ANDESFIT SA B23 Users Manual
GSH R5228 Owner’s Manual
Language: English
Unit: mg/dL
Market: US
VERSION Date By
M01A1Draft 2016/11/01 Karen Yeh
from FDA Single Patient version
BMU5228BTB_5228BT-M02A4

IMPORTANT SAFETY INFORMATION
Federal Communication Commission Interference Statement
This equipment has been tested and found to comply with the limits for a Class B
digital device, pursuant to Part 15 of the FCC Rules. These limits are designed to
provide reasonable protection against harmful interference in a residential installation.
This equipment generates, uses and can radiate radio frequency energy and, if not
installed and used in accordance with the instructions, may cause harmful
interference to radio communications. However, there is no guarantee that
interference will not occur in a particular installation. If this equipment does cause
harmful interference to radio or television reception, which can be determined by
turning the equipment off and on, the user is encouraged to try to correct the
interference by one of the following measures:
- Reorient or relocate the receiving antenna.
- Increase the separation between the equipment and receiver.
- Connect the equipment into an outlet on a circuit different from that to which the
receiver is connected.
- Consult the dealer or an experienced radio/TV technician for help.
FCC Caution: Any changes or modifications not expressly approved by the party
responsible for compliance could void the user's authority to operate this equipment.
This device complies with Part 15 of the FCC Rules. Operation is subject to the
following two conditions: (1) This device may not cause harmful interference, and (2)
this device must accept any interference received, including interference that may
cause undesired operation.
IMPORTANT NOTE:
Radiation Exposure Statement:
The product comply with the US portable RF exposure limit set forth for an

uncontrolled environment and are safe for intended operation as described in this
manual. The further RF exposure reduction can be achieved if the product can be
kept as far as possible from the user body or set the device to lower output power if
such function is available.
This transmitter must not be co-located or operating in conjunction with any other
antenna or transmitter.
Please use this device only for the intended use described in this user guide.
Before using this system to test your blood glucose, please read instructions
thoroughly and practice the test when you first use this system. Do a quality
check on the system by following the instructions and consult with healthcare
professionals for questions or problems.
Be aware of the safety of young children or handicapped persons near you
when you conduct a glucose test using this system.
Please keep the test strip vial away from children. The test strips and vial cap
can present a choking hazard.
GSH test strips can only be used with the GSH glucose meter.
Precaution
The meter and lancing device are for single patient use. Do not share them with
anyone including other family members!
Do not use on multiple patients!
All parts of the kit are considered biohazardous and can potentially transmit
infectious diseases, even after all cleaning and disinfection procedures have
been performed.
For further information, please refer to below:
FDA Public Health Notification: Use of Fingerstick Devices on More than One
Person Poses Risk for Transmitting Bloodborne Pathogens: Initial
Communication” (2010)
http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/
ucm224025.htm
CDC Clinical Reminder: Use of Fingerstick Devices on More than One Person
Poses Risk for Transmitting Bloodborne Pathogens” (2010)
http://www.cdc.gov/injectionsafety/Fingerstick-DevicesBGM.html
Lancet
Never share a lancet with anyone. Always use a new, sterile lancet; lancets are

for single use only.
Lancing Devices
Lancing devices should never be used for more than one person. Lancing
devices are re-useable, and the lifetime cleaning does not affect its
performance.
Test Strips
GSH Glucose test strips are intended for single use only. They should be
disposed of in an appropriate container according to your healthcare
provider’s instructions.
Meter
GSH Blood Glucose Monitoring System Model R5228 should only be used for
testing single patient.
Please test your blood glucose using the meter and the test strips with the
same reference number as labeled on the outer box of meter and the test
strips.

Limitations
1. Do not use for neonatal blood glucose testing or use the system for the
diagnosis or screening of diabetes.
2. Alternate site testing should not be used for CGM calibration or for insulin
dose calculations.
3. Hematocrit in the range of 20~60% does not affect the glucose results.
4. Cholesterol level up to 500 mg/dL(12.9 mmol/L) and Triglycerides up to 3,000
mg/dL(99.3mmol/L) have been shown not to affect glucose results.
5. Interference was observed for therapeutic levels of Glutathione.
6. Certain substances occurring in the blood naturally, such as uric acid, or from
normal therapeutic treatments (Ascorbic acid, L-Dopa, Tolazamide and Gentisic
acid) will not significantly affect results. However, higher concentrations in
blood may cause incorrect results.
7. There is no significant interference in the presence of galactose, maltose, or
fructose observed in blood glucose test.
8. Do not test blood glucose during or soon after xylose absorption test. Xylose
in the blood can give falsely elevated results.
9. The test strips may be used at altitudes up to 10,744 feet (3,275 m) without an
effect on test results.
10. Persons suffering from severe dehydration should not be tested using a
capillary whole blood sample.
11. Test results below 70 mg/dL(3.9 mmol/L) indicate low blood glucose
(hypoglycemia). Test results greater than 240 mg/dL (13.3 mmol/L )
indicate high blood glucose (hyperglycemia). If you get results below 70
mg/dL(3.9 mmol/L)or above 240 mg/dL(13.3 mmol/L), repeat the test, and
if the results are still below 70 mg/dL(3.9 mmol/L)or above 240 mg/dL(13.3
mmol/L), please consult your healthcare professional immediately2.
12. Not for use on critically ill patients, patients in shock, dehydrated patients or
hyper-osmolar patients.

TABLE OFCONTENTS
Understanding Your New System
Introduction 2-3
Test Principle 3
Appearance and Key Functions of the Meter 4
LCD Screen Overview 5
Installing/Replacing the Batteries 6
Settings (Date/Time/Alarm/Measuring Unit/Memory Deletion) 7-9
Performing a Control Test
About Control Solution Tests 10
Running a Control Solution Test 11-12
Testing Your Blood Sugar
Alternate Site Testing 13
How to Obtain a Blood Sample 14-15
Running a Blood Glucose Test 16-17
Recalling the Memory and Viewing the Average 18-19
Expected Values 20
Transmitting Results 20
Maintenance and Troubleshooting
Maintenance of Your System 21-24
Troubleshooting 25-26
Customer Service 27
Important Additional Information
Specifications 28
Ordering Supplies 28
Warranty Information 29
NOTE: For information about the name of the manufacturer of the lancing device
and the lancets, please refer to the package inserts that came with your
starter kits.

Understanding Your New System
Introduction
Intended Use / Indications for Use
The GSH Blood Glucose Monitoring System (Model R5228) is intended to be used for
the quantitative measurement of glucose in fresh capillary whole blood drawn from
the fingertips or forearm. The GSH Blood Glucose Monitoring System (Model R5228)
is intended to be used by a single person and should not be shared.
The GSH Blood Glucose Monitoring System (Model R5228) is intended for
self-testing outside the body(in vitro diagnostic use) by people with diabetes at
home as an aid in monitoring the effectiveness of a diabetes control program.
The GSH Blood Glucose Monitoring System (Model R5228) should not be used for
the diagnosis of or screening of diabetes or for neonatal use. Alternative site testing
should be done only during steady state times (when glucose is not changing rapidly).
The GSH Test Strips are for use with the GSH Blood Glucose Meter to quantitatively
measure glucose in fresh capillary whole blood drawn from the fingertips or
forearm.
The GSH Control Solutions are for use with the GSH Blood Glucose Monitoring
System (Model R5228) as a quality control check to verify that the meter and test
strips are working together properly.
Contents of the System
This User Guide has been prepared to describe the proper use of the GSH Blood
Glucose Monitoring System Model R5228. Please read this User Guide and the
package insert that comes with the GSH Glucose Test Strips before testing. The
system is available either as a meter alone or as a kit. If you have a meter only, you
can purchase the system supplies from your system provider. Please provide to your
system provider with the 4 digit system reference number marked on the packaging
box when you purchase supplies.

The starter kit of GSH Blood Glucose Monitoring System Model R5228 includes the
following items:
1. GSH Glucose Meter with 2 AAA batteries
2. User Guide
3. Log Book
4. Carrying Case
5. GSH Glucose Test Strips
6. GSH Control Solution (Level II)
7. Sterile Lancets
8. Lancing Device
9. Package Inserts for Test Strips, Control Solution, and Lancets/Lancing Device
A single level control solution (Level II) is provided in the "Starter Kit," and no control
solution is included in the "Meter Only" kit. You may purchase GSH Control Solution
Level I, Level II or III from your system provider if needed.
The GSH Glucose Meter uses GSH Glucose Test Strips. Neither the meter nor the test
strips will work when used with any other brand of glucose products.
Test Principle
When glucose reacts with the reagents on the test strips, an electrical current is
produced, which is proportional to the glucose concentration in the blood sample.
The glucose concentration is calculated by the meter and based on the current
measured.
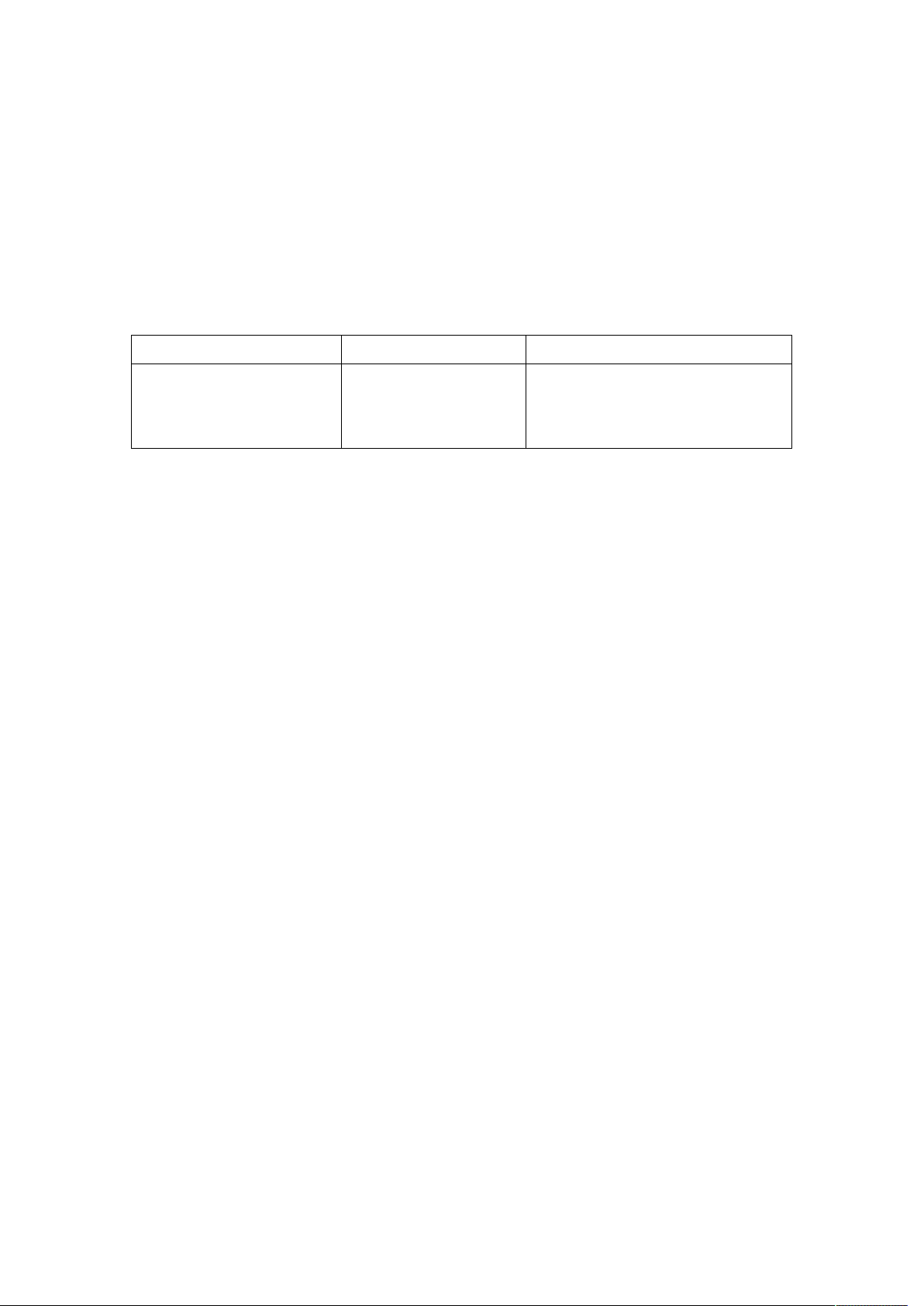
Appearance and Key functions of the meter
1. Test strip slot - When the strip is inserted into the slot, the meter will
automatically turn on.
2. LCD Display - Guide you through the test using symbols and simple messages.
3. M Key - Power ON/OFF, also for memory recalling mode, please refer to
manual for detailed function description.
4. C Key - Setting mode, please refer to manual for detailed function description.
5. Battery Compartment - Where batteries are located.
6. Ejector - Remove used strip.
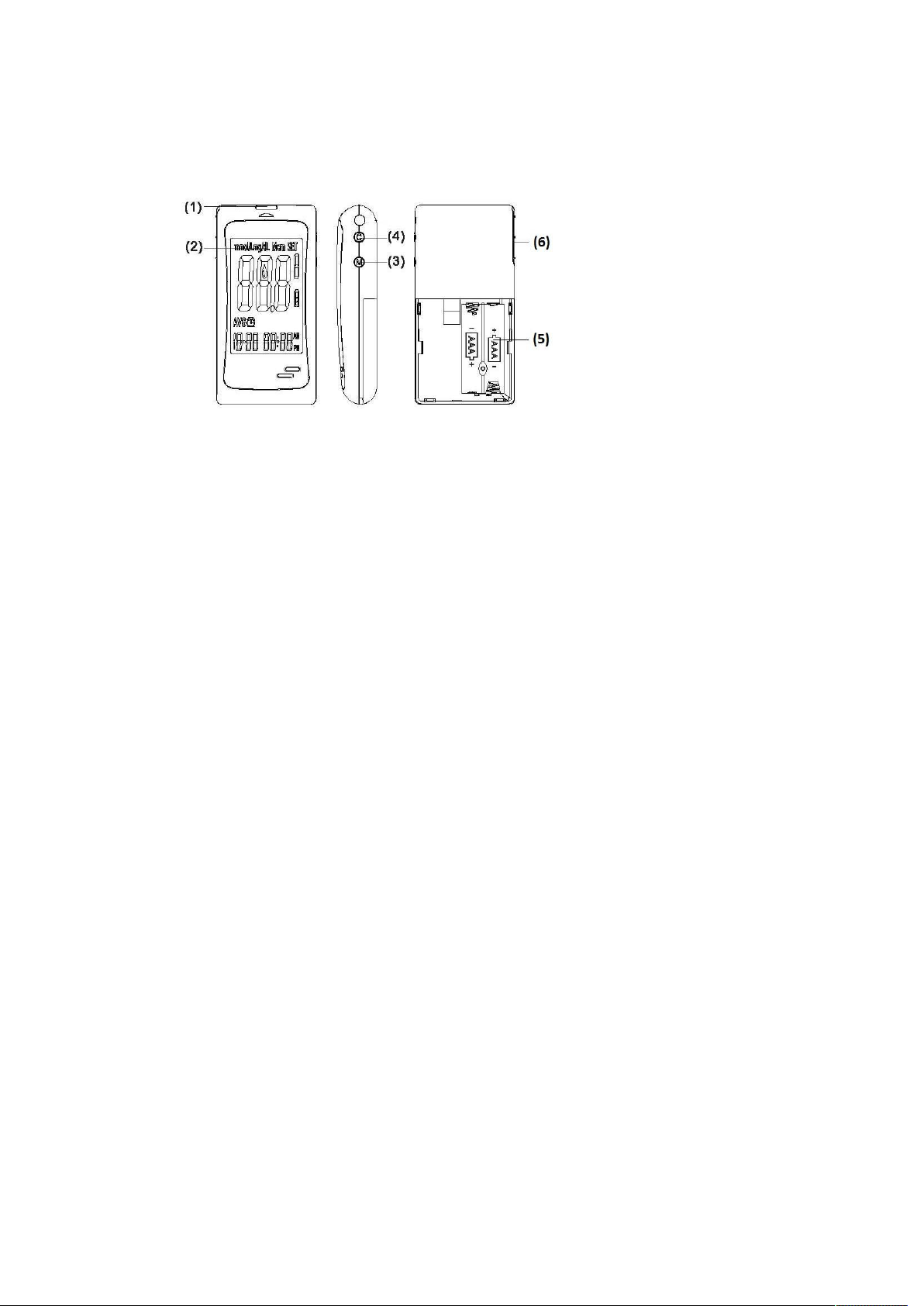
LCD Screen Overview
1. Appears with the test result either in mg/dL or in mmol/L.
2. Appears when you recall the memory.
3. Appears when you are in setting mode.
4. Appears for control solution test flag.
5. Indicates the meter is ready to take the blood sample when it flashes.
6. Indicates the battery status.
7. Indicates current displayed result is an average.
8. Appears when alarm is on.
9. Month
10. Day
11. Hour
12. Minute
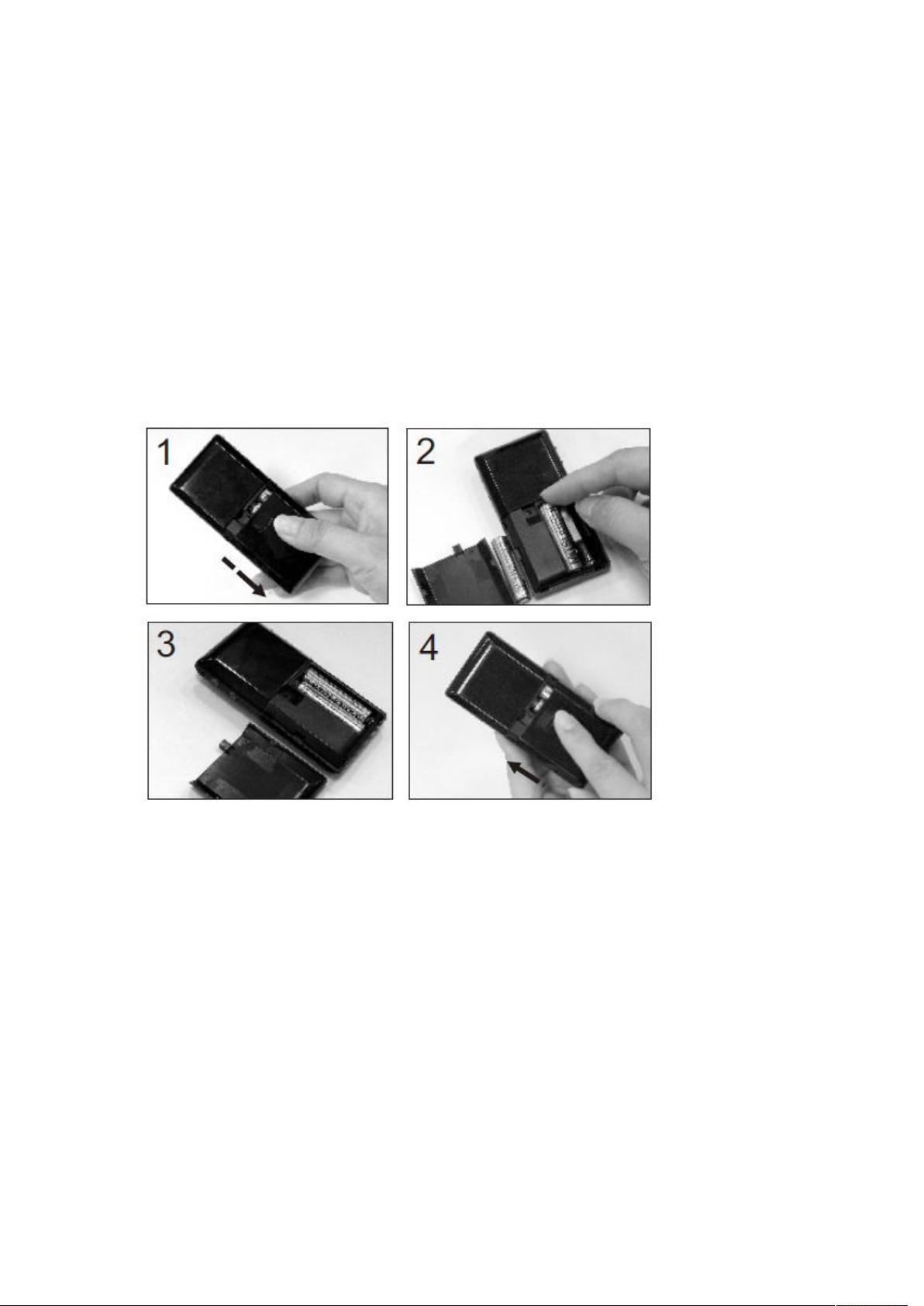
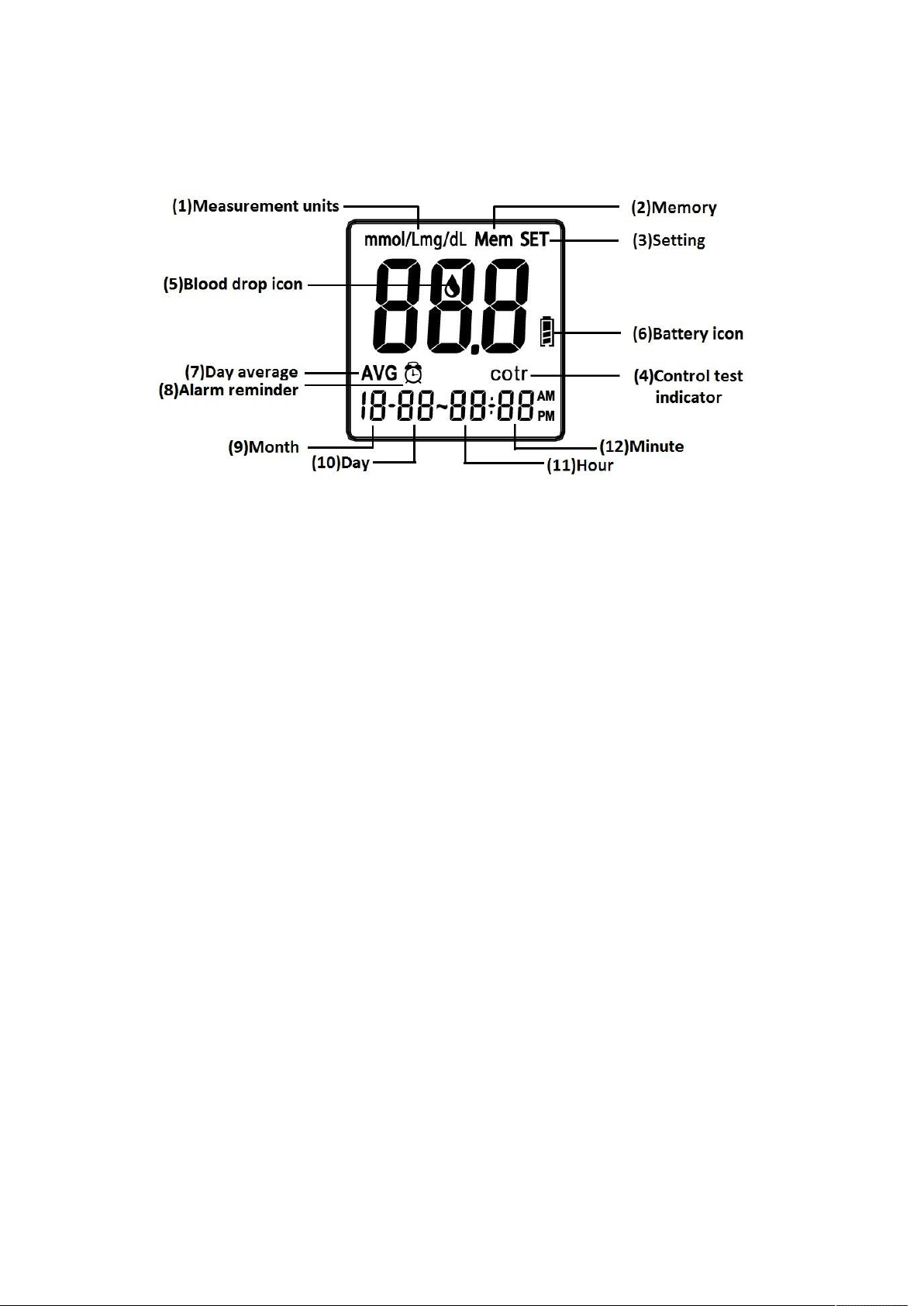
Installing/Replacing the Batteries
1. Turn off the meter by pressing “M” button and hold for 2 seconds before
replacing the batteries. Slide down the battery cover on the back of the meter
by pushing the cover in the direction of the arrow and put the cover aside.
2. Remove the two used batteries.
3. Insert 2 new batteries with correct +/- direction.
(Battery: AAA 1.5V 2 Alkaline 24A LR03)
4. Put the battery cover back in place by pushing the cover in the opposite
direction of the arrow to close the cover into position.
NOTE:
1. It is suggested that batteries need to be replaced when the battery icon
appears empty. If you keep using the meter, the battery icon will start
flashing and an error message E_3 will appear on the screen.
2. Note that replacing the battery will not reset the stored testing results.
3. You need to reset the time and date after the batteries are replaced.
4. If there is any unexpected symbol on the display, please follow the above
procedure to replace the batteries again.
5. Please dispose of batteries according to your local ordinances.
 Loading...
Loading...