Page 1
Series diode array detector (DAD)
is below 5 pmol with a signal-tonoise ratio of 2. For the Agilent
1100 Series fluorescence detector
(FLD) the limit of detection is
below 100 fmol except for Cys-SSCys. Due to better selectivity we
recommend the use of the FLD
below 100 pmol. The linearity correlation factor for well resolved
amino acids is between 0.99998
and 0.99999 in the range from
10 pmol up to 1000 pmol for DAD.
A special amino acid report is
shown and hints for maintenance
and troubleshooting are included.
Ordering information about
columns, standards, chemicals
and capillaries, and Agilent Application Services is also included.
Angelika Gratzfeld-Huesgen
Sensitive and Reliable Amino Acid
Analysis in Protein Hydrolysates using
the Agilent 1100 Series HPLC
Pharmaceutical
Technical Note
Agilent Technologies
Innovating the HP Way
Abstract
This technical note demonstrates
the performance of amino acids
analysis on the Agilent 1100 Series
modules and systems for LC.
Detailed information regarding
instrumental and chromatographic
conditions and performance are
given. The precision for retention
times over 10 runs is as low as
0.2 % RSD, and the RSD of areas is
between 0.6 and 5 %. The limit of
detection for the Agilent 1100
Page 2

Introduction
Since the introduction of automated amino acid analysis on the
HP 1090 Series HPLC system
using a two step precolumn
derivatization, this technique has
become a well accepted and routine method for the analysis of primary and secondary amino acids
in protein hydrolysates. This technical note will demonstrate that
amino acid analysis can be done
on the Agilent 1100 Series modules and systems for LC using the
binary pump to obtain even better
performance than was achieved
with the HP 1090 Series. The
conditions used are the same,
that were used on the HP AminoQuant 2 based on the HP 1090
Series system.
Experimental
HPLC Instrumentation:
The following Agilent 1100 series
modules were used:
• high pressure gradient pump
order number: G1312A
• online vacuum degasser
order number: G1322A
• autosampler
order number: G1313A ( or
thermostatted autosampler
order number: G1327A)
• thermostatted column
compartment
order number: G1316A
• diode array detector for
concentrations above 100 pmol
order number: G1315A
• fluorescence detector for
concentrations below 100 pmol
order number: G1321A
• Agilent ChemStation
order number: G1319A
• Software for amino acid reports
part number G1300-10013
(user contributed software).
One modification was made for
the standard high pressure
gradient pump. The solvent mixer
(part number G1312-87330) was
replaced by a capillary (part
number 1090-87610) or Upchurch
mixer to reduce delay volume.
Amino acid Standards
Five different concentrations of
amino acid standards were used
for evaluating the precision of
retention times and areas, and the
limit of detection and linearity.
These were 10, 25, 100, 250 and
1000 pmol/µl. The standards
contained the following
compounds:
Asp Aspartic acid
Glu Glutamic acid
Ser Serine
His Histidine
Gly Glycine
Thr Threonine
Ala Alanine
Arg Arginine
Tyr Tyrosine
Cys-SS-Cys Cystine
Val Valine
Met Methionine
Phe Phenylalanine
Ile Isoleucine
Leu Leucine
Lys Lysine
Pro Proline
Derivatization Reagents
The online derivatization was
performed using ortho-phthalaldehyde (OPA) for the primary amino
acids and 9-fluorenylmethyl
chloroformate (FMOC) for the
secondary amino acids. A 0.4 N
borate buffer was used with pH
10.4.
Preparing mobile phases,
standards and derivatization
reagents
Mobile phase A:
1. Weigh 1.36 ± 0.025 g of sodium
acetate tri-hydrate and transfer
it into a 800 ml glass beaker.
2. Add 500 ml of purified water
and stir until all crystals are
completely dissolved.
3. Add 90 µl of triethylamine
(TEA) and mix.
4. Adjust the pH to 7.20 ± 0.05 by
adding a few drops of 1–2 %
acetic acid.
5. Add 1.5 ml of tetrahydrofuran
(THF) and mix.
Page 3

FMOC reagent
1. Place several microvials in the
microvial rack.
2. Open one ampule of the FMOC
reagents. This is the approximate amount needed for ten
days.
3. Pipette between 50 and 100 µl
into each microvial.
4. Cap all vials. Store vials which
are not directly used in the
refrigerator.
Borate buffer
Place 1 ml of borate buffer into
a 2 ml vial.
Water
Place 1 ml purified water into a
2 ml vial.
Preparing internal standards of
5 nmol and 500 pmol
The secondary amino acids were
quantified using sarcosine as an
internal standard. The primary
amino acids can be quantitated by
either using external standard
procedures or by using norvaline
as an internal standard. If you are
working with DTDPA to convert
cystine and cysteine to the stable
Cys-MPA during hydrolysis, then
make up your ISTD stock solutions in borate buffer instead of
HCl to avoid solubility problems.
Mobile phase B:
1. Weigh 1.36 g ± 0.025 g of
sodium acetate trihydrate and
transfer it into a 200 ml glass
beaker.
2. Add 100 ml of purified water
and stir until all crystals are
dissolved.
3. Adjust pH to 7.20 ± 0.05 by
adding a few drops of 1–2 %
acetic acid.
4. Add this solution into a
mixture of 200 ml acetonitrile
and 200 ml methanol and mix.
Derivatization reagents:
For convenience use microvial kit
from Agilent (part number 9301-
1388)
OPA reagent
1. Place several microvials in the
microvial rack.
2. Open one ampule of the OPA
reagents. This is the approximate amount needed for ten
days. Preparing more could
cause problems with oxidation.
3. Pipette between 50 and 100 µl
into each microvial.
4. Cap all vials. Store vials which
are not directly used in the
refrigerator.
1. 5 nmol ISTD standard:
Weigh 22.3 mg sarcosine
(optional 29.3 mg norvaline)
and dissolve in 50 ml
0.1 N HCl.
2. 500 pmol ISTD standard:
Take 5 ml of 5 nmol ISTD and
dilute with 50 ml 0.1N HCl.
Preparing the calibration
standards
For standard sensitivity eight
different concentration were used:
1. 900 pmol/µl amino acid standards with 500 pmol/µl internal
standards.
Place 900 µl from standard
with 1000 pmol amino acids
and 100 µl from ISTD (5 nmol)
in a 2 ml vial. Mix and place
100 µl each in microvials
2. 225 pmol/µl amino acid standards with 500 pmol/µl
internal standards. Use 250
pmol amino acid standard
and follow the same procedure
as under 1.
3. 90 pmol/µl amino acid
standards with 500 pmol/µl
internal standards.
Use 100 pmol amino acid
standard and follow the same
procedure as under 1 of this
section.
Page 4

For high sensitivity three
different concentrations were
used:
1. 90 pmol/µl amino acid
standards with 50 pmol/µl
internal standards.
Place 900 µl from standard
with 100 pmol amino acids and
100 µl from ISTD (500 pmol) in
2 ml vial. Mix and place 100 µl
each in microvials.
2. 22.5 pmol/µl amino acid
standards with 50 pmol/µl
internal standards.
Use 25 pmol amino acid
standard and follow the same
procedure as under 1.
3. 9 pmol/µl amino acid standards
with 50pmol/µl internal standards.
Use 10 pmol amino acid
standard and follow the same
procedure as under 1.
Extended Amino Acids (EAA)
In addition to the previously
analyzed 17 amino acids which
can be found in hydrolysates (see
figure 1), the amino acid supplement kit contains the following
amino acids as solids, which are
of interest for amino acid analysis
in food:
norvaline
sarcosine
asparagine
glutamine
tryptophan
and hydroxyproline
Table 1 shows the stock solutions
used for different purposes.
Use these stock solutions in place
of the 5 nmol ISTD or 500 pmol
ISTD stock solutions previously
described, to prepare your calibration standards.
Preparing internal standards of
10 nmol and 1 nmol
The secondary amino acids were
quantitated using sarcosine as an
internal standard. The primary
amino acids can be quantitated by
either using external standard
procedures or by using norvaline
as an internal standard. If you are
working with DTDPA to convert
cystine and cysteine to the stable
Cys-MPA during hydrolysis, then
make up your ISTD stock
solutions in a borate buffer
instead of HCl to avoid solubility
problems.
1. 10 nmol ISTD standard:
Weigh 44.6 mg sarcosine
(optional 58.6 mg norvaline)
and dissolve in 50 ml
0.1 N HCl.
2. 1 nmol ISTD standard:
Take 5 ml of 10 nmol ISTD and
dilute with 50 ml 0.1 N HCl.
Standard sensitivity
9 nmol/µl extended amino acid with 5 nmol/µl internal standards
2.25 nmol/µl extended amino acid with 5 nmol/µl internal standards
900 pmol/µl extended amino acid with 5nmol/µl internal standards
High sensitivity
900 pmol/µl extended amino acid with 500 pmol/µl internal standards
225 pmol/µl extended amino acid with 500 pmol/µl internal standards
90 pmol/µl extended amino acid with 500 pmol/µl internal standards
Table 1
Stock solutions
Page 5

Preparing the stock solution of
the extended amino acid
standards
For standard sensitivity an
18 nmol/µl concentration was
used:
Weigh
59.5 mg asparagine
65.7 mg glutamine
91.8 mg tryptophan
59.0 mg 4-hydroxyproline
into a 25 ml graduated flask.
For high sensitivity a 1.8 nmol
concentration was used:
5 ml of the 18 nmol EAA was
placed into a 50 ml flask and
made up to 50 ml with
0.1N HCl
Table 2 details the preparation of
the calibration standards for standard sensitivity, with table 3 showing the calibration standards for
high sensitivity.
Concentration 90 pmol 22.5 pmol 9 pmol
of final solution
Amount of 18 nmol EAA 5 ml 5 ml 5 ml
Dilute with 0,1N HCl — 15 ml 45 ml
Amount of diluted solution from above 5 ml 5 ml 5 ml
Amount of 10 nmol ISTD 5 ml 5 ml 5 ml
Amount of mixed solution from above 100 µl 100 µl 100 µl
Amount of 1000 pmol AA standard 900 µl — —
Amount of 250 pmol AA standard — 900 µl —
Amount of 100 pmol AA standard — — 900 µl
Table 2
Preparing the calibration standards for standard sensitivity
Concentration 90 pmol 22.5 pmol 9 pmol
of final solution
Amount of 18 nmol EAA 5 ml 5 ml 5 ml
Dilute with 0,1N HCl — 15 ml 45 ml
Amount of diluted solution from above 5 ml 5 ml 5 ml
Amount of 1 nmol ISTD 5 ml 5 ml 5 ml
Amount of mixed solution from above 100 µl 100 µl 100 µl
Amount of 100 pmol AA standard 900 µl — —
Amount of 25 pmol AA standard — 900 µl —
Amount of 10 pmol AA standard — — 900 µl
Table 3
Preparing the calibration standards for high sensitivity
Page 6

Results
The analysis of amino acids using
precolumn online derivatization
with OPA and FMOC can be
accomplished using UV (figure 1)
or fluorescence detection
(figure 2). We recommend the use
of fluorescence detection if the
concentration of amino acids is
below 100 pmol.
Chromatographic conditions
Column: 200 × 2.1 mm AA column
and guard column
Mobile phase: A = 20 mMol NaAc + 0.018 %
TEA adjusted to pH 7.2
with 1-2 % acetic acid,
B = 20 % of 100 mMol NaAc
adjusted to pH 7.2 with 1-2 %
acetic acid + 40 % ACN
and 40 % MeOH
Flow rate 0.45 ml/min
Compressibility A 50 x 10-6bar
Compressibility B 115 x 10
-6
bar
Stroke: A and B Auto
Gradient: start with 100%A, at 17 min
60%B, at 18 min
100%B, at 18.1 min flow 0.45,
at 18.5 min flow 0.8,
at 23.9 min flow 0.8,
at 24 min 100%B and flow 0.45,
at 25 min 0%B
DAD UV Detector
Signal A= 338/10 nm,
Ref= 390/20 nm;
Signal B= 262/16 nm,
Ref= 324/8 nmat;
15 min signal A =262/16 nm,
Ref =324/8 nm
Oven temp: 40 ºC
Post time: 5 min
Injector program
1 Draw 5.0 µl from vial 10—borate buffer
2 Draw 1.0 µl from vial 11—OPA reagent
3 Draw 0.0 µl from vial 12—water
4 Draw 1.0 µl from sample
5 Draw 0.0 µl from vial 12—water
6 Mix 8 µl in air, max speed, six times
7 Draw 1.0 µl from vial 14—FMOC
8 Draw 0.0 µl from vial 12—water
9 Mix 9 µl in air, max speed, 3 times
10 Inject
FLD Setting Agilent 1100 Series
Excitation 340 nm
Emission 450 nm
PTM gain 12
at 14.5 min
Excitation = 266 nm
Emission = 305 nm
PTM Gain 11
Time [min]
2 4 6 8 10 12 14 16
0
Absorbance
[mAU]
0
10
20
30
40
50
60
70
80
1
2
3
4
5
6
7
8
9
10
11 12
13
14 15
16
2 Glu
1 Asp
3 Ser
4 His
5 Gly
6 Thr
7 Ala
8 Arg
9 Tyr
10 Cys-SS-Cys
11 Val
15 Leu
16 Lys
17 Pro
12 Met
14 Ile
13 Phe
Figure 1
Analysis of 250 pmol/µl amino acid standard with precolumn online derivatization and using
DAD-UV detection
Time [min]
0
2
4
6
8
10
12
14
16
18
LU
0
25
50
75
100
125
175
Asp
Glu
Ser
His
Gly
Thr
Ala
Arg
Try
Cys-SS-Cys
Val
Met
Phe
Ile
Leu
Lys
Pro
150
10 pmol standard
Figure 2
Analysis of 10 pmol/µl amino acids with fluorescence detection
Page 7
The limits of detection (LOD) for
both detectors is given in table 4.
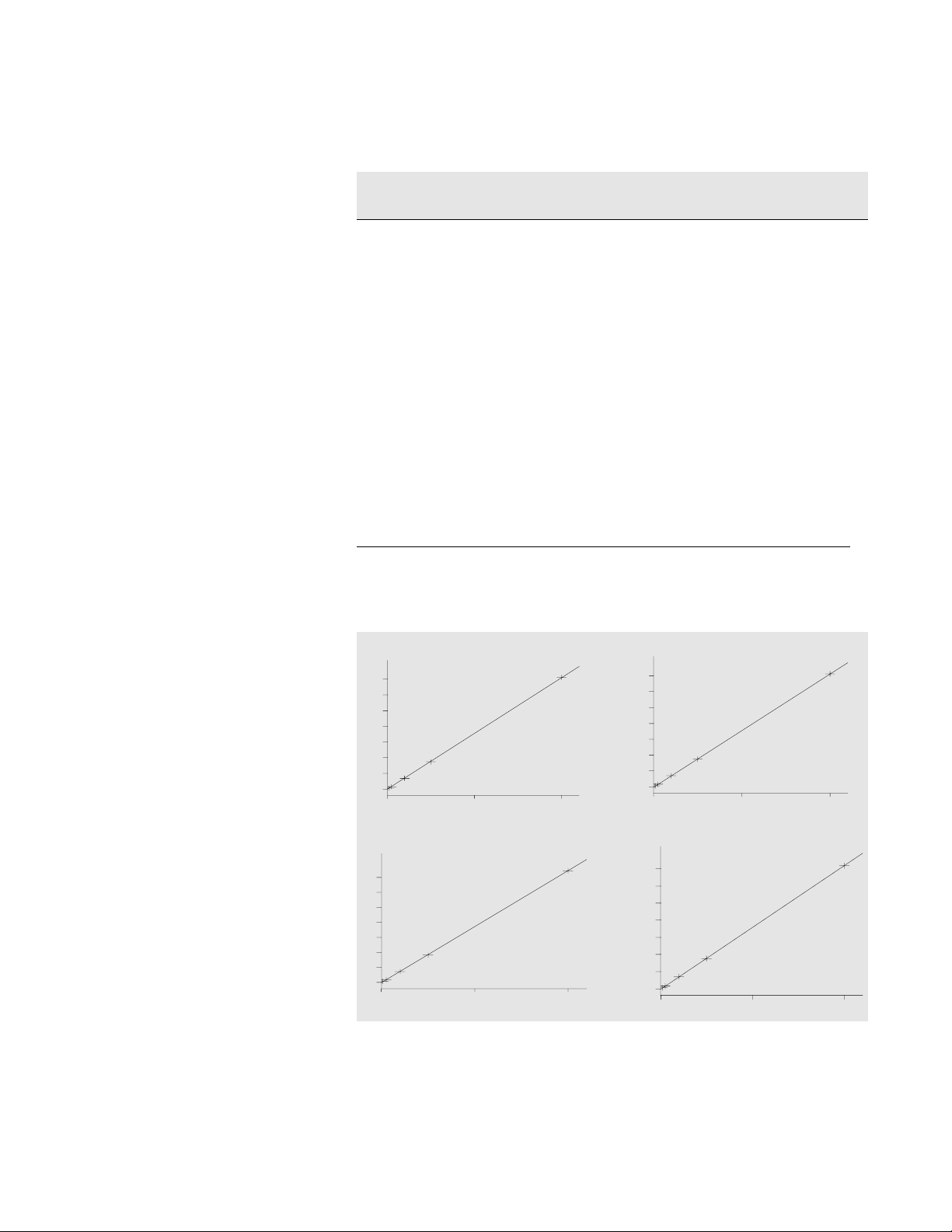
Linearity for the Agilent Series
1100 DAD is excellent for well
resolved peaks in the range from
10 to 1000 pmol/µl using standards
(figure 3). In figure 3 the correlation coefficient for four amino
acids is between 0.99998 and
0.99999.
Compound LOD for DAD LOD for FLD
(pmol) (pmol) (fmol)
Asp 0.6 19
Glu 0.5 18
Ser 0.9 21
His 2.1 29
Gly 1.6 21
Thr 1.3 21
Ala 1.3 20
Arg 1.2 17
Tyr 1.2 19
Cys-SS-Cys 1.7 not measured
Val 1.3 17
Met 1.2 16
Phe 1.4 17
Ile 1.4 16
Leu 1.4 18
Lys 1.2 57
Pro 2.4 22
Amount [pmol]
0
500
Area
0
100
200
300
400
500
600
700
1
2
3
4
5
17, DAD1 A
Correlation: 0.99999
for Leu
Rel. Res%(1): 13.424
Area = 0.7193022*Amt -1.1877714
Amount [pmol]
0 500
Area
0
100
200
300
400
500
600
700
1
2
3
4
5
7, DAD1 A
Correlation: 0.99999
for Thr
Rel. Res%(1): 28.342
Area = 0.71286557*Amt -2.0083654
Amount [pmol]
0 500
Area
0
100
200
300
400
500
600
700
1
2
3
4
5
2, DAD1 A
Correlation: 0.99999
for Glu
Rel. Res%(1): 23.525
Area = 0.7101211*Amt -1.3335603
Amount [pmol]
0
500
Area
0
100
200
300
400
500
600
700
1
2
3
4
5
13, DAD1 A
Correlation: 0.99998
for Cys-SS-Cys
Rel. Res%(1): 24.287
Area = 0.74520727*Amt -1.3516723
Table 4
LOD’s for fluorescence and UV detection
Figure 3
Linearity over the concentration range from 10 pmol/µl to 1000 pmol/µl
Page 8

Retention time and area precision
for both detectors are shown in
table 5. Retention time precision
over 6 runs is for both detectors
below 0.2 %. Area precision for the
DAD over 6 runs is below 5 % for
a 100 pmol/µl amino acid
standard. For the fluorescence
detector the precision of areas is
close to 5 %.
Software
The standard software of the
Agilent Chemstation can be used,
and no additional macros are
needed. A template for the special
amino acids report is available in
the Amino Acids Reports software
with all necessary information and
documentation.
Compound RSD RT RSD Area
DAD FLD DAD FLD
Asp 0.05 0.139 0.58 0.924
Glu 0.14 0.155 0.62 0.576
Ser 0.17 0.156 0.84 1.015
His 0.12 0.155 1.67 1.778
Gly 0.12 0.118 1.05 1.124
Thr 0.11 0.113 0.80 0.739
Ala 0.12 0.120 0.82 0.767
Arg 0.08 0.094 0.67 0.905
Tyr 0.06 0.062 3.18 1.614
Cys-SS-Cys 0.05 not measured 1.99 not measured
Val 0.05 0.058 0.84 0.919
Met 0.05 0.045 0.91 1.236
Phe 0.04 0.048 0.90 1.079
Ile 0.04 0.050 1.18 0.759
Leu 0.04 0.040 1.05 0.952
Lys 0.05 0.060 4.93 5.107
Pro 0.04 0.044 4.14 4.379
Table 5
Precision of retention times and areas of DAD and FLD over 6 runs
Page 9

Amino acid reports
The following report can be
obtained using the special method
and report template of the Amino
acid software kit (G1300-10013).
The following information is
included on the supplied disk:
• Installation procedure for the
method
• Checkout example for the
report template
• Instrument optimization
procedure for amino acid
analysis
• Customizing supplied operation
• Calculation specifications and
template and method details
• Common errors
A detailed description on how to
obtain and interpret this report is
included on the disk as a Word for
Windows document which can be
printed out. An example of the
printout is shown in figure 4.
Figure 4
An example of the report printout
Amino Acid Analysis Report
Acquisition method name: DATA:AAS.M Seq. Line: 6
Aquisition operator name: RG Vial#: 35
Sequence name: TOXIC.S Injection#: 1
Sample name: LYSOZYME
Data file name: C:\HPCHEM\1\DATA\1411F35A.D
Data analysis method name: C:\HPCHEM\1\METHODS\DEFAA.M
Injected on: 15 Nov 91 9:28 am
# Compound Ret time Area Height Width Symmetry
[min] [mAU*s] [mAU] [min]
1 ASP 1.285 226.5 80.78 0.043 0.666
2 GLU 1.477 65.8 38.79 0.025 0.468
3 SER 4.401 102.1 23.48 0.065 0.859
4 HIS 5.328 5.8 1.17 0.074 0.947
5 GLY 5.524 113.5 21.59 0.081 0.760
6 THR 5.902 74.5 14.91 0.077 0.811
7 CYS 6.094 73.4 14.86 0.075 0.847
8 ALA 7.199 139.0 28.94 0.074 0.739
9 ARG 7.459 122.1 25.03 0.075 0.786
10 TYR 8.833 27.9 5.51 0.077 0.705
11 VAL 10.730 59.1 11.67 0.077 0.770
12 MET 10.955 14.4 2.70 0.081 0.811
13 NVA 11.333 281.2 52.08 0.083 0.650
14 PHE 12.380 30.9 6.02 0.079 0.803
15 ILE 12.595 60.2 10.69 0.086 0.734
16 LEU 13.270 88.6 15.94 0.085 0.716
17 LYS 13.875 31.1 5.51 0.086 0.700
18 SAR 16.957 256.7 39.76 0.100 0.849
19 PRO 17.579 21.8 1.80 0.171 1.073
# Compound Ret time Amount Amount Residues Relative to
[min] [pmol] [ng] mole % / mole ALA
________________________________________________________________________
1 ASP 1.285 511.34 58.8 18.4 24.49 21.72
2 GLU 1.477 141.22 18.2 5.1 6.76 6.00
3 SER 4.401 243.88 21.2 8.8 11.68 10.36
4 HIS 5.328 15.56 2.1 0.6 0.75 0.66
5 GLY 5.524 261.22 14.9 9.4 12.51 11.10
6 THR 5.902 169.82 17.2 6.1 8.13 7.21
7 CYS 6.094 231.97 28.1 8.3 11.11 9.85
8 ALA 7.199 282.51 20.1 10.2 13.53 12.00
9 ARG 7.459 247.77 38.7 8.9 11.87 10.52
10 TYR 8.833 65.62 10.7 2.4 3.14 2.79
11 VAL 10.730 116.89 11.6 4.2 5.60 4.97
12 MET 10.955 25.44 3.3 0.9 1.22 1.08
13 NVA 11.333 500.00 - - - -
14 PHE 12.380 62.91 9.2 2.3 3.01 2.67
15 ILE 12.595 119.58 13.5 4.3 5.73 5.08
16 LEU 13.270 188.84 21.3 6.8 9.05 8.02
17 LYS 13.875 74.50 9.5 2.7 3.57 3.16
18 SAR 16.957 500.00 - - - -
19 PRO 17.579 22.71 2.2 0.8 1.09 0.96
Expected molecular weight (AA_ProteinMW) : 14400
Calculated minimum molecular weight : 12770
Expected MW / Calculated MW : 1.1277
min
2 4 6 8 1012141618
Norm.
0
100
200
300
400
500
DAD1 A, Sig=338,10 Ref=390,20 of 1411F35A.D
ASP
GLU
SER
HIS
GLY
THR CYS
ALA
ARG
TYR
VAL
MET
NVA
PHE
ILE
LEU
LYS
DAD1 B, Sig=262,16 Ref=324,8 of 1411F35A.D
SAR
PRO
Page 10

Maintenance
In order to keep the system running smoothly we recommend to
do the following:
Daily
1. Replace derivatization
reagents, borate buffer, amino
acid standards and wash water,
which are placed in the
autosampler tray.
2. Recalibration of retention
times and response factors
daily.
3. Check column and guard
column performance using system suitability report.
Every 2 days
1. Replace mobile phases A and B
with freshly-made solvents.
High column pressure occurs
1. Exchange guard column.
2. If this does not help, exchange
analytical column.
Troubleshooting
Changes in retention times—
amino acids elute earlier
• Poor resolution of His and Gly
is caused by absence of triethylamine.
• AA’s between Ser and Tyr elute
earlier, concentration of THF
too high. 0.4 % is already influencing the RT’s.
• Low concentration of sodium
acetate is influencing the later
peaks more than the earlier
ones.
• pH too high e.g. 7.6, specially
the peaks between 10 and 14
min are eluting too early.
• Oven temperature too high.
Nearly all peaks are eluting
earlier.
• High concentration of sodium
acetate decreases the resolution between Ala/Arg and
Val/Met.
• High concentration of acetonitrile lets peaks above 10 min
elute earlier.
• High concentration of
methanol decreases resolution
between Val/Met.
• If concentration of triethylamine is too low resolution
between Cys-Cys/Val is getting
poor.
Amino acids elute later
• THF absence is strongly
marked throughout the chromatogram. Note especially the
splitting of the Glu peak . As
the concentration of THF is
increased, the peak splitting
decreases and the retention
time improve.
• If the pH is too low, e.g. 6.8,
loss in resolution for Val/Met
and Cys-Cys/Val/Met ( for
DAD) occurs.
• If the oven temperature is too
low e.g. 38ºC all peaks are eluting later.
Poor chromatographic
resolution
• Exhausted guard column.
• Damaged analytical column.
• Post column band broadening
due to too long connections.
Low Intensity chromatogram.
• OPA reagent has deteriorated.
• FMOC reagent has
deteriorated.
• Glycine contamination.
Page 11

Ordering
Table 6 contains details for
ordering instrumentation and
supplies.
Amini acid standards
Each amino acid standard contains the following amino acids:
Asp Aspartic acid
Glu Glutamic acid
Ser Serine
His Histidine
Gly Glycine
Thr Threonine
Ala Alanine
Arg Arginine
Tyr Tyrosine
Cys-SS-Cys Cystine
Val Valine
Met Methionine
Phe Phenylalanine
Ile Isoleucine
Leu Leucine
Lys Lysine
Pro Proline
Order Number Description
Instrumentation
G1312A Agilent 1100 Series binary pump
G1312-67301 0.12 mm id capillary
or
A-330 Upchurch semi prep filter 5022-2165
G1313A Agilent 1100 Series autosampler
#011 Vial kit
or
G1327A Agilent 1100 Series thermostatted autosampler
#011 Vial kit
Optimum performance of the thermostatted autosampler requires the following capillary connections:
G1312-67305 (Pump to autosampler) Outlet capillary
5021-1823 (Autosampler to column compartment) Flexible tubing 400 mm, 0.12 mm id (no fittings)
G1316-87303 (Column compartment to inlet column) Capillary, 70 mm, 0.12 mm id
5021-1823 (Outlet column to DAD) Flexible tubing 400 mm, 0.12 mm id (no fittings)
G1322A Agilent 1100 Series vacuum degasser
G1316A Agilent 1100 Series column compartment
G1315A Agilent 1100 Series diode-array detector
#018 Standard flow cell (10 mm, 13 µl)
G1321A Agilent 1100 Series fluorescence detector
G1319A Agilent ChemStation
Supplies
5063-6588 Amino Acid Separation Kit contains:
79916AA-572 Column for amino acid analysis, 200 × 2.1 mm
79916KT-110 Guard columns, Hypersil ODS, 20 × 2.1 (3 per pack)
79900CH-010 Guard cartridge holder
79841-87609 Column connector, 35 mm, 0.12 mm id.
9301-1388 Crimpcap microvials (100 per pack)
5181-1210 Crimpcaps, butyl rubber (100 per pack)
Amino Acid Standards
5061-3330 1 nmol/µl AA standard 10 × 1 ml ampules
5061-3331 250 pmol/µl AA standard 10 × 1 ml ampules
5061-3332 100 pmol/µl AA standard 10 × 1 ml ampules
5061-3333 25 pmol/µl AA standard 10 × 1 ml ampules
5061-3334 10 pmol/µl AA standard 10 × 1 ml ampules
5062-2478 Supplemental Amino Acid Kit includes 1 g each norva
line, sarcosine, asparagine, glutamine, tryptophan
and 4-hydroxyproline
Reagents for amino acid analysis
5061-3335 OPA reagent, 10 mg/ml each in 0.4 M borate buffer
o-phthalaldehyde (OPA) and 3-mercaptoproprionic
acid, 6 ampules
5061-3337 FMOC reagent, 2.5 mg/ml in acetonitrile, 9-fluorenyl
methylchloroformate, 1 ml
5061-3339 Borate buffer, 100 ml
5062-2479 DTDPA (dithiodipropionic) reagent for analysis of
cysteine, 5 g
Software
G1300-10013 Software for special method set up and reporting—
software description included as document file
(User contributed software)
5968-5796E Sensitive and reliable amino acid analysis in protein
hydrolysates using the Agilent 1100 Series HPLC
Support
(not included in kit
)
H1170A Application Optimization Service—onsite, including
travel from 1–10 continuous days
Table 6
Ordering information
Page 12

Agilent chemical analysis
professional services for
chromatography
If you do not have the technical
recources available to set up new
applications and methods, you
may choose to use Agilent’s Application Optimization Service
(AOS).
Our application experts are
specialists in implementing and
optimizing applications,
integrating new analytical
technologies into the laboratory,
and complying with global quality
and regulatory standards.
Each application expert has a
minimum of five years practical
laboratory experience and is
factory trained—at an R&D level.
Most have advanced degrees in
science or technology.
With the Agilent Technologies
AOS application experts at your
side, you can:
• accelerate integration of new
analytical technologies into
your laboratory operations to
remain competitive
• decrease research and development cycle time to reduce the
time it takes to get your
products to market
• establish a benchmark for
instrument performance to
ensure confidence in analytical
results.
Additionally, all of our application
experts adhere to ISO-registered
quality processes.
For the latest information and services visit
our world wide web site:
http://www.agilent.com/chem
Agilent Technologies
Innovating the HP Way
Copyright © 1998, 1999 Agilent Technologies
All Rights Reserved. Reproduction, adaptation
or translation without prior written permission
is prohibited, except as allowed under the
copyright laws.
Publication Number 5968-5658E
 Loading...
Loading...