Page 1
Symbicort®
000001
Product Manual
4. Battery Check Button
5. Pair LED
6. Pair Button
7. USB Port
Compatible MDI Devices
The SmartTouch is designed to work only with
the MDI and medication indicated on the
device label:
• For use with Symbicort
®
MDIs.
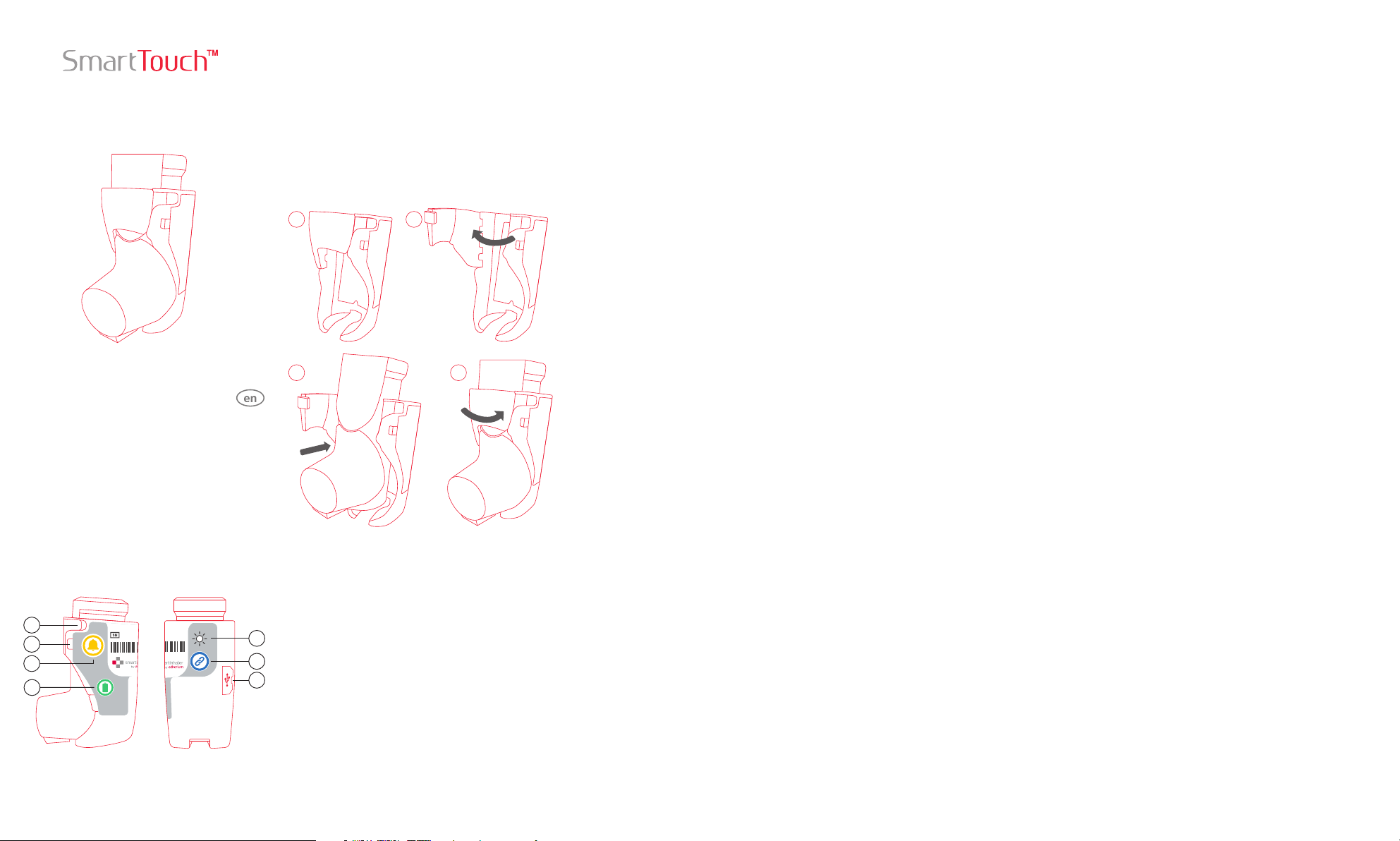
Installing or Removing an MDI
1 2
1) Follow the communications device
instructions to put it in pairing mode.
2) On the SmartTouch press the Pair Button
3 times to enter pairing mode.
The Pair LED will ash blue when the
SmartTouch is in pairing mode.
3) Follow any communications device
prompts to select and accept pairing with
the SmartTouch. The Pair LED will ash
green to indicate pairing is successful.
Note: If pairing is not successful within 60
seconds the Pair LED will ash red. The
pairing process can be repeated if necessary.
Note: The SmartTouch can only be paired with
a single communications device at a time.
Starting the pairing process removes the
current paired device.
cancelled by holding down the Reminder
Button.
Reviewing SmartTouch Usage Data
The MDI usage history can be automatically
uploaded via a compatible paired
communications device such as a
smartphone.
The upload of new information will occur
automatically as long as the SmartTouch is
within range (5 meters or 16 feet) of the
communication device.
Note: Refer to the instruction manual for your
specic communication device for more
information on this process.
• No ash – the battery is at, or the
SmartTouch device has failed, e.g. due to
liquid immersion or mechanical stress.
Cleaning the SmartTouch and MDI
Check the instructions from the MDI
manufacturer for keeping the mouthpiece
clean. Keep the SmartTouch clean and free
of chemicals, steam, water and dust. Clean
the outside plastic enclosure with a lightly
dampened cloth. Leave it to dry in a warm
place that is less than 30°C.
Warning: The SmartTouch is a battery
powered electronic device. Do not immerse
the SmartTouch in water.
Do not use the SmartTouch if it is not in
good condition.
Preparation for First Use
Before rst use remove the Battery Saver
Pull Tab.
If you plan to use Smartinhaler Connection
Center™ to review usage data, please refer
to instructions on SmartinhalerLive.com
before rst use.
SmartTouch Components
1
2
3
4
Key
1. Door Latch
2. Light Emitting Diode (LED)
3. Reminder Button
5
6
7
3 4
Installation
To install the inhaler, open the door of the
SmartTouch by pulling the Door Latch. Push
the MDI into the SmartTouch. Close the door
so it clicks into place.
Removal
To remove the inhaler, pull the Door Latch to
open the door of the SmartTouch. Pull the MDI
to remove it from the SmartTouch. Close the
door so it clicks into place.
Setting Up Bluetooth
Communications
The SmartTouch must be paired with a
compatible Bluetooth communications device
in order to upload stored usage data. To pair
the SmartTouch with a communications
device:
Delivering a Dose of Medication
Caution: This manual does not provide
information on how to use prescription MDI
medication, and is not intended to replace the
advice provided by a healthcare professional.
Directions for using prescription medication
should be obtained from a healthcare
professional and followed accordingly. Any
questions related to prescription medication
should be referred to a healthcare
professional. Please refer to the labeling
provided with the inhaler for instructions on
use.
The SmartTouch LED ashes after detecting a
medication dose has been actuated.
Dose Check
You can check if the prescribed dose has
been taken within the last 6 hours by pressing
the Reminder Button once. If the LED ashes
green the prescribed dosage has not been
taken, if the LED ashes red no doses are
overdue.
Reminders
Audio-visual reminders can be set up from a
Smartinhaler app or via Smartinhaler
Connection Center.
If enabled, the medication reminders are
generated with increasing frequency for up to
24 minutes until either the prescribed
medication dose is taken or the Reminder is
Manually Uploading Stored
Information
If required to ensure all current data has been
transferred, the SmartTouch can manually
upload information to a paired Bluetooth
communications device. To manually upload
stored information from the SmartTouch:
1) Hold down the Pair Button for 3 seconds
until the Pair LED shows white, then
release.
2) The Pair LED will ash white while the
SmartTouch attempts to upload, then
ash green if the upload is successful.
Note: The SmartTouch Pair LED will ash red if
the upload is not successful. Check the
SmartTouch is within range of the paired
communications device, and the
communications device has Bluetooth®
communications enabled.
Reviewing the Battery Level
The LED on the main face indicates the
battery level of the SmartTouch after
medication is actuated, or when the Battery
Check Button is pressed:
• Green – the battery level is good, and the
SmartTouch is monitoring actuations.
• Orange – the battery level is getting low.
The SmartTouch is still monitoring
actuations.
• Red – the battery is at. The SmartTouch
has stopped monitoring actuations.
Storing the SmartTouch
To help maintain battery capacity, store your
SmartTouch below 30°C. Keep out of direct
sunlight and avoid extreme temperatures.
Troubleshooting
If the SmartTouch is not responding to
Bluetooth communications, check the
SmartTouch battery level as per Reviewing
the Battery Level in this manual.
Other wireless communications equipment
such as wireless home network devices,
mobile phones, cordless telephones and
their base stations, and walkie-talkies can
affect the SmartTouch. Increase the
separation distance between the
SmartTouch and any such devices if this
could be causing problems.
If this does not help, contact the supplier or
manufacturer for further assistance.
Servicing
Please contact the supplier or manufacturer
for device servicing. Do not attempt to open
or service the SmartTouch. Tampering with
the device voids the warranty.
Disposal
Dispose or recycle the SmartTouch in
accordance with regulations for your
country, as applicable for electronic devices
containing a lithium polymer battery.
Page 2
EU only: Do not dispose of the SmartTouch
as unsorted municipal waste.
The SmartTouch must be recycled in
accordance with Directives 2012/19/EU and
2006/66/EC. To arrange for return or disposal
of the SmartTouch contact your local
supplier.
Warnings, Cautions, and Notes
Warnings identify actions or situations that
could lead to personal injury. Take note of all
warnings before using the SmartTouch.
Cautions identify actions or situations that
could damage the SmartTouch, or other
equipment, or affect the accuracy or
availability of compliance data.
Notes contain advisory information about
some aspect of the SmartTouch or its use.
Terms and Abbreviations
MDI Metered Dose Inhaler
SmartTouch Intended Use
The SmartTouch is intended for
single-patient use as a medication reminder
and electronic data capture accessory for
recording actuations of prescribed MDI
usage. This may be used in the following
applications:
• In clinical trials, where researchers need to
know when a patient has actuated their
trial MDI medication.
• In clinical practice, where specialists,
general practitioners, nurses, and
educators need to know if a patient has
actuated their prescribed MDI medication.
• In self-management, where patients need
to track their medication use as part of
their management plan.
The SmartTouch is not intended to indicate
remaining quantity of medication in an MDI
and does not include a dose counting
function.
How the SmartTouch Works
The SmartTouch is a small battery- powered
electronic data logger that attaches to an
MDI. The SmartTouch contains an electronic
clock and calendar that is used to log the
date and time of MDI actuation.
The SmartTouch registers MDI actuations.
Usage data can be uploaded via a wireless
Bluetooth connection.
Safety and Usage Information
Warning: To ensure your inhaler functions
correctly, do not use the SmartTouch with any
other MDI or medication than that indicated
on the device label.
Warning: The SmartTouch does not contain a
dose counter. Do not use data collected by
the SmartTouch to determine the number of
doses remaining in a medication canister.
Caution: For hygiene and data integrity
reasons, do not use the SmartTouch with
more than one patient.
Caution: Take care to not spill liquids on the
SmartTouch or immerse it in water.
Note: The SmartTouch does not detect or
record inhalation by the user. The SmartTouch
does not record the quantity of medication
delivered by the MDI.
Symbols
Manufacturer: Adherium (NZ) Ltd.
Serial Number
Year of Manufacture
Keep Dry
Non-Rechargeable Device
EU only: Do not dispose of the
SmartTouch as unsorted municipal
waste
US only: Caution - Federal law restricts
this device to sale by or on the order
of a physician
Warranty
The SmartTouch includes a 12 month
warranty against manufacturing defects from
date of rst use. This warranty may be voided
under the following circumstances: damage to
the SmartTouch including dropping, water
damage resulting from condensation or
immersion, tampering, attempts to service, or
other forms of abuse.
The SmartTouch warranty expires 5 years
from date of manufacture.
Specications
Weight
Size
Actuation Log
Precision
Actuation Log
Capacity
Internal Clock
Accuracy
Compatible
With
Wireless
Technology
Battery Type
Shelf Life
Service Life
Battery Life
Operating
Temperature
Storage
Temperature
Operating /
Storage
Humidity
24 grams, excluding MDI
41 x 36 x 66mm (L x W x H)
excluding MDI
1 second
6144 actuations and device
status records
± 1 hour after 12 months
Note: the SmartTouch clock
is updated every time data is
uploaded
iOS 7.1 and above, running
on:
• iPhone 4 and later
• iPad 3 and later
• iPad mini 1st Gen and
later
• iPod touch 5th Gen and
later
Android v4.4 and above
Bluetooth 4.0: 2.40-2.48 GHz,
2.5 mW Low Energy
Lithium Coin Cell,
Non-rechargeable
3 years
1 year
12 months, depending on
usage
0 to 40ºC (32 to 104ºF)
-20 to 60ºC (-4 to 140ºF)
15 to 95% RH
(non-condensing)
FCC Statement
This device complies with Part 15 of the FCC
Rules. Operation is subject to the following
two conditions:
1) This device may not cause harmful
interference, and
2) This device must accept any interference
received, including interference that may
cause undesired operation.
Note: The Grantee is not responsible for any
changes or modications not expressly
approved by the party responsible for
compliance. Such modications could void
the user’s authority to operate the equipment.
IC Statement
This device complies with Industry
Canada’s licence-exempt RSSs.
Operation is subject to the following two
conditions:
1) This device may not cause interference;
and
2) This device must accept any interference,
including interference that may cause
undesired operation of the device.
Le présent appareil est conforme aux CNR
d'Industrie Canada applicables aux appareils
radio exempts de licence. L'exploitation est
autorisée aux deux conditions suivantes :
1) l'appareil ne doit pas produire de brouillage,
et
2) l'utilisateur de l'appareil doit accepter tout
brouillage radioélectrique subi, même si le
brouillage est susceptible d'en
compromettre le fonctionnement.
RF Exposure Statement
This equipment complies with the RSS-102
radiation exposure limits set forth for an
uncontrolled environment.
Declaration of Conformity
Adherium (NZ) Ltd declares that this
SmartTouch is in compliance with the
essential requirements and other relevant
provisions of Directives 1999/5/EC and
93/42/EEC.
Email contact@smartinhaler.com for a copy of
the declaration of conformity.
No part of this document may be reproduced
or transmitted in any form or by any means,
electronic, mechanical, photocopying,
recording, or otherwise, without the prior
written permission of Adherium (NZ) Ltd.
Product specications may change without
notice.
Product covered by a NZ Design Application
No. 421072.
Patent pending.
Wellkang Ltd
29 Harley Street, Suite B
London W1G 9QR
United Kingdom
Australian Sponsor
Market Access Australia Pty Ltd
810 Pacic Highway
Gordon NSW 2072
Australia
SmartTouch™ Symbicort is manufactured
by:
Adherium (NZ) Ltd
Level 2, 204 Quay Street
Auckland 1010
New Zealand
Phone : +64 9 307 2771
contact@smartinhaler.com
www.smartinhaler.com
SmartTouch™ Symbicort
Manual
Part Number ND0274
Version 2
Issue Date 2 March 2016
Symbicort® is a registered trade mark of
AstraZeneca.
All product names and brand names in this
document are trademarks or registered
trademarks of their respective holders.
© Copyright Adherium Ltd 2016. All rights
reserved.
®
Product
 Loading...
Loading...