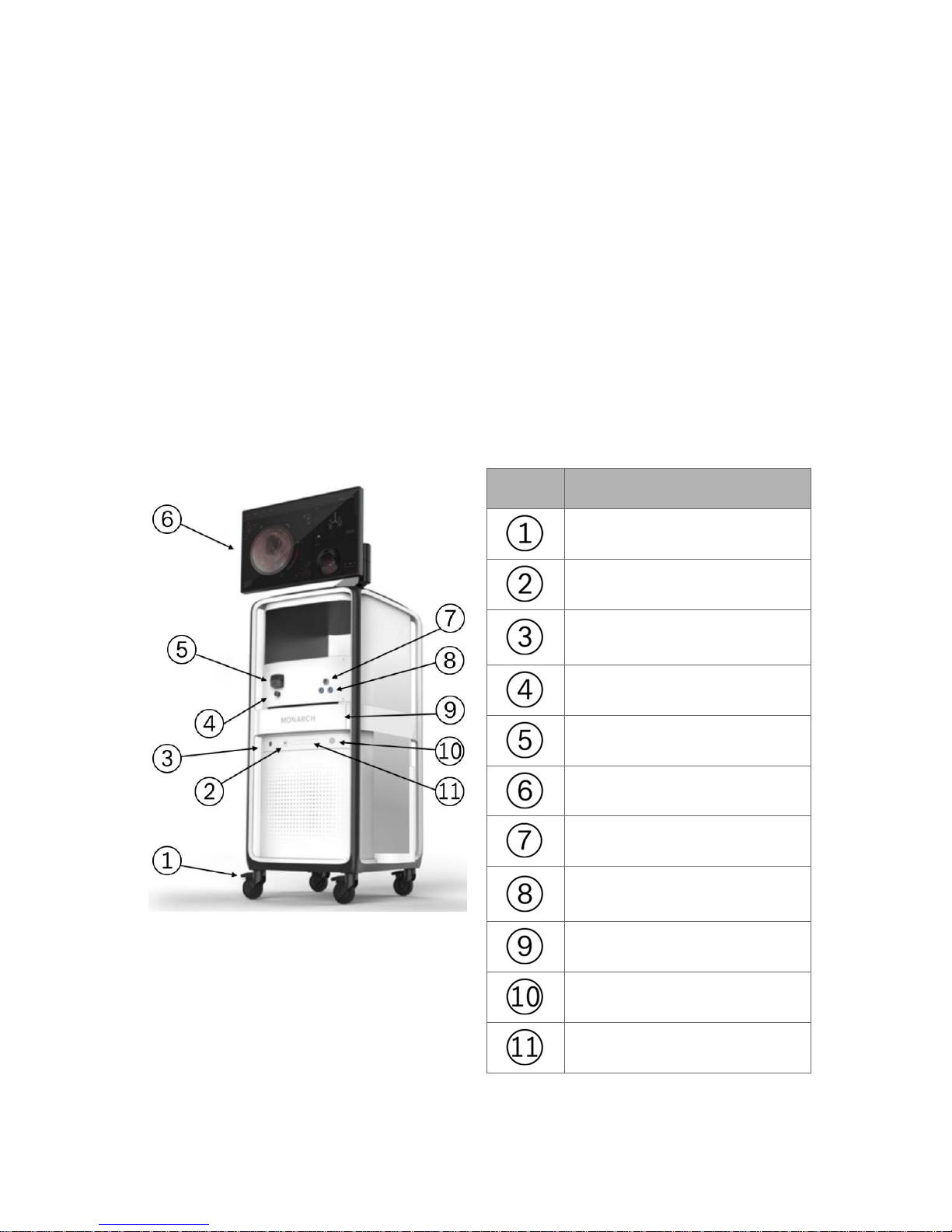
Item Description
Caster lock
USB port
Monarch Navigation Field
Generator port
Pinch valve
Peristaltic pump
Monarch Touchscreen
Bronchoscope umbilical port
Monarch Navigation Patient
Sensors port
Drawer
Power button
Optical disc drive (DVD)
300-002547-00 rev7 7
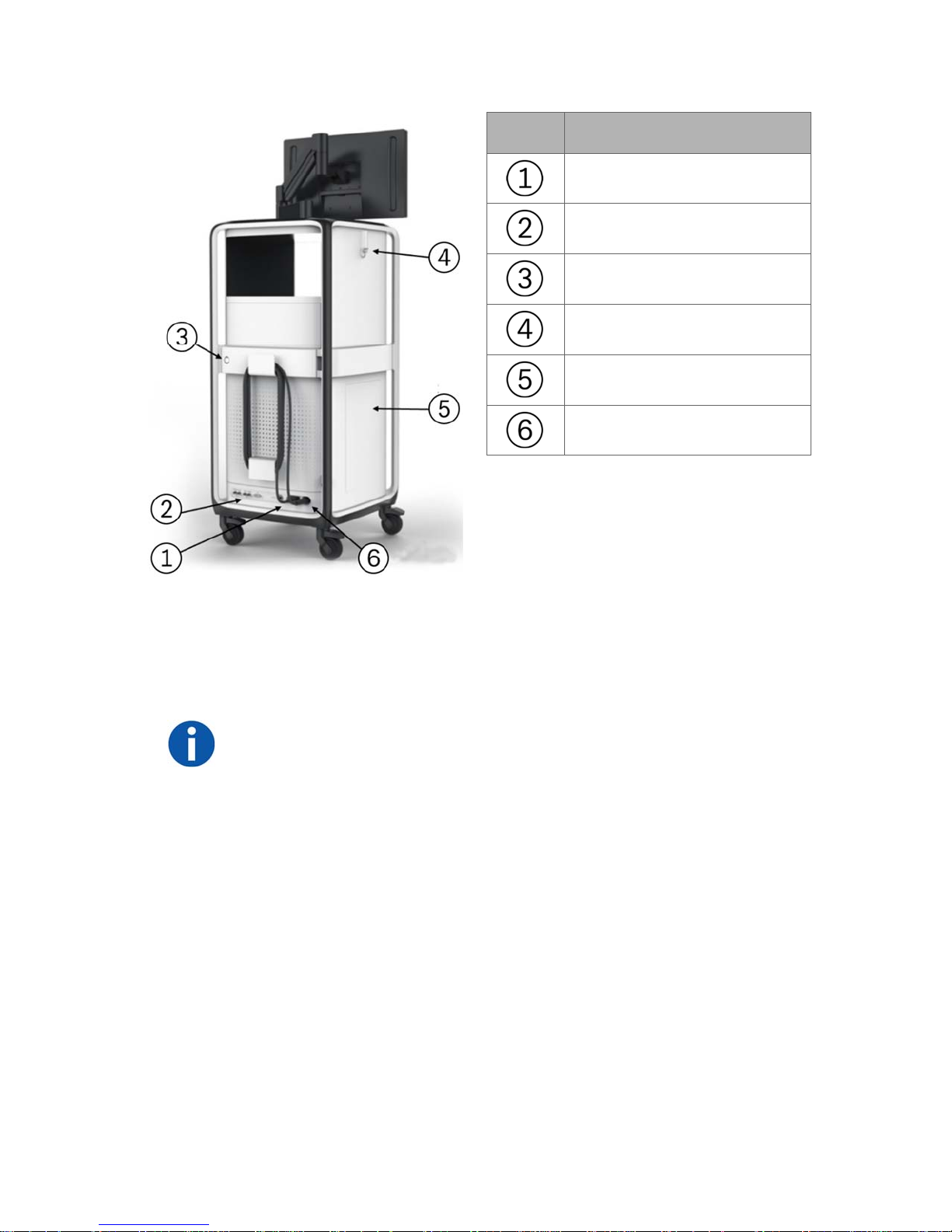
Item Description
Power cable
Video ports
Service door lock
Hook
Side storage door
Umbilical cable
The following components are included in the Monarch Tower: non-real-time
computer, real-time computer, endoscopic controller, camera, Monarch
Navigation Field Generator and power distribution unit (PDU) box. The PDU
contains the power supply to the tower only, as well as the batteries and UPS
which provide auxiliary power in the event of system fault.
NOTE: The Monarch Platform incorporates an uninterruptible power supply
backup. In the event of a power outage, the backup supply provides a five-minute
operating window, which allows the operator to safely remove the Monarch
Bronchoscope System and shut down the Monarch Platform.
The tower includes the Monarch Controller that allows the clinician to control the
system during a procedure. Two joysticks are used to drive and articulate the
Monarch Bronchoscope while various buttons are used to control irrigation,
aspiration, Tower user interface, light, bronchoscope/sheath selection, and image
capture.
8 300-002547-00 rev7
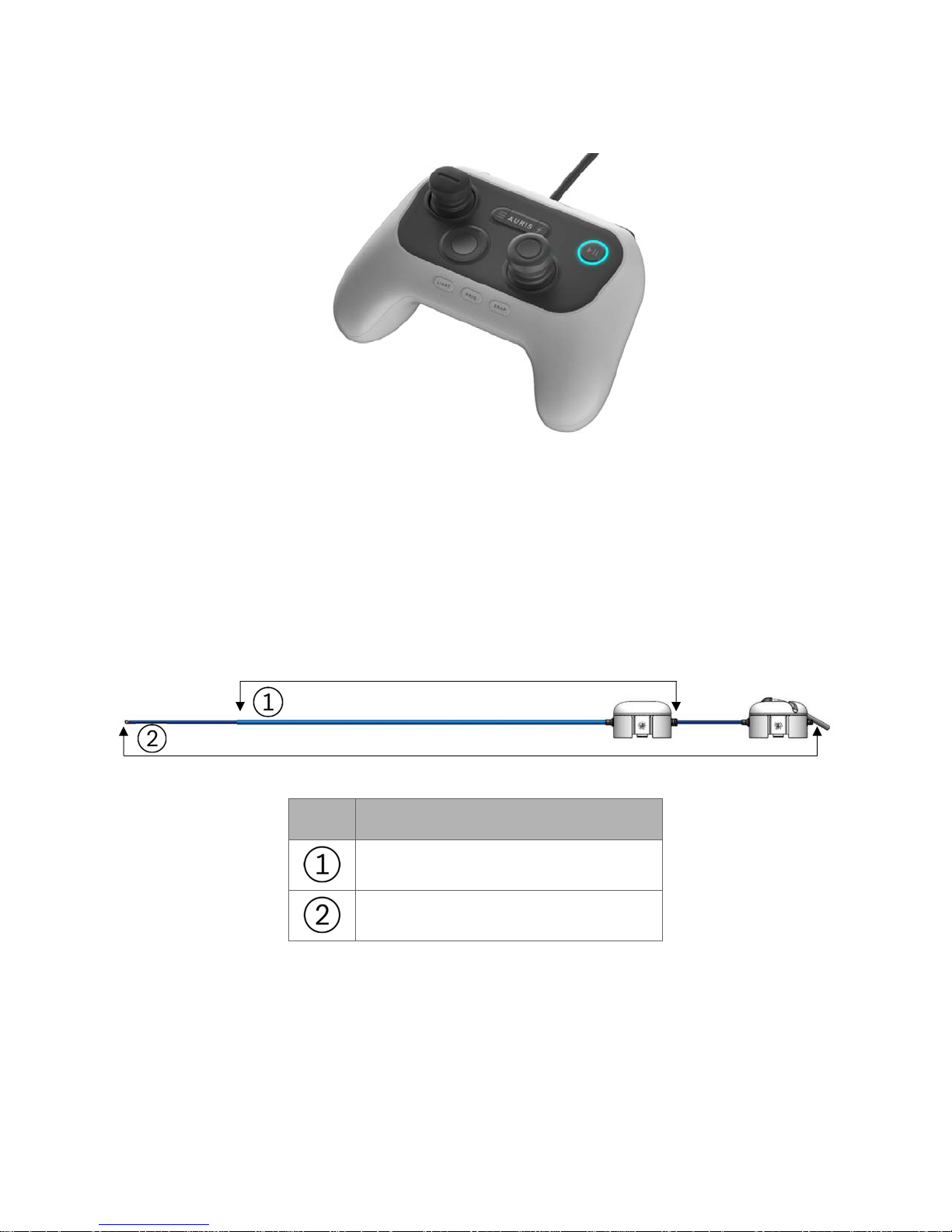
The following image shows the Monarch Controller:
Monarch Bronchoscope System
The Monarch Bronchoscope System is comprised of two collinear and concentric
devices, the inner Monarch Bronchoscope and the outer Monarch Bronchoscope
Sheath, both of which possess 4-way steering control. This configuration enables
the capability of telescoping, which enhances the Monarch Bronchoscope
System stability and access capability.
The following figure images show the Monarch Bronchoscope System and the
handles:
Item Equipment
Monarch Bronchoscope Sheath
Monarch Bronchoscope
The Monarch Bronchoscope includes a camera that provides the operative
perspective, an integrated light source in the handle, and a 2.1mm inner diameter
working channel for the passing of manually controlled tools.
The Monarch Bronchoscope System has a distal section capable of achieving
articulation in any direction and any combination of the two to enable precise
control while driving the Monarch Bronchoscope.
The Fluidics Tubing is equipped
300-002547-00 rev7 9
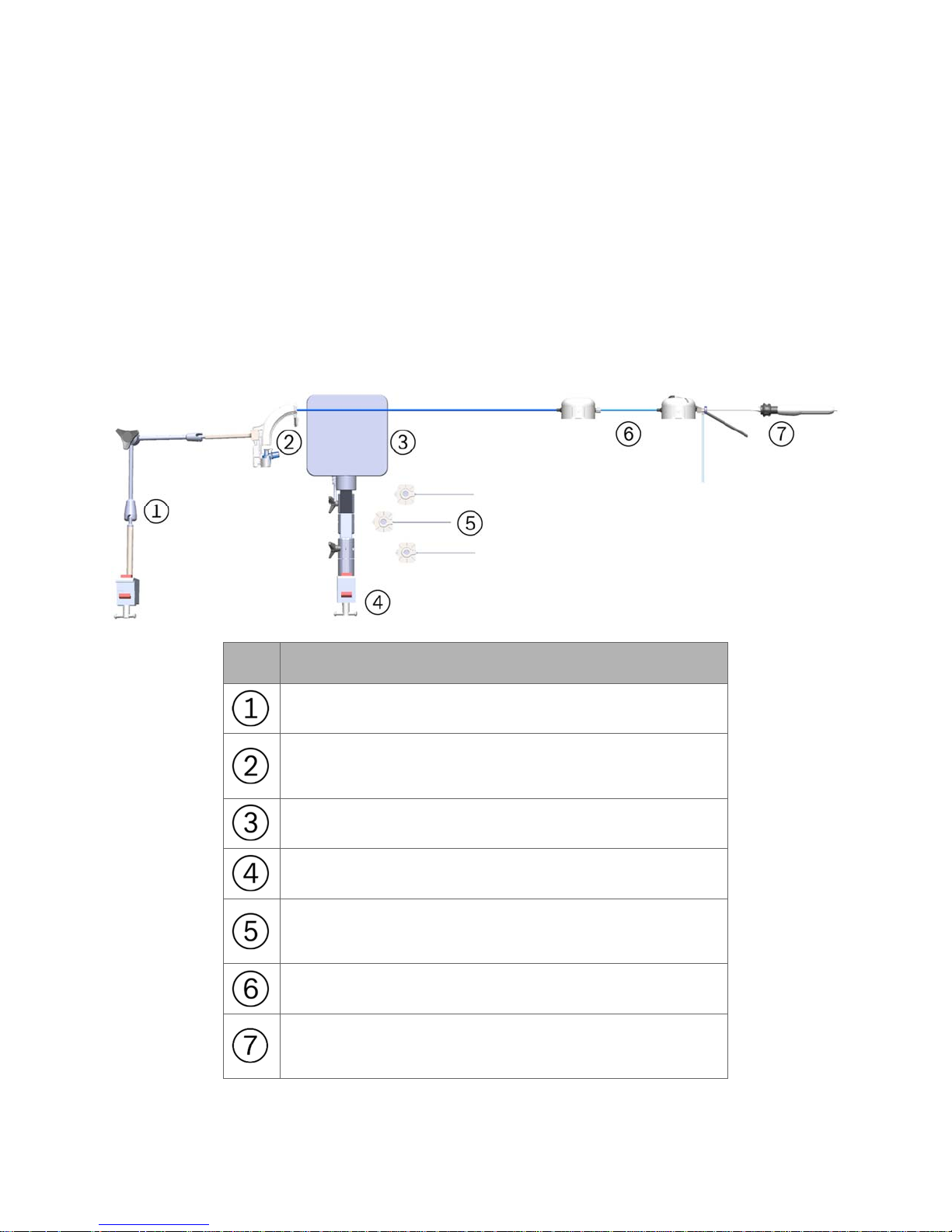
with a valve at the end
devices, such as the Aspirating Biopsy Needle. Additionally, the proximal section
routes irrigation and aspiration to the shared working channel.
to facilitate the insertion and sealing of various ancillary
Working Channel Instruments
The following single-use manually controlled Auris instruments compatible with
the Monarch Platform are provided sterilized: Aspirating Biopsy Needle, Biopsy
Forceps, and Cytology Brush. Refer to individual Instructions for Use included
with each working channel instrument for additional information.
Accessories
Item Equipment
Bronchoscope Patient Introducer Mount
Bronchoscope Patient Introducer with Bronchoscope
Swivel Adapter
Monarch Navigation Field Generator
Monarch Navigation Field Generator Mount
Monarch Navigation Patient Sensors and single-use
Navigation Patient Patches
Monarch Bronchoscope System and Sheath Valve
Working channel instrument (needle shown) inserted
through Fluidics Tubing valve
10 300-002547-00 rev7

Disposable Accessories
The system features sterilized single-use accessories necessary to perform
bronchoscopic procedures. These accessories are:
Bronchoscope Patient Introducer: A tube that guides the Bronchoscope
System through the Bronchoscope Swivel Adapter and into the endotracheal
tube.
Bronchoscope Swivel Adapter: Standard bronchoscope accessory that
attaches to the endotracheal tube to allow passage of a bronchoscope while
maintaining ventilation pressure. It is packaged with the Bronchoscope
Patient Introducer.
Bronchoscope Fluidics Tubing: Separate tubing lines for saline irrigation and
vacuum aspiration which converge at a valve that attaches to the
bronchoscope working channel. Saline irrigation and aspiration lines connect
to the tower. A valve is connected to the end of the Bronchoscope Fluidics
Tubing and provides a means of introducing working channel instruments,
irrigation, and aspiration into the working channel.
Bronchoscope Sheath Valve: Attaches to the proximal hub of the sheath and
provides a seal around the bronchoscope to reduce ventilation leakage.
Navigation Patient Patches: Single-use disposable adhesive patches that are
used to attach the Monarch Navigation Patient Sensors to the patient’s chest.
The patches are not provided sterile.
Non-Disposable Accessories
The following non-disposable accessories are included and stored with the tower.
Bronchoscope Patient Introducer Mount: Adjustable mount that attaches to a
bed rail and holds the Bronchoscope Patient Introducer.
Monarch Navigation Patient Sensors: Attaches to the patient to provide an
estimate of patient positioning for alignment of the Monarch Navigation Field
Generator.
Monarch Navigation Field Generator: Provides an electromagnetic field to be
sensed by scope and patient sensors to provide locational information. The
Monarch Navigation Field Generator plugs into the Monarch Tower.
Monarch Navigation Field Generator Mount: Adjustable mounts that attaches
to a bed rail and holds the Monarch Navigation Field Generator.
Classifications
According to Directive 2017/745, this product is a Class IIa Medical Device.
According to CISPR 11 (a publication of the IEC committee on radio
interference), this product is Group 1, Class A ISM Equipment.
300-002547-00 rev7 11

The Monarch Platform is classified by the following:
Protection against electric shock: Class 1 (grounded equipment).
Applied part(s): All Applied Parts of the Monarch System are Type BF Applied
Parts.
Protection against harmful ingress of water: Rated IPX0 (ordinary equipment,
not specially protected).
Methods of sterilization or disinfection: Ethylene oxide processing.
Mode of operation: The Monarch System is considered Continuous
equipment as defined by IEC 60601-1.
Monarch Platform Labels
Symbol Name Meaning
Type BF applied part
symbol is in accordance
with IEC 60601-1.
ON/OFF This indicates the power on state of the system.
Protective Earth This indicates a protective earth (grounding)
Manufacturer’s Serial
Number
Equipment Manufacturer
and date of Manufacture
Waste Electrical and
Electrical Equipment
Used to indicate Type BF certified components.
This power switch does not isolate the mains
supply.
terminal.
This symbol appears adjacent to the
manufacturer’s serial number.
This symbol appears adjacent to the name and
address of the equipment manufacturer.
This symbol indicates that this equipment has
been designed as electrical and electronic
equipment that is not to be disposed of as
unsorted municipal waste. EEE contains
substances that may present hazards to human
and to the environment. It must be recovered,
reused, recycled, or otherwise treated, and
properly disposed of.
12 300-002547-00 rev7
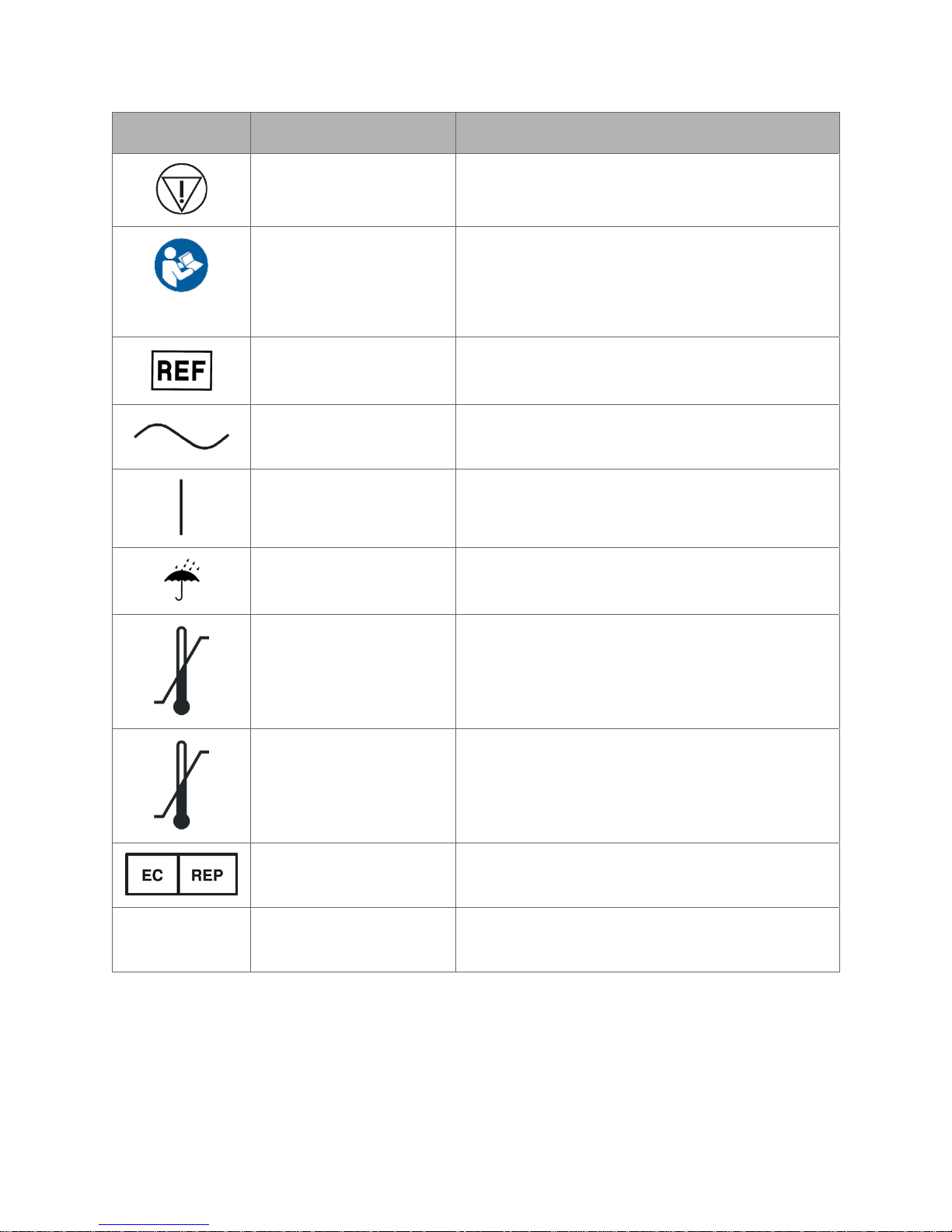
Symbol Name Meaning
Emergency Stop This symbol indicates an emergency stop
control device.
Read instructions prior
to use
This symbol is used to alert the user to refer to
the user manual or other instructions when
complete information cannot be provided on the
label.
Reference Number This symbol appears adjacent to Catalog or
Assembly Number.
Alternating Current This symbol indicates that the equipment is
suitable for alternating current only.
ON This symbol indicates connection to the mains.
Keep dry This symbol indicates to store in a dry place.
Temperature limit 10°C
to 25°C
This symbol indicates the operating temperature
limits of the device.
IPX0
Temperature limit -25°C
to 70°C
This symbol indicates the non-operating
temperature limits of the device.
Authorized EU
Representative
No special protection
against water
This symbol indicates the name and address of
authorized EU representative.
This symbol indicates the degree of protection
the device has against the ingress of water.
300-002547-00 rev7 13

Symbol Name Meaning
Single-use only Used to warn the user that the piece of
Use by date This symbol indicates that the device should not
equipment with this label is for single-use only
and it must not be used more than once.
be used after the date accompanying the
symbol.
Sterilized using ethylene
oxide
Rx only Federal (USA) law restricts this device to sale
Do not re-sterilize This symbol indicates that the device should not
Caution This symbol indicates that caution is necessary
Do not use if package is
damaged
This symbol indicates that the device is
provided sterile and has been sterilized using
ethylene oxide.
by or on the order of a physician.
be re-sterilized after it once has been sterilized.
when operating the device or control close to
where the symbol is placed, or to indicate that
the current situation needs operator awareness
or operator action in order to avoid undesirable
consequences.
This symbol indicates that the device must not
be used if the package holding the device is
damaged.
14 300-002547-00 rev7
 Loading...
Loading...