Page 1
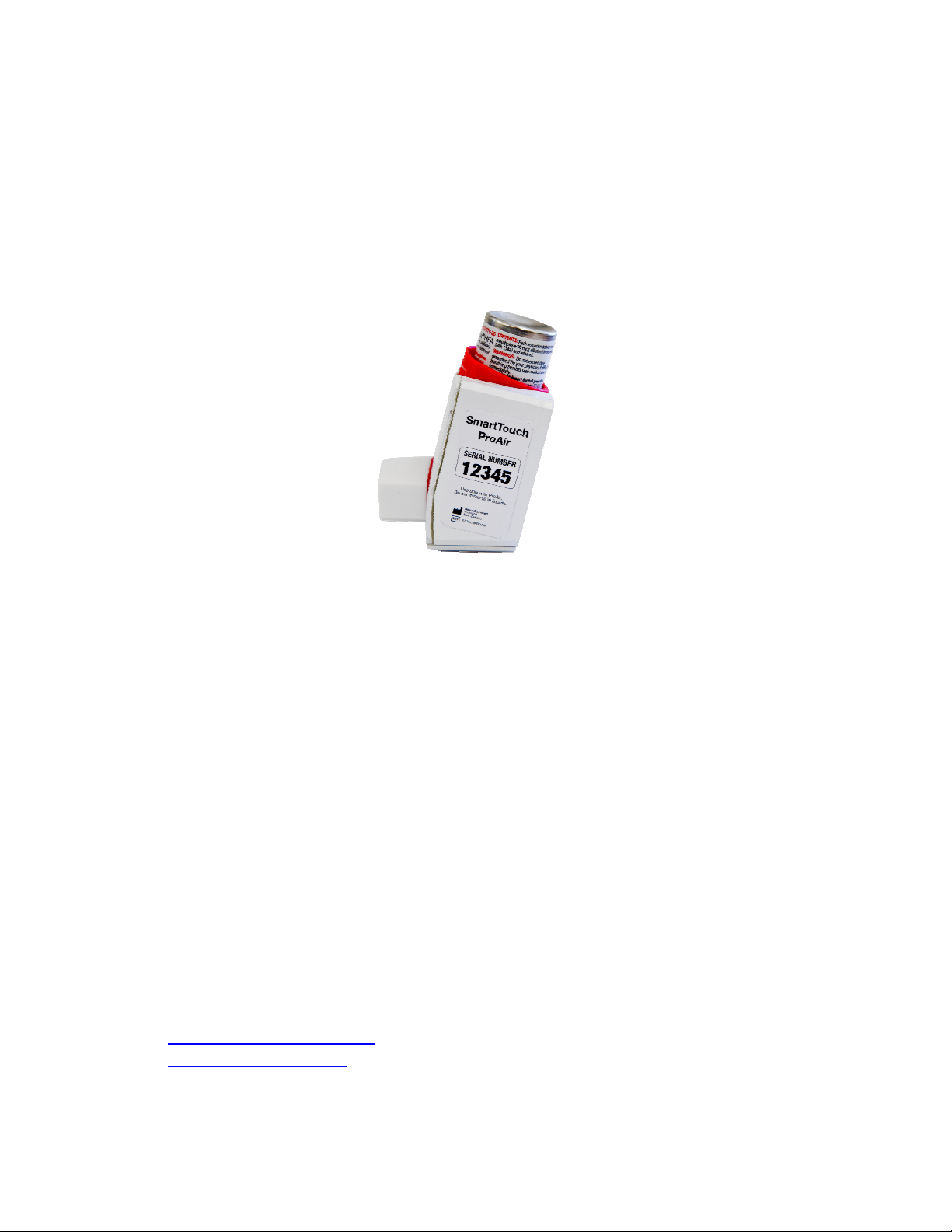
SmartTouch
All product names and brand names in this document are
. All rights reserved.
No part of this document may be reproduced
electronic, mechanical, photocopying, recording, or otherwise, without the prior written
Product specifications may change without notice.
is manufactured by:
contact@smartinhaler.com
Product Manual
NL0034 SmartTouch ProAir Product Manual (US Letter)
Copyright © 2012 Nexus6 Ltd. All rights reserved
trademarks or registered trademarks
or transmitted in any form or by any means,
- v5.1.docx
ProAir Product Manual
of their respective holders.
© Copyright Nexus6 Ltd 2012
permission of Nexus6 Ltd.
SmartTouch ProAir
Nexus6 Ltd
8 Commerce Street, Suite 205
Auckland 1010
New Zealand
Phone +64 9 307 2771
Fax +64 9 307 2773
Email
Web www.smartinhaler.com
SmartTouch ProAir
Page 1
Page 2

NL0034 SmartTouch ProAir Product Manual (US Letter) - v5.1.docx
pMDI
Pressurized Metered Dose Inhaler
Serial number
EU only:
D
o not dispose of the
US only: Caution
- Federal law restricts
Part Number NL0034
Revision 5.1
Issue Date 23 October 2012
Product Version STOUCHPROAIR
1 Who this Product Manual is intended for
This Product Manual is intended for users of the SmartTouch ProAir device, namely clinical trials
investigators, healthcare professionals, patients with respiratory conditions, and patient
caregivers.
2 Warnings, Cautions, and Notes
Warnings identify actions or situations that could lead to personal injury. Take note of all
warnings before using the SmartTouch ProAir.
Cautions identify actions or situations that could damage the SmartTouch ProAir, or other
equipment, or affect the accuracy or availability of compliance data.
Notes contain advisory information about some aspect of the SmartTouch ProAir or its use.
3 Terms and Abbreviations
4 Symbols
Consult instructions for use
Manufacturer
SmartTouch ProAir or used batteries
as unsorted municipal waste.
this device to sale by or on the order
of a physician.
Page 2 Copyright © 2012 Nexus6 Ltd. All rights reserved
Page 3

Intended Use
is intended for
actuation
In clinical trials, where researchers need to know when a patient has actuated their trial
practice, where specialists, general practitioners, and nurse educators need to
know if a patient has actuated their prescribed pMDI medication.
In self management, where patients need to track their medication
ProAir
is a small battery powered
internal
clock and calendar that is used to log the date and time
registers pMDI actuations.
Components
is designed
NL0034 SmartTouch ProAir Product Manual (US Letter)
Copyright © 2012 Nexus6 Ltd. All rights reserved
lectronic
d
The SmartTouch
contains an internal electronic
The SmartTouch
Bluetooth
Light Emitting Diode (LED)
Upload Button
- v5.1.docx
5 SmartTouch ProAir
The SmartTouch ProAir device
accessory for recording
applications:
•
pMDI medication;
• In clinical
•
management plan.
6 How the SmartTouch
The SmartTouch ProAir
ProAir is powered by an
ProAir
connection.
single-patient use as an e
s of prescribed pMDI usage. This may be use
use
Works
electronic data logger.
non-rechargeable battery, and
of pMDI actuation.
Usage data can be uploaded via a wireless
data capture
in the following
as part of their
7 SmartTouch ProAir
8 Compatible pMDI Devices
The SmartTouch ProAir
Main face
to work with ProAir pMDI.
Page 3
Page 4

NL0034 SmartTouch ProAir Product Manual (US Letter) - v5.1.docx
9 Safety and Usage Information
Warning: The SmartTouch ProAir does not contain a dose counter. Do not use information
collected by the SmartTouch ProAir to determine the number of doses remaining in a
medication canister.
Caution: For hygiene and data integrity reasons, do not use the SmartTouch ProAir with more
than one patient.
Caution: Take care to not spill liquids on the SmartTouch ProAir or immerse it in water.
Note: The SmartTouch ProAir does not detect or record patient inhalation within the pMDI it
is attached to. The SmartTouch ProAir does not record the quantity of medication
delivered by the pMDI.
10 Installing or Removing a pMDI
Installation
To install the SmartTouch ProAir, align the pMDI with the back of the SmartTouch
ProAir, and push the pMDI firmly into the SmartTouch, ensuring the mouth piece of the
pMDI goes through the front window on the SmartTouch.
Removal
While holding each side of the SmartTouch ProAir, gently push the mouthpiece of the
pMDI to remove the pMDI from the SmartTouch ProAir.
Page 4 Copyright © 2012 Nexus6 Ltd. All rights reserved
Page 5

NL0034 SmartTouch ProAir Product Manual (US Letter) - v5.1.docx
11 Delivering a Dose of Medication
Caution: This manual does not provide information on how to use prescription pMDI
medication. This manual is not intended to replace the advice provided by a
healthcare professional. Directions for using prescription medication should be
obtained from a healthcare professional and followed accordingly. Any questions
related to prescription medication should be referred to a healthcare professional.
Please refer to the labeling provided with the ProAir inhaler for instructions.
12 Uploading SmartTouch ProAir data
Data logged in the SmartTouch ProAir can be uploaded to the Smartinhaler Connection Center
Desktop software for review. Data is transferred to the Connection Center Desktop software
via Bluetooth wireless communication.
The Smartinhaler Connection Center Desktop software will need to be already installed onto
the computer before data can be reviewed. The software can be downloaded from the
SmartinhalerLive website (www.smartinhalerlive.com). Please follow the instructions on the
SmartinhalerLive website to download and install the Smartinhaler Connection Center Desktop
software.
12.1 Upload via Bluetooth
To upload data stored on the SmartTouch ProAir using Bluetooth communications, firstly click
the Upload button on the Connection Center software. Press the Upload button located on the
center of the SmartTouch ProAir main face (refer to image in Section 7). The button needs to
be held down for 2 seconds before data communications commences. The SmartTouch ProAir
searches for a Bluetooth device that it can communicate with, for example, Nexus6’s SmartKey.
While communicating, the LED on the top right corner of the main flashes blue. If the LED does
not flash after pressing the Upload button, either:
• the battery is flat, or
• the SmartTouch ProAir device has failed, e.g. due to liquid immersion or mechanical
stress.
Caution: Do not initiate wireless Bluetooth communications when travelling on an aircraft.
13 Reviewing SmartTouch ProAir Usage Data on Smartinhaler Connection Center Desktop
Page 5 Copyright © 2012 Nexus6 Ltd. All rights reserved
Page 6

NL0034 SmartTouch ProAir Product Manual (US Letter) - v5.1.docx
At a convenient time, the pMDI usage history can be uploaded to the Smartinhaler Connection
Center Desktop software for review. This can be done as often as required. However, it is
recommended that uploads are performed at least every 6 weeks. The SmartTouch ProAir can
store up to one year of medication usage data in its memory.
14 Reviewing the battery level of your SmartTouch ProAir
The SmartTouch ProAir contains an internal non-rechargeable battery. The LED on the top right
corner of the main face of the device allows you to review the battery level of the SmartTouch
ProAir. The LED on the SmartTouch ProAir flashes one of the following three colours when you
actuate the SmartTouch:
• Green – Indicates that the battery level is good, and that the monitoring of actuations is
being performed by the SmartTouch ProAir.
• Orange – Indicates that the battery level is getting low. The SmartTouch ProAir is still
monitoring actuations.
Note: Do not deploy the SmartTouch ProAir to a new patient if the LED has switched to
orange.
• Red – Indicates that the battery is flat. The SmartTouch ProAir has stopped monitoring
actuations.
Note: Do not deploy the SmartTouch ProAir to a new patient.
• No flash – the battery is flat, or, the SmartTouch ProAir device has failed, e.g. due to
liquid immersion or mechanical stress.
The SmartTouch ProAir has a shelf life of 3 years from its Use By date and an active service life
of 1 year (at an average daily use of two puffs twice daily).
15 Cleaning the SmartTouch ProAir and pMDI
Keep the SmartTouch ProAir clean and free of chemicals, steam, water and dust. Clean the
SmartTouch ProAir by wiping the outside plastic enclosure with a lightly dampened cloth.
Leave it to dry in a warm place that is less than 30°C.
Warning: The SmartTouch ProAir is a battery powered electronic device. Do not submerge
the SmartTouch ProAir in water. Do not use the SmartTouch ProAir if it is not in good
condition.
16 Storing the SmartTouch ProAir
Store your SmartTouch ProAir below 30°C. Keep out of direct sunlight and avoid extreme
temperatures. Keep the SmartTouch ProAir out of reach of children.
Page 6 Copyright © 2012 Nexus6 Ltd. All rights reserved
Page 7

NL0034 SmartTouch ProAir Product Manual (US Letter) - v5.1.docx
17 Trouble Shooting
If the SmartTouch ProAir is not responding to communications via Bluetooth, check the
SmartTouch ProAir battery level as per section 14 of this manual. If this does not help, contact
the supplier or manufacturer for further assistance.
18 Servicing
Please do not attempt to open or service the SmartTouch ProAir. Tampering with the device
voids the warranty.
19 Disposal
Dispose or recycle the SmartTouch ProAir in accordance with regulations for your country.
EU only: Do not dispose of the SmartTouch ProAir as unsorted municipal waste at the end of
the product’s lifetime. The SmartTouch ProAir must be recycled in accordance with the WEEE
Directive 2002/96/EC. To arrange for return or disposal of the SmartTouch ProAir contact your
local supplier.
EU only: Do not dispose of waste batteries as unsorted municipal waste. Device batteries must
be recycled in accordance with the Battery Directive 2006/66/EC. Return waste batteries to an
appropriate local collection point or contact your local supplier for disposal information.
Page 7 Copyright © 2012 Nexus6 Ltd. All rights reserved
Page 8

NL0034 SmartTouch ProAir Product Manual (US Letter) - v5.1.docx
Weight
20 grams including battery, excluding
Size 50mm High × 38mm Wide x 35mm
Actuation Log
1 second
Actuation Log
4096 actuations and device status
Internal
Clock
± 20 minutes
after
12 months
Bluetooth
Bluetooth Low Energy
(BLE)
v4.0
Battery Type
Lithium
Battery Life
3 year shelf life and 1 year service life
Operating
0 to 40 °C (32 to 104 °F)
Operating
25 to 90% at 40 °C (non
-
condensing)
Storage
-
20 to 60 °C (
-
4 to 140 °F)
Storage
25 to 95% at 40 °C (non
-
condensing)
20 Warranty
The SmartTouch ProAir includes a 24 month warranty against manufacturing defects. This
warranty may be voided under the following circumstances: damage to the SmartTouch
including dropping, water damage resulting from condensation or immersion, tampering,
attempts to service, or other forms of abuse.
21 Specifications
pMDI
Long excluding the pMDI
resolution
22 FCC Statement:
THIS DEVICE COMPLIES WITH PART 15 OF THE FCC RULES. OPERATION
IS SUBJECT TO THE FOLLOWING TWO CONDITIONS: (1) THIS DEVICE MAY NOT CAUSE
HARMFUL INTERFERENCE, AND (2) THIS DEVICE MUST
ACCEPT ANY INTERFERENCE RECEIVED, INCLUDING INTERFERENCE THAT MAY CAUSE
UNDESIRED OPERATION.
NOTE: THE GRANTEE IS NOT RESPONSIBLE FOR ANY CHANGES OR MODIFICATIONS NOT
EXPRESSLY APPROVED BY THE PARTY RESPONSIBLE FOR COMPLIANCE. SUCH
MODIFICATIONS COULD VOID THE USER’S AUTHORITY TO OPERATE THE EQUIPMENT.
Capacity
Accuracy
Temperature
Humidity
Temperature
Humidity
records
Note: the SmartTouch ProAir clock is
updated every time data is uploaded
to Nexus6 software.
Page 8 Copyright © 2012 Nexus6 Ltd. All rights reserved
 Loading...
Loading...