Atos Medical ProTrach DualCare Instructions For Use Manual

Instructions for Use
Unregistered copy
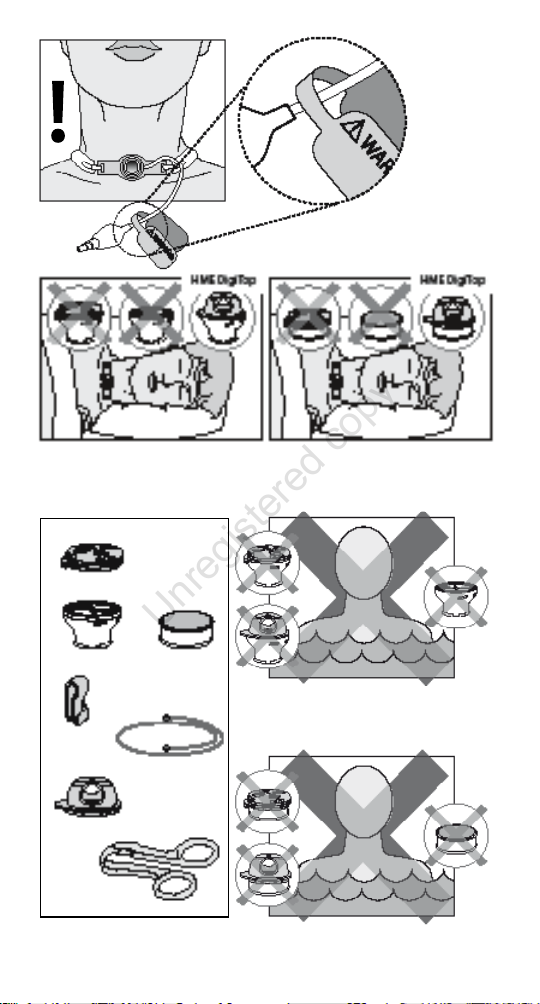
Figure 1
Unregistered copy
Figure 2a Figure 2b
a
b1 b2
c d
e
f
Figure 4a
Figure 4bFigure 3
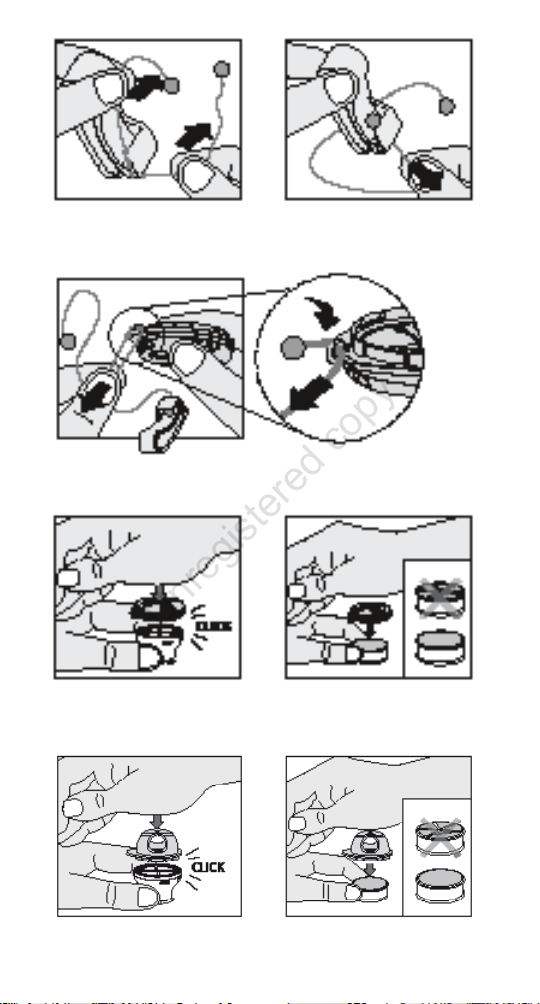
Figure 5a Figure 5b
Unregistered copy
Figure 5c
Figure 6a
Figure 6c
Figure 6b
Figure 6d
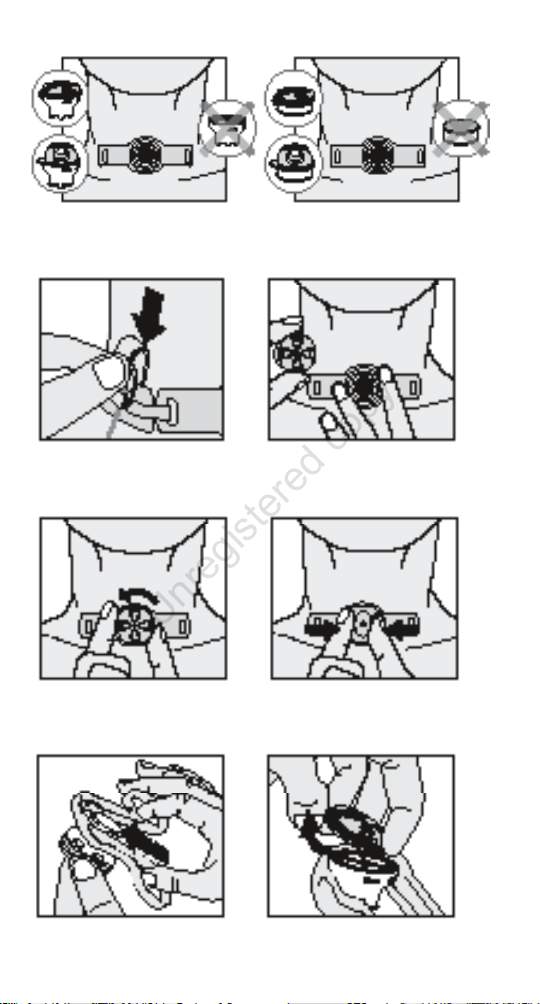
Figure 7a
Unregistered copy
Figure 7b
Figure 8
Figure 10 Figure 11
Figure 12a Figure 12b
Figure 9
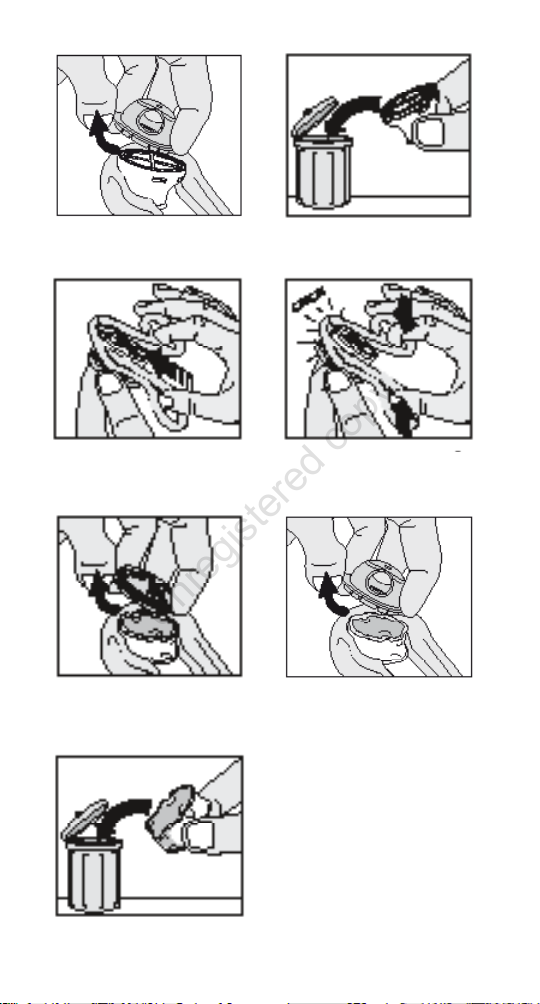
Figure 12dFigure 12c
Unregistered copy
Figure 13a
Figure 13c Figure 13d
Figure 13e
Figure 13b
15 min
Unregistered copy
Figure 14a
Figure 14c
Figure 14b
2 h
Figure 14d
2 h
10 min
Figure 15bFigure 15a
Figure 16

Prescription information
Unregistered copy
CAUTION: United States Federal law restricts this device
to sale, distribution and use by or on order of a physician or a
licensed practitioner. The availability of this product without
prescription outside the United States may vary from country
to countr y.
Disclaimer
Atos Medical offers no warranty – neither expressed nor
implied – to the purchaser hereunder as to the lifetime of the
product delivered, which may vary with individual use and
biological conditions. Furthermore, Atos Medical offers no
warranty of merchantability or tness of the product for any
particular purpose.
Patent and trademarks
ProTr a ch® is a registered trademark and DualCare™ is a trademark
of Atos Medical AB. The products are protected by US patent
no. US 6,921,417, JP patent no. 4,156,832 as well as other patents.

Contents
Unregistered copy
ENGLISH ..........................................................................9
DEUTSCH ..................................................................... 24
NEDERLANDS ............................................................. 41
FRANÇAIS .................................................................... 58
ITALIANO ...................................................................... 75
ESPAÑOL ...................................................................... 92
PORTUGUÊS ..............................................................109
SVENSKA .................................................................... 127
DANSK ......................................................................... 142
NORSK ......................................................................... 158
SUOMI ......................................................................... 173
ORDERING INFORMATION ..................................189

ENGLISH
Unregistered copy
The Instructions for Use, which accompanies this product, may
be revised from time to time and must therefore be reviewed
prior to each procedure in which the product is used.
Contents
1. Descriptive information ............................................................10
1.1 Intended use ..........................................................................10
1.2 CONTRAINDICATIONS ........................................................10
1.3 Description of the devices ................................................11
1.4 WARNINGS ..............................................................................14
1.5 PRECAUTIONS .......................................................................16
2. Instructions for use .....................................................................17
2.1 Preparation and assembly of the speaking
devices ............................................................................................17
2.2 Insertion and usage of Speaking Valve
with HME 15 / 22 .........................................................................18
2.3 Insertion and usage of HME DigiTop and HME
DigiTop O
2.4 Removal of the HME from the speaking devices......20
2.5 Pediatric Use ..........................................................................20
2.6 Cleaning and disinfection ................................................21
2.6.1 Cleaning of the speaking devices ..........................21
2.6.2 Disinfection of the speaking devices ....................22
2.7 Storage instructions ............................................................23
2.8 Device lifetime ......................................................................23
2.9 Disposal ...................................................................................23
2.10 Accessories ..........................................................................23
3. Additional information .............................................................23
3.1 Compatibility with MRI Examination ............................23
3.2 Ordering information .........................................................23
3.3 User assistance information .............................................23
......................................................................................19
2
Symbol explanation
Keep away from direct sunlight and heat.
Keep dry and away from rain and other moisture.
9

1. Descriptive information
Unregistered copy
1.1 Intended use
ProTrach DualCare is a combined Speaking Valve and Heat
and Moisture Exchanger (HME) intended for spontaneously
breathing tracheostomized patients using a tracheostomy
tube with a deated cuff, or a tracheostomy tube without cuff.
In HME-mode the device conditions inhaled air by retaining
heat and moisture from the exhaled air.
By turning the lid of the Speaking Valve into speaking mode
air is re-directed to enable speech.
The entire device is for single patient use and the HME-part
is for single use.
Patient Population: For spontaneously breathing tracheostomized
patients (adults and pediatric patients greater than 10 kg in
weight) using a tracheostomy tube with a deated cuff, or a
tracheostomy tube without cuff.
Environment of Use: Hospitals, ICU, sub-acute care institutions
and home.
1.2 CONTRAINDICATIONS
General
ProTrach HME 15 or 22 in combination with either ProTrach
DualCare Speaking Valve or ProTrach HME DigiTop are
contraindicated for:
- Use in combination with an in-line ventilator.
- Patients who are unable to handle or remove the device
themselves when needed, unless the patient is under
constant supervision of a clinician or a trained caregiver.
For example, patients who are unable to move their arms,
patients with decreased levels of consciousness, or patients
with diseases that put them at risk for unpredictable periodic
loss of consciousness.
- Patients who cannot tolerate the added dead space of 5 ml,
or who cannot tolerate the added breathing resistance
of 170 Pa / 1.7 cm H
clinician.
- Unresponsive or sedated patients. The patient must be
responsive and attempting to communicate in order to use
the device. The patient should be able to follow instructions.
O. This should be evaluated by a
2
10

The HME 15 or 22 in combination with either Speaking Valve
Unregistered copy
or HME DigiTop must NOT be used on a single lumen tube
(tube without an inner tube), unless the patient or caregiver is
able to reinsert the tube themselves after emergency removal.
Speaking Valve specic
The use of S peak ing Valve (in combination with HME 15 or 22)
is additionally contraindicated for the following patient groups:
- Laryngectomized patients since the device will prevent the
ability to exhale if the Speaking Valve is unintentionally set
to speaking mode.
- Patients suffering from severe aspiration.
- Patients with severe obstruction in the area of the
tracheostomy tube or in the upper airways, such as tracheal
and/or laryngeal stenosis, since this may cause air trapping.
- Patients with very thick and copious secretions which might
block the device.
DO NOT use the Speaking Valve:
- In combination with a tracheostomy tube with the cuff
inated. The cuff must be completely deated before
placing and during all use of the Speaking Valve.
- In combination with a tracheostomy tube with a foam cuff.
- In combination with a tracheostomy tube with a self-
inating cuff.
- When the size of the tracheostomy tube does not allow for
airow through the upper airways.
- In combination with an endotracheal tube.
Use of the Speaking Valve in these circumstances can restrict
exhalation through the upper airways and cause suffocation!
DO NOT use the Speaking Valve during sleep since the airway
could be blocked unintentionally. During sleep the HME
DigiTop (in combination with HME 15 or 22) should be used
instead (Figure 2a or 2b).
1.3 Description of the devices
The Speaking Valve, the HME DigiTop, and the HME
Dig iTop O2 are all used to enable speaking. In this Instructions
for Use they will therefore be referenced to as “speaking devices”.
Speaking Valve and HME 15 / 22
The reusable Speaking Valve is used with a single use 15 mm
or 22 mm Heat and Moisture Exchanger (HME). The HME is
placed so that it prevents direct contact between the Speaking
11

Valve and the airways. This prevents the Speaking Valve from
Unregistered copy
being clogged or soiled by mucus.
The Speaking Valve has two modes: speaking mode and
HME mode.
In speaking mode, a exible membrane is positioned in
the airow openings and acts as a one way valve. It opens
during inhalation so the patient can inhale through the device.
During exhalation, the membrane remains closed and the air
is re-directed through the upper airways and the vocal folds.
Thereby the patient is able to speak. In speaking mode the
inhaled air does not get conditioned since the exhaled air goes
out through the upper airways.
In HME mode the membrane is moved out of the way of the
ai r ow so that the patient both inhales and ex hales th rough the
device. The inhaled air is conditioned by the heat and moisture
that is retained from the exhaled air in the impregnated HME
media. In HME mode, speaking is not possible.
The device is switched between the modes by rotating the lid
of the Speaking Valve until it clicks into the desired position.
The ProTrach HME 15 is available in two versions: the XtraMoist
with better humidication properties and the Regular with lower
breathing resistance. The XtraMoist should be used when the
slightly higher breathing resistance is tolerated. The Regular
should be used when lower breathing resistance is required,
for example when rst getting used to HME use or during
physical activity.
HME DigiTop and HME DigiTop O
The HME DigiTop and the HME DigiTop O2 enable use of
the HME without the Speaking Valve, and can manually be
occluded to enable speaking.
The HME DigiTop O2 provides possibility to connect
oxygen tubing of 1/8 inch (3.2 mm) diameter to the Oxygen port
connector for patients requiring additional Oxygen (Figure 16).
12
2
ProTrach DualCare Set 15 / Set 22 include:
Unregistered copy
See Figure 3
(a) 1 pc ProTrach DualCare Speaking Valve
(b1 or b2) 30 pcs (b1) ProTrach HME 15 Regular or
(b2) ProTrach HME 22 Regular
(c) 1 pc ProTrach Connection Strap clip
(d) 1 pc ProTrach Connection Strap string
(e) 1 pc ProTrach HME DigiTop
(f) 1 pc Removal Aid
1 pc ProTrach DualCare Instructions for Use
ProTrach DualCare Speaking Valve / Speaking Valve
Blue package include:
See Figure 3
(a) 1 pc ProTrach DualCare Speaking Valve
or Speaking Valve Blue
(c) 1 pc ProTrach Connection Strap clip
(d) 1 pc ProTrach Connection Strap string
(e) 1 pc ProTrach HME DigiTop or DigiTop Blue
1 pc ProTrach DualCare Instructions for Use
Technical data ProTrach DualCare
Speaking Valve with HME 15 Speaking Valve with HME 22
Pressure drop at 30 l/min in
speaking mode, HME 15 Regular:
Pressure drop at 30 l/min in HME
mode, HME 15 Regular:
Pressure drop at 60 l/min in HME
mode, HME 15 Regular:
Pressure drop at 30 l/min
in speaking mode, HME 15
XtraMoist:
Pressure drop at 30 l/min in HME
mode, HME 15 XtraMoist:
Pressure drop at 60 l/min in HME
mode, HME 15 XtraMoist:
Dead space:
(Speaking Valve incl HME)
* During increased physical activity that requires lower breathing resistance the Regular HME
is recommended. Therefore, the XtraMoist is not tested in these conditions.
Oxygen concentration measured at:
Tidal volume 1 L, breathing frequency 10/min, O
DigiTop O2 with HME 15 70%
DigiTop O
with HME 22 71%
2
Pressure drop at 30 l/min in
125 Pa
speaking mode:
Pressure drop at 30 l/min in
35 Pa
HME mode:
Pressure drop at 60 l/min in
123 Pa
HME mode:
166 Pa
78 Pa
*
Dead space:
4.05 ml
(Speaking Valve incl HME)
2
136 Pa
48 Pa
166 Pa
4.62 ml
ow 10 L/min
13
1.4 WARNINGS
Unregistered copy
Speaking Valve specic
(Speaking Valve in combination with HME 15 or 22).
WARNING
If a tracheostomy tube with a cuff is used, the cuff must
be completely deated before placing, and during all use
of the Speaking Valve. The patient cannot breathe if the
cuff is not completely deated. There should be sufcient
airow around the tracheostomy tube and the deated cuff.
DO NOT use the Speaking Valve for patients who use a
tracheostomy tube with a self-inating or foam-lled cuff.
Use of Warning Labels: Attach warning labels provided
with Speaking Valve to the tracheostomy tube pilot balloon
ination line (Figure 1).
Monitoring: Patients with a tracheostomy tube with a cuff
using the Speaking Valve must be monitored according to
physician direction. Continuous observation is needed to make
sure that the patient can exhale through the upper airway.
Education: To ensure correct use of the Speaking Valve,
the patient, family, caregivers, and all personnel working
with the patient must be instructed on how to use Speaking
Valve, including contraindications, warnings, warning labels
and precautions.
Do
- Do make sure the patient has the respiratory ability to
exhale around the tracheostomy tube and out of the nasal
and oral cavities.
- Do make sure the patient has been cleared by the physician
for cuff deation. Cuff deation is mandatory with the
Speaking Valve to allow exhaled air to pass around the
tracheostomy tube and upper airways.
- Do make sure the tracheostomy tube is sized to allow
for sufcient airow around the tracheostomy tube to
facilitate speech. The cuff on the tube can also generate an
obstruction even when deated and this should be taken
into consideration during airway patency assessment.
- Do evaluate, per physician direction, if changing to a
smaller tracheostomy tube is indicated to provide sufcient
airow to allow use of the Speaking Valve.
14

- Do evaluate patients with thick unmanageable secretions
Unregistered copy
that may cause airway obstructions for use of the Speaking
Valve.
- Do evaluate patient experiencing difculty utilizing the
Speaking Valve, as the patient may have airway obstructions
due to stenosis, tissue mass, tracheomalacia, granulation,
vocal cord paralysis in the midline position, secretions,
or a tracheostomy tube that is oversized for the patient’s
trachea. If obstruction is corrected, the patient should be
re-evaluated for the Speaking Valve.
General
(HME 15 and 22 in combination with either Speaking Valve
or HME DigiTop).
Do
- Do make sure that patients with severe pulmonary diseases,
e.g. lung emphysema, severe asthma etc. are examined and
evaluated by a clinician to ensure suitability before they
start using the device.
- Do make sure that the patient or caregiver always has the
ability to remove the device quickly from the tracheostomy
tube if needed. Coughing up a lot of mucus may suddenly
block or nearly block the tube or HME.
- Do make sure that the patient, caregiver and others
understand the closing function and the use of the holes.
Do explain that blocking the holes in the Speaking Valve
or the HME DigiTop by clothing, for example, may cause
difculty in breathing or suffocation.
Do not
- Do not bathe or swim with the device (Figure 4a or 4b).
This could cause water to enter the airways. The speaking
devices do not prevent this in any mode.
- Do not use the device if it has been damaged or contaminated.
- Do not remove the foam from the HME. Using the
device without the foam increases the risk of aspiration
of small device parts, it may also negatively impact valve
functionality due to direct mucus contact, and also without
the foam the important HME function is lost.
- Do not reuse the HME, e.g. by washing it. By washing the
HME the important HME function is lost. Reuse can also
increase the risk of infection.
15

- Do not use any part of the device for more than one patient
Unregistered copy
since this may cause cross contamination. The device is
intended for single patient use only.
HME 22 specic
(HME 22 in combination with either Speaking Valve or HME
Dig iTop).
Do
- If the Speaking Valve comes loose from the HME 22
during the removal of the device, always remove the inner
tracheostomy tube or the single lumen tube together with
the HME instead of trying to remove the HME from the
tube connector.
Attempts to remove the HME 22 from the connector
while the tube is still in place is difcult, and the airways
may become blocked by mucus.
1.5 PRECAUTIONS
Do
- Do always check before each use that the Speaking Valve
rotates properly between the two modes. The Speaking
Valve may get stuck in one of the modes due to the presence
of mucus. If this occurs, clean the device thoroughly and if
the problem persists stop using the device and replace it.
- Do check the device if you experience increased breathing
resistance as this might be a sign of blockage by (e.g., by
mucus).
- Do consult your clinician if there is a problem in connection
with the device.
Do not
- Do not use humidiers or heated humidied oxygen via a
mask over the tracheostoma while using the device since it
will cause the HME to become too wet. If oxygen therapy
is required, use only non-heated humidied oxygen.
- Do not administer medicated nebulizer treatment over
the tracheostoma while using the device. The medication
can become deposited in the HME and medications may
also adversely affect the Speaking Valve membrane. If the
device is inadvertently used during a medicated nebulizer
treatment, the device must be removed immediately. The
HME must be discarded, and the Speaking Valve and the
16

HME DigiTop must be cleaned thoroughly to remove all
Unregistered copy
medication residues.
2. Instructions for use
The speaking devices (Speaking Valve, HME DigiTop and HME
Dig iTop O2) are reusable. The HME cassettes are disposable
and must be exchanged at least every 24 hours.
2.1 Preparation and assembly of the
speaking devices
CAUTION: Use only original accessories with the device.
Use of other accessories may cause product malfunction and
harm the patient.
When the Speaking Valve in combination with a HME is not
advisable, evaluate the possibility of using the ProTrach HME
DigiTop in combination with a HME.
Before each use, please check that the speaking device (Speaking
Valve, HME DigiTop, or HME DigiTop O2) and HME are
undamaged and that the Speaking Valve functions as intended,
e.g., it clicks into the HME mode and speaking mode. If the
device does not function as intended or seems damaged, do
not use the device and replace it.
Attach the ProTrach Connection Strap
The ProTrach Connection Strap can be used to secure the
Speaking Valve to the neckband (optional). This is done to avoid
that the device is lost, for example, when coughing or sneezing.
1. Insert the string of the Connection Strap into the clip
(Figure 5a and 5b).
2. Insert the string of the Connection Strap to the Speaking
Valve (Figure 5c).
Note: If the Connection Strap is pulled with a certain force,
the string will loosen from the clip. This is a security feature to
prevent injury if the string accidentally is caught up somewhere.
If this occurs, just insert the string again in the clip (Figure
5a and 5b).
CAUTION: Do not attempt to glue or otherwise permanently
attach the string to the clip or Speaking Valve.
17

Assemble speaking device and HME
Unregistered copy
CAUTION: Always assemble the HME with the speaking
device (Speaking Valve, HME DigiTop, or HME DigiTop O2)
before use (Figure 6a, 6b, 6c, 6d, 7a, and 7b). If the device is
not properly assembled, the ProTrach HME 22 can get stuck
in the tracheostomy tube and be difcult to remove in case
of blockage.
1. Place the HME on a at surface:
For ProTrach HME 15 with the narrow part down and the
open foam surface up (Figure 6a or 6c).
For HME 22 with the plastic grid down and the open foam
surface up (Figure 6b or 6d).
2. Put the speaking device on top of the HME and push it
down until it clicks in place.
3. Check that the speaking device is rmly attached to the
HME.
2.2 Insertion and usage of Speaking Valve
with HME 15 / 22
If the Connection Strap is used, attach the clip to the neckband
(Figure 8).
Attach the combined Speaking Valve and HME to the
connector of the tracheostomy tube (Figure 9). Caution:
HME 15 users should always attach the combined Speaking
Valve and HME 15 to the connector gently, using a 1/4 twist
in a clockwise motion. If the device is pushed too hard it can
get stuck on the tracheostomy tube and be difcult to remove
in case of blockage.
If you have not used HMEs previously, you should be aware
that the device may increase breathing resistance to some extent.
You can now switch between speaking mode and HME
mode based on your speaking needs, humidication needs,
and breathing resistance needs (Figure 10). Caution: When
changing modes, make sure that you only rotate the lid of
the Speaking Valve, not the entire device. Rotation of the
whole device might cause uncomfortable movement of the
tracheostomy tube.
In case you feel increased breathing resistance after coughing,
take out the device and wipe off the mucus, or replace the
HME with a new one. Also clear the tracheostomy tube from
mucus when necessary. If you feel the need to cough heavily,
it is recommended that you try to remove the device from the
tube prior to coughing. This prevents the device from falling
18

off and prevents the HME from getting clogged by mucus. If the
Unregistered copy
membrane pops out through the holes in the lid of the Speaking
Valve when coughing, rotate the lid, and the membrane will
get back to the correct position.
Speaking mode
The Speaking Valve is put in speaking mode by rotating the
lid until it clicks into the speaking mode position (the exible
membrane covers the openings of the lid). In speaking mode
the membrane only opens when you breathe in. It is closed
when you breathe out, so you will breathe out through your
mouth and nose, and you will be able to speak. You may feel
a somewhat higher breathing resistance in speaking mode
than in HME mode due to the membrane. You can now try to
speak some words, beginning with low volume and pressure.
HME mode
The Speaking Valve is put in HME mode by rotating the lid
until it clicks into the HME mode position (i.e., the exible
membrane does NOT cover the openings of the lid). HME
mode allows you to both breathe in and out through the HME,
thereby conditioning the inhaled air.
Note that increased mucus production may appear during
the rst weeks of HME use.
With Speaking Valve Blue (REF 7755), the colored membrane
makes it easier to see whether the valve is in speaking mode
or in HME mode.
2.3 Insertion and usage of HME DigiTop
and HME DigiTop O
Assemble and insert the speaking device as described in 2.1.
If you use the HME DigiTop O2 always assemble and
disassemble the oxygen tubing while the device is disconnected
from the trach-tube. This is to avoid uncomfortable movement
of the trach-tube.
Make sure that the oxygen tubing is securely attached; it
should be assembled beyond the conical edge (Figure 15).
If you have not used HMEs previously, you should be aware
that the device may increase breathing resistance to some extent.
When you occlude the two holes on the sides with your
ngers, exhaled air will be redirected through your upper
airways and you will be able to speak (Figure 11). Releasing
the nger occlusion will allow you to inhale again through the
2
19

device. The two openings in the HME DigiTop / HME DigiTop
Unregistered copy
O2 allow you to breathe in and out through the device, thereby
conditioning the inhaled air continuously.
Note that increased mucus production may appear during
the rst weeks of HME use.
2.4 Removal of the HME from the speaking
devices
If you use the HME DigiTop O2 remove the oxygen tubing as
described above before you start to remove the HME. The HME
is intended for single use, and must be replaced at least every
24 hours, or more often if needed. It should be removed before
cleaning the speaking devices and should also be replaced if
it becomes clogged by mucus.
CAUTION: Do not wash the HME since the important HME
function then is lost.
HME 15
1. Remove the combined speaking device and HME 15
(without separating them) from the tracheostomy tube
with a gentle pulling twist in a clockwise motion.
2. Hold the speaking device with one hand, and pull the
Removal Aid over the HME with the other hand (Figure
12a).
3. Pull the speaking device apart from the HME with a steady
grip (Figure 12b or 12c).
4. Discard the HME 15 (Figure 12d).
HME 22
1. Remove the combined speaking device and HME 22
(without separating them) from the tracheostomy tube.
2. Hold the speaking device with one hand, and pull the
Removal Aid over the HME with the other hand (Figure
13a)
3. Press the handles together (Figure 13b) and the HME 22
will crack and loosen from the speaking device (Figure
13c or 13d).
4. Discard the HME 22 (Figure 13e).
2.5 Pediatric Use
If selected properly, ProTrach DualCare can be used by children
of all ages, provided that it ts the local anatomy. This can
change as the child grows. In children with a short neck or a
high tracheostomy site, the device may contact the chin which
20

may be uncomfortable and can cause the Speaking Valve to
Unregistered copy
twist modes inadvertently. A tracheostomy tube extender may
prevent this from occurring. The ability to handle the device
independently depends on the dexterity and developmental
maturity of the child. Until the child is able to handle the
device independently, supervision by a parent or caregiver
while using the device is obligatory (see Contraindications).
The indications and contra-indications for the use of the HME
DigiTop and the HME DigiTop O2 are the same for adults and
children. However, manual occlusion for speaking may not be
possible if the ngers of the child are too small. In such a case,
the supervising parent or caregiver can occlude the device.
The indications and contra-indications for the use of the
Speaking Valve are the same for adults and children. The
ability to connect and disconnect the device from the tube,
assemble and disassemble the device, and twist the device
between modes depends on the dexterity and developmental
maturity of the child. These tasks need to be performed by the
supervising parent or caregiver until the child is able to handle
the device independently.
2.6 Cleaning and disinfection
2.6.1 Cleaning of the speaking devices
In order to keep the speaking devices (Speaking Valve and
HME DigiTop) clean and in a good working condition, they
should be cleaned daily. If used, the Connection Strap can be
cleaned together with the Speaking Valve.
CAUTION: Do NOT boil the device and do NOT use hydrogen
peroxide for disinfection since this will damage the device.
1. Remove the HME from the speaking device as described
above.
2. Flush the oxygen port connector of HME DigiTop O
(if used), with oxygen or air to remove any debris.
3. Rinse the speaking device carefully on both sides under
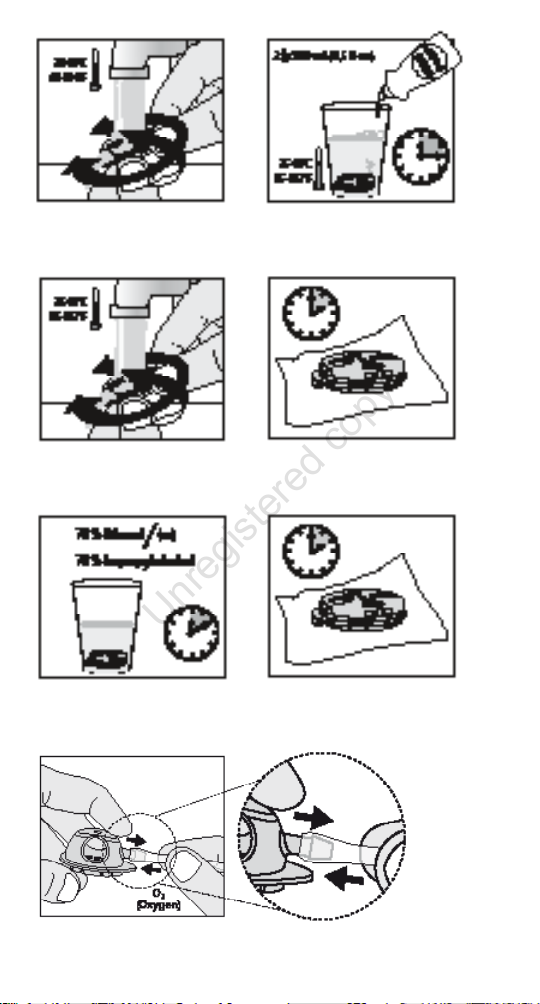
lukewarm (20-40 °C / 68-104 °F) drinking water. Rotate
the Speaking Valve lid back and forth during the rinse.
Rinse the speaking device for 2 minutes (Figure 14a).
4. Mix 2 drops of dish soap in 250 ml warm (35-45 °C /
95-113 °F) drinking water. Do not use boiling water since
this may damage the device.
5. Place the speaking device in the mixture for 15 minutes
(Figure 14b). Make sure that the Speaking Valve is in
2
21

speaking mode (with the membrane covering the openings
Unregistered copy
of the lid) before submerging it.
6. Rinse the speaking device on both sides in warm (35-45 °C
/ 95-113 °F) drinking water for 15 seconds. In order to reach
all parts of the Speaking Valve, be sure that it is opened
and closed a couple of times during rinsing (Figure 14c).
7. Inspect the device with respect to mucus and debris residues.
Repeat the steps above if necessary.
8. Flush the oxygen port connector of HME DigiTop O2 with
oxygen or air to remove any remaining water.
9. After cleaning, put the speaking device on a clean gauze
and leave it to air-dry for at least 2 hours (Figure 14d).
Make sure that the Speaking Valve is in speaking mode.
10. When the speaking device is completely dry, it can either
be used again or placed in a clean, closed container for
storage.
2.6.2 Disinfection of the speaking devices
It is recommended that the device is disinfected at least once
a week or before use if it looks dirty or there has been a risk
of contamination. A risk of contamination could exist if the
device has been dropped on the oor or if it has been in contact
with a pet, someone with a respiratory infection, or any other
gross contamination. If disinfection is needed always clean the
device rst as described above and then disinfect by using the
following procedure:
1. First follow the cleaning procedure as described in 2.6.1,
without step 9 and 10. After cleaning, put the Speaking
Valve in speaking mode and submerge it for 10 minutes
either in ethanol 70% or isopropyl alcohol 70% (Figure
15a).
CAUTION: Do NOT use hydrogen peroxide.
2. After cleaning and disinfection, put the speaking device
on a clean gauze and leave it to air-dry for at least 2 hours
(Figure 15b). Make sure that the Speaking Valve is in
speaking mode.
3. When the speaking device is completely dry, it can either be
used again or placed in a clean, closed container for storage.
CAUTION: Do not use the speaking device until it is
completely dry. Inhalation of disinfectant fumes can cause
severe coughing and irritation of the airways. The HME
Dig iTop O
oxygen or air before use to remove any disinfection residuals.
oxygen port connector shall be ushed with
2
22

2.7 Storage instructions
Unregistered copy
When not in use, clean and disinfect the Speaking Valve as
described above, and then store it in a clean and dry container
at room temperature. Protect from direct sunlight.
2.8 Device lifetime
The HME is for single use and must be replaced at least every
24 hours, or more often if needed.
The Speaking Valve, HME DigiTop, HME DigiTop O2, and
Connection Strap may be used for a maximum of 2 months as
long as they are intact and are functioning as intended.
2.9 Disposal
Always follow medical practice and national requirements
regarding biohazard when disposing of a used medical device.
2.10 Accessories
ProTrach Connection Strap: Can be used to attach the
Speaking Valve to the neckband.
Removal Aid: Can be used to help to remove the HME from
the speaking device.
3. Additional information
3.1 Compatibility with MRI Examination
MR-Safe: This device does not contain any metallic elements
and has no potential for interaction with the MRI eld.
3.2 Ordering information
See end of this Instructions for Use.
3.3 User assistance information
For additional help or information, please see back cover of
this Instructions for Use for contact information.
23

DEUTSCH
Unregistered copy
Die diesem Produkt beiliegende Gebrauchsanweisung unterliegt
gelegentlichen Änderungen und ist deshalb vor jedem Verfahren,
bei dem das Produkt verwendet wird, durchzusehen.
Inhalt
1. Beschreibende Informationen ...............................................25
1.1Verwendungszweck ............................................................25
1.2KONTRAINDIKATIONEN .....................................................25
1.3 Beschreibung des Produkts .............................................27
1.4WARNHINWEISE .................................................................... 29
1.5VORSICHTSMASSNAHMEN ...............................................32
2. Gebrauchsanweisung ...............................................................33
2.1 Vorbereitung und Montage der Sprechprodukte ....33
2.2 Einführung und Gebrauch des Sprechventils
mit HME 15 / 22 ...........................................................................34
2.3 Einführung und Gebrauch der HME DigiTop
und der HME DigiTop O
2.4 Entfernen des HME von den Sprechprodukten ........36
2.5Einsatz in der Pädiatrie .......................................................37
2.6 Reinigung und Desinfektion ...........................................38
2.6.1 Reinigung der Sprechprodukte ..............................38
2.6.2 Desinfektion der Sprechprodukte .........................39
2.7 Lagerungshinweise .............................................................39
2.8 Nutzungsdauer des Produkts ..........................................40
2.9 Entsorgung.............................................................................40
2.10Zubehör ................................................................................40
3. Zusätzliche Informationen ......................................................40
3.1Kompatibilität mit MRT-Untersuchungen ..................40
3.2Ordering information (Bestellinformationen) ...........40
3.3Anwenderunterstützung ..................................................40
..........................................................35
2
Symbolerklärung
Vor direkter Sonneneinstrahlung und Wärme schützen.
Vor Nässe, Regen und sonstiger Feuchtigkeit schützen.
24

1. Beschreibende Informationen
Unregistered copy
1.1Verwendungszweck
ProTrach DualCare ist eine Kombination aus Sprechventil und
Wärme- und Feuchtigkeitsaustauscher (Heat and Moisture
Exchanger, HME) und ist für spontan atmende tracheotomierte
Patienten bestimmt, die über eine Trachealkanüle mit
entblocktem Cuff oder eine Trachealkanüle ohne Cuff atmen.
Im HME-Modus konditioniert das Produkt die eingeatmete
Luft, indem es Wärme und Feuchtigkeit aus der ausgeatmeten
Luft zurückhält.
Wenn der Deckel des Sprechventils in den Sprechmodus gedreht
wird, wird die Luft umgeleitet und das Sprechen ermöglicht.
Das gesamte Produkt ist zum Gebrauch für einen einzigen
Patienten bestimmt, während die HME-Kasette zum einmaligen
Gebrauch bestimmt ist.
Patientenpopulation: Für spontan atmende tracheotomierte
Patienten (Erwachsene und Kinder mit einem Körpergewicht von
mehr als 10 kg), die über eine Trachealkanüle mit entblocktem
Cuff oder eine Trachealkanüle ohne Cuff atmen.
Verwendungsbereiche: Krankenhäuser, Intensivstationen,
subakute Pegeeinrichtungen und häusliche Pege.
1.2KONTRAINDIKATIONEN
Allgemeines
ProTrach HME 15 oder 22 in Kombination mit entweder dem
Sprechventil ProTrach DualCare Speaking Valve oder dem
Produkt ProTrach HME DigiTop sind kontraindiziert für:
- Nutzung in Kombination mit einem Inline-Respirator.
- Patienten, die das Produkt bei Bedarf nicht selbst handha-
ben oder entfernen können, wenn sie nicht unter ständiger
Aufsicht eines Arztes bzw. einer ausgebildeten Pegekraft
stehen. Dazu gehören zum Beispiel Patienten, die ihre Arme
nicht bewegen können, Patienten mit eingeschränktem Bewusstsein oder Patienten mit Krankheiten, mit denen ein
Risiko unvorhersehbarer, periodischer Bewusstseinsverluste assoziiert wird.
- Patienten, die den zusätzlichen Totraum von 5 ml bzw. den
zusätzlichen Atemwiderstand von 170 Pa/1,7 cm H
vertragen. Dies sollte ein Arzt beurteilen.
- Nicht ansprechbare oder sedierte Patienten. Der Patient
muss ansprechbar und kommunikationsbereit sein, um das
O nicht
2
25

Produkt zu benutzen. Der Patient muss in der Lage sein,
Unregistered copy
Anweisungen zu befolgen.
Der HME 15 oder 22 in Kombination mit entweder dem
Sprechventil oder dem HME DigiTop dürfen NICHT an einer
einlumigen Kanüle (d. h. einer Kanüle ohne Innenkanüle)
angebracht werden, es sei denn, der Patient oder die Pegekraft
ist in der Lage, die Kanüle wieder einzuführen, wenn die
Kanüle im Notfall entfernt werden muss.
Sprechventil-spezisch
Die Verwendung des Sprechventils (in Kombination mit HME
15 oder 22) ist zusätzlich bei den folgenden Patientengruppen
kontraindiziert:
- Laryngektomierte Patienten, da das Produkt die Ausatmung
verhindert, falls das Sprechventil versehentlich in den
Sprechmodus geschaltet wird.
- Patienten, die an schwerer Aspiration leiden.
- Patienten mit hochgradigen Obstruktionen im Bereich der
Trachealkanüle oder der oberen Atemwege, z. B. eine Trachea- oder Larynxstenose, da dies zu Lufteinschlüssen führen kann.
- Patienten mit starker, zähüssiger Sekretproduktion, durch
die das Produkt blockiert werden könnte.
Das Sprechventil NICHT verwenden:
- In Kombination mit einer Trachealkanüle mit geblocktem
Cuff. Der Cuff muss vor der Platzierung und während der
gesamten Verwendung des Sprechventils vollständig entblockt sein.
- In Kombination mit einer Trachealkanüle mit SchaumstoffCuff.
- In Kombination mit einer Trachealkanüle mit selbstaufblasendem Cuff.
- Wenn die Größe der Trachealkanüle das Passieren von Luft
durch die oberen Atemwege nicht zulässt.
- In Kombination mit einem Endotrachealtubus.
Die Verwendung des Sprechventils unter diesen Bedingungen
kann die Ausatmung durch die oberen Atemwege behindern
und ein Ersticken verursachen!
Das Sprechventil darf NICHT während des Schlafs verwendet
werden, da die Atemwege versehentlich blockiert werden
können. Während des Schlafs sollte stattdessen das HME
DigiTop (in Kombination mit HME 15 oder 22) verwendet
werden (Abbildung 2a oder 2b).
26

1.3 Beschreibung des Produkts
Unregistered copy
Das Sprechventil, das HME DigiTop sowie das HME DigiTop O2
werden verwendet, um das Sprechen zu ermöglichen. In dieser
Gebrauchsanweisung werden diese daher als „Sprechprodukte“
bezeichnet.
Sprechventil und HME 15 / 22
Das wiederverwendbare Sprechventil wird zusammen mit einem
Wärme- und Feuchtigkeitsaustauscher (Heat and Moisture
Exchanger, HME) der Größe 15 mm oder 22 mm verwendet,
der zum einmaligen Gebrauch bestimmt ist. Der HME wird so
platziert, dass er den direkten Kontakt des Sprechventils mit
den Atemwegen verhindert. Dadurch wird verhindert, dass das
Sprechventil durch Schleim verstopft oder verschmutzt wird.
Das Sprechventil verfügt über zwei Modi: den Sprechmodus
und den HME-Modus.
Im Sprechmodus wird eine flexible Membran in den
Luftstromöffnungen positioniert, die als wirksames EinwegVentil fungiert. Es öffnet sich beim Einatmen, sodass der Patient
durch das Produkt einatmen kann. Während der Ausatmung
bleibt die Membran geschlossen und die Luft wird über die
oberen Atemwege und die Stimmbänder umgeleitet. Dies
ermöglicht dem Patienten das Sprechen. Im Sprechmodus wird
die eingeatmete Luft nicht konditioniert, da die ausgeatmete
Luft durch die oberen Atemwege entweicht.
Im HME-Modus wird die Membran aus dem Luftstrom weg
gedreht, sodass der Patient durch das Produkt sowohl ein- als
auch ausatmet. Die eingeatmete Luft wird mit der Wärme und
Feuchtigkeit konditioniert, die aus der ausgeatmeten Luft im
imprägnierten Medium des HME zurückgehalten werden. Im
HME-Modus ist das Sprechen nicht möglich.
Das Produkt wird zwischen den beiden Modi umgeschaltet,
indem man am Deckel des Sprechventils dreht, bis er in der
gewünschten Stellung einrastet.
Der ProTrach HME 15 ist in zwei Ausführungen erhältlich: als
XtraMoist mit verbesserter Befeuchtung und als Regular mit
geringerem Atemwiderstand. XtraMoist sollte zum Einsatz
kommen, wenn der leicht höhere Atemwiderstand vertragen
wird. Regular sollte verwendet werden, wenn ein geringerer
Atemwiderstand erforderlich ist, z. B. im Rahmen der ersten
Gewöhnung an die HME-Verwendung oder bei körperlicher
Anstrengung.
27

HME DigiTop und HME DigiTop O
Unregistered copy
2
Die Produkte HME DigiTop und HME DigiTop O2 ermöglichen
die Verwendung des HME ohne das Sprechventil. Sie können
manuell verschlossen werden, um das Sprechen zu ermöglichen.
Für Patienten, die zusätzlichen Sauerstoff benötigen, bietet
das HME DigiTop O2 die Möglichkeit, einen Sauerstoffschlauch
mit einem Durchmesser von 3,2 mm (1/8 Inch) an den
Sauerstoffanschlusskonnektor anzubringen (Abbildung 16).
ProTrach DualCare Set 15/Set 22 enthält:
Siehe Abbildung 3
(a) 1 Stück ProTrach DualCare Speaking Valve
(b1 oder b2) 30 Stück (b1) ProTrach HME 15 Regular oder
(b2) ProTrach HME 22 Regular
(c) 1 Stück Clip für das ProTrach Connection
Strap (Halteband)
(d) 1 Stück Faden des ProTrach Connection Strap
(Halteband)
(e) 1 Stück ProTrach HME DigiTop
(f) 1 Stück Removal Aid (Entfernungshilfe)
1 Stück ProTrach DualCare –
Gebrauchsanweisung
ProTrach DualCare Speaking Valve/Paket Speaking
Valve Blue enthält:
Siehe Abbildung 3
(a) 1 Stück ProTrach DualCare Speaking Valve
oder Speaking Valve Blue
(c) 1 Stück Clip für das ProTrach Connection
(d) 1 Stück Faden des ProTrach Connection Strap
(e) 1 Stück ProTrach HME DigiTop oder DigiTop
1 Stück ProTrach DualCare –
Strap (Halteband)
(Halteband)
Blue
Gebrauchsanweisung
Technische Daten für ProTrach DualCare
Sprechventil mit HME 15 Sprechventil mit HME 22
Druckabfall bei 30 l/min im
Sprechmodus, HME 15 Regular:
Druckabfall bei 30 l/min im
HME-Modus, HME 15 Regular:
28
Druckabfall bei 30 l/min im
125 Pa
Sprechmodus:
Druckabfall bei 30 l/min im
35 Pa
HME-Modus:
136 Pa
48 Pa
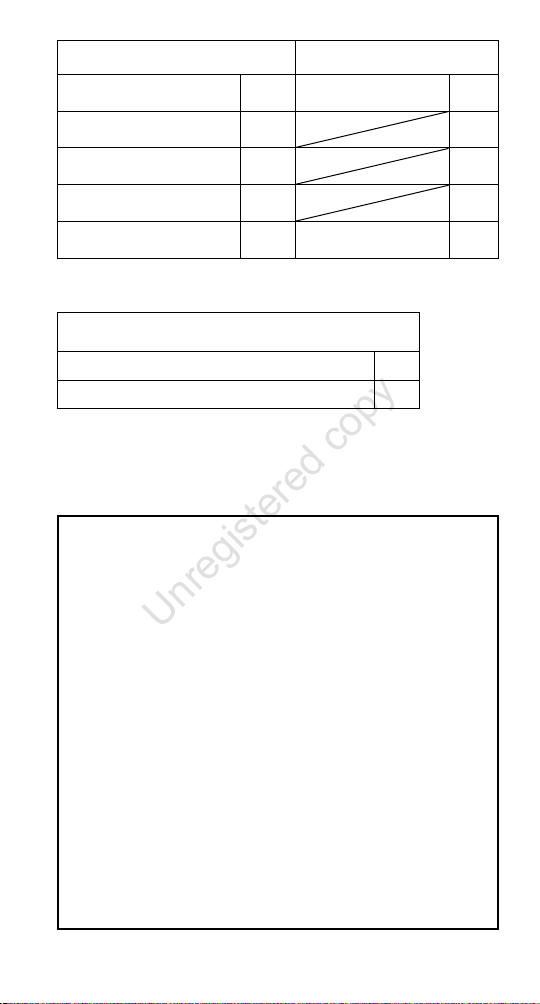
Sprechventil mit HME 15 Sprechventil mit HME 22
Unregistered copy
Druckabfall bei 60 l/min im
HME-Modus, HME 15 Regular:
Druckabfall bei 30 l/min im
Sprechmodus, HME 15 XtraMoist:
Druckabfall bei 30 l/min im
HME-Modus, HME 15 XtraMoist:
Druckabfall bei 60 l/min im
HME-Modus, HME 15 XtraMoist:
Totraum:
(Sprechventil einschl. HME)
* Bei höherer körperlicher Anstrengung, bei der ein geringerer Atemwiderstand erforderlich
ist, wird der Regular HME empfohlen. Daher wurde der XtraMoist nicht unter diesen
Bedingungen getestet.
Sauerstokonzentration gemessen bei:
Atemzugvolumen 1l, Atemfrequenz 10/min, O
DigiTop O2 mit HME 15 70%
DigiTop O
mit HME 22 71%
2
Druckabfall bei 60 l/min im
123 Pa
HME-Modus:
166 Pa
78 Pa
*
Totraum:
4,05 ml
(Sprechventil einschl. HME)
-Zufuhr 10l/min
2
166 Pa
4,62 ml
1.4WARNHINWEISE
Sprechventil-spezisch
(Sprechventil in Kombination mit HME 15 oder 22).
WARNHINWEIS
Wenn eine Trachealkanüle mit Cuff verwendet wird, muss
der Cuff vollständig entblockt sein, bevor das Sprechventil
platziert und während es verwendet wird. Der Patient
kann nicht atmen, wenn der Cuff nicht vollständig geleert
ist. Rund um die Trachealkanüle und den entblockten
Cuff muss ein ausreichender Luftstrom bestehen. Das
Sprechventil darf nicht bei Patienten verwendet werden,
die über eine Trachealkanüle mit selbstaufblasendem oder
schaumstoffgefülltem Cuff atmen.
Verwendung von Warnetiketten: Die dem Sprechventil
beiliegenden Warnetiketten sind am Zuleitungsschlauch
des Kontrollballons der Trachealkanüle anzubringen
(Abbildung 1).
Überwachung: Patienten mit einer Trachealkanüle mit Cuff,
die das Sprechventil verwenden, müssen gemäß Anordnung
des Arztes überwacht werden. Eine kontinuierliche
Beobachtung ist erforderlich, um sicherzustellen, dass der
Patient über die oberen Atemwege ausatmen kann.
29

WARNHINWEIS
Unregistered copy
Aufklärung: Um die korrekte Anwendung des Sprechventils
zu gewährleisten, müssen der Patient, die Angehörigen,
das Pegepersonal und alle mit dem Patienten arbeitenden
Personen in den Gebrauch des Sprechventils eingewiesen
und über Kontraindikationen, Warnhinweise, Warnetiketten
und Vorsichtsmaßnahmen informiert werden.
Zu beachten
- Sicherstellen, dass das Atmungssystem des Patienten für
die Ausatmung rund um die Trachealkanüle und aus der
Nasen- und Mundhöhle geeignet ist.
- Sicherstellen, dass der Arzt der Entblockung des Cuffs
beim Patienten zugestimmt hat. Der Cuff muss bei der
Verwendung des Sprechventils entblockt werden, sodass
die ausgeatmete Luft rund um die Trachealkanüle und
durch die oberen Atemwege strömen kann.
- Zum leichteren Sprechen sicherstellen, dass die Größe der
Trachealkanüle so gewählt wurde, dass ein ausreichender
Luftstrom rund um die Trachealkanüle möglich ist. Der
Cuff an der Kanüle kann auch im entblockten Zustand ein
Hindernis darstellen, was bei der Beurteilung der Durchgängigkeit der Atemwege berücksichtigt werden sollte.
- Auf Anordnung des Arztes beurteilen, ob ein Wechsel auf
eine kleinere Trachealkanüle indiziert ist, um einen für die
Verwendung des Sprechventils ausreichenden Luftstrom zu
erhalten.
- Bei Patienten mit zähüssigem, nicht beherrschbarem Se-
kret, das Atemwegsobstruktionen verursachen kann, muss
die Verwendbarkeit des Sprechventils beurteilt werden.
- Beurteilen, ob bei Patienten, die Schwierigkeiten bei der
Verwendung des Sprechventils haben, Atemwegsobstruktionen aufgrund von Stenosen, Gewebemassen, Tracheomalazie, Granulation, Stimmbandlähmung in der Mittellinienposition, Sekret oder einer für die Trachea des Patienten
zu großen Trachealkanüle vorliegen können. Wenn die Obstruktion behoben wurde, sollte erneut beurteilt werden, ob
das Sprechventil für den Patienten infrage kommt.
Allgemeines
(HME 15 und 22 in Kombination mit Sprechventil oder HME
Dig iTop).
30
 Loading...
Loading...