Page 1
Operating Instructions
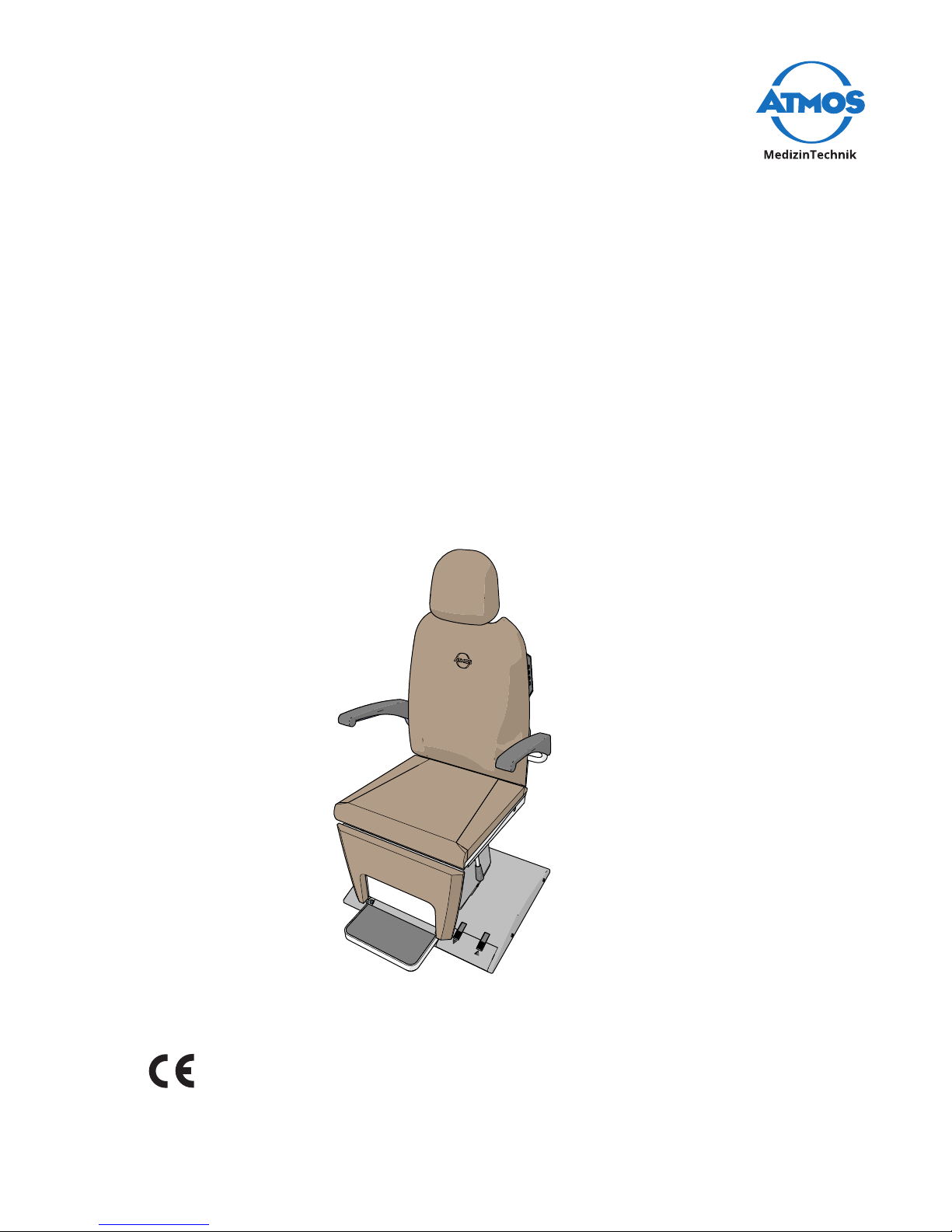
ATMOS® Chair
Professional
English
GA1GB.130220.0
2018-11 Index 01
Page 2

2
Table of contents
1 Introduction.......................................................................................................3
1.1 Notices on the operating instructions .....................................................................3
1.2 Explanation of pictures and symbols ......................................................................4
1.3 Intended use ...............................................................................................................5
1.4 Function .......................................................................................................................6
1.5 Intended users ...........................................................................................................6
1.6 Scope of delivery ........................................................................................................6
1.7 Transport and storage ...............................................................................................6
2 Notices for your safety .....................................................................................7
2.1 General safety information .......................................................................................7
2.2 Danger for users, patients and third parties ..........................................................7
2.3 Avoid damage to the device......................................................................................8
3 Setting up and starting up ...............................................................................9
3.1 Device overview ..........................................................................................................9
3.2 Connecting the device ............................................................................................ 10
4 Operation .........................................................................................................11
4.1 Ambient conditions during operation .................................................................. 11
4.2 Positioning the patient ........................................................................................... 11
4.3 Adjusting the Patient Chair .................................................................................... 11
4.3.1 Adjusting the arm rests ..................................................................................... 11
4.3.2 Adjusting the foot support ............................................................................... 12
4.3.3 Adjusting the backrest....................................................................................... 12
4.3.4 Adjusting the headrest ...................................................................................... 12
4.3.5 Adjusting the seat height .................................................................................. 13
4.3.6 Turning the Patient Chair .................................................................................. 13
4.3.7 Turning the seating surface (optional) ............................................................ 13
4.4 Adjusting the shock position ................................................................................. 14
4.5 Moving the Patient Chair (Mobile/Complete versions) ....................................... 14
4.6 Activating and deactivating the operation lock ................................................... 15
5 Cleaning and Disinfection ..............................................................................16
5.1 Cleaning and disinfecting the surfaces ................................................................ 16
5.2 Cleaning and disinfecting the upholstery ............................................................ 16
5.3 Recommended disinfectants ................................................................................. 17
6 Maintenance and service ...............................................................................18
6.1 Period tests .............................................................................................................. 18
6.2 Function check ......................................................................................................... 18
6.3 Lubricating moving parts ....................................................................................... 18
6.4 Sending in the device .............................................................................................. 18
7 Troubleshooting ..............................................................................................20
8 Disposal ............................................................................................................21
9 Technical data .................................................................................................22
10 Notices on EMC ................................................................................................23
Page 3

3Introduction
1 Introduction
1.1 Notices on the operating instructions
These operating instructions contain important instructions on how to
operate your product safely, correctly and eectively.
These operating instructions are designed for training and instructing
new operating personnel in the use of the system, and also for use as a
reference manual. This document may only be reprinted, either in part
or in whole, with written permission from ATMOS.
These operating instructions must always be kept to hand near
the device.
Care, period tests, regular cleaning and proper application are
essential. They ensure the operational safety and usability of the
product.
Maintenance, repairs and period tests may only be carried out by
persons who have the appropriate technical knowledge and are
familiar with the product. The person must possess the necessary test
devices and original spare parts required to carry out these measures.
Read chapter “2 Notices for your safety” on page 7 before using
the product for the rst time. This will help you to avoid potentially
dangerous situations.
The product bears the CE marking CE in accordance with EC Directive
93/42/EEC of the Council concerning medical devices and meets the
basic requirements of Appendix I of the directive.
The product complies with all the applicable requirements of Directive
2011/65/EC on the restriction of the use of certain hazardous
substances in electrical and electronic equipment (“RoHS”).
The Declaration of Conformity and our general standard terms and
conditions can be viewed on our website at www.atmosmed.com.
The quality management system at ATMOS has been certied
according to international standard EN ISO 13485.
These operating instructions are valid for the following devices:
• ATMOS® Chair Professional basic 535.5200.0
• ATMOS® Chair Professional mobile 535.5400.0
• ATMOS® Chair Professional electrical 535.5300.0
• ATMOS® Chair Professional complete 535.5500.0
Page 4
4
Introduction
1.2 Explanation of pictures and symbols
In the operating instructions
DANGER
Warning of a danger that results in immediate fatal or serious injury. Observe the
necessary measures.
WARNING
Warning of a danger that can cause fatal or serious injury. Observe the necessary
measures.
CAUTION
Warning of a danger that can cause minor injury. Observe the necessary measures.
ATTENTION
Notice of a danger that can damage the product or other objects. Observe the
necessary measures.
Warning of a danger that can cause fatal or serious injury.
Notice of potential material damage.
Useful information on the handling of the device.
1.
Action. Proceed step by step.
»
Result of an action.
Move/plug in this direction.
click
Engage, check correct t.
On device and type plate
Follow operating instructions (blue)
Warning; take extra care to observe
This product complies with the relevant requirements of the EU Directives.
Manufacturer
Manufacturing date
SN
Serial number
REF
Order number
Application part type B
Page 5

5Introduction
For indoor use only
Professional disposal
Equipotential bonding
Alternating current
Earth
Max.
Maximum load
Do not transport any weights or persons on the chair (Mobile/Complete
versions).
1.3 Intended use
Intended use
• As a treatment chair for treatment and examination on patients who are seated or
lying down
• In clinical areas, medical practices, company doctors, dentists (prophylaxis)
• As an appropriate and comfortable surface for supporting the patient during
treatment/examination. As an aid for the persons performing the procedure (in
order to improve ergonomics and quality during the work)
• Intended users: exclusively for personnel in the appropriate department who have
received the appropriate training and instruction, i.e. doctors, assistants, nurses,
carers, etc.
• Installation and start-up may need to be carried out by the operating company’s
technical personnel, or those of an authorised specialist dealer
NON-intended use
• In the vicinity of high-frequency surgical devices or debrillators
• In environments where ammable or explosive gases or vapours (e.g. anaesthetics)
may occur
• As a work chair
• As an operating chair/table
• As a substitute for a bed
Page 6

6
Introduction
1.4 Function
The ATMOS® patient chair can be adjusted to suit the individual patient. The seat height
can be adjusted electrically using a built-in or separate foot switch, and the height and
angle of the headrest can be altered manually. The backrest can be tilted manually or
electrically until completely horizontal; the leg rest will move in synchronisation with the
backrest. The top section of the chair can be rotated, and the arm rests can be folded up
individually.
An operation lock can be set to prevent the chair from being tampered with or operated
accidentally. The chair may also have an optional turning seating surface.
Versions
Basic Mobile Electrical Complete
Electrically adjustable seat height
x x x x
Manually adjustable backrest
x x
Electrically adjustable backrest
x x
Synchronised leg rest
x x x x
Mobile
x x
Rotary seating surface Optional Optional Optional Optional
1.5 Intended users
Exclusively for personnel in the appropriate department who have received the
appropriate training and instruction, i.e. doctors, assistants, nurses, carers, etc.
1.6 Scope of delivery
ATMOS® Chair
Professional
Operating
Instructions
1.7 Transport and storage
Only transport the device in a shipping carton that is padded and oers sucient
protection.
If damage occurs during transport:
1. Document and report the transport damage.
2. Send the device to ATMOS, see Chapter “3. Check whether all the xing screws are
fully tightened.” on page 18.
Ambient conditions for transport and storage:
• Temperature: -10...+50°C
• Relative humidity: 20...95% without condensation
• Air pressure: 700...1060hPa
Page 7

7Notices for your safety
2 Notices for your safety
The safety of the ATMOS® Chair Professional corresponds to the recognised technical
regulations and the guidelines in the German Medical Devices Act.
Please read and pay attention to the safety instructions prior to using the product.
2.1 General safety information
Activate the operation lock if you need to leave your patients alone, or if there is a risk of
buttons and foot switches being operated unintentionally. Never leave elderly persons,
children, injured persons, persons with disabilities or other patients who require
assistance alone on the patient chair.
The Declaration of Conformity shall be rendered null and void if any changes are made
to the product by the customer or any third parties. This includes, but is not limited to:
conversions of all types, the use of third-party accessories, and the removal of warning
and information signs.
If any signs are missing from your device or no longer legible, contact ATMOS and
replace the signs in question.
2.2 Danger for users, patients and third parties
Electric shock due to unsuitable mains connection, incorrect handling of the
product or damage to product components.
Risk of burns, cardiac arrhythmias and even fatal injury.
• Prior to each use, check for damage to the device and the power cable. Do
not operate the device if you notice any damage. In this case, mark the device
accordingly, clean and disinfect it, and send it in to ATMOS for repair.
• If the patient chair rolls over the power cable or the power cable is otherwise
subjected to strain or loads: check that the power cable is not damaged. Do not
operate the patient chair if you notice any damage.
• Do not clean the device in a washing plant or using a high-pressure cleaner.
• Disconnect the device from the mains power supply prior to cleaning or disinfection.
• The device can only be disconnected from the power supply by pulling out the power
plug.
• Position the device so that it can always be disconnected from the mains power
supply easily.
• Never connect the device to a mains power supply without a protective conductor.
• Take care to ensure that no liquid gets into the device. If liquid gets into the device,
operation of the device must cease. In this case, clean and disinfect the device and
send it to ATMOS for repair.
• Only use original accessories and original spare parts from ATMOS.
• Observe the specications regarding period tests in chapter “6.1 Period tests” on
page 18.
• Assembly, new settings, alterations, extensions and repairs may only be carried out
by authorised persons.
• Do not modify the device without the manufacturer’s permission.
Page 8

8
Notices for your safety
Keep the device fully functional at all times.
Malfunctions could cause injury to you and your patients.
• Prior to each use, check whether the device is damaged. Do not operate the device if
you notice any damage.
• Perform a function check at least once a week, and also at any other time if you have
any concerns with regard to the safety of the device.
• Please observe the notices on the electromagnetic compatibility (EMC) of the device.
• Observe the specications regarding period tests in chapter “6.1 Period tests” on
page 18.
• Only use original accessories and original spare parts from ATMOS.
• Assembly, new settings, modications, extensions and repairs may only be carried
out by persons who are authorised by ATMOS.
Risk of tipping due to overloading on one side.
The patient chair may tip over, injuring you or your patient.
• Ensure that the patient sits in the middle of the seating surface. The patient must
not be allowed to sit on the arm rest, backrest or leg rest.
• Move the patient chair to the bottom position and the backrest to an upright
position before the patient sits down or stands up.
Risk of infection due to patient secretion on the device!
Risk of diseases being transmitted.
• Clean and disinfect the device after every use.
• Clean and disinfect the device in accordance with the operating instructions.
Explosion and re hazard!
There is a risk of burns and injuries.
• Never operate the device in potentially explosive areas or areas which are
oxygenated.
• Only use original accessories and original spare parts from ATMOS.
Only a fully functional product meets the safety requirements of users, patients and
third parties. As such, observe the following instructions regarding the product.
2.3 Avoid damage to the device
Damage caused by rolling the patient chair in contravention of intended use.
There is a risk of damage to the patient chair and the power cable.
• Do not transport any weights or persons on the patient chair.
• Do not roll the patient chair over the power cable.
Damage due to excessive load.
There is a risk of damage to the foot support.
• Do not allow patients to place their full weight on the foot support.
Page 9

9Setting up and starting up
3 Setting up and starting up
3.1 Device overview
Front view
1
2
4
5
6
7
9
10
11
12
13
1
2
4
5
6
7
8
9
10
11
12
13
1
Headrest
2
Backrest
3
Control panel (Electrical/
Complete versions)
4
Arm rest
5
Backrest release lever (Basic/
Mobile versions)
6
Equipotential bonding
connection
7
Release lever for top section
of chair
8
Roller foot pedal (Mobile/
Complete versions)
9
Base
10
Foot switch
11
Foot support
12
Leg rest
13
Seating surface
Basic Mobile
1
2
4
6
7
9
10
11
12
13
3
1
2
3
4
6
7
8
9
10
11
12
13
Electrical Complete
Rear view
3
1
2
4
5
6
1
Control panel (Electrical/Complete versions)
2
Handle for turning seating surface (optional)
3
Release lever for top section of chair
4
Power cable
5
Headrest locking screw (height)
6
Headrest locking screw (angle)
Page 10

10
Setting up and starting up
Control panel (Electrical/Complete versions)
1
2
3
4
1
Backrest Back
button
2
Backrest forward
button
3
chair up
button
4
chair down
button
3.2 Connecting the device
1. Clean and disinfect the product prior to rst use.
2. Connect the equipotential bonding if required.
The equipotential bonding must be connected if required by the spatial environment
in which the product is going to be used. Refer to the specications of standard IEC
EN 60601-1.
3. Check whether the mains voltage and frequency of the device match the voltage and
frequency of the mains power supply .
The device's specications can be found on the type plate.
4. If the mains voltage and mains frequency match: Connect the device to a mains
power supply with a protective conductor using the supplied power cable.
5. Perform a function check; see Chapter “6.2 Function check” on page 18.
Page 11

11Operation
4 Operation
4.1 Ambient conditions during operation
The product is intended exclusively for use in buildings and under normal ambient
conditions. Do not subject the upholstery to high temperatures.
• Temperature: +10...+35°C
• Relative humidity: 20...95% without condensation
• Air pressure: 700...1060hPa
The device can only be disconnected from the power supply by pulling out the power
plug. Position the device so that it can always be disconnected from the mains power
supply easily.
4.2 Positioning the patient
Ensure that the patient sits in the middle of the seating surface. The patient must not be
allowed to sit on the arm rest, backrest or leg rest. Do not allow patients to place their
full weight on the foot support.
1
2
Risk of tipping due to overloading on one side.
1. Move the backrest
1
to an upright position.
2. Move the chair
2
to its bottom position.
3. Do not let the patient sit down or stand up until
this is done.
4.3 Adjusting the Patient Chair
Risk of crushing by moving parts.
There is a risk of the patient’s ngers being crushed.
• The patient's arms must remain on the arm rest at all times while you are adjusting
the patient chair.
1
4.3.1 Adjusting the arm rests
Folding the arm rests up
1. Fold the arm rest
1
up in a controlled manner.
Folding the arm rests down
The arm rests can be damaged if allowed to drop
down under their own weight.
1. Fold the arm rest
1
down in a controlled manner.
CAUTION
Page 12

12
Operation
1
4.3.2 Adjusting the foot support
Unfolding the foot support
1. Pull the handle and fold the foot support down.
Folding the foot support away
1. Fold the foot support up.
1
4.3.3 Adjusting the backrest
Mechanical
1. Push the backrest release lever
1
down, pushing
or pulling on the backrest at the same time.
2. Let go of the backrest release lever
1
as soon as
the backrest reaches the required position.
» The backrest is xed in place.
1
2
Electrical (Electrical/Complete versions)
1. Press the
Backrest Back 1 button and hold it down
until the backrest reaches the required position.
The leg rest will move in synchronisation with the
backrest.
2. To move the backrest back to an upright position,
press the
Backrest forward
button 2.
1
4.3.4 Adjusting the headrest
Angle
1. Undo the headrest locking screw (angle)
1
and
move the headrest to the required position.
2. Tighten the headrest locking screw (angle)
1
.
» The headrest is xed in place at the current
angle.
Page 13

13Operation
1
Height
1. Undo the headrest locking screw (height)
1
and
move the headrest to the required position.
2. Tighten the headrest locking screw (height)
1
.
» The headrest is xed in place at the current
height.
1
2
4.3.5 Adjusting the seat height
1. Press the corresponding foot switch 1.
Electrical/Comfort versions: you can also use the
appropriate button on the backrest
2
.
1
4.3.6 Turning the Patient Chair
1. Pull the release lever for rotary movement 1
forwards.
2. Turn the top section of the chair.
3. Push the release lever for rotary movement
1
back.
» The patient chair is xed in place.
1
4.3.7 Turning the seating surface
(optional)
1. Turn the seating surface in the required direction
using the two handles 1.
» The seating surface will latch into place.
Page 14

14
Operation
4.4 Adjusting the shock position
1. Adjust the angle of the backrest up to a fully
horizontal position.
4.5 Moving the Patient Chair (Mobile/Complete versions)
Damage caused by moving the patient chair in contravention of intended use.
There is a risk of damage to the patient chair and the power cable.
• Do not transport any weights or persons on the patient chair.
• Do not roll the patient chair over the power cable.
1
1. Move the chair to its bottom position.
2. Move the backrest to an upright position.
3. Pull the power plug out of the socket and place
the power cable on the patient chair.
4. Place the foot switch on the patient chair.
5. Press the foot pedal
1
down to extend the
rollers.
6. Move the patient chair to the required position.
7. Press the foot pedal
1
up to retract the rollers.
ATTENTION
Page 15

15Operation
4.6 Activating and deactivating the operation lock
Activate the operation lock if you need to leave your patients alone, or if there is a
risk of buttons and foot switches being operated unintentionally. Never leave elderly
persons, children, injured persons, persons with disabilities or other patients who
require assistance alone on the patient chair.
1
2
Foot switch
1. Press both foot switches simultaneously and
hold down for ve seconds
1
.
» A signal will sound. The operation lock will be
activated or deactivated.
Control panel (Electrical/Comfort versions)
1. Press the
chair up
and
chair down
buttons 2 at the
same time and hold them down for ve seconds.
» A signal will sound. The operation lock will be
activated or deactivated.
Page 16

16
Cleaning and Disinfection
5 Cleaning and Disinfection
We recommend that you always document all maintenance work and part replacements
in writing.
It is the responsibility of the user to ensure that the required results for cleaning and
disinfection are adhered to. Validation and routine monitoring of the procedure will
generally be necessary.
Electric shock due to liquid inside the device.
Risk of burns, cardiac arrhythmias and even fatal injury.
• Do not clean the device in a washing plant or using a high-pressure cleaner.
• Make sure that the cleaning cloth is only damp, not wet.
• Clean and disinfect the device in accordance with the operating instructions.
Risk of infection due to secretion on the product and accessories.
Risk of diseases being transmitted.
• Always wear disposable gloves during all cleaning operations.
• Clean the product after each use.
• Clean and disinfect the device in accordance with the operating instructions.
5.1 Cleaning and disinfecting the surfaces
Clean the product after each use. Disinfect the patient chair if it becomes contaminated
by secretion or other substances, at least once per day is necessary.
1. Move the chair to its bottom position.
2. Disconnect the chair from the mains power supply.
3. Moisten a clean, non-linting cloth with lukewarm, soapy water or a conventional
plastic cleaning agent.
4. Clean the surfaces with the damp cloth.
5. Disinfect the surfaces with a clean, non-linting cloth and a recommended
disinfectant.
6. Leave the device to dry before using it again.
5.2 Cleaning and disinfecting the upholstery
Always remove any oil, grease or sweat from the upholstery immediately. Clean the
patient chair after each use. Disinfect the patient chair if it becomes contaminated by
secretion or other substances, at least once per day is necessary.
1. Move the chair to its bottom position.
2. Disconnect the chair from the mains power supply.
3. Use warm, mild, soapy water.
4. Clean the upholstery using a damp micro-bre cloth or a soft brush.
5. Disinfect the upholstery with a clean, non-linting cloth and a recommended
disinfectant. Do not allow the disinfectant to soak in for longer than necessary.
WARNING
CAUTION
Page 17

17Cleaning and Disinfection
5.3 Recommended disinfectants
Unsuitable cleaning agents and disinfectants.
Risk of damage to the device surface, corrosion damage and stress cracks.
• Do not use cleaning agents or disinfectants that contain the following ingredients:
• Irritants
• Substances that may damage plastics or steel components or dissolve lubricants.
Unsuitable cleaning agents and disinfectants.
There is a risk of damage to the upholstery.
• Do not use cleaning agents or disinfectants that contain the following ingredients
for the upholstery:
• Solvents or chlorides
• Polish or wax polish
• Chemical cleaning agents
• Oils, greases or alcohol
• Use cleaning agents with a pH value of between 6 and 8.
• Observe the manufacturer’s specications with regard to concentration and soaking-
in time.
Use a suitable surface disinfectant.
ATTENTION
ATTENTION
Page 18

18
Maintenance and service
6 Maintenance and service
Maintenance, repairs and period tests may only be carried out by persons who have the
appropriate technical knowledge and are familiar with the product. The person must
possess the necessary test devices and original spare parts required to carry out these
measures.
ATMOS recommends: Work should be carried out by an authorised ATMOS service
partner. This ensures that the repairs and testing are carried out professionally, that
original spare parts are used and that warranty claims remain unaected.
6.1 Period tests
Please comply with the country-specic guidelines regarding regular testing, especially
with regard to electrical safety.
ATMOS recommends performing a test every 24 months.
6.2 Function check
Perform a function check at least once a week, and also at any other time if you have
any concerns with regard to the safety of the device.
1. Check whether the chair or power cable are damaged. Look particularly closely for
cracks and kinks.
2. Move the chair to its bottom position, paying attention to any unusual noises or
odours.
3. Electrical/Complete versions: Adjust the backrest, paying attention to any unusual
noises or odours.
4. Check that all the moving parts can be adjusted without any problems:
• Backrest
• Arm rests
• Headrest
• Foot support
• Optional: Rotary seating surface
5. Check whether the chair can be turned without any problems.
6. Mobile versions only: Check whether the chair can be moved without any problem.
6.3 Lubricating moving parts
Lubricate the moving parts at regular intervals to keep them fully functional.
1. Remove any dirt from the moving parts.
2. Lubricate the axes and joints using commercially available oil or grease.
3. Check whether all the xing screws are fully tightened.
6.4 Sending in the device
1. Clean and disinfect the product and accessories in accordance with the operating
instructions.
2. Place used accessories with the product.
3. Fill in the QD 434 “Delivery complaint/return shipment” form and the corresponding
decontamination certicate.
This form is enclosed with each delivery and can be found at www.atmosmed.com .
Page 19

19Maintenance and service
4. The device must be well padded and packed in suitable packaging.
5. Place the QD 434 “Delivery complaint/return shipment” form and the corresponding
decontamination certicate in an envelope.
6. Ax the envelope to the outside of the package.
7. Send the product to ATMOS or your dealer.
Page 20

20
Troubleshooting
7 Troubleshooting
The product has been subjected to a thorough quality control in the factory. If a fault
should occur despite this care, you may be able to resolve it yourself.
Error symptom Possible cause Remedy
The patient chair
is no longer
responding when
buttons are
pressed.
Operation lock active 1. Deactivate the operation lock;
see page 15.
The patient chair
is no longer
responding when
buttons are
pressed; a pulsing
signal sounds.
The system has crashed,
e.g. due to uctuations in
the mains voltage
1. Press the
chair up
and
chair down
buttons at the same time and
keep them held down until the
pulsing signal tone sounds and
then stops again.
» The system has been reset.
Inexplicable noises
detected.
Motor defective or worn 1. Disconnect the device from the
mains power supply.
2. Contact an authorised ATMOS
service partner.
The motor is
becoming hot.
Page 21

21Disposal
8 Disposal
Packaging
1. Please recycle the packing.
ATMOS® Chair Professional
Do not dispose of the product together with household waste.
Basic/Mobile versions: The product contains oil. Oil must be disposed
of professionally, and must not be allowed to penetrate into the soil or
water.
1. Clean and disinfect the device.
2. In Germany: Send the product in to ATMOS or your specialist dealer. They will
dispose of the device professionally.
3. In other countries: Dispose of the product professionally and according to the
country-specic laws and regulations.
In Germany, the device is excluded from the Electrical and Electronic Equipment Act
(ElektroG) in accordance with the National Register for waste electric equipment
because it may be contaminated. Do not dispose of the device in the electrical waste.
The housing is fully recyclable. Observe to the country-specic laws and regulations.
Gas springs (Basic/Mobile versions)
Observe the following instructions if you are unable to return the product to ATMOS or
your specialist dealer for disposal.
Risk of injury and crushing due to high internal pressure in the gas springs and
sudden movements of the piston rods.
There is a risk of injury to the eyes, face and hands.
• Wear suitable protective equipment.
• Cover the vent.
• Take extra care when operating the release mechanism after the gas springs have
been removed.
1. Depressurise the gas spring. Always observe the gas spring manufacturer’s
specications. The manufacturer is named on the type plate.
2. Drain the oil used for the gas spring.
3. Dispose of the oil properly and professionally.
4. Recycle the gas spring.
CAUTION
Page 22

22
Technical data
9 Technical data
Voltage 230V~ ± 10%; 50/60Hz
Current consumption Max. 2A (230V)
Power consumption Max. 460W
Fuses T 6.3 A (for 230V~, 50/60Hz)
Seat height 58cm (rigid seat upholstery)
Max. lifting capacity 200kg (safe working load)
Vertical speed Max. 18mm/s
Height of backrest Approx. 600mm (without headrest)
Approx. 780mm (headrest in bottom position)
Rotation
• Basic/Mobile versions 360°
• Electrical/Complete versions +/-165°
Backrest inclination +7° up to -90°
Operating time Intermittent operation: 2min/18min
Protective earth conductor resistance Max. 0.2Ω
Earth leakage current Max. 0.5mA
Housing leakage current Max. 0.1mA
Patient leakage current Max. 0.1mA
Ambient conditions for transport/
storage
• Temperature -10...+50°C
• Humidity without condensation 20...95%
• Air pressure 700...1060hPa
Ambient conditions for operation
• Temperature +10...+35°C
• Humidity without condensation 20...95%
• Air pressure 700...1060hPa
Maximum operational altitude ≤2000m
Contamination level Class 1
Overvoltage category II
Dimensions (LxWxH)
• Basic/Electrical versions 730x 700x 1300mm (foot support folded
away, headrest in bottom position)
• Mobile/Complete versions 730x 705x 1300mm (foot support folded
away, headrest in bottom position)
Weight
• Basic/Electrical versions 98kg
• Mobile/Complete versions 110kg
Period tests Recommended: Test every 24 months.
Protection class (EN 60601-1) I
Degree of protection Application parts type B
Type of protection IP X4
Page 23

23Notices on EMC
Classication according to Appendix
IX, EC Directive 93/42/EEC
Class 1
In accordance with Regulations 1& 12, Section
3
CE marking CE
UMDNS code 10-794
ID No. (REF) 535.5200.0 Basic
535.5300.0 Electrical
535.5400.0 Mobile
535.5500.0 Complete
10 Notices on EMC
As soon as the patient chair is connected to the mains power supply and highly sensitive
measuring equipment is in use at the same time, it is impossible to guarantee that the
patient chair is not electro-magnetically aecting the measurement results.
Page 24

ATMOS MedizinTechnik GmbH & Co. KG
Ludwig-Kegel-Straße 16
79853 Lenzkirch/Germany
Phone: +49 7653 689-0
atmos@atmosmed.de
GREINER GmbH
Wettestrasse 1
74385 Pleidelsheim, Germany
Phone: +49 7144 8112-0
info@greiner-gmbh.de
www.atmosmed.com www.greiner-gmbh.de
 Loading...
Loading...