Page 1
SureSelect XT HS2 DNA
System
DNA Library Preparation and Target
Enrichment for the Illumina Platform
Protocol
Version D0, April 2021
SureSelect platform manufactured with Agilent
SurePrint Technology
For Research Use Only. Not for use in diagnostic
procedures.
Agilent Technologies
Page 2

Notices
© Agilent Technologies, Inc. 2020, 2021
No part of this manual may be reproduced in
any form or by any means (including electronic storage and retrieval or translation
into a foreign language) without prior agreement and written consent from Agilent
Technologies, Inc. as governed by United
States and international copyright laws.
Manual Part Number
G9983-90000
Edition
Version D0, April 2021
Printed in USA
Agilent Technologies, Inc.
5301 Stevens Creek Blvd
Santa Clara, CA 95051 USA
Acknowledgment
Oligonucleotide sequences © 2006, 2008,
and 2011 Illumina, Inc. All rights reserved.
Only for use with the Illumina sequencer
systems and associated assays.
Technical Support
For US and Canada
Call (800) 227-9770 (option 3,4,4)
Or send an e-mail to:
ngs.support@agilent.com
For all other regions
Agilent’s world-wide Sales and Support
Center contact details for your location can
be obtained at
www.agilent.com/en/contact-us/page.
Warranty
The material contained in this
document is provided “as is,” and
is subject to being changed, without notice, in future editions. Further, to the maximum extent
permitted by applicable law, Agilent disclaims all warranties,
either express or implied, with
regard to this manual and any
information contained herein,
including but not limited to the
implied warranties of merchantability and fitness for a particular
purpose. Agilent shall not be liable for errors or for incidental or
consequential damages in connection with the furnishing, use,
or performance of this document
or of any information contained
herein. Should Agilent and the
user have a separate written
agreement with warranty terms
covering the material in this document that conflict with these
terms, the warranty terms in the
separate agreement shall control.
Technology Licenses
The hardware and/or software described in
this document are furnished under a license
and may be used or copied only in accordance with the terms of such license.
Restricted Rights Legend
U.S. Government Restricted Rights. Software and technical data rights granted to
the federal government include only those
rights customarily provided to end user customers. Agilent provides this customary
commercial license in Software and technical data pursuant to FAR 12.211 (Technical
Data) and 12.212 (Computer Software) and,
for the Department of Defense, DFARS
252.227-7015 (Technical Data - Commercial
Items) and DFARS 227.7202-3 (Rights in
Commercial Computer Software or Computer Software Documentation).
Notice to Purchaser
This product is provided under an agreement between Bio-Rad Laboratories and
Agilent Technologies Inc., and the manufacture, use, sale or import of this product is
subject to U.S. Pat. No. 6,627,424 and EP
Pat. No.1 283 875 81, owned by Bio-Rad
Laboratories, Inc. Purchase of this product
conveys to the buyer the non-transferable
right to use the purchased amount of the
product and components of the product in
PCR (but not including real-time PCR) in the
Research Field (including all Applied
Research Fields, including but not limited to
forensics, animal testing, and food testing)
and in real-time PCR in the Diagnostics and
Prognostics Fields. No rights are granted for
use of this product for real-time PCR in the
Research Field, including all Applied
Research Fields (including but not limited to
forensics, animal testing and food testing).
2 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 3
Safety Notices
CAUTION
A CAUTION notice denotes a hazard. It calls attention to an operating procedure, practice, or the like that, if not
correctly performed or adhered to, could result in damage to the product or loss of important data. Do not proceed
beyond a CAUTION notice until the indicated conditions are fully understood and met.
WARNING
A WARNING notice denotes a hazard. It calls attention to an operating procedure, practice, or the like that, if
not correctly performed or adhered to, could result in personal injury or death. Do not proceed beyond a
WARNING notice until the indicated conditions are fully understood and met.
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 3
Page 4

In this Guide...
This guide provides an optimized protocol for preparation of
target- enriched Illumina paired- end multiplexed sequencing
libraries using the SureSelect XT HS2 DNA system.
1 Before You Begin
This chapter contains information that you should read and
understand before you start an experiment.
2 Preparation and Fragmentation of Input DNA
This chapter describes the steps to prepare and fragment
gDNA samples, using either mechanical shearing or
enzymatic fragmentation, prior to library preparation.
3 Library Preparation
This chapter describes the steps to prepare dual- indexed,
molecular- barcoded gDNA sequencing libraries for target
enrichment.
4 Hybridization and Capture
This chapter describes the steps to hybridize and capture
the prepared DNA library using a SureSelect or ClearSeq
Probe Capture Library.
5 Post-Capture Sample Processing for Multiplexed Sequencing
This chapter describes the steps for post- capture
amplification and guidelines for sequencing sample
preparation.
6 Appendix: Using FFPE-derived DNA Samples
This chapter describes the protocol modifications for gDNA
isolated from FFPE samples.
7 Reference
This chapter contains reference information, including
component kit contents and index sequences.
4 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 5

What’s New in Version D0
• Support for SureSelect XT HS Human All Exon V8 Probe.
• Updates to instructions in the “Hybridization and
• New footnotes to Table 11 on page 25 and Table 14 on
• Update to Figure 4 on page 46 and associated text on
• Update to downstream sequencing support information in
• Update to description of flat strip caps in Table 8 on
See Table 3 on page 14 for ordering information and see
Table 28 on page 51 for the hybridization thermal cycling
program recommended for this probe with the SureSelect
XT HS2 DNA system. Also see troubleshooting
information on page 101 and updates to the Quick
Reference Protocol on page 103.
Capture” chapter on page 49 through page 56. Updates
include provision of two separate hybridization thermal
cycler programs (Table 28 and Table 29 on page 51) and
related changes throughout the chapter.
page 28 on FFPE sample initial fragment size impacts on
library fragment size distribution.
page 47.
Table 41 on page 71.
page 19.
What’s New in Version C1
• Updates to index pair sequence tables (page 87 through
page 94) including updates to P5 index platform
descriptions and correction of well position typographical
errors
• Updates to downstream sequencing support information
(see Table 41 on page 71 and Note on page 86)
• Updates to molecular barcode and associated dark base
information (see Figure 8 on page 70) and instructions
for processing using the Agilent Genomics NextGen
Toolkit (see page 72)
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 5
Page 6

• Addition of hybridization temperature considerations for
What’s New in Version C0
• Support for revised SureSelect custom probe products,
• Updates to DNA library quantitation/qualification
• Updates to thermal cycler and plasticware
• Updates to Materials Required including updated
• Updates to Optional Materials in Table 8 on page 19,
• Updates to “SureSelect XT HS2 Index Primer Pair
probes designed for use with the SureSelect XT system
(see Table 28 on page 51)
produced using an updated manufacturing process
beginning August, 2020 (see Table 3 on page 14). Custom
probes produced using the legacy manufacturing process
are also fully supported by the protocols in this
document. Probe information was reorganized (see
Table 3 on page 14), and probe nomenclature throughout
document was updated.
guidelines including increased support for the Agilent’s
Fragment Analyzer platform and streamlined sample
analysis guidelines (see Table 6 on page 17 and see
page 44 through page 46 and page 65 through page 67).
recommendations and usage instructions (see Caution
and Table 5 on page 16 and see Note on page 34).
ordering information for Dynabeads MyOne Streptavidin
T1 beads (Table 4 on page 15) and for Eppendorf
ThermoMixer C and Qubit Fluorometer (Table 5 on
page 16).
including removal of ethylene glycol supplier information
(see page 25 for related update to DNA shearing set up
instructions).
Information” section (page 86 through page 97) including
addition of P5 primer sequences oriented for the NextSeq,
HiSeq 4000 and HiSeq 3000 platforms and reorganization
of section content.
6 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 7

Content
1 Before You Begin 9
Overview of the Workflow 10
Procedural Notes 12
Safety Notes 12
Materials Required 13
Optional Materials 19
2 Preparation and Fragmentation of Input DNA 21
Step 1. Prepare and analyze quality of genomic DNA samples 22
Preparation of high-quality gDNA from fresh biological samples 22
Preparation and qualification of gDNA from FFPE samples 22
Step 2. Fragment the DNA 25
Method 1: Mechanical DNA Shearing using Covaris 25
Method 2: Enzymatic DNA Fragmentation 28
3 Library Preparation 31
Step 1. Prepare the Ligation master mix 33
Step 2. Repair and dA-Tail the DNA ends 34
Step 3. Ligate the molecular-barcoded adaptor 36
Step 4. Purify the sample using AMPure XP beads 37
Step 5. Amplify the adaptor-ligated library 39
Step 6. Purify the amplified library with AMPure XP beads 42
Step 7. Assess quality and quantity 44
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 7
Page 8

Contents
4 Hybridization and Capture 49
Step 1. Hybridize DNA libraries to the probe 50
Step 2. Prepare streptavidin-coated magnetic beads 55
Step 3. Capture the hybridized DNA using streptavidin-coated beads 56
5 Post-Capture Sample Processing for Multiplexed Sequencing 59
Step 1. Amplify the captured libraries 60
Step 2. Purify the amplified captured libraries using AMPure XP beads 63
Step 3. Assess sequencing library DNA quantity and quality 65
Step 4. Pool samples for multiplexed sequencing 68
Step 5. Prepare sequencing samples 70
Step 6. Do the sequencing run and analyze the data 72
Sequence analysis resources 77
6 Appendix: Using FFPE-derived DNA Samples 79
Protocol modifications for FFPE Samples 80
Methods for FFPE Sample Qualification 80
Sequencing Output Recommendations for FFPE Samples 81
7 Reference 83
Kit Contents 84
SureSelect XT HS2 Index Primer Pair Information 86
Troubleshooting Guide 98
Quick Reference Protocol 102
8 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 9
SureSelect XT HS2 DNA System Protocol
1
Before You Begin
Overview of the Workflow 10
Procedural Notes 12
Safety Notes 12
Materials Required 13
Optional Materials 19
Make sure you read and understand the information in this chapter and
have the necessary equipment and reagents listed before you start an
experiment.
NOTE
NOTE
This protocol differs from the Illumina Multiplexed Paired-End sequencing manual and
other SureSelect protocols at several steps. Pay close attention to the primers used for
each amplification step and the blocking agents used during hybridization.
Agilent guarantees performance and provides technical support for the SureSelect
reagents required for this workflow only when used as directed in this Protocol.
Agilent Technologies
9
Page 10
1 Before You Begin
Overview of the Workflow
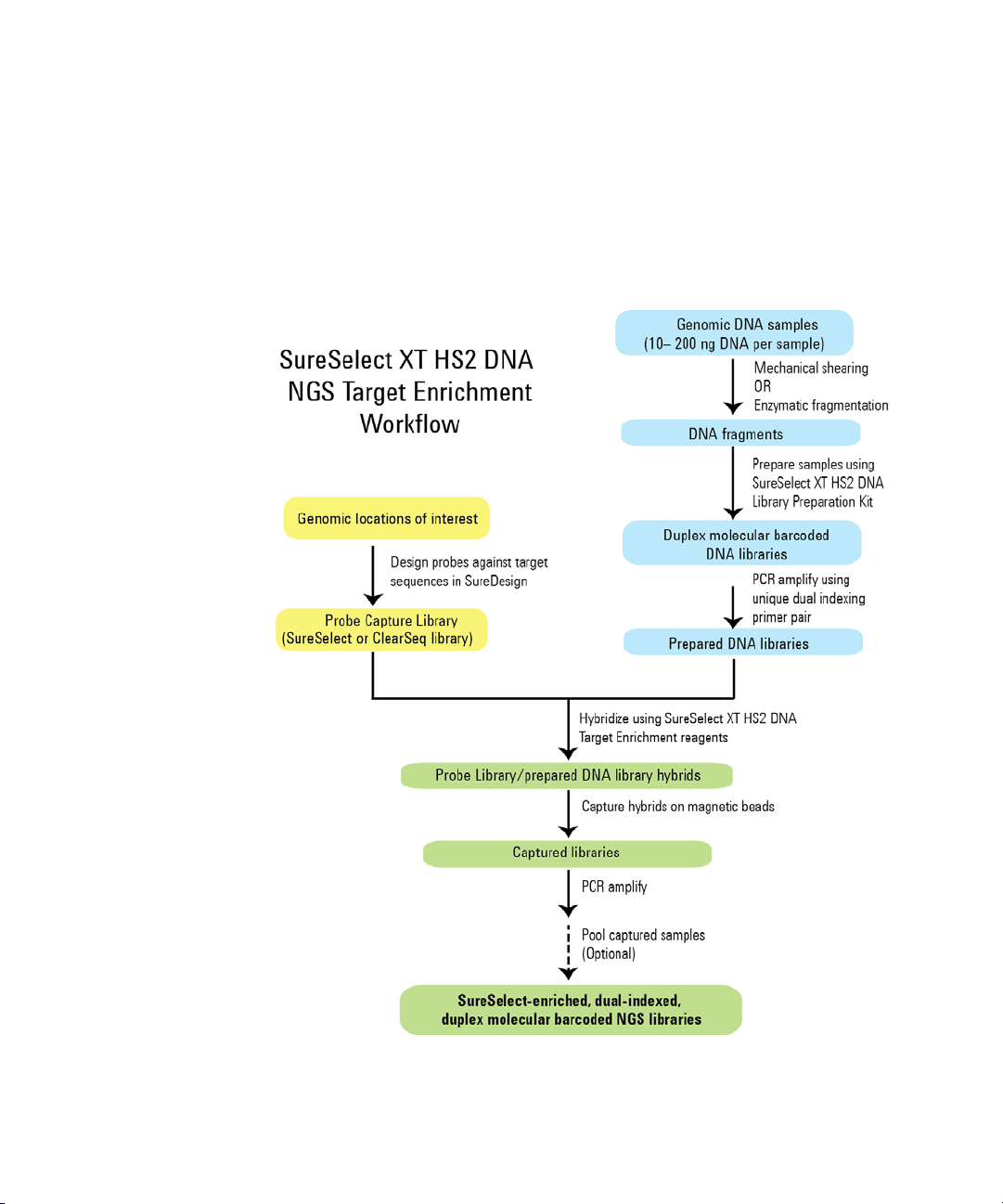
Overview of the Workflow
The SureSelect XT HS2 DNA workflow is summarized in Figure 1. The
estimated time requirements for each step are summarized in Table 1.
Figure 1 Overall target-enriched sequencing sample preparation workflow.
10 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 11

Overview of the Workflow
Table 1 Estimated time requirements (up to 16 sample run size)
Step Time
Library Preparation 3.5 hours
Hybridization and Capture 3.5 hours
Post-capture amplification 1 hour
Before You Begin 1
QC using Bioanalyzer or TapeStation platform and sample
pooling
1.5 hours
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 11
Page 12

1 Before You Begin
Procedural Notes
Procedural Notes
• To prevent contamination of reagents by nucleases, always wear
powder- free laboratory gloves and use dedicated solutions and pipettors
with nuclease- free aerosol- resistant tips.
• Use best- practices to prevent PCR product contamination of samples
throughout the workflow:
1 Assign separate pre- PCR and post- PCR work areas and use
dedicated equipment, supplies, and reagents in each area. In
particular, never use materials designated to post- PCR work areas for
pre- PCR segments of the workflow.
2 Maintain clean work areas. Clean pre- PCR surfaces that pose the
highest risk of contamination daily using a 10% bleach solution, or
equivalent.
3 Always use dedicated pre- PCR pipettors with nuclease- free
aerosol- resistant tips to pipette dedicated pre- PCR solutions.
4 Wear powder- free gloves. Use good laboratory hygiene, including
changing gloves after contact with any potentially- contaminated
surfaces.
• For each protocol step that requires removal of tube cap strips, reseal
the tubes with a fresh strip of caps. Cap deformation may result from
exposure of the cap strips to the heated lid of the thermal cycler and
from other procedural steps. Reuse of strip caps can cause sample loss,
sample contamination, or imprecision in sample temperatures during
thermal cycler incubation steps.
• In general, follow Biosafety Level 1 (BSL1) safety rules.
• Possible stopping points, where samples may be stored at –20°C, are
marked in the protocol. Do not subject the samples to multiple
freeze/thaw cycles.
Safety Notes
CAUTION
12 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
• Wear appropriate personal protective equipment (PPE) when working in the
laboratory.
Page 13
Before You Begin 1
Materials Required
Materials Required
Materials required to complete the SureSelect XT HS2 protocol will vary
based on the following considerations:
• SureSelect XT HS2 DNA Reagent Kit format preference, where some
options include ancillary reagent modules
• DNA sample type: high- quality gDNA derived from fresh/fresh- frozen
samples vs. FFPE- derived gDNA samples
• DNA fragmentation method used in workflow: mechanical
(Covaris- mediated) shearing vs. enzymatic fragmentation
To determine the materials required for your unique needs, first select the
preferred kit format from Table 2 below and a compatible target
enrichment probe from Table 3. Then refer to Table 4 through Table 7 for
additional materials needed to complete the protocols using the selected
kit format/DNA sample type/fragmentation method.
Table 2 SureSelect XT HS2 DNA Reagent Kit Varieties
Description Kit Part Number
16 Reaction Kit
SureSelect XT HS2 DNA Reagent Kit G9981A (with Index Pairs 1–16) G9983A (with Index Pairs 1–96)
Reagent Kits with additional component modules
SureSelect XT HS2 DNA Starter Kit
Includes the following modules:
SureSelect XT HS2 DNA Reagent Kit
SureSelect Enzymatic Fragmentation Kit
®
SureSelect DNA AMPure
SureSelect Streptavidin Beads
SureSelect XT HS2 DNA Reagent Kit with
®
AMPure
XP/Streptavidin Beads
XP Beads
‡
G9982A (with Index Pairs 1–16) Not applicable
Not applicable G9984A (with Index Pairs 1–96)
*
96 Reaction Kit
G9983B (with Index Pairs 97–192)
G9983C (with Index Pairs 193–288)
G9983D (with Index Pairs 289–384)
G9984B (with Index Pairs 97–192)
G9984C (with Index Pairs 193–288)
G9984D (with Index Pairs 289–384)
†
* 16-reaction kits contain enough reagents for 2 runs containing 8 samples per run.
† 96-reaction kits contain enough reagents for 4 runs containing 24 samples per run.
‡ AMPure, Beckman, and Beckman Coulter are trademarks or registered trademarks of Beckman Coulter, Inc.
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 13
Page 14

1 Before You Begin
Materials Required
Table 3 Compatible Probes
Probe Capture Library 16 Reactions 96 Reactions
Pre-designed Probes
SureSelect XT HS Human All Exon V8 5191-6873 5191-6874
SSel XT HS and XT Low Input Human All Exon V7 5191-4028 5191-4029
SureSelect XT Clinical Research Exome V2 5190-9491 5190-9492
SureSelect XT Mouse All Exon 5190-4641 5190-4642
ClearSeq Comprehensive Cancer XT 5190-8011 5190-8012
ClearSeq Inherited Disease XT 5190-7518 5190-7519
Custom Probes
SureSelect Custom Tier1 1–499 kb Please visit the SureDesign website to design
SureSelect Custom Tier2 0.5 –2.9 Mb
SureSelect Custom Tier3 3 –5.9 Mb
SureSelect Custom Tier4 6 –11.9 Mb
SureSelect Custom Tier5 12–24 Mb
Pre-designed Probes customized with additional Plus custom content
SSel XT HS and XT Low Input Human All Exon V7 Plus 1
SSel XT HS and XT Low Input Human All Exon V7 Plus 2
SureSelect XT Clinical Research Exome V2 Plus 1
SureSelect XT Clinical Research Exome V2 Plus 2
ClearSeq Comprehensive Cancer Plus XT
ClearSeq Inherited Disease Plus XT
*
Custom SureSelect probes and obtain ordering
information. Contact the SureSelect support
team (see page 2) or your local representative if
you need assistance. Custom probes are also
available in a 480 Reaction package.
Please visit the SureDesign website to design
the customized Plus content and obtain ordering
information. Contact the SureSelect support
team (see page 2) or your local representative if
you need assistance.
* Custom Probes designed August 2020 or later are produced using an updated manufacturing process; design-size
Tier is shown on labeling for these products. Custom Probes designed and ordered prior to August 2020 may be reordered, with these probes produced using the legacy manufacturing process; design-size Tier is not shown on labeling
for the legacy-process products. Custom Probes of both categories use the same optimized target enrichment protocols detailed in this publication.
14 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 15

Table 4 Required Reagents--All Sample Types/Fragmentation Methods
Description Vendor and Part Number Notes
Before You Begin 1
Materials Required
1X Low TE Buffer Thermo Fisher Scientific p/n
12090-015, or equivalent
100% Ethanol (Ethyl Alcohol, 200 proof) Millipore p/n EX0276 —
Qubit BR dsDNA Assay Kit
100 assays
500 assays
Nuclease-free Water Thermo Fisher Scientific p/n
®
AMPure
5 ml
60 ml
450 ml
Dynabeads MyOne Streptavidin T1
2 ml
10 ml
50 ml
XP Kit
Thermo Fisher Scientific
p/n Q32850
p/n Q32853
AM9930
Beckman Coulter Genomics
p/n A63880
p/n A63881
p/n A63882
Thermo Fisher Scientific
p/n 65601
p/n 65602
p/n 65604D
10 mM Tris-HCl, pH 7.5-8.0, 0.1 mM
EDTA
—
Water should not be DEPC-treated
Separate purchase not required for
use with SureSelect XT HS2 DNA
Reagent Kits that include
SureSelect DNA AMPure
Beads and SureSelect Streptavidin
Beads (Agilent p/n G9982A,
G9984A, G9984B, G9984C, or
G9984D)
®
XP
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 15
Page 16

1 Before You Begin
Materials Required
CAUTION
Sample volumes exceed 0.2 ml in certain steps of this protocol. Make sure that the
plasticware
used with the selected thermal cycler holds 0.25 ml per well.
Table 5 Required Equipment--All Sample Types/Fragmentation Methods
Description Vendor and Part Number
Thermal Cycler with 96-well, 0.2 ml block Various suppliers
Plasticware compatible with the selected thermal cycler:
96-well plates or 8-well strip tubes
Tube cap strips, domed
Nucleic acid analysis system (instrument and consumables) Select one system from Table 6 on page 17
Qubit Fluorometer Thermo Fisher Scientific p/n Q33238
Qubit Assay Tubes Thermo Fisher Scientific p/n Q32856
DNA LoBind Tubes, 1.5-ml PCR clean, 250 pieces Eppendorf p/n 022431021 or equivalent
Microcentrifuge Eppendorf microcentrifuge, model 5417C or equivalent
Plate or strip tube centrifuge Labnet International MPS1000 Mini Plate Spinner, p/n
96-well plate mixer Eppendorf ThermoMixer C, p/n 5382000023 and Eppendorf
Magnetic separator Thermo Fisher Scientific p/n 12331D or equivalent
Multichannel pipette Rainin Pipet-Lite Multi Pipette or equivalent
Single channel pipettes (10-, 20-, 200-, and 1000-µl capacity) Rainin Pipet-Lite Pipettes or equivalent
Sterile, nuclease-free aerosol barrier pipette tips general laboratory supplier
Vortex mixer general laboratory supplier
Ice bucket general laboratory supplier
Powder-free gloves general laboratory supplier
Consult the thermal cycler manufacturer’s
recommendations
C1000 (requires adapter, p/n C1000-ADAPT, for use with
strip tubes) or equivalent
SmartBlock 96 PCR, p/n 5306000006, or equivalent
*
* Select a magnetic separator configured to collect magnetic particles on one side of each well. Do not use a magnetic sep-
arator configured to collect the particles in a ring formation.
16 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 17

Materials Required
Table 6 Nucleic Acid Analysis Platform Options--Select One
Analysis System Vendor and Part Number Information
Agilent 4200/4150 TapeStation Instrument
Consumables:
96-well sample plates
96-well plate foil seals
8-well tube strips
8-well tube strip caps
D1000 ScreenTape
D1000 Reagents
High Sensitivity D1000 ScreenTape
High Sensitivity D1000 Reagents
Agilent 2100 Bioanalyzer Instrument
Agilent 2100 Expert SW Laptop Bundle (optional)
Consumables:
DNA 1000 Kit
High Sensitivity DNA Kit
Agilent 5200/5300/5400 Fragment Analyzer Instrument
Consumables:
NGS Fragment Kit (1-6000 bp)
HS NGS Fragment Kit (1-6000 bp)
Agilent p/n G2991AA/G2992AA
p/n 5042-8502
p/n 5067-5154
p/n 401428
p/n 401425
p/n 5067-5582
p/n 5067-5583
p/n 5067-5584
p/n 5067-5585
p/n G2939BA
p/n G2953CA
p/n 5067-1504
p/n 5067-4626
Agilent p/n M5310AA/M5311AA/M5312AA
p/n DNF-473-0500
p/n DNF-474-0500
Before You Begin 1
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 17
Page 18
1 Before You Begin
Materials Required
Table 7 Additional Required Materials based on DNA Sample Type/Fragmentation Method
Description Vendor and Part Number Notes
Required for preparation of high-quality DNA samples (not required for FFPE DNA sample preparation)
High-quality gDNA purification system, for example:
QIAamp DNA Mini Kit
50 Samples
250 Samples
Required for preparation of FFPE DNA samples (not required for high-quality DNA sample preparation)
QIAamp DNA FFPE Tissue Kit, 50 Samples Qiagen p/n 56404 —
Deparaffinization Solution Qiagen p/n 19093 —
FFPE DNA integrity assessment system:
Agilent NGS FFPE QC Kit
16 reactions
96 reactions
OR
TapeStation Genomic DNA Analysis Consumables:
Genomic DNA ScreenTape
Genomic DNA Reagents
Required for mechanical shearing of DNA samples (not required for workflows with enzymatic fragmentation)
Covaris Sample Preparation System Covaris model E220
Covaris microTUBE sample holders Covaris p/n 520045
Required for enzymatic fragmentation of DNA samples (not required for workflows with mechanical shearing)
SureSelect Enzymatic Fragmentation Kit Agilent
Qiagen
p/n 51304
p/n 51306
Agilent
p/n G9700A
p/n G9700B
Agilent
p/n 5067-5365
p/n 5067-5366
p/n 5191-4079 (16 reactions)
p/n 5191-4080 (96 reactions)
—
—
Not required for use with
SureSelect XT HS2 DNA Starter
Kit (Agilent p/n G9982A)
18 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 19

Before You Begin 1
Optional Materials
Optional Materials
Table 8 Supplier Information for Optional Materials
Description Vendor and Part Number Purpose
Tween 20 Sigma-Aldrich p/n P9416-50ML Sequencing library
storage (see page 69)
8× flat strip caps Consult the thermal cycler
manufacturer’s recommendations
MicroAmp Clear Adhesive Film Thermo Fisher Scientific p/n 4311971 Improved sealing for flat
PlateLoc Thermal Microplate Sealer with Small
Hotplate and Peelable Aluminum Seal for PlateLoc
Sealer
* Flat strip caps may be used instead of domed strip caps for protocol steps performed outside of the hybridization/capture
segment of the protocol. Adhesive film may be applied over the flat strip caps for improved sealing properties.
Please contact the SureSelect support
team (see page 2) or your local
representative for ordering information
Sealing wells for protocol
steps outside of
hybridization/capture
strip caps*
Sealing wells for protocol
steps performed inside or
outside of the thermal
cycler
*
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 19
Page 20

1 Before You Begin
Optional Materials
20 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 21

SureSelect XT HS2 DNA System Protocol
2
Preparation and Fragmentation of Input
DNA
Step 1. Prepare and analyze quality of genomic DNA samples 22
Preparation of high-quality gDNA from fresh biological samples 22
Preparation and qualification of gDNA from FFPE samples 22
Step 2. Fragment the DNA 25
Method 1: Mechanical DNA Shearing using Covaris 25
Method 2: Enzymatic DNA Fragmentation 28
This chapter describes the steps to prepare, quantify, qualify, and fragment
input DNA samples prior to SureSelect XT HS2 library preparation and
target enrichment. Protocols are provided for two alternative methods of
DNA fragmentation–mechanical shearing or enzymatic DNA fragmentation.
The library preparation protocol is compatible with both high- quality
gDNA prepared from fresh or fresh- frozen samples and lower- quality DNA
prepared from FFPE samples. Modifications required for FFPE samples are
included throughout the protocol steps. For a summary of modifications
for FFPE samples see Chapter 6, “Appendix: Using FFPE- derived DNA
Samples” on page 79.
The library preparation protocol requires 10 ng to 200 ng of input DNA,
with adjustments to DNA input amount or quantification method required
for some FFPE samples. For optimal sequencing results, use the maximum
amount of input DNA available within the recommended range.
Agilent Technologies
21
Page 22

2 Preparation and Fragmentation of Input DNA
Step 1. Prepare and analyze quality of genomic DNA samples
Step 1. Prepare and analyze quality of genomic DNA samples
Preparation of high-quality gDNA from fresh biological samples
1 Prepare high- quality gDNA using a suitable purification system, such as
Qiagen’s QIAamp DNA Mini Kit, following the manufacturer’s protocol.
NOTE
NOTE
Make sure genomic DNA samples are of high quality with an OD 260/280 ratio ranging
from 1.8 to 2.0.
2 Use the Qubit BR dsDNA Assay Kit to determine the concentration of
each gDNA sample. Follow the manufacturer’s instructions for the
instrument and assay kit.
Additional qualification of DNA samples is not required for DNA derived
from fresh biological samples. Proceed to “Step 2. Fragment the DNA” on
page 25.
Preparation and qualification of gDNA from FFPE samples
1 Prepare gDNA from FFPE tissue sections using Qiagen’s QIAamp DNA
FFPE Tissue Kit and Qiagen’s Deparaffinization Solution, following the
manufacturer’s protocol. Elute the final gDNA samples from the
MinElute column in two rounds, using 30 µl Buffer ATE in each round,
for a final elution volume of approximately 60 µl.
If tissue lysis appears incomplete after one hour of digestion with Proteinase K, add an
additional 10 µl of Proteinase K and continue incubating at 56°C, with periodic mixing, for
up to three hours.
Store the gDNA samples on ice for same- day library preparation, or at
–20°C for later processing.
2 Assess the quality (DNA integrity) for each FFPE DNA sample using one
of the methods below.
22 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 23

Preparation and Fragmentation of Input DNA 2
Preparation and qualification of gDNA from FFPE samples
Option 1: Qualification using the Agilent NGS FFPE QC Kit (Recommended
Method)
The Agilent NGS FFPE QC Kit provides a qPCR- based assay for DNA
sample integrity determination. Results include a Cq DNA integrity
score and the precise quantity of amplifiable DNA in the sample,
allowing direct normalization of DNA input for each sample. DNA input
recommendations based on Cq scores for individual samples are
summarized in Table 9.
a Use the Qubit BR dsDNA Assay Kit to determine the concentration of
each gDNA sample. Follow the manufacturer’s instructions for the
instrument and assay kit.
b Remove a 1 µl aliquot of the FFPE gDNA sample for analysis using
the Agilent NGS FFPE QC Kit to determine the Cq DNA integrity
score. See the kit user manual at www.agilent.com for more
information.
c For all samples with Cq DNA integrity score
Qubit- based gDNA concentration determined in step a, above, to
determine volume of input DNA needed for the protocol.
d For all samples with Cq DNA integrity score >1, use the
qPCR- based concentration of amplifiable gDNA, reported by the
Agilent NGS FFPE QC Kit results, to determine amounts of input
DNA for the protocol.
≤1, use the
Table 9 SureSelect XT HS2 DNA input modifications based on Cq DNA integrity score
Protocol Parameter non-FFPE Samples FFPE Samples
*
ΔΔCq≤1
DNA input for Library
Preparation
* FFPE samples with Cq scores 1 should be treated like non-FFPE samples for DNA input amount determinations. For sam-
ples of this type, make sure to use the DNA concentration determined by the Qubit Assay, instead of the concentration determined by qPCR, to calculate the volume required for 10–200 ng DNA.
10 ng to 200 ng DNA,
based on Qubit Assay
10 ng to 200 ng DNA, based
on Qubit Assay
ΔΔCq >1
10 ng to 200 ng of amplifiable DNA,
based on qPCR quantification
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 23
Page 24
2 Preparation and Fragmentation of Input DNA
Preparation and qualification of gDNA from FFPE samples
Option 2: Qualification using Agilent’s Genomic DNA ScreenTape assay DIN
score
Agilent’s Genomic DNA ScreenTape assay, used in conjunction with
Agilent’s TapeStation, provides a quantitative electrophoretic assay
for DNA sample integrity determination. This assay reports a DNA
Integrity Number (DIN) score for each sample which is used to
estimate the appropriate normalization of DNA input required for
low- integrity DNA samples.
a Use the Qubit BR dsDNA Assay Kit to determine the concentration of
each gDNA sample. Follow the manufacturer’s instructions for the
instrument and assay kit.
b Remove a 1 µl aliquot of the FFPE gDNA sample and analyze using
the Genomic DNA ScreenTape assay. See the user manual at
www.agilent.com for more information.
c Using the DIN score reported for each sample in the Genomic DNA
ScreenTape assay, consult Table 10 to determine the recommended
amount of input DNA for the sample.
Table 10 SureSelect XT HS2 DNA input modifications based on DNA Integrity Number (DIN) score
Protocol
Parameter
DNA input
for Library
Preparation
* FFPE samples with DIN>8 should be treated like non-FFPE samples for DNA input amount determinations.
non-FFPE
Samples
10 ng to 200 ng
DNA, quantified
by Qubit Assay
*
DIN > 8
10 ng to 200 ng DNA,
quantified by Qubit
Assay
FFPE Samples
DIN 3–8 DIN<3
Use at least 15 ng for more
intact samples and at least
40 ng for less intact samples.
Use the maximum amount of
DNA available, up to 200 ng, for
all samples. Quantify by Qubit
Assay.
Use at least 50 ng for more
intact samples and at least
100 ng for the least intact
samples. Use the maximum
amount of DNA available, up to
200 ng, for all samples. Quantify
by Qubit Assay.
24 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 25
Step 2. Fragment the DNA
Method 1: Mechanical DNA Shearing using Covaris
In this step, gDNA samples are sheared using conditions optimized for
either high- quality or FFPE DNA in a 50- µl shearing volume.
The target fragment size and corresponding shearing conditions may vary
for workflows using different NGS read lengths. See Table 11 for
guidelines. Complete shearing instructions are provided on page 26.
Table 11 Covaris shearing duration based on NGS read length
Preparation and Fragmentation of Input DNA 2
Step 2. Fragment the DNA
NOTE
NGS read length
requirement
2 ×100 reads 150 to 200 bp 2 × 120 seconds 240 seconds
2 ×150 reads 180 to 250 bp 2 × 60 seconds 240 seconds
* For FFPE DNA samples, initial DNA fragment size may impact the post-shear fragment size
distribution, resulting in fragment sizes shorter than the target ranges listed in this table. All
FFPE samples should be sheared for 240 seconds to generate fragment ends suitable for library construction. Libraries prepared from FFPE samples should be analyzed using an NGS
read length suitable for the final library fragment size distribution.
Target
fragment size
Shearing duration for
high-quality DNA samples
Shearing duration for
FFPE DNA samples
*
Shearing protocols have been optimized using a Covaris model E220 instrument and the
130-l Covaris microTUBE. Consult the manufacturer’s recommendations for use of other
Covaris instruments or sample holders to achieve the desired target DNA fragment size.
1 Set up the Covaris E220 instrument. Refer to the instrument user guide.
a Check that the water in the Covaris tank is filled with fresh
deionized water to the appropriate fill line level according to the
manufacturer’s recommendations.
b Check that the water covers the visible glass part of the tube.
c On the instrument control panel, push the Degas button. Degas the
instrument according to the manufacturer’s recommendations,
typically 30–60 minutes.
d Set the chiller temperature to between 2°C to 5°C to ensure that the
temperature reading in the water bath displays 5°C. Consult the
manufacturer’s recommendations for addition of coolant fluids to
prevent freezing.
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 25
Page 26
2 Preparation and Fragmentation of Input DNA
Method 1: Mechanical DNA Shearing using Covaris
2 Prepare the DNA samples for the run by diluting 10–200 ng of each
gDNA sample with 1X Low TE Buffer (10 mM Tris-
HCl, pH 7.5- 8.0,
0.1 mM EDTA) to a final volume of 50 µl. Vortex well to mix, then spin
briefly to collect the liquid. Keep the samples on ice.
NOTE
Do not dilute samples to be sheared using water. Shearing samples in water reduces the
overall library preparation yield and complexity.
3 Complete the DNA shearing steps below for each of the gDNA samples.
a Transfer the 50-
µl DNA sample into a Covaris microTUBE, using a
tapered pipette tip to slowly transfer the sample through the
pre- split septum of the cap.
b Spin the microTUBE for 30 seconds to collect the liquid and to
remove any bubbles from the bottom of the tube.
c Secure the microTUBE in the tube holder and shear the DNA with
the settings in Table 12.
Table 12 Shear settings for Covaris E-series instrument (SonoLab software v7 or later)
Setting High-quality DNA
for 2 × 100 read NGS
Duty Factor 10% 10% 10%
Peak Incident Power (PIP) 175 175 175
Cycles per Burst 200 200 200
Treatment Time 2 × 120 seconds 2 × 60 seconds 240 seconds
Bath Temperature 2° to 8° C 2° to 8° C 2° to 8° C
High-quality DNA
for 2 × 150 read NGS
FFPE DNA
(2 × 100 or 2 × 150 read NGS)
d Use the steps below for two- round shearing of high- quality DNA
samples only:
•Shear for 120 or 60 seconds (see Table 12)
•Spin the microTUBE for 10 seconds
•Vortex the microTUBE at high speed for 5 seconds
•Spin the microTUBE for 10 seconds
•Shear for additional 120 or 60 seconds
•Spin the microTUBE for 10 seconds
•Vortex the microTUBE at high speed for 5 seconds
•Spin the microTUBE for 10 seconds
26 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 27
NOTE
Preparation and Fragmentation of Input DNA 2
Method 1: Mechanical DNA Shearing using Covaris
e After completing the shearing step(s), put the Covaris microTUBE
back into the loading and unloading station.
f While keeping the snap- cap on, insert a pipette tip through the
pre- split septum, then slowly remove the sheared DNA.
g Transfer the sheared DNA sample (approximately 50 µl) to a 96-
plate or strip tube sample well. Keep the samples on ice.
h After transferring the DNA sample, spin the microTUBE briefly to
collect any residual sample volume. Transfer any additional collected
liquid to the sample well used in step g.
It is important to avoid loss of input DNA at this step, especially for low-abundance DNA
samples. Visually inspect the microTUBE to ensure that all of the sample has been
transferred. If droplets remain in the microTUBE, repeat step h.
The 50- µl sheared DNA samples are now ready for NGS sequencing library
preparation, beginning with end repair/dA-
Preparation” on page 31.
tailing. Proceed to “Library
well
NOTE
This is not a stopping point in the workflow, and analysis of the sheared samples is not
required before they are used for library preparation. Proceed directly to end-repair and
dA-tailing.
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 27
Page 28
2 Preparation and Fragmentation of Input DNA
Method 2: Enzymatic DNA Fragmentation
Method 2: Enzymatic DNA Fragmentation
In this step, gDNA samples are fragmented using Agilent’s SureSelect
Enzymatic Fragmentation Kit for ILM (Pre PCR).
1 In wells of a thermal cycler- compatible strip tube or PCR plate, dilute
10 ng to 200 ng of each gDNA sample with nuclease- free water to a
final volume of 7 µl.
2 Thaw the vial of 5X SureSelect Fragmentation Buffer, vortex, then place
on ice.
3 Preprogram a thermal cycler with the program in Table 13. Immediately
pause the program, and keep paused until samples are loaded in step 7.
Table 13 Thermal cycler program for enzymatic fragmentation
Step Temperature Time
Step 1 37°C Varies–see Table 14
Step 2 65°C 5 minutes
Step 3 4°C Hold
* Use a reaction volume setting of 10 l, if required for thermal cycler set up.
*
Optimal fragmentation conditions may vary based on the NGS read
length to be used in the workflow. Refer to Table 14 below for the
duration at 37°C appropriate for your sample type and required NGS
read length.
Table 14 Fragmentation duration based on sample type and NGS read length
NGS read length
requirement
2 ×100 reads 150 to 200 bp 15 minutes 15 minutes
2 ×150 reads 180 to 250 bp 10 minutes 15 minutes
* For FFPE DNA samples, initial DNA fragment size may impact the post-fragmentation size distribu-
tion, resulting in fragment sizes shorter than the target ranges listed in this table. All FFPE samples
should be incubated at 37°C for 15 minutes to generate fragment ends suitable for library construction. Libraries prepared from FFPE samples should be analyzed using an NGS read length suitable
for the final library fragment size distribution.
Target
fragment size
Duration of 37°C incubation step (Table 13)
High-quality DNA samples FFPE DNA samples
*
28 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 29

4 Prepare the appropriate volume of Fragmentation master mix by
combining the reagents in Table 15.
Mix well by pipetting up and down 20 times or seal the tube and vortex
at high speed for 5–10 seconds. Spin briefly to remove any bubbles and
keep on ice.
Table 15 Preparation of Fragmentation master mix
Preparation and Fragmentation of Input DNA 2
Method 2: Enzymatic DNA Fragmentation
Reagent Volume for 1 reaction Volume for 8 reactions
(includes excess)
5X SureSelect Fragmentation Buffer 2 µl 18 µl 50 µl
SureSelect Fragmentation Enzyme 1 µl 9 µl 25 µl
Total 3 µl 27 µl 75 µl
Volume for 24 reactions
(includes excess)
5 Add 3 µl of the Fragmentation master mix to each sample well
containing 7 µl of input DNA.
6 Mix well by pipetting up and down 20 times or cap the wells and
vortex at high speed for 5–10 seconds. Spin the samples briefly.
7 Immediately place the plate or strip tube in the thermal cycler and
resume the thermal cycling program in Table 13.
8 When the program reaches the 4°C Hold step, remove the samples from
the thermal cycler, add 40 µl of nuclease- free water to each sample,
and place the samples on ice.
The 50- µl reactions are now ready for NGS sequencing library preparation,
beginning with end repair/dA- tailing. Proceed to “Library Preparation” on
page 31.
NOTE
This is not a stopping point in the workflow, and analysis of the enzymatically-fragmented
samples is not required before they are used for library preparation. Proceed directly to
end-repair and dA-tailing.
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 29
Page 30

2 Preparation and Fragmentation of Input DNA
Method 2: Enzymatic DNA Fragmentation
30 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 31

SureSelect XT HS2 DNA System Protocol
3
Library Preparation
Step 1. Prepare the Ligation master mix 33
Step 2. Repair and dA-Tail the DNA ends 34
Step 3. Ligate the molecular-barcoded adaptor 36
Step 4. Purify the sample using AMPure XP beads 37
Step 5. Amplify the adaptor-ligated library 39
Step 6. Purify the amplified library with AMPure XP beads 42
Step 7. Assess quality and quantity 44
This chapter describes the steps to prepare DNA libraries for sequencing
using the Illumina paired- read platform. For each sample to be sequenced,
an individual dual- indexed and molecular- barcoded library is prepared.
For an overview of the SureSelect XT HS2 NGS sample preparation
workflow, see Figure 1 on page 10.
The NGS library preparation protocol that begins here is used for
fragmented DNA samples produced by mechanical shearing (as detailed on
page 25 to page 27) or produced by enzymatic fragmentation (as detailed
on page 28 to page 29). Samples produced by either method should
contain 10–200 ng of DNA fragments in a volume of 50 µl.
Protocol steps in this section use the components listed in Table 16. Thaw
and mix each component as directed in Table 16 before use (refer to the
Where Used column). Remove the AMPure XP beads from cold storage and
equilibrate to room temperature for at least 30 minutes in preparation for
use on page 37. Do not freeze the beads at any time.
To process multiple samples, prepare reagent mixtures with overage at
each step, without the cDNA library sample. Mixtures for preparation of
8 or 24 samples (including excess) are shown in tables as examples.
Agilent Technologies
31
Page 32

3 Library Preparation
Table 16 Reagents thawed before use in protocol
Kit Component Storage Location Thawing Conditions Mixing Method Where Used
End Repair-A Tailing Buffer
(yellow cap or bottle)
Ligation Buffer (purple cap
or bottle)
End Repair-A Tailing
Enzyme Mix (orange cap)
T4 DNA Ligase (blue cap) SureSelect XT HS2 Library
SureSelect XT HS2 Adaptor
Oligo Mix (clear cap)
SureSelect XT HS2 Library
Preparation Kit for ILM (Pre PCR),
–20°C
SureSelect XT HS2 Library
Preparation Kit for ILM (Pre PCR),
–20°C
SureSelect XT HS2 Library
Preparation Kit for ILM (Pre PCR),
–20°C
Preparation Kit for ILM (Pre PCR),
–20°C
SureSelect XT HS2 Library
Preparation Kit for ILM (Pre PCR),
–20°C
Thaw on ice (may
require >20 minutes)
then keep on ice
Thaw on ice (may
require >20 minutes)
then keep on ice
Place on ice just before
use
Place on ice just before
use
Thaw on ice then keep
on ice
Vortexing page 35
Vortexing page 33
Inversion page 35
Inversion page 33
Vortexing page 36
32 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 33

Library Preparation 3
Step 1. Prepare the Ligation master mix
Step 1. Prepare the Ligation master mix
Prepare the Ligation master mix to allow equilibration to room
temperature before use on page 36. Initiate this step before starting the
End Repair/dA- tailing protocol; leave samples on ice while completing this
step.
1 Vortex the thawed vial of Ligation Buffer for 15 seconds at high speed
to ensure homogeneity.
CAUTION
The Ligation Buffer used in this step is viscous. Mix thoroughly by vortexing at high
speed for 15 seconds before removing an aliquot for use. When combining with other
reagents, mix well by pipetting up and down 15–20 times using a pipette set to at least
80% of the mixture volume or by vortexing at high speed for 10–20 seconds.
Use flat top vortex mixers when vortexing strip tubes or plates throughout the protocol.
If reagents are mixed by vortexing, visually verify that adequate mixing is occurring.
2 Prepare the appropriate volume of Ligation master mix by combining
the reagents in Table 17.
Slowly pipette the Ligation Buffer into a 1.5- ml Eppendorf tube,
ensuring that the full volume is dispensed. Slowly add the T4 DNA
Ligase, rinsing the enzyme tip with buffer solution after addition. Mix
well by slowly pipetting up and down 15–20 times or seal the tube and
vortex at high speed for 10–20 seconds. Spin briefly to collect the
liquid.
Keep at room temperature for 30–45 minutes before use on page 36.
Table 17 Preparation of Ligation master mix
Reagent Volume for 1 reaction Volume for 8 reactions
(includes excess)
Ligation Buffer (purple cap or bottle) 23 µl 207 µl 575 µl
*
Volume for 24 reactions
(includes excess)
†
T4 DNA Ligase (blue cap) 2 µl 18 µl 50 µl
Total 25 µl 225 µl 625 µl
* The minimum supported run size for 16-reaction kits is 8 samples per run, with kits containing enough reagents for 2 runs of
8 samples each.
† The minimum supported run size for 96-reaction kits is 24 samples per run, with kits containing enough reagents for 4 runs
of 24 samples each.
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 33
Page 34

3 Library Preparation
Step 2. Repair and dA-Tail the DNA ends
Step 2. Repair and dA-Tail the DNA ends
1 Preprogram a thermal cycler with the program in Table 18 for the End
Repair and dA- Tailing steps. Immediately pause the program, and keep
paused until samples are loaded in step 5.
NOTE
CAUTION
Table 18 Thermal cycler program for End Repair/dA-Tailing
Step Temperature Time
Step 1 20°C 15 minutes
Step 2 72°C 15 minutes
Step 3 4°C Hold
* Use a reaction volume setting of 70 l, if required for thermal cycler set up.
When using Agilent’s SureCycler 8800 thermal cycler, the heated lid may be left on (default
setting) throughout the library preparation incubation steps. The heated lid must be on
during the hybridization and amplification steps on page 40, page 51 and page 61.
2 Vortex the thawed vial of End Repair- A Tailing Buffer for 15 seconds at
high speed to ensure homogeneity. Visually inspect the solution; if any
solids are observed, continue vortexing until all solids are dissolved.
*
The End Repair-A Tailing Buffer used in this step must be mixed thoroughly by
vortexing at high speed for 15 seconds before removing an aliquot for use. When
combining with other reagents, mix well either by pipetting up and down 15–20 times
using a pipette set to at least 80% of the mixture volume or by vortexing at high speed
for 5–10 seconds.
3 Prepare the appropriate volume of End Repair/dA- Tailing master mix,
by combining the reagents in Table 19.
Slowly pipette the End Repair- A Tailing Buffer into a 1.5- ml Eppendorf
tube, ensuring that the full volume is dispensed. Slowly add the End
Repair- A Tailing Enzyme Mix, rinsing the enzyme tip with buffer
solution after addition. Mix well by pipetting up and down 15–20 times
or seal the tube and vortex at high speed for 5–10 seconds. Spin briefly
to collect the liquid and keep on ice.
34 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 35

Table 19 Preparation of End Repair/dA-Tailing master mix
Library Preparation 3
Step 2. Repair and dA-Tail the DNA ends
Reagent Volume for 1 reaction Volume for 8 reactions
(includes excess)
End Repair-A Tailing Buffer (yellow cap or bottle) 16 µl 144 µl 400 µl
End Repair-A Tailing Enzyme Mix (orange cap) 4 µl 36 µl 100 µl
Total 20 µl 180 µl 500 µl
Volume for 24 reactions
(includes excess)
4 Add 20 µl of the End Repair/dA- Tailing master mix to each sample well
containing approximately 50 µl of fragmented DNA. Mix by pipetting up
and down 15–20 times using a pipette set to 50 µl or cap the wells and
vortex at high speed for 5–10 seconds.
5 Briefly spin the samples, then immediately place the plate or strip tube
in the thermal cycler and resume the thermal cycling program in
Table 18.
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 35
Page 36

3 Library Preparation
Step 3. Ligate the molecular-barcoded adaptor
Step 3. Ligate the molecular-barcoded adaptor
1 Once the thermal cycler reaches the 4°C Hold step, transfer the samples
to ice while setting up this step.
2 Preprogram a thermal cycler with the program in Table 20 for the
Ligation step. Immediately pause the program, and keep paused until
samples are loaded in step 5.
NOTE
Table 20 Thermal cycler program for Ligation
Step Temperature Time
Step 1 20°C 30 minutes
Step 2 4°C Hold
* Use a reaction volume setting of 100 l, if required for thermal cycler set up.
3 To each end- repaired/dA- tailed DNA sample (approximately 70 µl), add
25 µl of the Ligation master mix that was prepared on page 33 and
kept at room temperature. Mix by pipetting up and down at least
10 times using a pipette set to 70 µl or cap the wells and vortex at
high speed for 5–10 seconds. Briefly spin the samples.
4 Add 5 µl of SureSelect XT HS2 Adaptor Oligo Mix (clear- capped tube)
to each sample. Mix by pipetting up and down 15–20 times using a
pipette set to 70 µl or cap the wells and vortex at high speed for 5–10
seconds.
Make sure to add the Ligation master mix and the Adaptor Oligo Mix to the samples in
separate addition steps as directed above, mixing after each addition.
5 Briefly spin the samples, then immediately place the plate or strip tube
in the thermal cycler and resume the thermal cycling program in
Table 20.
*
NOTE
Stopping Point If you do not continue to the next step, seal the sample wells and store
36 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Unique molecular barcode sequences are incorporated into both ends of each library DNA
fragment at this step.
overnight at either 4°C or –20°C.
Page 37

Library Preparation 3
Step 4. Purify the sample using AMPure XP beads
Step 4. Purify the sample using AMPure XP beads
1 Verify that the AMPure XP beads were held at room temperature for at
least 30 minutes before use.
2 Prepare 400 µl of 70% ethanol per sample, plus excess, for use in
step 8.
NOTE
The freshly-prepared 70% ethanol may be used for subsequent purification steps run on the
same day. The complete Library Preparation protocol requires 0.8 ml of fresh 70% ethanol
per sample.
3 Mix the AMPure XP bead suspension well so that the reagent appears
homogeneous and consistent in color.
4 Add 80 µl of homogeneous AMPure XP beads to each DNA sample
(approximately 100 µl) in the PCR plate or strip tube. Pipette up and
down 15–20 times or cap the wells and vortex at high speed for
5–10 seconds to mix.
5 Incubate samples for 5 minutes at room temperature.
6 Put the plate or strip tube into a magnetic separation device. Wait for
the solution to clear (approximately 5 to 10 minutes).
7 Keep the plate or strip tube in the magnetic stand. Carefully remove
and discard the cleared solution from each well. Do not touch the beads
while removing the solution.
8 Continue to keep the plate or strip tube in the magnetic stand while
you dispense 200 µl of freshly- prepared 70% ethanol in each sample
well.
9 Wait for 1 minute to allow any disturbed beads to settle, then remove
the ethanol.
10 Repeat step 8 to step 9 once.
11 Seal the wells with strip caps, then briefly spin the samples to collect
the residual ethanol. Return the plate or strip tube to the magnetic
stand for 30 seconds. Remove the residual ethanol with a P20 pipette.
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 37
Page 38

3 Library Preparation
Step 4. Purify the sample using AMPure XP beads
12 Dry the samples by placing the unsealed plate or strip tube on the
thermal cycler, set to hold samples at 37°C, until the residual ethanol
has just evaporated (typically 1–2 minutes).
NOTE
NOTE
Do not dry the bead pellet to the point that the pellet appears cracked during any of the
bead drying steps in the protocol. Elution efficiency is significantly decreased when the
bead pellet is excessively dried.
13 Add 35 µl nuclease- free water to each sample well.
14 Seal the wells with strip caps, then mix well on a vortex mixer and
briefly spin the plate or strip tube to collect the liquid.
15 Incubate for 2 minutes at room temperature.
16 Put the plate or strip tube in the magnetic stand and leave for
approximately 5 minutes, until the solution is clear.
17 Remove the cleared supernatant (approximately 34 µl) to a fresh PCR
plate or strip tube sample well and keep on ice. You can discard the
beads at this time.
It may not be possible to recover the entire 34-µl supernatant volume at this step; transfer
the maximum possible amount of supernatant for further processing. To maximize recovery,
transfer the cleared supernatant to a fresh well in two rounds of pipetting, using a P20
pipette set at 17 µl.
38 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 39

Library Preparation 3
Step 5. Amplify the adaptor-ligated library
Step 5. Amplify the adaptor-ligated library
This step uses the components listed in Table 21. Before you begin, thaw
the reagents listed below and keep on ice.
Table 21 Reagents for pre-capture PCR amplification
Component Storage Location Mixing Method Where Used
Herculase II Fusion DNA
Polymerase (red cap)
5× Herculase II Buffer with
dNTPs (clear cap)
SureSelect XT HS2 Index Primer
Pairs
* Indexing primer pairs are provided in individual wells of strip tubes (16 reaction kits) or plates (96 reaction kits).
SureSelect XT HS2 Library Preparation
Kit for ILM (Pre PCR), –20°C
SureSelect XT HS2 Library Preparation
Kit for ILM (Pre PCR), –20°C
SureSelect XT HS2 Index Primer Pairs
for ILM (Pre PCR),
*
–20°C
Pipette up and down
15–20 times
Vortexing page 41
Vortexing page 41
page 41
1 Determine the appropriate index pair assignment for each sample. See
Table 51 through Table 58 in the “Reference” chapter for sequences of
the 8 bp index portion of the primers used to amplify the DNA libraries
in this step.
Use a different indexing primer pair for each sample to be sequenced in
the same lane.
CAUTION
The SureSelect XT HS2 Index Primer Pairs are provided in single-use aliquots. To avoid
cross-contamination of libraries, do not retain and re-use any residual volume in wells
for subsequent experiments.
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 39
Page 40

3 Library Preparation
Step 5. Amplify the adaptor-ligated library
2 Preprogram a thermal cycler with the program in Table 22. Immediately
pause the program, and keep paused until samples are loaded in step 6.
Table 22 Pre-Capture PCR Thermal Cycler Program
Segment Number of Cycles Temperature Time
1 1 98°C 2 minutes
2
3 1 72°C 5 minutes
4 1 4°C Hold
* Use a reaction volume setting of 50 l, if required for thermal cycler set up.
See Table 23 for cycle number
*
98°C 30 seconds
60°C 30 seconds
72°C 1 minute
Table 23 Pre-capture PCR cycle number recommendations
Quality of Input DNA Quantity of Input DNA Cycles
Intact DNA from fresh sample 100 to 200 ng 8 cycles
50 ng 9 cycles
10 ng 11 cycles
FFPE sample DNA 100 to 200 ng
50 ng* 12 cycles
10 ng* 14 cycles
* qPCR-determined quantity of amplifiable DNA or DIN value-adjusted amount of input DNA
*
11 cycles
40 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 41

Library Preparation 3
Step 5. Amplify the adaptor-ligated library
CAUTION
To avoid cross-contaminating libraries, set up PCR reactions (all components except
the library DNA) in a dedicated clean area or PCR hood with UV sterilization and
positive air flow.
3 Prepare the appropriate volume of pre- capture PCR reaction mix, as
described in Table 24, on ice. Mix well on a vortex mixer.
Table 24 Preparation of Pre-Capture PCR Reaction Mix
Reagent Volume for 1 reaction Volume for 8 reactions
(includes excess)
5× Herculase II Buffer with dNTPs (clear cap) 10 µl 90 µl 250 µl
Herculase II Fusion DNA Polymerase (red cap) 1 µl 9 µl 25 µl
Total 11 µl 99 µl 275 µl
Volume for 24 reactions
(includes excess)
4 Add 11 µl of the PCR reaction mixture prepared in Table 24 to each
purified DNA library sample (34 µl) in the PCR plate wells.
5 Add 5 µl of the appropriate SureSelect XT HS2 Index Primer Pair to
each reaction.
Cap the wells then vortex at high speed for 5 seconds. Spin the plate
or strip tube briefly to collect the liquid and release any bubbles.
6 Before adding the samples to the thermal cycler, resume the thermal
cycling program in Table 22 to bring the temperature of the thermal
block to 98°C. Once the cycler has reached 98°C, immediately place the
sample plate or strip tube in the thermal block and close the lid.
CAUTION
The lid of the thermal cycler is hot and can cause burns. Use caution when working
near the lid.
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 41
Page 42

3 Library Preparation
Step 6. Purify the amplified library with AMPure XP beads
Step 6. Purify the amplified library with AMPure XP beads
1 Verify that the AMPure XP beads were held at room temperature for at
least 30 minutes before use.
2 Prepare 400 µl of 70% ethanol per sample, plus excess, for use in
step 8.
3 Mix the AMPure XP bead suspension well so that the reagent appears
homogeneous and consistent in color.
4 Add 50 µl of homogeneous AMPure XP beads to each 50- µl
amplification reaction in the PCR plate or strip tube. Pipette up and
down 15–20 times or cap the wells and vortex at high speed for
5–10 seconds to mix.
5 Incubate samples for 5 minutes at room temperature.
6 Put the plate or strip tube into a magnetic separation device. Wait for
the solution to clear (approximately 5 minutes).
7 Keep the plate or strip tube in the magnetic stand. Carefully remove
and discard the cleared solution from each well. Do not touch the beads
while removing the solution.
8 Continue to keep the plate or strip tube in the magnetic stand while
you dispense 200 µl of freshly- prepared 70% ethanol into each sample
well.
9 Wait for 1 minute to allow any disturbed beads to settle, then remove
the ethanol.
10 Repeat step 8 and step 9 step once.
11 Seal the wells with strip caps, then briefly spin the samples to collect
the residual ethanol. Return the plate or strip tube to the magnetic
stand for 30 seconds. Remove the residual ethanol with a P20 pipette.
12 Dry the samples by placing the unsealed plate or strip tube on the
thermal cycler, set to hold samples at 37°C, until the residual ethanol
has just evaporated (typically 1–2 minutes).
13 Add 15 µl nuclease- free water to each sample well.
14 Seal the wells with strip caps, then mix well on a vortex mixer and
briefly spin the plate or strip tube to collect the liquid.
15 Incubate for 2 minutes at room temperature.
16 Put the plate or strip tube in the magnetic stand and leave for 2 to
3 minutes, until the solution is clear.
42 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 43

Library Preparation 3
Step 6. Purify the amplified library with AMPure XP beads
17 Remove the cleared supernatant (approximately 15 µl) to a fresh PCR
plate or strip tube sample well and keep on ice. You can discard the
beads at this time.
NOTE
It may not be possible to recover the entire 15-µl supernatant volume at this step; transfer
the maximum possible amount of supernatant for further processing.
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 43
Page 44

3 Library Preparation
Step 7. Assess quality and quantity
Step 7. Assess quality and quantity
Analyze a five- fold dilution of each sample using one of the platforms
listed in Table 25. Follow the instructions in the linked user guide
provided for each assay in Table 25, after reviewing the SureSelect library
qualification steps on page 45. Each analysis method provides an
electropherogram showing the size distribution of fragments in the sample
and tools for determining the concentration of DNA in the sample. See
Table 26 for fragment size distribution guidelines for various sample types.
Representative electropherograms generated using the TapeStation system
are provided to illustrate typical results for libraries prepared from several
types of input DNA.
Table 25 Pre-capture library analysis options
Analysis platform Assay used at this step Link to assay instructions Amount of library
sample to analyze
Agilent 4200 or 4150 TapeStation
system
Agilent 2100 Bioanalyzer system DNA 1000 Kit Agilent DNA 1000 Kit Guide 1 µl of five-fold
Agilent 5200, 5300, or 5400
Fragment Analyzer system
D1000 ScreenTape Agilent D1000 Assay Quick
Guide
NGS Fragment Kit (1-6000 bp) Agilent NGS Fragment Kit
(1-6000 bp) Kit Guide
1 µl of five-fold
dilution
dilution
2 µl of five-fold
dilution
Table 26 Pre-capture library qualification guidelines
NGS read length for
fragmentation protocol selection
2 ×100 reads Mechanical shearing Intact DNA 300 to 400 bp
2 ×150 reads Mechanical shearing Intact DNA 330 to 450 bp
Fragmentation method Input DNA type Expected library DNA
fragment size peak position
FFPE DNA 200 to 400 bp
Enzymatic fragmentation Intact DNA 300 to 400 bp
FFPE DNA 200 to 400 bp
FFPE DNA 200 to 450 bp
Enzymatic fragmentation Intact DNA 330 to 450 bp
FFPE DNA 200 to 450 bp
44 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 45

Library Preparation 3
Step 7. Assess quality and quantity
1 Set up the instrument as instructed in the appropriate user guide (links
provided in Table 25).
2 Prepare samples for analysis by diluting 1 µl of each prepared library
sample in 4 µl of nuclease- free water.
3 Prepare the diluted samples for analysis and set up the assay as
instructed in the appropriate user guide. Load the analysis assay into
the instrument and complete the run.
4 Verify that the electropherogram shows the expected DNA fragment size
peak position (see Table 26 for guidelines). Sample TapeStation system
electropherograms are shown for libraries prepared from sheared DNA
designed for 2 ×100 bp reads in Figure 2 (high- quality DNA), Figure 3
(medium- quality FFPE DNA), and Figure 4 (low- quality FFPE DNA).
Electropherograms obtained using the other analysis platform options
listed in Table 25 are expected to show similar fragment size profiles.
5 Determine the concentration of the library DNA by integrating under
the peak.
Figure 2 Pre-capture library prepared from a sheared high-quality gDNA sample ana-
lyzed using a D1000 ScreenTape assay.
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 45
Page 46

3 Library Preparation
Step 7. Assess quality and quantity
Figure 3 Pre-capture library prepared from a typical FFPE gDNA sample (fragmented by
shearing) analyzed using a D1000 ScreenTape assay.
Figure 4 Pre-capture library prepared from a low-quality FFPE gDNA sample (fragment-
ed by shearing) analyzed using a D1000 ScreenTape assay.
46 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 47

Library Preparation 3
Step 7. Assess quality and quantity
Observation of a low molecular weight peak, in addition to the expected
library fragment peak, indicates the presence of adaptor- dimers in the
library. It is acceptable to proceed to target enrichment with library
samples for which adaptor- dimers are observed in the electropherogram at
low abundance, similar to that seen in the example electropherogram in
Figure 4. See Troubleshooting on page 100 for additional considerations.
NOTE
Stopping Point If you do not continue to the next step, seal the sample wells and store at
For libraries being prepared for whole-genome sequencing (not specifically supported by
this user guide), samples with an adaptor-dimer peak must be subjected to an additional
round of SPRI-purification. To complete, first dilute the sample to 50 µl with nuclease free
water, then follow the SPRI purification procedure on page 42.
4°C overnight or at –20°C for prolonged storage.
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 47
Page 48

3 Library Preparation
Step 7. Assess quality and quantity
48 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 49

SureSelect XT HS2 DNA System Protocol
4
Hybridization and Capture
Step 1. Hybridize DNA libraries to the probe 50
Step 2. Prepare streptavidin-coated magnetic beads 55
Step 3. Capture the hybridized DNA using streptavidin-coated beads 56
This chapter describes the steps to hybridize the prepared gDNA libraries
with a target- specific probe. After hybridization, the targeted molecules
are captured on streptavidin beads. Each DNA library sample is hybridized
and captured individually.
The standard single- day SureSelect XT HS2 protocol includes the
hybridization step immediately followed by capture and amplification
steps. If required, the hybridized samples may be held overnight with
capture and amplification steps completed the following day by using the
simple protocol modifications noted on page 52.
CAUTION
The ratio of probe to prepared gDNA library is critical for successful capture.
Agilent Technologies
49
Page 50

4 Hybridization and Capture
Step 1. Hybridize DNA libraries to the probe
Step 1. Hybridize DNA libraries to the probe
In this step, the prepared gDNA libraries are hybridized to a
target- specific probe using probe- specific hybridization conditions. For
each sample library prepared, do one hybridization and capture. Do not
pool samples at this stage.
The hybridization reaction requires 500- 1000 ng of prepared DNA in a
volume of 12 µl. Use the maximum amount of prepared DNA available in
this range.
This step uses the components listed in Table 27. Thaw each component
under the conditions indicated in the table. Vortex each reagent to mix,
then spin tubes briefly to collect the liquid.
Table 27 Reagents for Hybridization
Kit Component Storage Location Thawing Conditions Where Used
SureSelect XT HS2 Blocker Mix
(blue cap)
SureSelect RNase Block (purple
cap)
SureSelect Fast Hybridization Buffer
(bottle)
Probe –80°C Thaw on ice page 53
SureSelect XT HS2 Target Enrichment Kit
ILM Hyb Module, Box 2 (Post PCR), –20°C
SureSelect XT HS2 Target Enrichment Kit
ILM Hyb Module, Box 2 (Post PCR), –20°C
SureSelect XT HS2 Target Enrichment Kit
ILM Hyb Module, Box 2 (Post PCR), –20°C
Thaw on ice page 52
Thaw on ice page 53
Thaw and keep at
Room Temperature
page 53
50 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 51

Hybridization and Capture 4
Step 1. Hybridize DNA libraries to the probe
1 Preprogram a thermal cycler (with heated lid ON) with the program in
Table 28 for the SureSelect XT HS Human All Exon V8 Probe or in
Table 29 for all other probes. Immediately pause the program, and keep
paused until samples are loaded in step 4 on page 52.
Table 28 Hybridization program for SureSelect XT HS Human All Exon V8 Probe
Segment # Number of Cycles Temperature Time
1 1 95°C 5 minutes
2 1 65°C 10 minutes
3 1 65°C 1 minute (Pause cycler here for reagent addition; see step 7 on page 54)
4 60 65°C 1 minute
37°C 3 seconds
5 1 65°C 60 minutes
6 1 65°C Hold briefly until ready to begin capture steps on page 56
* Use a reaction volume setting of 30 l (final volume of hybridization reactions in Segment 4).
Table 29 Hybridization program for all other probes
Segment # Number of Cycles Temperature Time
1 1 95°C 5 minutes
2 1 65°C 10 minutes
3 1 65°C 1 minute (Pause cycler here for reagent addition; see step 7 on page 54)
4 60 65°C
51
†
37°C 3 seconds
†
65°C
*
1 minute
Hold briefly until ready to begin capture steps on page 56
*
* Use a reaction volume setting of 30 l (final volume of hybridization reactions during cycling in Segment 4).
† Hybridization at 65°C is optimal for probes designed for the SureSelect XT HS2/XT HS/XL Low Input platforms. Reducing the
hybridization temperature (Segments 4 and 5) may improve performance for probes designed for the SureSelect XT platform,
including SureSelect XT Human All Exon V6 (62.5°C), SureSelect XT Clinical Research Exome V2 (62.5°C) and custom probes
originally designed for use with SureSelect XT system (60°C–65°C).
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 51
Page 52

4 Hybridization and Capture
Step 1. Hybridize DNA libraries to the probe
NOTE
CAUTION
The Hybridization reaction may be run overnight with the following protocol modifications:
• In the final segment of the thermal cycler program (Table 28 or Table 29), replace the
65°C Hold step with a 21°C Hold step.
• The hybridized samples may be held at 21°C for up to 16 hours. Begin the capture
preparation steps on page 55 on day 2, after the overnight hold.
2 Place 1000 ng of each prepared gDNA library sample into the
hybridization plate or strip tube wells and then bring the final volume
in each well to 12 µl using nuclease- free water. If 1000 ng DNA is not
available for any of the samples, use the maximum amount available,
within the 500–1000 ng range.
3 To each DNA library sample well, add 5 µl of SureSelect XT HS2
Blocker Mix (blue cap). Seal the wells then vortex at high speed for
5 seconds. Spin briefly to collect the liquid and release any bubbles.
The lid of the thermal cycler is hot and can cause burns. Use caution when working
near the lid.
4 Transfer the sealed sample plate or strip to the thermal cycler and
resume the thermal cycling program (Table 28 or Table 29 on page 51),
allowing the cycler to complete Segments 1 and 2 of the program.
Important: The thermal cycler must be paused during Segment 3 to
allow additional reagents to be added to the Hybridization wells in
step 7 on page 54.
During Segments 1 and 2 of the thermal cycling program, begin
preparing the additional hybridization reagents as described in step 5
and step 6 below. If needed, you can finish these preparation steps
after pausing the thermal cycler in Segment 3.
52
SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 53

5 Prepare a 25% solution of SureSelect RNase Block (1 part RNase Block
to 3 parts water) according to Table 30. Prepare the amount required
for the number of hybridization reactions in the run, plus excess. Mix
well and keep on ice.
Table 30 Preparation of RNase Block solution
Hybridization and Capture 4
Step 1. Hybridize DNA libraries to the probe
Reagent Volume for 1 reaction Volume for 8 reactions
(includes excess)
SureSelect RNase Block 0.5 µl 4.5 µl 12.5 µl
Nuclease-free water 1.5 µl 13.5 µl 37.5 µl
Total 2 µl 18 µl 50 µl
NOTE
Prepare the mixture described in step 6, below, just before pausing the thermal cycler in
Segment 3. Keep the mixture at room temperature briefly until the mixture is added to the
Volume for 24 reactions
(includes excess)
DNA samples in step 7 on page 54. Do not keep solutions containing the probe at room
temperature for extended periods.
6 Prepare the Probe Hybridization Mix appropriate for your probe design
size. Use Table 31 for probes 3 Mb or Table 32 for probes <3 Mb.
Combine the listed reagents at room temperature. Mix well by
vortexing at high speed for 5 seconds then spin down briefly. Proceed
immediately to step 7.
Table 31 Preparation of Probe Hybridization Mix for probes≥3 Mb
Reagent Volume for 1 reaction Volume for 8 reactions
(includes excess)
Volume for 24 reactions
(includes excess)
25% RNase Block solution (from step 5) 2 µl 18 µl 50 µl
Probe (with design 3 Mb) 5 µl 45 µl 125 µl
SureSelect Fast Hybridization Buffer 6 µl 54 µl 150 µl
Total 13 µl 117 µl 325 µl
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 53
Page 54
4 Hybridization and Capture
Step 1. Hybridize DNA libraries to the probe
Table 32 Preparation of Probe Hybridization Mix for probes<3 Mb
Reagent Volume for 1 reaction Volume for 8 reactions
(includes excess)
25% RNase Block solution (from step 5) 2 µl 18 µl 50 µl
Probe (with design<3 Mb) 2 µl 18 µl 50 µl
SureSelect Fast Hybridization Buffer 6 µl 54 µl 150 µl
Nuclease-free water 3 µl 27 µl 75 µl
Total 13 µl 117 µl 325 µl
Volume for 24 reactions
(includes excess)
7 Once the thermal cycler starts Segment 3 (1 minute at 65°C), pause the
program. With the cycler paused, and while keeping the DNA + Blocker
samples in the cycler, transfer 13 µl of the room- temperature Probe
Hybridization Mix from step 6 to each sample well.
Mix well by pipetting up and down slowly 8 to 10 times.
The hybridization reaction wells now contain approximately 30 µl.
8 Seal the wells with fresh domed strip caps. Make sure that all wells are
completely sealed. Vortex briefly, then spin the plate or strip tube
briefly to remove any bubbles from the bottom of the wells. Immediately
return the plate or strip tube to the thermal cycler.
9 Resume the thermal cycling program to allow hybridization of the
prepared DNA samples to the probe.
CAUTION
Wells must be adequately sealed to minimize evaporation, or your results can be
negatively impacted.
Before you do the first experiment, make sure the plasticware and capping method are
appropriate for the thermal cycler. Check that no more than 4 µl is lost to evaporation
under the conditions used for hybridization.
54 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 55

Hybridization and Capture 4
Step 2. Prepare streptavidin-coated magnetic beads
Step 2. Prepare streptavidin-coated magnetic beads
The remaining hybridization capture steps use the reagents in Table 33.
NOTE
Table 33 Reagents for Capture
Kit Component Storage Location Where Used
SureSelect Binding Buffer SureSelect Target Enrichment Kit, ILM Hyb Module, Box 1
SureSelect Wash Buffer 1 SureSelect Target Enrichment Kit, ILM Hyb Module, Box 1
SureSelect Wash Buffer 2 SureSelect Target Enrichment Kit, ILM Hyb Module, Box 1
SureSelect Streptavidin Beads OR
Dynabeads MyOne Streptavidin T1 Beads
If performing same-day hybridization and capture, begin the bead preparation steps below
approximately one hour after starting hybridization in step 9 on page 54. If performing
next-day capture after an overnight hold at 21°C, begin the bead preparation steps below on
day 2, just before you are ready to start the capture steps on page 56.
page 55
(Post PCR), RT
page 56
(Post PCR), RT
page 56
(Post PCR), RT
4°C page 55
1 Vigorously resuspend the vial of streptavidin beads on a vortex mixer.
The magnetic beads settle during storage.
2 For each hybridization sample, add 50 µl of the resuspended beads to
wells of a fresh PCR plate or strip tube.
3 Wash the beads:
a Add 200 µl of SureSelect Binding Buffer.
b Mix by pipetting up and down 20 times or cap the wells and vortex
at high speed for 5–10 seconds then spin down briefly.
c Put the plate or strip tube into a magnetic separator device.
d Wait 5 minutes or until the solution is clear, then remove and
discard the supernatant.
e Repeat step a through step d two more times for a total of 3 washes.
4 Resuspend the beads in 200 µl of SureSelect Binding Buffer.
NOTE
If you are equipped for higher-volume magnetic bead captures, the streptavidin beads may
instead be batch-washed in an Eppendorf tube or conical vial.
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 55
Page 56

4 Hybridization and Capture
Step 3. Capture the hybridized DNA using streptavidin-coated beads
Step 3. Capture the hybridized DNA using streptavidin-coated
beads
1 After all streptavidin bead preparation steps are complete and with the
hybridization thermal cycling program in the final hold segment (see
Table 28 or Table 29 on page 51), transfer the samples to room
temperature.
2 Immediately transfer the entire volume (approximately 30 µl) of each
hybridization mixture to wells containing 200 µl of washed streptavidin
beads using a multichannel pipette.
Pipette up and down 5–8 times to mix then seal the wells with fresh
caps.
3 Incubate the capture plate or strip tube on a 96- well plate mixer,
mixing vigorously (at 1400–1900 rpm), for 30 minutes at room
temperature.
Make sure the samples are properly mixing in the wells.
4 During the 30- minute incubation for capture, prewarm SureSelect Wash
Buffer 2 at 70°C as described below.
a Place 200- µl aliquots of Wash Buffer 2 in wells of a fresh 96- well
plate or strip tubes. Aliquot 6 wells of buffer for each DNA sample in
the run.
b Cap the wells and then incubate in the thermal cycler held at 70°C
until used in step 9.
5 When the 30- minute capture incubation period initiated in step 3 is
complete, spin the samples briefly to collect the liquid.
6 Put the plate or strip tube in a magnetic separator to collect the beads.
Wait until the solution is clear, then remove and discard the
supernatant.
7 Resuspend the beads in 200 µl of SureSelect Wash Buffer 1. Mix by
pipetting up and down 15–20 times, until beads are fully resuspended.
8 Put the plate or strip tube in the magnetic separator. Wait for the
solution to clear (approximately 1 minute), then remove and discard the
supernatant.
56 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 57

Hybridization and Capture 4
Step 3. Capture the hybridized DNA using streptavidin-coated beads
CAUTION
It is important to maintain bead suspensions at 70°C during the washing procedure
below to ensure specificity of capture.
Make sure that the SureSelect Wash Buffer 2 is pre-warmed to 70°C before use.
Do not use a tissue incubator, or other devices with significant temperature
fluctuations, for the incubation steps.
9 Remove the plate or strip tubes from the magnetic separator and
transfer to a rack at room temperature. Wash the beads with Wash
Buffer 2, using the protocol steps below.
a Resuspend the beads in 200 µl of 70°C prewarmed Wash Buffer 2.
Pipette up and down 15–20 times, until beads are fully resuspended.
b Seal the wells with fresh caps and then vortex at high speed for
8 seconds. Spin the plate or strip tube briefly to collect the liquid
without pelleting the beads.
Make sure the beads are in suspension before proceeding.
c Incubate the samples for 5 minutes at 70°C on the thermal cycler
with the heated lid on.
d Put the plate or strip tube in the magnetic separator at room
temperature.
e Wait 1 minute for the solution to clear, then remove and discard the
supernatant.
f Repeat step a through step e five more times for a total of 6 washes.
10 After verifying that all wash buffer has been removed, add 25 µl of
nuclease- free water to each sample well. Pipette up and down 8 times
to resuspend the beads.
Keep the samples on ice until they are used on page 62.
NOTE
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 57
Captured DNA is retained on the streptavidin beads during the post-capture amplification
step.
Page 58

4 Hybridization and Capture
Step 3. Capture the hybridized DNA using streptavidin-coated beads
58 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 59

SureSelect XT HS2 DNA System Protocol
5
Post-Capture Sample Processing for
Multiplexed Sequencing
Step 1. Amplify the captured libraries 60
Step 2. Purify the amplified captured libraries using AMPure XP beads 63
Step 3. Assess sequencing library DNA quantity and quality 65
Step 4. Pool samples for multiplexed sequencing 68
Step 5. Prepare sequencing samples 70
Step 6. Do the sequencing run and analyze the data 72
This chapter describes the steps to amplify, purify, and assess quality and
quantity of the captured libraries. Sample pooling instructions are
provided to prepare the indexed, molecular barcoded samples for
multiplexed sequencing. Sequencing run setup and sequencing data
analysis steps will vary according to your NGS platform and data analysis
pipeline; guidelines for these downstream steps are also provided in this
chapter.
Agilent Technologies
59
Page 60

5 Post-Capture Sample Processing for Multiplexed Sequencing
Step 1. Amplify the captured libraries
Step 1. Amplify the captured libraries
In this step, the SureSelect- enriched DNA libraries are PCR amplified.
This step uses the components listed in Table 34. Before you begin, thaw
the reagents listed below and keep on ice. Remove the AMPure XP beads
from cold storage and equilibrate to room temperature for at least
30 minutes in preparation for use on page 63. Do not freeze the beads at
any time.
Table 34 Reagents for post-capture PCR amplification
Component Storage Location Mixing Method Where Used
Herculase II Fusion DNA
Polymerase (red cap)
5× Herculase II Buffer with
dNTPs (clear cap)
SureSelect Post-Capture Primer
Mix (clear cap)
Prepare one amplification reaction for each DNA library.
CAUTION
To avoid cross-contaminating libraries, set up PCR mixes in a dedicated clean area or
PCR hood with UV sterilization and positive air flow.
SureSelect XT HS2 Target Enrichment
Kit ILM Hyb Module, Box 2 (Post PCR),
–20°C
SureSelect XT HS2 Target Enrichment
Kit ILM Hyb Module, Box 2 (Post PCR),
–20°C
SureSelect XT HS2 Target Enrichment
Kit ILM Hyb Module, Box 2 (Post PCR),
–20°C
Pipette up and down
15–20 times
Vortexing page 62
Vortexing page 62
page 62
60 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 61

Post-Capture Sample Processing for Multiplexed Sequencing 5
Step 1. Amplify the captured libraries
1 Preprogram a thermal cycler with the program in Table 35. Immediately
pause the program, and keep paused until samples are loaded in step 5.
Table 35 Post-capture PCR Thermal Cycler Program
Segment Number of Cycles Temperature Time
1 1 98°C 2 minutes
2 10–16
(See Table 36 for hybridization probe
design size-based cycle number
recommendations)
3 1 72°C 5 minutes
4 1 4°C Hold
* Use a reaction volume setting of 50 l, if required for thermal cycler set up.
*
98°C 30 seconds
60°C 30 seconds
72°C 1 minute
Table 36 Post-capture PCR cycle number recommendations
Probe Capture Library Size Cycles
Probes <0.2 Mb 16 cycles
Probes 0.2–3 Mb 12–16 cycles
Probes 3–5 Mb 11–12 cycles
Probes >5 Mb (including Human All Exon probes) 10–11 cycles
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 61
Page 62

5 Post-Capture Sample Processing for Multiplexed Sequencing
Step 1. Amplify the captured libraries
2 Prepare the appropriate volume of PCR reaction mix, as described in
Table 37, on ice. Mix well on a vortex mixer.
Table 37 Preparation of post-capture PCR reaction mix
Reagent Volume for 1 reaction Volume for 8 reactions
(includes excess)
Nuclease-free water 13 µl 117 µl 325 µl
5× Herculase II Buffer with dNTPs (clear cap) 10 µl 90 µl 250 µl
Herculase II Fusion DNA Polymerase (red cap) 1 µl 9 µl 25 µl
SureSelect Post-Capture Primer Mix (clear cap) 1 µl 9 µl 25 µl
Total 25 µl 225 µl 625 µl
Volume for 24 reactions
(includes excess)
3 Add 25 µl of the PCR reaction mix prepared in Table 37 to each sample
well containing 25 µl of bead- bound target- enriched DNA (prepared on
page 57 and held on ice).
4 Mix the PCR reactions well by pipetting up and down until the bead
suspension is homogeneous. Avoid splashing samples onto well walls; do
not spin the samples at this step.
5 Place the plate or strip tube in the thermal cycler and resume the
thermal cycling program in Table 35.
6 When the PCR amplification program is complete, spin the plate or
strip tube briefly. Remove the streptavidin- coated beads by placing the
plate or strip tube on the magnetic stand at room temperature. Wait
2 minutes for the solution to clear, then remove each supernatant
(approximately 50 µl) to wells of a fresh plate or strip tube.
The beads can be discarded at this time.
62 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 63

Post-Capture Sample Processing for Multiplexed Sequencing 5
Step 2. Purify the amplified captured libraries using AMPure XP beads
Step 2. Purify the amplified captured libraries using
AMPure XP beads
1 Verify that the AMPure XP beads were held at room temperature for at
least 30
minutes before use.
2 Prepare 400 µl of fresh 70% ethanol per sample, plus excess, for use in
step 8.
3 Mix the AMPure XP bead suspension well so that the suspension
appears homogeneous and consistent in color.
4 Add 50 µl of the homogeneous AMPure XP bead suspension to each
amplified DNA sample (approximately 50 µl) in the PCR plate or strip
tube. Mix well by pipetting up and down 15–20 times or cap the wells
and vortex at high speed for 5–10 seconds.
Check that the beads are in a homogeneous suspension in the sample
wells. Each well should have a uniform color with no layers of beads or
clear liquid present.
5 Incubate samples for 5 minutes at room temperature.
6 Put the plate or strip tube on the magnetic stand at room temperature.
Wait for the solution to clear (approximately 3 to 5 minutes).
7 Keep the plate or tubes in the magnetic stand. Carefully remove and
discard the cleared solution from each well. Do not disturb the beads
while removing the solution.
8 Continue to keep the plate or tubes in the magnetic stand while you
dispense 200 µl of freshly- prepared 70% ethanol in each sample well.
9 Wait for 1 minute to allow any disturbed beads to settle, then remove
the ethanol.
10 Repeat step 8 and step 9 once for a total of two washes. Make sure to
remove all of the ethanol at each wash step.
11 Seal the wells with strip caps, then briefly spin to collect the residual
ethanol. Return the plate or strip tube to the magnetic stand for
30 seconds. Remove the residual ethanol with a P20 pipette.
12 Dry the samples by placing the unsealed plate or strip tube on the
thermal cycler, set to hold samples at 37°C, until the residual ethanol
has just evaporated (typically 1–2 minutes).
13 Add 25 µl of Low TE to each sample well.
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 63
Page 64

5 Post-Capture Sample Processing for Multiplexed Sequencing
Step 2. Purify the amplified captured libraries using AMPure XP beads
14 Seal the sample wells with strip caps, then mix well on a vortex mixer
and briefly spin to collect the liquid without pelleting the beads.
15 Incubate for 2 minutes at room temperature.
16 Put the plate or strip tube in the magnetic stand and leave for
2 minutes or until the solution is clear.
17 Remove the cleared supernatant (approximately 25 µl) to a fresh well.
You can discard the beads at this time.
64 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 65

Post-Capture Sample Processing for Multiplexed Sequencing 5
Step 3. Assess sequencing library DNA quantity and quality
Step 3. Assess sequencing library DNA quantity and quality
Analyze each library using one of the platforms listed in Table 38. Follow
the instructions in the linked user guide provided for each assay in
Table 38, after reviewing the post- capture library qualification steps on
page 66. See Table 39 for fragment size distribution guidelines for various
sample types. Representative electropherograms generated using the
TapeStation system are provided to illustrate typical results for
post- capture libraries prepared from selected sample types.
Table 38 Post-capture library analysis options
Analysis platform Assay used at this step Link to assay instructions Amount of library
sample to analyze
Agilent 4200 or 4150
TapeStation system
Agilent 2100 Bioanalyzer
system
Agilent 5200, 5300, or 5400
Fragment Analyzer system
High Sensitivity D1000
ScreenTape
High Sensitivity DNA Kit Agilent High Sensitivity DNA Kit
HS NGS Fragment Kit
(1-6000 bp)
Agilent High Sensitivity D1000
Assay Quick Guide
Guide
Agilent HS NGS Fragment Kit
(1-6000 bp) Kit Guide
2 µl
1 µl
2 µl
Table 39 Post-capture library qualification guidelines
NGS read length for fragmentation
protocol selection
2 ×100 reads Intact DNA 200 to 400 bp (see Figure 5 for sample electropherogram)
2 ×150 reads Intact DNA 230 to 450 bp
Input DNA type Expected DNA fragment size peak position
FFPE DNA 200 to 400 bp (see Figure 6 and Figure 7 for sample
electropherograms)
FFPE DNA 200 to 450 bp
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 65
Page 66

5 Post-Capture Sample Processing for Multiplexed Sequencing
Step 3. Assess sequencing library DNA quantity and quality
1 Set up the instrument as instructed in the appropriate user guide (links
provided in Table 38).
2 Prepare the samples for analysis and set up the assay as instructed in
the appropriate user guide. Load the analysis assay into the instrument
and complete the run.
3 Verify that the electropherogram shows the expected DNA fragment size
peak position (see Table 39 for guidelines). Sample TapeStation system
electropherograms are shown for libraries prepared from sheared DNA
designed for 2 ×100 bp reads in Figure 5 (high- quality input DNA),
Figure 6 (medium- quality FFPE input DNA), and Figure 7 (low- quality
FFPE input DNA).
Electropherograms obtained using the other analysis platform options
listed in Table 38 are expected to show similar fragment size profiles.
4 Determine the concentration of the library DNA by integrating under
the entire peak.
Figure 5 Post-capture library prepared from a high-quality gDNA sample analyzed using
a High Sensitivity D1000 ScreenTape assay.
66 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 67

Post-Capture Sample Processing for Multiplexed Sequencing 5
Step 3. Assess sequencing library DNA quantity and quality
Figure 6 Post-capture library prepared from a typical FFPE gDNA sample analyzed us-
ing a High Sensitivity D1000 ScreenTape assay.
Figure 7 Post-capture library prepared from a low-quality FFPE gDNA sample analyzed
using a High Sensitivity D1000 ScreenTape assay.
Stopping Point If you do not continue to the next step, seal the plate and store at 4°C
overnight or at –20°C for prolonged storage.
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 67
Page 68

5 Post-Capture Sample Processing for Multiplexed Sequencing
Step 4. Pool samples for multiplexed sequencing
Step 4. Pool samples for multiplexed sequencing
The number of indexed libraries that may be multiplexed in a single
sequencing lane is determined by the output specifications of the platform
used, together with the amount of sequencing data required for your
research design. Calculate the number of indexes that can be combined
per lane, according to the capacity of your platform and the amount of
sequencing data required per sample.
Combine the libraries such that each index- tagged sample is present in
equimolar amounts in the pool using one of the following methods:
Method 1: Dilute each sample to be pooled to the same final
concentration (typically 4 nM–15 nM, or the concentration of the most
dilute sample) using Low TE, then combine equal volumes of all
samples to create the final pool.
Method 2: Starting with samples at different concentrations, add the
appropriate volume of each sample to achieve equimolar
concentration in the pool, then adjust the pool to the desired final
volume using Low TE. The formula below is provided for
determination of the amount of each indexed sample to add to the
pool.
Volume of Index
where V(f) is the final desired volume of the pool,
C(f) is the desired final concentration of all the DNA in the pool
(typically 4 nM–15 nM or the concentration of the most dilute
sample)
# is the number of indexes, and
C(i) is the initial concentration of each indexed sample
Table 40 shows an example of the amount of 4 index- tagged samples
(of different concentrations) and Low TE needed for a final volume of
20 µl at 10 nM DNA.
68 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Vf Cf
---------------------------------=
# Ci
Page 69

Post-Capture Sample Processing for Multiplexed Sequencing 5
Step 4. Pool samples for multiplexed sequencing
Table 40 Example of volume calculation for total volume of 20 µl at 10 nM concentration
Component V(f) C(i) C(f) # Volume to use (µl)
Sample 1 20 µl 20 nM 10 nM 4 2.5
Sample 2 20 µl 10 nM 10 nM 4 5
Sample 3 20 µl 17 nM 10 nM 4 2.9
Sample 4 20 µl 25 nM 10 nM 4 2
Low TE 7.6
If you store the library before sequencing, add Tween 20 to 0.1% v/v and
store at - 20°C short term.
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 69
Page 70

5 Post-Capture Sample Processing for Multiplexed Sequencing
Step 5. Prepare sequencing samples
Step 5. Prepare sequencing samples
The final SureSelect XT HS2 library pool is ready for direct sequencing
using standard Illumina paired- end primers and chemistry. Each fragment
in the prepared library contains one target insert surrounded by sequence
motifs required for multiplexed sequencing using the Illumina platform, as
shown in Figure 8.
Figure 8 Content of SureSelect XT HS2 sequencing library. Each fragment contains one
target insert (blue) surrounded by the Illumina paired-end sequencing elements (black), unique dual sample indexes (red and green), duplex molecular
barcodes (brown) and the library PCR primers (yellow).
Libraries can be sequenced on the Illumina HiSeq, MiSeq, NextSeq or
NovaSeq platform using the run type and chemistry combinations shown
in Table 41.
Proceed to cluster amplification using the appropriate Illumina Paired- End
Cluster Generation Kit. See Table 41 for kit configurations compatible with
the recommended read length.
The optimal seeding concentration for SureSelect XT HS2 target- enriched
libraries varies according to sequencing platform, run type, and Illumina
kit version. See Table 41 for guidelines. Seeding concentration and cluster
density may also need to be optimized based on the DNA fragment size
range for the library and on the desired output and data quality. Begin
optimization using a seeding concentration in the middle of the range
listed in Table 41.
Follow Illumina’s recommendation for a PhiX control in a
low- concentration spike- in for improved sequencing quality control.
70 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 71

Post-Capture Sample Processing for Multiplexed Sequencing 5
Step 5. Prepare sequencing samples
Table 41 Illumina Kit Configuration Selection Guidelines
Platform Run Type Read Length SBS Kit Configuration Chemistry Seeding
Concentration
HiSeq 2500 Rapid Run 2 × 100 bp 200 Cycle Kit v2 9–10 pM
HiSeq 2500 High Output 2 × 100 bp 250 Cycle Kit v4 12–14 pM
MiSeq All Runs 2 × 100 bp or
2 × 150 bp
MiSeq All Runs 2 × 75 bp 150 Cycle Kit v3 12–16 pM
NextSeq 500/550 All Runs 2 × 100 bp or
2 × 150 bp
HiSeq 3000/4000 All Runs 2 × 100 bp or
2 × 150 bp
NovaSeq 6000 Standard
Workflow Runs
NovaSeq 6000 Xp Workflow
Runs
2 × 100 bp or
2 × 150 bp
2 × 100 bp or
2 × 150 bp
300 Cycle Kit v2 9–10 pM
300 Cycle Kit v2.5 1.2–1.5 pM
300 Cycle Kit v1 230–240 pM
300 Cycle Kit v1.0 or v1.5 300–600 pM
300 Cycle Kit v1.0 or v1.5 200–400 pM
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 71
Page 72

5 Post-Capture Sample Processing for Multiplexed Sequencing
Step 6. Do the sequencing run and analyze the data
Step 6. Do the sequencing run and analyze the data
The guidelines below provide an overview of SureSelect XT HS2 library
sequencing run setup and analysis considerations. Links are provided for
additional details for various NGS platforms and analysis pipeline options.
• Each of the sample- level indexes requires an 8- bp index read. For
complete index sequence information, see Table 51 on page 87 through
Table 58 on page 94.
• For the HiSeq, NextSeq, and NovaSeq platforms, set up the run using
the instrument’s user interface, following the guidelines on page 73.
• For the MiSeq platform, set up the run using Illumina Experiment
Manager (IEM) using the steps detailed on page 73 to page 76 to
generate a custom sample sheet.
• Demultiplex using Illumina’s bcl2fastq software to generate paired end
reads based on the dual indexes and remove sequences with incorrectly
paired P5 and P7 indexes.
• The MBC sequence and dark bases are located at the 5’ end of both
Read 1 and Read 2. Use the Agilent Genomics NextGen Toolkit (AGeNT)
for molecular barcode extraction and trimming (see page 77 for more
information). If your sequence analysis pipeline excludes MBCs and is
incompatible with AGeNT, you can trim or mask the first five bases
from each read before alignment as described in the Note on page 77.
• Before aligning reads to the reference genome, Illumina adaptor
sequences should be trimmed from the reads using Agilent’s AGeNT
trimmer module, which properly accounts for the degenerate MBCs in
the adaptor sequence. See page 77 for more information. Do not use the
adaptor trimming options in Illumina Experiment Manager (IEM). Make
sure any IEM adaptor trimming option checkboxes are cleared
(deselected) when setting up the sequencing run.
72 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 73

Post-Capture Sample Processing for Multiplexed Sequencing 5
Step 6. Do the sequencing run and analyze the data
HiSeq/NextSeq/NovaSeq platform sequencing run setup guidelines
Set up sequencing runs using the instrument control software interface,
using the settings shown in Table 42. For HiSeq runs, select Dual Index
on the Run Configuration screen of the instrument control software
interface and enter the Cycles settings in Table 42.
For the NextSeq or NovaSeq platform, open the Run Setup screen of the
instrument control software interface and enter the Read Length settings
in Table 42. In the Custom Primers section, clear (do not select) the
checkboxes for all primers (Read 1, Read 2, Index 1 and Index 2).
Table 42 Run settings
Run Segment Cycles/Read Length
Read 1 100 or 150
Index 1 (i7) 8
Index 2 (i5) 8
Read 2 100 or 150
MiSeq platform sequencing run setup guidelines
Use the Illumina Experiment Manager (IEM) software to generate a custom
Sample Sheet according to the guidelines below. Once a Sample Sheet has
been generated, index sequences need to be manually changed to the
SureSelect XT HS2 indexes used for each sample. See page 87 through
page 94 for nucleotide sequences of the SureSelect XT HS2 index pairs.
Set up a custom Sample Sheet:
1 In the IEM software, create a Sample Sheet for the MiSeq platform
using the following Workflow selections.
• Under Category, select Other.
• Under Application, select FASTQ Only.
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 73
Page 74

5 Post-Capture Sample Processing for Multiplexed Sequencing
Step 6. Do the sequencing run and analyze the data
2 On the Workflow Parameters screen, enter the run information, making
sure to specify the key parameters highlighted below. In the Library
Prep Workflow field, select TruSeq Nano DNA. In the Index Adapters
field, select TruSeq DNA CD Indexes (96 Indexes). Clear both
adaptorSettings, since adaptor trimming must be performed using Agilent’s
AGeNT software (see page 77).
If TruSeq Nano DNA is not available in the Sample Prep Kit field,
instead select TruSeq HT.
trimming checkboxes under FASTQ Only Workflow- Specific
3 Using the Sample Sheet Wizard, set up a New Plate, entering the
required information for each sample to be sequenced. In the
I7 Sequence column, assign each sample to any of the Illumina i7
indexes. The index will be corrected to the i7 sequence from the
SureSelect XT HS2 index pair at a later stage.
74 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 75

Post-Capture Sample Processing for Multiplexed Sequencing 5
Step 6. Do the sequencing run and analyze the data
Likewise, in the I5 Sequence column, assign any of the Illumina i5
indexes, to be corrected to the i5 sequence from the SureSelect XT HS2
index pair at a later stage.
4 Finish the sample sheet setup tasks and save the sample sheet file.
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 75
Page 76

5 Post-Capture Sample Processing for Multiplexed Sequencing
Step 6. Do the sequencing run and analyze the data
Editing the Sample Sheet to include SureSelect XT HS2 dual indexes
• Open the Sample Sheet file in a text editor and edit the i7 and i5 index
information for each sample in columns 5–8 (highlighted below). See
page 87 through page 94 for nucleotide sequences of the SureSelect XT
HS2 indexes.
• In column 5 under I7_Index_ID, enter the SureSelect XT HS2 index
pair number assigned to the sample. In column 6 under index, enter
the corresponding P7 index sequence.
• In column 7 under I5_Index_ID, enter the SureSelect XT HS2 index
pair number assigned to the sample. In column 8 under index2, enter
the corresponding P5 index sequence.
• If the run includes more than 96 samples, the sample sheet may be
edited to include additional sample rows containing the assigned
SureSelect XT HS2 index pair sequences in column 6 (P7 index) and
column 8 (P5 index).
Figure 9 Sample sheet for SureSelect XT HS2 library sequencing
5 Save the edited Sample Sheet in an appropriate file location for use in
the run.
76 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 77

NOTE
Post-Capture Sample Processing for Multiplexed Sequencing 5
Sequence analysis resources
Sequence analysis resources
Guidelines are provided below for typical NGS analysis pipeline steps
appropriate for SureSelect XT HS2 library data analysis. Your NGS
analysis pipeline may vary.
Use the Illumina bcl2fastq software to generate paired end reads by
demultiplexing sequences based on the dual indexes and to remove
sequences with incorrectly paired P5 and P7 indexes.
The demultiplexed FASTQ data needs to be pre- processed to remove
sequencing adaptors and extract the molecular barcode (MBC) sequences
using the Agilent Genomics NextGen Toolkit (AGeNT). AGeNT is a set of
Java- based software modules that provide MBC pre- processing adaptor
trimming and duplicate read identification. This toolkit is designed to
enable building, integrating, maintaining, and troubleshooting internal
analysis pipelines for users with bioinformatics expertise. For additional
information and to download this toolkit, visit the AGeNT page at
www.agilent.com.
If your sequence analysis pipeline excludes MBCs, you can remove the first 5 bases from
Read 1 and Read 2 by either masking or trimming before proceeding to further analysis. To
remove during demultiplexing via masking, include the base mask N5Y*,I8,I8,N5Y* (where *
may be replaced with the actual read length, matching the read length value in the
RunInfo.xml file). Alternatively, the first 5 bases may be trimmed from the demultiplexed
fastq files using a suitable processing tool of your choice, such as seqtk. Alternatively, the
AGeNT trimmer module can be used to remove the MBCs and properly remove adaptor
sequences as well. Standard adaptor trimmers will fail to remove the MBC sequences from
the opposite adaptor (refer to Figure 8).
The trimmed reads should be aligned, and MBC tags added to the aligned
BAM file using a suitable tool such as the BWA- MEM. Once alignment and
tagging are complete, the AGeNT LocatIt module may be used to generate
consensus reads and mark or remove duplicates. The resulting BAM files
are ready for downstream analysis including variant discovery.
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 77
Page 78

5 Post-Capture Sample Processing for Multiplexed Sequencing
Sequence analysis resources
78 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 79

SureSelect XT HS2 DNA System Protocol
6
Appendix: Using FFPE-derived DNA
Samples
Protocol modifications for FFPE Samples 80
Methods for FFPE Sample Qualification 80
Sequencing Output Recommendations for FFPE Samples 81
This chapter summarizes the protocol modifications to apply to FFPE
samples based on the integrity of the FFPE sample DNA.
Agilent Technologies
79
Page 80

6 Appendix: Using FFPE-derived DNA Samples
Protocol modifications for FFPE Samples
Protocol modifications for FFPE Samples
Protocol modifications that should be applied to FFPE samples are
summarized in Table 43.
Table 43 Summary of protocol modifications for FFPE samples
Workflow Step and page Parameter Condition for non-FFPE Samples Condition for FFPE Samples
gDNA Sample
Preparation page 22
DNA input for Library
Preparation page 22
DNA Shearing page 26 Mode of DNA
Pre-capture PCR page 40 Cycle number 8–11 11–14
Sequencing page 81 Output augmentation Per project requirements 1× to 10× based on determined
Qualification of DNA
Integrity
Input amount and
means of
quantification
Shearing
Not required Required
10 ng to 200 ng, quantified by Qubit
assay
2 × 120 seconds (for 2 × 100 reads)
2 × 60 seconds (for 2 × 150 reads)
Based on determined DNA
integrity (see Table 9 on page 23
and Table 10 on page 24)
240 seconds (continuous, for all
read lengths)
DNA integrity (see Table 44 and
Table 45 on page 81)
Methods for FFPE Sample Qualification
DNA integrity may be assessed using the Agilent NGS FFPE QC Kit or
using the Agilent TapeStation system with the Genomic DNA ScreenTape.
The Agilent NGS FFPE QC Kit provides a qPCR- based assay for DNA
sample integrity determination. Results include the precise quantity of
amplifiable DNA in the sample to allow direct normalization of input DNA
amount and a Cq DNA integrity score used to design other protocol
modifications.
The Agilent TapeStation instrument, combined with the Genomic DNA
ScreenTape assay, provides an automated electrophoresis method for
determination of a DNA Integrity Number (DIN) score used to estimate
amount of input DNA required for sample normalization and to design
other protocol modifications.
80 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 81

Appendix: Using FFPE-derived DNA Samples 6
Sequencing Output Recommendations for FFPE Samples
Sequencing Output Recommendations for FFPE Samples
After determining the amount of sequencing output required for intact
DNA samples to meet the goals of your project, use the guidelines below
to determine the amount of extra sequencing output required for FFPE
DNA samples.
Samples qualified using ΔΔCq: For samples qualified based on the Cq DNA
integrity score, use the guidelines in Table 44. For example, if your
workflow demands 100 Mb output for intact DNA samples to achieve the
required coverage, an FFPE sample with Cq score of 1 requires 200–
400 Mb of sequencing output to achieve the same coverage.
Table 44 Recommended sequencing augmentation for FFPE-derived DNA samples
ΔΔCq value Recommended fold increase for FFPE-derived sample
<0.5 No extra sequencing output
between 0.5 and 2 Increase sequencing allocation by 2× to 4×
>2 Increase sequencing allocation by 5× to 10× or more
Samples qualified using DIN: For samples qualified based on the Genomic
DNA ScreenTape assay DIN integrity score, use the guidelines in Table 45.
For example, if your workflow demands 100 Mb output for intact DNA
samples to achieve the required coverage, an FFPE sample with DIN score
of 4 requires approximately 200–400 Mb of sequencing output to achieve
the same coverage.
Table 45 Recommended sequencing augmentation for FFPE-derived DNA samples
DIN value Recommended fold increase for FFPE-derived sample
8 No extra sequencing output
between 3 and 8 Increase sequencing allocation by 2× to 4×
<3 Increase sequencing allocation by 5× to 10× or more
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 81
Page 82

6 Appendix: Using FFPE-derived DNA Samples
Sequencing Output Recommendations for FFPE Samples
82 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 83

SureSelect XT HS2 DNA System Protocol
7
Reference
Kit Contents 84
SureSelect XT HS2 Index Primer Pair Information 86
Troubleshooting Guide 98
Quick Reference Protocol 102
This chapter contains reference information, including component kit
contents, index sequences, troubleshooting information, and a
quick- reference protocol for experienced users.
Agilent Technologies
83
Page 84

7 Reference
Kit Contents
Kit Contents
SureSelect XT HS2 Target Enrichment System Reagent Kits include the component kits listed
in Table 46. Detailed contents of each of the multi- part component kits listed in Table 46
are shown in Table 47 through Table 50 on the following pages.
Table 46 Component Kits
Component Kit Name Storage
Condition
Standard Component Modules
SureSelect XT HS2 Library
Preparation Kit for ILM (Pre PCR)
SureSelect XT HS2 Index Primer
Pairs for ILM (Pre PCR)
SureSelect Target Enrichment Kit,
ILM Hyb Module, Box 1 (Post PCR)
SureSelect XT HS2 Target
Enrichment Kit ILM Hyb Module, Box
2 (Post PCR)
Optional Component Modules
SureSelect DNA AMPure® XP Beads +4°C 5191-5739*
SureSelect Streptavidin Beads +4°C 5191-5741* 5191-5742
SureSelect Enzymatic Fragmentation
Kit
–20°C 5500-0146 5500-0147
–20°C 5191-5687 (Index Pairs 1–16) 5191-5688 (Index Pairs 1–96),
Room
Temperature
–20°C 5191-6686 5191-6688
–20°C 5191-4079* 5191-4080
Component Kit Part Number
16 Reaction Kits 96 Reaction Kits
5191-5689 (Index Pairs 97–192),
5191-5690 (Index Pairs 193–288), OR
5191-5691 (Index Pairs 289–384)
5190-9685 5190-9687
5191-5740
†
†
‡
* Provided with the 16-Reaction SureSelect XT HS2 DNA Starter Kit, p/n G9982A.
† Provided with 96-Reaction Reagent Kit part numbers G9984A, G9984B, G9984C, G9984D.
‡ Purchased separately; not included with SureSelect XT HS2 DNA Reagent Kits, but use is supported by the protocols in this
publication.
84 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 85

Reference 7
Kit Contents
Table 47 SureSelect XT HS2 Library Preparation Kit for ILM (Pre PCR) Content
Kit Component 16 Reaction Kit Format 96 Reaction Kit Format
End Repair-A Tailing Enzyme Mix tube with orange cap tube with orange cap
End Repair-A Tailing Buffer tube with yellow cap bottle
T4 DNA Ligase tube with blue cap tube with blue cap
Ligation Buffer tube with purple cap bottle
SureSelect XT HS2 Adaptor Oligo Mix tube with white cap tube with white cap
Herculase II Fusion DNA Polymerase tube with red cap tube with red cap
5× Herculase II Reaction Buffer with dNTPs tube with clear cap tube with clear cap
Table 48 SureSelect XT HS2 Index Primer Pairs for ILM (Pre PCR) Content
Kit Component 16 Reaction Kit Format 96 Reaction Kit Format
SureSelect XT HS2 Index
Primer Pairs for ILM (Pre PCR)
Blue 8-well strip tube (index pairs 1-8), AND
White 8-well strip tube (index pairs 9-16)
Orange 96-well plate (index pairs 1–96), OR
Blue 96-well plate (index pairs 97–192), OR
Green 96-well plate (index pairs 193–288), OR
Red 96-well plate (index pairs 289–384)
Table 49 SureSelect Target Enrichment Kit, ILM Hyb Module Box 1 (Post PCR) Content
Kit Component 16 Reaction Kit Format 96 Reaction Kit Format
SureSelect Binding Buffer bottle bottle
SureSelect Wash Buffer 1 bottle bottle
SureSelect Wash Buffer 2 bottle bottle
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 85
Page 86

7 Reference
SureSelect XT HS2 Index Primer Pair Information
Table 50 SureSelect XT HS2 Target Enrichment Kit, ILM Hyb Module Box 2 (Post PCR) Content
Kit Component 16 Reaction Kit Format 96 Reaction Kit Format
SureSelect Fast Hybridization Buffer bottle bottle
SureSelect XT HS2 Blocker Mix tube with blue cap tube with blue cap
SureSelect RNase Block tube with purple cap tube with purple cap
SureSelect Post-Capture Primer Mix tube with clear cap tube with clear cap
Herculase II Fusion DNA Polymerase tube with red cap tube with red cap
5× Herculase II Reaction Buffer with dNTPs tube with clear cap tube with clear cap
SureSelect XT HS2 Index Primer Pair Information
The SureSelect XT HS2 Index Primer Pairs are provided pre- combined.
Each member of the primer pair contains a unique 8- bp P5 or P7 index,
resulting in dual- indexed NGS libraries. The nucleotide sequence of the
index portion of each primer is provided in Table 51 through Table 58.
See page 72 for sequencing run setup requirements for sequencing
libraries using 8- bp indexes.
NOTE
P7 indexes are shown in a single orientation, applicable to any of the supported Illumina
platforms. P5 indexes are shown in two orientations for different platforms; check the table
column headings carefully before selecting the P5 sequences.
The first P5 index orientation is applicable to the supported platforms NovaSeq 6000 with
v1.0 chemistry, MiSeq, and HiSeq 2500. This orientation is also applicable to the HiSeq 2000
platform that is not specifically supported in this user manual.
The second P5 index orientation is applicable to the supported platforms NovaSeq 6000
with v1.5 chemistry, NextSeq 500/550, HiSeq 4000 and HiSeq 3000. This orientation is also
applicable to the iSeq 100, MiniSeq, and HiSeq X platforms that are not specifically
supported in this user manual.
One primer pair is provided in each well of 8- well strip tubes (16 reaction
kits; see Figure 10 for a map) or of 96- well plates (96 reaction kits; see
page 96 through page 97 for plate maps). Each well contains a single- use
aliquot of a specific pair of forward plus reverse primers.
86 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 87

Reference 7
SureSelect XT HS2 Index Primer Pair Information
Table 51 SureSelect XT HS2 Index Primer Pairs 1–48, provided in orange 96-well plate or in strip tubes
Primer
Well P7 Index P5 Index
Pair #
NovaSeq (v1.0
chemistry),
MiSeq,
HiSeq 2500
1 A01 CAAGGTGA ATGGTTAG CTAACCAT 25 A04 AGATGGAT TGGCACCA TGGTGCCA
2 B01 TAGACCAA CAAGGTGA TCACCTTG 26 B04 GAATTGTG AGATGGAT ATCCATCT
3 C01 AGTCGCGA TAGACCAA TTGGTCTA 27 C04 GAGCACTG GAATTGTG CACAATTC
4 D01 CGGTAGAG AGTCGCGA TCGCGACT 28 D04 GTTGCGGA GAGCACTG CAGTGCTC
5 E01 TCAGCATC AAGGAGCG CGCTCCTT 29 E04 AATGGAAC GTTGCGGA TCCGCAAC
6 F01 AGAAGCAA TCAGCATC GATGCTGA 30 F04 TCAGAGGT AATGGAAC GTTCCATT
7 G01 GCAGGTTC AGAAGCAA TTGCTTCT 31 G04 GCAACAAT TCAGAGGT ACCTCTGA
8 H01 AAGTGTCT GCAGGTTC GAACCTGC 32 H04 GTCGATCG GCAACAAT ATTGTTGC
9 A02 CTACCGAA AAGTGTCT AGACACTT 33 A05 ATGGTAGC GTCGATCG CGATCGAC
10 B02 TAGAGCTC CTACCGAA TTCGGTAG 34 B05 CGCCAATT ATGGTAGC GCTACCAT
11 C02 ATGTCAAG TAGAGCTC GAGCTCTA 35 C05 GACAATTG CGCCAATT AATTGGCG
12 D02 GCATCATA ATGTCAAG CTTGACAT 36 D05 ATATTCCG GACAATTG CAATTGTC
13 E02 GACTTGAC GCATCATA TATGATGC 37 E05 TCTACCTC ATATTCCG CGGAATAT
14 F02 CTACAATG GACTTGAC GTCAAGTC 38 F05 TCGTCGTG TCTACCTC GAGGTAGA
15 G02 TCTCAGCA CTACAATG CATTGTAG 39 G05 ATGAGAAC TCGTCGTG CACGACGA
16 H02 AGACACAC TCTCAGCA TGCTGAGA 40 H05 GTCCTATA ATGAGAAC GTTCTCAT
17 A03 CAGGTCTG AGACACAC GTGTGTCT 41 A06 AATGACCA GTCCTATA TATAGGAC
18 B03 AATACGCG CAGGTCTG CAGACCTG 42 B06 CAGACGCT AATGACCA TGGTCATT
19 C03 GCACACAT AATACGCG CGCGTATT 43 C06 TCGAACTG CAGACGCT AGCGTCTG
20 D03 CTTGCATA GCACACAT ATGTGTGC 44 D06 CGCTTCCA TCGAACTG CAGTTCGA
21 E03 ATCCTCTT CTTGCATA TATGCAAG 45 E06 TATTCCTG CGCTTCCA TGGAAGCG
22 F03 GCACCTAA ATCCTCTT AAGAGGAT 46 F06 CAAGTTAC TATTCCTG CAGGAATA
23 G03 TGCTGCTC GCACCTAA TTAGGTGC 47 G06 CAGAGCAG CAAGTTAC GTAACTTG
24 H03 TGGCACCA TGCTGCTC GAGCAGCA 48 H06 CGCGCAAT CAGAGCAG CTGCTCTG
P5 Index
NovaSeq (v1.5
chemistry),
NextSeq,
HiSeq 4000,
HiSeq 3000
Primer
Pair #
Well P7 Index P5 Index
NovaSeq (v1.0
chemistry),
MiSeq,
HiSeq 2500
P5 Index
NovaSeq (v1.5
chemistry),
NextSeq,
HiSeq 4000,
HiSeq 3000
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 87
Page 88

7 Reference
SureSelect XT HS2 Index Primer Pair Information
Table 52 SureSelect XT HS2 Index Primer Pairs 49–96, provided in orange 96-well plate
Primer
Pair #
Well P7 Index P5 Index
NovaSeq (v1.0
chemistry),
MiSeq,
HiSeq 2500
P5 Index
NovaSeq (v1.5
chemistry),
NextSeq,
HiSeq 4000,
HiSeq 3000
Primer
Pair #
Well P7 Index P5 Index
NovaSeq (v1.0
chemistry),
MiSeq,
HiSeq 2500
P5 Index
NovaSeq (v1.5
chemistry),
NextSeq,
HiSeq 4000,
HiSeq 3000
49 A07 TGAGGAGT CGCGCAAT ATTGCGCG 73 A10 AACGCATT ATAGTGAC GTCACTAT
50 B07 ATGACGAA TGAGGAGT ACTCCTCA 74 B10 CAGTTGCG AACGCATT AATGCGTT
51 C07 TACGGCGA ATGACGAA TTCGTCAT 75 C10 TGCCTCGA CAGTTGCG CGCAACTG
52 D07 AGCGAGTT TACGGCGA TCGCCGTA 76 D10 AAGGCTTA TGCCTCGA TCGAGGCA
53 E07 TGTATCAC AGCGAGTT AACTCGCT 77 E10 GCAATGAA AAGGCTTA TAAGCCTT
54 F07 GATCGCCT TGTATCAC GTGATACA 78 F10 AAGAACCT GCAATGAA TTCATTGC
55 G07 GACTCAAT GATCGCCT AGGCGATC 79 G10 CTGTGCCT AAGAACCT AGGTTCTT
56 H07 CAGCTTGC GACTCAAT ATTGAGTC 80 H10 TACGTAGC CTGTGCCT AGGCACAG
57 A08 AGCTGAAG CAGCTTGC GCAAGCTG 81 A11 AAGTGGAC TACGTAGC GCTACGTA
58 B08 ATTCCGTG AGCTGAAG CTTCAGCT 82 B11 CAACCGTG AAGTGGAC GTCCACTT
59 C08 TATGCCGC ATTCCGTG CACGGAAT 83 C11 CTGTTGTT CAACCGTG CACGGTTG
60 D08 TCAGCTCA TATGCCGC GCGGCATA 84 D11 GCACGATG CTGTTGTT AACAACAG
61 E08 AACTGCAA TCAGCTCA TGAGCTGA 85 E11 GTACGGAC GCACGATG CATCGTGC
62 F08 ATTAGGAG AACTGCAA TTGCAGTT 86 F11 CTCCAAGC GTACGGAC GTCCGTAC
63 G08 CAGCAATA ATTAGGAG CTCCTAAT 87 G11 TAGTCTGA CTCCAAGC GCTTGGAG
64 H08 GCCAAGCT CAGCAATA TATTGCTG 88 H11 TTCGCCGT TAGTCTGA TCAGACTA
65 A09 TCCGTTAA GCCAAGCT AGCTTGGC 89 A12 GAACTAAG ATACGAAG CTTCGTAT
66 B09 GTGCAACG TCCGTTAA TTAACGGA 90 B12 AAGCCATC GAGATTCA TGAATCTC
67 C09 AGTAACGC GTGCAACG CGTTGCAC 91 C12 AACTCTTG AAGCCATC GATGGCTT
68 D09 CATAGCCA AGTAACGC GCGTTACT 92 D12 GTAGTCAT AACTCTTG CAAGAGTT
69 E09 CACTAGTA CATAGCCA TGGCTATG 93 E12 CTCGCTAG GTAGTCAT ATGACTAC
70 F09 TTAGTGCG CACTAGTA TACTAGTG 94 F12 AGTCTTCA CAGTATCA TGATACTG
71 G09 TCGATACA TTAGTGCG CGCACTAA 95 G12 TCAAGCTA CTTCGTAC GTACGAAG
72 H09 ATAGTGAC TCGATACA TGTATCGA 96 H12 CTTATCCT TCAAGCTA TAGCTTGA
88 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 89

SureSelect XT HS2 Index Primer Pair Information
Table 53 SureSelect XT HS2 Index Primer Pairs 97–144, provided in blue 96-well plate
Reference 7
Primer
Pair #
Well P7 Index P5 Index
NovaSeq (v1.0
chemistry),
MiSeq,
HiSeq 2500
P5 Index
NovaSeq (v1.5
chemistry),
NextSeq,
HiSeq 4000,
HiSeq 3000
Primer
Pair #
Well P7 Index P5 Index
NovaSeq (v1.0
chemistry),
MiSeq,
HiSeq 2500
P5 Index
NovaSeq (v1.5
chemistry),
NextSeq,
HiSeq 4000,
HiSeq 3000
97 A01 TCATCCTT CTTATCCT AGGATAAG 121 A04 CAGGCAGA AGACGCCT AGGCGTCT
B01 AACACTCT TCATCCTT AAGGATGA 122 B04 TCCGCGAT CAGGCAGA TCTGCCTG
98
C01 CACCTAGA AACACTCT AGAGTGTT 123 C04 CTCGTACG TCCGCGAT ATCGCGGA
99
100
101
102
103
104
105
106
107
108
109
110
111
112
113
114
115
116
117
118
119
120
D01 AGTTCATG CACCTAGA TCTAGGTG 124 D04 CACACATA CTCGTACG CGTACGAG
E01 GTTGGTGT AGTTCATG CATGAACT 125 E04 CGTCAAGA CACACATA TATGTGTG
F01 GCTACGCA GTTGGTGT ACACCAAC 126 F04 TTCGCGCA CGTCAAGA TCTTGACG
G01 TCAACTGC GCTACGCA TGCGTAGC 127 G04 CGACTACG TTCGCGCA TGCGCGAA
H01 AAGCGAAT TCAACTGC GCAGTTGA 128 H04 GAAGGTAT CGACTACG CGTAGTCG
A02 GTGTTACA AAGCGAAT ATTCGCTT 129 A05 TTGGCATG GAAGGTAT ATACCTTC
B02 CAAGCCAT GTGTTACA TGTAACAC 130 B05 CGAATTCA TTGGCATG CATGCCAA
C02 CTCTCGTG CAAGCCAT ATGGCTTG 131 C05 TTAGTTGC CGAATTCA TGAATTCG
D02 TCGACAAC CTCTCGTG CACGAGAG 132 D05 GATGCCAA TTAGTTGC GCAACTAA
E02 TCGATGTT TCGACAAC GTTGTCGA 133 E05 AGTTGCCG GATGCCAA TTGGCATC
F02 CAAGGAAG TCGATGTT AACATCGA 134 F05 GTCCACCT AGTTGCCG CGGCAACT
G02 ATTGATGC AGAGAATC GATTCTCT 135 G05 ATCAAGGT GTCCACCT AGGTGGAC
H02 TCGCAGAT TTGATGGC GCCATCAA 136 H05 GAACCAGA ATCAAGGT ACCTTGAT
A03 GCAGAGAC TCGCAGAT ATCTGCGA 137 A06 CATGTTCT GAACCAGA TCTGGTTC
B03 CTGCGAGA GCAGAGAC GTCTCTGC 138 B06 TCACTGTG CATGTTCT AGAACATG
C03 CAACCAAC CTGCGAGA TCTCGCAG 139 C06 ATTGAGCT TCACTGTG CACAGTGA
D03 ATCATGCG CAACCAAC GTTGGTTG 140 D06 GATAGAGA ATTGAGCT AGCTCAAT
E03 TCTGAGTC ATCATGCG CGCATGAT 141 E06 TCTAGAGC GATAGAGA TCTCTATC
F03 TCGCCTGT TCTGAGTC GACTCAGA 142 F06 GAATCGCA TCTAGAGC GCTCTAGA
G03 GCGCAATT TCGCCTGT ACAGGCGA 143 G06 CTTCACGT GAATCGCA TGCGATTC
H03 AGACGCCT GCGCAATT AATTGCGC 144 H06 CTCCGGTT CTTCACGT ACGTGAAG
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 89
Page 90

7 Reference
SureSelect XT HS2 Index Primer Pair Information
Table 54 SureSelect XT HS2 Index Primer Pairs 145–192, provided in blue 96-well plate
Primer
Pair #
Well P7 Index P5 Index
NovaSeq (v1.0
chemistry),
MiSeq,
HiSeq 2500
P5 Index
NovaSeq (v1.5
chemistry),
NextSeq,
HiSeq 4000,
HiSeq 3000
Primer
Pair #
Well P7 Index P5 Index
NovaSeq (v1.0
chemistry),
MiSeq,
HiSeq 2500
P5 Index
NovaSeq (v1.5
chemistry),
NextSeq,
HiSeq 4000,
HiSeq 3000
145 A07 TGTGACTA CTCCGGTT AACCGGAG 169 A10 CGCTCAGA CTAACAAG CTTGTTAG
146
147
148
149
150
151
152
153
154
155
156
157
158
159
160
161
162
163
164
165
166
167
168
B07 GCTTCCAG TGTGACTA TAGTCACA 170 B10 TAACGACA CGCTCAGA TCTGAGCG
C07 CATCCTGT GCTTCCAG CTGGAAGC 171 C10 CATACTTG TAACGACA TGTCGTTA
D07 GTAATACG CATCCTGT ACAGGATG 172 D10 AGATACGA CATACTTG CAAGTATG
E07 GCCAACAA GTAATACG CGTATTAC 173 E10 AATCCGAC AGATACGA TCGTATCT
F07 CATGACAC GCCAACAA TTGTTGGC 174 F10 TGAAGTAC AATCCGAC GTCGGATT
G07 TGCAATGC CATGACAC GTGTCATG 175 G10 CGAATCAT TGAAGTAC GTACTTCA
H07 CACATTCG TGCAATGC GCATTGCA 176 H10 TGATTGGC CGAATCAT ATGATTCG
A08 CAATCCGA CACATTCG CGAATGTG 177 A11 TCGAAGGA TGATTGGC GCCAATCA
B08 CATCGACG CAATCCGA TCGGATTG 178 B11 CAGTCATT TCGAAGGA TCCTTCGA
C08 GTGCGCTT CATCGACG CGTCGATG 179 C11 CGCGAACA CAGTCATT AATGACTG
D08 ATAGCGTT GTGCGCTT AAGCGCAC 180 D11 TACGGTTG CGCGAACA TGTTCGCG
E08 GAGTAAGA ATAGCGTT AACGCTAT 181 E11 AGAACCGT TACGGTTG CAACCGTA
F08 CTGACACA GAGTAAGA TCTTACTC 182 F11 AGGTGCTT AGAACCGT ACGGTTCT
G08 ATACGTGT CTGACACA TGTGTCAG 183 G11 ATCGCAAC AGGTGCTT AAGCACCT
H08 GACCGAGT ATACGTGT ACACGTAT 184 H11 GCCTCTCA ATCGCAAC GTTGCGAT
A09 GCAGTTAG GACCGAGT ACTCGGTC 185 A12 TCGCGTCA GCCTCTCA TGAGAGGC
B09 CGTTCGTC GCAGTTAG CTAACTGC 186 B12 GAGTGCGT TCGCGTCA TGACGCGA
C09 CGTTAACG CGTTCGTC GACGAACG 187 C12 CGAACACT GCATAAGT ACTTATGC
D09 TCGAGCAT CGTTAACG CGTTAACG 188 D12 TAAGAGTG AGAAGACG CGTCTTCT
E09 GCCGTAAC TCGAGCAT ATGCTCGA 189 E12 TGGATTGA TAAGAGTG CACTCTTA
F09 GAGCTGTA GCCGTAAC GTTACGGC 190 F12 AGGACATA TGGATTGA TCAATCCA
G09 AGGAAGAT GAGCTGTA TACAGCTC 191 G12 GACATCCT AGGACATA TATGTCCT
H09 CTAACAAG AGGAAGAT ATCTTCCT 192 H12 GAAGCCTC GACATCCT AGGATGTC
90 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 91

SureSelect XT HS2 Index Primer Pair Information
Table 55 SureSelect XT HS2 Index Primer Pairs 193–240, provided in green 96-well plate
Reference 7
Primer
Pair #
Well P7 Index P5 Index
NovaSeq (v1.0
chemistry),
MiSeq,
HiSeq 2500
P5 Index
NovaSeq (v1.5
chemistry),
NextSeq,
HiSeq 4000,
HiSeq 3000
Primer
Pair #
Well P7 Index P5 Index
NovaSeq (v1.0
chemistry),
MiSeq,
HiSeq 2500
P5 Index
NovaSeq (v1.5
chemistry),
NextSeq,
HiSeq 4000,
HiSeq 3000
193 A01 GTCTCTTC GAAGCCTC GAGGCTTC 217 A04 GCGGTATG CACGAGCT AGCTCGTG
194 B01 AGTCACTT GTCTCTTC GAAGAGAC 218 B04 TCTATGCG GCGGTATG CATACCGC
195 C01 AGCATACA AGTCACTT AAGTGACT 219 C04 AGGTGAGA TCTATGCG CGCATAGA
196 D01 TCAGACAA AGCATACA TGTATGCT 220 D04 CACAACTT AGGTGAGA TCTCACCT
197 E01 TTGGAGAA TCAGACAA TTGTCTGA 221 E04 TTGTGTAC CACAACTT AAGTTGTG
198 F01 TTAACGTG TTGGAGAA TTCTCCAA 222 F04 TCACAAGA TTGTGTAC GTACACAA
199 G01 CGTCTGTG TTAACGTG CACGTTAA 223 G04 GAAGACCT TCACAAGA TCTTGTGA
200 H01 AACCTAAC CGTCTGTG CACAGACG 224 H04 AGTTCTGT GAAGACCT AGGTCTTC
201 A02 AGAGTGCT AACCTAAC GTTAGGTT 225 A05 GCAGTGTT AGTTCTGT ACAGAACT
202 B02 TTATCTCG AGAGTGCT AGCACTCT 226 B05 AGGCATGC GCAGTGTT AACACTGC
203 C02 CATCAGTC TTATCTCG CGAGATAA 227 C05 AAGGTACT AGGCATGC GCATGCCT
204 D02 AAGCACAA CATCAGTC GACTGATG 228 D05 CACTAAGT AAGGTACT AGTACCTT
205 E02 CAGTGAGC AAGCACAA TTGTGCTT 229 E05 GAGTCCTA CACTAAGT ACTTAGTG
206 F02 GTCGAAGT CAGTGAGC GCTCACTG 230 F05 AGTCCTTC GAGTCCTA TAGGACTC
207 G02 TCTCATGC GTCGAAGT ACTTCGAC 231 G05 TTAGGAAC AGTCCTTC GAAGGACT
208 H02 CAGAAGAA TCTCATGC GCATGAGA 232 H05 AAGTCCAT TTAGGAAC GTTCCTAA
209 A03 CGGATAGT CAGAAGAA TTCTTCTG 233 A06 GAATACGC AAGTCCAT ATGGACTT
210 B03 CACGTGAG CGGATAGT ACTATCCG 234 B06 TCCAATCA GAATACGC GCGTATTC
211 C03 TACGATAC CACGTGAG CTCACGTG 235 C06 CGACGGTA TCCAATCA TGATTGGA
212 D03 CGCATGCT TACGATAC GTATCGTA 236 D06 CATTGCAT CGACGGTA TACCGTCG
213 E03 GCTTGCTA CGCATGCT AGCATGCG 237 E06 ATCTGCGT CATTGCAT ATGCAATG
214 F03 GAACGCAA GCTTGCTA TAGCAAGC 238 F06 GTACCTTG ATCTGCGT ACGCAGAT
215 G03 ATCTACCA GAACGCAA TTGCGTTC 239 G06 GAGCATAC GTACCTTG CAAGGTAC
216 H03 CACGAGCT ATCTACCA TGGTAGAT 240 H06 TGCTTACG GAGCATAC GTATGCTC
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 91
Page 92

7 Reference
SureSelect XT HS2 Index Primer Pair Information
Table 56 SureSelect XT HS2 Index Primer Pairs 241–288, provided in green 96-well plate
Primer
Pair #
Well P7 Index P5 Index
NovaSeq (v1.0
chemistry),
MiSeq,
HiSeq 2500
P5 Index
NovaSeq (v1.5
chemistry),
NextSeq,
HiSeq 4000,
HiSeq 3000
Primer
Pair #
Well P7 Index P5 Index
NovaSeq (v1.0
chemistry),
MiSeq,
HiSeq 2500
P5 Index
NovaSeq (v1.5
chemistry),
NextSeq,
HiSeq 4000,
HiSeq 3000
241 A07 AAGAGACA TGCTTACG CGTAAGCA 265 A10 CAATGCTG CATGAATG CATTCATG
242 B07 TAGCTATG AAGAGACA TGTCTCTT 266 B10 CTTGATCA CAATGCTG CAGCATTG
243 C07 TCTGCTAC TAGCTATG CATAGCTA 267 C10 GCGAATTA CTTGATCA TGATCAAG
244 D07 GTCACAGA TCTGCTAC GTAGCAGA 268 D10 GTTCGAGC GCGAATTA TAATTCGC
245 E07 CGATTGAA GTCACAGA TCTGTGAC 269 E10 GCCAGTAG GTTCGAGC GCTCGAAC
246 F07 GAGAGATT CGATTGAA TTCAATCG 270 F10 AAGGTCGA GCCAGTAG CTACTGGC
247 G07 TCATACCG GAGAGATT AATCTCTC 271 G10 AGTGAAGT CACTTATG CATAAGTG
248 H07 TCCGAACT TCATACCG CGGTATGA 272 H10 GTTGCAAG ATAACGGC GCCGTTAT
249 A08 AGAGAGAA TCCGAACT AGTTCGGA 273 A11 AGCCGGAA GTTGCAAG CTTGCAAC
250 B08 GATCGTTA AGAGAGAA TTCTCTCT 274 B11 AACAGCCG AGCCGGAA TTCCGGCT
251 C08 GCGCTAGA GATCGTTA TAACGATC 275 C11 CTAGTGTA AACAGCCG CGGCTGTT
252 D08 ATGACTCG GCGCTAGA TCTAGCGC 276 D11 GAGGCTCT CTAGTGTA TACACTAG
253 E08 CAATAGAC ATGACTCG CGAGTCAT 277 E11 CTCCGCAA GAGGCTCT AGAGCCTC
254 F08 CGATATGC CAATAGAC GTCTATTG 278 F11 CGCTATTG CTCCGCAA TTGCGGAG
255 G08 GTCAGAAT CGATATGC GCATATCG 279 G11 GTGTTGAG CGCTATTG CAATAGCG
256 H08 CATAAGGT GCACTACT AGTAGTGC 280 H11 TCACCGAC GTGTTGAG CTCAACAC
257 A09 TGTTGGTT GATTCGGC GCCGAATC 281 A12 CGGTAATC TCACCGAC GTCGGTGA
258 B09 ATACTCGC TGTTGGTT AACCAACA 282 B12 GTGACTGC CGGTAATC GATTACCG
259 C09 AATGCTAG ATACTCGC GCGAGTAT 283 C12 CGACTTGT GTGACTGC GCAGTCAC
260 D09 GCCTAGGA AATGCTAG CTAGCATT 284 D12 GATAGGAC CGACTTGT ACAAGTCG
261 E09 GCAACCGA GCCTAGGA TCCTAGGC 285 E12 AAGTACTC GATAGGAC GTCCTATC
262 F09 ATACTGCA GCAACCGA TCGGTTGC 286 F12 GCTCTCTC AAGTACTC GAGTACTT
263 G09 TCTCCTTG ATACTGCA TGCAGTAT 287 G12 CTACCAGT GCTCTCTC GAGAGAGC
264 H09 CATGAATG TCTCCTTG CAAGGAGA 288 H12 GATGAGAT CTACCAGT ACTGGTAG
92 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 93

SureSelect XT HS2 Index Primer Pair Information
Table 57 SureSelect XT HS2 Index Primer Pairs 289–336, provided in red 96-well plate
Reference 7
Primer
Pair #
Well P7 Index P5 Index
NovaSeq (v1.0
chemistry),
MiSeq,
HiSeq 2500
P5 Index
NovaSeq (v1.5
chemistry),
NextSeq,
HiSeq 4000,
HiSeq 3000
Primer
Pair #
Well P7 Index P5 Index
NovaSeq (v1.0
chemistry),
MiSeq,
HiSeq 2500
P5 Index
NovaSeq (v1.5
chemistry),
NextSeq,
HiSeq 4000,
HiSeq 3000
289 A01 AGATAGTG GATGAGAT ATCTCATC 313 A04 AGCTACAT GATCCATG CATGGATC
290 B01 AGAGGTTA AGATAGTG CACTATCT 314 B04 CGCTGTAA AGCTACAT ATGTAGCT
291 C01 CTGACCGT AGAGGTTA TAACCTCT 315 C04 CACTACCG CGCTGTAA TTACAGCG
292 D01 GCATGGAG CTGACCGT ACGGTCAG 316 D04 GCTCACGA CACTACCG CGGTAGTG
293 E01 CTGCCTTA GCATGGAG CTCCATGC 317 E04 TGGCTTAG GCTCACGA TCGTGAGC
294 F01 GCGTCACT CTGCCTTA TAAGGCAG 318 F04 TCCAGACG TGGCTTAG CTAAGCCA
295 G01 GCGATTAC GCGTCACT AGTGACGC 319 G04 AGTGGCAT TCCAGACG CGTCTGGA
296 H01 TCACCACG GCGATTAC GTAATCGC 320 H04 TGTACCGA AGTGGCAT ATGCCACT
297 A02 AGACCTGA TCACCACG CGTGGTGA 321 A05 AAGACTAC TGTACCGA TCGGTACA
298 B02 GCCGATAT AGACCTGA TCAGGTCT 322 B05 TGCCGTTA AAGACTAC GTAGTCTT
299 C02 CTTATTGC GCCGATAT ATATCGGC 323 C05 TTGGATCT TGCCGTTA TAACGGCA
300 D02 CGATACCT CTTATTGC GCAATAAG 324 D05 TCCTCCAA TTGGATCT AGATCCAA
301 E02 CTCGACAT CGATACCT AGGTATCG 325 E05 CGAGTCGA TCCTCCAA TTGGAGGA
302 F02 GAGATCGC CTCGACAT ATGTCGAG 326 F05 AGGCTCAT CGAGTCGA TCGACTCG
303 G02 CGGTCTCT GAGATCGC GCGATCTC 327 G05 GACGTGCA AGGCTCAT ATGAGCCT
304 H02 TAACTCAC CGGTCTCT AGAGACCG 328 H05 GAACATGT GACGTGCA TGCACGTC
305 A03 CACAATGA TAACTCAC GTGAGTTA 329 A06 AATTGGCA GAACATGT ACATGTTC
306 B03 GACTGACG CACAATGA TCATTGTG 330 B06 TGGAGACT AATTGGCA TGCCAATT
307 C03 CTTAAGAC GACTGACG CGTCAGTC 331 C06 AACTCACA TGGAGACT AGTCTCCA
308 D03 GAGTGTAG CTTAAGAC GTCTTAAG 332 D06 GTAGACTG AACTCACA TGTGAGTT
309 E03 TGCACATC GAGTGTAG CTACACTC 333 E06 CGTAGTTA GTAGACTG CAGTCTAC
310 F03 CGATGTCG TGCACATC GATGTGCA 334 F06 CGTCAGAT CGTAGTTA TAACTACG
311 G03 AACACCGA CGATGTCG CGACATCG 335 G06 AACGGTCA CGTCAGAT ATCTGACG
312 H03 GATCCATG AACACCGA TCGGTGTT 336 H06 GCCTTCAT AACGGTCA TGACCGTT
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 93
Page 94

7 Reference
SureSelect XT HS2 Index Primer Pair Information
Table 58 SureSelect XT HS2 Index Primer Pairs 337–384, provided in red 96-well plate
Primer
Pair #
Well P7 Index P5 Index
NovaSeq (v1.0
chemistry),
MiSeq,
HiSeq 2500
P5 Index
NovaSeq (v1.5
chemistry),
NextSeq,
HiSeq 4000,
HiSeq 3000
Primer
Pair #
Well P7 Index P5 Index
NovaSeq (v1.0
chemistry),
MiSeq,
HiSeq 2500
P5 Index
NovaSeq (v1.5
chemistry),
NextSeq,
HiSeq 4000,
HiSeq 3000
337 A07 TGAGACGC GCCTTCAT ATGAAGGC 361 A10 CTGAGCTA GCACAGTA TACTGTGC
338 B07 CATCGGAA TGAGACGC GCGTCTCA 362 B10 CTTGCGAT CTGAGCTA TAGCTCAG
339 C07 TAGGACAT CATCGGAA TTCCGATG 363 C10 GAAGTAGT CTTGCGAT ATCGCAAG
340 D07 AACACAAG TAGGACAT ATGTCCTA 364 D10 GTTATCGA GAAGTAGT ACTACTTC
341 E07 TTCGACTC AACACAAG CTTGTGTT 365 E10 TGTCGTCG GTTATCGA TCGATAAC
342 F07 GTCGGTAA TTCGACTC GAGTCGAA 366 F10 CGTAACTG TGTCGTCG CGACGACA
343 G07 GTTCATTC GTCGGTAA TTACCGAC 367 G10 GCATGCCT CGTAACTG CAGTTACG
344 H07 AAGCAGTT GTTCATTC GAATGAAC 368 H10 TCGTACAC GCATGCCT AGGCATGC
345 A08 ATAAGCTG AAGCAGTT AACTGCTT 369 A11 CACAGGTG TCGTACAC GTGTACGA
346 B08 GCTTAGCG ATAAGCTG CAGCTTAT 370 B11 AGCAGTGA CACAGGTG CACCTGTG
347 C08 TTCCAACA GCTTAGCG CGCTAAGC 371 C11 ATTCCAGA AGCAGTGA TCACTGCT
348 D08 TACCGCAT TTCCAACA TGTTGGAA 372 D11 TCCTTGAG ATTCCAGA TCTGGAAT
349 E08 AGGCAATG TACCGCAT ATGCGGTA 373 E11 ATACCTAC TCCTTGAG CTCAAGGA
350 F08 GCCTCGTT AGGCAATG CATTGCCT 374 F11 AGACCATT ATACCTAC GTAGGTAT
351 G08 CACGGATC GCCTCGTT AACGAGGC 375 G11 CGTAAGCA AGACCATT AATGGTCT
352 H08 GAGACACG CACGGATC GATCCGTG 376 H11 TCTGTCAG CGTAAGCA TGCTTACG
353 A09 AGAGTAAG GAGACACG CGTGTCTC 377 A12 CACAGACT TCTGTCAG CTGACAGA
354 B09 AGTACGTT AGAGTAAG CTTACTCT 378 B12 GTCGCCTA CACAGACT AGTCTGTG
355 C09 AACGCTGC AGTACGTT AACGTACT 379 C12 TGCGCTCT GTCGCCTA TAGGCGAC
356 D09 GTAGAGCA AACGCTGC GCAGCGTT 380 D12 GCTATAAG TGCGCTCT AGAGCGCA
357 E09 TCCTGAGA GTAGAGCA TGCTCTAC 381 E12 CAACAACT GCTATAAG CTTATAGC
358 F09 CTGAATAG TCCTGAGA TCTCAGGA 382 F12 AGAGAATC CTCTCACT AGTGAGAG
359 G09 CAAGACTA CTGAATAG CTATTCAG 383 G12 TAATGGTC AGACGAGC GCTCGTCT
360 H09 GCACAGTA CAAGACTA TAGTCTTG 384 H12 GTTGTATC TAATGGTC GACCATTA
94 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 95

Index Primer Pair Strip Tube and Plate Maps
Index Primer Pair Strip Tube and Plate Maps
SureSelect XT HS2 Index Primer Pairs 1- 16 (provided with 16 reaction
kits) are supplied in a set of two 8- well strip tubes as detailed below.
Figure 10 Map of the SureSelect XT HS2 Index Primer Pairs for ILM (Pre PCR) strip tubes
provided with 16 reaction kits
The blue strip contains Index Primer Pairs 1- 8, with pair #1 supplied in
the well proximal to the numeral 1 etched on the strip’s plastic end tab.
The white strip contains Index Primer Pairs 9- 16, with pair #9 supplied in
the well proximal to the numeral 9 etched on the strip’s plastic end tab.
When using the strip tube- supplied index primer pairs in the library
preparation protocol, re- seal any unused wells using the fresh foil seal
strips provided with the index strip tubes.
Reference 7
See Table 59 on page 96 through Table 62 on page 97 for plate maps
showing positions of the SureSelect XT HS2 Index Primer Pairs provided
with 96 reaction kits.
CAUTION
The SureSelect XT HS2 Index Primer Pairs are provided in single-use aliquots. To avoid
cross-contamination of libraries, use each well in only one library preparation reaction.
Do not retain and re-use any residual volume for subsequent experiments.
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 95
Page 96
7 Reference
Index Primer Pair Strip Tube and Plate Maps
Table 59 Plate map for SureSelect XT HS2 Index Primer Pairs 1-96, provided in orange plate
1 2 3 4 5 6 7 8 9 10 11 12
A 1 9 17 25 33 41 49 57 65 73 81 89
B 2 10182634 42 50 58 66 74 82 90
C 3 11192735 43 51 59 67 75 83 91
D 4 12202836 44 52 60 68 76 84 92
E 5 13212937 45 53 61 69 77 85 93
F 6 14223038 46 54 62 70 78 86 94
G 7 15233139 47 55 63 71 79 87 95
H 8 16243240 48 56 64 72 80 88 96
Table 60 Plate map for SureSelect XT HS2 Index Primer Pairs 97-192, provided in blue plate
1 2 3 4 5 6 7 8 9 10 11 12
A 97 105 113 121 129 137 145 153 161 169 177 185
B 98 106 114 122 130 138 146 154 162 170 178 186
C 99 107 115 123 131 139 147 155 163 171 179 187
D 100 108 116 124 132 140 148 156 164 172 180 188
E 101 109 117 125 133 141 149 157 165 173 181 189
F 102 1110 118 126 134 142 150 158 166 174 182 190
G 103 111 119 127 135 143 151 159 167 175 183 191
H 104 112 120 128 136 144 152 160 168 176 184 192
96 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 97

Reference 7
Index Primer Pair Strip Tube and Plate Maps
Table 61 Plate map for SureSelect XT HS2 Index Primer Pairs 193-288, provided in green plate
1 2 3 4 5 6 7 8 9 10 11 12
A 193 201 209 217 225 233 241 249 257 265 273 281
B 194 202 210 218 226 234 242 250 258 266 274 282
C 195 203 211 219 227 235 243 251 259 267 275 283
D 196 204 212 220 228 236 244 252 260 268 276 284
E 197 205 213 221 229 237 245 253 261 269 277 285
F 198 206 214 222 230 238 246 254 262 270 278 286
G 199 207 215 223 231 239 247 255 263 271 279 287
H 200 208 216 224 232 240 248 256 264 272 280 288
Table 62 Plate map for SureSelect XT HS2 Index Primer Pairs 289-384, provided in red plate
1 2 3 4 5 6 7 8 9 10 11 12
A 289 297 305 313 321 329 337 345 353 361 369 377
B 290 298 306 314 322 330 338 346 354 362 370 378
C 291 299 307 315 323 331 339 347 355 363 371 379
D 292 300 308 316 324 332 340 348 356 364 372 380
E 293 301 309 317 325 333 341 349 357 365 373 381
F 294 302 310 318 326 334 342 350 358 366 374 382
G 295 303 311 319 327 335 343 351 359 367 375 383
H 296 304 312 320 328 336 344 352 360 368 376 384
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 97
Page 98

7 Reference
Troubleshooting Guide
Troubleshooting Guide
If recovery of gDNA from samples is low
✔ Using excess tissue for gDNA isolation can reduce yield. Use only the
amount of each specific tissue type recommended by the gDNA isolation
protocol.
✔ Tissue sample lysis may not have been optimal during gDNA isolation.
Monitor the extent of sample lysis during the Proteinase K digestion at
56°C by gently pipetting the digestion reaction every 20–30 minutes,
visually inspecting the solution for the presence of tissue clumps. If
clumps are still present after the 1- hour incubation at 56°C, add
another 10 µl of Proteinase K and continue incubating at 56°C, with
periodic mixing and visual inspections, for up to two additional hours.
When the sample no longer contains clumps of tissue, move the sample
to room temperature until lysis is complete for the remaining samples.
Do not over- digest. Individual samples may be kept at room
temperature for up to 2 hours before resuming the protocol. Do not
exceed 3 hours incubation at 56°C for any sample.
If yield of pre-capture libraries is low
✔ The library preparation protocol includes specific thawing, temperature
control, pipetting, and mixing instructions which are required for
optimal performance of the highly viscous buffer and enzyme solutions
used in the protocol. Be sure to adhere to all instructions when setting
up the reactions.
✔ Ensure that the ligation master mix (see page 33) is kept at room
temperature for 30–45 minutes before use.
✔ PCR cycle number may require optimization. Repeat library preparation
for the sample, increasing the pre- capture PCR cycle number by 1 to 2
cycles. If a high molecular weight peak (>500 bp) is observed in the
electropherogram for a sample with low yield, the DNA may be
overamplified. Repeat library preparation for the sample, decreasing the
pre- capture PCR cycle number by 1 to 3 cycles.
✔ DNA isolated from degraded samples, including FFPE tissue samples,
may be over- fragmented or have modifications that adversely affect
library preparation processes. Use the Agilent NGS FFPE QC Kit to
determine the precise quantity of amplifiable DNA in the sample and
allow direct normalization of input DNA amount.
98 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
Page 99

Reference 7
Troubleshooting Guide
✔ Performance of the solid- phase reversible immobilization (SPRI)
purification step may be poor. Verify the expiration date for the vial of
AMPure XP beads used for purification. Adhere to all bead storage and
handling conditions recommended by the manufacturer. Ensure that the
beads are kept at room temperature for at least 30 minutes before use.
Use freshly- prepared 70% ethanol for each SPRI procedure.
✔ DNA elution during SPRI purification steps may be incomplete. Ensure
that the AMPure XP beads are not overdried just prior to sample
elution.
If solids observed in the End Repair-A Tailing Buffer
✔ Vortex the solution at high speed until the solids are dissolved. The
observation of solids when first thawed does not impact performance,
but it is important to mix the buffer until all solutes are dissolved.
If pre-capture library fragment size is larger than expected in electropherograms
✔ Shearing may not be optimal. For intact, high- quality DNA samples,
ensure that shearing is completed using the two- round shearing
protocol provided, including all spinning and vortexing steps.
✔ Any bubbles present on the microTUBE filament may disrupt complete
shearing. Spin the microTUBE for 30 seconds before the first round of
shearing to ensure that any bubbles are released.
If pre-capture library fragment size is different than expected in
electropherograms
✔ FFPE DNA pre- capture libraries may have a smaller fragment size
distribution due to the presence of DNA fragments in the input DNA
that are smaller than the target DNA shear size.
✔ DNA fragment size selection during SPRI purification depends upon
using the correct ratio of sample to AMPure XP beads. Before removing
an aliquot of beads for the purification step, mix the beads until the
suspension appears homogeneous and consistent in color and verify
that you are using the bead volume recommended for pre- capture
purification on page 42.
SureSelect XT HS2 DNA Library Preparation and Target Enrichment 99
Page 100

7 Reference
Troubleshooting Guide
If low molecular weight adaptor-dimer peak is present in pre-capture library
electropherograms
✔ The presence of a low molecular weight peak, in addition to the
expected peak, indicates the presence of adaptor- dimers in the library.
It is acceptable to proceed to target enrichment with library samples for
which adaptor- dimers are observed in the electropherogram at low
abundance, similar to the example shown in Figure 4 on page 46. The
presence of excessive adaptor- dimers in the samples may be associated
with reduced yield of pre- capture libraries. If excessive adaptor- dimers
are observed, verify that the adaptor ligation protocol is being
performed as directed on page 36. In particular, ensure that the
Ligation master mix is mixed with the sample prior to adding the
Adaptor Oligo Mix to the mixture. Do not add the Ligation master mix
and the Adaptor Oligo Mix to the sample in a single step.
✔ For whole- genome sequencing (not specifically supported by this
protocol), samples with an adaptor- dimer peak must be subjected to an
additional round of SPRI- purification. To complete, first dilute the
sample to 50 µl with nuclease free water, then follow the SPRI
purification procedure on page 42.
If yield of post-capture libraries is low
✔ PCR cycle number may require optimization. Repeat library preparation
and target enrichment for the sample, increasing the post- capture PCR
cycle number by 1 to 2 cycles.
✔ The RNA Probe used for hybridization may have been compromised.
Verify the expiration date on the Probe vial or Certificate of Analysis.
Adhere to the recommended storage and handling conditions. Ensure
that the Capture Library Hybridization Mix is prepared immediately
before use, as directed on page 53, and that solutions containing the
Probe are not held at room temperature for extended periods.
If post-capture library fragment size is different than expected in
electropherograms
✔ DNA fragment size selection during SPRI purification depends upon
using the correct ratio of sample to AMPure XP beads. Before removing
an aliquot of beads for the purification step, mix the beads until the
suspension appears homogeneous and consistent in color and verify
that you are using the bead volume recommended for post- capture
purification on page 63.
100 SureSelect XT HS2 DNA Library Preparation and Target Enrichment
 Loading...
Loading...