Aircast VenaFlow 30A, VenaFlow 30AXL, VenaFlow 30AXXL User manual
VenaFlow
®
system 30A, 30AXL, 30AXXL
Operator’s Manual
prophylaxis for DVT and associated PE
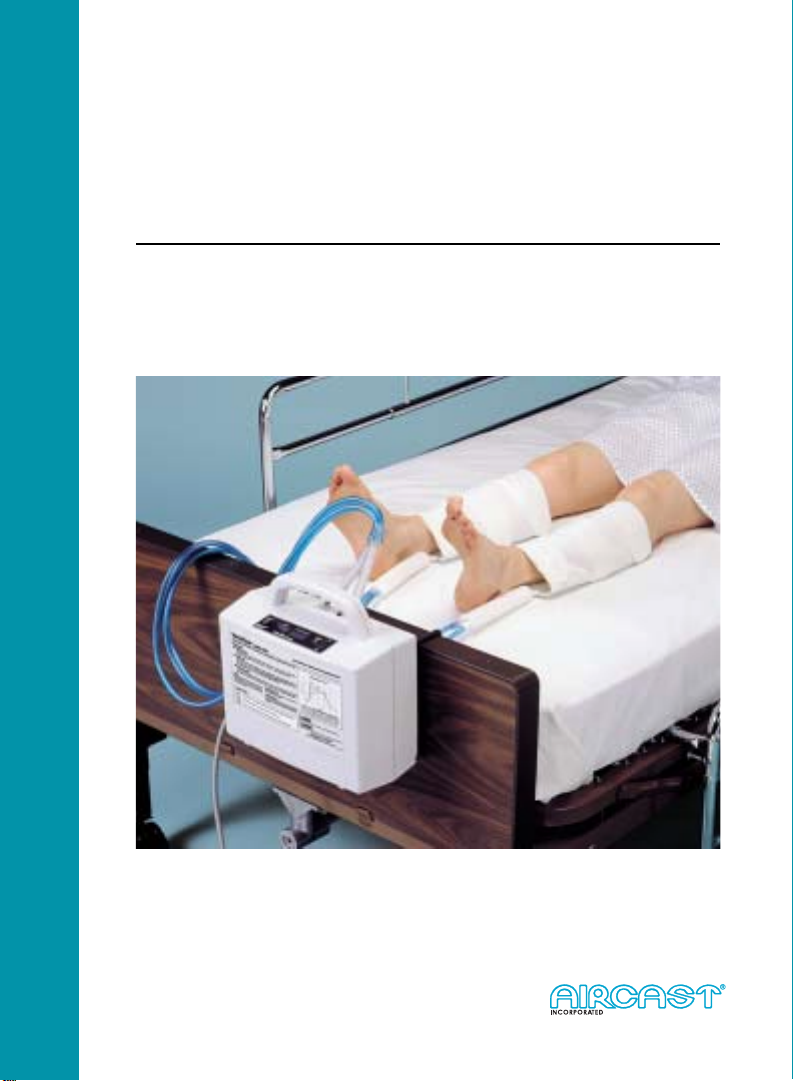
VenaFlow system with Calf Cuff


VenaFlow
®
system
Contents
Introduction. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Design Philosophy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Function . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Indications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Contraindications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Cuff Features . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Pump Features . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Application . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7–10
Ambulation. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Single Cuff Application . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Top Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Top Panel Displays/Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Service . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Warranty . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Pressure Verification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Optional Functional Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
Sizing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Components and Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Latex . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Ordering . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Notes. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
1
2
VenaFlow
®
system
Introduction
Aircast® pioneered graduated, pneumatic compression for the functional
management of orthopedic injuries. The VenaFlow system evolved from this
unique experience, and from performance principles established by earlier
researchers in pneumatic compression. VenaFlow provides intermittent
pneumatic compression as a prophylaxis for deep vein thrombosis. This
graduated, sequential, compression accelerates blood velocity and enhances fibrinolysis.
4,9,12,13
Design Philosophy
The VenaFlow system is designed to move blood faster and be more comfortable than any other known device.
Studies show that the Aircast VenaFlow system increases venous velocity
significantly more than other intermittent pneumatic compression (IPC) systems. This is because the compression is extremely rapid, graduated and
sequential — principles for optimal DVT prophylaxis demonstrated by Kamm,
et al, at MIT.
and Foot Cuffs, reduces the risk of pooling of blood between compartments
that is seen with conventional segmented cuffs.13 In addition, the VenaFlow
system’s compression is asymmetric for more effective emptying of the veins.
The unique cuffs are designed to enhance patient compliance and comfort,
and are available in three designs (Calf, Foot, and Thigh) to suit individual
physician preferences.
6,7,10
The patented Duplex™ aircell design, employed in the Calf
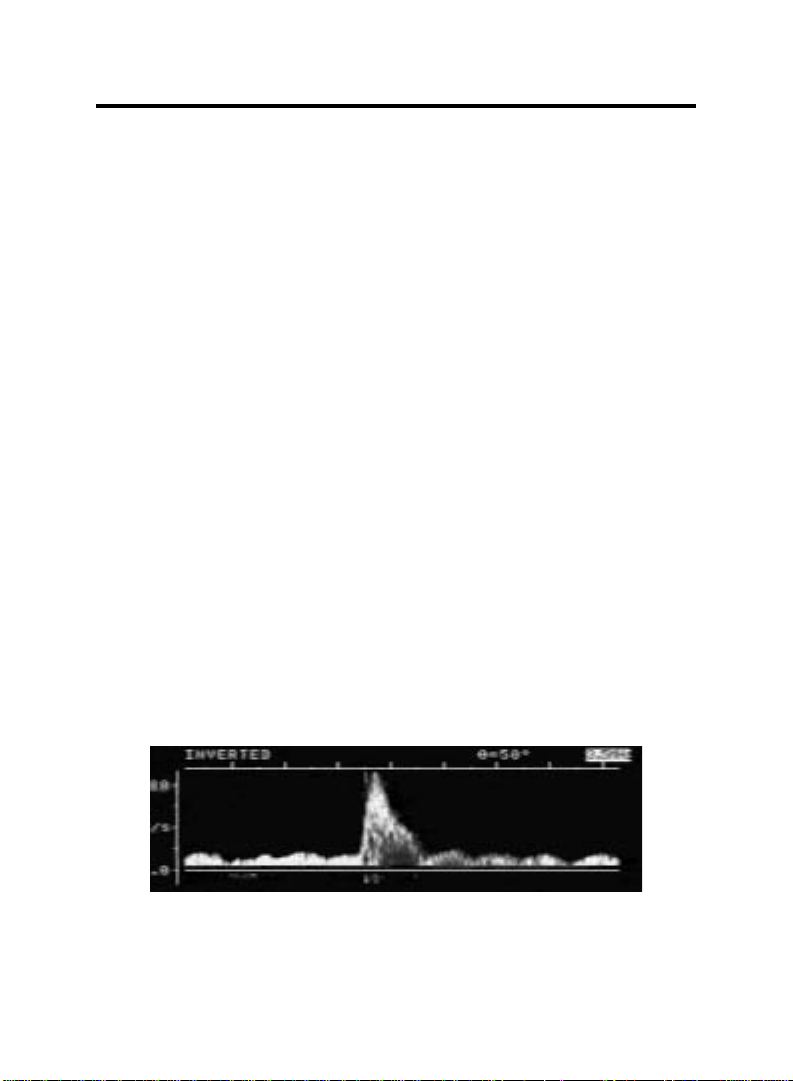
VenaFlow system’s rapid, graduated compression causes a three to four fold increase
in femoral vein velocity (Calf Cuff). In a typical Doppler trace, precompression velocity
is .19 meters per second, the peak during compression, .92 m/s — an increase of 384%.
This compares with the 80% to 140% increase found with conventional systems.
3,9
VenaFlow
®
system
3
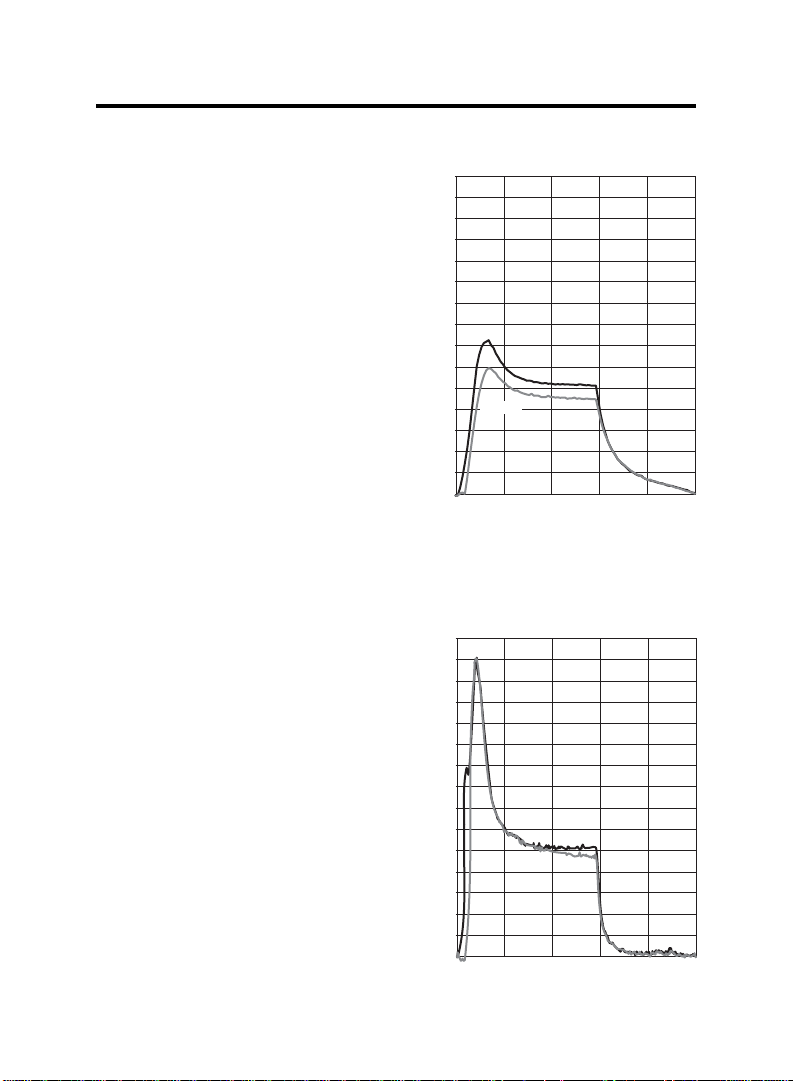
Function
The cuffs are applied, pump and
tube assembly connected, and
pump turned on. Compression is
rapid, graduated and sequential
for maximum effectiveness. The
distal aircell inflates rapidly, then
settles to 52 mmHg ±10%, and the
proximal aircell follows in .3 seconds to settle at 45 mmHg ±10%.
After 6 seconds of compression,
the cuffs deflate. This cycle repeats every minute.
When the pump is activated, the
peak pressures gradually rise
through the first five minutes of operation. This allows the patient to
gradually adjust to the treatment.
The VenaFlow system pump
automatically adjusts compression for each style of cuff. While
peak pressures vary with each
cuff style, the settle pressures
remain consistent for all cuffs.
Compression Curve Using Calf Cuff
150
140
130
120
110
100
90
80
70
60
Calf Compression mmHg
50
Proximal
40
30
20
10
0
0
2
Distal
46
Seconds
8
Compression Curve Using Foot Cuff
150
140
130
120
110
100
90
80
70
60
50
Foot Compression mmHg
40
30
20
10
0
0246810
Distal
Proximal
Seconds
10

4
VenaFlow
®
system
Indications
The N.I.H., Consensus Development Conference Panel on Prevention of
Venous Thrombosis and Pulmonary Embolism (Volume 6, Number 2)
that DVT is a marker for PE, and that risk of DVT is high following major
high risk surgery: general, orthopedic, urological, neurological, gynecological, and trauma. The Panel found external pneumatic compression an
effective and safe modality in these surgical categories.
8
found
Contraindications
The VenaFlow system should not be used by persons with known or suspected deep vein thrombosis, congestive heart failure, pulmonary edema,
thrombophlebitis, severe arteriosclerosis, or active infection. Do not use on
extremities which are not sensitive to pain, where cuff will interfere with gangrene, on patients with vein ligation or recent skin grafts, or extreme deformity
of the leg. Do not use the VenaFlow system where increased venous or
lymphatic return is undesirable.
VenaFlow
®
system
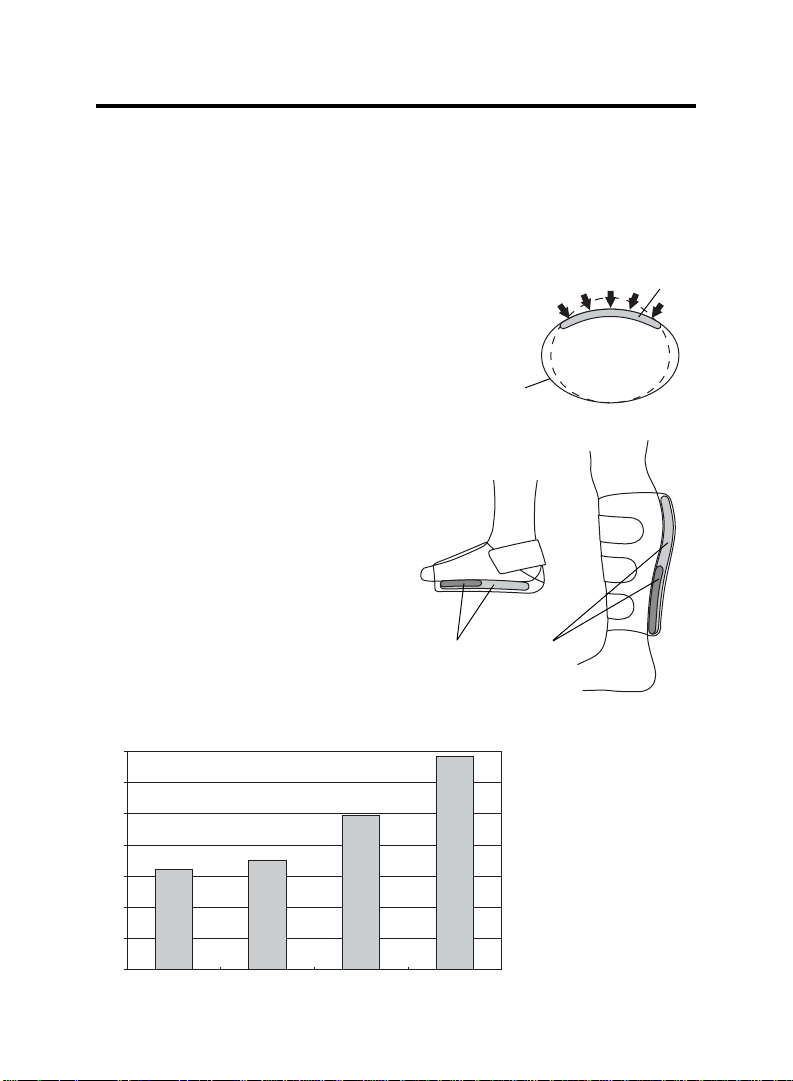
Cuff Features
• Comfort All cuffs are made from lightweight, hypoallergenic fabric. This
material is very breathable and encourages evaporation to keep the patient cool and comfortable.
• Asymmetric Compression All cuff designs ap-
ply focused pressure, maximizing blood velocity
and total flow.
• Seamless Duplex™ Aircell design
In both the Calf and Foot Cuff designs, the proximal aircell overlaps
the distal, providing consistent, uninterrupted compression.
(see Evaporation Rate Chart below)
Breathable Fabric
.
Aircell
5
14
12
10
8
Hours
6
4
2
0
Evaporation Rate for Calf Cuff
Sock Only
VenaFlow Brand X Brand Y
Duplex Aircell Design
In tests to compare
evaporation, a wet
sock with 50 cc’s of
moisture was placed
on an artificial leg.
With nothing covering
the sock, it took 6.4
hours to lose half its
moisture. The test
was repeated on each
of the three products.
6
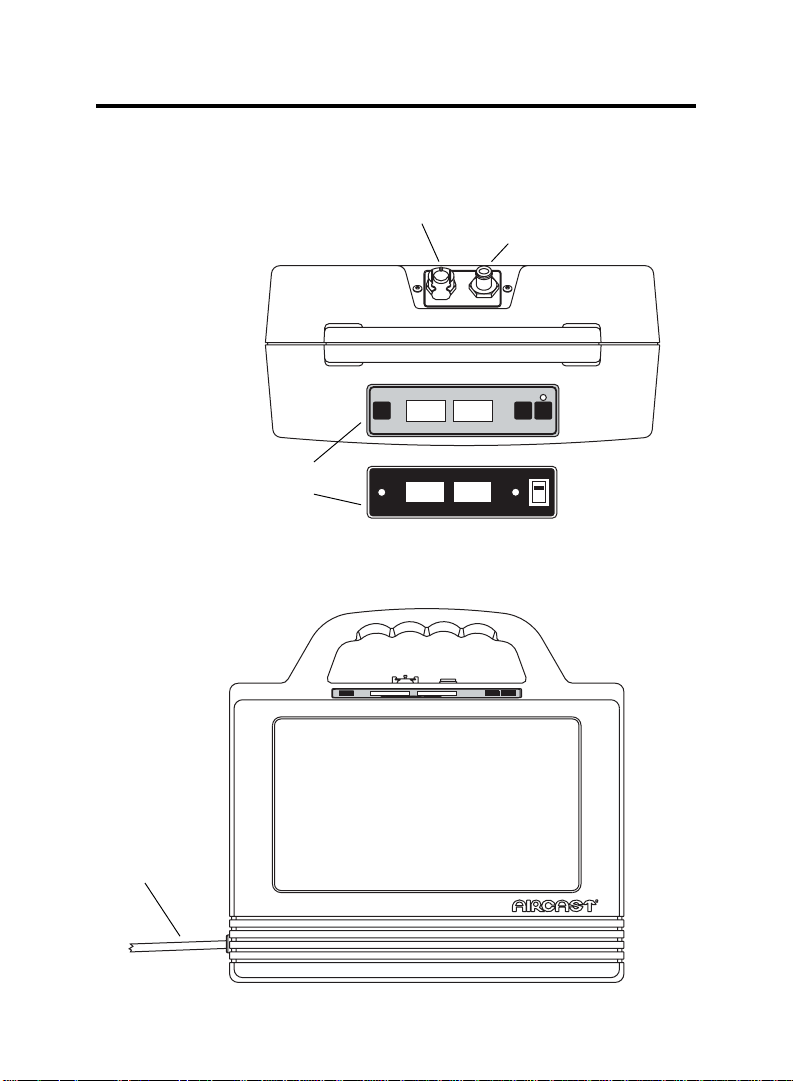
Pump Features
VenaFlow
®
system
Top View
Top Panel
Front View
with gray face
{
or
with black face
Proximal Output
Distal Output
COUNTER
COUNTER
VenaFlow
COUNTER
VenaFlow
PRESSURE-mmHg/ALARM
®
system
PRESSURE-mmHg / ALARM
®
system
COUNTER
RESET
RESET
ON / OFF
ALARM
RESET
ON
ALARM
RESET
OFF
Power Cord
 Loading...
Loading...