Page 1
Agilent XF Substrate Oxidation Stress Test Kits
User Manual
XF Long Chain Fatty Acid Oxidation Stress Test Kit (103672-100)
XF Glucose/Pyruvate Oxidation Stress Test Kit (103673-100)
XF Glutamine Oxidation Stress Test Kit (103674-100)
XF Palmitate Oxidation Stress Test Kit (103693-100)
Page 2
Notices
CAUTION
WARNING
© Agilent Technologies 2020
No part of this manual may be reproduced in
any form or by any means (including electronic
storage and retrieval or translation into a
foreign language) without prior agreement and
written consent from Agilent Technologies,
Inc. as governed by United States and
international copyright laws.
Manual Part Number
5994-1164EN
Rev B0
Edition
First edition, May 2020
Printed in USA
Agilent Technologies
1834 Hwy 71 West
Cedar Creek, TX 78612
Product is made in UK.
Warranty
The material contained in this document is
provided “as is,” and is subject to being
changed, without notice, in future editions.
Further, to the maximum extent permitted by
applicable law, Agilent disclaims all
warranties, either express or implied, with
regard to this manual and any information
contained herein, including but not limited to
the implied warranties of merchantability and
fitness for a particular purpose. Agilent shall
not be liable for errors or for incidental or
consequential damages in connection with
the furnishing, use, or performance of this
document or of any information contained
herein. Should Agilent and the user have a
separate written agreement with warranty
terms covering the material in this document
that conflict with these terms, the warranty
terms in the separate agreement shall
control.
Technology Licenses
The hardware and/or software described in
this document are furnished under a license
and may be used or copied only in
accordance with the terms of such license.
Restricted Rights Legend
U.S. Government Restricted Rights. Software
and technical data rights granted to the
federal government include only those rights
customarily provided to end user customers.
Agilent provides this customary commercial
license in Software and technical data
pursuant to FAR 12.211 (Technical Data) and
12.212 (Computer Software) and, for the
Department of Defense, DFARS
252.227-7015 (Technical Data -Commercial
Items) and DFARS 227.7202-3 (Rights in
Commercial Computer Software or Computer
Software Documentation).
Safety Notices
A CAUTION notice denotes a
hazard. It calls attention to an
operating procedure, practice, or the
like that, if not correctly performed
or adhered to, could result in
damage to the product or loss of
important data. Do not proceed
beyond a CAUTION notice until the
indicated conditions are fully
understood and met.
A WARNING notice denotes a
hazard. It calls attention to an
operating procedure, practice, or the
like that, if not correctly performed
or adhered to, could result in
personal injury or death. Do not
proceed beyond a WARNING notice
until the indicated conditions are
fully understood and met.
Page 3

Contents
1Introduction
Assay Background 6
XF Substrate Oxidation Stress Test - Standard Assay 8
XF Palmitate Oxidation Stress Test - Advanced Assay 12
Glossary 16
References 18
2 Kit Information
Kit Contents 20
Kit Shipping and Storage 21
Additional Agilent Products Required 21
3 Assay Workflow for Standard Substrate Oxidation Stress Test
One Day Prior to Assay 24
Day of Assay 24
Data Analysis Using Agilent Seahorse Analytics 30
Examples of Data 32
4 Assay Workflow for Palmitate Oxidation Stress Test - Advanced Assay
Two Days Prior to Assay 36
One Day Prior to Assay 36
Day of Assay 37
Data Analysis Using Agilent Seahorse Analytics 42
Examples of Data 44
5 Frequently Asked Questions
6 Context of Substrate Oxidation Tests Among Other XF Kits and Applications
Agilent XF Substrate Oxidation Stress Test Kits 3
Page 4

4 Agilent XF Substrate Oxidation Stress Test Kits
Page 5

1 Introduction
Assay Background 6
XF Substrate Oxidation Stress Test - Standard Assay 8
XF Palmitate Oxidation Stress Test - Advanced Assay 12
Glossary 16
References 18
Agilent XF Substrate Oxidation Stress Test Kits 5
Page 6
Assay Background
MPC
Glucose
ATP
ATP
O
2
e
-
Glucose
Glycolysis
Glutamate
Malate
Acetyl-CoA
Ⱥ-KG
ADP + Pi
ADP + Pi
ETC
Acyl-
CoA
TCA
cycle
Amino
acids
UK5099
Pyruvate
Pyruvate
Glutamine
LCFAs
BPTES
Eto
Gluconeogenesis
LCFAs
Glutamine
CPT1a
CPT2
Ȼ-Oxidation
GLS1
Gln transporter
Lactate
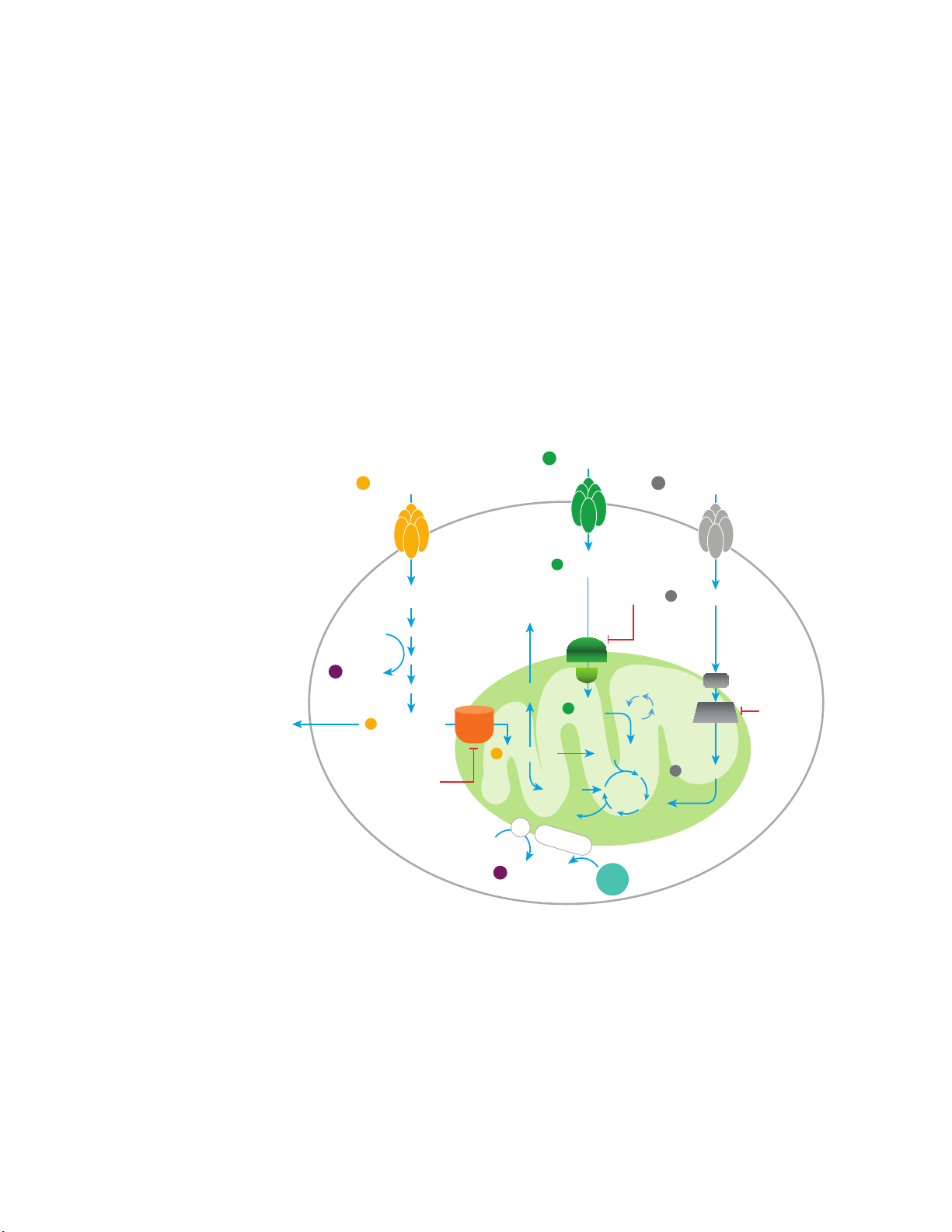
Assay Background
Agilent Seahorse XF technology measures energy metabolism in live cells in real time, providing
critical functional information that relates directly to cellular health and fitness. The Seahorse XF
Substrate Oxidation Stress Test Assay provides key metrics to facilitate the assessment of
specific mitochondrial substrates that are relevant or required for cellular phenotype and function.
Agilent provides a suite of optimized, rapid solutions for measuring cellular substrate oxidation by
assessing changes in oxygen consumption (OCR) using live cells. XF Substrate Oxidation Stress
Test Kits allow for both a sensitive measure of mitochondrial function, and the interrogation of
three primary substrates that fuel mitochondria: long-chain fatty acids (LCFAs), glucose/pyruvate
(G/P), and/or glutamine (Q) (Figure 1). These kits facilitate the convenient investigation of specific
substrate oxidation processes and the central role they play in the fundamental cellular functions
of activation, proliferation, and differentiation; as well as in better characterizing cellular responses
to genetic manipulation, pharmaceutical interventions, or specific disease-relevant
microenvironments.
6 Agilent XF Substrate Oxidation Stress Test Kits
Figure 1. Primary metabolic pathways, including glycolysis, the TCA cycle, electron transport (ETC),
and oxidative phosphorylation (OXPHOS). Glucose/pyruvate, glutamine and long chain fatty
The XF Substrate Oxidation Stress Tests combine the substrate pathway specific inhibitors
(Figure 1): etomoxir for LCFAs through inhibition of carnitine palmitoyl transferase 1a (CPT1a)
UK5099 for glucose and/or pyruvate through inhibition of the mitochondrial pyruvate carrier
(MPC)
Mito Stress Test (MST). The MST, a powerful and well-accepted tool for the interrogation of
mitochondrial function, in conjunction with these inhibitors, can be used to reveal dependence on
a specific metabolic substrate. Basal and maximal respiration rates are key metrics of
acid oxidation are highlighted. Red lines denote relevant inhibitors of glucose/pyruvate, LCFA,
and glutamine transport, which in turn, specifically limits oxidation of that respective
substrate.
2
; and BPTES for inhibition of glutamine through glutaminase 1 (GLS-1)3, with the XF Cell
1
;
Page 7
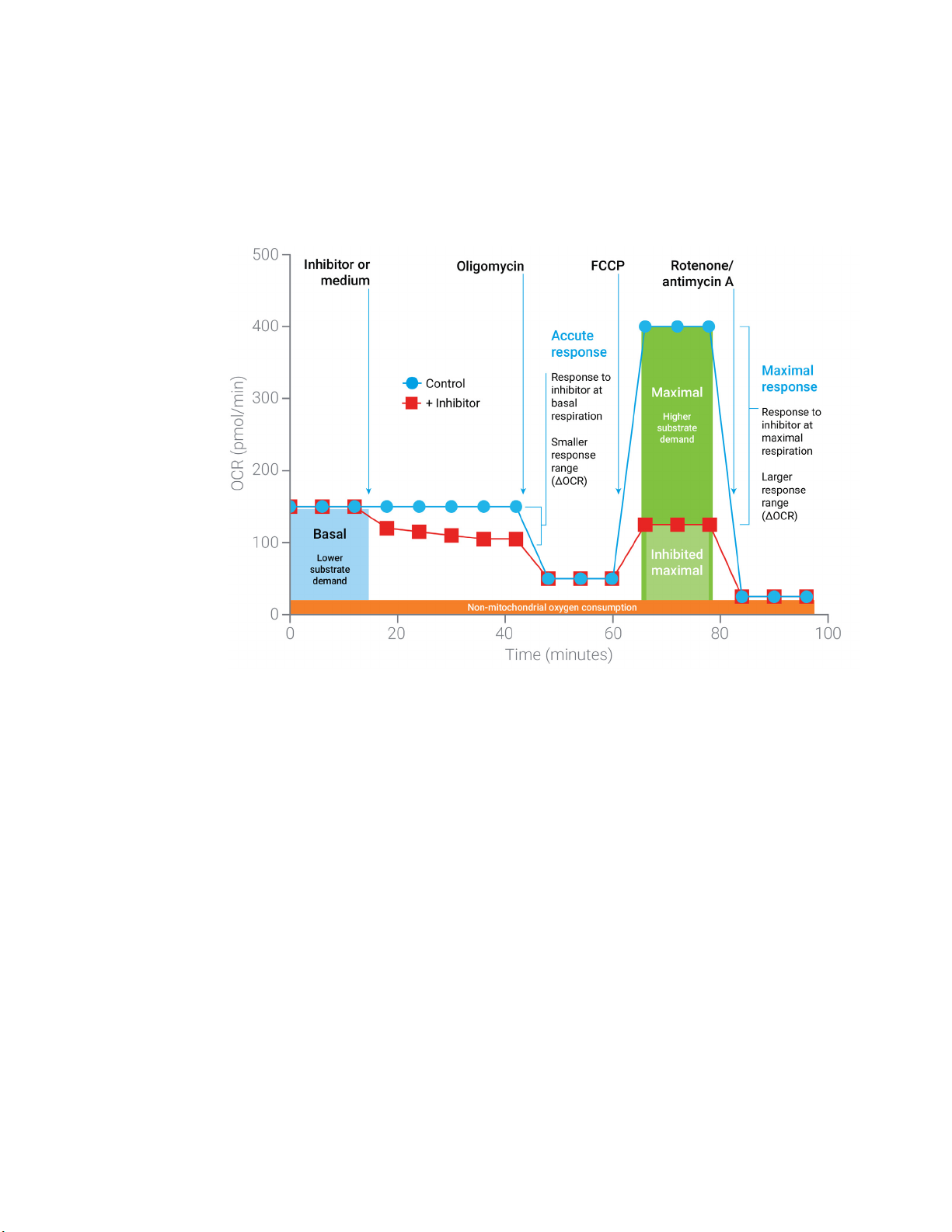
Assay Background
mitochondrial function reported by the MST. In the context of substrate oxidation, the basal, and in
particular, the maximal respiration rates are largely impacted by cells capacity to transport and
oxidize available substrates.
4
This method is ideally suited to the assessment for cellular
substrate demand both under basal conditions, and in response to elevated substrate demand
(maximal respiration). Figure 2 outlines the kinetic profile of a standard substrate oxidation assay
and relevant assay parameters.
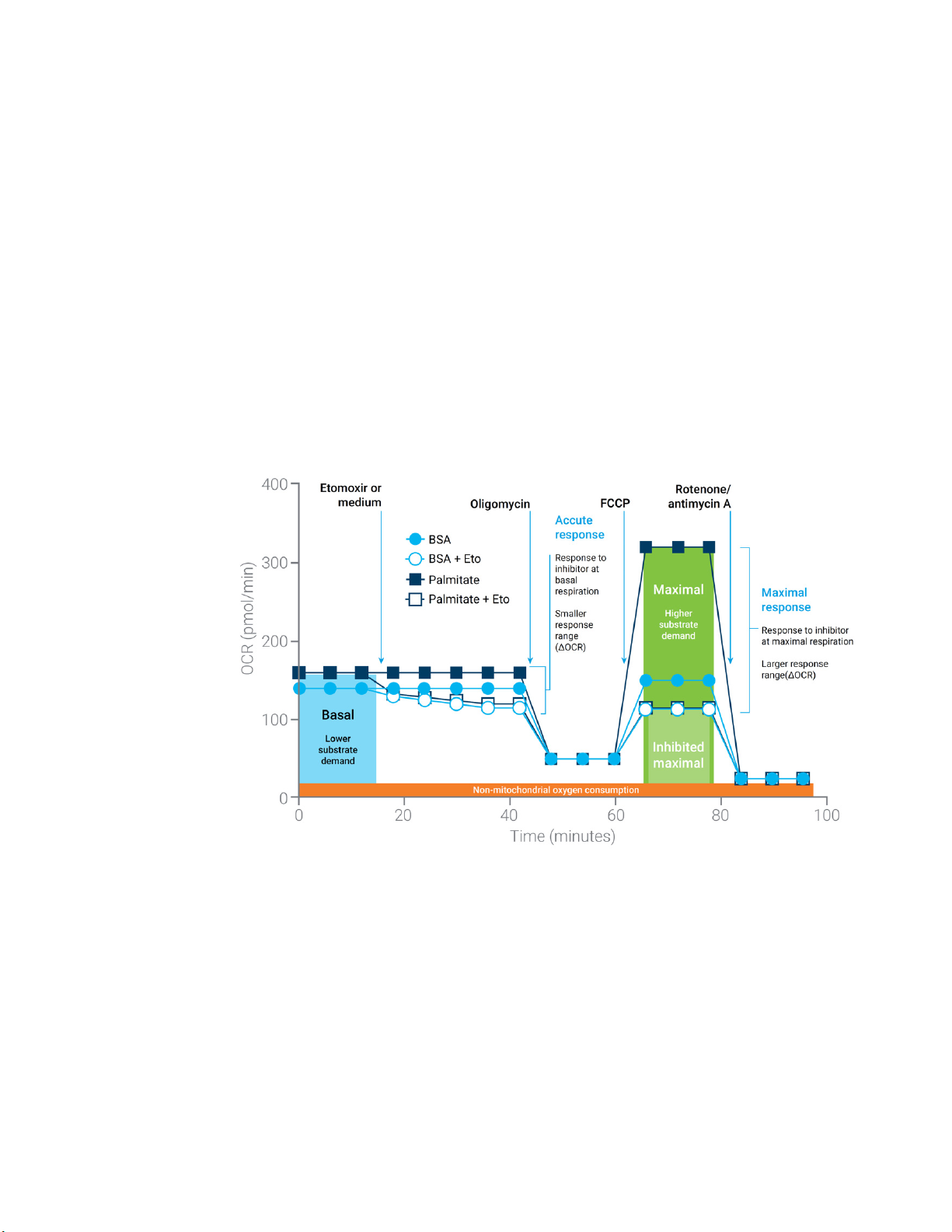
Figure 2. Agilent Seahorse XF Substrate Oxidation Stress Test profile of the respiration parameters
critical for substrate demand. Sequential compound injections measure basal respiration,
acute response to an inhibitor (etomoxir or UK5099 or BPTES), and maximal respiration in the
absence and presence of inhibitor. Note that while minimal changes may be measured under
basal conditions, such as the acute response; much larger responses are often revealed
under conditions of high substrate demand (for example, FCCP), thus revealing differences in
the ability of the cells to oxidize the substrate in question.
Measurements are highly informative, as the design described provides information on basal
respiration and the impact of pathway inhibition under conditions of basal substrate demand,
while also characterizing the impact of pathway inhibitions on maximal respiration, reflecting the
cell's sensitivity to impairment of a specific metabolic pathway under conditions of high substrate
demand. Due to the metabolic plasticity of many cell types, it is often more informative to assess
substrate dependence when demand is high as this reveals the cell's capacity to use substrates.
When mitochondria are uncoupled (such as FCCP exposure), substrate oxidation is increased to
generate the reducing equivalents needed for the increased respiration rate. By comparing the
maximum respiration rates of control versus inhibited cells, insights can be gained into how much
a cell will rely on a specific substrate to meet this high energy demand.
This manual details how to perform all assays in the XF Substrate Oxidation Stress Test suite. In
general, there are two distinct assay types: Standard and Advanced. The XF LCFA Oxidation
Stress Test, XF Glutamine Oxidation Stress Test and Glucose/Pyruvate Oxidation Stress Test kits
are to be carried out using the Standard Assay workflow, using a single inhibitor in the presence of
multiple substrates). Conversely, the XF Palmitate Oxidation Stress Test Kit requires users to use
the Advanced Assay protocol as additional steps are required (Figures 5, 6, and 10), and is carried
out using palmitate as the long chain fatty acid substrate and etomoxir as the inhibitor. The
following are the principles and designs of these assays.
Agilent XF Substrate Oxidation Stress Test Kits 7
Page 8
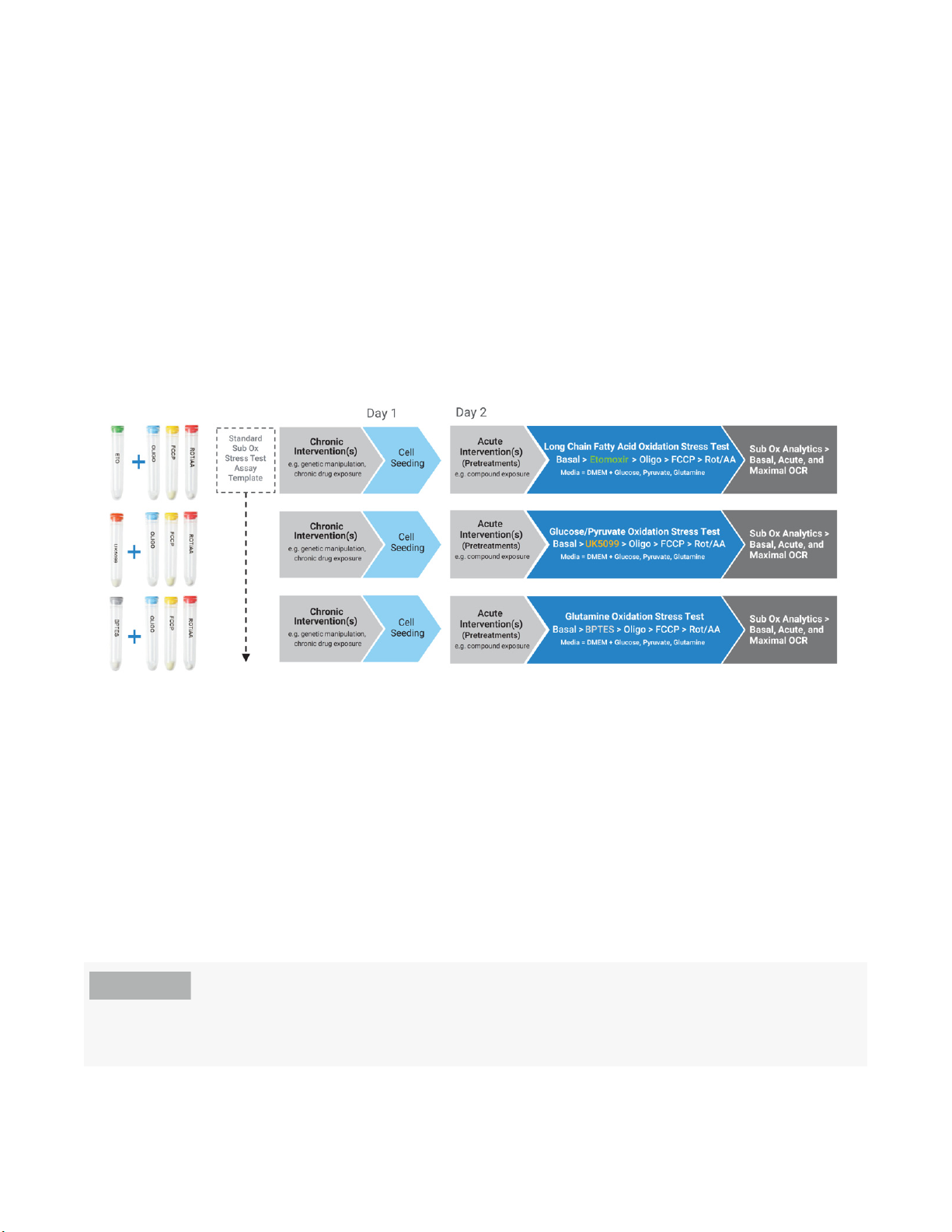
XF Substrate Oxidation Stress Test - Standard Assay
NOTE
XF Substrate Oxidation Stress Test - Standard Assay
Relevant kits include:
XF Long Chain Fatty Acid Oxidation Stress Test Kit (p/n 103672-100)
XF Glucose/Pyruvate Oxidation Stress Test Kit (p/n 103673-100)
XF Glutamine Oxidation Stress Test Kit (p/n 103674-100)
To perform a Standard Substrate Oxidation Stress Test, basal respiration is first established,
followed by injection of the relevant pathway inhibitor. The acute response to the inhibitor is
monitored over several measurement cycles (typically six). Then, the standard XF Cell Mito Stress
Test reagents, such as oligomycin, FCCP, and rotenone/antimycin A are injected sequentially,
similar to the assay scheme used for the XF Cell Mito Stress Test. Figure 3 outlines an overview of
the experimental workflow for each kit.
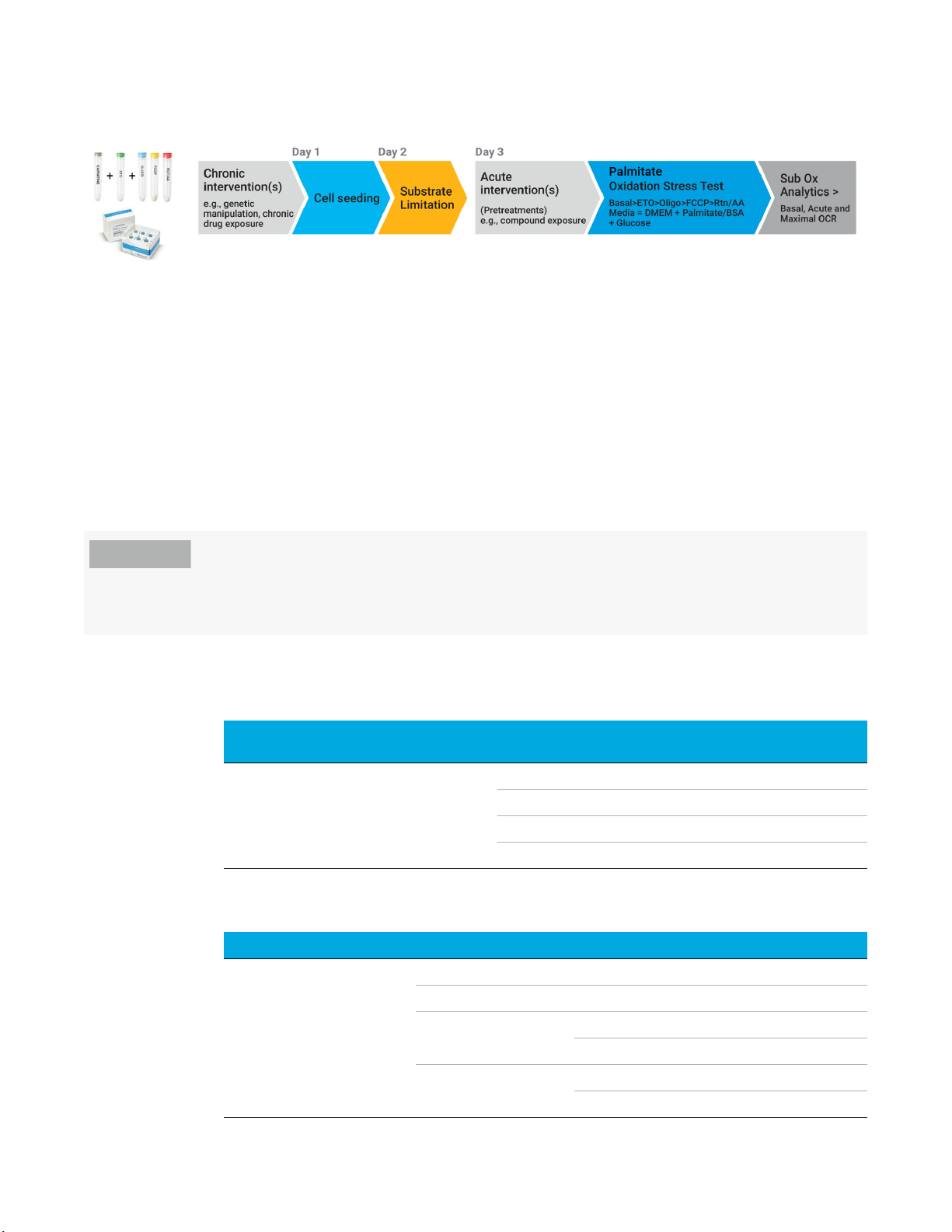
Figure 3. Agilent XF Substrate Oxidation Stress Test - Standard Assay Design. Each kit is focused on testing a single substrate
using the relevant inhibitor. Chronic or acute interventions (genetic manipulations/drug exposure) may be performed
upstream of the assay to understand the effects of these modulations on the oxidation of specific mitochondrial
substrates.
Each kit is focused on testing a single substrate using the optimized concentration of relevant
inhibitor: etomoxir (4 µM) to inhibit oxidation of LCFAs, UK5099 (2 µM) to inhibit the oxidation of
glucose and/or pyruvate, and BPTES (3 µM) to inhibit oxidation of glutamine (final
concentrations). These three assays are designed to be performed under conditions of saturating
substrates with respect to glucose (10 mM), pyruvate (1 mM) and glutamine (2 mM) in the assay
media. The source of long chain fatty acids is any endogenous stores of lipid/LCFAs in the cell
used, and is cell-type dependent. Figure 3 shows that the standard methods and assay conditions
for each of the XF Substrate Oxidation Stress Tests are identical with the exception of the identity
of the inhibitor used.
Users must first establish both the optimal cell seeding density and optimal FCCP
concentration for ideal assay performance and resulting data. Typically, these are the same
conditions established for a cell type in an XF Cell Mito Stress Test. For information on how to
optimize cell seeding density and FCCP concentrations, please visit the Agilent Cell Analysis
Learning Center website.
8 Agilent XF Substrate Oxidation Stress Test Kits
Page 9
XF Substrate Oxidation Stress Test - Standard Assay
In these Standard Assays, decreased respiration rates in response to an inhibitor suggest that the
cells have a demand or preference for that particular substrate under the experimental conditions
established. In general, these assays can be used to facilitate investigation to address the
following types of questions:
• Does the cell have a demand for a particular substrate or substrates?
• Is the cell highly reliant on a specific substrate, or can other substrates satisfy cellular
demands?
• How is mitochondrial substrate demand and/or reliance affected if an intervention, such as
genetic manipulation or drug exposure, is applied to the cell?
Like many XF assays, the Substrate Oxidation Stress Tests are typically performed subsequent to
a pretreatment, or intervention, as designed by the researcher. This is shown as either a chronic
intervention to the cells (for example, a genetic manipulation or long-term drug exposure), hours
to days upstream of XF assay, or as an acute intervention (for example, drug exposure) just prior
to the XF assay (Figure 3). In some cases, both chronic and acute interventions may be used (for
example, rescue of genetic dysfunction through compound exposure). The kits may be used
individually (such as focusing on one specific substrate) for investigating how a series of
interventions or compounds may affect oxidation of that particular substrate; or in combination
(such as focusing on two or more substrates) to elucidate overall effects of a given intervention
with respect to substrate oxidation and mitochondrial function.
For examples of the design of assay templates for XF Substrate Oxidation Stress Tests - Standard
Assays, refer to Figure 4.
Agilent XF Substrate Oxidation Stress Test Kits 9
Page 10
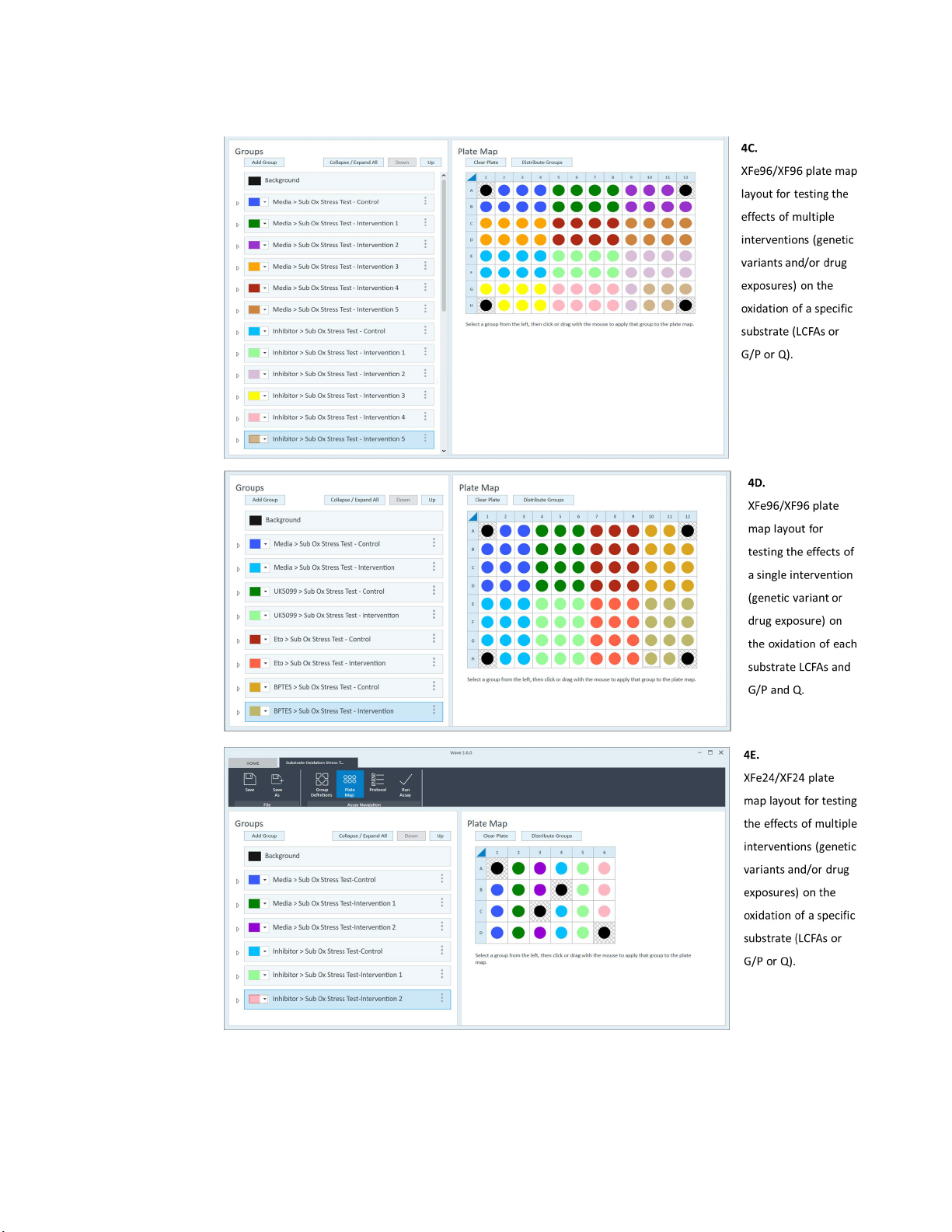
XF Substrate Oxidation Stress Test - Standard Assay
10 Agilent XF Substrate Oxidation Stress Test Kits
Page 11
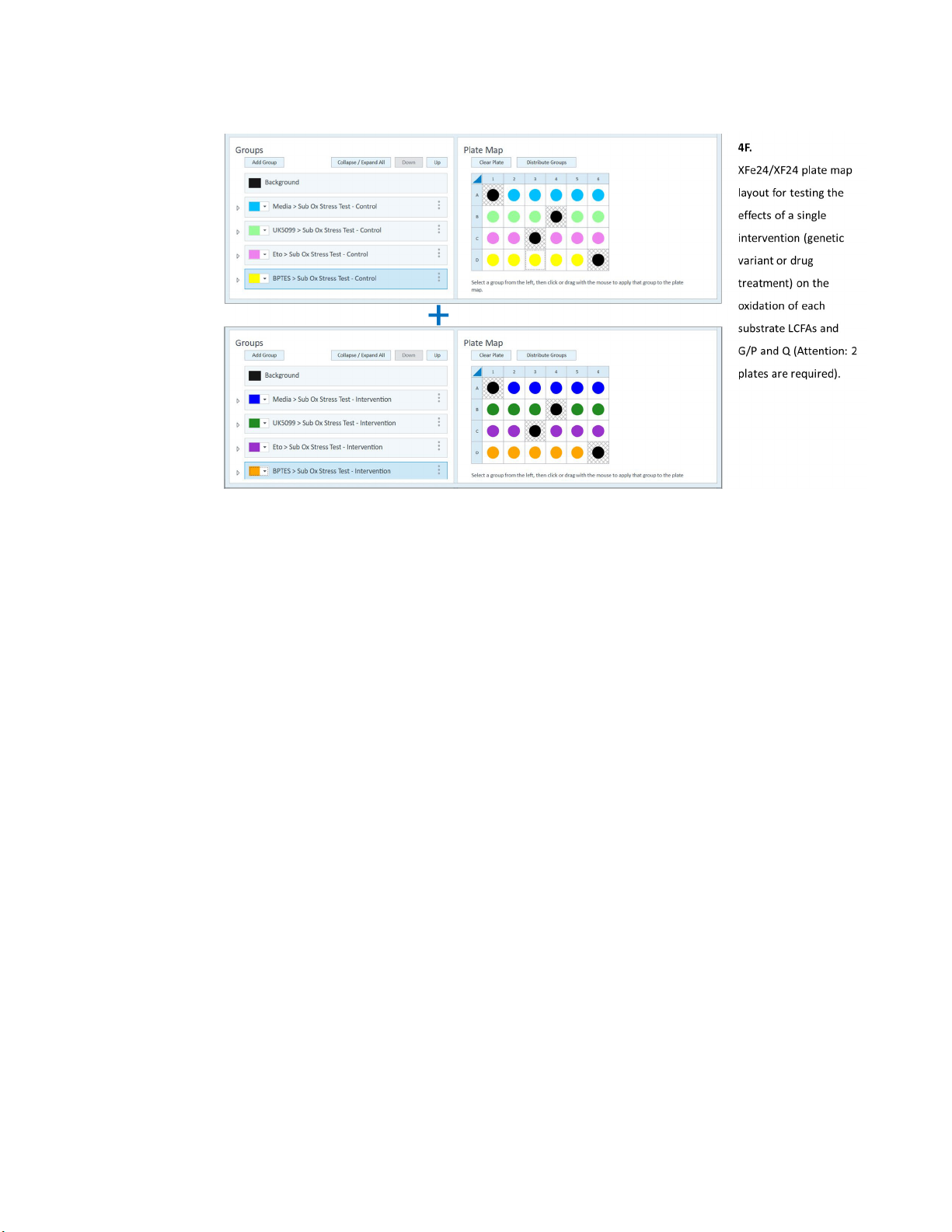
XF Substrate Oxidation Stress Test - Standard Assay
Figure 4. Agilent Seahorse XF Substrate Oxidation Stress Test - Assay Design Examples. These
examples are suggested designs, and the user is encouraged to modify the generic Standard
Substrate Oxidation Stress Test Assay Template file to accommodate the specific
requirements of the experimental design.
Agilent XF Substrate Oxidation Stress Test Kits 11
Page 12
XF Palmitate Oxidation Stress Test - Advanced Assay
XF Palmitate Oxidation Stress Test - Advanced Assay
Relevant kit includes:
XF Palmitate Oxidation Stress Test Kit (p/n 103693-100)
The XF Palmitate Oxidation Stress Test Advanced Assay details the workflow to specifically
analyze the long chain fatty acid oxidation pathway in live cells. The kit includes the XF
Palmitate-BSA FAO Substrate, L-carnitine, etomoxir, as well as oligomycin, FCCP, and
rotenone/antimycin A, and is designed to determine the intrinsic rate and capacity of a cell to
oxidize palmitate in the absence or limitation of other exogenous substrates. This workflow is
best applied when investigating how interventions (genetic manipulations and drug exposure)
specifically affect the LCFA oxidation process and can be a complementary and/or follow-up
assay to the Standard Assay workflow for XF Long Chain Fatty Acid Oxidation Stress Test
discussed in the previous section. The kinetic profile of the Advanced Palmitate oxidation assay
and relevant assay parameters is outlined in Figure 5, and an overview of the experimental
workflow for the Advance Assay is outlined in Figure 6.
Figure 5. Agilent Seahorse XF Palmitate Oxidation Stress Test profile of the respiration parameters
critical for palmitate demand. Sequential compound injections measure basal respiration,
acute response to etomoxir, and maximal respiration in the absence and presence of
etomoxir. Note that while minimal changes may be measured under basal conditions, such
as the acute response, much larger responses are often revealed under conditions of high
substrate demand (for example, FCCP), revealing differences in the ability of the cells to
oxidize palmitate.
12 Agilent XF Substrate Oxidation Stress Test Kits
Page 13
XF Palmitate Oxidation Stress Test - Advanced Assay
NOTE
Figure 6. Agilent XF Palmitate Oxidation Stress Test - Advanced Assay. This assay is specifically focused on determining the
intrinsic rate and capacity of a cell to oxidize palmitate in the absence or limitation of other exogenous substrates,
using inhibitor etomoxir to inhibit oxidation of palmitate.
The Palmitate Oxidation Stress Test is designed to be performed under conditions of saturating
palmitate (as palmitate-BSA) and L-carnitine, limited to no substrates provided with respect to
glucose, pyruvate, and glutamine in the assay media. It is best applied when the experimental
design calls for the cells to exclusively oxidize a long chain fatty acid, such as palmitate, to
investigate effects of interventions specifically on the long chain fatty acid oxidation process.
As shown in Figure 6, this workflow often requires substrate limitation before and/or during the
assay to condition the cells to obtain robust responses to the palmitate substrate added to the
assay media. The suggested initial substrate-limited growth media conditions can be found in
Table 1.
Users must first establish the optimal cell density and optimal FCCP concentration for best
assay performance and resulting data. Typically, these are the same conditions established for
a cell type in an XF Cell Mito Stress Test, except the FCCP concentration may need to be
re-optimized in the presence of BSA. For information on how to optimize cell density and FCCP
concentrations, please visit the Agilent Cell Analysis Learning Center website.
Table 1 Suggested substrate limitation conditions for performing the XF Palmitate Oxidation
Stress Test Advanced Assay with base growth media. Initial suggested time of
incubation under substrate limitation is overnight (16 to 24 hours).
Base growth media
DMEM or RPMI
without glucose, pyruvate,
glutamine, or GlutaMAX
Growth media supplement
Glucose 0.5 mM
Glutamine or GlutaMAX 1.0 mM
Serum (for example, FBS) 1%
Seahorse L-Carnitine 0.5 mM
Suggested initial concentration in
substrate-limited growth media
Table 2 Suggested substrate limitation conditions for performing the XF Palmitate Oxidation
Stress Test Advanced Assay with XF Assay Media.
XF Assay Media Assay Media supplement Suggested initial concentration in assay media
XF DMEM medium, pH 7.4 or
XF RPMI medium, pH 7.4
XF Glucose 2.0 mM
XF L-Carnitine 0.5 mM
XF Palmitate-BSA
*
96 well 30 µL/well
24 well 85 µL/well
XF BSA Control
* Note that XF Palmitate-BSA and XF BSA control are added directly to the XF cell culture plate wells just prior to starting the
assay. See pages 37 - 38 for further information.
*
96 well 30 µL/well
24 well 85 µL/well
Agilent XF Substrate Oxidation Stress Test Kits 13
Page 14
XF Palmitate Oxidation Stress Test - Advanced Assay
NOTE
Optimal limited substrate concentrations and optimal time of incubation are cell-dependent
and should be empirically determined for the cell type of interest.
Instead of examination of substrate demand and reliance, this Advanced Assay is designed to be
used when asking the following type of question:
• How an intervention, such as genetic manipulation or drug exposure, specifically affects the
oxidation of long chain fatty acids when applied to the cell?
This assay is most often performed under some pretreatment condition, or intervention, as
designed by the researcher. This is shown as either a chronic intervention to the cells (for
example, a genetic manipulation or long-term drug exposure), hours to days upstream of XF
assay, or as an acute intervention (for example, drug exposure) just prior to the XF assay
(Figure 6). In some cases, both chronic and acute interventions may be used (for example, rescue
of genetic dysfunction through compound treatment). For examples of the assay template design
for XF Palmitate Oxidation Stress Test - Advanced Assay, refer to Figure 7.
There are critical and distinct difference between this Advanced Assay workflow and the
Standard Assay workflow described in the previous section, including:
• Cells usually require a period of substrate limitation to measure oxidation of palmitate
substrate provided, especially established cell lines. The specific conditions will be cell type
dependent, and should be determined empirically for each cell type tested, particularly for
primary type cells.
• An analogous BSA control group must be included for each condition tested to ensure that the
observed responses are associated with Palmitate-BSA in the assay media. Therefore, the
number of groups used doubles compared to a Standard Substrate Oxidation Stress Test. For
examples of Palmitate Oxidation Stress Test assay templates, refer to Figure 6.
• L-carnitine is included in substrate-limited medium and assay medium to ensure that it is not a
rate limiting factor in the assay.
• The conditions for substrate limitation and performance of the Advanced Assay are only
validated for use with the XF Palmitate-BSA substrate and BSA control.
14 Agilent XF Substrate Oxidation Stress Test Kits
Page 15

XF Palmitate Oxidation Stress Test - Advanced Assay
Figure 7. Agilent XF Palmitate Oxidation Stress Test - Plate Layout Map Examples.
Agilent XF Substrate Oxidation Stress Test Kits 15
Page 16

Glossary
Glossary
• Basal Respiration: Oxygen consumption used to meet cellular ATP demand and resulting from
mitochondrial proton leak. Shows substrate demand of the cell under baseline conditions.
• Acute Response: Change in oxygen consumption rate due to an injection of substrate
oxidation inhibitor (etomoxir, UK5099, BPTES). Reported as a change in OCR
(i.e., ∆OCR pmol/min).
• Maximal Respiration: The maximal oxygen consumption rate attained by adding the
uncoupler FCCP. FCCP increases a substrate demand by stimulating the respiratory chain to
operate at maximum capacity, which causes rapid oxidation of substrates (sugars, fats, and
amino acids) to meet this metabolic challenge. Shows substrate demand of the cell under
maximal respiration conditions.
• Non-mitochondrial Respiration: Oxygen consumption that persists due to a subset of cellular
enzymes that continue to consume oxygen after rotenone/antimycin A addition. This is
important for getting an accurate measure of mitochondrial respiration.
• Standard Substrate Oxidation Stress Test Workflow: Three complementary assays, each
focused on testing cellular demand of a single substrate (LCFAs or G/P or Q) using the
optimized concentration of relevant inhibitor: etomoxir or UK5099 or BPTES. Designed to be
performed under conditions of saturating substrates with respect to glucose, pyruvate, and
glutamine in the assay media (Figures 2, 3, 4, and 8).
• Advanced XF Palmitate Oxidation Stress Test: A single assay designed to determine the
intrinsic rate and capacity of a cell to oxidize palmitate in the absence or limitation of other
exogenous substrates. Performed under conditions of saturating palmitate (as
palmitate-BSA) and L-carnitine, in the assay media. It is best applied when the experimental
design calls for the cells to exclusively oxidize a long-chain fatty acid, such as palmitate, in
order to investigate effects of interventions specifically on the long chain fatty acid oxidation
process (Figures 5, 6, 7, 12, and Tab l e 1).
•MST: XF Cell Mito Stress Test, a well-recognized assay that provides a comprehensive view of
mitochondrial function by reporting multiple parameters for mitochondrial respiration. For
more information, visit the Agilent Seahorse XF Cell Mito Stress Test product website.
•FAO: Fatty acid oxidation.
•Etomoxir: An inhibitor of long chain fatty acid oxidation. Etomoxir inhibits carnitine
palmitoyl-transferase 1a (CPT1a), which is critical for translocating long chain fatty acids from
the cytosol into the mitochondria for beta oxidation. Note that concentrations in excess of
4 µM (final in assay) result in substantial off-target effects on mitochondrial respiration
• UK5099: An inhibitor of the glucose oxidation pathway. UK5099 inhibits the mitochondrial
pyruvate carrier (MPC), which transports pyruvate into the mitochondria. Cells convert glucose
to pyruvate through glycolysis. Pyruvate can be transported into the mitochondria and
oxidized by the TCA cycle.
•BPTES: An inhibitor of the glutamine oxidation pathway. BPTES is an allosteric inhibitor of
glutaminase (GLS1). Glutaminase converts glutamine to glutamate, glutamate is then
converted to alpha-ketoglutarate, and oxidized by the TCA cycle. Note that BPTES does not
inhibit GLS2.
•LCFAs: Long chain fatty acids.
•G/P: Glucose/pyruvate.
•Q: Glutamine.
16 Agilent XF Substrate Oxidation Stress Test Kits
Page 17

Glossary
• L-Carnitine: A supplement provided in the Palmitate Oxidation Stress Kit for use in substrate
limited growth media and substrate limited assay media. Ensures concentrations of
L-carnitine are saturating and not limiting rates of palmitate oxidation.
•Growth Media: Fully supplemented cell culture media appropriate for the specific cell type.
• Substrate Limitation: A condition used in the Palmitate Oxidation Stress Test - Advanced
Assay. It usually refers to a period of time (e.g., overnight) in which key substrates (typically
glucose, pyruvate, GlutaMAX, and serum) are reduced in concentration in the cell culture
media.
• Substrate-limited growth media: Cell culture media with key substrates (typically glucose,
pyruvate, GlutaMAX, and serum) reduced in concentration (see Table 1).
• Substrate-limited assay media: Assay media with key substrates (typically glucose, pyruvate,
and glutamine) reduced in concentration and/or omitted (see Table 2).
Agilent XF Substrate Oxidation Stress Test Kits 17
Page 18

References
References
1 Divakaruni, A. S.; et al. 2018. 'Etomoxir Inhibits Macrophage Polarization by Disrupting CoA
2 Divakaruni, A. S.; et al. 2014. 'Analysis and interpretation of microplate-based oxygen
3 Hildyard, J. C.; et al. 2005. 'Identification and characterisation of a new class of highly specific
4 Robinson, Mary M.; et al. 2007. 'Novel mechanism of inhibition of rat kidney-type glutaminase
Homeostasis', Cell Metab, 28: 490-503.e7.
consumption and pH data', Methods Enzymol, 547: 309-54.
and potent inhibitors of the mitochondrial pyruvate carrier', Biochim Biophys Acta, 1707:
221-30.
by bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES)', Biochemical Journal,
406: 407-14.
18 Agilent XF Substrate Oxidation Stress Test Kits
Page 19

2 Kit Information
Kit Contents 20
Kit Shipping and Storage 21
Additional Agilent Products Required 21
Agilent XF Substrate Oxidation Stress Test Kits 19
Page 20

Kit Contents
Kit Contents
Products relevant to the Seahorse XF Substrate Oxidation Stress Tests include four Kits. Each kit
contains reagents sufficient for three complete XF Substrate Oxidation Stress Tests in either a
96- or 24-well Agilent Seahorse XF Cell Culture Microplate. The contents or components for each
kit are different, depending on the substrate being examined, and are listed in Table 3.
Table 3 Agilent Seahorse XF Substrate Oxidation Stress Test Kits contents are shown per pouch,
Compound Cap color Qty per vial
Agilent Seahorse XF Long Chain Fatty Acid Oxidation Stress Test kit contents (p/n 103672-100)
Etomoxir Green 112 nmol
Oligomycin Blue 63 nmol
FCCP Yellow 72 nmol
Rotenone/Antimycin A Red 27 nmol each
Agilent Seahorse XF Glucose/Pyruvate Oxidation Stress Test kit contents (p/n 103673-100)
UK5009 Orange 56 nmol
Oligomycin Blue 63 nmol
three (3) pouches or vials (Palmitate and BSA control) per kit.
FCCP Yellow 72 nmol
Rotenone/Antimycin A Red 27 nmol each
Agilent Seahorse XF Glutamine Oxidation Stress Test kit contents (p/n 103674-100)
BPTES Grey 84 nmol
Oligomycin Blue 63 nmol
FCCP Yellow 72 nmol
Rotenone/Antimycin A Red 27 nmol each
Agilent Seahorse XF Palmitate Oxidation Stress Test kit contents (p/n 103693-100)
Etomoxir Green 112 nmol
Oligomycin Blue 63 nmol
FCCP Yellow 72 nmol
Rotenone/Antimycin A Red 27 nmol each
XF Palmitate-BSA - 2 mL
XF BSA control - 2 mL
Seahorse L-carnitine Brown 10 mg
20 Agilent XF Substrate Oxidation Stress Test Kits
Page 21

Kit Shipping and Storage
Kit Shipping and Storage
Products are shipped at ambient temperature, and can be stored at room temperature, except for
XF Palmitate BSA FAO substrate. The XF Palmitate BSA FAO substrate is shipped with ice packs,
and should be stored at -20 °C upon arrival. All kits are stable for one year from the date of
manufacture, while XF L-carnitine is stable for two years from the date of manufacture. The kit
expiration date is printed on the label of the kit box. Depending on the shipping date, the actual
shelf life of the kit in the user's hand can vary between 3 and 12 months.
Additional Agilent Products Required
The following products are also required for performing the Seahorse XF Substrate Oxidation
Stress Tests but not supplied with the kits. For a complete list of materials required to perform an
XF assay, please visit the Agilent Cell Analysis Assay Learning Center website.
Table 4 Additional required items.
Item Vendor Part number
Seahorse XF96, XF24, XFe96 or XFe24 Analyzer Agilent Technologies
Seahorse XF DMEM Medium, pH 7.4
Seahorse XF RPMI Medium, pH 7.4
Seahorse XF FluxPak for the Analyzer being used Agilent Technologies Various
Seahorse XF 1.0 M Glucose solution Agilent Technologies 103577-100
Seahorse XF 100 mM Pyruvate solution
Seahorse XF 200 mM Glutamine solution
* XF DMEM or RPMI media can also be purchased together with the supplements listed in this table as bundled products
(p/n 103680-100 and 103681-100). For a full list of all medium types and our recommendation for each assay kit, please refer
to the Seahorse XF Media Selection Guide.
† Not required when performing the Seahorse XF Palmitate Oxidation Stress Test kit (p/n 103693-100).
*
or
*
†
†
Agilent Technologies 103575-100
Agilent Technologies 103576-100
Agilent Technologies 103578-100
Agilent Technologies 103579-100
Agilent XF Substrate Oxidation Stress Test Kits 21
Page 22

Additional Agilent Products Required
22 Agilent XF Substrate Oxidation Stress Test Kits
Page 23

3 Assay Workflow for Standard Substrate
Standard XF Substrate Oxidation Stress Test
XF Long Chain Fatt y Acid Oxidation Stress Test Kit (p/n 103672-10 0)
XF Glucose/Pyru vate Oxidation S tress T est Kit (p/n 103673-10 0)
XF Glutamine Oxidation Str ess Test Kit. (p/n 103674-100)
and/or
and/or
NOTE
Oxidation Stress Test
One Day Prior to Assay 24
Day of Assay 24
Data Analysis Using Agilent Seahorse Analytics 30
Examples of Data 32
Figure 8. Agilent Seahorse XF Substrate Oxidation Stress Test-Standard Assay Workflow.
Optimal cell seeding density and FCCP concentration should be empirically determined for
your cell type prior to the assay. For more details, please refer to the Basic Procedures on
Agilent Cell Analysis Learning Center website.
The Cell Line Reference Database is an excellent resource for finding information regarding the
cell type of interest.
Agilent XF Substrate Oxidation Stress Test Kits 23
Page 24

One Day Prior to Assay
NOTE
One Day Prior to Assay
1 Turn on the Seahorse XFe/XF96 or XFe/XF24 Analyzer, and let it warm up overnight to allow
the temperature to stabilize (minimum five hours).
2 Hydrate a sensor cartridge in sterile or distilled water at 37 °C in a non-CO
overnight. For more information, refer to the Basic Procedure, “Hydrating the sensor cartridge”,
on the Agilent Cell Analysis Learning Center website.
3 For adherent cells, plate cells at a pre-determined density in the Seahorse XF Microplate using
the appropriate cell culture growth medium. Refer to the Agilent Cell Analysis Learning
Center website for details.
For suspension cells, see section: Day of Assay/Prepare Seahorse XF Cell Culture
Microplate for Assay.
4 Create an Assay Template file with Wave Desktop software using the Standard Substrate
Oxidation Stress Test Assay Template. Make any necessary group modifications to the
template for your specific assay design.
Day of Assay
Complete hydration of sensor cartridge by removing water, adding the appropriate volume of
XF calibrant to each well, and placing in a 37 °C non-CO
Prepare assay media
1 Prepare Standard Substrate Oxidation Stress Test assay media by supplementing 97 mL of
Seahorse XF DMEM Medium, pH 7.4 with 1.0 mL each of XF glucose, XF pyruvate, and
XF glutamine (10 mM, 1 mM, and 2 mM final in assay media, respectively). These are the
recommended initial conditions. However, the desired assay medium composition can be
modified if needed.
2
incubator for 60 minutes.
2
incubator
Table 5 Standard Substrate Oxidation Stress Test Assay Media.
Component Volume Final concentration
Agilent Seahorse XF DMEM or RPMI Medium, pH 7.4 97 mL -
XF 1.0 M Glucose Solution 1.0 mL 10 mM
XF 100 mM Pyruvate Solution 1.0 mL 1 mM
XF 200 mM Glutamine Solution 1.0 mL 2 mM
2 Warm media to 37 °C until it is ready to use.
No pH adjustment to the assay media is necessary when recommended supplement
concentrations are used.
24 Agilent XF Substrate Oxidation Stress Test Kits
Page 25

Prepare Seahorse XF Cell Culture Microplate for assay
CAUTION
Prepare Seahorse XF Cell Culture Microplate for assay
For adherent cells
1 Remove cell culture microplate from 37 °C cell culture incubator and examine cells under
microscope to confirm consistent plating and proper cell morphology.
2 Remove the growth medium from the cell culture microplate. Wash once with warmed assay
medium. Add assay medium to a final volume of 180 µL/well for 96-well plates and
500 µL/well for 24-well plates. Refer to the Agilent Cell Analysis Learning Center for more
details.
3 Incubate cell plates with assay medium at 37 °C in a non-CO
prior to the assay.
4 Before starting the XF assay, remove the assay medium AGAIN and add fresh, warm assay
medium to each well. Total volume should be 180 µL/well for 96-well plates and 500 µL/well
for 24-well plates.
incubator for 45 to 60 minutes
2
For suspension cells
1 Pellet cells out of their growth medium and resuspend in warm assay medium.
2 Count cells and suspend at a concentration such that seeding 50 µL (XF96/XFe96) or
100 µL (XFe24) of cells contains the desired cell number per well, leaving four wells without
cells as background correction wells.
3 Add desired cells/well, then centrifuge gently to adhere.
4 Gently add assay medium to each well. Total well volume should be 180 µL/well for 96-well
plates and 500 µL/well for 24-well plates.
5 Incubate the plate at 37 °C in a non-CO
Refer to the Agilent Cell Analysis Learning Center for more information on preparation of
suspension cells for XF assays.
incubator for 45 to 60 minutes prior to the assay.
2
Prepare compound stock solutions and working solutions
Agilent XF Substrate Oxidation Stress Test Kits 25
Use compounds on the same day they are reconstituted. Do not freeze and reuse. Discard any
remaining compound solutions.
1 Remove one foil pouch and the decapper from the kit box.
2 Open pouch and remove all four vials: Oligomycin (blue cap), FCCP (yellow cap),
Rotenone/Antimycin A (red cap), and the inhibitor vial (specific to each kit; either etomoxir
(green cap), UK5099 (orange cap), or BPTES (grey cap)).
3 Tap down the vials to ensure powder is on the bottom of the vial before removing the vial cap
using the decapper provided with the kits.
Page 26

Prepare compound stock solutions and working solutions
4 Resuspend the content in each vial with the appropriate volume of prepared assay medium as
described in Table 6. Vortex ~1 minute to ensure full resuspension of compounds. These are
compound stock solutions.
Table 6 Stock solutions.
Compound
Volume of assay
medium
Stock concentration
Etomoxir from XF Long Chain Fatty Acid Oxidation Stress Test kit or
UK5099 from XF Glucose/Pyruvate Oxidation Stress Test kit or
BPTES from XF Glutamine Oxidation Stress Test kit
Oligomycin 420 µL 150 µM
FCCP 720 µL 100 µM
Rotenone/Antimycin A 540 µL 50 µM
700 µL 160 µM
700 µL 80 µM
700 µL 120 µM
5 Using the compound stock solutions to prepare working solutions for loading into the injection
ports on sensor cartridges.
6 Prepare 2 or 3 mL working solutions for each compound in assay medium as indicated in
Table 7 for XF/XFe96 sensor cartridges and Table 8 for XF/XFe24 sensor cartridges. It is
recommended to use 1.5 µM of oligomycin and 0.5 µM rotenone/antimycin A (final
concentration) for most cells. Optimal FCCP concentration should be determined prior to the
assay.
Table 7 Compound preparation for loading to XFe/XF96 sensor cartridges. Starting assay
medium volume for cell plate is 180 µL per well.
Final well
µM
Stock
solution
volume (µL)
Medium
volume (µL)
10× (Port)
µM
Volume
added to port
(µL)
Port A: Etomoxir or
UK5009 or
BPTES
Port B: Oligomycin 1.5 300 2,700 15 22
Port C: FCCP 0.25 75 2,925 2.5 25
Port D: Rotenone/Antimycin A 0.5 300 2,700 5 27
4 500 1,500 40 20
2 500 1,500 20 20
3 500 1,500 30 20
0.5 150 2,850 5 25
1.0 300 2,700 10 25
2.0 600 2,400 20 25
26 Agilent XF Substrate Oxidation Stress Test Kits
Page 27

Load the injection ports on sensor cartridge
Table 8 Compound preparation for loading to XFe/XF24 sensor cartridges. Starting assay
medium volume for cell plate is 500 µL per well.
Stock
Final well
µM
Port A: Etomoxir or
UK5009 or
BPTES
Port B: Oligomycin 1.5 300 2,700 15 62
Port C: FCCP 0.25 75 2,925 2.5 69
Port D: Rotenone/Antimycin A 0.5 300 2,700 5 75
4 500 1,500 40 56
2 500 1,500 20 56
3 500 1,500 30 56
0.5 150 2,850 5 69
1.0 300 2,700 10 69
2.0 600 2,400 20 69
solution
volume (µL)
Medium
volume (µL)
10× (Port)
µM
Volume
added to port
(µL)
Load the injection ports on sensor cartridge
Proper port loading techniques can be found in Basic Procedure “Loading the Sensor Cartridge
with Compounds”, on the Agilent Cell Analysis Learning Center website.
Please read the information prior to loading compounds. Ensure that the sensor cartridge is
properly hydrated prior to use.
For the location of the ports, please refer to Figure 9.
Figure 9. Layout of injection ports on sensor cartridges for XFe96, XF96, and XFe24 sensor cartridges.
Note the XF24 sensor cartridges have a different layout. Please consult the XF Assay
Learning Center for XF24 injection port layout.
Agilent XF Substrate Oxidation Stress Test Kits 27
Page 28

Load template onto the Seahorse XFe Analyzer
NOTE
Refer to Tab l e 9 for loading volume and port designation for compounds in different types of
assays.
Table 9 Recommended injection volumes for Substrate Oxidation Stress Test injection schemes.
Starting assay medium volume is 180 µL per well for 96-well cell plates and 500 µL per
well for 24-well cell plates.
Port Compound Port concentration 96 well 24 well
A Inhibitor (etomoxir, UK5099, or BPTES)
B Oligomycin 10× 22 µL 62 µL
C FCCP 10× 25 µL 69 µL
D Rotenone/Antimycin A 10× 27 µL 75 µL
* For controls, assay medium should be used in port A instead of inhibitors.
Load template onto the Seahorse XFe Analyzer
Add to port volume
*
10× 20 µL 56 µL
If templates(s) are already present on the XFe analyzer, skip this step.
Personal computer (internet access required)
1 Use the hyperlink below to download the Seahorse XF Substrate Oxidation Stress Test -
Standard Template (for Long Chain Fatty Acid, and/or Glucose/Pyruvate, and/or Glutamine
Oxidation Stress Tests).
www.agilent.com/cs/library/software/public/software-agilent-seahorse-xf-subox-assay-te
mplate-files-cell-analysis-agilent.zip
2 Unzip the folder, then copy and paste the assay template files to a USB drive or network drive
(if Seahorse XFe Analyzer is networked).
Seahorse XFe96/XFe24 Analyzer
1 Insert USB drive in front USB port and wait ~10 seconds.
2 Click Import (bottom of the New Assay view).
3 Locate the Assay Template file to import on the USB or network drive.
4 Click Open in the Windows dialogue box. The imported Assay template will be available for
selection from the Templates view on the XFe Analyzer.
5 Repeat for next template, if applicable.
6 The imported Assay template(s) will now be available for selection in the list of available
templates.
Running the XF substrate oxidation stress test assay
1 Select the Seahorse XF Substrate Oxidation Stress Test-Standard template from the list of
available templates and click Open File (or double-click the template).
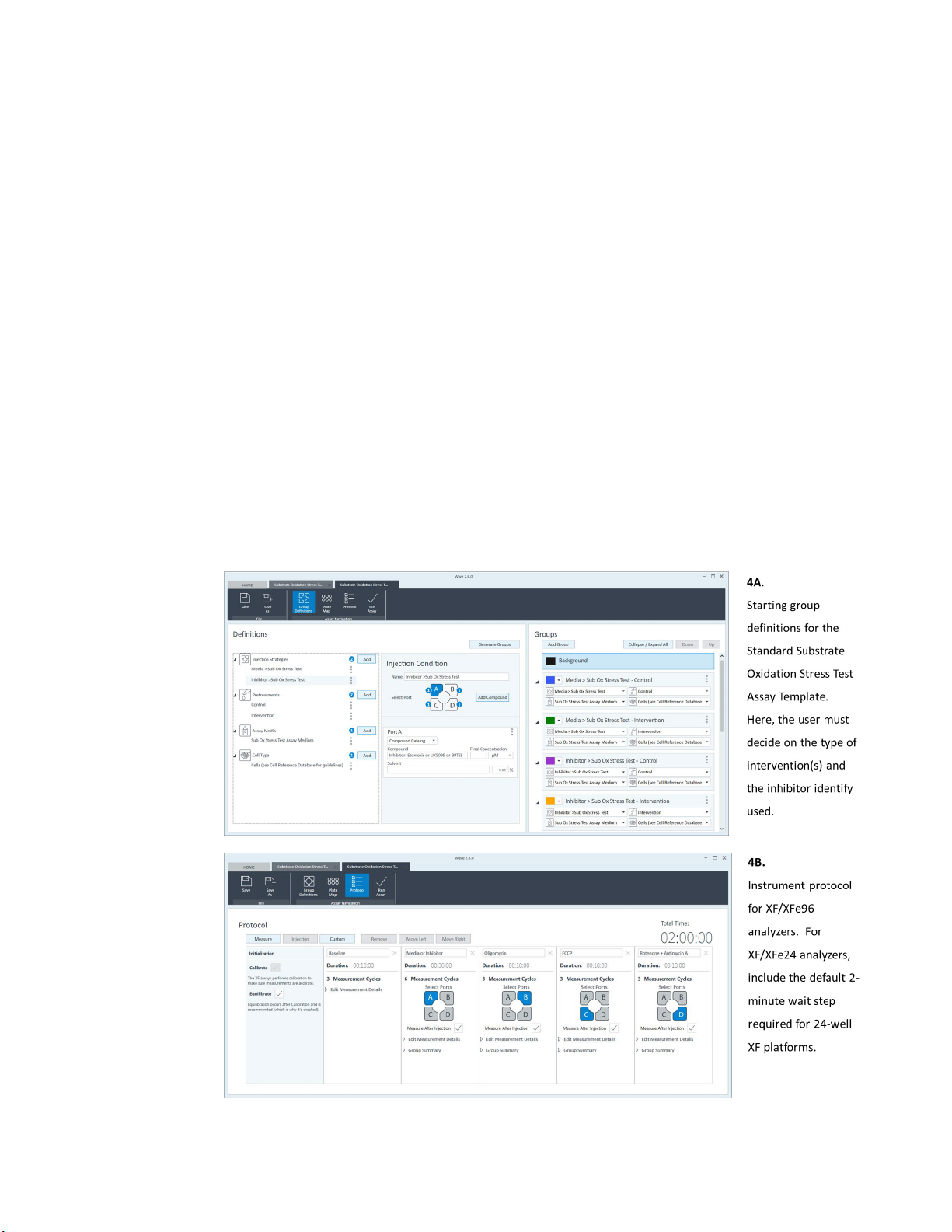
2 Group Definitions: confirm or modify the default groups and conditions for your assay.
3 Plate Map: confirm or modify the plate layout map for your assay.
28 Agilent XF Substrate Oxidation Stress Test Kits
Page 29

Running the XF substrate oxidation stress test assay
4 Protocol: No action required - confirm or modify the Instrument Protocol for additional
measurements cycles during the assay.
5 Run Assay: Click Start Run when ready.
6 When prompted, remove the cartridge lid and place the loaded sensor cartridge with the utility
plate on the thermal tray of the Seahorse XFe Analyzer. Ensure correct plate orientation and
the cartridge lid has been removed. Then, click I'm Ready. Calibration takes approximately
15 to 30 minutes.
7 After completing Calibration, Wave Controller will display the Load Cell Plate dialog. Click
Open Tray to eject Utility Plate and load the Cell Plate. Ensure the lid is removed from Cell Plate
before Loading.
8 Click Load Cell Plate to run the assay.
Agilent XF Substrate Oxidation Stress Test Kits 29
Page 30

Data Analysis Using Agilent Seahorse Analytics
Data Analysis Using Agilent Seahorse Analytics
Agilent Seahorse Analytics is a new, web-based software platform that provides a simple,
streamlined data analysis workflow for the XF Substrate Oxidation Stress Test assay. Seahorse
Analytics automatically calculates the Substrate Oxidation Stress Test Parameters: basal
respiration, acute response to inhibitor, and maximal respiration. Visit
https://seahorseanalytics.agilent.com to register or log in to your Seahorse Analytics account.
1 After the assay is completed, transfer your assay result file to your personal computer using a
USB drive or network drive.
2 Go to https://seahorseanalytics.agilent.com to register or log in to your Seahorse Analytics
account.
30 Agilent XF Substrate Oxidation Stress Test Kits
Page 31

Data Analysis Using Agilent Seahorse Analytics
3 Import the assay result file to your account.
4 Open the assay result file, and select the XF Substrate Oxidation Stress Test analysis view
found under the Assay Kit Companion views menu.
5 Select groups to add to the analysis view, then click Add View.
Agilent XF Substrate Oxidation Stress Test Kits 31
Page 32

Examples of Data
Examples of Data
Figure 10 shows example Standard Substrate Oxidation Assay data from Seahorse Analytics for
A549 cells.
Figure 10. Standard Substrate Oxidation Assay data derived from Agilent Seahorse Analytics for
A549 cells.
32 Agilent XF Substrate Oxidation Stress Test Kits
Page 33

Examples of Data
Figure 11 shows example data from experiments with A549 (Panel A), C2C12 (Panel B), and
HepG2 cells (Panel C).
Control
Etomoxir (LCFAs)
UK5099 (Glucose/Pyruvate)
AB C
A549 Mitochondrial respiration
10
Inhibitor Oligo FCCP Rot/AA
9
8
7
6
5
4
3
2
1
OCR (pmol/min/1,000 cells)
0
0 24487296120
Time (min)
C2C12 Mitochondrial respiration
18
Inhibitor Oligo FCCP Rot/AA
15
12
9
6
3
OCR (pmol/min/1,000 cells)
0
0 24 48 72 96 120
Time (min)
30
25
20
15
10
5
OCR (pmol/min/1,000 cells)
0
0 24 48 72 96 120
BPTES (Glutamine)
HepG2 Mitochondrial respiration
Inhibitor Oligo FCCP Rot/AA
Time (min)
A549 Basal (rate 3)
8
7
6
5
4
3
2
1
OCR (pmol/min/1,000 cells)
0
A549 Acute response (rate 9)
1
0
-1
-2
-3
-4
-5
-6
∆OCR (pmol/min/1,000 cells)
-7
0.5
0
-0.5
-1.0
-1.5
A549 Maximal respiration (rate 14)
8
7
6
5
4
3
2
1
OCR (pmol/min/1,000 cells)
0
C2C12 Basal (rate 3)
16
14
12
10
8
6
4
2
OCR (pmol/min/1,000 cells)
0
C2C12 Acute response (rate 9)
1
-3
0.2
-7
0
-0.2
-0.4
-11
∆OCR (pmol/min/1,000 cells)
-15
-0.6
-0.8
-1.0
C2C12 Maximal respiration (rate 14)
16
14
12
10
8
6
4
2
OCR (pmol/min/1,000 cells)
0
HepG2 Basal (rate 3)
24
20
16
12
8
4
OCR (pmol/min/1,000 cells)
0
HepG2 Acute response (rate 9)
2
-2
-6
-10
-14
-18
∆OCR (pmol/min/1,000 cells)
-22
1
0
-1
-2
-3
-4
HepG2 Maximal respiration (rate 15)
24
20
16
12
8
4
OCR (pmol/min/1,000 cells)
0
Figure 11. Comparing substrate oxidation in A549, C2C12 and HepG2 cells using XF Glucose Pyruvate,
Long Chain Fatty Acid, and Glutamine Oxidation Stress Tests. A549 (A), C2C12 (B), and
HepG2 (C) cells were seeded in XF96 Cell Culture Plates and grown overnight. Cells were
subject to XF Substrates Oxidation Stress Tests using injections of assay media (control),
etomoxir (LCFA oxidation), UK5099 (glucose/pyruvate oxidation) or BPTES (Glutamine
oxidation), followed by common sequential injections of oligomycin, FCCP, and Rtn/AA. XF
Assay Media = XF DMEM, pH 7.4 supplemented with 10 mM glucose, 1 mM pyruvate and
2 mM glutamine. Each cell type was assayed on three individual days, with result data being
compiled and processed through Seahorse Analytics. Error is reported as ± SEM. Similar
results for all cell types tested were obtained using an XFe24 Analyzer (data not shown).
Agilent XF Substrate Oxidation Stress Test Kits 33
Page 34

Examples of Data
Inspection of the Basal Respiration parameter for each cell type reveals the expected results with
all conditions showing identical basal OCRs before inhibitors are added. Upon injection of inhibitor,
each cell type displays a detectable, but relatively small, acute response with respect to respiration
under basal assay conditions. However, under conditions of maximal respiration (such as under
conditions of higher substrate demand by the mitochondria), responses to inhibitors are
significantly increased, and different responses to different inhibitors become apparent across the
different cell types tested. Please see the Agilent application note: Revealing Cellular Metabolic
Phenotype and Function Using Agilent XF Substrate Oxidation Stress Tests for further discussion
regarding interpretation of substrate demand for each cell type, as well as suggestions for
Standard Substrate Oxidation Stress Test experimental design in the context of cancer cell and
immune cell biology and drug discovery.
34 Agilent XF Substrate Oxidation Stress Test Kits
Page 35

4 Assay Workflow for Palmitate
Advanced XF Palmitate Oxidation Stress Test Method
XF Palmitate Oxidation Stress Test Kit (p/n 103693-100)
Change to
Substrate Limited
CultureMedia
Seed cells in
Culture Media
Design assay
Hydrate
cartridge
Change cells to
assay medium
Run assay,
analyze results
Prepare assay
components
y
s
One day prior to assay
Two days prior to assay
Day of assay
Oxidation Stress Test - Advanced
Assay
Two Days Prior to Assay 36
One Day Prior to Assay 36
Day of Assay 37
Data Analysis Using Agilent Seahorse Analytics 42
Examples of Data 44
Figure 12. Agilent Seahorse XF Palmitate Oxidation Stress Test Workflow - Advanced Assay workflow.
onent
com
Prepare assa
Agilent XF Substrate Oxidation Stress Test Kits 35
Page 36

Two Days Prior to Assay
Two Days Prior to Assay
For adherent cells, plate cells at a predetermined density in the Seahorse XF Tissue Culture plate
using the appropriate cell culture growth medium. Refer to the Agilent Cell Analysis Learning
Center website for details.
One Day Prior to Assay
Prepare substrate-limited growth media
1 Remove one foil pouch from Agilent Seahorse L-Carnitine box. Open pouch and remove the
L-carnitine vial. Bring to a cell culture hood.
2 Add 100 µL sterile, tissue culture-grade water to the L-carnitine vial. The resulting stock
concentration is 0.5 M.
3 Aliquot 18.5 mL unsupplemented growth media (without glucose, pyruvate, glutamine, or
GlutaMAX) to a sterile 50 mL conical tube in a cell culture hood.
4 Supplement with 0.5 mM glucose, 1 mM glutamine or GlutaMAX, and 1% fetal bovine serum
(final concentrations). Also add 20 µL of carnitine prepared in Step 2. This is the
substrate-limited growth media with a final carnitine concentration of 0.5 mM.
5 Place the remainder of carnitine stock solution at 4 °C after use. This carnitine solution will
also be used to supplement the assay media the next day.
Prepare cell plates
1 For both adherent cells or suspension cells, replace the cell growth media with
substrate-limited growth media. It is recommended to wash the cell plate one time with either
PBS or substrate-limited growth media to ensure the complete removal of residual growth
media.
2 Add 100 µL of substrate-limited growth media in each well for 96-well plates and 250 µL in
each well for 24-well plates.
3 Place the cell plates back to 37 °C cell culture incubator.
Other preparation
1 Turn on the Seahorse XFe/XF96 or XFe/XF24 Analyzer and let it warm up overnight to allow
the temperature to stabilize (minimum five hours).
2 Hydrate a sensor cartridge in sterile or distilled water at 37 °C in a non-CO
overnight. For more information, refer to the Basic Procedure, “Hydrating the sensor cartridge”,
on the Agilent Cell Analysis Learning Center website.
incubator
2
3 Create an Assay Template file with Wave Desktop software using the Palmitate Oxidation
Stress Test Advanced Assay Template. Make any necessary group modifications to the
template for your specific assay design.
36 Agilent XF Substrate Oxidation Stress Test Kits
Page 37

Day of Assay
NOTE
Day of Assay
Complete Hydration of sensor cartridge: remove water and add the appropriate volume of XF
calibrant to each well and replace in a 37 °C non-CO
Prepare assay media
1 Prepare Advanced Palmitate Oxidation Stress Test assay media by supplementing 75 mL of
Seahorse XF DMEM or RPMI Medium, pH 7.4 with 2 mM of XF glucose and 0.5 mM L-carnitine
(no pyruvate or glutamine). This is the recommended initial condition for glucose
concentration and can be modified if needed.
Table 10 Advanced Palmitate Oxidation Stress Test Assay Media.
Component Volume Final concentration
Agilent Seahorse XF DMEM or RPMI Medium, pH 7.4 75 mL -
XF Glucose 150 µL 2.0 mM
Seahorse L-Carnitine 75 µL 0.5 mM
incubator for 60 minutes.
2
2 Warm up at 37 °C until ready to use. (Palmitate-BSA substrate will be added immediately
before the assay.)
No pH-adjustment to the assay medium is necessary when recommended supplement
concentrations are used. Also, remove Palmitate-BSA and BSA control from the freezer. Warm
to 37 °C before use.
Prepare Seahorse XF Cell Culture Microplate for assay
For Adherent Cells
1 Remove cell culture microplate from 37 °C CO2 incubator and examine cells under
microscope to confirm consistent plating and proper cell morphology.
2 Remove the substrate-limited growth medium from the cell culture microplate. Wash once
with warmed assay medium using. Add assay medium to a final volume of 180 µL/well for
96-well plates and 500 µL/well for 24-well plates. Refer to the Agilent Cell Analysis Learning
Center for more details.
3 Incubate cell plates with substrate limited assay medium at 37 °C in a non-CO
45 to 60 minutes prior to the assay.
4 Before starting the XF assay, remove the assay medium from cell plates again and add fresh,
warm assay medium to each well to a volume of 150 µL for 96-well plates and 415 µL for
24-well plates.
incubator for
2
5 Finally, just prior to starting the assay, add 30 µL of 1X Palmitate-BSA or 30 µL of 1X BSA
control to the appropriate wells in 96-well plates. Or add 85 µL of 1X Palmitate-BSA or 85 µL of
1X BSA control to the appropriate wells in 24-well plates. The total volumes before the assay
are 180 µL/well for 96-well plates and 500 µL/well for 24-well plates.
Agilent XF Substrate Oxidation Stress Test Kits 37
Page 38

Prepare compound stock solutions and working solutions
CAUTION
For Suspension Cells
1 Pellet cells out of their growth medium and resuspend in warm assay medium.
2 Count cells and suspend at a concentration such that seeding 50 µL (XF96/XFe96) or
100 µL (XFe24) of cells contains the desired cell number per well, leaving four wells without
cells as background correction wells.
3 Add desired cells/well then centrifuge gently to adhere.
4 Gently add assay medium to each well: 100 uL for XF96 well plates and 315 µL for XF24 well
plate. Total well volume should now be 150 µL for XF96 well plates and 415 µL for XF24 well
plates.
5 Incubate the plate at 37 °C in a non-CO
incubator for 45 to 60 minutes prior to the assay.
2
6 Finally, just prior to starting the assay, add 30 µL of 1X Palmitate-BSA or 30 µL of 1X BSA
control to the appropriate wells in 96-well plates. Alternatively, add 85 µL of 1X Palmitate-BSA
or 85 µL of 1X BSA control to the appropriate wells in 24-well plates. The total volumes before
the assay are 180 µL/well for 96-well plates and 500 µL/well for 24-well plates.
Refer to the Agilent Cell Analysis Learning Center for details on preparation of suspension cells for
XF assays.
Prepare compound stock solutions and working solutions
Use compounds on the same day they are reconstituted. Do not freeze and reuse. Discard any
remaining compound solutions.
1 Remove one foil pouch and decapper from the XF Oxidation Stress Test kit box.
2 Open the pouch and remove all four vials: Oligomycin (blue cap), FCCP (yellow cap),
Rotenone/Antimycin A (red cap) and Etomoxir (green cap).
3 Tap down the vials to ensure powder is on the bottom of the vial before removing the vial cap
using the decapper provided with the kits.
4 Resuspend the content in each vial with the appropriate volume of prepared assay medium as
described in Table 11. Vortex ~one minute to ensure full resuspension of compounds. These
are compound stock solutions.
38 Agilent XF Substrate Oxidation Stress Test Kits
Table 11 Stock solutions.
Compounds from XF Long Chain Fatty Acid Oxidation
Stress Test kit box
Etomoxir 700 µL 160 µM
Oligomycin 420 µL 150 µM
FCCP 720 µL 100 µM
Rotenone/Antimycin A 540 µL 50 µM
Volume of assay
medium
Stock concentration
5 Use the compound stock solutions to prepare working solutions for loading into the injection
ports on sensor cartridges.
Page 39

Load the injection ports on sensor cartridge
6 Prepare 2 or 3 mL working solutions for each compound in Palmitate Oxidation Stress Test
assay medium, using the volumes indicated in Tabl e 12 for XF/XFe96 sensor cartridges and
Table 13 for XF/XFe24 sensor cartridges. It is recommended to use 1.5 µM of oligomycin and
0.5 µM Rotenone/Antimycin A (final concentration) for most cells. Optimal FCCP
concentration should be determined prior to the assay.
Table 12 Compound preparation for loading to XFe/XF96 sensor cartridges. Starting assay
medium volume for cell plate is 180 L per well.
Stock
Final well
µM
Port A: Etomoxir 4 500 1,500 40 20
Port B: Oligomycin 1.5 300 2,700 15 22
Port C: FCCP 0.25 75 2,925 2.5 25
0.5 150 2,850 5 25
1.0 300 2,700 10 25
2.0 600 2,400 20 25
Port D: Rotenone/Antimycin A 0.5 300 2,700 5 27
solution
volume (µL)
Medium
volume (µL)
10× (Port)
µM
Volume
added to
port (µL)
Table 13 Compound preparation for loading to XFe/XF24 sensor cartridges. Starting assay
medium volume for cell plate is 500 L per well.
Stock
Final well
µM
Port A: Etomoxir 4 500 1,500 40 56
Port B: Oligomycin 1.5 300 2,700 15 62
Port C: FCCP 0.25 75 2,925 2.5 69
solution
volume (µL)
Medium
volume (µL)
10× (Port)
µM
Volume
added to
port (µL)
0.5 150 2,850 5 69
1.0 300 2,700 10 69
2.0 600 2,400 20 69
Port D: Rotenone/Antimycin A 0.5 300 2,700 5 75
Load the injection ports on sensor cartridge
Proper port-loading techniques can be found on the Agilent Cell Analysis Learning Center
website.
Please read the information prior to loading compounds. Ensure that the sensor cartridge is
properly hydrated prior to use.
For the location of the ports, please refer to Figure 9 on page 27.
Refer to Tab l e 14 for loading volume and port designation for compounds in different types of
assays.
Agilent XF Substrate Oxidation Stress Test Kits 39
Page 40

Load template onto the Seahorse XFe Analyzer
NOTE
Table 14 Recommended injection volumes for Substrate Oxidation Stress Test injection schemes.
Starting assay medium volume is 180 µL per well for 96-well cell plates and 500 µL per
well for 24-well cell plates.
Port Compound Port concentration 96 well 24 well
AEtomoxir
B Oligomycin 10× 22 µL 62 µL
C FCCP 10× 25 µL 69 µL
D Rotenone/Antimycin A 10× 27 µL 75 µL
* For controls, assay medium should be used in port A instead of inhibitors.
*
Load template onto the Seahorse XFe Analyzer
If templates(s) are already present on the XFe analyzer, skip this step.
Add to port volume
10× 20 µL 56 µL
Personal Computer (internet access required)
1 Use the hyperlink below to download the Seahorse XF Substrate Oxidation Stress Test -
Advanced Template (for Palmitate Oxidation Stress Tests).
www.agilent.com/cs/library/software/public/software-agilent-seahorse-xf-subox-assay-te
mplate-files-cell-analysis-agilent.zip
2 Unzip the folder, then copy and paste the assay template files to a USB drive or network drive
(if Seahorse XFe Analyzer is networked).
Seahorse XFe96/XFe24 Analyzer
1 Insert a USB drive in the front USB port, and wait ~10 seconds.
2 Click Import (bottom of the New Assay view).
3 Locate the Assay Template file to import on the USB or network drive.
4 Click Open in the Windows dialogue box. The imported Assay Template will be available for
selection from the Templates view on the XFe Analyzer.
5 Repeat for next template, if applicable.
6 The imported Assay Template(s) will now be available for selection in the list of available
templates.
Running the XF Palmitate Oxidation Stress Test assay
1 Select the Seahorse XF Palmitate Oxidation Stress Test-Advanced template from the list of
available templates, and click Open File (or double-click the template).
2 Group Definitions: confirm or modify the default groups and conditions for your assay.
3 Plate Map: confirm or modify the plate layout map for your assay.
4 Protocol: No action required - confirm or modify the Instrument Protocol for additional
measurements cycles during the assay.
40 Agilent XF Substrate Oxidation Stress Test Kits
Page 41

Running the XF Palmitate Oxidation Stress Test assay
5 Run Assay: Click Start Run when ready.
6 When prompted, remove the cartridge lid, and place the loaded sensor cartridge with the utility
plate on the thermal tray of the Seahorse XFe Analyzer. Ensure correct plate orientation and
the cartridge lid has been removed. Then, click I'm Ready. Calibration takes approximately
15 to 30 minutes.
7 After completing Calibration, Wave Controller will display the Load Cell Plate dialog. Click
Open Tray to eject Utility Plate and load the Cell Plate. Ensure the lid is removed from Cell Plate
before Loading.
8 Click Load Cell Plate to run the assay.
Agilent XF Substrate Oxidation Stress Test Kits 41
Page 42

Data Analysis Using Agilent Seahorse Analytics
Data Analysis Using Agilent Seahorse Analytics
Agilent Seahorse Analytics is a new, web-based software platform that provides a simple,
streamlined data analysis workflow for the XF Substrate Oxidation Stress Test assay. Seahorse
Analytics automatically calculates the XF Palmitate Oxidation Stress Test parameters: basal
respiration, acute response to inhibitor, and maximal respiration. Visit
https://seahorseanalytics.agilent.com to register or log-in to your Seahorse Analytics account.
1 After the assay is completed, transfer your assay result file to your personal computer using a
USB drive or network drive.
2 Go to https://seahorseanalytics.agilent.com to register or log in to your Seahorse Analytics
account.
42 Agilent XF Substrate Oxidation Stress Test Kits
Page 43

Data Analysis Using Agilent Seahorse Analytics
3 Import the assay result file to your account.
4 Open the assay result file and select the XF Substrate Oxidation Stress Test analysis view
found under the Assay Kit Companion views menu.
5 Select groups to add to the analysis view, then click Add View.
Agilent XF Substrate Oxidation Stress Test Kits 43
Page 44

Examples of Data
Examples of Data
Figure 13 shows example Advanced Palmitate Oxidation Assay data from Seahorse Analytics for
HepG2 cells.
Figure 13. Advanced Palmitate Oxidation Assay data derived from Seahorse Analytics for HepG2 cells.
44 Agilent XF Substrate Oxidation Stress Test Kits
Page 45

Examples of Data
0
1
2
3
4
OCR (pmol/min/1,000 cells)
0.0
0.5
1.0
1.5
2.0
2.5
OCR (pmol/min/1,000 cells)
Basal
0.0
0.5
1.0
1.5
2.0
2.5
OCR (pmol/min/1,000 cells)
Maximal respiration
-2.5
-2.0
-1.5
-1.0
-0.5
0.0
-0.2
-0.4
-0.6
-0.8
-1.0
0.0
-0.2
-0.4
-0.6
-0.8
-1.0
0.0
∆OCR (pmol/min/1,000 cells)
Acute response
A549 Mitochondrial respiration
Etomoxir Oligo FCCP Rot/AA
Time (min)
BSA control + Eto
BSA control – Eto
Palmitate + Eto
Palmitate – Eto
0 24487296120
0
1
2
3
4
5
6
7
8
OCR (pmol/min/1000 cells)
0
1
2
3
4
5
6
OCR (pmol/min/1000 cells)
Basal
OCR (pmol/min/1000 cells)
0
1
2
3
4
5
6
Maximal respiration
Etomoxir Oligo FCCP Rot/AA
HepG2 Mitochondrial respiration
Time (min)
0 24487296120
-6
-5
-4
-3
-2
-1
0
Acute response
∆OCR (pmol/min/1,000 cells)
Figure 14 shows example data from experiments with A549 (Panel A) and HepG2 cells (Panel B).
Agilent XF Substrate Oxidation Stress Test Kits 45
Figure 14. Palmitate oxidation in A549 and HepG2 cells using XF Palmitate Oxidation Stress Tests.
A549 (A) and HepG2 (B) cells were seeded in XF96 Cell Culture Plates and grown overnight in
appropriate cell growth media. The next day growth media was exchanged for substrate
limited media (Table 1) and cultured for an additional 16 to 24 hours. Cells were then subject
to XF Palmitate Oxidation Stress Tests using injections of assay media (control), or etomoxir
(LCFA oxidation), followed by common sequential injections of oligomycin, FCCP, and
Rtn/AA. XF Assay Media = XF DMEM, pH 7.4 + 2 mM glucose, + 0.5 mM L-carnitine. Each cell
type was assayed on three individual days, with result data being compiled and processed
through Seahorse Analytics. Error is reported as ± SEM. Similar results for both cell types
tested were obtained using an XFe24 Analyzer (data not shown).
Page 46

Examples of Data
Inspection of the Basal Respiration parameter for each cell type shows the expected results for
the given condition: the Palmitate groups show slightly increased OCR relative to BSA control
groups, which are sensitive to etomoxir, with inhibition decreasing rates to those observed for the
BSA control - Etomoxir groups (Rate 9, ~55 minutes). This indicates some palmitate is being
oxidized in the basal (lower substrate demand) state. However, upon addition of FCCP, substrate
demand is increased, and the Palmitate - Etomoxir groups show significant increases in maximal
respiration compared to the groups with either BSA and/or +etomoxir. This indicates that these
cell types not only have the ability to oxidize palmitate under the experimental conditions
established, but that palmitate is the bulk of the substrate oxidized when forced to respire at a
maximal rate (compare Palmitate ± Etomoxir groups). This data suggests that A549 and HepG2
cells are good candidate cell types for investigating how an intervention, such as genetic
manipulation or drug exposure, applied to the cell specifically affect the oxidation of long chain
fatty acids.
46 Agilent XF Substrate Oxidation Stress Test Kits
Page 47

5 Frequently Asked Questions
NOTE
Will these assays work for my cell type? Most likely. The basis of the Substrate Oxidation Stress
Tests is the XF Cell Mito Stress, which has been used successfully with numerous cell types,
including cell lines and primary cells. Please visit the Agilent Cell Analysis Publication Database
to search for cell types used in publications.
Will these assays work for suspension, primary and/or immune, cells? Yes. However, please
ensure proper experimental and assay procedures are followed for the cell type of interest.
Do I need to optimize cell seeding density? Yes. Please visit the XF Assay Learning Center and
the Agilent Cell Analysis Publication Database for further information on basic cell density
optimization procedures. Note that for the Advanced Palmitate Oxidation Stress Test, the cell
density seeded may need to be decreased if cells are to be subject to substrate limitation, as this
step requires an extra day of cell culture.
Do I need to optimize FCCP concentration? Yes. For the Standard Substrate Oxidation Stress
Tests, this will likely be the same FCCP concentration used if the cell type of interest has been
previously subject to a Cell Mito Stress Test.
For the Advanced Palmitate Oxidation Stress Test, the concentration of FCCP may need to be
increased due to the presence of BSA in the assay media. This should be performed before or
during your initial Advanced Palmitate Oxidation Stress Test. Please visit the XF Assay
Learning Center and the Agilent Cell Analysis Publication Database for further information on
basic FCCP optimization procedures.
How do I know the inhibitors are at the correct concentration? The optimal concentration of each
inhibitor is based on both testing at Agilent as well as information provided for etomoxir, UK5099
and BPTES in the following references, respectively: Divakaruni et al. 20181; Hildyard et al. 20052;
and Robinson et al. 20073.
What does it mean if there is no response to an inhibitor? Biologically, this suggests that the cell
has no demand or reliance on that particular substrate under the experimental conditions defined.
Note that it is common to see low to no responses to these inhibitors under basal respiration
conditions (such as conditions of lower substrate demand).
Should I increase the concentration of the inhibitor? No. Each inhibitor is optimized for the
correct final concentration: 4 µM for etomoxir, 2 µM for UK5099, and 3 µM for BPTES. Increased
concentrations of inhibitors, especially etomoxir, will result in off-target effects, including effects
on mitochondrial respiration.
What is the difference between the Long Chain Fatty Acid Oxidation Stress Test and the Palmitate
Oxidation Stress Test? The Long Chain Fatty Acid Oxidation Stress Test is one of the three
Standard Substrate Oxidation Stress Tests. This assay is focused on testing cellular demand of
endogenous LCFAs under conditions of saturating substrates with respect to glucose, pyruvate
and glutamine in the assay media, and best applied to answer questions about substrate demand
and reliance with regard to LCFAO.
Agilent XF Substrate Oxidation Stress Test Kits 47
Page 48

The Advanced Palmitate Oxidation Stress Test is designed to be performed under conditions of
saturating palmitate (as Palmitate-BSA) and L-carnitine, with limited to no substrate provision with
respect to glucose, pyruvate, and glutamine in the assay media. It is best applied when the
experimental design calls for the cells to exclusively oxidize a long chain fatty acid, such as
palmitate, to investigate effects of interventions specifically on the long chain fatty acid oxidation
process.
When should I use Standard Assay workflow? The standard substrate oxidation assay is
designed to be used when asking the following types of questions:
• Does the cell have a demand for a particular substrate or substrates?
• Is the cell highly reliant on a specific substrate, or can other substrates satisfy cellular
demands?
• How is mitochondrial substrate demand and/or reliance affected if an intervention, such as a
genetic manipulation or drug exposure, is applied to the cell?
What is the source of LCFAs for Standard LCFA assays? The source of long chain fatty acids are
any endogenous stores of lipid/LCFAs in the cells used, and thus is cell-type dependent.
Can/Should I add Palmitate-BSA (source of FAs) to Standard Substrate Oxidation Stress Test
assays? It is not recommended to add Palmitate-BSA to Standard Substrate Oxidation Stress
Tests, as previous investigation has shown that cells typically will not respond to exogenous
palmitate under conditions of fully supplemented cell culture media and subsequent saturating
concentrations of glucose, pyruvate and glutamine in the XF assay media. No enhanced response
to etomoxir has been demonstrated for cells supplemented with palmitate under these same
conditions. For specific investigation of palmitate oxidation by cells, the Advance Assay for
Palmitate Oxidation Stress Test is recommended
Can I alter the composition of the substrate oxidation stress test assay media (with regard to
substrates)? It is recommended to begin the investigation with suggested substrate
concentrations to establish cell behavior under saturating substrate concentrations. Final
concentrations of supplements of glucose, pyruvate and glutamine may be altered, but care
should be demonstrated when designing experiments and subsequent interpretation of data.
How is this different from the Seahorse XF Mito Fuel Flux Test? The XF Substrate Oxidation
Stress Tests combine substrate pathway specific with the XF Cell Mito Stress Test (MST). Basal
and maximal respiration rates are key metrics of mitochondrial function reported by the MST. In
the context of substrate oxidation, the basal, and in particular, the maximal respiration rates are
largely impacted by cells capacity to transport and oxidize available substrates
4
. This method is
therefore ideally suited, and thus different from the mFFT, in that assessment for cellular
substrate oxidation under conditions of both basal and elevated substrate demand (maximal
respiration) where critical substrate dependence/reliance is more often revealed.
Do the Seahorse XF Mito Fuel Flex Test (mFFT) parameters (dependency, flexibility, capacity) apply
here? No. The mFFT quantitative parameters do not apply to these assays. The well-recognized
and accepted MST parameters (basal respiration, acute response and maximal respiration) are
used for both Standard and Advanced Substrate Oxidation Stress Tests. Note, however, that
terms such as substrate demand and reliance are used here qualitatively to describe cellular and
mitochondrial function.
Can two (or more) inhibitors be injected at the same time? It is recommended to begin the
investigation with the use of a single inhibitor to establish cell behavior. Two or more inhibitors
can be applied at the same time to the same group of cells, but care should be taken when
designing experiments and subsequent interpretation of data.
48 Agilent XF Substrate Oxidation Stress Test Kits
Page 49

When should I use the Advanced Assay? This advanced assay is designed to be used when
asking the following type of question: How an intervention, such as genetic manipulation or drug
exposure, applied to the cell specifically affect the oxidation of long chain fatty acids?
What is the optimal nutrient deprivation media composition for my cell type (Advanced
Procedure)? It is recommended to empirically determine the optimal nutrient deprivation media
composition and time of exposure for the cell type of interest. Common parameters to test are
decreased glucose, pyruvate, serum, and GlutaMAX concentrations in the substrate-limited
growth media, as well as the length of time the cells are provided with this substrate-limited
growth media before performing the assay.
Is the Palmitate saturating in concentration? Yes. The concentration of palmitate is saturating
when used as recommended.
Can/Should I omit glucose from XF Palmitate Oxidation Stress Test assay media? The final
glucose concentration in the XF Palmitate Oxidation Stress Test assay media should be
empirically tested. Complete removal of glucose is not recommended as low concentrations of
glucose are required by the cell to ensure proper function of glycolysis and the TCA cycle, which
are interrelated to and can impact optimal oxidation of palmitate.
Can the Advanced Assay be applied to glucose/pyruvate and/or glutamine oxidation? Currently,
the Advanced Assay method is validated only for use with the Palmitate Oxidation Stress Test Kit.
While it is possible to apply the Advanced Assay method to specifically investigate
glucose/pyruvate, and/or glutamine oxidation in the absence of other substrates, the potential
interference between glucose and glutamine demand can be substantial when lowering either or
both to sub-saturating levels in the substrate-limited growth and/or substrate-limited assay
media. Initial suggestions for optimization of advanced experimental conditions would include
cross titrations of glucose and glutamine concentrations across a range of both saturating and
subsaturating values for primary analysis.
Agilent XF Substrate Oxidation Stress Test Kits 49
Page 50

50 Agilent XF Substrate Oxidation Stress Test Kits
Page 51

6 Context of Substrate Oxidation Tests
Among Other XF Kits and Applications
Agilent offers a variety of XF Assay Kits that provide a full spectrum of information, from broad
assessment of cellular function down to specific details of metabolic mechanism (Figure 15).
Beginning with the XF Real-Time ATP Rate Assay, consider this as a compass that can point to or
uncover phenotypic shifts, metabolic switching, pathway liabilities, and more, through basic
information about cellular energetic phenotype. This assay also indicates a suitable follow-up XF
assay to delve deeper in metabolic mechanisms and/or the influence of a specific pathway on
cellular function. For example, the XF Cell Mito Stress Test and XF Glycolytic Rate Assay are
designed to interrogate mitochondrial and glycolytic function, respectively, in the context of the
entire cell. Once a change in mitochondrial function has been identified, often the next steps in the
investigation are to understand what may be responsible for this change, including the effects of
oxidation of glucose/pyruvate, glutamine, and long-chain fatty acids. Subsequently, this
information can be used to develop strategies for control of cell function and phenotype through
metabolic programming and engineering.
Figure 15. Agilent offers a variety of XF Assay Kits that provide a full spectrum of information, from broad assessment of
cellular function down to specific details of metabolic mechanism. Once a change in mitochondrial function has
been identified, the next steps in the investigation are to understand what may be responsible for this change,
including the effects of oxidation of glucose/pyruvate, glutamine, and long chain fatty acids.
Agilent XF Substrate Oxidation Stress Test Kits 51
Page 52

52 Agilent XF Substrate Oxidation Stress Test Kits
Page 53

Agilent XF Substrate Oxidation Stress Test Kits 53
Page 54

www.agilent.com
Agilent Technologies 2020
First edition, May 2020
For Research Use Only. Not for use in diagnostics procedures.
*5994-1164EN*
5994-1164EN
Rev B0
 Loading...
Loading...