ArthroCare Coblator II User Manual

COBLATOR® II (RF80000E) SYSTEM
Coblator® II
(RF8000E) System
User’s Manual
User’s Manual
1
ENGLISH

2
COBLATOR® II (RF8000E) SYSTEM
User’s Manual

COBLATOR® II (RF80000E) SYSTEM
User’s Manual
This equipment has been tested and found to comply with the limits for medical devices to IEC/EN 60601-1-2:2007.
These limits are designed to provide reasonable protection against harmful interference in a typical medical
installation. This equipment generates, uses, and can radiate radiofrequency energy and, if not installed and used
in accordance with the instructions, may cause harmful interference to other devices in the vicinity. If this equipment
does cause harmful interference to other devices, which can be verified by turning the equipment off and on, the
user is encouraged to try to correct the interference by one or more of the following measures:
• Reorient or relocate the receiving device,
• Increase the separation between the affected equipment and the Controller,
• Connect the affected equipment to an outlet or circuit different from that to which the Controller is connected, or
• Consult the manufacturer or field service technician for help.
High frequency surgical unit with respect to electrical shock, fire and mechanical hazards only in accordance with:
UL 60601-1/CAN/CSA C22.2 No. 601.1, IEC/EN 60601-2-2.
3
ENGLISH

4
COBLATOR® II (RF8000E) SYSTEM
User’s Manual

COBLATOR® II (RF80000E) SYSTEM
User’s Manual
Table of Contents
Description, Indications for Use, and Contraindications . . . . . . . . . . . . . . . . . . . . . . 7
Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Indications for Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Contraindications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
System Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
Connection Diagram . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
Principle of Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Warnings, Precautions, and Adverse Events . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10
Warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10
Precautions. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10
Adverse events. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12
Controls, Indicators, and Alarms. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12
Controls & Indicators . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12
Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Diagram of Controls, Indicators, and Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
5
ENGLISH
Unpacking, Assembly, and System Check. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15
Unpacking . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15
Assembly and System Check . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15
Instructions for Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
Operator Training Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .16
General System Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
Voltage Outputs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
System Preparation and Care . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .18
System Preparation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .18
Wand Selection. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .19
System Shut Down . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .19
System Storage and Transport . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .19
Equipment Disposal . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19

6
COBLATOR® II (RF8000E) SYSTEM
User’s Manual
Cleaning and Disinfection ..............................................................................................................................20
Controller, Flow Control Valve Unit, and Flow Control Cable ..................................................................................... 20
Foot Control ............................................................................................................................................................... 20
Wand..........................................................................................................................................................................20
Maintenance and Troubleshooting ............................................................................................................. 21
Maintenance .............................................................................................................................................................. 21
Fuse Replacement ..................................................................................................................................................... 21
Troubleshooting Guide ............................................................................................................................................... 21
Product Specifications .................................................................................................................................... 23
Technical Specifications ............................................................................................................................................. 23
Controller Output Graphs ........................................................................................................................................... 24
Electromagnetic Environment Specifications ............................................................................................................. 27
Controller Classification and Safety Verification .................................................................................31
Classification .............................................................................................................................................................. 31
Safety Verification ...................................................................................................................................................... 31
Customer Service .............................................................................................................................................. 32
Warranty Information ..................................................................................................................................................32
Product Complaints .................................................................................................................................................... 28
Symbols Key ........................................................................................................................................................ 33

COBLATOR® II (RF80000E) SYSTEM
User’s Manual
Description, Indications for Use, and
Contraindications
Description
The Coblator® II (RF8000E) is a bipolar, radiofrequency (RF) electrosurgical system designed for use in
otorhinolaryngology (ENT) surgery. The System consists of the following components:
1) an electrosurgical radiofrequency controller;
2) a reusable, non-sterile Power Cord;
3) a reusable, non-sterile Foot Control;
4) a reusable, non-sterile Flow Control Valve Unit;
5) a reusable, non-sterile Flow Control Cable;
6) a single use, disposable, sterile Wand
The Controller is the power source that delivers radiofrequency (RF) energy to the treatment site via the Wand.
The sterile disposable Wand device is available in various single or multi-tip configurations and is supplied separately.
Indications for Use
The Coblator II (RF8000E) is indicated for ablation, resection, and coagulation of soft tissue and hemostasis of
blood vessels in otorhinolaryngology (ENT) surgery.
7
ENGLISH
Contraindications
The Coblator II (RF8000E) is contraindicated in any procedures where a conductive solution is not used.
The Coblator II (RF8000E) is also contraindicated for patients who have cardiac pacemakers or other electronic
implants. Please refer to the Wand Instructions for Use for a more comprehensive list of contraindications regarding
specific procedures.
8
COBLATOR® II (RF8000E) SYSTEM
User’s Manual
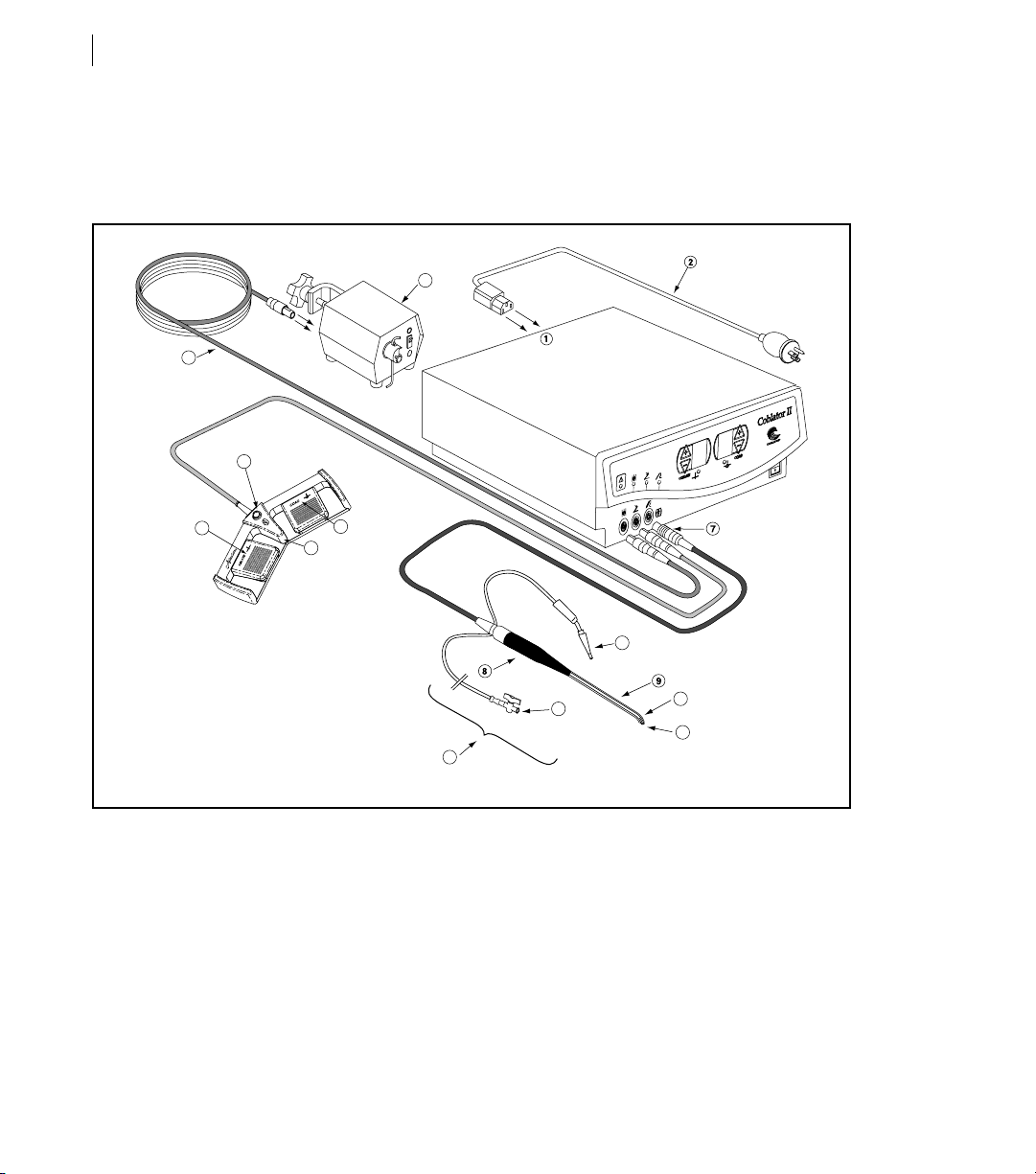
System Overview
Connection Diagram
15
6
16
4
5
3
12
13
14
10
11
1. Controller 09. Shaft
2. Power Cord 10. Return Electrode
3. Foot Control 11. Active Electrode Tip
4. Ablate Pedal 12. Suction Tube (Optional on Wand Style)
5. Coagulation Pedal 13. Irrigant Tube (Optional on Wand Style)
6. Coblate Set Point Adjustment Button 14. Wand
7. Controller Connectore 15. Flow Control Cable
8. Handle 16. Flow Control Valve Unit

COBLATOR® II (RF80000E) SYSTEM
User’s Manual
Principle of Operation
The Coblator II (RF8000E) System Controller is designed to deliver RF energy to the electrode elements located at
the distal end of the sterile single-use Wands. Current flows between the active electrode element and the return
electrode element, providing a localized energy field. The result of this arrangement is controlled energy delivery
with minimal collateral tissue damage.
The Coblator II (RF8000E) System works by passing RF energy through a conductive solution (such as normal
saline) in close proximity to or in contact with the tissue to be treated. The conductive solution forms a thin layer
between the active and return electrode elements. In the Coblate mode, when sufficient energy is applied, the
conductive solution is converted into a vapor layer (plasma) containing energized charged particles. When the highenergy charged particles come in contact with tissue, they cause its disintegration through molecular dissociation.
This mode of operation results in relatively low treatment site temperatures when compared to conventional
electrosurgical systems, thus yielding limited collateral thermal damage to the surrounding untreated tissue.
The function of the device is different when a lower voltage is applied between the active electrode(s) and the target
tissue. In this case, the electrical field is below the threshold required to create a plasma layer and resistive tissue
heating occurs. This mode is useful when a greater thermal effect is needed, i.e. for coagulation of blood vessel
or other vascular tissues. The appropriate voltage setting will depend on the design of Wand used, tissue type, and
desired tissue effect.
9
ENGLISH
In contrast, monopolar systems only have an active electrode on the device tip. The current path travels from the
active electrode through the patient’s body to a return pad affixed to the patient’s body. This results in significantly
more energy passing through the patient’s body and through the surrounding tissue.

10
COBLATOR® II (RF8000E) SYSTEM
User’s Manual
Warnings, Precautions, and Adverse Events
The following is a list of Warnings and Precautions that apply to the general operation of the Coblator II (RF8000E).
For specific warnings and precautions, please refer to the Wand and the Patient Cable Instructions for Use.
WARNINGS
• Failure to follow all applicable instructions may result in serious surgical consequences.
• Explosion Hazard: The following substances will contribute to increased fire and explosion hazards in the
operating room: flammable substances (such as alcohol-based skin prepping agents and tinctures), flammable
anesthetics, naturally occurring flammable gases which may accumulate in body cavities such as the bowel,
oxygen enriched atmospheres, and oxidizing agents such as nitrous oxide (N
• Fire Hazard: DO NOT place active accessories near or in contact with flammable materials (such as gauze or
surgical drapes).
• Electrosurgical accessories, which are activated or hot from use, can cause a fire.
• Accessory tips may remain hot enough to cause burns after the electrosurgical current is deactivated.
• Inadvertent activation or movement of Wands outside the field of vision may result in injury to the patient.
• Localized burns to the patient or physician may result from electrosurgical current carried through other
instruments and conductive objects.
• Electrosurgical current may be generated in conductive objects by direct contact with the active electrode or by
the active or return electrode being in close proximity to a conductive object.
• If excessive heating or physical forces cause damage to the Wand tip, foreign body fragments may result,
possibly requiring extended surgery for removal.
• DO NOT use the Coblator II (RF8000E) System with non-conductive media (e.g. sterile water, dextrose, air,
gas, glycine, etc.). Use only conductive media such as normal saline or Ringer’s lactate.
• Electric Shock Hazard: DO NOT connect wet accessories to the Controller.
2
O) atmospheres.
PRECAUTIONS
• Prior to initial use, ensure that all package inserts, warnings, precautions, and Instructions for Use are read and
understood.
• Safe and effective electrosurgery is dependent not only on equipment design, but also, to a large extent,
on factors under the user’s control. Only persons having adequate training and familiarity with orthopedic,
arthroscopic, spinal and neurosurgical surgeries should perform procedures with the Coblator II (RF8000E).
• Consult medical literature relative to techniques, complications, and hazards prior to performance of any procedure.
• Evaluate patients for predisposing medical problems that may be aggravated by the stress of surgery.
• A thorough understanding of the principles and techniques involved in electrosurgical procedures is essential to
avoid shock and burn hazards to both patient and medical personnel and damage to the device and other medical
instruments. Ensure that insulation or Controller grounding is not compromised.
• When instruments and accessories from different manufacturers are employed together in a procedure, verify
compatibility prior to initiation of the procedure.
• When not in use, remove the Wand from the surgical site and place outside of the operative field away from metallic
objects. Wands should remain separated from other electrosurgical equipment to avoid inadvertent electrical coupling
between devices. Otherwise, inadvertent activation may cause injury to patient and/or user or equipment damage.

COBLATOR® II (RF80000E) SYSTEM
User’s Manual
• DO NOT wrap Wand cables around metal objects. Wrapping cords around metal objects may induce currents
that could lead to shocks, fires, or injury to the patient or surgical personnel.
• Use caution when using Wand tips to probe or manipulate tissue. Forceful contact between Wand tips and tissue
or other instruments may result in damage to the instrument.
• DO NOT allow fluid to contact any electrical connectors on the Wands, Controller, or Cables during use.
• Maintain the lowest power setting necessary to achieve the desired tissue effect.
• DO NOT allow patient contact with grounded metal objects, such as a surgical table frame or an instrument
table, to avoid risk of shock. Grounding pads should not be used.
• DO NOT contact metal objects with an activated Wand.
• Observe fire precautions at all times. Sparking and heating associated with electrosurgery may be an ignition
source.
• DO NOT use flammable agents for cleaning and disinfection of the Controller or Cables.
• As with other electrosurgical units, electrodes and Cables can provide paths for high frequency current. Position
the cables to avoid contact with the patient or other electrical leads.
• High frequency (HF) electrosurgical equipment such as the Coblator II (RF8000E) System may adversely affect
the operation of other electronic equipment.
• Electrodes should remain separated from other electrosurgical equipment to avoid inadvertent electrical coupling
between devices.
• Monitoring electrodes should be positioned as far as possible from the surgical electrodes when HF surgical
equipment and physiological monitoring equipment are used simultaneously on a patient. Needle Monitoring
electrodes are not recommended.
• Monitoring equipment incorporating high frequency current-limiting devices is recommended.
• DO NOT remove the cover of the Controller. Refer servicing to qualified personnel.
• DO NOT obstruct the exhaust fan (located at rear of Controller).
• DO NOT touch the Controller’s fan and/or speaker while touching the patient.
• Before each use, check that all Controller indicator lights and audio signals are functional. Make sure that the
power cable plug is properly connected to the Controller receptacle.
• To avoid risk of fire, only replace the Controller fuses with the same type and rating.
• Controller failure could result in an unintended increase in output power.
• The Coblator II (RF8000E) System is designed to be operated exclusively as a unit. Only use accessories
provided by ArthroCare
• The Coblator II (RF8000E) System should not be used adjacent to or stacked with other equipment. If the system
is used adjacent to or stacked with other equipment, the system should be verified that it is operating in its intended
configuration.
• DO NOT use other ArthroCare Foot Controls. Use only the Foot Control provided with the Coblator II
(RF8000E) System.
• When endoscopes are used with endoscopically-used accessories, the patient leakage currents may be additive.
• The Controller may cause radio interference or may disrupt the operation of nearby equipment. It may be
necessary to take mitigation measures, such as reorienting or relocating the Controller or shielding the location.
• The controller is not intended to be used with a neutral electrode.
• DO NOT reuse ArthroCare PlasmaWands that have been designed for single use to avoid failure or
unanticipated performance.
11
ENGLISH
 Loading...
Loading...