Aesculap Saw GP125 Service manual
Aesculap Power Systems
Service Manual
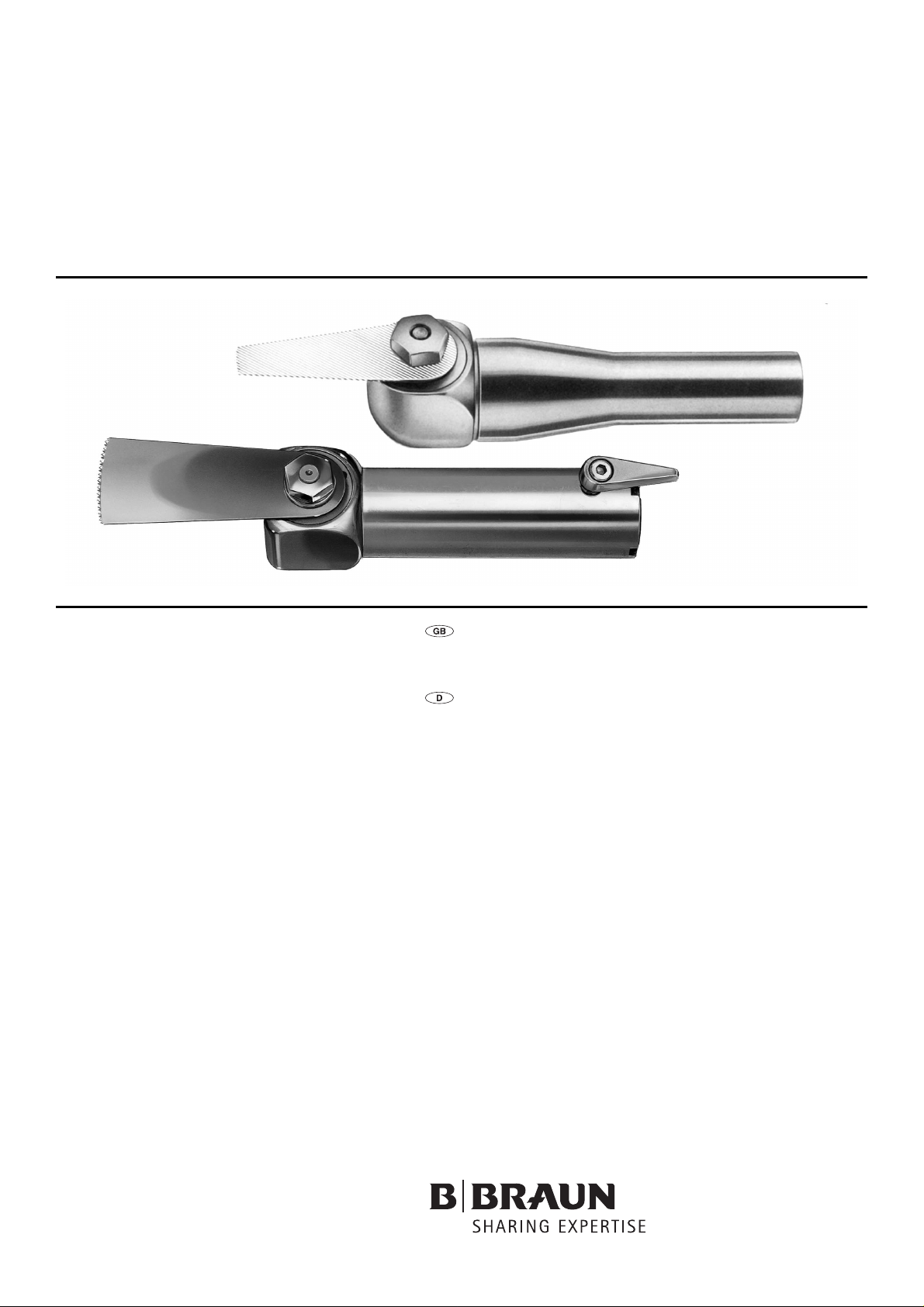
Oscillating depth saw handpiece GB125R/Micro pendulum
saw GB129
Service-Manual
Oszillierendes Tiefensägehandstück GB125R/Mikro-Pendelsäge GB129
TA004110
GB129R
GB125211
GB125213
GB125804
TA005307
TA003907
GB125305
GB125302
GB125304
GB125306
TA003222
GB129301
TA005385
GB129302
TA1003131
TA003909
TA003907
TA003983
TA005387
TA006659
TA003131
GB129200
GB125
GB125211
TA005307
GC650R
GB125804
GB125213
GB115801
TA004110
TA003907
GB125305
GB125304
TA003916
GB125302
TA003131
GB125306
TA003909
TA003904
GB125805
TA004527
GB125307
GB125312
TA004692

Aesculap Power Systems
Oscillating depth saw handpiece GB125R/Micro pendulum saw GB129
Copyright inform ation
Copyright© 2006
Aesculap AG & Co. KG®
All rights reserved 09/ 06
This service information is copyrighted. This service information must not
be copied or reproduced by other means either in whole or in part without
the express permission of Aesculap AG & Co. KG.
This manual is intended for informational purposes only. Ownership of this
manual alone does not constitute or imply authorization to service the
product.
The technical information, illustrations and dimensions contained in this
manual are non-binding. No claims may be made on the basis of the
information contained here in. We reserve the ri ght to make improvements
without altering this documentation. We reserve the right to make
technical changes without prior notice.
Main address for servicing:
Aesculap Technischer Service (ATS)
Am Aesculap-Platz
78532 Tuttlingen / Germany
Phone: +49 7461 95-2700
Fax: +49 7461 16-2887
E-mail: ats@aesculap.de
Other service addresses can be obtained through the address indicated
above.
Manufacturer’s liability
We expressly point out that we can accept responsibility for any effects
on the safety, reliability, and performance of our medical products, if and
only if:
• any assembly, extensions, readjustments, modifications, or repairs are
performed by technically experienced, knowledgeable and trained
personnel and
• the medical products are used as set forth in the instructions for use.
It is possible to learn to service the products through an appropriate
course of instruction given on the premises of Aesculap about the r elevant
medical products. To arrange for such a course, contact Aesculap
Technical Service (ATS).
To ensure that your Aesculap warranty remains valid, we recommend that
only spare parts that have been factory tested by Aesculap be used for
repairs. Spare parts, as well as the relevant tools, can be ordered from
Aesculap Technical Service (ATS). Any unauthorized opening and/or
alterations of the medical product by third parties lead to the exclusion of
our liability, as far as a fault is attributable to such unauthorized opening
and/or alteration of the product. Aesculap cannot accept responsibility for
the use of unsuitable spare parts, tools or devices. After repairs, dropping,
severe damage or misuse, the product should be inspected by a qualified
person.
This manual refers to the product as it was when the manual was
prepared. Technical changes may be made at any time, particularly in
software.
General advisory
This service manual contains illustrations and explanations. Each
explanation covers the following: modes of operation, spare parts list,
assembly, disassembly, functionality test, and the section tools/devices (if
applicable).
For further information about a specific product (e.g., troubleshooting list,
accessories) consult the relevant user instructions.
Literature Art. no.
Instructions for use of GB125 TA005327
Instructions for use of GB129 TA005320
It is crucial that extreme cleanliness be maintained when the products
described below are repaired. All products must undergo a functionality
test after being repaired. If, in the course of a repair, a labeled component
is replaced, the labeling must be transferred to the spare part. Either the
original label should be used or the spare part bearing the relevant label
should be ordered. To this end, be sure to include the applicable
information when ordering.
Unless otherwise indicated, all medical products should be inspected
annually.
General information about motors and handpieces
It is critically important that ball bearings be inspected annually. Even the
slightest defect or soiling can lead to overheating during use, which would
render the product unusable. If for the repair of, e.g., a motor a ball
bearing has to be replaced, it is best in most cases to replace all other ball
bearings as well.
If it is necessa ry to heat up adhes ive joints during disassembly, a hot-air
blower is the appropriate tool. The smallest possible nozzle should be used
so as to avoid damaging other components. When gluing parts together,
make certain that the surfaces to be joined are absolutely clean and fr ee
of grease. For cleaning we recommend Loctite Rapid Cleaner 7063 (to be
ordered under WS. no. 520001750). Only use adhesives listed in this
manual. Follow the glue manufacturer’s recommendations.
Lubricate components with the recommended Aesculap product only.
Following disassembly, clean all components thoroughly and inspect them
to ensure that they are undamaged. Do not install any component of
whose status you are uncertain. Products must always be test-run for
several minutes after being repaired.
2
Contents
1. Safe handling. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
2. Tools, auxiliary materials, supplies. . . . . . . . . . . . . . . . . . . . . . . . . . . 3
2.1 Tools . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
2.2 Auxiliary materials . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
2.3 Supplies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
3. Expendable parts/replacement parts . . . . . . . . . . . . . . . . . . . . . . . . . 4
3.1 GB125R . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
3.2 GB129. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
4. Basic preparations and maintenance. . . . . . . . . . . . . . . . . . . . . . . . . 4
4.1 Preparations. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
4.2 Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
5. Troubleshooting list. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
5.1 Oscillating depth saw handpiece GB125R. . . . . . . . . . . . . . . . . . . . . 5
5.2 Micro pendulum saw GB129 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
6. Disassembling . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
6.1 Disassembling the saw head (GB125R). . . . . . . . . . . . . . . . . . . . . . . 8
6.2 Removing the drive unit from the housing (GB125R) . . . . . . . . . . . 8
6.3 Disassembling the drive unit (GB125R) . . . . . . . . . . . . . . . . . . . . . . 8
6.4 Disassembling the saw head (GB129) . . . . . . . . . . . . . . . . . . . . . . . . 8
6.5 Removing the drive unit from the housing (GB129) . . . . . . . . . . . . 8
6.6 Disassembling the drive unit (GB129) . . . . . . . . . . . . . . . . . . . . . . . 8
7. Assembling. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
7.1 Preliminary work . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
7.2 Assembling the drive unit (GB125R) . . . . . . . . . . . . . . . . . . . . . . . . . 9
7.3 Assembling the housing (GB125R) . . . . . . . . . . . . . . . . . . . . . . . . . . 9
7.4 Assembling the saw head (GB125R) . . . . . . . . . . . . . . . . . . . . . . . . . 9
7.5 Assembling the drive unit (GB129) . . . . . . . . . . . . . . . . . . . . . . . . . . 9
7.6 Installing the drive unit in the housing (GB129) . . . . . . . . . . . . . . . 9
7.7 Assembling the saw head (GB129) . . . . . . . . . . . . . . . . . . . . . . . . . . 9
8. Postmaintenance. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
8.1 Surface inspection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
8.2 Function tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
9. Parts list. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
9.1 GB125R . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
9.2 GB129. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
1. Safe handling
2. Tools, auxiliary materials, supplies
2.1 Tools
Designation Art. no.
Wrench WW3 DIN 911 TA004801
Box wrench, angled, WW14 TA010100
Press-in device, s aw handpiece W-81000180
Press-in punch for axial shaft seal
TA004527
Press-in punch for bearing ring seal
TA004527
Punch W-90000026 no. 72
Auxiliary tool W-90000030 no. 66
Wrench for bearing bush GB125211 W-99010115
2.2 Auxiliary materials
Designation Art. no.
Instructions fo r us e of GB125R TA005327
Instructions for use of GB129 TA005320
2.3 Supplies
Designation WS. no.
Barrierta L25DL 537001568
Special grease MI-setral-FKR 2 537001602
Loctite 275 516001814
Loctite 572 560001817
Loctite 640 560001820
Eloxal Cleaner JG601
W-90000005
W-90000026
! Operate oscillating depth saw handpiece GB125 and micro pendulum
saw GB129 with authentic Aesculap accessories only.
! To avoid damage to the products, do not knock depth saw handpiece
GB125 and micro pendulum saw GB129 against hard object s .
3
Aesculap Power Systems
Oscillating depth saw handpiece GB125R/Micro pendulum saw GB129
3. Expendable parts/Replacement parts
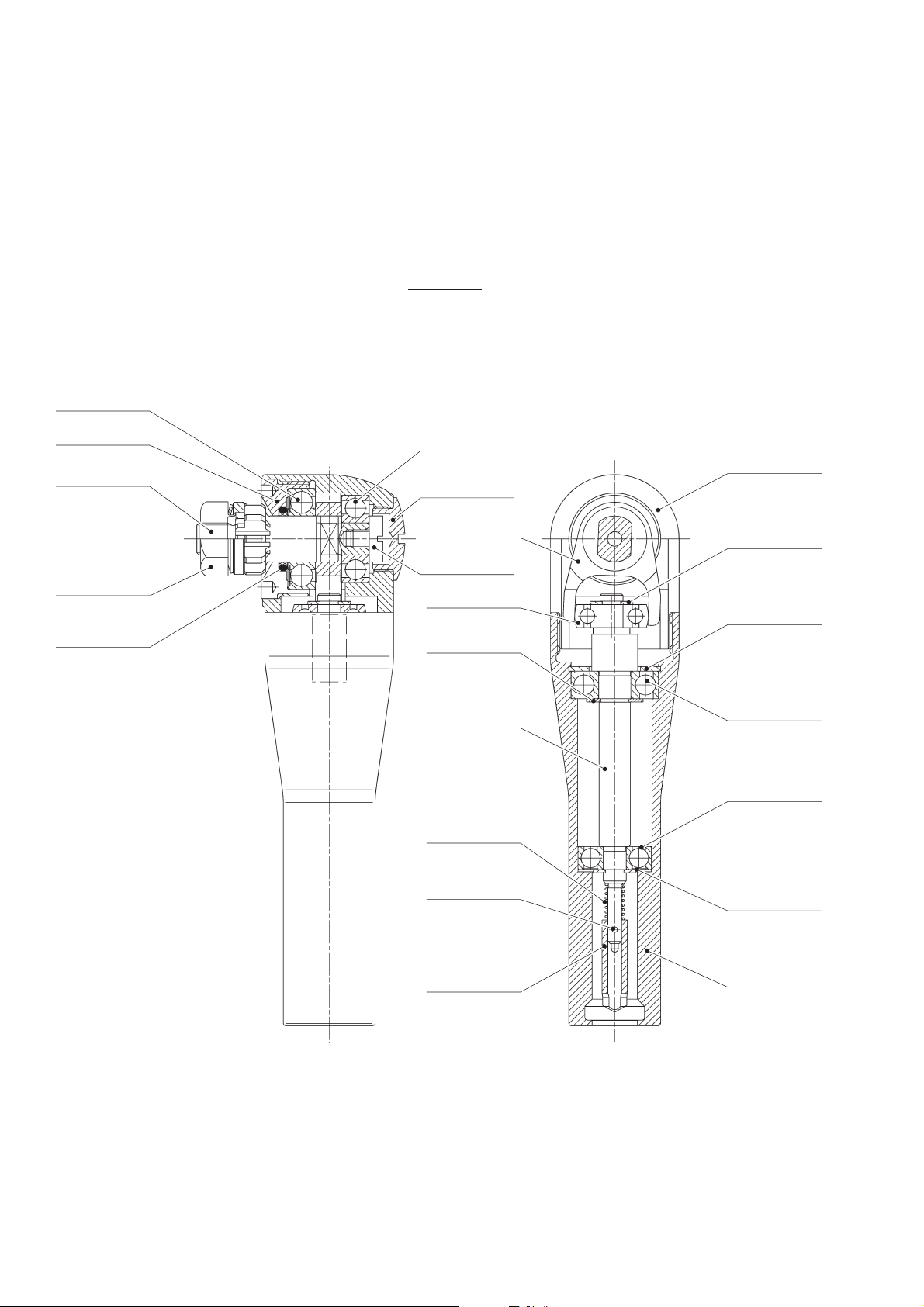
3.1 GB125R
Designation Art. no.
Saw blade axle GB125213
Rocker arm GB125302
Calotte bearing GB125306
Eccentric shaft GB125307
Nut with pressure plate GB125804
Housing, compl ete GB125805
Locking washer TA003131
Simmerring TA003257
Locking washer (x2) TA003904
Radial ball bearing TA003907
Radial ball bearing (x2) TA003916
Radial ball bearing TA004110
Shaft seal, axial TA004527
Bearing ring seal TA005307
4. Basic preparations and maintenance
4.1 Preliminary work
Function tests
! Switch on the oscillating depth saw handpiece GB125R/micro
pendulum saw GB129.
! Make certain that the motor runs smoothly.
! Couple saw blade GC650R.
! Test the power of oscillating depth saw handpiece GB125R/micro
pendulum saw GB129 by sawing into a block of wood.
4.2 Maintenance
In order to ensure reliable operation, Aesculap recommends maintenance
after every 300 reprocessing cycles or at least once a year.
! Lubricate the gears and all ball bearings.
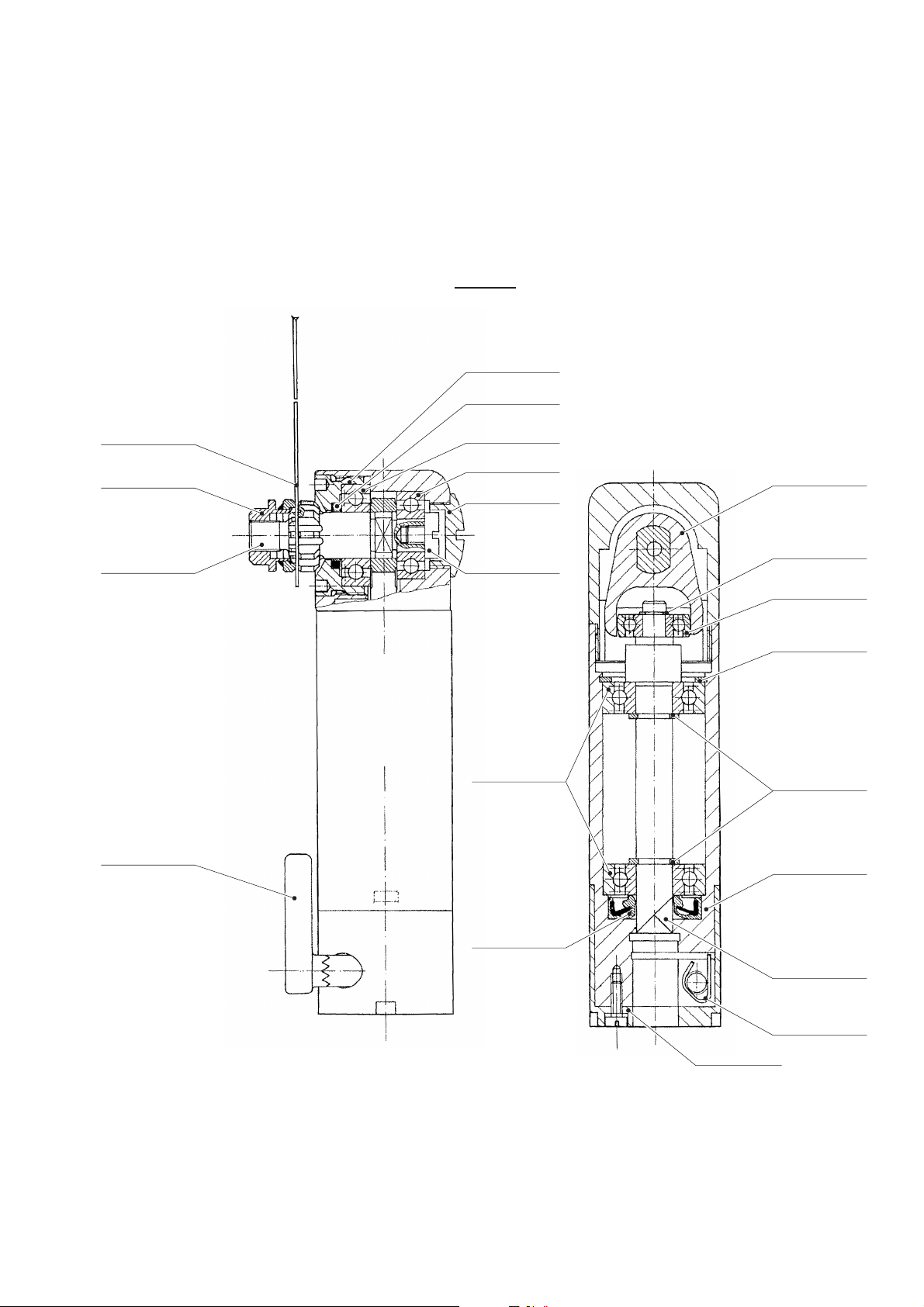
3.2 GB129
Designation Art. no.
Saw blade axle GB125213
Calotte bearing GB125306
Nut with pressure plate GB125804
Radial ball bearing (x2) TA003907
Radial ball bearing TA003983
Radial ball bearing TA004110
Bearing ring seal TA005307
4
5. Troubleshooting list
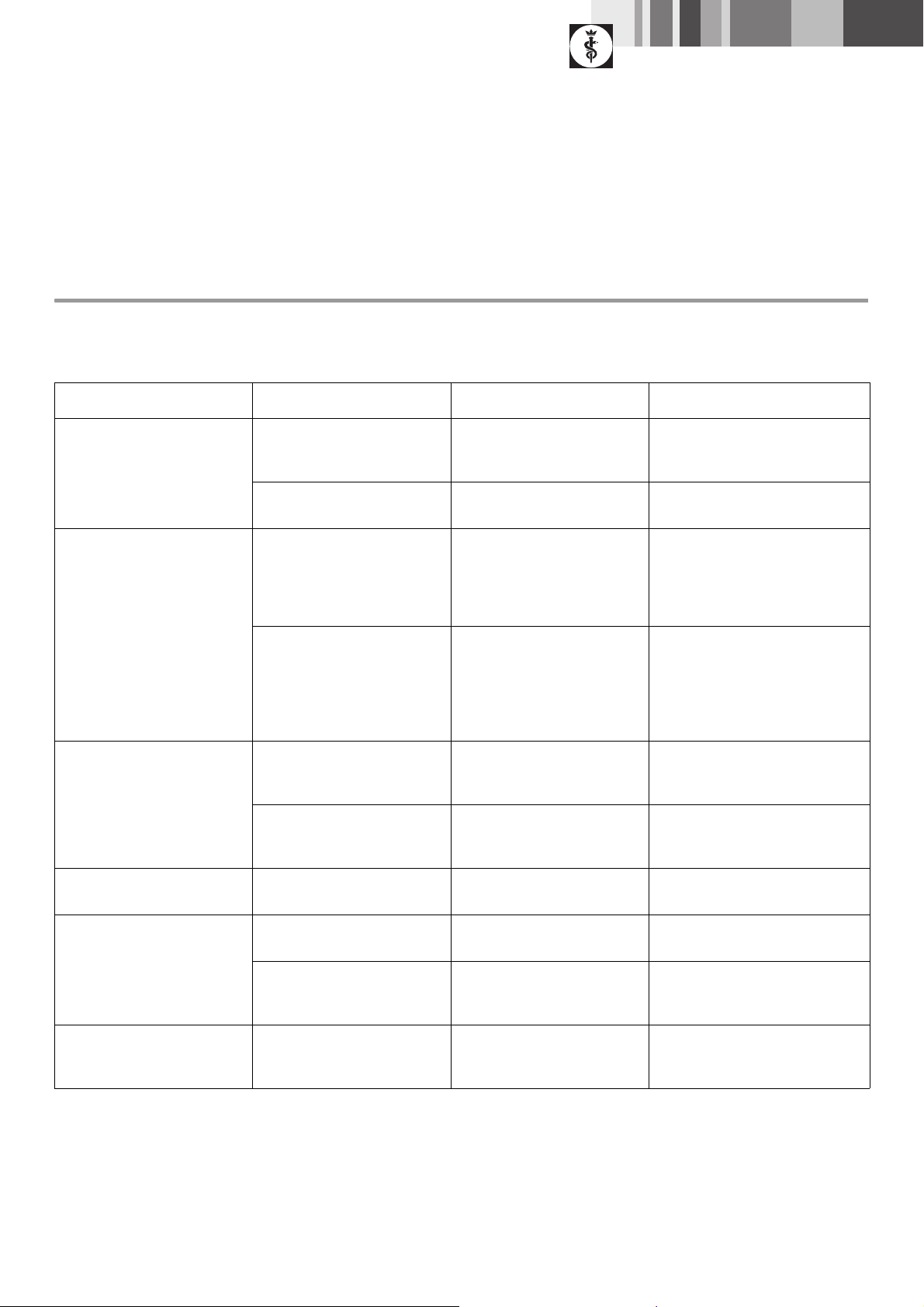
5.1 Oscillating depth saw handpiece GB125R
Malfunction Finding Cause Remedy
Loud running noise Major slack of saw blade axle
GB125213 with the drive shaft
fixated
Major slack of eccentric shaft
GB125307
Excessive heat-up Hot saw blade axle GB125213 Bearing ring seal TA005307
Hot oscillating depth saw
handpiece GB125R
Saw blade fails to move Major slack of saw blade axle
GB125213 with the drive shaft
fixated
Driver pivot broken Drive unit defective Repair drive unit (ELAN-E/EC-
Worn gearing Replace ball bearing TA004110/
Worn or defective bearing Replace bearing of eccentric shaft
overheated
Oscillating depth saw handpiece
GB125R overload ed
Worn saw blade coupling Replace ball bearing TA004110/
rocker arm GB12 530 2
GB125307
Replace bearing ring seal TA005307
Sterilize oscillating depth saw
handpiece GB125R
Allow bearing ring seal TA005307 to
run in
Move oscillating depth saw
handpiece GB125R back and forth
during sawing
Allow oscillating depth saw handpiece
GB125R to cut free
Do not cant the saw blade
rocker arm GB12 530 2
flexible shaft/pneumatic motor
GA200)
Deficient cutting performance Traces of wear on the saw blades Blunt saw blades Insert a new tool
Check instrument sets
Oscillating depth saw handpiece
GB125R cannot be coupled to
drive unit
Oscillating depth saw handpiece
GB125R cannot be fixed on the
drive unit
Dirty or damaged drive pivots/
drive hole of the saw
Bent, damaged or broken
eccentric shaft GB125307
Dirty or damaged drive pivots/
drive hole of the saw
Dirty or damaged drive pivots/
drive hole of the saw
Drive pinion damaged Repair drive unit (ELAN-E/EC-
Worn drive Replace drive unit/housing adapter
Clean or smoothen the surfaces of
the pivots/hole
flexible shaft/pneumatic motor
GA200)
5
Aesculap Power Systems
Oscillating depth saw handpiece GB125R/Micro pendulum saw GB129
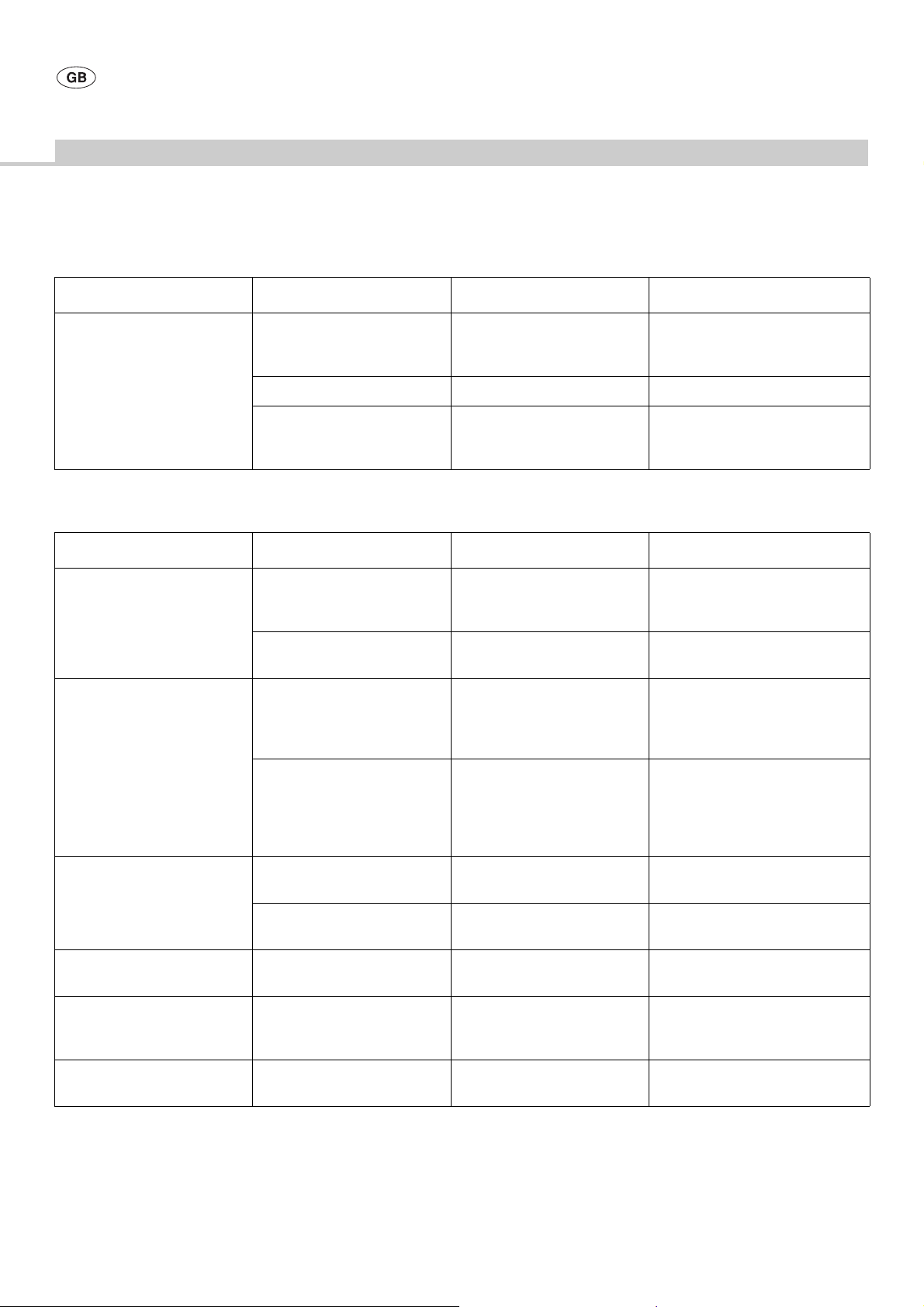
Malfunction Finding Cause Remedy
Tool cannot be coupled Dirty tool/ adapter chuck Dirt Couple a new tool
Take off nut GB125804 and clean
saw blade axle GB125213
Defective or broken driver (star) Deformation Replace saw blade axle GB125213
Wrong tool/
coupling shape mismatch
Wrong tool Check art. no. and compare to the
following information: Catalog no.
O17599, Burrs&Blades
5.2 Micro pendulum saw GB129
Malfunction Finding Cause Remedy
Loud running noise Major slack of saw blade axle
GB125213 with the drive shaft
fixated
Major slack of eccentric shaft
GB125307
Excessive heat-up Hot saw blade axle GB125213 Bearing ring seal TA005307
Hot micro pendulum saw GB129 Micro pendulum saw GB129
Worn gearing Replace ball bearing TA004110/
rocker arm GB12 530 2
Worn or defective bearing Replace bearing of eccentric shaft
GB125301
Replace bearing ring seal TA005307
overheated
overloaded
Sterilize micro pendulum saw GB129
Allow bearing ring seal TA005307 to
run in
Move micro pendu l um saw GB129
back and forth during sawing
Allow micro pe ndulum saw GB12 9 to
cut free
Do not cant the saw blade
Saw blade fails to move Grooved pin TA005387 broken Overloaded/ worn grooved pin Replace grooved pin TA005387/
coupling piece TA006659
Broken drive pin Drive unit defective Repair drive u nit (ELAN-E/EC-flexible
shaft/pneumatic motor GA200)
Deficient cutting performance Traces of wear on the saw blades Blunt saw blades Insert a new tool
Check instrument sets
Micro pendulum saw GB129
cannot be coupled to the drive
unit
Micro pendulum saw GB129
cannot be fixed on the drive unit
6
Dirty or damaged drive pivots/
drive hole of the saw
Locking on the drive pivot
(INTRA) defective
Dirty or damaged drive pivots/
drive hole of the saw
Worn drive Replace locking mechanism
Clean or smoothen the surfaces of
pivots/hole
 Loading...
Loading...