Page 1

Prepare to Meet the Challenges
of a Regulated PFAS Landscape
Page 2

Page 3

A Public Health Crisis
Decades in the Making
Per- and polyfluoroalkyl substances (PFAS) are man-made substances widely used
in industry and manufacturing because of their unique properties. These compounds
have been used for several decades in many applications like nonstick cookware,
stain-repellent clothing, food packaging materials, detergents, cleaning products,
and firefighting foams. The widespread use of these compounds has led to their
ubiquity in the environment. Studies have indicated that PFAS are also present
in most humans. Research on these compounds has identified them as being
persistent and bioaccumulative (especially PFAS with a carbon chain length >C7).
Toxic effects, including tumors and thyroid disruption, have also been attributed to
some of them.1 This has resulted in regulatory guidance for water and soil, as well
as accelerated monitoring and identification of these compounds.
1
US EPA. 2020. Basic Information On PFAS | US EPA.
Firefighting foams often contain PFAS to assist in dousing flames.
3
Page 4

Regulatory Overview
There are >4,000 known PFAS compounds that have been created for commercial use. Regulatory guidance
and restrictions exist for just a few of them, leading to unrestricted use for the rest.
– In 2009, perfluorooctanesulfonic acid (PFOS) and its salts were listed as persistent organic pollutants
(POPs) under the Stockholm Convention. All parties are required to eliminate the release of these
compounds into the environment.
– The European Union (EU) Water Framework Directive lists PFOS as a priority hazardous substance that poses
a “significant risk to the aquatic environment.” It has established an annual average environmental quality
standard (AA-EQS) of 0.65 ng/L in inland surface waters, and an AA-EQS of 0.13 ng/L for other surface
waters (Directive 2013/39/EU). The EU drinking water directive aims to have routine monitoring of up to 20
PFAS compounds in drinking water from 2021.
– The UK Chemical Investigation Program requires measurement of PFOS and perfluorooctanoic acid (PFOA)
down to 0.09 ng/L. Similarly, in October 2018, the EU Parliament approved a proposal to recast its Drinking
Water Directive with revised limits for monitoring PFOS and PFOA in drinking water. The new limits are
100 ng/L, with combined PFAS concentrations not to exceed 500 ng/L (COM (2017) 753 1.2.2018).
– Several European countries, including those in the Nordic region, have guidance levels for PFAS in drinking
water and surface water. In Sweden, recommended levels for a sum of 11 PFAS in drinking water should
not exceed 90 ng/L.
– In the United States, the US EPA has established a drinking water health advisory level for PFOS and PFOA
at a combined 70 ppt (ng/L). Several states have their own advisories for PFOA, PFOS, and other PFAS—
such as perfluorononanoic acid (PFNA) and GenX—at the low ppt range. Other initiatives are also in the
works. These include the PFAS Action Act (Jan 2019), the US EPA PFAS Plan (Feb 2019), and the US EPA
Commitment to PFAS Drinking Water Standards (Feb 2019).
– Australia, China, and several other countries are implementing restrictions (or establishing maximum
amounts) for drinking water and receiving water regulations for PFOA, PFOS, and newer PFAS detected
at low ng/L to pg/L levels.
As newer PFAS are identified in the environment, and as more toxicological information becomes available,
further guidelines and regulations are almost certain.
4
Page 5
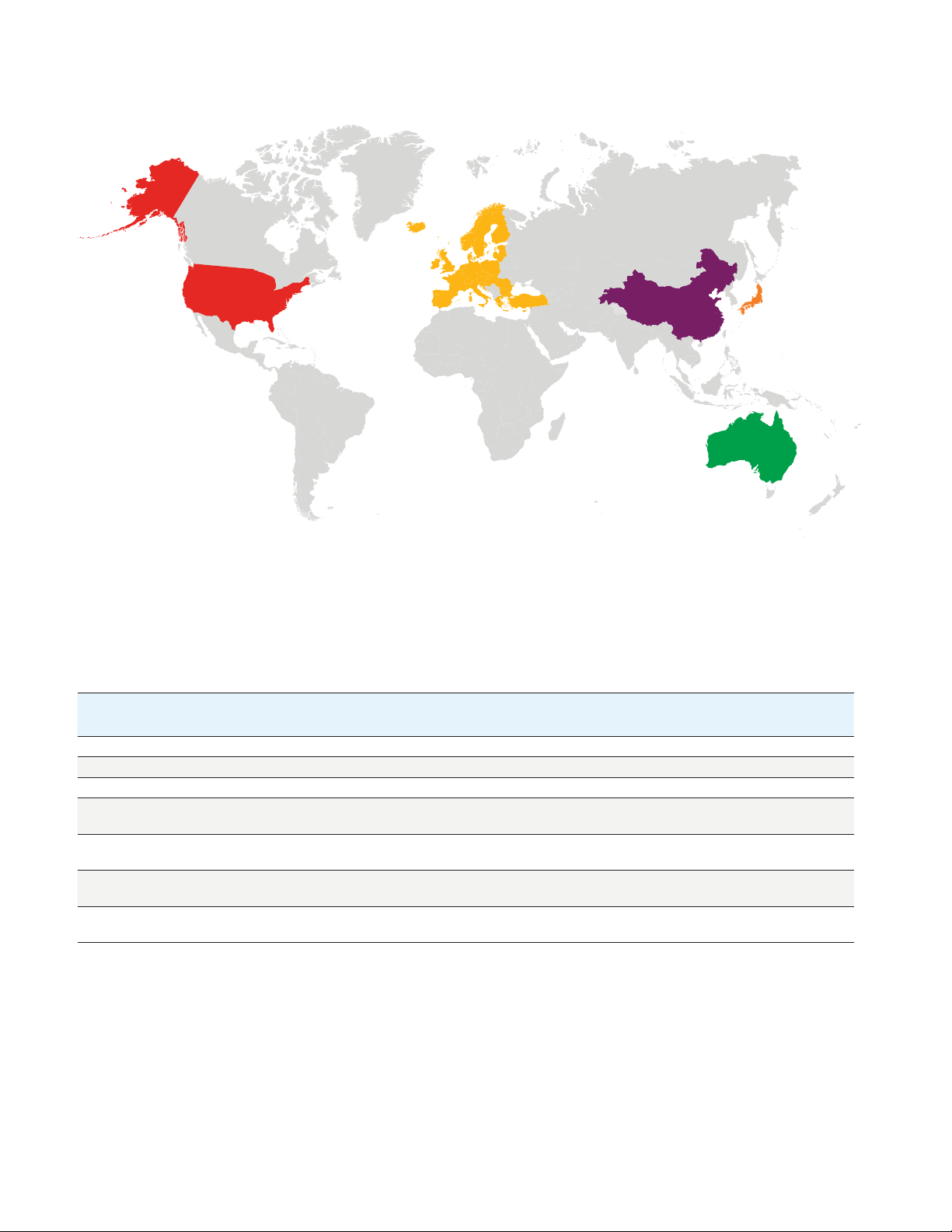
USA
Europe
Worldwide regions where strict PFAS monitoring regulations are being enacted.
Current Standards and Consensus methods for PFAS analysis in the environment.
Japan
China
Australia
Method Matrix Tested No. of
EPA 533 Drinking water 25 Solid phase extraction Isotope dilution
EPA 537 Drinking water 14 Solid phase extraction Internal standard correction
EPA 537.1 Drinking water 18 Solid phase extraction Internal standard correction
EPA 8327 (draft) Surface water, groundwater,
wastewater influent and effluent
ASTM 7979 Surface water, groundwater,
wastewater influent and effluent
ASTM 7968 Soil and solids 21 Organic extraction
ISO/DIS 21675 Drinking water, sea water,
fresh water, wastewater (<0.2% solids)
Analytes
24 Dilute and shoot External calibration (isotope dilution also
21 Dilute and shoot External calibration (isotope dilution also
30 Solid phase extraction Internal standard correction
Sample Preparation
Procedure
with MeOH
Quantification Technique
allowed)
allowed)
External calibration
5
Page 6

Sample Preparation Techniques to Maximize
PFAS Recoveries and Minimize Contamination
Due to their widespread use in clothing, protective gear, and consumer products, PFAS have been
found in human and environmental samples in all regions of the world. Use these best practices
to avoid contaminating your sample with PFAS during collection and storage.
6
Page 7

Do:
– Wear well-washed lab coats and nitrile lab gloves.
– Use high-density polyethylene (HDPE) or polypropylene (PP) containers and caps as recommended
in US EPA and ASTM methods.
– Refrigerate samples below 6 ºC during storage.
Don’t:
– Wear personal care products (such as sunscreen and hand creams) during sampling.
– Wear waterproof clothing or shoes that may be lined with PFAS or stain-repellent material.
– Use sample collection apparatus that may contain polytetrafluoroethylene (PTFE) or other
plastics containing PFAS.
– Use aluminum foil to cover openings of sample containers, as PFAS can be transferred from foil.
Proper sample cleanup and concentration
are essential to robust, accurate, and reliable
analysis. As the world’s chromatography leader,
Agilent supports your efforts with innovative LC
columns, solid phase extraction (SPE) cartridges,
vials, and filters manufactured to demanding
specifications. All are tested under strict
conditions for reliable analysis of PFAS.
7
Page 8
Extracting PFAS from water
Several regulatory methods, including EPA 537 and
533, call for extracting PFAS from drinking water
using SPE cartridges followed by LC/TQ analysis.
Typically, a weak anion exchange (WAX) cartridge is
suggested due to its ability to extract both shorterand longer-chain PFAS with good recoveries as
done in EPA 533 and ISO methods. EPA 537 uses an
Agilent Bond Elut LMS cartridge, which provides high
recoveries for medium- and long-chain PFAS.
From instruments, columns, and supplies, to
fast, worldwide delivery, to decades of method
development expertise—Agilent supports your
entire workflow for PFAS testing.
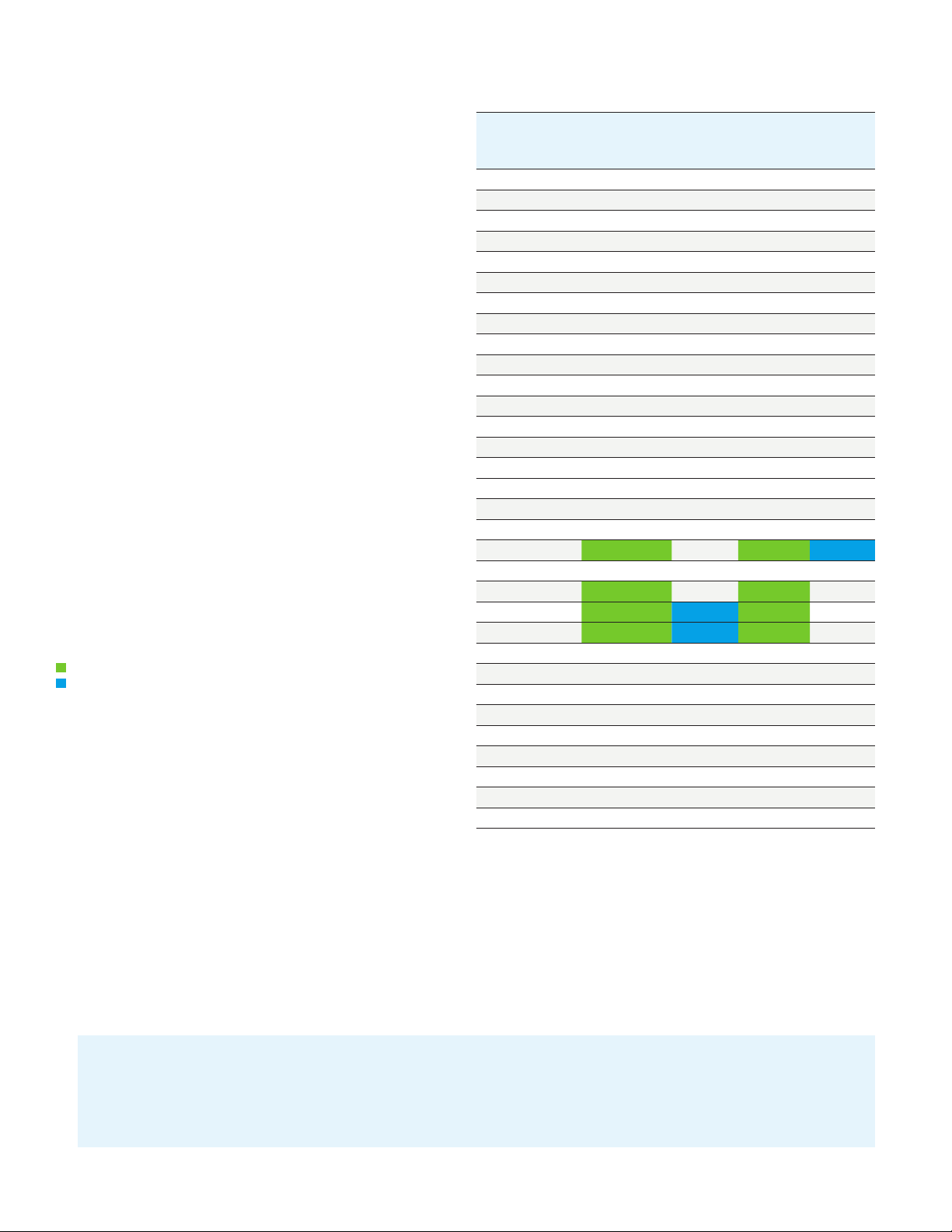
Average recovery is between 40% and 70%
RSD is greater than 19%
All 30 PFAS were recovered, and US EPA 537 compounds had recoveries
between 70 and 130% with RSD <15% for both water qualities. These
results comply with US EPA method QA/QC requirements. In addition,
only 4 of the 30 compounds had recoveries below 70%, but all 4 were
above 40%. The 14 compounds in the US EPA method produced
acceptable recovery using the Agilent WAX SPE car tridge. The 16 other
PFAS, including compounds on the ASTM list, also had good recoveries
and can be analyzed with this method.
Name LC Grade Water
Average
Recovery
EPA 537 Compounds
PFBS 85 14 99 1
PFDA 101 5 95 6
PFDoA 86 3 88 2
PFHpA 105 10 101 3
PFHxS 97 15 102 1
PFHxA 104 8 107 2
PFNA 100 5 104 3
PFOS 92 13 94 3
PFOA 102 10 106 2
PFTrDA 91 3 103 15
PFUdA 100 6 102 3
PFTrDA 91 3 103 15
N-MeFOSAA 84 11 79 10
N-EtFOSAA 84 9 89 3
Additional Compounds
PFDoA 86 3 88 2
PFTeDA 96 10 86 8
FOSA 57 14 67 20
FHEA 107 6 90 8
FOEA 61 13 52 14
FDEA 56 21 58 15
PFHpPA 47 27 46 14
4-2 FTS 91 10 91 13
6-2 FTS 87 16 100 6
8-2 FTS 104 10
6-2 FTUA 118 8 106 3
8-2 FTUA 96 11 78 13
PFPeS 97 15 104 2
PFHpS 83 11 83 3
PFNS 93 12 91 8
PFDS 85 4 81 6
LC Water
RSD (%)
Tap Water
Average
Recovery
101 10
Tap Water
RSD (%)
Want a closer look?
Read application note 5994-0250EN: Extraction of Per/Polyfluoroalkyl Substances in Water Using Agilent Offline
Solid Phase Extraction
8
Page 9
N MeFOSAA
5 µg/L spike
Recovery (%)
Acquisition time (min)
Extracting PFAS in biota
PFAS compounds are readily absorbed into animal
and human tissue. Since these substances have
been used in industry for many years, their existence
in human blood and serum—as well as presence
in fish, salmon, and other wildlife—is pervasive.
Research has shown that longer chain PFAS (>C7)
have the potential to bioaccumulate, increasing both
the need and urgency to test and analyze for PFAS
levels in biota and biological fluids.
125
100
20 µg/L spike
75
50
25
SPE and supported liquid extraction (SLE) can be
time consuming and complicated to perform on
biological samples. Agilent Captiva EMR—Lipid
makes it easy to remove interferences, particularly
phospholipids, in minutes without PFAS loss. Its
pass-through format is fast, repeatable, and delivers
a clean extract with minimal ion suppression,
extending column life and reducing the frequency
of MS cleaning.
0
PFBA
6:2 FTA
PFPeA
PFHxA
PFBS
8:2 FTA
PFHpA
PFOA
PFHxS
PFNA
10:2 FTA
PFDA
N EtFOSAA
PFOS
PFUdA
PFDoA
PFTrDA
PFTeDA
PFAS recovery fell between 70 and 130% with most compounds having
an extraction recovery of >90%. The full procedure for extraction with
Agilent Captiva EMR—Lipid is detailed in application note 5991-8656EN,
and shows how this simple technique can be performed in minutes.
Protein precipitation
EMR-Lipid cleanup
Relative abundance
0.5
1.0
1.5
2.0
2.5
3.0
3.5
4.0
4.5
5.0
5.5
6.0
6.5
7.0
Chromatographic baseline of EMR—Lipid phospholipid cleanup
compared to protein precipitation. We reduced MS cleaning and
increased column life without sacrificing PFAS recovery.
Want a closer look?
Read application note 5991-8656EN: Analysis of Per- and Polyfluoroalkyl Substances (PFAS) in Biological Fluid Using a Novel
Lipid Removing Sorbent and LC/TQ
For Research Use Only. Not for use in diagnostic procedures.
9
Page 10

Configure Your LC for Success:
PFAS Elimination
Fluoropolymers are commonly found in many industrial products, including those used in the lab for
analysis, extraction, and cleanup. Lab supplies such as solvents, syringes, pipettes, and SPE devices
can contain trace PFAS levels that may contaminate your samples and interfere with your results.
Some LC instrument parts can also contribute to trace-level contamination.
InfinityLab LC instruments:
Efficiency for any application and budget
Agilent InfinityLab HPLC and UHPLC systems feature the
latest technology while maintaining full compatibility with
legacy LC instrumentation.
– The Agilent 1260 Infinity II LC is the flexible choice for
operational efficiency.
– The Agilent 1290 Infinity II LC is the next generation of
liquid chromatography—delivering ultrahigh performance
for superior analytical results.
Is PFC background noise hurting your PFAS analysis?
The InfinityLab PFC-free HPLC conversion kit for the Agilent 1290
Infinity II LC helps keep your flow path free from perfluorinated
compounds, so you can minimize PFAS background noise and
meet stringent regulations.
To request your kit, simply use our easy ordering guide that links
to a prefilled shopping cart at the Agilent online store.
10
Page 11
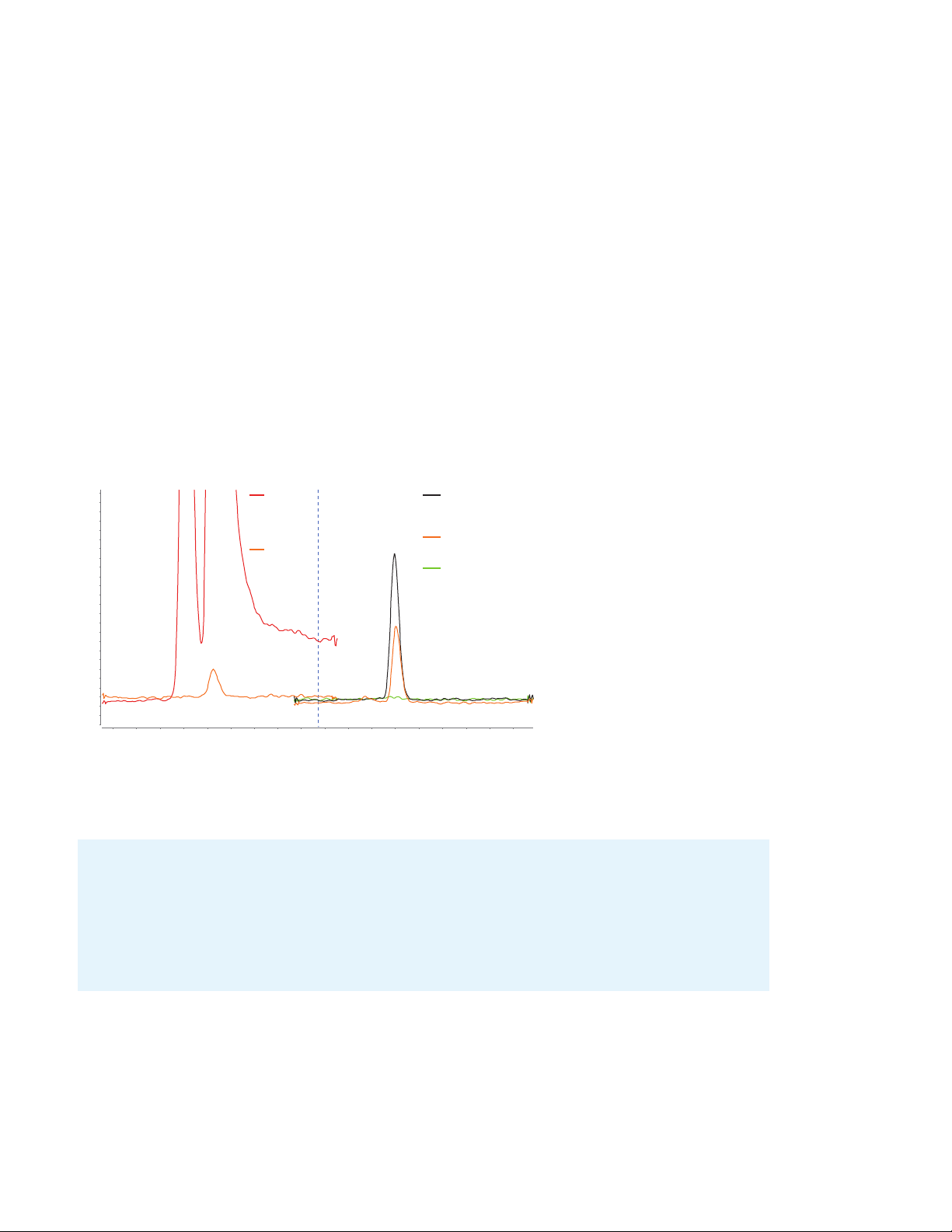
Consumables solutions for analyzing trace-level PFAS with confidence
Counts
Agilent’s new InfinityLab PFC-free HPLC conversion kit includes everything needed to ensure that the
1290 Infinity II HPLC systems and high-speed pumps are free of PFAS contamination. This includes
various replacement parts, tubing, inline filters, solvent bottle assembly, and an InfinityLab Delay
column with Quick Connect HPLC fitting.
Several regulatory methods, including EPA 537 and 533, call for extracting PFAS from drinking water
using SPE cartridges followed by LC/MS/MS analysis. Agilent’s SPE cartridge portfolio includes both
ultraclean divinylbenzene sorbent for EPA 537, as well as the weak anion exchange or WAX cartridge
required for analyzing shorter-chain and emerging PFAS compounds. Captiva syringe filters with
regenerated cellulose (RC) membranes are ideal for PFAS analysis described in EPA 8327 and ASTM
D7979 methods for non-potable waters.
Agilent ZORBAX RRHD columns, based on a fully porous particle, increase resistance to strong
sample injection solvents and allow for high-volume injections, making them ideal for analyzing PFAS
compounds in water.
2
×10
5
Without
delay column
4
3
2
1
0
3.5 3.6 3.7 3.8 3.9 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 4.9 5.0 5.1 5.2
Blank std
LC setup
(isomers
separated)
Blank
PFC-free
LC setup
Acquisition time (min)
With
delay column
Injection of 100 fg
of sample on column
PFC-free LC setup
Blank
std LC setup
Blank
PFC-free LC setup
PFHpA blank and sample
chromatograms from
different LC system setups.
We tested Agilent PFCfree conversion kits using
38 analytes representing
standard and regulatory
methods—including US EPA
533, 537.1, 8327, and ISO
21675. No background was
detected for 36 out of the
38 analytes.
Want a closer look?
Read these application notes:
5994-2291EN: Reduce PFAS Background with the Agilent PFC-Free HPLC Conversion Kit
5994-2151EN: Filtration for the Analysis of Per- and Polyfluoroalkyl Substances in Environmental Extracts
5994-0250EN: Extraction of Per/Polyfluoroalkyl Substances in Water Using Agilent Offline Solid Phase Extraction
11
Page 12

Meet Regulatory Guidance Levels
with Robust Results
Global regulations for PFOS and PFOA
PFOS and PFOA are the two most commonly
measured PFAS and have regulatory guidance levels
in regions including China, Europe, the United States,
Japan, and Australia.
PFOS and its formulations are listed as priority
substances in the Stockholm Convention. The EPA safe
drinking water guidelines for PFOS and PFOA are not
to exceed 70 ng/L. However, several regulatory and
municipal bodies require monitoring and detection of
PFAS at low single-digit ng/L levels in water and soil.
General PFAS analysis with SPE cleanup
The sensitivity, robustness, and reliability of Agilent
triple quadrupole LC/MS systems (LC/TQ) make them
ideal for analyzing general and regulated PFAS targets.
An Agilent Ultivo can quantify low levels (ppt) of PFOA
and PFAS in water and soil using WAX SPE cartridge
concentration and cleanup.
Here, we used Agilent WAX SPE cartridges to extract
PFOA and PFOS from water and soil.
Counts (cps)
Counts (cps)
Counts (cps)
×10
4
3
2
1
0
×10
2.0
1.5
1.0
0.5
0
×10
6
4
2
1
A
PFOS
499.0
S/N = 120
3
PFOA
413.0 369.0
S/N = 1,193
2.0
1
B
PFOS
499.0 99.0
S/N = 226
3.805
99.0
2.5 3.0 3.5 4.0 4.5 5.0
Retention time (min)
3.836
The Agilent InfinityLab Poroshell 120 EC-C18
(2.1 x 100 mm, 2.7 μm) column allows for effective
separation of linear and branched isomers for PFOA.
Recoveries between 80 and 120% for both compounds
in surface water, wastewater (2.5, 40, and 200 ng/L),
soil, and sediment (0.5, 5, and 20 ng/g) were achieved
at different spiking levels.
0
2
×10
PFOA
8
413.0 369.0
S/N = 1,382
6
4
Counts (cps )
2
0
2.0
Chromatograms for PFOA and PFOS spiked at 2.5 ng/L in water
(A) and 0.5 µg/kg in blank soil (B). Note: Only a quantitative ion
chromatogram is shown for each compound.
2.791
2.5 3.0 3.5 4.0 4.5 5.0
Retention time (min)
12
Page 13

US EPA drinking water methods
US EPA method 537.1 analyzes 18 PFAS, including PFOS and PFOA, in drinking water using SPE
followed by LC/TQ analysis. EPA 537 uses an Agilent Bond Elut LMS solid phase extraction cartridge
(p/n 12255021) to extract 500 mL of drinking water. This process is followed by evaporation and
creation of the final extract with ~96% methanol. The following chromatogram shows the separation
and detection of PFAS using an Agilent ZORBAX Eclipse Plus C18, 3.0 × 50 mm, 1.8 μm column
(p/n 959757-302). Samples were analyzed on the Agilent Ultivo triple quadrupole LC/MS.
3
x10
1.05
1
0.95
0.85
0.8
0.75
0.7
0.65
0.6
0.75
0.5
0.45
0.4
0.35
0.3
0.25
0.2
0.15
0.1
0.05
0
4 4.5 5 5.5 6 6.5 7 7.5 8 8.5 9 9.5 10 10.5 11 11.5 12 12.5 13
PFBA
PFPeA
PFHxA
PFBS
GenX
Counts vs. acquisition time (min)
PFHxS
PFHpA
NMeFOSAA
Adona
NEtFOSAA
PFOA
PFOS
PFUnA
PFNA
9Cl-PF3NOS
11Cl-PF3OUdS
PFDA
PFTA
PFTrDA
PFDoA
Want a closer look?
Read application note 5994-0437EN: LC/TQ Determination of PFOS and PFOA in Water and Soil Matrices
13
Page 14

In addition to meeting and exceeding reporting levels required by the US EPA method, you must
also achieve robustness across a batch of real water samples. Here, we show a relative response
in area counts for 11 continuous calibration water samples run over a 26-hour batch for EPA 537.
The Ultivo LC/TQ system allows you to continuously run routine EPA methods for PFAS with the
desired sensitivity and reproducibility. Its small footprint lets you fit approximately three Ultivo
systems in the space needed for one traditional LC/TQ instrument.
1.6
1.4
1.2
1.0
0.8
Relative response
0.6
0.4
0.2
0
0 4 8 12 16 20 24 28
Time (hrs)
RSD
1.2%
1.9%
3.5%
2.1%
3.0%
1.9%
3.6%
PFBA
PFHxA
PFOA
PFOS
N-MeFOSAA
PFUdA
PFTrDA
Want a closer look?
Read application note 5991-8969EN: Analysis of Per/Polyfluoroalkyl Substances (PFAS) in Drinking Water
Using the Agilent Ultivo Triple Quadrupole LC/MS
14
Page 15
Eliminating sample preparation for US EPA 537
SPE sample preparation and extraction is a critical step for cleanup and interference removal.
However, it is time consuming and tedious, and can introduce sources of PFAS contamination
into the sample.
For labs looking to monitor the PFAS listed on EPA method 537, the Agilent 6495 LC/TQ system
is an ideal choice. It eliminates the hassle of sample preparation in clean water samples and gets
you from sample collection to data reporting quickly. The 6495 offers excellent sensitivity and
increased throughput without any sacrifice in method reporting levels.
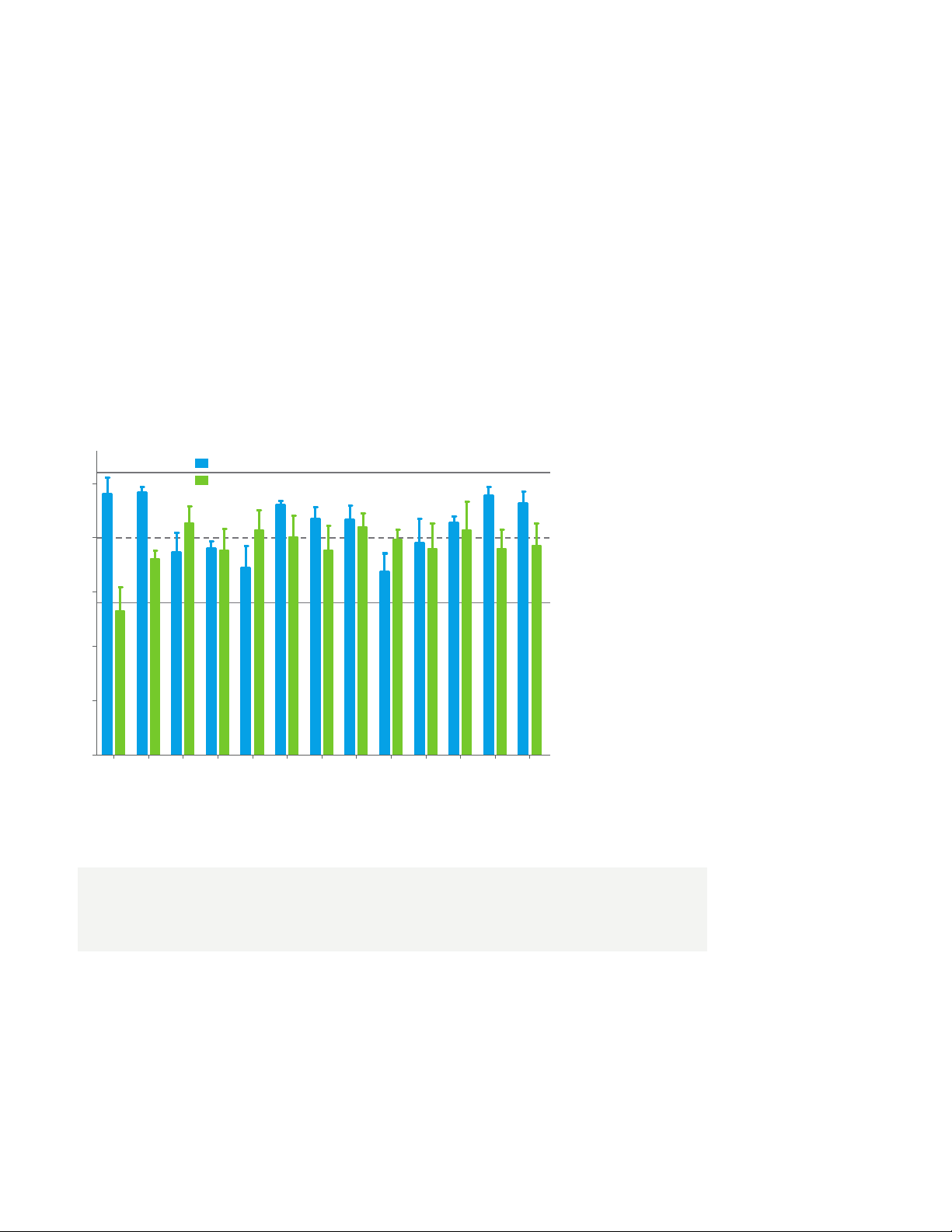
A method developed on the 6495 LC/TQ quantified 14 PFAS present in EPA 537. The analysis
took less than six minutes, with a direct injection of 80 µL of sample diluted slightly with methanol
to achieve detection levels of 0.83 to 3.3 ng/L.
The following graph shows the recovery of these PFAS at two spiking levels.
Mid-level spike
125
100
Low-level spike
75
50
25
Spike recoveries of PFAS
at low level (10 ng/L; 2
ng/L for PFOS and PFOA)
and mid level (40 ng/L) in
0
PFBA
PFBS
PFDA
PFDS
PFHpA
PFHpS
PFHxA
PFHxS
PFNA
PFNS
PFOA
PFOS
PFPeS
drinking water samples.
To learn more about the challenges and mitigation strategies in detection of PFAS in water, watch
this on-demand webinar by Dr. Arjun Venkatesan, Stony Brook University.
15
Page 16
Analyzing challenging compounds with EPA 533
EPA method 533 addresses some of the challenging compounds in EPA methods 537 and 537.1 for C4
acids and sulfonates. It also limits chain length to C12 acids, while adding some emerging PFAS.
and C
5
Agilent’s sample preparation, consumables, and Ultivo LC/TQ provide the tools needed to achieve the
detection limits required by EPA 533 while offering ultimate robustness and easy-maintenance features
for novice mass spectrometry users too.
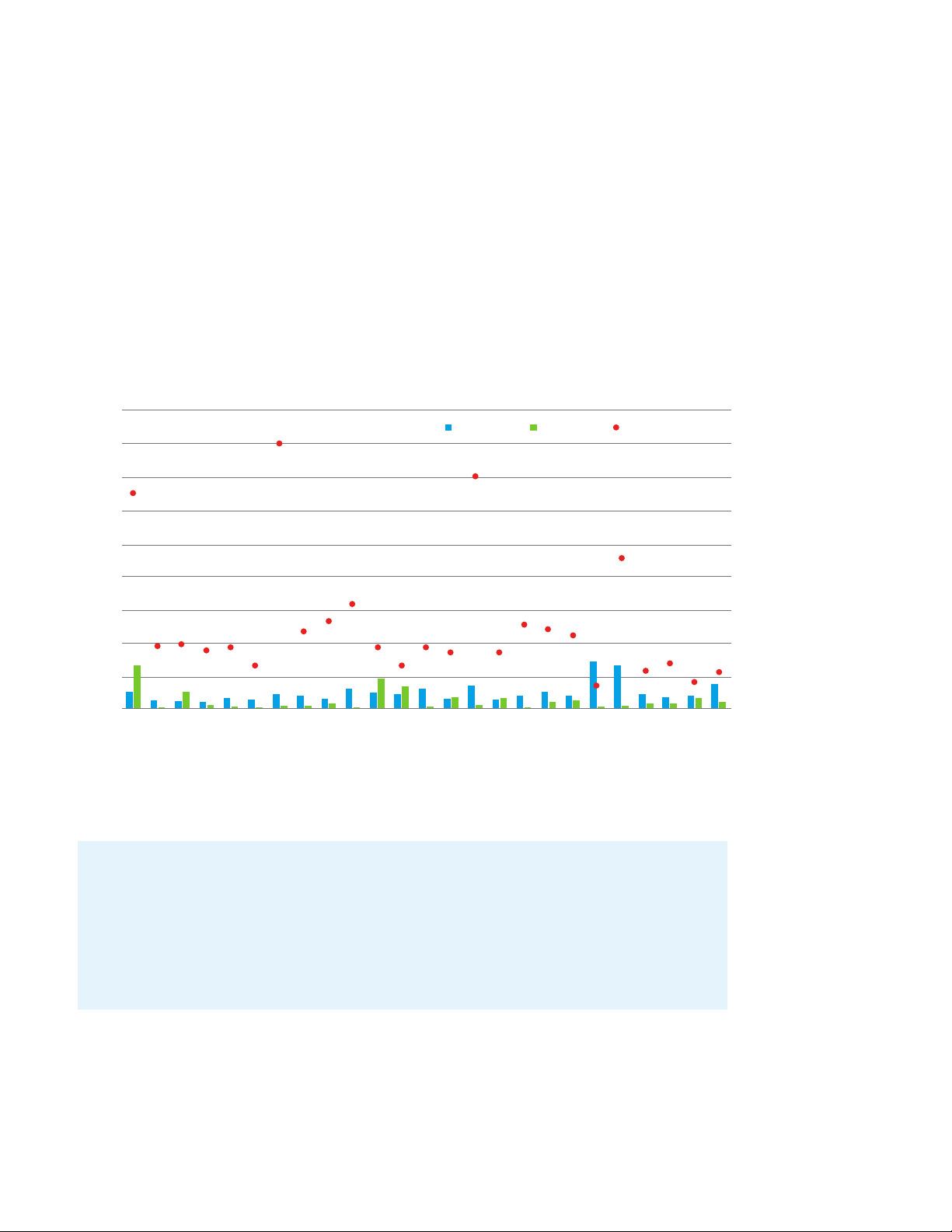
An Agilent 6470 LC/TQ with Agilent Jet Stream ESI source delivers sensitivity, precision, and accuracy
exceeding EPA requirements, allowing users to future-proof their lab against dropping regulatory limits
that could be expected. Recoveries were between 87% and 103% for all PFAS in reagent and tap water.
LCMRLs calculated according to EPA for all analytes in EPA 533 with Ultivo and 6470 LC/MS compared to EPA single lab LCMRL.
18
Ultivo LCMRL
16
14
12
10
6470 LCMRL EPA Single Lab LCMRL
8
LCMRL (ng/L)
6
4
2
0
PFBA
PFMPA
PFPeA
PFBS
PFMBA
PFEESA
NFDHA
4:2FTS
PFHxA
PFPeS
HFPO-DA
PFHpA
PFHxS
ADONA
6:2FTS
PFOA
PFHps
PFNA
PFOS
9CI-PF3ONS
8:2FTS
Want a closer look?
Read these application notes:
5994-1628EN: EPA Method 533 for Analysis of Per/Polyfluoroalkyl Substances in Drinking Water
Using Agilent 6470 LC/TQ
5994-1920: Analysis of Per/Polyfluoroalkyl Substances (PFAS) in Drinking Water by EPA 537.1 and
EPA 533 Using the Agilent Ultivo Triple Quadrupole LC/MS
PFDA
PFUnA
11CI-PF3OUdS
PFDoA
16
Page 17
EU regulations
The EU has an environmental quality standard of 0.65 ng/L in water bodies for PFOS, and
researchers anticipate similarly low reporting levels for PFOA in water. The high sensitivity of
the Agilent 6495 triple quadrupole LC/TQ lets you achieve these low levels by direct aqueous
injection and run your analysis in a “dilute-and-shoot” manner, so you can skip laborious,
time-consuming sample concentration.
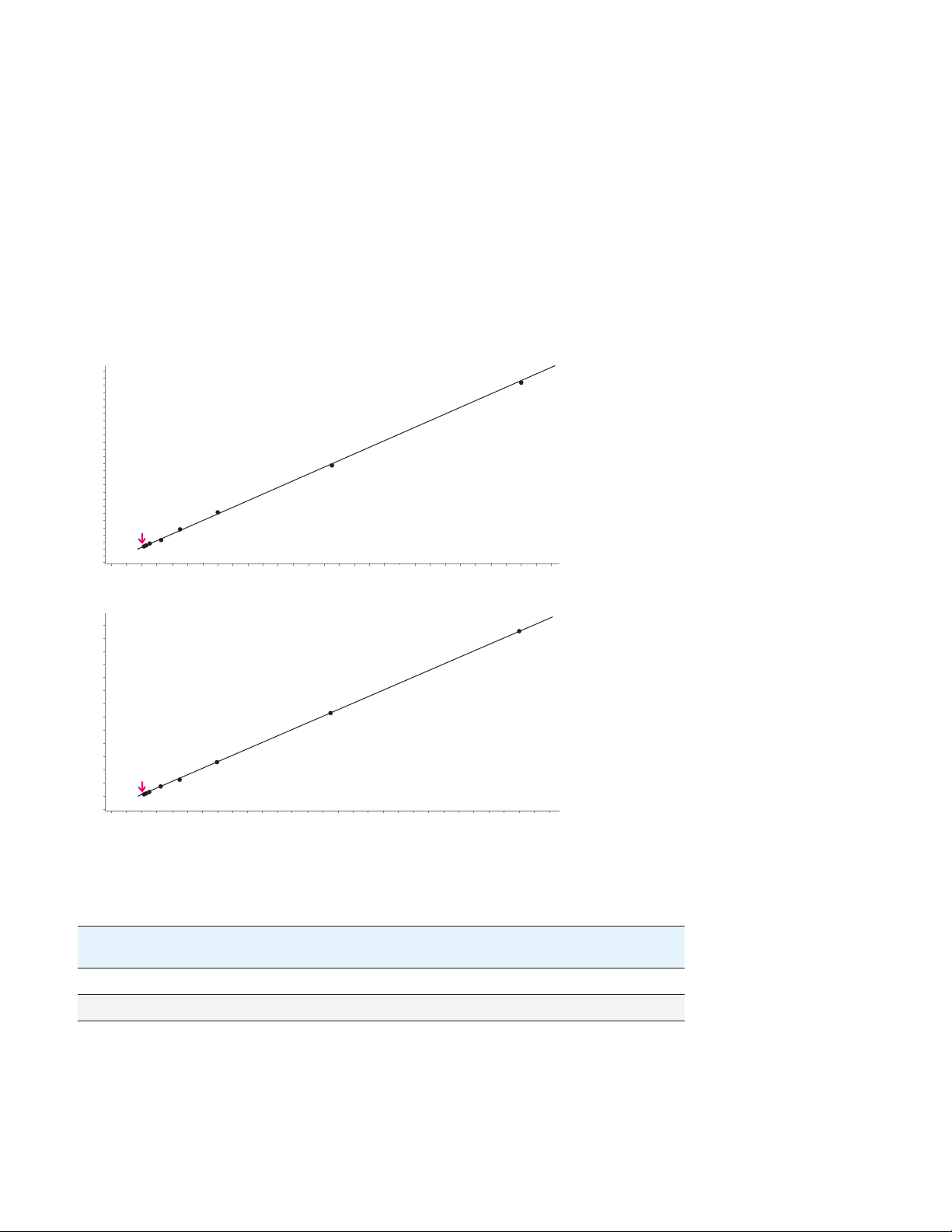
We generated these linear calibration curves for PFOS and PFOA standards (0.25, 0.5, 1, 2.5, 5,
2
10, 25, and 50 ng/L with R
PFOA — 8 Levels, 8 Levels used, 8 Points, 8 Points used, 0 QCs
4
x10
y = 233.309613* x
R^2 = 0.99841616
1.2
Type: Linear, Origin: Ignore, Weight: 1/x
1.1
1
0.9
0.8
0.7
0.6
0.5
Responses
0.4
0.3
0.2
0.1
0
-4 -2 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44 46 48 50 52 54
PFOA — 8 Levels, 8 Levels used, 8 Points, 8 Points used, 0 QCs
4
x10
y = 496.082875* x
R^2 = 0.99971471
2.6
Type: Linear, Origin: Ignore, Weight: 1/x
2.4
2.2
2
1.8
1.6
1.4
1.2
1
Responses
0.8
0.6
0.4
0.2
0
-0.2
-4 -2 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44 46 48 50 52 54
+
130.582233
+
213.285758
> 0.99) using direct aqueous injection on the 6495 LC/TQ.
Concentration (ng/L)
Concentration (ng/L)
Calibration curves for PFOS and PFOA, standards at 0.25, 0.5, 1, 2.5, 5, 10, 25, and 50 ng/L.
0.5 ng/L Standard 1.0 ng/L Standard
Compound Average Area RSD (%) Average Area RSD (%)
PFOS 423.3 5.3 668.3 4.9
PFOA 280.1 5.0 387.7 4.4
Enhanced sensitivity allows you to measure PFAS at extremely low levels without any loss in reproducibility
or robustness. The RSD for both PFOS and PFOA at a 0.5 ng/L concentration was <6%.
17
Page 18

ASTM and EPA 8327 methods
ASTM method 7979-17 analyzes 21 PFAS in nonpotable waters—including surface water,
groundwater, and wastewater. It uses a simplified methanol dilution and large-volume (30 μL)
injection onto an LC/TQ. EPA 8327 follows identical sample preparation and analytical protocols.
Briefly, the method requires 5 mL of sample to be diluted with 5 mL of methanol, acidified, filtered,
and injected onto an LC/TQ. This sample preparation technique increases throughput and reduces
contamination sources. Reporting levels for most compounds in this method are 10 ng/L with an
expected calibration range of up to 400 ng/L. Analysis at low ng/L levels demands a sensitive mass
spectrometer with good robustness to deal with different water matrices. It also requires strategies
to eliminate any PFAS contamination present in glass containers and consumables.
A simple technique for background removal or background delay
To perform the ASTM 7979 efficiently, we eliminated PFAS contamination quickly and easily using
an Agilent delay column and replacing any PTFE-containing tubing with PEEK. The Agilent 6470
LC/TQ achieved the detection limits required for all 21 PFAS in ASTM 7979. It also detected seven
other PFAS, including ADONA and 9Cl-PF3ONS (component in F53-B), that are being monitored
as “emerging” PFAS.
The following table shows recoveries for 28 PFAS, including those in the ASTM 7979 and EPA 8327
methods, at the low limit of quantification (LLOQ) levels of 10 and 20 ng/L with the Agilent 6470
LC/TQ. It includes six replicates with recoveries between 50% and 150%, as stated in the ASTM
method for all compounds. Most compounds showed recoveries between 80% to 120%, and the
relative standard deviation (RSD) was below 20% for all compounds.
18
Page 19

Compound
11Cl-PF3OUdS 119 1.8 116 3.6
4:2 FTS 115 5.8 96 8.6
8:2 FTS 103 15.2 114 0.8
9Cl-PF3ONS 110 10.5 108 6.8
ADONA 107 11.1 102 4.6
N-EtFOSAA 122 12.3 111 2.7
N-MeFOSAA 118 16.4 117 17.1
PFBA 101 10.7 104 10.6
PFBS 108 8.4 117 3.2
PFDA 110 17.1 107 6.6
PFDoA 101 13.3 106 13.3
PFDS 65 36.0 90 16.7
PFEESA 55 2.3 125 6.1
PFHpA 125 15.4 114 13.6
PFHpS 83 15.5 129 8.7
PFHxA 104 5.0 118 11.7
PFHxS 114 12.6 100 11.3
PFMBA 141 4.2 125 4.6
PFNA 102 18.8 106 9.4
PFNS 100 19.4 106 18.2
PFOA 121 11.1 108 9.1
PFOS 97 9.9 116 16.3
PFOSA 94 11.1 94 10.0
PFPeA 105 3.9 111 2.9
PFPeS 106 9.3 99 15.6
PFTreA 100
PFTriA 105 10.1 106 6.6
PFUnA 116 12.4 106 1.1
Spike Recovery, % at 10 ng/L Spike Recovery, % at 20 ng/L
Average RSD (%) Average RSD (%)
8.0 102 8.4
Spike recoveries and RSD (%) at 10 ng/L and 20 ng/L for all 28 PFAS, including those in ASTM 7979.
Consult application note 5994-0678EN for full method parameters.
Want a closer look?
Read application note 5994-0678EN: Simplified and Fast Analysis of Per- and Polyfluoroalkyl
Substances in Nonpotable Waters
19
Page 20

Future-Proof Your Lab to Address
the Growing List of PFAS
More than 4,000 PFAS are currently known to have been used, and this list continues to expand.
But current regulatory monitoring lists globally only look at a very limited subset of PFAS. While
regulatory lists may vary across regions, it is safe to assume that many additional PFAS will be
monitored across the globe in time.
The following table shows method detection and method quantification levels (MDLs and MQLs)
of more than 50 PFAS representing 16 different classes of legacy and emerging PFAS analyzed in
water. We used a single analytical method that covered both legacy and emerging PFAS, including
diPAPs, ADONA, and PFESAs (components of F-53B). Samples were analyzed on the 6495 LC/TQ,
making use of its enhanced sensitivity to analyze all these PFAS in one injection without
compromising detection levels.
The results can be summarized as follows:
– Instrument detection limits (IDLs) ranged from 2.5 to 469 fg on column for all compounds.
– Calculated IDLs were below 10 fg on column for 22 compounds from the classes PFCA, PFSA,
FTS, FOSAA, and Cl-PFAES—plus the compounds FOSA, diSAmPAP, and ADONA.
– MDLs ranged from 0.28 to 18 ng/L. MQLs ranged from 0.35 to 26 ng/L,
with 46 PFAS having quantification levels below 5 ng/L.
– Run time was less than 12 minutes.
MDLs for the 53 PFAS were calculated based on US EPA 40 CFR Part 136, Appendix B, Revision 2.
Seven 250 mL aliquots of ultrapure water were spiked at 5 ng/L for all compounds except FTCAs,
FOSEs, and PFDPA, which were spiked at 20 ng/L.
20
Page 21

PFAS analysis using SPE extraction and triple quadrupole LC/MS.
Compound MDL (ng/L) MQL (ng/L) Extraction Method
PFBA 0.59 0.75 93 4
PFPeA 0.71 0.89 92 5
PFHxA 0.87 1.1 90 6
PFHpA 0.84 1.1 96 6
PFOA 0.28 0.35 93 2
PFNA 0.61 0.77 98 4
PFDA 0.71 0.89 98 4
PFUnA 0.80 1.0 85 6
PFDoA 1.2 1.5 93 8
PFTrA 1.4 1.8 78 12
PFTeA 0.67 0.84 93 5
PFBS 0.49 0.62 89 3
PFPeS 1.2 1.5 100 9
PFHxS 0.69 0.88 91 5
PFHpS 0.79 1.0 99 6
PFOS 0.78 1.0 95 5
PFNS 1.0 1.3 87 7
PFDS 1.1 1.3 83 8
PFDoS 1.4 1.8 72 13
ADONA 0.82 1.0 88 6
6:2 FTCA 13 17 103 16
8:2 FTCA 16 19 92 23
10:2 FTCA 17 21 67 28
6:2 FTUCA 1.7 2.1 121 9
8:2 FTUCA 1.6 2.0 111 10
10:2 FTUCA
3:3 FTCA 1.4 1.7 118 7
2.8 3.6 87 19
Accuracy (%)
Method
Precision RSD (%)
Want a closer look?
Read application note 5994-0919EN: Analysis of >50 Legacy and Emerging PFAS in Water
Using the Agilent 6495C LC/TQ
21
Page 22

Ultrapure water blanks (n=7) were extracted alongside method validation samples. Method accuracy
was expressed as the mean recovery of method validation samples for the expected concentration as
a percentage and relative standard deviation.
The versatility, accuracy, and robustness of our LC/TQ systems let you add several PFAS to your
method without sacrificing sensitivity or throughput. If you need to add new or novel PFAS to your
methods, Agilent LC/MS compound optimizer automates the process and provides reliable compound
parameters in minutes.
In addition, Agilent consultants can help make your PFAS analysis hassle free. Our decades of
experience, combined with our MRM and method data capabilities, can get your lab up to speed quickly.
PFAS analysis using SPE extraction and triple quadrupole LC/MS.
Compound MDL (ng/L) MQL (ng/L) Extraction Method
5:3 FTCA 1.8 2.3 103 11
7:3 FTCA 2.4 3.1 75 20
PFHxPA 2.9 3.4 104 17
PFOPA 4.6 5.8 100 26
PFDPA 18 26 82 10
6:2 diPAP 1.9 2.4 81 14
6:2/8:2 diPAP 1.9 2.4 123 11
8:2 diPAP 0.83 1.1 93 6
6:2 Cl-PFESA 1.3 1.7 88 9
8:2 Cl-PFESA 1.1 1.4 80 9
4:2 FTS 2.7 3.4 93 16
6:2 FTS 0.56 0.7 90 4
8:2 FTS 1.3 1.7 87 9
10:2 FTS 1.4 1.8 66 13
FOSA 0.76 1.0 70 7
MeFOSA 4.0 5.0 127 18
EtFOSA 2.1 2.7 80 19
FOSAA 3.2 4.0 91 17
MeFOSAA 1.4 1.7 106 8
EtFOSAA 1.5 1.9 93 10
MeFOSE 2.9 3.7 96 5
EtFOSE 4.9 6.2 93 9
6:6 PFPiA 1.2 1.5 74 10
6:8 PFPiA 1.8 2.3 95 12
8:8 PFPiA 3.1 4.0 138 11
diSAmPAP 3.3
3.0 76 19
Accuracy (%)
Method Precision
RSD (%)
22
Page 23

For more information on the
analysis, fate, and removal of
PFAS in water treatment plants
using the 6495C LC/TQ, please
watch this on-demand webinar by
Prof. Bradley Clarke, University of
Melbourne, Australia.
23
Page 24
Automate PFAS sample preparation with online SPE
Solid phase extraction for PFAS can be time consuming and tedious, and offers the potential of
adding contamination while reducing reproducibility of the overall analysis. Automated online SPE
can provide similar detection limits while using a fraction of the water sample while completely
automating sample extraction and analysis which reduces labor time, increases accuracy of
results and drastically improves throughput. Further, it also reduces costs of expensive and
environmentally unfriendly solvent usage and the amount of isotopically labelled standards needed.
While automation can seem complicated, Agilent’s online SPE setup coupled to the most reliable
and robust Agilent mass spectrometers provide seamless software interface to make the process
much simpler, letting you reduce the loads of doing manual and tiresome offline extractions.
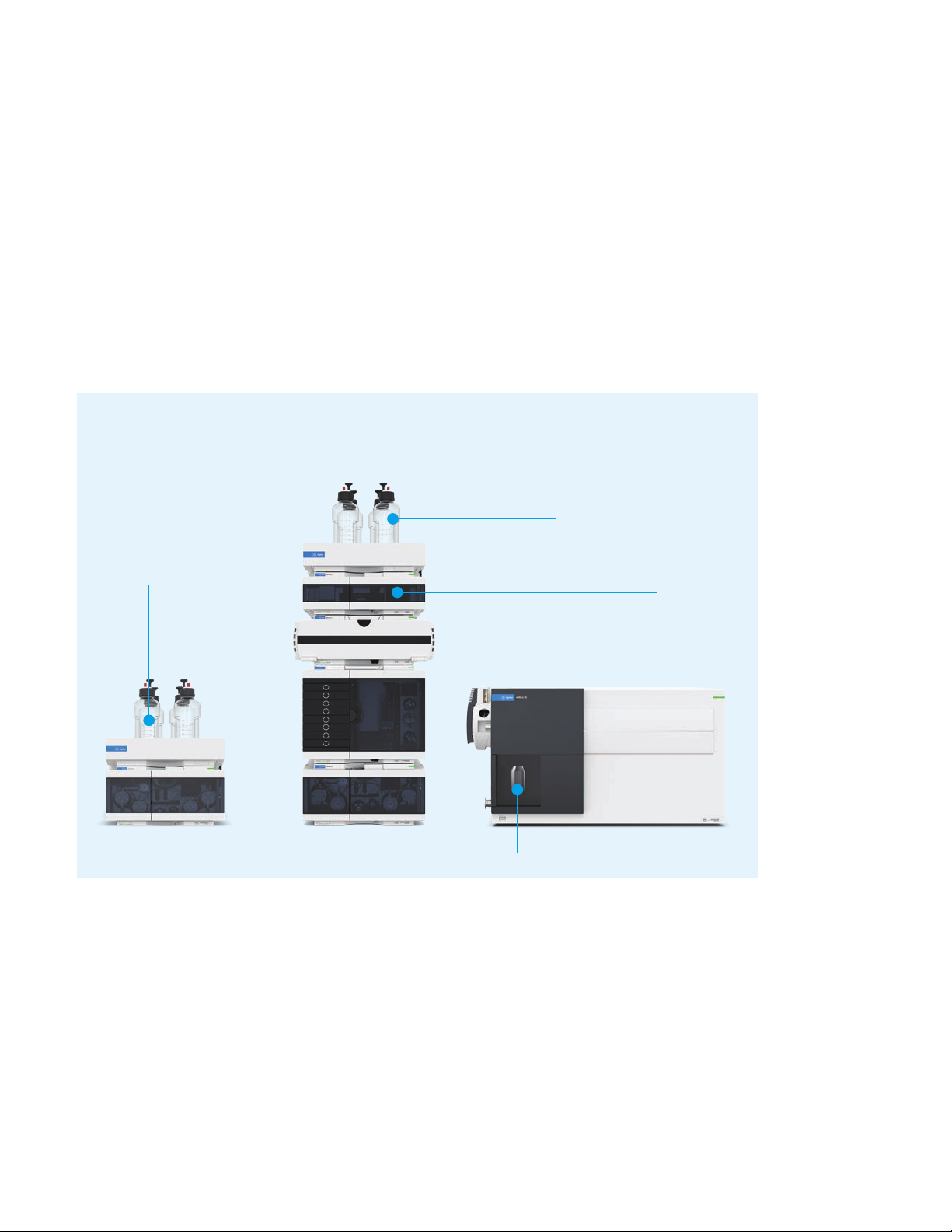
Agilent InfinityLab Online SPE solution for analysis of PFAS and emerging
contaminants in water.
Solvents for LC
Solvents required
for SPE rinsing
and conditioning
1290 Infinity Flexible Cube
The host of the switching valves
Triple quad MS for quantification
24
Page 25
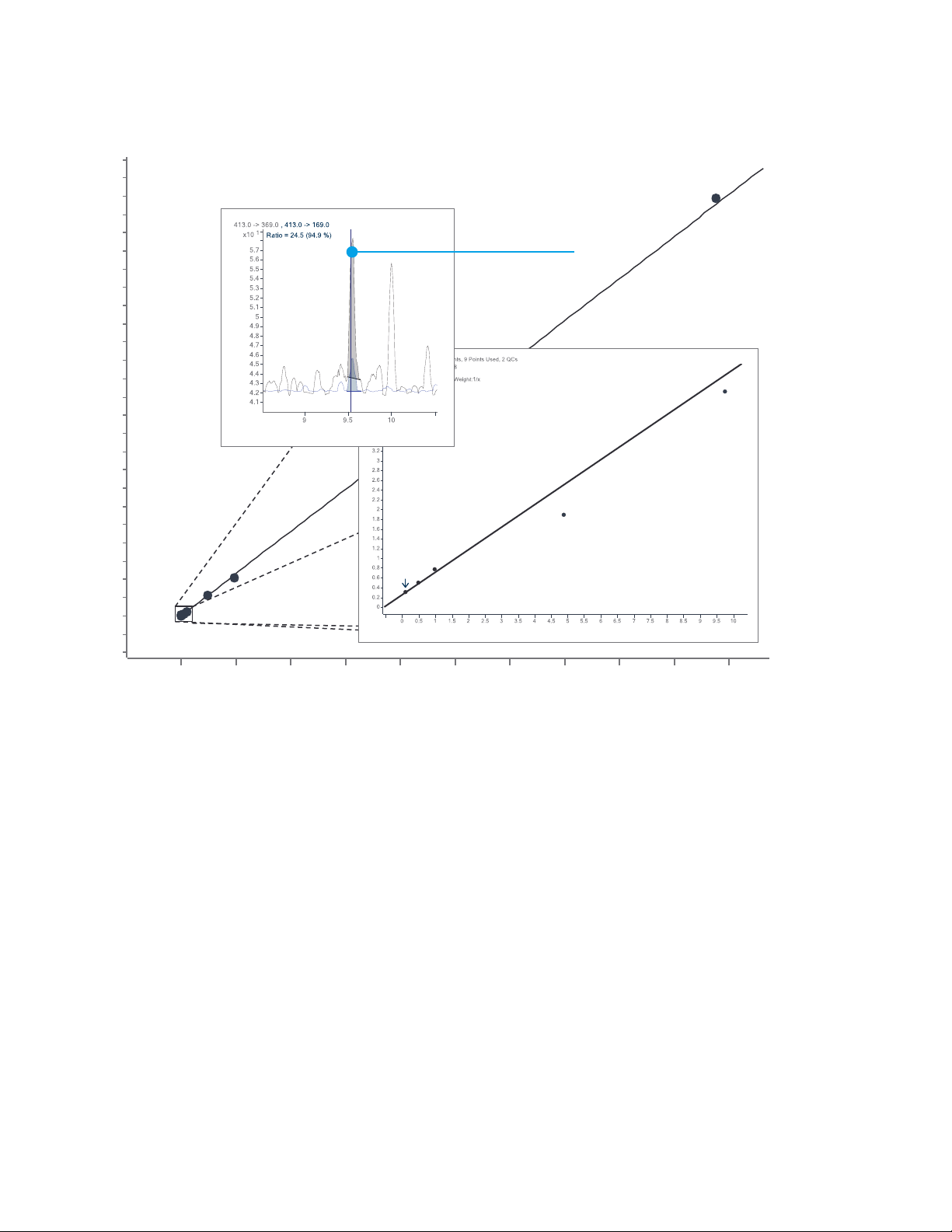
Linearity, dynamic range (0.1-1000 ng/L), and sensitivity of PFOA analysis using Agilent InfinityLab Online SPE with a 6470 LC/MS/MS.
PFOA - 9 Levels, 9 Levels Used, 9 Points, 9 Points Used, 7 QCs
1
y = 0.046187 * x + 0.025018
x10
4.8
R^2 = 0.99905565
Type:Linear, Origin:Ignore, Weight:1/x
4.6
4.4
4.2
4
3.8
3.6
3.4
3.2
2.8
2.6
2.4
responses
2.2
Relative
1.8
1.6
1.4
1.2
0.8
0.6
0.4
0.2
-0.2
-0.4
3
2
1
0
0 100 200 300 400 500 600 700 800 900 1000
Counts
Acquisition time (min)
Concentration (ng/L)
0.1 ng/L or 0.1 ppt
Relative concentration
25
Page 26

Discovering New PFAS Using
High-Resolution LC/Q-TOF
Triple quadrupole LC/MS is a great tool for measuring known PFAS at extremely low levels. However,
several thousand PFAS are used in commerce, and many have yet to be identified in the environment.
This is where a quadrupole time-of-flight (Q-TOF) mass spectrometer shows its value.
LC/Q-TOF instruments allow you to perform untargeted analysis and to identify unknown PFAS.
You can also use libraries to screen for PFAS without the need for analytical reference standards.
In addition, LC/Q-TOF technology lets you acquire data over a wide dynamic range. That means
you can analyze trace sample amounts next to abundant peaks in the same spectrum. Together
with simultaneous high sensitivity and high resolution, Agilent LC/Q-TOF instruments are ideal for
identifying unknown PFAS in environmental samples.
26
Page 27

In the following spectra, we identified 6:2 FtS with high mass accuracy and isotope fidelity in a
wastewater sample. The compound was later verified with a reference standard to have the same
retention time by LC/Q-TOF.
Identification of PFAS PCDL + Std ID.
WWTP1 Final Effluent
Cpd 11: 6:2 FTS / C2H4-perfluorooctane sulfonate: -ESI EIC (426.96790, 854.94309, 855.94614) Scan Frag=350.0V ETP FEFF 1.d
4
10
4
3.5
3
2.5
2
6:2 FTS
Mass: 427.97458
Mass diff: -1.41 ppm
Score: 98.11
1.5
1
0.5
0
4.5 5 5.5 6 6.5 7 7.5 8 8.5 9 9.5 10 10.5 11
Counts vs. acquisition time (min)
Standard
Cpd 22: 6:2 FTS / C2H4-perfluorooctane sulfonate: -ESI EIC (426.96790, 854.94309, 855.94614) Scan 6-2 FTS.d
4
10
2.25
2
1.75
1.5
1.25
1
0.75
0.5
0.25
0
4.5 5 5.5 6 6.5 7 7.5 8 8.5 9 9.5 10 10.5 11
Counts vs. acquisition time (min)
4
10
2.6
2.4
2.2
2
1.8
1.6
1.4
1.2
1
0.8
0.6
0.4
0.2
0
424 425 426 427 428 429 430 431 432
Count s vs. Mass-to -charge (m/z)
27
Page 28

Since LC/Q-TOF collects all ionizable species in the analysis, you are able to retroactively mine for
data and identify compounds without sample reinjection. Here, 6:2 FtS samples were identified at
all treatment stages of the wastewater plant samples. Therefore, the abundance of this compound
could be plotted to track efficacy in the wastewater treatment plant.
Alternatively, an LC/Q-TOF system can be used for fingerprinting PFAS sources, or for studying
PFAS composition in aqueous film-forming foams (AFFF).
Mass Profiler Professional (MPP) software can be used to identify new PFAS and perform
source appropriation and fingerprinting studies. It includes statistical profiling tools like principal
component analysis (PCA), hierarchical clustering, and Kendrick mass defect plots.
F
A and B:
Compounds removed
C, D, and E:
Compounds formed
F:
Compounds unchanged
A
C
D
B
E
Molecular feature abundance
normalized intensity (Log2)
Color range
-11. 2 0 11.2
28
Page 29

Capture, Analyze, and Share Water Quality Data
The advanced data mining and processing tools in the Agilent MassHunter software suite let you
quickly and accurately extract available information from the analytes in your samples.
– Take advantage of powerful data collection, processing, and reporting tools. Breeze through
application-specific workflows with comprehensive support of GC, GC/MS, and LC/MS technology.
– Use one software for all Agilent GC, GC/MS, and LC/MS instruments, including single quadrupole,
tandem quadrupole, and Q-TOF.
– Analyze complex environmental samples with compound-based analysis and reporting workflows,
using Agilent MassHunter Quantitative Analysis with Quant-My-Way customization.
– Simplify target and suspect screening with highly confident compound identification using Agilent
Personal Database and Libraries.
– Quickly build targeted screening and quantitation acquisition methods using the Agilent PFAS MRM
database for triple quadrupole LC/MS of a few—or a few dozen—PFAS compounds.
29
Page 30

Agilent CrossLab services
CrossLab is an Agilent capability that integrates services and consumables to
support workflow success and important outcomes like improved productivity
and operational efficiency. Through CrossLab, Agilent strives to provide insight
in every interaction to help you achieve your goals. CrossLab offers method
optimization, flexible service plans, and training for all skill levels. We have many
other products and services to help you manage your instruments and your lab
for best performance.
Learn more about Agilent CrossLab and see examples of insight that leads to
great outcomes at www.agilent.com/crosslab
Learn more:
www.agilent.com/chem/environmental
Get your PFAS ordering guide:
www.agilent.com/chem/pfas
Find a local Agilent customer center
in your country:
www.agilent.com/chem/contactus
U.S. and Canada
1-800-227-9770
agilent_inquiries@agilent.com
Europe
info_agilent@agilent.com
Asia Pacific
inquiry_lsca@agilent.com
RA.44141.2106018519
This information is subject to change without notice.
© Agilent Technologies, Inc. 2020
Published in the USA, November 24, 2020
5994-1322EN
 Loading...
Loading...