Page 1
Recommendations for use:
Test
Concentration
Contact time
VAH – Hand Disinfection | DGHM / EN Standard Methods
VAH – hygienic hand disinfection
DGHM / EN 1500
undiluted
30
s
VAH – surgical hand disinfection
DGHM / EN 12791
undiluted
1,5
min
tuberculocidal
undiluted
30
s
DVV / RKI-Guideline – virucidal efficacy
limited virucidal* (inactivation of enveloped viruses)
(incl. HIV / HBV / HCV)
undiluted
15
s
Vacciniavirus
undiluted
15
s
BVDV (Bovine Viral Diarrhea Virus)
undiluted
15
s
virucidal* (inactivation of enveloped and non-enveloped viruses)
undiluted
60
s
Adenovirus
undiluted
30
s
Poliovirus
undiluted
60
s
SV40 (Simian-Virus 40)
undiluted
30
s
Rotavirus
undiluted
15
s
Coronavirus (SARS)
undiluted
30
s
EN 14476 – virucidal efficacy
virucidal
undiluted
30
s
Poliovirus
undiluted
30
s
Adenovirus
undiluted
30
s
Norovirus (MNV)a
undiluted
30
s
Influenza A and B
undiluted
15
s
EN 14348 – tuberculocidal efficacy
tuberculocidal
undiluted
30
s
RKI List
Range of efficacy A
undiluted
30
s
limited virucidal* (inactivation of enveloped viruses)
undiluted
30
s
Range of efficacy B
undiluted
90
s
*according to RKI recommendation, Bundesgesundheitsblatt 01/2004
a
murine Norovirus = surrogate virus for human Norovirus
© Antiseptica 2018-09-21 GB
Fields of application:
While enveloped viruses can be inactivated
quite easily, the inactivation of nonenveloped viruses, e. g. the poliovirus,
still poses a demanding challenge to
hygienic applications.
Despite high-percentage alcohol (e. g.
Ethanol 90 - 95 %) offers the desired
effect, it highly stresses the skin and can
only be stored in small quantities due to
its low flash point.
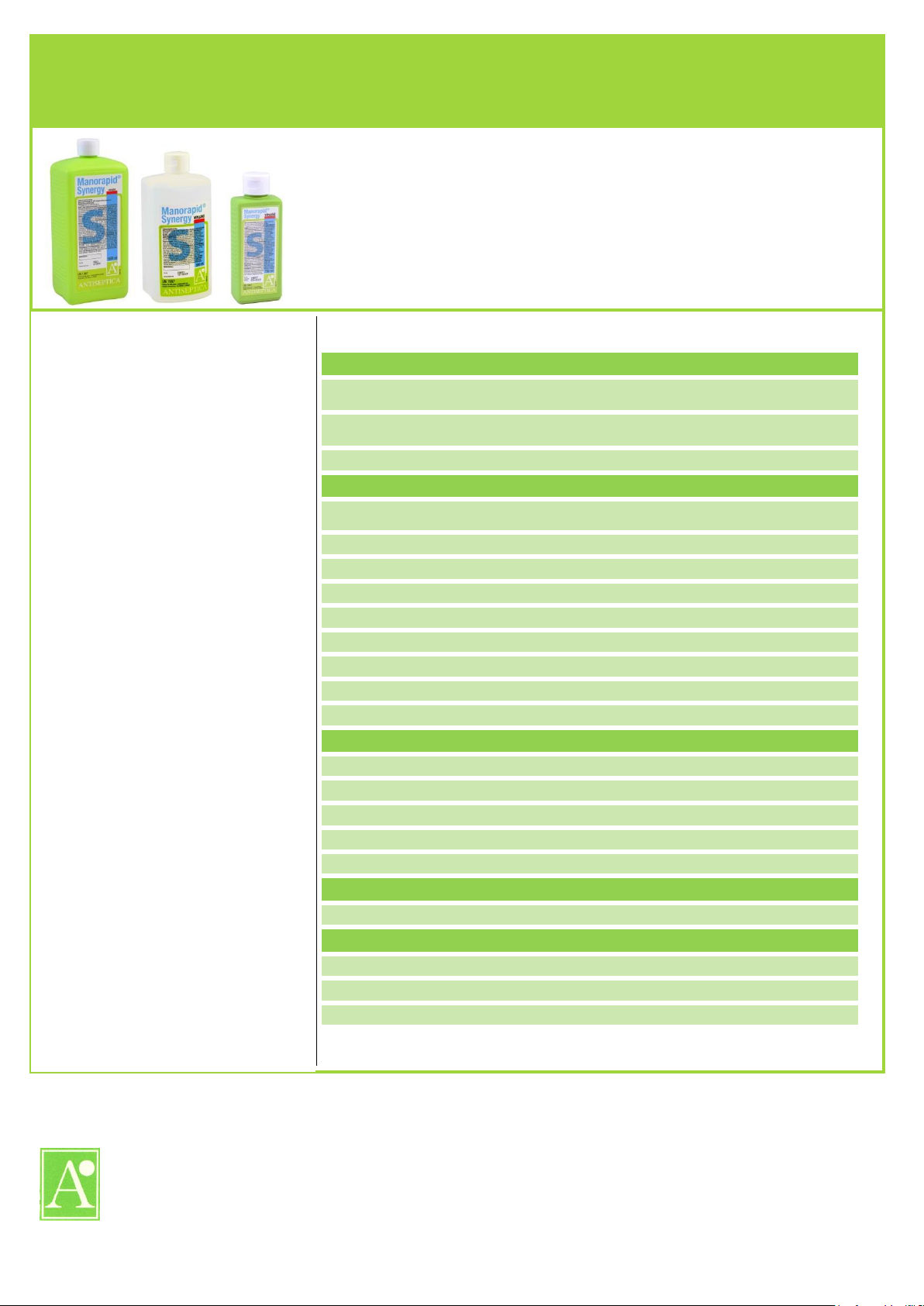
Manorapid® Synergy is a ready to use,
alcoholic solution for the hygienic and
surgical hand disinfection.
It contains a low amount of alcohol and
thus offers a good skin compatibility
which is further enhanced by the addition
of caring agents. Coincident, the product
exhibits an excellent efficacy against
enveloped and non-enveloped viruses.
Manorapid® Synergy provides powerful
and comprehensive support for the
prevention and control of infections,
particularly in areas of an increased
risk of virus infections.
Special characteristics:
very broad, virucidal spectrum of
efficacy
strong caring properties
pleasant scent
Spectrum of efficacy:
bactericidal
yeasticidal
tuberculocidal
virucidal*
Ready to use solution for the hygienic
and surgical hand disinfection in
areas with an increased risk of virus
contamination
Manorapid® Synergy
ANTISEPTICA
The company is certified according to EN ISO 13485
and meets the Council's requirements of Directive 93/42/EEC
on medical devices.
ANTISEPTICA Dr. Hans-Joachim Molitor GmbH
Legal successor of ANTISEPTICA chem.-pharm. Produkte GmbH
D-50259 Pulheim, Carl-Friedrich-Gauß-Straße 7
Tel.: +49-2234-984 66-0, Fax: +49-2234-984 66-11
E-Mail: info@antiseptica.com, www.antiseptica.com
ANTISEPTICA chem.-pharm. Produkte GmbH
A-1020 Wien, Handelskai 388 / Top 641
Tel.: +43-1-374 66 00, Fax: +43-1-374 66 00-66
E-Mail: office@antiseptica.at, www.antiseptica.at
Antisepsis by Antiseptica – Antisepsis mit Vernunft
page 1
Page 2
Forms of delivery:
carton of 30 x 150 ml bottles
carton of 20 x 500 ml bottles
carton of 10 x 1 L bottles
carton of 2 x 5 L canister
also available:
wall dispenser for 500 ml bottles
wall dispenser for 1 L bottles
dosing pump for 500 ml bottles
dosing pump for 1 L bottles
dosing pump for 5 L canister
The product is manufactured according
to the international Quality Management
System EN ISO 13485.
When handling the product, please note
the information according to the
Hazardous Substances Ordinance given
in the safety data sheet.
Further information is provided by your
expert advisor upon request or on our
website.
Use disinfectants safely. Always read the
label and product information before use.
Biocidal product according to BPR
BAuA Reg.-No.: N-45599
Special advice:
In case of contact with eyes, rinse
immediately with plenty of water and
seek medical advice.
Do not apply to mucous membranes
or open wounds.
Do not swallow or let it enter into the
blood circulation.
Please remember to use skin care
products, particularly upon frequent
application of alcoholic hand
disinfectants.
Contains alcohol, flammable.
Always keep biocidal products out of
the reach of children.
Pregnancy and breast feeding:
Manorapid® Synergy can safely be used
during pregnancy and breast feeding
when the provided instructions for use
are strictly implemented.
Manorapid® Synergy
© Antiseptica 2018-09-21 GB
Application:
For hygienic hand disinfection:
Rub undiluted product into hands and
keep them moist for 30 seconds.
For surgical hand disinfection:
Rub undiluted product into hands and
lower arms and keep them moist for
1.5 minutes.
For virus inactivation:
Allow Manorapid® Synergy to operate
according to the contact times given in
the recommendations for use.
Composition (active ingredients):
100 g contain:
54.1 g Ethanol
10.0 g Propan-1-ol
Listing:
VAH-list
ÖGHMP-list
RKI-list (Range of efficacy A, limited
virucidal* and B)
Physical and chemical properties:
Appearance: clear, colourless liquid
Scent: alcoholic, scented
pH-value: approx. 3.0 at 20 °C
Density: approx. 0.89 g/cm³
at 20 °C
Flash point: 22 °C according to
DIN 51755
ANTISEPTICA
The company is certified according to ISO 9001 and EN ISO 13485
and meets the Council's requirements of Directive 93/42/EEC
on medical devices.
ANTISEPTICA Dr. Hans-Joachim Molitor GmbH
Legal successor of ANTISEPTICA chem.-pharm. Produkte GmbH
D-50259 Pulheim, Carl-Friedrich-Gauß-Straße 7
Tel.: +49-2234-984 66-0, Fax: +49-2234-984 66-11
E-Mail: info@antiseptica.com, www.antiseptica.com
ANTISEPTICA chem.-pharm. Produkte GmbH
A-1020 Wien, Handelskai 388 / Top 641
Tel.: +43-1-374 66 00, Fax: +43-1-374 66 00-66
E-Mail: office@antiseptica.at, www.antiseptica.at
Antisepsis by Antiseptica – Antisepsis mit Vernunft
page 2
 Loading...
Loading...