
GXA-13 Density Determination Kit
1WMPD4003565A

© 2017 A&D Company, Limited. All rights reserved.
No part of this publication may be reproduced, transmitted, transcribed, or translated
into any language in any form by any means without the written permission of A&D
Company, Limited.
The contents of this manual and the specifications of the instrument covered by this
manual are subject to change for improvement without any notice and obligation on
the part of the manufacturer.

Contents
1. Introduction ............................................................................................................................................ 2
1.1. Precautions ....................................................................................................................................... 2
2. Packing List ........................................................................................................................................... 3
3. Density Measurement Principle ........................................................................................................... 4
3.1. Density .............................................................................................................................................. 4
3.2. Specific Gravity ................................................................................................................................. 4
3.3. Density Measurement Principle ...................................................................................................... 4
3.4. Density of a Solid .............................................................................................................................. 4
3.5. Density of a Liquid ............................................................................................................................ 4
4. Error Factors .......................................................................................................................................... 5
4.1. Buoyancy of Air ................................................................................................................................. 5
4.2. Temperature of a Liquid ................................................................................................................... 5
4.3. Volume of Float ................................................................................................................................. 5
4.4. Influence of Wire ............................................................................................................................... 5
4.5. Surface Tension ................................................................................................................................ 6
4.6. Bubbles ............................................................................................................................................. 6
5. Measuring the Density of a Solid ......................................................................................................... 8
5.1. Assembling the Kit ............................................................................................................................ 8
5.1.1. Assembling the Kit Using Glass Breeze Break (GXA-10) ....................................................... 9
5.2. Measuring the Density of a Solid ................................................................................................... 10
5.2.1. Density of Water ........................................................................................................................ 10
6. Measuring the Density of a Liquid...................................................................................................... 11
6.1. Assembling the Kit .......................................................................................................................... 11
6.2. Measuring the Density of a Liquid ................................................................................................. 11
7. Density Measurement Using the GX-A/GF-A Balance ............................................................... 12
7.1.1. Preparation Before Measurement (Settings of the Function Table) ................................... 12
7.1.2. Measuring Solid Density (Specific Gravity), Function Table ....................................... 14
7.1.3. Specifying Liquid Density ......................................................................................................... 15
7.1.4. Measuring Liquid Density (Specific Gravity), Function Table ...................................... 16
7.1.5. Specifying Volume of Float ....................................................................................................... 17
8. Frequently Asked Questions and Answers ....................................................................................... 18
1

1. Introduction
Thank you for your A&D purchase! This is the instruction manual for the density determination kit.
The density determination kit can be easily used to measure the density of solid or liquid when it is
combined with the following electronic balances.
GX-203A, GX-303A, GX-403A, GX-603A, GX-1003A, GX-1603A,
GF-203A, GF-303A, GF-403A, GF-603A, GF-1003A, GF-1603A
Before using the density determination kit, please read this instruction manual thoroughly.
1.1. Precautions
This density determination kit allows the density or specific gravity of a solid or liquid to be measured.
However, the results of measurements may be affected by various factors that could cause errors,
therefore we cannot guarantee them to be 100 % accurate.
Do not use the density determination kit for measuring the density of chemically active substances.
After using the density determination kit, clean all surfaces to remove rust and oxides.
The balance is precision instrument, so avoid shocks or excessive loads.
To improve measuring accuracy, allow the temperature of liquid (water) and sample (solid) to
equalize to the ambient room temperature and perform measurement in a stable environment.
The breeze break provided with the balance cannot be used with the density determination kit.
To perform an accurate density measurement, use the glass breeze break available as an option
( GXA-10).
2
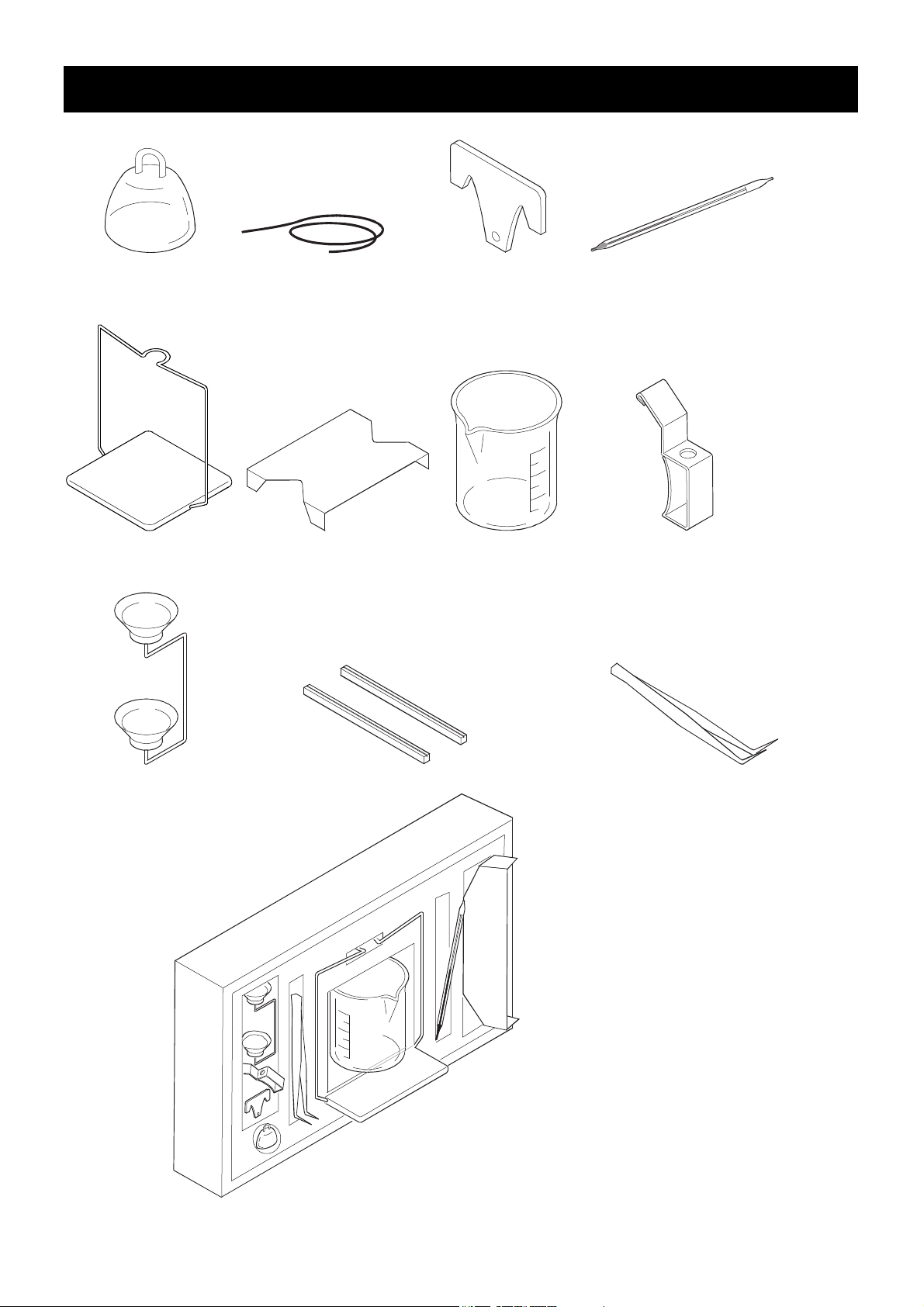
2. Packing List
φ0.2
Float Wire Float hook Thermometer
Density pan stand Beaker stand Beaker Thermometer clamp
Density pan Beaker stand spacer 2 pieces Tweezers
Kit box
3
3. Density Measurement Principle
3.1. Density
Density means the amount of mass of a sample per unit volume. ( Unit [g/cm3] and others )
ρ = ρ: Density [g/cm3] M: Mass [g] V: Volume [cm3]
3.2. Specific Gravity
Specific gravity means the ratio of the density of a sample to the density of pure water (with the
same volume as that of the sample) at 4 °C at 1013.25 hPa.
ρ = ρ: Specific gravity [ No unit ] M: Mass [g] V: Volume [cm3]
ρ4: Density of water at 4 [°C], 0.99997 [g/cm3] 1.000 [g/cm3]
M
V
M
V•ρ4
3.3. Density Measurement Principle
The GXA-13 combined with an authorized balance can measure the density of a sample based on
the Archimedes’ principle.
Archimedes’ Principle of Density Measurement
A body immersed in a liquid (gas) is subject to an upward force equal to the weight of the
liquid (gas) it displaces. The upward force is buoyancy.
3.4. Density of a Solid
The density of a solid can be obtained according to the sample weight in air, sample weight in liquid
and the density of the liquid.
ρ = ×ρ0 ρ: Density of sample [g/cm3] A: Sample weight in air [g]
ρ0: Density of liquid [g/cm3] B: Sample weight in liquid [g]
A
A - B
3.5. Density of a Liquid
The density of a liquid can be obtained according to the weight of the float in air, the weight of the
float in a liquid, and the known volume of the float.
ρ = +d ρ: Density of liquid [g/cm3] A: Weight of the float in air [g]
V: Volume of the float [cm3] B: Weight of the float in liquid [g]
d: Density of air ( approximately 0.001 [g/cm3] )
4
A - B
V

4. Error Factors
There are several error factors that may affect the accuracy of a density measurement.
4.1. Buoyancy of Air
When the liquid density is measured, an upward force, 0.0010 to 0.0014 [g/cm3], is exerted in
proportion to the volume of the liquid. The buoyancy in air per 1 [cm3] is found by:
d = × d: Air density [g/cm3] t: Air temperature [°C]
P: Air pressure [hPa]
When obtaining measurement results down to the third decimal place, 0.001 [g/cm3] is added to
the measured value to compensate for an error in air density.
0.0012932
1+0.0036728×t
P
1013.25
4.2. Temperature of a Liquid
When taking a density measurement using a liquid, the density measurement will change depending on
the temperature. Obtain the density of a liquid by comparing its temperature with the values in "Table 1
Temperature and Density of Water" for distilled water or provided in other reference documents for
other liquids.
4.3. Volume of Float
The tolerance of the measured value of the volume of the float is ±0.01 [cm3].
4.4. Influence of Wire
When a solid sample is placed on the density pan for measurement, which is immersed in a liquid,
the liquid surface position rises. At this time, the wire (1 mm) connecting the upper and lower pans
is subject to the buoyancy whose magnitude is equal to the weight of the raised liquid. 1 mm raise of
the liquid surface exerts a buoyancy of approx. 0.8 mg to the wire. To minimize a measurement error
caused by the buoyancy, adjust the sample size so that it will not raise the liquid surface, or perform
a mathematical correction.
When measuring the density of a liquid, the float-suspending wire (2 mm) immersed in the liquid
affects the measurement. Immersing the wire by 10 mm exerts a buoyancy of approx. 0.3 mg to the
wire. However, this buoyancy can be ignored because it is divided by the volume of the float when
the density of the liquid is obtained.
5
4.5. Surface Tension
When the density of a solid is measured, a force of approx. 5 mg (surface tension) is applied to the
density pan, between the wire (1 mm) of the density pan and the surface of the liquid.
The surface tension can be reduced to approx. 1 mg by adding a surfactant (for example, a water
droplet preventive solution as used for developing photographs). Adding 0.1 mL (1.2 [g/cm3]
density) of a surfactant to 200 mL of water will increase the density of the water by approx. 0.0001
[g/cm3].
When the density of a liquid is measured, a force of about 1 mg is exerted by the wire of 0.2 mm in
diameter. However, this force can be ignored because it is divided by the volume of the float.
4.6. Bubbles
The buoyancy of a bubble of 1 mm in diameter is approx. 0.5 mg. Stickiness of bubbles depends on
the shape and material of a sample. Make measurements considering the characteristic of bubbles.
When the density of a solid is measured, we recommend adding a surfactant to reduce the influence
of surface tension and bubbles.
6
 Loading...
Loading...