Page 1
Best Practices for Using an Agilent LC System
Technical Note
This technical note describes best practices for using an Agilent LC system.
Housekeeping 2
Daily / Weekly Tasks 3
Power Up / Shut Down the System 4
Recommendations for Degassers 6
Recommendations for Pumps 7
Recommendations for Samplers 11
Recommendations for Pumps and Samplers With Optional Inline Filter 13
Recommendations for Columns 14
Recommendations for Detectors 15
Recommendations for Bio-Compatible and Bio-Inert Systems 16
Additional Information for 1290 Infinity and 1290 Infinity II Pumps 17
Best Practices - Technical Note
Page 2
Housekeeping
Housekeeping
How to deal with solvents
• Use clean borosilicate glass bottles only.
• Rinse bottle with desired solvent before refilling it.
• Bottles can get contaminated with detergents from the dishwasher.
• Use solvent inlet filters to protect the system from incoming particles.
• Exchange water-based solvents daily.
• Algae growth may block the degasser or filters.
• Precipitation of insoluble salts may block filters or capillaries.
• Prepare solvent volume to be consumed within 1 – 2 days.
• Use only HPLC-grade solvents and water.
• All prepared organic solvents, mixtures and aqueous buffers must be filtered through
0.2 µm filter.
• Residues or contaminations may block filters or capillaries.
• Label bottles correctly with bottle content, filling date and expiry date.
• Reduce risk of algae growth: use brown bottles for aqueous solvents, avoid direct sunlight or
wrap the bottles in aluminium foil.
NOT E
CAUTI O N
Extra measures with Acetonitrile (ACN)
• ACN and other organic solvents can be filtered using a 0.2 µm PTFE filter membrane (e.g.
5191-4339).
Filtering through nylon filters is not recommended for High Sensitivity LCMS.
• Use brown bottles and fill them with the ACN volume to be consumed within 1-2 days to
prevent photochemical reactions and oxidation.
How to prepare samples
Possible sample precipitation
Ensure the sample is completely soluble in both sample diluent and mobile phase.
Match the sample diluent as closely as possible to the initial mobile phase in order to avoid
precipitation, pressure spikes and solvent peaks on the chromatogram.
• Filtering is the preferred method to remove the insoluble fraction from samples and avoid
blockages in the system. If not possible, centrifuge samples vigorously and be careful not to
contaminate the supernatant with precipitates while decanting or aspirating it.
• Make sure the sample diluent is also free of particles.
Best Practices - Technical Note 2
Page 3

Daily / Weekly Tasks
Daily / Weekly Tasks
Daily Tasks
• Replace solvents and solvent bottles for mobile phases based on water/buffer.
• Replace solvents and solvent bottles for organic mobile phase latest every second day.
• Check presence of seal wash solvent.
• Purge each channel with fresh solvent at 2.5 – 3 mL/min for 5 min before operation.
• Equilibrate the system with composition and flow rate of subsequent method.
Weekly Tasks
• Change seal wash solvent (10 % isopropanol in water) and bottle.
• Inspect solvent filters for dirt or blockages. Exchange if no flow is coming out of the solvent
line when removed from the degasser inlet.
CAUTI O N
Contamination of seal wash solvent
Damage to pistons and seals
Do not recycle seal wash solvent.
Exchange seal wash solvent weekly.
Typical solvent consumption: 0.5 L per week.
Use of Seal Wash Bottle Head Kit (5067-6131) is strongly recommended.
Best Practices - Technical Note 3
Page 4

Power Up / Shut Down the System
Power Up / Shut Down the System
Power Up the System
• Ensure that all modules are in an environment with stable temperature protected from direct
sun light.
• Switch on all modules and proceed to prepare them as described below:
Prepare the Pump
• Use fresh or different mobile phase (as required).
• Purge each channel with 2.5 – 3 mL/min for 5 min. Open the manual purge valve or use the
purge command, depending on the pump type.
Prepare the Sampler
• Avoid using needle wash and seat backflush with organic solvents with buffer applications,
because this can cause salt precipitation in the needle seat.
• When using needle wash and/or seat backflush:
• Always use fresh solvents.
• Methanol, ACN, isopropanol, water, and their mixtures are good options.
• Do not use not miscible or precipitating wash solutions.
• When using a Sample Cooler / Sample Thermostat:
• Turn on cooler/thermostat and wait until the target temperature is reached and stable.
This can be done while the detector lamp warms up (see “Prepare the Detector” on page 4).
• Ensure that the vials contain enough sample solution for all injections.
Prepare the Detector
• Warm up the lamp for at least 1 h to guarantee baseline stability.
• For refractive index (RI) detectors only: switch on the heater and flush the reference and
sample sides with fresh solvent used for the current application.
Equilibrate the System
• While the detector lamp is warming up, equilibrate your system (column and detector
included) using the composition of your application for at least 15 min, until pressure and
detector baseline signal are stable.
Best Practices - Technical Note 4
Page 5

Power Up / Shut Down the System
Shut Down the System
• Flush the column with the appropriate solvents and store it according to column manual
instructions (be sure that the flushing solvent is compatible with the solvent present in the
system to avoid precipitation).
• Install a union or a restriction capillary and flush the system extensively with water, especially
after using buffers. For details, see “Flushing Procedure” on page 16.
• Flush and store the system in 50 % methanol or 50 % isopropanol in water, without additives.
• Remove all samples from the autosampler and store them according to good laboratory
practices.
• Power off all modules.
Best Practices - Technical Note 5
Page 6
Recommendations for Degassers
Recommendations for Degassers
CAUTI O N
Liquid inside the degasser
In case a low boiling point solvent condensates or there is a leak, liquid can accumulate inside
the degasser chambers, and this will compromise performance.
When this happens,
Purge all solvent channels with isopropanol.
Keep unused channels filled with isopropanol.
• Check compatibility of solvents with degasser and application
• Use the standalone standard degassers (G1322A or G7122A) for RI applications, flow rates
higher than 5 mL/min, with low boiling point solvents (<60 °C) and with hexane,
tetrahydrofuran and any halogenated solvents.
• Use integrated or a standalone high-performance degasser (G4225A) for all other
applications.
• If enough vacuum for the optimal degassing performance cannot be reached or maintained
(as indicated by yellow or red status LED in standalone degassers, or specific error messages
on integrated degassers), power cycle the module.
• If, after power cycling, vacuum still cannot be reached or maintained on integrated degassers,
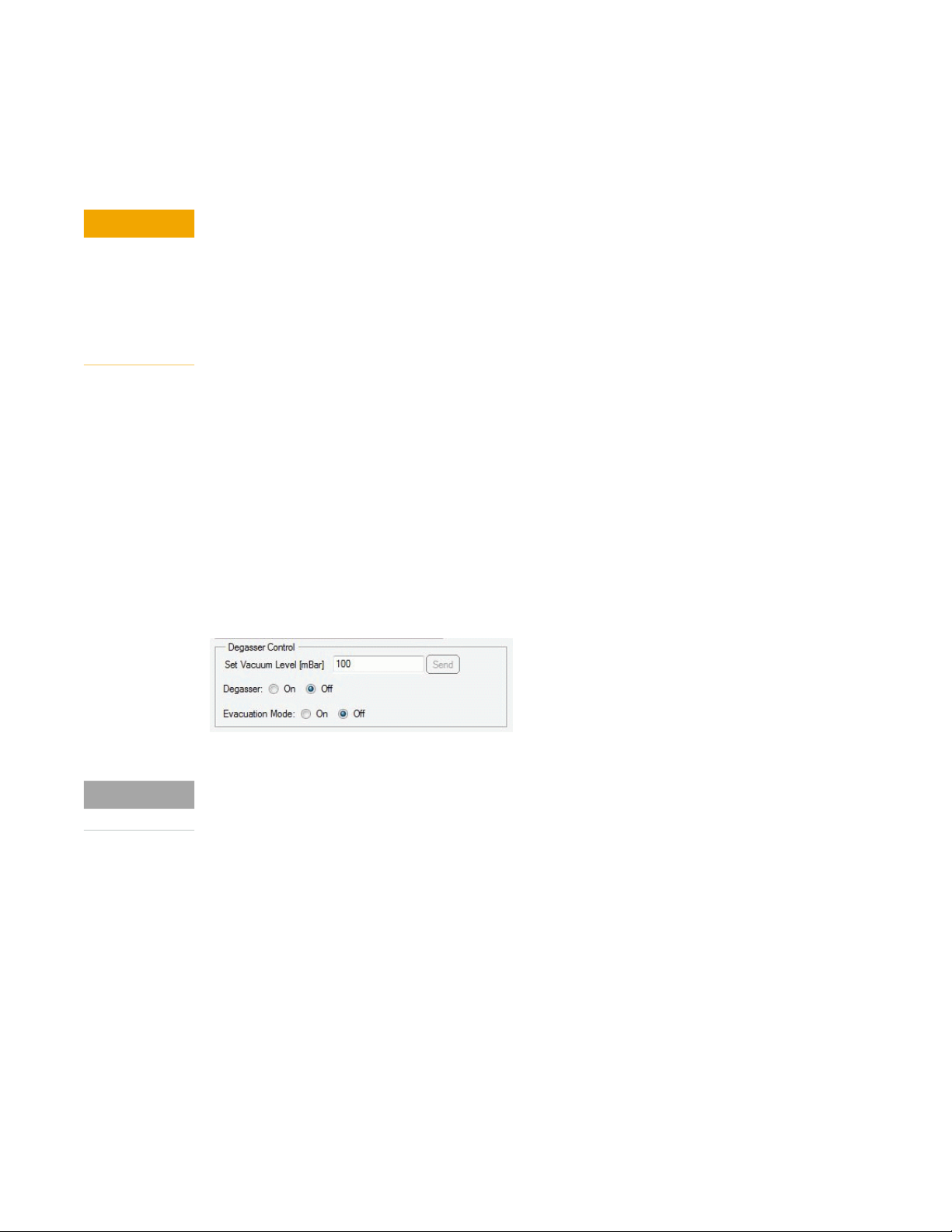
use the Evacuation Mode, available in the instrument control screen of Agilent Lab Advisor.
Figure 1 Degasser Control for internal degassers in Agilent Lab Advisor
NOT E
Best Practices - Technical Note 6
Follow the instructions prompted on the screen when starting the Evacuation Mode.
Page 7

Recommendations for Pumps
Recommendations for Pumps
• Check pump performance on a regular basis by monitoring the pressure signal.
• Perform preventive maintenance within the recommended usage interval.
• Prepare the pump as described in the power up section to ensure optimal performance and
best lifetime.
• When exchanging solvents, ensure the new solvent is miscible with the previous one (if
necessary, use an additional miscible solvent as intermediate).
• Use the seal wash function as recommended to ensure optimal performance and best lifetime,
see “Seal Wash (Usage Mandatory When Installed)” on page 8.
Recommendations for Pumps with MCGV
Select Channels for Multi-Channel Gradient Valve (MCGV)
NOT E
• Use lower channels (A and/or D) for buffer solutions.
• Regularly flush all MCGV channels with 200 mL of water to remove possible salt deposits.
• Check compatibility of buffers and organic solvents to avoid precipitation in the MCGVs mixing
chamber.
When mixing incompatible solvents, salts can precipitate at the point of mixing blocking the
downstream flow path and damaging parts.
Best Practices - Technical Note 7
Page 8

Recommendations for Pumps
Seal Wash (Usage Mandatory When Installed)
Seal Wash (G4204A, G4220A, All 1260 Pumps)
CAUTI O N
Contamination of seal wash solvent
Damage to pistons and seals
Do not recycle seal wash solvent.
Exchange seal wash solvent weekly.
Typical solvent consumption: 0.5 L per week.
Use of Seal Wash Bottle Head Kit (5067-6131) is strongly recommended.
Using the seal wash function is mandatory when installed and when using buffers and other
non-volatile solvents or additives that could deposit on pistons and seals. The seal wash function
regularly cleans these parts automatically.
Benefits of Seal Wash Operation:
• Removal of particles, salt crystals and other non-volatile residues from the pistons and seals,
which have the potential to damage the piston and piston seals
• Lubrication of seal/piston interface
• Cooling of pistons
Seal Wash Dialog in your CDS
The dialog can be found under the control screen, it is recommended to use the settings displayed
in Figure 2 on page 9.
Be aware that:
• The seal wash settings are NOT method parameters (as Instrument Control settings, they are
applied to all methods run in a particular instrument).
• The seal wash has to be turned on again manually after:
• An ERROR has been cleared.
• Power on.
Seal Wash Operation:
• PERIODIC operation, for example 0.5 min every 7 min.
• Setting can be changed in the Control screen, see Figure 2 on page 9.
The settings are available via the context menu, see Figure 4 on page 9.
• Typical solvent flow is 0.7 mL/min which corresponds to an approximate consumption of
3 mL/h of or 0.5 L/week at constant operation.
• Use
• 10 % isopropanol in water.
• 100 % isopropanol for normal phase applications.
Best Practices - Technical Note 8
Page 9

Recommendations for Pumps
• Position wash solvent bottle above and waste bottle below instrument.
• Do not refill seal wash solvent bottle, always use properly cleaned, fresh bottles.
• For pumps not equipped with seal wash sensor, test peristaltic pump.
• Touch the peristaltic pump with your finger to check that the pump is working, or confirm
seal wash solvent flow by watching seal wash solvent to drop out of the tubing.
Table 1 Seal wash dialog and operation
G4204A, G4220A all 1260 Pumps
Figure 2 Seal wash settings (1290 Infinity Pumps) Figure 3 Seal wash settings (all 1260 pumps)
Figure 4 Context Menu (1290 Infinity Pumps) Figure 5 Context Menu (all 1260 pumps)
Best Practices - Technical Note 9
Page 10

Recommendations for Pumps
Seal Wash (G7104A, G7104C, G7120A and G7132A)
CAUTI O N
Contamination of seal wash solvent
Damage to pistons and seals
Do not recycle seal wash solvent.
Exchange seal wash solvent weekly.
Typical solvent consumption: 0.5 L per week.
Use of Seal Wash Bottle Head Kit (5067-6131) is strongly recommended.
The seal wash pump is operating not only when the analytic pump is moving solvent, but also
when it is on standby or not-ready states. The seal wash function regularly cleans pistons and
seals from deposits automatically.
The seal wash sensor will constantly check the performance of the seal wash system and warn
the user in case an anomaly has been detected.
Seal Wash Operation:
• The seal wash interval is set to 30 s on every 7 min.
• The flow is set to 500 µL/min.
• The integrity of the seal wash system is checked at regular intervals.
• Typical solvent usage is about 0.5 L per week.
• Use
• 10 % isopropanol in water.
• 100 % isopropanol for normal phase applications.
• Position wash solvent bottle above and waste bottle below instrument.
• DO NOT refill the seal wash solvent, always use properly cleaned, fresh bottles.
• DO NOT recycle seal wash solvent bottles.
• The EMF symbol will turn yellow once the pumps seal wash sensor detects an irregularity.
• Change the seal wash solvent and trigger the Seal Wash Prime function from the context
menu (see Figure 6 on page 10).
Figure 6 Context Menu
• Check seal wash tubing and filter for kinks, leaks, or blockages.
• Check for blockages of the waste tubings, make sure that the solvent waste can drain off
freely:
• If solvent waste is building up in the tube, the sensor does not perform correctly.
Best Practices - Technical Note 10
Page 11

Recommendations for Samplers
Recommendations for Samplers
• Prior to shut down or a long standby, always
• Remove buffer with HPLC grade water (see “Flushing Procedure” on page 16).
• Perform at least 15 min flush of the sampler with water (both needle external wash and
seat backflush for Multiwash option).
• Perform visual check, if necessary clean the salt residuals manually.
• Remove contaminating substances with a strong solvent, for example pure ACN.
• Use the Auto-clean function to flush the sampler while switching the injection valve back
and forth.
• Always use fresh wash solvent for the needle or seat backflush function.
• Place the wash solvent reservoir for needle wash or seat backflush into the solvent cabinet.
• Use an appropriate solvent based on the sample and mobile phase properties.
NOT E
NOT E
Best Practices - Technical Note 11
The composition of the needlewash solvent should be the most solubilizing compatible solvent
(your strongest diluent). Selecting wash solvents is part of the method development. A mixture of
50 – 100 % organic solvent in distilled water is a good choice for many applications.
• Check the drainage routing of the washport outlet into a waste container.
• Fill each vial with enough sample solution for all injections.
• Use Agilent recommended vials only.
• Do not overfill the vials, fill each vial up to 90 % only.
• Use pre-slitted septa when drawing large volumes or multiple times from the same vial.
• Filter, decant, or centrifuge sample to separate from insoluble solid.
Sample solvent should be free of particles, preferably always filtered.
• Take care that the sample solvents match the proposed mobile phase as closely as possible.
Page 12

Recommendations for Samplers
Multiwash
Multiwash option is designed for the low carryover and can be installed in any Agilent
Multisampler.
This option reduces carryover for critical samples by allowing:
• External needle wash with up to three different solvents
• Seat backflush with up to three different solvents
The use of the Multiwash option is not recommended with salt-containing mobile phases, as salt
will crystallize on the needle and needle seat. Due to the flow path of the Multiwash hydraulic box,
when the metering device moves to the home position before injection, the mobile phase will
come out from the needle tip and might fall into the seat. This is expected behavior, independent
of the Injection Path Cleaning settings, and does not indicate leakage.
If the Multiwash is installed on the system with salt-containing mobile phases, to avoid salt
build-up on the needle and the seat, it is recommended to flush the Multisampler daily with water
for 15 min and visually inspect the needle and seat. If necessary, use a lint-free tissue with distilled
water to manually clean the needle, seat, and other parts that still contain salt residue.
When should the Multiwash option be used?
• If the system is intended for reversed-phase applications only and no precipitating compounds
are present in the mobile phase.
• If the system is intended for applications with salt-containing mobile phases, the Multiwash
option is not recommended. If the option is however installed, crystallization on the needle and
the seat can take place. The following practices should be followed:
• Perform daily a 15 min flushing with water to remove salt residue.
• Perform external needle wash and seat backflush.
• Visually control needle/seat/washport for salt residue.
• Manually clean needle/seat/washport if necessary.
NOT E
If these procedures are not followed, it will lead to needle and seat blockages.
• If the system is intended for alternating applications with salt-containing mobile phases AND
reversed-phase applications, Multiwash option is not supported. There is a high risk that salt
residue will be continuously released and either interfere with reversed-phase chromatography
or even cause clogging of the system.
Best Practices - Technical Note 12
Page 13

Recommendations for Pumps and Samplers With Optional Inline Filter
Recommendations for Pumps and Samplers With Optional
Inline Filter
Usually, the lifetime limiting factor for UHPLC columns is high backpressure. Particulate matter in
the sample is collected on the inlet frit of the column, which causes an increase in the
backpressure until the pressure limit of the system is reached. Using the inline filter is
recommended to prevent blockages of the column frit, especially when sample preparation does
not allow for filtering or the sample potentially forms precipitate.
The following modules can be equipped with an extra inline filter:
• Quaternary pumps (G7104A, G7104C, G4204A):
• Inline Filter Assembly (5067-5407)
• 1290 Infinity and 1290 Infinity II Agilent autosamplers (except G5668A, G7137A):
• 1290 Infinity II Inline Filter Kit (5067-6189)
These inline filters with a nominal pore size of 0.3 µm protect the UHPLC column from clogging by
filtering particulate matter from samples or the UHPLC system.
Advantages of the inline filter:
• Very small internal volume
• Delay volume with rigid capillary 1.3 µL
• Delay volume with flexible capillary 1.6 µL
• Specified for working at high pressures (max. operating pressure 1300 bar)
Installing the inline filter into the G4204A or G7104A/C is recommended to protect the
downstream system from blockages in the following cases:
• When solvent combinations are used that can form precipitates after mixing,
• When running applications with buffers or additives while using columns with small particle
sizes
General hints for effective use of the inline filter:
• Filter solvents before use.
• Follow best practices.
• For G4204A, G7104A/C pumps, backflush the filter in the pump weekly (start Flush Filter from
the context menu).
CAUTI O N
NOT E
Best Practices - Technical Note 13
Damage to the valve
Use the filter flush mode only if the inline filter is installed, otherwise the pressure pulse
might damage the multipurpose valve.
• For inline filters installed on the sampler, exchange the filter frit (Frit 0.3 µm for inline filter, 5/pk
(5023-0271)) every 1000 injections or when the back-pressure rises by 15 %.
See Technote G7167-90130 for further reference.
Page 14

Recommendations for Columns
Recommendations for Columns
• Use columns only in the marked direction.
• Always use suitable fittings for the specific column.
• Columns from different vendors require different fitting dimensions.
• Using an inappropriate fitting may result in peak dispersion or even damage the column.
• Agilent recommends using InfinityLab fittings to overcome fitting incompatibilities when
using columns from different vendors.
• Always adhere to operating and application limits, as put forth in the column user guide.
• Equilibrate the column with 10 – 20 column volumes before use.
• It is advisable to do an intermediate flush with a mobile phase of the correct composition
without additives before equilibrating to the final solvent with additives.
• The use of a guard column is recommended to protect your column and increase its lifetime.
NOT E
Long-term storage of columns should always be in the appropriate storing solvent, for more
details on the column in use, see the User Guide inserted in the column package.
Best Practices - Technical Note 14
Page 15

Recommendations for Detectors
Recommendations for Detectors
CAUTI O N
NOT E
Frequently turning the lamp on and off
Reduced lamp lifetime
Avoid unnecessarily switching on/off the lamp.
There is a safety period/wait time before a lamp can be re-ignited after it has been turned off.
• Warm-up the lamp at least 1 h.
• Keep environment and ambient temperature stable for all modules, especially for the detector.
• Do not expose the detector to direct sun light.
• Do not expose the detector to too much air current from the HVAC.
• Install pressure relieve valve (p/n G4212-68001 when using DAD detectors G4212A/B and
G7117A/B/C, or p/n 0100-3150 when using FLD detectors) when connecting a second
detector after the Max-Light cartridge cell.
• Use the recommended waste lines for each detector type. Avoid pinching the waste tube after
the cell outlet.
• Ensure that the detector flow cell is bubble free by flushing with isopropanol or other organic
solvent until a stable baseline is achieved.
• For RI detectors only: flush the reference and sample sides with fresh solvent used for the
current application.
• Flush the flow cell after use.
• Use HPLC grade water to remove salts.
• Use isopropanol to remove organic solvents.
• Before removing a flow cell for storage, fill it with isopropanol to prevent algae growth.
Best Practices - Technical Note 15
Page 16

Recommendations for Bio-Compatible and Bio-Inert Systems
Recommendations for Bio-Compatible and Bio-Inert
Systems
• Make sure all supplies (fittings, capillaries, inline filters, columns, etc.) are bio-inert /
bio-compatible.
• Be aware that some columns for bio-related applications have a stainless steel case and
can introduce iron and other metal ions in the flow path. This may lead to adsorption of
susceptible samples like phosphorylated nucleotides. In this case, use PEEK-lined columns.
• After using the system with high salt concentrations, flush it extensively with water to prevent
blockages caused by salt crystals.
• Reliable operation of 1290 pumps during analysis cannot be guaranteed if pressure falls below
20 bar. For optimal results, pressure should be at least 50 bar continuously. Therefore, when
using columns that create low backpressure (<50 bar, such as SEC columns with 1290 LC
systems), install a restriction capillary between the pump and the sampler, to achieve at least
50 bar.
• Perform daily flush of the Multisampler with water if the Multiwash option is installed (see
“Multiwash” on page 12).
CAUTI O N
Agilent Bio-inert and Bio LC systems should not be subject to passivation or similar
procedures
This can cause irreversible damage to the system's internal surfaces
Do not perform passivation or similar procedures on bio-inert and bio-compatible systems.
Flushing Procedure
This procedure should be used when salt-containing mobile phases are used. It has to be
performed regularly, at least once a week, or prior a long standby or off time, to remove salt
deposits from the flow path and surfaces in contact with the solvents. How to prepare the
system for shutdown see “Shut Down the System” on page 5.
The procedure is mandatory for switching from salt-containing mobile phase to reversed
phase applications (or any applications running with high organics), where the precipitation of
salt can occur.
• Flush the column with recommended storage solvent, be sure that this solvent is compatible
with current mobile phase and cannot cause precipitation.
• Replace the column with a union, replace the salt-containing solvent bottle with a new bottle of
HPLC-grade water at room temperature.
• Clean the bottle head assembly using lint-free wipes to minimize carryover of remaining salt
solution into the new water bottle.
• Autosampler: perform at least 15 min purge with water to remove salt residues from all lines,
both needle wash and seat backflush for Multiwash option, visually control
needle/seat/washport for salt residues, if necessary manually clean needle/seat/washport.
• Purge each pump channel that has pumped buffer separately, for at least 10 min at 5 mL/min.
• Flush the entire system flow path with water for at least 10 min at 2 mL/min. During this step,
switch the injection valve and the column selection valve (if installed) position every 1 min, and
repeat this until every position has been selected for at least 5 times.
• Replace water with fresh solvent bottles to minimize salt carryover.
Best Practices - Technical Note 16
Page 17

Additional Information for 1290 Infinity and 1290 Infinity II Pumps
Additional Information for 1290 Infinity and 1290 Infinity II
Pumps
The Agilent 1290 Infinity and 1290 Infinity II Pumps are equipped with automatic purge valves.
This enables a variety of additional functions not available in Agilent Pumps with manual purge
valves. It is possible to prepare the pump (set paramaters and start the functions Purge, Condition,
or Prime) with the software.
Purge
Use the Purge function to:
• Fill the system with fresh or different solvent.
• Ensure that the new solvent is miscible with the previous solvent.
• Prevent damage to the degasser or pump by using an intermediate step with a co-miscible
solvent, if necessary.
• Remove air bubbles in tubes and pump heads.
• After the pump has been idle for a few hours or more (air may have diffused into the solvent
lines).
As soon as the purge procedure ends the module automatically switches to analytical conditions
again.
Condition
If micro air bubbles persist in the pump head, the overall pump performance may be
compromised and flow accuracy / precision may be negatively affected. This may be visible as
increased pressure and / or detector baseline ripple. A reliable indicator of such situation is a
strongly negative but slowly increasing tuning signal value (below –1). To remove the air
efficiently, the Condition function can be used. During conditioning, the pump is delivering flow
into the system (column) and the last used method settings, like flow, composition and max
pressure are used. It is not possible to perform sample analysis while conditioning.
Use a reasonable flow rate (for example 1.5 mL/min), composition setting (for example A: 50 % B:
50 %) and backpressure (>200 bar) to ensure efficient air bubble removal from all pump heads.
Condition the pump if you see:
• Excessive pressure ripple.
• Excessive composition ripple (baseline noise/mixing noise – noise level changes with the
composition), when you are sure that the solvent type is correctly set, and there is no evidence
of a leak in the pump.
Conditioning may be necessary:
• After a long period of standby
• After running out of solvent
• After service or repair
CAUTI O N
Best Practices - Technical Note 17
Filling empty solvent lines
Damage to the seals
Use a syringe or the Purge function to fill empty solvent lines.
Do not use the Prime procedure to fill empty solvent lines.
Page 18

Additional Information for 1290 Infinity and 1290 Infinity II Pumps
1.
2.
Prime
The Prime function is helpful if air has entered the pump heads and cannot be removed by
conditioning for 15 minutes. The module draws solvent at a high speed with all pump drives
simultaneously and dispenses it into the waste position of the automatic purge valve. This is done
20 times and is stressful to the valve and rotor seal. Therefore, it should be performed only as a
last resource, before forcefully filling the pump heads with a syringe or attempting to repair the
pump heads.
Use the Prime function to:
• Free a potentially stuck valve.
The described functions can be triggered from the driver interface:
• 1290 Infinity and 1290 Infinity II
NOT E
For parameter settings, see Figure 2 on page 9.
Figure 7 Prepare the pump (1290 Infinity).
1. Right click on the module dashboard
2. Select the appropriate function to start the procedure
Best Practices - Technical Note 18
Page 19

The user-optimized Prepare Pump context menu replaces the classical menu:
1.
2.
3.
4.
• 1290 Infinity II Pumps
Figure 8 Prepare the pump (1290 Infinity II pumps)
1. Right click on the module dashboard
2. Select Prepare Pump...
3. Select the procedure and fill in adequate parameters
4. Click Start to run the selected procedure
www.agilent.com
Agilent Technologies Inc. 2016-2020
Edition: 08/2020
Document No: SD-29000194 Rev. B
 Loading...
Loading...