Page 1

P-1000 PENDANT TAG
P-1000 PENDANT TAG
OPERATIONAL DESCRIPTION
Theory of operation
Operational Description 1
Page 2

P-1000 PENDANT TAG
Table of Contents
Overview ................................................... 3
Tag Descriptions ........................................... 4
P-1000 Tag Features ........................................ 4
Beaconing and Bi-directional Communication ............... 4
RF Transmitter operation ................................. 4
Battery .................................................. 5
Small Form Factor with Ergonomic Design .................. 5
Egress Point Detection ................................... 5
Rugged Performance ....................................... 5
Visual Indication ........................................ 5
Tag Management ........................................... 5
Call Button .............................................. 5
Tag Management ............................................. 6
Configuring and Updating Tags ............................ 6
Updating Tag Firmware and Configuration .................. 6
Tag LED Indications ........................................ 6
Tag Maintenance ............................................ 8
Battery Capacity ......................................... 8
Cleaning the Tag ......................................... 8
Specifications ............................................. 8
Tag Specifications ....................................... 8
Safety, Warnings and Warranty ............................. 10
Operational Description 2
Page 3
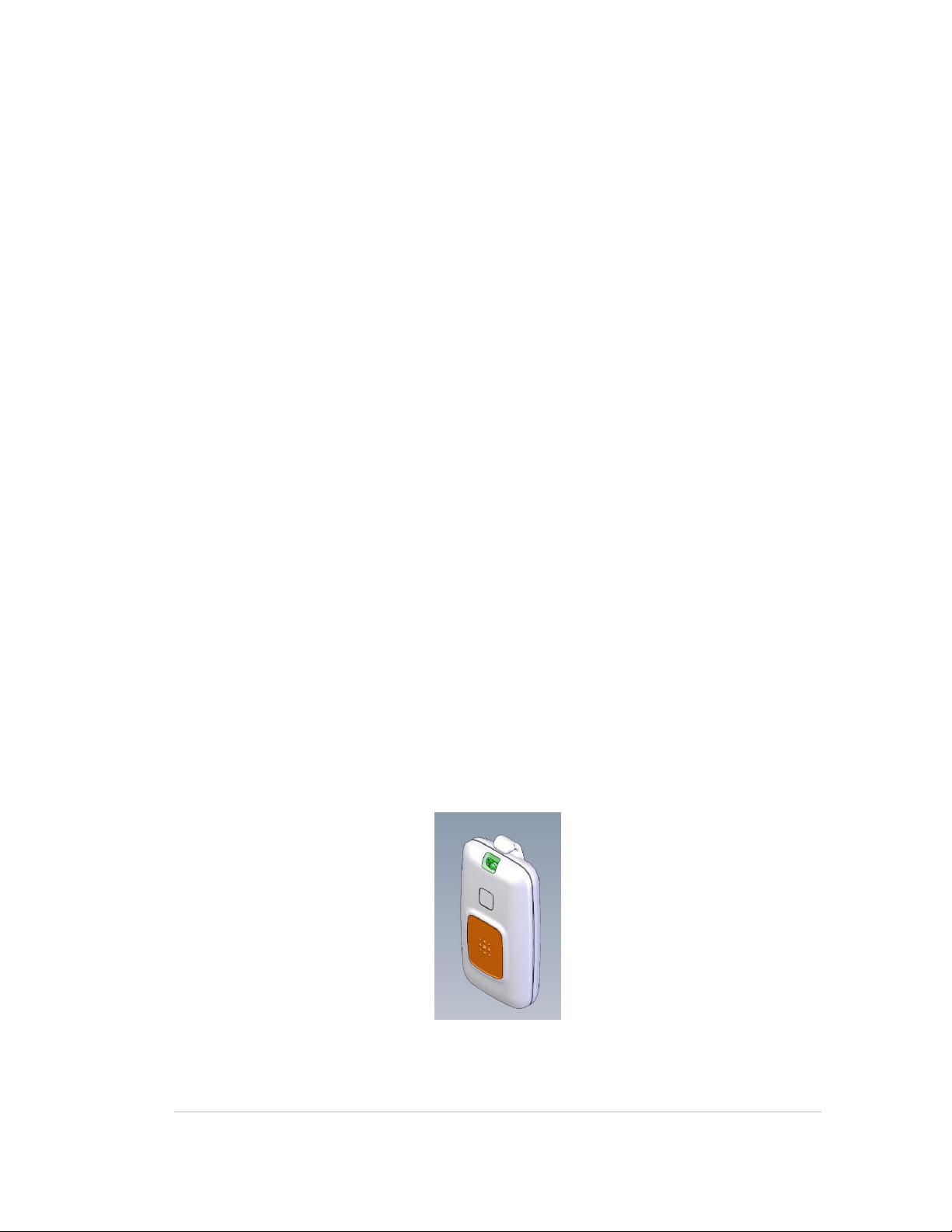
P-1000 PENDANT TAG
Overview
STANLEY Healthcare’s Patient Safety Monitoring solution is a Class 1 FDA medical
device registered, HIPAA-compliant, cloud-based solution that safely and effectively
monitors a group of patients at all times while allowing healthcare professionals to
perform their daily duties. Rely on us to:
Reduce the risk of hospital-acquired pressure ulcers by knowing when to turn
patients
Effectively monitor patients at risk for patient falls, reducing need for patient
sitters
Obtain reliable information to determine individual care plans
Provide patient care with dignity with undetectable technology
Eliminate patient discomfort with a completely touch-free solution
The STANLEY Healthcare P-1000 Bi-directional Tag is a
component of the enterprise-level visibility solution based on
standard Wi-Fi communication for location-based applications.
The P-1000 Tag adds further flexibility and scalability to
locate patients and staff across a wide variety of
applications.
Once deployed, the Tag uses Wi-Fi bi-directional communication
to receive firmware and configuration updates from MobileView.
This removes the need to manually collect, update and redeploy tags in the field.
P-1000 Tags optionally come with a Call Button and an embedded
Low frequency (125KHz) Receiver.
Figure 1: P-1000 Pendant Tag
User Guide 3
Page 4

P-1000 PENDANT TAG
Front View
Back View
LED Indicator
Buzzer
Call Button
Rear panel
with label
Tag Descriptions
The following describes the parts of the P-1000 Tag:
Figure 2: P-1000 Tag Descriptions
P-1000 Tag Features
Beaconing and Bi-directional Communication
The P-1000 Pendant Tag utilizes lightweight beaconing
communication (for standard messages) and bi-directional Wi-Fi
communication with full network association and authentication
(for advanced applications). This unique combination provides
a flexible and scalable solution for advanced applications.
The Tag can operate with up to four different network SSIDs in
a secure or non-secure mode and is able to store up to two
network IPs. The Tag also supports both static IP
configuration and DHCP.
RF Transmitter operation
In normal (regular) mode the Tag sends Wi-Fi beacon –
short message includes Tag unique ID. The RF transmission
duration is about 60 usec. Beaconing interval varies from
up to 5 minutes and depends of following events
processing- Motion, Call button pressing or LF receiver
activation. The AeroScout Location Engine receives
information from Wi-Fi-based Active RFID Tags and
standard Wi-Fi networking devices, and applies multiple
complex algorithms to produce highly accurate and
reliable location and status data in both indoor and
outdoor environments.
User Guide 4
Page 5

P-1000 PENDANT TAG
Battery
The P-1000 Tag has replaceable battery that provides up to one
year battery life*. The Tag is able to report its battery
level which is displayed in MobileView. The P-1000 Tag also
has a motion sensor to conserve battery life when the Tag is
not in motion
*Battery life depends on Tag configuration.
Small Form Factor with Ergonomic Design
The P-1000 Tag’s small and ergonomic design provides ease of
use for patient.
Egress Point Detection
When combined with STANLEY Healthcare Exciters, the P-1000 Tag
provides instant notification when a tagged patient passes
through an egress point, such as a gate, doorway or other
tightly defined area. Additionally the Tags behavior can be
automatically modified while passing through an egress point,
such as activating / deactivating the Tag or changes
transmission rates to accommodate different usage scenarios.
Rugged Performance
The P-1000 Tag enclosure is designed for durability against
significant impacts and is water and dust resistant*.
*See product specifications for IP rating.
Visual Indication
P-1000 Tags include a dual-color LED which enables distinct
visual indications for specific use cases.
Tag Management
P-1000 Tags are easily configured and activated wirelessly via
the Tag Manager BD application and a Tag Activator device. The
Tags can then be reprogrammed using bi-directional
communication via MobileView.
Call Button
The Tags includes a call button that can trigger an event in
MobileView. MobileView events can be defined according to
specific button press patterns, such as long press, short
press and double press.
User Guide 5
Page 6

P-1000 PENDANT TAG
Action
LED Indication
Button Presses
Short Press
1 short green blink ( - )
Long Press
1 long green blink (—)
Double Press
2 short green blinks ( -
- )
Tag Management
The P-1000 Bi-directional Tag firmware contains pre-defined
sets of parameters. Each parameter set is called a static
configuration and has a unique number. Static configurations
cannot be edited. Only one (1) configuration can be selected
and used for the Tag.
Configuring and Updating Tags
P-1000 Tags must be initially configured using the TMBD
application (Tag Manager Bi-Directional application) before
use. The TMBD application allows you to activate and configure
the Tags according to your sites infrastructure.
Once the Tags have been initially configured using the TMBD
application, future configurations can then be done via
MobileView.
For more details on configuring and updating the Tags, see the
Tag Manager BD User Guide.
Updating Tag Firmware and Configuration
MobileView supports the option to update the P-1000 Tag
configuration and firmware over the air (wirelessly). A group
of Tags or Tags associated to a specific category can be
configured simultaneously.
For more details see the Tag Manager BD User Guide.
Tag LED Indications
P-1000 Tags include a dual-color LED (red and green) for
visual indications. These indications are described in the
table below.
The following symbols are used:
Long blink
Short blink
Quick flash
User Guide 6
Page 7

P-1000 PENDANT TAG
Action
LED Indication
Low Battery
Alternating red and green
short blinks every minute.
( -- -- --
)
Message received
from LF or
Ultrasound Exciter
1 quick green flash ( . )
Message
transmission
(periodic or
triggered)
1 quick red flash ( . )
Bi-directional
session
Start
1 short red blink ( - )
End
1 Short red blink followed
by 2 quick red flashes.
( -.. )
User Guide 7
Page 8

P-1000 PENDANT TAG
WARNING: This device contains a lithium battery. Do
not force open, heat to 212°F (100°C), or dispose of
in fire.
Tag Maintenance
Battery Capacity
The Tag’s battery capacity is 1200mamp/h.The reduction in
capacity is much slower when the battery is partly discharged.
When the operating time of a charged battery is significantly
reduced, the Tag should be disposed of according to facility
procedures in your jurisdiction.
Cleaning the Tag
Cleaning the external surface of the Tag’s housing should be
done using Alcohol or Chloride based wipers only and dried
completely.
Specifications
Tag Specifications
Range
Outdoor range: Up to 200m (650 feet)
Indoor range: Up to 80m (260 feet)
Physical and Mechanical
Dimensions: 2.5 x 1.4 x 0.6 inch (65x 36 X 15 mm)
Weight: 20g (0.7oz)
Radio
802.11 radio (2.4 GHz); b/g/n compliant
Low frequency receiver for chokepoint detection (125kHz)
Transmission power: up to +16.2dBm (~41mW)
Patented clear channel sensing avoids interference with
wireless networks
User Guide 8
Page 9

P-1000 PENDANT TAG
Security protocol: WPA2 security with AES encryption
Environmental Specifications
Temperature: 0°C to 50°C (32°F to 122°F)
Humidity: 0% to 95% RH non-condensing
Atmospheric Pressure: 80kPa to 120kPa
Ingress Protection Rating: IP-67
3V Lithium Manganese Dioxide 1.2Ah
Battery life: up to one year, depending on Tag type and
configuration.
Programmability
Tag configurations
Transmission channels
IP Settings
Certification
Radio:
FCC Part 15, sub-part C class B, sub-part B
EN 300-328, EN 300-330, EN 301-489, RSS 210 (Canada)
Safety:
CE, cTUVus (EN60950)
EN 60601-1-Rev3
User Guide 9
Page 10

P-1000 PENDANT TAG
WARNING: This device complies with Part 15 of the FCC Rules and RSS-210 of Industry and
Science Canada. Operation is subject to the following two conditions: (1) This device may not cause
harmful interference, and (2) this device must accept any interference received, including
interference that may cause undesired operation.
This device complies with Industry Canada license-exempt RSS standard(s). Operation is subject to
the following two conditions: (1) this device may not cause interference, and (2) this device must
accept any interference, including interference that may cause undesired operation of the device.
Le présent appareil est conforme aux CNR d'Industrie Canada applicables aux appareils radio
exempts de licence. L'exploitation est autorisée aux deux conditions suivantes : (1) l'appareil ne
doit pas produire de brouillage, et (2) l'utilisateur de l'appareil doit accepter tout brouillage
radioélectrique subi, même si le brouillage est susceptible d'en compromettre le fonctionnement.
Safety, Warnings and Warranty
FCC STATEMENT
Operation is subject to the following two conditions:
a) This device may not cause harmful interference
b) This device must accept any interference received, including interference
that may cause undesired operation.
FCC Warning
Modifications not expressly approved by the manufacturer could void the user
authority to operate the equipment under FCC Rules.
User Guide 10
Page 11

P-1000 PENDANT TAG
STANLEY Healthcare (“STANLEY”) Standard Warranty and Disclaimer
For STANLEY Healthcare AeroScout® Products (“Products”)
Limited Warranty and Disclaimer. STANLEY warrants that commencing from the date of
delivery to Customer and continuing for a period of one (1) year thereafter (the “Warranty Period”), the
hardware components of STANLEY Healthcare AeroScout® Products (the “Hardware”) will be free from
defects in material and workmanship under normal use subject to the terms hereof. The date of
shipment of the Hardware by STANLEY is set forth on the packaging material in which the Hardware is
shipped. This limited warranty extends only to the original user of the Hardware. Customer's sole and
exclusive remedy and the entire liability of STANLEY and its suppliers under this limited warranty will
be, at STANLEY’s or its service center's option, shipment of replacement Hardware components within
the Warranty Period or a refund of the purchase price if the Hardware is returned to the party supplying
it to Customer, if different than STANLEY, freight and insurance prepaid. STANLEY replacement parts
used in Hardware repair may be new or equivalent to new, and STANLEY reserves the right to provide
replacement Hardware components of similar form and function, as long as the functionality is equal or
better than Customer’s original Hardware components. STANLEY’s obligations hereunder are
conditioned upon the return of affected Hardware in accordance with STANLEY’s then-current Return
Material Authorization (RMA) procedures. Notwithstanding the foregoing, the warranty for TAG
Hardware specifically designated for sterilization via autoclave or other sterilization methods shall have
a warranty period of 350 sterilization cycles from the date of delivery; provided, however, that
sterilization outside of environmental specifications approved in any applicable user documentation
voids all warranties.
Extended Warranty: STANLEY offers an extended warranty, for a fee, on AeroScout products.
Within the one (1) year of the standard warranty, additional warranty of two (2) years may be
purchased. Additional warranty years may only be purchased once within the first one (1) year, or prior
to warranty expiration. A maximum of three (3) total warranty years are available for Hardware.
Exclusions: The warranty set forth above will not apply if the Hardware or the Product (i) has
been altered, except by STANLEY, (ii) has not been installed, operated, repaired, or maintained in
accordance with instructions supplied by STANLEY, (iii) has been subjected to abnormal physical or
electrical stress, misuse, negligence, or accident; or (iv) is provided for beta, evaluation, testing, or
demonstration purposes for which STANLEY does not receive a payment of purchase price or license
fee.
In addition, this warranty shall not cover the following:
Batteries (other than DOA -Dead On Arrival).
Plastics (including defects in appearance, cosmetics, decorative or structural items including
framing and non-operative parts).
Tag Calibration.
Expenses related to removing or reinstalling the Products.
Defects or damage that result from the use of Non-STANLEY certified Products, Accessories,
Software or other peripheral equipment.
Defects or damages resulting from service, testing, adjustment, installation, maintenance,
alteration, or modification in any way by any party other than STANLEY, or its authorized
service partners.
All software contained in or otherwise part of STANLEY Healthcare AeroScout®
Products, which is covered by STANLEY’s separate software warranty contained in the
separate software license agreement with respect to such Products.
User Guide 11
Page 12

P-1000 PENDANT TAG
The warranty set forth above shall not be enlarged and no obligation or liability shall arise out
of STANLEY’s rendering of technical advice, facilities or service in connection with Customer's
purchase of the STANLEY Healthcare AeroScout® Products.
Except for the foregoing warranties, which shall be the exclusive warranties with respect to any
Products, STANLEY MAKES NO WARRANTY OR REPRESENTATION OF ANY KIND, EXPRESS
OR IMPLIED, WRITTEN OR ORAL, REGARDING INFORMATION GIVEN OR THE PRODUCTS OR
SERVICES SUPPLIED AND EXPRESSLY DISCLAIMS ALL EXPRESS AND IMPLIED
WARRANTIES, REPRESENTATIONS AND CONDITIONS, INCLUDING WITHOUT LIMITATION ALL
WARRANTIES AND CONDITIONS OF QUALITY, NON-INFRINGEMENT, MERCHANTABILITY AND
SUITABILITY OR FITNESS FOR A PARTICULAR PURPOSE TO THE EXTENT PERMITTED BY
LAW. STANLEY WILL NOT BE LIABLE FOR CONSEQUENTIAL, INCIDENTAL, INDIRECT OR
PUNITIVE DAMAGES FOR ANY CAUSE OF ACTION, WHETHER IN CONTRACT, TORT OR
OTHERWISE. Consequential, incidental and indirect damages include, but are not limited to,
lost profits, lost revenue and loss of business opportunity, whether or not STANLEY was aware
or should have been aware of the possibility of these damages.
User Guide 12
Page 13

About STANLEY Healthcare
STANLEY Healthcare provides over 5,000 acute care hospitals and 12,000 long-term
care organizations with enterprise solutions that transform safety, security and
operational efficiency. The STANLEY Healthcare solution set enables customers to
achieve organizational excellence and superior care in five critical areas:
Security & Protection, Safety, Environmental Monitoring, Clinical Operations &
Workflow, and Supply Chain & Asset Management. These solutions are complemented
by consulting, training, implementation and integration services. STANLEY
Healthcare is proud to be part of Stanley Black & Decker, Inc. For more
information, visit: www.stanleyhealthcare.com
STANLEY Healthcare
130 Turner Street
Building 3
Waltham, MA 02453
Tel: +1-888-622-6992
North America
E-mail: stanleyhealthcare@sbdinc.com
Asia-Pacific
E-mail: stanleyhealthcareasiapac@sbdinc.com
Europe
E-mail: shs-uk@sbdinc.com
Latin America
E-mail: stanleyhealthcarelatam@sbdinc.com
Middle East
E-mail: stanleyhealthcare-MEA@sbdinc.com
 Loading...
Loading...